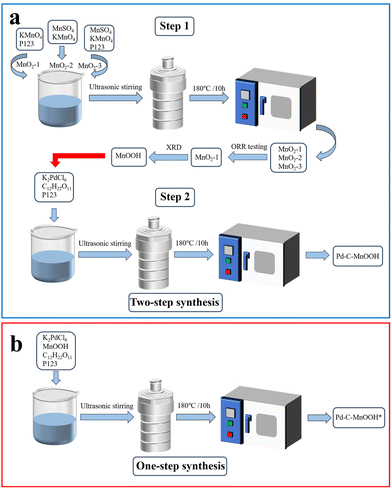
Synthesis of MnOOH and its application in a supporting hexagonal Pd/C catalyst for the oxygen reduction reaction†
Zheng
Cheng
,
Wei
Cheng
,
Xin-Ning
Lin
,
Rong-Hua
Zhang
 *,
Luo-Yi
Yan
,
Gui-Xian
Tian
,
Xiao-Yu
Shen
and
Xin-Wen
Zhou
*
*,
Luo-Yi
Yan
,
Gui-Xian
Tian
,
Xiao-Yu
Shen
and
Xin-Wen
Zhou
*
College of Materials and Chemical Engineering, China Three Gorges University, Yichang 443002, China. E-mail: rhzhang@ctgu.edu.cn; xwzhou@ctgu.edu.cn
First published on 23rd November 2023
Abstract
With the expansion of global energy problems and the deepening of research on oxygen reduction reaction (ORR) in alkaline media, the development of low cost and high electrocatalytic performance catalysts has become a research hotspot. In this study, a hexagonal Pd-C-MnOOH composite catalyst was prepared by using the triblock copolymer P123 as the reducing agent and protective agent, sucrose as the carbon source and self-made MnOOH as the carrier under hydrothermal conditions. When the Pd load is 20% and the C/MnOOH ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the 20% Pd-C-MnOOH-1
1, the 20% Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst obtained by the one-step method has the highest ORR activity and stability in the alkaline system. At 1600 rpm, the limiting diffusion current density and half-wave potential of the 20% Pd-C-MnOOH-1
1 catalyst obtained by the one-step method has the highest ORR activity and stability in the alkaline system. At 1600 rpm, the limiting diffusion current density and half-wave potential of the 20% Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrocatalyst are −4.78 mA cm−2 and 0.84 V, respectively, which are better than those of the commercial 20%Pd/C catalyst. According to the Koutecky–Levich (K–L) equation and the linear fitting results, the electron transfer number of the 20%Pd-C-MnOOH-1
1 electrocatalyst are −4.78 mA cm−2 and 0.84 V, respectively, which are better than those of the commercial 20%Pd/C catalyst. According to the Koutecky–Levich (K–L) equation and the linear fitting results, the electron transfer number of the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrocatalyst for the oxygen reduction reaction is 3.8, which is similar to that of a 4-electron process. After 1000 cycles, the limiting diffusion current density of the 20%Pd-C-MnOOH-1
1 electrocatalyst for the oxygen reduction reaction is 3.8, which is similar to that of a 4-electron process. After 1000 cycles, the limiting diffusion current density of the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst is −4.61 mA cm−2, which only decreases by 3.7%, indicating that the 20%Pd-C-MnOOH-1
1 catalyst is −4.61 mA cm−2, which only decreases by 3.7%, indicating that the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst has good stability. The reason for the improvement of the ORR performance of the Pd-C-MnOOH composite catalyst is the improvement of the conductivity of the carbon layer formed by original carbonization, the regular hexagonal highly active Pd particles and the synergistic catalytic effect between Pd and MnOOH. The method of introducing triblock copolymers in the synthesis of oxides and metal–oxide composite catalysts is expected to be extended to other electrocatalysis fields.
1 catalyst has good stability. The reason for the improvement of the ORR performance of the Pd-C-MnOOH composite catalyst is the improvement of the conductivity of the carbon layer formed by original carbonization, the regular hexagonal highly active Pd particles and the synergistic catalytic effect between Pd and MnOOH. The method of introducing triblock copolymers in the synthesis of oxides and metal–oxide composite catalysts is expected to be extended to other electrocatalysis fields.
1. Introduction
With the rapid development of society, the demand for energy in industries, such as the manufacturing industry and the transportation industry is increasing, and the energy crisis has become one of the major issues of global concern in this century.1 Traditional nonrenewable resources such as oil, natural gas, coal, etc., with limited reserves and high carbon emissions have caused irreversible damage to the ecological environment and global climate.2,3 The excessive exploitation and use of fossil fuels have led to a series of problems such as serious environmental pollution, global climate change, and energy shortage.4 In view of the harm caused by fossil energy, it is necessary to develop new energy sources as substitutes to achieve the purpose of reducing carbon dioxide emissions and reducing pollution of the ecological environment.5 New energy sources should have the characteristics of being green, having high efficiency, and being economical. Fuel cells have become one of the promising, hot research, and widely used new energy sources due to their high energy density, environmental friendliness, economic portability, convenient and safe storage and transportation.6At present, commercialized Pt/C catalysts are mainly used in acidic media, but acidic media will corrode these catalysts and reduce their service life; the cost of platinum metal is high and the storage amount is small, which further limit the large-scale commercialization of platinum-based catalysts.7–9 In order to avoid the shortcomings of platinum-based catalysts in acidic media, developing a catalyst with high efficiency and high stability that can replace platinum-based catalysts in alkaline media has become a hot spot in ORR electrocatalysis.10–12 Nonprecious metals can be used as catalysts for alkaline membrane fuel cells, which are abundant in reserves and low in cost and are potential commercial metal catalysts with research prospects.13–15 However, in alkaline medium, using platinum as an example, the electrode reaction kinetics decreased by 2 to 3 orders of magnitude compared to acidic medium, which seriously hindered the development of alkaline membrane fuel cells and the alkaline water electrolysis technology.16–18 Many non-precious metals and non-precious metal oxides have some ORR catalytic activity, limited by the material itself, and their catalytic performance is generally low.19 Since non-precious metals and their oxides have certain catalytic activity, the performance of the catalyst can be improved by loading a certain amount of precious metals and doping conductive substances.20 Therefore, the aim of the present research is to develop an electrocatalyst with high catalytic activity and stability for catalyzing the oxygen reduction reaction in alkaline media.
Mn-based oxides have more than 20 kinds of crystal forms, and their non-stoichiometric composition and multivalent nature make them more complicated than other metal oxides.21 Manganese dioxide (MnO2), as a typical oxide of manganese, has rich crystal structure characteristics due to the different connection modes of [MnO6] octahedra. Due to its rich crystal structure, the electrochemical properties of manganese dioxide are unique and investigational, which provides the possibility for it to be used as a carrier of electrocatalysts.22,23 At the same time, manganese dioxide has the advantages of rich reserves, low preparation cost, environmental friendliness (low toxicity), etc.24 At present, a large number of publications have reported that manganese dioxide crystals with different particle sizes and specific morphologies can be obtained by adjusting the conditions of experimental preparation.25 Manganese dioxide nanomaterials have complex morphological characteristics such as lines, flowers, tubes, balls, etc. Due to their different crystal structures and morphologies, their catalytic properties as carriers are different, and their synthesis methods are also different.26 Manganese dioxide can exist stably under alkaline conditions and can be used as a carrier material of the electrocatalyst for the oxygen reduction reaction in an alkaline medium.27 In addition, manganese dioxide itself has a certain catalytic activity, and whether the catalytic performance of 1 + 1 > 2 can be achieved by loading metal catalysts on its surface is the main source of ideas under this topic.
In recent years, there have been many reports on the use of MnO2 with different structures as electrocatalysts or catalyst carriers. A carbon quantum dot (CD) embedded in MnO2 nanoflowers for oxygen evolution reaction (OER) efficient catalysts in alkaline media was investigated.28 The catalyst was synthesized by the microwave heating method and has the advantages of a high surface area, strong conductivity, fast charge transfer rate, and efficient catalytic activity and stability for the OER. In 2020, I Cruz Reyes et al. prepared a bi-functional Pd-MnO2 electrocatalyst by a hydrothermal method, which has catalytic activity for oxygen reduction and hydrogen oxidation in alkaline fuel cells, and the catalytic activity increased significantly with the increase of palladium content.29 A reduced graphene oxide (rGO)/gamma-MnO2 compound could be prepared by electrophoretic deposition reduction of graphene oxide, which has a very high surface area and has catalytic activity for the oxygen reduction reaction.30 A Mn/Mn3O4 catalyst with a monomeric core–shell structure shows good catalytic activity and durability for ORR, which is a potential non-precious metal electrocatalyst.31 The above research results show that how to prepare a single crystalline and stable MnO2-based catalyst carrier and improve the electrocatalytic performance of the catalyst is a difficult and hot topic in this field, and further research should be carried out.
