DOI:
10.1039/D4NJ03947E
(Paper)
New J. Chem., 2024,
48, 19044-19059
Smart chitosan Schiff base ferrite: a dual-action visible light photocatalyst for dye degradation and antimicrobial defense†
Received
8th September 2024
, Accepted 11th October 2024
First published on 22nd October 2024
Abstract
This study presents the novel fabrication of a new visible-light-driven SBXIII@NiFe photocatalytic material, which was then used to photodegrade two key exemplar water pollutants, methyl orange and rhodamine-B. The structure of the material, chemistry, and optical characteristics of the SBXIII@NiFe nanocomposites were thoroughly examined using various characterization techniques. Scanning electron microscopy reveals that SBXIII molecules are incorporated into the NiFe particles. Spectrophotometry was used to quantify the rate of decolorization from the remaining quantities. Similar studies were carried out with different pH (2–12), catalyst quantity (0.25–2.0 g L−1), and electrolyte concentration (0.1–2 mol L−1). The experiments showed that the SBXIII@NiFe photocatalyst resulted in the highest decolorization (more than 90%) of dyes, with MO degrading the most at pH 4 and RH-B at pH 9. Among the synthesized NiFe and its SBXIII@NiFe composite, the latter exhibits higher photocatalytic efficiency. This finding was ascribed to the synergistic impact of SBXIII and NiFe, with SBXIII acting as an electron trap site, reducing electron–hole recombination and inducing visible light absorption. The active entity trapping studies identify (OH˙) and (O2˙−) radicals as the significant reactive agents involved in the oxidative photodegradation of dye contaminants. Based on these findings, a potential photocatalytic pathway has been postulated. Furthermore, the engineered photocatalyst was tested for antibacterial efficacy towards Gram-positive (S. aureus) and Gram-negative (P. vulgaris) bacteria. The inclusion of chitosan, as well as the synergistic actions of ferrite and anthrone, increased the antibacterial activity significantly.
1. Introduction
Environmental difficulties and energy-related challenges have emerged as significant obstacles to the sustainable development of human society, exacerbated by rapid industrialization. The ecological environment is under severe strain due to economic and technological pressures, and meeting the substantial energy demands of human civilization remains a formidable task.1 Water contamination is particularly concerning, as the current speed of industrial waste discharge poses significant threats to the environment and human health while adversely affecting industrial growth. Industrial waste comprises diverse contaminants, including personal and pharmaceutical, dyes, and other organic pollutants.2,3 These substances are highly toxic and resistant to degradation by conventional methods, posing serious risks to both ecological and human health.4,5 Organic dye pollutants, for instance, can have numerous harmful effects depending on the type and concentration of the dye. Potential health impacts include skin irritation, respiratory problems including breathing difficulties and coughing, allergic responses like anaphylaxis, and long-term risks such as cancer, neurotoxicity (manifesting as headaches, dizziness, and confusion), and reproductive problems and toxicity (including infertility and birth defects). Organic dye pollutants harm human health, including the kind of dye, exposure amount, length of exposure, and individual sensitivity.5,6 Minimizing exposure through safer products and adherence to better safety protocols in industries utilizing these dyes is essential to mitigate these risks. Globally, water contamination requires immediate, low-energy, ecologically friendly solutions. The search for sustainable water contamination treatment methods is currently a major focus of international attention.6,7
However, using pristine chitosan as a catalyst for wastewater treatment is not ideal due to several limitations, including the generation of colloids in water, susceptibility to chemical degradation, poor mechanical strength, and acidic environment dissociation.8 Various strategies have been employed to address these issues and develop chitosan-based catalysts with enhanced physicochemical properties. These include chemical modification through crosslinking reactions,9 combining with metal oxide nanoparticles10 and incorporation with multi-walled carbon nanotubes.11 Among these methods, the crosslinking reaction of chitosan is particularly favored as it significantly improves its mechanical strength and stability in acidic conditions. Modifications of chitosan, especially those involving Schiff base reactions and its derivatives, have garnered considerable attention for their effectiveness in degrading pollutants in water.12–14
Recently, nanostructured ferrites have garnered significant interest due to their wide range of applications.15,16 Studies have shown that numerous organic dyes can be effectively degraded using ferrites through photocatalytic methods.17–20 For instance, it was found that over 40% of rhodamine B dye degraded within four hours when exposed to visible light in the presence of bismuth ferrite (BiFe2O4) microcrystals.21 Sun et al. compared the photocatalytic efficiency of ZnFe2O4 synthesized via the solution combustion method with that of ZnFe2O4 produced using the traditional solid-state technique.22 Co0.7Zn0.3Fe2O4 nanorods, synthesized via the co-precipitation method using a hydrazine precursor, have demonstrated significant photocatalytic efficacy in the degradation of dyes such as malachite green, methyl red, rose Bengal and rhodamine B when exposed to solar irradiation over various time intervals.23 In another investigation, Ru-doped cobalt ferrite nanomaterials (CoRuxFe2−xO4; x = 0.0, 0.02, 0.06) showed remarkable photocatalytic degradation of Remazol deep red, with CoRu0.06Fe1.94O4 achieving 94% degradation efficiency.24 Spinel ferrites, including NiFe1.98RE0.02O4 (RE = La, Sm, Gd, or Dy), were synthesized via a sol–gel auto-combustion method utilizing maleic acid as a fuel.25 The La-doped variant exhibited the highest catalytic activity, with the reaction rate constant being five times greater than that of undoped NiFe2O4. Nano spinel ferrites (MFe2O4), characterized by their cubic close-packed oxygen lattice and the occupation of tetrahedral (A) and octahedral (B) sites by M2+ and Fe3+ ions, have emerged as highly promising catalysts. These materials offer several advantages, including high stability, narrow bandgap, superior adsorption properties, low production costs, facile renewability, environmental compatibility, and a relatively small bandgap of approximately 2.0 eV, rendering them ideal candidates for photocatalytic applications.26–30
Semiconductors, such as ZnO, ZnS, TiO2, Fe3O4, CdS, and C3N4, are widely utilized as photocatalysts.31–36 Among these, TiO2 and ZnO are particularly well-studied. However, these semiconductors present challenges, including wide bandgaps and nonmagnetic properties, limiting their use to UV radiation and complicating their removal post-degradation. These issues can be mitigated by employing spinel catalysts with narrow bandgaps, specifically ferrite spinels, which exhibit superior magnetic properties compared to traditional semiconductors.37–39 Although spinels may not be as effective as semiconductors under UV radiation alone, combining the two can offer a promising solution to the limitations inherent in each type of catalyst. Semiconductor-spinel composites benefit from easy separation, the ability to harness both UV and visible spectra of sunlight (which together comprise nearly 50% of the sun), increased surface area, and a more significant number of active sites, making them highly efficient for photocatalytic applications.40–42
A comparatively new and promising method known as advanced oxidation processes (AOPs) has been developed and employed for the treatment of dye-contaminated wastewater effluents.43 AOPs generally involve the utilization of oxidizing solid species, such as hydroxyl (OH˙) radicals generated in situ, which trigger a series of reactions that degrade macromolecules into smaller, less harmful substances, often resulting in their complete decomposition into water and carbon dioxide.44 AOPs encompass a variety of techniques, including methods utilizing ultrasound,45 plasma,46 and electrohydraulic discharge.47 Additionally, semiconductor photocatalysis (e.g., TiO2/UV) and photo-redox reactions of transition metal complexes are part of AOPs. Despite the excellent activity of some oxides like TiO2, ZnO, and ferrites, their commercial use has been limited by low surface area, poor thermal stability, and inadequate mechanical properties.33
Chitosan Schiff bases (CSBs) have been widely explored for various applications. The unique behavior of the imine bond in CSBs under different pH conditions makes them effective sensors for external stimuli. This pH-responsive property has also been leveraged in drug delivery systems. Additionally, due to their strong chelation ability, CSBs have proven valuable in developing stable metal catalysts for heterogeneous catalysis. However, CSB-based catalysts still require improvements in reusability. The formation of inorganic–organic hybrids by incorporating CSBs with materials like silica, titania, and magnetite has been proposed to address this. These hybrid supports not only enhance the stability of the catalysts but also prevent the leaching of the catalytic sites, thereby improving reusability. Moreover, CSBs have shown promise in heavy metal adsorption for wastewater treatment due to their chelating properties. Looking ahead, renewable CSB-based membrane filters could serve as efficient heavy metal adsorbents for industrial applications.
Given the strong adhesive capacity of chitosan biopolymers towards a wide range of solid substrates and its tendency to incorporate many metals and metal oxide nanoparticles into its matrix—thanks to its amine and hydroxyl groups—this study aims to exploit the chelating properties of chitosan-based Schiff-base and the bandgap energy of NiFe2O4. The goal is to evaluate the effectiveness of visible light-mediated photocatalytic degradation of organic dyes.
2. Experimental
2.1. Materials and methods
Anthrone (97%), chitosan (>85%), glacial acetic acid (>99%), Fe(NO3)3·9H2O (>98%), NiCl2·6H2O (>98%), sodium hydroxide (>98%), ethanol (99.9%), rhodamine B (>95%), and methyl orange (85%) were bought from Sigma-Aldrich and were utilized as received without further purification of any sort.
