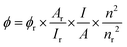Nitrogen-doped carbon quantum dots for fluorescence sensing, anti-counterfeiting and logic gate operations†
Li
Xu
,
Yi
Qian
,
Lei
Bao
 ,
Wei
Wang
,
Wei
Wang
 ,
Nengmei
Deng
,
Li
Zhang
,
Guanglin
Wang
,
Xucheng
Fu
,
Nengmei
Deng
,
Li
Zhang
,
Guanglin
Wang
,
Xucheng
Fu
 * and
Wei
Fu
* and
Wei
Fu
 *
*
Key Laboratory of Biomimetic Sensor and Detecting Technology of Anhui Province, West Anhui University, Lu’an 237012, China. E-mail: fxc8307@wxc.edu.cn; w-fu17@tsinghua.org.cn
First published on 21st November 2023
Abstract
The low quantum yields (QY) of carbon quantum dots (CQDs) not only limit their sensitivity as sensors, but also hinder their application in fluorescent anti-counterfeiting materials. To develop highly fluorescent CQDs, several factors, like a large conjugated double bond system, a rigid plane structure and electron-donating substituent group incorporation on the aromatic ring need to be considered. With this in mind, herein, highly fluorescent nitrogen-doped CQDs (N-CQDs) were successfully fabricated by a solvothermal method using ammonia, citric acid and phloroglucinol as precursors. The sensing platform based on the obtained N-CQDs was constructed to determine Fe3+ and ascorbic acid (AA) via the “on–off–on” fluorescence principle. The fluorescence of the N-CQDs was effectively quenched upon addition of Fe3+, and then, restored by AA. The detection limits for Fe3+ and AA were determined to be 0.28 μM and 0.81 μM within the respective ranges of 1–10 μM and 30–90 μM. It is worth noting that the proposed strategy was successfully applied to monitor AA in fruit samples with satisfactory results. In addition, we successfully applied N-CQDs to information anti-counterfeiting and molecular logic gate operations, which confirmed their great potential application prospects.
1. Introduction
Ascorbic acid (AA) is indeed an essential cofactor with important biological functions in the human body, participating in various biological processes. Abnormal levels of AA are associated with several diseases and health issues, such as cardiovascular diseases,1 kidney stones,2 iron-deficiency anemia,3 stomach convulsion4 and atherosclerosis.5 Furthermore, due to its antioxidant properties, AA was widely used in the fields of food, beverages, pharmaceutical preparations, and cosmetics.6 Thus, the importance of accurately measuring AA levels underscores the need for the development of convenient and effective quantitative methods. Iron ions (Fe3+) are not only essential metal ions in organisms but can also be contaminants as heavy metals in water. Excess or deficiency of Fe3+ can cause severe physiological harm and lead to biological disorders, including cancer, diabetes, Parkinson's disease, hemochromatosis, and Alzheimer's disease.7 According to the regulations set by the United States Environmental Protection Agency (USEPA), the maximum allowable concentration of iron ions in drinking water is approximately 5.4 μM.8 In this context, the development of a convenient analytical method for rapid detection of Fe3+ holds significant practical significance.Currently, various technologies are established for the determination of Fe3+ and AA, such as electrochemical methods, colorimetry, chromatography, and fluorescence spectroscopy.9,10 Among these methods, fluorescence detection technology has attracted more and more attention due to its high sensitivity, selectivity, and ability to overcome the shortcomings of sophisticated equipment, complicated processes, high costs, and time-consuming procedures.11 Fluorescent chemosensors utilize fluorescent molecules to undergo specific interactions with target analytes, resulting in changes in their fluorescence properties.12–14 These sensors typically consist of a receptor that can selectively interact with the target analyte. Upon binding to the target analyte, the fluorescence properties such as the intensity, lifetime, or wavelength of the fluorescent molecules undergo modifications. By measuring these changes, the presence and concentration of the analyte can be determined. Chemodosimeters are a special type of fluorescence sensor that typically contains a reactive group.15,16 This probe undergoes an irreversible reaction with the target analyte, leading to changes in its fluorescence properties. Generally, chemodosimeters are characterized by their specificity and irreversibility. However, their design can be challenging, and the reaction is not sensitive enough, resulting in limited use.
