Open Access Article
Open Access ArticleLithium-ion batteries with fluorinated mesogen-based liquid-crystalline electrolytes: molecular design towards enhancing oxidation stability†
Shingo
Takegawa
 a,
Kazuma
Hamaguchi
a,
Eiji
Hosono
a,
Kazuma
Hamaguchi
a,
Eiji
Hosono
 b,
Shunsuke
Sato
c,
Go
Watanabe
b,
Shunsuke
Sato
c,
Go
Watanabe
 cde,
Junya
Uchida
cde,
Junya
Uchida
 a and
Takashi
Kato
a and
Takashi
Kato
 *af
*af
aDepartment of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan. E-mail: kato@chiral.t.u-tokyo.ac.jp
bGlobal Zero Emission Research Center, National Institute of Advanced Industrial Science and Technology (AIST), 16-1 Onogawa, Tsukuba, Ibaraki 305-8569, Japan
cDepartment of Physics, School of Science, Kitasato University, 1-15-1 Kitazato, Minami-ku, Sagamihara, Kanagawa 252-0373, Japan
dDepartment of Data Science, School of Frontier Engineering, Kitasato University, 1-15-1 Kitazato, Minami-ku, Sagamihara, Kanagawa 252-0373, Japan
eKanagawa Institute of Industrial Science and Technology (KISTEC), 705-1 Shimoimaizumi, Ebina, Kanagawa 242-0435, Japan
fInstitute for Aqua Regeneration, Shinshu University, 4-17-1 Wakasato, Nagano 380-8553, Japan
First published on 14th October 2024
Abstract
Two-dimensional (2D) nanostructured liquid crystals containing fluorinated cyclohexylphenyl and cyclic carbonate moieties have been developed as quasi-solid-state self-organized electrolytes for safe lithium-ion batteries. We have designed lithium ion-conductive liquid-crystalline (LC) materials with fluorine substituents on mesogens for improved oxidation stability. Computational studies suggest that the fluorination of mesogens lowers the highest occupied molecular orbital (HOMO) level of LC molecules and improves their oxidation resistance as electrolytes. The LC molecule complexed with lithium bis(trifluoromethanesulfonyl)imide exhibits smectic A LC phases with 2D ion transport pathways over wide temperature ranges. Cyclic voltammetry measurements of the fluorinated mesogen-based LC electrolytes indicate that they are electrochemically stable above 4.0 V vs. Li/Li+. Lithium half-cells composed of fluorinated LC electrolytes show higher discharge capacity and coulombic efficiency than those containing non-fluorinated analogous LC molecules. Combining molecular dynamics simulations with the experimental results, it is revealed that the fluorination of the mesogen effectively enhances the electrochemical stability of the LC electrolytes without significantly disrupting ionic conductivities and the LC order.
Introduction
Nanostructured liquid-crystalline (LC) materials1–10 have attracted considerable attention as transport materials for ions,1–3,5,6,11–26 electrons,1,2,6–8,27–31 and molecules1,4,9,32–39 because they form well-organized molecular-based nanostructures for potential applications in energy, environment, resources, and separation materials. LC molecules having block structures consisting of incompatible moieties form nanosegregated structures. The transport pathways formed by nanosegregation are expected to enable the efficient transport of ions, electrons, or molecules even in quasi-solid states. The dynamic and soft properties of LC materials also contribute to switching,1–4,6,10,23,37 flexibility,1–10,16,21 processability,1,2,8,27,31 or interfacial compatibility.2,3,8,21The aim of this study is to develop nanostructured LC electrolytes for lithium-ion batteries (Fig. 1). We successfully prepared lithium-ion batteries with an LC electrolyte for the first time using a smectic rod-like LC molecule complexed with a lithium salt.11 This LC electrolyte showed a smectic phase to construct two-dimensional (2D) ion-conduction pathways. Ionic conductivities in the order of 10−6–10−4 S cm−1 were achieved with these LC electrolytes in the quasi-solid states. These LC molecules need to be designed to meet the requirements for lithium-ion battery operations including electrochemical stability.
 | ||
| Fig. 1 Schematic illustration of the lithium-ion battery containing the LC electrolytes in the ideal state. | ||
Conventional electrolytes for lithium-ion batteries consist of organic low-molecular-weight carbonates which have inherent safety issues, such as leakage, volatility, and flammability.40–42 To ensure the safety of lithium-ion batteries, various electrolytes, such as solid inorganic electrolytes,43–46 solid polymer electrolytes,43,47–52 gel polymer electrolytes,53 or ionic liquid electrolytes,54 have been studied. Solid inorganic electrolytes, represented by oxides, have ordered structures and exhibit high ionic conductivities; however, they experience high resistance at grain boundaries or instability with lithium metal anodes.43,44 Polymer electrolytes are promising candidates owing to their lightness, processability, and flexibility; however, the ionic conductivities in the amorphous region are relatively lower.43,47,48 Nanostructured ion-conductive liquid crystals1–3,5,6,11–23 also show potential as quasi-solid electrolytes because they provide dynamic ordered structures, which may contribute to safety, good compatibility with electrodes, and efficient ion transport through self-assembly pathways. Furthermore, it was reported that ordered LC structures could effectively suppress the growth of lithium dendrites which cause capacity fading and short circuits, hindering the practical application of lithium metal anodes.18–21
In the present study, we focus on the fluorination of aromatic mesogens as a new approach for the enhancement of the oxidation resistance of LC electrolytes. The electrochemical stability of electrolytes is an important factor determining the efficiency and cyclability of batteries.55–57 Fluorine atoms are characterized by small volume, high electronegativity, low polarizability, and the formation of stable C–F bonds. Associated with the nature of fluorine atoms, fluorinated organic electrolytes49–52,58–65 possess superior electrochemical and physicochemical properties, such as higher oxidation stability,49–52,58–65 nonflammability,58,59,63–65 and low-temperature performance.58,59,64 Conversely, the physicochemical and LC properties of a variety of fluorinated liquid crystals14,17,66–72 have been reported. Fluorinated liquid crystals have often been studied for display applications that modulate their physicochemical properties.66–71
Herein, we report the effects of fluorination of LC molecules on the electrochemical and self-assembly properties of compound 1. The performance of lithium-ion batteries based on the LC electrolytes of 1 and lithium salts using half-cells comprising LiFePO4, Li metal, and LC electrolytes is described.