2.2. Characterizations
The FT-IR spectrum was acquired using a PerkinElmer FT-IR spectrophotometer (KBr disks, 4000–400 cm−1). A TGA/DTA Mettler-Toledo 3+ GmbH analyzer provided the Thermal analysis data. A Rigaku Smart Lab XRD was used to record XRD data. A ZEISS Gemini SEM 500 FE-SEM and Octane Elect EDS were used for the SEM and EDAX analysis. Utilizing a PerkinElmer Lambda 365 UV-Visible Spectrophotometer, DR-UV investigations were performed. Zeta potential measurement was carried out using a Particle Analyzer (LitesizerTM 500). XPS analysis was performed using a Thermoscientific NEXA Surface Analyzer. The photoelectrochemical capacity of the materials was assessed using an electrochemical workstation (Bio-Logic SP-200). The nitrogen adsorption–desorption isotherms were investigated using a Quanta chrome NOVA 4200 analyzer operating at 77 K. Each sample was degassed at 200 °C overnight. The Brunauer–Emmett–Teller (BET) analysis technique has determined the surface area. The photoluminescence (PL) spectra analysis for all samples was conducted utilizing a Hitachi fluorescence spectrophotometer F-7000 with the illumination of a Xenon lamp.
2.3. Synthesis of magnetic nickel ferrite (NiFe)
The synthesis was done in a 100 mL stainless-steel autoclave with a Teflon liner under hydrothermal conditions. During the typical synthesis process, 40 mL of deionized distilled water (DDW) was used to dissolve 0.02 moles of Fe(NO3)3·9H2O and 0.01 moles of NiCl2·6H2O, maintaining a Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe molar ratio of 1
Fe molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The resulting solution was clear. Next, while stirring magnetically, 10 mL of 8 M NaOH solution was gradually added. The mixture was transferred into an autoclave, which was then sealed and heated to 180 °C for 10 hours. After the reaction was completed, the solid products were filtered, rinsed thoroughly with acetone and DDW, and vacuum-dried at 50 °C.48
2. The resulting solution was clear. Next, while stirring magnetically, 10 mL of 8 M NaOH solution was gradually added. The mixture was transferred into an autoclave, which was then sealed and heated to 180 °C for 10 hours. After the reaction was completed, the solid products were filtered, rinsed thoroughly with acetone and DDW, and vacuum-dried at 50 °C.48
2.4. Synthesis of Schiff-base (SBXIII)
A solution of 1 g chitosan was prepared in 100 mL of a 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (v/v) ethanol-glacial acetic acid mixture and stirred. After ten minutes, 10 mL of an ethanolic solution of anthrone (1 g) was gradually added. The reaction mixture was then stirred and heated to 80 °C until an orange-yellow solid precipitate formed. Once cooled, the solid was filtered, rinsed with ethanol, and dried at room temperature for several days.49
5 (v/v) ethanol-glacial acetic acid mixture and stirred. After ten minutes, 10 mL of an ethanolic solution of anthrone (1 g) was gradually added. The reaction mixture was then stirred and heated to 80 °C until an orange-yellow solid precipitate formed. Once cooled, the solid was filtered, rinsed with ethanol, and dried at room temperature for several days.49
2.5. Synthesis of the Schiff-base ferrite composite (SBXIII@NiFe)
While stirring, one gram of chitosan Schiff-base (SBXIII) was dissolved in 50 milliliters of DDW. After that, a 50 mL aqueous solution of nickel ferrite (1 g) was gradually added to the mixture, maintaining it at 50 °C. The reaction was vigorously stirred for 5 hours at room temperature. After the reaction, the resulting yellow gel-like product was separated and purified by washing multiple times with DDW and absolute ethanol. Finally, the desired product was dried in a thermal oven at 60 °C and manually ground into a powder (Scheme 1).
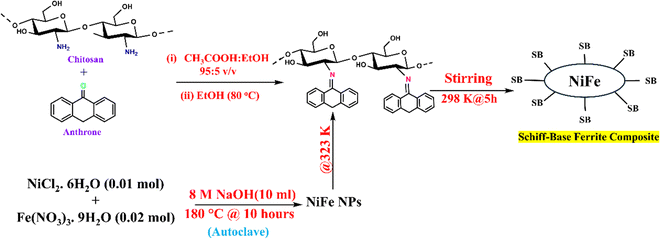 |
| | Scheme 1 Synthesis of the Schiff-base ferrite composite. | |
2.6. Photocatalytic activity
The photocatalytic ability of the nanocomposite was tested by degrading two dyes, namely methyl orange (MO) and rhodamine-B (RH-B), under photo-irradiation from a 200 W Xenon lamp at room temperature. At room temperature, 50 mL of the reaction solution containing 10 mg L−1 catalyst and dye (10 mg L−1 in neutral condition) was put in a 100 mL beaker and agitated with a magnetron. Before illumination, the combined solution was gently stirred for an hour in the dark to achieve adsorption–desorption equilibrium on the catalyst's surface. During the photodegradation study, a tiny sample of the reaction solution was taken out at regular intervals to evaluate dye concentration, which was calculated by determining the solution's absorbance at a set wavelength using an ultraviolet-visible spectrophotometer. The reaction solution was centrifuged for 10 minutes to remove the catalyst at 4000 rpm before measuring the absorbance. To evaluate the photocatalytic stability of the SBXIII@NiFe composite material, recycling studies for dye degradation employing the composites were carried out. After the first cycle, the photocatalyst was centrifuged, rinsed with water, and dried. The recovered photocatalyst was added to a new dye solution for the second cycle of the photocatalytic experiment, which was carried out under identical circumstances. The rate of degradation (D) is computed using the equation:| |  | (1) |
C
o is the preliminary concentration, and Ct is the concentration at time intervals.
2.7. Photoelectrochemical measurement
The photoelectrochemical potential of the nanocomposite was evaluated using a Bio-Logic SP-200 electrochemical workstation. A standard three-electrode configuration was employed for electrochemical impedance spectroscopy (EIS). The working electrode was plated on ITO with an active area of 1 cm2. Pt wire was the counter electrode, and Ag/AgCl was the reference electrode. As the electrolyte, 0.5 M NaOH solution was used. An open-circuit voltage bias was supplied at a frequency of 0.4 Hz and a current amplitude of 10 mV. The identical workstation and settings were used to create the Mott–Schottky graphs.
2.8. Antibacterial studies
The antibacterial properties of the Schiff-base and its derivative against P. vulgaris and S. aureus were investigated using the agar-well diffusion technique, as described in earlier publications. Overnight cultures of the desired bacteria (50 μL) were distributed on a Mueller Hinton (MH) agar matrix containing beef extract, acid casein hydrolysate, starch, and agar. Agar was perforated with a cork borer to make wells measuring 6 mm in diameter. 30 μL of the investigated drugs were added. The plates were chilled at 4 °C for two hours to let the ingredients permeate into the medium. After that, the plates were incubated for 24 hours at 37 °C. Then, an inspection was performed, and photos of bacterial inhibitions were captured.
3. Results and discussion
3.1. Structural analysis
3.1.1. FT-IR.
Fig. 1 presents the FT-IR spectrum of nickel ferrite, chitosan Schiff-base, and their nickel ferrite composite. In Fig. 1(a), a characteristic band at approximately 573 cm−1 is observed, corresponding to the stretching vibration of the Fe–O bond, which confirms the formation of magnetic spinel-type NiFe2O4 nanoparticles. The FT-IR spectra of nickel ferrite (NiFe), chitosan Schiff-base (SBXIII), and the SBXIII@NiFe composite are depicted in Fig. 1(a), (b) and (c), respectively. A broad characteristic band appears around 3645 cm−1, indicating overlapping N–H and O–H groups of chitosan. Peaks at 1658 cm−1 in SBXIII and 1652 cm−1 in SBXIII@NiFe are attributed to the ionic bond of the –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– group. The peaks around 1533, 1309, and 1015 cm−1 in the FT-IR spectra of SBXIII and SBXIII@NiFe correspond to C
N– group. The peaks around 1533, 1309, and 1015 cm−1 in the FT-IR spectra of SBXIII and SBXIII@NiFe correspond to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrational modes. The glycoside C–O, C–O–C, and C–C bonds and stretching in the chitosan's pyranose ring polymer chain appear as several wide peaks between 1000 and 1300 cm−1. In the FT-IR spectrum of SBXIII@NiFe, new weak bands appear at around 665 and 1319 cm−1, associated with the Fe–O bond.49
C vibrational modes. The glycoside C–O, C–O–C, and C–C bonds and stretching in the chitosan's pyranose ring polymer chain appear as several wide peaks between 1000 and 1300 cm−1. In the FT-IR spectrum of SBXIII@NiFe, new weak bands appear at around 665 and 1319 cm−1, associated with the Fe–O bond.49
 |
| | Fig. 1 FTIR spectra of (a) NiFe, (b) SBXIII and (c) SBXIII@NiFe; XRD graphs of (x) SBXIII@NiFe (y) and SBXIII (z) NiFe. | |
