Visible-light-active type-II heterojunction CdS@Cu0.5Mg2.5SnS4 composites for the efficient removal of brilliant green dye†
Ashmalina
Rahman
 a,
Fazlurrahman
Khan
a,
Fazlurrahman
Khan
 bcd,
James Robert
Jennings
bcd,
James Robert
Jennings
 ef,
Young-Mog
Kim
ef,
Young-Mog
Kim
 cdg and
Mohammad Mansoob
Khan
cdg and
Mohammad Mansoob
Khan
 *af
*af
aChemical Sciences, Faculty of Science, Universiti Brunei Darussalam, Jalan Tungku Link, Gadong, BE 1410, Brunei Darussalam. E-mail: mmansoobkhan@yahoo.com; mansoob.khan@ubd.edu.bn
bOcean and Fisheries Development International Cooperation Institute, Pukyong National University, Busan 48513, Republic of Korea
cMarine Integrated Biomedical Technology Center, The National Key Research Institutes in Universities, Pukyong National University, Busan 48513, Republic of Korea
dResearch Center for Marine Integrated Bionics Technology, Pukyong National University, Busan 48513, Republic of Korea
eApplied Physics, Faculty of Science, Universiti Brunei Darussalam, Jalan Tungku Link, Gadong, BE 1410, Brunei Darussalam
fOptoelectronic Device Research Group, Universiti Brunei Darussalam, Jalan Tungku Link, Gadong, BE 1410, Brunei Darussalam
gDepartment of Food Science and Technology, Pukyong National University, Busan 48513, Republic of Korea
First published on 4th November 2024
Abstract
Copper magnesium tin sulfide (CMTS) is a multifunctional material that has recently generated a lot of interest as a potential new photocatalyst for organic pollutant degradation. Some of the desirable characteristics of CMTS are its narrow band gap energy, good chemical and photochemical stability, and high relative abundance of its constituent elements. In this work, CMTS (with the empirical formula Cu0.5Mg2.5SnS4) and type-II heterojunction CdS@CMTS composites with varying CdS contents have been synthesized using a cost-effective and energy-efficient microwave-assisted method. X-ray diffraction analysis confirmed the presence of kesterite CMTS and hexagonal CdS in the composites. After the addition of CdS, the effective band gap energy of CMTS increased from 2.07 to 2.40 eV. The composites exhibited a uniform distribution of CdS on the CMTS surface. Photocatalytic studies reveal that CdS@CMTS exhibits higher photocatalytic activity for the degradation of brilliant green (BG) dye under visible light irradiation in comparison to pristine CMTS. Among the synthesized materials, the 30% CdS@CMTS exhibited the highest photocatalytic activity, with up to 95.8 ± 1.45% degradation of BG within 5 h. Thus, CdS@CMTS prepared by microwave-assisted synthesis has shown potential to degrade organic pollutants for wastewater remediation.
1. Introduction
Quaternary chalcogenides contain four different ions: three metal cations and a chalcogen anion.1,2 Quaternary chalcogenides Cu2XSnS4 (X = Zn, Fe, Mg, Co, or Mn) are particularly attractive for photocatalytic applications such as wastewater remediation due to their light absorption characteristics and because they comprise only earth-abundant elements with low toxicity.3–7 Zhong and co-workers have studied the structural and thermodynamic stabilities of Cu2MSnX4 (M = Mg, and Ca, X = S and Se) and reported that Mg can serve as a good substitute for Zn due to their thermodynamically stable nature in comparison to Cu2CaSnS4 and Cu2CaSnSe4.8 To date, there are very few reports available on the fabrication of copper magnesium tin sulfide (CMTS). The methods that have been employed for CMTS synthesis include pulsed laser deposition,9 sol–gel spin-coating followed by sulfuration,10 spray pyrolysis,11 co-precipitation,12 hot-injection,13 and the sol–gel method.3In a study carried out by Wei and co-workers, Cu2MgSnS4 was synthesized using the hot injection method.13 They reported that the synthesized CMTS exhibited the kesterite phase with a band gap energy of 1.63 eV. Mg2+ and Zn2+ have similar ionic radii; therefore, they can take the place of Zn in Cu2ZnSnS4 (CZTS) without much alteration in the crystal structure to form CMTS. CMTS may become a new earth-abundant element material for solar cells. Yang and co-workers have synthesized CMTS via a sol–gel spin-coating route followed by sulfuration.10 The synthesized material yielded a zinc-blende CMTS structure with a band gap energy of 1.5 eV.
In a different study, Ali and co-workers showed that kesterite CMTS modified with tetragonal BaTiO3 exhibits remarkably strong bacterial inhibition for both Escherichia coli and Staphylococcus aureus.14 Computational studies have also provided insights into the structural, elastic, and thermodynamic properties of CMTS, as carried out by Bekki and co-workers.15 Based on their calculations, CMTX (X = S, Se, or Te) can exhibit stannite, kesterite, primitive-mixed CuAu, and mixed-phase wurtzite-stannite structures, and the stannite phase is the most stable among them.
Compared with other synthesis methods, the use of microwave irradiation for CMTS synthesis offers distinct advantages over conventional heating methods.16–18 The microwave irradiation accelerates the reaction kinetics by enhancing the nucleation process and overcoming energy barriers, leading to reduced synthesis times.19 Microwave heating also ensures uniform temperature distribution throughout the reaction vessel, minimizing thermal gradients.20 Consequently, microwave heating proves to be more cost-effective, energy-efficient, faster, and more economical compared to traditional methods.21 CMTS has garnered attention as a potential candidate for photocatalysis in energy conversion and environmental remediation. However, the photocatalytic performance of CMTS is limited and not widely explored. On the other hand, pristine CdS has drawn a lot of attention due to its suitable 2.4 eV direct band gap, which can be excited by visible light to produce charge carriers.22 However, it is unable to satisfy the requirements of practical applications due to photo-corrosion and the rapid recombination of photogenerated charge carriers.23,24 This issue can be effectively tackled by forming heterojunction composites with photocatalysts with matched energy bands.25 This strategy is expected to significantly improve its photocatalytic performance.
In this study, a rapid and energy-efficient microwave-assisted method has been utilized to synthesize CMTS and CdS@CMTS using ethylene glycol as a solvent for the first time. To the best of the authors' knowledge, there are no reported studies on type-II heterojunction CdS@CMTS composites. The influence of CdS on the structural, optical, and morphological properties of CMTS was investigated. The photocatalytic degradation of brilliant green (BG) dye using CMTS and CdS@CMTS was achieved with a low dosage of photocatalyst. More importantly, an in-depth study on the scavenging/trapping of active species was carried out for the first time using CdS@CMTS to determine the major species involved in the BG degradation process.