Results and discussion
Compound 1 has a fluorinated rod-like mesogen and a cyclic carbonate group at the extremities of the alkyl spacer (Fig. 1). Compound 1 is a fluorinated analogue of compound 2. Compound 2 complexed with a lithium salt was previously used as an LC electrolyte for lithium-ion battery studies.11 However, the cells with compound 2 based LC electrolytes were reported to deteriorate gradually in repeated charge–discharge reactions due to the insufficient oxidation resistance of compound 2.11,12 Fluorine substituents were introduced to the lateral part of the mesogen to enhance the oxidation resistance. We expected that the electron-withdrawing fluorine atoms would stabilize the highest occupied molecular orbital (HOMO), which is localized at the cyclohexylphenyl moiety. The density functional theory (DFT) calculations (B3LYP/6-31G(d), Spartan'18) confirmed that compound 1 has a HOMO level of −6.07 eV, which is lower than that of compound 2 (Fig. 2). The stabilization of the HOMO was envisioned to result in the enhanced oxidation resistance of the LC electrolytes and stable charge–discharge reactions. From a view of self-assembling properties, complexes of compound 1 and lithium salts were expected to form 2D ion-conduction pathways where the lithium ions should interact with the carbonate moieties,11,12,15,17 such as compound 2. Due to the wider 2D pathways of the smectic phases, the conduction pathways would be partially connected, even in polydomain states.11–13,38 The laterally fluorinated mesogens may suppress excessive intermolecular packing that decreases conductivity and compatibility with electrodes.66,69,73Compound 1 was synthesized from commercially available 1-ethoxy-2,3-difluoro-4-(trans-4-pentylcyclohexyl)benzene as the starting material (Scheme S1†). Compound 1 was characterized by 1H and 13C nuclear magnetic resonance spectroscopy, matrix-assisted laser desorption ionization time-of-flight mass spectrometry, and elemental analysis (Fig. S1 and S2†).
The LC properties of compound 1 were studied by differential scanning calorimetry (DSC), polarized optical microscopy (POM), and X-ray diffraction (XRD) measurements (Table 1, Fig. S3–S5†). Compound 1 showed an enantiotropic smectic A (SmA) phase with an isotropization temperature of 65 °C. No LC phases other than the SmA phase were observed for compound 1 when cooled to −20 °C. Compound 1 did not exhibit a smectic B (SmB) phase, while compound 2 exhibited an SmB phase as well as an SmA phase.11 The fluorine atoms of compound 1 may disturb the ordered packing of mesogens in the layer plane.66,69,73 The effects of the lateral fluorine substituents on liquid crystallinity will be discussed later in combination with molecular dynamics simulations.
Complexes of 1/lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) were prepared at molar ratios of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 80
10, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 70
20, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, and 60
30, and 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40. The interactions between 1 and LiTFSI were examined using Fourier transform infrared (FT-IR) spectroscopy (Fig. 3 and S6†). Compound 1 showed a characteristic stretching vibrational band due to the C
40. The interactions between 1 and LiTFSI were examined using Fourier transform infrared (FT-IR) spectroscopy (Fig. 3 and S6†). Compound 1 showed a characteristic stretching vibrational band due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in the cyclic carbonate moiety at 1796 cm−1 at 30 °C. Upon addition of LiTFSI, the band of the C
O group in the cyclic carbonate moiety at 1796 cm−1 at 30 °C. Upon addition of LiTFSI, the band of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of the cyclic carbonate coordinated to lithium ions was observed at 1777 cm−1. These observations supported the hypothesis that 1 and LiTFSI formed complexes. These band shifts derived from the interactions between lithium ions and the C
O stretching vibration of the cyclic carbonate coordinated to lithium ions was observed at 1777 cm−1. These observations supported the hypothesis that 1 and LiTFSI formed complexes. These band shifts derived from the interactions between lithium ions and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group have also been reported for complexes 2/LiTFSI.15
O group have also been reported for complexes 2/LiTFSI.15
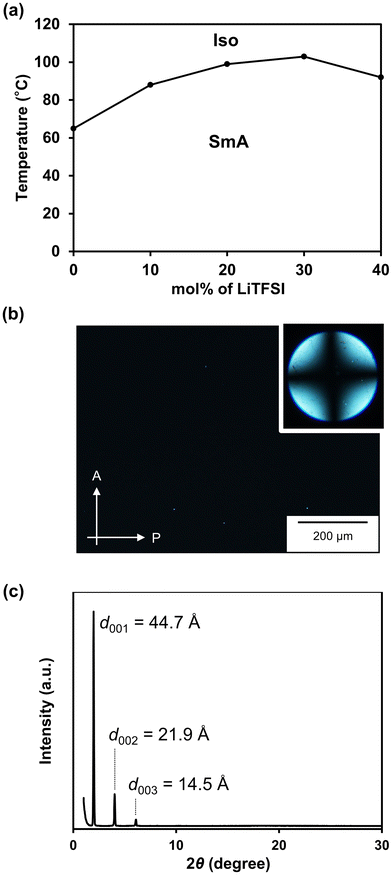
Fig. 4a shows a phase diagram of 1/LiTFSI complexes (Fig. S7 and S8†). The 1/LiTFSI complex at a molar ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 showed a homeotropic alignment in the SmA phase, which was confirmed by the dark POM image and the cross pattern of the conoscopic image (Fig. 4b). The XRD pattern of the 1/LiTFSI complex at a molar ratio of 80
20 showed a homeotropic alignment in the SmA phase, which was confirmed by the dark POM image and the cross pattern of the conoscopic image (Fig. 4b). The XRD pattern of the 1/LiTFSI complex at a molar ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 at 60 °C shows the formation of a layered structure with a d-spacing of approximately 44 Å (Fig. 4c). A diffusion halo is observed in the wide-angle region. Considering the molecular length of compound 1 (23 Å) obtained by structural optimization using DFT, these results suggest the formation of the SmA phase with a bilayer structure (Fig. 5). The isotropization temperatures of 1/LiTFSI complexes were higher than that of compound 1 (Fig. 4a). The nanosegregated layered assemblies in the SmA phases were stabilized by ion–dipole interactions between the polar carbonate moieties of 1 and the lithium ions, as observed from the FT-IR measurements. The isotropization temperature of 1/LiTFSI at a molar ratio of 60
20 at 60 °C shows the formation of a layered structure with a d-spacing of approximately 44 Å (Fig. 4c). A diffusion halo is observed in the wide-angle region. Considering the molecular length of compound 1 (23 Å) obtained by structural optimization using DFT, these results suggest the formation of the SmA phase with a bilayer structure (Fig. 5). The isotropization temperatures of 1/LiTFSI complexes were higher than that of compound 1 (Fig. 4a). The nanosegregated layered assemblies in the SmA phases were stabilized by ion–dipole interactions between the polar carbonate moieties of 1 and the lithium ions, as observed from the FT-IR measurements. The isotropization temperature of 1/LiTFSI at a molar ratio of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 was lower than that at 70
40 was lower than that at 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, resulting from the destabilization of the layered structures by the bulky TFSI anions.