3.1.2. XRD.
The crystal structure, phase purity, and particle size of the produced nanocomposite were examined by powder X-ray diffraction (XRD). Fig. 1(z) portrays nickel ferrite's ambient temperature (300 K) X-ray diffraction (XRD) patterns. The X-ray diffractogram shows the (311) plane of NiFe2O4 nanoparticles (inverse spinel structure) with the most substantial peak at 2θ = 35.62° and plane spacing d = 2.494 Å. Crystalline NiFe2O4 has diffraction peaks at 30.14°, 35.50°, 37.25°, 43.17°, 53.70°, 56.97°, and 63.70°, corresponding to crystal planes (220), (311), (222), (400), (422), (511), and (440). According to the JCPDS standard (Card No. 01-087-2337), it may be classified as cubic structure NiFe2O4.50 A diffraction peak of the (104) plane at 31.65 °C shows the synthesis of a minimal quantity of α-Fe2O3, but no peak was identified for the NiO impurity. The resulting ferrite is nano-sized, broadening the powder XRD peaks when considering Scherrer's relation and the widening reflections of (311). The following formula may be used to determine the crystallite size of NiFe2O4:| |  | (2) |
where λ is the wavelength of X-rays used (1.5406 Å), the value of K is fixed as 0.89, θ is the diffraction angle, and β depicts the full width at half maximum (FWHM); the average crystallite size is found to be 33.34 nm.48 The Schiff-base (Fig. 1y) showed a characteristic peak at 11.53 °C and 22.50 °C, corresponding to the presence of chitosan in the SBXIII. The other peaks in the SBXIII are at 17.02 °C, 19.18 °C, 25.19 °C, 32.86 °C, 36.82 °C, 41.20 °C, and 44.63 °C. The peaks have been matched with JCPDS (Card no. 00-027-1593). All the prominent peaks in the monomers (SBXIII and NiFe) have been incorporated into the SBXIII@NiFe composite, indicating the successful synthesis of the desired material. The chemical modification of ferrite using Schiff-base has led to the formation of amorphous nature in SBXIII@NiFe. The reduction in the crystalline nature is due to the loss of amino groups in the parent Schiff-base due to the incorporation of Schiff base into ferrite nanoparticles. The crystallite size of the prepared crystals was calculated using the Debye Scherrer equation and was found to be 62.05 nm.51
3.1.3. XPS.
Fig. 2 illustrates the surface chemical states of the SBXIII@NiFe composite as revealed by X-ray photoelectron spectroscopy (XPS). The SBXIII@NiFe XPS survey spectrum (Fig. 2a) reveals the characteristic Ni, Fe, O, N, and C peak positions consistent with the EDAX mapping results. As shown in Fig. 2b, the high-resolution XPS spectrum of carbon in the region of C 1s shows three distinctive peaks at 285.0, 286.4, and 287.1 eV, which correspond to the C–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and C
C, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively. This insight enables us to design an effective coating of chitosan Schiff base on the surface of NiFe2O4. Fig. 2c depicts the XPS curve of N 1s in the 394 to 410 eV range. The C
O bonds, respectively. This insight enables us to design an effective coating of chitosan Schiff base on the surface of NiFe2O4. Fig. 2c depicts the XPS curve of N 1s in the 394 to 410 eV range. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–N bonds in the Schiff base ligand components on the surface of NiFe2O4 are attributed to binding energies 399.9 and 402.6 eV, respectively.52Fig. 2d depicts the XPS curve of O 1s, with two peaks occurring at 532.8 and 530 eV, corresponding to the O–H and M–O bonds, respectively. Fig. 2e depicts the Fe 2p scan of the prepared material, which shows two peaks at 712.6 and 725.4 eV, respectively. The appearance of these two binding energy peaks is ascribed to Fe 2p3/2 and Fe 2p1/2, suggesting that the material has a NiFe2O4 core. The comparatively low strength of these two peaks shows that NiFe2O4 was efficiently coated, resulting in the synthesis of the Schiff base and its NiFe composite. The Ni 2p spectra (Fig. 2f) reveal the peaks of Ni 2p3/2 at BE of 847.54 and 850.4, as well as Ni 2p1/2 at BE of 856.45 and 863.59 eV, confirming the presence of Ni2+ and Ni3+ in the sample.53
N and C–N bonds in the Schiff base ligand components on the surface of NiFe2O4 are attributed to binding energies 399.9 and 402.6 eV, respectively.52Fig. 2d depicts the XPS curve of O 1s, with two peaks occurring at 532.8 and 530 eV, corresponding to the O–H and M–O bonds, respectively. Fig. 2e depicts the Fe 2p scan of the prepared material, which shows two peaks at 712.6 and 725.4 eV, respectively. The appearance of these two binding energy peaks is ascribed to Fe 2p3/2 and Fe 2p1/2, suggesting that the material has a NiFe2O4 core. The comparatively low strength of these two peaks shows that NiFe2O4 was efficiently coated, resulting in the synthesis of the Schiff base and its NiFe composite. The Ni 2p spectra (Fig. 2f) reveal the peaks of Ni 2p3/2 at BE of 847.54 and 850.4, as well as Ni 2p1/2 at BE of 856.45 and 863.59 eV, confirming the presence of Ni2+ and Ni3+ in the sample.53
 |
| | Fig. 2 (a) XPS survey spectrum XPS spectra of (b) C1s, (c) N1s, (d) O1s, (e) Fe 2p and (f) Ni 2p. | |
4. Morphological analysis
4.1. SEM/TEM/EDAX
SEM inspection showed the surface structure of pure NiFe and its SBXIII@NiFe composite. Pure SBXIII displayed a sheet-like form with rough, rugged features on the Schiff-base exterior, while no other distinct shape was observed for the organic moiety.54 Upon the addition of nickel ferrite to chitosan, spherical arrangements within the chitosan Schiff-base matrix were created by the Schiff-base, indicating a well-dispersed composition that led to increased surface area, as shown in Fig. 3. Additionally, integrating ferrite particles into the chitosan, Schiff-base resulted in a nanocomposite surface containing numerous small, elongated structures, which enhanced its potential for the degradation of organic pollutants through photocatalysis. Moreover, TEM analysis has been carried out to study the internal structure of the prepared photocatalyst (Fig. S9, ESI†). EDAX analysis of SBXIII and SBXIII@NiFe is displayed in ESI† in Fig. S1 and S2, respectively. We noted peaks for C, N, and O at peak positions of 0.3, 0.4, and 0.5 keV, respectively, in the SBXIII sample (Fig. S1e, ESI†) and persisting in the SBXIII@NiFe NP sample (Fig. S2g, ESI†) as well. This demonstrates that the corresponding samples include the components C, N, and O. Likewise, the presence of elemental peaks for Ni and Fe in the SBXIII@NiFe (Fig. S2g, ESI†) samples confirms the availability of these elements. This provides evidence for the successful integration of nickel ferrite particles into the SBXIII@NiFe nanocomposite.10,52
 |
| | Fig. 3 SEM analysis of (a) and (d) SBXIII, (b) and (e) NiFe, and (c) and (f) SBXIII@NiFe. | |
4.1.1. BET.
Using the BET method, the N2 adsorption–desorption isotherm was employed to determine the SBET of the prepared sample. According to the Brunauer–Emmett–Teller (BET) analysis in Fig. 4(a), the SBXIII@NiFe catalyst exhibits a surface area of 21.883 m2 g−1, enhancing the catalyst's interaction with the environment. The SBXIII@NiFe catalyst has a pore diameter (average) of 3.83 nm and a total pore volume of 0.044 cc g−1. This is due to the active adsorption properties of the imine, hydroxyl, amino groups, and nickel ferrite nanoparticles, as indicated by the type-III adsorption–desorption isotherm shown in Fig. 4(b). Furthermore, the aggregates of the SBXIII@NiFe catalyst are identified as having a slit-like morphology based on the diagram in Fig. 4(b). The hysteresis loop of the sample is in the range of 0.8–1.0. Relative pressure has shifted towards a higher P/Po region, indicating mesoporous material. It first depicts monolayer formation followed by multilayer filling and consecutively capillary condensation. The shift to the right results from wider pores requiring more pressure to fill the pores because multilayer formation occurs on low-energy sites away from the adsorbent.10
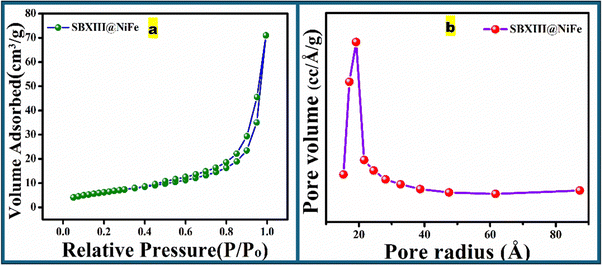 |
| | Fig. 4 (a) N2 adsorption–desorption isotherm and (b) pore size distribution of SBXIII@NiFe. | |
4.2. Thermal analysis
4.2.1. TGA/DTA.
The TGA and DTA curves of SBXIII@NiFe were examined, which is apparent in Fig. 5. Table 1 shows critical thermal analysis findings for the prepared materials SBXIII@NiFe, including weight losses, residual percentages, and temperature ranges. The initial period of weight loss below 100 °C might be ascribed to the loss of adsorbed molecules of alcohol or moisture onto the chitosan chain and weak hydrogen bonds with chitosan. The title material, SBXIII@NiFe, loses 8% of its weight. This finding validated the adsorption of molecules of alcohol or moisture onto the SBXIII@NiFe chitosan network. In the second stage, the compounds show a mass loss of 45.06% of the polymer weight, with SBXIII@NiFe occurring between 150 and 280 °C. This is related to the thermal (temperature) breakdown of the chitosan Schiff-base chain and the evaporation of volatile chemicals, including CH3CH2O–, –OH, and CH2OH [27]. Lastly, SBXIII@NiFe exhibits a mass loss of ≈ 43.55% between 300 and 560 °C, which might be related to the thermal deterioration of the pyranose ring of chitosan and the phenyl ring of ketone. The remaining percentage of SBXIII@NiFe at 550 °C, the end of thermal degradation, is 4.17%. The nickel ferrite nanoparticles (NiFe2O3) in SBXIII@NiFe are responsible for the more notable residual value. The magnetic analysis of nickel ferrite (NiFe) has been provided in the ESI† (Fig. S6).
