Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Aza-BODIPY-based polymeric nanoparticles for photothermal cancer therapy in a chicken egg tumor model†
Kantapat
Chansaenpak
 *a,
Gong Yi
Yong
b,
Anawin
Prajit
c,
Peraya
Hiranmartsuwan
a,
Shaamini
Selvapaandian
b,
Bongkot
Ouengwanarat
c,
Tunyawat
Khrootkaew
c,
Piyanut
Pinyou
*a,
Gong Yi
Yong
b,
Anawin
Prajit
c,
Peraya
Hiranmartsuwan
a,
Shaamini
Selvapaandian
b,
Bongkot
Ouengwanarat
c,
Tunyawat
Khrootkaew
c,
Piyanut
Pinyou
 c,
Chin Siang
Kue
*b and
Anyanee
Kamkaew
c,
Chin Siang
Kue
*b and
Anyanee
Kamkaew
 *c
*c
aNational Nanotechnology Center, National Science and Technology Development Agency, Thailand Science Park, Pathum Thani, Thailand 12120. E-mail: kantapat.cha@nanotec.or.th
bFaculty of Health and Life Sciences, Management and Science University, Seksyen 13, Shah Alam, Selangor, Malaysia 40100. E-mail: cskue@msu.edu.my
cSchool of Chemistry, Institute of Science, Suranaree University of Technology, Nakhon Ratchasima, Thailand 30000. E-mail: anyanee@sut.ac.th
First published on 27th October 2023
Abstract
A new push–pull aza-BODIPY (AZB-CF3) derivative comprised of dimethylamino groups and trifluoromethyl moieties was successfully synthesized. This derivative exhibited broad absorption in the near-infrared region in the range from 798 to 832 nm. It also exhibited significant near-infrared (NIR) signals in low-polar solvents with emission peaks around 835–940 nm, while non-fluorescence in high-polar environments due to the twisted intramolecular charge transfer (TICT) phenomenon. The nanoprecipitation of this compound with phospholipid-based polyethylene glycol (DSPE-PEG) yielded AZB-CF3@DSPE-PEG nanoparticles (NPs) with a hydrodynamic size of 70 nm. The NPs exhibited good photostability, colloidal stability, biocompatibility, and excellent photothermal (PTT) competence with a conversion efficiency (η) of 44.9%. These NPs were evaluated in vitro and in ovo in a 4T1 breast cancer cell line for NIR light-trigger photothermal therapy. Proven in the chicken egg tumor model, AZB-CF3@DSPE-PEG NPs induced severe vascular damage (∼40% vascular destruction), showed great anticancer efficacy (∼75% tumor growth inhibition), and effectively inhibited distant metastasis via photothermal treatment. As such, this PTT-based nanocarrier system could be a potential candidate for a clinical cancer therapy approach.
Introduction
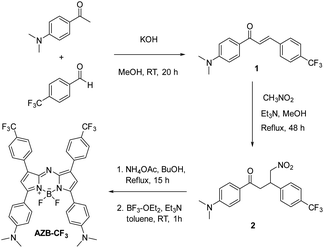
Cancer is still a serious menace to human health among human non-communicable diseases (NCD) despite many methods have been developed to conquer its risk.1–4 Due to the fast proliferation of tumor cells, traditional cancer treatments, including radiation therapy, chemotherapy, surgery, cannot cure cancer effectively.5–7 Recently, phototherapies, such as photodynamic therapy (PDT) and photothermal therapy (PTT), have received growing attention for tumor ablation owing to their low toxicity, low side effects, and no drug resistance.8–10 The PTT technique is a potent and minimally invasive cancer treatment that can induce apoptosis through the elevation of the tissue temperature.11–13 The thermal effect is generated artificially when near-infrared (NIR) light is applied in the presence of the PTT agent. The NIR light benefits PTT in terms of high penetration depth and minimal cytotoxicity due to the low photon absorption of endogenous biomolecules in the NIR wavelength.14–16 In addition, PTT treatments rely on the administration of tumor-targeting photosensitizers (PS), followed by local irradiation at the tumor location. The formulation of those PS into nanoscale materials can lead to a high accumulation of PS in the tumor sites via enhanced permeability and retention (EPR) effects, thus enhancing PTT efficiency.17–19Azadipyrromethene boron difluoride (aza-BODIPY) derivatives are recently developed PS that possess suitable properties for cancer phototherapeutic applications, including near-infrared absorption, high extinction coefficients, and excellent photostability.20–26 Most importantly, their absorption range and photothermal conversion efficiency can be nicely tuned through structural modifications of the aza-BODIPY core by varying the electronic-tuning substituents. Recently, our group has successfully introduced the push–pull electronic effect into the aza-BODIPY backbone consisting of electron-donating dimethylamino groups (push moiety), as well as the strong electron-withdrawing cyano group (pull moiety), which can adjust its absorption range to 850–887 nm in various solvents.27 Furthermore, adding dimethylamino units to aza-BODIPY has been shown to encourage photothermal conversion as well as significantly decrease fluorescence quantum yield via non-radiative relaxation.27,28
As part of our continued investigation on PTT-based aza-BODIPY, we have synthesized a new push–pull aza-BODIPY analog containing dimethylamino groups and trifluoromethyl groups (AZB-CF3). Similar to other aza-BODIPY derivatives in the literature,29–31 the hydrophobic AZB-CF3 needs to be formulated into nanoparticles to enhance the water suspendability and tumor-targetableity of the compound. In this work, the amphiphilic phospholipid–polymer conjugate, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-poly(ethylene glycol) (DSPE-PEG) was selected as the encapsulating material due to its biocompatibility, biodegradability, and great colloidal stability in biological matrices.32–34 After nanoparticle formulation via the nanoprecipitation method, the PTT efficiency of the prepared nanoparticles for cancer treatment was then examined in the chicken egg tumor model as demonstrated in Scheme 1. In this model, the tumor cells can be efficiently engrafted on the vascularized chorioallantoic membrane (CAM) due to the natural immunodeficiency of the avian embryo. This in ovo model can serve as an alternative for mammalian in vivo models to investigate the characteristics of tumor growth, metastasis, angiogenesis, and efficacy of the cancer phototherapies of the nanoparticles.35–37
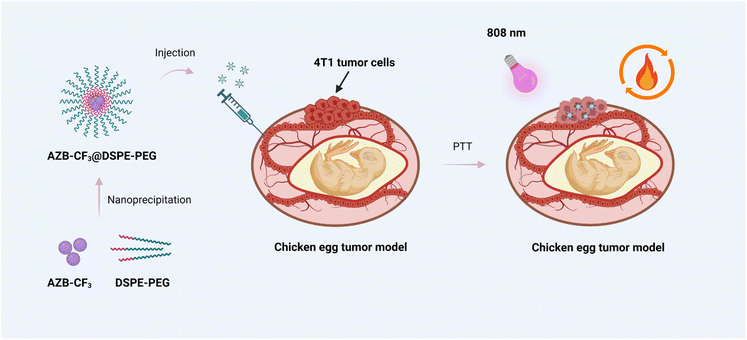 | ||
| Scheme 1 The overall concept of the aza-BODIPY-based polymeric nanoparticles for photothermal cancer therapy in the chicken egg tumor model. | ||
Experimental section
Materials and instruments
