Open Access Article
Open Access ArticleA green method for the synthesis of lubricating ester oil using a bi-functional ionic liquid†
Yanan
Wang
 ab,
Qilong
Zhao
ab,
Jun
Yin
ab,
Huaigang
Su
ab,
Qilong
Zhao
ab,
Jun
Yin
ab,
Huaigang
Su
 ab,
Hongyuan
Yu
ab and
Wenjing
Lou
ab,
Hongyuan
Yu
ab and
Wenjing
Lou
 *ab
*ab
aState key laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, No. 18 Tianshui Middle Road, Lanzhou 730000, China. E-mail: wjlou@licp.cas.cn; ynwang@licp.cas.cn
bQingdao Key Laboratory of Lubrication Technology for Advanced Equipment, Qingdao Center of Resource Chemistry&New Materials, 266100, China
First published on 8th November 2024
Abstract
A bi-functional ionic liquid 1-(benzotriazole-1-methylene)-3-methylimidazole bis(2-ethylhexyl) phosphate ([BTAMIM][DEHP]) was prepared. It exhibited high activity and selectivity for the esterification of pentaerythritol with caproic acid to pentaerythritol tetra-hexanoate (PETH). Meanwhile, the friction reduction and anti-wear performance of pentaerythritol tetra-hexanoate were improved significantly by [BTAMIM][DEHP].
Synthetic ester oil is a lubricant that has emerged with the development of aero-engine technology and the demand for high-performance lubricating materials. It has been playing an indispensable role as a key component of aviation lubricating oil in the aviation field owing to its excellent high and lowtemperature properties, tribological properties and other good comprehensive properties.1–3 One of the advantages of synthetic ester as a lubricating material is the adjustability of its molecular structure, and its various properties, including thermal stability, oxidation stability, hydrolysis stability, viscosity grade and viscosimetric temperature performance, lubrication, solubility, and biodegradability, show great differences with changes in its molecular composition and structure. At the same time, because of its renewable raw material source and environmental friendliness, it has significant potential applications in automobile, metallurgy, cement and other industries. A lot of research work has been carried out worldwide on the structure–activity relationship, preparation process, properties and applications of synthetic ester, but the depth and systematization of the research still need to be further deepened and enhanced.4–7 Therefore, the development of high-performance synthetic ester lubricating base oil is the key factor for solving the abovementioned problems.
Generally, the synthetic ester oil is synthesized through esterification using an acid catalyst.8–13 However, the residue of the catalyst always has an adverse effect on many properties of the synthetic ester: The corrosion resistance of the synthetic ester is greatly reduced by the residual liquid acid, and the friction reduction and anti-wear properties of the synthetic ester are adversely affected in the presence of solid catalyst particles. Therefore, the development of a new type of catalyst that not only acts as a catalyst for synthesizing the ester oil but also serves as a lubricant additive after the esterification without separation is an effective way to solve the problem arising from the harmful residue of the reaction catalyst.
Ionic liquids exhibit unique physical and chemical properties compared to traditional solids and liquids due to their special structure and composition, exhibiting characteristics such as vapor pressure approximately equal to zero, non-volatility, non-flammability, non-explosiveness, and high chemical and thermodynamic stability. In addition, ionic liquids have a wide liquid temperature range, good solubility for organic and inorganic compounds, wide electrochemical window, designable structure and adjustable performance and many other characteristics.14–19 Ionic liquids have shown not only good catalytic performance in the field of organic synthesis and catalysis20–22 but also excellent tribological properties as a lubricant.23–26 However, to date, there is no report on the application of ionic liquids, which can be used as both a catalyst and a lubricant additive in the process of synthesis of ester lubricants. Herein, we document the first demonstration of an efficient bi-functional ionic liquid that can be used as a catalyst for the ester oil synthesis and then as a lubricant additive for the ester oil after the reaction without separation from the reaction system.
