3,5-Bis(trifluoromethyl)benzyl modified triazine-based covalent organic frameworks suppressing the shuttle effect of polysulfides in lithium-sulfur batteries†
Shirui
Pang
,
Yuxin
Liu
,
Zhe
Zhang
,
Yuxin
Li
,
Chunguang
Li
,
Zhan
Shi
 * and
Shouhua
Feng
* and
Shouhua
Feng

State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, People's Republic of China. E-mail: zshi@mail.jlu.edu.cn
First published on 23rd November 2023
Abstract
The shuttle effect of polysulfides poses a significant obstacle to the commercialization of lithium-sulfur (Li–S) batteries. Covalent organic frameworks have been widely used in Li–S batteries as porous crystalline materials to suppress the shuttle effect. Herein, a 3,5-bis(trifluoromethyl)benzyl (BTFMB-TzDa) modified triazine-based covalent organic framework was synthesized. The high electronegativity and large steric hindrance of the BTFMB-TzDa modified separator successfully suppressed the diffusion of polysulfides, leading to improved capacity and cyclic stability of Li–S batteries. Cells with the BTFMB-TzDa modified separator exhibited a high initial capacity of 1205 mA h g−1 at 0.2C and 657 mA h g−1 at 3.0C. Furthermore, the capacity still remained at 501 mA h g−1 after 500 cycles. The BTFMB-TzDa modified separator provides an alternative strategy for the construction of high-performance Li–S batteries.
Energy storage has become a central issue for economic and social development. Therefore, exploring high-energy-density batteries is a major requirement within the field of energy storage. To meet this requirement, lithium-ion batteries with high performance have been evaluated. Li–S batteries have been used as next-generation energy storage devices due to their high theoretical specific capacity (∼1675 mA h g−1) and high energy density (∼2600 W h kg−1).1,2 However, the development of Li–S batteries is limited by the polysulfide shuttle effect, poor conductivity of sulfur, volume expansion, and lithium dendrite growth.3,4 Many studies have tried to solve the shuttle effect of Li–S batteries. During the discharge process, sulfur molecules dissolve in electrolytes, producing polysulfides and shuttling to the lithium anode, causing irreversible capacity loss. A variety of compounds, such as porous carbon, metal–organic frameworks (MOFs),5–10 and covalent organic frameworks (COFs),11–13 have been employed to reduce the shuttle effect caused by polysulfides.
COFs have been extensively employed in adsorption,14 catalysis,15–17 and energy storage18–20 applications, due to their high porosity,21,22 low density,23 insolubility, and robust stability as a crystalline material for decades. The high flexibility and high thermal and chemical stability make COFs promising anode materials for Li–S batteries.24,25 Meanwhile, pores in 2D stacked COFs facilitate the transportation of ions. Previous studies have shown that COFs inhibit the shuttle effect of polysulfide in Li–S batteries. Wang et al.26 reported a 2D COF, CTF-1, as a host material with a remarkable positive effect on the capacity retention of Li–S batteries. Wang and co-workers27 demonstrated that sulfonate-rich COF-modified separators deliver an ultralow attenuation rate of 0.047% per cycle over 800 cycles at 1.0C.
We designed and synthesized a highly crystalline covalent organic framework BTFMB-TzDa. The conjugated triazine structure provides fast electron transfer, while the highly electronegative and large sterically hindered group can effectively inhibit the shuttle effect of polysulfides. By coating the commercial separator Celgard 2500 with BTFMB-TzDa, the BTFMB-TzDa modified separator was achieved and its electrochemical performance was examined. The batteries with the BTFMB-TzDa modified separator exhibited a high initial capacity of 1205 mA h g−1 at 0.2C, and 657 mA h g−1 at 3.0C. Additionally, the batteries with the BTFMB-TzDa modified separator still retained 501 mA h g−1 after 500 cycles. It is evident that the acquired capacity under 0.2C is competitive with those of batteries based on other separators reported previously (Table S1, ESI†).