Boron trifluoride etherate (BF3OEt2) was purchased from Sigma Aldrich. 1,2-Distearoyl-sn-glycero-3-phosphoethanol amine-N-[methoxyl-(polyethylene glycol)-2000] (DSPE-PEG2000) was purchased from Avanti Polar Lipids, Inc. 4-Dimethylaminoacetophenone, 4-(trifluoromethyl) benzaldehyde, nitromethane (CH3NO2), triethylamine (Et3N), and ammonium acetate (NH4OAc) were purchased from Tokyo Chemical Industry (TCI). All the chemicals and solvents were used without further purification. UV-vis absorption spectra were acquired from a Cary Series UV-Vis-NIR spectrophotometer (Agilent Tech, Santa Clara, CA, USA). Emission spectra were obtained using a PTI QuantaMaster 500 Near Infra-Red Photoluminescence System (HORIBA Scientific). The details for UV-Vis-NIR and fluorescence measurements and quantum yield calculations are listed in the SI. Dynamic light scattering (DLS) measurements were performed using the Zetasizer Nano series (Malvern Panalytical). Scanning electron microscopy (SEM) images were obtained using a SU-8030 Field-Emission Scanning Electron Microscope (Hitachi). Transmission electron microscopy (TEM) images were acquired from a JEM- 2100-Plus Transmission Electron Microscope (JEOL). Nuclear magnetic resonance (NMR) spectra were obtained on a Bruker NMR 500 spectrometer operating at 500 and 125 MHz for 1H and 13C NMR, respectively. Electrospray ionization mass spectra were collected from a Bruker micrOTOF spectrometer.Synthetic procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1
1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the AZB-CF3 as a dark purple powder (40.6 mg, 28.3%). 1H NMR (500 MHz, DMSO-d6) δ: 8.43 (d, J = 8.1 Hz, 4H), 8.30 (d, J = 9.2 Hz, 4H), 7.99 (d, J = 8.3 Hz, 4H), 7.85 (s, 2H), 6.97 (d, J = 9.2 Hz, 4H), 3.20 (s, 12H). 13C NMR (125 MHz, DMSO-d6) δ: 154.61, 152.09, 144.26, 136.88, 136.28, 132.48, 131.90, 130.43, 129.08, 128.75, 128.60, 125.41, 120.14, 117.44, 116.90, 112.07, 111.90, 111.74, 29.28, 28.97. HRMS (ESI), m/z calcd for C38H31BF3N4O2 ([M + H]+): 719.2429, found: 719.2444.
1) to give the AZB-CF3 as a dark purple powder (40.6 mg, 28.3%). 1H NMR (500 MHz, DMSO-d6) δ: 8.43 (d, J = 8.1 Hz, 4H), 8.30 (d, J = 9.2 Hz, 4H), 7.99 (d, J = 8.3 Hz, 4H), 7.85 (s, 2H), 6.97 (d, J = 9.2 Hz, 4H), 3.20 (s, 12H). 13C NMR (125 MHz, DMSO-d6) δ: 154.61, 152.09, 144.26, 136.88, 136.28, 132.48, 131.90, 130.43, 129.08, 128.75, 128.60, 125.41, 120.14, 117.44, 116.90, 112.07, 111.90, 111.74, 29.28, 28.97. HRMS (ESI), m/z calcd for C38H31BF3N4O2 ([M + H]+): 719.2429, found: 719.2444.
Electrochemical characterization
A three-electrode setup with a PalmSens 4 potentiostat/galvanostat (PalmSens, Houten, the Netherlands) and PSTrace 5.9 software was used to investigate the electrochemical behavior of AZB-CF3. The three-electrode setup included a Pt-disk working electrode (3 mm diameter), an Ag/Ag+ non-aqueous reference electrode (0.01 M AgNO3), and a Pt plate counter electrode. Under an argon-saturated atmosphere, the measurements were carried out by dissolving the compound in dichloromethane with 0.1 M tetrabutylammonium hexafluorophosphate (TBAPF6) as a supporting electrolyte.Theoretical calculations
The ground state and the first singlet excited state geometries were optimized using the density functional theory (DFT) and the time-dependent (TD) methods. Both methods were applied with the B3LYP hybrid functional and 6-311G basis set (abbreviated B3LYP/6-311G). All the calculations were performed using the TURBOMOLE software package.Preparation of AZB-CF3@DSPE-PEG NPs
The AZB-CF3@DSPE-PEG NPs were prepared by the nanoprecipitation method adapted from the literature.38 A THF solution (2 mL) containing phospholipid-poly(ethylene glycol) hybrid polymer, DSPE-PEG2000, (1.5 mg), and three different amounts of AZB-CF3 (0.6, 0.8, and 1.0 mg) was added to de-ionized (DI) water (20 mL) under continuous stirring. The solution mixture was sonicated for 5 min using a sonicator (CREST) before being uninterruptedly stirred overnight to remove the organic solvent (THF). The NP suspension was filtered through a 0.22 μm filter resulting in a deep purple AZB-CF3@DSPE-PEG suspension. The resulting NP aqueous solutions were freeze-dried and stored at 4 °C for further use. The detailed protocols for the determination of encapsulation efficiency (%EE), dye loading percentage, and colloidal stability of the NPs are displayed in the (ESI†).Bioapplications of AZB-CF3@DSPE-PEG NPs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The cell mixture at the volume of 25 μL per embryo was loaded on the targeted CAM (EDD-10) enriched with the blood vessels. The eggs were sealed with parafilm and returned to the incubator post-tumor implantation. On EDD-14, the tumor mass grown on CAMs was monitored and randomly selected for the antitumor study. AZB-CF3@DSPE-PEG NPs, DSPE-PEG, and AZB-CF3 (equivalent dose as described above) were intravenously administered into the chick embryos at 20 μL per embryo (n = 5 per group). At 2 min post-administration, tumor tissue was irradiated with an 808 nm laser at 1.0 W cm−2 for 60 s. The real-time temperature at the irradiated tumor site was monitored throughout the 60 s of photo-irradiation by using the IR Thermal Imager. The tumor volume was measured using calipers at 24, 48, and 72 h post-PTT. The tumor volume (mm3) was calculated according to the equation of volume [(tumor width)2 × tumor length/2]. The chick embryos were euthanised under hypothermic conditions (4 °C) for histopathological analysis.
1. The cell mixture at the volume of 25 μL per embryo was loaded on the targeted CAM (EDD-10) enriched with the blood vessels. The eggs were sealed with parafilm and returned to the incubator post-tumor implantation. On EDD-14, the tumor mass grown on CAMs was monitored and randomly selected for the antitumor study. AZB-CF3@DSPE-PEG NPs, DSPE-PEG, and AZB-CF3 (equivalent dose as described above) were intravenously administered into the chick embryos at 20 μL per embryo (n = 5 per group). At 2 min post-administration, tumor tissue was irradiated with an 808 nm laser at 1.0 W cm−2 for 60 s. The real-time temperature at the irradiated tumor site was monitored throughout the 60 s of photo-irradiation by using the IR Thermal Imager. The tumor volume was measured using calipers at 24, 48, and 72 h post-PTT. The tumor volume (mm3) was calculated according to the equation of volume [(tumor width)2 × tumor length/2]. The chick embryos were euthanised under hypothermic conditions (4 °C) for histopathological analysis.