30, resulting from the destabilization of the layered structures by the bulky TFSI anions.
 | ||
Fig. 5 Schematic illustration of the assembled structure of 1/LiTFSI at a molar ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 in the SmA phase. 20 in the SmA phase. | ||
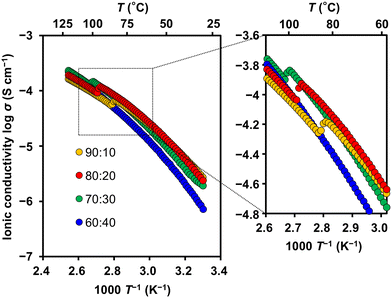
The ionic conductivities of 1/LiTFSI complexes were measured by an alternative current impedance method using comb-shaped gold electrodes deposited on a glass substrate (Fig. 6 and S9†). LC electrolytes 1/LiTFSI exhibited ionic conductivities of 10−6–10−4 S cm−1 in the SmA phases. A sudden increase in ionic conductivity was observed in isotropic (Iso)–SmA phase transitions upon cooling of 1/LiTFSI complexes at molar ratios of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 80
10, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, and 70
20, and 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (Fig. 6). These observations and the XRD results (Fig. 4c) suggest that the formation of aligned 2D pathways leads to more efficient ion transport between the electrodes (Fig. 5). Higher ionic conductivities were obtained for 1/LiTFSI at molar ratios of 90
30 (Fig. 6). These observations and the XRD results (Fig. 4c) suggest that the formation of aligned 2D pathways leads to more efficient ion transport between the electrodes (Fig. 5). Higher ionic conductivities were obtained for 1/LiTFSI at molar ratios of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 80
10 and 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 than at other ratios. In our previous study,12 compound 3 was designed to improve the oxidation resistance of compound 2 (Fig. 7). The cyclohexylphenyl mesogen of compound 2 was changed to the bicyclohexyl mesogen of compound 3. Flexibility was increased by introducing an oligo(ethylene oxide) spacer. The phase transition temperatures of 1/LiTFSI and the LC electrolytes based on 2
20 than at other ratios. In our previous study,12 compound 3 was designed to improve the oxidation resistance of compound 2 (Fig. 7). The cyclohexylphenyl mesogen of compound 2 was changed to the bicyclohexyl mesogen of compound 3. Flexibility was increased by introducing an oligo(ethylene oxide) spacer. The phase transition temperatures of 1/LiTFSI and the LC electrolytes based on 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11,15 and 3
11,15 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 used for previous lithium-ion battery studies are listed in Table 2. The conductivities of 1/LiTFSI were compared with those reported in previous studies (Fig. 8).11,12 The conductivities of 1/LiTFSI at a molar ratio of 80
12 used for previous lithium-ion battery studies are listed in Table 2. The conductivities of 1/LiTFSI were compared with those reported in previous studies (Fig. 8).11,12 The conductivities of 1/LiTFSI at a molar ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 were comparable to those of 2/LiTFSI11 and 3/LiTFSI,12 suggesting the applicability of 1/LiTFSI to the electrolytes of lithium-ion batteries. The fluorination of the mesogen did not decrease the ionic conductivities. Comparing ionic conductivities at 60 °C, where lithium-ion batteries with LC electrolytes were operated in our previous report, 1/LiTFSI at a molar ratio of 80
20 were comparable to those of 2/LiTFSI11 and 3/LiTFSI,12 suggesting the applicability of 1/LiTFSI to the electrolytes of lithium-ion batteries. The fluorination of the mesogen did not decrease the ionic conductivities. Comparing ionic conductivities at 60 °C, where lithium-ion batteries with LC electrolytes were operated in our previous report, 1/LiTFSI at a molar ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 showed the highest values among 1/LiTFSI mixtures. Therefore, 1/LiTFSI at a molar ratio of 80
20 showed the highest values among 1/LiTFSI mixtures. Therefore, 1/LiTFSI at a molar ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 was employed as the LC lithium-ion electrolyte for the following electrochemical measurements.
20 was employed as the LC lithium-ion electrolyte for the following electrochemical measurements.
