Open Access Article
Open Access Article2,2′-Bipyridine-4,4′-dicarboxylic acid ligand engineered CsPbBr3 perovskite nanocrystals for enhanced photoluminescence quantum yield with stable display applications†
Ankit
Kumar
a,
Sukanya
Ghosh
a,
Ankush
Saini
b,
Sumit
Kumar
a,
Monojit
Bag
 *b and
Prasenjit
Kar
*b and
Prasenjit
Kar
 *a
*a
aDepartment of Chemistry, Indian Institute of Technology Roorkee, Roorkee, Uttarakhand, India. E-mail: kar.prasen@gmail.com; prasenjit.kar@cy.iitr.ac.in
bDepartment of Physics, Indian Institute of Technology Roorkee, Roorkee, Uttarakhand, India. E-mail: monojit.bag@ph.iitr.ac.in
First published on 30th May 2024
Abstract
Cesium lead halide perovskite nanocrystals (PNCs) have emerged over the last decade as a promising candidate for optoelectronic devices, owing to their exceptional optical and color-tunable properties. Despite this, the instability of these materials and reduction in the photoluminescence (PL) properties with time prevents them from reaching their potential applications in the real world. The degradation in the PL properties of PNCs is due to surface defects caused by the removal of surface ligands. Herein, we have used a bidentate ligand, namely 2,2′-bipyridine-4,4′-dicarboxylic acid (BPY), to improve the optical properties of green-emitting CsPbBr3 PNCs such as PL, and photoluminescence quantum yield (PLQY). The surface defects are reduced by the coordination of the carboxyl group of the bidentate BPY ligand with under-coordinated lead atoms. The PLQY of pristine CsPbBr3 PNCs increased from 64 ± 2% to 88 ± 2% for BPY-CsPbBr3 PNCs. In addition, a down-converted green light-emitting diode (LED) was fabricated by utilizing BPY-CsPbBr3 PNCs, which shows its potential in display applications. Thus, our results will promote these inorganic PNCs in the commercial development of optoelectronic devices.
Introduction
Recently, lead halide perovskites (LHPs) have grabbed significant attention from the scientific community.1,2 The scrutiny towards LHPs is due to their exceptional optical and electrical properties, such as high PLQY,3,4 broad absorption over the whole visible region, intense PL with a very narrow width of the emission,5 tunable band gap,6 high charge-carrier diffusion,7 and high tolerance factor,8etc. With all these superior properties, LHPs have been extensively used in optoelectronic devices, such as light emitting diodes (LEDs),9 solar cells,10 photodetectors,11 lasers,12 and wide color gamut displays.13 Additionally, the low fabrication cost and easy synthetic approach make these materials more suitable for use in the field of optoelectronic devices. However, with all these exceptional achievements, LHPs are not able to achieve practical applications because of their toxicity and instability towards heat, moisture, and light.14There are numerous methods to enhance the stability of LHPs, such as encapsulation with other semiconductor material shells, encapsulation with mesoporous materials like metal–organic frameworks and covalent organic frameworks, and surface passivation with different types of ligands.15–19 Recently, it was found that surface passivation with capping ligands is an efficient way to enhance the stability and optical properties of LHPs.20,21 The bonding between the surface of nanocrystals and the capping ligands is highly dynamic in nature; due to this, perovskite materials suffer from intrinsic chemical instability.22 The ligands detach from the surface of perovskite nanocrystals with aging, washing, and dilution. All these processes lead to the generation of defects on the surface of nanocrystals.23 These surface defects are responsible for nonradiative recombination. The combination of these defects leads to a decrease in the optical properties, stability, and performance of the device. Therefore, it is critical to understand the role of surface defects in limiting device performance and developing new routes to passivate the surface defects to increase device performance.
Oleic acid (OA) and oleylamine (OAm) are one of the most familiar acid–base pairs of capping ligands that have been regularly used by a number of research groups in the synthesis of perovskite nanomaterials.24,25 OA capped the surface of the LHPs by the coordination of its carboxyl group with Pb, while OAm capped the surface through hydrogen bonding with halogen (X) by its protonated ammonium group. However, this pair of ligands acts as an insulating layer on the surface of nanocrystals, which hampers the charge carriers and reduces the performance of the device.26 Nowadays, along with OA and OAm, different capping ligands are used during different passivation techniques, such as post-synthesis and in situ synthesis.27–29 Alivisatos et al. demonstrate that post-treatment of CsPbBr3 nanocrystals with thiocyanate salt increases the PLQY as well as the stability of the synthesized nanocrystal.30 The combination of trioctylphosphine with PbI2 (TOP-PbI2) is used as a precursor for the synthesis of phase-stable CsPbBr3 nanocrystals with unity PLQY.31 Pan et al. have synthesized CsPbI3 nanocrystals with improved stability and near-unity PLQY with post-synthesis passivation by the use of a bidentate ligand 2,2′-iminodibenzoic acid.26 Zhu et al. have improved the PLQY near unity via the post-synthetic modification with ZnBr2.32 Pradhan et al. brought the PLQY of all LHPs closer to unity by developing a simple synthetic method based on the use of alkylammonium halide salt, which serves both as a source of halides and as a capping ligand at the same time.33
Here, we have reported the synthesis of BPY-CsPbBr3 PNCs using OAm and a bidentate ligand (BPY) in place of conventionally used OA as a capping ligand (Scheme 1). Bidentate ligands strongly bind with the Pb2+ atom as compared to the monodentate ligands.26,34 As a result, the stability of the nanocrystals increased, which further improved the optical properties of the perovskite nanocrystals. The effect of the BPY ligand on the optical properties was studied by performing optical studies. The BPY ligand enhances the optical properties and the BPY-CsPbBr3 PNCs show PLQY (88 ± 2%) that is much higher than that of pristine CsPbBr3 PNCs (64 ± 2%). Furthermore, the stability of BPY-CsPbBr3 PNCs in the ambient environment and against heat was investigated. Finally, a down-converted green LED was fabricated using BPY-CsPbBr3 PNCs.
Experimental details
Materials
Lead bromide (PbBr2, 99.99%), oleylamine (OAm, technical grade, 70%), and octadecene (ODE, technical grade, 70%) were procured from Sigma-Aldrich. Hexane and hydrochloric acid (HCl) were purchased from Rankem. Oleic acid (OA, 90%, Alfa Aesar), sodium dichromate (Na2Cr2O7·2H2O, Himedia), sulfuric acid (H2SO4, Finar), 4,4′-dimethyl-2,2′-bipyridine (Loba Chemicals), sodium hydroxide (NaOH, SRL), and cesium carbonate (Cs2CO3, Avra chemicals, 98%) were ordered from local vendors. All the chemicals were utilized without being further purified.Synthesis of 2,2′-bipyridine-4,4′-dicarboxylic acid (BPY)
0.50 g (2.71 mmol) of 4,4′dimethyl-2,2′-bipyridine was gradually added to a solution of 1.81 g (6.09 mmol) of Na2Cr2O7·2H2O in 7 mL of concentrated sulfuric acid under constant magnetic stirring. The resulting mixture became orange, and after a few minutes, the color changed to green, and the reaction was accomplished after 30 minutes. After that, the reaction mixture was poured into 100 mL of cold water, resulting in a light-yellow precipitate. Following filtration and drying, the solid was dissolved in an alkaline 10% NaOH aqueous solution, which was then slowly acidified using a 10% aqueous HCl solution. This recrystallization produced the desired product, which was free from Cr (+3) ions. After filtration and drying in a vacuum, the final white solid was collected.Preparation of cesium oleate
Cs2CO3 (0.407 g) was loaded into a 50 ml two-neck flask along with ODE (15 ml), and then degassed at 120 °C for 20 minutes. After that, 1.5 ml OA was injected, and the reaction mixture was heated under an N2 atmosphere at 120 °C for 2 h. Cs-oleate was preheated at 80 °C before injection because it is precipitated out from ODE at room temperature.Synthesis of BPY-CsPbBr3 PNCs
ODE (5 ml), PbBr2 (0.0367 g, 0.1 mmol), and BPY (20 mg) were degassed for 1 hour at 120 °C in a 50 ml three-neck flask. OAm (0.5 ml) was injected under N2 at 120 °C. After the complete dissolution of PbBr2, the temperature of the reaction was increased to the required temperature of 160 °C. Under N2, preheated (80 °C) Cs-oleate (0.4 ml) was injected, and the reaction mixture was heated for 20 s. The reaction mixture was cooled to room temperature in an ice bath. The supernatant was removed after centrifuging the crude solution at 6000 rpm for 10 minutes, and the remaining solid precipitate was redispersed in hexane. After repeating this procedure, the solid precipitate was redispersed in hexane for further characterization.Synthesis of pristine CsPbBr3 PNCs
ODE (5 ml) and PbBr2 (0.0367 g, 0.1 mmol) were degassed for 1 hour at 120 °C in a 50 ml three-neck flask. OAm (0.5 ml) and OA (0.5 ml) were injected under N2 at 120 °C. After that, all the remaining steps are the same as above.LED device fabrication
A down-converted green LED was fabricated by coating BPY-CsPbBr3 PNCs on a commercially available blue LED (λex = 395 nm).Material characterization
The optical properties of the synthesized nanocrystals were recorded by performing UV-vis, PL, PLQY, and TCSPC studies. The formation of the nanocrystals was confirmed by XRD analysis. Morphology, crystallinity, and crystal size were confirmed by using TEM analysis. Furthermore, SEM studies support the TEM analysis. The presence of BPY ligand on the nanocrystal surface was confirmed by performing FTIR and XPS studies. For the stability data, time-dependent XRD and PL studies were performed. For thermal stability, the TGA technique was utilized. We also synthesized the BPY ligand and the formation of BPY was confirmed by FTIR and NMR analysis (Fig. S1 and S2, ESI†).Results and discussion
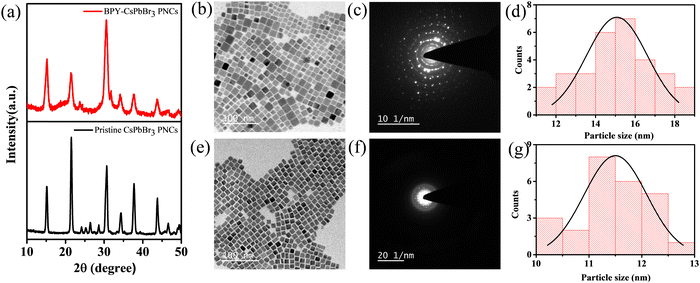
The synthesis of CsPbBr3 PNCs was carried out using the widely recognized synthetic technique of hot injection (HI).35 Cs-oleate and PbBr2 were used as precursors along with OAm, OA, and BPY as capping ligands. The as-synthesized CsPbBr3 PNCs were redispersed in hexane after purification for further characterization. CsPbBr3 PNCs were synthesized utilizing OA and OAm represented as pristine CsPbBr3 PNCs, while CsPbBr3 PNCs were synthesized utilizing BPY and OAm represented as BPY-CsPbBr3 PNCs.Fig. 1(a) depicts the XRD patterns of pristine CsPbBr3 PNCs and BPY-CsPbBr3 PNCs. The XRD analysis implies that the XRD patterns of both the nanocrystals are well indexed with the cubic phase.36 All the diffraction peaks obtained in the XRD pattern at 2θ = 15.20°, 21.45°, 30.55°, 34.18°, 37.65°, and 43.67°, well resemble the corresponding planes of (100), (110), (200), (210), (211), and (220), respectively. XRD analysis confirmed that no structural changes occur between the pristine CsPbBr3 PNCs and BPY-CsPbBr3 PNCs. Additionally, similarities in structural properties were further supported by transmission electron microscopy (TEM) analysis. The TEM images of BPY-CsPbBr3 PNCs and pristine CsPbBr3 PNCs are depicted in Fig. 1(b) and (e), respectively.
The TEM images indicate that both the nanocrystals exhibit a uniform cubic morphology. The high crystallinity of the nanocrystals was confirmed by using selected area electron diffraction (SAED) (Fig. 1(c) and (f)). The average crystal size of pristine CsPbBr3 PNCs and BPY-CsPbBr3 PNCs is ∼11.5 and ∼15 nm, respectively (Fig. 1(d) and (g)). The morphology of the nanocrystals was further examined by scanning electron microscopy (SEM). The SEM images also reveal that nanocrystals acquire a cubic morphology. The SEM images of the pristine CsPbBr3 PNCs and BPY-CsPbBr3 PNCs are presented in Fig. S3 and S4 (ESI†), respectively. The energy-dispersive X-ray spectroscopy (EDX) analysis indicates the presence of all respective elements, as depicted in Fig. S3 and S4 (ESI†). Elemental mapping of the SEM images demonstrates the uniform distribution of all the elements throughout the surface of the nanocrystals (Fig. S3 and S4 (ESI†)). These studies prove that there are no changes between the structural properties of pristine CsPbBr3 PNCs and BPY-CsPbBr3 PNCs.
However, we observed a significant change in the optical properties between pristine CsPbBr3 PNCs and BPY-CsPbBr3 PNCs. The absorbance and PL spectra of pristine CsPbBr3 PNCs and BPY-CsPbBr3 PNCs are presented in Fig. 2(a) and (b), respectively. The characteristic absorption and emission peaks of CsPbBr3 PNCs are exhibited at 504 and 519 nm, respectively. There is an improvement in the PL and PLQY for BPY-CsPbBr3 PNCs. The PLQY improved from 64 ± 2% for pristine CsPbBr3 PNCs to 88 ± 2% for BPY-CsPbBr3 PNCs (Fig. S5, ESI†). To understand the origin of the PL enhancement of BPY-CsPbBr3 PNCs, time-correlated single-photon counting (TCSPC) analysis was used. Fig. 2(c) shows the TCSPC curves of both the nanocrystals in hexane using a 450 nm LED as an excitation source. All the TCSPC curves are well-fitted with an exponential function (eqn (1)).
Fit = B + A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2 exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) + A3 exp(−t/τ2) + A3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ3) exp(−t/τ3) | (1) |
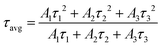 | (2) |
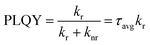 | (3) |
 | ||
| Fig. 2 (a) UV-Vis; (b) PL (inset shows the optical images of 1 pristine CsPbBr3 PNCs and 2 BPY-CsPbBr3 PNCs); (c) TCSPC spectra of pristine CsPbBr3 PNCs and BPY-CsPbBr3 PNCs. | ||
| Sample | A 1 (%) | τ 1 (ns) | A 2 (%) | τ 2 (ns) | A 3 (%) | τ 3 (ns) | τ avg (ns) | PLQY (%) | K r (ns−1) | K nr (ns−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| Pristine CsPbBr3 PNCs | 56.80 | 2.22 | 36.09 | 10.00 | 7.11 | 41.59 | 20.67 | 64 | 0.030 | 0.016 |
| BPY-CsPbBr3 PNCs | 32.45 | 3.44 | 55.47 | 13.94 | 12.08 | 46.57 | 25.80 | 88 | 0.034 | 0.004 |
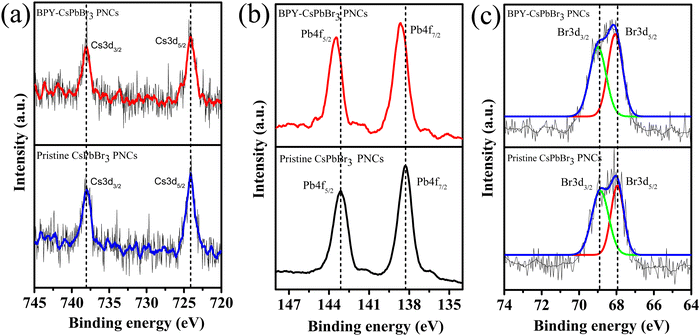
To gain insight into the presence of the BPY ligand on the surface of the nanocrystals, the FTIR technique was employed. Fig. S6 (ESI†) displays the FTIR spectrum of both BPY and BPY-CsPbBr3 PNCs. The peaks at 2855 and 2926 cm−1 are the characteristic peaks of C–H stretching vibrations of (–CH2) and (–CH3) groups of OAm, respectively. The peak at 2975 cm−1 is attributed to the stretching vibration of the aromatic C–H bond. The peak at 1640 cm−1 is due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C functional group. The peak at 1384 cm−1 is due to the COO− functional group.41 The peak at 1564 cm−1 corresponds to the C–N stretching vibration. The characteristic peaks at 720–910 cm−1 are due to aromatic bending vibrations.42 The prominent peak at 1468 cm−1 in the BPY ligand spectrum is due to the OH stretching vibration of the carboxylic acid, but the intensity of this peak decreased in BPY-CsPbBr3 PNCs.43 These results indicate that the BPY ligand binds with the Pb atom through oxygen atoms of the carboxylic group which effectively passivates the surface of the BPY-CsPbBr3 PNCs. To further confirm the presence of BPY ligand on the surface of BPY-CsPbBr3 PNCs, X-ray photoelectron spectroscopy (XPS) analysis was performed. The full spectrum scan exhibits the representative peaks of all the constituent elements Cs, Pb, Br, C, and O, as shown in Fig. S7 (ESI†). The two peaks at 724.13, and 738.07 eV correspond to the Cs 3d5/2 and Cs 3d3/2 orbitals (Fig. 3(a)), respectively, and show no shift in the peak position, which is consistent with previous reports.44
C functional group. The peak at 1384 cm−1 is due to the COO− functional group.41 The peak at 1564 cm−1 corresponds to the C–N stretching vibration. The characteristic peaks at 720–910 cm−1 are due to aromatic bending vibrations.42 The prominent peak at 1468 cm−1 in the BPY ligand spectrum is due to the OH stretching vibration of the carboxylic acid, but the intensity of this peak decreased in BPY-CsPbBr3 PNCs.43 These results indicate that the BPY ligand binds with the Pb atom through oxygen atoms of the carboxylic group which effectively passivates the surface of the BPY-CsPbBr3 PNCs. To further confirm the presence of BPY ligand on the surface of BPY-CsPbBr3 PNCs, X-ray photoelectron spectroscopy (XPS) analysis was performed. The full spectrum scan exhibits the representative peaks of all the constituent elements Cs, Pb, Br, C, and O, as shown in Fig. S7 (ESI†). The two peaks at 724.13, and 738.07 eV correspond to the Cs 3d5/2 and Cs 3d3/2 orbitals (Fig. 3(a)), respectively, and show no shift in the peak position, which is consistent with previous reports.44
In the Pb 4f orbital (Fig. 3(b)), we observed a shift of 0.36 eV towards higher binding energy, which is relatively due to the strong interaction of the carboxyl group of BPY with the Pb2+ atom.45 Furthermore, a slight shift of 0.13 eV in the Br 3d orbitals (Fig. 3(c)) confirms the strong interaction of the BPY ligand.42 Thus, these results indicate an improvement in the interaction in the presence of the BPY ligand.
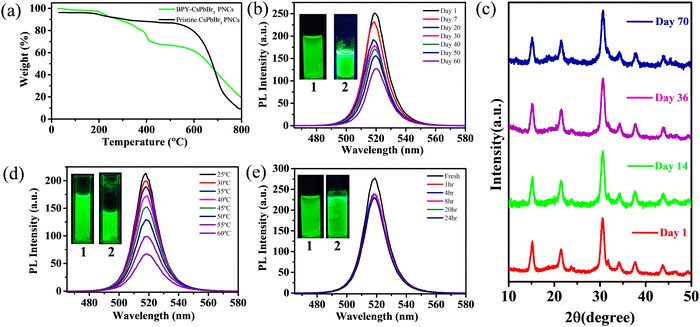
The stability of PNCs is an integral part of device fabrication. In this context, we performed stability experiments for BPY-CsPbBr3 PNCs. To determine the comparative thermal stability of pristine and BPY-CsPbBr3 PNCs, a thermogravimetric analysis (TGA) was carried out. TGA spectra of pristine and BPY-CsPbBr3 PNCs are depicted in Fig. 4(a). The spectra demonstrate that the majority of the weight of nanocrystals is lost in three distinct stages. Evaporation of the water that is present on the surface of the nanocrystals is responsible for the first-minute weight loss that occurs close to 100 °C. The second decomposition step starts from 180 °C and extends up to 450 °C. The release of surface-bound ligands BPY (boiling point >300 °C), OAm (boiling point ∼350 °C), and OA (boiling point ∼360 °C) is the cause of this weight loss. The third and most substantial weight loss indicates that BPY-CsPbBr3 PNCs are stable up to 550 °C. After that temperature, the material loses its functional stability. TGA analysis shows that both the PNCs have almost comparative thermal stability. Furthermore, to find out more about the stability of BPY-CsPbBr3 PNCs in ambient conditions, we stored the nanocrystals in the laboratory and performed PL and XRD studies with time. Fig. 4(b) displays the time-dependent PL spectra, which reveal that the BPY-CsPbBr3 PNCs are still fluorescent after two months. Fig. 4(c) depicts a time-dependent XRD analysis, indicating that there are no observable alterations in the XRD pattern over time. We further investigated the stability of the BPY-CsPbBr3 PNCs under hydrothermal conditions by adding 0.1 mL of water to 3 mL colloidal solution of BPY-CsPbBr3 PNCs and recorded the PL spectra at elevated temperatures up to 60 °C. The BPY-CsPbBr3 PNCs maintained their PL after heating up to 60 °C (Fig. 4(d)). For photostability, BPY-CsPbBr3 PNC colloidal solution is kept under continuous UV irradiation (λex = 365 nm) for 24 h. We found that PL is preserved even after 24 h of continuous UV irradiation (Fig. 4(e)). The results validate that the BPY-CsPbBr3 PNCs under investigation have exhibited stability in normal environmental conditions for a duration exceeding two months as well as in different harsh conditions. Furthermore, we have listed the different bidentate ligands used along with their variable properties in Table S1 (ESI†) to demonstrate a comparison of our work with the previously reported literature.
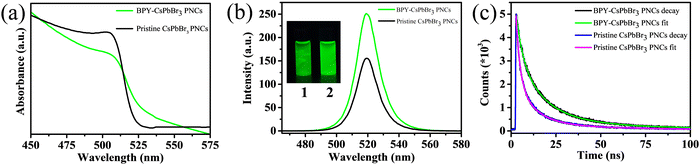
To further investigate the potential of green emissive BPY-CsPbBr3 PNCs for optoelectronic applications. We fabricate a down-converted green LED by coating BPY-CsPbBr3 PNCs on a commercially available blue LED (λex = 395 nm). The down-converted LED device shows a strong PL emission centered at 521 nm with a narrow full width at half maxima (fwhm) of 19 nm (Fig. 5(a)). In the inset of Fig. 5(a), the photograph of the green LED was taken inside a dark room. Fig. S8 (ESI†) shows the photograph of the LED under daylight. The time-dependent PL spectra of BPY-CsPbBr3 PNCs were recorded under continuous working conditions from initial 0 h to 3 h, as shown in Fig. 5(b). The nanocrystals retain 86% of their initial PL after 3 h. The Commission Internationale de l’Eclairage (CIE) color coordinates of the LED are (0.17, 0.74) and the CIE chromaticity plot is given in Fig. 5(c). These results demonstrate that the synthesized nanocrystals have color stability after working for 3 h with no shift in the wavelength and are promising for optoelectronic applications.
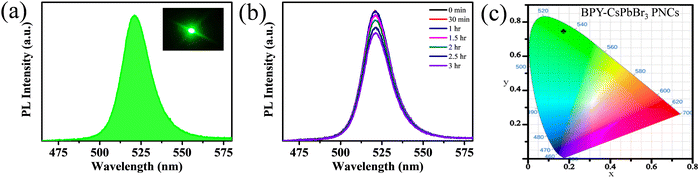 | ||
| Fig. 5 (a) PL spectra of a down-converted LED (inset shows the photograph), (b) PL spectra from 0 h to 3 h, and (c) CIE chromaticity coordinates of the down-converted LED. | ||
Conclusion
In conclusion, bidentate BPY and OAm were used as capping ligands for the synthesis of highly green-emitting BPY-CsPbBr3 PNCs. We studied the effect of BPY on the optical properties of BPY-CsPbBr3 PNCs and found that BPY significantly boosts the optical properties. The BPY ligand effectively reduced the surface defects by strong interaction of the carboxyl group with lead atoms, which eventually enhanced the optical properties which is evidenced by performing PL, PLQY, and TCSPC characterization techniques. FTIR and XPS studies confirmed the presence of BPY on the BPY-CsPbBr3 PNC surface. Meanwhile, XRD, SEM, and TEM studies reveal that there are no structural or morphological changes observed in the BPY-CsPbBr3 PNCs compared to the pristine CsPbBr3 PNCs. The stability-related studies prove that BPY-CsPbBr3 PNCs are stable in the ambient environment for more than two months. Finally, a green down-converted LED was fabricated by drop-casting BPY-CsPbBr3 PNCs on a blue LED. These results demonstrate the potential of BPY-CsPbBr3 PNCs in light-emitting applications.Conflicts of interest
There is no conflict of interest to declare for this work.Acknowledgements
P. K. acknowledges Science and Engineering Research Board (CRG/2020/000702), New Delhi, India. A. K. and S. K. acknowledge CSIR, India, and S. G. and A. S. acknowledge MoE for their doctoral fellowship. M. B. acknowledges the Science and Engineering Research Board, India (CRG/2021/001744). The authors acknowledge Institute Instrumental Centre (IIC), and IIT Roorkee for instrumental facilities.References
- D. Zhang, S. W. Eaton, Y. Yu, L. Dou and P. Yang, J. Am. Chem. Soc., 2015, 137, 9230–9233 CrossRef CAS PubMed.
- X. Li, F. Cao, D. Yu, J. Chen, Z. Sun, Y. Shen, Y. Zhu, L. Wang, Y. Wei, Y. Wu and H. Zeng, Small, 2017, 13, 1603996 CrossRef PubMed.
- F. Liu, Y. Zhang, C. Ding, S. Kobayashi, T. Izuishi, N. Nakazawa, T. Toyoda, T. Ohta, S. Hayase, T. Minemoto, K. Yoshino, S. Dai and Q. Shen, ACS Nano, 2017, 11, 10373–10383 CrossRef CAS PubMed.
- A. Jha, H. Shankar and P. Kar, New J. Chem., 2022, 46, 844–850 RSC.
- A. Swarnkar, R. Chulliyil, V. K. Ravi, M. Irfanullah, A. Chowdhury and A. Nag, Angew. Chem., Int. Ed., 2015, 127, 15644–15648 CrossRef.
- J. A. Sichert, Y. Tong, N. Mutz, M. Vollmer, S. Fischer, K. Z. Milowska, R. García Cortadella, B. Nickel, C. Cardenas-Daw, J. K. Stolarczyk, A. S. Urban and J. Feldmann, Nano Lett., 2015, 15, 6521–6527 CrossRef CAS PubMed.
- C. Wehrenfennig, G. E. Eperon, M. B. Johnston, H. J. Snaith and L. M. Herz, Adv. Mater., 2014, 26, 1584–1589 CrossRef CAS PubMed.
- M. V. Kovalenko, L. Protesescu and M. I. Bodnarchuk, Science, 2017, 358, 745–750 CrossRef CAS PubMed.
- J. Song, J. Li, X. Li, L. Xu, Y. Dong and H. Zeng, Adv. Mater., 2015, 27, 7162–7167 CrossRef CAS PubMed.
- N. Ahn, D. Y. Son, I. H. Jang, S. M. Kang, M. Choi and N. G. Park, J. Am. Chem. Soc., 2015, 137, 8696–8699 CrossRef CAS PubMed.
- L. Dou, Y. M. Yang, J. You, Z. Hong, W. H. Chang, G. Li and Y. Yang, Nat. Commun., 2014, 5, 5404 CrossRef CAS PubMed.
- Y. Fu, H. Zhu, C. C. Stoumpos, Q. Ding, J. Wang, M. G. Kanatzidis, X. Zhu and S. Jin, ACS Nano, 2016, 10, 7963–7972 CrossRef CAS PubMed.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS PubMed.
- A. H. Slavney, R. W. Smaha, I. C. Smith, A. Jaffe, D. Umeyama and H. I. Karunadasa, Inorg. Chem., 2017, 56, 46–55 CrossRef CAS PubMed.
- Q. Zhong, M. Cao, H. Hu, D. Yang, M. Chen, P. Li, L. Wu and Q. Zhang, ACS Nano, 2018, 12, 8579–8587 CrossRef CAS PubMed.
- H. Li, C. Jia, H. Li and X. Meng, Chem. Commun., 2018, 54, 6300–6303 RSC.
- F. Krieg, S. T. Ochsenbein, S. Yakunin, S. Ten Brinck, P. Aellen, A. Süess, B. Clerc, D. Guggisberg, O. Nazarenko, Y. Shynkarenko, S. Kumar, C. J. Shih, I. Infante and M. V. Kovalenko, ACS Energy Lett., 2018, 3, 641–646 CrossRef CAS PubMed.
- H. Shankar, W. W. Yu, Y. Kang and P. Kar, Sci. Rep., 2022, 12, 7848 CrossRef CAS PubMed.
- P. Kour and S. P. Mukherjee, J. Mater. Chem. A, 2021, 9, 6819–6826 RSC.
- L. Yang, B. Fu, X. Li, H. Chen and L. Li, J. Mater. Chem. C, 2021, 9, 1983–1991 RSC.
- M. Qin, H. Xue, H. Zhang, H. Hu, K. Liu, Y. Li, Z. Qin, J. Ma, H. Zhu, K. Yan, G. Fang, G. Li, U. S. Jeng, G. Brocks, S. Tao and X. Lu, Adv. Mater., 2020, 32, 2004630 CrossRef CAS PubMed.
- J. de Roo, M. Ibáñez, P. Geiregat, G. Nedelcu, W. Walravens, J. Maes, J. C. Martins, I. van Driessche, M. V. Kovalenko and Z. Hens, ACS Nano, 2016, 10, 2071–2081 CrossRef CAS PubMed.
- J. Pan, S. P. Sarmah, B. Murali, I. Dursun, W. Peng, M. R. Parida, J. Liu, L. Sinatra, N. Alyami, C. Zhao, E. Alarousu, T. K. Ng, B. S. Ooi, O. M. Bakr and O. F. Mohammed, J. Phys. Chem. Lett., 2015, 6, 5027–5033 CrossRef CAS PubMed.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS PubMed.
- K. Vighnesh, S. Wang, H. Liu and A. L. Rogach, ACS Nano, 2022, 16, 19618–19625 CrossRef CAS PubMed.
- J. Pan, Y. Shang, J. Yin, M. de Bastiani, W. Peng, I. Dursun, L. Sinatra, A. M. El-Zohry, M. N. Hedhili, A. H. Emwas, O. F. Mohammed, Z. Ning and O. M. Bakr, J. Am. Chem. Soc., 2018, 140, 562–565 CrossRef CAS PubMed.
- J. Qiu, W. Xue, W. Wang and Y. Li, Dyes Pigm., 2022, 198, 109806 CrossRef CAS.
- J. Y. Woo, Y. Kim, J. Bae, T. G. Kim, J. W. Kim, D. C. Lee and S. Jeong, Chem. Mater., 2017, 29, 7088–7092 CrossRef CAS.
- S. Ghosh and P. Kar, Inorg. Chem., 2022, 61, 10079–10088 CrossRef CAS PubMed.
- B. A. Koscher, J. K. Swabeck, N. D. Bronstein and A. P. Alivisatos, J. Am. Chem. Soc., 2017, 139, 6566–6569 CrossRef CAS PubMed.
- F. Liu, Y. Zhang, C. Ding, S. Kobayashi, T. Izuishi, N. Nakazawa, T. Toyoda, T. Ohta, S. Hayase, T. Minemoto, K. Yoshino, S. Dai and Q. Shen, ACS Nano, 2017, 11, 10373–10383 CrossRef CAS PubMed.
- Y. Yue, S. Liu, B. Qi, Z. Su, G. Li, C. Wang and D. Zhu, ACS Appl. Mater. Interfaces, 2021, 13, 21645–21652 CrossRef CAS PubMed.
- A. Dutta, R. K. Behera, P. Pal, S. Baitalik and N. Pradhan, Angew. Chem., Int. Ed., 2019, 131, 5608–5612 CrossRef.
- Y. Li, M. Cai, M. Shen, Y. Cai and R. J. Xie, J. Mater. Chem. C, 2022, 10, 8356–8363 RSC.
- D. Chen, P. K. Ko, C. H. A. Li, B. Zou, P. Geng, L. Guo and J. E. Halpert, ACS Energy Lett., 2023, 8, 410–416 CrossRef CAS.
- M. Liu, Q. Wan, H. Wang, F. Carulli, X. Sun, W. Zheng, L. Kong, Q. Zhang, C. Zhang, Q. Zhang, S. Brovelli and L. Li, Nat. Photonics, 2021, 15, 379–385 CrossRef CAS.
- J. Stachurski, S. Tamariz, G. Callsen, R. Butté and N. Grandjean, Light: Sci. Appl., 2022, 11, 114 CrossRef CAS PubMed.
- Y. Xing, L. Wang, D. Yang, Z. Wang, Z. Hao, C. Sun, B. Xiong, Y. Luo, Y. Han, J. Wang and H. Li, Sci. Rep., 2017, 7, 45082 CrossRef CAS PubMed.
- J. Y. Woo, Y. Kim, J. Bae, T. G. Kim, J. W. Kim, D. C. Lee and S. Jeong, Chem. Mater., 2017, 29, 7088–7092 CrossRef CAS.
- X. Zhu, Z. Pan, T. Xu, X. Shao, Z. Gao, Q. Xie, Y. Ying, W. Pei, H. Lin, J. Wang, X. Tang, W. Chen and Y. Liu, Inorg. Chem., 2023, 62, 9190–9198 CrossRef CAS PubMed.
- D. Patra and S. P. Singh, J. Phys. Chem. C, 2023, 127, 9397–9406 CrossRef CAS.
- V. G. V. Dutt, S. Akhil, R. Singh, M. Palabathuni and N. Mishra, J. Phys. Chem. C, 2022, 126, 9502–9508 CrossRef CAS.
- P. S. Calefi, A. O. Ribeiro, A. M. Pires and O. A. Serra, J. Alloys Compd., 2002, 344, 285–288 CrossRef CAS.
- K. A. Huynh, S. R. Bae, T. Van Nguyen, H. H. Do, D. Y. Heo, J. Park, T. W. Lee, Q. Van Le, S. H. Ahn and S. Y. Kim, ACS Photonics, 2021, 8, 1979–1987 CrossRef CAS.
- V. G. V. Dutt, S. Akhil, R. Singh, M. Palabathuni and N. Mishra, ACS Appl. Nano Mater., 2022, 5, 5972–5982 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: FTIR spectra and 1H NMR spectrum of BPY, SEM images, elemental mapping, EDX spectra of pristine CsPbBr3 PNCs and BPY-CsPbBr3 PNCs, and FTIR and PLQY spectra of BPY-CsPbBr3 PNCs. XPS survey scan of pristine CsPbBr3 PNCs and BPY-CsPbBr3 PNCs, and photographs of the down-converted LED under daylight. See DOI: https://doi.org/10.1039/d4ma00124a |
| This journal is © The Royal Society of Chemistry 2024 |