Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Non-covalent attachment of BODIPY-caged amines to graphene and their localized photocleavage†
Erich
See‡
,
Elsa
Korhonen‡
 ,
Maija
Nissinen
,
Maija
Nissinen
 and
Mika
Pettersson
and
Mika
Pettersson
 *
*
Nanoscience Center, Department of Chemistry, University of Jyvaskyla, P.O. BOX 35, 40014, Finland. E-mail: mika.j.pettersson@jyu.fi
First published on 27th November 2023
Abstract
We describe the synthesis of a novel boron-dipyrromethene (BODIPY)-caged amine capable of adhering to a pristine graphene surface via non-covalent bonding and demonstrate the release of the amine with green (530–540 nm) light both in solution and adhered to a graphene surface.
The functionalization of graphene is the primary method of altering and tuning its properties, such as dispersibility, band-gap and affinity for specific biomolecules, among others.1 While some techniques exist for selective oxidation,2–4 covalent functionalization,5,6 or targeted metallization7 of selected areas of pristine graphene, most functionalization methods are uncontrolled, random, or require the use of chemical or physical masks to ‘protect’ areas of the graphene.1 Spatially-selective functionalization is of interest because it allows for more complex variation, such as regional control of hydrophobicity, specifically selected chemical attachment points, the ability to guide cell growth via surface group functionalization,8 and other tailored surface properties.
A possible solution for selective functionalization is utilizing photoprotecting groups (PPGs) to create a programmable surface. PPGs have been previously used to create functionalized surfaces that can be selectively altered by applying light at the appropriate wavelength after deposition.9–12 Additionally, once the entire surface has been functionalized with a PPG, it can be patterned later without additional chemicals, and different areas of a PPG-coated surface can be activated in multiple successive photopatternings if different chemical conjugations are desired. However, despite their widespread use in other fields, we have found no literature discussing the use of photoprotecting groups (PPGs) in conjunction with the functionalization of graphene. PPG-capped linker molecules could also prove a possible solution to the recently discovered problem of 2-D material contamination, by providing linkers capped by a photoreactive moiety to protect the surface from contamination until chemical conjugation is desired.13
A relatively recent but extensively studied addition to the field of PPGs is boron-dipyrromethene (BODIPY)-based PPGs, which have shown to be biocompatible, easily tuneable, and highly selective in their photocleavage range.14–21 Additionally, they can cleave in optical ranges that are less harmful to living cells, such as the green and red-light regions. Further, BODIPY PPGs can be used to cap a wide range of cargo, including primary amines, such as dopamine and histamine, and carboxylic acids, such as nitrophenylacetic acid.14 PPG-capped amines have been used, for example, in controlled nanoparticle self-assembly22 and altering the beating frequency of cardiomyocytes.17 This makes them an ideal choice for proof-of-principle PPGs to cap a graphene-attaching moiety and for surface functionalization, extending this flexibility and versatility to graphene surfaces.
In order to add a new spatially-selective method to the toolbox of functionalizing graphene, we set out to synthesize a BODIPY-based photocleavable PPG that protects a primary amine and is capable of adhering to a pristine graphene substrate. The proposed method is a proof-of-concept approach toward creating selected areas of free amines on a graphene surface at will, without requiring any additional chemical moieties after the initial functionalization.
The photocleavable compound 5 was designed for non-covalent graphene functionalization by π–π stacking of the phenyl ring with the graphene surface (Scheme 1). A phenyl ring was selected as an attachment point to graphene, as based on previous theoretical and experimental studies, a phenyl ring is enough to induce π–π stacking interaction with graphene.23–25 After photoexposure, the BODIPY group could be cleaved, releasing free amines (5a) attached to the graphene surface via weak interactions. An amine group was selected as the target functionality due to its key role in a variety of biological compounds and its potential for targeted binding.8,17,22
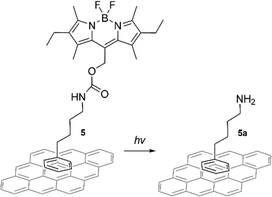 | ||
| Scheme 1 Schematic presentation of the proposed photocleavage route. Only one possible π–π stacking orientation on the graphene surface via phenyl ring is shown for clarity. Other possible stacking modes are shown in ESI,† Fig. S22. | ||
The synthesis of BODIPY-capped amine 5 is shown in Scheme 2 and described in detail in the ESI.† The success of the synthesis of target compound 5 was confirmed by NMR spectroscopy and HR-MS (see ESI,† Fig. S4–S11). The existence of two different conformations (anti and syn) was confirmed by 2D NOESY NMR spectroscopy (ESI,† Fig. S10) of which the anti conformation is more favourable.
