Open Access Article
Open Access ArticleA micro-scale humanized ventilator-on-a-chip to examine the injurious effects of mechanical ventilation†
Basia
Gabela-Zuniga
ab,
Vasudha C.
Shukla
ab,
Christopher
Bobba
ab,
Natalia
Higuita-Castro
abc,
Heather M.
Powell
ade,
Joshua A.
Englert‡
 *bf and
Samir N.
Ghadiali‡
*bf and
Samir N.
Ghadiali‡
 *abf
*abf
aDepartment of Biomedical Engineering, The Ohio State University, Columbus, Ohio, USA. E-mail: ghadiali.1@osu.edu
bThe Dorothy M. Davis Heart and Lung Research Institute, The Ohio State Wexner Medical Center, Columbus, Ohio, USA
cDepartment of Neurosurgery, The Ohio State University Wexner Medical Center, Columbus, Ohio, USA
dDepartment of Materials Science and Engineering, The Ohio State University, Columbus, Ohio, USA
eScientific Staff, Shriners Children's Ohio, Dayton, Ohio, USA
fDivision of Pulmonary, Critical Care, and Sleep Medicine, Department of Internal Medicine, The Ohio State University Wexner Medical Center, Columbus, Ohio, USA
First published on 7th August 2024
Abstract
Patients with compromised respiratory function frequently require mechanical ventilation to survive. Unfortunately, non-uniform ventilation of injured lungs generates complex mechanical forces that lead to ventilator induced lung injury (VILI). Although investigators have developed lung-on-a-chip systems to simulate normal respiration, modeling the complex mechanics of VILI as well as the subsequent recovery phase is a challenge. Here we present a novel humanized in vitro ventilator-on-a-chip (VOC) model of the lung microenvironment that simulates the different types of injurious forces generated in the lung during mechanical ventilation. We used transepithelial/endothelial electrical impedance measurements to investigate how individual and simultaneous application of mechanical forces alters real-time changes in barrier integrity during and after injury. We find that compressive stress (i.e. barotrauma) does not significantly alter barrier integrity while over-distention (20% cyclic radial strain, volutrauma) results in decreased barrier integrity that quickly recovers upon removal of mechanical stress. Conversely, surface tension forces generated during airway reopening (atelectrauma), result in a rapid loss of barrier integrity with a delayed recovery relative to volutrauma. Simultaneous application of cyclic stretching (volutrauma) and airway reopening (atelectrauma), indicates that the surface tension forces associated with reopening fluid-occluded lung regions are the primary driver of barrier disruption. Thus, our novel VOC system can monitor the effects of different types of injurious forces on barrier disruption and recovery in real-time and can be used to interogate the biomechanical mechanisms of VILI.
Introduction
Mechanical ventilation (MV) is a critical form of life support for patients with respiratory failure and patients that require general anesthesia for surgical procedures.1 Patients with compromised respiratory function require MV to prevent hypoxemia, maintain acid–base balance, and alleviate the increased work of breathing associated with a pulmonary or systemic injury.2 While lifesaving, positive pressure MV can exacerbate existing lung injuries and lead to ventilator induced lung injury (VILI).3 VILI arises from three primary biomechanical forces including alveolar over-distention (volutrauma), high transpulmonary pressure (barotrauma), and cyclic collapse/reopening of lung units (atelectrauma).4 Lung protective strategies that utilize low tidal volumes and modulate the positive end expiratory pressure (PEEP) have been employed to minimize VILI. However, patients requiring MV often have heterogeneous lung injury, and non-uniform ventilation makes it difficult to eliminate the biomechanical forces that cause VILI.5,6 Although animal models have been used to simulate VILI, these in vivo models are limited because they do not allow investigators to control and/or determine the relative importance of different biomechanical forces during VILI, cannot be used to monitor barrier function in real-time, and do not use human cells. Therefore, the development of novel in vitro systems/devices that can be used to independently investigate the effect of the biomechanical forces that cause VILI, utilize primary human cells, and account for the clinical course of disease are needed.Historically, in vitro models of VILI have utilized monoculture systems with a single cell type that allow for compressive stress7,8 (barotrauma) or injurious strain via a flexible membrane9,10 (volutrauma). Investigators have also modeled the surface tension and fluid shear stress (atelectrauma) that occur during airway/alveolar reopening by propagating an air–liquid interface over an epithelium cultured on a static, rigid membrane.10–12 While these models provide valuable insight into injury patterns during MV, they have significant limitations including the lack of a fibrous extracellular matrix that mimics the basement membrane, lack of epithelial–endothelial interactions, and an inability to monitor barrier integrity in real-time. Furthermore, previous systems could not model multiple forms of VILI simultaneously and cannot monitor repair and recovery following injury.
Over the last several years investigators have developed microfluidic lung-on-a-chip (LOC) systems that recapitulate the alveolar microenvironment. Many of these models use co-cultures of transformed or neoplastic epithelial and endothelial cells and cyclically stretch a non-fibrous, homogenous, flexible membrane to mimic physiologic breathing.9,13 These systems have been used to study lung cancer,14,15 chronic obstructive pulmonary disease16 and asthma.17 Ongoing advancements in micromachining technology have allowed investigators to use techniques like electrospinning to create nanofiber meshes that reflect the fibrous architecture of the native lung basement membrane.18–20 In parallel, other investigators have developed models of the alveolar microenvironment that mimic the alveolar geometry.21,22 Although these advanced LOC models can simulate the physiologic structure and environment of the alveolus, none of them have been used to model the injurious physical forces that occur during mechanical ventilation. In addition, it is well known that tissue repair is a critical component of recovery following lung injury. However, previous LOC systems do not allow for investigation of how mechanical forces alter the degree of repair and/or the long-term recovery of barrier integrity. Therefore, the goal of this study was to develop a microfluidic ventilator-on-a-chip (VOC) model of the lung microenvironment that uses primary human cells, recapitulates the topography alveolar basement membrane, allows for the independent and concurrent simulation of injurious physical forces that occur during mechanical ventilation, and enables monitoring of barrier recovery during and following injury.
Methods
Fabrication & assembly of the ventilator-on-a-chip
The device consists of two identical molds made of polydimethylsiloxane (PDMS; Sylgard 184, Dow Corning, Midland, MI) formed with a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 base to curing agent ratio. As shown in Fig. 1A–C, the design features two concentric chambers separated by a 1 mm thick wall. The outer compartment is 5 mm wide and serves as the vacuum chamber which contains a single 18-gauge port that enables stretching of an electrospun membrane via negative pressure. The inner chamber, where cells are cultured, is 14 mm in diameter (Fig. 1C) and features two 18-gauge ports placed 2–3 mm from the chamber wall on opposite sides. The depth of both chambers, including the 1 mm thick inner chamber wall, is 4 mm unless stated otherwise. A thin ring of PDMS was applied to the top surface of the inner chamber wall to ensure a full seal between the apical and basolateral chambers (red, Fig. 1D). Platinum electrodes were embedded into the device at the ceiling of both apical and basolateral chambers in a four-electrode configuration (Fig. 1E and F). Wires were inserted through the top of each PDMS mold, bent at a right angle and secured to the top of their respective PDMS mold. Electrodes were arranged approximately 4 mm a part and equidistance from the ports. Fig. 1F demonstrates that the working electrode and the counter electrode were placed on the apical and basolateral chambers, respectively. Two reference electrodes were also used and placed according to manufacturer's recommendation with the sensing electrode on the apical chamber and the reference electrode on the basolateral chamber. During assembly, both the apical and basolateral molds were oxygen plasma treated and the electrospun membrane (see below) was attached to one half of the device. Both halves were then sealed together and allowed to cure, with additional PDMS as described above, undisturbed for at least 2 hours before sterilization. Devices were sterilized by exposure to ultraviolet light for 10 minutes on each side. Devices were then flooded with 70% ethanol and left overnight. Prior to cell seeding, ethanol was removed, and devices were flooded with sterile PBS to ensure full hydration of the membrane overnight. For a subset of experiments simulating atelectrauma or combined volutrauma/atelectrauma, both sides of the electrospun membrane were collagen coated with type 1 bovine collagen (Advanced Biomatrix; Carlsbad, CA) overnight at 60 μg mL−1.
1 base to curing agent ratio. As shown in Fig. 1A–C, the design features two concentric chambers separated by a 1 mm thick wall. The outer compartment is 5 mm wide and serves as the vacuum chamber which contains a single 18-gauge port that enables stretching of an electrospun membrane via negative pressure. The inner chamber, where cells are cultured, is 14 mm in diameter (Fig. 1C) and features two 18-gauge ports placed 2–3 mm from the chamber wall on opposite sides. The depth of both chambers, including the 1 mm thick inner chamber wall, is 4 mm unless stated otherwise. A thin ring of PDMS was applied to the top surface of the inner chamber wall to ensure a full seal between the apical and basolateral chambers (red, Fig. 1D). Platinum electrodes were embedded into the device at the ceiling of both apical and basolateral chambers in a four-electrode configuration (Fig. 1E and F). Wires were inserted through the top of each PDMS mold, bent at a right angle and secured to the top of their respective PDMS mold. Electrodes were arranged approximately 4 mm a part and equidistance from the ports. Fig. 1F demonstrates that the working electrode and the counter electrode were placed on the apical and basolateral chambers, respectively. Two reference electrodes were also used and placed according to manufacturer's recommendation with the sensing electrode on the apical chamber and the reference electrode on the basolateral chamber. During assembly, both the apical and basolateral molds were oxygen plasma treated and the electrospun membrane (see below) was attached to one half of the device. Both halves were then sealed together and allowed to cure, with additional PDMS as described above, undisturbed for at least 2 hours before sterilization. Devices were sterilized by exposure to ultraviolet light for 10 minutes on each side. Devices were then flooded with 70% ethanol and left overnight. Prior to cell seeding, ethanol was removed, and devices were flooded with sterile PBS to ensure full hydration of the membrane overnight. For a subset of experiments simulating atelectrauma or combined volutrauma/atelectrauma, both sides of the electrospun membrane were collagen coated with type 1 bovine collagen (Advanced Biomatrix; Carlsbad, CA) overnight at 60 μg mL−1.
Electrospinning
A 5 weight/volume% polyurethane (PU; ChronoFlex C; AdvanSource; Wilmington, MA) solution was made by dissolving PU pellets into hexafluoroisopropanol (HFP, Sigma-Aldrich; St. Louis, MO). A 10 mL syringe filled with the polymer solution and an 18-gauge flat tipped stainless-steel needle was placed in a syringe pump. The tip of the needle was 20 cm from the collector covered with aluminum foil (3′′ × 3′′), which was connected to a high voltage generator while the needle tip was grounded. When 20 kV of positive voltage was applied, the polymer solution formed a Taylor cone that produced randomly aligned fibers (Fig. 2A). The syringe pump was programmed with a flow rate of 2.0 mL h−1 and scaffolds were spun for 24 minutes. Temperature and relative humidity were kept constant at 65–70 °F and 20–25%, respectively.Transepithelial/endothelial electrical impedance measurements
A Vertex.One.EIS potentiostat (Ivium Technologies, the Netherlands) was used to record impedance spectra. Four platinum electrodes were used to obtain four-point impedance measurements by applying a 1 Hz, 10 μA AC current to the current leads and measuring voltage in real-time via the voltage leads. Impedance was calculated by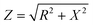 where Z is the magnitude of the impedance in ohms, R is the real part and X is the imaginary part of the complex valued impedance measurement. A majority of the impedance data is presented as a percent change of impedance (% ΔZ), where values obtained at each time point were normalized to the 0 h timepoint. Note that absolute Z values and the corresponding percent change for volutrauma, barotrauma, atelectrauma and combined injury models can be found in Fig. S2–S4.†
where Z is the magnitude of the impedance in ohms, R is the real part and X is the imaginary part of the complex valued impedance measurement. A majority of the impedance data is presented as a percent change of impedance (% ΔZ), where values obtained at each time point were normalized to the 0 h timepoint. Note that absolute Z values and the corresponding percent change for volutrauma, barotrauma, atelectrauma and combined injury models can be found in Fig. S2–S4.†
Since impedance measured at high frequency (≥10 kHz) is theoretically governed by solution resistance while impedance at low frequency (<100 Hz) is dominated by the resistance across the barrier,23 we assumed that our 1 Hz impedance measurements are representative of commonly reported transepithelial/endothelial electrical resistance (TEER). To validate this assumption, we obtained impedance vs. frequency data for a small set of devices not exposed to VILI forces. We then used a nonlinear regression algorithm to fit this data to a previously published model. Based on work by Henry et al.,23 this model consists of a solution resistor (Rsol) in series with a TEER resistor (RTEER) and a constant phase element (CPE) that is in parallel with the TEER resistor (see inset Fig. 3E). The mathematical expression of the CPE impedance is:
 | (1) |
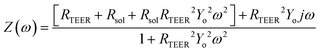 | (2) |
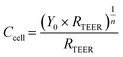 | (3) |
Membrane characterization
Morphology of the electrospun fibers was examined using scanning electron microscopy (SEM). The SEM was operated at an accelerating voltage of 2 kV and current of 50 pA. The 2D pore size and fiber diameter were quantified in ImageJ using three samples (n = 1 per sample) and 30–40 measurements per image. Pore size was assessed by measuring the inter-fiber distance, e.g. the distance between opposing sides of two fibers that formed a single 2D pore. Membrane thickness was characterized using a Helios focused ion beam (FIB) laser. The laser was used to mill into the fibers to create a trench that exposed a clean interface between the fiber mesh and the underlying aluminum foil. The SEM was then used to measure the thickness of the fiber scaffold. Three measurements from three random regions were selected and measured.Tensile testing
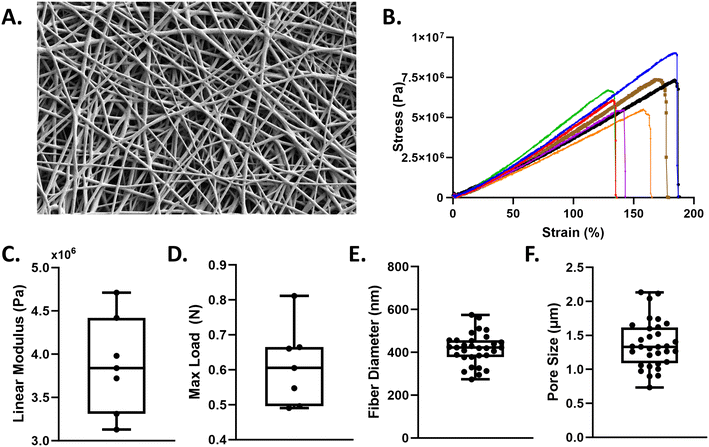
The mechanical properties of the electrospun fibers membrane were quantified via tensile testing. A dog-bone shaped punch, 3 mm width and 27 mm length, was used for all samples. Samples were hydrated in PBS prior to strain tests. Samples were strained at a grip speed of 2 mm s−1 until failure using a tensile tester (TestResources 100R; Shakopee, MN). Stress–strain curves were generated for n = 7 samples across two different scaffolds. Cross-sectional area of the membrane and the slope of the stress–strain curve in the 50–70% strain region (i.e. linear region) was used to determine the linear elastic modulus.Cell-culture
Primary human alveolar epithelial cells (pneumocytes) were obtained from Cell Biologics and cultured in human epithelial cell (HEC) growth media supplemented with a human epithelial supplement kit. Primary human lung microvascular endothelial cells (HMVEC-L) were obtained from Lonza and cultured in endothelial cell basal medium (EBM-2) and supplemented with a microvascular endothelial cell growth media (EGM2-MV) kit. All cell types and donors were non-smokers and grown according to manufacturer's recommendations. Prior to cell seeding in the VOC, ‘day 0’ impedance measurements were obtained to calibrate baseline impedance of the nanofiber membrane. Once cells were grown to confluence on tissue culture plastic, pneumocytes were collected and injected into the apical chamber at a density of 3.5 × 105 cells per mL. Cells were allowed to adhere under static conditions for 4–5 hours or overnight. The device was then inverted, and the HMVEC-Ls were injected into the basolateral chamber at a density of 6 × 104 cells per mL and allowed to adhere overnight under static conditions. Cells were grown under a submerged cultured at 37 °C and media was manually changed every other day unless otherwise stated. Media was allowed to equilibrate for 15–20 minutes at 37 °C prior to taking impedance measurements. Barrier formation was assessed via impedance measurements taken every other day (during media changes) until impedance measurements reached a plateau (±100 Ω cm2) over 2–4 days indicating the formation of a complete barrier.Simulation of ventilator-induced lung injury
Prior to all experiments, fresh media was introduced to both apical and basolateral chambers and allowed to equilibrate for 15–20 minutes at 37 °C. All experiments were completed at 37 °C.Immunofluorescence microscopy
To visualize tight junction formation, cells were fixed in 4% PFA for 10 min and permeabilized with 0.1% Triton X-100 in PBS for 10 min. Cells were blocked with 1% BSA/5% goat serum solution for 1 hour at room temperature and then stained with anti-ZO-1 antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200; Invitrogen; Carlsbad, CA) for pneumocytes and anti-VE-cadherin (1
200; Invitrogen; Carlsbad, CA) for pneumocytes and anti-VE-cadherin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100; Invitrogen; Carlsbad, CA) for HMVEC-Ls overnight at 4 °C. The following day, an Alexa Fluor 488 conjugated secondary antibody (1
100; Invitrogen; Carlsbad, CA) for HMVEC-Ls overnight at 4 °C. The following day, an Alexa Fluor 488 conjugated secondary antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, goat anti-rabbit; Thermo Fisher, Waltham, MA) was added for 1 hour and cells were counterstained with Hoechst (1
500, goat anti-rabbit; Thermo Fisher, Waltham, MA) was added for 1 hour and cells were counterstained with Hoechst (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). The membrane was then removed from the device and mounted to a glass coverslip. Images were taken with a Leica Stellaris 5 confocal microscope at 120×. To visualize monolayer formation for both epithelial and endothelial cells, a phalloidin stain was employed and counterstained with Hoechst. Cells were fixed in 4% PFA, permeabilized with Triton X-100, blocked with 1% BSA/5% goat serum and stained at 1
000). The membrane was then removed from the device and mounted to a glass coverslip. Images were taken with a Leica Stellaris 5 confocal microscope at 120×. To visualize monolayer formation for both epithelial and endothelial cells, a phalloidin stain was employed and counterstained with Hoechst. Cells were fixed in 4% PFA, permeabilized with Triton X-100, blocked with 1% BSA/5% goat serum and stained at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 phalloidin for 1 hour. Cells were then counterstained with Hoechst (1
40 phalloidin for 1 hour. Cells were then counterstained with Hoechst (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and the membrane was mounted to a glass coverslip. Images were taken with a Leica Stellaris 5 confocal microscope at 60×.
000) and the membrane was mounted to a glass coverslip. Images were taken with a Leica Stellaris 5 confocal microscope at 60×.
Statistics
Statistical analysis was performed using GraphPad Prism 10. All statistical analyses were performed on impedance measurements normalized to zero hour timepoint measurements. Outliers were identified using the ROUT method with a 1% threshold.8 Data were tested for normality using a Shapiro–Wilk test. For data that were normally distributed, a one-way ANOVA was performed to compare multiple groups. For experiments with two independent variables, a two-way ANOVA was performed. If a significant effect was found, a Tukey or Dunnett's post hoc test for group comparisons was performed. A p-value of ≤ 0.05 was considered statistically significant. Error bars for all data presented in line and bar graphs is the standard deviation. Error bars for data presented as a box and whisker plot are min to max.Results
Electrospun polyurethane scaffold characterization
Medical grade, thermoplastic polyurethane was chosen for its biocompatibility and deformablity28 and electrospun to create fibrous scaffolds (Fig. 2A). Scaffold characterization via uniaxial tensile testing (Fig. 2B) reveals polyurethane membranes have an average linear modulus of 3.8 MPa ± 0.11 (Fig. 2C). Our nanofiber mesh can withstand an average maximum load of 0.6 N ± 0.56 (Fig. 2D) and exhibited an average fiber diameter of 420 nm ± 99.43 (Fig. 2E) and an average 2D pore size of 1.35 μm ± 436 nm. (Fig. 2F).The ventilator-on-a-chip (VOC) recapitulates the function of the alveolar-capillary barrier
Barrier formation was assessed by immunostaining for ZO-1 and VE-cadherin in epithelial and endothelial cells respectively. Confocal images in Fig. 3A and B indicate the presence of tight junctions and adherens junctions following 14 days of primary cell co-culture in the VOC. To visualize monolayer formation for both cell types, a z-stack image was obtained after staining both epithelial and endothelial cells with phalloidin to label actin (Fig. 3C). These images demonstrate monolayer formation and separation between the apical and basolateral cell monolayers from the electrospun membrane. To assess barrier function we measured change in barrier resistance23 by obtaining impedance measurements every 2 days for 14 days in culture (Fig. 3D). Under co-culture conditions, impedance measurements began increasing at day 6–8 and reached >5000 Ω cm2 after 10 days. Interestingly, when only epithelial cells were cultured in the device, impedance measurements only increased to ∼2000 Ω cm2 by 6–8 days which was significantly lower than impedance measured under co-culture conditions. In addition, when only endothelial cells were cultured in the device, we observed no increase in impedance measurements. These data indicate that although epithelial and endothelial cells form monolayers (Fig. 3A and B), cell–cell communication under co-culture conditions is required to obtain an electrically tight barrier in our VOC device. Since calculated impedance measurements are impacted by channel geometry (solution resistance, capacitance of the system, etc.), we determined actual TEER values by fitting frequency response data to an assumed electrical circuit model. As shown in Fig. 3D and E, RTEER values obtained over 14 days for both co-culture and monoculture conditions (Fig. 3E) closely followed impedance measurements at 1 Hz the over the same time period and conditions (Fig. 3D). This indicates that impedance measurements at 1 Hz can be used as surrogate measure of TEER. We also performed an analysis of how device geometry alone alters impedance values by comparing steady-state (i.e. after 14 days of culture) impedance measurements in devices with either 2 mm and 4 mm apical channel heights. As shown in Fig. S1B,† the median impedance values at 1 Hz are significantly larger in devices with a 2 mm channel height compared to a 4 mm channel height (median impedance value = 811.5 and 2588 ohms cm2 for 4 mm and 2 mm apical channel height, respectively). Therefore, we do not directly compare impedance measurements between devices of different configurations/channel heights.Volutrauma leads to magnitude dependent barrier disruption and subsequent recovery
We used the VOC to investigate how different cyclic radial strain magnitudes alter both barrier disruption (reduction in impedance) and recovery (increase in impedance after injury). As shown in Fig. 4A, 20% radial strain results in a statistically significant drop in impedance relative to the 0 h timepoint after 30 minutes and impedance measurements remain low throughout 4 hours of injury. Stopping the injurious 20% radial strain resulted in an increased impedance value that was not statistically different from the zero-hour timepoint indicating improved barrier function within one hour. At 10% radial strain, we observed a transient decrease in barrier function following stretch (Fig. 4A) at 30 minutes and a recovery to baseline during the injury period. Static controls resulted in non-significant fluctuations in impedance measurements. Comparisons between different strain magnitudes (Fig. 4C) reveal a significant difference between all groups after 0.5 hours of injury with 20% radial strain exhibiting more injury than 10% radial strain. Following four hours of stretch, barrier integrity is significantly lower at 20% radial strain compared to 10% radial strain while the injury level at 10% radial strain is not different than static controls. By 5 and 24 hours (1 and 23 hours of recovery) there is no statistically significant difference in injury among the groups indicating that the alveolar-capillary barrier function rapidly recovers following volutrauma at all strain magnitudes.Barotrauma does not cause significant barrier disruption
As shown in Fig. 4B, we used the VOC to investigate how different oscillatory transmural pressure magnitudes alter barrier disruption. Application of high cyclic transmural pressure (20 cm H2O) resulted in a small but statistically significant decrease in relative impedance at the 1 h time point compared to the zero-hour timepoint. However, the relative impedance at the 3, 6 and 24 h timepoints were not statistically different than the zero timepoint. In addition, 10 cm H2O resulted in no significant differences in relative impedance over 24 hours of injury. Comparisons between the different levels of oscillatory transmural pressure only indicated a significant difference between 20 cm H2O and static controls following three hours of cyclical pressure (Fig. 4D). These data indicate that this degree of barotrauma is less injurious than volutrauma in the VOC system.Atelectrauma leads to a significant loss of barrier integrity with a delayed recovery compared to volutrauma
We used the VOC to investigate how different airway/alveolar reopening velocities/frequencies during atelectrauma alter both barrier disruption and recovery. As shown in Fig. 5A, atelectrauma induces a very large and statistically significant reduction in relative impedance relative to the zero-hour timepoint within 30 minutes and this rapid and dramatic loss of barrier function is sustained during two hours of atelectrauma for both high and low frequencies. Interesting, after the cessation of atelectrauma, the relative impedance remains low where the impedance at the 3- and 4-hour timepoints remain statistically lower than the zero-hour impedance. Only after 22 to 46 hours of recovery (24 and 48 h timepoints) do we observe an improvement in barrier integrity and relative impedances that are not statistically different than the zero-hour timepoint. Comparisons between groups (Fig. 5B) reveal that for all injury time points and after one hour of recovery (3 h timepoint), both reopening frequencies resulted in significantly more barrier disruption compared to control conditions. However, the relative impedance measured at 0.125 Hz and 0.25 Hz during injury and recovery was not statistically different. After 22 hours of recovery, there were no statistically significant differences between any group. Since previous investigators have demonstrated that atelectrauma causes significant cell injury/plasma membrane rupture,11,12 we used a live/dead assay to investigate how atelectrauma in the VOC influenced cell injury/plasma membrane rupture. As shown in Fig. 5C, we found significantly increased cell death at both frequency conditions with the slower frequency (0.125 Hz) resulting in significantly more plasma membrane rupture/cell death than the static control and faster frequency (0.25 Hz). Representative live/dead images support this quantitative data (Fig. 5D). These data indicate that in our VOC model, atelectrauma rapidly disrupts the barrier and causes cell death which delays repair/recovery after this form of VILI.Barrier disruption during simultaneous volutrauma and atelectrauma is driven by atelectrauma
Since patients undergoing MV are exposed to multiple injurious physical forces concurrently, we used the VOC to investigate how simultaneous application of volutrauma and atelectrauma impact barrier disruption. First, we simultaneously applied 10% radial strain and atelectrauma at 0.25 Hz in our VOC. This resulted in a rapid and statistically significant decrease in barrier function at 30 minutes which remained statistically lower than the zero-hour timepoint during the 2 hours of injury (Fig. 6A). In addition, after cessation of simultaneous volutrauma/atelectrauma, the relative impedance remained statistically lower than the zero-hour impedance for the first 2 hours of recovery. Impedance was not statistically different than the zero-hour timepoint after 24–48 hours of recovery. Although similar trends were observed when we applied atelectrauma only (Fig. 6A), the application of volutrauma only (Fig. 6A) resulted in a different pattern. Specifically, volutrauma alone led to a small but statistically significant decrease in relative impedance at the 0.5 and 1-hour injury timepoints. However, impedance values at 1.5 and 2 hours were not statistically different than the zero-hour timepoint and recovery was rapid with all impedance values during recovery not statistically different than the zero-hour timepoint. We then repeated these experiments at 0.125 Hz and observed similar trends (Fig. 6B). One important difference in the lower frequency experiments was that volutrauma did not induce a significant reduction in impedance at any injury or recovery time point when compared to the zero-hour control and both atelectrauma and combined conditions resulted in more barrier disruption as evidence by relative impedance values <10%. Note that the data presented for atelectrauma only in Fig. 6 is the same data from Fig. 5. | ||
| Fig. 6 Injury frequency leads to variable impact on barrier function under combined injury conditions. Barrier integrity as assessed by percent change of impedance (% ΔZ) at A. 0.25 and B. 0.125 Hz during 2 h of injury and 48 h of recovery for volutrauma only (10% strain at 2 mm channel height; n = 4), atelectrauma only (data from Fig. 4a; n = 5) and combined volutrauma & atelectrauma (n = 3). All data are normally distributed by Shapiro–Wilk test. Data were analyzed via repeated measures one-way ANOVA with Dunnett's multiple comparison test. Data are normalized to 0 h time point. *p < 0.05. Additional multiple comparisons via Tukey's test were completed following a repeated measures two-way ANOVA for C. 0.25 Hz and D. 0.125 Hz. *p < 0.05, **p < 0.005, ***p < 0.001. All data presented as means with standard deviation. Comb – combined volutrauma and atelectrauma; Vol only – volutrauma only; Atel only – atelectrauma only. | ||
When comparing groups at the higher 0.25 Hz frequency, Fig. 6C reveals that atelectrauma and the combined conditions cause significantly more barrier disruption (lower impedance) than volutrauma at the 0.5-hour timepoint and that atelectrauma induced significantly more barrier disruption than volutrauma at 2 hours. We did not observe any significant differences in relative impedance during recovery between conditions at 0.25 Hz. At a slower frequency (0.125 Hz), simultaneous injuries and atelectrauma alone both resulted in significantly more barrier disruption than volutrauma after 2 hours of injury (Fig. 6D) and during the initial recovery phase (3 h). Importantly, there were no statistically significant differences between the atelectrauma and combined conditions at any injury or recovery timepoint. Together, this data indicates that under the conditions used in this study, atelectrauma is the driving physical force causing barrier disruption and that slower reopening of fluid occluded lung units may be the most important physical force to mitigate when trying to reduce the degree of VILI.
Discussion
In this study, we developed a novel ventilator-on-a-chip (VOC) system that was specifically designed to investigate the complex biomechanics associated with ventilation induced lung injury (VILI). The VOC recapitulates the functional properties of the alveolar capillary barrier including tight junction formation in epithelial cells, adherens junction formation in endothelial cells (Fig. 3A and B), monolayer formation (Fig. 3C), and the development of an electrically tight barrier (Fig. 3D and E). The electrospun nanofiber membrane used in the VOC exhibits several characteristics of the basal lamina, including pore size (Fig. 2F) and a fiber diameter (Fig. 2E) that are consistent with measurements of human collagen fibrils.29,30 Importantly, the VOC utilizes primary human epithelial and endothelial cells and exposes these cells to different types of physical forces that occur during the mechanical ventilation of injured lungs. To our knowledge, this is the first in vitro model of lung injury that accounts for primary human cell-based co-culture, the fibrous texture of the lung basement membrane, exposure of cells to the individual or concurrent physical forces responsible for VILI and real-time measurements of barrier properties during both injury and recovery.Advancements in micromachining technology have allowed investigators to develop sophisticated organ-on-a-chip systems to study a wide array of diseases. Huh et al. was the first to develop a lung-on-a-chip (LOC) platform with the capability to generate physiologic levels of uniaxial strain (5–15%) using primary endothelial cells and transformed lung epithelial cells cultured on a thin PDMS membrane.31 Several investigators have developed enhanced LOC models which can replicate negative pressure diaphragmatic breathing,13,32 mimic the in vivo 3D geometry of alveolar sacs22 and incorporate nanofiber membranes to study transepithelial molecular transport.19 However, none of these previous systems were designed to investigate how the complex biophysical forces generated during mechanical ventilation cause lung injury and although previous systems used impedance measurements to monitor barrier function,23 these systems did not assess real-time changes in barrier function due to biomechanical injury, especially in the context of VILI. Therefore, the VOC represents a significant advance since it allows for the real-time assessment of how the biophysical forces associated with VILI alter barrier disruption.
The VOC accounts for several of the key components required to form a functional alveolar-capillary barrier including primary cell co-culture and tight junction formation.9,31 The data shown in Fig. 2 and 3 validates that incorporating these components leads to a functional alveolar-capillary barrier. We then used this validated system to investigate the biomechanical mechanisms of VILI. First, we found that four hours of cyclic injurious stretch (20% radial strain, volutrauma) results in a significant loss of tight junction integrity as measured by electrical impedance (Fig. 4A). These results are consistent with previous studies which demonstrated that high cyclic stretch magnitudes result in a loss of barrier function as measured by albumin levels and decreased tight junction protein content.33–36 In addition, Birukov et al. demonstrated that the deleterious effects of cyclic stretch are most severe within the first few minutes of cycling35 which is also consistent with our data where injurious levels of radial strain result in a significant decline in tight junction integrity within 30 minutes (Fig. 4A). After cessation of volutrauma, we observed a rapid increase in impedance measurements within 2 hours indicating that once injurious stretching is halted the barrier is capable of rapid recovery. In support of this conclusion, previous studies have shown that following high tidal ventilation, lungs are able to reverse plasma membrane rupture, lung permeability and inflammation.37,38
Atelectrauma is another well studied form of VILI and although previous studies investigated how atelectrauma alters cell death and cell adhesion,18,25,39 our novel VOC can for the first time monitor the effect of atelectrauma on transepithelial/endothelial barrier resistance (via impedance measurements) in real-time during both injury and recovery. Both high and low frequency lung reopening resulted in rapid and significant drop in barrier function as measured by impedance (Fig. 5A) and barrier disruption was maintained during 2 hours of atelectrauma. In contrast to volutrauma, where repair began within one hour following the cessation of injury, the epithelium repairs more slowly following atelectrauma. Specifically, impedance measurements after cessation of atelectrauma continue to be statistically lower than the zero-hour timepoints for up to 2 hours (Fig. 5B). This data suggest that atelectrauma induces a more severe injury that cannot recover as rapidly. Much work has been done by our group and others to investigate how cell matrix, temperature, cytoskeletal remodeling, and cell confluence impact cell injury during atelectrauma.11,12,18,24,40,41 A common finding in these studies is that lower reopening velocities (lower frequency) lead to more plasma membrane disruption and cell death than atelectrauma at higher velocities/frequencies. Although we confirmed this inverse relationship between cell death and reopening velocity/frequency in this study (Fig. 5C), we did not observe a statistically significant difference in barrier disruption as measured by impedance between the high and low frequencies (Fig. 5B). Atelectrauma involves the generation of several complex mechanical forces and previous studies24,42 have demonstrated that the large pressure gradients generated during slow airway reopening cause more plasma membrane disruption. However, the data in Fig. 5B indicate that these pressure gradients may not be the forces responsible for disrupting tight junctions. Future studies could therefore use computational technqiues43 to determine which mechanical force generated during airway reopening is responsible for barrier disruption. Furthermore, the occlusion fluid used in all atelectrauma experiments was media containing 10% FBS, which did not contain a lipid based surfactant. Our model of atelectrauma was intended to simulate a high surface tension occlusion fluid as found in mechanically ventilated lungs where surfactant deficiency and/or surfactant in-activation occurs due to underlying ARDS conditions.44 However, future studies should explore how occlusion fluids with a range of surface tensions/surfactant properties alter susceptibility to VILI.
While many investigators have studied how volutrauma or atelectrauma alone alter cell injury in vitro,45 to our knowledge Takayama et al.46 are the only group that used in vitro microfluidics to evaluate cell injury during the simultaneous application of volutrauma and atelectrauma. Although the device used in that study utilized monocultures of transformed human or murine epithelial cells, a homogenous PDMS membrane, a short duration of injury (<5 min) and could only measure cell morphology/detachment, it did indicate that atelectrauma results in more cell detachment than volutrauma.46 In this study, we developed a novel VOC that makes significant improvements in capturing important physiological and clinical aspects of VILI including the use of a deformable fibrous membrane that mimics the extracellular matrix, co-culture of primary human (non-transformed) epithelial and endothelial cells, real-time measurement of a clinically relevant parameter (barrier integrity) and application of injurious forces on clinically relevant time scales (i.e. hours to days). In addition, the VOC allows for important advantages as it can measure barrier function in real-time during both injury and recovery. Using the VOC, we demonstrated that concurrent application of volutrauma and atelectrauma for several hours results in barrier disruption and recovery patterns that are similar to atelectrauma alone (Fig. 6A and B). In addition, barrier disruption due to atelectrauma only and combined volutrauma and atelectrauma were not statistically different at any time point (Fig. 6C and D). Therefore, our data indicate that atelectrauma is driving mechanical forces responsible for injury/barrier disruption under the conditions used in this study and that atelectrauma is more injurious than volutrauma. This conclusion is consistent with previous work by Hussein et al. who found that ventilation of partially fluid filled lungs (atelectrauma) causes more injury than over-distending an air-filled lung (volutrauma).41 Furthermore, there is evidence that cyclic reopening (atelectrauma) and overdistention (volutrauma) synergistically induce injury. In vivo, Seah et al. demonstrated that a combination of high tidal volume and no PEEP result in a dramatic decrease in lung recruitability as measured by lung stiffness.47 More recently, a computational model suggests that the stresses of lung recruitment/decruitment are the primary instigating damage mechanism due to disruption of cell–cell and cell–substrate adhesions48 and that when coupled with overdistension, it leads to exacerbation of injury. This may have important therapeutic implications since previous studies have documented the cell mechanical changes needed to prevent atelectrauma.12,43
Although the VOC models several important aspects of alveolar-capillary barrier and can measure how VILI impacts cell injury and recovery, this system does not replicate all aspects of in vivo lung physiology. First, we found that an air–liquid interface (ALI) culture was not necessary for tight junction formation and therefore used a submerged co-culture system to model alveolar flooding during ARDS. However, future studies could utilize ALI culture conditions. While we utilized a 20–30 μm thick nanofiber mesh for ease of handling, we acknowledge that the basal lamina thickness in vivo is ∼2–10 μm. Given that the VOC forms a stronger barrier (i.e. higher TEER) under co-culture conditions as compared to monoculture conditions (Fig. 3c) the membrane thickness we used does not appear to inhibit cell–cell communication across the membrane. Although the nanofiber mesh used in this study has fiber diameters and pore sizes (420 nm and 1.35 μm respectively) that are similar to the fiber diameter and pore size observed in the lung basement membrane,29,30 the nanofiber membrane is stiffer (∼4 MPa) than normal human lung tissue (1–10 kPa (ref. 49)). However, this nanofiber membrane is still softer than other LOC scaffold fibers19 and future studies could use a more biocompatible polymer to lower the scaffold stiffness. Finally, to be consistent with previous studies from our group7 we used a model of barotrauma where the applied pressures do not cause membrane deflection. Although this allows us to independently investigate the effect of hydrostatic pressure on barrier permeability, we acknowledge that in vivo application of transmural pressure may also cause distension of the alveolar-capillary barrier. Future experiments could be performed with the bottom ports opened to explore how simultaneous changes in transmural pressure and membrane deflection (barotrauma/volutrauma) alter barrier permeability.
Conclusion
In summary, we have developed a novel ventilator-on-a-chip (VOC) device that utilizes primary human epithelial and endothelial cells, a deformable nanofiber membrane, and microfluidics to simulate the complex mechanical forces that are responsible for lung injury during mechanical ventilation. We have used this system to demonstrate that atelectrauma is more injurious than volutrauma and that atelectrauma drives the injury response under simultaneous force application conditions. The VOC represents a powerful tool to study the mechanisms of ventilator induced lung injury and may also serve as a novel platform for drug discovery.Data availability
Data for this article, including impedance measurements and imaging data are available at Dyad at https://doi.org/10.5061/dryad.qjq2bvqqt.Author contributions
Conceptualization: BGZ, JAE, SNG. Data curation: BGZ, JAE, SNG. Formal analysis: BGZ, JAE, SNG. Funding acquisition: JAE, SNG. Investigation: BGZ, VS. Methodology: BGZ, VS, CB, NHC, HP, JAE, SNG. Project administration: JAE, SNG. Resources: NHC, HP, JAE, SNG. Software: BGZ, SNG. Supervision: JAE, SNG. Validation: BGZ, VS. Visualization: BGZ. Writing – original draft: BGZ, JAE, SNG. Writing – review & editing: BGZ, VS, CB, NHC, HP, JAE, SNG.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by NIH Grant R56 HL142767 (J. A. E., S. N. G.), R01 HL142767 (J. A. E., S. N. G.), and a Department of Defense Grant W81XWH-19-1-0210 (S. N. G., J. A. E.). We would like to acknowledge the contributions of the Center for Electron Microscopy and Analysis (CEMAS) at The Ohio State University for their technical assistance.Notes and references
- T. Pham, L. J. Brochard and A. S. Slutsky, Mayo Clin. Proc., 2017, 92, 1382–1400 CrossRef.
- E. C. Goligher, N. D. Ferguson and L. J. Brochard, Lancet, 2016, 387, 1856–1866 CrossRef.
- J. C. Parker, L. A. Hernandez and K. J. Peevy, Crit. Care Med., 1993, 21, 131–143 CrossRef CAS PubMed.
- A. S. Slutsky and V. M. Ranieri, N. Engl. J. Med., 2013, 369, 2126–2136 CrossRef CAS.
- L. N. Tremblay and A. S. Slutsky, Intensive Care Med., 2006, 32, 24–33 CrossRef PubMed.
- L. Gattinoni, P. Caironi, M. Cressoni, D. Chiumello, V. M. Ranieri, M. Quintel, S. Russo, N. Patroniti, R. Cornejo and G. Bugedo, N. Engl. J. Med., 2006, 354, 1775–1786 CrossRef CAS.
- Y. Huang, M. Crawford, N. Higuita-Castro, P. Nana-Sinkam and S. N. Ghadiali, FASEB J., 2012, 26, 3351–3364 CrossRef CAS PubMed.
- C. M. Bobba, Q. Fei, V. Shukla, H. Lee, P. Patel, R. K. Putman, C. Spitzer, M. Tsai, M. D. Wewers, R. J. Lee, J. W. Christman, M. N. Ballinger, S. N. Ghadiali and J. A. Englert, Nat. Commun., 2021, 12, 289 CrossRef CAS PubMed.
- D. Huh, B. D. Matthews, A. Mammoto, M. Montoya-Zavala, H. Y. Hsin and D. E. Ingber, Science, 2010, 328, 1662–1668 CrossRef CAS.
- H. Lee, Q. Fei, A. Streicher, W. Zhang, C. Isabelle, P. Patel, H. C. Lam, A. Arciniegas-Rubio, M. Pinilla-Vera, D. P. Amador-Munoz, D. Barragan-Bradford, A. Higuera-Moreno, R. K. Putman, L. M. Sholl, E. P. Henske, C. M. Bobba, N. Higuita-Castro, E. M. Shalosky, R. D. Hite, J. W. Christman, S. N. Ghadiali, R. M. Baron and J. A. Englert, JCI Insight, 2021, 6(14), e137708 CrossRef.
- N. Higuita-Castro, V. C. Shukla, C. Mihai and S. N. Ghadiali, Ann. Biomed. Eng., 2016, 44, 3632–3644 CrossRef CAS.
- H. C. Yalcin, K. M. Hallow, J. Wang, M. T. Wei, H. D. Ou-Yang and S. N. Ghadiali, Am. J. Physiol., 2009, 297, L881–L891 CAS.
- A. O. Stucki, J. D. Stucki, S. R. Hall, M. Felder, Y. Mermoud, R. A. Schmid, T. Geiser and O. T. Guenat, Lab Chip, 2015, 15, 1302–1310 RSC.
- B. A. Hassell, G. Goyal, E. Lee, A. Sontheimer-Phelps, O. Levy, C. S. Chen and D. E. Ingber, Cell Rep., 2018, 23, 3698 CrossRef.
- A. Zuchowska, E. Jastrzebska, M. Chudy, A. Dybko and Z. Brzozka, Anal. Chim. Acta, 2017, 990, 110–120 CrossRef CAS.
- K. H. Benam, R. Novak, J. Nawroth, M. Hirano-Kobayashi, T. C. Ferrante, Y. Choe, R. Prantil-Baun, J. C. Weaver, A. Bahinski, K. K. Parker and D. E. Ingber, Cell Syst., 2016, 3, 456–466 CrossRef CAS PubMed , e454.
- K. H. Benam, R. Villenave, C. Lucchesi, A. Varone, C. Hubeau, H. H. Lee, S. E. Alves, M. Salmon, T. C. Ferrante, J. C. Weaver, A. Bahinski, G. A. Hamilton and D. E. Ingber, Nat. Methods, 2016, 13, 151–157 CrossRef CAS.
- N. Higuita-Castro, M. T. Nelson, V. Shukla, P. A. Agudelo-Garcia, W. Zhang, S. M. Duarte-Sanmiguel, J. A. Englert, J. J. Lannutti, D. J. Hansford and S. N. Ghadiali, Sci. Rep., 2017, 7, 11623 CrossRef CAS PubMed.
- A. Nishiguchi, S. Singh, M. Wessling, C. J. Kirkpatrick and M. Möller, Biomacromolecules, 2017, 18, 719–727 CrossRef CAS PubMed.
