Open Access Article
Open Access ArticleIn situ growth of copper oxide on MXene by combustion method for electrochemical ammonia production from nitrate†
Sagar
Ingavale
 a,
Phiralang
Marbaniang
b,
Manoj
Palabathuni
a and
Nimai
Mishra
a,
Phiralang
Marbaniang
b,
Manoj
Palabathuni
a and
Nimai
Mishra
 *c
*c
aDepartment of Chemistry, SRM University-AP, Andhra Pradesh, Neerukonda, Guntur (Dt), Andhra Pradesh 522240, India
bDepartment of Chemistry, Indian Institute of Technology Madras, Chennai 600036, India
cInstitute of Chemical Technology Mumbai, IOC Odisha Campus Bhubaneswar, Bhubaneswar, Odisha 751013, India. E-mail: n.mishra@iocb.ictmumbai.edu.in
First published on 24th November 2023
Abstract
The elimination of the nitrogen pollutant nitrate ions through the electrochemical synthesis of ammonia is an important and environment friendly strategy. Electrochemical nitrate reduction requires highly efficient, selective, and stable catalysts to convert nitrate to ammonia. In this work, a composite of copper oxide and MXene was synthesized using a combustion technique. As reported, nitrate ions are effectively adsorbed by CuxO (CuO & Cu2O) nanoparticles. Herein, MXene is an excellent assembly for anchoring CuxO on its layered surface because it has a strong support structure. Powder X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), and transmission electron microscopy (TEM) analyses show the presence of oxidation states of metal ions and the formation of CuxO nanofoam anchors on the surface of MXene (Ti3C2Tx). The optimized CuxO/Ti3C2Tx composite exhibits an improved nitrate reduction reaction. The electrochemical studies of CuxO/Ti3C2Tx show an interesting nitrate reduction reaction (NO3RR) with a current density of 162 mA cm−2. Further, CuxO/Ti3C2Tx shows an electrocatalytic activity with an ammonia production of 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 982 μg h−1 mcat−1 and its faradaic efficiency is 48% at −0.7 V vs. RHE. Thus, such performance by CuxO/Ti3C2Tx indicates a well-suitable candidate for nitrate ion conversion to ammonia.
982 μg h−1 mcat−1 and its faradaic efficiency is 48% at −0.7 V vs. RHE. Thus, such performance by CuxO/Ti3C2Tx indicates a well-suitable candidate for nitrate ion conversion to ammonia.
1. Introduction
Ammonia is a basic nitrogen source for industrial applications widely used for manufacturing fertilizers, pharmaceuticals, textiles, and plastics.1,2 Ammonia is mostly synthesized by the traditional Haber–Bosch process, requiring high temperature and pressure to convert nitrogen and hydrogen gases to ammonia in the presence of a iron-based catalyst.3 Though the preparation of ammonia is carbon-free, it consumes >1% of the global energy supply (the energy obtained by burning huge amounts of fossil fuels resulting in hundreds of millions of tons of CO2 release).4 The electrochemical conversion of nitrogen to ammonia at ambient conditions is the best approach as an alternative and environmentally friendly method.5 However, the direct electrochemical reduction of N2 to ammonia is hindered due to the high energy barrier necessary for breaking the inert N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond, the poor contact between non-polar N2 and active sites of catalysts, and the low solubility of N2 in water.6–8 In contrast, nitrates have special benefits as nitrogen sources for the electro-synthesis of NH3, as the polar N–O bond has a bond energy that is four times lower than the non-polar N
N bond, the poor contact between non-polar N2 and active sites of catalysts, and the low solubility of N2 in water.6–8 In contrast, nitrates have special benefits as nitrogen sources for the electro-synthesis of NH3, as the polar N–O bond has a bond energy that is four times lower than the non-polar N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond (204 kJ mol−1), allowing the N–O bond to be easily activated at lower energies.9 Nitrate is widely distributed in the environment and builds up over time as a result of industrial and agricultural production activities, mostly having negative impacts on aquatic ecosystems and human health, including methemoglobinemia development, thyroid effects, and cancer.10–12 Hence, the electroreduction of nitrate for NH3 generation not only complies with energy sustainability but also serves as a strategy to reduce pollution.
N triple bond (204 kJ mol−1), allowing the N–O bond to be easily activated at lower energies.9 Nitrate is widely distributed in the environment and builds up over time as a result of industrial and agricultural production activities, mostly having negative impacts on aquatic ecosystems and human health, including methemoglobinemia development, thyroid effects, and cancer.10–12 Hence, the electroreduction of nitrate for NH3 generation not only complies with energy sustainability but also serves as a strategy to reduce pollution.
