Heterobimetallic iridiumIII–europiumIII complex: the role of donor energy on sensitising the EuIII ion†
Felipe da Silva Manrique
Canisares
 ab,
Renan Caike
Silva
ab,
Renan Caike
Silva
 ac,
Marian Rosaly
Davolos
b,
Ana Maria
Pires
ac,
Marian Rosaly
Davolos
b,
Ana Maria
Pires
 abc and
Sergio Antonio Marques
Lima
abc and
Sergio Antonio Marques
Lima
 *abc
*abc
aSão Paulo State University (Unesp), School of Technology and Sciences, Presidente Prudente-SP, Brazil. E-mail: sergio.lima@unesp.br
bSão Paulo State University (Unesp), Institute of Chemistry, Araraquara-SP, Brazil
cSão Paulo State University (Unesp), Institute of Biosciences, Humanities and Exact Sciences, São José do Rio Preto-SP, Brazil
First published on 4th December 2023
Abstract
In luminescent devices, the combination of red, green, and blue luminophores (RGB system) allows the creation of other colours in the electromagnetic spectrum, including the perception of white light. Among the three fundamental colours necessary to create the RGB system, normally, the red colour has received great attention compared with the others because red luminophores present lower Φ due to the energy gap law. Red emitters are easily obtained using EuIII ion, however, although the red emitters based on the EuIII ion have high colour purity, the Φ normally is lower compared with red emitters based on the IrIII ion, even though the colour purity of the latter is not so high. Thus, to produce efficient red-emitting complexes, a strategy that has been developed is the synthesis of heterobimetallic d–f complexes to join the high Φ of the d-metal complexes with the high colour purity of the EuIII ion. Herein, a novel dual bimetallic emitter was synthesised and fully characterised, the [{Ir(dfppy)2(μ-bpdc)}3Eu2]Cl3·nH2O·mCH3OH (dfppy is the 2-(2,4-difluorophenyl)pyridine cyclometallating ligand, and the ancillary ligand bpdc is the 2,2′bipyridine-3,3′-dicarboxylic acid). This complex was immobilised in a polymeric PMMA film presenting a high Φ value, 42.2 ± 4.2%, in the yellow spectral region. Its precursor, the novel green-emitting IrIII complex, [Ir(dfppy)2(bpdc)], showed 97.2 ± 9.7% of Φ after doped in PMMA matrix. Both films were used to coat a UV-LED chip to investigate the photophysical properties when these complexes are applied to create phosphor-converted LEDs. The bimetallic prototype exhibited higher radiant photostability after 18 h of operation compared with the heteroleptic IrIII complex-doped film. For the first time, the lowest triplet energy (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 103 cm−1) from an IrIII complex that efficiently sensitises the EuIII ion in an IrIII–EuIII heterobimetallic complex has been determined using voltage modulation in a UV-coated LED prototype, which can be used to engineer new pure red-emitting d–f bimetallic systems.
103 cm−1) from an IrIII complex that efficiently sensitises the EuIII ion in an IrIII–EuIII heterobimetallic complex has been determined using voltage modulation in a UV-coated LED prototype, which can be used to engineer new pure red-emitting d–f bimetallic systems.
1. Introduction
Screen devices such as televisions, cell phones, and computers, as well as public and domestic lighting, are responsible for a large share of energy consumption in developed countries,1–3 which is directly connected to the low efficiency of these devices in converting electricity into light. In this scenario, the search for luminescent materials with high emission quantum yields (Φ), to reduce energy consumption has been incessant. Compounds based on metal complexes with a large spin–orbit coupling constant (ξ), such as IrIII,4 PtII,5 OsII,6 and ReI,7 have been reported with high values of Φ's. However, these complexes have broad emission bands attributed to a metal-to-ligand charge transfer (MLCT), a ligand-centered (LC), or a hybrid (MLCT-LC) emissive state.8 As a consequence, the colour purity of d-metal complexes is quite low, which is a problem for imaging devices because higher colour purity enables the production of images with greater colour gamut and contrast. Therefore, a convenient strategy to increase the colour purity of luminophores is the use of lanthanide ions that exhibit narrow emission bands. In addition, their emission properties are less dependent on the chemical environment in which they are inserted.9In luminescent devices, the combination of red, green, and blue luminophores (RGB system) allows the perception of all other colours, including white light.10 Among the three fundamental colours necessary to create the RGB system, red causes great apprehension in the scientific community because red luminophores normally exhibit lower Φ due to the energy gap law than the other two.11
The red emission colour, in turn, can be easily obtained through the coordination of organic molecules to the EuIII ion. Photophysical and energy transfer processes of EuIII complexes have been deeply investigated,12 allowing the construction of an energy map between the excited donor state of the ligand (D) and the excited acceptor state (A) of EuIII ion.13 These studies report that the dominant energy transfer path is singlet state (S) → triplet state (T) → EuIII, and thus, the only important parameter is considered to be the gap between the feeding triplet state of the ligand (D) and the excited states of the EuIII ion.14 This process is called the antenna effect. Normally, the best energy transfer rates in monocentered EuIII complexes are found when the triplet state has a slightly higher energy than the acceptor excited states of EuIII ion. It is agreed that a safe energy difference between D and A states to avoid high energy back-transfer rates and promote high Φ is observed about 2500 and 3000 cm−1 above the 5D0.14 While 5D0 is the main emitter state of the EuIII ion, 5D1 is the main acceptor state for most of the ligand classes, including β-diketones,15 exceptions to this rule are Schiff bases that sensitise the 5D0 emitting state better,16 and polyaminocarboxylates, in which the best acceptor is the 5D2 excited state.17 These rationalisations are obtained through the analysis of an enormous number of different complexes with ligands having different triplet energies, making this strategy time and resources consuming.
