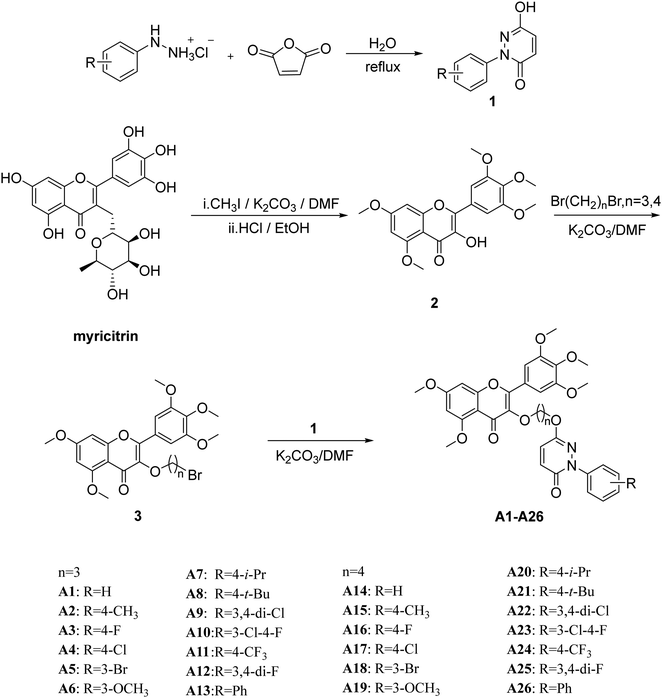
Design, synthesis, and antiviral activities of myricetin derivatives containing pyridazinone†
Li
Xing
a,
Youshan
An
a,
Yishan
Qin
a,
Hui
Xin
a,
Tianyu
Deng
a,
Kaini
Meng
a,
Da
Liu
*b and
Wei
Xue
 *a
*a
aNational Key Laboratory of Green Pesticide, Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Center for R&D of Fine Chemicals of Guizhou University, Guiyang, 550025, China. E-mail: wxue@gzu.edu.cn; Fax: +86-0851-88292090; Tel: +86-0851-88292090
bHunan Engineering Laboratory for Preparation Technology of Polyvinyl Alcohol (PVA) Fiber Material, Huaihua University, Huaihua, Hunan 418008, China. E-mail: liuda@hhtc.edu.cn
First published on 1st December 2023
Abstract
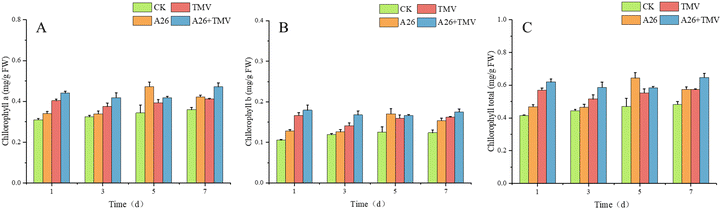
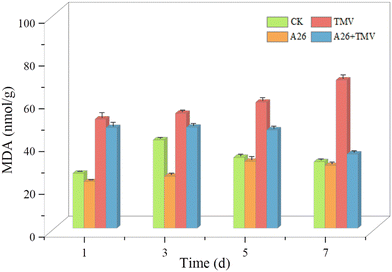
26 derivatives of myricetin containing pyridazinone were designed and synthesized from the natural product myricitrin, which were structurally characterized by nuclear magnetic resonance (NMR) spectroscopy and high-resolution mass spectrometry (HRMS), and the structure of A14 was further determined using an X-ray single crystal diffractometer. The in vivo anti-tobacco mosaic virus (TMV) activity was tested by the half-leaf method, and the results showed that A4, A23 and A26 possessed better curative activity with effective concentration 50% (EC50) of 131.6, 138.5 and 118.9 μg mL−1, which were superior to that of ningnanmycin (NNM) (235.6 μg mL−1), respectively. A24 and A26 possessed better protective activity with EC50 values of 117.4 and 162.5 μg mL−1, superior to that of NNM (263.2 μg mL−1). Microcalorimetric thermophoresis (MST) and molecular docking assays showed that A23 and A26 had strong binding ability with TMV-CP. After treatment with A26, the chlorophyll content in tobacco was significantly higher than that in the control group, and the growth rate of malondialdehyde content was slowed down. This further confirmed that the drug molecules have good antiviral activity.
1. Introduction
Plant virus diseases have always been a major threat to agriculture, where crop infection with viruses leads to shortened lifespan and reduced yields, causing huge yield losses worldwide. Among them, tobacco mosaic virus (TMV) has caused the most serious yield losses and economic losses.1,2 TMV was the first virus to be discovered, and being the first among the top ten plant viruses,3 it is present stably in high concentrations in infected plants.4,5 It mainly infects not only tobacco plants, but also a variety of lycopene plants such as tomato and pepper.6 Characterized by a wide host range, good persistent stability, high resistance, and natural plant inoculation, the virus infects plants and affects the photosynthetic system of the host plant, causes defects such as greenish discoloration.7 In addition, once the plant is infected, the virus destroys the cellular structure, deprives it of nutrients and affects its normal physiological conditions. It usually shows retarded development, leaf curling, necrosis, wilting and other developmental abnormalities, which can lead to plant death in severe cases.8,9 The main defense at present is the use of plant virus suppression pesticides; however, drugs designed to suppress viruses may also cause damage to the host, which makes the control of plant virus diseases difficult.10 In addition, the long-term use of pesticides can lead to the development of plant resistance and residues of pesticides and their metabolites, which can also have adverse effects on human health and the ecological environment.11–13 Therefore, it is important to explore the development of new, efficient and low-toxicity green antiviral agents for the control of TMV.Plant-derived pesticides have great potential for development as antiviral drugs because they are environmentally friendly, efficient and safe, and not easy to develop drug resistance.14 As one of the plant-derived natural products, myricetin is a flavonoid compound from a wide range of sources and is found in plants such as onion, red wine,15 tea, vegetables,16 raspberry,17etc. It possesses a variety of biological activities such as antimicrobial, antioxidant, antimutagenic, anticarcinogenic, anti-inflammatory, antiallergic, analgesic activities and so on,18,19 and thus has attracted a great deal of attention from researchers. In the previous studies of our group, the derivatives of myricetin were found to possess a variety of biological activities such as anticancer,20 antiviral,21–23 antibacterial,24–26 and antifungal activities,27,28 which provides a new idea for the discovery of new green pesticides.
Pyridazinones are an important class of nitrogen-containing small molecules that have been widely studied and applied in the fields of pesticides, pharmaceuticals, and chemical dyes due to the diversity of their biological activities. Pyridazinones exhibit biocidal, insecticidal, acaricidal, herbicidal, and plant growth regulating activities in the field of pesticides,29–31 and anticancer,32,33 antibacterial,34 antiviral,35 hypoglycemic,36 anti-inflammatory,37,38 and antipsychotic39 activities in the field of pharmaceuticals. Therefore, research on pyridazinones has been of great interest. Some of the pesticides containing pyridazinone are listed in Fig. 1.
Based on this, a low-toxicity and highly efficient derivative of myricetin was expected to be obtained through the principle of active splicing, using myricetin as the lead compound and introducing pyridazinone active small molecules. To the best of our knowledge, this is the first report on the synthesis and evaluation of the antiviral activity of myricetin derivatives containing a fraction of pyridazinone.
2. Methods and materials
2.1 Chemicals and instruments
Melting points were determined using a binocular micro melting point tester, an X-4B instrument (Shanghai INESA Co., Ltd), which was not calibrated. The structural characterization of the target compounds was obtained using ASCEND400 (Bruker, Germany) and JEOL-ECX500 (Tokyo, Japan) proton NMR spectrometers using CDCl3 as the solvent and TMS as the internal standard. High resolution spectra were obtained using the Thermo scientific Q Exactive mass spectrometer (Missour, USA). Crystal data were obtained using the Bruker D8-QUEST diffractometer (Bruker, Germany). Microscale thermophoresis was performed using a NanoTemp Monolith NT.115 microscale thermophoresis instrument (NanoTemper, Germany). The MDA content assay kit was purchased from Solarbio, and the OD values were determined using a Gen5 enzyme labeling instrument (Bio-Tek, Germany). The reagents used in the experiments were analytically pure and did not require further purification and drying.2.2 Chemical compound synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 1
ethyl acetate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v) to obtain the target compounds. The specific synthetic route is detailed in Scheme 1.
4, v/v) to obtain the target compounds. The specific synthetic route is detailed in Scheme 1.
6-(3-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)propoxy)-2-phenylpyridazin-3(2H)-one (A1). White solid (56%), m.p.: 114.9–116.3 °C. 1H NMR (400 MHz, chloroform-d) δ 7.63 (d, J = 7.6 Hz, 2H, Ph–H), 7.44 (t, J = 7.9 Hz, 2H, Ph–H), 7.34 (t, J = 7.4 Hz, 1H, Ph–H), 7.31 (s, 2H, Ph–H), 6.98 (d, J = 9.7 Hz, 1H, Ph–H), 6.92 (d, J = 9.7 Hz, 1H, Ph–H), 6.49 (d, J = 2.2 Hz, 1H, Ph–H), 6.36 (d, J = 2.3 Hz, 1H, Ph–H), 4.31 (t, J = 6.3 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 4.16 (t, J = 6.3 Hz, 2H, –O–CH2CH2
CH2CH2–O–), 4.16 (t, J = 6.3 Hz, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.96 (s, 3H, Ph–OCH3), 3.90 (d, J = 4.4 Hz, 6H, Ph–OCH3), 3.86 (s, 6H, Ph–OCH3), 2.18 (p, J = 6.3 Hz, 2H, –O–CH2
–O–), 3.96 (s, 3H, Ph–OCH3), 3.90 (d, J = 4.4 Hz, 6H, Ph–OCH3), 3.86 (s, 6H, Ph–OCH3), 2.18 (p, J = 6.3 Hz, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 173.98, 164.08, 161.04, 158.83, 152.99, 152.70, 141.58, 140.61, 139.92, 133.98, 128.69, 127.67, 126.86, 125.99, 125.06, 109.41, 105.84, 95.91, 92.46, 69.19, 64.24, 61.04, 56.49, 56.29, 55.90, 29.74. HRMS (ESI) calcd for C33H32N2O10 [M + H]+: 617.21297, found 617.21295.
CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 173.98, 164.08, 161.04, 158.83, 152.99, 152.70, 141.58, 140.61, 139.92, 133.98, 128.69, 127.67, 126.86, 125.99, 125.06, 109.41, 105.84, 95.91, 92.46, 69.19, 64.24, 61.04, 56.49, 56.29, 55.90, 29.74. HRMS (ESI) calcd for C33H32N2O10 [M + H]+: 617.21297, found 617.21295.
6-(3-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)propoxy)-2-(p-tolyl)pyridazin-3(2H)-one (A2). White solid (59%), m.p.: 148.6–150.5 °C. 1H NMR (500 MHz, chloroform-d) δ 7.48 (d, J = 8.3 Hz, 2H, Ph–H), 7.31 (s, 2H, Ph–H), 7.23 (d, J = 8.2 Hz, 2H, Ph–H), 6.96 (d, J = 9.8 Hz, 1H, Ph–H), 6.90 (d, J = 9.7 Hz, 1H, Ph–H), 6.49 (d, J = 2.2 Hz, 1H, Ph–H), 6.35 (d, J = 2.1 Hz, 1H, Ph–H), 4.29 (t, J = 6.3 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 4.16 (t, J = 6.3 Hz, 2H, –O–CH2CH2
CH2CH2–O–), 4.16 (t, J = 6.3 Hz, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.96 (s, 3H, Ph–OCH3), 3.90 (d, J = 4.2 Hz, 6H, Ph–OCH3), 3.86 (s, 6H, Ph–OCH3), 2.37 (s, 3H, Ph–CH3), 2.17 (p, J = 6.3 Hz, 2H, –O–CH2
–O–), 3.96 (s, 3H, Ph–OCH3), 3.90 (d, J = 4.2 Hz, 6H, Ph–OCH3), 3.86 (s, 6H, Ph–OCH3), 2.37 (s, 3H, Ph–CH3), 2.17 (p, J = 6.3 Hz, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–). 13C NMR (126 MHz, chloroform-d) δ 174.01, 164.12, 161.09, 158.89, 158.87, 153.04, 152.71, 152.65, 140.67, 139.98, 139.19, 137.66, 133.93, 129.34, 126.75, 126.04, 124.95, 109.45, 105.91, 95.94, 92.50, 69.24, 64.23, 61.07, 56.52, 56.33, 55.93, 29.78, 21.25. HRMS (ESI) calcd for C34H34N2O10 [M + H]+: 631.22862, found 631.22845.
CH2–O–). 13C NMR (126 MHz, chloroform-d) δ 174.01, 164.12, 161.09, 158.89, 158.87, 153.04, 152.71, 152.65, 140.67, 139.98, 139.19, 137.66, 133.93, 129.34, 126.75, 126.04, 124.95, 109.45, 105.91, 95.94, 92.50, 69.24, 64.23, 61.07, 56.52, 56.33, 55.93, 29.78, 21.25. HRMS (ESI) calcd for C34H34N2O10 [M + H]+: 631.22862, found 631.22845.