2. Experimental section
2.1. Chemicals used
All reagents were used without further purification. For the synthesis, copper nitrate trihydrate (Cu(NO3)2·3H2O) was purchased from Alfa Aesar as the Cu source. Cadmium sulfate (CdSO4·8/3H2O), thiourea (NH2CSNH2), and ethylene glycol were purchased from Merck, Germany. The distilled water was purified using a water still from Aquatron, England. Ethanol was purchased from Duksan Pure Chemicals Co. Ltd, South Korea. Magnesium nitrate (Mg(NO3)2) tin tetrachloride pentahydrate (SnCl4·5H2O) and BG dye were obtained from Sigma-Aldrich. For the free radical scavenging/trapping experiments, isopropanol and benzoquinone were obtained from Acros, and ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA) was purchased from Fluka.2.2. Characterization
CMTS and type-II heterojunction CdS@CMTS composites were synthesized using an Anton-Paar microwave reactor (Monowave 400, Austria). A Malvern PANalytical Aeris Research Edition (United Kingdom) benchtop X-ray diffraction (XRD) instrument was utilized to determine the crystal structure of the CMTS, CdS, 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS composites. X-ray photoelectron spectroscopy (XPS) was performed on a Kratos Analytical AXIS Nova XPS instrument to determine the elemental composition and chemical states of the CMTS and CdS@CMTS composites. Fourier transform infrared (FT-IR) spectroscopy was used to identify the vibrational modes present in the as-synthesized materials. The FT-IR spectra of these materials were recorded using an IRspirit Fourier transform infrared spectrometer (Shimadzu, Japan) in the range of 400–4000 cm−1 through the ATR method. The Raman spectra of CMTS and type-II heterojunction CdS@CMTS composites were obtained using a Micro Raman spectrometer (JASCO NRS-5100) equipped with a 532.06 nm laser. The optical band gaps of the synthesized materials were determined using ultraviolet–visible diffuse reflectance spectroscopy (UV-visible DRS, Shimadzu UV-2600i, Japan) and BaSO4 was used as a reference. The morphology and crystallographic information of the synthesized materials were analyzed using field-emission transmission electron microscopy (FE-TEM), energy dispersive X-ray spectroscopy (EDS) elemental mapping, and selected area electron diffraction (SAED) conducted with a JEM-F200 (JEOL Ltd., Tokyo, Japan) instrument. Indexing of the SAED patterns was performed using ImageJ software. The surface areas of CMTS, 10% CdS@CMTS, and 30% CdS@CMTS were measured using a surface area analyzer (Quantachrome autosorb-iQ, Austria). The photocatalytic degradation of brilliant green (BG) dye using pristine CMTS, 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS composites was carried out in a Toption (TOPT-V) photochemical reactor equipped with a water-jacketed 300 W Xe lamp with a wavelength >350 nm (light intensity at the position of the reaction vessel ∼14 mW cm−2) at room temperature.2.3. Synthesis of CMTS
CMTS was prepared using a simple microwave-assisted synthesis method. Typically, appropriate amounts of Cu(NO3)2·3H2O, Mg(NO3)2, SnCl4·5H2O, and thiourea were added into 20 mL of ethylene glycol and the mixture was loaded into a 30 mL quartz vessel (G30 vial). The vessel was purged with N2 gas and sealed with a septum then rapidly heated to 200 °C with 850 W microwave irradiation for 30 min with continuous stirring. After the reaction was complete, the product was obtained by centrifugation (3500 rpm, 5 min per wash), washed three times with distilled water and ethanol, and dried at 80 °C for 4 h to yield a black powder of CMTS.2.4. Synthesis of type-II heterojunction CdS@CMTS composites
Appropriate amounts of CdSO4·8/3H2O and thiourea were added into a suspension of 0.1 g of CMTS in ethylene glycol for the preparation of type-II heterojunction CdS@CMTS composites. The mixtures were purged with N2 gas and heated at 200 °C with a microwave power and reaction time of 850 W and 30 min, respectively. The obtained products were washed three times with distilled water and ethanol then dried at 80 °C for 4 h and labelled as either 10% CdS@CMTS, 20% CdS@CMTS, or 30% CdS@CMTS. The composites were synthesized with the CdS content in increments of ten revealing that the percentage degradation peaked at 30%, surpassing the performance of pure CdS (100%). The composites were only synthesized up to 30% of CdS to minimize toxicity while maintaining the high efficiency of the material.2.5. Photocatalytic degradation of brilliant green dye
The photocatalytic performance of the CMTS and type-II heterojunction CdS@CMTS composites was studied via the photocatalytic degradation of 10 ppm BG (pH 6.10) in an aqueous solution under UV-visible light irradiation. Typically, 10 mg of the as-synthesized material was dispersed and sonicated in 50 mL of BG aqueous solution. Then, to achieve adsorption–desorption equilibrium between the BG dye and the synthesized material, the suspension was kept in the dark for 3 min with constant stirring. Upon light irradiation, a specific amount of suspension (3 mL) was collected regularly every 60 min for 5 h and centrifuged to separate the photocatalyst from the BG dye. The clear aliquots were analyzed using a UV–vis spectrophotometer in the range of 200–800 nm, and the photocatalytic activities of pristine CMTS, 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS were estimated by measuring the percentage of dye degradation using the following relation (1):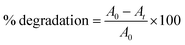 | (1) |
2.6. Free radical scavenging/trapping experiments
As photocatalysis involves the formation of different reactive species, it is crucial to determine the role of these species in the degradation process. Free radical scavenging/trapping experiments were carried out in order to understand the main reactive species responsible for the photocatalytic degradation of BG using CMTS, 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS composites. Different scavenging/trapping agents were used in the experiments to investigate the inhibitory effect of these scavenging/trapping agents during the photocatalytic reaction under the same experimental conditions as described in sub-heading 2.5. These experiments were carried out in the presence of three typical scavenging/trapping agents: isopropanol, benzoquinone, and EDTA, which are utilized as scavenging/trapping agents of hydroxyl (˙OH), superoxide radicals (O2˙−), and holes (h+), respectively. These agents were added to the aqueous BG dye solution at the beginning of the photocatalytic reaction.3. Results and discussion
3.1. Powder X-ray diffraction analysis
