Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Quantification of technetium-99 in wastewater by means of automated on-line extraction chromatography – anion-exchange chromatography – inductively coupled plasma-mass spectrometry†
Maximilian
Horstmann
 a,
C. Derrick
Quarles
Jr
a,
C. Derrick
Quarles
Jr
 b,
Steffen
Happel
c,
Michael
Sperling
b,
Steffen
Happel
c,
Michael
Sperling
 d,
Andreas
Faust
d,
Andreas
Faust
 e,
David
Clases
e,
David
Clases
 *f and
Uwe
Karst
*f and
Uwe
Karst
 *a
*a
aUniversity of Münster, Institute of Inorganic and Analytical Chemistry, Münster, Germany. E-mail: uk@uni-muenster.de
bElemental Scientific Inc., Omaha, NE, USA
cTrisKem International SAS, Bruz, France
dEuropean Virtual Institute for Speciation Analysis (EVISA), Münster, Germany
eEuropean Institute for Molecular Imaging (EIMI), Münster, Germany
fUniversity of Graz, Institute of Chemistry, Graz, Austria. E-mail: david.clases@uni-graz.at
First published on 27th September 2024
Abstract
With more than 30 million medical applications annually, 99mTc is the most widely used radioisotope. Nevertheless, the discharge of 99mTc and its radioactive nuclear isomer 99Tc through medical facilities into the aqueous environments is mostly unknown. This is related to the low absolute Tc mass used in medical examinations and consequent trace levels of Tc emitted from respective facilities. In this work, a new approach employing automated on-line extraction chromatography and anion-exchange chromatography was developed and coupled to inductively coupled plasma-mass spectrometry. The extraction column was filled with TK201 resin to preconcentrate, purify and elute pertechnetate ([99Tc]TcO4−) as the most relevant Tc species. The eluting 99Tc fraction was focused and separated on an anion-exchange chromatography column and directly calibrated into a mass flow using an on-line isotope dilution-like approach named isobaric dilution analysis. To accommodate ultra-trace levels in strong matrices with high grades of particulate contamination, the automated method was complemented by a newly developed manual filtration and preconcentration workflow with TK201 impregnated filter disks, reaching a combined preconcentration factor as high as 4615. An aerosol desolvation nebulization system was used additionally to boost sensitivity, achieving an ultimate limit of detection (LOD) as low as 0.70 ± 0.02 fg kg−1. As proof of concept, a wastewater sample from a retention basin of a local university hospital was collected. This wastewater contained 99Tc emitted in diagnostic procedures and levels were determined to be as low as 89 ± 4 fg kg−1.
Introduction
Environmental emission of 99Tc
Since its discovery in 1937, Tc, specifically its most prominent isotope 99Tc, has been a significant contributor to anthropogenic radionuclide emission into the environment.1 Today, multiple different emission pathways result in a consistent discharge of 99Tc mainly into aquatic ecosystems.2 Under aerobic, oxidizing conditions, [99Tc]TcO4− is the most stable chemical species of 99Tc.3,4 It is widely known for its high ecological mobility and can be taken up and transformed by microorganisms, plants, and animals, ultimately entering the food chain.5,6 Due to its properties and its high fission yield in nuclear power plants, 99Tc is a crucial radionuclide in assessing the risk of radioactivity in the environment, in decommissioning nuclear facilities, in management of nuclear waste, and for calculating the long-term collective future dose to the population.7,8Initially, only a small number of locally restricted nuclear sites released the majority of 99Tc, which occurred as a by-product of nuclear weapons development and deployment as well as nuclear power production.9 Since the 1960s, the two primary nuclear fuel reprocessing facilities in Europe, Sellafield located in northwestern England and La Hague in northern France, alone, were accountable for a total emission of more than 2.8 tons, until the annual release was restricted to 140 kg in 2000.7,9–11 Still, as the global demand for electricity, much of which is generated through nuclear power, continues to rise, so does the need for nuclear fuel reprocessing. With decisions on future reprocessing campaigns often pending, in some countries spent nuclear fuel is again stacking up waiting to be reprocessed.11,12 Its contribution to the environmental emission of 99Tc is, however, decreasing due to new environmental restrictions and novel approaches for eliminating 99Tc from the resulting radioactive wastewater.2,13,14 Between 2010 and 2021, both plants combined emitted only about 25 kg of 99Tc to the marine environment, due to even lower current emission restrictions.11,12
