Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Bridging the gap: an in-depth comparison of CVT-grown layered transition metal dichalcogenides for supercapacitor applications†
Muhammad
Habib‡
 *a,
Zahir
Muhammad‡
b,
Yasir A.
Haleem
*a,
Zahir
Muhammad‡
b,
Yasir A.
Haleem
 *c,
Sajid
Farooq
*c,
Sajid
Farooq
 d,
Raziq
Nawaz
e,
Adnan
Khalil
c,
Fozia
Shaheen
f,
Hamza
Naeem
g,
Sami
Ullah
h and
Rashid
Khan
*i
d,
Raziq
Nawaz
e,
Adnan
Khalil
c,
Fozia
Shaheen
f,
Hamza
Naeem
g,
Sami
Ullah
h and
Rashid
Khan
*i
aDepartment of Physics, COMSATS University Islamabad, Lahore Campus, Lahore, Pakistan. E-mail: muhammad.habib@cuilahore.edu.pk
bHefei Innovation Research Institute, School of Integrated Circuit Science & Engineering, Beihang University, Hefei 230013, P. R. China
cInstitute of Physics, Khwaja Fareed University of Engineering & Information Technology, Rahim Yar Khan 64200, Pakistan. E-mail: yasir.haleem@kfueit.edu.pk; hiyasir@mail.ustc.edu.cn
dCentre for Lasers and Applications, Instituto de Pesquisas Energéticas e Nucleares, IPEN—CNEN, Sao Paulo 05508-000, Brazil
eCAS Key Laboratory of Ion-beam Engineering, Institute of Intelligent Machines, Hefei Institutes of Physical Science, Chinese Academy of Sciences, Hefei 230031, P. R. China
fDepartment of Physics, Government College (GC) University, Lahore 54000, Pakistan
gDepartment of Physics, Division of Science & Technology, University of Education, Lahore 54000, Pakistan
hK.A.CARE Energy Research & Innovation Centre (ERIC), King Fahd University of Petroleum & Minerals (KFUPM), Dhahran 31261, Saudi Arabia
iZhejiang Provincial Key Laboratory of Advanced Chemical Engineering Manufacture Technology, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, P. R. China. E-mail: rashid@mail.ustc.edu.cn
First published on 12th December 2023
Abstract
Layered transition metal dichalcogenides (TMDCs) have garnered immense interest in supercapacitor energy storage applications. Despite the growing reports on TMDCs in the context of electrochemical supercapacitor studies, the prevailing use of carbon-based additives often obscures their correct analysis and overshadows their intrinsic behavior. In this work, we meticulously analyzed supercapacitor characteristics of distinct TMDC materials without using carbon or any other conductive, revealing their pure intrinsic behavior, specifically focusing on highly crystalline 2H phase tantalum (Ta), tungsten (W) and zirconium (Zr)-based TMDCs, grown using the chemical vapor transport (CVT) technique. The grown materials were characterized using cutting-edge techniques like X-ray diffraction (XRD), Raman spectroscopy, and high-resolution transmission electron microscopy (HRTEM), ensuring a comprehensive perspective of the synthesized TMDCs. To delve into the electrochemical properties of the prepared electrodes, extensive analysis using cyclic voltammetry (CV), galvanostatic charge–discharge (GCD) and electrochemical impedance spectroscopy (EIS) was performed. The obtained results were further supported with density functional theory (DFT) calculations to get insights regarding the charge transfer mechanism and electronic density distribution proximate to the Fermi levels. The synergy between the experimental results and theoretical calculations significantly improved the validity of our findings, thus probing the comprehension and optimization avenues of TMDCs for superior supercapacitor performance.
1 Introduction
Layered transition metal dichalcogenides (TMDCs) have attained so much consideration due to their diverse properties, which make them valuable materials for optical, electronic, and magnetic applications.1–4 Qualitatively, TMDC crystals usually have highly pure, clearly defined crystal structures. The crystal growth parameters are critical and include growth rate, temperature, and pressure, etc. For high-quality crystals with consistent qualities, these parameters must be optimized. The electronic characteristics of these materials must be measured quantitatively in order to customize them for energy-related devices. The conductivity of TMDC crystals is largely determined by their electronic states. For electrical devices and energy storage systems to efficiently transmit charge, high charge carrier mobility is preferred. Comprehending and managing these characteristics is crucial for optimizing TMDC crystal performance in energy-related applications. TMDCs are also successfully applied as an active material in energy harvesting applications such as batteries, fuel cells and supercapacitors.5–7 Among electrochemical energy storage devices, supercapacitors have emerged with multiple intriguing characteristics of high-power density, stability, fast charging time and high capacitance values. There are abundant reports on electrochemical supercapacitor devices utilizing TMDCs as an active electrode material including their hybrids and composites with other materials.8–10For supercapacitor measurements it has become a common trend to add conductive additives to the active electrode materials. Generally, carbon black,11 conductive polymer,12 acetylene black13 and graphite14 are used as an additive material and typically added in 7 to 10% of the total weight of the active electrode material. There are dedicated reports that have analysed the role of these additives and evaluated their performance.15–17 These additives render overall improved device performance, thus increasing the stability and capacitance values manifold compared to the original value.18–20 Besides improving performance, these additives may obscure intrinsic features and factual characteristics of the active electrode materials.
In this work, a comparative analysis was performed to reveal the intrinsic supercapacitor properties of tantalum, tungsten and zirconium-based 2H phase TMDC materials without using conductive additives. Six different TMDC materials, namely TaS2, TaSe2, WS2, WSe2, ZrS2 and ZrSe2, were synthesized and tested for electrochemical property studies without using conductive additives. Although TMDCs can be synthesized with CVD, hydrothermal, microwave-assisted or solution phase techniques,21–24 we opted for the chemical vapor transport (CVT) technique due to its high purity crystalline nature yield.25 The CVT technique is highly suitable for our work as it only requires distinctive constituent materials to grow a particular TMDC compound and does not require unnecessary precursors as in the case of other techniques. Other synthesis techniques may result in materials with impurity remnants, surface functional groups or defects26,27 that make a profound contribution in determining the supercapacitor efficiency and obscure the original behaviour of the active electrode materials. This comparative analysis work may cast new insight into the fabrication and rational design of TMDC-based electrode materials.