The formation and the crystal structure of pristine CMTS, CdS 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS were confirmed by XRD analysis. The XRD pattern of CMTS does not match with any pattern in the ICDD database and was simulated based on the kesterite CZTS crystal structure. As shown in Fig. 1, the XRD pattern of CMTS with 2θ peaks at 28.3, 50.0, and 56.9° can be assigned to the (112), (200), and (312) planes of kesterite CMTS (indexed in black).26 The formation of a secondary phase can be observed from the appearance of an additional peak around 16.1°, which is attributed to the (110) plane of Cu2SnS3 (ICDD 01-086-5306: indexed in red).27 As the CdS decoration increases from 10% to 30%, the peak intensity decreases. The weak diffraction peaks of CMTS are in good agreement with previous reports and may be weak due to its amorphous nature (incomplete crystallization) because of the absence of a post-annealing process under inert conditions.26 The diffraction peaks of CdS are located at 24.9, 26.5, 28.2, 36.7, 43.8, 47.9, and 51.8°, which correspond to the (100), (002), (101), (102), (110), (103), and (112) planes of hexagonal CdS (ICDD 01-074-9665: indexed in orange), as shown in Fig. S1 (ESI†).28 The diffraction peaks around 25 to 30° for 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS were observed to broaden (shown in Fig. S2, ESI†) as the amount of CdS increases from 10% to 30%. Moreover, the peaks at 44.5, 47.4, and 50.2° began to appear in 30% CdS@CMTS, which suggests the successful decoration of CdS on CMTS.3.2. X-ray photoelectron spectroscopy
XPS analysis of CMTS and type-II heterojunction CdS@CMTS composites was conducted to investigate the elemental composition and chemical states of the elements present in the materials. The survey scan spectrum of CMTS shown in Fig. S3 (ESI†) confirmed the presence of Cu, Mg, Sn, and S.13 Similar peaks were observed in the 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS survey spectra with the addition of a Cd peak.29In the Cu 2p spectrum of CMTS shown in Fig. 2(a), two peaks at 951.4 eV (Cu 2p1/2) and 931.6 (Cu 2p3/2) with a splitting of 19.8 eV is the characteristic of Cu+.10 While the peaks are observed at 950.2/930.3 eV, 949.8/929.9, and 949.7/929.8 eV for 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS, respectively. The Cu 2p peaks shifted to lower binding energy in the composites, and this may be due to the presence of CdS on the surface of CMTS. Fig. 2(b) shows the spectrum of Mg 1s of CMTS with a peak located at 1303.5 eV, which is associated with the presence of Mg.12 With CdS decoration, the Mg 1s peak appeared at 1302.5, 1302.2, and 1301.5 eV for 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS, respectively. The lower binding energy portion of the Mg XPS scan is shown in the inset of Fig. 2(b), which clearly provides convincing evidence for the presence of Mg 2p at 451.6 eV in CMTS. The Mg 2p peaks of 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS appeared at 46.7 eV, which is consistent with the literature values reported for an Mg2+ state.30
 | ||
| Fig. 2 XPS spectra of CMTS, 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS; (a) Cu 2p, (b) Mg 1s (inset: Mg 2p), (c) Sn 3d, (d) S 2p, (e) Cd 3d, and (f) C 1s. | ||
The two characteristic Sn 3d peaks in CMTS appeared at 494.2 and 485.8 eV, which can be assigned to Sn 3d3/2 and Sn 3d5/2 of Sn4+, respectively, as shown in Fig. 2(c).31 The binding energy peaks of Sn appeared at 493.2/484.8, 492.8/484.4, and 492.9/484.5 eV for 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS, respectively. The peak splitting of Sn 3d was 8.4 eV, which suggested the presence of Sn4+ in the synthesized materials.4 The S 2p peaks of CMTS observed at 162.0 and 160.8 eV can be assigned to the S 2p1/2 and S 2p3/2 of S2−, respectively (Fig. 2(d)).32 Moreover, the binding energies of S 2p in 10% CdS@CMTS are located at 160.8 and 159.6 eV with a peak splitting of 1.2 eV. However, the peaks appeared at 160.4/159.3 and 160.3/159.3 eV for 20% CdS@CMTS and 30% CdS@CMTS, respectively. The two peaks at 410.0 and 403.2 eV in the Cd 3d spectrum of 10% CdS@CMTS were attributed to Cd 3d3/2 and Cd 3d5/2, respectively, as shown in Fig. 2(e). Moreover, the peaks were located at 409.6/402.9 and 409.6/402.8 eV with a binding energy difference of 6.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) eV for 20% CdS@CMTS and 30% CdS@CMTS, respectively. These peaks are characteristic of Cd2+.33
eV for 20% CdS@CMTS and 30% CdS@CMTS, respectively. These peaks are characteristic of Cd2+.33
Furthermore, the XPS analysis revealed interesting changes in the chemical states of the elements upon the addition of CdS. The high-resolution XPS spectra showed a shift in the binding energies of the Cu, Mg, Sn, and S peaks compared to the pure CMTS sample. The shifts suggest a potential interaction between the CdS and CMTS. In conclusion, the XPS analysis of the CMTS and type-II heterojunction CdS@CMTS composites not only confirmed the elemental composition and chemical states of the constituent elements but also provided evidence of the successful decoration of CdS onto CMTS.
3.3. Fourier transform infrared spectroscopy
FT-IR was carried out to examine the different vibrational modes present in the pristine CMTS, 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS. As shown in Fig. 3(a), CMTS exhibits a band located at ∼530–550 cm−1 which can be assigned to metal–S stretching vibrations,34 while the bands around 1040 and 2340 cm−1 can be ascribed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and S–H vibrations of thiourea, respectively.35 For pristine CdS, the bands around 600, 850, and 1640 cm−1 can be attributed to the characteristic Cd–S bonding36,37 and the band around 1400 cm−1 belongs to the C–N of thiourea.38 The broadening of the band around 530 cm−1 was observed in the FTIR spectra after the addition of CdS in 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS. The composites also display the characteristic bands corresponding to CMTS and CdS. This confirms the presence of both CMTS and CdS. Thus, the FT-IR measurements also support the successful formation of CdS and CMTS in the type-II heterojunction composites.
S and S–H vibrations of thiourea, respectively.35 For pristine CdS, the bands around 600, 850, and 1640 cm−1 can be attributed to the characteristic Cd–S bonding36,37 and the band around 1400 cm−1 belongs to the C–N of thiourea.38 The broadening of the band around 530 cm−1 was observed in the FTIR spectra after the addition of CdS in 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS. The composites also display the characteristic bands corresponding to CMTS and CdS. This confirms the presence of both CMTS and CdS. Thus, the FT-IR measurements also support the successful formation of CdS and CMTS in the type-II heterojunction composites.