Although red emitters based on the EuIII ion have high colour purity, the Φ is normally lower than that of red emitters based on IrIII complexes. Strategically, the synthesis of heterobimetallic d–f complexes or d–f metal organic frameworks (MOFs) has been explored, combining the high Φ of the d-metal complexes with the high colour purity of the lanthanideIII ions. In this context, systems based on IrIII complexes coordinated to EuIII ions are the most investigated aiming for synergic results for a good luminophore.18–20
In an IrIII–EuIII heterobimetallic complex, the IrIII complex itself acts as a sensitising ligand, and the fundamental idea is that the triplet excited overpopulated state, resulting from the high spin–orbit coupling (SOC) of the IrIII ion (ξ = 4430 cm−1),21 transfers its energy to EuIII ion.22 As in monocentered EuIIIcomplexes, in heterobimetallic IrIII–EuIII complexes, the sensitisation efficiency depends on the energy difference between D and A; however, for d–f systems, this energy gap still needs to be further studied.
Data from the literature reveal that the energy transfer efficiency between the D state of IrIII complex and the A state of EuIII ion is not so obvious, because in this case, the D state is also an efficient and long-lived emitter state. Consequently, in such systems, three different situations can occur, as depicted in Fig. 1. (i) When the D state is energetically lower than the emissive state of EuIII ion, the sensitisation does not occur, as expected, and the IrIII complex is not a good antenna for the EuIII ion. This is the case of [{(ppy)2Ir(μ-pmc)}3EuCl3] heterobimetallic complex reported by Lian et al.,23 in which the D state is at approximately 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 666 cm−1, and it is situated below the 5D0 emitting level of the EuIII ion (17
666 cm−1, and it is situated below the 5D0 emitting level of the EuIII ion (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 225 cm−1),24 accordingly only the emission from the IrIII complex component is detected. By increasing the D energy state to be energetically higher than 5D0, the sensitisation efficiency increases until it reaches its maximum. (ii) If the D state is higher but close to the A state, the energy might be transferred partially, characterising an emission spectrum with both emissive components, a broad band from IrIII component, and narrow emission bands from EuIII. (iii) If the D state is higher than the A state to avoid energy back-transfer, the emission spectrum will show only the narrow emission bands from EuIII. A few heterobimetallic complexes with efficient energy transfer to the EuIII ion have been reported, such as [{(dfppy)2Ir(μ-phen5f)}3EuCl]Cl2 published by Chen et al.,25 where the D state is centred at 20,408 cm−1; Jiang et al.18 also reported a heterobimetallic complex with efficient energy transfer, i.e., the [{Ir(dfppy)2(cbphen)}3ClEu]Cl2, in which the D state is situated at 19
225 cm−1),24 accordingly only the emission from the IrIII complex component is detected. By increasing the D energy state to be energetically higher than 5D0, the sensitisation efficiency increases until it reaches its maximum. (ii) If the D state is higher but close to the A state, the energy might be transferred partially, characterising an emission spectrum with both emissive components, a broad band from IrIII component, and narrow emission bands from EuIII. (iii) If the D state is higher than the A state to avoid energy back-transfer, the emission spectrum will show only the narrow emission bands from EuIII. A few heterobimetallic complexes with efficient energy transfer to the EuIII ion have been reported, such as [{(dfppy)2Ir(μ-phen5f)}3EuCl]Cl2 published by Chen et al.,25 where the D state is centred at 20,408 cm−1; Jiang et al.18 also reported a heterobimetallic complex with efficient energy transfer, i.e., the [{Ir(dfppy)2(cbphen)}3ClEu]Cl2, in which the D state is situated at 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230 cm−1 at room temperature in dichloromethane solution. In both cases solely emission from the EuIII component is observed. If the D-state energy is much higher than the 5D0 energy, emission from both components will occur due to the poor energy transfer efficiency, and a spectrum-like situation (ii) is expected, which has been reported for several IrIII–EuIII bimetallic systems.23,26,27 In such studies, D-state values above 21
230 cm−1 at room temperature in dichloromethane solution. In both cases solely emission from the EuIII component is observed. If the D-state energy is much higher than the 5D0 energy, emission from both components will occur due to the poor energy transfer efficiency, and a spectrum-like situation (ii) is expected, which has been reported for several IrIII–EuIII bimetallic systems.23,26,27 In such studies, D-state values above 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 690 cm−1 make the sensitisation process inefficient. Systems based on multiple emissive states find several applications, such as white light for general lighting,28 or in luminescent ratiometric probes for analyses in environmental29 and biomedical fields.30 However, a clear boundary between a situation in which there is only emission from the EuIII component or a mixture of light from components has never been established. In this work, two films of PMMA with [Ir(dfppy)2(bpdc)] heteroleptic complex (Ir-p), or [{Ir(dfppy)2(μ-bpdc)}3Eu2]Cl3·nH2O·mCH3OH heterobimetallic complex (Ir-p–Eu) were used to coat a UV-LED chip to create two phosphor-converted LED (PC-LED) prototypes. The results acquired by modulating the voltage applied to the Ir-p–Eu:LED prototype allowed us to establish the minimum energy required to solely observe the emission of EuIII ions.
690 cm−1 make the sensitisation process inefficient. Systems based on multiple emissive states find several applications, such as white light for general lighting,28 or in luminescent ratiometric probes for analyses in environmental29 and biomedical fields.30 However, a clear boundary between a situation in which there is only emission from the EuIII component or a mixture of light from components has never been established. In this work, two films of PMMA with [Ir(dfppy)2(bpdc)] heteroleptic complex (Ir-p), or [{Ir(dfppy)2(μ-bpdc)}3Eu2]Cl3·nH2O·mCH3OH heterobimetallic complex (Ir-p–Eu) were used to coat a UV-LED chip to create two phosphor-converted LED (PC-LED) prototypes. The results acquired by modulating the voltage applied to the Ir-p–Eu:LED prototype allowed us to establish the minimum energy required to solely observe the emission of EuIII ions.