 |
| | Fig. 5 TGA/DTA graphs of SBXIII@NiFe. | |
Table 1 Thermal analysis information for SBXIII@NiFe
| Sample |
Temperature range (°C) |
Weight loss (%) |
| SBXIII@NiFe |
25–100 |
8.0 |
| 150–280 |
45.06 |
| 300–560 |
43.55 |
4.3. Optical studies
4.3.1. DR-UV.
The optical properties of the material in photocatalysis are crucial since they help determine the bandgap and light absorption properties. Measurements of UV-vis diffuse reflectance were performed to examine the degree to which different samples absorbed UV and visible light, as shown in Fig. 6(b). To comprehend the optical characteristics of the prepared materials, the absorption spectra of NiFe, SBXIII, and SBXIII@NiFe were acquired within the 200–800 nm wavelength range. The primary absorption bands of the Schiff-Base (SB) UV-VIS spectra were 232 and 288 nm. The n–π* transition is associated with the band at 288 nm, while the π–π* transition is related to the band at 232 nm.55 The two bands in SBXIII@NiFe experienced a redshift (265 and 372 nm) because of metal and Schiff base interactions, as seen in Fig. 6(b), suggesting the synthesis of the composite. The Kubelka–Munk function was utilized to compute the band gap energy of the photocatalyst. The results are displayed in Fig. 6(d–f) to facilitate more investigation. The nanocomposite's band gap energy is smaller (2.44 eV) than SBXIII. The band gap energy of SBXIII@NiFe is less than that of SBXIII (3.2 eV), possibly due to the presence of nickel ferrite. Consequently, it can also be demonstrated that the nanocomposite was synthesized successfully and is an excellent match for the photodegradation of organic dyes.56
 |
| | Fig. 6 (a) Nyquist plots of NiFe and SBXIII@NiFe; (b) UV-vis absorption spectra; (c) PL spectra; (d)–(f) Tauc plots of SBXIII, NiFe, and SBXIII@NiFe. | |
4.4. Photocatalytic activity
The photocatalytic effectiveness of the prepared photocatalyst was tested by degrading two dyes, RH-B and MO, in aqueous solutions. At the same time, they were exposed to visible light at room temperature. Fig. 7(a and d) depicts the steady development of the UV-vis spectra of RH-B and MO solutions over a set length of visible light irradiation. The delayed breakdown rates of NiFe and SBXIII demonstrate a decreased photocatalytic effectiveness for these two monomers. Combining improves the degradation rates, and the SBXIII@NiFe composites degrade at greater than 75%. The findings suggest that adding chemicals can significantly improve the photocatalytic ability of NiFe.57 The intensity of the peaks for absorption decreased gradually throughout the entire spectra. This decrease in absorbance is most likely caused by the degradation of the dye chromogen, which indicates that SBXIII@NiFe efficiently breaks down the conjugated xanthene ring in RH-B. RH-B degradation under simulated visible light involves the gradual breakdown of the dye's chromophores and aromatic ring structure as the duration of exposure increases. In short, the dye molecules were damaged rather than simply losing color. The color of MO, an azo dye, is controlled by azo bonds (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) and associated auxochromes and chromophores. Throughout the deterioration process, the color of the MO solution faded progressively. The photocatalytic elimination of methyl orange was distinct from that of rhodamine B. As an azo dye, the principal chromophore of methyl orange comprises the azo group and aryl rings in a conjugated system; hence, the fading of methyl orange's color was predominantly caused by damage to this conjugated system.58 We also studied the degradation kinetics of dyes employing two monomers (SBXIII and NiFe) independently and in combination (SBXIII@NiFe). All these components degraded dyes using pseudo-first-order kinetics, with SBXIII@NiFe having the most significant reaction rate constant, as shown in Fig. 7(b, c, e, f). Table 2 also shows the parameters for pseudo-first-order kinetics. Table 3 compares the photocatalytic degradation of dyes by the present binary composite with the already reported similar composite materials.
N–) and associated auxochromes and chromophores. Throughout the deterioration process, the color of the MO solution faded progressively. The photocatalytic elimination of methyl orange was distinct from that of rhodamine B. As an azo dye, the principal chromophore of methyl orange comprises the azo group and aryl rings in a conjugated system; hence, the fading of methyl orange's color was predominantly caused by damage to this conjugated system.58 We also studied the degradation kinetics of dyes employing two monomers (SBXIII and NiFe) independently and in combination (SBXIII@NiFe). All these components degraded dyes using pseudo-first-order kinetics, with SBXIII@NiFe having the most significant reaction rate constant, as shown in Fig. 7(b, c, e, f). Table 2 also shows the parameters for pseudo-first-order kinetics. Table 3 compares the photocatalytic degradation of dyes by the present binary composite with the already reported similar composite materials.
 |
| | Fig. 7 (a) and (d) Photodegradation of MO and RHB dyes using the SBXIII@NiFe catalyst with time; (b), (c), (e) and (f) kinetic curves of the prepared catalyst. | |
Table 2 Rate constants (pseudo-first order) and the associated R2 values of all prepared photocatalysts
| S No. |
Photocatalysts |
k (min−1) |
R
2
|
k (min−1) |
R
2
|
| RH-B |
MO |
| 01 |
Blank |
0.000 |
1.000 |
0.000 |
1.000 |
| 02 |
SBXIII |
0.019 |
0.978 |
0.018 |
0.983 |
| 03 |
NiFe |
0.027 |
0.982 |
0.027 |
0.979 |
| 04 |
SBXIII@NiFe |
0.108 |
0.986 |
0.113 |
0.995 |
Table 3 Comparison table
| S. No. |
Photocatalyst |
Pollutant |
Radiation time (min) |
Degradation (%) |
Ref. |
| 01 |
TiO2-graphene |
RH-B |
80 |
98.00 |
59
|
| 02 |
Chitosan/TiO2-aerogel |
RH-B |
100 |
95.00 |
60
|
| 03 |
g-C3N4@CS-MoS2 |
RH-B |
40 |
99.00 |
61
|
| 04 |
Gr/CS/Fe3O4 |
RH-B/MO |
120/150 |
>90.00 |
62
|
| 05 |
ChdSb/CuO |
RH-B |
30 |
98.9 |
63
|
| 06 |
Ch/Fe3O4/α-MoO3 |
RH-B |
75 |
96.30 |
64
|
| 07 |
TiO2, CdS, ZnO, CdO |
MO/RH-B |
100/240 |
>90.00 |
58
|
| 08 |
PMBMNOPhen/TiO2/SiO2 |
MO |
60 |
60–80 |
65
|
| 09 |
DyVO4/AgBr |
MO |
90 |
79.80 |
66
|
| 11 |
SBXIII@NiFe |
MO/R-HB |
70/90 |
98.6/84.6 |
Present work |
4.5. Physical parameters
4.5.1. pH and zeta potential.
The pH of the solution has an important influence on the photodegradation of dyes. Fig. 8(d) illustrates the influence of pH on the degradation of MO and RH-B dyes using the SBXIII@NiFe catalyst. The optimal pH for MO degradation was 7, achieving a degradation percentage of 96.8%, while for RH-B, the optimal pH was 9, with a degradation percentage of 91%. At pH values of 2, 4, 6, 9, and 11, the degradation percentages for both dyes decreased, underscoring the significance of pH in the degradation process. The point of zero charge for the SBXIII@NiFe catalyst is 7.6, with a zeta potential of −28 mV around pH 9. Consequently, the catalyst surface is protonated at neutral pH, exhibiting a positive net charge. Methyl orange (MO) is an anionic dye featuring an N–H group, which interacts readily with the positively charged catalyst surface, creating a strong electrostatic connection between the positively charged catalyst and negatively charged MO molecules and encouraging MO degradation under neutral circumstances. Conversely, the catalyst surface is negatively charged in an alkaline environment, making it effective for degrading cationic dyes like rhodamine B (RH-B). At pH 9, the catalyst surface achieves maximum negative charge, enhancing its interaction with RH-B through strong electrostatic attraction. In neutral and acidic situations, the catalyst's surface has a positive charge, while in alkaline circumstances, it has a negative charge. Zeta potential investigations of the SBXIII@NiFe nanocomposite, as shown in Fig. 8(c), corroborate these results. Consequently, the catalyst and dye molecules repel each other at pH values below 3 or above 9, reducing the catalyst's ability to adsorb the dye and lowering the photocatalytic efficiency. Taking these factors into account, the catalyst exhibited the highest dye degradation at pH 7 for MO and pH 9 for RH-B.67
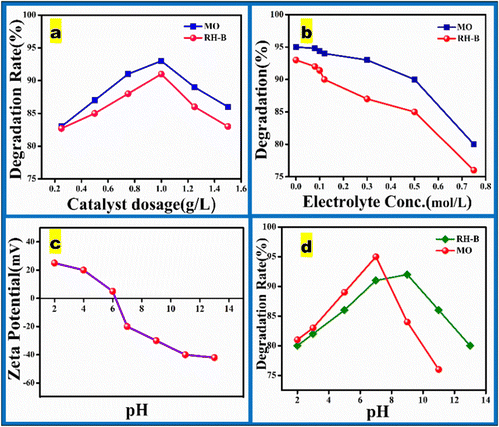 |
| | Fig. 8 Effect of different reaction conditions on the photodegradation of dyes: (a) catalyst dosage, (b) ionic strength, (c) pH and (d) zeta potential of SBXIII@NiFe at different pH. | |