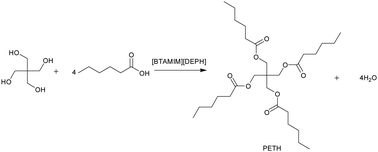
In the current study, an efficient and novel bi-functional ionic liquid 1-(benzotriazole-1-methylene)-3-methyl Imidazole bis(2-ethylhexyl) phosphate ([BTAMIM]DEHP]) was prepared (ESI†) and the 1H NMR and 13C NMR of [BTAMIM]DEHP] are provided in the ESI† (Fig S2 and S3). Pentaerythritol esters of the straight-chain saturated aliphatic acids have good lubricity and thermal stability.27 By applying the esterification of pentaerythritol with caproic acid as a model reaction, the catalytic activity of [BTAMIM][DEHP] was tested, and the results are shown in Table 1. By comparing the acid number of products catalyzed with and without the catalyst, the acid number of the reaction product catalyzed by [BTAMIM][DEHP] is much lower than that of the reaction product catalyzed without the catalyst, and as such the esterification of pentaerythritol is much higher (98%) than that in the reaction proceeded without the catalyst. Meanwhile, the yield of pentaerythritol tetra-hexanoate was up to 91% under mild reaction conditions, and the 1H NMR was prepared as per the procedure in the ESI† (Fig. S4). The results demonstrated that the bi-functional ionic liquid [BTAMIM][DEHP] has good catalytic properties for the ester oil synthesis. The thermogravimetric (TG) of [BTAMIM][DEHP] was tested, and the result is shown in Fig. 1. The decomposition of [BTAMIM][DEHP] started around 229.1 °C and was completed at 290.5 °C. Meanwhile, the esterification was tested at 160 °C, which was much lower than the start of the decomposition temperature of [BTAMIM][DEHP], as such the bi-functional ionic liquid [BTAMIM][DEHP] could exhibit good stability during the reaction. X-ray photoelectron spectroscopy (XPS) analysis was measured using a K-alpha-surface analysis instrument with Al Kα radiation (1486.8 eV) to further investigate the thermal stability of [BTAMIM][DEHP]. The spectra of N 1s and P 2p of [BTAMIM][DEHP] and PETH + [BTAMIM][DEHP] (the product after the esterification without catalyst separation) are shown in Fig. 2. It could be found that the binding energies of N 1s and P 2p of [BTAMIM][DEHP] are consistent with those of PETH + [BTAMIM][DEHP]. It illustrates that the [BTAMIM][DEHP] is stable during the esterification, and the result is verified by TG. According to the Fourier transform infrared spectroscopy (FT-IR) spectra (Fig. 3) we can see that the peak at 1150 cm−1 is attributed to the P–O–C stretching vibration, and the peak at 1360 cm−1 is assigned to P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration.28 The position of the two adsorption peaks of [BTAMIM][DEHP] did not change after the reaction, which reveals that the structure of [BTAMIM][DEHP] is stable. This result verified the conclusion of TG and XPS.
O stretching vibration.28 The position of the two adsorption peaks of [BTAMIM][DEHP] did not change after the reaction, which reveals that the structure of [BTAMIM][DEHP] is stable. This result verified the conclusion of TG and XPS.
| Entry | Catalyst | ANb/(mg KOH per g) | Erc/% |
|---|---|---|---|
| a Reaction conditions (the reaction device was shown in Fig. S1, ESI): 0.00013 mol (0.0681 g) [BTAMIM][DEHP], 0.013 mol (1.770 g) pentaerythritol, 0.052 mol (6.040 g) caproic acid, 2 mL toluene, temperature: 160 °C, Time: 7 h. b AN: acid number (quantity of base, expressed in milligrams of potassium hydroxide (KOH)per gram of sample that is required to titrate the acid constituents present in 1 g of sample when titrated under prescribed conditions). c Er: Esterification. | |||
| 1 | None | 80.61 | 78 |
| 2 | [BTAMIM][DEHP] | 9.17 | 98 |
The results of kinematic viscosity, viscosity index and copper strip test of PETH and PETH + [BTAMIM][DEHP] are shown in Table 2. It can be seen that the viscosity at 40 °C and 100 °C of PETH+ [BTAMIM][DEHP] only slightly increases. There is not any color change of PETH and PETH+ [BTAMIM][DEHP] compared with the fresh copper during the copper strip tests. This indicates that the bifunctional ionic liquid [BTAMIM][DEHP] qualifies as an additive in PETH.