2 Experimental
2.1 Transition metal dichalcogenide growth
For TMDC sample growth, research grade powder materials such as tantalum, tungsten, zirconium, sulphur and selenium were purchased from Sigma-Aldrich and used without applying further purification steps. For the sample preparation, stoichiometric amounts of the desired powders were mixed with 100 mg iodine as a transport agent. After thoroughly mixing, these materials were placed into a clean quartz tube. A quartz ampoule comprising 5 mm inner diameter and 25 cm length was used for growth purposes. Prior to use, ampoule tubes were thoroughly cleaned with diluted nitric acid and vacuum dried later. After putting powder materials in the clean tubes and right before sealing off, a vacuum of 10−3 torr was created in the tubes to get rid of air and to avoid oxidation of the sample during the growth process. Sealed tubes were carefully placed in a two-zone split-type heat furnace with the sample-containing side in a higher temperature zone, while the other side of the tube where crystals were to be grown was in a lower temperature zone. The growth temperatures for tantalum-based crystals (TaS2 & TaSe2) were 950 and 850 °C, for tungsten-based crystals (WS2 & WSe2) were 1030 and 930 °C and for zirconium-based crystals (ZrS2 & ZrSe2) were 950 and 850 °C for the sample and crystal growth zones, respectively. After cooling down of the furnace, the quartz ampoules were taken out and bulk form crystals were collected cautiously by breaking the ampoule. In order to get rid of any unreacted powder particles and iodine traces, the obtained materials were gently washed with acetone and isopropyl alcohol.2.2 Electrode preparation and electrochemical measurements
The grown TMDC crystals were in a bulk form and could not be utilized directly for electrochemical measurements. For this purpose, crystals were thoroughly ground for about two hours to obtain the fine powdered form. For solution preparation, 2 mg of the ground powder was used in 750 μl DI water, 250 μl isopropyl alcohol and 10 μl Nafion was also added as a binding agent and the whole solution was sonicated for four hours. From the prepared solution, 5 μl solution was poured onto a glassy carbon electrode of 3 mm diameter and used for electrochemical measurements after drying at room temperature. Electrochemical measurements were taken at room temperature in 1 M H2SO4 electrolyte solution using a standard three-electrode configuration system. For measurements, a platinum mesh-shaped electrode was used as a counter electrode and Ag/AgCl (3 M KCl) was employed as a reference electrode.2.3 Material characterizations and calculations
The crystallographic structures of the prepared transition metal dichalcogenides were studied with Panalytical X'Pert PRO MPD X-ray diffraction with Cu Kα radiation (λ = 1.54178 Å). Raman spectroscopy was performed with an XploRa ONE Raman microscope using a 532 nm laser and structural morphology was analysed with HRTEM images obtained using JEOL JEM2010. All electrochemical measurements were accomplished with a 600E potentiostat electrochemical analyser by CH Instruments. All Density Functional Theory (DFT) results were derived by employing the Vienna Ab initio Simulation Package (VASP) code.1–3 The exchange–correlation functional4 by using the General Gradient Approximation (GGA) of Perdew–Burke–Ernzerhof (PBE)5 with the Projector Augmented Wave (PAW) technique6,7 was used to generate all the DFT results while taking into account spin polarization. The six compounds, TaS2, TaSe2, WS2, WSe2, ZrS2 and ZrSe2 were formed by combining transition metals (Ta, W and Zr) and chalcogens (S and Se). The applied PAW–PBE pseudopotentials were selected according to the valence electrons (i.e., Ta & W: 5p6s5d, Zr: 4s4p5s4d and S & Se: 2s4p) and a suitable cutoff energy was set for each compound. The cutoff energy of 520 eV for the plane wave basis set, the force convergence of 0.01 eV Å−1, and the energy convergence criterion of 10−6 eV were used. The Brillouin-zone was modelled by 7 × 7 × 1 Monkhorst–Pack k-points for sufficient geometric optimization and total energy calculation. The recommended Γ-centred grid for the hexagonal phase with an appropriate k-points set and an accurate condition of forces (below 0.0001 > eV) are used for structural relaxation. As a pseudopotential method, the effective core potentials, in which some degree of relativistic corrections were introduced into the core electrons, were employed.3 Results and discussion