2. Experimental
2.1. Chemicals
Methanol (CH3OH, Synth, 99.5%), iridiumIII chloride hydrate (IrCl3·xH2O, Aldrich, 99.99%), 2 ethoxyethanol (C4H10O2, Aldrich, 99.0%), dichloromethane (CH2Cl2, Synth, 99.99%), 2-(2,4-difluorophenyl)pyridine (dfppy – C11H7F2N, Aldrich, 97%), 2,2′-bipyridine-3,3′-dicarboxylic acid (bpdc – C12H8N2O4, Aldrich, 97%), and PMMA ([CH2C(CH3)(CO2CH3)]n, Aldrich) were used as start reactants without any further purification.2.2. Complex syntheses
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 24 h. The second step was iridium complex synthesis. The ancillary ligand bpdc (2,2′ bipyridine-3,3′-dicarboxylic acid) (54.2 mg – 0.22 mmol) was dissolved in water, then a solution of [(dfppy)2Ir(μ-Cl)2Ir(dfppy)2] (112.2 mg–0.09 mmol) in CH2Cl2 (5 mL) was added to the ancillary ligand solution, resulting in a biphasic system; then, CH3OH (5 mL) was added resulting in a monophasic system. The mixture was stirred for 4 h at room temperature; after this time, the solvents were reduced by heating and the complex was precipitated by adding water, filtered, and dried in a dissector. Yield: 88.7% (78.7 mg). FTIR – ATR (vmax/cm−1): 1602 (υCOO−)ass, 1572, 1558 (υC
1) for 24 h. The second step was iridium complex synthesis. The ancillary ligand bpdc (2,2′ bipyridine-3,3′-dicarboxylic acid) (54.2 mg – 0.22 mmol) was dissolved in water, then a solution of [(dfppy)2Ir(μ-Cl)2Ir(dfppy)2] (112.2 mg–0.09 mmol) in CH2Cl2 (5 mL) was added to the ancillary ligand solution, resulting in a biphasic system; then, CH3OH (5 mL) was added resulting in a monophasic system. The mixture was stirred for 4 h at room temperature; after this time, the solvents were reduced by heating and the complex was precipitated by adding water, filtered, and dried in a dissector. Yield: 88.7% (78.7 mg). FTIR – ATR (vmax/cm−1): 1602 (υCOO−)ass, 1572, 1558 (υC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)pyridine, 1374 (υCOO−)sym. MALDI-TOF for C34H20F4IrN4O4m/z found(calcd.) of 817.1(817.1), representing [[Ir(dfppy)2(bpdc)] + H]+. UV-Vis (in DCM, 6.48 × 10−6 mol L−1); λmax 254 and εmax 41203 mol−1 dm3 cm−1, others λ nm (ε, mol−1 dm3 cm−1): 300 (19444), 350 (6327), 417 (1234), 488 (308). Fluorescence: excitation λmax (in aerated DCM solution, 6.48 × 10−6 mol L−1) 488 nm, emission 555 nm, Stokes shift 67 nm, quantum yield (Φfl) 10.4%.
N)pyridine, 1374 (υCOO−)sym. MALDI-TOF for C34H20F4IrN4O4m/z found(calcd.) of 817.1(817.1), representing [[Ir(dfppy)2(bpdc)] + H]+. UV-Vis (in DCM, 6.48 × 10−6 mol L−1); λmax 254 and εmax 41203 mol−1 dm3 cm−1, others λ nm (ε, mol−1 dm3 cm−1): 300 (19444), 350 (6327), 417 (1234), 488 (308). Fluorescence: excitation λmax (in aerated DCM solution, 6.48 × 10−6 mol L−1) 488 nm, emission 555 nm, Stokes shift 67 nm, quantum yield (Φfl) 10.4%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)pyridine, 1383 (υCOO−)sym. Elemental analysis; found(calcd.) C: 39.45%(39.39%), H: 2.87%(2.86%), N: 5.90%(5.30%) to C104H90Cl3Eu2F12Ir3N12O28 ({[Ir(dfppy)2(μ-bpdc)]3Eu2}Cl3·14H2O·2CH3OH), MALDI-TOF found(calcd.) [{[(dfppy)2Ir(μ-bpdc)]3Eu2}Cl·13H2O·2CH3OH – (2Cl− + H2O)]2+m/z of 1541.2(1541.1). UV-Vis (in DCM, 4.62 × 10−6 mol L−1); λmax 254 and εmax 56
N)pyridine, 1383 (υCOO−)sym. Elemental analysis; found(calcd.) C: 39.45%(39.39%), H: 2.87%(2.86%), N: 5.90%(5.30%) to C104H90Cl3Eu2F12Ir3N12O28 ({[Ir(dfppy)2(μ-bpdc)]3Eu2}Cl3·14H2O·2CH3OH), MALDI-TOF found(calcd.) [{[(dfppy)2Ir(μ-bpdc)]3Eu2}Cl·13H2O·2CH3OH – (2Cl− + H2O)]2+m/z of 1541.2(1541.1). UV-Vis (in DCM, 4.62 × 10−6 mol L−1); λmax 254 and εmax 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 277 mol−1 dm3 cm−1, others λ nm (ε, mol−1 dm3 cm−1): 300 (31168), 350 (10606), 417 (3246), 488 (1082). Fluorescence: excitation λmax (in aerated DCM solution, 4.62 × 10−6 mol L−1) 451 nm, emission IrIII component 560 nm, EuIII component 616 nm. Quantum yield (Φfl) 9.7%.
277 mol−1 dm3 cm−1, others λ nm (ε, mol−1 dm3 cm−1): 300 (31168), 350 (10606), 417 (3246), 488 (1082). Fluorescence: excitation λmax (in aerated DCM solution, 4.62 × 10−6 mol L−1) 451 nm, emission IrIII component 560 nm, EuIII component 616 nm. Quantum yield (Φfl) 9.7%.
 | ||
| Fig. 2 Molecular structure of the complexes synthesised in this study. For clarity, water- and methanol-coordinated molecules were omitted in the representation of the bimetallic complex. | ||
Preparation of PMMA films with Ir-p and Ir-p–Eu complexes. Solutions of the complexes in DCM were prepared and mixed with PMMA dissolved in DCM to obtain films containing 0.5 wt% of the complex. The mixture was stirred and heated to reduce the solvent volume to approximately 0.5 mL, and the mixture was then deposited on a 22 × 22 mm glass substrate and covered with a Petri dish in an atmosphere saturated with DCM vapour.