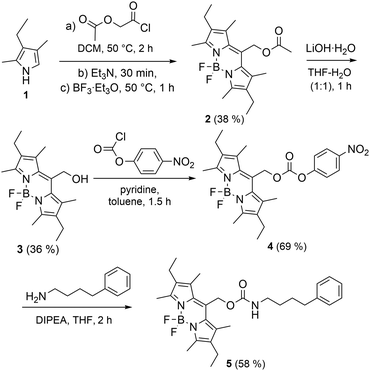 | ||
| Scheme 2 The synthesis of BODIPY derivative 5. The yields of each step are shown in parenthesis. Only anti conformation of 5 is shown. | ||
Photochemical activity and photocleaving properties of the BODIPY compound 5 were first investigated by fluorescence and absorption spectroscopy in 1 μM acetonitrile–water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) solution (see ESI†). The absorption and fluorescence spectra (Fig. 1) were measured from the native solution and then after illumination with a 520 nm LED (50 mW) every five minutes (0–35 min). As expected, the absorption peak for the BODIPY core at 542 nm and the fluorescence intensity decreased with increasing illumination time, signifying its decomposition. The change is typical for this type of photodissociation process and for BODIPY molecules in general.15,26 A decrease in the 350–450 nm band in the absorption spectra after approximately 20 min exposure corresponds with the measurements of similar BODIPY compounds.15,17 We also observed an increase in absorption peak at approximately 210 nm and a broad increase in the 250–300 nm range, which correlates to the free amine based on the measured reference spectrum (ESI,† Fig. S13). These results indicate successful photocleavage in solution and are in accordance with the earlier results with similar compounds.15 In addition, the success of photocleavage (release of amine) was confirmed by NMR and HR-MS experiments (ESI,† Fig. S14–S20). Similarly to the previous studies of BODIPY dyes,27–29 NMR studies indicate the photodegradation of the BODIPY core: after irradiation, peaks related to BODIPY core disappeared and new peaks potentially derived from the degradation products arose (ESI,† Fig. S14).
3) solution (see ESI†). The absorption and fluorescence spectra (Fig. 1) were measured from the native solution and then after illumination with a 520 nm LED (50 mW) every five minutes (0–35 min). As expected, the absorption peak for the BODIPY core at 542 nm and the fluorescence intensity decreased with increasing illumination time, signifying its decomposition. The change is typical for this type of photodissociation process and for BODIPY molecules in general.15,26 A decrease in the 350–450 nm band in the absorption spectra after approximately 20 min exposure corresponds with the measurements of similar BODIPY compounds.15,17 We also observed an increase in absorption peak at approximately 210 nm and a broad increase in the 250–300 nm range, which correlates to the free amine based on the measured reference spectrum (ESI,† Fig. S13). These results indicate successful photocleavage in solution and are in accordance with the earlier results with similar compounds.15 In addition, the success of photocleavage (release of amine) was confirmed by NMR and HR-MS experiments (ESI,† Fig. S14–S20). Similarly to the previous studies of BODIPY dyes,27–29 NMR studies indicate the photodegradation of the BODIPY core: after irradiation, peaks related to BODIPY core disappeared and new peaks potentially derived from the degradation products arose (ESI,† Fig. S14).