The optical images of the CVT synthesized crystals are shown in Fig. 1(a). It can be noticed distinctly that the synthesized crystals exhibit random shapes within the millimetre scale. X-ray diffraction patterns of the CVT grown TMDCs are shown in Fig. 1(b). It can be observed that all TMDC materials showed narrow and sharp peaks, which is evidence of high-quality crystal growth. High intensity sharp peaks, observed in the range around 13 to 16°, reveal the layered growth of our samples, which is a typical feature of transition metal dichalcogenide materials. In the XRD profiles of TaS2 and TaSe2, major peaks were found at ∼14.8 and ∼13.8° which referred to the (001) and (004) planes, respectively. The peaks at around 35 and 46.6° were well matched with the (101) and (1010) peaks of TaS2 and TaSe2, respectively. In the case of WS2 and WSe2, it can be seen that major peaks appeared at around 14.3 and 13.7°, which corresponded to the (002) peaks. Similarly, the peaks observed at ∼39.5 and ∼37.8° were ascribed as (103) peaks of both materials. Likewise, two intense peaks at around 15 and 14.4° can be stated as (001) peaks of the XRD profiles of ZrS2 and ZrSe2, respectively, while at ∼34°, the (101) peak of ZrS2 at around 31°, and (102) peak of ZrSe2 were identified. X-ray diffraction peak analysis confirms the hexagonal crystallographic structure of the CVT-synthesized TMDC samples. It was further investigated that the synthesized TaS2 and TaSe2 matched with the JCPDS card numbers 02-0137 and 21-1200, and WS2 and WSe2 with 08-0237 and 38-1388, while ZrS2 and ZrSe2 with 11-0679 and 03-065-3376, respectively.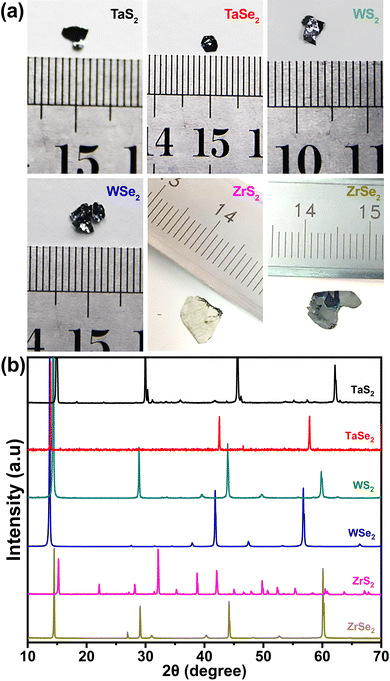 | ||
| Fig. 1 (a) Optical images and (b) X-ray diffraction patterns of the grown transition metal dichalcogenides. | ||
The structure of the synthesized materials was further analysed by means of their vibrational modes using Raman spectroscopy given in Fig. 2. The Raman spectra of TaS2 exhibited one in-plane vibrational mode E2a (or represented by E12a as in ref. 28) at ∼280 cm−1 and one out-of-plane mode A1g at ∼388 cm−1. The broader peak that appeared at around 212 cm−1 is attributed to the second order scattering. Similarly, three peaks were observed in the TaSe2 measured spectra at ∼148, 206 and 236 cm−1 which correspond to E1a, E12a and A1a modes, respectively. The measured Raman modes for TaS2 and TaSe2 are in good agreement with the reported work.29,30 In the WS2 spectra, two peaks appeared at ∼354 and ∼422 cm−1 that correspond to E12a and A1a modes, respectively, whereas one peak was observed in the WSe2 spectra at ∼245 cm−1 which matches with its standard E12a in-plane vibrational mode.31 In the case of ZrS2, the observed peaks were ascribed to Eg (in-plane) and A1g (out-of-plane) vibrational modes that appeared at ∼247.5 and ∼333.5 cm−1 respectively. Correspondingly, in the ZrSe2 Raman curve, one major peak was found at ∼193 cm−1 which was attributed to A1g and a small peak appeared at ∼144 cm−1 associated with the Eg vibrational mode. Another small peak was seen at ∼263 cm−1 that possibly appeared due to the density of the two phonon states.32 The observed peaks and corresponding values in the spectra matched well with the reported literature.33,34
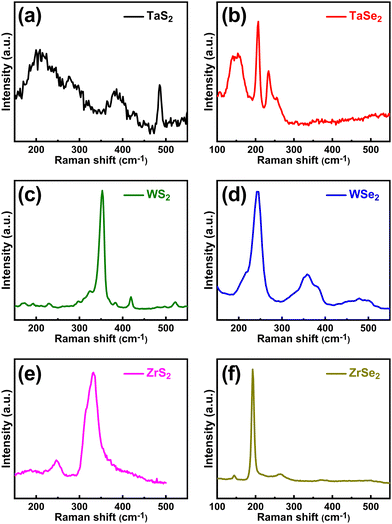 | ||
| Fig. 2 Raman spectra of the CVT grown TMDCs. (a) TaS2 and (b) TaSe2, (c) WS2 and (d) WSe2, (e) ZrS2 and (f) ZrSe2. | ||
Fig. 3 shows the high-resolution transmission electron microscopy (HRTEM) images of the synthesized TMDCs. The results (Fig. 3(a)–(f)) reveal that bulk TMDC materials were grown in multiple layer stacking. The measured layer spacing for all TMDC materials was determined with their corresponding XRD peaks found at around 13 to 16°. In more detail, the (001) and (004) planes of the TaS2 and TaSe2 XRD data were closely matched with the HRTEM measured ∼0.602 and ∼0.636 nm interlayer spacings of these materials. Similarly, the assessed layer spacings of 0.618 nm for WS2 and 0.638 nm for WSe2 corresponded to the (002) peak of their XRD data. For ZrS2 and ZrSe2, using HRTEM, the interlayer spacings appeared to be 0.661 and 0.668 nm, which can be referred to their (001) XRD peak. Correspondingly, lattice plane spacings were also marked according to their matched X-ray diffraction peaks. The appeared (101) and (1010) XRD peaks of TaS2 and TaSe2 correspond to their lattice spacings of around 0.264 and 0.191 nm, respectively. Similarly, the ∼0.231 and ∼0.228 nm plane spacings of WS2 and WSe2 matched with the (103) peak. Relatedly, the plane spacings of the ZrS2 and ZrSe2 electrode materials can be referred to their (101) and (102) peaks. Dissimilar inter-layer spacing values observed in the HRTEM images are obvious due to the different atomic radii of the various transition metals and chalcogens used in this study. The obtained HRTEM images clearly revealed the layered structures and high crystalline quality of the grown TMDC materials. The layered structure of these materials is one of the key factors to determine their supercapacitive performance. Wider layer spacing is favourable for the ions to interact and swiftly flow through these layers, which will consequently increase the specific capacitance. On the other hand, narrower layer spacing restricts the flow of ions, which results in the reduction of the performance.
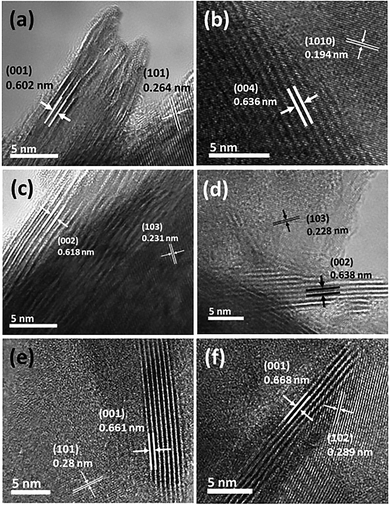 | ||