Among all luminescent materials, quantum dots (QDs) are considered excellent fluorescent probes due to their remarkable photophysical and photochemical stability. Carbon quantum dots (CQDs) have attracted widespread attention from researchers due to their wide availability of raw materials, environmental safety, low toxicity, high water solubility, and good biocompatibility.17,18 However, CQDs used for sensors often have relatively low quantum yields (QY), which limit their sensitivity of detection. After tireless pursuit and continuous exploration, it is generally believed that strong fluorescent substances often have the following characteristics: first, the presence of a large conjugated double bond system in CQDs, which allows for efficient electron delocalization and promotes fluorescence. This can be achieved by controlling the synthesis conditions and introducing suitable precursors with extended conjugation. Second, a rigid plane structure is needed, which is beneficial for maintaining the electronic structure and minimizing non-radiative energy loss. Third, there are electron-donating substituent groups on the aromatic ring, which can facilitate charge transfer and reduce non-radiative decay pathways, thus increasing the QY. Therefore, methods to improve the QY of CQDs include surface passivation with electron donating groups,19 heteroatom doping,20,21 constructing large π systems,22 and using molecules with sp2-conjugation domains as raw materials.23 For example, nitrogen, phosphorus co-doped carbon quantum dots (N,P-CQDs) derived from beer were successfully synthesized, achieving a high QY of 21.7%.24 The Sargent group reported that amine-based modification can eliminate oxygen-containing functional groups, contribute to narrow-linewidth emission, and enable high-purity emission of CQDs.19 Lin's team used phenylenediamine with a benzene ring structure and electron-donating groups as a precursor, successfully synthesizing full-color emission, strongly fluorescent CQDs with a QY of 20.6%.25 These approaches effectively modulate the inherent characteristics of CQDs, such as photoelectric and photochemical properties, and QY, resulting in unexpected effects in practical applications.
Counterfeit goods have a significant economic impact and also pose threats to health and safety. Thus, adopting anti-counterfeiting tags as a solution provides significant opportunities for complex and intelligent information encryption. Nowadays, luminescent materials play a crucial role in encoding information for anti-counterfeiting. CQDs, due to the wide range of sources and excellent luminescent properties, offer a new class of ink materials for anti-counterfeiting, and achieved satisfactory results.26–28
Based on the above research and analysis, herein, a new and facile strategy was developed for the fabrication of highly fluorescent nitrogen doped CQDs (N-CQDs) via the solvothermal treatment of ammonia, citric acid and phloroglucinol. The obtained N-CQDs showed a significant enhancement in the fluorescence emission properties with a QY of 25.9%. Further research showed that the N-CQD based sensor is highly sensitive to Fe3+ and AA with excellent linearity and the detection limits are 0.28 μM and 0.81 μM, respectively. Moreover, the chemosensor can be successfully applied for the quantitative detection of AA in fruit samples. In addition, the obtained N-CQDs were also applied for information encryption and decryption and logic gate operations, and the results indicated that the N-CQDs displayed good application potential.
2. Experimental
2.1 Materials
Ethanol and ammonium hydroxide were produced by Macklin Biochemical Co., Ltd. Metal chlorides (Mg2+, Ba2+, Cu2+, Na+, Ca2+, K+, Fe3+, Ni2+, Zn2+, and Cr3+) with AR purity degree were purchased from Meryer Co., Ltd. Cysteine (Cys), glycine (Gly), glutathione (GSH), malic acid (MA), tartaric acid (TA), D-fructose (Fru), citric acid (CA), glucose (Glu), methionine (Met) and anhydrous phloroglucinol were provided by Aladdin, Shanghai.2.2 Characterization methods
The structure and morphology of the N-CQDs were examined using a transmission electron microscope (TEM) on H-7650B at 80 kV. High-resolution transmission electron microscopy (HRTEM) images were obtained with a JEM-2100F microscope. The crystalline structure of the N-CODs was determined by X-ray diffraction (XRD, LabX XRD-6000) and Raman spectroscopy (532 nm, Horiba-Jobin-Yvon Raman system). Fourier transform infrared spectroscopy (Nicolet iS5 FTIR spectrophotometer, Thermo Scientific) and X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi) were used to determine the surface chemical composition and chemical states of the N-CQDs. The ultraviolet-visible (UV-vis) absorption properties of the N-CQDs were determined using a Lambda 750S spectrometer (PerkinElmer). The photoluminescence (PL) spectra were acquired using an F-2710 spectrofluorometer (Hitachi) at room temperature.2.3 Synthesis of N-CQDs
N-CQDs were prepared using a one-pot solvothermal synthesis method, as illustrated in Scheme 1. First, anhydrous phloroglucinol (126 mg), citric acid (210 mg), ethanol (15 mL) and ammonium hydroxide (5 mL) were blended well and subjected to ultrasonic treatment for 30 min. Subsequently, the mixture was poured into a 50 mL Teflon-lined stainless autoclave and maintained at 180 °C for 6 h. After cooling the Teflon-lined stainless autoclave to room temperature, the raw N-CQD solution was filtered through a 0.22 μm microporous membrane. The resulting solution was poured into a dialysis bag with a retained molecular weight of 3500 Da, and water was exchanged every 12 h for 3 days. Finally, N-CQDs were obtained by centrifugation (10 min at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm).
000 rpm).
2.4 Quantum yield measurements
The quantum yield (QY) of N-CQDs was calculated using the following equation:where ϕ represents the QY of the sample, I denotes the integrated fluorescence under excitation at 350 nm, n is the refractive index of the solvent, and A is the UV-vis absorbance. The subscript (r) refers to the quinine sulfate reference substance (QY = 54% in 0.1 mol L−1 H2SO4 solution). To reduce the absorption effect, the absorbance was kept below 0.05.
2.5 Detection of Fe3+ and AA
To verify the selectivity of Fe3+, metal ions of the same concentration (100 μM) were added to the N-CQD solution (1 mg mL−1), and fluorescence changes were observed. In addition, competitive experiments in the presence of other metal ions were conducted. A solution of N-CQDs (1 mg mL−1) and various analytes (300 μM) was added to Fe3+ (100 μM), and their fluorescence changes were recorded.For AA detection, N-CQDs (0.5 mg mL−1) and Fe3+ (200 μM) were mixed; then, different concentrations of AA were added to the mixture and finally brought to a constant volume of 3 mL to obtain the fluorescence curves for different concentrations of AA. At the same time, we tested the interference of different substances, including cysteine (Cys), glycine (Gly), glutathione (GSH), malic acid (MA), tartaric acid (TA), D-fructose (Fru), citric acid (CA), glucose (Glu) and methionine (Met) on AA detection. The limit of detection (LOD) was calculated according to the following equation: LOD = 3σ/K, where σ represents the standard deviation for ten blank measurements and K is the slope of the straight-line equation.
2.6 Quantitative analysis of AA in fruit samples
Based on previous literature reports,24,29 the process of extracting AA from fruits is as follows: 50 g of each fruit sample (apple, grape, pear, tomato, and citrus) was weighed, and then the fruits were ground in order to better extract AA from them. In addition, to stabilize AA and prevent its degradation during the subsequent analysis, 50 mL of oxalic acid (0.02 g L−1) was added to the fruit samples. After thorough stirring, solutions containing AA were obtained by centrifugation (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 minutes). AA was detected by the standard curve method and the results were validated using the standard UV-vis method.
000 rpm for 5 minutes). AA was detected by the standard curve method and the results were validated using the standard UV-vis method.
2.7 Application in information anti-counterfeiting
N-CQDs (5 mg mL−1) were used as a fluorescent anti-counterfeiting ink for information encryption, and commercially available filter papers were employed as hand-writing substrates. Photographs were taken under natural and UV light (365 nm). During the decryption process, Fe3+ (0.5 mM) was first added to the CQD ink before hand-writing, followed by spraying with an AA (5 mM) solution.3. Results and discussion
3.1 Characterization of N-CQDs
Phloroglucinol, citric acid and ammonia were used as raw materials to prepare N-CQDs, and the synthesis process is shown in Scheme 1. In order to obtain information on the microstructures of the N-CQDs, TEM and HR-TEM were used to characterize the obtained products. As shown in the TEM image (Fig. 1a) the N-CQDs exhibited good monodispersity and a uniform particle size distribution. Meanwhile, as can be seen from the HR-TEM image (inset of Fig. 1a), N-CQDs showed a clear lattice spacing distance of 0.21 nm, which is consistent with the (100) plane of graphite.30 In addition, statistical analysis was conducted on the size of N-CQDs according to the TEM images (Fig. S1, ESI†), the average particle size of N-CQDs is 3.27 ± 0.69 nm. Using XRD, Raman spectroscopy, XPS and FT-IR characterization methods, we further investigated the structure and composition of N-CQDs. The XRD pattern of N-CQDs is shown in Fig. 1b, where a typical single diffraction peak is observed at around 2θ = 24.3°, which corresponds to the (002) plane. Raman spectra with an excitation wavelength of 532 nm were used to determine the structure of N-CQDs. As shown in Fig. 1c, the two typical peaks at 1359 cm−1 and 1580 cm−1 can be attributed to the D and G bands. The D (A1g) peak indicates the presence of sp3 defects, and the G peak (E2g) explains the phonon mode of sp2 carbon atoms;31 therefore, the Raman peak intensity IG/ID ratio is used to evaluate the quality of synthetic materials,32 and the IG/ID for N-CQDs is about 1.34, indicating that sp2 carbons are the main component. | ||
| Fig. 1 (a) TEM and HRTEM images of N-CQDs (inset); (b) XRD pattern of N-CQDs; (c) Raman spectra of N-CQDs; and high-resolution XPS spectra of (d) C 1s, (e) N 1s, and (f) O 1s. | ||
XPS was used to further study the chemical composition of N-CQDs. Fig. S2 (ESI†) shows the full XPS spectrum of N-CQDs, which indicates the presence of C, N and O in N-CQDs. The corresponding high-resolution spectra of C 1s, N 1s and O 1s are displayed in Fig. 1d–f. As shown in Fig. 1d, the C 1s spectrum can be deconvoluted into three peaks, corresponding to C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (284.7 eV), C–O/C–N (286.2 eV) and C
C (284.7 eV), C–O/C–N (286.2 eV) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.1 eV).33 The high-resolution N 1s spectrum fitted well with two peaks at 399.5 eV and 401.1 eV (Fig. 1e), which could be attributed to N–H and C–N–C, indicating that nitrogen was doped into CQDs.34 As illustrated in Fig. 1f, the peak divides into three peaks, corresponding to 531.4 eV, 533.0 eV and 533.2 eV, which could be assigned to C
O (288.1 eV).33 The high-resolution N 1s spectrum fitted well with two peaks at 399.5 eV and 401.1 eV (Fig. 1e), which could be attributed to N–H and C–N–C, indicating that nitrogen was doped into CQDs.34 As illustrated in Fig. 1f, the peak divides into three peaks, corresponding to 531.4 eV, 533.0 eV and 533.2 eV, which could be assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O and O–C
O, C–O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.35
O, respectively.35
On the basis of FT-IR spectra (Fig. S3, ESI†), abundant functional groups were detected in N-CQDs. The peak at ∼3477 cm−1 corresponds to the stretching vibration of O–H/N–H and the peak at ∼1162 cm−1 represents the tensile vibration of C–N.20,36 Furthermore, the peak at ∼1660 cm−1 confirms the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, the peak at ∼1357 cm−1 corresponds to the C
O, the peak at ∼1357 cm−1 corresponds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibration of sp2 hybridization, and the peak at ∼1080 cm−1 corresponds to the stretching vibration of C–O. Additionally, the peak at ∼1581 cm−1 indicates the presence of an NH2 group, and the peak that appears at ∼2827 cm−1 belongs to the tensile vibration of C–H.21,37,38 The above findings demonstrate that the surface of N-CQDs contains a large number of oxygen-containing functional groups, which make them highly water-soluble.
C vibration of sp2 hybridization, and the peak at ∼1080 cm−1 corresponds to the stretching vibration of C–O. Additionally, the peak at ∼1581 cm−1 indicates the presence of an NH2 group, and the peak that appears at ∼2827 cm−1 belongs to the tensile vibration of C–H.21,37,38 The above findings demonstrate that the surface of N-CQDs contains a large number of oxygen-containing functional groups, which make them highly water-soluble.
To reveal the optical properties of N-CQDs, the PL spectra of N-CQDs with different excitation wavelengths were recorded, as shown in Fig. 2a. The results indicated that the fluorescence peak position changed with the variation of the excitation wavelength (from 300 to 400 nm), and the maximum fluorescence emission was observed at 442 nm with excitation at 340 nm. The excitation wavelength dependent fluorescence behavior confirmed the presence of multiple emission centers in the N-CQDs, which may be due to the synergistic effect of quantum confinement and surface emission states. The UV-vis absorption spectrum was collected, and is presented in Fig. 2b. Two absorption peaks were observed at ∼236 and 330 nm. The peak at ∼236 nm was assigned to the π–π* transition of the aromatic ring C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond in the carbon core,39 which is called the core state absorption. The strong absorption of the long wavelengths at ∼330 nm was assigned to the n–π* transitions of the C
C double bond in the carbon core,39 which is called the core state absorption. The strong absorption of the long wavelengths at ∼330 nm was assigned to the n–π* transitions of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds.37 In addition, the PLQY of the as-prepared N-CQDs was found to be 25.9% based on the standard relative method using quinine sulfate as the reference substance (Fig. S4, S5 and Table S1, ESI†), which is better than or comparable to that of previous literature (Table S2). Meanwhile, the stability of N-CQDs was explored, as shown in Fig. S6 (ESI†), and the test results demonstrate the excellent stability of N-CQDs.
O bonds.37 In addition, the PLQY of the as-prepared N-CQDs was found to be 25.9% based on the standard relative method using quinine sulfate as the reference substance (Fig. S4, S5 and Table S1, ESI†), which is better than or comparable to that of previous literature (Table S2). Meanwhile, the stability of N-CQDs was explored, as shown in Fig. S6 (ESI†), and the test results demonstrate the excellent stability of N-CQDs.
3.2 Fluorescence responses for Fe3+
In recent years, due to their multiple properties including environmental friendliness, low toxicity, tunable fluorescence and facile synthesis, CQDs have attracted widespread attention as an alternative fluorescent sensing material. Therefore, the fluorescence quenching efficiencies of various metal ions on the N-CQDs were investigated. Different cations such as K+, Fe3+, Ni2+, Zn2+, Cr3+, Mg2+, Ba2+, Cu2+, Na+, and Ca2+, each at a concentration of 100 μM, were added to a 1 mg mL−1 N-CQD solution. As depicted in Fig. 2c, among these metal ions, Fe3+ exhibited the strongest PL quenching effect and the relative change in the PL intensity was approximately 90% compared to the blank. Other metal ions had a slight impact on the fluorescence intensity (Fig. S7, ESI†), indicating that N-CQDs exhibit good selectivity for the detection of Fe3+. Furthermore, the effect of the coexisting metal ions on the fluorescence intensity of the N-CQDs (quenched by Fe3+) was determined (Fig. S8, ESI†). The results showed that the fluorescence remained almost unchanged in the presence of other interfering ions. To further explore the detection performance of N-CQDs for Fe3+, various concentrations of Fe3+ranging from 0 to 100 μM were added to the N-CQD solution, and the fluorescence of N-CQDs was gradually quenched as the Fe3+ concentration increased (Fig. 2d and Fig. S9, ESI†). The variation curve of the F0/F values with Fe3+ concentration is shown in Fig. 2e, where F0 and F represent the PL intensities with and without Fe3+ addition, respectively. Fig. 2f shows the standard curve between F0/F and Fe3+concentration, which exhibits a good linear relationship (R2 = 0.9914) in the range of 1–10 μM with a low limit of detection (LOD, 3σ/K) of 0.28 μM. The LOD of the as-prepared N-CQD nanoprobe was superior to or comparable to those reported in previous literature (Table S3, ESI†). In addition, there was a good linear correlation between the fluorescence intensity of N-CQDs and the concentration of Fe3+ ions in the range of 30–100 μM (R2 = 0.9958) with the LOD calculated to be 0.35 μM (Fig. S10, ESI†). It is worth noting that prior to analyzing and detecting Fe3+ and then AA, we conducted a test to establish the correlation between the fluorescence intensity and time after adding the analytes, as illustrated in Fig. S11 and S12 (ESI†). Accordingly, based on the test results, we proceeded to test Fe3+ and AA after 800 s and 650 s, respectively. In addition, based on the highest fluorescence of quantum dots synthesized at pH 9(Fig. S13, ESI†), we meticulously prepared a buffer solution with a pH of 9.18 for determining Fe3+. The results of this experiment are shown in Fig. S14 (ESI†). There was no discernible linear relationship between the fluorescence intensity and Fe3+ concentration. This finding conclusively indicates that the N-CQDs employed in our study are incapable of effectively detecting Fe3+ under alkaline conditions.3.3 Exploration of fluorescence quenching mechanism
PL quenching mechanisms can be divided into dynamic and static quenching. Therefore, in our work, to obtain further insight into the PL quenching mechanism, the UV-vis absorption spectrum of Fe3+ and the excitation and fluorescence spectra of the N-CQDs were measured. As shown in Fig. 3a, the absorption spectra of Fe3+ have a large overlap with the excitation spectrum of N-CQDs, which suggests that the fluorescence quenching caused by Fe3+ may be due to the inner filter effect (IFE), namely, the absorption of the excitation spectra of N-CQDs by Fe3+, which is a result of static quenching. In addition, due to the presence of open d orbitals, Fe3+ is prone to chelation with electron donating groups. According to the above characterization (XPS and FT-IR) results, there are numerous hydroxyl, carboxyl and amine groups on the surface of the N-CQDs, which can chelate with Fe3+ and quench fluorescence based on the electronic transition from the excited state of N-CQDs to the d orbital of Fe3+ ions.40 Thus, we measured the time-resolved fluorescence spectra of the N-CQDs to study the exciton behaviour before and after quenching, as shown in Fig. 3b. The fluorescence decay curves indicated that the average lifetime of the N-CQDs decreased significantly after reacting with Fe3+. These results indicate that PL quenching may be due to nonradiative electron transfer, which demonstrates dynamic quenching. Therefore, the PL quenching mechanism of N-CQDs by Fe3+ involves both dynamic and static quenching, which also explains the high selectivity of N-CQDs to Fe3+.3.4 Fluorescence responses for AA
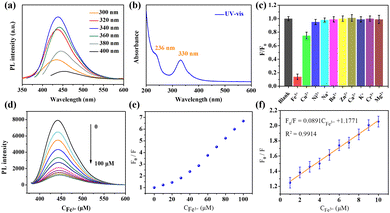