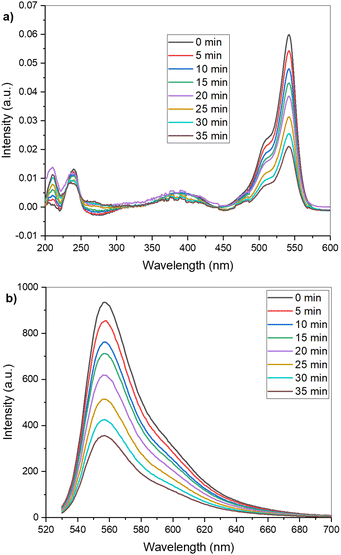 | ||
| Fig. 1 Evolution of the absorption (a) and fluorescence (b) spectra of 5 over time under 520 nm illumination. | ||
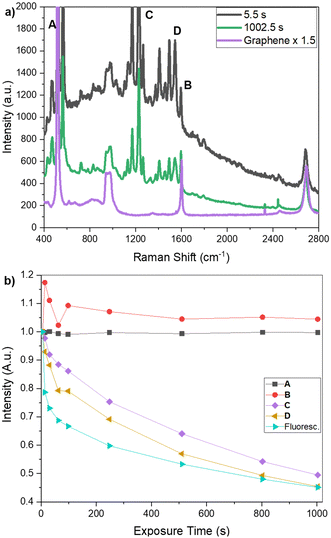
Raman spectroscopy was used both to characterize and cleave compound 5 on graphene after surface functionalization via the evaporation method. The surface was probed with a 532 nm laser, and the spectra were measured after increasing exposure times (Fig. 2). A wavelength capable of photocleavage was selected to ensure that the measured spot would be the photoexposed position. Comparing the Raman spectra of bare graphene (enhanced 1.5× for scale) to the spectra of graphene functionalized with 5 (Fig. 2a), we can see a clear difference, confirming that 5 adhered to the surface. The Raman peaks not belonging to graphene (1600, 2690 cm−1) or silicon (520 cm−1, the broad band 930–990 cm−1) were assigned to 5. At only 5.5 s of exposure, the Raman spectrum showed a broad fluorescence emission in addition to sharp Raman peaks. As compound 5 is strongly fluorescent, the Raman spectrum would normally be very difficult to observe due to the overwhelming intensity of fluorescence. However, when compound 5 is bound on graphene, the fluorescence is strongly quenched, presumably by surface energy transfer, revealing the Resonance Raman (RR) scattering peaks in the spectrum.
During the increasing exposure, most of the peaks and the overall fluorescence decreased in intensity, while the graphene and Si peaks remained relatively unchanged. As the change in the baseline fluorescence made it difficult to clearly see the stability of the Si and graphene peaks compared to the expected photoreactivity of the peaks attributed to 5, we integrated the area under a set of selected peaks (Fig. 2; Si peak (A: 520 cm−1), a graphene peak (B: 1600 cm−1), and two peaks attributed to 5 (C: 1220 cm−1 and D: 1544 cm−1)) using a point-to-point baseline, excluding the fluorescence from the calculation. We also calculated the area beneath the piecewise fit used to calculate fluorescence at increasing exposure times. These values are charted in Fig. 2b as a fraction of their initial value to quantitatively demonstrate the change with increasing exposure times. A and B – the Si and graphene peaks – were at approximately 1 and 1.05 times their original values, respectively, after 1000 s of exposure. Conversely, the peaks assigned to 5 – C and D – and the overall fluorescence, had dropped by over 50% after 1000 s of exposure, ending at 0.49, 0.45, and 0.45 their initial values, respectively. This indicates the photodegradation of 5 on the stable graphene surface and qualitatively shows that this was not simply the photobleaching of the graphene but the adhesion of a photoreactive molecule to the graphene substrate. The increase in peak B over its initial value can be readily attributed to small errors in the fitting of the fluorescence at earlier timepoints. These peaks were selected to demonstrate the photoreactive effect but should not be considered a full analysis.
Because the primary absorption peak of the free amine is in the 260 nm range, we do not expect to see it in the RR spectrum excited at 532 nm. However, we can deduce the existence of the free amine indirectly by looking closely at the G band (peak B). The G band showed a downshift shift in the peak position for all samples where compound 5 had been applied, which is expected for a molecule binding to graphene, and remains after 1002.5 s of photoexposure. This is evidence that the amine remained attached on the surface, even after photocleavage. Further, recent studies have demonstrated that while photocleavage of similarly-structured BODIPY-based PPGs in air and aerated solutions results in a reduced quantum yield, photocleavage still proceeds.19,20
Subsequent measurements after 2 and 6 weeks of deposition (see ESI,† Fig. S21) show that compound 5 remained both bound to the surface and photoreactive, even after being stored in a standard atmosphere, demonstrating its potential for long-term use.