 | ||
| Fig. 7 Molecular structure, HOMO structure, and the HOMO energy level (DFT, B3LYP/6-31G(d), Spartan'18) of compound 3 bearing a bicyclohexyl mesogen. | ||
 | ||
Fig. 8 Comparison of the ionic conductivities of 1/LiTFSI at an 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 molar ratio (red circle), 2/LiTFSI at a 90 20 molar ratio (red circle), 2/LiTFSI at a 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 molar ratio11 (grey square), and 3/LiTFSI at an 80 10 molar ratio11 (grey square), and 3/LiTFSI at an 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 molar ratio12 (black triangle). 20 molar ratio12 (black triangle). | ||
| LC electrolyte | Phase transition behaviora | ||||
|---|---|---|---|---|---|
| a Phase transition temperature (°C) determined by DSC during the cooling process. Scanning rate: 10 °C min−1. b Ref. 11 and 15. c Ref. 12. Iso: isotropic; SmA: smectic A; and SmB: smectic B. | |||||
1/LiTFSI (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) 20) |
Iso | 99 | SmA | ||
2/LiTFSI (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10)b 10)b |
Iso | 114 | SmA | 0 | SmB |
3/LiTFSI (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20)c 20)c |
Iso | 109 | SmA | 99 | SmB |
To evaluate the electrochemical stability of the complex, cyclic voltammetry (CV) of 1/LiTFSI at a molar ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 was performed using a stainless-steel plate as the working electrode and lithium metal as the counter and reference electrodes at 60 °C (Fig. 9). The fluorinated cyclohexylphenyl-based LC electrolyte 1/LiTFSI was stable in the range from −0.2 V to 4.2 V vs. Li/Li+ (Fig. 9a). Considering that 2/LiTFSI without fluorine substituents on the benzene ring electrochemically decomposed at 4.0 V vs. Li/Li+ (Fig. 9b),12 the fluorination of mesogens was effective for improving the electrochemical stability of LC lithium-ion electrolytes. The electrochemical decomposition of the LC electrolyte 1/LiTFSI was observed above 4.3 V. The decomposition potential based on the CV measurements of 1/LiTFSI is seen between those of 2/LiTFSI and 3/LiTFSI12 (Fig. 9b and S10†), which is consistent with the order of the HOMO levels of the respective liquid crystal molecules calculated using DFT (Fig. 2 and 7). These results suggest that the introduction of fluorine substituents reduces the HOMO level and improves oxidation stability. It is known that the electrochemical stability of conventional organic liquid electrolytes can be enhanced by high concentrations of lithium salts.57,74 To exclude the effect of LiTFSI concentrations on the stability of LC electrolytes, we conducted CV measurement for 1/LiTFSI at a molar ratio of 90
20 was performed using a stainless-steel plate as the working electrode and lithium metal as the counter and reference electrodes at 60 °C (Fig. 9). The fluorinated cyclohexylphenyl-based LC electrolyte 1/LiTFSI was stable in the range from −0.2 V to 4.2 V vs. Li/Li+ (Fig. 9a). Considering that 2/LiTFSI without fluorine substituents on the benzene ring electrochemically decomposed at 4.0 V vs. Li/Li+ (Fig. 9b),12 the fluorination of mesogens was effective for improving the electrochemical stability of LC lithium-ion electrolytes. The electrochemical decomposition of the LC electrolyte 1/LiTFSI was observed above 4.3 V. The decomposition potential based on the CV measurements of 1/LiTFSI is seen between those of 2/LiTFSI and 3/LiTFSI12 (Fig. 9b and S10†), which is consistent with the order of the HOMO levels of the respective liquid crystal molecules calculated using DFT (Fig. 2 and 7). These results suggest that the introduction of fluorine substituents reduces the HOMO level and improves oxidation stability. It is known that the electrochemical stability of conventional organic liquid electrolytes can be enhanced by high concentrations of lithium salts.57,74 To exclude the effect of LiTFSI concentrations on the stability of LC electrolytes, we conducted CV measurement for 1/LiTFSI at a molar ratio of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (Fig. S11†). The stability of 1/LiTFSI was comparable with different LiTFSI concentrations, supporting the hypothesis that the fluorination of mesogens enhances oxidation stability.
10 (Fig. S11†). The stability of 1/LiTFSI was comparable with different LiTFSI concentrations, supporting the hypothesis that the fluorination of mesogens enhances oxidation stability.
 | ||
Fig. 9 (a) Cyclic voltammograms of the LC electrolyte 1/LiTFSI at an 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 molar ratio in the voltage region of −0.2–3.9 V (red), −0.2–4.3 V (blue), and −0.2–5.0 V (black) vs. Li/Li+ at 60 °C. (b) Enlarged CV curves in the high voltage region of 1/LiTFSI at an 80 20 molar ratio in the voltage region of −0.2–3.9 V (red), −0.2–4.3 V (blue), and −0.2–5.0 V (black) vs. Li/Li+ at 60 °C. (b) Enlarged CV curves in the high voltage region of 1/LiTFSI at an 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 molar ratio (black solid line), 2/LiTFSI at a 90 20 molar ratio (black solid line), 2/LiTFSI at a 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 molar ratio12 (black dashed line), and 3/LiTFSI at an 80 10 molar ratio12 (black dashed line), and 3/LiTFSI at an 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 molar ratio12 (black dotted line). 20 molar ratio12 (black dotted line). | ||
The performance of lithium-ion batteries based on the LC electrolyte 1/LiTFSI at a molar ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 was studied using half-cells of Li/LC electrolytes/LiFePO4 (Fig. 10 and 11). Fig. 10a shows the charge–discharge curves for the cell based on the 1/LiTFSI complex in the potential range of 2.5–3.9 V and at a current density of 5 mA g−1 for the initial 30 cycles at 60 °C. Half-cells containing the LC electrolyte 1/LiTFSI exhibited a discharge capacity of 140 mA h g−1, whereas the theoretical capacity of LiFePO4 is 170 mA h g−1. The charge–discharge curves of the cell with 1/LiTFSI were almost the same within 30 cycles (Fig. 10a). The charge–discharge curves of the cell using 1/LiTFSI showed small polarization and a clear plateau corresponding to the lithium-ion insertion/extraction of LiFePO4 (Fig. 10b red line), while those for the 2/LiTFSI cell exhibited greater polarization (Fig. 10b grey line), presumably due to the decomposition of the LC electrolyte. The stable discharge capacities were obtained for the half-cell using 1/LiTFSI (Fig. 11a). In contrast, the capacity of the half-cell with 2/LiTFSI11 gradually decreased, resulting in a lower capacity at the 30th cycle. The coulombic efficiency of the half-cell using 1/LiTFSI was approximately 95% (Fig. 11b), which was also higher than that of the cell using 2/LiTFSI. These results show that the fluorination of the mesogen effectively enhances the oxidation resistance of the LC electrolyte to suppress the decomposition of the LC electrolytes during the charge–discharge reactions. The cell using 3/LiTFSI,12 which was the most electrochemically stable among 1–3, also showed a high coulombic efficiency of approximately 95% (Fig. 11b). However, the cell using 3/LiTFSI showed the smallest capacity among the cells due to the cell resistance resulting from the strong packing of the bicyclohexyl mesogens at an operation temperature of 60 °C (Fig. 11a). Compared to the modification of the molecular framework, the introduction of the fluorine substituents to the mesogenic moieties could enhance the oxidation resistance without inducing closer mesogen packing or significant changes in the LC order, resulting in improved battery performance.