4.5.2. Electrolyte.
Aqueous environments often include electrolytes, and several studies have shown that the anions in these electrolytes may impact how pollutants degrade via photodegradation. It is well-recognized that producing OH radicals in an aqueous solution is the first step in photodegradation. The oxidation of molecular water or hydroxyl ions on the catalyst surface in a photocatalytic system led to the creation of this very potent oxidizing agent, which these equations may describe:
| TiO2 + h+ + OH− → TiO2 + OH˙ |
| TiO2 + h+ + H2O → TiO2 + H+ + OH˙ |
The degradation rate was investigated using NaCl as a representative inorganic salt component at various concentrations. This study aimed to understand how different levels of NaCl affect the degradation process under the given conditions. As seen in Fig. 8(b), salt significantly impacts how well degradation occurs. Lesser concentrations barely impact the process of degradation. In contrast, the breakdown rate dramatically drops when the NaCl level surpasses 0.25 mol L−1.
This tendency might be related to the competition between ions and dye adsorption. Furthermore, earlier research suggests that active species in the solution may oxidize some ions, such as Cl−, raising the possibility that they may compete for active sites and decrease the pace at which dyes degrade.68
| TiO2 + h+ + Cl− → TiO2 + Cl˙ |
4.5.3. Dosage.
In addition to pH, the catalyst's potency needs to be considered, as it minimizes production costs and decreases waste. Fig. 8(a) depicts the results of varying the quantity of catalyst added to investigate the influence on the dosage without affecting the dye solution's pH. The extent of degradation increases with dosage when the dose is less than 1 g L−1, which makes sense since more catalysts might yield more reactive regions and active species. Once the concentration surpasses 1 g L−1, the rate of breakdown decreases. This result may have happened because the solution became opaque due to excess catalyst, which slowed the degradation rate and reduced light absorption. In conclusion, the optimal concentration of the catalyst is 1 g L−1.
4.5.4. Stability analysis of the SBXIII@NiFe composite.
Besides demonstrating significant photocatalytic prowess, an exceptional photocatalyst must also exhibit exceptional reusability. The composite underwent recyclability testing for up to four cycles, with each catalyst cycle being filtered and cleaned to assess the photocatalytic stability of SBXIII@NiFe. The results of these experiments on the composite are presented in Fig. 9. The fourth reusability test indicated that the efficiency remained above 85%. The decrease in degrading efficiency was attributed to particle aggregation of the catalyst and subsequent weight loss during filtration and washing procedures. After five successive cycles, XRD, FTIR, and XPS characterizations verified the catalyst's stability. The appearance and structure of the SBXIII@NiFe utilized in Fig. S3–S5 (ESI†) did not alter much. The XRD patterns of the utilized sample displayed no extra peaks and preserved the distinctive diffraction peaks of SBXIII@NiFe, as shown in Fig. S3 (ESI†). Similarly, Fig. S4 (ESI†) shows that following five cycles of dye photodegradation, the FTIR spectra of the SBXIII@NiFe composite did not change. Moreover, Fig. S5 (ESI†) demonstrates that the used SBXIII@NiFe XPS spectrum had Fe 2p, C 1s, N 1s, Ni 2p, and O 1s peaks, along with no modifications or extra peaks.
 |
| | Fig. 9 Reusability test of SBXIII@NiFe. | |
4.6. MECHANISM
Before photodegradation, dye molecules in an aqueous solution compete for active sites on the SBXIII@NiFe photocatalyst surface with water molecules and dissolved oxygen. The SBXIII@NiFe photocatalyst and these dye molecules interact until an equilibrium between adsorption and desorption is reached. Upon exposure to visible light in the photocatalytic reactor, the SBXIII@NiFe surface undergoes a sequence of photocatalytic and photosensitization reactions. The valence band (VB) and conduction band (CB) of the SBXIII@NiFe photocatalyst are activated during the photocatalytic process, creating an electron in the CB and a hole in the VB. This process produces pairs of electrons and holes. The holes (h+) in the VB can then initiate a series of photocatalytic processes by directly oxidizing chemisorbed dye molecules or by oxidizing chemisorbed water (H2O) to form reactive oxygen species (ROS). Meanwhile, oxygen molecules that have adsorbed on the surface may steal electrons from the CB. Additionally, visible light can be absorbed by chemisorbed dye molecules on the catalyst surface. This will excite the dye and cause an electron to be ejected into the CB, initiating photosensitization. Finally, the photodegradation products can undergo total degradation. It is known that the heteroatoms in dye molecules undergo oxidation during photodegradation, transforming them into a variety of minuscule neutral chemicals and ions. The oxidation of dyes during photodegradation creates tiny molecules such as CO2, NO3−, SO42−, H2O, and others.
Trapping experiments (Fig. 10) clarified the photocatalytic reaction mechanism and identified the primary active species involved in the photocatalytic degradation process. In these experiments, ethanol, benzoquinone (BQ), silver nitrate (AgNO3), and ammonium oxalate (AO) were used as scavengers for photo-generated hydroxyl radicals (˙OH), superoxide radical anions (O2˙−), electrons (e−), and holes (h+), respectively. Fig. 10 illustrates that adding ethanol significantly suppressed the degradation of MO and RH-B dye solutions, indicating that ˙OH radicals are crucial in the photocatalytic reaction process. The presence of BQ reduced the degradation rate, suggesting that O2˙− also plays a significant role in the decomposition of MO and RH-B pollutants under visible light irradiation. When AgNO3 and AO were used in the photocatalysis experiments, the degradation efficiency was only slightly hindered, indicating that electrons and holes (e− and h+) were not the primary active species and played a minimal role. Based on these results, the enhancement mechanism of the photocatalytic activity of the SBXIII@NiFe nanocomposite can be described as follows:
| | | NiFe + hv → NiFe(e−) + h+ | (3) |
| | | NiFe(e−) + SBXIII → NiFe + SBXIII(e−) | (4) |
| | | SBXIII(e−) + O2 → O2˙− + SBXIII | (5) |
| | | NiFe(h+) + H2O → NiFe + OH˙− + H+ | (6) |
| | | O2˙− + H2O → HO2˙ + OH˙ | (7) |
| | | O2˙− + OH˙ + dye solution → Degradation products | (9) |
 |
| | Fig. 10 Scavenging experiments. | |
The probable mechanism of photodegradation can be clarified by various processes as explained below. The intense emission peak observed for SBXIII at 485 nm suggests an efficient recombination rate of photogenerated carriers. However, in the case of SBXIII@NiFe, there was a noticeable quenching of photoluminescence compared to SBXIII and NiFe alone, as shown in Fig. 6(c). This indicates a reduced recombination rate of photogenerated holes and electrons in the SBXIII@NiFe nanocomposite. Thus, by forming a nanocomposite between NiFe and SBXIII, the rate of recombination of the photogenerated charge carriers is significantly lowered. As a result, the photogenerated e−/h+ pairs in SBXIII@NiFe are more efficiently transferred at the nanocomposite interface, leading to enhanced photocatalytic performance compared to pure NiFe and SBXIII.
The effect of the SBXIII@NiFe nanocomposite on the electron–hole pair separation efficiency was also examined using electrochemical impedance spectroscopy (EIS). The EIS changes of the SBXIII@NiFe and NiFe electrodes are displayed in Fig. 6(a). A narrower arc often denotes a lower electrode surface charge-transfer resistance in an EIS Nyquist diagram. The relative arc diameters for the two electrodes are in the order NiFe > SBXIII@NiFe, according to the results in Fig. 6(a). This suggests that the most efficient composite for charge separation and electron transport is the SBXIII@NiFe one. The appropriate VB, CB, separation, and interface interaction were primarily responsible for the performance enhancement of the nanocomposites in earlier investigations. The CB position of the NiFe sample was examined using Mott–Schottky technology in Fig. 11, and the flat band position for NiFe is 0.31 V. The CB position for NiFe is 0.35 V since the absolute CB position is around 0.04 V, which is more positive than the flat band potential. NiFe and SBXIII have band gaps of 1.90 and 3.2 V in the UV-vis DRS spectrum, respectively. The band gap and CB positions are used to determine the VB position for NiFe, which comes out to 2.61 V. Based on the above analysis of the band structure of NiFe, it is speculated that under visible light irradiation, photogenerated electrons are produced on the VB of NiFe, which we're excited to transfer to the CB, and holes (h+) remain in the VB. The possible photoreaction mechanism is shown in Scheme 2. According to the suggested photoreaction process in Scheme 2, electrons are excited from the VB to the CB of NiFe and then transferred to the SBXIII when exposed to visible light, leaving holes in the VB. Since conjugation in Schiff-base helps overcome the recombination of electrons and holes, as also seen in the PL spectra, this increases the total photocatalytic efficiency.