Today, in addition to the emission as a by-product of nuclear fission, 99Tc is also being emitted following medical examinations (Fig. 1). This significantly lower yet more widespread release is based on the use of its nuclear isomer, 99mTc, as a radiotracer for diagnostic imaging in scintigraphy.15 Here, the distinct physical attributes of the employed metastable 99mTc (half-life ∼6 h), combined with its convenient availability through generators in the hospital, have established its prominent position among commercially administered radionuclides.15–17 The versatile redox chemistry of 99mTc enables a robust and rapid synthesis of specific complexes in commercial labeling kits.18 The radiotracers obtained can subsequently be used to target specific organs and structures within the patient's body in techniques such as single photon emission computed tomography (SPECT).18,19 Every year, around 30 million administrations of 99mTc-based tracers are carried out globally, accounting for about 85% of all conducted scintigraphy examinations.16
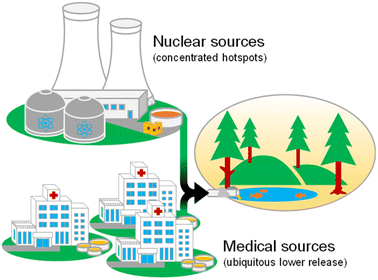 | ||
| Fig. 1 Schematic depiction of the two presented emission pathways of 99Tc into the aqueous environment. | ||
With global administration numbers still increasing, medical production of 99Tc has been predicted to be responsible for an overall annual release of approximately 7 g in 2023, derived from the assumption that global production for its parent isotope 99Mo has met its predicted amounts for the last 3 years.20,21 Despite the small absolute mass released, with other emission sources continuously declining, the future role of ubiquitously released medical 99Tc will increase, especially regarding its discharge in previously unaffected freshwater systems. The expected concentrations for monitoring this pathway, however, lie beyond current analytical capabilities and can therefore not be determined experimentally. This prevents endeavors to evaluate the emission and to confirm theoretical interpolations.22
Environmental monitoring of 99Tc
Commonly, the analysis of radionuclides in aqueous samples is performed by recording their activity through methods like liquid scintillation (LS) or Geiger–Müller (GM) counting. Radiometric counting strategies are very sensitive for the γ-emitting 99mTc isotope and enable absolute quantification of the analyte without requiring reference materials. However, the situation is different for the less active ground state, 99Tc, which possesses a half-life of about 211![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 years.23 Its weak β−-emission challenges current counting methods and environmental applications requiring extensive manual analyte preconcentration steps as well as long recording times.22 In a previous study, Chen et al. achieved a detection limit (LOD) of 5 fg L−1 (3 mBq m−3), but required 500 L of seawater and a multi-day preconcentration workflow to enable β-detection with a GM-counter.24 Similar studies investigated options to reduce sample volume, preparation efforts and recording time, but did not achieve comparable LODs.25,26
100 years.23 Its weak β−-emission challenges current counting methods and environmental applications requiring extensive manual analyte preconcentration steps as well as long recording times.22 In a previous study, Chen et al. achieved a detection limit (LOD) of 5 fg L−1 (3 mBq m−3), but required 500 L of seawater and a multi-day preconcentration workflow to enable β-detection with a GM-counter.24 Similar studies investigated options to reduce sample volume, preparation efforts and recording time, but did not achieve comparable LODs.25,26
Currently, most approaches are developed to target 99Tc in marine environments and information from the medical emission pathway is scarce. Using β-counting, Villar et al. detected 99Tc in hospital waste, while Fischer et al. targeted 99mTc in the inflow, effluent, primary sludge, and sewage sludge of a wastewater treatment plant using gamma spectroscopy.27,28 The latter managed to uncover correlations between the concentrations of 99mTc and scintigraphy numbers conducted in the catchment area of the surveyed wastewater treatment plant.28 Here, however, it must be taken into account that the actual total 99Tc concentration has to be significantly higher due to the short half-life of 99mTc, resulting in gamma spectroscopy only detecting a small fraction of the entire emission. To study the medical discharge of 99Tc in its entirety as well as its long-term environmental behavior, accurate quantification requires sensitive mass spectrometric methods, which are dedicated to address the needs of this emission pathway.