20 was studied using half-cells of Li/LC electrolytes/LiFePO4 (Fig. 10 and 11). Fig. 10a shows the charge–discharge curves for the cell based on the 1/LiTFSI complex in the potential range of 2.5–3.9 V and at a current density of 5 mA g−1 for the initial 30 cycles at 60 °C. Half-cells containing the LC electrolyte 1/LiTFSI exhibited a discharge capacity of 140 mA h g−1, whereas the theoretical capacity of LiFePO4 is 170 mA h g−1. The charge–discharge curves of the cell with 1/LiTFSI were almost the same within 30 cycles (Fig. 10a). The charge–discharge curves of the cell using 1/LiTFSI showed small polarization and a clear plateau corresponding to the lithium-ion insertion/extraction of LiFePO4 (Fig. 10b red line), while those for the 2/LiTFSI cell exhibited greater polarization (Fig. 10b grey line), presumably due to the decomposition of the LC electrolyte. The stable discharge capacities were obtained for the half-cell using 1/LiTFSI (Fig. 11a). In contrast, the capacity of the half-cell with 2/LiTFSI11 gradually decreased, resulting in a lower capacity at the 30th cycle. The coulombic efficiency of the half-cell using 1/LiTFSI was approximately 95% (Fig. 11b), which was also higher than that of the cell using 2/LiTFSI. These results show that the fluorination of the mesogen effectively enhances the oxidation resistance of the LC electrolyte to suppress the decomposition of the LC electrolytes during the charge–discharge reactions. The cell using 3/LiTFSI,12 which was the most electrochemically stable among 1–3, also showed a high coulombic efficiency of approximately 95% (Fig. 11b). However, the cell using 3/LiTFSI showed the smallest capacity among the cells due to the cell resistance resulting from the strong packing of the bicyclohexyl mesogens at an operation temperature of 60 °C (Fig. 11a). Compared to the modification of the molecular framework, the introduction of the fluorine substituents to the mesogenic moieties could enhance the oxidation resistance without inducing closer mesogen packing or significant changes in the LC order, resulting in improved battery performance.
 | ||
Fig. 10 Charge–discharge curves of half-cells of Li/LC electrolytes/LiFePO4 using 1/LiTFSI at an 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 molar ratio, 2/LiTFSI at a 90 20 molar ratio, 2/LiTFSI at a 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 molar ratio,11 and 3/LiTFSI at an 80 10 molar ratio,11 and 3/LiTFSI at an 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 molar ratio12 at 60 °C. Potential range: 2.5–3.9 V for 1/LiTFSI and 2/LiTFSI, 2.7–3.8 V for 3/LiTFSI; current density: 5 mA g−1. (a) Charge–discharge curves of 1/LiTFSI for the initial 30 cycles. (b) Representative charge–discharge curves of 1/LiTFSI (red line), 2/LiTFSI (grey line), and 3/LiTFSI (black line) at the 30th cycle. 20 molar ratio12 at 60 °C. Potential range: 2.5–3.9 V for 1/LiTFSI and 2/LiTFSI, 2.7–3.8 V for 3/LiTFSI; current density: 5 mA g−1. (a) Charge–discharge curves of 1/LiTFSI for the initial 30 cycles. (b) Representative charge–discharge curves of 1/LiTFSI (red line), 2/LiTFSI (grey line), and 3/LiTFSI (black line) at the 30th cycle. | ||
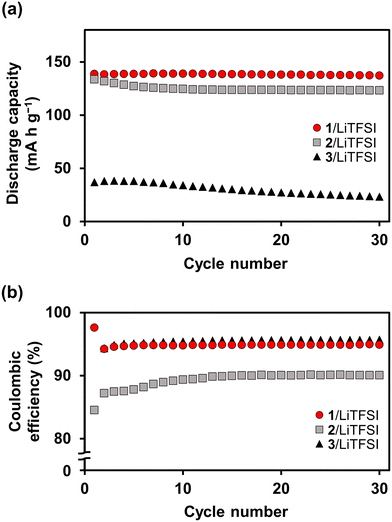 | ||
Fig. 11 Cycling performance of half-cells of Li/LC electrolytes/LiFePO4 using 1/LiTFSI at an 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 molar ratio (red circle), 2/LiTFSI at a 90 20 molar ratio (red circle), 2/LiTFSI at a 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 molar ratio11,12 (grey square), and 3/LiTFSI at an 80 10 molar ratio11,12 (grey square), and 3/LiTFSI at an 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 molar ratio12 (black triangle) at 60 °C. Potential range: 2.5–3.9 V for 1/LiTFSI and 2/LiTFSI, and 2.7–3.8 V for 3/LiTFSI; current density: 5 mA g−1. (a) Discharge capacity; (b) coulombic efficiency. 20 molar ratio12 (black triangle) at 60 °C. Potential range: 2.5–3.9 V for 1/LiTFSI and 2/LiTFSI, and 2.7–3.8 V for 3/LiTFSI; current density: 5 mA g−1. (a) Discharge capacity; (b) coulombic efficiency. | ||
To study the effects of the lateral fluorine groups of the cyclohexylphenyl mesogen on the molecular packing and LC order, molecular dynamics (MD) simulations were performed for compounds 1 and 2 (Fig. 12, 13, and S12–S14†). We recently reported unusual smectic phase transitions of analogous carbonate-based molecules,75 which were examined using large-scale MD simulations. In the present study, the models and methods of MD simulations75 were applied to compounds 1 and 2. The MD simulations of compounds 1 and 2 revealed bilayer structures at 30 °C (Fig. 12). The simulated interlayer distances of compounds 1 (44 Å, Fig. 12a) and 2 (46 Å, Fig. 12b) are consistent with the interlayer distances estimated by the XRD measurements of 1 (43 Å, Fig. S5†) and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 (46 Å), respectively. The order parameters and electron density profiles of the LC phases of compounds 1 and 2 were calculated from the results of MD simulations (Fig. 13 and S14†). We confirmed that the order parameters of the rigid-rod mesogen and the alkyl spacer of compounds 1 and 2 were almost the same in the SmA phases (Fig. S14†). These results indicated that the LC order of compound 1 was not significantly disrupted by the lateral fluorine groups of the cyclohexylphenyl mesogen. In addition, the high-electron-density region (red region in Fig. 12 left) of compound 1 in the SmA phase was wider than that of compound 2 (Fig. 13). Fluorinated LC molecule 1 exhibits dynamic and ordered 2D ion transport pathways in the SmA phase and improved electrochemical stability. These properties of compound 1 led to higher performances of lithium-ion batteries using LC electrolytes (Fig. 10 and 11).