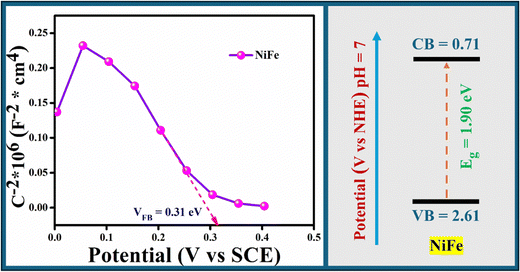 |
| | Fig. 11 Mott–Schottky plots and band edge potentials of NiFe. | |
 |
| | Scheme 2 Probable photodegradation mechanism. | |
4.7. Mineralization studies of SBXIII@NiFe
The COD percentage change was examined for dye samples with an initial concentration of 25 mg L−1 under optimized conditions. For MO, a catalyst dose of 1 g L−1 at pH 7 was used, and for RHB, a catalyst dose of 0.5 g L−1 at pH 9 was applied. This study measured the COD reduction as a function of exposure time under visible light. The findings are displayed in Table 4. Using visible light, MO and RH-B produced 82% and 76% COD reductions, respectively, within 90 minutes. The COD decrease is less than % degradation, which might be attributed to the production of tiny colorless byproducts. As a result, longer irradiation times are necessary for complete dye mineralization.
Table 4 Chemical oxygen demand reduction studies of dyes (%)
| Irradiation time (min) |
MO |
RH-B |
| 0 |
0 |
0 |
| 10 |
23 |
22 |
| 30 |
37 |
39 |
| 60 |
54 |
51 |
| 90 |
82 |
76 |
4.8. Antibacterial activity
Over the past several years, we have seen an increase in focus on the antimicrobial properties of chitosan and its derivatives. The emergence of microorganism mutations resistant to antimicrobial agents has propelled researchers to develop novel materials with antimicrobial potential against evolving pathogenic microorganisms.69 The antimicrobial efficacy of Schiff-base and its ferrite composite was initially assessed using the agar-well diffusion method against typical Gram-positive bacteria (S. aureus) and Gram-negative bacteria (P. vulgaris) (Fig. S7, ESI†). Compared with streptomycin as a benchmark antibiotic, the activity of chitosan and its derivatives was evaluated, with DMSO as the negative control. The zones of inhibition for antimicrobial activities were quantified and detailed in Table 5. The results indicate that chitosan and its derivatives exhibited significant antimicrobial activity against the tested bacteria, as shown in Fig. 12. Notably, the chitosan Schiff-base derivatives displayed greater efficacy than the original chitosan against the indicator bacteria. These findings suggest that the Schiff-base nanocomposite (SBXIII@NiFe) displayed superior activity against the examined microorganisms, irrespective of their structures. The enhanced antibacterial effectiveness of SBXIII@NiFe, surpassing its counterparts, can be linked to its increased surface area, enhanced ROS generation, and photo-induced electron–hole pair recombination inhibition.70 Due to inherent characteristics, including its source and molecular weight, which influence penetration into bacteria and the creation of novel chitosan derivatives with improved antimicrobial capabilities, the antimicrobial effects of chitosan might differ. Based on differences in cell wall structure and metabolic pathways, different hypothesized mechanisms explain how chitosan interacts with different microbes. The first method releases intracellular components through an electrostatic contact between the nanocomposite's positively charged amine groups (NH3+) and different negatively charged microbial cell walls. The second mechanism highlights how the molecular weight of chitosan regulates its penetration into microbial nuclei, binding with DNA and repressing mRNA expression to inhibit protein synthesis. Thirdly, metal ions such as Ca2+, Mg2+, and Zn2+, essential for microbial growth and metabolic processes, including spore production in Gram-positive bacteria, are attributed to the chelating capacity of chitosan. Utilizing reactive oxygen species (ROS) to interact with pathogens presents an effective germicidal strategy for bacterial destruction.71Scheme 2 illustrates how a photocatalytic material undergoes an external redox process that produces ROS from the photo-induced e–h pair and chemisorbed oxygen (O2) and water (H2O). The ROS kills the bacterium created through contact and the disintegration of the bacterial cell wall. Therefore, it is suggested that the earlier processes might be coupled to execute the antibacterial efficacy of the synthesized chitosan modifications. These characteristics were achieved in the new chitosan. The fabrication of different Schiff bases to increase their antimicrobial activity has been documented in earlier investigations; the novel compounds have shown exceptional antibacterial properties. This demonstrates how the Schiff-base composite's combinatorial improvement of chitosan activity against the investigated bacteria is superior to the pristine chitosan.72,73
Table 5 Inhibition indices of chitosan, chitosan Schiff-base (SBXIII) and its nanocomposite (SBXIII@NiFe) against P. vulgaris and S. aureus
| Bacteria |
Inhibition zone (mm) |
| Streptomycin |
Chitosan |
SBXIII |
SBXIII@NiFe (10 mg) |
SBXIII@NiFe (20 mg) |
SBXIII@NiFe (30 mg) |
|
P. vulgaris
|
26 |
16 |
32 |
32 |
38 |
40 |
|
S. aureus
|
20 |
15 |
34 |
24 |
29 |
33 |
 |
| | Fig. 12 Zone of inhibition (mm) of the prepared catalyst depicting the antibacterial activity. | |
Based on the band structure analysis of NiFe, it is proposed that under visible light irradiation, photogenerated electrons are excited from the valence band (VB) to the conduction band (CB) of NiFe, leaving behind holes (h+) in the VB. The suggested photoreaction mechanism, illustrated in Scheme 2, shows that these excited electrons are transferred from the CB of NiFe to SBXIII upon exposure to visible light, while holes remain in the VB. The conjugated structure of the Schiff base effectively reduces electron–hole recombination, as corroborated by the photoluminescence (PL) spectra, thereby significantly enhancing the overall photocatalytic performance.
In terms of antimicrobial activity, the reactive oxygen species (ROS), specifically superoxide radicals (O2˙−) and hydroxyl radicals (OH˙), interact directly with the microbial cell membrane, leading to cell damage. These ROS and the charge carriers breach the microbial cell wall, allowing them to penetrate the cytoplasm. Once inside, they oxidize enzymes and other cellular components, rendering them toxic. This disruption destabilizes the cell structure, ultimately causing cell death. The efficient transfer of electrons to the CB of NiFe, facilitated by its low bandgap and the electron delocalization in the Schiff base, effectively reduces the electron–hole recombination rate in the SBXIII@NiFe composite, enhancing both its photocatalytic and antibacterial efficacy.
5. Conclusion
In conclusion, this study successfully developed a novel visible-light-driven SBXIII@NiFe photocatalyst, achieving over 90% decolorization of two major water pollutants, methyl orange (MO) and rhodamine-B (RH-B). The maximum photocatalytic activity was observed at pH 4 for MO, with a degradation rate of 95%, and at pH 9 for RH-B, reaching a 92% degradation rate. The SBXIII@NiFe composite exhibited significantly higher photocatalytic efficiency compared to pure NiFe, attributed to the incorporation of SBXIII, which acts as an electron trap, reducing electron–hole recombination by over 60% and enhancing visible light absorption by approximately 40%. Active species trapping studies identified hydroxyl radicals (OH˙) and superoxide radicals (O2˙−) as the primary reactive agents responsible for oxidative degradation. Furthermore, the photocatalyst demonstrated superior antibacterial efficacy, reducing the growth of Gram-positive (S. aureus) and Gram-negative (P. vulgaris) compared to control samples. This enhanced antibacterial performance is likely due to the combined effects of chitosan, ferrite, and anthrone, which boost the catalyst's activity together. These findings suggest that the SBXIII@NiFe composite is a promising candidate for environmental remediation and antimicrobial applications.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Due to privacy and ethical restrictions, the data have not been deposited in a public repository. For any additional information or inquiries, please contact the corresponding author at https://musaibchem48@gmail.com.
Author contributions
Shakeel A. Shah: supervision, verification, resources, review, and editing; Musaib Y. Wani: analysis, data classification, methodology, writing, review, and editing; conducted experimental work and data curation.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
The authors acknowledged the instrumentation assistance and technical insights provided by NIT Srinagar as having significantly contributed to this research project. Furthermore, Musaib Y. Wani expresses gratitude to the Ministry of Education for providing financial assistance to carry out this work.
References
- D. Foray and A. Grübler, Technol. Forecase. Soc., 1996, 53, 3–13 CrossRef.
- B. Shen, C. Dong, J. Ji, M. Xing and J. Zhang, Chin. Chem. Lett., 2019, 30, 2205–2210 CrossRef CAS.
- Q. Liu, W. Xu, S. Lu, J. Jiang, J. Zhou, Z. Shao, X. Liu, L. Xu, Y. Xiong and H. Zheng, Front. Med., 2018, 12, 3–22 CrossRef PubMed.
- T. Islam, M. R. Repon, T. Islam, Z. Sarwar and M. M. Rahman, Environ. Sci. Pollut. Res., 2023, 30, 9207–9242 CrossRef CAS PubMed.
- A. Tkaczyk, K. Mitrowska and A. Posyniak, Sci. Total Environ, 2020, 717, 137222 CrossRef CAS PubMed.
- K.-T. Chung, J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev., 2016, 34, 233–261 CrossRef CAS PubMed.
-
K. Singha, P. Pandit, S. Maity and S. R. Sharma, in Green Chemistry for Sustainable Textiles, Elsevier, 2021, pp. 153–164 Search PubMed.
- N. N. Bahrudin, M. A. Nawi and Z. Zainal, Int. J. Biol. Macromol., 2020, 165, 2462–2474 CrossRef CAS PubMed.