Preparation of PC-UV LED prototypes. The 0.5 wt% doped PMMA films were glued onto the surface of a UV-LED chip (λmax = 395 ± 10 nm, Shenzhen Chang Long Technology Co., Ltd, type SMD) with cyanoacrylate and allowed to dry overnight. The emission spectrum of the LED prototype was acquired operating at 2.98 V and 54 mA, and the photostability was monitored for 18 h in a PerkinElmer model LS55 spectrometer using an emission slit width of 3 nm. To evaluate the functionality of the fabricated devices at different voltages, the LED prototype spectra were recorded by varying the voltage from 2.90 V to 3.05 V in intervals of 0.01 V. The power supply to turn on the LED was an ICEL PS- 1500 pack.
2.3. Instrumental
 | (1) |
| I = I0·e−t/τ | (2) |
Depopulation can occur by radiative (Arad) or non-radiative (Anrad) paths. The total decay rate (At = Arad + Anrad) is given by the inverse of the lifetime, as shown in eqn (3):36
| τ−1 = Arad + Anrad | (3) |
The radiative decay rate is calculated by the sum of the photons emitted in each 5D0 → 7FJ (J = 0, 1, 2, 3, and 4) set of transitions, as shown in eqn (4) and (5):
| Arad = A00 + A01 + A02 + A03 + A04 | (4) |
 | (5) |
A 01 is the spontaneous decay rate referring to the magnetic dipole allowed transition 5D0 → 7F1, used as an internal standard because it is not influenced by the electric field over the system. S01:0J and v01:0J are the area and barycenter of the 5D0 → 7F1 and 5D0 → 7FJ transitions (J = 0, 2, 3, and 4), respectively. Using the radiative and nonradiative decay rates, it is possible to determine the intrinsic emission quantum yield (ΦEuEu) of the 5D0 emissive state of the complex, as shown in eqn (6):37
 | (6) |
3. Results and discussion
3.1. Structural characterisation of the complexes
The zwitterionic heteroleptic IrIII complex Ir-p, which was used as the ligand, was synthesised according to the method described in the literature.31 In this complex, dfppy is the 2-(2,4-difluorophenyl)pyridine cyclometallating ligand, and the ancillary ligand bpdc is the 2,2′-bipyridine-3,3′-dicarboxylic acid. The stoichiometry of IrIII complex was confirmed by MALDI-TOF analysis, [M + H]+m/z found(calcd.) of 817.1(817.1) (Fig. S1, ESI†); FTIR (Fig. S2, ESI†) and UV-Vis (Fig. S3, ESI†) spectroscopies were also performed to complete the elucidation.The heterobimetallic Ir-p–Eu complex was synthesised in methanolic solution with the aforementioned synthesised IrIII complex and europiumIII chloride (EuCl3). The formation of the heterobimetallic complex was confirmed by elemental analysis, found(calcd.) C: 39.45%(39.39%), H: 2.87%(2.86%), N: 5.90%(5.30%) to C104H90Cl3Eu2F12Ir3N12O28 – [{Ir(dfppy)2(μ-bpdc)}3Eu2]Cl3·14H2O·2CH3OH. The stoichiometry of the Ir-p–Eu complex was confirmed by MALDI TOF analysis, in which the spectra display the molecular ion peak with the isotopic signature of the IrIII and EuIII ions, as shown in Fig. 3. In the Ir-p–Eu spectrum, it is possible to observe the molecular ion signal at 1541.2 m/z, which is assigned to the ionised structure ([{[(dfppy)2Ir(μ-bpdc)]3Eu2}Cl·13H2O·2CH3OH – (2Cl− + H2O)]2+), with a chemical formula of C104H88Eu2F12Ir3N12O27Cl2+ and a molar weight of 3081.91 g mol−1. The chemical formula of the neutral species is C104H90Eu2F12Ir3N12O28Cl3 with a molar weight of 3170.82 g mol−1. Signal peaks associated with other ionised species were detected at 1497.1 and 1480.1, these values indicate a lower number of water molecules associated with the complex. The FTIR spectra (Fig. S4, ESI†) show that the IrIII complex is coordinated by the oxygen atoms of the carboxylate groups into the EuIII ion. The antisymmetric-stretching of the carboxylic group, ν(COO−)ass, shifted from 1601 cm−1 to 1598 cm−1, indicating a lower oscillator energy and now matches with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching. On the other hand, the symmetric-stretching, ν(COO−)sym, shifted to higher energy, from 1374 cm−1 to 1384 cm−1. Other bands related to C
N stretching. On the other hand, the symmetric-stretching, ν(COO−)sym, shifted to higher energy, from 1374 cm−1 to 1384 cm−1. Other bands related to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds were not influenced by coordination with LnIII ions, as shown in Fig. S4 (ESI†). Furthermore, water and methanol molecules can be in both coordinated and hydrated forms, as the coordination number of EuIII ions can vary from 6 to 12. The elucidation of the structure of the Ir-p–Eu complex was based on the article published by Calefi et al.,38 in which dinuclear EuIII and TbIII complexes with 2, 2′-bipyridine-4,4′-dicarboxylic acid, a ligand similar to bpdc used here, and the synthesis was also carried out via wet route. Normally, the bpdc ligand forms metal organic frameworks (MOF) when the compound is prepared by the hydrothermal route.39 From the FTIR data, it was possible to assume that carboxylic groups from Ir-p moisture are coordinated by the monodentate binding mode to EuIII, since the energy difference between νass(COO−) and νsym(COO−) in Ir-p–Eu (Δ = 213 cm−1) is higher than that measured for the ionic ligand40 (Na2bpdc: Δ = 205 cm−1), Fig. S5 (ESI†). UV-Vis spectra were recorded, and they are presented in the ESI† Fig. S6. Both complexes showed absorption bands extending from the deep UV to visible region. The thermal stability of the complexes was determined by differential scanning calorimetry (DSC), which displays an exothermic event with onset at 368 °C for both complexes. These temperatures were attributed to the initial degradation of these complexes, probably starting at the Ir component, as shown in Fig. S7 (ESI†). The coordination of the Ir-p complex with the EuIII ion did not influence the thermal stability of the final compound.