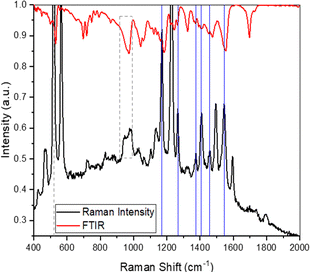
The RR spectrum of graphene-bound 5 is shown in Fig. 3 with the Fourier transform infrared (FTIR) spectrum of the compound. Because of the difficulty of sorting the resonance peaks from the ‘real’ peaks, a full assignment of the RR spectrum of 5 is beyond the scope of this paper. However, several peaks match reasonably well with the FTIR spectrum of the solid compound. The list of matched peaks is not exhaustive but still reinforces the conclusion that the BODIPY compound 5 is indeed successfully bound to the graphene substrate. It is of note that the RR spectrum of 5 and the FTIR spectrum do not have to match exactly because the selection rules and relative intensities of the peaks are different.
Our proof-of-concept results demonstrate that we successfully synthesized a BODIPY-based photocleavable compound 5 that cleaves in the 520–540 nm exposure range. We have shown its capacity to bind to a graphene surface, photoreact when bound, and demonstrated its presence on the surface at least one month after functionalization. Our future aim is to explore other BODIPY derivatives with larger aromatic anchor groups on the graphene surface, characterize and compare their ability to form self-assembling monolayers (SAMs) on graphene and photocleave selectively. This work also sets the stage for biocompatibility tests, controlled nanoparticle adhesion, and targeted surface functionalization.
Author contributions
E. S. is responsible for the concept, Raman, absorbance and fluorescence measurements with E. K., data analysis and visualization, and the writing. E. K. is responsible for design, synthesis, and characterization 5, preparing the samples, data analysis, visualization, and the original draft of the synthetic parts. M. N. and M. P. are responsible for the supervision, design of the photocleavable compound with E. K. and expert insight into how best to proceed with the project. All authors contributed to manuscript review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Mr Olli Rissanen for preparing and transferring the graphene samples. This project was made possible by funding from the Research Council of Finland, Project # 343370 (E. S.).Notes and references
- V. Georgakilas, M. Otyepka, A. B. Bourlinos, V. Chandra, N. Kim, K. C. Kemp, P. Hobza, R. Zboril and K. S. Kim, Chem. Rev., 2012, 112, 6156–6214 CrossRef CAS.
- J. Koivistoinen, L. Sládková, J. Aumanen, P. Koskinen, K. Roberts, A. Johansson, P. Myllyperkiö and M. Pettersson, J. Phys. Chem. C, 2016, 120, 22330–22341 CrossRef CAS.
- J. Aumanen, A. Johansson, J. Koivistoinen, P. Myllyperkiö and M. Pettersson, Nanoscale, 2015, 7, 2851–2855 RSC.
- A. Johansson, H.-C. Tsai, J. Aumanen, J. Koivistoinen, P. Myllyperkiö, Y.-Z. Hung, M.-C. Chuang, C.-H. Chen, W. Y. Woon and M. Pettersson, Carbon, 2017, 115, 77–82 CrossRef CAS.
- K. F. Edelthalhammer, D. Dasler, L. Jurkiewicz, T. Nagel, S. Al-Fogra, F. Hauke and A. Hirsch, Angew. Chem., Int. Ed., 2020, 59, 23329–23334 CrossRef CAS.
- A. Piñeiro-García, S. M. Vega-Díaz, F. Tristan, D. Meneses-Rodríguez and V. Semetey, FlatChem, 2022, 33, 100349 CrossRef.
- K. Xia, W.-Y. Chiang, C. J. Lockhart de la Rosa, Y. Fujita, S. Toyouchi, H. Yuan, J. Su, H. Masuhara, S. De Gendt, S. De Feyter, J. Hofkens and H. Uji-i, Nanoscale, 2020, 12, 11063–11069 RSC.
- M. Liu, C. Huang, Z. Jia, Z. Zhao, X. Xiao, A. Wang, P. Li, X. Guan, G. Zhou and Y. Fan, ACS Chem. Neurosci., 2020, 11, 604–614 CrossRef CAS.
- A. P. Pelliccioli and J. Wirz, Photochem. Photobiol. Sci., 2002, 1, 441–458 CrossRef.
- A. Bardhan and A. Deiters, Curr. Opin. Struct. Biol., 2019, 57, 164–175 CrossRef CAS.
- C. Daengngam, S. B. Thorpe, X. Guo, S. V. Stoianov, W. L. Santos, J. R. Morris and H. D. Robinson, J. Phys. Chem. C, 2013, 117, 14165–14175 CrossRef CAS.
- U. Jonas, A. del Campo, C. Krüger, G. Glasser and D. Boos, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 5034–5039 CrossRef CAS PubMed.
- A. Pálinkás, G. Kálvin, P. Vancsó, K. Kandrai, M. Szendrő, G. Németh, M. Németh, Á. Pekker, J. S. Pap, P. Petrik, K. Kamarás, L. Tapasztó and P. Nemes-Incze, Nat. Commun., 2022, 13, 6770 CrossRef.
- N. Rubinstein, P. Liu, E. W. Miller and R. Weinstain, Chem. Commun., 2015, 51, 6369–6372 RSC.
- K. Sitkowska, B. L. Feringa and W. Szymański, J. Org. Chem., 2018, 83, 1819–1827 CrossRef CAS.
- D. Kand, P. Liu, M. X. Navarro, L. J. Fischer, L. Rousso-Noori, D. Friedmann-Morvinski, A. H. Winter, E. W. Miller and R. Weinstain, J. Am. Chem. Soc., 2020, 142, 4970–4974 CrossRef CAS.
- K. Sitkowska, M. F. Hoes, M. M. Lerch, L. N. Lameijer, P. van der Meer, W. Szymański and B. L. Feringa, Chem. Commun., 2020, 56, 5480–5483 RSC.
- J. A. Peterson, C. Wijesooriya, E. J. Gehrmann, K. M. Mahoney, P. P. Goswami, T. R. Albright, A. Syed, A. S. Dutton, E. A. Smith and A. H. Winter, J. Am. Chem. Soc., 2018, 140, 7343–7346 CrossRef CAS.
- T. Slanina, P. Shrestha, E. Palao, D. Kand, J. A. Peterson, A. S. Dutton, N. Rubinstein, R. Weinstain, A. H. Winter and P. Klán, J. Am. Chem. Soc., 2017, 139, 15168–15175 CrossRef CAS.
- P. Shrestha, D. Kand, R. Weinstain and A. H. Winter, J. Am. Chem. Soc., 2023, 145, 17497–17514 CrossRef CAS PubMed.
- P. K. Singh, P. Majumdar and S. P. Singh, Coord. Chem. Rev., 2021, 449, 214193 CrossRef CAS.
- E. M. See, C. L. Peck, W. L. Santos and H. D. Robinson, Langmuir, 2017, 33, 10927–10935 CrossRef CAS PubMed.
- Z. Pei, L. Li, L. Sun, S. Zhang, X. Shan, S. Yang and B. Wen, Carbon, 2013, 51, 156–163 CrossRef CAS.
- X. Zhang and J. Guo, Appl. Surf. Sci., 2022, 571, 151376 CrossRef CAS.
- A. Rochefort and J. D. Wuest, Langmuir, 2009, 25, 210–215 CrossRef CAS PubMed.
- A. M. Schulte, G. Alachouzos, W. Szymański and B. L. Feringa, J. Am. Chem. Soc., 2022, 144, 12421–12430 CrossRef CAS.
- S. Mula, A. K. Ray, M. Banerjee, T. Chaudhuri, K. Dasgupta and S. Chattopadhyay, J. Org. Chem., 2008, 73, 2146–2154 CrossRef CAS PubMed.
- P. Rybczynski, A. Smolarkiewicz-Wyczachowski, J. Piskorz, S. Bocian, M. Ziegler-Borowska, D. Kędziera and A. Kaczmarek-Kędziera, Int. J. Mol. Sci., 2021, 22(13), 6735 CrossRef CAS PubMed.
- G. B. Guseva, E. N. Nuraneeva, M. B. Berezin and E. V. Antina, J. Photochem. Photobiol., A, 2022, 423, 113620 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nj04776h |
| ‡ Both authors share equal credit for this work. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2024 |