Results and discussion
Synthesis and photophysical properties of AZB-CF3
AZB-CF3 was synthesized via the conventional synthetic methods for aza-BODIPY as demonstrated in Scheme 2.41–43 In the procedure, the chalcone derivative 1 was simply prepared from an aldol condensation reaction between 4-(trifluoromethyl)benzaldehyde and 4-dimethylaminoacetophenone. The resulting chalcone was then converted to the γ-nitro-substituted ketone product (2) through the Michael addition of nitromethane. Next, compound 2 was reacted with an excess amount of ammonium acetate followed by BF2-complexation, yielding AZB-CF3 as the final product. The structures of AZB-CF3 and its intermediates were confirmed by 1H and 13C NMR spectroscopic techniques as displayed in the NMR spectra (Fig. S1–S6) in the ESI.† The detection of the molecular ions by positive-mode electrospray mass spectrometry at m/z = 342.1074, 403.1238, and 719.2444 additionally confirmed the identities of 1, 2, and AZB-CF3, respectively (Fig. S7–S9†).As indicated in Table 1 and Fig. 1A and B, the photophysical characteristics of AZB-CF3 were examined using a Vis-NIR and fluorescence spectrophotometer in different solvents. The near-infrared region of AZB-CF3 showed wide absorption with a peak at a wavelength between 798 and 832 nm. Interestingly, the dye showed strong fluorescence signals in low-polar solvents with emission maxima at 835, 901, and 937 nm in hexane, toluene, and dichloromethane (DCM), respectively. In contrast to its cyano-analogue,27AZB-CF3 showed higher fluorescence quantum yields (Φf) in low-polar solvents (hexane and toluene) and variable Stokes shifts ranging from 37 to 107 nm. AZB-CF3 exhibited weak fluorescence signals in polar solvents, including, acetone, acetonitrile, and methanol, indicating a twisted intramolecular charge transfer (TICT) phenomenon upon excitation.44,45 As the concentration of EtOH was increased in the hexane-EtOH systems, AZB-CF3 likewise displayed a red shift in emission maxima as well as decreased fluorescence, confirming the molecule's solvatochromic nature as a result of the TICT effect (Fig. 1C).
| Solventa | λ max (nm) | ε (M−1 cm−1) | λ emiss (nm) | Δλ (nm) | Φ f |
|---|---|---|---|---|---|
| a DCM = dichloromethane, MeOH = methanol. b The solutions were excited at 810 nm. c Relative indocyanine green (ICG) in DMSO (Φf = 0.13). d NF = no fluorescence. | |||||
| Hexane | 798 | 7.4 × 104 | 835 | 37 | 0.13 (±0.02) |
| Toluene | 826 | 8.3 × 104 | 901 | 75 | 0.14 (±0.02) |
| DCM | 830 | 8.4 × 104 | 937 | 107 | 0.04 (±0.01) |
| Acetone | 832 | 6.8 × 104 | NFd | ||
| Acetonitrile | 828 | 6.3 × 104 | NF | ||
| MeOH | 824 | 6.3 × 104 | NF | ||
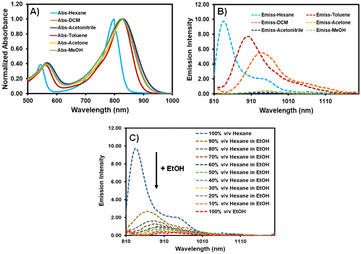 | ||
| Fig. 1 Absorption (A) and emission (B) spectra of AZB-CF3 in various solvents and (C) emission spectra of AZB-CF3 in hexane–EtOH mixtures (excitation wavelength = 810 nm). | ||
Electrochemical properties and computational studies of AZB-CF3
The electronic properties of AZB-CF3 were studied by the cyclic voltammetry (CV) technique. As depicted in Fig. S15,† the cyclic voltammogram of AZB-CF3 exhibits two distinct reversible reduction waves for the cathodic scan (−0.65 and −1.20 V) attributed to the one-electron transfer process from the formation of stable radical anions and di-anions.27 For the anodic scan, two oxidation peaks were observed. The first oxidation wave at 0.28 V is associated with a one-electron oxidation process, yielding the cation radical. The second oxidation wave coud be attributed to the unstable oxidized form from the first oxidation, which undergoes one-electron oxidation.46Next, the electrochemical bandgap was determined by calculating the first oxidation and reduction potentials obtained from the CV. These potentials provide the energy levels for the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) with reference to the energy levels of the Fc/Fc+ redox couple (−4.8 eV relative to the vacuum level). The HOMO and LUMO levels of AZB-CF3 can be calculated using the half-wave potential (E1/2) of Fc/Fc+ (0.26 V), as presented in Table 2. The small energy gap of 0.93 eV between the LUMO and HOMO obtained from CV is consistent with the absorption and emission spectra of AZB-CF3, which are aligned in a NIR region.
| E red2 (V) | E red1 (V) | E ox1 (V) | E ox2 (V) | LUMO (eV) | HOMO (eV) | ΔEg (eV) |
|---|---|---|---|---|---|---|
| a E LUMO = −4.8 + [E1/2(Fc) − Ered] eV, EHOMO = −4.8 + [E1/2(Fc) − Eox] eV, ΔEg = ELUMO − EHOMO. | ||||||
| −1.20 | −0.65 | 0.28 | 0.47 | −3.89 | −4.82 | 0.93 |
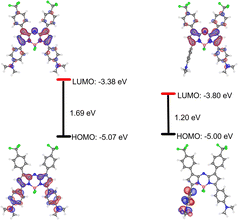
The electronic phenomenon of this aza-BODIPY derivative was further investigated by density functional theory (DFT) and time-dependent (TD) DFT calculations using the B3LYP/6-311G level of theory. The optimized structures, as well as frontier molecular orbitals (HOMO/LUMO) of AZB-CF3 in both the ground state and excited state, are demonstrated in Fig. 2. In the excited state, AZB-CF3 demonstrated obvious electron cloud migration from the dimethylaminophenyl moiety in the HOMO to the aza-BODIPY backbone in the LUMO, while their planes were arranged perpendicular to each other, indicating the TICT state.47 The low energy gap of 1.20 eV obtained from this calculated method agrees well with that acquired from the experimental method (0.93 eV).