- A. H. Jawad and A. S. Abdulhameed, Colloids Surf., A, 2020, 605, 125329 CrossRef CAS.
- A. Reghioua, D. Barkat, A. H. Jawad, A. S. Abdulhameed and M. R. Khan, Sustainable Chem. Pharm., 2021, 20, 100379 CrossRef CAS.
- Z. Wu, W. Feng, Y. Feng, Q. Liu, X. Xu, T. Sekino, A. Fujii and M. Ozaki, Carbon, 2007, 45, 1212–1218 CrossRef CAS.
- R. Kumar, A. Sudhaik, P. Raizada, A. Singh, T. Ahamad, A. A. P. K. Khan, S. Rangabhashiyam and P. Singh, Biomater. Polym. Horiz., 2022, 1, 3 Search PubMed.
- M. A. M. Adnan, B. L. Phoon and N. M. Julkapli, J. Cleaner Prod., 2020, 261, 121190 CrossRef.
- I. Aadnan, O. Zegaoui, I. Daou and J. C. G. E. da Silva, J. Environ. Chem. Eng., 2020, 8, 104260 CrossRef CAS.
- S. Hazra and N. N. Ghosh, J. Nanosci. Nanotechnol., 2014, 14, 1983–2000 CrossRef CAS PubMed.
- O. F. Odio and E. Reguera, Magn. Spinels: Synth., Prop. Appl., 2017, 185–216 CAS.
- J. F. J. Sherin, T. C. Bessy, S. Asha, C. V. Kumar, D. Huessien, M. R. Bindhu, R. A. Rasheed and K. M. Alarjani, Environ. Res., 2022, 208, 112687 CrossRef PubMed.
- J. López, A. A. Ortíz, F. Muñoz-Muñoz, D. Dominguez, J. N. D. de León, J. T. E. Galindo, T. Hogan, S. Gómez, H. Tiznado and G. Soto–Herrera, J. Phys. Chem. Solids, 2021, 150, 109869 CrossRef.
- E. Casbeer, V. K. Sharma and X.-Z. Li, Sep. Purif. Technol., 2012, 87, 1–14 CrossRef CAS.
- P. Shah, K. Joshi, M. Shah, A. Unnarkat and F. J. Patel, Environ. Sci. Pollut. Res., 2022, 29, 78255–78264 CrossRef CAS PubMed.
- T. K. Dixit, S. Sharma and A. S. K. Sinha, Inorg. Chem. Commun., 2020, 117, 107945 CrossRef CAS.
- S. Sun, X. Yang, Y. Zhang, F. Zhang, J. Ding, J. Bao and C. Gao, Prog. Nat. Sci.: Mater. Int., 2012, 22, 639–643 CrossRef.
- L. R. Gonsalves, V. M. S. Verenkar and S. C. Mojumdar, J. Therm. Anal. Calorim., 2009, 96, 53–57 CrossRef CAS.
- S. Singh and S. Singhal, Mater. Today: Proc., 2019, 14, 453–460 CAS.
- M. Dhiman and S. Singhal, Mater. Today: Proc., 2019, 14, 435–444 CAS.
- S. Kanithan, N. A. Vignesh, K. M. Katubi, P. S. Subudhi, E. Yanmaz, J. A. Dhanraj, N. S. Alsaiari, M. Sukumar, M. Sundararajan and S. Baskar, J. Mol. Struct., 2022, 1265, 133289 CrossRef CAS.
- S. A. Jadhav, S. B. Somvanshi, M. V. Khedkar, S. R. Patade and K. M. Jadhav, J. Mater. Sci.: Mater. Electron., 2020, 31, 11352–11365 CrossRef CAS.
- G. Padmapriya, A. Manikandan, V. Krishnasamy, S. K. Jaganathan and S. A. Antony, J. Mol. Struct., 2016, 1119, 39–47 CrossRef CAS.
- M. I. A. A. Maksoud, G. S. El-Sayyad, A. H. Ashour, A. I. El-Batal, M. A. Elsayed, M. Gobara, A. M. El-Khawaga, E. K. Abdel-Khalek and M. M. El-Okr, Microb. Pathog., 2019, 127, 144–158 CrossRef CAS PubMed.
- S. Meena, K. S. Anantharaju, Y. S. Vidya, L. Renuka, B. Uma, S. C. Sharma and S. S. More, Ceram. Int., 2021, 47, 14760–14774 CrossRef CAS.
- T. Hisatomi, J. Kubota and K. Domen, Chem. Soc. Rev., 2014, 43, 7520–7535 RSC.
- K. Zhang and L. Guo, Catal. Sci. Technol., 2013, 3, 1672–1690 RSC.
- M. Nemiwal, T. C. Zhang and D. Kumar, Sci. Total Environ., 2021, 767, 144896 CrossRef CAS PubMed.
- G. Sharma, A. Kumar, S. Sharma, M. Naushad, P. Dhiman, D.-V. N. Vo and F. J. Stadler, Mater. Lett., 2020, 278, 128359 CrossRef CAS.
- H. Cui, B. Li, Z. Li, X. Li and S. Xu, Appl. Surf. Sci., 2018, 455, 831–840 CrossRef CAS.
- C. Van Tran, D. D. La, P. N. T. Hoai, H. D. Ninh, P. N. T. Hong, T. H. T. Vu, A. K. Nadda, X. C. Nguyen, D. D. Nguyen and H. H. Ngo, J. Hazard. Mater., 2021, 420, 126636 CrossRef PubMed.
- R. Suresh, S. Rajendran, P. S. Kumar, D.-V. N. Vo and L. Cornejo-Ponce, Chemosphere, 2021, 274, 129734 CrossRef CAS PubMed.
- S. Pansambal, A. Roy, H. E. A. Mohamed, R. Oza, C. M. Vu, A. Marzban, A. Chauhan, S. Ghotekar and H. C. A. Murthy, J. Nanomater., 2022, 2022, 8560069 CrossRef.
- B. Alqassem, F. Banat, G. Palmisano and M. A. Haija, Sustainable Mater. Technol., 2024, e00961 CrossRef CAS.
- M. B. Gawande, Y. Monga, R. Zboril and R. K. Sharma, Coord. Chem. Rev., 2015, 288, 118–143 CrossRef CAS.
- J. Govan and Y. K. Gun’ko, Nanomaterials, 2014, 4, 222–241 CrossRef PubMed.
-
C. P. Vinod, A. B. Vysakh and S. Sreedhala, Metal Nanoparticles and Clusters: Advances in Synthesis, Properties and Applications, 2018, 165–199 Search PubMed.
- A. Zuorro and R. Lavecchia, Desalin. Water Treat., 2014, 52, 1571–1577 CrossRef CAS.
- T. An, H. Yang, G. Li, W. Song, W. J. Cooper and X. Nie, Appl. Catal., B, 2010, 94, 288–294 CrossRef CAS.
- N. S. Deshmukh and M. P. Deosarkar, Mater. Today: Proc., 2022, 57, 1575–1584 CAS.
- C. Chen, C. Ma, Y. Yang, X. Yang, K. Demeestere, A. Nikiforov and S. Van Hulle, Water Res., 2023, 235, 119881 CrossRef CAS PubMed.
- A. Krosuri, S. Wu, M. A. Bashir and M. Walquist, J. Water Process Eng., 2021, 40, 101926 CrossRef.
- K. Nejati and R. Zabihi, Chem. Cent. J., 2012, 6, 1–6 CrossRef PubMed.
- J. E. dos Santos, E. R. Dockal and É. T. G. Cavalheiro, Carbohydr. Polym., 2005, 60, 277–282 CrossRef CAS.
- J. Huo and M. Wei, Mater. Lett., 2009, 63, 1183–1184 CrossRef CAS.
- A. Z. Moghaddam, E. Ghiamati, A. Pourashuri and A. Allahresani, Int. J. Biol. Macromol., 2018, 120, 1714–1725 CrossRef PubMed.
- E. M. Abd El-Monaem, M. S. Ayoup, A. M. Omer, E. N. Hammad and A. S. Eltaweil, Appl. Water Sci., 2023, 13, 67 CrossRef CAS.
- M. Hua, L. Xu, F. Cui, J. Lian, Y. Huang, J. Bao, J. Qiu, Y. Xu, H. Xu and Y. Zhao, J. Mater. Sci., 2018, 53, 7621–7636 CrossRef CAS.
- A. H. Jawad, N. S. A. Mubarak and A. S. Abdulhameed, Int. J. Biol. Macromol., 2020, 142, 732–741 CrossRef CAS PubMed.
- Z. Li, H. Li, X. Zeng, S. Liu and Y. Yang, Chem. Eng. J., 2023, 458, 141455 CrossRef CAS.
- T. Ahamad, A. A. Chaudhary, M. Naushad and S. M. Alshehri, Int. J. Biol. Macromol., 2019, 134, 180–188 CrossRef CAS PubMed.
- P. Ramadevi, R. Shanmugavadivu, R. Venkatesan, J. Mayandi and S. Sagadevan, Inorg. Chem. Commun., 2023, 150, 110532 CrossRef CAS.
- S. K. Kansal, M. Singh and D. Sud, J. Hazard. Mater., 2007, 141, 581–590 CrossRef CAS PubMed.
- F. Wang and K. Zhang, J. Mol. Catal. A: Chem., 2011, 345, 101–107 CrossRef CAS.