Over the last decades, elemental mass spectrometric methods, particularly inductively coupled plasm-mass spectrometry (ICP-MS), have substantially advanced and now provide new strategies for the detection of 99Tc.23 Compared to the detection of β-emission, it offers better sensitivity, higher matrix tolerance, and faster results with fewer requirements to account for interfering radioisotopes.29–31 ICP-MS is becoming the method of choice for the ultra-trace analysis of many nuclides and instrumental LOD below the parts-per-trillion mark are possible.32 However, to cope with the extremely low environmental Tc levels, method LODs were decreased further, which was achieved using dedicated sample preparation techniques and preconcentration resins. Employing an automated sample preconcentration setup, Shi et al. managed to achieve an LOD of 12 fg L−1 (7.5 mBq m−3) by analyzing the precipitate obtained from 200 L of seawater via extraction chromatography (ExC) coupled to ICP-MS.33 In an entirely automated approach, without any prior manual precipitation to preconcentrate 99Tc, Matsueda et al. used on-line solid-phase extraction (SPE) in combination with ICP-MS, reaching an LOD of 9.3 pg L−1 (5.9 mBq L−1) in a sample of 50 mL. In the study they managed to further enhance sensitivity by employing an automated off-line anion exchange preconcentration to address larger sample volumes of up to 40 L, subsequently lowering the LOD to 70 fg L−1 (44 mBq m−3).34 A comparison of studies using ICP-MS for the detection of 99Tc in aqueous samples, achieved LODs as well as analyzed samples is given in Table 3 of the ESI.†
A challenge for the determination of 99Tc with ICP-MS is the limited availability of accessible elemental standards.23 To overcome this challenge, Clases et al. proposed a new internal quantification strategy for the internal calibration of transient 99Tc signals.35 Based on isotope dilution analysis (IDA), isobaric dilution analysis (IBDA) was developed as both, off-line and on-line technique, harnessing the similarity and isotopic overlap of 99Ru and 99Tc.35 Here, a Ru-spiked solution is either directly spiked into the sample manually (off-line) or added continuously after the chromatographic separation (on-line), whilst accounting for differences in elemental sensitivities.15 Especially in an on-line approach, IBDA allowed the robust and rapid calibration of any eluting Tc species.15 The interested reader will find more details on IBDA, its derivation, and application elsewhere.15,35
In this study, this internal quantification strategy is combined with a newly developed, modular and fully automated method for on-line preconcentration and separation of [99Tc]TcO4−. This approach overcomes challenges which are related to extensive sample preparation and manual labor. As such, it offers an opportunity to achieve feasible and repeatable monitoring of trace amounts of Tc in complex environments. To investigate the emission of 99Tc through a local medical facility and specifically to gain experimental insight into its contribution to the medical emission pathway, a sample was drawn from a retention basin of the local university hospital. To enable the analysis of ultra-trace levels of [99Tc]TcO4−, an on-line ExC equipped with TK201 resin was used in conjunction with ion exchange chromatography (IC) coupled to ICP-MS. With dissolved as well as undissolved contaminations highly present in the extremely complex wastewater matrix of nuclear medical wastewater, the on-line method was complemented by an additional off-line integrated filtration, clean-up and preconcentration procedure performed for sample preparation and a simultaneous further increase in sensitivity.
Materials and methods
Chemicals and consumables
Bi-distilled water was produced in an Aquatron water still purification system model A4000D (Barloworld Scientific, Nemours, France). Elemental standards of Ru and In (each 1000 mg L−1) as well as a PTFE-coated vacuum filter holder set for 47 mm membranes were both obtained from Merck KGaA (Darmstadt, Germany). Nitric acid (65%, AnalaR NORMAPUR®, (w/v)) and sulfuric acid (95%, AnalaR NORMAPUR, (w/v)) were acquired from VWR International (Radnor, PA, USA) and ammonium nitrate (p.a.) was purchased from AppliChem GmbH (Darmstadt, Germany). Ammonium hydroxide solution (25%, Analytical Reagent Grade, (w/v)) was acquired from Fluka Chemie GmbH (Buchs, Switzerland). Syringe filters (0.45 μm, 25 mm, hydrophilic PTFE) were purchased from BGB Analytik AG (Boeckten, Switzerland) and Omnipore filter disks (0.45 μm, 47 mm, hydrophilic PTFE) were bought from Merck Millipore (Burlington, MA, USA). TK201 ExC resin in powder form (50–100 μm particle size), as well as filter disks (47 mm) impregnated with the TK201 extractant, were provided by Triskem International SAS (Bruz, France). Empty columns for ExC were provisioned by Elemental Scientific, Inc. (Omaha, NE, USA). Polypropylene sample tubes (15, 50 mL and 250 mL) were acquired from Th. Geyer (Renningen, Germany).Chromatographic separation