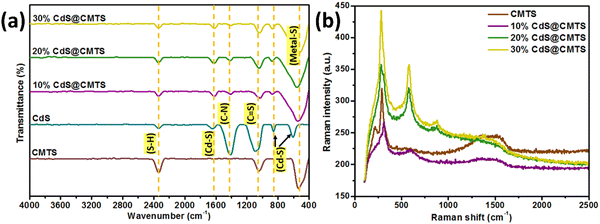 | ||
| Fig. 3 (a) FT-IR spectra and (b) Raman spectra of pristine CMTS, 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS. | ||
3.4. Raman spectroscopy
Raman spectroscopy was carried out to further confirm the synthesis of the CMTS and type-II heterojunction CdS@CMTS composites. As shown in Fig. 3(b), the Raman spectrum of pristine CMTS exhibited a strong peak at 287 cm−1 and another one with a lower intensity at 218 cm−1, which corresponds to the kesterite phase of CMTS.39 Moreover, CdS exhibited characteristic peaks located at around 300, 600, and 900 cm−1,40 which are associated with the phonon vibrational peaks of the 1 longitudinal optical (LO), 2LO, and 3LO modes of hexagonal CdS, respectively.41 The peak at around 900 cm−1 confirmed the presence of CdS in the type-II heterojunction CdS@CMTS composites. The intensity of the CdS peak increases as the amount of CdS decoration increases from 10% to 30%. The Raman spectra of the CdS@CMTS composites showed the characteristic features of CMTS and CdS. Therefore, Raman analysis also confirms the coexistence of CMTS and CdS in the composites and the formation of type-II heterojunction CdS@CMTS composites.3.5. UV-visible diffuse reflectance spectroscopy
The diffuse reflectance spectra of CMTS and type-II heterojunction CdS@CMTS composites were measured in the wavelength range of 200 to 1000 nm. The optical band gap energies of pristine CMTS, 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS were estimated from Tauc plots constructed from the diffuse reflectance spectra transformed using the Kubelka–Munk function. Fig. 4 shows the Tauc plot of [F(R)hv]2vs. photon energy. The band gap energy of CMTS was found to be 2.07 eV, which is higher compared to the previously reported values of about ∼1.5 eV.11 This may be influenced by the amount of Cu. As reported by Souli and co-workers, the band gap energy of CMTS increases as the concentration of Cu added decreases.39 As shown in Fig. S4 (ESI†), the band gap energy of CdS was estimated to be 2.50 eV. Moreover, the estimated effective band gap energies of the 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS composites were found to be 2.23, 2.34, and 2.40 eV, respectively. This signifies their potential to harvest visible light.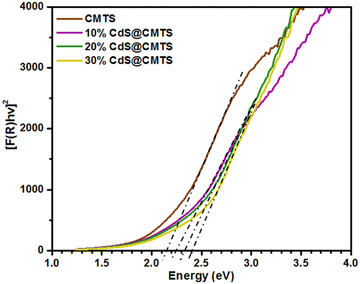 | ||
| Fig. 4 Tauc plots constructed from Kubelka–Munk transformed diffuse reflectance spectra for pristine CMTS, 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS. | ||
3.6. Transmission electron microscopy
TEM was employed to determine the morphologies of CMTS, 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS. Fig. 5(a1) shows that the CMTS adopted a sheet-like morphology. The TEM images of 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS shown in Fig. 5(b1)–(d1), respectively, show that the spherical CdS was uniformly dispersed on the surface of the CMTS. Moreover, the close contact of CdS and CMTS resulted in the formation of a type-II heterojunction CdS@CMTS composite, which is helpful for fast interfacial charge carrier transfer.42 Thus, the photogenerated charge carriers can be utilized effectively, thereby enhancing the photocatalytic performance. | ||
| Fig. 5 TEM images and SAED patterns of (a) CMTS, (b) 10% CdS@CMTS, (c) 20% CdS@CMTS, and (d) 30% CdS@CMTS, respectively. | ||
The selective area electron diffraction (SAED) pattern of CMTS in Fig. 5(a2) confirmed the presence of the (112) and (220) planes of kesterite CMTS.13,26 The SAED patterns of the 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS composites in Fig. 5(b2)–(d2) showed concentric rings corresponding to the (112) and (220) diffraction planes of kesterite CMTS in addition to the (101) and (103) planes of hexagonal CdS.26,43 Additional (100) and (110) planes of hexagonal CdS were observed in 30% CdS@CMTS, probably due to the presence of a higher amount of CdS compared to the low percentage composites (10% and 20%). These results are in accordance with the XRD results, which also confirm the successful decoration of CdS onto CMTS.
3.7. EDS mapping
The spatial distribution of elements in the CMTS, 10% CdS@CMTS, and 30% CdS@CMTS was determined using EDS mapping. As shown in Fig. 6, all the synthesized materials contained uniformly distributed Cu, Mg, Sn, and S elements.13 While the 10% and 30% CdS@CMTS composites exhibited additional uniform Cd mapping. Thus, EDS mapping also confirmed the formation of CMTS and type-II heterojunction CdS@CMTS composites. | ||
| Fig. 6 The respective elemental mapping patterns of Cu, Mg, Sn, S, and Cd in the (a1)–(e1) CMTS, (a2)–(f2) 10% CdS@CMTS, and (a3)–(f3) 30% CdS@CMTS. | ||
3.8. Brunauer–Emmett–Teller surface area analysis
Fig. 7 shows the Brunauer–Emmett–Teller (BET) analysis using N2 adsorption–desorption isotherms of the synthesized materials. As is seen, the synthesized CMTS and type-II heterojunction CdS@CMTS composites exhibit a type IV curve (according to IUPAC classification) with a hysteresis loop.44 The surface areas of 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS were measured and are found to be 142.23, 76.11, and 73.45 m2 g−1, respectively which are all higher than that of the pristine CMTS (40.94 m2 g−1). As the CdS decoration increases from 10% to 30%, the BET surface area of the CdS@CMTS composite decreases. At a higher percentage of CdS (30%), the particles may have a higher tendency to agglomerate. These agglomerates may form larger particles that further reduce the overall surface area, reducing the available sites for N2 molecules to adsorb. This suggests that the enhanced photocatalytic activity of 30% CdS@CMTS does not depend on the surface area but results from the effective separation of the photogenerated e− and h+. | ||
| Fig. 7 N2 adsorption and desorption isotherms of CMTS, 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS. | ||
4. Photocatalytic activity of CMTS and type-II heterojunction CdS@CMTS composites
4.1. Photocatalytic degradation of brilliant green dye