| Fig. 3 High resolution TEM images showing layer and plane spacings of the CVT grown TMDCs. (a) TaS2 and (b) TaSe2, (c) WS2 and (d) WSe2, (e) ZrS2 and (f) ZrSe2. | ||
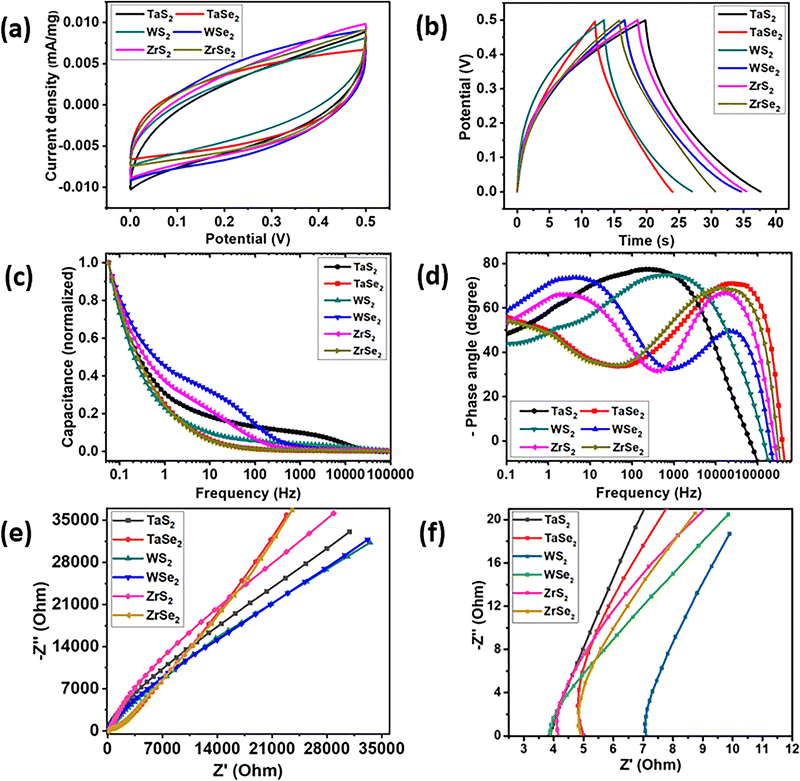
Cyclic voltammogram (CV) curves, drawn from 0 to 0.5 V potential range at 100 mV s−1 scan rate, for all six electrode materials are shown in Fig. 4(a) while Fig. 4(b) illustrates the galvanostatic charge–discharge (GCD) curves, taken at a current density of 0.3 A g−1. Specific capacitance values for the prepared electrode materials were calculated with the GCD curves by using the formula given in eqn (1).35
| C = IΔt/AΔV | (1) |
The calculated specific capacitance values for TaS2, TaSe2, WS2, WSe2, ZrS2 and ZrSe2 were 151.11, 102.6, 116.1, 152.3, 141.8 and 127.3 F cm−2, respectively. The comparison of the calculated gravimetric specific capacitances from both CV and GCD is given in Table S1 of the ESI.† All CVT grown samples were electrochemical double-layer capacitor (EDLC) type with nearly rectangular shapes and the absence of redox peaks in the CV curves.36,37 Similar non-faradaic observations were witnessed in the charge–discharge curves as well. It is most probable that faradaic reactions occur at low scan rates and current densities. Therefore, to further affirm the nature of the prepared electrode materials CV curves at the low and higher scan rates of 5, 10, 20 and 40 mV s−1 were conducted, which are given in Fig. S1 in the ESI.† Similarly charge–discharge measurements, performed at 0.2, 0.4 and 0.5 A g−1 current densities, are also provided in Fig. S2 of the ESI.† The nonappearance of redox peaks, particularly in the low scan rate CV curves, and the absence of any plateau in the GCD curves are manifestations of EDLC behaviour. These observations are reflected in the measurements due to the absence of functional groups at the surface of the electrode materials. The exhibition of double layer (EDLC) supercapacitor behaviour by transition metal dichalcogenides is already reported in the literature.21,38,39
Fig. 4(c) represents a plot where the trend of the electrode's capacitance (normalized) against a range of frequencies can be found. It can be observed that the WSe2 electrode possessed the highest capacitance value followed by ZrS2 up to around 500 Hz. On the contrary, although the TaS2 electrode encompasses relatively less capacitance, it holds it for a wide frequency range that is ∼15 kHz. Other electrodes demonstrated almost a similar trend of capacitance versus frequency range. A typical supercapacitor (SC) device features its nature at low frequencies and resistive behaviour at higher frequencies, which are measured in the form of Bode plots.40Fig. 4(d) illustrates phase angle versus frequency Bode plots of the prepared electrode that were calculated using eqn (2).41
| C = −1/2πfZ′′ | (2) |
The Nyquist plot of electrochemical impedance spectroscopy (EIS) is another tool to analyse the intrinsic characteristics of the synthesized electrode materials. EIS was performed in a frequency range of 0.01 Hz to 1 MHz with a 0.005 (system generated) AC voltage signal. The higher straight line in the low frequency region corresponds to Warburg impedance and reflects the ideal supercapacitor behavior.42,43 From the Nyquist plots of Fig. 4(e), it can be observed that the TaSe2 and ZrSe2 electrodes showed higher straight lines at low frequencies that eventually lessened while moving towards the higher frequency region. The TaS2 electrode, which showed a high phase angle at relatively higher frequencies, can also be differentiated in the EIS graphs with its high slope even after the mid frequency region. Fig. 4(f) represents the higher frequency region, which highlighted the x-axis (Z′) intercepts of the EIS curves to determine the interface resistance (Rs) of the corresponding electrode materials. It is clear from the Z′ intercept that TaS2 and WSe2 materials showed similar interface resistance values of 3.8 Ohm followed by a comparable value of 4.1 Ohm for the ZrS2. The WS2 electrode showed a relatively high interface resistance value of ∼7.1 Ohm among all electrode materials. To further reveal the conductivity and to determine the contribution of the charge transfer resistance (Rct) in our electrode materials, we have plotted individual impedance spectroscopy graphs in Fig. S3 of the ESI.† EIS plots are also supplemented with the inset circuit models constructed after EIS curve fitting. From the fitted data values, it was observed that the TaS2, WSe2 and ZrS2 electrodes showed low resistance values of around 32, 50 and 175 Ohm, respectively, as compared to 300, 238 and 530 Ohm for the TaSe2, WS2 and ZrSe2 electrodes, respectively. The interface resistance values obtained from the simulated curves are comparable to the values obtained at the high frequency region of the Nyquist plots. The EIS findings are further verified with the density of states calculations in the coming discussions.