The newly developed modular and fully automated method for on-line preconcentration and separation of [99Tc]TcO4− was performed using a single platform system for total metal analysis and syringe-driven chromatography (prepFAST IC, Elemental Scientific, Inc., Omaha, NE, USA), which was modified to meet the particular requirements of the described analysis (Fig. 2). In comparison to the conventional setup, which is designed to perform automatic dilution of standards and samples in two separate loops, only one large sample loop of 11.035 mL was used.36,37 Instead of the second loop, an ExC column, which had been densely packed in-house with approximately 100 mg of particulate TK201 resin was installed for preconcentration. As Matsueda et al. previously stated, this resin alone does not fully reach the necessary discrimination ratios to differentiate 99Tc from its isobaric interferences potentially present in environmental samples and thus requires other additional separation techniques.34 In their work, they suggested a method using an "Oxygen Dynamic Reaction", to overcome the lack in specificity, allowing a discrete separation between 99Tc, Mo and Ru, while still exploiting the beneficial softer elution conditions required for TK201 resin.34 In the present study, an IC method was introduced to increase specificity and to separate Tc from potential interferences. The column enabled the separation of [99Tc]TcO4− from potential interference residues trapped on the ExC column and was added to the system on the third available chromatography valve (Fig. 2).Before loading the sample, both columns were rinsed. The ExC column was rinsed for 3 minutes, changing between HNO3 (0.02 M, 700 μL min−1) and NH4OH (0.5 M, 700 μL min−1) after every 30 s. The IC column was rinsed with NH4NO3 solution (0.15 M, set to pH 9.2 with NH4OH solution, 3 mL, 650 μL min−1) for 1 minute. After the prerinsing procedure and an essential conditioning step of the resin (HNO3, 0.01 M, 2.8 mL min−1), a total sample volume of 110.35 mL was loaded onto the ExC column within 10 cycles, with untrapped elements and sample matrix being immediately discharged into a waste container. Employing a cyclic loading approach enabled adapting the method to a multitude of sample volumes, each depending on necessary preconcentration factors and the overall time of the analysis. This modular character of platform and method allows to match preconcentration effort with individual requirements of the sample, thereby offering potential to save both time and reagents during the analysis. Loading the sample loop and injecting the sample onto the ExC column was performed using the combined carrier syringes 1 and 2 (0.01 M, each 6 mL) at a total flow of 3.04 mL min−1. The ExC column was subsequently washed (0.1 M HNO3, 1 cycle, 11.035 mL, 3.4 mL min−1) to reduce potential contaminations. The elution was performed via the subsequent IC column (IonPac AG9-SC, 4 × 50 mm, Thermo Fisher Scientific, Waltham, MA, USA) in a 2-step gradient with NH4OH solution (0.5 M, 650 μL min−1, 430 s) and NH4NO3 solution (0.15 M, set to pH 9.2 with NH4OH solution, 650 μL min−1, 140 s) to focus the peaks and to separate 99Tc from occurring interferences. The entire automatized method including initialization, pre-cleaning, column conditioning, loading, washing, elution and post-cleaning took an overall time of about 75 minutes per sample, which can be lowered by applying fewer sample loading cycles.
For quantification of transient 99Tc signals by means of IBDA, an internal Ru standard (1 μg kg−1 in 0.01 M HNO3, 50 μL min−1) was added post-column to reach a total flow rate of 700 μL min−1. LOD and limit of quantification (LOQ) were determined by injecting diluted solutions (each 110.35 mL load volume) of an in-house prepared and counter-quantified 99Tc standard, without the added post-column internal Ru standard, through an external calibration curve.38,39 The 99Tc standard was prepared from decayed medical 99mTc-generator eluate and quantified using total reflection X-ray fluorescence analysis.39 The interested reader can find more on the preparation of this standard in a recent publication.39 IBDA recovery of the overall on-line ExC-IC method was checked by analyzing similar diluted spiked solutions of the standard (each 110.35 mL load volume) with the internal standard added to the chromatographic flow and comparing the concentrations calculated by IBDA with the prepared standard concentrations. Individual recovery data for ExC column, IC-column and chemically regenerated cation-suppressor were checked similarly and are listed in the ESI.† Operation, method design, and optimization were conducted using the software ESI SC (Version 2.9.0.496, Elemental Scientific, Inc., Omaha, NE, USA).