TzDa28 and BTFMB-TzDa were synthesized as shown in Scheme 1. Crystal structures of Tzda and BTFMB-TzDa were characterized by powder X-ray diffraction (PXRD) (Fig. 1a). A strong peak indicates the high crystallinity of TzDa and BTFMB-TzDa, which was at 2θ = 2.80°, 4.95°, 5.67°, and 7.50°, and 2.80° and 4.95°, respectively. IR spectra are displayed in Fig. 1b. The strong C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrational stretching and C–F vibrational stretching were observed at 1750 cm−1 and 1281 cm−1 respectively, indicating the BTFMB-TzDa successful synthesis. The solid state 13C NMR spectra are shown in Fig. S1 and S2 (ESI†). The chemical shift on 232 ppm is typical for C
O vibrational stretching and C–F vibrational stretching were observed at 1750 cm−1 and 1281 cm−1 respectively, indicating the BTFMB-TzDa successful synthesis. The solid state 13C NMR spectra are shown in Fig. S1 and S2 (ESI†). The chemical shift on 232 ppm is typical for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbon. The results indicate the successful formation of the ester.
O carbon. The results indicate the successful formation of the ester.
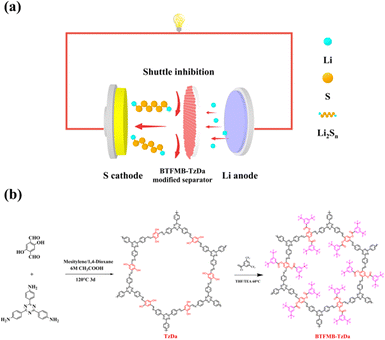 | ||
| Scheme 1 (a) BTFMB-TzDa modified separator that suppresses LiPSs shuttling. (b) Schematic of the synthesis of TzDa and BTFMB-TzDa. | ||
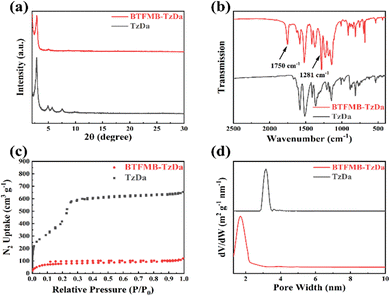 | ||
| Fig. 1 (a) PXRD; (b) FT-IR spectra; (c) N2 adsorption–desorption isotherms and (d) pore size distribution of the TzDa and BTFMB-TzDa. | ||
The 77 K nitrogen sorption isotherm curves reveal that the Brunauer–Emmett–Teller (BET) surface area of TzDa is 2704 m2 g−1 (Fig. 1c). TzDa has a pore size of 3.2 nm (Fig. 1d). BTFMB-TzDa has a BET surface area of 230 m2 g−1 with a 1.7 nm pore size. The large sterically hindered BTFMB group leads to a significant decrease in pore size, which will help inhibit the shuttle effect of polysulfides. TGA results show that TzDa displays 96% weight retention at 400 °C and still maintains 67% at 800 °C under a nitrogen atmosphere, while BTFMB-TzDa presents thermal stability with 88% retention at 400 °C and 40% retention at 800 °C (Fig. S3, ESI†). The difference in thermal stability between TzDa and BTFMB-TzDa may be attributed to the decomposition of the ester group in BTFMB-TzDa. The morphology of Tzda and BTFMB-TzDa was investigated by scanning electron microscopy (SEM) (Fig. S4, ESI†) and transmission electron microscopy (TEM) (Fig. S5, ESI†) measurements. Compared to Tzda, even though BTFMB-TzDa exhibits a reduced particle size, the hierarchical structure remains.
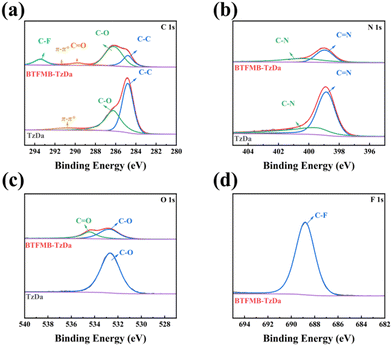
The surface elemental compositions of TzDa and BTFMB-TzDa were analyzed by X-ray photoelectron spectroscopy (XPS). As shown in Fig. 2a, the binding energies at 286.18, 289.78, and 293.38 eV are indexed to C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–F, respectively. For the N 1s (Fig. 2b), both TzDa and BTFMB-TzDa exhibit characteristic peaks of C–N and C
O and C–F, respectively. For the N 1s (Fig. 2b), both TzDa and BTFMB-TzDa exhibit characteristic peaks of C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N. In addition, the binding energies at 534.48 (Fig. 2c), and 688.78 eV (Fig. 2d) are indexed to C
N. In addition, the binding energies at 534.48 (Fig. 2c), and 688.78 eV (Fig. 2d) are indexed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–F, respectively. The results indicate the successful synthesis of BTFMB-TzDa.