- X. Yang, K. Li, X. Zhang, C. Liu, B. Guo, W. Wen and X. Gao, Lab Chip, 2018, 18, 486–495 RSC.
- D. Baptista, L. Moreira Teixeira, D. Barata, Z. Tahmasebi Birgani, J. King, S. van Riet, T. Pasman, A. A. Poot, D. Stamatialis, R. J. Rottier, P. S. Hiemstra, A. Carlier, C. van Blitterswijk, P. Habibović, S. Giselbrecht and R. Truckenmüller, ACS Biomater. Sci. Eng., 2022, 8, 2684–2699 CrossRef CAS.
- D. Huang, T. Liu, J. Liao, S. Maharjan, X. Xie, M. Perez, I. Anaya, S. Wang, A. Tirado Mayer, Z. Kang, W. Kong, V. L. Mainardi, C. E. Garciamendez-Mijares, G. Garcia Martinez, M. Moretti, W. Zhang, Z. Gu, A. M. Ghaemmaghami and Y. S. Zhang, Proc. Natl. Acad. Sci. U. S. A., 2021, 118(19), e2016146118 CrossRef CAS PubMed.
- O. Y. F. Henry, R. Villenave, M. J. Cronce, W. D. Leineweber, M. A. Benz and D. E. Ingber, Lab Chip, 2017, 17, 2264–2271 RSC.
- H. C. Yalcin, S. F. Perry and S. N. Ghadiali, J. Appl. Physiol., 2007, 103, 1796–1807 CrossRef CAS PubMed.
- A. M. Jacob and D. P. Gaver, J. Appl. Physiol., 2012, 113, 1377–1387 CrossRef PubMed.
- A. Davies and C. Moores, Respir. Syst., 2010, 11–28, DOI:10.1016/B978-0-7020-3370-4.00002-5.
- D. J. Tschumperlin and S. S. Margulies, Am. J. Physiol., 1998, 275, L1173–L1183 CAS.
- C. Akduman, I. Özgüney and E. P. A. Kumbasar, Mater. Sci. Eng., C, 2016, 64, 383–390 CrossRef CAS PubMed.
- S. S. Sobin, Y. C. Fung and H. M. Tremer, J. Appl. Physiol., 1988, 64, 1659–1675 CrossRef CAS.
- W. J. Howat, J. A. Holmes, S. T. Holgate and P. M. Lackie, Am. J. Pathol., 2001, 158, 673–680 CrossRef CAS.
- D. Huh, D. C. Leslie, B. D. Matthews, J. P. Fraser, S. Jurek, G. A. Hamilton, K. S. Thorneloe, M. A. McAlexander and D. E. Ingber, Sci. Transl. Med., 2012, 4, 159ra147 Search PubMed.
- J. D. Stucki, N. Hobi, A. Galimov, A. O. Stucki, N. Schneider-Daum, C. M. Lehr, H. Huwer, M. Frick, M. Funke-Chambour, T. Geiser and O. T. Guenat, Sci. Rep., 2018, 8, 14359 CrossRef.
- K. J. Cavanaugh, J. Oswari and S. S. Margulies, Am. J. Respir. Cell Mol. Biol., 2001, 25, 584–591 CrossRef CAS.
- K. J. Cavanaugh and S. S. Margulies, Am. J. Physiol., 2002, 283, C1801–C1808 CrossRef CAS PubMed.
- K. G. Birukov, J. R. Jacobson, A. A. Flores, S. Q. Ye, A. A. Birukova, A. D. Verin and J. G. Garcia, Am. J. Physiol., 2003, 285, L785–L797 CAS.
- D. J. Tschumperlin, J. Oswari and A. S. Margulies, Am. J. Respir. Crit. Care Med., 2000, 162, 357–362 CrossRef CAS PubMed.
- O. Gajic, J. Lee, C. H. Doerr, J. C. Berrios, J. L. Myers and R. D. Hubmayr, Am. J. Respir. Crit. Care Med., 2003, 167, 1057–1063 CrossRef PubMed.
- A. González-López, A. Astudillo, E. García-Prieto, M. S. Fernández-García, A. López-Vázquez, E. Batalla-Solís, F. Taboada, A. Fueyo and G. M. Albaiceta, Am. J. Physiol., 2011, 301, L500–L509 Search PubMed.
- E. Yamaguchi, J. Yao, A. Aymond, D. B. Chrisey, G. F. Nieman, J. H. T. Bates and D. P. Gaver, Biosensors, 2022, 12(6), 390 CrossRef CAS.
- N. Higuita-Castro, C. Mihai, D. J. Hansford and S. N. Ghadiali, J. Appl. Physiol., 2014, 117, 1231–1242 CrossRef CAS.
- O. Hussein, B. Walters, R. Stroetz, P. Valencia, D. McCall and R. D. Hubmayr, Am. J. Physiol., 2013, 305, L478–L484 CAS.
- S. S. Kay, A. M. Bilek, K. C. Dee and D. P. Gaver, 3rd, J. Appl. Physiol., 2004, 97, 269–276 CrossRef.
- H. L. Dailey and S. N. Ghadiali, Biomech. Model. Mechanobiol., 2010, 9, 263–279 CrossRef CAS PubMed.
- A. Dushianthan, M. P. W. Grocott, G. S. Murugan, T. M. A. Wilkinson and A. D. Postle, Diagnostics, 2023, 13(18), 2964 CrossRef CAS.
- R. K. Albert, Am. J. Respir. Crit. Care Med., 2022, 205, 152–160 CrossRef CAS PubMed.
- N. J. Douville, P. Zamankhan, Y. C. Tung, R. Li, B. L. Vaughan, C. F. Tai, J. White, P. J. Christensen, J. B. Grotberg and S. Takayama, Lab Chip, 2011, 11, 609–619 RSC.
- A. S. Seah, K. A. Grant, M. Aliyeva, G. B. Allen and J. H. Bates, Ann. Biomed. Eng., 2011, 39, 1505–1516 CrossRef PubMed.
- J. H. T. Bates, G. F. Nieman, M. Kollisch-Singule and D. P. Gaver, Ann. Biomed. Eng., 2023, 51, 1052–1062 CrossRef PubMed.
- S. R. Polio, A. N. Kundu, C. E. Dougan, N. P. Birch, D. E. Aurian-Blajeni, J. D. Schiffman, A. J. Crosby and S. R. Peyton, PLoS One, 2018, 13, e0204765 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4lc00143e |
| ‡ Denotes equal contribution. |
| This journal is © The Royal Society of Chemistry 2024 |