C bonds were not influenced by coordination with LnIII ions, as shown in Fig. S4 (ESI†). Furthermore, water and methanol molecules can be in both coordinated and hydrated forms, as the coordination number of EuIII ions can vary from 6 to 12. The elucidation of the structure of the Ir-p–Eu complex was based on the article published by Calefi et al.,38 in which dinuclear EuIII and TbIII complexes with 2, 2′-bipyridine-4,4′-dicarboxylic acid, a ligand similar to bpdc used here, and the synthesis was also carried out via wet route. Normally, the bpdc ligand forms metal organic frameworks (MOF) when the compound is prepared by the hydrothermal route.39 From the FTIR data, it was possible to assume that carboxylic groups from Ir-p moisture are coordinated by the monodentate binding mode to EuIII, since the energy difference between νass(COO−) and νsym(COO−) in Ir-p–Eu (Δ = 213 cm−1) is higher than that measured for the ionic ligand40 (Na2bpdc: Δ = 205 cm−1), Fig. S5 (ESI†). UV-Vis spectra were recorded, and they are presented in the ESI† Fig. S6. Both complexes showed absorption bands extending from the deep UV to visible region. The thermal stability of the complexes was determined by differential scanning calorimetry (DSC), which displays an exothermic event with onset at 368 °C for both complexes. These temperatures were attributed to the initial degradation of these complexes, probably starting at the Ir component, as shown in Fig. S7 (ESI†). The coordination of the Ir-p complex with the EuIII ion did not influence the thermal stability of the final compound.
3.2. Luminescence characterisation
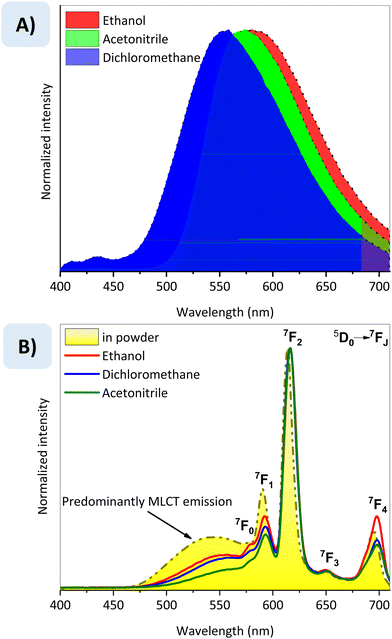
The luminescent behaviour of the complexes was analysed in an aerated solution of ethanol (EtOH), dichloromethane (DCM), and acetonitrile (ACN). The Ir-p–Eu was also analysed in the solid state. The emission spectra of Ir-p are dominated by a MLCT broadband in all three solvents analysed and are strongly influenced by solvent polarisation properties, as shown in Fig. 4(A). Ir-p can be excited in a wide range from 250 nm to over 500 nm, Fig. S8 (ESI†). The Ir–p–Eu complex emits from green to orange because of the reminiscent emission from the Ir–p complex, which partially sensitises the EuIII, along with narrow emission bands of the 5D0 → 7FJ (J from 0 to 4) transitions of EuIII, Fig. 4(B). The maximum emission wavelength and the relative emission intensity of the MLCT band in both complexes change under the influence of the solvent, while the f–f transitions remain almost unchanged for the Ir–p–Eu complex, as expected. This shows that the sensitisation of the EuIII ion is dependent on the environment. The spectral changes in the emission spectra of both samples reflect the displacement of the colour coordinates (CIE-1931 x; y), as follows: Ir-p in DCM: (0.496; 0.497), ACN: (0.500; 0.487) and EtOH: (0.424; 0.529); Ir-p–Eu in DCM: (0.564; 0.422), ACN: (0.599; 0.394), EtOH: (0.554; 0.434) and solid state: (0.500; 0.475). The relative emission quantum yield (Φ) was analysed for all tested solutions, and for both complexes, the same behaviour was observed greatest value being found in the DCM solution and the lowest in the EtOH solution. For Ir-p, the following values were found, DCM: 10.4%, ACN: 2.3%, and EtOH: 1.3%; for Ir-p–Eu, the following values were found: DCM: 9.7%, ACN: 6.6%, and EtOH: 4.4%. The method used to calculate the relative emission quantum yield is described in the Experimental Section, which was obtained from the UV-Vis and emission spectra (Fig. S9 and S10, ESI†). The lifetime (τ), Fig. S11 (ESI†), and intrinsic emission quantum yield (ΦEuEu) of the 5D0 level of the EuIII ion were determined, and the following values were found; DCM: τ = 0.477 ms, and ΦEuEu = 18.1%; ACN: τ = 0.567 ms, and ΦEuEu = 26.8%; EtOH: τ = 0.263 ms, and ΦEuEu = 10.1%, and in the solid state: τ = 0.237 ms, and ΦEuEu = 7.4%. The lifetime values were obtained using a monoexponential decay fit and are presented in Fig. S11 (ESI†).To evaluate the potential application of Ir-p and Ir-p–Eu complexes as coatings on PC-LEDs, they were immobilised into PMMA films. The emission spectra of both films are shown in Fig. 5 compared to the emission spectra of the respective complexes in the DCM solution. By comparison, it is possible to observe that the immobilisation in the PMMA matrix resulted in a blue shift of the MLCT emission band of the IrIII component due to the rigidochromic effect.41 The overall emission quantum yield of the doped PMMA films was measured using an integrated sphere, and the values were 97.2% (± 9.7%) for Ir-p and 42.2% (± 4.2%) for Ir-p–Eu doped films. The lower value found for the bimetallic-doped film evidences the strong influence of the PMMA matrix in the radiative and non-radiative pathways of EuIII emission, since this ion is more susceptible to vibronic coupling or multiphonon relaxation.42 The measured Φ for the bimetallic system in dichloromethane solution was 9.7%, a value lower than the values already reported in the literature considering an efficient energy transfer between the IrIII moiety and the EuIII ion, in which pure red-emitting compounds were reported, such as the complex {[(dfppy)2Ir(μ-phen5f)]3EuCl}Cl2, with 17% of Φ25 or {[Ir(dfppy)2(cbphen)]3ClEu}Cl2 and Ir(dfppy)2(cbphen)Eu(TTA)3 with 10% and 44%, respectively.18 It is a consensus that dual-emitting IrIII–EuIII bimetallic complexes have a