Our previous work has shown that the tricomplete copolymer can be used as both a protective agent and a reducing agent to regulate the structure and morphology of precious metal Pt and Pd-based catalysts due to its special polyhydroxyl chain structure.32–36 P123 (PEO20–PPO70–PEO20), poly(ethylene oxide)–poly(ethylene oxide)–poly(ethylene oxide), is the most typical representative of trisomeric copolymers. Sucrose can be directly carbonized into nano-carbon under certain hydrothermal reaction conditions, which is a common means to introduce carbon sources to improve the conductivity of catalysts and thus improve their electrocatalytic activity.37 In this study, on the basis of previous work, we innovatively extended P123 and sucrose to the synthesis of Pd/C/MnO2 composite catalysts and discussed their ORR properties. The MnOOH support with a single structure and the highest ORR activity was first screened, then Pd nanoparticles were loaded onto MnOOH by a one-step method and a two-step method respectively, and carbon materials were introduced in situ to obtain composite catalysts. The effects of different synthesis methods, the Pd loading capacity and carbon incorporation on the ORR performance of the catalyst were systematically discussed.
2. Experimental section
2.1 Synthetic process
| Experiment number | KMnO4 (g) | MnSO4 (g) | P123 (g) |
|---|---|---|---|
| MnO2-1 | 0.63 | — | 0.13 |
| MnO2-2 | 0.63 | 0.67 | — |
| MnO2-3 | 0.63 | 0.67 | 0.13 |
For the synthesis of MnO2-1, as shown in Fig. 1a, 0.63 g of KMnO4 was poured into a solution of 20 mL of water dissolved in 0.13 g of P123 and stirred ultrasonically for 5 minutes. Then the reaction liquid was transferred to a polytetrafluoroethylene lining and reacted in a high temperature reactor at 180 °C for 10 hours. The product was obtained after centrifugation, washing and drying. If all other reaction conditions remain the same, without adding P123, only KMnO4 and MnSO4 are needed, and MnO2-2 can be obtained. MnO2-3 can be prepared by adding KMnO4, MnSO4 and P123 at the same time. Subsequent tests showed that the obtained MnO2-1 was actually a hydroxyl manganese oxide (MnOOH) and showed the highest ORR activity. Therefore, in the following experiments, MnOOH was selected as the carrier of Pd.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the synthesis of the catalyst with 20% Pd load is taken as an example. 0.016 g MnOOH, 0.04 g sucrose and 0.03 g K2PdCl6 were added to a 20 mL aqueous solution containing dissolved P123 (0.5 g). After ultrasonic mixing, they were transferred to a high temperature reactor and reacted at 180 °C for 12 hours. The obtained product is 20% Pd-C-MnOOH-1
1, and the synthesis of the catalyst with 20% Pd load is taken as an example. 0.016 g MnOOH, 0.04 g sucrose and 0.03 g K2PdCl6 were added to a 20 mL aqueous solution containing dissolved P123 (0.5 g). After ultrasonic mixing, they were transferred to a high temperature reactor and reacted at 180 °C for 12 hours. The obtained product is 20% Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 after centrifugation, washing and vacuum drying. When the Pd loading is adjusted to 10% and 40% respectively, the catalysts obtained are recorded as 10%Pd-C-MnOOH-1
1 after centrifugation, washing and vacuum drying. When the Pd loading is adjusted to 10% and 40% respectively, the catalysts obtained are recorded as 10%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 40%Pd-C-MnOOH-1
1 and 40%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively.
1, respectively.
When the Pd load was 20%, in order to investigate the effect of the Pd/MnOOH ratio on the performance of the electrocatalyst, we synthesized catalysts with Pd/MnOOH ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 respectively for comparative analysis. Two catalysts, 20% Pd-C-MnOOH-1
1 respectively for comparative analysis. Two catalysts, 20% Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 20% Pd-C-MnOOH-2
2 and 20% Pd-C-MnOOH-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, can be obtained by keeping all the synthesis conditions unchanged and changing only the ratio of Pd and Mn precursors.
1, can be obtained by keeping all the synthesis conditions unchanged and changing only the ratio of Pd and Mn precursors.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1*.
In the two-step synthesis of Pd-C-MnOOH, the reaction conditions of the synthesis of MnOOH in the first step are consistent with the loading and carbonization process in the second step. Therefore, for comparative analysis, we try to combine the two-step method into one step to synthesize Pd-C-MnOOH. As shown in Fig. 1b, the raw materials used are directly mixed together in a fixed proportion. Under the same conditions, the product obtained by the one-step hydrothermal method is labeled as 20%Pd-C-MnOOH-1
1*.
In the two-step synthesis of Pd-C-MnOOH, the reaction conditions of the synthesis of MnOOH in the first step are consistent with the loading and carbonization process in the second step. Therefore, for comparative analysis, we try to combine the two-step method into one step to synthesize Pd-C-MnOOH. As shown in Fig. 1b, the raw materials used are directly mixed together in a fixed proportion. Under the same conditions, the product obtained by the one-step hydrothermal method is labeled as 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1*.
1*.
2.2 Physical characterization
The X-ray diffraction pattern was obtained using a Rigaku SmartLab with operating parameters of 200 mA and 45 kV with Cu kα radiation. The morphology and microstructure of the catalyst were characterized using the following instruments: a transmission electron microscope (TEM), a high resolution TEM (HRTEM), and an energy dispersive spectrometer (EDS). The composition of the catalyst was measured by X-ray photoelectron spectroscopy (PHI Quantum 2000 XPS system).2.3 Electrochemical experiment
Electrochemical performance tests in this work were carried out on a three-electrode system using an electrochemical workstation model CHI 760E (made in Chenhua, Shanghai). The three-electrode system for electrochemical testing is composed of a working electrode, a reference electrode and a counter electrode. The working electrode is a rotating disk electrode (RDE, Φ5 mm), the reference electrode is a saturated calomel electrode (SCE), the salt bridge solution is saturated potassium nitrate, and the platinum plate electrode is the counter electrode. The LSV curve of the oxygen reduction reaction was measured with 0.1 mol L−1 KOH solution as the electrolyte and a pine modulated speed rotator (AFMSRCE) was used.Before the electrochemical test, the working electrode needs to be polished and cleaned. The specific process is as follows: first, apply an appropriate amount of 0.05 μm Al2O3 powder to the polishing cloth, add deionized water, and polish the electrode for 15 minutes. Then the polished electrode is ultrasonically cleaned 3 times with deionized water, and the stain and residual Al2O3 powder on the electrode surface are washed away. Then 3.0 mg of the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst was dispersed into 1.0 ml solution (the volume ratio of deionized water to ethanol was 7
1 catalyst was dispersed into 1.0 ml solution (the volume ratio of deionized water to ethanol was 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), then 10 μL of 5% Nafion solution was added, and the material required for preparing the working electrode was obtained by ultrasonic mixing evenly. Then 10.0 μL of the mixture was taken, slowly and evenly dropped on the clean surface of the RDE, and dried naturally to obtain the working electrode.
3), then 10 μL of 5% Nafion solution was added, and the material required for preparing the working electrode was obtained by ultrasonic mixing evenly. Then 10.0 μL of the mixture was taken, slowly and evenly dropped on the clean surface of the RDE, and dried naturally to obtain the working electrode.