11 (46 Å), respectively. The order parameters and electron density profiles of the LC phases of compounds 1 and 2 were calculated from the results of MD simulations (Fig. 13 and S14†). We confirmed that the order parameters of the rigid-rod mesogen and the alkyl spacer of compounds 1 and 2 were almost the same in the SmA phases (Fig. S14†). These results indicated that the LC order of compound 1 was not significantly disrupted by the lateral fluorine groups of the cyclohexylphenyl mesogen. In addition, the high-electron-density region (red region in Fig. 12 left) of compound 1 in the SmA phase was wider than that of compound 2 (Fig. 13). Fluorinated LC molecule 1 exhibits dynamic and ordered 2D ion transport pathways in the SmA phase and improved electrochemical stability. These properties of compound 1 led to higher performances of lithium-ion batteries using LC electrolytes (Fig. 10 and 11).
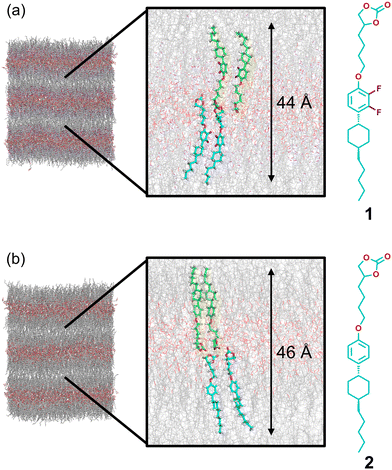 | ||
| Fig. 12 Side views of MD simulation snapshots after 500 ns of the equilibration run at 30 °C: (a) compound 1 and (b) compound 2. | ||
 | ||
| Fig. 13 Electron density profiles along the layer normal at 500 ns: (a) compound 1 and (b) compound 2. | ||
Conclusions
Nanostructured ion-conductive liquid crystals with high oxidation resistance have been designed based on the approach of mesogen fluorination. Fluorinated LC molecule 1 consisting of cyclohexyl-2,3-difluorophenyl and cyclic carbonate moieties is miscible with LiTFSI, and 1/LiTFSI complexes exhibit SmA phases over a wide temperature range, including room temperature. The fluorination of the mesogens imparts high oxidation resistance to the LC electrolytes without significantly changing the molecular framework and LC order, resulting in more stable cycling properties and a higher coulombic efficiency of the Li/LC electrolytes/LiFePO4 half-cells. The introduction of fluorine atoms into LC molecules has been used primarily in the field of informational displays to tune the dielectric constant, viscosity, and photophysical properties.66–71 In the present study, we have demonstrated that this approach can also be applied to liquid crystals for energy devices. The synthesis of electrochemically stable LC molecules via fluorination opens up the possibility of their use as LC electrolyte materials in safe and high-performance lithium-ion batteries.Author contributions
T. K. conceived and designed the study. T. K., J. U., S. T., and K. H. designed the experiments. S. T. and K. H. performed the experiments. S. T. and E. H. performed the cyclic voltammetry and charge–discharge experiments. S. S. and G. W. performed the MD simulations. S. T., K. H., J. U., S. S., G. W., and T. K. wrote the manuscript. All the authors discussed the results and edited the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was partially supported by JSPS KAKENHI JP19H05715 and JP19H05718 (Grant-in-Aid for Scientific Research on Innovative Areas of Aquatic Functional Materials) and the JST SPRING grant (JPMJSP2108). K. H. is grateful for the financial support from the Japan Society for the Promotion of Science (JSPS) Research Fellowship for Young Scientists (JP21J10984).References
- J. Uchida, B. Soberats, M. Gupta and T. Kato, Adv. Mater., 2022, 34, 2109063 CrossRef CAS PubMed.
- T. Kato, M. Yoshio, T. Ichikawa, B. Soberats, H. Ohno and M. Funahashi, Nat. Rev. Mater., 2017, 2, 17001 CrossRef.
- Q. Ruan, M. Yao, D. Yuan, H. Dong, J. Liu, X. Yuan, W. Fang, G. Zhao and H. Zhang, Nano Energy, 2023, 106, 108087 CrossRef CAS.
- H. K. Bisoyi and Q. Li, Chem. Rev., 2022, 122, 4887–4926 CrossRef CAS PubMed.
- N. Kapernaum, A. Lange, M. Ebert, M. A. Grunwald, C. Haege, S. Marino, A. Zens, A. Taubert, F. Giesselmann and S. Laschat, ChemPlusChem, 2022, 87, e202100397 CrossRef CAS PubMed.
- T. Kato, M. Gupta, D. Yamaguchi, K. P. Gan and M. Nakayama, Bull. Chem. Soc. Jpn., 2021, 94, 357–376 CrossRef CAS.
- W. Pisula and K. Müllen, in Handbook of Liquid Crystals, ed. J. W. Goodby, P. J. Collings, T. Kato, C. Tschierske, H. Gleeson and P. Raynes, Wiley-VCH, Weinheim, 2nd edn, 2014, vol. 8, ch. 20, pp. 627–673 Search PubMed.
- M. Kumar and S. Kumar, Polym. J., 2017, 49, 85–111 CrossRef CAS.
- J. Kloos, N. Joosten, A. Schenning and K. Nijmeijer, J. Membr. Sci., 2021, 620, 118849 CrossRef CAS.
- Y. Shen and I. Dierking, Appl. Sci., 2019, 9, 2512 CrossRef CAS.
- J. Sakuda, E. Hosono, M. Yoshio, T. Ichikawa, T. Matsumoto, H. Ohno, H. Zhou and T. Kato, Adv. Funct. Mater., 2015, 25, 1206–1212 CrossRef CAS.
- A. Kuwabara, M. Enomoto, E. Hosono, K. Hamaguchi, T. Onuma, S. Kajiyama and T. Kato, Chem. Sci., 2020, 11, 10631–10637 RSC.
- T. Onuma, E. Hosono, M. Takenouchi, J. Sakuda, S. Kajiyama, M. Yoshio and T. Kato, ACS Omega, 2018, 3, 159–166 CrossRef CAS PubMed.
- T. Onuma, M. Yoshio, M. Obi, K. Kashiwagi, S. Tahara and T. Kato, Polym. J., 2018, 50, 889–898 CrossRef CAS.
- Y. Mizumura, D. Högberg, K. Arai, J. Sakuda, B. Soberats, M. Yoshio and T. Kato, Bull. Chem. Soc. Jpn., 2019, 92, 1226–1233 CrossRef CAS.
- R. L. Kerr, S. A. Miller, R. K. Shoemaker, B. J. Elliott and D. L. Gin, J. Am. Chem. Soc., 2009, 131, 15972–15973 CrossRef CAS PubMed.