O and C–F, respectively. The results indicate the successful synthesis of BTFMB-TzDa.
The BTFMB-TzDa modified separator was fabricated by coating the slurry containing BTFMB-TzDa, carbon black, and PVDF onto the Celgard 2500 separator with a loading amount of 0.68 mg cm−2. The BTFMB-TzDa modified separator coating layer was dense and compact after the folding test as shown in Fig. S6 (ESI†). Contact angle measurements (Fig. S8, ESI†) reveal that after BTFMB-TzDa modification, the wettability of the separator was improved in the case of using 1M LiTFSI in a mixture of 1,2-dimethoxyethane (DME) and 1,3-dioxolane (DOL) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) with 2 wt% LiNO3 as the electrolyte. The SEM (Fig. S9, ESI†) image of the pristine Celgard 2500 exhibits a typical polymer morphology. The BTFMB-TzDa modified separator exhibits a porous structure, and the cross-sectional SEM image (Fig. S10, ESI†) shows that the thickness of Celgard 2500 is 18.5 μm and that of the coating layer is 13.4 μm.
1, v/v) with 2 wt% LiNO3 as the electrolyte. The SEM (Fig. S9, ESI†) image of the pristine Celgard 2500 exhibits a typical polymer morphology. The BTFMB-TzDa modified separator exhibits a porous structure, and the cross-sectional SEM image (Fig. S10, ESI†) shows that the thickness of Celgard 2500 is 18.5 μm and that of the coating layer is 13.4 μm.
The CV curves of different separators at varied scanning rates from 0.1 to 0.5 mV s−1 were also investigated and the corresponding relationship between the peak current and the square root of scanning rate at different redox peaks is presented in Fig. 3. The slope of the fitting line corresponds to DLi+, which positively reflects the mobility of Li+ within the battery (Fig. S11, ESI†). Obviously, the BTFMB-TzDa modified separator delivered much higher DLi+ than the Celgard 2500 separator, confirming the significantly facilitated Li+ diffusion behavior. The galvanostatic intermittent titration technique (GITT) was employed to analyze Li+ diffusion (Fig. S12a, ESI†). There were more steps in the galvanostatic and relaxation patterns of the BTFMB-TzDa cell than TzDa during the discharge voltage plateau, which could be attributed to the enhanced capture and conversion of LiPSs. The Li+ diffusion coefficients of TzDa and BTFMB-TzDa were calculated (Fig. S12b, ESI†). The results showed that the Li+ diffusion coefficient of BTFMB-TzDa was higher than that of TzDa.