Preparation and characterizations of AZB-CF3 nanoparticles
Due to the undesirable hydrophobicity of the aza-BODIPY derivative, AZB-CF3 was formulated into nanoparticles (NPs) using 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxyl-(polyethylene glycol)-2000] (DSPE-PEG), the phospholipid-based PEG polymer, by the nanoprecipitation method (Fig. 3A). To prepare the water-suspendable AZB-CF3 NPs (AZB-CF3@DSPE-PEG), a THF solution of AZB-CF3 (0.6, 0.8, or 1.0 mg) and DSPE-PEG (1.5 mg) was slowly added to 20 mL water under continuous stirring. The mixture was sonicated for 5 min before being stirred overnight to remove THF. The resulting purple suspension was then passed through a 0.22 μm filter yielding AZB-CF3@DSPE-PEG NPs. The dried NPs can be thoroughly suspended in a phosphate buffer solution (PBS, pH 7) as shown in Fig. 3B (vial ii), which is different from the free dye (vial i), suggesting the great water-suspendability of the AZB-CF3-encapsulated nanomaterials.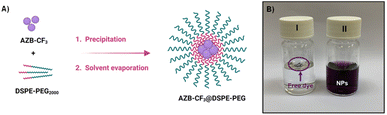 | ||
| Fig. 3 (A) Scheme showing the preparation of AZB-CF3@DSPE-PEG NPs, (B) vials containing free dye AZB-CF3 (I) and AZB-CF3@DSPE-PEG NPs (II) suspended in PBS. | ||
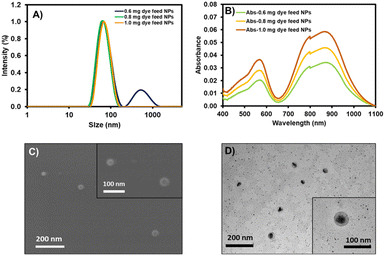
The physical characteristics of NPs were investigated by dynamic light scattering (DLS), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). In the DLS size distributions, the NPs with 0.6 mg of dye feed displayed two groups of size distributions yielding an average size of 87.7 ± 3.1 nm (Fig. 4A and Table 3). The larger size distribution could be assigned to the excess of DSPE-PEG residue, which did not involve dye encapsulation. When the amounts of dye feed were increased to 0.8 and 1.0 mg, only a size distribution peak was observed at 70.0 ± 0.7 nm and 71.7 ± 2.4 nm for the NPs with 0.8 and 1.0 mg of dye feed, respectively. SEM demonstrated the spherical-shaped morphology of the prepared nanoparticles, while TEM revealed the core–shell architecture of the NPs consisting of a dense lyophobic aza-BODIPY core and a soft lyophilic PEG shell (Fig. 4C and D). The absorption spectra of AZB-CF3@DSPE-PEG NPs showed a broad absorption band peaking at 870 nm, which agrees well with the absorption spectra of the free AZB-CF3, while the intensities of the absorption peak are directly proportional to the amounts of dye feed (Fig. 4B). The AZB-CF3@DSPE-PEG NPs, on the other hand, showed no fluorescence in PBS, demonstrating non-radiative relaxation via the photothermal effect. This PTT event could be caused by intramolecular charge transfer (ICT) from an electron-donating group, NMe2, to the aza-BODIPY center, which serves as the electron-accepting component.40,48–50
| Amount of dye feed (mg) | % Dye loadingb (n = 3) | % EEc (n = 3) | DLS size (nm) (n = 3) | PDI (n = 3) | Zeta (ζ) potential (mV) (n = 3) |
|---|---|---|---|---|---|
| a Characteristics in each entry were derived from three different batches of nanoparticle preparation (n = 3). b Dye-loading percentages were obtained from the (mass of AZB-CF3 found in dried nanoparticles/total mass of dried nanoparticles) × 100. c Encapsulation Efficiencies (EE) were calculated from the (amount of dye encapsulated in nanoparticle/amount of dye feed) × 100. | |||||
| 0.6 | 27.4 (±0.4) | 94.1 (±1.8) | 87.7 (±3.1) | 0.351 (±0.028) | −5.81 (±0.86) |
| 0.8 | 33.8 (±0.7) | 95.8 (±2.9) | 70.0 (±0.7) | 0.250 (±0.029) | −5.88 (±1.27) |
| 1.0 | 39.3 (±0.5) | 97.1 (±2.3) | 71.7 (±2.4) | 0.214 (±0.030) | −5.85 (±0.83) |
The additional characteristics of the AZB-CF3-based nanoparticles are demonstrated in Table 3. The lower polydispersity indices (PDIs) of 0.250 and 0.214 were observed in the NPs with 0.8 and 1.0 mg dye feed as only one size distribution curve was detected. The low negative values of zeta-potentials for all conditions (from −5.81 to −5.88 mV) suggested the non-ionic nature of the lipid-based PEG polymeric materials. The great encapsulation efficacies (%EE) of 94.1–97.1% indicated the good efficiency of this nanoprecipitation method, which could hold up to 39.3% of the AZB-CF3 dye within the NPs. Next, the AZB-CF3@DSPE-PEG NPs with 1.0 mg of dye feed were selected for further studies on photothermal efficacy, colloidal stability, and cancer treatment applications due to their highest photosensitizer content.
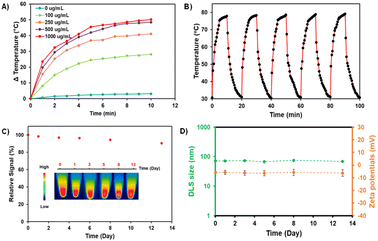
To investigate the photothermal effect of the AZB-CF3@DSPE-PEG NPs, 1.0 mL of NP suspension in DI water was exposed to 808 nm laser radiation (1.0 W cm−2) by varying the irradiation periods (1 to 10 min) and nanoparticle contents (100–1000 μg mL−1). As shown in Fig. 5A, the temperature of the AZB-CF3@DSPE-PEG in aqueous solutions increased with extended irradiation times in a dose-dependent manner. The NP solution at 1000 μg mL−1 exhibited the temperature alteration (ΔT) of 50 °C within 10 min of irradiation, indicating excellent photothermal properties. Next, we examined the photostability of the AZB-CF3@DSPE-PEG NPs by continuing ON/OFF cycles of irradiation. As displayed in Fig. 5B, the AZB-CF3 NPs still sustained a high photothermal effect after five ON/OFF cycles. There were no significant changes in absorbance intensity or the heat signal of the NPs over 13 days (Fig. 5C). After laser irradiation, the size and zeta potential of AZB-CF3@DSPE-PEG were assessed to investigate the thermal stability of the particles in contrast to the NPs before irradiation. The results indicated that the structural integrity of the NPs was preserved during photothermal activity based on their size and zeta potential in cell culture media and serum (Fig. S12†). Furthermore, the photothermal conversion efficiency of the nanoparticles was calculated as 44.9% (Fig. S11†), which is comparable to those of other reported aza-BODIPY-based photothermal agents (Table S1†).22,23,40,51–53 The generation of singlet oxygen by AZB-CF3@DSPE-PEG NPs in an aqueous solution was also investigated. Using ICG as a standard, the results revealed that AZB-CF3@DSPE-PEG has a singlet oxygen quantum yield of 0.17 (Fig. S10†). This implies that only photothermal conversion is an important part of phototherapy in this work.
Understanding the colloidal stabilities of the NPs in different biological environments is critical for the further clinical translation of AZB-CF3@DSPE-PEG nanomaterials. To demonstrate the colloidal and chemical stabilities of our NPs, we used four different aqueous solutions, including DI water, phosphate-buffered saline (PBS) at pH = 5 and pH = 7, Roswell Park Memorial Institute (RPMI) 1640 culture medium, and fetal bovine serum (FBS). Water and PBS solutions are the most commonly used aqueous solutions in biological research because PBS has the same osmolarity and ion concentrations as the human body. Furthermore, the cell culture medium is dependent on cell type; RPMI-1640 is one of the most common cell culture media that we used to grow 4T1 cells. FBS is also the most widely used growth supplement for cell culture media due to its high content of embryonic growth factors. As shown in Fig. S13,† when dispersed in DI, RPMI, and FBS, AZB-CF3@DSPE-PEG demonstrated comparable stability behaviors for up to 24 h to those in PBS at pH = 5 and pH = 7, where the size was slightly larger. This could be due to the competitive binding of DSPE-PEG and phosphate saline. In polymer-stabilized nanoparticle systems, such competitive association and dissociation are common.54 When the NPs were dispersed in different solutions for up to 24 h, the Vis-NIR absorbance did not vary considerably, indicating that the dyes inside the particles were stable. The colloidal stability of the NPs was evaluated in 0.1 M PBS at pH = 7.4, for a longer incubation time. As seen in Fig. 5D, the DLS sizes and zeta-potentials of the NPs were negligibly changed in the medium up to day 13 of incubation, suggesting high colloidal stability.
