DOI:
10.1039/D4NJ03546A
(Paper)
New J. Chem., 2025,
49, 109-123
Structural characterization and tuning of magnetic properties of CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) nanomaterials synthesized via a modified sol–gel autocombustion method
Received
8th August 2024
, Accepted 16th November 2024
First published on 18th November 2024
Abstract
We report magnetic properties of CoVδFe2−δO4 nanomaterials synthesized via a modified sol–gel autocombustion method. Lattice parameters obtained from X-ray diffraction (XRD) studies varied from 8.348 to 8.285 Å for δ = 0.0–0.9. Crystallite sizes estimated using Williamson–Hall (W–H) plots were found to be in the range of 52–30 nm (δ = 0.0–0.9). Lattice strain varied from 1.34 × 10−3 to 2.00 × 10−3 for δ = 0.0–0.9. Fourier transform infrared (FTIR) studies revealed peak positions in the ranges of 589.2–602.9 and 409.3–419.5 cm−1 for tetrahedral (Td) and octahedral (Oh) coordination, respectively. Raman spectra showed peaks corresponding to T2g(3), Eg(2), Eg(1), T2g(2), T2g(1), A1g(2) and A1g(1) modes. Particle sizes obtained from field emission scanning electron microscopy (FESEM) analysis varied from 56 to 34 nm for δ = 0.0–0.9. Values of saturation magnetization (Ms) showed significant variation from 82.5 to 47.3 emu g−1 for δ = 0.0–0.9. Coercivity (Hc) values (at 300 K) decreased from 1180 (δ = 0.0) to 886 Oe (δ = 0.9). The estimated values of Ms and Hc (at 5 K) varied from 74.8 to 48.9 emu g−1 (δ = 0.0 to 0.9) and from 7772 to 7078 Oe (δ = 0.0 to 0.9), respectively. XPS study confirmed the redistribution of Co2+ and Fe3+ ions in Oh and Td sites, agreeing well with the magnetic behaviour. The observed decrease in Hc values were also related to a decrease in anisotropy constant (K1), i.e. from 1.44 × 104 to 1.19 × 104 J m−3 for δ = 0.0–0.9.
1. Introduction
Over the past few decades, spinel ferrites have gained significant attention owing to their numerous potential technological applications, such as targeted drug delivery, biomedical applications, spintronic devices, wastewater treatment, gas-sensing devices, and microwave devices.1–4 Among the various properties of spinel ferrites, magnetic properties have been well researched and reported by material scientists. Alterations of intrinsic magnetic characteristics, such as coercivity (Hc) and saturation magnetization (Ms), of ferrite materials are significant. However, to achieve desired changes in the magnetic parameters of ferrites, it is important to control their particle size, morphology, surface properties, etc. This can be achieved by developing novel synthesis methods to obtain certain set of properties that may help in various application designs. In this regard, the development of newer synthesis routes for substitution of aliovalent dopants in ABO3 oxide materials in order to obtain enhanced optical and magnetic properties is significant.5–7 The effect of substituent elements in spinel ferrites has also been widely studied by materials scientists over the past few decades for the above stated purposes.8–10 One of the most studied and prominent ferrite materials is cobalt ferrite (CoFe2O4).11–13 Magnetic and electrical properties shown by CoFe2O4 make it a suitable candidate for technological applications.14,15 Excellent properties shown by CoFe2O4 materials at their bulk level include high Hc (230 Oe at 298 K), high Ms (80 emu g−1) and moderate anisotropy constant (2.65 × 106 to 5.1 × 10 6 erg cm−3).16–18 At the nano-scale level, the above-mentioned properties can be altered substantially, which can be attributed to the manifestation of surface effects having the canted spin structure. For example, a large coercivity value (Hc) of 4.3 kOe at room temperature was observed for CoFe2O4 with a particle size of 30 nm.19
Several synthesis methods have been used for the synthesis of un-substituted and substituted cobalt ferrite materials. Among the various synthesis methods, micro-emulsion, co-precipitation, green synthesis, and hydrothermal methods are noteworthy.20,21 Also, a recently reported vanadium-doped cobalt ferrite material synthesized via a solid-state method at 1100 °C showed interesting potential for high-frequency applications.22 Cobalt ferrite materials synthesized via green synthesis methods were reported that would be suitable for therapeutic applications, such as drug carrier and delivery agents.20 In the crystal structure of spinel ferrites of the type AB2O4, the metal cations occupy the tetrahedral and octahedral positions, i.e. A and B sites, respectively.23 Various properties of cobalt ferrite (e.g. magnetic properties) can be influenced by numerous factors, such as the synthesis method, annealing temperature, different substituent elements in the crystal lattice, and particle size and shape.15 Therefore, the substitution of different elements in CoFe2O4 (either at Fe3+ sites or Co2+ sites) is an appropriate approach to modify the magnetic properties for application purposes.15,24,25
Substituting non-magnetic d-block transition elements (e.g. vanadium) in the CoFe2O4 crystal lattice has attracted much attention because they can induce changes in the values of Ms and Hc, which are of technological importance. Some of the technological applications include magnetic recording, magnetic resonance imaging, and magnetic hyperthermia.18 Alteration of the magnetic properties as a result of substitution of V5+ ion in CoFe2O4 has been well studied.22,26,27 The effect of vanadium substitution in cobalt ferrite CoVxFe2−xO4 (0.0 ≤ x ≤ 0.9) on the magnetic properties of pure CoFe2O4 has already been studied from different perspectives, including using a modified synthesis strategy, a smaller particle size ranging between 30–8 nm, and cation redistribution in materials.22,26,27
Reports on synthesizing phase-pure CoVδFe2−δO4 materials having a higher vanadium content, δ (i.e. 0.25 ≤ δ ≤ 0.9), at relatively lower temperature (700 °C) are rare. Substituting various vanadium ions in CoVδFe2−δO4 can change the magnetic properties substantially by inducing cation distribution. Further, it may further reduce the crystallite size and induce lattice strain. These deformations can lead to the generation of larger Hc values in the materials. In addition, low-temperature magnetic studies on vanadium-substituted cobalt ferrite have rarely been reported, if even at all. Therefore, studies on the variation of Ms and Hc in CoVδFe2−δO4 materials performed in a systematic way are merited. Therefore, in this paper, we report the synthesis of pure-phase CoVδFe2−δO4 nanomaterials (0.0 ≤ δ ≤ 0.9) at 700 °C using a modified sol–gel autocombustion method. The effect of vanadium insertion in CoVδFe2−δO4 nanomaterials was studied specifically with regards to structural modifications of the spinel lattice. We have attempted exhaustive spectroscopic and electron microscopic investigations for materials characterizations. Finally, a correlation of structure of materials is done with the observed room temperature (300 K) and low temperature (5 K) magnetic properties.
2. Experimental
2.1 Synthesis of CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) nanomaterials
Synthesis of the CoVδFe2−δO4 nanomaterials (δ = 0.0, 0.1, 0.3, 0.5, 0.7, and 0.9) was carried out using a modified citric acid-assisted sol–gel autocombustion method.26 To be noted, the synthesis procedure adopted in this study has some similarities with the Pechini sol–gel process.5,6 In summary, Fe (NO3)3·9H2O (99%, Molychem, India) and CoCl2·6H2O (99%, Molychem, India) were taken in desired stoichiometric ratios and dissolved in a minimum quantity of distilled water in separate beakers. V2O5 (99% Fisher Scientific, India) was dissolved in water using 1 M NaOH solution, followed by maintaining an acidic pH of 1.5 using 1 M HNO3. These solutions containing V5+, Co2+, and Fe3+ ions were mixed together quantitatively, then transferred to a round-bottom (RB) flask and diluted to 400 mL using distilled water. Citric acid (10.0 g) was added at this stage and the reaction mixture was stirred for 10 min. By the addition of 1 M aq. HNO3, the pH of the solution mixture was maintained at 1.33. The final solution was then refluxed at 80 °C for 3 h. Next, a viscous gel was formed at 90 °C by the simple process of evaporation using a hot plate. The resulting gel was transformed into a swollen gel when heat treated at 90 °C in a hot air oven for a duration of 6 h. The resulting swollen gel was then crushed and combusted at 400 °C in a muffle furnace (air atmosphere) for a period of 3 h. The resulting black-coloured powdered product was then digested using distilled water (approximately 100 mL) for nearly 10 min, and then the suspension was filtered and washed with distilled water several times. This final pure vanadium-substituted cobalt ferrite product was subjected to annealing at 700 °C for 3 h in a muffle furnace in an air atmosphere, and the final product was used for the subsequent characterization.
2.2 Characterization techniques
CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) nanomaterials, annealed at 700 °C, were characterized using the powder X-ray diffraction (XRD) technique (Bruker, D8 Advance X-ray diffractometer), field emission scanning electron microscopy (FESEM) with energy dispersive spectroscopy (EDS) (QUANTA, FEG 250), Fourier transform infrared spectroscopy (FTIR) (Shimadzu, IRAffinity-1S), Raman spectroscopy (LAB RAM HR Horiba France), and X-ray photoelectron spectroscopy (XPS) (ThermoFisher Scientific). The XPS instrument was equipped with a monochromatic Al Kα X-ray source (hν = 1486.6 eV). The samples were sputtered with an Ar+ ion gun for 1 min using 500 eV energy before recording the XPS spectra. The XRD patterns were recorded using CuKα radiation with a 3° min−1 scan rate. The crystallite sizes were calculated using Scherrer's formula (D = (0.9λ)/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ)), where D is the average crystallite size, λ is the wavelength of the X-rays, β is the full width at half maximum expressed in radian (FWHM), and θ is the diffraction angle in radian.28 Also, Williamson–Hall (WH) plots (i.e. β
θ)), where D is the average crystallite size, λ is the wavelength of the X-rays, β is the full width at half maximum expressed in radian (FWHM), and θ is the diffraction angle in radian.28 Also, Williamson–Hall (WH) plots (i.e. β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(θ) vs. 4
cos(θ) vs. 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin(θ)) were used to determine the lattice strain and crystallite size of the materials. In particular, the WH plots were linearly fitted in order to obtain the slope and Y-intercept values, from which the lattice strain (ε) and crystallite sizes were estimated for the CoVδFe2−δO4 lattice, respectively. To be noted, the Williamson–Hall plot is based on the expression, [(β
sin(θ)) were used to determine the lattice strain and crystallite size of the materials. In particular, the WH plots were linearly fitted in order to obtain the slope and Y-intercept values, from which the lattice strain (ε) and crystallite sizes were estimated for the CoVδFe2−δO4 lattice, respectively. To be noted, the Williamson–Hall plot is based on the expression, [(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) = (0.9λ/D) + (ε4
θ) = (0.9λ/D) + (ε4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ)], where ε represents the lattice strain, θ is the diffraction angle, β is full width at half maximum (FWHM) expressed in radian, D is the crystallite size, and λ is the wavelength of X-ray radiation.29 The FESEM images were analysed for obtaining the morphology and particle sizes of the CoVδFe2−δO4 nanomaterials under study. The average particle sizes were estimated using ImageJ software with the maximum number of particles distributed throughout the FESEM image. EDS was carried out for confirming the presence of V, Fe, Co, and O in CoVδFe2−δO4 materials. The elemental compositions were also investigated through the EDS spectra and were in good agreement with the stoichiometry of the materials. For the FTIR studies, the samples were ground with solid KBr powder (in an approximately 1
θ)], where ε represents the lattice strain, θ is the diffraction angle, β is full width at half maximum (FWHM) expressed in radian, D is the crystallite size, and λ is the wavelength of X-ray radiation.29 The FESEM images were analysed for obtaining the morphology and particle sizes of the CoVδFe2−δO4 nanomaterials under study. The average particle sizes were estimated using ImageJ software with the maximum number of particles distributed throughout the FESEM image. EDS was carried out for confirming the presence of V, Fe, Co, and O in CoVδFe2−δO4 materials. The elemental compositions were also investigated through the EDS spectra and were in good agreement with the stoichiometry of the materials. For the FTIR studies, the samples were ground with solid KBr powder (in an approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 ratio) and formed into transparent discs. The powdered CoVδFe2−δO4 samples were used directly for the Raman spectroscopic measurements. For the magnetic properties studies, vibrating sample magnetometry (VSM) was used (Quantum Design, PPMS Evercool II) and the measurements were carried out at room temperature (300 K) and at low temperature (5 K) using a maximum field strength of 2T.