Previous works have demonstrated that Fe3+ can be reduced to Fe2+ in the presence of AA,10,41 on the basis of which a “turn-on” chemosensor platform has been constructed for determining AA. As shown in Fig. 4a, the PL of the quenched N-CQDs/Fe3+ probe was gradually restored by the addition of AA (Fig. S15, ESI†). The plot of F2/F1vs. AA concentration ranging from 30–90 μM showed good linearity with a regression value of around 0.9920, which can be fitted to F2/F1 = 0.0135CAA + 0.9903 (Fig. 4b). The LOD = 0.81 μM, which was calculated according to the formula LOD = 3σ/K. These results suggest that the LOD of the N-CQD/Fe3+ sensor is comparable to or better than those reported in previous literature (Table S4, ESI†). Furthermore, selectivity tests of the N-CQD/Fe3+ sensing platform for AA were performed. Different biological molecules including Cys, Gly, GSH, MA, TA, Fru, CA, Glu, Met and AA at concentrations of 100 μM were mixed with N-CQDs/Fe3+ solutions. As shown in Fig. 4c, the fluorescence intensity of N-CQDs/Fe3+ was significantly increased by the addition of AA, while other biomolecules did not cause obvious changes in fluorescence (Fig. S16, ESI†). To further assess the selectivity of N-CQDs/Fe3+ towards AA, the fluorescence spectra of N-CQDs/Fe3 in AA solution were recorded in the presence of other interfering biological molecules. As shown in Fig. 4d, these common biological molecules had negligible effects on the fluorescence intensity of N-CQDs/Fe3+. Thus, the N-CQDs/Fe3+ could act as a sensitive and selective sensor for AA detection.To gain insight into the fluorescence detection mechanism of AA, the interaction between AA and Fe3+ in the N-CQDs/Fe3+ was investigated. To accomplish this, 1,10-phenanthroline was introduced into both N-CQDs/Fe3+ and N-CQDs/Fe3+ + AA. Remarkably, the presence of 1,10-phenanthroline in N-CQDs/Fe3+ + AA resulted in a distinct absorption peak at approximately 510 nm, which differed from the absorption spectrum of N-CQDs/Fe3+ (Fig. S17, ESI†). Furthermore, the solution underwent a noticeable color change to yellow. This transformation can be attributed to the formation of a novel orange-red complex between Fe2+ and 1,10-phenanthroline,36 indicating the involvement of Fe2+ in the fluorescence detection of AA. Consequently, the introduction of AA effectively minimized the non-radiative electron transfer from the N-CQDs to Fe3+, leading to the recovery of the photoluminescence intensity of the N-CQDs.
3.5 Application to real samples
In order to evaluate the practicality of the N-CQDs/Fe3+ based chemosensor platform, the N-CQDs/Fe3+ sensor was applied for detecting AA in fruits (tomato, citrus, pear, grape and apple). As listed in Table 1, the concentrations detected by the N-CQDs/Fe3+ system using the standard curve method were consistent with those detected by UV-vis spectroscopy, with a low relative standard deviation (RSD) of less than 3.48%. These results indicate that the proposed switchable sensor presents remarkable practicability for monitoring the AA content in fruits.| Samples | UV-vis spectroscopy (mg/100 g) | N-CQDs/Fe3+ system (mg/100 g) | RSD by the SQDs/Fe3+ system (%, n = 5) |
|---|---|---|---|
| Apple | 0.42 ± 0.01 | 0.44 ± 0.67 | 2.36 |
| Pear | 4.89 ± 0.11 | 5.06 ± 0.18 | 3.48 |
| Grape | 22.98 ± 1.09 | 21.73 ± 1.12 | 2.37 |
| Citrus | 33.17 ± 0.54 | 35.05 ± 0.97 | 3.07 |
| Tomato | 27.03 ± 0.96 | 25.96 ± 0.56 | 2.84 |
3.6 Information encryption and decryption applications
Based on the on–off–on fluorescence detection mechanism, we applied the obtained N-CQDs to information security encryption and anti-counterfeiting applications. Words ‘‘Anhui University” and “West” were written using N-CQDs and N-CQDs-Fe3+ ink, respectively. As shown in Fig. 5a, the words were invisible under natural light. However, the word ‘‘Anhui University” was visible under 365 nm UV light (Fig. 5b), indicating that N-CQDs can be used as an invisible ink to achieve information anti-counterfeiting. When AA was sprayed, the word “West” appeared under UV light because the fluorescence from N-GQDs was recovered by AA (Fig. 5c). | ||
| Fig. 5 Words “West Anhui University”, written on commercially available filter paper: (a) daylight; (b) UV light; and (c) after spraying AA solution under UV light. | ||
3.7 Logic gate operations
According to the fluorescence phenomenon of on–off–on, we successfully designed a logic gate. As illustrated in Fig. 6a, the two inputs were Fe3+ and AA, and the output was fluorescence emission.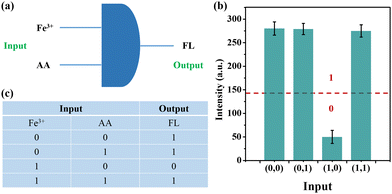 | ||
| Fig. 6 (a) Symbol of logic gate operation; (b) significance of truth table in fluorescence intensity; and (c) truth table. | ||
First, for the outputs, we set the fluorescence intensity value to 140 as the threshold, as shown in Fig. 6b. For the intensity below the threshold, indicating the absence of fluorescence, the output was defined as “0”, while for the intensity above the threshold indicating the presence of fluorescence, the output was defined as “1”. Second, for the inputs, the presence of Fe3+ or AA was defined as “1” and their absence as “0”. Therefore, there are four possible input modes, namely (0,0), (0,1), (1,0) and (1,1), as shown in Fig. 6c. According to the truth table, only when Fe3+ was added and AA was absent (1,0), the fluorescence intensity was below the threshold (Fig. 6b). Therefore, based on the experimental phenomenon, a molecular logic gate was constructed.
4. Conclusions
In summary, we have demonstrated a facile route for the synthesis of highly fluorescent N-CQDs using a solvothermal method. The as-synthesized N-CQDs were successfully used as a highly sensitive and selective sensor for Fe3+ based on the fluorescence “on–off” switch strategy. Moreover, the quenched fluorescence can be recovered by AA; thus, the fluorescence “off–on” system based on N-CQD/Fe3+ has been used to detect AA. In addition, the N-CQD/Fe3+ system can also be used to determine AA in fruit samples with acceptable accuracy and precision. Therefore, the present work offered a convenient alternative method for the detection of Fe3+ and AA according to the “on–off–on” principle. Furthermore, we used the prepared N-CQDs as a fluorescence ink for anti-counterfeiting and designed a molecular logic gate.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the High-level Talent Project of West Anhui University (WGKQ2022085) and the Excellent Research and Innovation Team Project of Anhui Province (2023AH010077). The authors would like to thank the Analytical and Testing Center, West Anhui University, for providing various characterization facilities.Notes and references
- Y. Liu, P. Wu, X. Wu, C. Ma, S. Luo, M. Xu, W. Li and S. Liu, Talanta, 2020, 210, 120649 CrossRef CAS.
- A. Li, J. Zhang, S. Sun, E. Ding, F. Chen, S. He, T. Li, S. Liu and B. Wang, Sens. Actuators, B, 2019, 296, 126636 CrossRef CAS.
- L. Guo, Y. Liu, R. Kong, G. Chen, Z. Liu, F. Qu, L. Xia and W. Tan, Anal. Chem., 2019, 91, 12453–12460 CrossRef CAS.
- H. Yu, Q. Liu, J. Li, Z. M. Su, X. Li, X. Wang, J. Sun, C. Zhou and X. Hu, J. Mater. Chem. C, 2021, 9, 562–568 RSC.
- G. Veerapandi, S. Meenakshi, S. Anitta, C. Arul, P. Ashokkumar and C. Sekar, Food Chem., 2022, 382, 132251 CrossRef CAS PubMed.
- L. V. de Faria, T. P. Lisboa, D. M. de Farias, F. M. Araujo, M. M. Machado, R. A. de Sousa, M. A. C. Matos, R. A. A. Muñoz and R. C. Matos, Food Chem., 2020, 319, 126509 CrossRef PubMed.
- Q. L. Wen, Z. F. Pu, Y. J. Yang, J. Wang, B. C. Wu, Y. L. Hu, P. Liu, J. Ling and Q. Cao, Microchem. J., 2020, 159, 105364 CrossRef CAS.
- R. R. Srivastava, V. K. Singh and A. Srivastava, Opt. Mater., 2020, 109, 110337 CrossRef CAS.
- H. Wang, X. Wang, R. M. Kong, L. Xia and F. Qu, Chin. Chem. Lett., 2021, 32, 198–202 CrossRef CAS.
- X. Yang, J. Yang, M. Zhang, Y. Wang, B. Zhang and X. Mei, Microchem. J., 2022, 174, 107048 CrossRef CAS.
- M. Sobiech, P. Luliński, P. P. Wieczorek and M. Marć, TrAC, Trends Anal. Chem., 2021, 142, 116306 CrossRef CAS.
- S. Zhang, M. Lai, Z. Gao, H. Li, X. Yang, Y. Wei, F. Luo, Z. You, D. Zhang and X. Ji, Sens. Actuators, B, 2023, 395, 134499 CrossRef CAS.
- X. Yang, Q. Zhang, S. Zhang, M. Lai, X. Ji, Y. Ye, H. Li and M. Zhao, Coord. Chem. Rev., 2023, 487, 215154 CrossRef CAS.
- X. Yang, S. Zhang, M. Lai, X. Ji, Y. Ye, J. Tang, X. Liu and M. Zhao, Talanta, 2023, 260, 124628 CrossRef CAS.
- S. Subedi, L. Neupane, H. Yu and K. H. Lee, Sens. Actuators, B, 2021, 338, 129814 CrossRef CAS.
- S. Dhiman, M. Ahmad, N. Singla, G. Kumar, P. Singh, V. Luxami, N. Kaur and S. Kumar, Coord. Chem. Rev., 2020, 405, 213138 CrossRef CAS.
- H. Ren, Y. Yuan, A. Labidi, Q. Dong, K. Zhang, E. Lichtfouse, A. A. Allam, J. S. Ajarem and C. Wang, Chin. Chem. Lett., 2023, 34, 107998 CrossRef.
- R. Qiang, H. Huang, J. Chen, X. Shi, Z. Fan, G. Xu and H. Qiu, ACS Appl. Mater. Interfaces, 2023, 15, 38653–38664 CrossRef CAS.
- F. Yuan, Y. K. Wang, G. Sharma, Y. Dong, X. Zheng, P. Li, A. Johnston, G. Bappi, J. Z. Fan, H. Kung, B. Chen, M. I. Saidaminov, K. Singh, O. Voznyy, O. M. Bakr, Z. H. Lu and E. H. Sargent, Nat. Photonics, 2020, 14, 171–176 CrossRef CAS.
- W. Dong, L. Wang, R. Zhang, C. Wen, R. Su, X. Gong and W. Liang, Dalton Trans., 2023, 52, 6551–6558 RSC.
- X. Kou, S. Jiang, S. J. Park and L. Y. Meng, Dalton Trans., 2020, 49, 6915–6938 RSC.
- F. Yuan, T. Yuan, L. Sui, Z. Wang, Z. Xi, Y. Li, X. Li, L. Fan, Z. A. Tan, A. Chen, M. Jin and S. Yang, Nat. Commun., 2018, 9, 2249 CrossRef.
- T. Yuan, F. Yuan, L. Sui, Y. Zhang, Y. Li, X. Li, Z. A. Tan and L. Fan, Angew. Chem., Int. Ed., 2023, 62, e202218568 CrossRef CAS.
- X. Li, C. Wang, P. Li, X. Sun, Z. Shao, J. Xia, Q. Liu, F. Shen and Y. Fang, Food Chem., 2023, 409, 135243 CrossRef CAS PubMed.
- K. Jiang, S. Sun, L. Zhang, Y. Lu, A. Wu, C. Cai and H. Lin, Angew. Chem., Int. Ed., 2015, 127, 5450–5453 CrossRef.
- Q. Wang, Z. Wang, Z. Pu, Y. Wang and M. Li, Lanthanum-doped carbon quantum dots (La-CQDs) for detection of Fe3+ in colorimetric test paper and information anti-counterfeiting, Opt. Mater., 2023, 137, 113630 CrossRef CAS.
- A. Tan, G. Yang and X. Wan, Spectrochim. Acta, Part A, 2021, 253, 119583 CrossRef CAS.
- B. Gollapelli, R. Suguru Pathinti and J. Vallamkondu, ACS Appl. Nano Mater., 2022, 5, 11912–11922 CrossRef CAS.
- H. Rao, H. Ge, Z. Lu, W. Liu, Z. Chen, Z. Zhang, X. Wang, P. Zou, Y. Wang, H. He and X. Zeng, Microchim. Acta, 2016, 183, 1651–1657 CrossRef CAS.
- M. R. Pallavolu, S. Prabhu, R. R. Nallapureddy, A. S. Kumar, A. N. Banerjee and S. W. Joo, Carbon, 2023, 202, 93–102 CrossRef CAS.
- A. C. Ferrari, J. C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K. S. Novoselov, S. Roth and A. K. Geim, Phys. Rev. Lett., 2006, 97, 187401 CrossRef CAS PubMed.
- L. Jiao, X. Wang, G. Diankov, H. Wang and H. Dai, Nat. Nanotechnol., 2010, 5, 321–325 CrossRef CAS.
- H. Li, T. Xu, Z. Zhang, J. Chen, M. She, Y. Ji, B. Zheng, Z. Yang, S. Zhang and J. Li, Chem. Eng. J., 2023, 453, 139722 CrossRef CAS.
- Y. Shen, M. Rong, X. Qu, B. Zhao, J. Zou, Z. Liu, Y. Bao, Y. He, S. Li, X. Wang, M. Chen, K. Chen, Y. Zhang and L. Niu, Talanta, 2022, 241, 123224 CrossRef CAS.
- P. Siahcheshm and P. Heiden, J. Photochem. Photobiol., A, 2023, 435, 114284 CrossRef CAS.
- X. Luo, W. Zhang, Y. Han, X. Chen, L. Zhu, W. Tang, J. Wang, T. Yue and Z. Li, Food Chem., 2018, 258, 214–221 CrossRef CAS.
- G. Wang, S. Zhang, J. Cui, W. Gao, X. Rong, Y. Lu and C. Gao, Anal. Chim. Acta, 2022, 1195, 339478 CrossRef CAS PubMed.
- G. S. Das, J. P. Shim, A. Bhatnagar, K. M. Tripathi and T. Y. Kim, Sci. Rep., 2019, 9, 15084 CrossRef PubMed.
- W. Liu, Q. Wang, Z. Liu and G. Ding, J. Colloid Interface Sci., 2022, 622, 21–30 CrossRef CAS.
- L. Zhu, D. Shen, Q. Liu, C. Wu and S. Gu, Appl. Surf. Sci., 2021, 565, 150526 CrossRef CAS.
- X. Gao, X. Zhou, Y. Ma, T. Qian, C. Wang and F. Chu, Appl. Surf. Sci., 2019, 469, 911–916 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nj04521h |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2024 |