Stability is an essential parameter of a supercapacitor which was conducted for 5000 charge–discharge cycles at 0.3 A g−1 current density for each electrode. In terms of capacitance retention, curves illustrating the stability of the individual electrode material are shown in Fig. 5(a)–(f). Some of the highest capacitance retention of about 98% was displayed by the WS2, WSe2, and TaSe2 electrodes. They were followed by Zr-based electrode overlapping curves that experienced about 8% loss in capacitance, while the TaS2 electrode showed around 19% capacitance loss. To elaborate a comparative view, combined stability curves for all electrodes are given in Fig. S4 of the ESI.† Careful analysis of the prepared materials revealed that almost all electrode materials exhibited stable supercapacitor features due to the least defects and highly crystalline nature obtained from the adopted chemical vapor transport technique. It can be interpreted that a loss in capacitance occurred for the first couple of hundred cycles and then the electrode materials possess very stable features up to the rest of the 5000 cycles. The early decrease in capacitance retention is attributed to the preliminary time taken by the system to get electrochemically stabilized. The initial capacitance reduction in the stability is due to the feeble interactions between the electrolyte and active electrode material and saturation of active sites. A similar trend of initial capacitance reduction was also observed in the previously published works.39,44,45
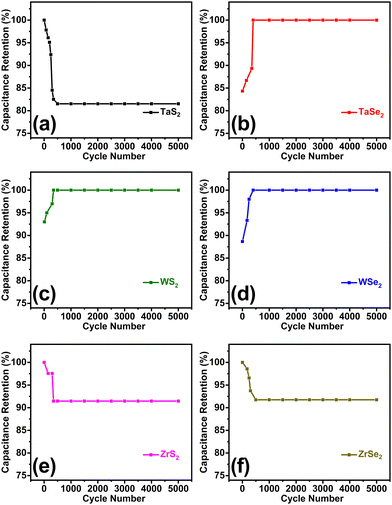 | ||
| Fig. 5 Stability curves of (a) TaS2, (b) TaSe2, (c) WS2, (d) WSe2, (e) ZrS2 and (f) ZrSe2 electrodes measured for the first 5000 cycles. | ||
Understanding how conductivity and electrochemical performance interplay is essential to comprehending material behaviour, especially in the context of devices like supercapacitors. As semiconductors, TMDCs have the capacity to contribute electrons to the conduction band and this capability has a direct impact on their conductivity. When TMDCs have high conductivity, they can readily donate or accept charges during electrochemical reactions. This ability is particularly important for the overall efficiency of the electrochemical processes occurring in the device. The electronic band structure and partial density of states (PDOS) were calculated for all the structures to see the contribution of orbitals in the conduction and valence band in the electronic structure of these materials and their conductivity related to the bandgaps is shown in Fig. 6. The electrochemical performance of these materials depends on the electronegativity of the anion and cation atoms that contributed near the Fermi level in the d-orbitals. Fig. 6(a) and (b) show the band structure and PDOS of TaS2 and TaSe2, respectively. It can be seen that for TaS2 the conduction band crossed the Fermi level, which belongs to Ta-d orbitals, while from the PDOS it is also observed that below the Fermi level the maximum density of states appeared contributed by the Ta-d orbital. The PDOS also further crossed the Fermi level and enhanced the conductivity of the TaS2. Whereas, for TaSe2 (Fig. 6(b)) the conduction band also crossed the Fermi level, however, the uneven distribution of PDOS is less deep in the conduction band than TaS2 (see Fig. 6(a)). This could be the reason for the better electrochemical properties of TaS2. Fig. 6(c) and (d) show the band structure and PDOS of WS2 and WSe2. Compared to WS2 (see Fig. 6(c)), the WSe2 has a lower energy gap (see Fig. 6(d)); therefore, WSe2 has better conductivity. Meanwhile, the PDOS distributions in the valence bands are closer to the Fermi level, with strong hybridization of the W-d orbitals near the Fermi level. Therefore, due to the smaller band gap energy and strong hybridization of the W-d orbitals adjacent to the Fermi level, the transfer of charge is easier in the case of WSe2, which causes better electrochemical performance. Similarly, Fig. 6(e) and (f) shows the band structure and PDOS of ZrS2 and ZrSe2, respectively. From the band structure of ZrSe2, it is shown that the valence band is closer to the Fermi level for ZrS2 as compared to ZrSe2, which reveals that the transfer of charge is much stronger for ZrS2. At the same time, it can also be seen that the PDOS near the Fermi level in ZrS2 is much higher as compared to ZrSe2, which makes ZrS2 a better conductive material than ZrSe2, having better performance for electrochemical properties. From these results, it is determined that the energy gap and carrier concentration near the Fermi level have a major role in the electronic conductivity of these TMDCs, which enhances the electrochemical performance of these materials due to the charge transfer mechanism.
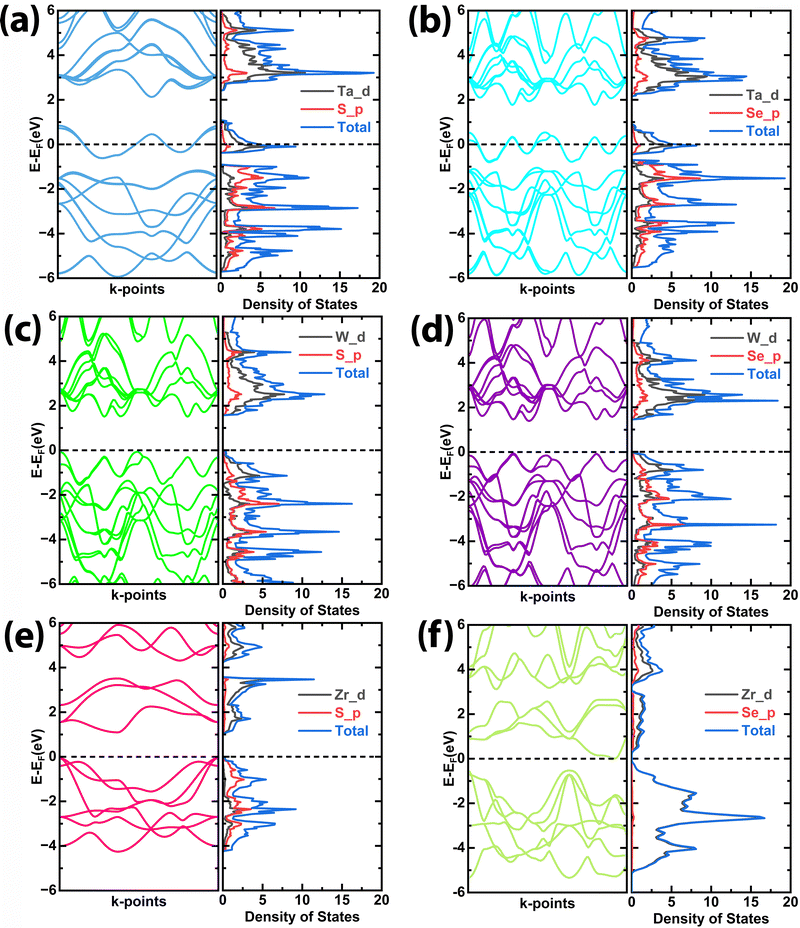 | ||
| Fig. 6 Calculated band structure and partial density of states (PDOS) of (a) TaS2, (b) TaSe2, (c) WS2, (d) WSe2, (e) ZrS2, and (f) ZrSe2, respectively. | ||
The materials with higher carrier concentration near the Fermi level are more appropriate to donate electrons or holes to the conduction band, which increases their conductivity and hence increases the electrochemical performance. Our calculation results are in agreement with the experimentally obtained EIS results and the predicted higher (comparative) conductivities of the TaS2, WSe2 and ZrS2 are verified with the charge transfer resistance value trend found from the EIS results. It is further mentioned that the performance of any electrode material is markedly influenced by factors like conductivity,46 surface area,47 electrolyte-type,48,49 defects50 and surface functional groups.51 In addition, we have made a comparison between our results and the previously published literature that focuses primarily on 2D materials; this comparison is shown in Table 1.