The photocatalytic activities of pristine CMTS, 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS were investigated by degradation of BG as a model dye pollutant under visible light irradiation. All measurements were performed under the same conditions. Fig. 8(a) shows control experiments carried out in the dark, which tracks the adsorption of the BG dye onto the surface of the synthesized materials by monitoring the absorbance at λmax of BG (620 nm) over a period of 5 h. In the absence of light, the adsorption capacities of CMTS, 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS reached up to 10.3 ± 0.81%, 31.5 ± 2.15%, 35.9 ± 0.65%, and 16.8 ± 2.85%, respectively, after 5 h. This shows that the materials were able to adsorb BG dye for the sequential photocatalytic degradation process, which is crucial because if the dye molecules cannot be adsorbed on the surface of the synthesized materials, the photocatalytic activity would be poor and not efficient.45Fig. 8(b) shows the photocatalytic degradation of pristine CMTS, 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS at different irradiation times. Moreover, CMTS exhibited poor photocatalytic performance of only about 18.9 ± 1.96% under irradiation by visible light, which may be ascribed to the rapid recombination of charge carriers. Within 5 h, 10% CdS@CMTS, 20% CdS@CMTS, and 30% CdS@CMTS were able to degrade about 42.5 ± 3.69%, 81.1 ± 0.26%, and 95.8 ± 1.45% of BG, respectively, under visible light irradiation. The photocatalytic performance of CMTS is significantly enhanced as the amount of CdS decoration increases from 10% to 30%. The photocatalytic activity of 30% CdS@CMTS was found to be 95.8 ± 1.45%, which is considerably higher than those of pristine CMTS, 10% CdS@CMTS, and 20% CdS@CMTS. The synthesis of composites stopped at 30% CdS because this is when the CdS@CMTS performance matches (and slightly exceeds) that of pure CdS (100%). A higher CdS content was not tested to minimize the amount of toxic Cd in the photocatalyst. The improved photocatalytic performance of 30% CdS@CMTS is likely to be due to the optimal band gap energy of 2.4 eV, which enables efficient utilization of light for photocatalytic processes and the effective transfer and separation of the charge carriers between CdS and CMTS, resulting in a higher degradation efficiency.46
Generally, organic pollutants can be degraded by photocatalytic processes where a series of reactive species including h+, O2˙−, and ˙OH radicals are responsible for the degradation reactions. To further investigate the main reactive species involved in the degradation of the aqueous BG dye, the highest performing composite, 30% CdS@CMTS was used to carry out the free radical scavenging/trapping experiments in the presence of different scavengers including isopropanol, benzoquinone, and EDTA to trap ˙OH, O2˙−, and h+, respectively. The significance of a particular active species in the degradation process is proportional to the corresponding inhibition (reduction in percentage photocatalytic degradation) in the presence of a scavenging/trapping agent. As shown in Fig. 8(c), the photocatalytic degradation of BG using 30% CdS@CMTS was only slightly affected by the addition of isopropanol (94.3 ± 1.51%) when compared to no scavenger (95.8 ± 1.45%). While in the presence of EDTA and benzoquinone, the photocatalytic degradation of BG was reduced from 95.8 ± 1.45% to 74.6 ± 2.81% and 68.2 ± 4.68%, respectively. Based on these inhibitions, it can be observed that on average benzoquinone was more inhibiting than EDTA, although there is only a slight difference between these scavengers. This suggests that O2˙− and h+ were the main reactive species during the photocatalytic degradation process.
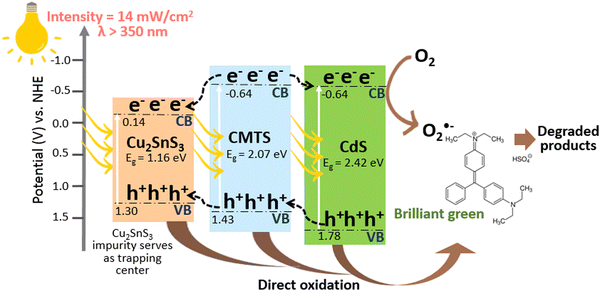
Based on the aforementioned characterization studies and the free radical scavenging/trapping experiments of the type-II heterojunction CdS@CMTS composite, a proposed mechanism for photocatalytic degradation is illustrated in Fig. 9. The addition of CdS could significantly enhance the photocatalytic degradation performance of CMTS. Although the target synthesis was pure, stoichiometric CMTS, the presence of a Cu2SnS3 secondary phase is nearly unavoidable (despite being detected only in XRD and not in other characterization studies). As a result, we have taken the role of Cu2SnS3 into account in the photocatalytic process. Based on the estimation of ECB and EVB using the atomic electron affinities and ionization energies as reported by previous literature,47–49 CMTS is estimated to have ECB = −0.63 and EVB = 1.43 V, while CdS has ECB = −0.63 V and EVB = 1.78 V. The reported band gap energy of Cu2SnS3 is 1.16 eV,50 and the estimated ECB and EVB values are found to be 0.14 and 1.30 V, respectively. Both CMTS and CdS could easily absorb and utilize visible light because of their narrow band gap energies. Upon visible light irradiation, CdS simultaneously generates e− and h+, in which photoinduced e− are excited to the conduction band (CB), leaving behind h+ in the valence band (VB). Subsequently, the photoinduced e− of CMTS migrates to the CB of CdS to take part in the reduction process. The photogenerated e− on the CB of CdS can reduce the dye directly or react with electron acceptors adsorbed on the surface of the photocatalyst, such as dissolved O2 in water to produce O2˙− (O2/O2˙− = −0.33 V) which is in accordance with the scavenging/trapping experiment results. In addition, since the VB of CMTS is higher than that of CdS, the photogenerated h+ can migrate from the VB of CdS to CMTS and further to the VB of Cu2SnS3 in the composite. The VB potential of Cu2SnS3 (1.30 V) is lower than the potentials of ˙OH/OH− (+2.38 V) and ˙OH/H2O (+2.72 V).51 Thus, the photogenerated h+ in the VB of Cu2SnS3 cannot oxidize OH− or H2O to ˙OH. However, the photogenerated h+ can oxidize the organic dyes directly into harmless end products. In principle, the presence of Cu2SnS3 as an impurity can also prolong the lifetime of these charge carriers by acting as a trapping center,52 delaying recombination and facilitating better separation of photoinduced e− and h+, thus improving the photocatalytic activity of the type-II heterojunction CdS@CMTS composites. Based on the results of the free radicals scavenging/trapping experiment, both benzoquinone and EDTA showed the highest inhibitions when compared to no scavenger. However, there is no significant difference between the inhibition caused by benzoquinone and EDTA, which indicates that both O2˙− and h+ play equally important roles in the photocatalytic degradation process. Moreover, isopropanol shows the lowest inhibition, implying the least importance of ˙OH taking part in BG degradation.
While this study has provided valuable insights into CdS@CMTS composites for the efficient removal of the BG dye, several avenues exist to further develop this promising photocatalyst system. For instance, further optimization of the synthesis process could be pursued to mitigate the formation of impurity phases, potentially through variation in sulfur source, solvent, microwave power, or reaction time. Additional studies could also investigate the point of zero charge of the synthesized materials to enhance the understanding of the surface charge, the effect of pH on the degradation performance, and the recycling stability of the composite for long-term applications. These extensions are recommended to improve the fundamental understanding of the degradation mechanism and to further optimize the photocatalytic performance of the CdS@CMTS system.
5. Conclusion
An efficient CdS@CMTS heterojunction was successfully prepared via a rapid and efficient microwave-assisted method. The structural, optical, and morphological properties of the synthesized materials were analyzed using different techniques. X-ray diffraction analysis confirmed the preparation of the kesterite phase of CMTS and the hexagonal phase of CdS. The effective bang gap energy was greatly influenced by the CdS content, in which an increase of the band gap energy from 2.07 to 2.40 eV was observed. Transmission electron microscopy images showed a uniform distribution of spherical CdS on the sheet-like CMTS in the type-II heterojunction CdS@CMTS composites. The photocatalytic degradation of BG by CMTS and CdS@CMTS photocatalysts was investigated. Based on the results, 30% CdS@CMTS showed the highest photocatalytic response of up to 95.8 ± 1.45% degradation of BG within 5 h. Based on the free radical scavenging/trapping experiment, the results revealed that O2˙− and h+ were the primary reactive species during the photocatalytic degradation process. Therefore, this study shows that CdS@CMTS has the potential to degrade organic pollutants and could be applied for environmental remediation.Author contributions
Ashmalina Rahman: methodology, investigation, data curation, and writing – original draft. Fazlurrahman Khan: data curation and formal analysis. James Robert Jennings: supervision, writing, and review & editing. Young-Mog Kim: resources, funding, and formal analysis. Mohammad Mansoob Khan: supervision, conceptualization, funding acquisition, writing, and review & editing.Data availability
All data generated or analyzed during this study are included in this article and its ESI† file.Conflicts of interest
The authors declare that there are no conflicts of interest.Acknowledgements
This research was funded by Universiti Brunei Darussalam through grant UBD/RSCH/1.4/FICBF(b)/2023/059 and was also supported by the Basic Science Research Program through the National Research Foundation (NRF) of Korea grants funded by the Ministry of Education (2022R1A2B5B01001998 and RS-2023-00241461).References
- M. M. Khan, Chalcogenide-Based Nanomaterials as Photocatalysts, Elsevier, 2021 DOI:10.1016/C2019-0-01819-5.
- A. Rahman and M. M. Khan, Chalcogenides as Photocatalysts, New J. Chem., 2021, 19622–19635, 10.1039/d1nj04346c.
- A. Sharma, P. Sahoo, A. Singha, S. Padhan, G. Udayabhanu and R. Thangavel, Efficient Visible-Light-Driven Water Splitting Performance of Sulfidation-Free, Solution Processed Cu2MgSnS4 Thin Films: Role of Post-Drying Temperature, Sol. Energy, 2020, 203, 284–295, DOI:10.1016/j.solener.2020.04.027.
- I. A. Mkhalid, R. M. Mohamed, M. Alhaddad, A. Basaleh, L. A. Al-Hajji and A. A. Ismail, Green Synthesis of Porous Cu2ZnSnS4/g-C3N4 Heterostructured for Promoted Photocatalytic Degradation of Trichloroethylene, Ceram. Int., 2022, 48(8), 11736–11746, DOI:10.1016/j.ceramint.2022.01.032.
- V. Dhiman, S. Kumar, M. Kaur, R. Sharma, T. Chandel, D. Bhardwaj and D. Prasher, Synergistic Effect of Stirring and Marigold Shaped Cu2FeSnS4 Nanostructure for the Enhanced Performance of Rhodamine B Degradation under Visible Light, Inorg. Chem. Commun., 2023, 154, 110923, DOI:10.1016/j.inoche.2023.110923.
- Z. Shi, W. Jin, Y. Sun, X. Li, L. Mao, X. Cai and Z. Lou, Interface Charge Separation in Cu2CoSnS4/ZnIn2S4 Heterojunction for Boosting Photocatalytic Hydrogen Production, Chin. J. Struct. Chem., 2023, 42(12), 100201, DOI:10.1016/j.cjsc.2023.100201.
- H. Guan, H. Hou, M. Li and J. Cui, Photocatalytic and Thermoelectric Properties of Cu2MnSnS4 Nanoparticles Synthesized via Solvothermal Method, Mater. Lett., 2017, 188, 319–322, DOI:10.1016/j.matlet.2016.09.018.
- G. Zhong, K. Tse, Y. Zhang, X. Li, L. Huang, C. Yang, J. Zhu, Z. Zeng, Z. Zhang and X. Xiao, Induced Effects by the Substitution of Zn in Cu2ZnSnX4 (X = S and Se), Thin Solid Films, 2016, 603, 224–229, DOI:10.1016/j.tsf.2016.02.005.
- G. L. Agawane, S. A. Vanalakar, A. S. Kamble, A. V. Moholkar and J. H. Kim, Fabrication of Cu2(ZnxMg1−x)SnS4 Thin Films by Pulsed Laser Deposition Technique for Solar Cell Applications, Mater. Sci. Semicond. Process., 2018, 76, 50–54, DOI:10.1016/j.mssp.2017.12.010.
- G. Yang, X. Zhai, Y. Li, B. Yao, Z. Ding, R. Deng, H. Zhao, L. Zhang and Z. Zhang, Synthesis and Characterizations of Cu2MgSnS4 Thin Films with Different Sulfuration Temperatures, Mater. Lett., 2019, 242, 58–61, DOI:10.1016/j.matlet.2019.01.102.
- A. Hammoud, A. Jrad, B. Yahmadi, M. Souli, F. Kouki, L. Ajili and N. Kamoun-Turki, Investigation on Cu2MgSnS4 Thin Film Prepared by Spray Pyrolysis for Photovoltaic and Humidity Sensor Applications, Opt. Mater., 2022, 127, 112296, DOI:10.1016/j.optmat.2022.112296.
- A. Ali, Y. Liang, S. Ahmed, B. Yang, B. Guo and Y. Yang, Mutual Contaminants Relational Realization and Photocatalytic Treatment Using Cu2MgSnS4 Decorated BaTiO3, Appl. Mater. Today, 2020, 18, DOI:10.1016/j.apmt.2019.100534.
- M. Wei, Q. Du, R. Wang, G. Jiang, W. Liu and C. Zhu, Synthesis of New Earth-Abundant Kesterite Cu2MgSnS4 Nanoparticles by Hot-Injection Method, Chem. Lett., 2014, 43(7), 1149–1151, DOI:10.1246/cl.140208.
- A. Ali, J. Zhao, R. Yao, S. Ahmed, L. Wang, B. Guo, W.-F. Rao and Y. Yang, Stimulated Piezotronical Decontamination Using Cu2MgSnS4 Modified BaTiO3, Mater. Today Energy, 2021, 21, 100717, DOI:10.1016/j.mtener.2021.100717.
- B. Bekki, K. Amara, N. Marbouh, F. Khelfaoui, Y. Benallou, M. Elkeurti and A. Bentayeb, Theoretical Study of Structural, Elastic and Thermodynamic Properties of Cu2MgSnX4 (X = S, Se and Te) Quaternary Compounds, Comput. Condens. Matter, 2019, 18, e00339, DOI:10.1016/j.cocom.2018.e00339.
- A. Rahman, F. Khan, J. R. Jennings, Y. M. Kim and M. M. Khan, Microwave-Assisted Synthesis of ZnS@CuInxSy for Photocatalytic Degradation of Coloured and Non-Coloured Pollutants, Sci. Rep., 2024, 14, 16155, DOI:10.1038/s41598-024-66100-2.
- A. Rahman, J. R. Jennings, A. L. Tan and M. M. Khan, Molybdenum Disulfide-Based Nanomaterials for Visible-Light-Induced Photocatalysis, ACS Omega, 2022, 22089–22110, DOI:10.1021/acsomega.2c01314.
- A. Rahman, J. R. Jennings and M. M. Khan, CuInS2 and CuInS2-Based Nanostructures as Photocatalysts, Mater. Sci. Semicond. Process., 2024, 169, 107930, DOI:10.1016/j.mssp.2023.107930.
- Mohammad Mansoob Khan, Theoretical Concepts of Photocatalysis, Elsevier, 2023 DOI:10.1016/C2021-0-01798-3.
- K. Matras-Postołek, A. Żaba, P. Dąbczyński, M. Marzec, A. Bernasik, J. Rysz, M. A. Borysiewicz and T. Wojtowicz, Microwave-Synthesized CZTS Nanoparticles and Their Surface Modification for the Model Hybrid Photovoltaic Devices, Chem. Eng. Process., 2023, 194, 109606, DOI:10.1016/j.cep.2023.109606.
- S. P. Kandare, S. D. Dhole, V. N. Bhoraskar and S. S. Dahiwale, Cu2ZnSnS4 Nanoflakes Prepared by One Step Microwave Irradiation Technique: Effect of Cu Concentration, in AIP Conference Proceedings, American Institute of Physics Inc., 2016, vol. 1731, p. 050084 DOI:10.1063/1.4947738.
- A. Rahman, F. Khan, J. R. Jennings, A. L. Tan, Y. M. Kim and M. M. Khan, Effect of CdS Loading on the Properties and Photocatalytic Activity of MoS2 Nanosheets, BMC Chem., 2024, 18, 135, DOI:10.1186/s13065-024-01250-y.
- J. Li, J. Chen, Y. Ao, X. Gao, H. Che and P. Wang, Prominent Dual Z-Scheme Mechanism on Phase Junction WO3/CdS for Enhanced Visible-Light-Responsive Photocatalytic Performance on Imidacloprid Degradation, Sep. Purif. Technol., 2022, 281, 119863, DOI:10.1016/j.seppur.2021.119863.
- Q. Zhang, T. Wu, H. Che, C. Tang, B. Liu and Y. Ao, In-Situ Growing Ni2P on CdS@g-C3N4 Composites for Highly Efficient Synergistically Photocatalytic H2 Evolution and Antibiotic Degradation, Surf. Interfaces, 2024, 47, 104205, DOI:10.1016/j.surfin.2024.104205.
- X. Gao, J. Chen, H. Che, Y. Ao and P. Wang, Rationally Constructing of a Novel Composite Photocatalyst with Multi Charge Transfer Channels for Highly Efficient Sulfamethoxazole Elimination: Mechanism, Degradation Pathway and DFT Calculation, Chem. Eng. J., 2021, 426, 131585, DOI:10.1016/j.cej.2021.131585.
- A. Hammoud, M. Souli, F. Kouki, L. Ajili, B. Bouricha and N. Kamoun, Investigation of Substrate Temperature Effect on Physical Properties of Sprayed Cu2MgSnS4 Thin Films for Solar Cells and Humidity Sensing Applications, J. Mater. Sci.: Mater. Electron., 2022, 33(9), 6926–6941, DOI:10.1007/s10854-022-07872-z.
- F. Yarur Villanueva, P. B. Green, C. Qiu, S. R. Ullah, K. Buenviaje, J. Y. Howe, M. B. Majewski and M. W. B. Wilson, Binary Cu2–xS Templates Direct the Formation of Quaternary Cu2ZnSnS4 (Kesterite, Wurtzite) Nanocrystals, ACS Nano, 2021, 15(11), 18085–18099, DOI:10.1021/acsnano.1c06730.
- S. Vadakkedath Gopi, N. Spalatu, M. Basnayaka, R. Krautmann, A. Katerski, R. Josepson, R. Grzibovskis, A. Vembris, M. Krunks and I. Oja Acik, Post Deposition Annealing Effect on Properties of CdS Films and Its Impact on CdS/Sb2Se3 Solar Cells Performance, Front. Energy Res., 2023, 11, DOI:10.3389/fenrg.2023.1162576.
- A. Rahman, F. Khan, J. R. Jennings, Y.-M. Kim and M. M. Khan, CdS@CuInS2 Nanocomposites for Enhanced Photocatalytic Activity under Visible Light Irradiation, Mater. Sci. Semicond. Process., 2024, 177, 108365, DOI:10.1016/j.mssp.2024.108365.
- D. Briggs, C. D. Wanger, W. M. Riggs, L. E. Davis, J. F. Moulder and G. E. Muilenberg, Handbook of X-Ray Photoelectron Spectroscopy, Perkin-Elmer Corporation Physical Electronics Division, Eden Prairie, Minnesota, USA, 1979 Search PubMed.
- X. Zhang, Y. Xu, J. Zhang, S. Dong, L. Shen, A. Gupta and N. Bao, Synthesis of Wurtzite Cu2ZnSnS4 Nanosheets with Exposed High-Energy (002) Facets for Fabrication of Efficient Pt-Free Solar Cell Counter Electrodes, Sci. Rep., 2018, 8(1), 248, DOI:10.1038/s41598-017-18631-0.
- C. Calderón, G. Gordillo, R. Becerra and P. Bartolo-Pérez, XPS Analysis and Characterization of Thin Films Cu2ZnSnS4 Grown Using a Novel Solution Based Route, Mater. Sci. Semicond. Process., 2015, 39, 492–498, DOI:10.1016/j.mssp.2015.05.064.