- J. Chen, H. Li, L. Ma, G. Jiang, D. Li, Y. Wu, X. Shi, X. Wang and H. Deng, Carbohydr. Polym., 2021, 273, 118559 CrossRef CAS PubMed.
- M. Nikitha, S. S. Elanchezhiyan and S. Meenakshi, Environ. Res., 2023, 238, 117032 CrossRef CAS PubMed.
- M. Maruthupandy, T. Muneeswaran, M. Anand and F. Quero, Int. J. Biol. Macromol., 2020, 153, 736–746 CrossRef CAS PubMed.
- V. S. Narayanan, P. V. Prasath, K. Ravichandran, D. Easwaramoorthy, Z. Shahnavaz, F. Mohammad, H. A. Al-Lohedan, S. Paiman, W. C. Oh and S. Sagadevan, Mater. Sci. Semicond. Process., 2020, 119, 105238 CrossRef.
- M. Vosough, G. R. Khayati and S. Sharafi, Environ. Res., 2024, 249, 118415 CrossRef CAS PubMed.
- N. L. Rajput, M. A. Mughal, A. Balouch, K. M. Khan, S. A. Tunio, Kanwal and S. Sohu, Int. J. Environ. Anal. Chem., 2022, 102, 3561–3575 CrossRef CAS.
- M. H. Khorasanizadeh, R. Monsef, M. Salavati-Niasari, H. S. Majdi, W. K. Al-Azzawi and F. S. Hashim, Arabian J. Chem., 2023, 16, 105020 CrossRef.
- R. Abdallah, B. Djamel, A. H. Jawad, A. A. Saud, S. Rangabhashiyam, M. R. Khan and Z. A. Alothman, J. Polym. Environ., 2021, 29, 3932–3947 CrossRef.
- N. A. Chopan, New J. Chem., 2023, 47, 15487–15505 RSC.
- A. Abd-El-Aziz, M. M. G. Fouda, C. M. Sharaby, O. Xiao, X. Zhang, Y. A. Alzahrany, S. A. Ahmed, N. Ma and A. S. Abd-El-Aziz, Macromol. Chem. Phys., 2024, 225, 2400123 CrossRef CAS.
- S. A. Jasim, K. Hachem, W. K. Abdelbasset, G. Yasin and W. Suksatan, Int. J. Biol. Macromol., 2022, 204, 644–651 CrossRef CAS PubMed.
- P. Rokicka-Konieczna and A. W. Morawski, Molecules, 2024, 29, 2221 CrossRef CAS PubMed.
- N. A. Chopan and A. H. Bhat, New J. Chem., 2023, 47, 15922–15941 RSC.
- S. Shokri, N. Shariatifar, E. Molaee-Aghaee, G. J. Khaniki, P. Sadighara, M. A. Faramarzi, M. Mohammadi and A. Rezagholizade-Shirvan, Sci. Rep., 2023, 13, 15777 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  * and
Shakeel A.
Shah
*
* and
Shakeel A.
Shah
*
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe molar ratio of 1
Fe molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The resulting solution was clear. Next, while stirring magnetically, 10 mL of 8 M NaOH solution was gradually added. The mixture was transferred into an autoclave, which was then sealed and heated to 180 °C for 10 hours. After the reaction was completed, the solid products were filtered, rinsed thoroughly with acetone and DDW, and vacuum-dried at 50 °C.48
2. The resulting solution was clear. Next, while stirring magnetically, 10 mL of 8 M NaOH solution was gradually added. The mixture was transferred into an autoclave, which was then sealed and heated to 180 °C for 10 hours. After the reaction was completed, the solid products were filtered, rinsed thoroughly with acetone and DDW, and vacuum-dried at 50 °C.48
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (v/v) ethanol-glacial acetic acid mixture and stirred. After ten minutes, 10 mL of an ethanolic solution of anthrone (1 g) was gradually added. The reaction mixture was then stirred and heated to 80 °C until an orange-yellow solid precipitate formed. Once cooled, the solid was filtered, rinsed with ethanol, and dried at room temperature for several days.49
5 (v/v) ethanol-glacial acetic acid mixture and stirred. After ten minutes, 10 mL of an ethanolic solution of anthrone (1 g) was gradually added. The reaction mixture was then stirred and heated to 80 °C until an orange-yellow solid precipitate formed. Once cooled, the solid was filtered, rinsed with ethanol, and dried at room temperature for several days.49

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– group. The peaks around 1533, 1309, and 1015 cm−1 in the FT-IR spectra of SBXIII and SBXIII@NiFe correspond to C
N– group. The peaks around 1533, 1309, and 1015 cm−1 in the FT-IR spectra of SBXIII and SBXIII@NiFe correspond to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrational modes. The glycoside C–O, C–O–C, and C–C bonds and stretching in the chitosan's pyranose ring polymer chain appear as several wide peaks between 1000 and 1300 cm−1. In the FT-IR spectrum of SBXIII@NiFe, new weak bands appear at around 665 and 1319 cm−1, associated with the Fe–O bond.49
C vibrational modes. The glycoside C–O, C–O–C, and C–C bonds and stretching in the chitosan's pyranose ring polymer chain appear as several wide peaks between 1000 and 1300 cm−1. In the FT-IR spectrum of SBXIII@NiFe, new weak bands appear at around 665 and 1319 cm−1, associated with the Fe–O bond.49

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and C
C, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively. This insight enables us to design an effective coating of chitosan Schiff base on the surface of NiFe2O4. Fig. 2c depicts the XPS curve of N 1s in the 394 to 410 eV range. The C
O bonds, respectively. This insight enables us to design an effective coating of chitosan Schiff base on the surface of NiFe2O4. Fig. 2c depicts the XPS curve of N 1s in the 394 to 410 eV range. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–N bonds in the Schiff base ligand components on the surface of NiFe2O4 are attributed to binding energies 399.9 and 402.6 eV, respectively.52Fig. 2d depicts the XPS curve of O 1s, with two peaks occurring at 532.8 and 530 eV, corresponding to the O–H and M–O bonds, respectively. Fig. 2e depicts the Fe 2p scan of the prepared material, which shows two peaks at 712.6 and 725.4 eV, respectively. The appearance of these two binding energy peaks is ascribed to Fe 2p3/2 and Fe 2p1/2, suggesting that the material has a NiFe2O4 core. The comparatively low strength of these two peaks shows that NiFe2O4 was efficiently coated, resulting in the synthesis of the Schiff base and its NiFe composite. The Ni 2p spectra (Fig. 2f) reveal the peaks of Ni 2p3/2 at BE of 847.54 and 850.4, as well as Ni 2p1/2 at BE of 856.45 and 863.59 eV, confirming the presence of Ni2+ and Ni3+ in the sample.53
N and C–N bonds in the Schiff base ligand components on the surface of NiFe2O4 are attributed to binding energies 399.9 and 402.6 eV, respectively.52Fig. 2d depicts the XPS curve of O 1s, with two peaks occurring at 532.8 and 530 eV, corresponding to the O–H and M–O bonds, respectively. Fig. 2e depicts the Fe 2p scan of the prepared material, which shows two peaks at 712.6 and 725.4 eV, respectively. The appearance of these two binding energy peaks is ascribed to Fe 2p3/2 and Fe 2p1/2, suggesting that the material has a NiFe2O4 core. The comparatively low strength of these two peaks shows that NiFe2O4 was efficiently coated, resulting in the synthesis of the Schiff base and its NiFe composite. The Ni 2p spectra (Fig. 2f) reveal the peaks of Ni 2p3/2 at BE of 847.54 and 850.4, as well as Ni 2p1/2 at BE of 856.45 and 863.59 eV, confirming the presence of Ni2+ and Ni3+ in the sample.53
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) and associated auxochromes and chromophores. Throughout the deterioration process, the color of the MO solution faded progressively. The photocatalytic elimination of methyl orange was distinct from that of rhodamine B. As an azo dye, the principal chromophore of methyl orange comprises the azo group and aryl rings in a conjugated system; hence, the fading of methyl orange's color was predominantly caused by damage to this conjugated system.58 We also studied the degradation kinetics of dyes employing two monomers (SBXIII and NiFe) independently and in combination (SBXIII@NiFe). All these components degraded dyes using pseudo-first-order kinetics, with SBXIII@NiFe having the most significant reaction rate constant, as shown in Fig. 7(b, c, e, f). Table 2 also shows the parameters for pseudo-first-order kinetics. Table 3 compares the photocatalytic degradation of dyes by the present binary composite with the already reported similar composite materials.
N–) and associated auxochromes and chromophores. Throughout the deterioration process, the color of the MO solution faded progressively. The photocatalytic elimination of methyl orange was distinct from that of rhodamine B. As an azo dye, the principal chromophore of methyl orange comprises the azo group and aryl rings in a conjugated system; hence, the fading of methyl orange's color was predominantly caused by damage to this conjugated system.58 We also studied the degradation kinetics of dyes employing two monomers (SBXIII and NiFe) independently and in combination (SBXIII@NiFe). All these components degraded dyes using pseudo-first-order kinetics, with SBXIII@NiFe having the most significant reaction rate constant, as shown in Fig. 7(b, c, e, f). Table 2 also shows the parameters for pseudo-first-order kinetics. Table 3 compares the photocatalytic degradation of dyes by the present binary composite with the already reported similar composite materials.