Photothermal efficiency of AZB-CF3 nanoparticles in vitro
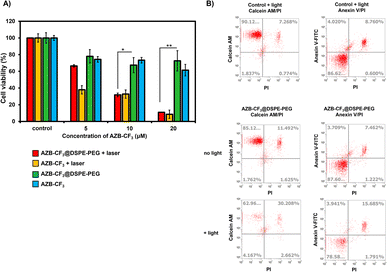
To demonstrate the efficacy of AZB-CF3@DSPE-PEG in cancer cell treatment via PTT, the murine mammary carcinoma cells (4T1) were treated with the NPs under light irradiation in comparison to the free dye (AZB-CF3) treatment, and their viability was assessed using the MTT assay.55 As shown in Fig. 6A, cell viability decreased as the concentration of AZB-CF3@DSPE-PEG or AZB-CF3 increased when exposed to 808 nm laser irradiation for 5 min (1.0 W cm−2). At 20 μM, light exposure reduced cell viability to approximately 10 percent, which is significantly lower than without laser illumination. However, at high doses, NPs and free dye exhibited some dark toxicity.Calcein-AM and propidium iodide (PI) staining were used to identify viable and dead cells. When calcein-AM enters live cells, it emits green fluorescence after being cleaved by intracellular esterase, whereas PI only interacts with dead cell nuclei and emits red fluorescence. As shown in Fig. 6B, after incubating cells with AZB-CF3@DSPE-PEG followed by light irradiation, many dead cells were observed from 4T1, while there were fewer live cells, with only 63% of live cells existing after light irradiation, where the percentage of dead cells was up to 30%. When compared to cells treated with NPs without light irradiation, viable and dead cells were 85 and 11%, respectively.
We used flow cytometry to investigate the photothermal-assisted apoptosis of cancer cells by our NPs. To identify apoptotic cells, an apoptosis detection kit containing Annexin V, fluorescein isothiocyanate (FITC), and PI was used. Fig. 6B shows that cells treated with AZB-CF3@DSPE-PEG followed by light irradiation encountered apoptosis at a rate of 16%, which is higher than cells not treated with light (7%).
These findings indicated that the dye, even after forming NPs, retained its light activity and its ability to destroy cancer cells through photothermal mechanisms.
Photothermal efficiency of AZB-CF3 nanoparticles in ovo
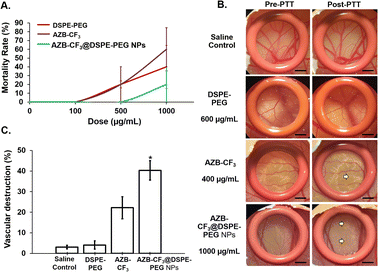
Next, the anti-angiogenesis ability of all the samples was compared post-PTT in the chick chorioallantoic membrane (CAM). As shown in Fig. 7B, the CAMs treated with saline and DSPE-PEG at 600 μg mL−1 (equivalent to the DSPE-PEG content in AZB-CF3@DSPE-PEG NPs at 1000 μg mL−1) did not induce any visible morphological changes in the blood vessel post-photo-irradiation. Conversely, both AZB-CF3 and AZB-CF3@DSPE-PEG NPs-treated CAMs showed vasculature destruction at 10 min post-PTT. AZB-CF3-treated CAM at 400 μg mL−1 (equivalent to the AZB-CF3 content in AZB-CF3@DSPE-PEG NPs at 1000 μg mL−1) exhibited thinning of blood vessels as compared to pre-PTT, while AZB-CF3@DSPE-PEG NPs induced severe vascular damage with a larger area of blood capillary disruption post-PTT.
Quantitatively, saline- and DSPE-PEG-treated CAMs displayed very minor or negligible anti-angiogenic activity of 3.08 ± 0.87% and 4.10 ± 1.98%, respectively. An enhanced and higher anti-angiogenic efficacy was demonstrated by AZB-CF3@DSPE-PEG NPs with vascular destruction of 40.47 ± 4.56% after photo-irradiation as compared to the parent drug, AZB-CF3 with 22.21 ± 5.34% destruction (p = 0.008) (Fig. 7C).
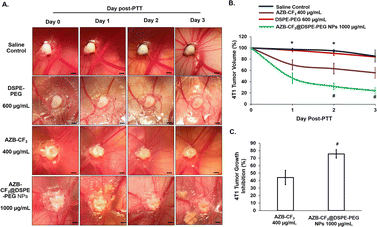
The tumor tissue in the CAM post-irradiation was observed daily, for 72 h. As shown in Fig. 8A, the saline- and DSPE-PEG-treated CAMs post-PTT showed comparable tumor volumes with pre-PTT, up to 72 h. Comparatively, AZB-CF3 and AZB-CF3@DSPE-PEG NPs-treated CAMs showed a significant decline in tumor volume as compared to pre-PTT.
The tumor growth curve was plotted across 3 days post-PTT. A comparable tumor volume was observed in both saline- and DSPE-PEG-treated CAMs as compared to pre-PTT. At an equivalent dose, AZB-CF3- and AZB-CF3@DSPE-PEG NPs-treated CAMs showed 44.19 ± 9.56% and 75.72 ± 5.75% reduction in the tumor volume, respectively, at 72 h post-PTT (p = 0.044) (Fig. 8B and C). This suggests that AZB-CF3@DSPE-PEG NPs have better efficacy in inhibiting tumor growth, which might be due to the accumulation of AZB-CF3@DSPE-PEG NPs at the tumor site by passive targeting. Similarly, the aza-BODIPY-derived probe (CB1) was synthesized and encapsulated with DSPE-PEG2000 by Yang et al. and showed an enhanced photothermal effect, inducing significant antitumor activity in the 4T1 grafted mice model as compared to the parent drug CB1.56 Other polymeric aza-BODIPY nanoparticles, such as an iodine-substituted aza-BODIPY (B4) coated with DSPE-PEG5000,22 an aza-BODIPY-based phototherapeutic agent (B-3) encapsulated with DSPE-PEG5000 and F108,57 and an aza-BODIPY with dimethylamino moieties (A1) constructed with DSPE-PEG5000,28 exhibited enhanced photothermal antitumor effects in vivo. These reports further prove the enhancement of the therapeutic outcome in the presence of nanocarriers.
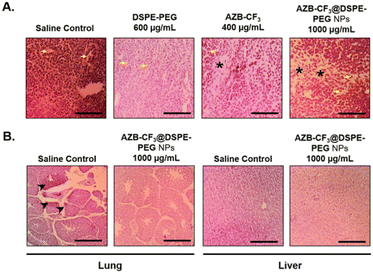
AZB-CF3@DSPE-PEG NPs were further evaluated in the 4T1 xenografted CAMs model for preventing spontaneous metastasize to the lung. Histopathological analysis of the lung tissue revealed that the topically xenografted murine 4T1 mammary carcinoma cells had metastasized to the lung of the developing chick embryo in the saline-treated CAMs, as indicated by the accumulation of neoplastic epithelial cells (arrowhead). However, this phenomenon is absent in the AZB-CF3@DSPE-PEG NPs-treated CAMs (Fig. 9B). This result confirms that AZB-CF3@DSPE-PEG NPs-treated CAMs post-PTT effectively shut down the vessels, reducing tumor volume and hence preventing the spread of the tumor to distant organs.
The liver is also the main detoxifying organ where the metabolism of drugs takes place, hence, liver tissue was evaluated histologically and compared between the saline- and AZB-CF3@DSPE-PEG NPs-treated CAMs. Normal liver morphology was observed in the AZB-CF3@DSPE-PEG NPs-treated CAMs, suggesting that the metabolism of the NPs did not induce cellular damage to the liver in the chick embryo (Fig. 9B). Altogether, these findings highlight the potential of AZB-CF3@DSPE-PEG NPs as a multifunctional photothermal cancer therapeutic by effectively inhibiting angiogenesis, cancer growth, and distant metastasis with remarkable biocompatibility.
Conclusions
A photothermal-based aza-BODIPY derivative (AZB-CF3) has been successfully synthesized and encapsulated into nanoparticles via the nanoprecipitation method. The obtained NPs exhibited a round shape with an average hydrodynamic size of 70 nm, as well as a broad Vis-NIR absorbance with a maximum at 870 nm. Although AZB-CF3 showed near-infrared absorption in a lipophilic environment, the formulated nanoparticles were non-fluorescent in aqueous media due to an aggregation-caused quenching phenomenon. In addition, the AZB-CF3@DSPE-PEG NPs exhibited great photostability and colloidal stability, good biocompatibility in vitro and in ovo, and high photothermal (PTT) efficacy, which are suitable for PTT-based cancer treatment. In the cell-based evaluation, the NPs (20 μM AZB-CF3 content) combined with 5 min of 808 nm laser irradiation led to about 10% viability of 4T1 breast cancer cells. The additional proof in the chicken egg 4T1-tumor model suggested that the AZB-CF3@DSPE-PEG NPs are good candidates for photothermal cancer therapeutic agents as they display excellent antitumor efficiency, anti-angiogenesis, and anti-metastasis properties.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financially supported by the National Research Council of Thailand (NRCT) through the Mid-Career Research Grant (N42A650389) co-funded by National Nanotechnology Center (P2250217) granted to KC. C. S. K. acknowledges Management and Science University Seed Research Funding (SG-026-022023-FHLS). AK acknowledges the SUT Research and Development Fund for her funding.References
- Q. Meng, J. Meng, W. Ran, J. Wang, Y. Zhai, P. Zhang and Y. Li, Light-Activated Core–Shell Nanoparticles for Spatiotemporally Specific Treatment of Metastatic Triple-Negative Breast Cancer, ACS Nano, 2018, 12, 2789–2802 CrossRef CAS.
- S. Bajpai, S. K. Tiwary, M. Sonker, A. Joshi, V. Gupta, Y. Kumar, N. Shreyash and S. Biswas, Recent Advances in Nanoparticle-Based Cancer Treatment: A Review, ACS Appl. Nano Mater., 2021, 4, 6441–6470 CrossRef CAS.
- Y. Yao, Y. Zhou, L. Liu, Y. Xu, Q. Chen, Y. Wang, S. Wu, Y. Deng, J. Zhang and A. Shao, Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance, Front. Mol. Biosci., 2020, 7, 193 CrossRef CAS PubMed.
- Z. Cheng, M. Li, R. Dey and Y. Chen, Nanomaterials for cancer therapy: current progress and perspectives, J. Hematol. Oncol., 2021, 14, 85 CrossRef.
- M. C. White, F. Babcock, N. S. Hayes, A. B. Mariotto, F. L. Wong, B. A. Kohler and H. K. Weir, The history and use of cancer registry data by public health cancer control programs in the United States, Cancer, 2017, 123, 4969–4976 CrossRef PubMed.
- E. M. Jaffee, C. V. Dang, D. B. Agus, B. M. Alexander, K. C. Anderson, A. Ashworth, A. D. Barker, R. Bastani, S. Bhatia, J. A. Bluestone, O. Brawley, A. J. Butte, D. G. Coit, N. E. Davidson, M. Davis, R. A. DePinho, R. B. Diasio, G. Draetta, A. L. Frazier, A. Futreal, S. S. Gambhir, P. A. Ganz, L. Garraway, S. Gerson, S. Gupta, J. Heath, R. I. Hoffman, C. Hudis, C. Hughes-Halbert, R. Ibrahim, H. Jadvar, B. Kavanagh, R. Kittles, Q.-T. Le, S. M. Lippman, D. Mankoff, E. R. Mardis, D. K. Mayer, K. McMasters, N. J. Meropol, B. Mitchell, P. Naredi, D. Ornish, T. M. Pawlik, J. Peppercorn, M. G. Pomper, D. Raghavan, C. Ritchie, S. W. Schwarz, R. Sullivan, R. Wahl, J. D. Wolchok, S. L. Wong and A. Yung, Future cancer research priorities in the USA: a Lancet Oncology Commission, Lancet Oncol., 2017, 18, e653–e706 CrossRef.
- D. Luo, K. A. Carter, D. Miranda and J. F. Lovell, Chemophototherapy: An Emerging Treatment Option for Solid Tumors, Advanced Science, 2017, 4, 1600106 CrossRef PubMed.
- X. Li, J. F. Lovell, J. Yoon and X. Chen, Clinical development and potential of photothermal and photodynamic therapies for cancer, Nat. Rev. Clin. Oncol., 2020, 17, 657–674 CrossRef.
- C. Dai, Y. Chen, X. Jing, L. Xiang, D. Yang, H. Lin, Z. Liu, X. Han and R. Wu, Two-Dimensional Tantalum Carbide (MXenes) Composite Nanosheets for Multiple Imaging-Guided Photothermal Tumor Ablation, ACS Nano, 2017, 11, 12696–12712 CrossRef CAS PubMed.
- L. Cheng, W. He, H. Gong, C. Wang, Q. Chen, Z. Cheng and Z. Liu, PEGylated Micelle Nanoparticles Encapsulating a Non-Fluorescent Near-Infrared Organic Dye as a Safe and Highly-Effective Photothermal Agent for In Vivo Cancer Therapy, Adv. Funct. Mater., 2013, 23, 5893–5902 CrossRef CAS.
- L. Zou, H. Wang, B. He, L. Zeng, T. Tan, H. Cao, X. He, Z. Zhang, S. Guo and Y. Li, Current Approaches of Photothermal Therapy in Treating Cancer Metastasis with Nanotherapeutics, Theranostics, 2016, 6, 762–772 CrossRef CAS PubMed.
- H. S. Jung, P. Verwilst, A. Sharma, J. Shin, J. L. Sessler and J. S. Kim, Organic molecule-based photothermal agents: an expanding photothermal therapy universe, Chem. Soc. Rev., 2018, 47, 2280–2297 RSC.
- L. Zhao, X. Zhang, X. Wang, X. Guan, W. Zhang and J. Ma, Recent advances in selective photothermal therapy of tumor, J. Nanobiotechnol., 2021, 19, 335 CrossRef CAS PubMed.
- L. Cheng, K. Yang, Q. Chen and Z. Liu, Organic Stealth Nanoparticles for Highly Effective in Vivo Near-Infrared Photothermal Therapy of Cancer, ACS Nano, 2012, 6, 5605–5613 CrossRef CAS PubMed.
- K. Yang, H. Xu, L. Cheng, C. Sun, J. Wang and Z. Liu, In Vitro and In Vivo Near-Infrared Photothermal Therapy of Cancer Using Polypyrrole Organic Nanoparticles, Adv. Mater., 2012, 24, 5586–5592 CrossRef CAS PubMed.
- Y. Liu, K. Ai, J. Liu, M. Deng, Y. He and L. Lu, Dopamine-Melanin Colloidal Nanospheres: An Efficient Near-Infrared Photothermal Therapeutic Agent for In Vivo Cancer Therapy, Adv. Mater., 2013, 25, 1353–1359 CrossRef CAS PubMed.
- C.-K. Lim, J. Shin, Y.-D. Lee, J. Kim, K. S. Oh, S. H. Yuk, S. Y. Jeong, I. C. Kwon and S. Kim, Phthalocyanine-Aggregated Polymeric Nanoparticles as Tumor-Homing Near-Infrared Absorbers for Photothermal Therapy of Cancer, Theranostics, 2012, 2, 871–879 CrossRef CAS PubMed.
- O. Taratula, C. Schumann, T. Duong, K. L. Taylor and O. Taratula, Dendrimer-encapsulated naphthalocyanine as a single agent-based theranostic nanoplatform for near-infrared fluorescence imaging and combinatorial anticancer phototherapy, Nanoscale, 2015, 7, 3888–3902 RSC.
- Y. Cai, P. Liang, Q. Tang, X. Yang, W. Si, W. Huang, Q. Zhang and X. Dong, Diketopyrrolopyrrole–Triphenylamine Organic Nanoparticles as Multifunctional Reagents for Photoacoustic Imaging-Guided Photodynamic/Photothermal Synergistic Tumor Therapy, ACS Nano, 2017, 11, 1054–1063 CrossRef CAS.
- J. Treekoon, K. Chansaenpak, G. Tumcharern, Z. S. Zaiman Zain, H. B. Lee, C. S. Kue and A. Kamkaew, Aza-BODIPY encapsulated polymeric nanoparticles as an effective nanodelivery system for photodynamic cancer treatment, Mater. Chem. Front., 2021, 5, 2283–2293 RSC.
- C. Wu, X. Huang, Y. Tang, W. Xiao, L. Sun, J. Shao and X. Dong, Pyrrolopyrrole aza-BODIPY near-infrared photosensitizer for dual-mode imaging-guided photothermal cancer therapy, Chem. Commun., 2019, 55, 790–793 RSC.
- M. Zhao, Y. Xu, M. Xie, L. Zou, Z. Wang, S. Liu and Q. Zhao, Halogenated Aza-BODIPY for Imaging-Guided Synergistic Photodynamic and Photothermal Tumor Therapy, Adv. Healthcare Mater., 2018, 7, 1800606 CrossRef.
- D. Chen, Q. Tang, J. Zou, X. Yang, W. Huang, Q. Zhang, J. Shao and X. Dong, pH-Responsive PEG–Doxorubicin-Encapsulated Aza-BODIPY Nanotheranostic Agent for Imaging-Guided Synergistic Cancer Therapy, Adv. Healthcare Mater., 2018, 7, 1701272 CrossRef.
- Z. Yu, J. Zhou, X. Ji, G. Lin, S. Xu, X. Dong and W. Zhao, Discovery of a Monoiodo Aza-BODIPY Near-Infrared Photosensitizer: in vitro and in vivo Evaluation for Photodynamic Therapy, J. Med. Chem., 2020, 63, 9950–9964 CrossRef CAS.
- Y. Tian, H. Zhou, Q. Cheng, H. Dang, H. Qian, C. Teng, K. Xie and L. Yan, Stable twisted conformation aza-BODIPY NIR-II fluorescent nanoparticles with ultra-large Stokes shift for imaging-guided phototherapy, J. Mater. Chem. B, 2022, 10, 707–716 RSC.
- Ł. Łapok, I. Cieślar, T. Pędziński, K. M. Stadnicka and M. Nowakowska, Near-Infrared Photoactive Aza-BODIPY: Thermally Robust and Photostable Photosensitizer and Efficient Electron Donor, ChemPhysChem, 2020, 21, 725–740 CrossRef PubMed.
- S. Rattanopas, K. Chansaenpak, K. Siwawannapong, K. Ngamchuea, S. Wet-osot, J. Treekoon, T. Pewklang, T. Jinaphon, K. Sagarik, R.-Y. Lai, L. Cheng and A. Kamkaew, Synthesis and Characterization of Push-Pull Aza-BODIPY Dyes Towards Application in NIR-II Photothermal Therapy, ChemPhotoChem, 2020, 4, 5304–5311 CrossRef CAS.
- Y. Xu, T. Feng, T. Yang, H. Wei, H. Yang, G. Li, M. Zhao, S. Liu, W. Huang and Q. Zhao, Utilizing Intramolecular Photoinduced Electron Transfer to Enhance Photothermal Tumor Treatment of Aza-BODIPY-Based Near-Infrared Nanoparticles, ACS Appl. Mater. Interfaces, 2018, 10, 16299–16307 CrossRef CAS PubMed.
- K. Chansaenpak, S. Tanjindaprateep, N. Chaicharoenaudomrung, O. Weeranantanapan, P. Noisa and A. Kamkaew, Aza-BODIPY based polymeric nanoparticles for cancer cell imaging, RSC Adv., 2018, 8, 39248–39255 RSC.
- Q. Tang, W. Si, C. Huang, K. Ding, W. Huang, P. Chen, Q. Zhang and X. Dong, An aza-BODIPY photosensitizer for photoacoustic and photothermal imaging guided dual modal cancer phototherapy, J. Mater. Chem. B, 2017, 5, 1566–1573 RSC.
- M. H. Y. Cheng, K. M. Harmatys, D. M. Charron, J. Chen and G. Zheng, Stable J-Aggregation of an aza-BODIPY-Lipid in a Liposome for Optical Cancer Imaging, Angew. Chem., Int. Ed., 2019, 58, 13394–13399 CrossRef CAS.
- J. Che, I. C. Okeke, Z.-B. Hu and J. Xu, DSPE-PEG: A Distinctive Component in Drug Delivery System, Curr. Pharm. Des., 2015, 21, 1598–1605 CrossRef CAS.
- K. Pu, J. Mei, J. V. Jokerst, G. Hong, A. L. Antaris, N. Chattopadhyay, A. J. Shuhendler, T. Kurosawa, Y. Zhou, S. S. Gambhir, Z. Bao and J. Rao, Diketopyrrolopyrrole-Based Semiconducting Polymer Nanoparticles for In Vivo Photoacoustic Imaging, Adv. Mater., 2015, 27, 5184–5190 CrossRef CAS.
- B. Guo, G. Feng, P. N. Manghnani, X. Cai, J. Liu, W. Wu, S. Xu, X. Cheng, C. Teh and B. Liu, A Porphyrin-Based Conjugated Polymer for Highly Efficient In Vitro and In Vivo Photothermal Therapy, Small, 2016, 12, 6243–6254 CrossRef CAS.