lower Φ than pure red-emitting bimetallic complexes, such as the complexes published by W. Jiang et al.,26 which Φ comprises between 11% and 28%; therefore, the bimetallic system presented here follows the default behaviour. Furthermore, it is important to mention that no article has reported the Φ of IrIII–EuIII bimetallic complexes after incorporation into polymeric films. Here, the immobilisation of the dyad in the PMMA matrix increased the Φ from 9.7% to 42.2%, indicating that incorporation into the PMMA film has boosted the Φ of the blend, qualifying it to be applied in coated UV-LEDs. All additional luminescent characterisations of the complexes can be found in the ESI,† from Fig. S8 to Fig. S12. From Fig. 4 (B) it is clear that the present system corresponds to a situation in which the triplet donor state of the IrIII component does not completely sensitise the EuIII ion; hence, dual emission is observed from both emitter states (3MLCT of the IrIII component and 5D0 of the EuIII ion).
 | ||
| Fig. 5 Comparison between emission spectra measured in DCM solution and immobilised in PMMA film, (A) heteroleptic Ir-p complex and (B) bimetallic Ir-p–Eu complex. | ||
3.3 PC-LED prototypes characterisation
The PMMA films were glued onto the surface of a UV-LED chip (λmax = 395 ± 10 nm, Shenzhen Chang Long Technology Co., Ltd, type SMD) with cyanoacrylate and allowed to dry overnight. Then, luminescence measurements were performed to monitor the radiant stability of the fabricated prototypes. In this construction the UV light is generated by electroluminescence, which then photoexcites the top films, thus the PMMA films are excited by photoluminescence. Photographs of the fabricated prototypes in operation are shown in Fig. 6(A). The emission band centred at 390 nm presented in both prototypes is assigned to the UV emission of the chip itself used as the excitation source Fig. 6(A). The emission spectrum of the Ir-p:LED prototype is dominated by a broadband in the green spectral region (λmax = 545 nm). The same broadband is observed for the Ir-p–Eu:LED prototype, however, the narrow emission bands of the EuIII ion in the red spectral region are also present, similar to what was observed in solution, once again indicating a dual emission from the device Ir-p–Eu:LED as the IrIII component can only partially transfer its energy to the EuIII component. The radiant photostability of the prototypes was analysed at 2.98 V over an uninterrupted 18 h of operation, and the measured emission spectra are shown in Fig. S13 (ESI†). The radiant profile of the Ir-p–Eu:LED prototype closely follows the bare LED curve, indicating high photostability of the Ir-p–Eu complex. On the other hand, the Ir-p:LED curve indicates lower photostability, losing 28% of the initial emission area after 10 h of operation; thereafter, the emission area remained almost constant. As the bare UV-LED loses approximately 13% of its initial area, it is pertinent to say that the decrease in the total emission area for the Ir-p–Eu:LED prototype is largely due to the decrease in the efficiency of the commercial UV LED chip used as the excitation source. By monitoring the emission intensity of the Ir-p–Eu:LED prototype over time, it was possible to observe that both Ir-p and EuIII emission components decrease; however, the EuIII component decreases faster than the Ir-p broadband emission, as can be seen in the normalised emission spectra in Fig. 6(B), Moreover, the maximum emission wavelength of the Ir-p component shifts to a lower energy, and consequently, the energy transfer efficiency to the EuIII ion decreases, leading to a lower efficient sensitisation. Thus, the decrease in the integrated emission area of the Ir-p–Eu:LED prototype is not only related to the degradation of the heterobimetallic complex, as expected. This may also be due to the decrease in the EuIII sensitisation efficiency over the operation time, probably due to the heating of the doped film by the UV-LED chip. To attest to this behaviour, the temperature was monitored before and after 18 h of operation using a digital thermometer, and the temperature showed an increase of 1.5 °C, from 27.2 °C to 28.7 °C. The Ir-p:LED prototype also exhibited the same behaviour, shifting the emission towards a lower energy region over the operating time (Fig. S14, ESI†). In order to evaluate the functionality of the fabricated devices at different voltages, the LED prototype spectra were recorded by varying the voltage from 2.90 V to 3.05 V in intervals of 0.01 V, Fig. 6(A). For both prototypes, a shift in the maximum emission wavelength of the Ir-p component towards higher energy was observed as the applied voltage increases (λmax 558 → 535 nm). In the Ir-p–Eu:LED, this phenomenon boosted the sensitisation of the EuIII ion when the voltage was increased, which can be observed through the normalised spectra obtained for each applied voltage, Fig. 7(A). Similar shifting behaviour for the Ir-p:LED prototype can be seen in Fig. S15 (ESI†). Fig. 7(B) shows a graphic of the ratio between the area under the emission curve of the IrIII component and under the emission narrow band assigned to 5D0 → 7F2 of the EuIII ion in the Ir-p–Eu:LED device as a function of the maximum emission energy of the IrIII component at the different tested voltages. A linear behaviour is obtained between 2.98 and 3.04 V. Below 2.98 V, the spectra exhibited low resolution and high noise/signal ratio (Fig. S16, ESI†), and for this reason, they were not considered. Above 3.04 V, the prototype emission saturates the detector, making it impossible to adequately quantify the photons. The emission areas of each component were determined using the deconvolution method for each emission spectrum obtained (Fig. S17–S23, ESI†). Through a linear fit, it was possible to propose eqn (7), which relates the IrIII/EuIII emission ratio as a function of the maximum emission energy of the IrIII component (assuming it as the donor state), and thus calculate the minimum energy required for the total sensitisation of the EuIII ion. | (7) |
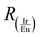 is the ratio of the integrated areas under the IrIII and EuIII components and EIr_max is the energy of the maximum wavelength of emission for the IrIII (D) component in the Ir-p–Eu:LED prototype, given in cm−1. In this way, when
is the ratio of the integrated areas under the IrIII and EuIII components and EIr_max is the energy of the maximum wavelength of emission for the IrIII (D) component in the Ir-p–Eu:LED prototype, given in cm−1. In this way, when 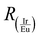 is null, only the emission of the EuIII component will be observed, and the energy necessary to satisfy this condition is 19
is null, only the emission of the EuIII component will be observed, and the energy necessary to satisfy this condition is 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 103 cm−1, approximately 1876 cm−1 above the EuIII ion 5D0 emitter level. This value is very close to those reported in the literature for total EuIII ion sensitisation in both monocentred EuIII
103 cm−1, approximately 1876 cm−1 above the EuIII ion 5D0 emitter level. This value is very close to those reported in the literature for total EuIII ion sensitisation in both monocentred EuIII![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 and heterobimetallic IrIII–EuIII complexes.20
13 and heterobimetallic IrIII–EuIII complexes.20
Conclusions
A novel dual-emitting IrIII–EuIII bimetallic complex, [{Ir(dfppy)2(μ-bpdc)}3Eu2]Cl3·nH2O·mCH3OH, was synthesised and fully characterised. This complex was immobilised in a polymeric PMMA film and presented high Φ value, 42.2 ± 4.2% in the yellow spectral region. Its precursor, Ir-p, showed 97.2 ± 9.7% of Φ after doped in PMMA matrix. The Ir-p–Eu bimetallic complex was applied as a PC-LED and showed great radiant stability, losing only 15% of the initial intensity after 18 h of operation. However, the dominant emission wavelength suffered a redshift because heating over the operation time decreased the energy of the donor 3MLCT state of IrIII component, decreasing the efficiency of the sensitisation process to the EuIII ion. In addition, with this study, it was possible to determine the minimum energy of the ligand triplet donor level to observe only the red emission of the EuIII ion in a heterobimetallic IrIII–EuIII complex, that is, 19103 cm−1. With this result, it is possible to predict whether an iridium heteroleptic complex has enough energy to efficiently sensitise an EuIII ion to produce pure red-emitting compounds.Author contributions
Felipe da Silva Manrique Canisares: conceptualization, data curation, formal analysis, and writing: original writing – original draft. Renan Caike Silva: formal analysis, writing – original draft. Marian Rosaly Davolos: writing – review & editing, supervision. Ana Maria Pires: writing – review & editing, supervision. Sergio Antonio Marques de Lima: conceptualization, writing – review & editing, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful to the São Paulo Research Foundation (FAPESP, Grant No. 2019/26103-7 and Grant No. 2015/03400-5) for supporting this research. We also acknowledge the Brazilian agencies for the concession of scholarships CNPq (A.M.P. for Grant No. 309448/2021-2 and S.A.M.L. for Grant No. 308868/2022-6), and CAPES (RCS for Grant no. 88887.686158/2022-00 and F.S.M.C. for Grant No. 88887.341772/2019-00.), and Fulbright Commission.Notes and references
- R. Saad, B. A. Portnov and T. Trop, Clean Technol. Environ. Policy, 2021, 23, 251–269 CrossRef
.
- Y. L. Chang and Z. H. Lu, J. Disp. Technol., 2013, 9, 459–468 CAS
.
- C. Te Lee and P. T. Ho, Electronics, 2020, 9, 1–13 Search PubMed
.
- S. Lamansky, P. Djurovich, D. Murphy, F. Abdel-Razzaq, R. Kwong, I. Tsyba, M. Bortz, B. Mui, R. Bau and M. E. Thompson, Inorg. Chem., 2001, 40, 1704–1711 CrossRef CAS
.
- B. Ma, P. I. Djurovich and M. E. Thompson, Coord. Chem. Rev., 2005, 249, 1501–1510 CrossRef CAS
.
- J. Kido and Y. Okamoto, Chem. Rev., 2002, 102, 2357–2368 CrossRef CAS PubMed
.
- A. Casula, V. Nairi, V. Fernández-Moreira, A. Laguna, V. Lippolis, A. Garau and M. Concepción Gimeno, Dalton Trans., 2015, 44, 18506–18517 RSC
.
- H. Yersin, A. F. Rausch, R. Czerwieniec, T. Hofbeck and T. Fischer, Coord. Chem. Rev., 2011, 255, 2622–2652 CrossRef CAS
.
- J. Kido and Y. Okamoto, Chem. Rev., 2002, 102, 2357–2368 CrossRef CAS
.
- N. Thejokalyani and S. J. Dhoble, Renew. Sustainable Energy Rev., 2014, 32, 448–467 CrossRef CAS
.
- Y. Zhang, D. Zhang, T. Huang, A. Gillett, Y. Liu, D. Hu, L. Cui, Z. Bin, G. Li, J. Wei and L. Duan, Angew. Chem., Int. Ed., 2021, 60, 20498–20503 CrossRef CAS
.
- K. Binnemans, Coord. Chem. Rev., 2015, 295, 1–45 CrossRef CAS
.
- J.-C. G. Bünzli, Coord. Chem. Rev., 2015, 293–294, 19–47 CrossRef
.