| Lubricant | PETH | PETH + [BTAMIM][DEHP] | |
|---|---|---|---|
| a The viscosity index was determined by ASTMT 2270-10. b The copper strip test was performed according to the ASTMD procedure 130–10 (temperature: 100 °C, time: 3 h). | |||
| Kinematic | 40 °C | 18.13 | 19.12 |
| Viscosity/(mm2 s−1) | 100 °C | 4.150 | 4.270 |
| Viscosity indexa | 132 | 130 | |
| Copper strip test/corrosion gradeb | 1a | 1a | |
Subsequently, the tribological properties of the PETH plus [BTAMIM][DEHP] were investigated. It is well known that a good lubricant usually has a low friction coefficient and small wear scar diameter. A lubricant with a low friction coefficient means that it can reduce the friction force during the mechanical operation, at the same time, a small wear scar diameter indicates that the lubricant can effectively reduce wear. The mean friction coefficient with time at a constant load of 50 N, temperature of 100 °C and frequency of 25 Hz for PETH and PETH + [BTAMIM][DEHP] is shown in Fig. 4 and the wear scar diameter of the upper running ball lubricated by PETH and PETH + [BTAMIM][DEHP] measured using an optical microscope(OLYMPUS DSX1000) is shown in Fig. 5, and the detail data are shown in Table 3. It can be seen that PETH + [BTAMIM][DEHP] shows a stable and much lower friction coefficient compared with the base oil PETH and the wear scar diameter of the upper running ball lubricated by PETH is 1.5 times more than that of lubricated by PETH + [BTAMIM][DEHP]. The friction coefficient of the base oil is reduced and meanwhile, the wear scar diameter is decreased after the addition of the bi-functional ionic liquid [BTAMIM][DEHP]. These results demonstrated that the introduction of the bi-functional ionic liquid [BTAMIM][DEHP] significantly improved the friction reduction and anti-wear performance of PETH.
 | ||
| Fig. 4 Evolution of the friction coefficient with time of PETH and PETH + [BTAMIM][DEHP]. (SRV load: 50 N, frequency:25 Hz, temperature: 100 °C, stroke: 1 mm). | ||
 | ||
| Fig. 5 The wear scar diameter of the upper running ball lubricated by (a) PETH, (b) PETH + [BTAMIM][DEHP]. (SRV load: 50 N, frequency: 25 Hz, temperature: 100 °C, stroke: 1 mm). | ||
| Lubricant | COF mean | WSD/mm |
|---|---|---|
| a SRV conditions: load: 50 N, frequency: 25 Hz, temperature: 100 °C, stroke: 1 mm. | ||
| PETH | 0.180 | 0.49 |
| PETH + [BTAMIM][DEHP] | 0.113 | 0.30 |
The morphologies of the worn surfaces of the lower steel disc lubricated by PETH and PETH + [BTAMIM][DEHP] were characterized by SEM (JSM-6701F), and the results are shown in Fig. 6. It was found that the worn surface of the steel disc lubricated by PETH was much wider and deeper than the surface lubricated by PETH + [BTAMIM][DEHP]. The wear scar of the lower steel disc lubricated by PETH + [BTAMIM][DEHP] was relatively narrow and the abrasion became smoother. This result is consistent with the characterization of the test of the upper running ball with the optical microscope.
 | ||
| Fig. 6 SEM morphologies of worn surfaces lubricated by (a) and (b) PETH, (c) and (d) PETH + [BTAMIM][DEHP]. (SRV load: 50 N, frequency:25 Hz, temperature: 100 °C, stroke: 1 mm). | ||
Conclusions
In summary, we prepared a bi-functional ionic liquid [BTAMIM][DEHP] which plays dual roles in the synthetic ester oil: synthetic catalyst and friction reduction and anti-wear additive. [BTAMIM][DEHP] exhibited good catalytic activity in the esterification reaction, and the conversion of caproic acid was up to 98% under the substrate ratio at stoichiometric conditions. Meanwhile, [BTAMIM][DEHP] plays a friction reduction and anti-wear additive role without the separation after the reaction and can significantly improve the tribological properties of the base oil pentaerythritol tetra-hexanoate, which shortened the preparation procedure of synthetic ester oil, which is beneficial to industrial production.Author contributions
Yanan Wang: experimental designing, data collection and analysis, writing the manuscript. Qilong Zhao: Investigation. Jun Yin: formal analysis. Huaigang Su: supervision. Hongyuan Yu: conceptualization. Wenjing Lou: editing. All authors have read and approved the final manuscript.Data availability
The data supporting this article have been included as part of the ESI.† Raw data for each experiment are available by contacting the corresponding author.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (52105226) and Taishan Scholar Youth Expert Program.Notes and references
- G. W. Rowe, R. Inst. Chem., Rev., 1968, 1, 135 RSC.