20 ratio) and formed into transparent discs. The powdered CoVδFe2−δO4 samples were used directly for the Raman spectroscopic measurements. For the magnetic properties studies, vibrating sample magnetometry (VSM) was used (Quantum Design, PPMS Evercool II) and the measurements were carried out at room temperature (300 K) and at low temperature (5 K) using a maximum field strength of 2T.
3. Results and discussion
3.1 X-Ray diffraction studies
In Fig. 1, the XRD patterns for the CoVδFe2−δO4 nanomaterials annealed at 700 °C are depicted. Analysis of the XRD patterns for the CoVδFe2−δO4 materials confirmed the single-phase product formation with an inverse cubic spinel ferrite crystal structure. The XRD lines were indexed to ICDD card no. 00-003-0864. The peak indexing for the XRD patterns included reflections from the (220), (311), (222), (400), (422), (511), and (440) planes. For pure CoFe2O4 materials annealed at 700 °C, reflections from the (220), (311), (222), (400), (422), (511), and (440) planes occurred at 2θ values of 30.25°, 35.70°, 37.34°, 43.31°, 53.74°, 57.29°, and 62.91°, respectively. As the V content increased in CoVδFe2−δO4, there was a shift in the XRD line positions. The XRD lines coinciding to the (311) plane as a function of increasing vanadium content, δ, for CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) are shown in Fig. 2. As the vanadium content (δ) increased in the lattice, there was a shift of the (311) diffraction line initially towards lower values of 2θ up to δ = 0.3, after which it shifted towards higher values of 2θ. In particular, for vanadium contents δ = 0.0, 0.1, 0.3, 0.5, 0.7, and 0.9, the corresponding peak positions (2θ) for the (311) lines were found at 35.70°, 35.61°, 35.64°, 35.80°, 35.72°, and 35.97°, respectively. The shift in the values of 2θ towards lower angles for δ = 0.0, 0.1 and 0.3 concurred with the corresponding values of the lattice parameters showing the lattice expansion, as can be noted in Table 1. Then, for the materials with a higher V content (i.e. δ = 0.5, 0.7 and 0.9), the shift in the values of 2θ were observed towards higher values of 2θ, with the lattice parameters showing a systematic decrease for δ = 0.5, 0.7, and 0.9 in CoVδFe2−δO4 (Table 1).
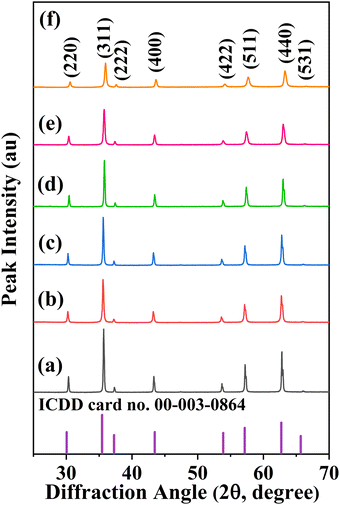 |
| | Fig. 1 XRD patterns of CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) nanomaterials annealed at 700 °C (air atmosphere) for different vanadium contents, δ: (a) 0.0, (b) 0.1, (c) 0.3, (d) 0.5, (e) 0.7 and (f) 0.9 together with the XRD pattern for ICDD card no. 00-003-0864. | |
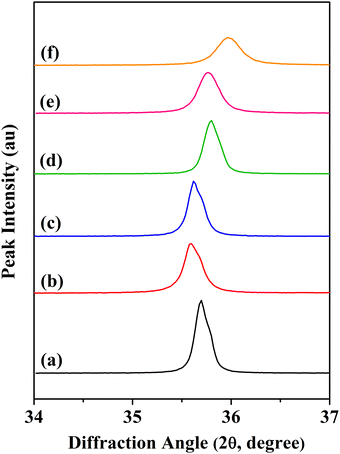 |
| | Fig. 2 Shift in the (311) line for CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) nanomaterials annealed at 700 °C for different vanadium contents, δ: (a) 0.0, (b) 0.1, (c) 0.3, (d) 0.5, (e) 0.7 and (f) 0.9. | |
Table 1 Material compositions, lattice parameters, lattice volume, crystallite sizes, lattice strain, and FESEM particle sizes of CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) nanomaterials annealed at 700 °C
| Material composition CoVδFe2−δO4 |
Lattice parameters, a (Å) |
Volume of unit cell, a3 (Å3) |
Crystallite size from Scherrer's formula (nm) |
Crystallite size from WH plot (nm) |
Lattice strain by WH plot (× 10−3) |
FESEM particle size (nm) |
|
δ = 0.0 |
8.348 |
581.76 |
43 |
52 |
1.34 |
56 |
|
δ = 0.1 |
8.365 |
585.33 |
34 |
39 |
1.44 |
37 |
|
δ = 0.3 |
8.361 |
584.49 |
40 |
49 |
1.77 |
48 |
|
δ = 0.5 |
8.326 |
577.18 |
40 |
48 |
1.57 |
41 |
|
δ = 0.7 |
8.324 |
576.76 |
31 |
38 |
2.01 |
37 |
|
δ = 0.9 |
8.285 |
568.69 |
25 |
30 |
2.00 |
34 |
The variation in crystallite sizes and lattice parameters with increasing vanadium content ‘δ’ (CoVδFe2−δO4, 0.0 ≤ δ ≤ 0.9) is depicted in Fig. 3. It could be observed that there was an overall decrease in the crystallite sizes and lattice parameters as the V content increased from δ = 0.0 to δ = 0.9 in CoVδFe2−δO4. A significant decrease in crystallite size from 43 nm for δ = 0.0 to 34 nm for δ = 0.1 was observed. Thereafter, it increased, showing a broad hump centred at δ = 0.3. Then, a drastic decrease in the crystallite sizes was observed, i.e. from 40 nm to 25 nm for δ = 0.5 to δ = 0.9, respectively, for the CoVδFe2−δO4 nanomaterials. An overall decrease in the lattice parameters was observed from 8.348 Å for δ = 0.0 to 8.285 Å for δ = 0.9 for the CoVδFe2−δO4 nanomaterials. Detailed and precise observation revealed that when the V content was increased from δ = 0.0 to δ = 0.1, there was a slight increase in the lattice parameters values from 8.348 Å to 8.365 Å, respectively. Next, no measurable variation was found for δ = 0.3. However, the values of the lattice parameters for CoVδFe2−δO4 showed a systematic decreasing trend from 8.361 Å to 8.285 Å for δ = 0.3 to δ = 0.9, respectively. These results agreed well with the similar trends in variation of the crystallite sizes and lattice parameters already reported for vanadium-doped cobalt ferrite systems.26
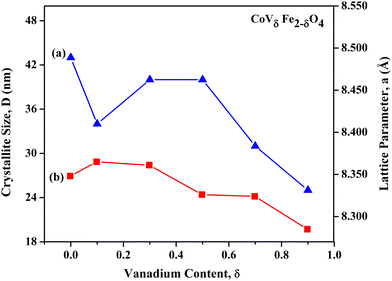 |
| | Fig. 3 Variation in (a) crystallite sizes, D, and (b) lattice parameters, a, with increasing V content ‘δ’ in CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) nanomaterials annealed at 700 °C in an air atmosphere. | |
In nanocrystalline systems, broadening of the XRD peaks occurs owing to the following reasons: (i) occurrence of nano-sized crystallites and (ii) strain induced line broadening due to lattice defects and dislocations. As the total broadening is the sum of the size and strain broadening, the Williamson–Hall (WH) method is employed for the separation of the same.30,31Fig. 4 shows the WH plots for all the compositions of CoVδFe2−δO4 nanomaterials considered here. Specifically, β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) cos(θ) vs. 4
cos(θ) vs. 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin(θ) were plotted and fitted with linear fit functions. The fitted values of the slope and intercept represent the lattice strain ‘ε’ and β
sin(θ) were plotted and fitted with linear fit functions. The fitted values of the slope and intercept represent the lattice strain ‘ε’ and β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(θ). The crystallite size (D) (in nm) was calculated using the formula D = (0.9λ)/(β
cos(θ). The crystallite size (D) (in nm) was calculated using the formula D = (0.9λ)/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(θ)). The simulated values of the positive lattice strains and the crystallite sizes obtained from the WH plots are provided in Table 1. There was a significant increase in the lattice strain from 1.34 × 10−3 for pure CoFe2O4 to 2.00 × 10−3 for CoV0.9Fe1.1O4. Such a kind of increasing trend in the values of lattice strains as a result of the progressive increase in large-sized dopant atoms in the spinel crystal structure has previously been reported in earlier studies.30,32 This shows the presence of tensile force in the CoVδFe2−δO4 lattice. It was observed that the crystallite sizes calculated with Scherrer's formula were relatively smaller compared to those calculated from the WH plots. These results can be rationalized as follows: Scherrer's method neglects the microstrain in the lattice that may originate from imperfections in the structure, whereas in the WH plot method, there is a separation of the instrumental and sample broadening effects in the calculation.
cos(θ)). The simulated values of the positive lattice strains and the crystallite sizes obtained from the WH plots are provided in Table 1. There was a significant increase in the lattice strain from 1.34 × 10−3 for pure CoFe2O4 to 2.00 × 10−3 for CoV0.9Fe1.1O4. Such a kind of increasing trend in the values of lattice strains as a result of the progressive increase in large-sized dopant atoms in the spinel crystal structure has previously been reported in earlier studies.30,32 This shows the presence of tensile force in the CoVδFe2−δO4 lattice. It was observed that the crystallite sizes calculated with Scherrer's formula were relatively smaller compared to those calculated from the WH plots. These results can be rationalized as follows: Scherrer's method neglects the microstrain in the lattice that may originate from imperfections in the structure, whereas in the WH plot method, there is a separation of the instrumental and sample broadening effects in the calculation.