ICP-MS detection
For mass spectrometric detection, the chromatography flow from eluent syringes 1 and 2 was connected to an Agilent 7700x ICP-MS (Agilent Technologies, Santa Clara, CA, USA), which – for the reason of improved longevity – was equipped with platinum sampler and skimmer cones. Operation of the mass spectrometer was performed using MassHunter software (MassHunter 4.6, Version C.01.06). Aerosol desolvation nebulization was employed using an Apex 2 High Sensitivity Desolvating System with a MicroFlow PFA ST nebulizer (both from Elemental Scientific, Inc., Omaha, NE, USA), to increase analyte transmission to the plasma compared to common sample introduction setups. This required the additional use of a Dionex ACRS 500 chemically regenerated suppressor (4 mm ID, Thermo Fisher Scientific, Waltham, MA, USA), preventing blockage through crystallization of non-volatile eluent compounds within the desolvation unit. Instrument parameters were optimized at the beginning of each day of analysis to maximize the signal-to-noise ratios of m/z 99 and 101 whilst introducing a Ru standard (c(Ru) 100 ng kg−1). Within the 99Tc mass flow calculations of IBDA, the in-house prepared 99Tc standard was used to directly correct for any differences in elemental sensitivity between 99Tc and 99Ru, included in the continuously added post-column Ru spike of natural isotopic composition. More on the approach of IBDA and its included correction layers can be found in recent publications.15,35,39Common instrument parameters for all components of the overall setup can be found in Table S1 (ESI).† Analyses were performed without a collision cell gas flow to maintain high ion transmission and therefore, highest sensitivity. To calibrate the detector dead time prior to each day of analysis, different concentrations of an elemental In standard (10 mg L−1 and 50 μg L−1) were used. Mass bias correction was performed with multiple isotopes of Ru (99, 100, 101, 102, 104) using the exponential method proposed by Rodriguez Gonzalez et al.40
Additional off-line filter disk preconcentration method
To overcome the issues caused by the strong matrix present in targeted samples of nuclear medical wastewater from hospital retention basins, an integrated filtration, clean-up and preconcentration procedure was developed. The workflow was based on specifically manufactured 47 mm filter disks, which have been impregnated by the manufacturer with the TK201 extractant similar to the material used in the ExC column of the on-line method. To prevent the disks from clogging caused by a significant quantity of undissolved matter present in the sample, a second filter made from hydrophilic PTFE was placed on top of the ExC disk, which could be replaced as soon as it was clogged (Fig. 3(1), blue filter).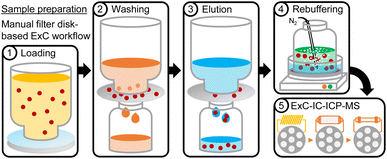 | ||
| Fig. 3 Schematic description of the integrated filtration, clean-up and preconcentration procedure, performed prior to the on-line ExC-IC-ICP-MS method, when samples required it. | ||
After two conditioning steps, first with ethanol (20%, 50 mL, ∼25 mL min−1) and then with HNO3 (0.01 M, 50 mL, ∼25 mL min−1), the entire sample was vacuum filtered (∼25 mL min−1) through the two filter layers, which simultaneously removed particles and preconcentrated [99Tc]TcO4−. Subsequently, only the ExC disk was washed (HNO3, 0.1 M, 20 mL, 10 mL min−1, Fig. 3(2)) and, by again applying vacuum, eluted into a new plastic container (NH4OH, 0.5 M, 100 mL, 25 mL min−1, Fig. 3(3)). The workflow was performed on a PTFE-coated vacuum filter holder set to prevent [99Tc]TcO4− from adsorbing on glass.
For combining the manual preparation workflow with the on-line ExC-IC-ICP-MS method, the eluate from the filter disk extraction was heated in a water bath set at 80 °C, while continuously bubbling N2 through the liquid for approximately 4 h. Together, this resulted in the evaporation of the dissolved NH3 (Fig. 3(4)). If required, the rebuffering step can be performed for multiple samples simultaneously. After a pH value of between 7 and 8 was reached, the remaining eluate was filtered with a hydrophilic syringe filter, diluted to a defined volume and subsequently acidified with HNO3 to a final concentration of 0.01 M. The obtained purified and preconcentrated sample could later be used for the automated on-line method, using as many loading cycles as required for the set sample volume. To rule out losses of 99Tc during the manual preparation workflow, its recovery was determined with a reference solution (10 pg kg−1) of the 99Tc standard. One aliquot of the solution (110.35 mL) was analyzed directly with the on-line ExC-IC method, while an equal aliquot was diluted with bi-distilled water to 1 L to perform the manual two-filter-based vacuum filtration workflow. Afterwards, the eluate from the ExC filter disk was diluted back to the initial volume of the aliquot and analyzed with the on-line ExC-IC-ICP-MS method.