- A. Eisele, K. Kyriakos, R. Bhandary, M. Schönhoff, C. M. Papadakis and B. Rieger, J. Mater. Chem. A, 2015, 3, 2942–2953 RSC.
- Y. Wang, C. J. Zanelotti, X. Wang, R. Kerr, L. Jin, W. H. Kan, T. J. Dingemans, M. Forsyth and L. A. Madsen, Nat. Mater., 2021, 20, 1255–1263 CrossRef CAS PubMed.
- Z. Ahmad, Z. Hong and V. Viswanathan, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 26672–26680 CrossRef CAS PubMed.
- D. Gopalakrishnan, S. Alkatie, A. Cannon, S. Rajendran, N. K. Thangavel, N. Bhagirath, E. M. Ryan and L. M. R. Arava, Sustainable Energy Fuels, 2021, 5, 1488–1497 RSC.
- S. Wang, X. Liu, A. Wang, Z. Wang, J. Chen, Q. Zeng, X. Jiang, H. Zhou and L. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 25273–25284 CrossRef CAS PubMed.
- L. Su, F. Lu, Y. Li, Y. Wang, X. Li, L. Zheng and X. Gao, ACS Nano, 2024, 18, 7633–7643 CrossRef CAS PubMed.
- B. Soberats, M. Yoshio, T. Ichikawa, X. Zeng, H. Ohno, G. Ungar and T. Kato, J. Am. Chem. Soc., 2015, 137, 13212–13215 CrossRef CAS PubMed.
- T. Kobayashi, Y. Li, A. Ono, X. Zeng and T. Ichikawa, Chem. Sci., 2019, 10, 6245–6253 RSC.
- S. Chai, F. Xu, R. Zhang, X. Wang, L. Zhai, X. Li, H. J. Qian, L. Wu and H. Li, J. Am. Chem. Soc., 2021, 143, 21433–21442 CrossRef CAS PubMed.
- B. Soberats, M. Yoshio, T. Ichikawa, S. Taguchi, H. Ohno and T. Kato, J. Am. Chem. Soc., 2013, 135, 15286–15289 CrossRef CAS PubMed.
- H. Iino, T. Usui and J. Hanna, Nat. Commun., 2015, 6, 6828 CrossRef CAS PubMed.
- B. X. Dong, Z. Liu, M. Misra, J. Strzalka, J. Niklas, O. G. Poluektov, F. A. Escobedo, C. K. Ober, P. F. Nealey and S. N. Patel, ACS Nano, 2019, 13, 7665–7675 CrossRef CAS PubMed.
- S. Yazaki, M. Funahashi, J. Kagimoto, H. Ohno and T. Kato, J. Am. Chem. Soc., 2010, 132, 7702–7708 CrossRef CAS PubMed.
- T. Yasuda, H. Ooi, J. Morita, Y. Akama, K. Minoura, M. Funahashi, T. Shimomura and T. Kato, Adv. Funct. Mater., 2009, 19, 411–419 CrossRef CAS.
- S. Inoue, K. Nikaido, T. Higashino, S. Arai, M. Tanaka, R. Kumai, S. Tsuzuki, S. Horiuchi, H. Sugiyama, Y. Segawa, K. Takaba, S. Maki-Yonekura, K. Yonekura and T. Hasegawa, Chem. Mater., 2022, 34, 72–83 CrossRef CAS.
- T. Kato, J. Uchida, Y. Ishii and G. Watanabe, Adv. Sci., 2024, 11, 2306529 CrossRef CAS PubMed.
- D. L. Gin, X. Lu, P. R. Nemade, C. S. Pecinovsky, Y. Xu and M. Zhou, Adv. Funct. Mater., 2006, 16, 865–878 CrossRef CAS.
- J. Kloos, N. Jansen, M. Houben, A. Casimiro, J. Lub, Z. Borneman, A. P. H. J. Schenning and K. Nijmeijer, Chem. Mater., 2021, 33, 8323–8333 CrossRef CAS PubMed.
- Y. Zhang, D. Kim, R. Dong, X. Feng and C. O. Osuji, Sci. Adv., 2022, 8, eabm5899 CrossRef CAS PubMed.
- T. Sakamoto, K. Asakura, N. Kang, R. Kato, M. Liu, T. Hayashi, H. Katayama and T. Kato, J. Mater. Chem. A, 2023, 11, 22178–22186 RSC.
- P. Li, C. Johnson, S. S. Dyer, C. O. Osuji and D. L. Gin, Adv. Mater. Interfaces, 2023, 10, 2201761 CrossRef CAS.
- D. Kuo, M. Liu, K. R. S. Kumar, K. Hamaguchi, K. P. Gan, T. Sakamoto, T. Ogawa, R. Kato, N. Miyamoto, H. Nada, M. Kimura, M. Henmi, H. Katayama and T. Kato, Small, 2020, 16, 2001721 CrossRef CAS PubMed.
- D. Kuo, T. Sakamoto, S. Torii, M. Liu, H. Katayama and T. Kato, Polym. J., 2022, 54, 821–825 CrossRef CAS PubMed.
- K. Xu, Chem. Rev., 2004, 104, 4303–4417 CrossRef CAS PubMed.
- J. Kalhoff, G. G. Eshetu, D. Bresser and S. Passerini, ChemSusChem, 2015, 8, 2154–2175 CrossRef CAS PubMed.
- Y. Chen, Y. Kang, Y. Zhao, L. Wang, J. Liu, Y. Li, Z. Liang, X. He, X. Li, N. Tavajohi and B. Li, J. Energy Chem., 2021, 59, 83–99 CrossRef CAS.
- Q. Zhao, S. Stalin, C. Z. Zhao and L. A. Archer, Nat. Rev. Mater., 2020, 5, 229–252 CrossRef CAS.
- A. Banerjee, X. Wang, C. Fang, E. A. Wu and Y. S. Meng, Chem. Rev., 2020, 120, 6878–6933 CrossRef CAS PubMed.
- A. Sakuda, T. Takeuchi and H. Kobayashi, Solid State Ionics, 2016, 285, 112–117 CrossRef CAS.
- A. Sakuda, A. Hayashi and M. Tatsumisago, Curr. Opin. Electrochem., 2017, 6, 108–114 CrossRef CAS.
- Z. Xue, D. He and X. Xie, J. Mater. Chem. A, 2015, 3, 19218–19253 RSC.