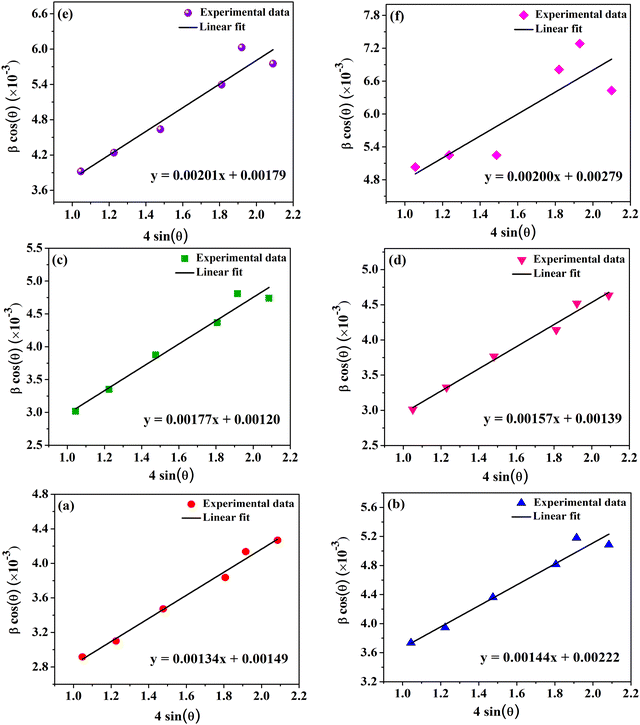 |
| | Fig. 4 Linearly fitted Williamson–Hall (WH) plots for CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) nanomaterials annealed at 700 °C for different vanadium contents, δ: (a) 0.0, (b) 0.1, (c) 0.3, (d) 0.5, (e) 0.7 and (f) 0.9. | |
In Fig. 5(a)–(c), the values of the XRD peak intensity ratios, i.e. I(422)/I(400), I(220)/I(400), and I(400)/I(440), are plotted against the vanadium content, δ, in CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9). The observed drastic variation in the peak intensity ratios indicated cation distributions as a result of the increasing V content in the CoFe2O4 lattice. The intensity ratio I(220)/I(400) decreased from 0.88 for δ = 0.0 to 0.72 for δ = 0.3, and then showed an increasing trend thereafter with a broad-hump peak at δ = 0. 7. Next, the peak intensity ratio of I(422)/I(400) plotted against the V content, first showed an initial sharp decrease up to δ = 0.1, i.e. from 0.61 for δ = 0.0 to 0.51 for δ = 0.1, then showed no measurable change from δ = 0.3 to δ = 0.9, for which the I(422)/I(400) ratio was found to be 0.52. Finally, considering the ratio of I(400)/I(440) as a function of V content, δ, there was an initial increase from 0.34 for δ = 0.0 to 0.37 for δ = 0.1, followed by an increasing trend, with a broad-hump peak at δ = 0. 7. These above-mentioned planes are known to be sensitive to the cation distribution between tetrahedral and octahedral holes in the spinel crystal lattice. Qualitatively, it could be anticipated that the alterations in the peak intensity ratios mentioned above may alter the magnetic structure of ferrite materials.21,33
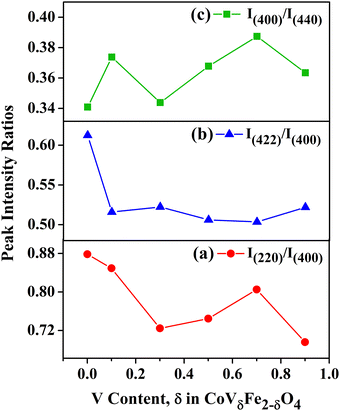 |
| | Fig. 5 XRD peak intensity ratios vs. vanadium content, δ, in CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) nanomaterials: (a) I(220)/I(400), (b) I(422)/I(400) and (c) I(400)/I(440). | |
3.2 FTIR spectroscopic analysis
Fig. 6 shows the FTIR spectra of the CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) nanomaterials recorded in the range of 4000–400 cm−1. The presence of M–O bonding, where M stands for metal (M = Co (Fe/V)), was confirmed by the presence of strong peaks located in the frequency ranges of 409–420 cm−1 and 454–460 cm−1 for the octahedral (Oh) (ν2) coordination, while the frequency range 589–603 cm−1 represented the tetrahedral (Td) (ν1) coordination.34,35 As the nanomaterials were obtained via the autocombustion of citrate gel at elevated temperatures, in addition to the above-mentioned characteristics ν2 and ν1 bands for the spinel structure, a few low intensity peaks (compared to the intensity of the ν2 and ν1 bands) corresponding to organic linkages were observed. The IR bands located in the frequency ranges of 839–888 cm−1 and 952–962 cm−1 are the signature of M–O-organic linkages.21,36 The IR peak near 1690 cm−1 was assigned to the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) group of citrate complexes. Also, the weak bands observed at 1545–1550 cm−1 and 1639–1641 cm−1 were assigned to M–(COO)-organic linkages.21,36 The medium to strong IR band located near 2346 cm−1 was assigned due to the presence of adsorbed CO2 and M–O–O-organic linkages.16 The IR bands observed at nearly 3445 cm−1 in almost all the compositions were related to possible O–H stretching of the adsorbed H2O (l).21,37
O) group of citrate complexes. Also, the weak bands observed at 1545–1550 cm−1 and 1639–1641 cm−1 were assigned to M–(COO)-organic linkages.21,36 The medium to strong IR band located near 2346 cm−1 was assigned due to the presence of adsorbed CO2 and M–O–O-organic linkages.16 The IR bands observed at nearly 3445 cm−1 in almost all the compositions were related to possible O–H stretching of the adsorbed H2O (l).21,37
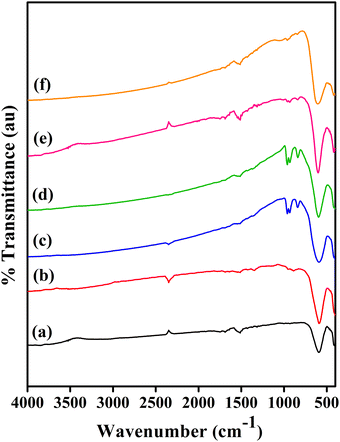 |
| | Fig. 6 FTIR spectra of CoVδFe2−δO4 nanomaterials annealed at 700 °C for different vanadium contents, δ: (a) 0.0, (b) 0.1, (c) 0.3, (d) 0.5, (e) 0.7 and (f) 0.9. | |
The variations in the IR frequencies corresponding to the octahedral and tetrahedral coordination for each composition of the CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) materials are presented in Fig. 7. For both coordinations, an initial decrease in the trend of ν1 and ν2 was observed down to a minimum at δ = 0.1. However, after attaining the minima, an increasing trend in the values of the ν1 and ν2 frequencies was then observed until reaching a saturation value at δ = 0.9. Thus, the number and nature of the metal ions that form bonds with oxygen in octahedral and tetrahedral coordinations were significantly with the progressive increase in vanadium (δ) content. These results, i.e. variation in the range of frequencies of ν1 and ν2, could be related to the cation distribution in the cobalt ferrite spinel lattice.38
 |
| | Fig. 7 Variation of IR frequencies as a function of vanadium content (δ) in CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) nanomaterials annealed at 700 °C for various coordination sites: (a) tetrahedral site and (b) octahedral site. | |
3.3 Raman spectroscopy studies
The Raman spectra for the CoVδFe2−δO4 nanomaterials annealed at 700 °C are shown in Fig. 8. The characteristic Raman shifts for the CoVδFe2−δO4 nanomaterials with varying the vanadium content (δ) are summarized in Table 2. Vibrational modes showing the asymmetric stretching, symmetric stretching, and symmetric bending of the bonds associated with A site or B site cations in the spinel structure (AB2O4) and O2− anions could be observed in the Raman spectra.39,40 Factor group analysis was used to predict the number of active Raman modes in the cubic spinel having space group Fd3m; such as A1g, Eg, and 3T2g. These modes are generated from the cations’ M–O vibrations occurring in octahedral and tetrahedral sites. The alterations of the peak positions, broadening, and intensities can be correlated to cation redistribution in the CoVδFe2−δO4 nanomaterials.41 The CoVδFe2−δO4 nanomaterials annealed at 700 °C showed Raman modes in the frequency ranges of 218 cm–229, 292–314, 454–472, 601–632, and 680–696 cm−1, which were assigned to the T2g(3), Eg(2), T2g(2), A1g(2), and A1g(1) modes, respectively.42,43 Plots of the Raman peak intensity ratios, i.e. A1g(2)/A1g(1) and A1g(2)/T2g(2) vs. vanadium content, δ, in the CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) nanomaterials are shown in Fig. 9. The peak intensity ratio A1g(2)/T2g(2) was considered to indicate the relative occupation of Co2+ in tetrahedral and octahedral sites; whereby a higher ratio indicates a lower degree of inversion in the structure, where more Co2+ ions migrate from octahedral to tetrahedral interstitials. Further, the intensity ratio A1g(2)/A1g(1) can also be related to the cation distribution; whereby a higher intensity ratio of A1g(2)/A1g(1) indicates a smaller inversion degree, with Fe3+ ions majorly occupying the tetrahedral sites. The peak intensity ratio A1g(2)/T2g(2) increase from 0.0148 to 0.2799 for δ = 0 to δ = 0.9, respectively, whereas for [A1g(2)/A1g(1)], the increase was steeper, i.e. 0.0225 to 0.6395 for δ = 0 to δ = 0.9, respectively. Thus, with more and more V5+ insertion in the spinel lattice, the above-mentioned ratios increased, indicating a lower degree of inversion.
 |
| | Fig. 8 Raman spectra for CoVδFe2−δO4 nanomaterials annealed at 700 °C for different vanadium contents, δ: (a) 0.0, (b) 0.1, (c) 0.3, (d) 0.5, (e) 0.7 and (f) 0.9. | |
Table 2 Characteristic Raman shifts for CoVδFe2−δO4 nanomaterials for different values of vanadium content (δ), along with the peak intensity ratios [A1g(2)/A1g(1)] and [A1g(2)/T2g(2)]
| V content, δ (CoVδFe2−δO4) |
Raman shift (cm−1) |
Peak intensity ratios |
| T2g(3) |
Eg(2) |
Eg(1) |
T2g(2) |
A1g(2) |
A1g(1) |
[A1g(2)/A1g(1)] |
[A1g(2)/T2g(2)] |
| 0.0 |
222 |
292 |
354 |
454 |
626 |
681 |
0.0225 |
0.0148 |
| 0.1 |
218 |
306 |
355 |
458 |
606 |
680 |
0.0251 |
0.0642 |
| 0.3 |
229 |
294 |
351 |
461 |
601 |
683 |
0.1839 |
0.1036 |
| 0.5 |
222 |
295 |
350 |
460 |
613 |
684 |
0.532 |
0.1886 |
| 0.7 |
225 |
306 |
338 |
472 |
632 |
696 |
0.3662 |
0.1356 |
| 0.9 |
226 |
314 |
339 |
468 |
629 |
693 |
0.6395 |
0.2799 |
 |
| | Fig. 9 Plots of Raman peak intensity ratios vs. vanadium content, δ, in CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) nanomaterials: (a) A1g(2)/A1g(1) and (b) A1g(2)/T2g(2). | |
3.4 FESEM studies
FESEM micrographs were obtained and are presented in Fig. 10(a)–(f). The morphology and histograms for the particle-size distribution (inset) of the products annealed at 700 °C are shown in Fig. 10. Spherical shapes along with aggregated particles forming a trigonal bipyramidal morphology could be observed. The FESEM particle sizes are given in Table 1. The FESEM micrographs depict anisotropic, poly-dispersed particles with the particle sizes of the CoVδFe2−δO4 materials varying from 56 nm to 34 nm for δ = 0.0 down to δ = 0.9, respectively. As can be seen from Table 1, the FESEM particle sizes agreed well with the crystallite sizes estimated from the WH plots. The presence of the elements, i.e. Co, Fe, V, and O, were confirmed by the EDS spectra, wherein the atomic percentages of these elements complied with the stoichiometry of the CoVδFe2−δO4 materials.