| S. no. | Material | Binder | Additive | No. of cycles | Capacitance retention (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | TaS2 | Nafion | None | 5000 | 81 | This work |
| 2 | TaSe2 | Nafion | None | 5000 | 98 | This work |
| 3 | WS2 | Nafion | None | 5000 | 98 | This work |
| 4 | WSe2 | Nafion | None | 5000 | 98 | This work |
| 5 | ZrS2 | Nafion | None | 5000 | 92 | This work |
| 6 | ZrSe2 | Nafion | None | 5000 | 92 | This work |
| 7 | Ti2C | PTFE | Conductive carbon | 6000 | 93 | Rakhi et al.52 |
| 8 | Ti3C2 | PVDF | Acetylene black | 6000 | 88 | Cao et al.53 |
| 9 | Nb2C | PVDF | Acetylene black | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
81 | Ayesha et al.54 |
| 10 | V2C | PTFE | Carbon black | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
90 | Yunfeng et al.55 |
| 11 | V2N | PVDF | Carbon black | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
96 | Sandhya et al.56 |
| 12 | MoS2 | PVDF | Carbon black | 1000 | 70 | Gupta et al.57 |
| 13 | MnO2 (nanoflake) | PVDF | Carbon black | 1000 | 50 | Sagar et al.58 |
| 14 | RGO@Cuf | None | None | 5000 | 92 | Taniya et al.59 |
The comparison clearly shows that the intrinsic performance of TMDC electrodes without additives approaches previously published studies on 2D materials.52–59
In the presented work, we kept the same electrolyte for the entire electrochemical measurements to get clear insights into the material's intrinsic behaviour and preferred the CVT technique that yields high quality crystalline materials with the least defect density. From CV and GCD observations, it is also concluded that no functional groups were present at the surface of the electrode materials, so the exhibited electrochemical behaviour of our TMDC electrode materials can be decisively regarded as due to their intrinsic conductivity and layer spacings. In our synthesized electrode materials, the highest capacitance of TaS2 can be dominantly attributed to its high conductivity, as substantiated with EIS and PDOS results, followed by ZrS2, which although has slightly large interfacial resistance, on the other hand also possesses comparatively high inter-layer spacing. On the contrary, though the WSe2 electrode keeps a small interface resistance value, it has moderate layer spacing which places it after TaS2 and ZrS2.
4 Conclusions
In summary, we have synthesized highly crystalline Ta-, W- and Zr-based 2H phase TMDCs using the chemical vapor transport technique and utilized them as an electrode material for electrochemical supercapacitors. The calculated specific capacitance values for the TaS2, TaSe2, WS2, WSe2, ZrS2 and ZrSe2 electrodes were 151.11, 102.6, 116.1, 152.3, 141.8 and 127.3 F cm−2 respectively. No functional groups were present on the surface of the electrode materials, and their capacitance values depend on the layer spacings and intrinsic conductivity. In the EIS results, it was observed that the WSe2 electrode possessed the highest capacitance value followed by ZrS2 up to around 500 Hz. On the contrary, the TaS2 electrode encompasses comparable capacitance but holds it for a wide frequency range that is ∼15 kHz. The high capacitance value of the TaS2 electrode was due to its high conductivity as revealed in the EIS analysis, which was further confirmed by the DFT calculations. Moreover, the supercapacitor behaviour was tested without using any type of conductive additive to confirm the intrinsic behaviour of the prepared crystalline TMDC electrode material. This study provides insights into the role of high inter-layer spacing and intrinsic conductivity of the TMDC electrode materials in the field of electrochemical energy storage devices.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank our collaborators, the Prof. Song Li group from University of Science and Technology of China, for their support and facilitation of the work. This research was supported by the National Natural Science Foundation of China (Grant No. 62150410438).References
- H. Duan, G. Li, H. Tan, C. Wang, Q. Li, C. Liu, Y. Yin, X. Li, Z. Qi and W. Yan, Sulfur-vacancy-tunable interlayer magnetic coupling in centimeter-scale MoS2 bilayer, Nano Res., 2022, 15, 881–888 CrossRef CAS.
- M. Habib, Z. Muhammad, R. Khan, C. Wu, Z. Ur Rehman, Y. Zhou, H. Liu and L. Song, Ferromagnetism in CVT grown tungsten diselenide single crystals with nickel doping, Nanotechnology, 2018, 29(11), 115701 CrossRef PubMed.
- S. Li, J. Hong, B. Gao, Y. C. Lin, H. E. Lim, X. Lu, J. Wu, S. Liu, Y. Tateyama and Y. Sakuma, Tunable Doping of Rhenium and Vanadium into Transition Metal Dichalcogenides for Two-Dimensional Electronics, Adv. Sci., 2021, 8(11), 2004438 CrossRef CAS PubMed.
- K. M. McCreary, M. Phillips, H.-J. Chuang, D. Wickramaratne, M. Rosenberger, C. S. Hellberg and B. T. Jonker, Stacking-dependent optical properties in bilayer WSe 2, Nanoscale, 2022, 14(1), 147–156 RSC.
- W. S. V. Lee, T. Xiong, X. Wang and J. Xue, Unraveling MoS2 and transition metal dichalcogenides as functional zinc-ion battery cathode: A perspective, Small Methods, 2021, 5(1), 2000815 CrossRef CAS PubMed.
- B. Li, Y. He, M. Xiao, Y. Zhang, Z. Wang, Z. Qin, B. Chai, J. Yan, J. Li and J. Li, A solar-light driven photocatalytic fuel cell for efficient electricity generation and organic wastewater degradation, Colloids Surf., A, 2022, 642, 128205 CrossRef CAS.
- D. Vikraman, S. Hussain, K. Karuppasamy, A. Kathalingam, E.-B. Jo, A. Sanmugam, J. Jung and H.-S. Kim, Engineering the active sites tuned MoS2 nanoarray structures by transition metal doping for hydrogen evolution and supercapacitor applications, J. Alloys Compd., 2022, 893, 162271 CrossRef CAS.
- S. Dutta and S. De, MoS2 nanosheet/rGO hybrid: an electrode material for high performance thin film supercapacitor, Mater. Today: Proc., 2018, 5(3), 9771–9775 CAS.
- G. Sun, J. Liu, X. Zhang, X. Wang, H. Li, Y. Yu, W. Huang, H. Zhang and P. Chen, Fabrication of ultralong hybrid microfibers from nanosheets of reduced graphene oxide and transition-metal dichalcogenides and their application as supercapacitors, Angew. Chem., Int. Ed., 2014, 53(46), 12576–12580 CrossRef CAS PubMed.
- S. Tanwar, N. Singh and A. Sharma, Structural and electrochemical performance of carbon coated molybdenum selenide nanocomposite for supercapacitor applications, J. Energy Storage, 2022, 45, 103797 CrossRef.
- D. Kesavan, V. K. Mariappan, P. Pazhamalai, K. Krishnamoorthy and S.-J. Kim, Topochemically synthesized MoS2 nanosheets: a high performance electrode for wide-temperature tolerant aqueous supercapacitors, J. Colloid Interface Sci., 2021, 584, 714–722 CrossRef CAS PubMed.