- J.-C. G. Bünzli and S. V. Eliseeva, Basics of Lanthanide Photophysics, in Lanthanide Luminescence, ed. P. Hänninen and H. Härmä, Springer Series on Fluorescence, Springer, Berlin, Heidelberg, 2010, vol. 7, pp. 1–45.
- S. Sato and W. Masanobu, Bull. Chem. Soc. Jpn., 1970, 43, 1955–1962 CrossRef CAS
.
- R. D. Archer, H. Chen and L. C. Thompson, Inorg. Chem., 1993, 12, 2089–2095 Search PubMed
.
- M. Latva, H. Takalo, V.-M. Mukkala, C. Matachescu, J. C. Rodriguez-Ubis and J. Kankare, J. Lumin., 1997, 75, 149–169 CrossRef CAS
.
- W. Jiang, C. Hong, H. Wei, Z. Wu, Z. Bian and C. Huang, Inorganica Chim. Acta, 2017, 459, 124–130 CrossRef CAS
.
- F. F. Chen, Z. Q. Chen, Z. Q. Bian and C. H. Huang, Coord. Chem. Rev., 2010, 254, 991–1010 CrossRef CAS
.
- G. Yu, Y. Xing, F. Chen, R. Han, J. Wang, Z. Bian, L. Fu, Z. Liu, X. Ai, J. Zhang and C. Huang, ChemPlusChem, 2013, 78, 852–859 CrossRef CAS
.
- K. P. S. Zanoni, A. Ito and N. Y. Murakami Iha, New J. Chem., 2015, 39, 6367–6376 RSC
.
- D. Sykes, A. J. Cankut, N. M. Ali, A. Stephenson, S. J. P. Spall, S. C. Parker, J. A. Weinstein and M. D. Ward, Dalton Trans., 2014, 43, 6414–6428 RSC
.
- P. Lian, H. Wei, C. Zheng, Y. Nie, J. Bian, Z. Bian and C. Huang, Dalton Trans., 2011, 40, 5476–5482 RSC
.
- G. Blasse, M. Buys and N. Sabbatini, Chem. Phys. Lett., 1986, 124, 538–542 CrossRef CAS
.
- F. F. Chen, Z. Q. Bian, Z. W. Liu, D. B. Nie, Z. Q. Chen and C. H. Huang, Inorg. Chem., 2008, 47, 2507–2513 CrossRef CAS PubMed
.
- W. Jiang, B. Lou, J. Wang, H. Lv, Z. Bian and C. Huang, Dalton Trans., 2011, 40, 11410–11418 RSC
.
- P. Coppo, M. Duati, V. N. Kozhevnikov, J. W. Hofstraat and L. De Cola, Angew. Chem., Int. Ed., 2005, 44, 1806–1810 CrossRef CAS
.
- A. G. Bispo-Jr, S. A. M. Lima, L. D. Carlos, A. M. Pires and R. A. S. Ferreira, Adv. Eng. Mater., 2020, 22, 2000422 CrossRef CAS
.
- T. Rasheed, C. Li, M. Bilal, C. Yu and H. M. N. Iqbal, Sci. Total Environ, 2018, 640–641, 174–193 CrossRef CAS
.
- Y. Zhuang, Q. Xu, F. Huang, P. Gao, Z. Zhao, X. Lou and F. Xia, ACS Sens., 2016, 1, 572–578 CrossRef CAS
.
- W. Jiang, Y. Gao, Y. Sun, F. Ding, Y. Xu, Z. Bian, F. Li, J. Bian and C. Huango, Inorg. Chem., 2010, 49, 3252–3260 CrossRef CAS
.
- M. Nonoyama, Bull. Chem. Soc. Jpn., 1974, 47, 767–768 CrossRef CAS
.
- M. Pannipara, A. M. Asiri, K. A. Alamry, M. N. Arshad and S. A. El-Daly, J. Fluoresc., 2014, 24, 1629–1638 CrossRef CAS
.
- F. S. M. Canisares, A. G. Bispo-Jr, A. M. Pires and S. A. M. Lima, Optik, 2020, 219, 164995 CrossRef CAS
.
-
J.-C. G. Bünzli and S. V. Eliseeva, Basics of Lanthanide Photophysics, in Lanthanide Luminescence, ed. P. Hänninen and H. Härmä, Springer Series on Fluorescence, Springer, Berlin, Heidelberg, 2010, vol. 7, pp. 1–45
.
- C. M. B. Leite Silva, A. G. Bispo-Jr, F. S. M. Canisares, S. A. Castilho, S. A. M. Lima and A. M. Pires, Luminescence, 2019, 34, 877–886 CrossRef CAS PubMed
.
- I. P. Assunção, A. N. Carneiro Neto, R. T. Moura, C. C. S. Pedroso, I. G. N. Silva, M. C. F. C. Felinto, E. E. S. Teotonio, O. L. Malta and H. F. Brito, Chem. Phys. Chem., 2019, 20, 1931–1940 CrossRef PubMed
.
- P. S. Calefi, A. O. Ribeiro, A. M. Pires and O. A. Serra, J. Alloys Compd., 2002, 344, 285–288 CrossRef CAS
.
- K. Fan, S. S. Bao, R. Huo, X. Da Huang, Y. J. Liu, Z. W. Yu, M. Kurmoo and L. M. Zheng, Inorg. Chem. Front., 2020, 7, 4580–4592 RSC
.
- G. B. Deacon and R. J. Phillips, Coord. Chem. Rev., 1980, 33, 227–250 CrossRef CAS
.
- P. N. Lai, C. H. Brysacz, M. K. Alam, N. A. Ayoub, T. G. Gray, J. Bao and T. S. Teets, J. Am. Chem. Soc., 2018, 140, 10198–10207 CrossRef CAS PubMed
.
-
E. Kreidt, C. Kruck and M. Seitz, Handbook on the Physics and Chemistry of Rare Earths, Elsevier B.V., 2018, vol. 53, pp. 35–79 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nj03161f |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2024 |