 |
| | Fig. 10 FESEM micrographs for CoVδFe2−δO4 nanomaterials annealed at 700 °C for different vanadium contents, δ: (a) 0.0, (b) 0.1, (c) 0.3, (d) 0.5, (e) 0.7 and (f) 0.9 (insets present histograms of particle-size distributions in the respective CoVδFe2−δO4 nanomaterials). | |
3.5 XPS studies
Fig. 11(a)–(d) present the XPS spectra for the chemical states of the various elements for the selected composition of CoVδFe2−δO4 nanomaterials, i.e. pure CoFe2O4 and CoV0.5Fe1.5O4 are shown. The binding energy values were calibrated using the observed adventitious C1s peak at 284.8. The observed peaks (in Fig. 11) majorly corresponded to the 2p3/2 and 2p1/2 states of Co2+, Fe3+, and V5+ ions, as well as the 1s states of the O2− ion. The changes in peak positions and intensities of the Co-2p3/2 and Fe-2p3/2 spectra corresponding to both their tetrahedral and octahedral coordination were analyzed and compared. Fig. 11(a) shows the distinct peaks corresponding to Co2+ ions in tetrahedral and octahedral environments were located at 782.1 and 779.5 eV (with an intensity ratio of ≈1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for CoFe2O4, respectively. For the CoV0.5Fe1.5O4 materials, peak positions of 780.4 and 779.32 eV (with an intensity ratio of ≈5.3
1) for CoFe2O4, respectively. For the CoV0.5Fe1.5O4 materials, peak positions of 780.4 and 779.32 eV (with an intensity ratio of ≈5.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were observed for Co2+ ions in tetrahedral (Td) and octahedral (Oh) environments, respectively. These results indicate the increase in Co2+ ion concentrations in the tetrahedral environment as a result of vanadium ion incorporation in the CoFe2O4 material. In a similar manner, Fig. 11(b) shows distinct peaks corresponding to Fe3+ ions in Td and Oh environments at 711.2 and 709.8 eV (with an intensity ratio of ≈1
1) were observed for Co2+ ions in tetrahedral (Td) and octahedral (Oh) environments, respectively. These results indicate the increase in Co2+ ion concentrations in the tetrahedral environment as a result of vanadium ion incorporation in the CoFe2O4 material. In a similar manner, Fig. 11(b) shows distinct peaks corresponding to Fe3+ ions in Td and Oh environments at 711.2 and 709.8 eV (with an intensity ratio of ≈1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) for CoFe2O4, respectively. For the CoV0.5Fe1.5O4 materials, peaks were observed located at 712.4 and 709.5 eV (with an intensity ratio of ≈0.8
2) for CoFe2O4, respectively. For the CoV0.5Fe1.5O4 materials, peaks were observed located at 712.4 and 709.5 eV (with an intensity ratio of ≈0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for Fe3+ ions in Td and Oh environments, respectively.39,44 In addition, we observed a small intensity peak at 707 eV for the vanadium-substituted product, which was attributed to the occurrence of the Fe2+ state in the material. The above results indicate the increase in Fe3+ concentration in the tetrahedral coordination. In the O1s spectrum, peaks corresponding to lattice oxygen and molecular oxygen were observed at binding energy values of 529.9 and 532.3 eV, respectively (Fig. 11(c)). The observation of two distinct peaks at binding energy values of 516.1 and 517.7 eV corresponded to V4+ and V5+ states, respectively.45 The existence of the V4+ state in the materials along with V5+ and the redistribution of Co2+ and Fe3+ ions in the octahedral and tetrahedral coordination confirmed the redistribution of cations as a result of vanadium incorporation.
1) for Fe3+ ions in Td and Oh environments, respectively.39,44 In addition, we observed a small intensity peak at 707 eV for the vanadium-substituted product, which was attributed to the occurrence of the Fe2+ state in the material. The above results indicate the increase in Fe3+ concentration in the tetrahedral coordination. In the O1s spectrum, peaks corresponding to lattice oxygen and molecular oxygen were observed at binding energy values of 529.9 and 532.3 eV, respectively (Fig. 11(c)). The observation of two distinct peaks at binding energy values of 516.1 and 517.7 eV corresponded to V4+ and V5+ states, respectively.45 The existence of the V4+ state in the materials along with V5+ and the redistribution of Co2+ and Fe3+ ions in the octahedral and tetrahedral coordination confirmed the redistribution of cations as a result of vanadium incorporation.
 |
| | Fig. 11 XPS spectra of CoVδFe2−δO4 nanomaterials (annealed at 700 °C) for (a) Co-2p, (b) Fe-2p, (c) O-1s for δ = 0 and (d) V-2p for δ = 0.5. | |
3.6 Magnetic properties of CoVδFe2−δO4 nanomaterials (0.0 ≤ δ ≤ 0.9)
3.6.1 Room-temperature magnetic properties.
Fig. 12 presents the of M–H hysteresis curves for CoVδFe2−δO4 nanomaterials recorded at room temperature (300 K). The values of saturation magnetization (Ms) were estimated using the fitted data obtained from the plot of (1/H2) vs. magnetization (M), where H is the applied field strength. The values of the saturation magnetization (Ms), coercivity (Hc) and remanent magnetization (Mr) at room temperature (300 K) for CoVδFe2−δO4 are summarized in Table 3. The values of Ms were estimated to be 82.5, 74.5, 71.1, 68.1, 56.9, and 47.3 emu g−1 for vanadium contents δ = 0.0, 0.1, 0.3, 0.5, 0.7, and 0.9, respectively. Similarly, the values of Hc obtained from the MH curve were 1180, 1151, 1065, 885, 946, and 886 Oe for V contents δ = 0.0, 0.1, 0.3, 0.5, 0.7, and 0.9, respectively. The values of Ms and coercivity (Hc) were plotted against the increasing content of vanadium ‘δ’ in CoVδFe2−δO4 (where δ = 0.0, 0.1, 0.3, 0.5, 0.7, 0.9), as depicted in Fig. 13. As can be observed from the plot, the variation in the values of Ms followed a systematic linear decrease from 82.5 emu g−1 down to 47.3 emu g−1 for δ = 0.0 and δ = 0.9, respectively. Similarly, a linear decrease in the values of Hc was observed, i.e. from 1180 Oe to 886 Oe for δ = 0.0 to δ = 0.9, respectively, besides the drastic decrease in the Hc value to 946 Oe at δ = 0.7.
Table 3 Materials composition (δ), saturation magnetization (Ms), coercivity (Hc), remanent magnetization (Mr), squareness ratio (Mr/Ms), magnetocrystalline anisotropic constant (K1), effective magnetic anisotropic constant (Keff) and surface anisotropy (Ks) measured at room temperature (300 K) for CoVδFe2−δO4 nanomaterials (annealed at 700 °C)
| Material composition, δ (CoVδFe2−δO4) |
Saturation magnetization, Ms (emu g−1) |
Coercivity, Hc (Oe) |
Remanent magnetization, Mr (emu g−1) |
Squareness ratio (Mr/Ms) |
K
1 (J m−3) (K1 × 104) |
K
eff (J m−3) (Keff × 104) |
K
s (J m−2) (Ks × 10−3) |
|
δ = 0.0 |
82.5 |
1180 |
36.3 |
0.4402 |
1.44 |
1.521 |
70.2 |
|
δ = 0.1 |
74.5 |
1151 |
33.2 |
0.4451 |
1.32 |
1.339 |
12.35 |
|
δ = 0.3 |
71.1 |
1065 |
31.6 |
0.4444 |
1.39 |
1.118 |
−22.213 |
|
δ = 0.5 |
68.1 |
885 |
30.6 |
0.4501 |
1.26 |
0.9416 |
−25.472 |
|
δ = 0.7 |
56.9 |
946 |
21.3 |
0.3740 |
1.22 |
0.8410 |
−24.003 |
|
δ = 0.9 |
47.3 |
886 |
17.1 |
0.3624 |
1.19 |
0.6548 |
−26.76 |
 |
| | Fig. 12 Plots of M vs. H (measured at 300 K) for CoVδFe2−δO4 nanomaterials annealed at 700 °C for different vanadium contents, δ: (a) 0.0, (b) 0.1, (c) 0.3, (d) 0.5, (e) 0.7 and (f) 0.9. | |
In spinel ferrites, magnetization originates from the vector sum of two unequal magnetic sublattices (A and B) arranged in an antiparallel manner.46–48 In the lattice structure, three kinds of interactions exist, namely: A–A, A–B, and B–B. The inter-atomic A–B super-exchange interactions are dominant over A–A and B–B interactions. In particular, in the inverse spinel CoFe2O4 system, the spins of Fe3+ at octahedral sites can be aligned antiparallel to the spins of Fe3+ at tetrahedral sites’ whereas the Co2+ spins remain aligned to Fe3+ at octahedral sites.17,49,50 Substitution of a transition metal ion Mn+ in place of an Fe3+ ion octahedral site can result in the redistribution of Co2+ in the tetrahedral and octahedral sites and a possible change in the degree of inversion in such materials.51 It has been observed that magnetization may first increase to a maximum value and then decrease as a result of metal ion substitution. This behaviour of an initial increase of magnetization and subsequent decrease can be attributed to the degree of inversion and weakening of the A–B superexchange interactions as a result of metal ion incorporation, respectively. The overall magnetic properties of CoVδFe2−δO4 nanomaterials depend on several factors, such as (i) cation distribution in the octahedral and tetrahedral sites, (ii) extent to which vanadium substitution occurs, (iii) shape, size, and surface effects, (iv) lattice strain, (v) magnetic nature of V substituent, and (vi) spin canting.12,52
The extent of the magnetic interactions depends on the inter-ionic separations and the angle between them.53 When V5+ is substituted in spinel ferrites, due to difference in ionic radii, the inter-ionic separations may change. This leads to an alteration of the magnetization of the materials. In addition, V5+ ions, having no d-orbital electrons, do not contribute towards the spin only magnetic moment of the sample, directly. However, the existence of V4+ in the material cannot be ruled out and XPS investigation supported its existence (Section 3.5). The substitution of V5+ in place of Fe3+ promoted a redistribution of the cations in the interstitial sites, as proven by our XPS study. To be noted, the research approach for this kind of idea has been tested successfully by substituting aliovalent dopants in BaSnO3 oxide material systems.6 Also, Co2+ can migrate from tetrahedral to octahedral interstitial sites, whereas varied fractions of Fe3+ can distribute themselves in suitable octahedral and tetrahedral positions. In addition, the presence of a minute quantity of Fe2+ ions can also contribute towards the magnetization. Because of the fact that Fe3+ ions situated in two different environments (Oh and Td) have differences in their intrinsic magnetic moments, the above-mentioned cation redistribution effect can lead to a significant change in the magnetization. The increasing trend in the plot of the Raman peak intensity ratios vs. vanadium content, y, in the CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) nanomaterials, i.e. A1g(2)/A1g(1) and A1g(2)/T2g(2), proved the above arguments (see Fig. 9). Such analysis of the cation redistribution leading to an alteration of the net magnetic moment has already been proven in ferromagnetic systems.40,52,54 In addition, the CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) nanomaterials studied in this research work for assessing their magnetic properties are nano-structured in nature, where size, strain, and surface effects, including spin canting, can substantially reduce the net magnetization.55,56 The above-mentioned factors explain the nearly linear decrease of Ms from 82.5 emu g−1 down to 47.3 emu g−1 with the increase in vanadium content, δ, in CoVδFe2−δO4 from δ = 0.0 to δ = 0.9, respectively. In a similar manner, the above inferences are strengthened by reports showing a drastic decrease in values of Ms observed in the Nb5+-doped NiZnCo ferrite system.57
The coercivity (Hc) for nanometre-scale ferrite materials can be expressed as:
| Hc = 3[(kTcK1/aMs)][(1/D)], |
where
Tc is the Curie temperature,
K1 is the first anisotropy constant, ‘
a’ is a lattice parameter,
Ms is the saturation magnetization, and ‘
D’ is the grain diameter.
58 The observed decrease in coercivities from 1180 Oe to 886 Oe for
δ = 0.0 to
δ = 0.9, respectively, in CoV
δFe
2−δO
4 (0.0 ≤
δ ≤ 0.9) could not be due to the increase in
Ms and/or increase in grain diameter as per the above-mentioned expression. To be noted,
Ms and the grain size decreased with the continuous increase in vanadium substitution in CoV
δFe
2−δO
4 (0.0 ≤
δ ≤ 0.9). Therefore, the decrease in coercivity can be majorly related to the reduction in the magnetic anisotropy constant with the increase in vanadium atom substitution in the CoV
δFe
2−δO
4 (0.0 ≤
δ ≤ 0.9) nanomaterials.