6-(3-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)propoxy)-2-(4-fluorophenyl)pyridazin-3(2H)-one (A3). White solid (54%), m.p.: 171.8–173.6 °C. 1H NMR (400 MHz, chloroform-d) δ 7.63 (d, J = 4.9 Hz, 2H, Ph–H), 7.30 (s, 2H, Ph–H), 7.12 (d, J = 8.3 Hz, 2H, Ph–H), 6.97 (d, J = 9.8 Hz, 1H, Ph–H), 6.92 (d, J = 9.7 Hz, 1H, Ph–H), 6.49 (d, J = 2.1 Hz, 1H, Ph–H), 6.35 (d, J = 2.0 Hz, 1H, Ph–H), 4.30 (t, J = 6.4 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 4.16 (t, J = 6.2 Hz, 2H, –O–CH2
CH2CH2–O–), 4.16 (t, J = 6.2 Hz, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–), 3.96 (s, 3H, Ph–OCH3), 3.90 (d, J = 3.1 Hz, 6H, Ph–OCH3), 3.87 (s, 6H, Ph–OCH3), 2.18 (p, J = 12.5, 6.2 Hz, 2H, –O–CH2
CH2–O–), 3.96 (s, 3H, Ph–OCH3), 3.90 (d, J = 3.1 Hz, 6H, Ph–OCH3), 3.87 (s, 6H, Ph–OCH3), 2.18 (p, J = 12.5, 6.2 Hz, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.01, 164.15, 161.10, 158.88, 158.80, 153.05, 152.79, 140.61, 140.02, 133.98, 127.03, 126.98, 126.95, 126.91, 126.88, 126.02, 115.63, 115.45, 109.45, 105.93, 95.96, 92.52, 69.15, 64.36, 61.07, 56.52, 56.38, 56.34, 55.93, 29.75. 19F NMR (376) MHz, chloroform-d) δ −113.84. HRMS (ESI) calcd for C32H31N2O10F [M + H]+: 635.20355, found 635.20355.
CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.01, 164.15, 161.10, 158.88, 158.80, 153.05, 152.79, 140.61, 140.02, 133.98, 127.03, 126.98, 126.95, 126.91, 126.88, 126.02, 115.63, 115.45, 109.45, 105.93, 95.96, 92.52, 69.15, 64.36, 61.07, 56.52, 56.38, 56.34, 55.93, 29.75. 19F NMR (376) MHz, chloroform-d) δ −113.84. HRMS (ESI) calcd for C32H31N2O10F [M + H]+: 635.20355, found 635.20355.
6-(3-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)propoxy)-2-(4-chlorophenyl)-pyridazin-3(2H)-one (A4). White solid (63%), m.p.: 177.9–179.8 °C. 1H NMR (400 MHz, chloroform-d) δ 7.63 (d, J = 8.8 Hz, 2H, Ph–H), 7.40 (d, J = 8.8 Hz, 2H, Ph–H), 7.30 (s, 2H, Ph–H), 6.97 (d, J = 9.8 Hz, 1H, Ph–H), 6.92 (d, J = 9.8 Hz, 1H, Ph–H), 6.49 (d, J = 2.2 Hz, 1H, Ph–H), 6.36 (d, J = 2.2 Hz, 1H, Ph–H), 4.31 (t, J = 6.4 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 4.16 (t, J = 6.2 Hz, 2H, –O–CH2CH2
CH2CH2–O–), 4.16 (t, J = 6.2 Hz, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.96 (s, 3H, Ph–OCH3), 3.90 (d, J = 3.2 Hz, 6H, Ph–OCH3), 3.87 (s, 6H, Ph–OCH3), 2.18 (p, J = 6.3 Hz, 2H, –O–CH2
–O–), 3.96 (s, 3H, Ph–OCH3), 3.90 (d, J = 3.2 Hz, 6H, Ph–OCH3), 3.87 (s, 6H, Ph–OCH3), 2.18 (p, J = 6.3 Hz, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 173.94, 164.06, 161.01, 158.80, 158.66, 152.96, 152.77, 152.72, 140.52, 139.99, 139.90, 133.96, 133.03, 128.72, 127.01, 126.17, 125.94, 109.37, 105.82, 95.89, 92.43, 69.05, 64.31, 61.00, 56.47, 56.26, 55.86, 29.73. HRMS (ESI) calcd for C33H31N2O10Cl [M + H]+: 651.17400, found 651.17395.
CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 173.94, 164.06, 161.01, 158.80, 158.66, 152.96, 152.77, 152.72, 140.52, 139.99, 139.90, 133.96, 133.03, 128.72, 127.01, 126.17, 125.94, 109.37, 105.82, 95.89, 92.43, 69.05, 64.31, 61.00, 56.47, 56.26, 55.86, 29.73. HRMS (ESI) calcd for C33H31N2O10Cl [M + H]+: 651.17400, found 651.17395.
6-(3-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)propoxy) 2-(3-bromophenyl)-pyridazin-3(2H)-one (A5). Yellow solid (43%), m.p.: 124.9–126.5 °C. 1H NMR (500 MHz, chloroform-d) δ 7.83 (t, 1H, Ph–H), 7.62 (d, J = 8.0 Hz, 1H, Ph–H), 7.44 (d, J = 9.1 Hz, 1H, Ph–H), 7.31 (s, 1H, Ph–H, 7.29 (s, 2H, Ph–H), 6.95 (d, J = 9.9 Hz, 1H, Ph–H), 6.91 (d, J = 9.8 Hz, 1H, Ph–H), 6.48 (d, J = 2.1 Hz, 1H, Ph–H), 6.34 (d, J = 2.2 Hz, 1H, Ph–H), 4.30 (t, J = 6.3 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 4.15 (t, J = 6.2 Hz, 2H, –O–CH2CH2
CH2CH2–O–), 4.15 (t, J = 6.2 Hz, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.95 (s, 3H, Ph–OCH3), 3.89 (d, J = 5.4 Hz, 9H, Ph–OCH3), 3.86 (s, 3H, Ph–OCH3), 2.20 (p, 2H, –O–CH2
–O–), 3.95 (s, 3H, Ph–OCH3), 3.89 (d, J = 5.4 Hz, 9H, Ph–OCH3), 3.86 (s, 3H, Ph–OCH3), 2.20 (p, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–). 13C NMR (126 MHz, chloroform-d) δ 173.99, 164.09, 161.05, 158.85, 158.67, 153.00, 152.83, 152.78, 142.58, 140.59, 139.93, 134.01, 130.62, 129.90, 128.06, 127.18, 125.99, 123.67, 122.01, 109.42, 105.87, 95.92, 92.47, 69.10, 64.32, 61.05, 56.50, 56.31, 55.90, 29.78. HRMS (ESI) calcd for C33H31N2O10Br [M + H]+: 695.12348, found 695.12341.
CH2–O–). 13C NMR (126 MHz, chloroform-d) δ 173.99, 164.09, 161.05, 158.85, 158.67, 153.00, 152.83, 152.78, 142.58, 140.59, 139.93, 134.01, 130.62, 129.90, 128.06, 127.18, 125.99, 123.67, 122.01, 109.42, 105.87, 95.92, 92.47, 69.10, 64.32, 61.05, 56.50, 56.31, 55.90, 29.78. HRMS (ESI) calcd for C33H31N2O10Br [M + H]+: 695.12348, found 695.12341.
6-(3-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)propoxy)-2-(3-methoxyphenyl)pyridazin-3(2H)-one (A6). White solid (41%), m.p.: 173.9–175.8 °C. 1H NMR (500 MHz, chloroform-d) δ 7.34 (t, J = 8.0 Hz, 1H, Ph–H), 7.31 (s, 2H, Ph–H), 7.22 (s, 1H, Ph–H), 7.20 (d, 1H, Ph–H), 6.97 (d, J = 9.8 Hz, 1H, Ph–H), 6.92 (d, J = 9.8 Hz, 1H, Ph–H), 6.88 (d, J = 8.3 Hz, 1H, Ph–H), 6.49 (s, 1H, Ph–H), 6.35 (s, 1H, Ph–H), 4.31 (t, J = 6.3 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 4.15 (t, J = 6.2 Hz, 2H, –O–CH2CH2
CH2CH2–O–), 4.15 (t, J = 6.2 Hz, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.96 (s, 3H, Ph–OCH3), 3.90 (d, J = 4.7 Hz, 6H, Ph–OCH3), 3.87 (s, 6H, Ph–OCH3), 3.82 (s, 3H, Ph–OCH3), 2.18 (p, J = 6.1 Hz, 2H, –O–CH2
–O–), 3.96 (s, 3H, Ph–OCH3), 3.90 (d, J = 4.7 Hz, 6H, Ph–OCH3), 3.87 (s, 6H, Ph–OCH3), 3.82 (s, 3H, Ph–OCH3), 2.18 (p, J = 6.1 Hz, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–). 13C NMR (126 MHz, chloroform-d) δ 172.88, 162.99, 159.94, 158.61, 157.74, 157.73, 151.90, 151.63, 151.52, 141.52, 139.52, 138.82, 132.89, 128.32, 125.78, 124.90, 116.38, 112.55, 109.85, 108.31, 104.75, 94.81, 91.37, 68.08, 63.14, 59.95, 55.39, 55.20, 54.80, 54.41, 28.63. HRMS (ESI) calcd for C34H34N2O11 [M + H]+: 647.22354, found 647.22321.
CH2–O–). 13C NMR (126 MHz, chloroform-d) δ 172.88, 162.99, 159.94, 158.61, 157.74, 157.73, 151.90, 151.63, 151.52, 141.52, 139.52, 138.82, 132.89, 128.32, 125.78, 124.90, 116.38, 112.55, 109.85, 108.31, 104.75, 94.81, 91.37, 68.08, 63.14, 59.95, 55.39, 55.20, 54.80, 54.41, 28.63. HRMS (ESI) calcd for C34H34N2O11 [M + H]+: 647.22354, found 647.22321.
6-(3-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)propoxy)-2-(4-isopropylphenyl)pyridazin-3(2H)-one (A7). Yellow solid (56%), m.p.: 151.2–152.1 °C. 1H NMR (400 MHz, chloroform-d) δ 7.52 (d, J = 8.5 Hz, 2H, Ph–H), 7.31 (s, 2H, Ph–H), 7.29 (d, J = 8.5 Hz, 2H, Ph–H), 6.97 (d, J = 9.7 Hz, 1H, Ph–H), 6.91 (d, J = 9.7 Hz, 1H, Ph–H), 6.49 (d, 1H, Ph–H), 6.36 (d, 1H, Ph–H), 4.30 (t, J = 6.3 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 4.16 (t, J = 6.3 Hz, 2H, –O–CH2CH2
CH2CH2–O–), 4.16 (t, J = 6.3 Hz, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.96 (s, 3H, Ph–OCH3), 3.90 (d, J = 4.3 Hz, 6H, Ph–OCH3), 3.86 (s, 6H, Ph–OCH3), 2.93 (p, J = 13.8, 7.0 Hz, 1H, Ph-
–O–), 3.96 (s, 3H, Ph–OCH3), 3.90 (d, J = 4.3 Hz, 6H, Ph–OCH3), 3.86 (s, 6H, Ph–OCH3), 2.93 (p, J = 13.8, 7.0 Hz, 1H, Ph-![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) (CH3)2), 2.18 (p, J = 6.3 Hz, 2H, –O–CH2
(CH3)2), 2.18 (p, J = 6.3 Hz, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–), 1.26 (d, J = 6.9 Hz, 6H, Ph–CH
CH2–O–), 1.26 (d, J = 6.9 Hz, 6H, Ph–CH![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) ). 13C NMR (101 MHz, chloroform-d) δ 173.95, 164.04, 161.01, 158.82, 158.79, 152.96, 152.65, 152.60, 148.34, 140.59, 139.89, 139.28, 133.86, 126.70, 126.67, 125.96, 124.87, 109.37, 105.81, 95.87, 92.42, 69.18, 64.16, 61.00, 56.46, 56.26, 55.86, 33.88, 29.72, 23.97. HRMS (ESI) calcd for C36H38N2O10 [M + H]+: 659.25992, found 659.25970.
). 13C NMR (101 MHz, chloroform-d) δ 173.95, 164.04, 161.01, 158.82, 158.79, 152.96, 152.65, 152.60, 148.34, 140.59, 139.89, 139.28, 133.86, 126.70, 126.67, 125.96, 124.87, 109.37, 105.81, 95.87, 92.42, 69.18, 64.16, 61.00, 56.46, 56.26, 55.86, 33.88, 29.72, 23.97. HRMS (ESI) calcd for C36H38N2O10 [M + H]+: 659.25992, found 659.25970.