Sample handling
The combination of manual preparation workflow and automated ExC-IC-ICP-MS method was validated in a proof of concept using a sample from a retention basin at the University Hospital of Münster. The sample contained a highly complex matrix with a significant amount of undissolved particulate matter, as it was composed of all combined wastewater collected from the therapy ward of the nuclear medicine department. Sampling took place when the basin underwent respective activity testing with a GM-counter prior to its potential release into the municipal wastewater system. The sample was tested below the required limit, which allowed treating it as common clinical wastewater. A total volume of 961 mL sample was acidified to a HNO3 concentration of 0.01 M and spiked with H2O2 to reach a final content of 2%. This served equally as a disinfection step for the potentially infectious sample, as well as an oxidation step to ensure that all Tc species were converted to [99Tc]TcO4−. Under these conditions [99Tc]TcO4− shows highly mobile and stable behavior to prevent it from forming clusters or complexes with matrix compounds or from sticking to container and instrument surfaces, which would otherwise impair quantitative recovery.39 The resulting sample volume of 1 L was stored at 8 °C until it was prepared using the off-line integrated filtration, clean-up and preconcentration workflow.Results and discussion
On-line method development and figures of merit
The extraction of Tc from complex samples as well as its purification and elution is often associated with harsh extractants with high ionic strength, low pH values and oxidation potentials. These conditions are not immediately compatible with ICP-MS and can be a pitfall for an on-line analysis. Therefore, instead of the frequently featured TEVA resin, this work used TK201 resin packed in an ExC column enabling milder elution conditions (0.5 M NH4OH solution). While this material provided lower selectivity and also broader peak-shapes, thus lowering ultimate signal-to-noise-ratios (S/N), it facilitated ICP-MS coupling substantially.34 To mitigate lower specificity and decreasing signal-to-noise ratios, an additional IC-column was introduced to the method. The column allowed separation and elution of [99Tc]TcO4− in a focused peak of less than 20 s (Fig. 4), compared to more than 80 s for the peak obtained when performing only ExC with TK201 resin. [99Tc]TcO4− eluted from the IC-column in a fraction of about 216 μL and thereby supported a preconcentration factor as high as 510 when loading a sample volume of 110.35 mL. The entire method including loading, washing and the elution took less than 75 min per sample and was fully automated simplifying preparation efforts substantially, thereby improving overall robustness of the analysis.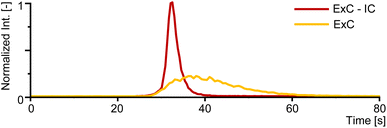 | ||
| Fig. 4 Comparison of the different peak shapes of ExC and ExC-IC from 1 pg kg−1 of 99Tc in 110.35 mL of test sample. | ||
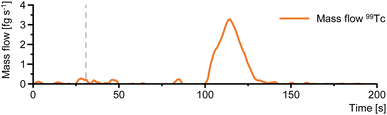
As 99Ru is a potential interference for the accurate determination of 99Tc, the capability to separate these two isotopes using the ExC-IC method was tested using a simulated set-up. As shown in Fig. 5, the two transiently monitored signals of m/z 99 and 101 show a peak for Ru emerging shortly after the eluent switch to NH4NO3 (dashed line) and the later eluting [99Tc]TcO4− at a retention time of 100 s (Fig. 5, upper part).
As the results in Fig. 5 demonstrate, the combination of ExC and IC avoided the co-elution of 99Tc with Ru potentially contained in the sample matrix and enabled the post-column addition of an external Ru-spike for the robust Tc quantification via IBDA. This allowed calculation of the transient 99Tc mass flow as shown in Fig. 5 (bottom), which was subsequently integrated to determine the absolute Tc mass and concentration. Within the mass flow of 99Tc, which was transiently calculated from the corrected isotope ratio of m/z 99 and 101, no indication of an elution of Ru at about 50 s was observed as the ratio between the two isotopic signals remained constant. This was critical as for the calibration of 99Tc, a potential coelution of the two elements, as observed without the added IC separation, would have affected the calculated result by disturbing the continuous Ru mass flow.