- Q. Xu, Z. Wang, H. Yang, Y. Xiang, G. Nie and W. Yue, Synthesis of Hierarchical Cu2CdSnS4 by Microwave-Assisted Transformation from Precursor for Photodegradation to Malachite Green, J. Alloys Compd., 2022, 904, 163966, DOI:10.1016/j.jallcom.2022.163966.
- M. Sahu, V. R. M. Reddy, B. Kim, B. Patro, C. Park, W. K. Kim and P. Sharma, Fabrication of Cu2ZnSnS4 Light Absorber Using a Cost-Effective Mechanochemical Method for Photovoltaic Applications, Materials, 2022, 15(5), 1708, DOI:10.3390/ma15051708.
- R. V. Digraskar, B. B. Mulik, P. S. Walke, A. V. Ghule and B. R. Sathe, Enhanced Hydrogen Evolution Reactions on Nanostructured Cu2ZnSnS4 (CZTS) Electrocatalyst, Appl. Surf. Sci., 2017, 412, 475–481, DOI:10.1016/j.apsusc.2017.03.262.
- N. Susha, K. Nandakumar and S. S. Nair, Enhanced Photoconductivity in CdS/Betanin Composite Nanostructures, RSC Adv., 2018, 8(21), 11330–11337, 10.1039/C7RA13116J.
- L. Ge, F. Zuo, J. Liu, Q. Ma, C. Wang, D. Sun, L. Bartels and P. Feng, Synthesis and Efficient Visible Light Photocatalytic Hydrogen Evolution of Polymeric G-C3N 4 Coupled with CdS Quantum Dots, J. Phys. Chem. C, 2012, 116(25), 13708–13714, DOI:10.1021/jp3041692.
- R. V. Digraskar, S. M. Mali, S. B. Tayade, A. V. Ghule and B. R. Sathe, Overall Noble Metal Free Ni and Fe Doped Cu2ZnSnS4 (CZTS) Bifunctional Electrocatalytic Systems for Enhanced Water Splitting Reactions, Int. J. Hydrogen Energy, 2019, 44(16), 8144–8155, DOI:10.1016/j.ijhydene.2019.02.054.
- M. Souli, R. Engazou, L. Ajili and N. Kamoun-Turki, Physical Properties Evolution of Sprayed Cu2MgSnS4 Thin Films with Growth Parameters and Vacuum Annealing, Superlattices Microstruct., 2020, 147, 106711, DOI:10.1016/j.spmi.2020.106711.
- P. Kumar, N. Saxena, R. Chandra, V. Gupta, A. Agarwal and D. Kanjilal, Nanotwinning and Structural Phase Transition in CdS Quantum Dots, Nanoscale Res. Lett., 2012, 7(1), 584, DOI:10.1186/1556-276X-7-584.
- L. Ma, T. Zhang, R. Song and L. Guo, In-Situ Raman Study of Relation between Microstructure and Photoactivity of CdS@TiO2 Core-Shell Nanostructures, Int. J. Hydrogen Energy, 2018, 43(30), 13778–13787, DOI:10.1016/j.ijhydene.2018.03.044.
- C. Patriarchea, I. Vamvasakis, E. D. Koutsouroubi and G. S. Armatas, Enhancing Interfacial Charge Transfer in Mesoporous MoS2/CdS Nanojunction Architectures for Highly Efficient Visible-Light Photocatalytic Water Splitting, Inorg. Chem. Front., 2022, 9(4), 625–636, 10.1039/D1QI01278A.
- Y. Li, S. Shang, L. Meng, F. Wang, H. Wu and H. Mei, Photoelectrochemical Sensor Based on Sn3O4/CdS Heterojunction for Detecting Glucose in the Body and Cu(II) Ion, J. Electroanal. Chem., 2024, 960, 118208, DOI:10.1016/j.jelechem.2024.118208.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report), Pure Appl. Chem., 2015, 87(9–10), 1051–1069, DOI:10.1515/pac-2014-1117.
- K. Michalec and A. Kusior, From Adsorbent to Photocatalyst: The Sensitization Effect of SnO2 Surface towards Dye Photodecomposition, Molecules, 2021, 26(23), 7123, DOI:10.3390/molecules26237123.
- M. Yuan, W.-H. Zhou, D.-X. Kou, Z.-J. Zhou, Y.-N. Meng and S.-X. Wu, Cu2ZnSnS4 Decorated CdS Nanorods for Enhanced Visible-Light-Driven Photocatalytic Hydrogen Production, Int. J. Hydrogen Energy, 2018, 43(45), 20408–20416, DOI:10.1016/j.ijhydene.2018.09.161.
- M. A. Butler and D. S. Ginley, Prediction of Flatband Potentials at Semiconductor-Electrolyte Interfaces from Atomic Electronegativities, J. Electrochem. Soc., 1978, 125(2), 228–232, DOI:10.1149/1.2131419.
- A. H. Nethercot, Prediction of Fermi Energies and Photoelectric Thresholds Based on Electronegativity Concepts, Phys. Rev. Lett., 1974, 33(18), 1088–1091, DOI:10.1103/PhysRevLett.33.1088.
- Z. U. Rehman, M. Bilal, F. K. Butt, S. Ur Rehman, Z. Asghar, K. Zheng, Y. Zhang, X. Xu, J. Hou and X. Wang, Selenium Doping to Improve Internal Electric Field for Excellent Photocatalytic Efficiency of G-C3N4 Nanosheets, Sep. Purif. Technol., 2024, 350, 128001, DOI:10.1016/j.seppur.2024.128001.
- S. B. Jathar, S. R. Rondiya, Y. A. Jadhav, D. S. Nilegave, R. W. Cross, S. V. Barma, M. P. Nasane, S. A. Gaware, B. R. Bade, S. R. Jadkar, A. M. Funde and N. Y. Dzade, Ternary Cu2SnS3: Synthesis, Structure, Photoelectrochemical Activity, and Heterojunction Band Offset and Alignment, Chem. Mater., 2021, 33(6), 1983–1993, DOI:10.1021/acs.chemmater.0c03223.
- M. Z. A. Warshagha and M. Muneer, Direct Z-Scheme AgBr/β-MnO2 Photocatalysts for Highly Efficient Photocatalytic and Anticancer Activity, ACS Omega, 2022, 7(34), 30171–30183, DOI:10.1021/acsomega.2c03260.
- J. Yan, G. Wu, N. Guan, L. Li, Z. Li and X. Cao, Understanding the Effect of Surface/Bulk Defects on the Photocatalytic Activity of TiO2: Anatase versus Rutile, Phys. Chem. Chem. Phys., 2013, 15(26), 10978, 10.1039/c3cp50927c.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nj03782k |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2024 |