To test the LSV curve of the oxygen reduction reaction, O2 should be fed to the electrolytic tank for 30 minutes in advance to ensure sufficient dissolved oxygen in the electrolyte. During the test, the scanning rate was 5 mV s−1, and the scanning potential range was −0.7 to 1.0 V (vs. SCE). Then the LSV curves of the oxygen reduction reaction were tested at 7 rotational speeds of 400, 625, 900, 1225, 1600, 2025 and 2500 rpm. When drawing an electrochemical test image, E(SCE) is converted to E(RHE) according to the following formula.
| E(RHE) = E(SCE) + 0.0591 pH + 0.24 |
In order to understand the catalytic process of the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst during the oxygen reduction reaction, we need to calculate its electron transfer number (n). The number of electron transfers can be calculated according to the linear fitting of the Koutecký–Levich (K–L) equation and test data.40,41
1 catalyst during the oxygen reduction reaction, we need to calculate its electron transfer number (n). The number of electron transfers can be calculated according to the linear fitting of the Koutecký–Levich (K–L) equation and test data.40,41
| 1/j = 1/jk + 1/jL = 1/nFkC0 + 1/Bω1/2 |
| B = 0.62nFD2/3ν−1/6C0 |
3. Results and discussion
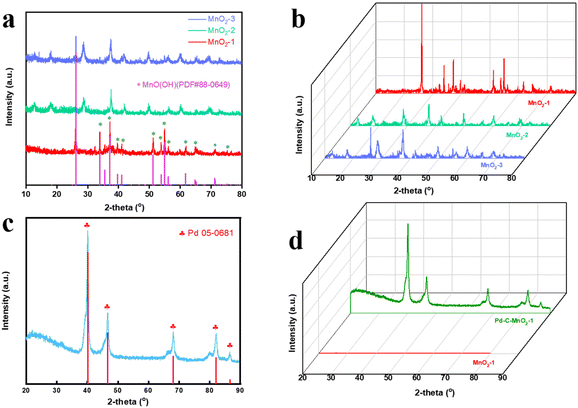
The XRD patterns of MnO2-1, MnO2-2, and MnO2-3 are shown in Fig. 2a and 2b. As can be seen from the figure, the diffraction peak intensity of MnO2-1 is relatively high, and the impurity peaks are relatively small, which can be well matched with the JADE PDF standard card (MnOOH 88-0649), so the synthesized MnO2-1 can be identified as MnOOH. The five strong diffraction peaks on the XRD spectrum of MnOOH correspond to the crystal plane diffraction peaks (111), (020), (002), (220), and (311), indicating that MnOOH has a polycrystalline structure.42,43 When the total area of MnOOH diffraction peaks is fitted to the area of the amorphous region, the crystallinity of MnOOH is calculated to be 81.5%, indicating that MnOOH crystals have large particles and good crystallinity. For MnO2-2 and MnO2-3, it can be seen from the diffraction peaks of the XRD pattern that their diffraction peak intensity is relatively low, there are many miscellaneous peaks, their crystallinity is not good, and their accurate configuration cannot be determined by standard cards. In Fig. 2a, where 2-theta equals around 26°, MnO2-3 has a strong diffraction peak, while MnO2-2 does not, which coincides with the position of MnOOH's strongest diffraction peak. P123 was added to MnO2-3 during the synthesis process, while MnO2-2 was not added. The other synthesis conditions were completely consistent with the reagents used. It is speculated that P123 added in the synthesis process has a certain regulatory effect, and P123 may affect the crystallization mode of the product.
Fig. 2c shows the XRD spectrum of the catalyst 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, using the standard card JADE PDF (Pd 05-0681) as a reference. The four strong diffraction peaks correspond to the crystal planes (1 1 1), (2 0 0), (2 2 0), and (3 1 1), respectively. It can be inferred that the catalyst has a face-centered cubic structure.44–46 As shown in Fig. 2d, the XRD spectra of MnO2-1 (MnOOH) and 20%Pd-C-MnOOH-1
1, using the standard card JADE PDF (Pd 05-0681) as a reference. The four strong diffraction peaks correspond to the crystal planes (1 1 1), (2 0 0), (2 2 0), and (3 1 1), respectively. It can be inferred that the catalyst has a face-centered cubic structure.44–46 As shown in Fig. 2d, the XRD spectra of MnO2-1 (MnOOH) and 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were placed on the same scale. Due to the high diffraction peak intensity of 20%Pd-C-MnOOH-1
1 were placed on the same scale. Due to the high diffraction peak intensity of 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the diffraction peak of MnO2-1 was almost invisible. This can explain why the diffraction peak of MnOOH cannot be found in the XRD pattern of the 20%Pd-C-MnOOH-1
1, the diffraction peak of MnO2-1 was almost invisible. This can explain why the diffraction peak of MnOOH cannot be found in the XRD pattern of the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst. In summary, palladium in the 20%Pd-C-MnOOH-1
1 catalyst. In summary, palladium in the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst exists in the form of elemental Pd, confirming that K2PdCl6 is completely reduced to elemental Pd during the synthesis process.
1 catalyst exists in the form of elemental Pd, confirming that K2PdCl6 is completely reduced to elemental Pd during the synthesis process.
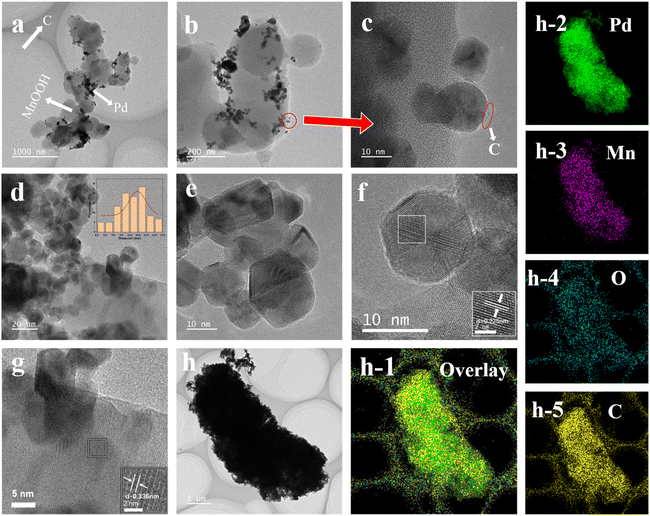
It can be seen from Fig. 3a–c that 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as a whole presents a spherical chain structure, and the spherical particles are connected to each other to form a chain structure. This kind of chain structure may come from the special chain structure of P123 itself. P123 can help to get the chain structure that has been demonstrated in previous experiments.34,36,47 The Pd nanoparticles and MnOOH adhere to the surface of the carbon spheres and exhibit a certain degree of aggregation. It can also be seen from Fig. 3a and b that there are components with different colors and structures, and the subsequent characterization can confirm that they are Pd, MnOOH and carbon, respectively, which can be observed more clearly in Fig. 3c.
1 as a whole presents a spherical chain structure, and the spherical particles are connected to each other to form a chain structure. This kind of chain structure may come from the special chain structure of P123 itself. P123 can help to get the chain structure that has been demonstrated in previous experiments.34,36,47 The Pd nanoparticles and MnOOH adhere to the surface of the carbon spheres and exhibit a certain degree of aggregation. It can also be seen from Fig. 3a and b that there are components with different colors and structures, and the subsequent characterization can confirm that they are Pd, MnOOH and carbon, respectively, which can be observed more clearly in Fig. 3c.
Fig. 3d shows that the average particle size of Pd nanoparticles is 10.1 nm, and there is no agglomeration among the particles, but simply stacking together. It can be intuitively seen that Pd has a polygonal morphology, which presents a hexagonal shape in general, with regular hexagons being the main shape (Fig. 3e and f). Fig. 3e and f show the HRTEM images of Pd nanoparticles. At this resolution, crystal lattice fringes of Pd can be clearly observed, indicating good crystallization. As shown in Fig. 3f, the image is analyzed and processed using Digital micrograph software, and the Fourier transform (FT) is calculated by computer to obtain the crystal plane spacing of Pd, which is 0.225 nm. Referring to the JADE PDF standard card (Pd 05-0681), the (111) crystal face with the highest diffraction peak intensity is consistent with the calculated Pd crystal face spacing of 0.225 nm, so it can be proved that the hexagonal crystal in the HRTEM image is crystal Pd nanparticles.48–50
By observing Fig. 3g, it can be seen that there is a large crystal in the lower right corner of the image that is different from the crystal lattice stripe of Pd. As shown in Fig. 3g, digital micrograph software was used to analyze and process the image, and the Fourier transform (FT) was calculated by computer to obtain the crystal plane spacing in this region, which was 0.336 nm. Referring to the standard card JADE PDF (MnOOH 88-0649), the crystal plane spacing with the highest diffraction peak intensity (111) is 0.340 nm, which corresponds well to the calculated value of 0.336 nm.51,52 Combined with the results of XRD, it can be determined that the crystal is MnOOH, and it adheres to the surface of the carbon sphere together with Pd nanoparticles. It should be noted that in order to analyze the distribution of elements in the catalyst more clearly, we selected a block with more serious agglomeration to analyze.
Fig. 3h shows the HAADF-STEM image of 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, where h2, h3, h4, and h5 are the elemental mappings of Pd, Mn, O, and C, respectively, and h1 is the overlapping image. It can be seen that the steps of various elements are relatively uniform, and there is no serious agglomeration phenomenon. In addition to the uniform mixing of elemental carbon and Pd/MnOOH, it can also be observed that the three-dimensional grid structure of carbon can be formed. This network structure can help stabilize the nanoparticles while improving the electrical conductivity between the particles, improving the activity and stability of the catalyst in the near term.
1, where h2, h3, h4, and h5 are the elemental mappings of Pd, Mn, O, and C, respectively, and h1 is the overlapping image. It can be seen that the steps of various elements are relatively uniform, and there is no serious agglomeration phenomenon. In addition to the uniform mixing of elemental carbon and Pd/MnOOH, it can also be observed that the three-dimensional grid structure of carbon can be formed. This network structure can help stabilize the nanoparticles while improving the electrical conductivity between the particles, improving the activity and stability of the catalyst in the near term.
Fig. 4 shows the XPS spectrum of catalyst 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the detected elements are Pd, Mn, O, and C. Fig. 4a shows the survey spectrum of the sample, and Fig. 4b–e correspond to the high resolution spectra of elements Pd, Mn, O, and C, respectively. The characteristic peaks of Pd 3d, Mn 2p and O 1s can be clearly seen in Fig. 4a. As shown in Fig. 4b, it can be clearly observed in the high-resolution spectrum of Pd that there are two characteristic peaks in the 3d region of Pd, which are at 335.6 eV (Pd 3d5/2) and 340.9 eV (Pd3d3/2), and these two peaks can be well fitted with Pd0.53,54 In Fig. 4b, there are three small peaks of Pd oxide, corresponding to PdO 342.5 eV (3d3/2), PdO 336.8 eV (3d5/2) and surface-adsorbed Pd 338.3 eV (PdOxCly, 3d5/2).55–57 The peak area of Pd0 and Pd oxides was calculated, and the content of Pd0 was 84.3%. In Fig. 4c, in the 2p region of Mn, the XPS spectrum shows that the binding energies of the two main peaks Mn 2p1/2 and Mn 2p3/2 are 654.2 eV and 642.3 eV, respectively, which are completely consistent with Mn(III) in MnOOH.58,59
1, and the detected elements are Pd, Mn, O, and C. Fig. 4a shows the survey spectrum of the sample, and Fig. 4b–e correspond to the high resolution spectra of elements Pd, Mn, O, and C, respectively. The characteristic peaks of Pd 3d, Mn 2p and O 1s can be clearly seen in Fig. 4a. As shown in Fig. 4b, it can be clearly observed in the high-resolution spectrum of Pd that there are two characteristic peaks in the 3d region of Pd, which are at 335.6 eV (Pd 3d5/2) and 340.9 eV (Pd3d3/2), and these two peaks can be well fitted with Pd0.53,54 In Fig. 4b, there are three small peaks of Pd oxide, corresponding to PdO 342.5 eV (3d3/2), PdO 336.8 eV (3d5/2) and surface-adsorbed Pd 338.3 eV (PdOxCly, 3d5/2).55–57 The peak area of Pd0 and Pd oxides was calculated, and the content of Pd0 was 84.3%. In Fig. 4c, in the 2p region of Mn, the XPS spectrum shows that the binding energies of the two main peaks Mn 2p1/2 and Mn 2p3/2 are 654.2 eV and 642.3 eV, respectively, which are completely consistent with Mn(III) in MnOOH.58,59
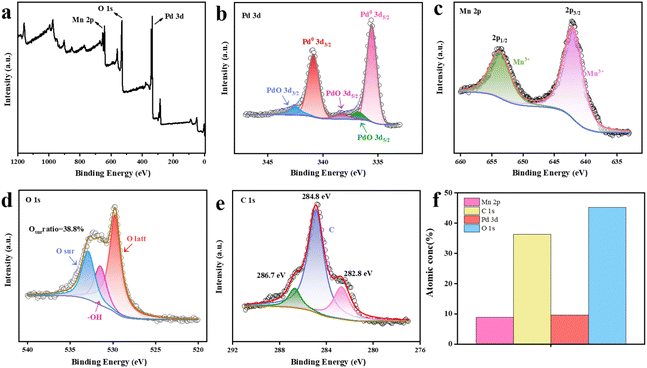 | ||
Fig. 4 (a) XPS survey spectrum of 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and high-resolution XPS spectra of (b) Pd3d, (c) Mn2p, (d) O1s and (e) C1s. (f) Atomic ratio obtained by XPS fitting. 1, and high-resolution XPS spectra of (b) Pd3d, (c) Mn2p, (d) O1s and (e) C1s. (f) Atomic ratio obtained by XPS fitting. | ||
Fig. 4d shows the spectrum of O 1s. Three peaks of O can be observed in the figure: Olatt, Osur, and –OH. Their corresponding electron binding energies are 529.7 eV, 532.5 eV and 531.8 eV, respectively. Olatt and Osur represent oxygen atoms and surface oxygen vacancies in the lattice skeleton, respectively, and the proportion of Osur is 38.8%.60–62 For metal oxides, oxygen vacancy can be used as an electron trapping site to regulate the coordination structure and electronic state of surface adsorbed substances, thus promoting the formation of surface active species and achieving the objective of improving electrocatalytic activity. Because of the addition of sucrose to the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst during the synthesis process, carbon spheres were generated after reduction.46 Therefore, the XPS spectra of C were also analyzed. As shown in Fig. 4e, there are three peaks in the spectrum of C 1s, whose binding energies are 282.8 eV, 284.9 eV, and 286.7 eV, respectively, which are the peaks of calibration material carbon used by testing institutions. The binding energy 284.9 eV is the strongest peak, which is also the characteristic peak of elemental C, so it can also be proved that sucrose is reduced to the elemental carbon sphere during the experiment. Fig. 4f shows the histogram of atomic concentrations of Mn, C, Pd and O, and the proportion of each element is close to the expected result.
1 catalyst during the synthesis process, carbon spheres were generated after reduction.46 Therefore, the XPS spectra of C were also analyzed. As shown in Fig. 4e, there are three peaks in the spectrum of C 1s, whose binding energies are 282.8 eV, 284.9 eV, and 286.7 eV, respectively, which are the peaks of calibration material carbon used by testing institutions. The binding energy 284.9 eV is the strongest peak, which is also the characteristic peak of elemental C, so it can also be proved that sucrose is reduced to the elemental carbon sphere during the experiment. Fig. 4f shows the histogram of atomic concentrations of Mn, C, Pd and O, and the proportion of each element is close to the expected result.
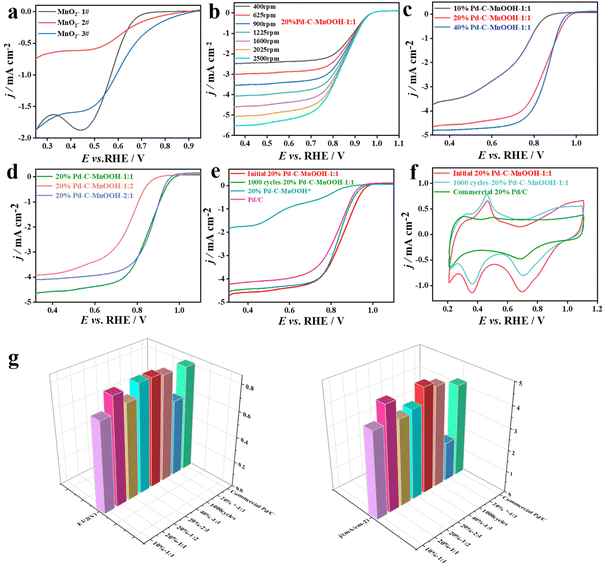
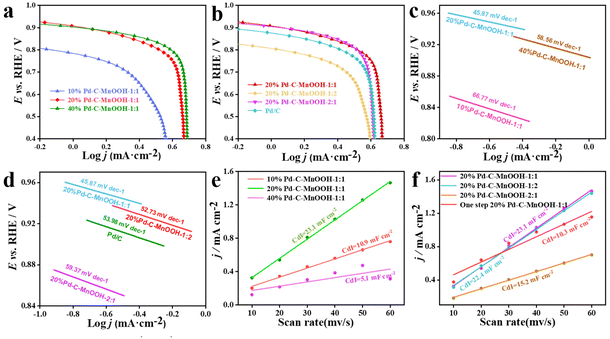
Mn-based oxides have certain ORR activity in alkaline systems. In order to filtrate the MnO2 carrier with the highest activity, the ORR activity of three MnO2 carriers was tested. The ORR activity of all catalysts in alkaline systems was evaluated by linear sweep voltammetry (LSV). The rotating disk electrode speeds are 400, 625, 900, 1225, 1600, 2025 and 2500 rpm, respectively. The scanning range is −0.7–0.1 V (vs. SCE), and the scanning rate was 5 mV s−1. The LSV test curves obtained are shown in Fig. S1a–c.† The three MnO2 catalysts all showed the characteristics of the LSV curve common to oxides, and all had certain ORR activity. Fig. 5a shows the LSV curves of the three MnO2 catalysts at the same speed (1600 rpm). It can be seen that although the initial reduction potential of the MnO2-1 catalyst is more negative, it has the highest limiting diffusion current density. Combined with the following experimental results, MnO2-1 (MnOOH) was selected as the subsequent catalyst carrier.
As shown in Fig. 5b, the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst exhibited the same LSV curve as the precious metal Pd-based ORR catalyst. At 1600 rpm, the jL and half-wave potential (E1/2) values are −4.78 mA cm−2 and 0.84 V, respectively. Under the same conditions, the LSV curves obtained when the Pd load is 10% (10% Pd-C-MnOOH-1
1 catalyst exhibited the same LSV curve as the precious metal Pd-based ORR catalyst. At 1600 rpm, the jL and half-wave potential (E1/2) values are −4.78 mA cm−2 and 0.84 V, respectively. Under the same conditions, the LSV curves obtained when the Pd load is 10% (10% Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 40% (40%Pd-C-MnOOH-1
1) and 40% (40%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) are shown in Fig. S1d and S1e,† respectively. Fig. 5c shows the ORR performance comparison of three Pd-C-MnOOH-1
1) are shown in Fig. S1d and S1e,† respectively. Fig. 5c shows the ORR performance comparison of three Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalysts with different Pd loads at 1600 rpm. When the Pd loading is increased from 10% to 20%, the ORR performance of the catalyst is obviously enhanced (Table 2). When the Pd content continues to increase from 20% to 40%, jL and E1/2 change from −4.78 mA cm−2 and 0.84 V to −4.80 mA cm−2 and 0.85 V, respectively. When the loading of Pd in Pd-C-MnOOH-1
1 catalysts with different Pd loads at 1600 rpm. When the Pd loading is increased from 10% to 20%, the ORR performance of the catalyst is obviously enhanced (Table 2). When the Pd content continues to increase from 20% to 40%, jL and E1/2 change from −4.78 mA cm−2 and 0.84 V to −4.80 mA cm−2 and 0.85 V, respectively. When the loading of Pd in Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 doubled, the jL only increased by 0.42%, and E1/2 was almost unchanged. Therefore, subsequent experiments were conducted to further explore the influence of other factors on the ORR performance of the 20%Pd-C-MnOOH-1
1 doubled, the jL only increased by 0.42%, and E1/2 was almost unchanged. Therefore, subsequent experiments were conducted to further explore the influence of other factors on the ORR performance of the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst.
1 catalyst.
| Catalyst | E 1/2 (V) | J (mA cm−−2) | Electron transfer number (n) | C dI (mF cm−2) |
|---|---|---|---|---|
10%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.71 | −3.97 | 3.7 | 10.9 |
20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.84 | −4.78 | 3.8 | 23.1 |
20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.75 | −3.94 | 3.4 | 22.4 |
20%Pd-C-MnOOH-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.85 | −4.10 | 2.1 | 10.3 |
40%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.85 | −4.80 | 3.8 | 5.1 |
20%Pd-C-MnOOH*-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.60 | −1.83 | 1.8 | 15.2 |
The Pd load was fixed at 20%, and the effect of changing the ratio of carbon and MnOOH on the ORR performance of the catalyst was further studied. Fig. S1f and 1g† show the LSV curves obtained when the mass ratio of C to MnOOH in the catalyst is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. When the content of C is low, the LSV characteristics of some oxides (MnOOH) still exist in the diffusion region of the LSV curve. When the content of C increases, the conductivity of the catalyst is enhanced, and the LSV curve is consistent with that of precious metals. Fig. 5d shows the effects of three different C to MnOOH mass ratios (1
1, respectively. When the content of C is low, the LSV characteristics of some oxides (MnOOH) still exist in the diffusion region of the LSV curve. When the content of C increases, the conductivity of the catalyst is enhanced, and the LSV curve is consistent with that of precious metals. Fig. 5d shows the effects of three different C to MnOOH mass ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 2
2, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on the ORR of the 20%Pd-C-MnOOH catalyst. The statistical results in Table 2 show that the jL values of the two catalysts with a mass ratio of C to MnOOH of 1
1) on the ORR of the 20%Pd-C-MnOOH catalyst. The statistical results in Table 2 show that the jL values of the two catalysts with a mass ratio of C to MnOOH of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 are similar, which are −3.94 mA cm−2 and −4.1 mA cm−2, respectively. However, their E1/2 is quite different (ΔE1/2 = 0.1 V). The reason for this result may be that when the amount of MnOOH is increased, the ORR activity of the metal oxide itself is limited and the conductivity is not as good as that of the carbon material, which leads to the decrease of E1/2 of the catalyst. Therefore, based on the above catalyst performance and cost analysis, it is shown that the 20%Pd-C-MnOOH-1
1 are similar, which are −3.94 mA cm−2 and −4.1 mA cm−2, respectively. However, their E1/2 is quite different (ΔE1/2 = 0.1 V). The reason for this result may be that when the amount of MnOOH is increased, the ORR activity of the metal oxide itself is limited and the conductivity is not as good as that of the carbon material, which leads to the decrease of E1/2 of the catalyst. Therefore, based on the above catalyst performance and cost analysis, it is shown that the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst has the best catalytic activity for ORR.
1 catalyst has the best catalytic activity for ORR.
Our previous studies have shown that if the preparation of the carrier and the catalyst occurs at the same time, the interaction force between the carrier and the catalyst will be stronger, thus improving its electrocatalytic performance.33,37,62 In this study, the preparation of the carrier MnOOH, the reduction of Pd salt and the carbonization of sucrose are completed under the same conditions. Will 20% Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1* obtained in one step show better ORR performance? Unfortunately, as can be seen from Fig. S1h† and Fig. 5e, the ORR activity of 20% Pd-C-MnOOH-1
1* obtained in one step show better ORR performance? Unfortunately, as can be seen from Fig. S1h† and Fig. 5e, the ORR activity of 20% Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1* obtained by the one-step method is much lower than that of 20% Pd-C-MnOOH-1
1* obtained by the one-step method is much lower than that of 20% Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 prepared by the two-step method. In the one-step synthesis, Pd reduction and MnO2 generation may not be carried out simultaneously, and Pd does not complete in situ loading. Furthermore, P123 is consumed in the Pd reduction process, which results in insufficient P123 to reduce KMnO4 to crystal form MnOOH, which may be related to the poor catalytic activity of the 20%Pd-C-MnOOH-1
1 prepared by the two-step method. In the one-step synthesis, Pd reduction and MnO2 generation may not be carried out simultaneously, and Pd does not complete in situ loading. Furthermore, P123 is consumed in the Pd reduction process, which results in insufficient P123 to reduce KMnO4 to crystal form MnOOH, which may be related to the poor catalytic activity of the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1* oxygen reduction reaction. However, we believe that the one-step method still has potential advantages, and this part of the work will be further explored in the follow-up research. At the same time, compared with the commercial 20%Pd/C catalyst (Fig. S1i†), the 20%Pd-C-MnOOH-1
1* oxygen reduction reaction. However, we believe that the one-step method still has potential advantages, and this part of the work will be further explored in the follow-up research. At the same time, compared with the commercial 20%Pd/C catalyst (Fig. S1i†), the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst shows higher ORR activity regarding both jL and E1/2 (Table 2).
1 catalyst shows higher ORR activity regarding both jL and E1/2 (Table 2).
In addition to the activity of the catalyst, the stability of the catalyst is also one of the important indicators to measure the performance of the catalyst. The ORR stability of the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst was studied by LSV and CV methods. Fig. 5e and f show the LSV curves and CV curves of the first and 1000th cycles of the 20% Pd-C-MnOOH-1
1 catalyst was studied by LSV and CV methods. Fig. 5e and f show the LSV curves and CV curves of the first and 1000th cycles of the 20% Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst in an alkaline system, respectively, and are compared with commercial Pd/C catalysts. It should be noted that the CV and LSV tests for the 20%Pd-C-MnOOH-1
1 catalyst in an alkaline system, respectively, and are compared with commercial Pd/C catalysts. It should be noted that the CV and LSV tests for the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst were performed on the same working electrode. After 1000 cycles of the 20%Pd-C-MnOOH-1
1 catalyst were performed on the same working electrode. After 1000 cycles of the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst, the jL value is −4.61 mA cm−2, ΔjL = 0.17 mA cm−2, E1/2 = 0.83 V, and ΔE1/2 = 0.01 V. The jL value of 20%Pd-C-MnOOH-1
1 catalyst, the jL value is −4.61 mA cm−2, ΔjL = 0.17 mA cm−2, E1/2 = 0.83 V, and ΔE1/2 = 0.01 V. The jL value of 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 attenuates only by 3.7%, and the E1/2 value decreases by 1.19%, indicating that the catalyst has good ORR stability. At the same time, the jL and E1/2 values of the 20% Pd-C-MnOOH-1
1 attenuates only by 3.7%, and the E1/2 value decreases by 1.19%, indicating that the catalyst has good ORR stability. At the same time, the jL and E1/2 values of the 20% Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst after 1000 cycles are better than those of the commercial Pd/C catalyst. In order to further compare the stability of the 20%Pd-C-MnOOH-1
1 catalyst after 1000 cycles are better than those of the commercial Pd/C catalyst. In order to further compare the stability of the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst with commercial Pd/C, chronoamperometry was used for 10 hours in a 0.1 M KOH electrolyte under a saturated oxygen atmosphere. The i–t curve (Fig. S1i†) shows that the initial current density of the two catalysts is similar, the Pd/C current density drops sharply around 21
1 catalyst with commercial Pd/C, chronoamperometry was used for 10 hours in a 0.1 M KOH electrolyte under a saturated oxygen atmosphere. The i–t curve (Fig. S1i†) shows that the initial current density of the two catalysts is similar, the Pd/C current density drops sharply around 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 seconds, and the current density of the two catalysts is almost the same after 21
000 seconds, and the current density of the two catalysts is almost the same after 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 seconds. Therefore, the stability of the 20% Pd-C-MnOOH-1
000 seconds. Therefore, the stability of the 20% Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst is almost the same as that of the Pd/C catalyst. At 1600 rpm, the ORR catalytic activity of the 20%Pd-C-MnOOH-1
1 catalyst is almost the same as that of the Pd/C catalyst. At 1600 rpm, the ORR catalytic activity of the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst was compared with that of a commercial 20%Pt/C catalyst. The limiting diffusion current density and half-wave potential of the 20%Pd-C-MnOOH-1
1 catalyst was compared with that of a commercial 20%Pt/C catalyst. The limiting diffusion current density and half-wave potential of the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst were higher than those of commercial Pt/C catalysts. The half-wave potential and limiting diffusion current densities of commercial Pt/C and 20%Pd-C-MnOOH-1
1 catalyst were higher than those of commercial Pt/C catalysts. The half-wave potential and limiting diffusion current densities of commercial Pt/C and 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalysts are 0.82 V, −4.48 mA cm−2 and 0.84 V, −4.78 mA cm−2, respectively. The ORR activity comparison diagram of the 20%Pd-C-MnOOH-1
1 catalysts are 0.82 V, −4.48 mA cm−2 and 0.84 V, −4.78 mA cm−2, respectively. The ORR activity comparison diagram of the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst and the commercial 20%Pt/C catalyst and the complete ORR image of the commercial 20%Pt/C catalyst are shown in Fig. S1k.† From the CV curve of the stability test of the 20% Pd-C-MnOOH-1
1 catalyst and the commercial 20%Pt/C catalyst and the complete ORR image of the commercial 20%Pt/C catalyst are shown in Fig. S1k.† From the CV curve of the stability test of the 20% Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst (Fig. 5f), it can be found that after 1000 cycles, the characteristic peak intensity corresponding to Pd is weakened to a certain extent, but it is very obvious. The performance statistics of various Pd-C-MnOOH catalysts are shown in Table 2 and Fig. 5g. For reference and comparison, the ORR catalytic activity data of similar Pd-based catalysts were collected, as shown in Table S1† in the attachment.
1 catalyst (Fig. 5f), it can be found that after 1000 cycles, the characteristic peak intensity corresponding to Pd is weakened to a certain extent, but it is very obvious. The performance statistics of various Pd-C-MnOOH catalysts are shown in Table 2 and Fig. 5g. For reference and comparison, the ORR catalytic activity data of similar Pd-based catalysts were collected, as shown in Table S1† in the attachment.
In order to further investigate the electrocatalytic activity of the catalyst, the Tafel slope test, electron transfer number fitting, and double layer capacitance testing were performed. The smaller the Tafel slope of the catalyst, the higher the activity of the catalyst and the faster the reaction rate.63 The Tafel plots for ORR are obtained by plotting the potential (E) vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) jK as shown in Fig. 6a and b. When ORR occurs on a Pd-based catalyst, two linear regions appear in the low and high current regions on the Tafel curve. The change of the linear region reveals the mechanism transformation and the improvement of the intrinsic activity of oxygen adsorption on Pd-based electrocatalysts.64 The Tafel slopes of 20% Pd-C-MnOOH-1
jK as shown in Fig. 6a and b. When ORR occurs on a Pd-based catalyst, two linear regions appear in the low and high current regions on the Tafel curve. The change of the linear region reveals the mechanism transformation and the improvement of the intrinsic activity of oxygen adsorption on Pd-based electrocatalysts.64 The Tafel slopes of 20% Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and Pd/C catalysts obtained from Fig. 6c and 6d are 45.87 mV dec−1 and 53.98 mV dec−1, respectively. The results show that the 20% Pd-C-MnOOH-1
1 and Pd/C catalysts obtained from Fig. 6c and 6d are 45.87 mV dec−1 and 53.98 mV dec−1, respectively. The results show that the 20% Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst has higher ORR activity, which is consistent with the results of the previous analysis.
1 catalyst has higher ORR activity, which is consistent with the results of the previous analysis.
The electrochemically active area (CdI) of the catalyst can be obtained by integrating the double layer in the CV curve of the specified interval (Fig. S2†). It can be seen from Fig. 6e and f that the CdI of the Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst is 23.1 mF cm−2, which is the largest among all obtained Pd-based catalysts. According to the K–L equation, the LSV curves at 400 rpm, 900 rpm, 1600 rpm and 2500 rpm were selected for linear fitting, and the electron transfer number of the 20%Pd-C-MnOOH-1
1 catalyst is 23.1 mF cm−2, which is the largest among all obtained Pd-based catalysts. According to the K–L equation, the LSV curves at 400 rpm, 900 rpm, 1600 rpm and 2500 rpm were selected for linear fitting, and the electron transfer number of the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst during the ORR was calculated. The fitted graph line is shown in Fig. S3b† and the slope of the linear equation K = 7.536 is obtained.65,66 The electron transfer number n = 3.8 is calculated by incorporating it into the K–L equation, which indicates that 20%Pd-C-MnOOH-1
1 catalyst during the ORR was calculated. The fitted graph line is shown in Fig. S3b† and the slope of the linear equation K = 7.536 is obtained.65,66 The electron transfer number n = 3.8 is calculated by incorporating it into the K–L equation, which indicates that 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 can be considered a 4-electron process in the ORR, and the O–O bond is broken directly to generate H2O in the 4-electron reaction with a high reaction efficiency.
1 can be considered a 4-electron process in the ORR, and the O–O bond is broken directly to generate H2O in the 4-electron reaction with a high reaction efficiency.
As a comparison, after completing the ORR test of the catalyst, TEM images were taken of the 20% Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst to observe whether the morphology and structure of the catalyst had changed before and after use. As shown in Fig. S4,† the 20%Pd-C-MnOOH-1
1 catalyst to observe whether the morphology and structure of the catalyst had changed before and after use. As shown in Fig. S4,† the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst exhibited slight agglomeration after use. Part of Pd is loaded on the surface of MnOOH, and the remaining Pd is wrapped inside MnOOH. On the whole, the morphology and structure of the 20%Pd-C-MnOOH-1
1 catalyst exhibited slight agglomeration after use. Part of Pd is loaded on the surface of MnOOH, and the remaining Pd is wrapped inside MnOOH. On the whole, the morphology and structure of the 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst did not change much before and after use and could still maintain the shape before use, which may be the reason for the good stability of the catalyst.
1 catalyst did not change much before and after use and could still maintain the shape before use, which may be the reason for the good stability of the catalyst.
Combined with relevant literature reports, the main reasons why 20%Pd-C-MnOOH showed enhanced ORR catalytic activity and stability may be as follows: (1) the co-catalytic effect of Pd and MnOOH. Many studies have shown that the chemical properties of support and metal–support interaction (MSI) strongly regulate the catalyst activity.67 The MnOOH carrier with a stable structure and certain ORR activity can effectively disperse Pd with a hexagonal structure, and its interaction can improve its ORR performance more effectively; (2) the chain structure, 3D carbon grid and carbon layer improve its electrical conductivity. The Pd-C-MnOOH composite catalyst is also a chain-like structure under the inductive effect of the chain-like P123 itself.34,47 During the loading process, the carbon network with a 3D mesh structure and a carbon layer formed by in situ carbonization of sucrose are conducive to improving the conductivity of the catalyst;33,37,68 (3) this method of simultaneous loading and in situ carbonization involving P123 and sucrose is also the reason for the improvement of catalyst performance. The method is expected to be extended to other electrocatalysis fields, and related work is also underway.
4. Conclusions
In summary, three kinds of manganese oxide were synthesized by the hydrothermal method. After structural characterization and the performance test, MnOOH with a definite structure and the highest ORR activity was selected as the carrier material. Under the action of P123 and sucrose, through the hydrothermal reaction, the reduction of the Pd precursor and sucrose carbonization occurred simultaneously, and a Pd-C-MnOOH composite catalyst was obtained. The effects of the synthesis method, the amount of Pd load and the C/MnOOH ratio on the performance of the catalyst were discussed. Finally, it was found that when the Pd load was 20% and the C/MnOOH ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the 20% Pd-C-MnOOh-1
1, the 20% Pd-C-MnOOh-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst prepared by the one-step reaction showed the highest ORR performance. At 1600 rpm, the jL value of 20% Pd-C-MnOOH-1
1 catalyst prepared by the one-step reaction showed the highest ORR performance. At 1600 rpm, the jL value of 20% Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is −4.78 mA cm−2 and the E1/2 value is 0.84 V, both of which are higher than those of commercial 20%Pd/C catalysts. After 1000 CV cycles, the jL value of 20%Pd-C-MnOOH-1
1 is −4.78 mA cm−2 and the E1/2 value is 0.84 V, both of which are higher than those of commercial 20%Pd/C catalysts. After 1000 CV cycles, the jL value of 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 only decreased by 3.7%, and the E1/2 value only decreased by 0.01 V, indicating that the stability of the catalyst was very high. In addition, when 20%Pd-C-MnOOH-1
1 only decreased by 3.7%, and the E1/2 value only decreased by 0.01 V, indicating that the stability of the catalyst was very high. In addition, when 20%Pd-C-MnOOH-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 participates in the ORR, the electron transfer number n = 3.8, which indicates approximately 4 electron processes, and it is a reaction with a high reaction rate and a high output voltage. The reason for the improvement of the ORR performance of the Pd-C-MnOOH composite catalyst may be the special chain structure introduced by P123, the carbon layer formed by in situ carbonization, and the co-catalysis effect between the metal and the oxide. This study provides a new idea for the synthesis of oxygen reduction catalysts in alkaline media and proves that oxides with certain catalytic properties can be used as support materials by loading and doping methods to improve the activity and stability of catalysts.
1 participates in the ORR, the electron transfer number n = 3.8, which indicates approximately 4 electron processes, and it is a reaction with a high reaction rate and a high output voltage. The reason for the improvement of the ORR performance of the Pd-C-MnOOH composite catalyst may be the special chain structure introduced by P123, the carbon layer formed by in situ carbonization, and the co-catalysis effect between the metal and the oxide. This study provides a new idea for the synthesis of oxygen reduction catalysts in alkaline media and proves that oxides with certain catalytic properties can be used as support materials by loading and doping methods to improve the activity and stability of catalysts.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The financial support from the National Natural Science Foundation of China (21503120, 21403126), the Hubei Provincial Natural Science Foundation of China (2018CFB659) and the 111 Project (D20015) is highly acknowledged.References
- X. Q. Mu, S. L. Liu, L. Chen and S. C. Mu, Small Struct., 2023, 4, 2200281 CrossRef CAS.
- A. Arora, P. Oswal, A. Datta and A. Kumar, Coord. Chem. Rev., 2022, 459, 214406 CrossRef CAS.
- J. M. Turnet, Science, 2022, 376, 6600 Search PubMed.
- A. Troeger, Sol. RRL, 2023, 7, 2201038 CrossRef.
- Y. Yurko and L. Elbaz, J. Am. Chem. Soc., 2023, 145, 2653–2660 CrossRef CAS PubMed.
- S. Ong, A. Al-Othman and M. Tawalbeh, Energy, 2023, 277, 127721 CrossRef CAS.
- T. Zhao, E. G. Luo, X. Wang, J. J. Ge, C. P. Liu and G. J. He, J. Electrochem., 2020, 26(1), 84–95 CAS.
- Y. Z. Chen, S. M. Zhang, J. C-Y. Jung and J. J. Zhang, Prog. Energy Combust. Sci., 2023, 98, 101101 CrossRef.
- Y. K. Kim, H. E. Bae, D. Lee, J. Kim and E. Lee, J. Power Sources, 2022, 533, 231378 CrossRef CAS.
- Y. Q. Kang, J. Q. Wang, Y. P. Wei, Y. L. Wu, D. S. Xia and L. Gan, Nano Res., 2022, 15, 6148–6155 CrossRef CAS.
- L. Hang, M. Wei, R. J. Qi, C. L. Dong, D. Dang, C. C. Yang, C. F. Xia and C. Chen, Nat. Commun., 2022, 13, 6703 CrossRef PubMed.
- T. J. Wang, Y. C. Jiang, J. W. He and F. M. Li, Carbon Energy, 2022, 4, 283–293 CrossRef CAS.
- Y. F. Zhang, F. Xiao, G. Y. Chen and M. H. Shao, J. Electrochem., 2020, 26(4), 563–572 CAS.
- L. Huang, S. H. Zaman, X. L. Tian, Z. T. Wang, W. S. Fang and B. Y. Xia, Acc. Chem. Res., 2021, 54, 311–322 CrossRef CAS PubMed.
- T. L. Xia, Y. Yang, Q. Song, M. C. Luo, M. Q. Xue, K. Ostrikov, Y. Zhao and F. W. Li, Nanoscale Horiz., 2023, 8, 146–157 RSC.
- F. Omobosede, A. Bello, T. Adebusuyi and S. Bindir, Curr. Opin. Electrochem., 2022, 36, 101132 CrossRef.
- G. L. Vecchio, X. Lyu, I. Gatto, B. Zulevi, A. Serov and V. Baglio, CNR-ITAE, J. Power Sources, 2023, 561, 232732 CrossRef.
- M. L. Wang, J. Zhao, J. J. Wang, J. M. Zhang, Y. Z. Tian and Z. Z. Yue, Rare Met., 2023, 42(5), 1516–1525 CrossRef CAS.
- T. B. Ferriday and P. H. Middleton, Int. J. Hydrogen Energy, 2021, 46, 18489–18510 CrossRef CAS.
- F. Xiao, Y. C. Wang, Z. P. Wu, G. Y. Chen, F. Yang, S. Q. Zhu, K. Siddharth, Z. J. Kong, A. Lu and J. C. Li, Adv. Mater., 2021, 33, 2006292 CrossRef CAS PubMed.
- Y. J. Tang, S. S. Zheng, S. Cao, H. G. Xue and H. Pang, J. Mater. Chem. A, 2022, 8, 18492–18514 RSC.
- X. Y. Chen, J. Chen, Y. J. Liu, Y. Liu, Y. Gao, S. W. Fan, X. X. He, X. H. Liu, C. Shen, Y. Jiang, L. L. Yun and Y. Qiao, ACS Appl. Mater. Interfaces, 2023, 15, 28106–28115 CrossRef CAS PubMed.
- P. Rui, A. Q. Zheng, B. He, Y. W. Xiong, F. S. Han, L. Wei, Q. W. Li, Q. C. Zhang, K. B. Yin, L. T. Sun and B. C. Sun, Nanoscale Horiz., 2022, 7, 1501–15123 RSC.
- X. K. Jin, M. H. He, F. J. Chen, K. Z. Li, J. Y. Min, Z. Y. Wang, J. H. Li and J. J. Chen, Chem. Eng. J., 2023, 464, 142712 CrossRef CAS.
- Y. Liu, S. Q. Zhu, Z. Zhang, Q. Sun, C. Wu, W. B. Gong, L. X. Kang and Y. Yang, J. Alloys Compd., 2023, 957, 170362 CrossRef CAS.
- C. Z. Wang, H. Yang, B. Wang, P. B. Ding, Y. Wan, W. J. Bao, Y. N. Li, S. Y. Ma, Y. Liu, Y. K. Lu and H. Hu, Nano Res., 2023, 16, 9488–9495 CrossRef CAS.
- H. Yan, B. W. Liu, X. Zhou, F. Y. Meng, M. Y. Zhao, Y. Pan, J. Liu, Y. N. Wu, H. Zhao, Y. B. Liu, X. B. Chen, L. N. Li and X. Feng, Nat. Commun., 2023, 14, 4509 CrossRef CAS PubMed.
- L. Tian, Z. Li, J. J. Wang, K. Wang, H. X. Wo, X. Wang, W. C. Zhuang, T. X. Li and X. H. Du, Carbon, 2022, 143, 457–466 CrossRef.
- I. Cruz-Reyes, B. Trujillo-Navarrete, K. García-Tapia, M. I. Salazar-GastélumAzuma, H. Tamagaki, F. Paraguay-Delgado and R. M. Félix-Navarro, Fuel, 2020, 279, 118470 CrossRef CAS.
- H. Purwaningsih, N. I. P. Suari, W. Widiyastuti and H. Setyawan, ACS Omega, 2022, 7, 6760–6767 CrossRef CAS PubMed.
- S. W. Chen, H. B. Wang, M. Y. Zhu, F. You, W. Lin, D. Chan, W. X. Lin, P. Li, Y. X. Tang and Y. Y. Zhang, Nanoscale Horiz., 2023, 8, 29–54 RSC.
- X. W. Zhou, Y. L. Gan, Z. X. Dai and R. H. Zhang, J. Electroanal. Chem., 2012, 685, 97–102 CrossRef CAS.
- Y. Yang, L. M. Luo, D. Chen, H. M. Liu, R. H. Zhang, Z. X. Dai and X. W. Zhou, Acta Phys.-Chim. Sin., 2017, 33(8), 1628–1634 CAS.
- W. Zhan, Z. Cheng, R. H. Zhang, L. Y. Yan, G. X. Tian, Z. X. Dai, S. Z. Xiao and X. W. Zhou, J. Electroanal. Chem., 2023, 942, 117547 CrossRef CAS.
- C. Fu, P. Wang, R. H. Zhang, L. Y. Yan, Z. Cheng, D. H. Lin and X. W. Zhou, Fuel, 2023, 332, 126105 CrossRef CAS.
- G. X. Tian, Y. Yang, R. H. Zhang, L. Y. Yan, Z. Cheng, D. H. Lin and X. W. Zhou, Adv. Energy Sustainability Res., 2023, 2300058 CrossRef.
- Y. Yang, L. M. Luo, J. J. Du, S. S. Li, R. H. Zhang, Z. X. Dai and X. W. Zhou, Int. J. Hydrogen Energy, 2016, 41, 12062–12068 CrossRef CAS.
- X. H. Cai, H. H. Li, H. H. Yan, Y. W. Liu, H. X. Yu and L. Yan, Ionics, 2021, 27, 3943–3950 CrossRef CAS.
- S. Yadav, A. S. Ghrera and A. Devi, J. Energy Storage, 2022, 56, 105949 CrossRef.
- S. C. Xu, Y. M. Kim, D. Higgins, M. Yusuf, T. F. Jaramillo and F. B. Prinz, Electrochim. Acta, 2017, 255, 99–108 CrossRef CAS.
- J. Masa, C. B. Mcauley, W. G. Cchuhmann and R. G. Compton, Nano Res., 2014, 7, 71–78 CrossRef CAS.
- A. Sathyaseelan, V. Elumalai, K. Krishnamoorthy, A. Sajeew and S. J. Kim, ACS Sustainable Chem. Eng., 2023, 11, 5345–5355 CrossRef CAS.
- X. S. Peng and I. Ichinose, Adv. Funct. Mater., 2011, 21, 2080–2087 CrossRef CAS.
- L. Wang and D. L. Wang, Electrochim. Acta, 2011, 56, 5010–5015 CrossRef CAS.
- Z. L. Cui and X. F. Bai, ACS Appl. Mater. Interfaces, 2022, 14, 9024–9035 CrossRef CAS PubMed.
- A. Sathyaseelan, V. Elumalai, K. Krishnamoorthy, A. Sajeew and S. J. Kim, ACS Sustainable Chem. Eng., 2023, 11, 5345–5355 CrossRef CAS.
- Q. Y. Hu, R. H. Zhang, D. Chen, Y. F. Guo, W. Zhan, L. M. Luo and X. W. Zhou, Int. J. Hydrogen Energy, 2019, 44, 16287–16296 CrossRef CAS.
- H. L. Li, S. Dai, Y. W. Wu, Q. Dong, J. J. Chen and H. T. Chen, Adv. Sci., 2023, 10, 2207109 CrossRef CAS PubMed.
- Y. Wang, J. L. Liu, H. J. Yuan, F. Liu, T. J. Hu and B. Q. Yang, Adv. Funct. Mater., 2023, 33, 2211909 CrossRef CAS.
- D. Bhalothia, C. Yan, N. Hiraoka, H. Ishii, Y. F. Liao, P. C. Chen, K. W. Wang and J. P. Chou, ACS Appl. Mater. Interfaces, 2023, 15, 16177–16188 CrossRef CAS PubMed.
- Y. B. Cao, Y. H. Xiao, Y. Y. Gong, C. F. Wang and F. Li, Electrochim. Acta, 2014, 127, 200–207 CrossRef CAS.
- G. P. Kim, D. Lim, I. Park, H. Park, S. E. Shim and S. H. Baeck, J. Power Sources, 2016, 324, 687–693 CrossRef CAS.
- D. Y. Wei, G. N. Xing, H. Q. Chen, X. Q. Xie, H. M. Huang, J. C. Dong, J. H. Tian, H. Zhang and J. F. Li, J. Colloid Interface Sci., 2023, 650, 1518–1524 CrossRef CAS PubMed.
- M. J. Ma, Y. Zhang, Y. J. Ji, Q. Shao, W. X. Zhu, J. J. Yang, F. Liao, Z. L. Fan, Y. Liu, Y. Y. Li, M. W. Shao and Z. H. Kang, Appl. Catal., B, 2022, 315, 121549 CrossRef CAS.
- V. H. Do, P. Prabhu, V. Jose, T. Yoshida, Y. T. Zhou, H. Miwa, T. Kaneko, T. Uruga, Y. Iwasawa and J. M. Lee, Adv. Mater., 2023, 35, 2208860 CrossRef CAS PubMed.
- X. H. Huang, Y. J. Xia, Y. J. Cao, X. S. Zheng, H. B. Pan, J. F. Zhu, C. Ma, H. W. Wang, J. J. Li, R. You, S. Q. Wei, W. X. Huang and J. J. Lu, Nano Res., 2017, 10(4), 1302–1312 CrossRef CAS.
- B. T. Hu, K. A. Sun, Z. W. Zhuang, Z. Chen, S. J. Liu, W. C. Cheong, C. Chen, M. Z. Hu, X. Cao, J. G. Ma, R. Y. Tu, X. S. Zheng, H. Xiao, X. Chen, Y. Cui, Q. Peng, C. Chen and Y. D. Li, Adv. Mater., 2022, 34, 2107721 CrossRef CAS PubMed.
- Y. Dong, Y. Liu, Y. Y. He, Z. Y. Chen, Y. Orikasa, Y. Uchimoto and X. M. Wang, ACS Sustainable Chem. Eng., 2023, 11, 9523–9527 CrossRef CAS.
- X. Hua, C. J. Mao, J. S. Chen, P. P. Chen and C. F. Zhang, J. Alloys Compd., 2019, 777, 749–758 CrossRef CAS.
- D. P. Liu, J. Tian, Y. G. Tang, J. S. Li, S. A. Wu, S. J. Yi and X. B. Huang, Chem. Eng. J., 2021, 406, 126772 CrossRef CAS.
- Y. L. Sun, Y. Lin, M. F. Yue, H. Q. Chen, H. J. Ze, Y. H. Wang, J. C. Dong, Z. Q. Tian and P. P. Fang, Anal. Chem., 2022, 94, 4779–4786 CrossRef CAS PubMed.
- Y. Yang, Y. F. Guo, C. Fu, R. H. Zhang, P. Wang, X. Zhang, Q. Wang and X. W. Zhou, J. Colloid Interface Sci., 2021, 595, 107–117 CrossRef CAS PubMed.
- C. Costentin and J. M. Savéant, ACS Appl. Mater. Interfaces, 2017, 23, 19894–19899 CrossRef PubMed.
- L. M. Luo, R. H. Zhang, D. Chen, Q. Y. Hu and X. W. Zhou, ACS Appl. Energy Mater., 2018, 1, 2619–2629 CrossRef CAS.
- Z. Y. Mei, S. Cai, G. F. Zhao, Q. Jing, X. L. Sheng, J. W. Jiang and H. Guo, Energy Storage Mater., 2022, 50, 12–20 CrossRef.
- X. H. Zhang, P. Lu, C. Zhang, X. Z. Cui, Y. F. Xu, H. Y. Qu and J. L. Shi, J. Catal., 2017, 356, 229–236 CrossRef CAS.
- V. Muravev, A. Parastaev, Y. Bosch, B. Ligt, N. Claes, S. Bals, N. Kosinov and E. J. M. Hensen, Science, 2023, 380, 1174–1178 CrossRef CAS PubMed.
- X. X. Huang, T. Shen, S. N. Sun and Y. L. Hou, ACS Appl. Mater. Interfaces, 2021, 13, 6989–7003 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nr04724e |
| This journal is © The Royal Society of Chemistry 2024 |