- A. Chowdhury, S. Biswas, D. Mandal and A. Chandra, Facile strategy of using conductive additive supported NaMnPO4 nanoparticles for delivering high performance Na-ion supercapacitors, J. Alloys Compd., 2022, 902, 163733 CrossRef CAS.
- S. Liu, C. Mao, Y. Niu, F. Yi, J. Hou, S. Lu, J. Jiang, M. Xu and C. Li, Facile synthesis of novel networked ultralong cobalt sulfide nanotubes and its application in supercapacitors, ACS Appl. Mater. Interfaces, 2015, 7(46), 25568–25573 CrossRef CAS PubMed.
- Z.-Y. Zhao, W.-B. Zhang, X.-J. Ma, K. Li, Y. Zhao, J.-F. Gao, L. Kang and L.-B. Kong, A novel capacitive negative electrode material of Fe3N, Nano, 2018, 13(01), 1850002 CrossRef CAS.
- M. Canal-Rodríguez, J. A. Menéndez, M. A. Montes-Morán, I. Martín-Gullón, J. B. Parra and A. Arenillas, The role of conductive additives on the performance of hybrid carbon xerogels as electrodes in aqueous supercapacitors, Electrochim. Acta, 2019, 295, 693–702 CrossRef.
- N. Jäckel, D. Weingarth, A. Schreiber, B. Krüner, M. Zeiger, A. Tolosa, M. Aslan and V. Presser, Performance evaluation of conductive additives for activated carbon supercapacitors in organic electrolyte, Electrochim. Acta, 2016, 191, 284–298 CrossRef.
- R. Na, N. Lu, L. Li, Y. Liu, J. Luan, G. Wang and A. Robust, Conductive Polymer Network as a Multi-Functional Binder and Conductive Additive for Supercapacitors, ChemElectroChem, 2020, 7(14), 3056–3064 CrossRef CAS.
- N. Guo, Y. Lin, Y. Cui, S. Su, H. Dai, J. Yang and X. Zhu, Effect of MWCNTs additive on preservation stability of rGO powder, J. Mater. Sci.: Mater. Electron., 2022, 33(9), 6766–6779 CrossRef CAS.
- Y. Niu, J. Wang, J. Zhang and Z. Shi, Graphene quantum dots as a novel conductive additive to improve the capacitive performance for supercapacitors, J. Electroanal. Chem., 2018, 828, 1–10 CrossRef CAS.
- J.-W. Wang, W.-K. Zhang, C. Jiao, F.-Y. Su, C.-M. Chen, C.-L. Guo, H. T. Chua and S. Eahon, Activated carbon based supercapacitors with a reduced graphene oxide additive: preparation and properties, J. Nanosci. Nanotechnol., 2020, 20(7), 4073–4083 CrossRef CAS PubMed.
- Z. Muhammad, Y. Wang, Y. Zhang, P. Vallobra, S. Peng, S. Yu, Z. Lv, H. Cheng and W. Zhao, Radiation-Tolerant Electronic Devices Using Wide Bandgap Semiconductors, Adv. Mater. Technol., 2023, 8(2), 2200539 CrossRef CAS.
- A. Khalil, Q. Liu, Q. He, T. Xiang, D. Liu, C. Wang, Q. Fang and L. Song, Metallic 1T-WS2 nanoribbons as highly conductive electrodes for supercapacitors, RSC Adv., 2016, 6(54), 48788–48791 RSC.
- Z. Liu, N. Li, C. Su, H. Zhao, L. Xu, Z. Yin, J. Li and Y. Du, Colloidal synthesis of 1T′ phase dominated WS2 towards endurable electrocatalysis, Nano Energy, 2018, 50, 176–181 CrossRef CAS.
- J. You, M. D. Hossain and Z. Luo, Synthesis of 2D transition metal dichalcogenides by chemical vapor deposition with controlled layer number and morphology, Nano Convergence, 2018, 5, 1–13 CrossRef PubMed.
- K. Ueno, Introduction to the growth of bulk single crystals of two-dimensional transition-metal dichalcogenides, J. Phys. Soc. Jpn., 2015, 84(12), 121015 CrossRef.
- Z. Lin, B. R. Carvalho, E. Kahn, R. Lv, R. Rao, H. Terrones, M. A. Pimenta and M. Terrones, Defect engineering of two-dimensional transition metal dichalcogenides, 2D Mater., 2016, 3(2), 022002 CrossRef.
- Y. Sun, M. Terrones and R. E. Schaak, Colloidal nanostructures of transition-metal dichalcogenides, Acc. Chem. Res., 2021, 54(6), 1517–1527 CrossRef CAS PubMed.
- W. McMullan and J. Irwin, Long-wavelength phonons in 2H-NbS2, 2H-TaS2, and AgxTaS2, Can. J. Phys., 1984, 62(8), 789–795 CrossRef CAS.
- M. Hangyo, S.-I. Nakashima and A. Mitsuishi, Raman spectroscopic studies of MX2-type layered compounds, Ferroelectrics, 1983, 52(1), 151–159 CrossRef CAS.
- X. Sun, Optoelectronic Properties and Raman Spectra of Layered TaWSe2 Ternary Alloys, MSc (EE), Vanderbilt University, 2021 Search PubMed.
- X. Zhang, X.-F. Qiao, W. Shi, J.-B. Wu, D.-S. Jiang and P.-H. Tan, Phonon and Raman scattering of two-dimensional transition metal dichalcogenides from monolayer, multilayer to bulk material, Chem. Soc. Rev., 2015, 44(9), 2757–2785 RSC.
- S. Mañas-Valero, V. García-López, A. Cantarero and M. Galbiati, Raman spectra of ZrS2 and ZrSe2 from bulk to atomically thin layers, Appl. Sci., 2016, 6(9), 264 CrossRef.
- L. Roubi and C. Carlone, Resonance Raman spectrum of HfS2 and ZrS2, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37(12), 6808 CrossRef CAS PubMed.
- X. Zhang, Q.-H. Tan, J.-B. Wu, W. Shi and P.-H. Tan, Review on the Raman spectroscopy of different types of layered materials, Nanoscale, 2016, 8(12), 6435–6450 RSC.
- M. Zhang, F. Heraly, M. Yi and J. Yuan, Multitasking tartaric-acid-enabled, highly conductive, and stable MXene/conducting polymer composite for ultrafast supercapacitor, Cell Rep. Phys. Sci., 2021, 2(6), 100449 CrossRef CAS.
- P. Sharma and T. Bhatti, A review on electrochemical double-layer capacitors, Energy Convers. Manage., 2010, 51(12), 2901–2912 CrossRef CAS.