Tc concentrations were expected to be below 100 pg kg−1 and therefore, [99Tc]TcO4− recoveries were determined for two concentrations at 1 and 10 pg kg−1 using a loading volume of 110.35 mL, respectively. For this purpose, an in-house prepared and characterized [99Tc]TcO4− standard was used to investigate the possibility to separate it from potentially confounding compounds. Linearity was tested by analyzing increasing levels of a known 99Tc concentration as shown in Fig. 6. The R2 value of the calibration curve was 0.9999. The calibration (Fig. 5, diagram insert) was used to estimate detection and quantification limits of the automated method to be 6.3 ± 0.1 fg kg−1 and 21 ± 2 fg kg−1, respectively.32 Compared to previous methods, this was a significant increase in detection power for [99Tc]TcO4− in aqueous samples, especially when considering the relatively low loading volume of only 110.35 mL.33,34 Besides the focused peak shapes enhancing S/N, the improved sensitivity was a result of the implementation of aerosol desolvation nebulization, which improved the aerosol transport efficiency significantly increasing S/N up to 7.5-fold for individual peaks compared to commonly applied sample introduction techniques with ICP-MS.
 | ||
| Fig. 6 Chromatograms of different injected diluted solutions of the in-house prepared [99Tc]TcO4− standard and the resulting calibration curve. LOD and LOQ were calculated by using the standard deviation of the blank (σ) and the slope of the calibration curve (S) applying 3 and 10σ criteria (LOD = 3 σ·S−1).38 The recovery of the overall automated method is given for two diluted solutions of the [99Tc]TcO4− standard from inside the targeted concentration range. | ||
Off-line filter disk method development
To address the general need for a removal of the undissolved matter present in the targeted samples and to conduct an additional selective matrix cleanup as well as a further analyte preconcentration, an integrated two-filter-based vacuum filtration workflow was developed for 1 L of sample. Since the identical ExC material (TK201) was used for disk and column, a rebuffering step was necessary to prevent [99Tc]TcO4− from passing through the ExC column when eluate from the manual disk-based preparation workflow was to be used for the on-line ExC-IC-method. Multiple approaches, based on different elution condition, eluate dilution as well as neutralization or preparative ion suppression were tested to work up the filter disk eluate, until the final rebuffering step was developed. During the rebuffering, all dissolved NH3 from the NH4OH eluent was removed by evaporating it in a heated water bath while applying a flow of gaseous N2 through the liquid sample. To investigate the recovery, especially during manual handling and rebuffering of the sample, a spiked reference solution (10 pg kg−1) of the generated 99Tc standard (Figure 1, ESI†) was tested. Since wastewater – especially of clinical origin – was expected to vary highly in its exact chemical composition, a reference blank of medical wastewater, which did not contain any [99Tc]TcO4−, but also originated from the same wastewater batch, could not be obtained. The recovery of the off-line workflow was calculated to be above 98%. Peak shapes and widths (<20 s) were not significantly affected by the previous sample handling procedure and the filter disk impregnated with the TK201 extractant had the necessary capacity to trap all loaded [99Tc]TcO4−.With the additional (manual) preconcentration step, sensitivity could be further increased by a factor of 9.06, reaching an ultimate LOD of 0.7 ± 0.1 fg kg−1. The overall preconcentration factor reached with the combination of manual preparation and automated ExC-IC-ICP-MS method was as high as 4615.
Application of the combined setup
In a proof of concept, the combination of manual filter disk extraction workflow and on-line ExC-IC-ICP-MS method was applied to a wastewater sample collected from a retention basin of the nuclear medical department of the University Hospital in Münster (Fig. 7).The sample presented a strong matrix with a significant amount of undissolved matter, as it included all combined wastewater from the therapy ward collected in a certain period of time. Therefore, the hydrophilic PTFE filter disk used above the ExC disk had to be replaced four times. This integrated filtration was crucial and ultimately enabled the loading of 1 L of sample onto the subjacent ExC disk to be analyzed later in the automated on-line method. This strong matrix composition in combination with the large sample volume of the analyzed wastewater sample caused slight broadening to the peak of [99Tc]TcO4. It, however, did not reach the peak widths obtained by solely using ExC methods with TK201 (Fig. 4) and still enabled a significant increase in S/N. In the analyzed sample, [99Tc]TcO4− could be determined at a 99Tc concentration of 89 fg kg−1. The available sample volume only allowed for one analysis to be performed, hence overall method uncertainty was estimated. The main contributors to overall uncertainty were identified to be the process of IBDA calculation, the accurate and repeatable loading of the sample loop and the recovery of each component of the method. The error introduced by the sensitivity correction between 99Tc and the post-column internal Ru standard added during IBDA was approximately 0.8% and estimated by introducing an in-house prepared standard of 99Tc.39 When developing the automated ExC preconcentration, repeatability of the cyclic loading steps of the large sample loop was examined by recording the exact volume of 10 loading cycles with an analytical balance. The volume of all 10 cycles showed a relative standard deviation of less than 0.05%. Individual recovery data of each component included in the automated method can be found in the ESI† and prove no substantial analyte loss taking place during the automated ExC-IC-ICP-MS method. By considering all those three individual sources of uncertainty, the overall method uncertainty was estimated to be less than 4%.39 Given the restricted access to samples in this case, we used this uncertainty budget and estimated the concentration the 99Tc in the analyzed wastewater sample to be 89 ± 4 fg kg−1.”