- D. Bresser, S. Lyonnard, C. Iojoiu, L. Picard and S. Passerini, Mol. Syst. Des. Eng., 2019, 4, 779–792 RSC.
- L. Tang, B. Chen, Z. Zhang, C. Ma, J. Chen, Y. Huang, F. Zhang, Q. Dong, G. Xue, D. Chen, C. Hu, S. Li, Z. Liu, Y. Shen, Q. Chen and L. Chen, Nat. Commun., 2023, 14, 2301 CrossRef CAS PubMed.
- Y. Su, X. Rong, A. Gao, Y. Liu, J. Li, M. Mao, X. Qi, G. Chai, Q. Zhang, L. Suo, L. Gu, H. Li, X. Huang, L. Chen, B. Liu and Y. S. Hu, Nat. Commun., 2022, 13, 4181 CrossRef CAS PubMed.
- H. Nguyen, G. Kim, J. Shi, E. Paillard, P. Judeinstein, S. Lyonnard, D. Bresser and C. Iojoiu, Energy Environ. Sci., 2018, 11, 3298–3309 RSC.
- A. Mayer, H. Nguyen, A. Mariani, T. Diemant, S. Lyonnard, C. Iojoiu, S. Passerini and D. Bresser, ACS Macro Lett., 2022, 11, 982–990 CrossRef CAS PubMed.
- X. Cheng, J. Pan, Y. Zhao, M. Liao and H. Peng, Adv. Energy Mater., 2018, 8, 1702184 CrossRef.
- M. Watanabe, M. L. Thomas, S. Zhang, K. Ueno, T. Yasuda and K. Dokko, Chem. Rev., 2017, 117, 7190–7239 CrossRef CAS PubMed.
- J. Liu, Z. Bao, Y. Cui, E. J. Dufek, J. B. Goodenough, P. Khalifah, Q. Li, B. Y. Liaw, P. Liu, A. Manthiram, Y. S. Meng, V. R. Subramanian, M. F. Toney, V. V. Viswanathan, M. S. Whittingham, J. Xiao, W. Xu, J. Yang, X. Q. Yang and J. G. Zhang, Nat. Energy, 2019, 4, 180–186 CrossRef CAS.
- W. Li, B. Song and A. Manthiram, Chem. Soc. Rev., 2017, 46, 3006–3059 RSC.
- K. Yoshida, M. Nakamura, Y. Kazue, N. Tachikawa, S. Tsuzuki, S. Seki, K. Dokko and M. Watanabe, J. Am. Chem. Soc., 2011, 133, 13121–13129 CrossRef CAS PubMed.
- Y. Wang, Z. Li, Y. Hou, Z. Hao, Q. Zhang, Y. Ni, Y. Lu, Z. Yan, K. Zhang, Q. Zhao, F. Li and J. Chen, Chem. Soc. Rev., 2023, 52, 2713–2763 RSC.
- N. von Aspern, G. V. Röschenthaler, M. Winter and I. Cekic-Laskovic, Angew. Chem., Int. Ed., 2019, 58, 15978–16000 CrossRef CAS PubMed.
- Z. Zhang, L. Hu, H. Wu, W. Weng, M. Koh, P. C. Redfern, L. A. Curtiss and K. Amine, Energy Environ. Sci., 2013, 6, 1806–1810 RSC.
- Y. Sasaki, M. Takehara, S. Watanabe, N. Nanbu and M. Ue, J. Fluorine Chem., 2004, 125, 1205–1209 CrossRef CAS.
- Q. Zheng, Y. Yamada, R. Shang, S. Ko, Y.-Y. Lee, K. Kim, E. Nakamura and A. Yamada, Nat. Energy, 2020, 5, 291–298 CrossRef CAS.
- Z. Yu, H. Wang, X. Kong, W. Huang, Y. Tsao, D. G. Mackanic, K. Wang, X. Wang, W. Huang, S. Choudhury, Y. Zheng, C. V. Amanchukwu, S. T. Hung, Y. Ma, E. G. Lomeli, J. Qin, Y. Cui and Z. Bao, Nat. Energy, 2020, 5, 526–533 CrossRef CAS.
- J. Xu, J. Zhang, T. P. Pollard, Q. Li, S. Tan, S. Hou, H. Wan, F. Chen, H. He, E. Hu, K. Xu, X. Yang, O. Borodin and C. Wang, Nature, 2023, 614, 694–700 CrossRef CAS PubMed.
- D. H. C. Wong, J. L. Thelen, Y. Fu, D. Devaux, A. A. Pandya, V. S. Battaglia, N. P. Balsara and J. M. DeSimone, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 3327–3331 CrossRef CAS PubMed.
- M. Hird, Chem. Soc. Rev., 2007, 36, 2070–2095 RSC.
- M. Bremer, P. Kirsch, M. Klasen-Memmer and K. Tarumi, Angew. Chem., Int. Ed., 2013, 52, 8880–8896 CrossRef CAS PubMed.
- Q. Chen and M. Hird, Liq. Cryst., 2015, 42, 877–886 CAS.
- G. W. Gray, M. Hird, D. Lacey and K. J. Toyne, J. Chem. Soc., Perkin Trans. 2, 1989, 2041–2053 RSC.
- H. F. Gleeson, Y. Wang, S. Watson, D. Sahagun-Sanchez, J. W. Goodby, M. Hird, A. Petrenko and M. A. Osipov, J. Mater. Chem., 2004, 14, 1480–1485 RSC.
- J. S. Gasowska, S. J. Cowling, M. C. R. Cockett, M. Hird, R. A. Lewis, E. P. Raynes and J. W. Goodby, J. Mater. Chem., 2010, 20, 299–307 RSC.
- C. Chen, M. Poppe, S. Poppe, C. Tschierske and F. Liu, Angew. Chem., Int. Ed., 2020, 59, 20820–20825 CrossRef CAS PubMed.
- M. A. Osman, Mol. Cryst. Liq. Cryst., 1985, 128, 45–63 CrossRef CAS.
- Y. Yamada, J. Wang, S. Ko, E. Watanabe and A. Yamada, Nat. Energy, 2019, 4, 269–280 CrossRef CAS.
- K. Hamaguchi, H. Lu, S. Okamura, S. Kajiyama, J. Uchida, S. Sato, G. Watanabe, Y. Ishii, H. Washizu, G. Ungar and T. Kato, ChemPhysChem, 2023, 24, e202200927 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr03559c |
| This journal is © The Royal Society of Chemistry 2024 |