21 These observations were confirmed by estimations of the magnetocrystalline anisotropy constant (
K1), effective magnetic anisotropy constant (
Keff), and surface anisotropy (
Ks) at 300 K.
59–61 The values of
K1 were estimated from the expression

, where
Ms and the ‘
b’ factor were obtained from linear fitting of the
M vs. (1/
H2) plot, which followed the expression

.
59,61 The values of
K1 showed a decreasing trend,
i.e. from 1.44 × 10
4 to 1.19 × 10
4 J m
−3 for
δ = 0.0 to
δ = 0.9, respectively (
Table 3). The values of
Keff were estimated using the expression
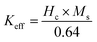
(
Table 3). The calculated values of
Keff were in the range of 1.521 × 10
4 to 6.548 × 10
3 J m
−3 for
δ = 0.0 to
δ = 0.9, respectively. Similarly, the values of
Ks were obtained using the expression
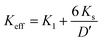
where
D′ is the diameter of the crystallite (
Table 3). It is clear from
Table 3 that the
Ks values varied significantly from 70.2 × 10
−3 to 12.35 × 10
−3 J m
−2 for
δ = 0.0 to
δ = 0.1, respectively. The
Ks values were found to be negative for
δ = 0.3 to
δ = 0.9, indicating different stress directions with respect to the magnetization axis. The squareness ratio increased initially from 0.4402 for
δ = 0.0 until it reached a maximum value of 0.4501 for
δ = 0.5, and thereafter showed a decreasing trend down to 0.3624 for
δ = 0.9. For the CoV
δFe
2−δO
4 (0.0 ≤
δ ≤ 0.9) nanomaterials, the
Mr/
Ms values were found to be in the range of 0.4501–0.3624 (
Table 3). These results can be explained by the possible existence of a surface spin canting effect as a consequence of the nanoparticle size and surface effects.
62
3.6.2 Low-temperature magnetic properties.
The M vs. H curves were recorded at low temperature, i.e. at 5 K, for CoVδFe2−δO4 nanomaterials and are presented in Fig. 14. The M vs. H plots indicated the nanomaterials had ferrimagnetic structures with large coercivities. With the increase in ‘V’ content in CoVδFe2−δO4, the values of Ms and Hc were found to alter quite significantly. The variation of the magnetic parameters, i.e. saturation magnetization (Ms) and coercivity (Hc), for the CoVδFe2−δO4 nanomaterials are shown in Fig. 15. Also, the values of Ms, Hc, and Mr, recorded at 5 K and estimated K1, Keff, Ks and squareness ratio (Mr/Ms) for various values of δ in CoVδFe2−δO4 are summarized in Table 4.
 |
| | Fig. 13 Variation in magnetic parameters at room temperature (300 K) for CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) nanomaterials: (a) saturation magnetization (Ms) and (b) coercivity (Hc). | |
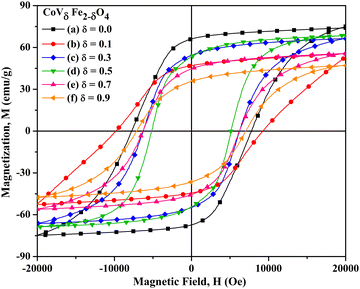 |
| | Fig. 14
M vs. H plots (measured at 5 K) for CoVδFe2−δO4 nanomaterials annealed at 700 °C for different contents of vanadium, δ: (a) 0.0, (b) 0.1, (c) 0.3, (d) 0.5, (e) 0.7 and (f) 0.9. | |
Table 4 Material compositions, Ms, Hc, and Mr recorded at 5 K, squareness ratio (Mr/Ms), magnetocrystalline anisotropic constant (K1), effective magnetic anisotropic constant (Keff) and surface anisotropy (Ks) of CoVδFe2−δO4 nanomaterials
| Material composition, δ (CoVδFe2−δO4) |
Saturation magnetization, Ms (emu g−1) |
Coercivity, Hc (Oe) |
Remanent magnetization, Mr (emu g−1) |
Squareness ratio (Mr/Ms) |
K
1 (J m−3) (K1 × 104) |
K
eff (J m−3) (Keff × 104) |
K
s (J m−2) (Ks × 10−5) |
|
δ = 0.0 |
74.8 |
7772 |
66.2 |
0.885 |
1.4254 |
9.0835 |
66.3702 |
|
δ = 0.1 |
57.5 |
9538 |
46.9 |
0.816 |
1.4882 |
8.5692 |
46.0265 |
|
δ = 0.3 |
67.9 |
6252 |
54.2 |
0.798 |
1.4396 |
6.6329 |
42.41195 |
|
δ = 0.5 |
70.0 |
5150 |
53.4 |
0.763 |
1.4167 |
5.6328 |
33.7288 |
|
δ = 0.7 |
57.3 |
6243 |
44.7 |
0.780 |
1.3276 |
5.5894 |
26.9914 |
|
δ = 0.9 |
48.9 |
7078 |
35.7 |
0.730 |
1.2945 |
5.4080 |
20.5675 |
The Ms values obtained from the fitted data obtained from the plot of (1/H2) vs. magnetization (M) for the CoVδFe2−δO4 nanomaterials varied from 74.8 to 48.9 emu g−1 for δ = 0.0 to δ = 0.9, respectively. The decrease in Ms as a function of the V content ‘δ’ has already been explained in subsection 3.5.1. As can be observed from the M vs. H plots, measured at 5 K, the approach towards saturation of the magnetization was quite flat compared with those recorded at a temperature of 300 K. These results can be rationalized due to superferromagnetic phase transition occurring at lower temperatures. Also, the variation in the values of Hc was observed to vary from 7772 to 7078 Oe for δ = 0.0 to δ = 0.9, respectively. The CoVδFe2−δO4 nanomaterials showed a significant increase in coercivity from 7772 Oe (δ = 0.0) to 9538 Oe (δ = 0.1). This observation of large coercivity may be explained on the basis of the larger magnetocrystalline anisotropy (K1) for CoV0.1Fe1.9O4 (Table 4), and the alterations in the size, shape, and surface effects.63,64 The estimated values of K1 showed a decreasing trend, i.e. from 1.42 × 104 to 1.29 × 104 J m−3 for δ = 0.0 to δ = 0.9, respectively (Table 4). The calculated values of Keff were in the range of 9.08 × 104 to 5.40 × 104 J m−3 for δ = 0.0 to δ = 0.9, respectively. It can be seen from Table 4 that the Ks values varied from 66.37 × 10−5 to 20.56 × 10−5 J m−2 for δ = 0.0 to δ = 0.9, respectively. The decreasing value of the magnetic anisotropy constant as a result of vanadium incorporation supports the observed decreasing trend for Hc. Alterations of the crystallite size and particle morphology in the CoVδFe2−δO4 nanomaterials as a function of the increasing vanadium content can be a major reason for the reduction of Hc. In addition, the nanomaterials synthesized by the current method displayed a superparamagnetic nature at 300 K. At lower temperatures, magnetocrystalline anisotropy dominated over the thermal energy, kT, whereby a large increase in Hc values could be seen with the single domain particles.12 It could also be noted that Mr showed a significant change as the V content increased in the cobalt ferrite lattice, with Mr observed to change from 66.2 to 35.7 emu g−1 for δ = 0.0 to δ = 0.9, respectively. Drastic variations in the values of Mr were observed from 66.2 emu g−1 for δ = 0.0 to 46.9 emu g−1 for δ = 0.1 in the CoVδFe2−δO4 nanomaterials. For the compositions δ = 0.3 and δ = 0.5, there was no significant change observed in the values of Mr, whereas for δ = 0.7 and δ = 0.9, the remanent magnetisation varied from 44.7 to 35.7 emu g−1, respectively. These values of remanent magnetization suggest uniaxial anisotropy of the nanoparticles, which is also strongly depended on the shapes and sizes of the nanomaterials.65 The squareness ratios were all significantly high and varied from 0.885 for δ = 0.0 to 0.730 for δ = 0.9 (Table 4 and Fig. 15).
 |
| | Fig. 15 Plots of the variation in (a) Ms and (b) Hc measured at 5 K for various V contents, δ = 0.0, 0.1, 0.3, 0.5, 0.7 and 0.9 in CoVδFe2−δO4 nanomaterials. | |
Finally, the observed magnetic parameters for one of the compositions of cobalt ferrites (CoV0.1Fe1.9O4) in the present work and those of some literature reported results are compared in Table 5, demonstrating that our system could succeed in getting larger values of coercivities, i.e. 1151 Oe and 9538 Oe at 300 K and 5 K, respectively.
Table 5 Comparison of some reported CoV0.1Fe1.9O4 nanomaterials with respect to their synthesis methods, average crystallite size, saturation magnetization and coercivity measured at room temperature and at low temperature
| Composition of material |
Synthesis method |
Annealing temperature (°C)/time (h) |
Average crystallite size (nm) |
Room temperature (298 K) magnetic properties |
Low temperature (5 K) magnetic properties |
|
M
s (emu g−1) |
H
c (Oe) |
M
s (emu g−1) |
H
c (Oe) |
| CoV0.1Fe1.9O4 (ref. 26) |
Sol–gel auto-combustion |
550 °C/3 h |
27 |
68.30 |
1451 |
— |
— |
| CoV0.1Fe1.9O4 (ref. 27) |
Sol–gel method |
700 °C/1 h |
23 |
69.85 |
1248.2 |
— |
— |
| Co0.9V0.1Fe2O4 (ref. 51) |
Sol–gel method |
800 °C/3 h |
35.8 |
60.6 |
859.7 |
— |
— |
| CoV0.1Fe1.9O4 (this work) |
Sol–gel method |
700 °C/3 h |
34 |
74.5 |
1151 |
57.5 |
9538 |
4. Conclusions
The synthesis of vanadium-substituted cobalt ferrite nanomaterials (CoVδFe2−δO4) via a citric acid-assisted sol–gel autocombustion method was carried out successfully at a relatively low temperature (400 °C). We successfully synthesized nanocrystalline pure-phase products with cubic spinel structures. The observed average values of the crystallite sizes varied from 43 nm (δ = 0.0) to 25 nm (δ = 0.9) for the CoVδFe2−δO4 nanomaterials. The values of lattice strain calculated from WH plots varied from 1.34 × 10−3 to 2.00 × 10−3 for δ = 0.0 to δ = 0.9, respectively. A significant decrease in the lattice parameters was noted from 8.348 Å to 8.285 Å for δ = 0.0 to δ = 0.9, respectively proving the lattice contraction in the materials with the higher content of vanadium in the lattice. These results indicate the successful insertion of V5+ in the spinel ferrite crystal structure. FESEM micrographs confirmed the nature of the spherical-shaped particles with narrow size distributions and average particle sizes varying from 56 nm to 34 nm for δ = 0.0 to δ = 0.9, respectively. The formation of M–O bonds was indicated by the occurrence of bands in the frequency ranges of 589.2–602.9 cm−1 and 409.3–419.5 cm−1 for the tetrahedral and octahedral sites, respectively. Raman spectroscopic studies confirmed the various sites occupancy and degree of inversion. The values of the Raman peak intensity ratios, i.e. A1g(2)/A1g(1) and A1g(2)/T2g(2), vs. vanadium content, δ, in CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) were used to verify the Co2+ occupancy, which can affect degree of inversion. XPS studies confirmed the existence of Fe2+ and V4+ states in the ferrite samples. Further, the existence of the V5+ state and redistribution of Co2+ and Fe3+ ions in the Oh and Td coordination evidenced the cation redistribution as a result of vanadium ion incorporation in a continuous manner. These results correlated with the observed decreasing trend in Ms values in the CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) materials with the increase in vanadium content. The hysteresis curves recorded at 300 K showed a systematic decreasing trend for saturation magnetization (Ms), i.e. from 82.5 to 47.3 emu g−1 for δ = 0.0 to δ = 0.9, respectively. Similarly, the values of Ms measured at 5 K varied from 74.8 to 48.9 emu g−1 for δ = 0.0 to δ = 0.9, respectively. The observed magnetic properties could be explained by the following factors: (i) cation distribution, (ii) substitution of ‘V’, (iii) lattice strain, (iv) shape, size, and surface effects, (vi) canted spin structure, etc. The values of coercivity recorded at 300 K showed a marginal decrease from 1180 to 886 Oe for δ = 0.0 to δ = 0.9, respectively. Also, at a low temperature of 5 K, alterations of Hc were observed, i.e. from 7772 to 7078 Oe for δ = 0.0 to δ = 0.9, respectively. The observation of a large coercivity of 9538 Oe for δ = 0.1 may be due to the occurrence of larger magnetocrystalline anisotropy in the sample. The overall decreasing trend in coercivity of the materials was correlated with the decreasing values of magnetocrystalline anisotropy as a result of the progressive increase in vanadium substitution in the CoFe2O4 materials. Also, there were significant changes in the values of Mr recorded at room temperature, which was observed to vary from 36.3 for δ = 0.0 to 17.1 emu g−1 for δ = 0.9, respectively in CoVδFe2−δO4. A variation in Mr/Ms ratios was observed from 0.4402 for δ = 0.0 up to a maximum value of 0.4501 for δ = 0.5 in CoVδFe2−δO4. Similarly, the values of Mr at 5 K changed from 66.2 for δ = 0.0 to 35.7 emu g−1 for δ = 0.9. The observed variations in the Mr/Ms ratio at 5 K (i.e. from 0.885 for δ = 0.0 to 0.730 for δ = 0.9) were quite significant for the nanomaterials for potential use in data-storage applications.