- M. Winter and R. J. Brodd, What are batteries, fuel cells, and supercapacitors?, Chem. Rev., 2004, 104(10), 4245–4270 CrossRef CAS PubMed.
- M. Habib, A. Khalil, Z. Muhammad, R. Khan, C. Wang, Z. Ur Rehman, H. T. Masood, W. Xu, H. Liu and W. Gan, WX2 (X = S, Se) single crystals: a highly stable material for supercapacitor applications, Electrochim. Acta, 2017, 258, 71–79 CrossRef CAS.
- H. Li, H. Li, Z. Wu, L. Zhu, Y. Huang, X. Zhu and Y. Sun, Phase engineering of Mo1−xWxS2 nanosheets for flexible supercapacitors, Scr. Mater., 2022, 208, 114346 CrossRef CAS.
- P. A. Basnayaka, M. K. Ram, L. Stefanakos and A. Kumar, Graphene/polypyrrole nanocomposite as electrochemical supercapacitor electrode: electrochemical impedance studies, Graphene, 2013, 2(2), 81–87 CrossRef CAS.
- C. Wang, D. Liu, S. Chen, Y. Sang, Y. A. Haleem, C. Wu, W. Xu, Q. Fang, M. Habib and J. Cao, All-carbon ultrafast supercapacitor by integrating multidimensional nanocarbons, Small, 2016, 12(41), 5684–5691 CrossRef CAS PubMed.
- R. Khan, M. Habib, M. A. Gondal, A. Khalil, Z. U. Rehman, Z. Muhammad, Y. A. Haleem, C. Wang, C. Q. Wu and L. Song, Facile synthesis of CuFe2O4–Fe2O3 composite for high-performance supercapacitor electrode applications, Mater. Res. Express, 2017, 4(10), 105501 CrossRef.
- P. Taberna, P. Simon and J.-F. Fauvarque, Electrochemical characteristics and impedance spectroscopy studies of carbon-carbon supercapacitors, J. Electrochem. Soc., 2003, 150(3), A292 CrossRef CAS.
- L. Cao, S. Yang, W. Gao, Z. Liu, Y. Gong, L. Ma, G. Shi, S. Lei, Y. Zhang and S. Zhang, Direct laser-patterned micro-supercapacitors from paintable MoS2 films, Small, 2013, 9(17), 2905–2910 CrossRef CAS PubMed.
- A. Winchester, S. Ghosh, S. Feng, A. L. Elias, T. Mallouk, M. Terrones and S. Talapatra, Electrochemical characterization of liquid phase exfoliated two-dimensional layers of molybdenum disulfide, ACS Appl. Mater. Interfaces, 2014, 6(3), 2125–2130 CrossRef CAS PubMed.
- P. Zhu, Y. Wang, M. Zhang, Y. Wu and W. Cai, Anions influence on the electrochemical performance of Co3X4 (X = O, Se) for supercapacitor: Experiments and theoretical calculations, Appl. Surf. Sci., 2022, 574, 151646 CrossRef CAS.
- L. Zhang, F. Zhang, X. Yang, G. Long, Y. Wu, T. Zhang, K. Leng, Y. Huang, Y. Ma and A. Yu, Porous 3D graphene-based bulk materials with exceptional high surface area and excellent conductivity for supercapacitors, Sci. Rep., 2013, 3(1), 1408 CrossRef PubMed.
- M. Acerce, D. Voiry and M. Chhowalla, Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials, Nat. Nanotechnol., 2015, 10(4), 313–318 CrossRef CAS PubMed.
- K. O. Oyedotun, T. M. Masikhwa, S. Lindberg, A. Matic, P. Johansson and N. Manyala, Comparison of ionic liquid electrolyte to aqueous electrolytes on carbon nanofibres supercapacitor electrode derived from oxygen-functionalized graphene, Chem. Eng. J., 2019, 375, 121906 CrossRef CAS.
- F. Zhang, T.-Y. Eom, M. Cho, H.-J. Lee and H. Pang, Fabrication of defect-rich bifunctional hollow NiTe2 nanotubes for high performance hydrogen evolution electrocatalysts and supercapacitors, J. Energy Storage, 2021, 42, 103098 CrossRef.
- X. Han, Z.-H. Huang, F. Meng, B. Jia and T. Ma, Redox-etching induced porous carbon cloth with pseudocapacitive oxygenic groups for flexible symmetric supercapacitor, J. Energy Chem., 2022, 64, 136–143 CrossRef CAS.
- R. B. Rakhi, B. Ahmed, M. N. Hedhili, D. H. Anjum and H. N. Alshareef, Effect of postetch annealing gas composition on the structural and electrochemical properties of Ti2CTx MXene electrodes for supercapacitor applications, Chem. Mater., 2015, 27(15), 5314–5323 CrossRef CAS.
- M. Cao, F. Wang, L. Wang, W. Wu, W. Lv and J. Zhu, Room temperature oxidation of Ti3C2 MXene for supercapacitor electrodes, J. Electrochem. Soc., 2017, 164(14), A3933 CrossRef CAS.
- A. Zaheer, S. A. Zahra, M. Z. Iqbal, A. Mahmood, S. A. Khan and S. Rizwan, Nickel-adsorbed two-dimensional Nb2C MXene for enhanced energy storage applications, RSC Adv., 2022, 12(8), 4624–4634 RSC.
- Y. Guan, S. Jiang, Y. Cong, J. Wang, Z. Dong and Q. Zhang, et al., A hydrofluoric acid-free synthesis of 2D vanadium carbide (V2C) MXene for supercapacitor electrodes, 2D Mater., 2020, 7(2), 025010 CrossRef CAS.
- S. Venkateshalu, J. Cherusseri, M. Karnan, K. S. Kumar, P. Kollu and M. Sathish, et al., New method for the synthesis of 2D vanadium nitride (MXene) and its application as a supercapacitor electrode, ACS Omega, 2020, 5(29), 17983–17992 CrossRef CAS PubMed.
- H. Gupta, S. Chakrabarti, S. Mothkuri, B. Padya, T. Rao and P. Jain, High performance supercapacitor based on 2D-MoS2 nanostructures, Mater. Today: Proc., 2020, 26, 20–24 CAS.
- S. Mothkuri, S. Chakrabarti, H. Gupta, B. Padya, T. Rao and P. Jain, Synthesis of MnO2 nano-flakes for high performance supercapacitor application, Mater. Today: Proc., 2020, 26, 142–147 CAS.
- T. Purkait, G. Singh, D. Kumar, M. Singh and R. S. Dey, High-performance flexible supercapacitors based on electrochemically tailored three-dimensional reduced graphene oxide networks, Sci. Rep., 2018, 8(1), 640 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ma00672g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |