Single-cell force spectroscopy of fluid flow-tuned cell adhesion for dissecting hemodynamics in tumor metastasis†
Jiajia
Wei
 abc,
Yanqi
Yang
abc and
Mi
Li
abc,
Yanqi
Yang
abc and
Mi
Li
 *abc
*abc
aState Key Laboratory of Robotics, Shenyang Institute of Automation, Chinese Academy of Sciences, Shenyang 110016, China. E-mail: limi@sia.cn
bInstitutes for Robotics and Intelligent Manufacturing, Chinese Academy of Sciences, Shenyang 110169, China
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 5th December 2023
Abstract
Cell adhesion plays an important role in regulating the metastasis of cancer cells, and atomic force microscopy (AFM)-based single-cell force spectroscopy (SCFS) has become an important method to directly measure the adhesion forces of individual cells. Particularly, bodily fluid flow environments strongly affect the functions and behaviors of metastatic cells for successful dissemination. Nevertheless, the interactions between fluidic flow medium environment and cell adhesion remain poorly understood. In this work, AFM-based SCFS was exploited to examine the effects of fluidic flow environment on cellular adhesion. A fluidic cell culture medium device was used to simulate the fluidic flow environment experienced by cancer cells during metastasis, which was combined with AFM-based SCFS assay. A single living cancer cell was attached to the AFM tipless cantilever to prepare the single-cell probe for performing SCFS experiments on the mesothelial cells grown under the fluidic flow medium conditions, and the effects of experimental parameters (retraction speed, contact time, loading force) on the measured cellular adhesion forces were analyzed. Experimental results of SCFS assay show that cellular adhesion forces significantly decrease after growth in fluidic flow medium, whereas cellular adhesion forces increase after growth in static culture medium. Experiments performed with the use of spherical probes coated with cell adhesion-associated biomolecules also show the weakening of cell adhesion after growth in fluidic flow cell culture medium, which was subsequently confirmed by the confocal fluorescence microscopy experiments of cell adhesion molecules, vividly illustrating the remarkable effects of fluidic flow environment on cellular adhesion. The study provides a new approach to detect adhesion force dynamics involved in the interactions between cells and the fluidic flow environment at the single-cell level, which will facilitate dissecting the role of hemodynamics in tumor metastasis.
1. Introduction
Cell adhesion plays a crucial role in the physiological and pathological processes of living organisms. Attachments of a cell to other cells (such as in epithelial tissues) and to extracellular matrix (ECM) (such as in connective tissues) control the orientation and behavior of the cell's cytoskeleton, thereby allowing cells to sense and respond to changes in the mechanical features of their environment which ultimately affect every aspect of the organization, function, and dynamics of multicellular structures.1 Cell adhesion molecules (e.g., integrin, cadherin, immunoglobulin, selectin, proteoglycan superfamilies) are important functional units that mediate binding interactions at the extracellular surface and determine the specificity of cell–cell and cell–ECM recognition.2 These cell adhesion molecules recognize and bind to either other cell adhesion molecules on adjacent cells or bind to proteins (e.g., proteoglycans, collagens, elastins, fibronectins, laminins3) of the ECM. Aberrant cell adhesion interactions often contribute to the pathological changes such as cancer. The two major hallmarks of cancer, loss of cell-to-cell adhesion and anchorage-independent growth, are both dependent on cell adhesion molecules.4 There are also substantial changes in the ECM components and features during tumor progression, which influence the adhesion behaviors of cancer cells.5 For the unicellular organisms, such as bacteria, cellular adhesion to form biofilm is critical for bacteria to survive in the hostile host environment.6 Accordingly, revealing the delicate events taking place during the adhesion behaviors of single cells7 is of great significance for unraveling the mysteries of life activities and will potentially promote the diagnosis and treatment of human diseases.Atomic force microscopy (AFM)-based single-cell force spectroscopy (SCFS)8,9 has become an important and standard method for characterizing and quantifying the adhesion of single cells. In SCFS, a living cell is attached to a tipless cantilever to prepare the single-cell probe which is then used to perform approach-dwell-retract cycles on a substrate (the substrate can be another cell or a specific surface depending on the type of cell adhesion to be detected) to obtain the force curves. The detailed detachment process of single cell from the substrate can be identified and the detachment force can be calculated from the acquired force curves. Now, several detailed protocols are available for the attachment of different types of cells (such as animal cells10 and microbial cells11) to the cantilever, facilitating SCFS-based studies. Studies with the use of AFM-based SCFS have yielded numerous novel insights into how mechanical forces regulate the physiological and pathological processes, which contribute much to the field of single-cell mechanobiology.12,13 Particularly, AFM-based SCFS has been applied to probe various adhesion behaviors of single cancer cells, such as adhesion between cancer cells and effector cells,14 adhesion between cancer cells and stem cells,15 effect of epidermal growth factor receptor on the adhesion of cancer cells,16 adhesion between cancer cells and endothelium cells,17 and adhesion between cancer cells and activated platelets.18 However, current studies of cancer cells using AFM-based SCFS are commonly performed in static medium, which cannot access the effects of fluidic flow environment on cancer cell adhesion. The bodily fluid flow environment (such as blood and lymphatic circulatory systems) plays a crucial role in the metastasis of cancer cells,19 which can influence the migration and dissemination of cancer cells.20 So far, knowledge about the adhesion of individual cancer cells in fluidic flow environment is quite poor and thus utilizing AFM-based SCFS to explore the effects of fluidic flow conditions on the adhesion of cancer cells will benefit dissecting the role of hemodynamics in tumor metastasis.
Previously, we have developed a method of integrating AFM with a fluidic medium device to reveal the effect of fluid mechanics in tuning the mechanical behaviors of single cells.21 In this work, we present a study of applying AFM-based SCFS to characterize how the fluidic medium (non-static/flow) environment affects the adhesion behaviors of cells at the single-cell level. Experimental results of AFM force spectroscopy show that fluidic environment can significantly weaken cellular adhesion, which were confirmed by confocal fluorescence microscopy experiments, adding to the understanding of fluid flow-tuned cell adhesion and providing additional possibilities for unraveling the underlying mechanisms guiding tumor invasion and metastasis.
2. Materials and methods
2.1. Cell culture
HMrSV5 cells (a human peritoneal mesothelial cell line), MGC-803 cells (a human gastric cancer cell line), and HGC-27 cells (a human undifferentiated gastric cancer cell line) were purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). The RPMI-1640 cell culture medium and phosphate-buffered saline (PBS) were purchased from DearyTech Company (Shanghai, China). The Leibovitz's L-15 medium, fetal bovine serum (FBS), and trypsin solution were purchased from Thermo Fisher Scientific Company (Waltham, Massachusetts, USA). The penicillin–streptomycin solution was purchased from Hyclone Laboratories (Logan, UT, USA). HMrSV5 cells, MGC-803 cells, and HGC-27 cells were cultured in RPMI-1640 medium containing 10% FBS and 1% penicillin–streptomycin at 37 °C (5% CO2 and 95% air). The cell culture medium was changed to the Leibovitz's L-15 medium containing 10% FBS and 1% penicillin–streptomycin when cells were cultured in the fluidic cell growth medium device.2.2. Reagents and preparation
The biotin-conjugated concanavalin A (ConA) powder was purchased from Sigma-Aldrich Company (St Louis, MO, USA). The biotin-conjugated bovine serum albumin (BSA) solution, streptavidin powder, and fibronectin solution (from human plasma) were purchased from Solarbio Science & Technology Company (Beijing, China). The Hank's balanced salt solution (HBSS) was purchased from Beyotime Biotechnology (Shanghai, China). The biotin–ConA powder was dissolved in PBS to prepare the stock solution (the final concentration is 0.4 mg mL−1), which was then stored at −20 °C. The biotin–BSA stock solution (the final concentration is 0.5 mg mL−1) was prepared by dissolving biotin–BSA solution in NaHCO3 buffer (100 mM, pH 8.6) and stored at −20 °C. The streptavidin stock solution (the final concentration is 0.5 mg mL−1) was prepared by dissolving streptavidin powder in PBS and stored at −20 °C. The fibronectin stock solution (the final concentration is 20 μg mL−1) was obtained by dissolving fibronectin solution in HBSS, which was then stored at 4 °C. For E-cadherin confocal fluorescence microscopy experiments, the E-cadherin mouse monoclonal antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA), and the Alexa Fluor 488-labeled donkey anti-mouse IgG secondary antibodies were purchased from Jackson ImmunoResearch (West Grove, PA, USA). Other reagents used in the confocal fluorescence microscopy experiments, including chemical fixation solution, Triton X-100, donkey blocking serum, and DAPI dye solution, were purchased from Solarbio Science & Technology Company (Beijing, China).2.3. Functionalization of AFM tipless cantilevers for SCFS
For AFM-based SCFS experiments, the tipless cantilevers (NP-O10-B, Bruker Company, Santa Barara, CA, USA) were modified with a layer of ConA according to a protocol.10 The material of the cantilever is silicon nitride and the nominal spring constant of the cantilever is 0.12 N m−1. For the functionalization of ConA, the tipless cantilevers were firstly placed in a biological safety cabinet (Thermo Fisher Scientific Company, Waltham, Massachusetts, USA) and sterilized by UV light overnight. Next, 20 μL of biotin–BSA solution was added to a fresh Petri dish, and the disinfected cantilevers were immersed in the solution and incubated overnight at 37 °C in a cell culture incubator (Thermo Fisher Scientific Company, Waltham, Massachusetts, USA). After the incubation, the cantilevers were washed three times with PBS to remove unbound molecules. The cantilevers were then immersed and incubated in 20 μL of streptavidin solution for 30 min at room temperature, after which the cantilevers were washed three times with PBS to remove unbound molecules. Subsequently, the cantilevers were incubated in 20 μL of biotin–ConA solution for 60 min at room temperature, and PBS was used to wash the cantilevers three times. The cantilevers could then be used for AFM-based SCFS experiments.2.4. Preparation of AFM spherical probes
The microsphere-modified probes were prepared with the use of AFM (JPK NanoWizard AFM, Bruker, Santa Barbara, CA, USA) manipulations under the guidance of an inverted optical microscopy (Eclipse Ti-U, Nikon, Tokyo, Japan) according to a previous protocol.21 Two types of microspheres (BaseLine Company, Tianjin, China) were used for the functionalization of different biomolecules, including silica microspheres (10 micron in diameter) and polystyrene microspheres (10 micron in diameter). The ConA molecules were coated onto the silica microspheres, and the fibronectin molecules were coated onto the polystyrene microspheres. The procedure of attaching a microsphere onto an AFM tipless cantilever (NP-O10-B, Bruker Company, Santa Barara, CA, USA) is following. Firstly, the two components of epoxy adhesives (3M, Maplewood, Minnesota, USA) were mixed on a bare area of a fresh glass slide. Subsequently, the microsphere powder was added to another bare area of the glass slide. Next, under the guidance of optical microscopy, the AFM probe was moved to lightly touch the epoxy adhesive, after which the AFM probe was controlled to retract from the adhesive. The adhesive-modified AFM probe was then controlled to touch a single microsphere, and the microsphere-modified probe was prepared. The microsphere-modified probe was placed in an AFM probe box at room temperature for 12 h for hardening of epoxy adhesive, after which the probe could be used in the subsequent molecular functionalization.2.5. Molecular functionalization of AFM spherical probes
The process of coating ConA molecules on the surface of microsphere (silica)-modified probes is the same as that of attaching ConA molecules onto the tipless cantilever described above. Fibronectin molecules were attached onto the microsphere (polystyrene)-modified probes according to the reagent supplier's manual. Briefly, 20 μL of fibronectin solution was added into a Petri dish, and then the spherical probes were immersed in the fibronectin solution and incubated for at least 1 hour at room temperature in air. After the incubation, the excess fibronectin solution was removed. The dried spherical probes could be immediately used in the subsequent experiments or stored at 4 °C for no more than 1 week.2.6. AFM-based SCFS experiments
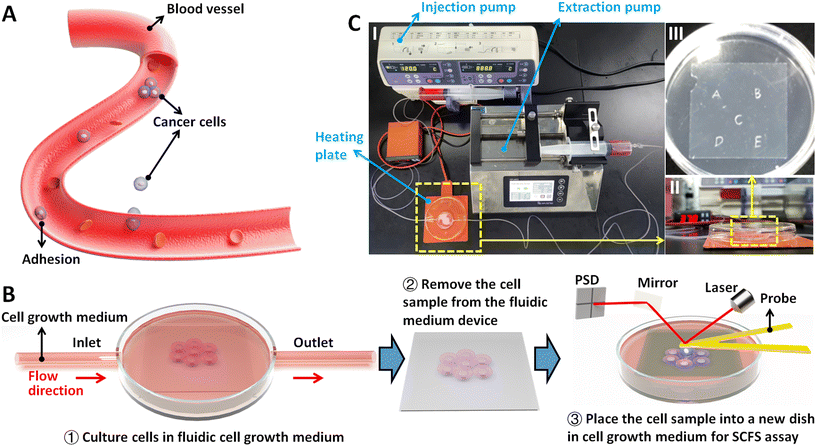
A commercial AFM called JPK NanoWizard AFM (Bruker, Santa Barbara, CA, USA) was used for AFM-based SCFS experiments, which has a heater system providing the temperature environment (37 °C) required for the growth and proliferation of living cells. A fluidic cell culture medium device21 was established to simulate the flowing fluid conditions for living cells, which includes an injection pump, an extraction pump, a heating plate, a side-opening Petri dish, and tubes (Fig. 1C). Under the guidance of optical microscopy, the prepared single-cell probes (described in the main text) or biomolecule-coated spherical probes were used to perform approach-dwell-retract cycles on individual living cells, during which force curves were recorded. In order to ensure the comparability of the results, the experimental parameters of obtaining force curves were kept the same: the loading force of probe was 5 nN, the retract speed of probe was 5 μm s−1, and the dwell time of probe on the cell after the approach process was 2 s. Before preparing the single-cell probe, the ConA-functionalized cantilever was used to obtain force curves on a fresh coverslip to calibrate the deflection sensitivity of the cantilever, which was then used to exactly calibrate the spring constant of the cantilever by using the thermal noise module of the AFM.In order to examine the changes of cellular adhesion forces under fluidic flow medium conditions, cells were seeded on the marked coverslips (physical marks on the bottom face of a coverslip were formed by using a silicon wafer knife). These marks are used to visually locate the cells to be measured by AFM. After the cells which were grown on the coverslip reached 60–80% confluence, the cell-seeded coverslip was placed in a fresh Petri dish containing L-15 medium. Under the guidance of the optical microscopy, 10 cells near the central region of the coverslip were selected for AFM measurements at 37 °C (0 hour group). For each cell, 10 force curves were obtained. After the measurements, the coverslip was placed in the fluidic cell culture medium device, and the injection pump and extraction pump were activated to create a fluidic environment on the cells at a constant flow rate of 120 mL h−1 for 4 h. Subsequently, the pumps were turned off, and the same 10 cells were measured again by AFM at 37 °C to obtain force curves (4 hours group). For control experiments (in static medium), the cell-seeded coverslip was placed in a Petri dish containing regular medium, and 10 cells were measured by AFM at 37 °C (0 hour group). The cells were then cultured in a cell culture incubator at 37 °C (5% CO2 and 95% air) for 4 h, after which the 10 cells were measured by AFM again at 37 °C (4 hours group).