Author contributions
Anagha B. Patil: conceptualization, data curation, formal analysis, investigation, methodology, writing – original draft and writing – review and editing, Rabi N. Panda: conceptualization, methodology, supervision, writing – review and editing.
Data availability
The data that support the findings of this study are available within the paper and additional data will be made available upon request.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
We are thankful to the Central Sophisticated Instrumentation Facility (CSIF), BITS Pilani KK Birla Goa Campus for the provision of XRD, FESEM and Raman Spectroscopy data procurements. We also thank the Department of Physics, BITS PILANI K K Birla Goa Campus and Department of Science and Technology (DST), Government of India for Department of Science and Technology Funds for Improvement of Science and Technology (DST-FIST) grant number SR/FST/PS-I/2017/21 for PPMS VSM measurements. The authors would also like to acknowledge the central analytical laboratory at BITS Pilani, Hyderabad Campus for XPS measurements. One of the authors, Anagha B. Patil is thankful for the SRF fellowship to BITS Pilani University, Pilani India.
References
- M. Amiri, M. Salavati-Niasari and A. Akbari, Magnetic nanocarriers: Evolution of spinel ferites for medical applications, Adv. Colloid Interface Sci., 2019, 265, 29–44 CrossRef CAS.
- H. Qin, Y. He, P. Xu, D. Huang, Z. Wang, H. Wang, Z. Wang, Y. Zhao, Q. Tian and C. Wang, Spinel ferrites (MFe2O4): Synthesis, improvement and catalytic application in environment and energy field, Adv. Colloid Interface Sci., 2021, 294, 102486 CrossRef CAS PubMed.
- P. Thakur, S. Taneja, D. Chahar, B. Ravelo and A. Thakur, Recent advances on synthesis, characterization and high frequency applications of Ni–Zn ferrite nanoparticles, J. Magn. Magn. Mater., 2021, 530, 167925 CrossRef CAS.
- T. N. Pham, T. Q. Huy and A. T. Le, Spinel ferrite (AFe2O4)-based heterostructured designs for lithium-ion battery, environmental monitoring, and biomedical applications, RSC Adv., 2020, 10, 31622–31661 RSC.
- W. Sharmoukh and S. M. Yakout, New spin-electronics compositions: Large ferromagnetic order of BaSn0.98−xFe0.02CuxO3 (x = 0.02, 0.04, 0.06) semiconductor, J. Alloys Compd., 2024, 993, 174664 CrossRef CAS.
- W. Sharmoukh, T. A. Hameed and S. M. Yakout, New nonmagnetic aliovalent dopants (Li+, Cu2+, In3+ and Ti4+): Optical and strong intrinsic room temperature ferromagnetism of perovskite BaSnO3, J. Alloys Compd., 2022, 925, 166702 CrossRef CAS.
- M. A. Wahba, S. M. Yakout, A. M. Youssef, W. Sharmoukh, A. M. E. Sayed and M. S. Khalil, Chelating Agents Assisted Rapid Synthesis of High Purity BiFeO3: Remarkable Optical, Electrical, and Magnetic Characteristics, J. Supercond. Novel Magn., 2022, 35, 3689–3704 CrossRef CAS.
- T. R. Tatarchuk, N. D. Paliychuk, M. Bououdina, B. Al-Najar, M. Pacia, W. Macyk and A. Shyichuk, Effect of cobalt on structural, elastic, magnetic and optical properties of zinc ferrite nanoparticles, J. Alloys Compd., 2018, 731, 1256–1266 CrossRef CAS.
- S. B. Narang and K. Pubby, Nickel Spinel Ferrites: A review, J. Magn. Magn. Mater., 2021, 519, 167163 CrossRef CAS.
- S. Zare, A. A. Ati, S. Dabagh, R. M. Rosnan and Z. Othaman, Synthesis, structural and magnetic behavior of Zn–Al substituted cobalt ferrite nanoparticles, J. Mol. Struct., 2015, 1089, 25–31 CrossRef CAS.
- G. S. Kumar, T. Raguram and K. S. Rajni, Synthesis and Characterization of Nickel-Substituted Cobalt Ferrite Nanoparticles Using Sol–Gel Auto-combustion Method, J. Supercond. Novel Magn., 2019, 32, 1715–1723 CrossRef CAS.
- S. I. Ahmad, Nano cobalt ferrites: Doping, Structural, Low-temperature, and room temperature magnetic and dielectric properties – A comprehensive review, J. Magn. Magn. Mater., 2022, 562, 169840 CrossRef CAS.
- L. Ajroudi, N. Mliki, L. Bessais, V. Madigou, S. Villain and C. Leroux, Magnetic, electric and thermal properties of cobalt ferrite nanoparticles, Mater. Res. Bull., 2014, 59, 49–58 CrossRef CAS.
- P. Thakur, N. Gahlawat, P. Punia, S. Kharbanda, B. Ravelo and A. Tahkur, Cobalt Nanoferrites: a Review on Synthesis, Characterization, and Applications, J. Supercond. Novel Magn., 2022, 35, 2639–2669 CrossRef CAS.
- S. Jauhar, J. Kaur, A. Goyal and S. Singhal, Tuning the properties of cobalt ferrite: a road towards diverse applications, RSC Adv., 2016, 6, 97694–97719 RSC.
- K. V. Chandekar and K. M. Kant, Strain induced magnetic anisotropy and 3d7 ions effect in CoFe2O4 nanoplatelets, Superlattices Microstruct., 2017, 111, 610–627 CrossRef CAS.
- S. Rasheed, R. A. Khan, F. Shah, B. Ismail, J. Nisar, S. M. Shah, A. Rahim and A. R. Khan, Enhancement of electrical and magnetic properties of cobalt ferrite nanoparticles by co-substitution of Li–Cd ions, J. Magn. Magn. Mater., 2019, 471, 236–241 CrossRef CAS.
- K. V. Chandekar and K. M. Kant, Estimation of the spin–spin relaxation time of surfactant coated CoFe2O4 nanoparticles by electron paramagnetic resonance spectroscopy, Phys. E, 2018, 104, 192–205 CrossRef CAS.
- A. Milutinović, Z. Ž. Lazarević, M. Šuljagić and L. Andjelković, Synthesis-Dependent Structural and Magnetic Properties of Monodomain Cobalt Ferrite Nanoparticles, Metals, 2024, 14, 833 CrossRef.
- S. Fiaz, M. Naeem Ahmed, I. Ul Haq, S. W. Ali Shah and M. Waseem, Green synthesis of cobalt ferrite and Mn doped cobalt ferrite nanoparticles: Anticancer, antidiabetic and antibacterial studies, J. Trace Elem. Med. Biol., 2023, 80, 127292 CrossRef CAS.
- B. G. Toksha, S. E. Shrisath, M. L. Mane, S. M. Patange, S. S. Jadhav and K. M. Jadhav, Autocombustion high- temperature synthesis, structural, and magnetic properties of CoCrxFe2−xO4 (0 ≤ x ≤ 1.0), J. Phys. Chem. C, 2011, 115, 20905–20912 CrossRef CAS.
- T. Zeeshan, S. Waseem, Z. Ejaz, Z. Kayani and T. E. Kuntsevich, Study of electrical conductance and dielectric properties of vanadium doped cobalt ferrites for high frequency applications, Inorg. Chem. Commun., 2024, 162, 111687 CrossRef CAS.
- S. Gaffar, A. Kumar and U. Riaz, Synthesis techniques and advanced applications of spinel ferrites: A short review, J. Electroceram., 2023, 51, 246–257 CrossRef CAS.
- M. Albino, E. Fantechi, C. Innocenti, A. L. Ortega, V. Bonanni, G. Campo, F. Pineider, M. Gurioli, P. Arosio, T. Orlando, G. Bertoni, C. de, J. Fernandez, A. Lascialfari and C. Sangregorio, Role of Zn2+ Substitution on the Magnetic, Hyperthermic, and Relaxometric Properties of Cobalt Ferrite Nanoparticles, J. Phys. Chem. C, 2019, 123, 6148–6157 CrossRef CAS.
- F. Sharifianjazi, M. Moradi, N. Parvin, A. Nemati, A. J. Rad, N. Sheysi, A. Abouchenari, A. Mohammadi, S. Karbasi, Z. Ahmadi, A. Esmaeilkhanian, M. Irani, A. Pakseresht, S. Sahmani and M. S. Asl, Magnetic CoFe2O4 nanoparticles doped with metal ions: A review, Ceram. Int., 2020, 46, 18391–18412 CrossRef CAS.
- A. B. Patil and R. N. Panda, Synthesis, characterizations and magnetic properties of nanoscale CoVxFe2−xO4 (0.0 ≤ x ≤ 0.9) materials synthesized via sol–gel autocombustion route, Mater. Chem. Phys., 2023, 307, 128215 CrossRef CAS.
- Z. K. Heiba, M. B. Mohamed and S. I. Ahmed, Cation distribution with correlated with magnetic properties of cobalt ferrite nanoparticles defective by vanadium doping, J. Magn. Magn. Mater., 2017, 441, 409–416 CrossRef CAS.
- T. Huang, Z. Qiu, Z. Hu and X. Lu, Novel method of preparing hierarchical porous CoFe2O4 by the citric acid-assisted sol–gel auto-combustion for supercapacitors, J. Energy Storage, 2021, 35, 102286 CrossRef.
- S. Upadhyay, K. Parekh and B. Pandey, Influence of crystallite size on the magnetic properties of Fe3O4 Nanoparticles, J. Alloys Compd., 2016, 678, 478–485 CrossRef CAS.
- K. V. Chandekar and K. M. Kant, Size-strain analysis and elastic properties of CoFe2O4 nanoplatlets by hydrothermal method, J. Mol. Struct., 2018, 1154, 418–427 CrossRef CAS.
- H. Irfan, R. K. Mohamed and S. Anand, Microstructural evaluation of CoAl2O4 nanoparticles by Williamson-Hall and size-strain plot methods, J. Asian Ceram. Soc., 2018, 6, 54–62 CrossRef.
- M. M. L. Sonia, S. Anand, V. M. Vinosel, M. A. Janifer, S. Pauline and A. Manikanda, Effect of lattice strain on structure, morphology and magneto-dielectric properties of spinel NiGdxFe2−xO4 ferrite nano-crystallites synthesized by sol–gel route, J. Magn. Magn. Mater., 2018, 466, 238–251 CrossRef CAS.
- G. Channagoudra, J. P. J. Nunez, R. L. Hadimani and V. Dayal, Study of cation distribution in La3+ and Eu3+ substituted cobalt ferrite and its effect on magnetic properties, J. Magn. Magn. Mater., 2022, 559, 169550 CrossRef CAS.
- W. Wenwei, C. Jinchao, W. Xuehang, L. Sen and H. Aigui, Co0.35Mn0.65Fe2O4 magnetic particles: Preparation and kinetics research of thermal process of the precursor, Powder Technol., 2012, 215–216, 200–205 CrossRef.
- P. V. V. Romanholo, T. E. P. Alves, J. Swapnalin, P. Banerjee and A. Franco, Tailoring the magnetic properties of Zn doped nickel, magnesium and cobalt ferrite ceramics, Mater. Chem. Phys., 2022, 284, 126072 CrossRef CAS.
- E. Puscasu, L. Sacarescu, L. Popescu-Lipan, V. Nica, M. Grigoras, A. Domocos, N. Lupu and D. Creanga, Study on the effect of some surface phenomena on the properties of citrate capped cobalt doped ferrites, Appl. Surf. Sci., 2019, 483, 1182–1191 CrossRef CAS.
- S. A. Al-Zahrani, A. Manikandan, K. Thanrasu, A. Dinesh, K. K. Raja, M. A. Almessiere, Y. Slimani, A. Baykal, S. Bhuminathan, S. Raghavendra Jayesh, J. Ahmed, H. S. Alorfi, M. A. Hussein, I. Khan and A. Khan, Influence of Ce3+ on the Structural, Morphological, Magnetic, Photocatalytic and Antibacterial Properties of Spinel MnFe2O4 Nanocrystallites Prepared by the Combustion Route, Crystals, 2022, 12, 268 CrossRef CAS.
- G. Sharada, N. P. Kumar, P. Sowjanya and D. Sreenivasu, Low concentration doping effects of holmium on structural and spectroscopic properties of cobalt ferrite, Mater. Today: Proc., 2023, 92, 440–444 CAS.
- R. S. Yadav, I. Kuřitka, J. Vilcakova, J. Havlica, J. Masilko, L. Kalina, J. Tkacz, J. Švec, V. Enev and M. Hajdúchová, Impact of grain size and structural changes on magnetic, dielectric, electrical, impedance and modulus spectroscopic characteristics of CoFe2O4 nanoparticles synthesized by honey mediated sol–gel combustion method, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2017, 8, 045002 Search PubMed.
- P. Chandramohan, M. P. Srinivasan, S. Velmurugan and S. V. Narasimhan, Cation distribution and particle size effect on Raman spectrum of CoFe2O4, J. Solid State Chem., 2011, 184, 89–96 CrossRef CAS.
- V. D’Ippolito, G. B. Andreozzi, D. Bersani and P. P. Lottici, Raman fingerprint of chromate, aluminate and ferrite spinels, J. Raman Spectrosc., 2015, 46, 1255–1264 CrossRef.
- L. E. Caldeira, C. S. Erhardt, F. R. Mariosi, J. Venturini, R. Y. S. Zampiva, O. R. K. Montedo, S. Arcaro, C. P. Bergmann and S. R. Bragança, Correlation of synthesis parameters to the structural and magnetic properties of spinel cobalt ferrites (CoFe2O4) – an experimental and statistical study, J. Magn. Magn. Mater., 2022, 550, 169128 CrossRef.
- N. Liu, P. Du, P. Zhou, R. G. Tanguturi, Y. Qi, T. Zhang and C. Zhuang, Annealing temperature effects on the cation distribution in CoFe2O4 nanofibers, Appl. Surf. Sci., 2020, 532, 147440 CrossRef CAS.
- P. N. Anantharamaiah and P. A. Joy, Effect of co-substitution of Co2+ and V5+ for Fe3+ on the magnetic properties of CoFe2O4, Phys. B, 2019, 554, 107–113 CrossRef CAS.
- C. N. Chervin, J. S. Ko, B. W. Miller, L. Dudek, A. N. Mansour, M. D. Donakowski, T. Brintlinger, P. Gogotsi, S. Chattopadhyay, T. Shibata, J. F. Parker, B. P. Hahn, D. R. Rolison and J. W. Long, Defective by design: vanadium-substituted iron oxide nanoarchitectures as cation-insertion hosts for electrochemical charge storage, J. Mater. Chem. A, 2015, 3, 12059–12068 RSC.
- G. F. Dionne, A Review of Ferrites for Microwave Applications, Proc. IEEE, 1975, 63, 777–789 CAS.
- H. Ghorbani, M. Eshraghi and A. A. S. Dodaran, Structural and magnetic properties of cobalt ferrite nanoparticles doped with cadmium, Phys. B, 2022, 634, 413816 CrossRef CAS.
- S. B. Dalavi, P. P. Mishra, T. Cherian, M. M. Raja and R. N. Panda, Magnetic and Mössbauer Studies on Nanostructured CoCrxFe2−xO4 (0 ≤ x ≤ 1) Spinel Ferrites Prepared by Sol–Gel Auto Combustion Method, J. Nanosci. Nanotechnol., 2020, 20, 983–990 CrossRef CAS.
- K. Bouferrache, Z. Charifi, H. Baaziz, G. Uğur, Ş. Uğur, B. Boyacıoğlu and H. Ünver, Cation distribution effect on electronic, magnetic structure and optic properties in cobalt ferrites (Co1−yFey)Tet (CoyFe2−y)Oct O4 with disordered spinel structure, Phys. Scr., 2020, 95, 105801 CrossRef CAS.
- M. George, S. S. Nair, K. A. Malini, P. A. Joy and M. R. Anantharaman, Finite size effects on the electrical properties of sol–gel synthesized CoFe2O4 powders: deviation from Maxwell–Wagner theory and evidence of surface polarization effects, J. Phys. D: Appl. Phys., 2007, 40, 1593–1602 CrossRef CAS.
- P. Imanipour, S. Hasani, A. Seifoddini, A. Farnia, F. Karimabadi, K. Jahanbani-Ardakani and F. Davar, The possibility of vanadium substitution on Co lattice sites in CoFe2O4 synthesized by sol–gel autocombustion method, J. Sol-Gel Sci. Technol., 2020, 95, 157–167 CrossRef CAS.
- R. S. Yadav, J. Havlica, J. Masilko, L. Kalina, J. Wasserbauer, M. Hajdúchová, V. Enev, I. Kuřitka and Z. Kožáková, Impact of Nd3+ in CoFe2O4 spinel ferrite nanoparticles on cation distribution, structural and magnetic properties, J. Magn. Magn. Mater., 2016, 399, 109–117 CrossRef CAS.
- Y. Yafet and C. Kittel, Antiferromagnetic Arrangements in Ferrites, Phys. Rev., 1952, 87, 290–294 CrossRef CAS.
- V. Vaithyanathan, K. Ugendar, J. A. Chelvane, K. K. Bharathi and S. S. R. Inbanathan, Structural and magnetic properties of Sn and Ti doped Co ferrite, J. Magn. Magn. Mater., 2015, 382, 88–92 CrossRef CAS.
- D. M. Ghone, V. L. Mathe, K. K. Patankar and S. D. Kaushik, Microstructure, lattice strain, magnetic and magnetostriction properties of holmium substituted cobalt ferrites obtained by co-precipitation method, J. Alloys Compd., 2018, 739, 52–61 CrossRef CAS.
- R. S. Ningthoujam, R. N. Panda and N. S. Gajbhiye, Variation of intrinsic magnetic parameters of single domain Co–N interstitial nitrides synthesized via hexa-ammine cobalt nitrate route, Mater. Chem. Phys., 2012, 134, 377–381 CrossRef CAS.
- X. Wu, J. Xu, X. Huo, J. Chen, Q. Zhang, F. Huang, Y. Lia, H. Su and L. Li, Nb2O5-doped NiZnCo ferrite ceramics with ultra-high magnetic quality factor and low coercivity for high-frequency electronic devices, J. Eur. Ceram. Soc., 2021, 41, 5193–5200 CrossRef CAS.
- T. Zhou, H. Zhang, C. Liu, L. Jin, F. Xu, Y. Liao, N. Jia, Y. Wang, G. Gan, H. Su and L. Jia, Li2O–B2O3–SiO2–CaO–Al2O3 and Bi2O3 co-doped gyromagnetic Li0.43Zn0.27Ti0.13Fe2.17O4 ferrite ceramics for LTCC Technology, Ceram. Int., 2016, 42, 16198–16204 CrossRef CAS.
- K. V. Chandekar and K. M. Kant, Relaxation phenomenon and relaxivity of cetrimonium bromide (CTAB) coated CoFe2O4 nanoplatelets, Phys. B, 2018, 545, 536–548 CrossRef CAS.
- K. V. Chandekar and S. P. Yadav, Comprehensive study of MFe2O4 (M = Co, Ni, Zn) nanostructures prepared by co-precipitation route, J. Alloys Compd., 2023, 960, 170838 CrossRef CAS.
- F. Bødker, S. Mørup and S. Linderoth, Surface effects in metallic iron nanoparticles, Phys. Rev. Lett., 1994, 72, 282–285 CrossRef.
- A. B. Patil and R. N. Panda, Magnetic Properties of CoNbyFe2−yO4 (0.00 ≤ y ≤ 0.08) Nanomaterials Synthesized via Modified Sol–gel Autocombustion Route, J. Supercond. Novel Magn., 2024, 37, 597–608 CrossRef CAS.
- D. Tomar and P. Jeevanandam, Synthesis of cobalt ferrite nanoparticles with different morphologies via thermal decomposition approach and studies on their magnetic properties, J. Alloys Compd., 2020, 843, 15515 CrossRef.
- K. V. Chandekar and K. M. Kant, Effect of size and shape dependent anisotropy on superparamagnetic property of CoFe2O4 nanoparticles and nanoplatelets, Phys. B, 2017, 520, 152–163 CrossRef CAS.
- H. Ghorbani, M. Eshraghi, A. A. S. Dodaran, P. Kameli, S. Protasowicki, C. Johnson and D. Vashaee, Effect of Yb doping on the structural and magnetic properties of cobalt ferrite nanoparticles, Mater. Res. Bull., 2022, 147, 111642 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *
*
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ)), where D is the average crystallite size, λ is the wavelength of the X-rays, β is the full width at half maximum expressed in radian (FWHM), and θ is the diffraction angle in radian.28 Also, Williamson–Hall (WH) plots (i.e. β
θ)), where D is the average crystallite size, λ is the wavelength of the X-rays, β is the full width at half maximum expressed in radian (FWHM), and θ is the diffraction angle in radian.28 Also, Williamson–Hall (WH) plots (i.e. β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(θ) vs. 4
cos(θ) vs. 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin(θ)) were used to determine the lattice strain and crystallite size of the materials. In particular, the WH plots were linearly fitted in order to obtain the slope and Y-intercept values, from which the lattice strain (ε) and crystallite sizes were estimated for the CoVδFe2−δO4 lattice, respectively. To be noted, the Williamson–Hall plot is based on the expression, [(β
sin(θ)) were used to determine the lattice strain and crystallite size of the materials. In particular, the WH plots were linearly fitted in order to obtain the slope and Y-intercept values, from which the lattice strain (ε) and crystallite sizes were estimated for the CoVδFe2−δO4 lattice, respectively. To be noted, the Williamson–Hall plot is based on the expression, [(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) = (0.9λ/D) + (ε4
θ) = (0.9λ/D) + (ε4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ)], where ε represents the lattice strain, θ is the diffraction angle, β is full width at half maximum (FWHM) expressed in radian, D is the crystallite size, and λ is the wavelength of X-ray radiation.29 The FESEM images were analysed for obtaining the morphology and particle sizes of the CoVδFe2−δO4 nanomaterials under study. The average particle sizes were estimated using ImageJ software with the maximum number of particles distributed throughout the FESEM image. EDS was carried out for confirming the presence of V, Fe, Co, and O in CoVδFe2−δO4 materials. The elemental compositions were also investigated through the EDS spectra and were in good agreement with the stoichiometry of the materials. For the FTIR studies, the samples were ground with solid KBr powder (in an approximately 1
θ)], where ε represents the lattice strain, θ is the diffraction angle, β is full width at half maximum (FWHM) expressed in radian, D is the crystallite size, and λ is the wavelength of X-ray radiation.29 The FESEM images were analysed for obtaining the morphology and particle sizes of the CoVδFe2−δO4 nanomaterials under study. The average particle sizes were estimated using ImageJ software with the maximum number of particles distributed throughout the FESEM image. EDS was carried out for confirming the presence of V, Fe, Co, and O in CoVδFe2−δO4 materials. The elemental compositions were also investigated through the EDS spectra and were in good agreement with the stoichiometry of the materials. For the FTIR studies, the samples were ground with solid KBr powder (in an approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 ratio) and formed into transparent discs. The powdered CoVδFe2−δO4 samples were used directly for the Raman spectroscopic measurements. For the magnetic properties studies, vibrating sample magnetometry (VSM) was used (Quantum Design, PPMS Evercool II) and the measurements were carried out at room temperature (300 K) and at low temperature (5 K) using a maximum field strength of 2T.
20 ratio) and formed into transparent discs. The powdered CoVδFe2−δO4 samples were used directly for the Raman spectroscopic measurements. For the magnetic properties studies, vibrating sample magnetometry (VSM) was used (Quantum Design, PPMS Evercool II) and the measurements were carried out at room temperature (300 K) and at low temperature (5 K) using a maximum field strength of 2T.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) cos(θ) vs. 4
cos(θ) vs. 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin(θ) were plotted and fitted with linear fit functions. The fitted values of the slope and intercept represent the lattice strain ‘ε’ and β
sin(θ) were plotted and fitted with linear fit functions. The fitted values of the slope and intercept represent the lattice strain ‘ε’ and β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(θ). The crystallite size (D) (in nm) was calculated using the formula D = (0.9λ)/(β
cos(θ). The crystallite size (D) (in nm) was calculated using the formula D = (0.9λ)/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(θ)). The simulated values of the positive lattice strains and the crystallite sizes obtained from the WH plots are provided in Table 1. There was a significant increase in the lattice strain from 1.34 × 10−3 for pure CoFe2O4 to 2.00 × 10−3 for CoV0.9Fe1.1O4. Such a kind of increasing trend in the values of lattice strains as a result of the progressive increase in large-sized dopant atoms in the spinel crystal structure has previously been reported in earlier studies.30,32 This shows the presence of tensile force in the CoVδFe2−δO4 lattice. It was observed that the crystallite sizes calculated with Scherrer's formula were relatively smaller compared to those calculated from the WH plots. These results can be rationalized as follows: Scherrer's method neglects the microstrain in the lattice that may originate from imperfections in the structure, whereas in the WH plot method, there is a separation of the instrumental and sample broadening effects in the calculation.
cos(θ)). The simulated values of the positive lattice strains and the crystallite sizes obtained from the WH plots are provided in Table 1. There was a significant increase in the lattice strain from 1.34 × 10−3 for pure CoFe2O4 to 2.00 × 10−3 for CoV0.9Fe1.1O4. Such a kind of increasing trend in the values of lattice strains as a result of the progressive increase in large-sized dopant atoms in the spinel crystal structure has previously been reported in earlier studies.30,32 This shows the presence of tensile force in the CoVδFe2−δO4 lattice. It was observed that the crystallite sizes calculated with Scherrer's formula were relatively smaller compared to those calculated from the WH plots. These results can be rationalized as follows: Scherrer's method neglects the microstrain in the lattice that may originate from imperfections in the structure, whereas in the WH plot method, there is a separation of the instrumental and sample broadening effects in the calculation.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) group of citrate complexes. Also, the weak bands observed at 1545–1550 cm−1 and 1639–1641 cm−1 were assigned to M–(COO)-organic linkages.21,36 The medium to strong IR band located near 2346 cm−1 was assigned due to the presence of adsorbed CO2 and M–O–O-organic linkages.16 The IR bands observed at nearly 3445 cm−1 in almost all the compositions were related to possible O–H stretching of the adsorbed H2O (l).21,37
O) group of citrate complexes. Also, the weak bands observed at 1545–1550 cm−1 and 1639–1641 cm−1 were assigned to M–(COO)-organic linkages.21,36 The medium to strong IR band located near 2346 cm−1 was assigned due to the presence of adsorbed CO2 and M–O–O-organic linkages.16 The IR bands observed at nearly 3445 cm−1 in almost all the compositions were related to possible O–H stretching of the adsorbed H2O (l).21,37



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for CoFe2O4, respectively. For the CoV0.5Fe1.5O4 materials, peak positions of 780.4 and 779.32 eV (with an intensity ratio of ≈5.3
1) for CoFe2O4, respectively. For the CoV0.5Fe1.5O4 materials, peak positions of 780.4 and 779.32 eV (with an intensity ratio of ≈5.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were observed for Co2+ ions in tetrahedral (Td) and octahedral (Oh) environments, respectively. These results indicate the increase in Co2+ ion concentrations in the tetrahedral environment as a result of vanadium ion incorporation in the CoFe2O4 material. In a similar manner, Fig. 11(b) shows distinct peaks corresponding to Fe3+ ions in Td and Oh environments at 711.2 and 709.8 eV (with an intensity ratio of ≈1
1) were observed for Co2+ ions in tetrahedral (Td) and octahedral (Oh) environments, respectively. These results indicate the increase in Co2+ ion concentrations in the tetrahedral environment as a result of vanadium ion incorporation in the CoFe2O4 material. In a similar manner, Fig. 11(b) shows distinct peaks corresponding to Fe3+ ions in Td and Oh environments at 711.2 and 709.8 eV (with an intensity ratio of ≈1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) for CoFe2O4, respectively. For the CoV0.5Fe1.5O4 materials, peaks were observed located at 712.4 and 709.5 eV (with an intensity ratio of ≈0.8
2) for CoFe2O4, respectively. For the CoV0.5Fe1.5O4 materials, peaks were observed located at 712.4 and 709.5 eV (with an intensity ratio of ≈0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for Fe3+ ions in Td and Oh environments, respectively.39,44 In addition, we observed a small intensity peak at 707 eV for the vanadium-substituted product, which was attributed to the occurrence of the Fe2+ state in the material. The above results indicate the increase in Fe3+ concentration in the tetrahedral coordination. In the O1s spectrum, peaks corresponding to lattice oxygen and molecular oxygen were observed at binding energy values of 529.9 and 532.3 eV, respectively (Fig. 11(c)). The observation of two distinct peaks at binding energy values of 516.1 and 517.7 eV corresponded to V4+ and V5+ states, respectively.45 The existence of the V4+ state in the materials along with V5+ and the redistribution of Co2+ and Fe3+ ions in the octahedral and tetrahedral coordination confirmed the redistribution of cations as a result of vanadium incorporation.
1) for Fe3+ ions in Td and Oh environments, respectively.39,44 In addition, we observed a small intensity peak at 707 eV for the vanadium-substituted product, which was attributed to the occurrence of the Fe2+ state in the material. The above results indicate the increase in Fe3+ concentration in the tetrahedral coordination. In the O1s spectrum, peaks corresponding to lattice oxygen and molecular oxygen were observed at binding energy values of 529.9 and 532.3 eV, respectively (Fig. 11(c)). The observation of two distinct peaks at binding energy values of 516.1 and 517.7 eV corresponded to V4+ and V5+ states, respectively.45 The existence of the V4+ state in the materials along with V5+ and the redistribution of Co2+ and Fe3+ ions in the octahedral and tetrahedral coordination confirmed the redistribution of cations as a result of vanadium incorporation.


 , where Ms and the ‘b’ factor were obtained from linear fitting of the M vs. (1/H2) plot, which followed the expression
, where Ms and the ‘b’ factor were obtained from linear fitting of the M vs. (1/H2) plot, which followed the expression  .59,61 The values of K1 showed a decreasing trend, i.e. from 1.44 × 104 to 1.19 × 104 J m−3 for δ = 0.0 to δ = 0.9, respectively (Table 3). The values of Keff were estimated using the expression
.59,61 The values of K1 showed a decreasing trend, i.e. from 1.44 × 104 to 1.19 × 104 J m−3 for δ = 0.0 to δ = 0.9, respectively (Table 3). The values of Keff were estimated using the expression 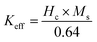 (Table 3). The calculated values of Keff were in the range of 1.521 × 104 to 6.548 × 103 J m−3 for δ = 0.0 to δ = 0.9, respectively. Similarly, the values of Ks were obtained using the expression
(Table 3). The calculated values of Keff were in the range of 1.521 × 104 to 6.548 × 103 J m−3 for δ = 0.0 to δ = 0.9, respectively. Similarly, the values of Ks were obtained using the expression 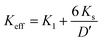 where D′ is the diameter of the crystallite (Table 3). It is clear from Table 3 that the Ks values varied significantly from 70.2 × 10−3 to 12.35 × 10−3 J m−2 for δ = 0.0 to δ = 0.1, respectively. The Ks values were found to be negative for δ = 0.3 to δ = 0.9, indicating different stress directions with respect to the magnetization axis. The squareness ratio increased initially from 0.4402 for δ = 0.0 until it reached a maximum value of 0.4501 for δ = 0.5, and thereafter showed a decreasing trend down to 0.3624 for δ = 0.9. For the CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) nanomaterials, the Mr/Ms values were found to be in the range of 0.4501–0.3624 (Table 3). These results can be explained by the possible existence of a surface spin canting effect as a consequence of the nanoparticle size and surface effects.62
where D′ is the diameter of the crystallite (Table 3). It is clear from Table 3 that the Ks values varied significantly from 70.2 × 10−3 to 12.35 × 10−3 J m−2 for δ = 0.0 to δ = 0.1, respectively. The Ks values were found to be negative for δ = 0.3 to δ = 0.9, indicating different stress directions with respect to the magnetization axis. The squareness ratio increased initially from 0.4402 for δ = 0.0 until it reached a maximum value of 0.4501 for δ = 0.5, and thereafter showed a decreasing trend down to 0.3624 for δ = 0.9. For the CoVδFe2−δO4 (0.0 ≤ δ ≤ 0.9) nanomaterials, the Mr/Ms values were found to be in the range of 0.4501–0.3624 (Table 3). These results can be explained by the possible existence of a surface spin canting effect as a consequence of the nanoparticle size and surface effects.62