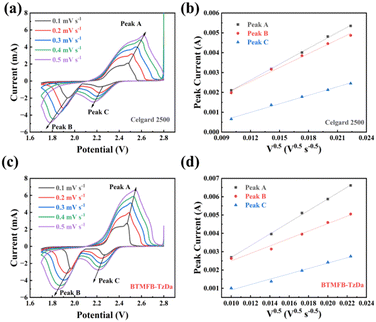 | ||
| Fig. 3 CV curves of (a) Celgard 2500 and (c) BTFMB-TzDa at different scan rates. Linear fitting diagram of the peak current of (b) celgard 2500 and (d) BTFMB-TzDa. | ||
Li–S batteries were assembled using lithium metal, and the hybrids of sulfur and carbon were used as the anode and cathode, respectively. Cyclic voltammetry (CV) was performed for the Li–S batteries with the modified separator at a scan rate of 0.1 mV s−1 within a voltage window of 1.7 to 2.8 V. The characteristics of the electrochemical reaction of sulfur with lithium are shown in Fig. 4a. Two major reduction peaks appeared during the cathodic scan, corresponding to the transformation of pristine S8 to soluble long-chain polysulfides Li2S4 and their subsequent reduction to insoluble short-chain Li2S2 to Li2S, respectively. Meanwhile, an oxidation peak observed during the positive scan could be attributed to the conversion of polysulfides to sulfur. Two obvious reduction peaks emerged at 1.94 V and 2.22 V, respectively with Celgard 2500. In comparison with Celgard 2500, the reduction peaks emerged at 1.97 V and 2.27 V,29 respectively, with the BTFMB-TzDa separator. Such a shifting trend makes the gap between oxidation and reduction potentials significantly decrease. This reveals a better conversion of sulfur in the presence of the BTFMB-TzDa modified separator. EIS (Fig. S13, ESI†) shows that smaller resistance of the BTFMB-TzDa modified separator facilitates the charge transfer and enhances the electrochemical performance. The small pore size of BTFMB-TzDa aids in retarding the diffusion of polysulfides. Then, the adjacent carbon black offer electrons to electrochemically reduce the fixed polysulfides into short-chain sulfur species. Without carbon black, electrical conduction was largely decreased, resulting in low efficiency of polysulfide transformation. As shown in Fig. S14 (ESI†), for batteries cycled for 100 times at 1C, another new semicircle related to the solid electrolyte interface film toward solid Li2S2/Li2S emerged. Based on the corresponding equivalent circuit model and fitting results, it can be inferred that the BTFMB-TzDa exhibits lower impedance values, demonstrating rapid redox reaction. The resistance became smaller after 100 cycles compared with those of fresh cells, which could be attributed to the gradual infiltration of the electrolyte into the electrode and the redispersion of sulfur species on the surface of the electrode.
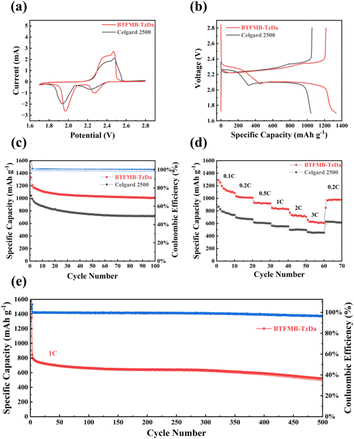 | ||
| Fig. 4 (a) CV curves, (b) the galvanostatic discharge and charge profiles at 0.2C; (c) the cycling performance at 0.2C; (d) discharge rate performance and (e) the long-term cycling performance at 1C. | ||
Performances of Li–S batteries were evaluated by using the BTFMB-TzDa modified separator and Celgard 2500, respectively. The charge–discharge voltage profiles of the BTFMB-TzDa modified separator at 0.2C (Fig. 4b) display a typical two–plateau charge–discharge curve according to the multistep sulfur redox reactions, which agrees with the CV results. As shown in the cycling performance curve (Fig. 4c), BTFMB-TzDa Li–S batteries exhibited a high initial capacity of 1205 mA h g−1 at a current density of 0.2C, and after 100 cycles, they retained a high capacity of 1003 mA h g−1. In contrast, the Celgard 2500 Li–S batteries exhibited lower initial capacities, 985 mA h g−1 at 0.2C, and 715 mA h g−1 after 100 cycles, respectively.
The rate performance of the Li–S batteries was evaluated at various current densities from 0.1 to 3.0C every 10 cycles, as shown in Fig. 4d. The BTFMB-TzDa modified Li–S batteries delivered 1288 mA h g−1, 1034 mA h g−1, 932 mA h g−1, 848 mA h g−1, 770 mA h g−1, and 657 mA h g−1 at 0.1C, 0.2C, 0.5C, 1.0C, 2.0C, and 3.0C, respectively, indicating the high rate capability. After cycling at high rates, the reversible capacity reached 964 mA h g−1 as the current density was back to 0.2C. In contrast, the Celgard 2500 Li–S batteries showed much lower capacities of 866 mA h g−1, 694 mA h g−1, 614 mA h g−1, 561 mA h g−1, 498 mA h g−1, and 460 mA h g−1 at 0.1–3.0C, respectively. After the high rate of cycling, the reversible capacity reached 629 mA h g−1 as the current density was back to 0.2C. Additionally, the charge–discharge voltage profiles of the different separators from 0.1 to 3.0C were also evaluated (Fig. S15, ESI†), and the results indicated that the cell using the BTFMB-TzDa modified separator exhibited better electrochemical performance than the cell using the Celgard 2500 separator. To verify the long-term cycling ability of cells with modified separators, the cycling performance over 500 cycles at 1.0C (Fig. 4e) was investigated. The battery with the BTFMB-TzDa modified separator presented an initial capacity of 905 mA h g−1at 1.0C and retained 501 mA h g−1 after 500 cycles.