2.7. Confocal fluorescence microscopy
Confocal fluorescence microscopy experiments to detect the expression levels of E-cadherin molecules on HMrSV5 cells after growth in static or fluidic medium were performed. For doing so, HMrSV5 cells from the same batch were seeded on two coverslips respectively and then cultured until cells reached 60–80% confluence. After that, one cell coverslip was cultured in static medium, and the other cell coverslip was cultured in fluidic medium. After growth in static or fluidic medium for 4 h, cells were chemically fixed for 30 min at room temperature. After washing cells with PBS for three times (5 min each time), the cell coverslip was treated by Triton X-100 for 10 min at room temperature. Subsequently, cells were incubated with donkey blocking serum for 30 min at 37 °C, after which cells were incubated with E-cadherin mouse monoclonal antibodies at 4 °C overnight. After washing cells with PBS for three times, cells were incubated with Alexa Fluor 488-labeled donkey anti-mouse IgG secondary antibodies for 1 h at 37 °C. After incubation, cells were washed by PBS for three times, and then DAPI solution was added and incubated for 5 min in the dark at room temperature. After washing cells with PBS for three times, the cell coverslip was attached onto a glass slide via the mounting medium, and then a confocal fluorescence microscope (A1R MP+, Nikon, Japan) was utilized to capture the fluorescent images of cells. Fluorescent images were captured with the identical instrumental parameters. For obtaining the fluorescent images of E-cadherin molecules, the laser excitation wavelength was 488 nm and the laser intensity was set to 70% of maximum. For obtaining the fluorescent images of cell nuclei, the laser excitation wavelength was 405 nm and the laser intensity was set to 70% of maximum. The laser was approximately focused onto the medium plane of cells, and then the focus plane was finely adjusted up or down through the z-axis displacement of the microscope to determine the cell apical surface or cell basal surface according to the obtained images.3. Results and discussion
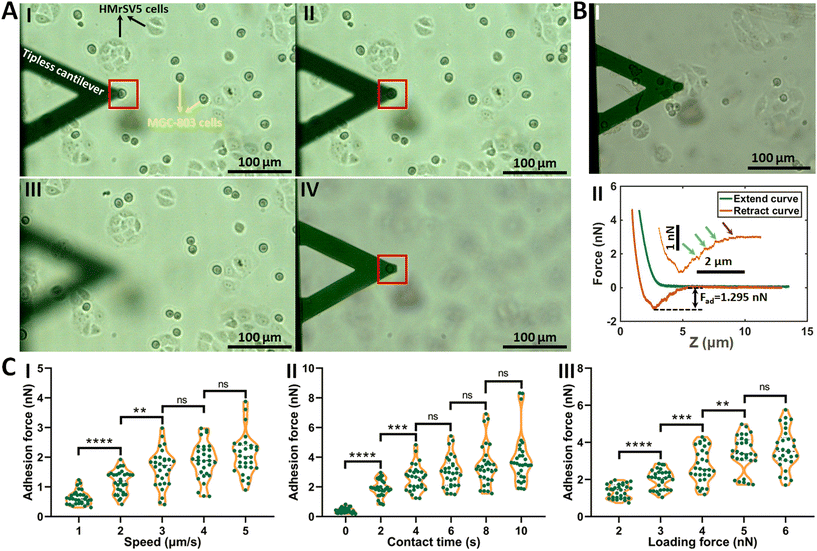
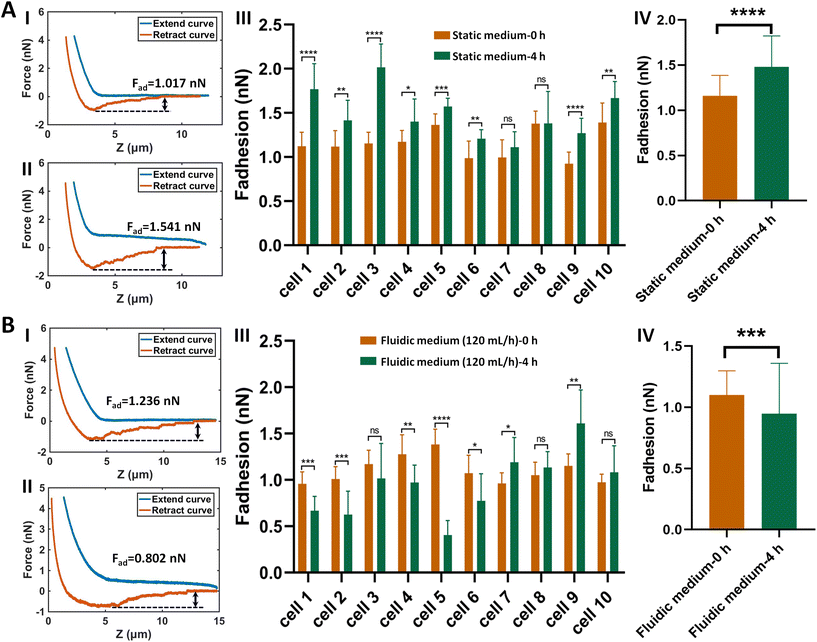
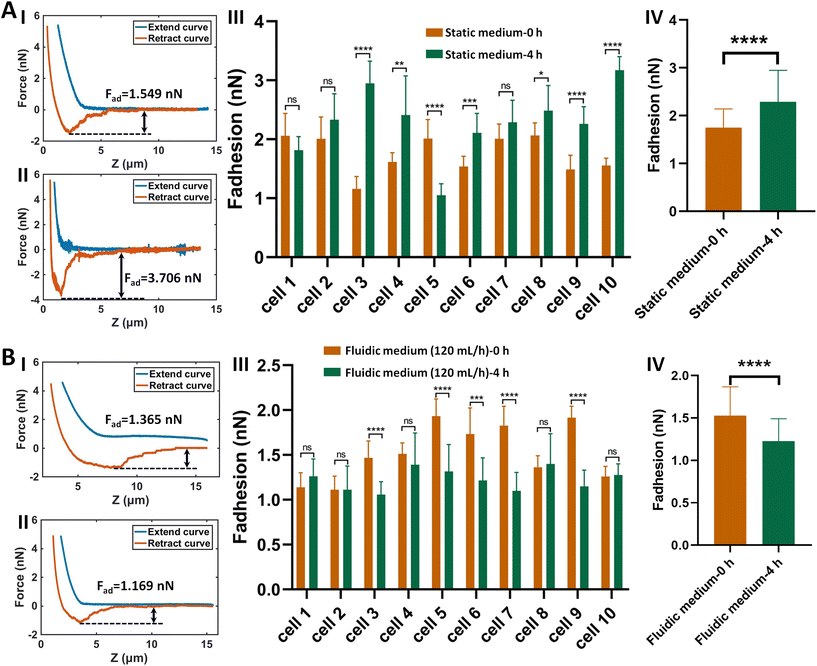
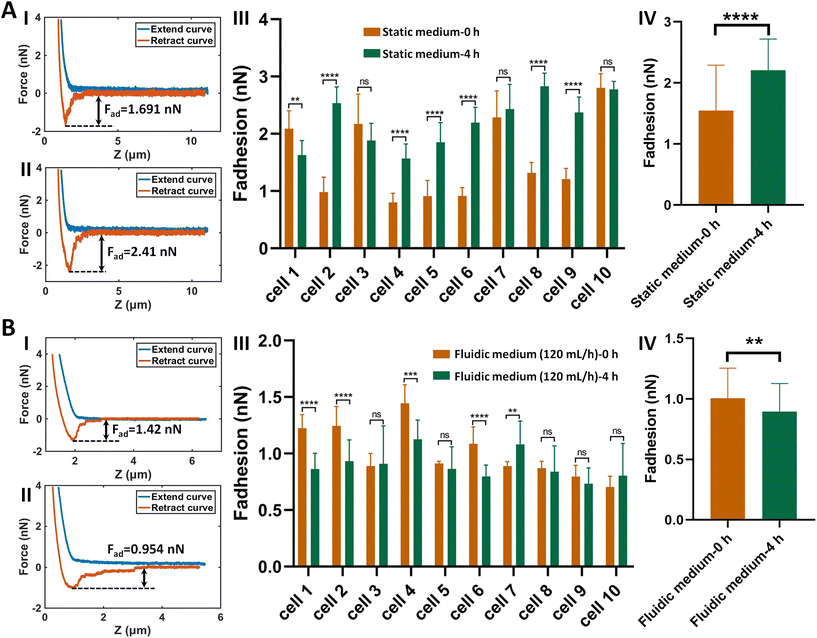
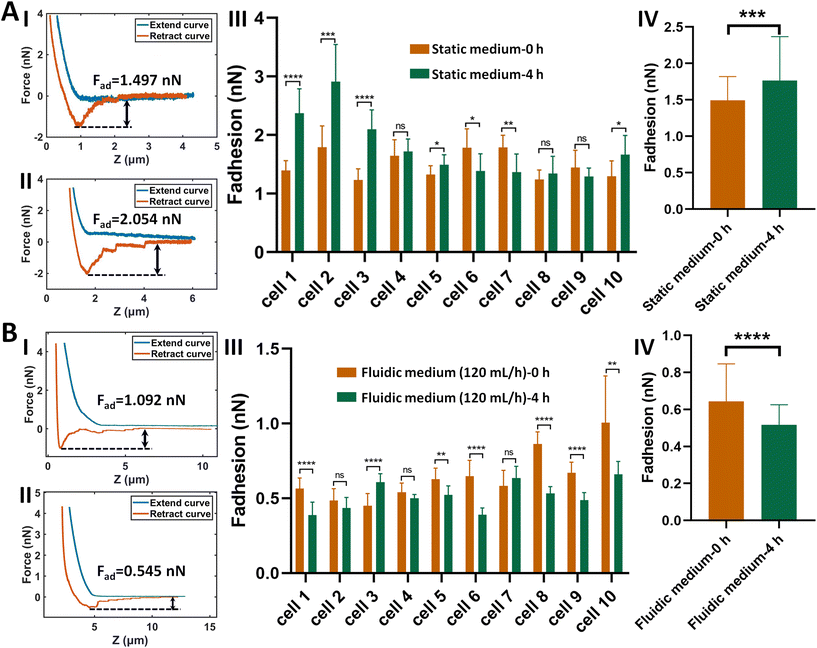
The procedure of applying AFM-based SCFS assay to detect cell–cell adhesion forces regulated by fluidic cell culture medium was firstly established. During the process of metastasis, tumor cells exit their primary sites of growth (local invasion, intravasation), translocate systemically (survival in the circulation, arrest at a distant organ site, extravasation), and adapt to survive and thrive in the foreign microenvironments of distant tissues (micrometastasis formation, metastatic colonization).22 Particularly, the extravasation stage involves the specific interactions of cancer cells with vascular endothelial cells via cell adhesion-related processes,23 which occur in the bloodstream environment (Fig. 1A). Here, AFM-based SCFS assay was exploited to explore and quantify the effect of fluid flow on cell adhesion at single-cell level (Fig. 1B). For doing so, a device allowing cell growth under fluidic cell culture medium conditions at 37 °C was used (Fig. 1C), which was combined with AFM-based SCFS assay to detect the changes of adhesion forces of cells after growth in fluidic flow environment. Since the device does not have the CO2 environment for cell growth, the CO2-independent cell culture medium (Leibovitz's L-15 medium) was used for the device. HMrSV5 cells are mesothelial cells which are able to form a monolayer (mesothelium) lining the serosal cavities (pleural, pericardial and peritoneal),24 and thus we used HMrSV5 cells as an example to mimic the cells lining blood vessels. In order to characterize the adhesion forces of HMrSV5 cells by AFM-based SCFS assay, AFM single-cell probes are required. MGC-803 cells and HGC-27 cells are both cancer cells, and here we used them as an example to mimic the cancer cells that enter blood vessels during tumor metastasis and these two types of cells were attached to AFM tipless cantilevers to prepare single-cell probes. The HMrSV5 cells were grown on the coverslip in the fluidic cell culture medium device. Another type of cancer cell (MGC-803 cells or HGC-27 cells in their digested states) was then added into the dish to prepare the single-cell probe (Fig. 2). Under the guidance of optical microscopy, a single living cell was attached onto the ConA-functionalized AFM tipless cantilever to prepare the single-cell probe (Fig. 2A), which was then used to perform SCFS experiments on the HMrSV5 cells (Fig. 2B). Two types of typical molecular unbinding events were clearly observed from the obtained force curves (Fig. 2BII and Fig. S1†), including the unbinding of cell adhesion receptors which were anchored to the cytoskeleton (event of force step) and the unbinding of cell adhesion receptors which were not anchored to the cytoskeleton (event of tether unbinding).8,10,25 The overall cell adhesion force (corresponding to the force between the maximum force peak and the baseline) was then calculated from the retract curve (denoted by the double arrow in Fig. 2BII). The impacts of experimental parameters on the measured adhesion forces during SCFS assay were also examined and optimized, showing that the measured cell adhesion forces significantly increased as the increase of probe retract speed/contact time/loading force (Fig. 2C). Hence, in the subsequent SCFS experiments, force curves were obtained with identical experimental parameters to make the results comparable with each other.The established procedure was then applied to probe the effect of fluidic medium conditions on the cell adhesion forces. Fig. 3 shows the results of the changes of the adhesion forces of HMrSV5 cells after 4 hours of growth in static cell culture medium (for control) or in fluidic cell culture medium. Before growth in static cell culture medium or fluidic cell culture medium, MGC-803 cells were added and SCFS experiments were performed on ten HMrSV5 cells using an AFM tipless cantilever carrying a living MGC-803 cell (0 h group). After the measurements, the trypsin solution was dropped onto the AFM cantilever to remove the attached MGC-803 cell. After growth for 4 h (in static medium or in fluidic medium), MGC-803 cells from the same batch were added and the same ten HMrSV5 cells were measured again (the positions of the HMrSV5 cells were confirmed by the physical markers on the bottom surface of the coverslip, as shown in Fig. S2†) using the same AFM tipless cantilever carrying another living MGC-803 cell (4 h group). For doing so, the changes of adhesion forces of the same HMrSV5 cells were directly revealed. For each set of experiments (0 h group and 4 h group), a notable point is that the two cells on the single-cell probes used in experiments were not the same. In order to overcome this issue, MGC-803 cells with similar shape and size were selected for the preparation of single-cell probes. Besides, before performing SCFS experiments on HMrSV5 cells, the prepared single-cell probes were used to obtain force curves in the bare area of the substrate, and the results show that the measured adhesion forces between single-cell probes and substrates were close (Fig. S3†), indicating that the single-cell probes were approximately identical in force measurements. Our previous results have shown that cells grown under fluidic cell culture medium (especially when the flow rate was 120 mL h−1) significantly softened and smoothened,21 and currently the maximum flow rate the fluidic medium device (Fig. 1C) can provide is 120 mL h−1. Therefore, here the flow rate of the fluidic medium was set to 120 mL h−1. The experimental results remarkably show that the cell adhesion forces increased significantly when cells were grown in the static medium (III and IV in Fig. 3A), whereas the cell adhesion forces decreased significantly when cells were grown in the fluidic medium (III and IV in Fig. 3B). In order to confirm that the changes of adhesion forces were from the HMrSV5 cells, we then used HGC-27 cells to prepare the single-cell probes to perform the experiments (Fig. S4†), and the experimental results (Fig. 4) again show the increased adhesion forces of cells grown in static medium (III and IV in Fig. 4A) and the decreased adhesion forces of cells grown in fluidic medium (III and IV in Fig. 4B), indicating that the fluid flow environment affects cell adhesion.
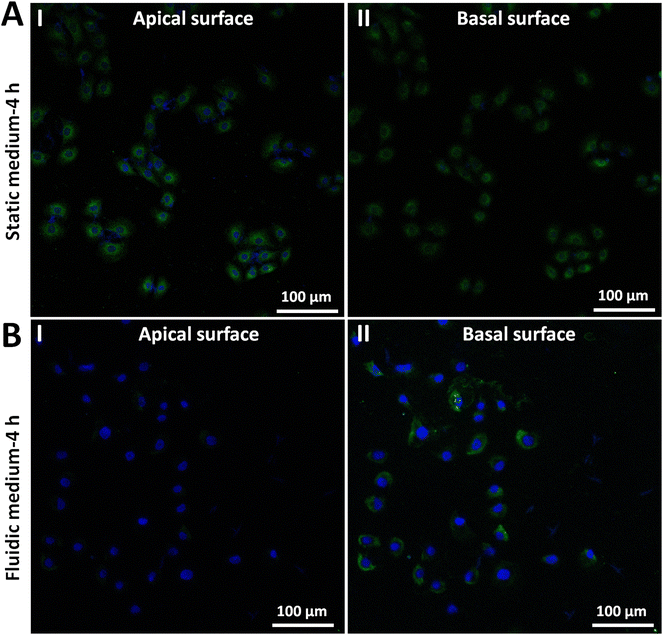
We further explored the molecular mechanisms involved in the adhesion force changes of cells grown in fluidic medium conditions. The spherical probe was prepared by attaching a microsphere to an AFM tipless cantilever (Fig. S5†). After coating the microsphere-modified cantilevers with cell adhesion-associated biomolecules, the probes were then used to measure the adhesion forces of HMrSV5 cells before and after growth in static/fluidic cell culture medium (Fig. S6 and S7†). Fig. 5 shows the results of the changes of the adhesion forces of HMrSV5 cells measured by the fibronectin-coated spherical probe. Before growth in static cell culture medium or fluidic cell culture medium, ten HMrSV5 cells were measured by a fibronectin-coated spherical probe. After growth in static cell culture medium or fluidic cell culture medium, the same ten HMrSV5 cells were measured by the same fibronectin-coated spherical probe. For doing so, the potential influence of different probes on the measured results could be avoided. The results show that the adhesion forces between HMrSV5 cells and fibronectin molecules increased significantly when cells were grown in static medium (III and IV in Fig. 5A), whereas the adhesion forces significantly decreased when cells were grown in fluidic medium (III and IV in Fig. 5B), which are consistent with the results obtained by single-cell probes (Fig. 3 and 4). Fig. 6 shows the results of the changes of the adhesion forces of HMrSV5 cells measured by the ConA-coated spherical probe. An interesting phenomenon is that force curves obtained in static medium using fibronectin-coated spherical probes have short and sharp force peaks (I and II in Fig. 5A), whereas force curves obtained in static medium using ConA-coated spherical probes have long force peaks with distinct tether unbinding events (I and II in Fig. 6A), which may be due to the differences in binding interactions between these two types of molecules and cells as well as the different functionalization methods used to attach these two types of molecules onto microspheres (fibronectin molecules were directly attached onto the polystyrene microsphere surface by physical adsorption, while ConA molecules were attached onto the silica microsphere surface via biotin–streptavidin linkers). The results obtained using ConA-coated spherical probes again show the increased adhesion forces for cells grown in static medium (III and IV in Fig. 6A) and the decreased adhesion forces for cells grown in fluidic medium (III and IV in Fig. 6B). Notably, detecting cell adhesion forces with a same molecule-coated spherical probe might cause the contamination of the probe. We then performed more measurements, and the results (Fig. S8†) overall show that the fluidic flow environment could result in reduced cell adhesion force compared with the static medium environment. Fibronectin is an important ECM protein which regulates cellular interactions with ECM,26 and ConA has also been widely used to attach living cells.10,27 The experimental results (Fig. 5 and 6) confirm that growth in fluidic cell culture medium could weaken the adhesion between cells and ECM proteins. In order to explore the molecular mechanisms involved in the changes of cell adhesion forces induced by fluidic flow environment, we then used confocal fluorescence microscopy to examine the changes of cell adhesion molecules (E-cadherin) after growth in static/fluidic cell culture medium, and the results are shown in Fig. 7. More confocal fluorescence microscopy images are shown in Fig. S9.† We can clearly see that the cells after growth in fluidic medium had significantly decreased adhesion molecules compared with cells after growth in static medium, particularly for the apical surface of cells. The adhesion molecules on the apical surface of the cell are responsible for the cell adhesion forces measured by AFM-based SCFS, and therefore we observed the decreased cell adhesion forces measured by AFM for cells after growth in fluidic medium. An interesting phenomenon is that cells after growth in fluidic medium possess dense adhesion molecules at the basal surface (II in Fig. 7B). Previous studies have shown the flow-enhanced adhesion of suspended cells in fluidic medium.28 Here, our results show the weakening of apical surface adhesion of adherent cells grown in fluidic medium, and the fluidic flow environment may result in the migration of cell adhesion molecules from the cell apical surface to the cell basal surface to regulate cell–ECM adhesion.29 In addition, the nuclei of cells grown in static medium were not visible in images obtained at cell basal surface (Fig. 7A), whereas the nuclei of cells grown in fluidic flow medium were visible in images obtained both at cell apical surface and basal surface (Fig. 7B), which might be due to the structural changes in cell nucleus caused by the fluidic flow medium.
Combining the above results, we can see that the fluidic flow environment can weaken both the cell–cell adhesion (Fig. 3 and 4) and cell–ECM adhesion (Fig. 5 and 6) through down-expression of adhesion molecules on the apical surface of the cell grown in the fluidic flow environment (Fig. 7). Notably, here only two types of cell adhesion-associated ECM proteins (fibronectin and ConA) and one type of cell adhesion molecules were measured during the experiments. There are about 300 proteins (e.g., collagens, proteoglycans, glycoproteins) which constitute the ECM,30 and also there are many other different types of proteins31,32 responsible for cell–cell and cell–ECM adhesion. Consequently, further studies to reveal the detailed molecular mechanisms regulating the adhesion dynamics of cells grown in fluidic flow environment will be quite meaningful for understanding the interactions between cell adhesion and fluid mechanics.
The experimental results obtained by utilizing AFM-based SCFS assay to quantify the fluid flow-tuned adhesion force dynamics of single cells will facilitate dissecting the role of hemodynamics in tumor metastasis. Metastasis causes more than 90% of cancer deaths, which poses a huge challenge to cancer treatment.33 Understanding the initiation and progression of metastasis is therefore crucial for the development of new therapeutic strategies to treat and prevent metastatic cancer.34 However, to date, the underlying mechanisms of tumor metastasis remain poorly understood. In recent years, single-cell analysis techniques have been considered as promising methods to reveal novel mechanisms directing tumor metastasis.35,36 Particularly, the role of the physical microenvironment in tumor development, progression, metastasis, and treatment is gaining appreciation.37 AFM-based SCFS assay has become a powerful tool to directly measure the forces driving cell–cell adhesion and cell–ECM interactions on a single cell basis.38,39 Current studies of utilizing AFM-based SCFS assay to detect the adhesive behaviors of cancer cells are commonly performed in static medium, which neglect the influence of fluidic flow medium environment on cell adhesion. To the best of our knowledge, studies of applying AFM-based SCFS assay to probe the fluid flow-tuned adhesive behaviors of cancer cells are still scarce. It has been shown that cancer cells can exploit the underlying physical forces within the bodily fluids to seed distant metastases.19 Probing the adhesive behaviors of cancer cells in an environment which is closer to the real situation of tumor metastasis undoubtedly benefits better characterizing the cellular behaviors. Here, we explored utilizing AFM-based SCFS to quantify the changes of adhesion forces of single cells grown in fluidic cell culture medium, and the results dramatically show the significant influence of fluidic medium condition on cell adhesion. The experimental results provide novel insights into the interactions between cell adhesion and fluid flow environment from the single-cell level, which therefore offer a feasible way to assess the role of hemodynamics in tumor metastasis from the perspective of single-cell adhesion. In future, developing devices which allow long-term growth of cells under fluidic medium condition,40 changing the medium components to resemble bodily fluids more closely,20 and utilizing the established methodology to detect the adhesion dynamics of more different types of cells, will further facilitate exploring the underlying mechanisms of fluid flow-tuned cell adhesion during the process of tumor metastasis.
4. Conclusions
This work has demonstrated the capability of AFM-based SCFS to measure the adhesion forces of single cells grown under fluidic flow environment by incorporating a fluidic cell culture medium device. Experiments performed with the use of single-cell probes show a significant decrease in cell adhesion force after growth in fluidic cell culture medium and experiments performed with ECM protein-functionalized spherical probes also show the weakening of cell adhesion after growth in fluidic medium, which were confirmed by confocal fluorescence microscopy experiments of cell adhesion molecules, evidencing the dramatic impact of fluid flow environment on cell–cell and cell–ECM adhesion. The experimental results improve our understanding of cellular adhesion behaviors in fluidic medium microenvironments at single-cell level, which provide additional possibilities for the studies of single-cell mechanobiology in response to fluidic flow stimuli to dissect the role of hemodynamics in tumor metastasis.Author contributions
M. L. conceived and designed the study. J. W. and Y. Y. carried out the experiments and collected experimental data. M. L. and J. W. wrote the paper. All authors reviewed the manuscript. M. L. supervised the work.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (62273330) and the Key Research Program of Frontier Sciences CAS (ZDBS-LY-JSC043).References
- B. Alberts, A. Johnson, J. Lewis, D. Morgan, M. Raff, K. Roberts and P. Walter, Molecular Biology of the Cell, Garland Science, New York, 6th edn, 2015 Search PubMed.
- B. M. Gumbiner, Cell adhesion: the molecular basis of tissue architecture and morphogenesis, Cell, 1996, 84(3), 345–357 CrossRef CAS PubMed.
- C. Frantz, K. M. Stewart and V. M. Weaver, The extracellular matrix at a glance, J. Cell Sci., 2010, 123(24), 4195–4200 CrossRef CAS PubMed.
- M. Janiszewska, M. C. Primi and T. Izard, Cell adhesion in cancer: beyond the migration of single cells, J. Biol. Chem., 2020, 295(8), 2495–2505 CrossRef CAS PubMed.
- T. R. Cox, The matrix in cancer, Nat. Rev. Cancer, 2021, 21(4), 217–238 CrossRef CAS PubMed.
- C. R. Arciola, D. Campoccia and L. Montanaro, Implant infections: adhesion, biofilm formation and immune evasion, Nat. Rev. Microbiol., 2018, 16(7), 397–409 CrossRef CAS PubMed.
- P. Kanchanawong, G. Shtengel, A. M. Pasapera, E. B. Ramko, M. W. Davidson, H. F. Hess and C. M. Waterman, Nanoscale architecture of integrin-based cell adhesion, Nature, 2010, 468(7323), 580–584 CrossRef CAS PubMed.
- J. Helenius, C. P. Heisenberg, H. E. Gaub and D. J. Muller, Single-cell force spectroscopy, J. Cell Sci., 2008, 121(11), 1785–1791 CrossRef CAS PubMed.
- A. Viljoen, M. Mathelie-Guinlet, A. Ray, N. Strohmeyer, Y. J. Oh, P. Hinterdorfer, D. J. Muller, D. Alsteens and Y. F. Dufrene, Force spectroscopy of single cells using atomic force microscopy, Nat. Rev. Methods Primers, 2021, 1, 63 CrossRef CAS.
- J. Friedrichs, J. Helenius and D. J. Muller, Quantifying cellular adhesion to extracellular matrix components by single-cell force spectroscopy, Nat. Protoc., 2010, 5(7), 1353–1361 CrossRef CAS PubMed.
- A. Beaussart, S. El-Kirat-Chatel, R. M. A. Sullan, D. Alsteens, P. Herman, S. Derclaye and Y. F. Dufrene, Quantifying the forces guiding microbial cell adhesion using single-cell force spectroscopy, Nat. Protoc., 2014, 9(5), 1049–1055 CrossRef CAS PubMed.
- M. Krieg, G. Flaschner, D. Alsteens, B. M. Gaub, W. H. Roos, G. J. L. Wuite, H. E. Gaub, C. Gerber, Y. F. Dufrene and D. J. Muller, Atomic force microscopy-based mechanobiology, Nat. Rev. Phys., 2019, 1(1), 41–57 CrossRef.
- Y. F. Dufrene and A. Persat, Mechanomicrobiology: how bacteria sense and respond to forces, Nat. Rev. Microbiol., 2020, 18(4), 227–240 CrossRef CAS PubMed.
- S. C. Hoffmann, A. Cohnen, T. Ludwig and C. Watzl, 2B4 engagement mediates rapid LFA-1 and actin-dependent NK cell adhesion to tumor cells as measured by single cell force spectroscopy, J. Immunol., 2011, 186(5), 2757–2764 CrossRef CAS PubMed.
- L. Andolfi, E. Bourkoula, E. Migliorini, A. Palma, A. Pucer, M. Skrap, G. Scoles, A. P. Beltrami, D. Cesselli and M. Lazzarino, Investigation of adhesion and mechanical properties of human glioma cells by single cell force spectroscopy and atomic force microscopy, PLoS One, 2014, 9(11), e112582 CrossRef PubMed.
- S. Azadi, M. Tafazzoli-Shadpour, R. Omidvar, L. Moradi and M. Habibi-Anbouhi, Epidermal growth factor receptor targeting alters gene expression and regulates the adhesion function of cancerous cells as measured by single cell force spectroscopy, Mol. Cell. Biochem., 2016, 423, 129–139 CrossRef CAS PubMed.
- L. Xie, Z. Sun, Z. Hong, N. J. Brown, O. V. Glinskii, K. Rittenhouse-Olson, G. A. Meininger and V. V. Glinsky, Temporal and molecular dynamics of human metastatic breast carcinoma cell adhesive interactions with human bone marrow endothelium analyzed by single-cell force spectroscopy, PLoS One, 2018, 13(9), e0204418 CrossRef PubMed.
- A. G. Liebsch and H. Schillers, Quantification of heparin's antimetastatic effect by single-cell force spectroscopy, J. Mol. Recognit., 2021, 34(1), e2854 CrossRef CAS PubMed.
- G. Follain, D. Herrmann, S. Harlepp, V. Hyenne, N. Osmani, S. C. Warren, P. Timpson and J. G. Goetz, Fluids and their mechanics in tumour transit: shaping metastasis, Nat. Rev. Cancer, 2020, 20(2), 107–124 CrossRef CAS PubMed.
- K. Bera, A. Kiepas, I. Godet, Y. Li, P. Mehta, B. Ifemembi, C. D. Paul, A. Sen, S. A. Serra, K. Stoletov, J. Tao, G. Shatkin, S. J. Lee, Y. Zhang, A. Boen, P. Mistriotis, D. M. Gilkes, J. D. Lewis, C. M. Fan, A. P. Feinberg, M. A. Valverde, S. X. Sun and K. Konstantopoulos, Extracellular fluid viscosity enhances cell migration and cancer dissemination, Nature, 2022, 611(7935), 365–373 CrossRef CAS PubMed.
- J. Wei and M. Li, Interplay of fluid mechanics and matrix stiffness in tuning the mechanical behaviors of single cells probed by atomic force microscopy, Langmuir, 2023, 39(3), 1309–1319 CrossRef CAS PubMed.
- S. Valastyan and R. A. Weinberg, Tumor metastasis: molecular insights and evolving paradigms, Cell, 2011, 147(2), 275–292 CrossRef CAS PubMed.
- N. Reymond, B. B. d'Agua and A. J. Ridley, Crossing the endothelial barrier during metastasis, Nat. Rev. Cancer, 2013, 13(12), 858–870 CrossRef CAS PubMed.
- S. E. Mutsaers, The mesothelial cell, Int. J. Biochem. Cell Biol., 2004, 36(1), 9–16 CrossRef CAS PubMed.
- J. Friedrichs, K. R. Legate, R. Schubert, M. Bharadwaj, C. Werner, D. J. Muller and M. Benoit, A practical guide to quantify cell adhesion using single-cell force spectroscopy, Methods, 2013, 60(2), 169–178 CrossRef CAS PubMed.
- R. Pankov and K. M. Yamada, Fibronectin at a glance, J. Cell Sci., 2002, 115(20), 3861–3863 CrossRef CAS PubMed.
- M. Huber, J. Casares-Arias, R. Fassler, D. J. Muller and N. Strohmeyer, In mitosis integrins reduce adhesion to extracellular matrix and strengthen adhesion to adjacent cells, Nat. Commun., 2023, 14, 2143 CrossRef CAS PubMed.
- C. Zhu, T. Yago, J. Lou, V. I. Zarnitsyna and R. P. McEver, Mechanisms for flow-enhanced cell adhesion, Ann. Biomed. Eng., 2008, 36(4), 604–621 CrossRef PubMed.
- R. Hadjisavva, O. Anastasiou, P. S. Ioannou, M. Zheltkova and P. A. Skourides, Adherens junctions stimulate and spatially guide integrin activation and extracellular matrix deposition, Cell Rep., 2022, 40(3), 111091 CrossRef CAS PubMed.
- C. Bonnans, J. Chou and Z. Werb, Remodelling the extracellular matrix in development and disease, Nat. Rev. Mol. Cell Biol., 2014, 15(12), 786–801 CrossRef CAS PubMed.
- D. S. Harburger and D. A. Calderwood, Integrin signalling at a glance, J. Cell Sci., 2009, 122(2), 159–163 CrossRef CAS PubMed.
- F. van Roy, Beyond E-cadherin: roles of other cadherin superfamily members in cancer, Nat. Rev. Cancer, 2014, 14(2), 121–134 CrossRef CAS PubMed.
- K. Ganesh and J. Massague, Targeting metastatic cancer, Nat. Med., 2021, 27(1), 34–44 CrossRef CAS PubMed.
- D. A. Lawson, N. R. Bhakta, K. Kessenbrock, K. D. Prummel, Y. Wu, K. Takai, A. Zhou, H. Eyob, S. Balakrishnan, C. Y. Wang, P. Yaswen, A. Goga and Z. Werb, Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells, Nature, 2015, 526(7571), 131–135 CrossRef CAS PubMed.
- I. Tirosh, B. Izar, S. M. Prakadan, M. H. Wadsworth, D. Treacy, J. J. Trombetta, A. Rotem, C. Rodman, C. Lian, G. Murphy, M. Fallahi-Sichani, K. Dutton-Regester, J. R. Liu, O. Cohen, P. Shah, D. Lu, A. S. Genshaft, T. K. Hughes, C. G. K. Ziegler, S. W. Kazer, A. Gaillard, K. E. Kolb, A. C. Willani, C. M. Johannessen, A. Y. Andreev, E. M. Van Allen, M. Bertagnolli, P. K. Sorger, R. J. Sullivan, K. T. Flaherty, D. T. Frederick, J. Jane-Valbuena, C. H. Yoon, O. Rozenblatt-Rosen, A. K. Shalek, A. Regev and L. A. Garraway, Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq, Science, 2016, 352(6282), 189–196 CrossRef CAS PubMed.
- H. W. Jackson, J. R. Fischer, V. R. T. Zanotelli, H. R. Ali, R. Mechera, S. D. Soysal, H. Moch, S. Muenst, Z. Varga, W. P. Weber and B. Bodenmiller, The single-cell pathology landscape of breast cancer, Nature, 2020, 578(7796), 615–620 CrossRef CAS PubMed.
- H. T. Nia, L. L. Munn and R. K. Jain, Physical traits of cancer, Science, 2020, 370(6516), eaaz0868 CrossRef CAS PubMed.
- D. J. Muller, J. Helenius, D. Alsteens and Y. F. Dufrene, Force probing surfaces of living cells to molecular resolution, Nat. Chem. Biol., 2009, 5(6), 383–390 CrossRef PubMed.
- Y. F. Dufrene, Sticky microbes: forces in microbial cell adhesion, Trends Microbiol., 2015, 23(6), 376–382 CrossRef CAS PubMed.
- Y. Jun, J. Lee, S. Choi, J. H. Yang, M. Sander, S. Chung and S. H. Lee, In vivo-mimicking microfluidic perfusion culture of pancreatic islet spheroids, Sci. Adv., 2019, 5(11), eaax4520 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nr04439d |
| This journal is © The Royal Society of Chemistry 2024 |