- S. N. M. Khazaai, G. P. Maniam, M. H. A. Rahim, M. M. Yusoff and Y. Matsumura, Ind. Crops Prod., 2017, 97, 191 CrossRef CAS.
- G. P. Barchan, G. G. Chigarenko and V. S. Bolotnikov, Patent, 1986, 1986–074965 Search PubMed.
- S. Boyde, Green Chem., 2002, 4, 293 RSC.
- X. Chen, G. Wen and Z. Guo, Mater. Horiz., 2020, 7, 1697 RSC.
- L. Koch, A. Guntermann, K. Hirschbicheler, C. Plass, T. Betke, L. Ma, T. Kilthau and H. Groger, Green Chem., 2023, 25, 6398 RSC.
- X. Zhang, T. Ren and Z. Li, J. Mater. Chem. A, 2023, 11, 9239 RSC.
- M. Stacey, E. J. Bourne, J. C. Tatlow and J. M. Tedder, Natrue, 1949, 4173, 705 CrossRef PubMed.
- A. Lodhi and K. C. Maheria, Catal. Commun., 2024, 187, 106883 CrossRef CAS.
- Y. Zeng, H. Chen, G. Hu, R. Cai, Y. Chenyang, Z. Huang and B. Han, Ind. Crops Prod., 2024, 216, 118778 CrossRef CAS.
- J. Zheng, X. Zhang, Z. Li and N. Zhong, J. Mol. Liq., 2024, 398, 124292 CrossRef CAS.
- L. Hu, Y. Liu, P. Zhang, H. Cui and J. He, J. Ind. Eng. Chem., 2024, 129, 321 CrossRef CAS.
- L. Jin, C. Zhou, S. Chen and P. Liu, Appl. Catal., A, 2024, 681, 119773 CrossRef CAS.
- M. L. Smith, Zh Hong and S. A. Asher, Analyst, 2014, 139, 6379 RSC.
- M. Vilas, M. A. A. Rocha, A. M. Fernandes, E. Tojo and L. M. N. B. G. Santos, Phys. Chem. Chem. Phys., 2015, 17, 2560 RSC.
- M. Smiglak, W. M. Reichert, J. D. Holbrey, J. S. Wilkers, L. Sun, J. S. Thrasher, K. Kirichenko, S. Singh, A. R. Kattritzky and R. D. Rogers, Chem. Commun., 2006, 2554 RSC.
- H. Liaw, C. Chen, Y. Chen, J. Chen, S. Huang and S. Liu, Green Chem., 2012, 14, 2001 RSC.
- F. Heym, B. J. M. Etzold, C. Kern and A. Jess, Green Chem., 2011, 13, 1453 RSC.
- K. R. J. Lovelock, J. P. Armstrong, P. Licence and R. G. Jones, Phys. Chem. Chem. Phys., 2014, 16, 1339 RSC.
- S. Sadiadi, J. Mol. Liq., 2021, 323, 114994 CrossRef.
- L. Naicker, M. Schörner, D. Kremitzl, H. B. Friedrich, M. Haumann and P. Wasserscheid, ChemCatChem, 2022, 14, e202200388 CrossRef CAS.
- O. Bartlewicz, I. Dabek, A. Szymanska and H. Maciejewski, Catal, 2020, 10, 1227 CAS.
- Y. Zhou and J. Qu, ACS Appl. Mater. Interfaces, 2017, 9, 3209 CrossRef CAS PubMed.
- E. Cigno, Ch Magagnoli, M. S. Pierce and P. Iglesias, Wear, 2017, 376–377, 756 CrossRef CAS.
- Y. Zhang, T. Cai, W. Shang, L. Sun, D. Liu and D. Tong, Tribol. Int., 2017, 115, 297 CrossRef CAS.
- H. Guo, A. R. Adukure and P. Iglesias, Coatings, 2019, 9, 713 CrossRef CAS.
- H. J. Taufen, J. Bradford and E. S. Moler, Ind. Eng. Chem., 1959, 51, A51 Search PubMed.
- Q. Liu, H. He, Z. Chao, J. Xie and E. Ruchenstein, New J. Chem., 2012, 36, 139 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and Fig. S1–S4. See DOI: https://doi.org/10.1039/d4nj03618b |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2024 |