In conclusion, we fabricated a BTFMB-TzDa modified separator to achieve high-performance Li–S batteries. The groups with high electronegativity and large steric hindrance enabled the BTFMB-TzDa modified separator to suppress the diffusion of polysulfides and promote the capacity, leading to high-rate performance. Benefiting from these advantages, batteries with the BTFMB-TzDa modified separator exhibited a high initial capacity of 1205 mA h g−1 at 0.2C. Moreover, the BTFMB-TzDa modified separator cell presented a high discharge capacity (657 mA h g−1) even at 3.0C. Meanwhile, the batteries with the BTFMB-TzDa modified separator retained 501 mA h g−1 after 500 cycles. Our work demonstrates a novel approach to design COFs to improve the performance of Li–S batteries.
Author contributions
Conceptualization: Shirui Pang, methodology: Shirui Pang, experiments: Shirui Pang, Yuxin Liu, Zhe Zhang and Yuxin Li, funding acquisition: Chunguang Li, Zhan Shi and Shouhua Feng, writing – original draft: Shirui Pang and Zhan Shi, writing – review and editing: all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22271114 and 21621001), the Foundation of Science and Technology Development of Jilin Province, China (20200801004GH) and the 111 Project (B17020). The authors also gratefully acknowledge the financial support from the program for JLU Science and Technology Innovative Research Team (JLUSTIRT).Notes and references
- A. Manthiram, Y. Fu, S. H. Chung, C. Zu and Y. S. Su, Chem. Rev., 2014, 114, 11751 CrossRef CAS.
- K. Shen, X. Chen, J. Chen and Y. Li, ACS Catal., 2016, 6, 5887 CrossRef CAS.
- S. Feng, Z. H. Fu, X. Chen and Q. Zhang, InfoMat, 2022, 4, 1 CrossRef.
- Y. Dong, D. Cai, T. Li, S. Yang, X. Zhou, Y. Ge, H. Tang, H. Nie and Z. Yang, ACS Nano, 2022, 16, 6414 CrossRef CAS.
- C. Qi, L. Xu, J. Wang, H. Li, C. Zhao, L. Wang and T. Liu, ACS Sustainable Chem. Eng., 2020, 8, 12968 CrossRef CAS.
- A. E. Baumann, D. A. Burns, J. C. Diaz and V. S. Thoi, ACS Appl. Mater. Interfaces, 2019, 11, 2159 CrossRef CAS.
- Z. Wang, B. Wang, Y. Yang, Y. Cui, Z. Wang, B. Chen and G. Qian, ACS Appl. Mater. Interfaces, 2015, 7, 20999 CrossRef CAS PubMed.
- X. J. Hong, C. L. Song, Y. Yang, H. C. Tan, G. H. Li, Y. P. Cai and H. Wang, ACS Nano, 2019, 13, 1923 CAS.
- Y. Fan, Z. Niu, F. Zhang, R. Zhang, Y. Zhao and G. Lu, ACS Omega, 2019, 4, 10328–10335 CrossRef CAS PubMed.
- N. Yuan, W. Sun, J. Yang, X. Gong and R. Liu, Adv. Mater. Interfaces, 2021, 8, 2001941 CrossRef CAS.
- F. Xu, S. Yang, G. Jiang, Q. Ye, B. Wei and H. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 37731 CrossRef CAS.
- X. Deng, Y. Li, L. Li, S. Qiao, D. Lei, X. Shi and F. Zhang, Nanotechnology, 2021, 32, 275708 CrossRef CAS.
- Y. Peng, Y. Huang, Y. Zhu, B. Chen, L. Wang, Z. Lai, Z. Zhang, M. Zhao, C. Tan, N. Yang, F. Shao, Y. Han and H. Zhang, J. Am. Chem. Soc., 2017, 139, 8698 CrossRef CAS PubMed.
- Y. Zhang, J. Duan, D. Ma, P. Li, S. Li, H. Li, J. Zhou, X. Ma, X. Feng and B. Wang, Angew. Chem., Int. Ed., 2017, 56, 16313 CrossRef CAS PubMed.
- H. Chen, W. Liu, A. Laemont, C. Krishnaraj, X. Feng, F. Rohman, M. Meledina, Q. Zhang, R. Van Deun, K. Leus and P. Van Der Voort, Angew. Chem., Int. Ed., 2021, 60, 10820 CrossRef CAS.
- Y. Li, R. Jin, Y. Xing, J. Li, S. Song, X. Liu, M. Li and R. Jin, Adv. Energy Mater., 2016, 6, 1601273 CrossRef.
- L. Liu, Z. Chen, J. Wang, D. Zhang, Y. Zhu, S. Ling, K. W. Huang, Y. Belmabkhout, K. Adil, Y. Zhang, B. Slater, M. Eddaoudi and Y. Han, Nat. Chem., 2019, 11, 622 CrossRef CAS PubMed.
- Y. Hu, N. Dunlap, S. Wan, S. Lu, S. Huang, I. Sellinger, M. Ortiz, Y. Jin, S. H. Lee and W. Zhang, J. Am. Chem. Soc., 2019, 141, 7518 CrossRef CAS PubMed.
- Z. Wu, L. Li, J. M. Yan and X. B. Zhang, Adv. Sci., 2017, 4, 1600382 CrossRef.
- S. Yuan, Y. H. Zhu, W. Li, S. Wang, D. Xu, L. Li, Y. Zhang and X. B. Zhang, Adv. Mater., 2017, 29, 1602469 CrossRef.
- D. G. Atinafu, S. J. Chang, K.-H. Kim, W. Dong and S. Kim, Chem. Eng. J., 2020, 389, 124430 CrossRef CAS.
- J. Liu, Q. Li, X. Xiao, F. Li, C. Zhao, Q. Sun, P. Qiao, J. Zhou, J. Wu, B. Li, H. Bao and B. Jiang, J. Colloid Interface Sci., 2021, 590, 1 CrossRef CAS PubMed.
- Z. Zhang, X. Dong, J. Yin, Z. G. Li, X. Li, D. Zhang, T. Pan, Q. Lei, X. Liu, Y. Xie, F. Shui, J. Li, M. Yi, J. Yuan, Z. You, L. Zhang, J. Chang, H. Zhang, W. Li, Q. Fang, B. Li, X. H. Bu and Y. Han, J. Am. Chem. Soc., 2022, 144, 6821 CrossRef CAS.
- J. Xu, F. Yu, J. Hua, W. Tang, C. Yang, S. Hu, S. Zhao, X. Zhang, Z. Xin and D. Niu, Chem. Eng. J., 2020, 392, 123694 CrossRef CAS.
- Y. Zhang, Y. Wu, Y. Liu and J. Feng, Chem. Eng. J., 2022, 428, 131040 CrossRef CAS.
- H. Liao, H. Ding, B. Li, X. Ai and C. Wang, J. Mater. Chem. A, 2014, 2, 8854 RSC.
- J. Xu, S. An, X. Song, Y. Cao, N. Wang, X. Qiu, Y. Zhang, J. Chen, X. Duan, J. Huang, W. Li and Y. Wang, Adv. Mater., 2021, 33, 2105178 CrossRef CAS.
- M. Huang, J. Chong, C. Hu and Y. Yang, Inorg. Chem. Commun., 2020, 119, 108094 CrossRef CAS.
- A. Manthiram, Y. Fu, S. H. Chung, C. Zu and Y. S. Su, Chem. Rev., 2014, 114, 11751 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nj03791f |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2024 |