The adsorption of nitrate ions, deoxygenation of the N-species, hydrogenation of the N-species, and desorption of the reduced species are the steps involved during the conversion of NO3− ions to NH3 on the electrode surface by the electroreduction process.13 A copper (Cu)-based catalyst was recently developed for nitrate reduction with >90% faradaic efficiency (FE).14 Cu-based composites with different particle sizes and oxidation states were studied and observed to have multiple active sites for ammonia production from nitrate ions.15 Generally, copper easily gets oxidized. The effect of the oxidation state of Cu on the activity and selectivity of NO3 electroreduction to NH3 is still unknown.15 According to Zhao et al., a Cu2O film formed on Cu foam exhibits catalytic activity for NO3RR. It is stated that the creation of a strong preferred (111) orientation and a nanopyramid terminated on the surface of the Cu2O film account for the increased efficiency and selectivity for ammonia production.16 Gong et al. reported that Cu2O, subjected to plasma treatment, provides a hydroxyl group-rich surface and a high concentration of oxygen vacancies, resulting in greater charge density near the Cu sites and an upshifted d-orbital.17 The treated Cu2O enhances electron transport between the surface and chemical intermediates, making NO3RR more favourable. Furthermore, to enhance electron transport, Zhang et al. generated electron-deficient Cu by dispersing Au particles on the surface of Cu (111), allowing electrons to move from Cu to Au atoms.1 Similarly, CuPd nanocubes favour NO3− ion adsorption because the Cu sites favour bridge-bidentate NO3− adsorption and increase ammonia production due to Pauli repulsion between *N and the d-orbital of Pd, which facilitates protonation of N-bonded species towards NH3.18 Cu nanowires supported by Ru (Ru–CuNW) also improve the adsorption of NO3− ions, allowing it to hydrogenate with N atoms better than CuNW.13 However, it is not preferred to use expensive metals, and Wang's group claims that oxidised Cu metal, i.e., CuO exhibits strong adsorption towards NO3− ion.19 Additionally, MXene (Ti3C2Tx: Tx = Cl−, OH− and F−) possesses excellent conductivity, hydrophilicity, large specific surface area, and exceptionally rich surface groups thanks to its distinctive structural characteristics. Because of its huge surface area and high concentration of surface groups, it serves as an excellent anchoring and dispersal medium for a wide variety of electrocatalysts.20,21
An interesting electrocatalytic activity towards NO3RR can be expected from a composite of CuxO and MXene due to CuxO potency at adsorbing nitrate ions and MXene potency at supporting CuxO on its surface. Therefore, in this study, we developed an efficient electrocatalyst for NH3 generation by in situ growing CuxO on MXene via a combustion approach. The presence of metal ion oxidation states and the creation of a CuxO nanofoam anchor on the surface of Ti3C2Tx are evidenced by powdered-XRD, XPS, SEM, and TEM analyses. The electrochemical reduction of nitrate by CuxO/Ti3C2Tx was demonstrated in the presence and absence of nitrate ions. The electrochemical studies of CuxO/Ti3C2Tx show interesting NO3RR with a current density of 162 mA cm−2. Further, CuxO/Ti3C2Tx shows an electrocatalytic activity with ammonia production of 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 982 μg h−1 mcat−1 and its faradaic efficiency is 48% at −0.7 V vs. RHE. Thus, such performance by CuxO/Ti3C2Tx indicates a well-suitable candidate for nitrate ion conversion to ammonia. In the future, similar composites with various synthesis techniques may be highlighted for NO3 reduction to ammonia.
982 μg h−1 mcat−1 and its faradaic efficiency is 48% at −0.7 V vs. RHE. Thus, such performance by CuxO/Ti3C2Tx indicates a well-suitable candidate for nitrate ion conversion to ammonia. In the future, similar composites with various synthesis techniques may be highlighted for NO3 reduction to ammonia.
2. Experimental section
2.1. Chemicals and materials
All chemicals purchased were of analytical grade (refer ESI†) and used without being tested for purity.2.2. Synthesis of MXene (Ti3C2Tx) from MAX phase (Ti3AlC2)
MXene (Ti3C2Tx) was synthesized from the MAX phase (Ti3AlC2) using the methods described in the recently published article. In brief, 500 mg of Ti3AlC2 powder was sonicated and stirred at room temperature for 24 hours in HF (40 wt%) solution. The solution was centrifuged at 5000 rpm and was rinsed in deionized water until the pH was neutral, resulting in nanosheets of Ti3C2Tx.2.3. Preparation of copper oxide and MXene composite
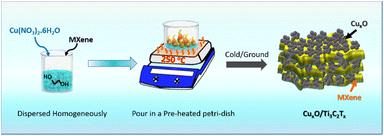
Copper oxide and MXene composites were synthesized using a simple combustion process. 500 mg of Cu(NO3)2·6H2O and 20 mg of MXene were mixed together in 1 ml of ethylene glycol for 15 minutes until a homogeneous dispersed solution was achieved. A Petri dish was pre-heated for 15 minutes at 250 °C. The solution is then poured into a Petri dish, where an immediate combustion reaction occurs with the effervescence to generate copper oxide and MXene composite. The product was collected and labelled as CuxO/Ti3C2Tx after cooling to ambient temperature. Similarly, composites were optimized using the same technique and by altering the weight ratio of Cu(NO3)2·6H2O by 250 mg and 750 mg, respectively, and are indicated as CuxO-1/Ti3C2Tx and CuxO-2/Ti3C2Tx. Similar steps were taken to synthesise CuxO, but in the absence of Ti3C2Tx.3. Results and discussion
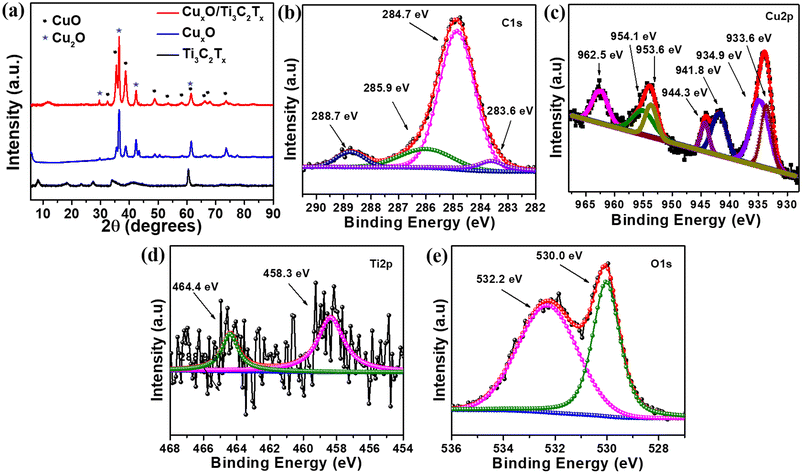
MXene (Ti3C2Tx) was synthesized from the MAX phase (Ti3AlC2) by etching Al in a 40% HF solution. The as-prepared Ti3C2Tx was first homogeneously dispersed in ethylene glycol with Cu(NO3)2·6H2O, followed by a combustion technique in order to self-assemble CuxO on Ti3C2Tx. MXene is a well-known material in the current research due to its excellent properties especially electrical conductivity, which is the highest among all synthesized 2D materials, more than ten times the conductivity of reduced graphene oxide (rGO) films. It is also a good supporter because of the consistent negative charge at the surface of Ti3C2Tx, which attracts the positively charged ions like Cu2+ and Cu+ on its surface. Fig. 1 depicts a schematic illustration of the CuxO/Ti3C2Tx synthesis.The crystal structures of CuxO/Ti3C2Tx composites were compared to those of Ti3C2Tx and CuxO using XRD analysis, as shown in Fig. 2(a). The presence of a peak at 8.4° suggests the production of exfoliated layers corresponding to the (002) plane.22 The peak at 8.4° moved to a broad peak at 11.6° along with the appearance of other CuxO peaks after CuxO self-assembled on Ti3C2Tx, confirming the composite creation of CuxO and Ti3C2Tx. CuxO/Ti3C2Tx composites show diffraction peaks for CuO at 32.5°, 35.5°, 38.6°, 48.7°, 53.6°, 58.1°, 61.5°, 66.2°, 68.0° and 73.7°, corresponding to Miller indices (110), (002), (111), (−202), (020), (202), (−113), (−311), (220), and (221), respectively (agreed with reference code JCPDS no. 01-089-5898). It is also observed that the peaks of Cu2O at 29.5° 36.4°, 42.3°, and 61.5° correspond to (110), (111), (200), and (220) planes, respectively (JCPDS no. 01-078-2076). Thus, from XRD analysis, it is observed that CuxO in the composite retains the same crystal structure as compared to the as-prepared CuxO. The comparative survey spectra of CuxO and CuxO/Ti3C2Tx are given in Fig. S1† to provide the relative abundance of elements. Both the catalysts display the presence of Cu 2p metal. XPS analysis was used to determine the chemical state of CuxO/Ti3C2Tx, and Gaussian fitting was used to deconvolute the C 1s, Ti 2p, Cu 2p, and O 1s core levels. Fig. 2(b) depicts the deconvoluted C 1s core level as four peaks at 283.6 eV, 284.7 eV, 285.9 eV, and 288.7 eV, which correspond to the C–Ti, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–O, and C
C, C–O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively.22 The deconvoluted peaks of Cu(II) and Cu(I) states are shown in Fig. 2(c). Peaks at 934.9 eV and 954.1 eV are assigned to Cu 2p3/2 and Cu 2p1/2, respectively, and the gap between these peaks is 19.2 eV, which coincides well with the creation of CuO on the composite.23 The peaks at 933.6 eV and 953.6 eV are attributed to Cu 2p3/2 and Cu 2p1/2, respectively, which represented Cu2O. The main Cu 2p3/2 peak at 934.9 eV is accompanied by two satellite peaks on the higher binding energy side at around 944.3 eV and 941.8 eV, indicating the presence of CuO.24,25Fig. 2(c) clearly depicts the main peak of Cu 2p1/2 at 954.1 eV and its satellite peak at 962.5 eV, which also confirms the presence of CuO. Because of the presence of the partially filled d9 shell arrangement in the ground state, the CuO (Cu(II)) spectrum shows prominent shake-up satellite features, whereas no shake-up satellite is seen for Cu2O (Cu(I)) due to its filled d10 arrangement in the ground state. Fig. 2(d) depicts the core level of Ti 2p deconvoluted into two peaks at 458.3 eV and 464.4 eV, which correspond to the Ti–C and Ti–O bonds, respectively.22 The appearance of Ti–O bonding could be due to oxidation during the combustion process, which converts unstable Ti–C bonds to Ti–O bonds. Furthermore, as seen in Fig. 2(e), the O 1s core level is deconvoluted into two peaks, 530.0 eV and 532.2 eV, which correspond to the metallic oxygen and O
O bonds, respectively.22 The deconvoluted peaks of Cu(II) and Cu(I) states are shown in Fig. 2(c). Peaks at 934.9 eV and 954.1 eV are assigned to Cu 2p3/2 and Cu 2p1/2, respectively, and the gap between these peaks is 19.2 eV, which coincides well with the creation of CuO on the composite.23 The peaks at 933.6 eV and 953.6 eV are attributed to Cu 2p3/2 and Cu 2p1/2, respectively, which represented Cu2O. The main Cu 2p3/2 peak at 934.9 eV is accompanied by two satellite peaks on the higher binding energy side at around 944.3 eV and 941.8 eV, indicating the presence of CuO.24,25Fig. 2(c) clearly depicts the main peak of Cu 2p1/2 at 954.1 eV and its satellite peak at 962.5 eV, which also confirms the presence of CuO. Because of the presence of the partially filled d9 shell arrangement in the ground state, the CuO (Cu(II)) spectrum shows prominent shake-up satellite features, whereas no shake-up satellite is seen for Cu2O (Cu(I)) due to its filled d10 arrangement in the ground state. Fig. 2(d) depicts the core level of Ti 2p deconvoluted into two peaks at 458.3 eV and 464.4 eV, which correspond to the Ti–C and Ti–O bonds, respectively.22 The appearance of Ti–O bonding could be due to oxidation during the combustion process, which converts unstable Ti–C bonds to Ti–O bonds. Furthermore, as seen in Fig. 2(e), the O 1s core level is deconvoluted into two peaks, 530.0 eV and 532.2 eV, which correspond to the metallic oxygen and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, respectively.
C bonds, respectively.
 | ||
| Fig. 2 (a) Comparative XRD patterns of Ti3C2Tx, CuxO and CuxO/Ti3C2Tx. Deconvoluted XPS spectrum for (b) C 1s, (c) Cu 2p, (d) Ti 2p and (e) O 1s of CuxO/Ti3C2Tx composite. | ||
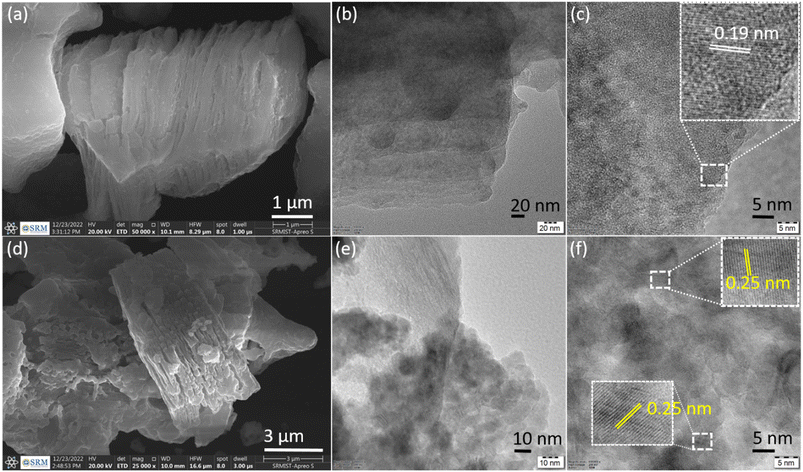
Surface morphologies and microstructures of Ti3C2Tx and CuxO/Ti3C2Tx samples were examined using SEM and TEM, respectively. Fig. 3(a) displays a typical Ti3C2Tx nanosheet layered structure with an accordion-like structure, and the separated layer of Ti3C2Tx nanosheets suggests successful aluminium etching from the MAX phase. The TEM image of Ti3C2Tx revealed exfoliated nanoflakes with distinct edges (Fig. 3(b)), and crystallinity was confirmed by the HRTEM image with interplanar spacing of 0.19 nm (Fig. 3(c)). The nanoflakes distinct edges of Ti3C2Tx may be required for in situ CuxO development on its surfaces, which may promote nitrate ion adsorption, charge transfer, and electron transport. Fig. 3(d) indicates that CuxO is evenly distributed on Ti3C2Tx, owing to the combustion process, which produces a smooth nanofoam of CuxO on the surface of MXene (a CuxO nanofoam structure is created as a result of nitrate ions escaping from the precursor during the combustion process). As shown in Fig. 3(f), the interplanar spacing values were 0.25 nm, which is in good agreement with the (111) plane of Cu2O. Consequently, the formation of CuxO/Ti3C2Tx is evident from the morphologies.
 | ||
| Fig. 3 SEM of (a) Ti3C2Tx and (d) CuxO/Ti3C2Tx composite. TEM image of (b and c) Ti3C2Tx and (e and f) CuxO/Ti3C2Tx composite. | ||
4. Electrochemical nitrate reduction by CuxO/Ti3C2Tx catalysts
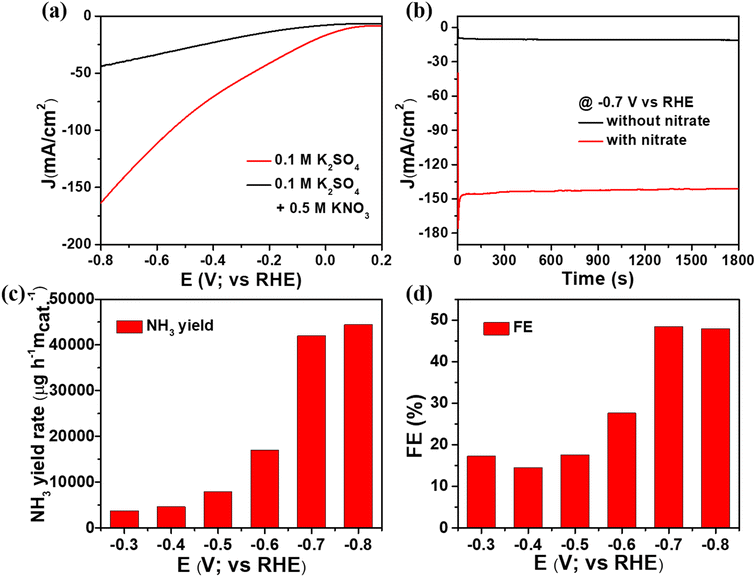
Electrochemical ammonia production is an environment friendly process because it converts nitrate waste into a valuable ammonia product. Consequently, electrochemical measurements were conducted on the CuxO/Ti3C2Tx composite, and the construction of electrodes is described in ESI.† Initially, we employed 0.1 M K2SO4 solution with and without nitrate ions (0.5 M KNO3) to conduct the electrochemical nitrate reduction to ammonia using the linear sweep voltammetry (LSV) technique. As shown in Fig. 4(a), the LSVs show that nitrate reduction occurs when compared to the competent hydrogen evolution reaction. The neutral electrolyte, 0.1 M K2SO4, aids in minimizing the HER reaction and improving nitrate reduction. The current density was determined to be around 162 mA cm−2 in the presence of nitrate solution and around 43 mA cm−2 in the absence of nitrate solution, demonstrating a successful electrochemical nitrate reduction reaction. After adding nitrate solution, the overpotential was reduced to approximately 310 mV to obtain a current density of 20 mA cm−2. Along with the optimized composite, the nitrate reduction of other controlled catalysts was analysed using LSV and shown in Fig. S2.† The significance of synergetic effects between CuxO and Ti3C2Tx composite was assessed by comparing the NO3RR catalytic activity of CuxO, Ti3C2Tx, and the optimised CuxO/Ti3C2Tx composite, as shown in Fig. S3.† At −0.7 V vs. RHE, the current density for CuxO, Ti3C2Tx, and CuxO/Ti3C2Tx is determined to be roughly 45 mA cm−2, 37 mA cm−2, and 162 mA cm−2, respectively (Fig. S3(a)†). According to a chronoamperometry investigation, the current density for CuxO, Ti3C2Tx, and CuxO/Ti3C2Tx was found to be approximately 28 mA cm−2, 32 mA cm−2, and 150 mA cm−2, respectively, at −0.7 V, as shown in Fig. S3(b).† It is obvious that the composite catalyst outperforms the CuxO or Ti3C2Tx catalysts by three times due to their synergy effect. This is because Ti3C2Tx solely (consisting of a negatively charged surface) is unfavourable to adsorb the electron-rich nitrate due to electrostatic repulsion. Whereas, CuxO is favourable but the poor conductivity properties decrease the electroreduction of nitrate ions. As a result, as compared to either CuxO or Ti3C2Tx alone, the combination of Ti3C2Tx and CuxO provides an excellent activity for the nitrate reduction reaction. Consequently, the NH3 yield and FE of the optimized composite were determined using the chronoamperometric method, which consisted of conducting experiments at varying potentials with time. The electrolyte was collected after 30 minutes of analysis, and indophenol blue was used to evaluate NH3 production and FE of catalysts (details of the indophenol blue method procedure are included in the ESI†). The confirmation of i–t measurements in 0.1 M K2SO4 electrolyte at −0.7 V vs. RHE to identify NH3 generated by nitrate ions is shown in Fig. 4(b).The electrochemical process of converting nitrate ions to ammonia requires eight electron transitions. We initially standardized ammonia and hydrazine so that we would know the precise concentration of ammonia and hydrazine solution, as shown in Fig. S4 and S5,† which is necessary for our final goal of ammonia manufacturing. In order to calculate the amount of nitrate converted by the CuxO/Ti3C2Tx composite, half-hour-long chronoamperometric measurements were recorded at several potentials while the solution included nitrate electrolyte. Fig. 4(c) and (d) displays the results of a spectroscopic investigation, which determined that the NH3 yield and FE at −0.7 V vs. RHE were approximately 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 982 μg h−1 mcat−1 and 48%, respectively. We found that the NH3 production was slightly less at −0.7 V vs. RHE as compared to −0.8 V vs. RHE, but the FE was the highest at −0.7 V vs. RHE; thus, we consider it as the optimized potential. Additionally, the nitrate reduction reaction comparison study of CuxO, Ti3C2Tx, and CuxO/Ti3C2Tx composite was examined at −0.7 V against RHE. The spectroscopic examination results for the composites are displayed in Fig. S3(c) and (d).† In the case of CuxO, the NH3 yield and FE at −0.7 V vs. RHE were approximately 3987 μg h−1 mcat−1 and 22%, respectively. The bare Ti3C2Tx delivered NH3 yield and FE at −0.7 V vs. RHE were approximately 3175 μg h−1 mcat−1 and 21%, respectively. Thus, the optimized CuxO/Ti3C2Tx composite delivered better NH3 yield and the highest FE compared to CuxO and Ti3C2Tx. Generally, another product, hydrazine, which could be generated during nitrate reduction, was investigated. As shown in Fig. S6(c) and (e),† no UV-Vis absorption peak was found at 460 nm, and there was no colour associated with hydrazine. As a result, the selective conversion of nitrate to NH3 is confirmed.
982 μg h−1 mcat−1 and 48%, respectively. We found that the NH3 production was slightly less at −0.7 V vs. RHE as compared to −0.8 V vs. RHE, but the FE was the highest at −0.7 V vs. RHE; thus, we consider it as the optimized potential. Additionally, the nitrate reduction reaction comparison study of CuxO, Ti3C2Tx, and CuxO/Ti3C2Tx composite was examined at −0.7 V against RHE. The spectroscopic examination results for the composites are displayed in Fig. S3(c) and (d).† In the case of CuxO, the NH3 yield and FE at −0.7 V vs. RHE were approximately 3987 μg h−1 mcat−1 and 22%, respectively. The bare Ti3C2Tx delivered NH3 yield and FE at −0.7 V vs. RHE were approximately 3175 μg h−1 mcat−1 and 21%, respectively. Thus, the optimized CuxO/Ti3C2Tx composite delivered better NH3 yield and the highest FE compared to CuxO and Ti3C2Tx. Generally, another product, hydrazine, which could be generated during nitrate reduction, was investigated. As shown in Fig. S6(c) and (e),† no UV-Vis absorption peak was found at 460 nm, and there was no colour associated with hydrazine. As a result, the selective conversion of nitrate to NH3 is confirmed.
The stability of catalysts can be evaluated by comparing the amount of NH3 produced to the number of cycles the catalyst has undergone. As a result, ten successive cycles of the CuxO/Ti3C2Tx catalyst were performed for half an hour at −0.7 V vs. RHE, as shown in Fig. 5(a). The detailed spectroscopic analysis of the NH3 production process is depicted in Fig. 5(b) and S5.† The NH3 production drops by as much as 60% after 10 cycles as the number of cycles increases. However, after 10 cycles, the average FE dropped by only 3% from the initial FE, as shown in Fig. 5(c) and (d). As shown in Fig. S7,† no UV-Vis absorption peak was found at 460 nm after 10 cycles as there was no colour associated with hydrazine. In addition, i–t measurements were performed for 8 hours on the CuxO/Ti3C2Tx catalyst at −0.7 V vs. RHE to get insight into the catalyst's stability (see Fig. S8†). After 8 hours, the current density slightly changed, demonstrating the composite's remarkable stability.
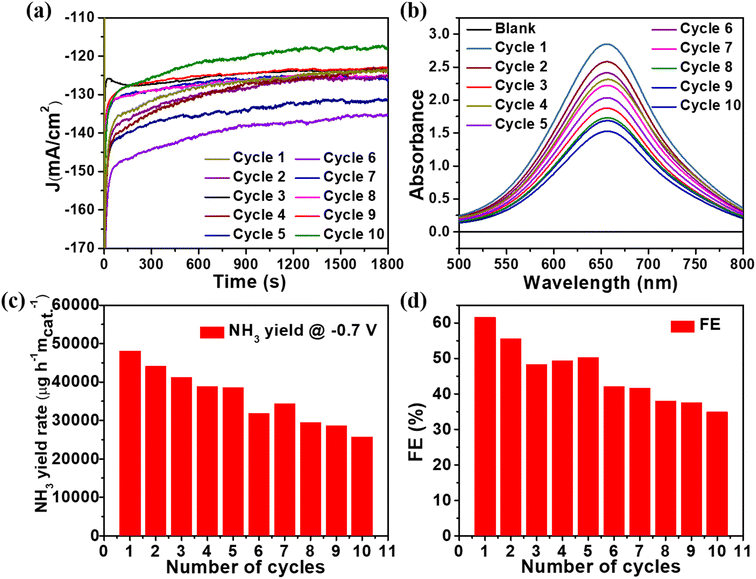 | ||
| Fig. 5 (a) Chronoamperometric measurements at −0.7 V vs. RHE (b) UV-Vis spectra (c) NH3 yield and (d) faradaic efficiency for NH3 production of CuxO/Ti3C2Tx catalyst. | ||
In addition, the electrochemically active surface area (ECSA) was analysed for CuxO, Ti3C2Tx, and CuxO/Ti3C2Tx composites as shown in Fig. S9(a) and (b).† Commonly, double layer capacitance is correlated to the capacitive current and the scan rate by the following equation:26
| Idl = Cdl × scan rate |
| ECSA = Cdl/Cs |
5. Conclusion
In summary, we successfully constructed a CuxO/Ti3C2Tx composite via a combustion approach for electrochemical NH3 synthesis. The development of CuxO nanofoam on the Ti3C2Tx surface improved the catalytic conversion of NO3− to NH3. This is because CuxO is an excellent candidate for adsorbing nitrate ions and reducing it to NH3. The improved nitrate reduction reaction with CuxO/Ti3C2Tx is considerably superior as compared to CuxO-2/Ti3C2Tx and CuxO-3/Ti3C2Tx. Electrochemical experiments of CuxO/Ti3C2Tx reveal an interesting NO3RR with a current density of 162 mA cm−2. Furthermore, the CuxO/Ti3C2Tx exhibits electrocatalytic activity with ammonia production of 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 982 μg h−1 mcat−1 and a faradaic efficiency of 48% at −0.7 V vs. RHE. As a result, the performance of CuxO/Ti3C2Tx indicates that it is a good candidate for nitrate ion conversion to ammonia. Furthermore, CuxO/Ti3C2Tx composites may be a promising candidates in the future since they meet the parameters for an electrocatalyst for NO3 reduction to ammonia.
982 μg h−1 mcat−1 and a faradaic efficiency of 48% at −0.7 V vs. RHE. As a result, the performance of CuxO/Ti3C2Tx indicates that it is a good candidate for nitrate ion conversion to ammonia. Furthermore, CuxO/Ti3C2Tx composites may be a promising candidates in the future since they meet the parameters for an electrocatalyst for NO3 reduction to ammonia.
Author contributions
Sagar Ingavale: conceptualization, investigation, methodology, writing – original draft, writing – review & editing. Phiralang Marbaniang: conceptualization, methodology, writing – original draft. Manoj Palabathuni: investigation, methodology. Nimai Mishra: funding acquisition, resources, supervision, writing – review & editing.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We acknowledge the HRTEM facility at SRMIST set up with support from MNRE (project no. 31/03/2014-15/PVSER&D), Government of India. S. I. thanks SRM University, Andhra Pradesh, for financial support.References
- Y. Zhang, X. Chen, W. Wang, L. Yin and J. C. Crittenden, Appl. Catal., B, 2022, 310, 121346 CrossRef CAS.
- S. Ingavale, P. Marbaniang, M. Palabathuni, V. N. Kale and N. Mishra, Nanoscale, 2023, 11497–11505 RSC.
- N. C. Kani, N. H. L. Nguyen, K. Markel, R. R. Bhawnani, B. Shindel, K. Sharma, S. Kim, V. P. Dravid, V. Berry, J. A. Gauthier and M. R. Singh, Adv. Energy Mater., 2023, 2204236 CrossRef CAS , early view..
- F. Jiao and B. Xu, Adv. Mater., 2019, 31, 1805173 CrossRef PubMed.
- J. Wang, S. Chen, Z. Li, G. Li and X. Liu, ChemElectroChem, 2020, 7, 1067–1079 CrossRef CAS.
- Y. Huang, D. D. Babu, Z. Peng and Y. Wang, Adv. Sci., 2020, 7, 1902390 CrossRef CAS PubMed.
- L. Li, C. Tang, H. Jin, K. Davey and S. Z. Qiao, Chem, 2021, 7, 3232–3255 CAS.
- H. Shen, C. Choi, J. Masa, X. Li, J. Qiu, Y. Jung and Z. Sun, Chem, 2021, 7, 1708–1754 CAS.
- J. Li, Y. Zhang, C. Liu, L. Zheng, E. Petit, K. Qi, Y. Zhang, H. Wu, W. Wang, A. Tiberj, X. Wang, M. Chhowalla, L. Lajaunie, R. Yu and D. Voiry, Adv. Funct. Mater., 2022, 32, 2108316 CrossRef CAS.
- H. Chen, Y. Pang, Y. Wei, X. He, Y. Zhang and L. Xie, Environ. Sci. Pollut. Res., 2022, 114, 1–10 Search PubMed.
- M. Varsha, P. S. Kumar and B. S. Rathi, Chemosphere, 2022, 287, 132270 CrossRef CAS PubMed.
- T. R. Kumaraswamy, S. Javeed, M. Javaid and K. Naika, Fresh Water Pollut. Dyn. Remediat., 2020, 1031, pp. 69–81 Search PubMed.
- F. Y. Chen, Z. Y. Wu, S. Gupta, D. J. Rivera, S. V. Lambeets, S. Pecaut, J. Y. T. Kim, P. Zhu, Y. Z. Finfrock, D. M. Meira, G. King, G. Gao, W. Xu, D. A. Cullen, H. Zhou, Y. Han, D. E. Perea, C. L. Muhich and H. Wang, Nat. Nanotechnol., 2022, 17, 759–767 CrossRef CAS PubMed.
- W. Jung and Y. J. Hwang, Mater. Chem. Front., 2021, 5, 6803–6823 RSC.
- J. Yuan, Z. Xing, Y. Tang and C. Liu, ACS Appl. Mater. Interfaces, 2021, 13, 52469–52478 CrossRef CAS PubMed.
- Q. Zhao, Z. Tang, B. Chen, C. Zhu, H. Tang and G. Meng, Chem. Commun., 2022, 58, 3613–3616 RSC.
- Z. Gong, W. Zhong, Z. He, Q. Liu, H. Chen, D. Zhou, N. Zhang, X. Kang and Y. Chen, Appl. Catal., B, 2022, 305, 121021 CrossRef CAS.
- Q. Gao, H. S. Pillai, Y. Huang, S. Liu, Q. Mu, X. Han, Z. Yan, H. Zhou, Q. He, H. Xin and H. Zhu, Nat. Commun., 2022, 13, 1–12 Search PubMed.
- Y. Wang, W. Zhou, R. Jia, Y. Yu and B. Zhang, Angew. Chem., Int. Ed., 2020, 59, 5350–5354 CrossRef CAS PubMed.
- K. Li, Y. Lei, J. Liao and Y. Zhang, Inorg. Chem. Front., 2021, 8, 1747–1761 RSC.
- L. X. Li, W. J. Sun, H. Y. Zhang, J. L. Wei, S. X. Wang, J. H. He, N. J. Li, Q. F. Xu, D. Y. Chen, H. Li and J. M. Lu, J. Mater. Chem. A, 2021, 9, 21771–21778 RSC.
- L. Wang, X. Yao, S. Yuan, Y. Gao, R. Zhang, X. Yu, S. T. Tu and S. Chen, RSC Adv., 2023, 13, 6264–6273 RSC.
- X. Su, G. Feng, L. Yu, Q. Li, H. Zhang, W. Song and G. Hu, J. Mater. Sci.: Mater. Electron., 2019, 30, 3545–3551 CrossRef CAS.
- W. Lv, L. Li, Q. Meng and X. Zhang, J. Mater. Sci., 2020, 55, 2492–2502 CrossRef CAS.
- J. Yuan, J. J. Zhang, M. P. Yang, W. J. Meng, H. Wang and J. X. Lu, Catalysts, 2018, 8, 171 CrossRef CAS.
- S. Ingavale, P. Marbaniang, B. Kakade and A. Swami, Catal. Today, 2021, 370, 55–65 CrossRef CAS.
- S. B. Ingavale, I. Patil, H. Parse, D. C. Sesu, P. Marbaniang, N. Ramgir, B. Kakade and A. Swami, Int. J. Hydrogen Energy, 2019, 44, 24922–24933 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The details of materials used for the synthesis of CuxO/Ti3C2Tx, physical characterization, electrochemical measurements and determination of the concentration of ammonia (including the faradaic efficiency and NH3 yield) are given. XPS survey spectra for CuxO and CuxO/Ti3C2Tx. LSVs curve for CuxO-2/Ti3C2Tx, CuxO/Ti3C2Tx and CuxO-3/Ti3C2Tx at 10 mV s−1 with nitrate ions in 0.1 M K2SO4 electrolyte. Comparative LSV pattern at 10 mV s−1 and chronoamperometric curve of CuxO, Ti3C2Tx, and CuxO/Ti3C2Tx with nitrate ions in 0.1 M K2SO4 electrolyte. NH3 yield and faradaic efficiency for NH3 production of CuxO, Ti3C2Tx, and CuxO/Ti3C2Tx catalysts at −0.7 V vs. RHE. UV-Vis spectra for indo-phenol determination of different known concentrations of NH4+ standards. Calibration curve obtained from linear fit. Photograph of standard NH4+ solution. UV-Vis spectra for Watt and Chrisp determination of different known concentrations of hydrazine standards. Calibration curve obtained from linear fit. Photograph of standard hydrazine solution. Electrocatalytic i–t experiments at various applied potentials of CuxO/Ti3C2Tx catalyst, UV-Vis spectra for indo-phenol determination of ammonia and Watt and Chrisp determination of hydrazine in electrolytes obtained from electrocatalytic CA experiments at different applied potentials respectively. Photograph of ammonia and hydrazine solution. UV-Vis spectra for Watt and Chrisp determination of hydrazine in electrolytes obtained from electrocatalytic CA experiments at −0.7 V vs. RHE. Chronoamperometric analysis of CuxO/Ti3C2Tx catalyst at −0.7 V vs. RHE in 0.5 M KNO3 + 0.1 M K2SO4 electrolyte. Comparative current vs. scan rate profile; (b) ECSA and roughness factor for CuxO, Ti3C2Tx, and CuxO/Ti3C2Tx electrocatalysts. See DOI: https://doi.org/10.1039/d3na00609c |
| This journal is © The Royal Society of Chemistry 2024 |