6-(3-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)propoxy)-2-(4-(tert-butyl)phenyl)-pyridazin-3(2H)-one (A8). White solid (44%), m.p.: 148.2–149.7 °C. 1H NMR (400 MHz, chloroform-d) δ 7.54 (d, J = 8.8 Hz, 2H, Ph–H), 7.45 (d, J = 8.7 Hz, 2H, Ph–H), 7.31 (s, 2H, Ph–H), 6.98 (d, J = 9.7 Hz, 1H, Ph–H), 6.91 (d, J = 9.7 Hz, 1H, Ph–H), 6.49 (d, J = 2.2 Hz, 1H, Ph–H), 6.36 (d, J = 2.2 Hz, 1H, Ph–H), 4.30 (t, J = 6.3 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 4.16 (t, J = 6.3 Hz, 2H, –O–CH2CH2
CH2CH2–O–), 4.16 (t, J = 6.3 Hz, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.96 (s, 3H, Ph–OCH3), 3.90 (d, J = 4.3 Hz, 6H, Ph–OCH3), 3.86 (s, 6H, Ph–OCH3), 2.18 (p, J = 6.3 Hz, 2H, –O–CH2
–O–), 3.96 (s, 3H, Ph–OCH3), 3.90 (d, J = 4.3 Hz, 6H, Ph–OCH3), 3.86 (s, 6H, Ph–OCH3), 2.18 (p, J = 6.3 Hz, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–), 1.33 (s, 9H, Ph–C(CH3)3). 13C NMR (101 MHz, chloroform-d) δ 173.95, 164.04, 161.01, 158.83, 158.79, 152.97, 152.64, 152.61, 150.53, 140.59, 139.89, 138.97, 133.87, 126.66, 125.97, 125.64, 124.48, 109.37, 105.81, 95.87, 92.42, 69.17, 64.17, 61.00, 56.46, 56.26, 55.86, 34.67, 31.33, 29.72. HRMS (ESI) calcd for C37H40N2O10 [M + H]+: 673.27557, found 673.27557.
CH2–O–), 1.33 (s, 9H, Ph–C(CH3)3). 13C NMR (101 MHz, chloroform-d) δ 173.95, 164.04, 161.01, 158.83, 158.79, 152.97, 152.64, 152.61, 150.53, 140.59, 139.89, 138.97, 133.87, 126.66, 125.97, 125.64, 124.48, 109.37, 105.81, 95.87, 92.42, 69.17, 64.17, 61.00, 56.46, 56.26, 55.86, 34.67, 31.33, 29.72. HRMS (ESI) calcd for C37H40N2O10 [M + H]+: 673.27557, found 673.27557.
6-(3-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)propoxy)-2-(4-(trifluoromethyl)phenyl)pyridazin-3(2H)-one (A9). White solid (27%), m.p.: 119.7–120.8 °C. 1H NMR (400 MHz, chloroform-d) δ 7.86 (d, J = 8.0 Hz, 2H, Ph–H), 7.70 (d, J = 8.3 Hz, 2H, Ph–H), 7.30 (s, 2H, Ph–H), 6.99 (d, J = 9.7 Hz, 1H, Ph–H), 6.94 (d, J = 9.8 Hz, 1H, Ph–H), 6.49 (d, J = 2.2 Hz, 1H, Ph–H), 6.36 (d, J = 2.2 Hz, 1H, Ph–H), 4.33 (t, J = 6.4 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 4.17 (t, J = 6.2 Hz, 2H, –O–CH2CH2
CH2CH2–O–), 4.17 (t, J = 6.2 Hz, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.96 (s, 3H, Ph–OCH3), 3.90 (d, J = 4.8 Hz, 6H, Ph–OCH3), 3.87 (s, 6H, Ph–OCH3), 2.18 (p, J = 6.3 Hz, 2H, –O–CH2
–O–), 3.96 (s, 3H, Ph–OCH3), 3.90 (d, J = 4.8 Hz, 6H, Ph–OCH3), 3.87 (s, 6H, Ph–OCH3), 2.18 (p, J = 6.3 Hz, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 173.94, 164.08, 161.01, 158.81, 158.70, 152.97, 152.92, 152.76, 144.24, 140.48, 139.91, 134.08, 127.29, 125.92, 125.78, 125.75, 124.96, 109.36, 105.83, 95.89, 92.44, 68.99, 64.40, 60.98, 56.46, 56.25, 55.86, 29.64. 19F NMR (376 MHz, chloroform-d) δ -62.48. HRMS (ESI) calcd for C34H31N2O10F3 [M + H]+: 685.20036, found 685.20032.
CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 173.94, 164.08, 161.01, 158.81, 158.70, 152.97, 152.92, 152.76, 144.24, 140.48, 139.91, 134.08, 127.29, 125.92, 125.78, 125.75, 124.96, 109.36, 105.83, 95.89, 92.44, 68.99, 64.40, 60.98, 56.46, 56.25, 55.86, 29.64. 19F NMR (376 MHz, chloroform-d) δ -62.48. HRMS (ESI) calcd for C34H31N2O10F3 [M + H]+: 685.20036, found 685.20032.
6-(3-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)propoxy)-2-(3,4-dichlorophenyl)-pyridazin-3(2H)-one (A10). White solid (73%), m.p.: 149.2–151.1 °C. 1H NMR (400 MHz, chloroform-d) δ 7.87 (d, J = 2.4 Hz, 1H, Ph–H), 7.59 (d, J = 2.5 Hz, 1H, Ph–H), 7.50 (s, 1H, Ph–H), 7.30 (s, 2H, Ph–H), 6.96 (d, J = 9.8 Hz, 1H, Ph–H), 6.92 (d, J = 9.8 Hz, 1H, Ph–H), 6.49 (d, J = 2.2 Hz, 1H, Ph–H), 6.35 (d, J = 2.2 Hz, 1H, Ph–H), 4.32 (t, J = 6.3 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 4.16 (t, J = 6.2 Hz, 2H, –O–CH2CH2
CH2CH2–O–), 4.16 (t, J = 6.2 Hz, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.96 (s, 3H, Ph–OCH3), 3.90 (d, J = 4.0 Hz, 6H, Ph–OCH3), 3.87 (s, 6H, Ph–OCH3), 2.18 (p, J = 6.2 Hz, 2H, –O–CH2
–O–), 3.96 (s, 3H, Ph–OCH3), 3.90 (d, J = 4.0 Hz, 6H, Ph–OCH3), 3.87 (s, 6H, Ph–OCH3), 2.18 (p, J = 6.2 Hz, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 173.93, 164.07, 161.00, 158.80, 158.55, 152.95, 152.86, 152.77, 140.63, 140.49, 139.89, 133.99, 132.35, 131.24, 130.14, 127.27, 126.66, 125.92, 124.11, 109.36, 105.83, 95.89, 92.44, 68.97, 64.35, 61.00, 56.46, 56.27, 55.86, 29.61. HRMS (ESI) calcd for C33H30N2O10Cl2 [M + H]+: 685.13503, found 685.13489.
CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 173.93, 164.07, 161.00, 158.80, 158.55, 152.95, 152.86, 152.77, 140.63, 140.49, 139.89, 133.99, 132.35, 131.24, 130.14, 127.27, 126.66, 125.92, 124.11, 109.36, 105.83, 95.89, 92.44, 68.97, 64.35, 61.00, 56.46, 56.27, 55.86, 29.61. HRMS (ESI) calcd for C33H30N2O10Cl2 [M + H]+: 685.13503, found 685.13489.
6-(3-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)propoxy)-2-(3,4-difluorophenyl)-pyridazin-3(2H)-one (A11). White solid (57%), m.p.: 166.9–168.7 °C. 1H NMR (400 MHz, chloroform-d) δ 7.62 (s, 1H, Ph–H), 7.48 (d, J = 9.0 Hz, 1H, Ph–H), 7.30 (s, 2H, Ph–H), 7.20 (d, 1H, Ph–H), 6.97 (d, J = 9.8 Hz, 1H, Ph–H), 6.92 (d, J = 9.7 Hz, 1H, Ph–H), 6.49 (d, J = 2.2 Hz, 1H, Ph–H), 6.36 (d, J = 2.2 Hz, 1H, Ph–H), 4.32 (t, J = 6.3 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 4.16 (t, J = 6.2 Hz, 2H, –O–CH2
CH2CH2–O–), 4.16 (t, J = 6.2 Hz, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–), 3.96 (s, 3H, Ph–OCH3), 3.90 (d, J = 3.7 Hz, 6H, Ph–OCH3), 3.88 (s, 6H, Ph–OCH3), 2.17 (p, J = 12.5, 6.3 Hz, 2H), –O–CH2
CH2–O–), 3.96 (s, 3H, Ph–OCH3), 3.90 (d, J = 3.7 Hz, 6H, Ph–OCH3), 3.88 (s, 6H, Ph–OCH3), 2.17 (p, J = 12.5, 6.3 Hz, 2H), –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–. 13C NMR (101 MHz, chloroform-d) δ 173.99, 164.11, 161.04, 158.85, 158.63, 153.00, 152.83, 152.80, 140.53, 139.94, 134.06, 127.21, 125.97, 121.14, 121.08, 117.00, 116.82, 114.73, 114.52, 109.39, 105.86, 95.93, 92.47, 69.01, 64.38, 61.02, 56.49, 56.29, 55.89, 29.66. 19F NMR (376 MHz, chloroform-d) δ −135.96 (s), −138.42 (s). HRMS (ESI) calcd for C33H30N2O10F2 [M + H]+: 653.19413, found 653.19391.
CH2–O–. 13C NMR (101 MHz, chloroform-d) δ 173.99, 164.11, 161.04, 158.85, 158.63, 153.00, 152.83, 152.80, 140.53, 139.94, 134.06, 127.21, 125.97, 121.14, 121.08, 117.00, 116.82, 114.73, 114.52, 109.39, 105.86, 95.93, 92.47, 69.01, 64.38, 61.02, 56.49, 56.29, 55.89, 29.66. 19F NMR (376 MHz, chloroform-d) δ −135.96 (s), −138.42 (s). HRMS (ESI) calcd for C33H30N2O10F2 [M + H]+: 653.19413, found 653.19391.
6-(3-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)propoxy) 2-(3-chloro-4-fluorophenyl)-pyridazin-3(2H)-one (A12). White solid (77%), m.p.: 178.0–179.4 °C. 1H NMR (400 MHz, chloroform-d) δ 7.79 (s, 1H, Ph–H), 7.59 (d, J = 4.7 Hz, 1H, Ph–H), 7.30 (s, 2H, Ph–H), 7.21 (d, J = 8.8 Hz, 1H, Ph–H), 6.96 (d, J = 9.8 Hz, 1H, Ph–H), 6.92 (d, J = 9.8 Hz, 1H, Ph–H), 6.49 (d, J = 2.2 Hz, 1H, Ph–H), 6.35 (d, J = 2.2 Hz, 1H, Ph–H), 4.31 (t, J = 6.3 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 4.16 (t, J = 6.2 Hz, 2H, –O–CH2CH2
CH2CH2–O–), 4.16 (t, J = 6.2 Hz, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.95 (s, 3H, Ph–OCH3), 3.90 (d, J = 3.3 Hz, 6H, Ph–OCH3), 3.88 (s, 6H, Ph–OCH3), 2.18 (p, J = 6.2 Hz, 2H, –O–CH2
–O–), 3.95 (s, 3H, Ph–OCH3), 3.90 (d, J = 3.3 Hz, 6H, Ph–OCH3), 3.88 (s, 6H, Ph–OCH3), 2.18 (p, J = 6.2 Hz, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 173.95, 164.08, 161.01, 158.82, 158.60, 152.97, 152.84, 152.76, 140.51, 139.91, 133.95, 127.32, 127.25, 125.95, 124.94, 124.87, 116.43, 116.21, 109.37, 105.83, 105.74, 95.89, 92.44, 68.99, 64.34, 61.00, 56.47, 56.27, 55.87, 29.63. 19F NMR (376 MHz, chloroform-d) δ −116.18. HRMS (ESI) calcd for C33H30N2O10ClF [M + H]+: 669.16479, found 669.16458.
CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 173.95, 164.08, 161.01, 158.82, 158.60, 152.97, 152.84, 152.76, 140.51, 139.91, 133.95, 127.32, 127.25, 125.95, 124.94, 124.87, 116.43, 116.21, 109.37, 105.83, 105.74, 95.89, 92.44, 68.99, 64.34, 61.00, 56.47, 56.27, 55.87, 29.63. 19F NMR (376 MHz, chloroform-d) δ −116.18. HRMS (ESI) calcd for C33H30N2O10ClF [M + H]+: 669.16479, found 669.16458.
6-(3-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)propoxy)-2-(naphthalen-2-yl)pyridazin-3(2H)-one (A13). White solid (27%), m.p.: 145.2–146.7 °C. 1H NMR (400 MHz, chloroform-d) δ 8.16 (d, J = 1.9 Hz, 1H, Ph–H), 7.90 (d, J = 9.0 Hz, 2H, Ph–H), 7.87 (s, 1H, Ph–H), 7.75 (d, J = 2.1 Hz, 1H, Ph–H), 7.49 (d, J = 9.5 Hz, 2H, Ph–H), 7.30 (s, 2H, Ph–H), 7.02 (d, J = 9.8 Hz, 1H, Ph–H), 6.96 (d, J = 9.7 Hz, 1H, Ph–H), 6.49 (d, J = 2.3 Hz, 1H, Ph–H), 6.36 (d, J = 2.3 Hz, 1H, Ph–H), 4.35 (t, J = 6.3 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 4.18 (t, J = 6.3 Hz, 2H, –O–CH2CH2
CH2CH2–O–), 4.18 (t, J = 6.3 Hz, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.96 (s, 3H, Ph–OCH3), 3.89 (d, J = 12.3 Hz, 6H, Ph–OCH3), 3.84 (s, 6H, Ph–OCH3), 2.20 (p, J = 6.3 Hz, 2H, –O–CH2
–O–), 3.96 (s, 3H, Ph–OCH3), 3.89 (d, J = 12.3 Hz, 6H, Ph–OCH3), 3.84 (s, 6H, Ph–OCH3), 2.20 (p, J = 6.3 Hz, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 173.95, 164.05, 161.01, 158.98, 158.80, 152.95, 152.78, 152.69, 140.58, 139.88, 138.99, 133.98, 133.07, 132.39, 128.42, 127.62, 126.86, 126.50, 126.42, 125.95, 123.62, 123.16, 109.38, 105.81, 95.87, 92.42, 69.15, 64.24, 60.99, 56.46, 56.25, 55.86, 29.71. HRMS (ESI) calcd for C37H34N2O10 [M + H]+: 667.22864, found 667.22862.
CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 173.95, 164.05, 161.01, 158.98, 158.80, 152.95, 152.78, 152.69, 140.58, 139.88, 138.99, 133.98, 133.07, 132.39, 128.42, 127.62, 126.86, 126.50, 126.42, 125.95, 123.62, 123.16, 109.38, 105.81, 95.87, 92.42, 69.15, 64.24, 60.99, 56.46, 56.25, 55.86, 29.71. HRMS (ESI) calcd for C37H34N2O10 [M + H]+: 667.22864, found 667.22862.
6-(4-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)butoxy)-2-phenylpyridazin-3(2H)-one (A14). White solid (66%), m.p.: 121.8–123.6 °C. 1H NMR (400 MHz, chloroform-d) δ 7.64 (d, J = 7.4 Hz, 2H, Ph–H), 7.44 (t, J = 7.8 Hz, 2H, Ph–H), 7.34 (s, 2H, Ph–H), 7.32 (d, J = 7.4 Hz, 1H, Ph–H), 6.99 (d, J = 9.7 Hz, 1H, Ph–H), 6.95 (d, J = 9.8 Hz, 1H, Ph–H), 6.50 (d, J = 2.3 Hz, 1H, Ph–H), 6.35 (d, J = 2.3 Hz, 1H, Ph–H), 4.16 (t, J = 6.0 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2CH2–O–), 4.06 (t, J = 5.9 Hz, 2H, –O–CH2CH2CH2
CH2CH2CH2–O–), 4.06 (t, J = 5.9 Hz, 2H, –O–CH2CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.96 (s, 3H, Ph–OCH3), 3.91 (d, J = 1.6 Hz, 9H, Ph–OCH3), 3.91 (s, 3H, Ph–OCH3), 1.90 (p, J = 5.2, 1.7 Hz, 2H, –O–CH2
–O–), 3.96 (s, 3H, Ph–OCH3), 3.91 (d, J = 1.6 Hz, 9H, Ph–OCH3), 3.91 (s, 3H, Ph–OCH3), 1.90 (p, J = 5.2, 1.7 Hz, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 1.89 (p, 2H, –O–CH2CH2
CH2CH2–O–), 1.89 (p, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–). 13C NMR (101 MHz, CDCl3) δ 174.03, 164.01, 161.02, 158.80, 152.95, 152.84, 152.66, 141.58, 140.59, 139.85, 133.91, 128.65, 127.63, 126.96, 126.08, 125.06, 109.40, 105.84, 95.84, 92.41, 71.83, 66.94, 61.03, 56.45, 56.30, 55.85, 26.94, 25.35. HRMS (ESI) calcd for C34H34N2O10 [M + H]+: 631.22862, found 631.22821.
CH2–O–). 13C NMR (101 MHz, CDCl3) δ 174.03, 164.01, 161.02, 158.80, 152.95, 152.84, 152.66, 141.58, 140.59, 139.85, 133.91, 128.65, 127.63, 126.96, 126.08, 125.06, 109.40, 105.84, 95.84, 92.41, 71.83, 66.94, 61.03, 56.45, 56.30, 55.85, 26.94, 25.35. HRMS (ESI) calcd for C34H34N2O10 [M + H]+: 631.22862, found 631.22821.
6-(4-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)butoxy)-2-(p-tolyl)pyridazin-3(2H)-one (A15). White solid (26%), m.p.: 141.6–143.4 °C. 1H NMR (400 MHz, chloroform-d) δ 7.50 (d, J = 8.3 Hz, 2H, Ph–H), 7.34 (s, 2H, Ph–H), 7.23 (d, J = 8.3 Hz, 2H, Ph–H), 6.98 (d, J = 9.7 Hz, 1H, Ph–H), 6.93 (d, J = 9.7 Hz, 1H, Ph–H), 6.49 (d, J = 2.1 Hz, 1H, Ph–H), 6.35 (d, J = 2.1 Hz, 1H, Ph–H), 4.14 (t, J = 6.1 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2CH2–O–), 4.06 (t, J = 6.0 Hz, 2H, –O–CH2CH2CH2
CH2CH2CH2–O–), 4.06 (t, J = 6.0 Hz, 2H, –O–CH2CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.96 (s, 3H, Ph–CH3), 3.91 (d, J = 2.2 Hz, 9H, Ph–OCH3), 3.90 (s, 3H, Ph–OCH3), 2.37 (s, 3H, Ph–CH3), 1.96 (p, 2H, –O–CH2
–O–), 3.96 (s, 3H, Ph–CH3), 3.91 (d, J = 2.2 Hz, 9H, Ph–OCH3), 3.90 (s, 3H, Ph–OCH3), 2.37 (s, 3H, Ph–CH3), 1.96 (p, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 1.88 (p, 2H, –O–CH2CH2
CH2CH2–O–), 1.88 (p, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.02, 164.00, 161.02, 158.85, 158.80, 152.95, 152.77, 152.65, 140.60, 139.84, 139.14, 137.62, 133.81, 129.28, 126.82, 126.08, 124.92, 109.41, 105.84, 95.83, 92.41, 71.85, 66.91, 61.03, 56.45, 56.30, 55.85, 26.95, 25.34, 21.19. HRMS (ESI) calcd for C35H36N2O10 [M + H]+: 645.24427, found 645.24359.
CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.02, 164.00, 161.02, 158.85, 158.80, 152.95, 152.77, 152.65, 140.60, 139.84, 139.14, 137.62, 133.81, 129.28, 126.82, 126.08, 124.92, 109.41, 105.84, 95.83, 92.41, 71.85, 66.91, 61.03, 56.45, 56.30, 55.85, 26.95, 25.34, 21.19. HRMS (ESI) calcd for C35H36N2O10 [M + H]+: 645.24427, found 645.24359.
6-(4-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)butoxy)-2-(4-fluorophenyl)pyridazin-3(2H)-one (A16). White solid (46%), m.p.: 175.6–177.6 °C. 1H NMR (400 MHz, chloroform-d) δ 7.62 (d, J = 4.9 Hz, 2H, Ph–H), 7.34 (s, 2H, Ph–H), 7.12 (d, J = 8.7 Hz, 2H, Ph–H), 6.98 (d, J = 9.7 Hz, 1H, Ph–H), 6.95 (d, J = 9.7 Hz, 1H, Ph–H), 6.49 (d, J = 2.0 Hz, 1H, Ph–H), 6.35 (d, J = 1.9 Hz, 1H, Ph–H), 4.16 (t, J = 6.2 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2CH2–O–), 4.05 (t, J = 6.1 Hz, 2H, –O–CH2CH2CH2
CH2CH2CH2–O–), 4.05 (t, J = 6.1 Hz, 2H, –O–CH2CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.95 (s, 3H, Ph–CH3), 3.91 (d, J = 2.2 Hz, 9H, Ph–CH3), 3.90 (s, 3H, Ph–CH3), 1.94 (p, 2H, –O–CH2
–O–), 3.95 (s, 3H, Ph–CH3), 3.91 (d, J = 2.2 Hz, 9H, Ph–CH3), 3.90 (s, 3H, Ph–CH3), 1.94 (p, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 1.88 (p, 2H, –O–CH2CH2
CH2CH2–O–), 1.88 (p, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.09, 164.11, 161.10, 158.88, 158.82, 153.04, 152.97, 152.74, 140.66, 139.97, 133.95, 127.18, 126.98, 126.91, 126.15, 115.63, 115.44, 109.47, 105.94, 95.92, 92.51, 71.83, 67.04, 61.09, 56.52, 56.38, 55.92, 26.96, 25.39. 19F NMR (471 MHz, chloroform-d) δ −113.87. HRMS (ESI) calcd for C34H33N2O10F [M + H]+: 649.21920, found 649.21906.
CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.09, 164.11, 161.10, 158.88, 158.82, 153.04, 152.97, 152.74, 140.66, 139.97, 133.95, 127.18, 126.98, 126.91, 126.15, 115.63, 115.44, 109.47, 105.94, 95.92, 92.51, 71.83, 67.04, 61.09, 56.52, 56.38, 55.92, 26.96, 25.39. 19F NMR (471 MHz, chloroform-d) δ −113.87. HRMS (ESI) calcd for C34H33N2O10F [M + H]+: 649.21920, found 649.21906.
6-(4-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)butoxy)-2-(4-chlorophenyl)-pyridazin-3(2H)-one (A17). White solid (26%), m.p.: 132.3–134.2 °C. 1H NMR (400 MHz, chloroform-d) δ 7.64 (d, J = 8.9 Hz, 2H, Ph–H), 7.39 (d, J = 8.9 Hz, 2H, Ph–H), 7.34 (s, 2H, Ph–H), 6.98 (d, J = 9.7 Hz, 1H, Ph–H), 6.94 (d, J = 9.7 Hz, 1H, Ph–H), 6.49 (d, J = 2.2 Hz, 1H, Ph–H), 6.35 (d, J = 2.2 Hz, 1H, Ph–H), 4.17 (t, J = 6.1 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2CH2–O–), 4.05 (t, J = 6.0 Hz, 2H, –O–CH2CH2CH2
CH2CH2CH2–O–), 4.05 (t, J = 6.0 Hz, 2H, –O–CH2CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.96 (s, 3H, Ph–OCH3), 3.91 (d, J = 2.0 Hz, 9H, Ph–OCH3), 3.90 (s, 3H, Ph–OCH3), 1.92 (p, J = 7.9, 5.5 Hz, 2H, –O–CH2
–O–), 3.96 (s, 3H, Ph–OCH3), 3.91 (d, J = 2.0 Hz, 9H, Ph–OCH3), 3.90 (s, 3H, Ph–OCH3), 1.92 (p, J = 7.9, 5.5 Hz, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 1.89 (p, 2H, –O–CH2CH2
CH2CH2–O–), 1.89 (p, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.07, 164.07, 161.05, 158.83, 158.72, 152.99, 152.72, 140.61, 140.05, 139.89, 133.95, 133.08, 128.76, 127.22, 126.25, 126.10, 109.41, 105.86, 95.89, 92.46, 71.76, 67.02, 61.06, 56.48, 56.33, 55.89, 26.90, 25.32. HRMS (ESI) calcd for C34H33N2O10Cl [M + H]+: 665.18976, found 665.18965.
CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.07, 164.07, 161.05, 158.83, 158.72, 152.99, 152.72, 140.61, 140.05, 139.89, 133.95, 133.08, 128.76, 127.22, 126.25, 126.10, 109.41, 105.86, 95.89, 92.46, 71.76, 67.02, 61.06, 56.48, 56.33, 55.89, 26.90, 25.32. HRMS (ESI) calcd for C34H33N2O10Cl [M + H]+: 665.18976, found 665.18965.
6-(4-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)butoxy)-2-(3-bromophenyl)-pyridazin-3(2H)-one (A18). Yellow solid (52%), m.p.: 115.9–117.8 °C. 1H NMR (400 MHz, chloroform-d) δ 7.83 (s, 1H, Ph–H), 7.64 (d, J = 8.1 Hz, 1H, Ph–H), 7.44 (d, J = 8.9 Hz, 1H, Ph–H), 7.34 (s, 2H, Ph–H), 7.29 (t, J = 8.1 Hz, 1H, Ph–H), 6.98 (d, J = 9.8 Hz, 1H, Ph–H), 6.94 (d, J = 9.8 Hz, 1H, Ph–H), 6.49 (d, J = 2.2 Hz, 1H, Ph–H), 6.35 (d, J = 2.2 Hz, 1H, Ph–H), 4.17 (t, J = 6.1 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2CH2–O–), 4.06 (t, J = 6.0 Hz, 2H, –O–CH2CH2CH2
CH2CH2CH2–O–), 4.06 (t, J = 6.0 Hz, 2H, –O–CH2CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.95 (s, 3H, Ph–OCH3), 3.91 (d, J = 2.9 Hz, 9H, Ph–OCH3), 3.90 (s, 3H, Ph–OCH3), 1.92 (p, J = 7.8, 5.7 Hz, 2H, –O–CH2
–O–), 3.95 (s, 3H, Ph–OCH3), 3.91 (d, J = 2.9 Hz, 9H, Ph–OCH3), 3.90 (s, 3H, Ph–OCH3), 1.92 (p, J = 7.8, 5.7 Hz, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 1.86 (p, J = 8.0, 5.9 Hz, 2H, –O–CH2CH2
CH2CH2–O–), 1.86 (p, J = 8.0, 5.9 Hz, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.03, 164.02, 161.02, 158.80, 158.64, 152.99, 152.96, 152.66, 142.58, 140.59, 139.85, 133.94, 130.57, 129.85, 128.04, 127.31, 126.09, 123.66, 121.99, 109.41, 105.83, 95.85, 92.42, 71.77, 67.10, 61.04, 56.46, 56.31, 55.86, 26.92, 25.33. HRMS (ESI) calcd for C34H33N2O10Br [M + H]+: 709.13913, found 709.13867.
CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.03, 164.02, 161.02, 158.80, 158.64, 152.99, 152.96, 152.66, 142.58, 140.59, 139.85, 133.94, 130.57, 129.85, 128.04, 127.31, 126.09, 123.66, 121.99, 109.41, 105.83, 95.85, 92.42, 71.77, 67.10, 61.04, 56.46, 56.31, 55.86, 26.92, 25.33. HRMS (ESI) calcd for C34H33N2O10Br [M + H]+: 709.13913, found 709.13867.
6-(4-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)butoxy)-2-(3-methoxyphenyl)pyridazin-3(2H)-one (A19). White solid (61%), m.p.: 124.8–126.5 °C. 1H NMR (400 MHz, chloroform-d) δ 7.34 (s, 2H), 7.31 (s, 1H, Ph–H), 7.24 (t, 1H, Ph–H), 7.20 (d, J = 2.2 Hz, 1H, Ph–H), 6.98 (d, J = 9.7 Hz, 1H, Ph–H), 6.94 (d, J = 9.7 Hz, 1H, Ph–H), 6.88 (d, J = 8.3 Hz, 1H, Ph–H), 6.49 (d, J = 2.0 Hz, 1H, Ph–H), 6.35 (d, J = 2.0 Hz, 1H, Ph–H), 4.15 (t, J = 5.9 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2CH2–O–), 4.06 (t, J = 5.9 Hz, 2H, –O–CH2CH2CH2
CH2CH2CH2–O–), 4.06 (t, J = 5.9 Hz, 2H, –O–CH2CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.95 (s, 3H, Ph–OCH3), 3.91 (d, J = 1.7 Hz, 9H, Ph–OCH3), 3.90 (s, 3H, Ph–OCH3), 3.81 (s, 3H, Ph–OCH3), 1.94 (m, 2H, –O–CH2
–O–), 3.95 (s, 3H, Ph–OCH3), 3.91 (d, J = 1.7 Hz, 9H, Ph–OCH3), 3.90 (s, 3H, Ph–OCH3), 3.81 (s, 3H, Ph–OCH3), 1.94 (m, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 1.88 (m, 2H, –O–CH2CH2
CH2CH2–O–), 1.88 (m, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.02, 164.02, 161.04, 159.70, 158.81, 158.79, 152.98, 152.77, 152.65, 142.65, 140.61, 139.87, 133.95, 129.39, 126.98, 126.10, 117.48, 113.59, 110.96, 109.42, 105.85, 95.85, 92.43, 71.84, 66.99, 61.04, 56.46, 56.31, 55.87, 55.47, 26.98, 25.37. HRMS (ESI) calcd for C35H36N2O11 [M + H]+: 661.23919, found 661.23914.
CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.02, 164.02, 161.04, 159.70, 158.81, 158.79, 152.98, 152.77, 152.65, 142.65, 140.61, 139.87, 133.95, 129.39, 126.98, 126.10, 117.48, 113.59, 110.96, 109.42, 105.85, 95.85, 92.43, 71.84, 66.99, 61.04, 56.46, 56.31, 55.87, 55.47, 26.98, 25.37. HRMS (ESI) calcd for C35H36N2O11 [M + H]+: 661.23919, found 661.23914.
6-(4-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)butoxy)-2-(4-isopropylphenyl)pyridazin-3(2H)-one (A20). Yellow solid (28%), m.p.: 123.1–123.8 °C. 1H NMR (400 MHz, chloroform-d) δ 7.53 (d, J = 8.5 Hz, 2H, Ph–H), 7.34 (s, 2H, Ph–H), 7.28 (d, J = 8.4 Hz, 2H, Ph–H), 6.98 (d, J = 9.7 Hz, 1H, Ph–H), 6.93 (d, J = 9.7 Hz, 1H, Ph–H), 6.49 (d, J = 2.2 Hz, 1H, Ph–H), 6.35 (d, J = 2.2 Hz, 1H, Ph–H), 4.15 (t, J = 6.0 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2CH2–O–), 4.06 (t, J = 6.0 Hz, 2H, –O–CH2CH2CH2
CH2CH2CH2–O–), 4.06 (t, J = 6.0 Hz, 2H, –O–CH2CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.95 (s, 3H, Ph–OCH3), 3.91 (d, J = 1.7 Hz, 9H, Ph–OCH3), 3.90 (s, 3H, Ph–OCH3), 2.94 (p, J = 13.8, 6.9 Hz, 1H, Ph-
–O–), 3.95 (s, 3H, Ph–OCH3), 3.91 (d, J = 1.7 Hz, 9H, Ph–OCH3), 3.90 (s, 3H, Ph–OCH3), 2.94 (p, J = 13.8, 6.9 Hz, 1H, Ph-![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) (CH3)2), 1.95 (p, 2H, –O–CH2
(CH3)2), 1.95 (p, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 1.87 (p, 2H, –O–CH2CH2
CH2CH2–O–), 1.87 (p, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–), 1.25 (d, J = 6.9 Hz, 6H, Ph–CH
CH2–O–), 1.25 (d, J = 6.9 Hz, 6H, Ph–CH![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) ). 13C NMR (101 MHz, chloroform-d) δ 174.03, 164.02, 161.05, 158.84, 158.82, 152.97, 152.80, 152.64, 148.33, 140.62, 139.86, 139.35, 133.86, 126.80, 126.72, 126.11, 124.91, 109.43, 105.85, 95.85, 92.43, 71.85, 66.92, 61.04, 56.47, 56.31, 55.87, 33.89, 26.97, 25.38, 23.98. HRMS (ESI) calcd for C37H40N2O10 [M + H]+: 673.27557, found 673.27557.
). 13C NMR (101 MHz, chloroform-d) δ 174.03, 164.02, 161.05, 158.84, 158.82, 152.97, 152.80, 152.64, 148.33, 140.62, 139.86, 139.35, 133.86, 126.80, 126.72, 126.11, 124.91, 109.43, 105.85, 95.85, 92.43, 71.85, 66.92, 61.04, 56.47, 56.31, 55.87, 33.89, 26.97, 25.38, 23.98. HRMS (ESI) calcd for C37H40N2O10 [M + H]+: 673.27557, found 673.27557.
6-(4-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)butoxy)-2-(4-(tert-butyl)phenyl)-pyridazin-3(2H)-one (A21). White solid (46%), m.p.: 104.3–105.6 °C. 1H NMR (400 MHz, chloroform-d) δ 7.55 (d, J = 8.7 Hz, 2H, Ph–H), 7.44 (d, J = 8.7 Hz, 2H, Ph–H), 7.34 (s, 2H, Ph–H), 6.98 (d, J = 9.7 Hz, 1H, Ph–H), 6.93 (d, J = 9.7 Hz, 1H, Ph–H), 6.49 (d, J = 2.2 Hz, 1H, Ph–H), 6.35 (d, J = 2.2 Hz, 1H, Ph–H), 4.15 (t, J = 6.0 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2CH2–O–), 4.06 (t, J = 6.0 Hz, 2H, –O–CH2CH2CH2
CH2CH2CH2–O–), 4.06 (t, J = 6.0 Hz, 2H, –O–CH2CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.95 (s, 3H, Ph–OCH3), 3.91 (d, J = 1.6 Hz, 9H, Ph–OCH3), 3.90 (s, 3H, Ph–OCH3), 1.93 (m, 2H, –O–CH2
–O–), 3.95 (s, 3H, Ph–OCH3), 3.91 (d, J = 1.6 Hz, 9H, Ph–OCH3), 3.90 (s, 3H, Ph–OCH3), 1.93 (m, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 1.87 (m, 2H, –O–CH2CH2
CH2CH2–O–), 1.87 (m, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–), 1.32 (s, 9H, Ph–C(CH3)3). 13C NMR (101 MHz, chloroform-d) δ 174.03, 164.01, 161.03, 158.85, 158.81, 152.96, 152.81, 152.64, 150.52, 140.61, 139.86, 139.02, 133.85, 126.79, 126.09, 125.64, 124.51, 109.41, 105.85, 95.84, 92.42, 71.84, 66.91, 61.02, 56.45, 56.30, 55.85, 34.67, 31.33, 26.95, 25.37. HRMS (ESI) calcd for C38H42N2O10 [M + H]+: 687.29122, found 687.29010.
CH2–O–), 1.32 (s, 9H, Ph–C(CH3)3). 13C NMR (101 MHz, chloroform-d) δ 174.03, 164.01, 161.03, 158.85, 158.81, 152.96, 152.81, 152.64, 150.52, 140.61, 139.86, 139.02, 133.85, 126.79, 126.09, 125.64, 124.51, 109.41, 105.85, 95.84, 92.42, 71.84, 66.91, 61.02, 56.45, 56.30, 55.85, 34.67, 31.33, 26.95, 25.37. HRMS (ESI) calcd for C38H42N2O10 [M + H]+: 687.29122, found 687.29010.
6-(4-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)butoxy)-2-(4-(trifluoromethyl)phenyl)pyridazin-3(2H)-one (A22). White solid (51%), m.p.: 110.8–111.7 °C. 1H NMR (400 MHz, chloroform-d) δ 7.86 (d, J = 8.4 Hz, 2H, Ph–H), 7.68 (d, J = 8.6 Hz, 2H, Ph–H), 7.33 (s, 2H, Ph–H), 6.99 (d, J = 9.8 Hz, 1H, Ph–H), 6.96 (d, J = 9.8 Hz, 1H, Ph–H), 6.49 (d, J = 2.2 Hz, 1H, Ph–H), 6.35 (d, J = 2.2 Hz, 1H, Ph–H), 4.19 (t, J = 6.2 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2CH2–O–), 4.06 (t, J = 6.1 Hz, 2H, –O–CH2CH2CH2
CH2CH2CH2–O–), 4.06 (t, J = 6.1 Hz, 2H, –O–CH2CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.94 (s, 3H, Ph–OCH3), 3.91 (d, J = 2.3 Hz, 9H, Ph–OCH3), 3.90 (s, 3H, Ph–OCH3), 1.98 (p, 2H, –O–CH2
–O–), 3.94 (s, 3H, Ph–OCH3), 3.91 (d, J = 2.3 Hz, 9H, Ph–OCH3), 3.90 (s, 3H, Ph–OCH3), 1.98 (p, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 1.86 (p, J = 8.1, 5.8 Hz, 2H, –O–CH2CH2
CH2CH2–O–), 1.86 (p, J = 8.1, 5.8 Hz, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.04, 164.06, 161.02, 158.81, 158.73, 153.12, 152.98, 152.69, 144.31, 140.58, 139.88, 134.05, 127.47, 126.08, 125.78, 125.74, 125.01, 109.39, 105.84, 95.87, 92.45, 71.67, 67.08, 61.02, 56.45, 56.30, 55.86, 26.86, 25.30. 19F NMR (376 MHz, chloroform-d) δ −62.49. HRMS (ESI) calcd for C35H33N2O10F3 [M + H]+: 699.21601, found 699.21497.
CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.04, 164.06, 161.02, 158.81, 158.73, 153.12, 152.98, 152.69, 144.31, 140.58, 139.88, 134.05, 127.47, 126.08, 125.78, 125.74, 125.01, 109.39, 105.84, 95.87, 92.45, 71.67, 67.08, 61.02, 56.45, 56.30, 55.86, 26.86, 25.30. 19F NMR (376 MHz, chloroform-d) δ −62.49. HRMS (ESI) calcd for C35H33N2O10F3 [M + H]+: 699.21601, found 699.21497.
6-(4-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)butoxy) 2-(3,4-dichlorophenyl)-pyridazin-3(2H)-one (A23). White solid (83%), m.p.: 146.2–147.8 °C. 1H NMR (400 MHz, chloroform-d) δ 7.86 (d, J = 2.4 Hz, 1H, Ph–H), 7.60 (d, J = 2.5 Hz, 1H, Ph–H), 7.49 (s, 1H, Ph–H), 7.34 (s, 2H, Ph–H), 6.97 (d, J = 9.3 Hz, 1H, Ph–H), 6.96 (s, 1H, Ph–H), 6.49 (d, J = 2.2 Hz, 1H, Ph–H), 6.35 (d, J = 2.2 Hz, 1H, Ph–H), 4.18 (t, J = 6.2 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2CH2–O–), 4.06 (t, J = 6.1 Hz, 2H, –O–CH2CH2CH2
CH2CH2CH2–O–), 4.06 (t, J = 6.1 Hz, 2H, –O–CH2CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.95 (s, 3H, Ph–OCH3), 3.92 (d, J = 3.3 Hz, 9H, Ph–OCH3), 3.90 (s, 3H, Ph–OCH3), 1.93 (p, J = 7.8, 5.8 Hz, 2H, –O–CH2
–O–), 3.95 (s, 3H, Ph–OCH3), 3.92 (d, J = 3.3 Hz, 9H, Ph–OCH3), 3.90 (s, 3H, Ph–OCH3), 1.93 (p, J = 7.8, 5.8 Hz, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 1.89 (p, 2H, –O–CH2CH2
CH2CH2–O–), 1.89 (p, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.03, 164.04, 161.02, 158.80, 158.58, 153.07, 152.97, 152.68, 140.66, 140.58, 139.87, 133.97, 132.38, 131.25, 130.14, 127.47, 126.69, 126.07, 124.15, 109.39, 105.84, 95.86, 92.44, 71.70, 67.14, 61.03, 56.45, 56.31, 55.86, 26.86, 25.30. HRMS (ESI) calcd for C34H32N2O10Cl2 [M + H]+: 699.15068, found 699.15021.
CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.03, 164.04, 161.02, 158.80, 158.58, 153.07, 152.97, 152.68, 140.66, 140.58, 139.87, 133.97, 132.38, 131.25, 130.14, 127.47, 126.69, 126.07, 124.15, 109.39, 105.84, 95.86, 92.44, 71.70, 67.14, 61.03, 56.45, 56.31, 55.86, 26.86, 25.30. HRMS (ESI) calcd for C34H32N2O10Cl2 [M + H]+: 699.15068, found 699.15021.
6-(4-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)butoxy) 2-(3,4-difluorophenyl)-pyridazin-3(2H)-one (A24). White solid (67%), m.p.: 119.2–120.8 °C. 1H NMR (400 MHz, chloroform-d) δ 7.61 (d, J = 1.6 Hz, 1H, Ph–H), 7.48 (s, 1H, Ph–H), 7.33 (s, 2H, Ph–H), 7.20 (d, J = 9.7 Hz, 1H, Ph–H), 6.97 (d, J = 9.8 Hz, 1H, Ph–H), 6.94 (d, J = 9.8 Hz, 1H, Ph–H), 6.49 (d, J = 2.2 Hz, 1H, Ph–H), 6.35 (d, J = 2.2 Hz, 1H, Ph–H), 4.18 (t, J = 6.2 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2CH2–O–), 4.05 (t, J = 6.0 Hz, 2H, –O–CH2CH2CH2
CH2CH2CH2–O–), 4.05 (t, J = 6.0 Hz, 2H, –O–CH2CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.95 (s, 3H, Ph–OCH3), 3.91 (d, J = 3.4 Hz, 9H, Ph–OCH3), 3.90 (s, 3H, Ph–OCH3), 1.93 (dd, J = 8.0, 5.8 Hz, 2H, –O–CH2
–O–), 3.95 (s, 3H, Ph–OCH3), 3.91 (d, J = 3.4 Hz, 9H, Ph–OCH3), 3.90 (s, 3H, Ph–OCH3), 1.93 (dd, J = 8.0, 5.8 Hz, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 1.87 (dd, J = 8.5, 4.0 Hz, 2H, –O–CH2CH2
CH2CH2–O–), 1.87 (dd, J = 8.5, 4.0 Hz, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.03, 164.04, 161.02, 158.80, 158.61, 152.97, 152.68, 140.57, 139.87, 133.99, 127.35, 126.07, 121.13, 121.10, 121.07, 116.95, 116.77, 114.71, 114.51, 109.38, 105.83, 95.85, 92.43, 71.68, 67.08, 61.02, 56.44, 56.29, 55.86, 26.85, 25.29. 19F NMR (376 MHz, chloroform-d) δ −136.01, −138.47. HRMS (ESI) calcd for C34H32N2O10F2 [M + H]+: 667.20978, found 667.20874.
CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.03, 164.04, 161.02, 158.80, 158.61, 152.97, 152.68, 140.57, 139.87, 133.99, 127.35, 126.07, 121.13, 121.10, 121.07, 116.95, 116.77, 114.71, 114.51, 109.38, 105.83, 95.85, 92.43, 71.68, 67.08, 61.02, 56.44, 56.29, 55.86, 26.85, 25.29. 19F NMR (376 MHz, chloroform-d) δ −136.01, −138.47. HRMS (ESI) calcd for C34H32N2O10F2 [M + H]+: 667.20978, found 667.20874.
6-(4-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)butoxy) 2-(3-chloro-4-fluorophenyl)-pyridazin-3(2H)-one (A25). White solid (82%), m.p.: 165.9–168.1 °C. 1H NMR (400 MHz, chloroform-d) δ 7.77 (s, 1H, Ph–H), 7.60 (d, J = 4.8 Hz, 1H, Ph–H), 7.33 (s, 2H, Ph–H), 7.19 (d, J = 8.7 Hz, 1H, Ph–H), 6.96 (s, 1H, Ph–H), 6.95 (d, J = 9.8 Hz, 1H, Ph–H), 6.49 (d, J = 2.2 Hz, 1H, Ph–H), 6.35 (d, J = 2.2 Hz, 1H, Ph–H), 4.17 (t, J = 6.1 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2CH2–O–), 4.05 (t, J = 6.0 Hz, 2H, –O–CH2CH2CH2
CH2CH2CH2–O–), 4.05 (t, J = 6.0 Hz, 2H, –O–CH2CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.95 (s, 3H, Ph–OCH3), 3.91 (d, J = 3.3 Hz, 9H, Ph–OCH3), 3.90 (s, 3H, Ph–OCH3), 1.94 (p, 2H, –O–CH2
–O–), 3.95 (s, 3H, Ph–OCH3), 3.91 (d, J = 3.3 Hz, 9H, Ph–OCH3), 3.90 (s, 3H, Ph–OCH3), 1.94 (p, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 1.88 (p, 2H, –O–CH2CH2
CH2CH2–O–), 1.88 (p, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.04, 164.05, 161.03, 158.81, 158.63, 153.04, 152.98, 152.68, 140.59, 139.88, 137.91, 133.92, 127.43, 127.34, 126.08, 124.96, 124.89, 116.41, 116.19, 109.40, 105.84, 95.86, 92.45, 71.71, 67.12, 61.03, 56.46, 56.31, 55.87, 26.88, 25.31. 19F NMR (376 MHz, chloroform-d) δ −116.34. HRMS (ESI) calcd for C34H32N2O10ClF [M + H]+: 683.18023, found 683.17963.
CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.04, 164.05, 161.03, 158.81, 158.63, 153.04, 152.98, 152.68, 140.59, 139.88, 137.91, 133.92, 127.43, 127.34, 126.08, 124.96, 124.89, 116.41, 116.19, 109.40, 105.84, 95.86, 92.45, 71.71, 67.12, 61.03, 56.46, 56.31, 55.87, 26.88, 25.31. 19F NMR (376 MHz, chloroform-d) δ −116.34. HRMS (ESI) calcd for C34H32N2O10ClF [M + H]+: 683.18023, found 683.17963.
6-(4-((5,7-Dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)butoxy)-2-(naphthalen-2-yl)pyridazin-3(2H)-one (A26). White solid (65.4%), m.p.: 129.4–130.7 °C. 1H NMR (400 MHz, chloroform-d) δ 8.16 (d, J = 1.9 Hz, 1H, Ph–H), 7.89 (d, J = 8.6 Hz, 1H, Ph–H), 7.86 (d, J = 3.8 Hz, 1H, Ph–H), 7.85 (t, 1H, Ph–H), 7.77 (t, 1H, Ph–H), 7.49 (d, J = 3.9 Hz, 1H, Ph–H), 7.47 (s, 1H, Ph–H), 7.33 (s, 2H, Ph–H), 7.04 (d, J = 9.7 Hz, 1H, Ph–H), 6.98 (d, J = 9.7 Hz, 1H, Ph–H), 6.49 (d, J = 2.2 Hz, 1H, Ph–H), 6.35 (d, J = 2.2 Hz, 1H, Ph–H), 4.20 (t, J = 6.1 Hz, 2H, –O–
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2CH2–O–), 4.06 (t, J = 6.0 Hz, 2H, –O–CH2CH2CH2
CH2CH2CH2–O–), 4.06 (t, J = 6.0 Hz, 2H, –O–CH2CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) –O–), 3.95 (s, 3H, Ph–OCH3), 3.90 (s, 6H, Ph–OCH3), 3.90 (d, J = 1.2 Hz, 6H, Ph–OCH3), 2.00 (p, 2H, –O–CH2
–O–), 3.95 (s, 3H, Ph–OCH3), 3.90 (s, 6H, Ph–OCH3), 3.90 (d, J = 1.2 Hz, 6H, Ph–OCH3), 2.00 (p, 2H, –O–CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2–O–), 1.88 (p, J = 8.0, 3.4 Hz, 2H, –O–CH2CH2
CH2CH2–O–), 1.88 (p, J = 8.0, 3.4 Hz, 2H, –O–CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.07, 164.04, 161.03, 159.04, 158.82, 153.01, 152.97, 152.71, 140.61, 139.87, 139.05, 133.95, 133.10, 132.42, 128.45, 128.42, 127.64, 127.04, 126.53, 126.44, 126.09, 123.69, 123.22, 109.41, 105.86, 95.86, 92.44, 71.86, 67.02, 61.04, 56.45, 56.31, 55.87, 26.94, 25.37. HRMS (ESI) calcd for C38H36N2O10 [M + H]+: 681.24427, found 681.24341.
CH2–O–). 13C NMR (101 MHz, chloroform-d) δ 174.07, 164.04, 161.03, 159.04, 158.82, 153.01, 152.97, 152.71, 140.61, 139.87, 139.05, 133.95, 133.10, 132.42, 128.45, 128.42, 127.64, 127.04, 126.53, 126.44, 126.09, 123.69, 123.22, 109.41, 105.86, 95.86, 92.44, 71.86, 67.02, 61.04, 56.45, 56.31, 55.87, 26.94, 25.37. HRMS (ESI) calcd for C38H36N2O10 [M + H]+: 681.24427, found 681.24341.
2.3 Biological activity testing
3. Results and discussion
3.1 Chemistry
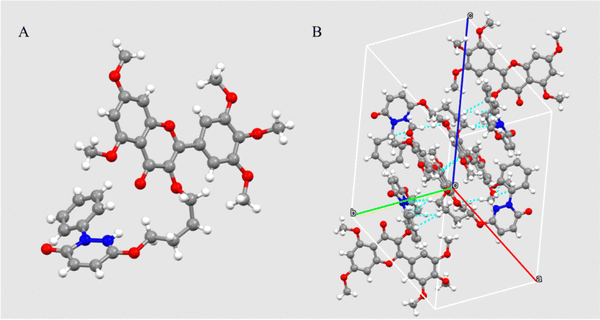
Twenty-six target compounds, numbered A1–A26, were synthesized according to the above experimental route. All compounds were structurally characterized by NMR spectroscopy and HRMS, and the detailed data are listed in the ESI.† To further clarify the structures of the target compounds, the crystal structure of A14 was cultured and analyzed by X-ray single crystal diffraction. The crystal structure and cell stacking of A14 (CCDC: 2247966) are shown in Fig. 2, and the detailed data are listed in the ESI.†3.2 In vivo antiviral activity
The antiviral activity of A1–A26 against TMV was determined using the half-leaf blotch method with NNM as the positive control. The detailed data are shown in Table 1. The curative activities of A4, A8, A23 and A26 against TMV were 74.8, 71.7, 73.5 and 76.8%, respectively, which were superior to that of NNM (68.0%), while the protective activities of A24 and A26 were 78.1 and 69.5%, respectively, which were superior to that of NNM (62.2%). The results of A26 and NNM against TMV were characterized on the leaf as shown in Fig. 3. The EC50 values of the compounds against TMV were tested and are shown in Table 2. The antiviral curative EC50 values of A4, A23 and A26 were 131.6, 138.5 and 118.9 μg mL−1, respectively, which were superior to that of the control drug NNM (235.6 μg mL−1). The antiviral protective EC50 values of A24 and A26 were 117.4 and 162.5 μg mL−1, respectively, which were superior to that of the control drug NNM (263.2 μg mL−1).| Compd. | n | R | Curative (%) | Protection (%) | Inactivation (%) |
|---|---|---|---|---|---|
| a Average of the three replicates. b Commercial antiviral agent NNM. | |||||
| A1 | 3 | H | 59.9 ± 6.1 | 36.6 ± 4.4 | 50.1 ± 5.8 |
| A2 | 3 | 4-CH3 | 51.6 ± 1.6 | 59.4 ± 7.1 | 61.3 ± 4.0 |
| A3 | 3 | 4-F | 39.8 ± 2.8 | 58.8 ± 9.8 | 37.3 ± 4.0 |
| A4 | 3 | 4-Cl | 74.8 ± 6.0 | 64.1 ± 4.1 | 50.1 ± 4.4 |
| A5 | 3 | 3-Br | 64.2 ± 3.8 | 67.9 ± 6.7 | 43.7 ± 8.7 |
| A6 | 3 | 3-OCH3 | 65.2 ± 4.2 | 68.0 ± 3.0 | 62.6 ± 1.5 |
| A7 | 3 | 4-i-Pr | 62.3 ± 2.3 | 56.0 ± 2.4 | 31.0 ± 0.9 |
| A8 | 3 | 4-t-Bu | 71.7 ± 0.6 | 47.2 ± 8.1 | 40.6 ± 5.2 |
| A9 | 3 | 4-CF3 | 55.2 ± 8.9 | 56.2 ± 2.2 | 49.6 ± 4.7 |
| A10 | 3 | 3,4-di-Cl | 57.0 ± 4.3 | 55.7 ± 5.9 | 54.3 ± 2.5 |
| A11 | 3 | 3,4-di-F | 47.5 ± 6.4 | 62.8 ± 4.2 | 47.8 ± 3.7 |
| A12 | 3 | 3-Cl-4-F | 49.7 ± 7.2 | 59.9 ± 4.0 | 50.6 ± 8.1 |
| A13 | 3 | Ph | 55.4 ± 3.5 | 67.4 ± 4.8 | 51.9 ± 2.7 |
| A14 | 4 | H | 67.0 ± 3.0 | 60.5 ± 7.2 | 47.9 ± 3.7 |
| A15 | 4 | 4-CH3 | 65.5 ± 1.3 | 62.0 ± 2.0 | 63.2 ± 7.0 |
| A16 | 4 | 4-F | 44.7 ± 4.7 | 67.1 ± 1.0 | 57.7 ± 6.9 |
| A17 | 4 | 4-Cl | 57.4 ± 4.0 | 57.2 ± 5.9 | 64.9 ± 8.6 |
| A18 | 4 | 3-Br | 66.7 ± 6.1 | 64.4 ± 3.0 | 49.4 ± 0.2 |
| A19 | 4 | 3-OCH3 | 58.6 ± 1.9 | 52.2 ± 6.9 | 52.3 ± 5.1 |
| A20 | 4 | 4-i-Pr | 61.2 ± 6.5 | 44.0 ± 6.1 | 54.3 ± 2.5 |
| A21 | 4 | 4-t-Bu | 54.5 ± 2.0 | 56.3 ± 4.2 | 30.2 ± 5.1 |
| A22 | 4 | 4-CF3 | 47.8 ± 2.4 | 60.8 ± 4.3 | 61.3 ± 8.2 |
| A23 | 4 | 3,4-di-Cl | 73.5 ± 5.6 | 62.9 ± 5.6 | 63.0 ± 2.9 |
| A24 | 4 | 3,4-di-F | 52.5 ± 3.4 | 78.1 ± 5.3 | 37.2 ± 5.6 |
| A25 | 4 | 3-Cl-4-F | 62.0 ± 3.3 | 54.5 ± 7.6 | 60.9 ± 1.2 |
| A26 | 4 | Ph | 76.8 ± 3.3 | 69.5 ± 9.6 | 67.9 ± 3.4 |
| NNM | — | 68.0 ± 6.0 | 62.2 ± 6.4 | 86.3 ± 4.0 | |
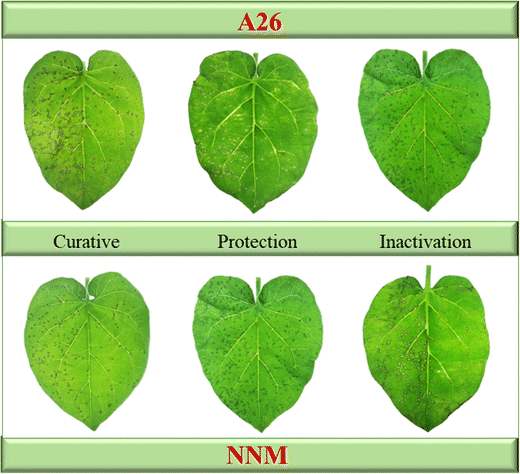 | ||
| Fig. 3 Tobacco leaf morphology effects of A26 and NNM against tobacco mosaic virus in vivo (left leaf: not treated with the compound; right leaf: smeared with the compound). | ||
| Compd | n | R | Regression equation | r | EC50 (μg mL−1) | |
|---|---|---|---|---|---|---|
| a Average of the three replicates. b Commercial antiviral agent NNM. | ||||||
| Curative activity | A4 | 3 | 4-Cl | y = 1.0702x + 2.7319 | 0.9888 | 131.6 |
| A8 | 3 | 4-t-Bu | y = 1.0736x + 2.6653 | 0.9961 | 149.5 | |
| A23 | 4 | 3,4-di-Cl | y = 1.0882x + 2.6698 | 0.9649 | 138.5 | |
| A26 | 4 | Ph | y = 1.1059x + 2.6962 | 0.9903 | 118.9 | |
| NNMb | — | — | y = 0.9651x + 2.7107 | 0.9629 | 235.6 | |
| Protection activity | A24 | 4 | 3,4-di-F | y = 1.1000x + 2.7234 | 0.9772 | 117.4 |
| A26 | 4 | Ph | y = 0.8650x + 3.0875 | 0.9755 | 162.5 | |
| NNMb | — | — | y = 0.9057x + 2.8079 | 0.9748 | 263.2 | |
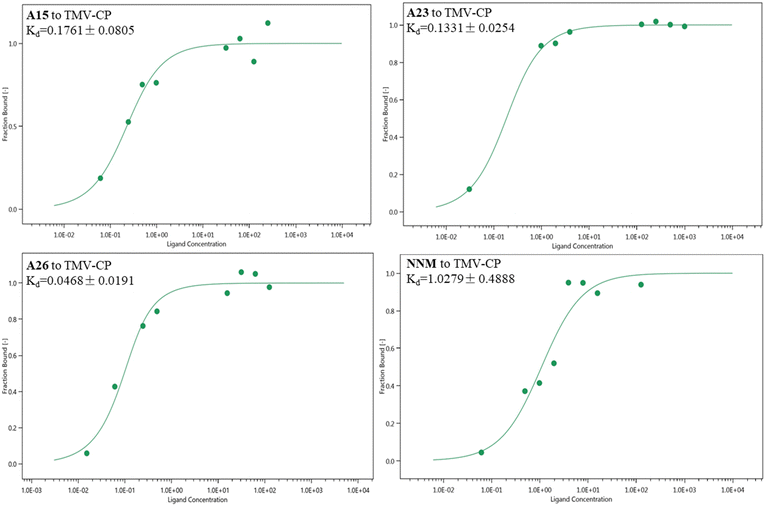
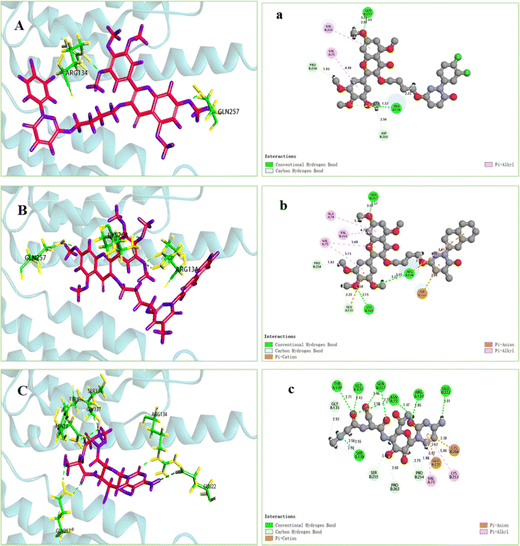
3.3 Microscale thermophoresis analysis
The affinity of the target compounds with TMV-CP was illustrated by the MST test, and the binding affinity was evaluated using the Kd value to accurately reflect the intermolecular interactions. The results of the MST test are shown in Fig. 4, and the Kd values of the binding affinities of A15, A23, A26 and NNM with TMV-CP were 0.176, 0.133, 0.047 and 1.028 μM, respectively, and the detailed data are listed in Table 3. It can be seen that the Kd values of A15, A23, and A26 with TMV-CP were significantly better than that of the control agent NNM, which indicated that the compounds had a stronger binding ability to TMV-CP than that of the control agent.3.4 Molecular docking analysis
The binding mode of the drug molecules was explored through molecular docking experiments to simulate the interaction of drugs A23 and A26 with TMV-CP, using NNM as a positive control. As shown in Fig. 5, both A23 and A26 exhibited hydrogen bonding interactions with key amino acid residues, GLN257 and ARG134 of TMV-CP. NNM exhibits hydrogen bonding interactions with several amino acid residues including GLN257 and ARG134. It could be seen that the bond lengths of the hydrogen bonds produced by the binding of A23 to GLN257 and ARG134 were 1.60 and 1.57 Å, respectively, and that of the hydrogen bond produced by the binding of A26 to GLN257 was 1.57 Å, whereas those of the hydrogen bonds produced by the binding of NNM to GLN257 and ARG134 were 2.74 and 1.85 Å, respectively. Although the docking of NNM to TMV-CP formed more hydrogen bonds, in terms of bond length, the drug molecules exhibited shorter hydrogen bond lengths and would bind more tightly to TMV-CP.3.5 Analysis of chlorophyll content in tobacco
The chlorophyll content in plants is closely related to the health status of plants, and chlorophyll not only plays a decisive role in photosynthesis but also improves the disease resistance of plants. As can be seen from Fig. 6, in the healthy group, the chlorophyll content in the CK control plants changed slightly over time, but the overall chlorophyll content in the tobacco leaves treated with A26 was higher than that in the CK group and peaked on the fifth day. The chlorophyll content in plants treated with A26 in the susceptible group was significantly higher than that in the TMV control group. The results showed that A26 could be involved in regulating the chlorophyll content in plants, promoting plant photosynthesis, mobilizing the plant's defense ability, and then improving the plant's disease resistance.3.6 Analysis of malondialdehyde content
The presence of unfavorable environments during plant growth triggers the action of oxygen radicals on lipids in the plant, resulting in membrane lipid peroxidation and the production of a complex series of compounds, including malondialdehyde. The degree of plant membrane damage and plant disease resistance was studied by testing the malondialdehyde content. As shown in Fig. 7, the overall malondialdehyde content in the leaves of the CK group showed an increasing trend with time and reached the peak on the 3rd day, which indicated that the plants contained antioxidant enzymes in the plant body during the senescence process, which had a certain regulatory effect. The malondialdehyde content in the A26-treated plants was significantly lower than that in the CK group, indicating that the drug molecules could inhibit tobacco from being subjected to adversity stress. The malondialdehyde content in plants of the TMV-sensitive group was significantly increased and was the highest among the groups, which fully responded to the serious damage caused by TMV to tobacco. The malondialdehyde content in plants of the A26 + TMV-sensitive group was significantly lower than that in the TMV-sensitive group, which showed that TMV was the most effective drug in the TMV-sensitive group, and that it could be used in the TMV-sensitive group. The drug molecules could inhibit the injury of TMV on leaves and reduce the destruction speed. In summary, the drug molecule A26 could inhibit TMV from infecting plants, participate in the regulation of membrane lipid peroxidation, inhibit the production of malondialdehyde in tobacco, and enhance the antioxidant capacity and disease resistance of plants.4. Conclusion
In summary, a series of myricetin derivatives containing pyridazinone were designed and synthesized. The results of antiviral activity tests showed that some of the target compounds exhibited good antiviral activity. Among them, A4, A23 and A26 exhibited good curative activity with EC50 values of 131.6, 138.5 and 118.9 μg mL−1, respectively, which were better than that of the control drug NNM (235.6 μg mL−1). A24 and A26 exhibited good protective activity with EC50 values of 117.4 and 162.5 μg mL−1, respectively, which were better than that of the control drug NNM (263.2 μg mL−1). The biological activities of A23 and A26 were further confirmed by microcalorimetric thermophoretic assays and molecular docking to investigate the mechanism of action of the drug molecules, which were tightly bound to TMV-CP with higher binding affinity and shorter hydrogen bonds to exert antiviral effects. In addition, the chlorophyll and malondialdehyde contents of tobacco plants were tested, and it was found that the drug molecules could be involved in regulating the chlorophyll content of plants, promoting plant photosynthesis, participating in the regulation of membrane lipid peroxidation, inhibiting the TMV-infected plants, mobilizing the plant's defense ability, and thus enhancing the antioxidant ability and disease resistance of plants. Therefore, myricetin derivatives containing pyridazinone offer a reference point for the development of novel antiviral pesticides with great potential for development.Author contributions
Wei Xue: conceived and designed the experiments. Provide experiment drawing software and method. Li Xing: performed the experiments, analyzed the data and writing-original draft preparation. Da Liu, Youshan An, Yishan Qin: evaluated the antiviral activities of the target compounds. Hui Xin, Tianyu Deng, Kaini Meng: provided the material for evaluating the antiviral activities. All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no competing financial interest. All authors have contributed to the work, read the manuscript, and agreed to be listed as authors.Acknowledgements
The authors gratefully acknowledge the Science Foundation of Guizhou Province (No. 20192452) and the Key Laboratory of Institute of Environment and Plant Protection (No. HZSKFKT202208).References
- G. P. Rao, et al., Overview of yield losses due to plant viruses, Appl. Plant Virol., 2020, 38, 531–562, DOI:10.1016/B978-0-12-818654-1.00038-4.
- A. Boualem, et al., The battle for survival between viruses and their host plants, Curr. Opin. Virol., 2016, 17, 32–38, DOI:10.1016/j.coviro.2015.12.001.
- X. Ding, et al., Marine sesquiterpenes for plant protection: discovery of laurene sesquiterpenes and their derivatives as novel antiviral and antiphytopathogenic fungal agents, J. Agric. Food Chem., 2022, 70, 6006–6014, DOI:10.1021/acs.jafc.2c00664.
- L. Verhage, How tobacco mosaic virus goes the distance, Plant J., 2021, 106, 894–895, DOI:10.1111/tpj.15309.
- M. J. Adams, et al., ICTV virus taxonomy profile: virgaviridae, J. Gen. Virol., 2017, 98, 1999–2000, DOI:10.1099/jgv.0.000884.
- M. C. Marqués, et al., Diagnostics of infections produced by the plant viruses TMV, TEV, and PVX with CRISPR-Cas12 and CRISPR-Cas13, ACS Synth. Biol., 2022, 11, 2384–2393, DOI:10.1021/acssynbio.2c00090.
- C. Y. Liu, et al., Transcriptome sequencing reveals that photoinduced gene IP-L affects the expression of PsbO to response to virus infection in Nicotiana benthamiana, Physiol. Mol. Plant Pathol., 2021, 114, 101613, DOI:10.1016/j.pmpp.2021.101613.
- M. B. Wang, et al., RNA silencing and plant viral diseases, Mol. Plant-Microbe Interact., 2012, 25, 1275–1285, DOI:10.1094/Mpmi-04-12-0093-Cr.
- L. Jiang, et al., Fabrication of an alginate-based ZhiNengCong gel showed an enhanced antiviral and plant growth promoting functions, Pestic. Biochem. Physiol., 2023, 191, 105373, DOI:10.1016/j.pestbp.2023.105373.
- L. Zhao, et al., Advances and prospects in biogenic substances against plant virus: a review, Pestic. Biochem. Physiol., 2017, 135, 15–26, DOI:10.1016/j.pestbp.2016.07.003.
- X. Zhao, et al., Development strategies and prospects of nano-based smart pesticide formulation, J. Agric. Food Chem., 2017, 66, 6504–6512, DOI:10.1021/acs.jafc.7b02004.
- C. J. Li, et al., The present situation of pesticide residues in China and their removal and transformation during food processing, Food Chem., 2021, 354, 129552, DOI:10.1016/j.foodchem.2021.129552.
- A. Basit, et al., Enhancement of resistance by poultry manure and plant hormones (salicylic acid & citric acid) against tobacco mosaic virus, Saudi J. Biol. Sci., 2021, 28, 3526–3533, DOI:10.1016/j.sjbs.2021.03.025.
- W. H. Guo, et al., Anti-TMV activity and mode of action of three alkaloids isolated from chelidonium majus, Pest Manage. Sci., 2021, 77, 510–517, DOI:10.1002/ps.6049.
- K. W. Lee, et al., Myricetin is a novel natural inhibitor of neoplastic cell transformation and MEK1, Carcinogenesis, 2007, 28, 1918–1927, DOI:10.1093/carcin/bgm110.
- Y. Tong, et al., Analgesic activity of myricetin isolated from myrica rubra sieb. et zucc. leaves, Arch. Pharm. Res., 2009, 32, 527–533, DOI:10.1007/s12272-009-1408-6.
- S. H. Häkkinen, et al., Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries, J. Agric. Food Chem., 1999, 47, 2274–2279, DOI:10.1021/jf9811065.
- Z. Javed, et al., Myricetin: targeting signaling networks in cancer and its implication in chemotherapy, Cancer Cell Int., 2022, 22, 239, DOI:10.1186/s12935-022-02663-2.
- S. Cho, et al., Synthesis and physicochemical characterization of acyl myricetins as potential anti-neuroexocytotic agents, Sci. Rep., 2023, 13, 5136, DOI:10.1038/s41598-023-32361-6.
- W. Xue, et al., Novel myricetin derivatives: design, synthesis and anticancer activity, Eur. J. Med. Chem., 2015, 97, 155–163, DOI:10.1016/j.ejmech.2015.04.063.
- M. Chen, et al., Antibacterial and antiviral activities and action mechanism of flavonoid derivatives with a benzimidazole moiety, J. Saudi Chem. Soc., 2021, 25, 101194, DOI:10.1016/j.jscs.2020.101194.
- X. Tang, et al., Synthesis and antiviral activity of novel myricetin derivatives containing ferulic acid amide scaffolds, New J. Chem., 2020, 44, 2374–2379, 10.1039/c9nj05867b.
- T. T. Liu, et al., Design, synthesis, antibacterial activity, antiviral activity, and mechanism of myricetin derivatives containing a quinazolinone moiety, ACS Omega, 2021, 6, 30826–30833, DOI:10.1021/acsomega.1c05256.
- Y. Chen, et al., Synthesis and antibacterial and antiviral activities of myricetin derivatives containing a 1,2,4-triazole Schiff base, RSC Adv., 2019, 9, 23045–23052, 10.1039/c9ra05139b.
- S. J. Su, et al., Design, synthesis, and antibacterial activity of novel myricetin derivatives containing sulfonate, Monats Chem., 2021, 152, 345–356, DOI:10.1007/s00706-021-02739-1.
- S. C. Jiang, et al., Design, synthesis and antibacterial activities against Xanthomonas oryzae pv. oryzae, Xanthomonas axonopodis pv. Citri and Ralstonia solanacearum of novel myricetin derivatives containing sulfonamide moiety, Pest Manage. Sci., 2020, 76, 853–860, DOI:10.1002/ps.5587.
- X. Cao, et al., Design, synthesis and bioactivity of myricetin derivatives for control of fungal disease and tobacco mosaic virus disease, RSC Adv., 2023, 13, 6459–6465, 10.1039/d2ra08176h.
- F. Liu, et al., Design, synthesis, biological activity evaluation and action mechanism of myricetin derivatives containing thiazolebisamide, Chem. Biodiversity, 2023, 20, e202201103, DOI:10.1002/cbdv.202201103.
- S. Cao, et al., Synthesis and antifeedant activity of new oxadiazolyl 3(2H)-pyridazinones, J. Agric. Food Chem., 2002, 51, 152–155, DOI:10.1021/jf0208029.
- M. Asif, Some recent approaches of biologically active substituted pyridazine and phthalazine drugs, Curr. Med. Chem., 2012, 19, 2984–2991, DOI:10.2174/092986712800672139.
- X. J. Zou, et al., Synthesis, fungicidal activity, and 3D-QSAR of pyridazinone-substituted 1,3,4-oxadiazoles and 1,3,4-thiadiazoles, J. Agric. Food Chem., 2002, 50, 3757–3760, DOI:10.1021/jf0201677.
- X. Zhang, et al., Design, synthesis and anti-tumor evaluation of 1,2,4-triazol-3-one derivatives and pyridazinone derivatives as novel CXCR2 antagonists, Eur. J. Med. Chem., 2021, 226, 113812, DOI:10.1016/j.ejmech.2021.113812.
- X. W. Wu, et al., Design, synthesis and biological evaluation of pyrazolo[3,4-d]pyridazinone derivatives as covalent FGFR inhibitors, Acta Pharm. Sin. B, 2021, 11, 781–794, DOI:10.1016/j.apsb.2020.09.002.
- M. Sonmez, et al., Synthesis, antibacterial and antifungal activity of some new pyridazinone metal complexes, Eur. J. Med. Chem., 2006, 41, 101–105, DOI:10.1016/j.ejmech.2005.10.003.
- Y. M. Youssef, et al., Synthesis and antioxidant, antimicrobial, and antiviral activity of some pyrazole-based heterocycles using a 2(3H)-furanone derivative, J. Iran. Chem. Soc., 2023, 20, 2203–2216, DOI:10.1007/s13738-023-02814-w.
- I. G. Rathish, et al., Synthesis and blood glucose lowering effect of novel pyridazinone substituted benzenesulfonylurea derivatives, Eur. J. Med. Chem., 2009, 44, 2673–2678, DOI:10.1016/j.ejmech.2008.12.013.
- I. Allart-Simon, et al., Pyridazinone derivatives as potential anti-inflammatory agents: synthesis and biological evaluation as PDE4 inhibitors, RSC Med. Chem., 2021, 12, 584–592, 10.1039/d0md00423e.
- Ł. Szczukowski, et al., Design, synthesis, biological evaluation and in silico studies of novel pyrrolo[3,4-d]pyridazinone derivatives with promising anti-inflammatory and antioxidant activity, Bio Chem., 2020, 102, 104035, DOI:10.1016/j.bioorg.2020.104035.
- X. D. Cao, et al., Synthesis and biological evaluation of new 6-hydroxypyridazinone benzisoxazoles: potential multi-receptor-targeting atypical antipsychotics, Eur. J. Med. Chem., 2016, 124, 713–728, DOI:10.1016/j.ejmech.2016.09.008.
- X. D. Cao, et al., Synthesis and biological evaluation of novel σ1 receptor ligands for treating neuropathic pain: 6-hydroxypyridazinones, J. Med. Chem., 2016, 59, 2942–2961, DOI:10.1021/acs.jmedchem.5b01416.
- R. S. Shainova, et al., Synthesis and biological evaluation of 3-O-substituted 1-benzyl-6-oxo-1,6-dihydropyridazine derivatives, J. Chem. Res., 2019, 43, 352–358, DOI:10.1177/1747519819866402.
- S. C. Jiang, et al., Antibacterial activities of novel dithiocarbamate-containing 4H-chro-men-4-one derivatives, J. Agric. Food Chem., 2020, 68, 5641–5647, DOI:10.1021/acs.jafc.0c01652.
- F. Peng, et al., Novel 1,3,4-oxadiazole sulfonate/carboxylate flavonoid derivatives: synthesis and biological activity, Pest Manage. Sci., 2022, 79, 274–283, DOI:10.1002/ps.7197.
- F. Peng, et al., Antibacterial and antiviral activities of 1,3,4-oxadiazole thioether 4H-chromen-4-one derivatives, J. Agric. Food Chem., 2021, 69, 11085–11094, DOI:10.1021/acs.jafc.1c03755.
- T. T. Liu, et al., Design, synthesis, biological activity evaluation and mechanism of action of myricetin derivatives containing thioether quinazolinone, Arab. J. Chem., 2022, 15, 104019, DOI:10.1016/j.arabjc.2022.104019.
- X. Zhou, et al., Design, synthesis and anti-TMV activity of novel alpha-aminophospho-nate derivatives containing a chalcone moiety that induce resistance against plant dise-ase and target the TMV coat protein, Pestic. Biochem. Physiol., 2021, 172, 104749, DOI:10.1016/j.pestbp.2020.104749.
- X. Cao, et al., Design, synthesis, bioactivity and mechanism of action of novel myricetin derivatives containing amide and hydrazide, Arab. J. Chem., 2023, 16, 104588, DOI:10.1016/j.arabjc.2023.104588.
- F. A. C. Soares, et al., Thiobarbituric acid reactive substances in dogs with spontaneous hypercortisolism, Domest. Anim. Endocrin., 2021, 77, 106634, DOI:10.1016/j.domaniend.2021.106634.
- N. Sun, et al., Design, synthesis, and bioactivity of chalcone derivatives containing indanone, ACS Omega, 2023, 8, 2556–2563, DOI:10.1021/acsomega.2c07071.
Footnote |
| † Electronic supplementary information (ESI) available: The NMR and HRMS data for A1–A26. CCDC 2247966. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3nj04902g |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2024 |