- L. Miebach, J. Berner and S. Bekeschus, In ovo model in cancer research and tumor immunology, Front. Immunol., 2022, 13, 1006064 CrossRef CAS PubMed.
- J. Eckrich, P. Kugler, C. R. Buhr, B. P. Ernst, S. Mendler, J. Baumgart, J. Brieger and N. Wiesmann, Monitoring of tumor growth and vascularization with repetitive ultrasonography in the chicken chorioallantoic-membrane-assay, Sci. Rep., 2020, 10, 18585 CrossRef CAS.
- A. Komatsu, K. Matsumoto, T. Saito, M. Muto and F. Tamanoi, Patient Derived Chicken Egg Tumor Model (PDcE Model): Current Status and Critical Issues, Cells, 2019, 8, 440 CrossRef CAS.
- J. Geng, Z. Zhu, W. Qin, L. Ma, Y. Hu, G. G. Gurzadyan, B. Z. Tang and B. Liu, Near-infrared fluorescence amplified organic nanoparticles with aggregation-induced emission characteristics for in vivo imaging, Nanoscale, 2014, 6, 939–945 RSC.
- C. S. Kue, K. Y. Tan, M. L. Lam and H. B. Lee, Chick embryo chorioallantoic membrane (CAM): an alternative predictive model in acute toxicological studies for anti-cancer drugs, Exp. Anim., 2015, 64, 129–138 CrossRef CAS.
- S. Kampaengsri, K. Chansaenpak, G. Y. Yong, P. Hiranmartsuwan, B. Uengwanarat, R.-Y. Lai, P. Meemon, C. S. Kue and A. Kamkaew, PEGylated Aza-BODIPY Nanoparticles for Photothermal Therapy, ACS Appl. Bio Mater., 2022, 5, 4567–4577 CrossRef CAS.
- Y. Ge and D. F. O'Shea, Azadipyrromethenes: from traditional dye chemistry to leading edge applications, Chem. Soc. Rev., 2016, 45, 3846–3864 RSC.
- Z. Shi, X. Han, W. Hu, H. Bai, B. Peng, L. Ji, Q. Fan, L. Li and W. Huang, Bioapplications of small molecule Aza-BODIPY: from rational structural design to in vivo investigations, Chem. Soc. Rev., 2020, 49, 7533–7567 RSC.
- E. Bodio, F. Denat and C. Goze, BODIPYS and aza-BODIPY derivatives as promising fluorophores for in vivo molecular imaging and theranostic applications, J. Porphyrins Phthalocyanines, 2019, 23, 1159–1183 CrossRef CAS.
- P. Hiranmartsuwan, X. Ma, J. Nootem, R. Daengngern, A. Kamkaew, P. Pinyou, W. Wattanathana, V. Promarak, Z. Li and K. Chansaenpak, Synthesis and properties of AIE-active Triazaborolopyridiniums toward fluorescent nanoparticles for cellular imaging and their biodistribution in vivo and ex vivo, Mater. Today Chem., 2022, 26, 101121 CrossRef CAS.
- C. Duangkamol, P. Muangsopa, S. Rattanopas, P. Wongsuwan, T. Khrootkae, P. Chueakwon, N. Niamnont, K. Chansaenpak and A. Kamkaew, Polarity and viscosity-sensitive fluorescence probes for lipid droplet imaging in cancer cells, Dyes Pigm., 2023, 111365, DOI:10.1016/j.dyepig.2023.111365.
- S. Pascal, L. Bucher, N. Desbois, C. Bucher, C. Andraud and C. P. Gros, Synthesis, Electrochemistry, and Photophysics of Aza-BODIPY Porphyrin Dyes, Chem.–Eur. J., 2016, 22, 4971–4979 CrossRef CAS.
- T. Debnath and H. N. Ghosh, An Insight of Molecular Twisting of Coumarin Dyes, ChemistrySelect, 2020, 5, 9461–9476 CrossRef CAS.
- Z. Shi, X. Han, W. Hu, H. Bai, B. Peng, L. Ji, Q. Fan, L. Li and W. Huang, Bioapplications of small molecule Aza-BODIPY: from rational structural design to in vivo investigations, Chem. Soc. Rev., 2020, 49, 7533–7567 RSC.
- Y. Xu, S. Wang, Z. Chen, R. Hu, S. Li, Y. Zhao, L. Liu and J. Qu, Highly stable organic photothermal agent based on near-infrared-II fluorophores for tumor treatment, J. Nanobiotechnol., 2021, 19, 37 CrossRef CAS PubMed.
- S. Rattanopas, K. Chansaenpak, K. Siwawannapong, K. Ngamchuea, S. Wet-osot, J. Treekoon, T. Pewklang, T. Jinaphon, K. Sagarik, R. Y. Lai, L. Cheng and A. Kamkaew, Synthesis and Characterization of Push-Pull Aza-BODIPY Dyes Towards Application in NIR-II Photothermal Therapy, ChemPhotoChem, 2020, 4, 5304–5311 CrossRef CAS.
- M. Zhao, Q. Zeng, X. Li, D. Xing and T. Zhang, Aza-BODIPY-based phototheranostic nanoagent for tissue oxygen auto-adaptive photodynamic/photothermal complementary therapy, Nano Res., 2022, 15, 716–727 CrossRef CAS.
- D. Chen, J. Zhang, Y. Tang, X. Huang, J. Shao, W. Si, J. Ji, Q. Zhang, W. Huang and X. Dong, A tumor-mitochondria dual targeted aza-BODIPY-based nanotheranostic agent for multimodal imaging-guided phototherapy, J. Mater. Chem. B, 2018, 6, 4522–4530 RSC.
- Y. Tian, D. Yin, Q. Cheng, H. Dang, C. Teng and L. Yan, Supramolecular J-aggregates of aza-BODIPY by steric and π–π interactions for NIR-II phototheranostics, J. Mater. Chem. B, 2022, 10, 1650–1662 RSC.
- T. A. Larson, P. P. Joshi and K. Sokolov, Preventing Protein Adsorption and Macrophage Uptake of Gold Nanoparticles via a Hydrophobic Shield, ACS Nano, 2012, 6, 9182–9190 CrossRef CAS PubMed.
- J. C. Stockert, R. W. Horobin, L. L. Colombo and A. Blázquez-Castro, Tetrazolium salts and formazan products in Cell Biology: viability assessment, fluorescence imaging, and labeling perspectives, Acta Histochem., 2018, 120, 159–167 CrossRef CAS PubMed.
- N. Yang, S. Song, C. Liu, J. Ren, X. Wang, S. Zhu and C. Yu, An aza-BODIPY-based NIR-II luminogen enables efficient phototheranostics, Biomater. Sci., 2022, 10, 4815–4821 RSC.
- Y. Xu, M. Zhao, L. Zou, L. Wu, M. Xie, T. Yang, S. Liu, W. Huang and Q. Zhao, Highly Stable and Multifunctional Aza-BODIPY-Based Phototherapeutic Agent for Anticancer Treatment, ACS Appl. Mater. Interfaces, 2018, 10, 44324–44335 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3na00718a |
| This journal is © The Royal Society of Chemistry 2024 |