The entire method consisting of the manual off-line workflow and automated on-line ExC-IC-ICP-MS has a high utility for detecting and quantifying 99Tc in the form of its most stable species, [99Tc]TcO4−, in samples from the medical emission pathway. The results from this proof of concept study provide future opportunities to target 99Tc in a variety of different aqueous samples. Not only medical emission but also other Tc sources andrelease pathways may be investigated.
Conclusions
As one of the most relevant environmental radiocontaminants today, 99Tc was targeted as its most stable species [99Tc]TcO4− in medical wastewater. The detection and quantification limits of the on-line method were determined at 6.3 ± 0.1 fg kg−1 and 21 ± 2 fg kg−1, respectively. This represents a noteworthy enhancement in detection capabilities for [99Tc]TcO4− in aqueous samples, particularly given the smaller loading volume of only 110.35 mL. In combination with a manual preparation workflow, the automated on-line ExC-IC method achieved a preconcentration factor as high as 4615. In a proof of concept, the combined setup facilitated the internal quantification of [99Tc]TcO4− in a sample from the retention basin from the therapy ward of the University Hospital Münster at a 99Tc concentration of 89 ± 4 fg kg−1. However, it is worth noting that access to medical wastewater is somewhat difficult, which limited sample availability and volume for analysis. While the aim of this study was to demonstrate a new automated preconcentration technique for ultra trace determination of Tc, it is likely that Tc concentration drastically change across different times and locations. To gain a more holistic understanding of the Tc release into the environment, more investigations at different sites and times are required.The analysis of Tc in the environment is challenged by extremely low trace amounts, complex matrices and a requirement for extensive sample preparations. The method demonstrated here allows to overcome long standing obstacles and facilitates preconcentration whilst reducing the required sample volume. As such, it offers an opportunity to drive the environmental monitoring of Tc and to better understand its discharge and distribution through different sources.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
All authors have given approval to the final version of the manuscript.Conflicts of interest
C. D. Quarles is an employee of Elemental Scientific, Inc. and S. Happel is an employee of TrisKem International SAS.Acknowledgements
M. Horstmann, U. Karst and A. Faust gratefully acknowledge the Deutsche Forschungsgemeinschaft (DFG) – CRC 1450 – 431460824 for funding. D. Clases acknowledges the financial support by the University of Graz.References
- C. Perrier and E. Segrè, Some Chemical Properties of Element 43, J. Chem. Phys., 1937, 5(9), 712–716, DOI:10.1063/1.1750105.
- R. V. Hercigonja, D. D. Maksin, A. B. Nastasović, S. S. Trifunović, P. B. Glodić and A. E. Onjia, Adsorptive Removal of Technetium-99 Using Macroporous Poly(GMA-Co-EGDMA) Modified with Diethylene Triamine, J. Appl. Polym. Sci., 2012, 123, 1273–1282, DOI:10.1002/app.34693.
- S. Gerland, B. Lind, M. Dowdall, M. Karcher and A. K. Kolstad, 99Tc in Seawater in the West Spitsbergen Current and Adjacent Areas, J. Environ. Radioact., 2003, 69(1–2), 119–127, DOI:10.1016/S0265-931X(03)00090-0.
- S. Walas, K. Kleszcz, A. Tobiasz, H. Mrowiec and J. W. Mietelski, Determination of Technetium-99 in Peat by Flow Injection–Inductively Coupled Plasma Mass Spectrometry, Anal. Lett., 2016, 49(17), 2755–2765, DOI:10.1080/00032719.2016.1156119.
- N. Ishii, K. Tagami and S. Uchida, Physicochemical Forms of Technetium in Surface Water Covering Paddy and Upland Fields, Chemosphere, 2004, 57(8), 953–959, DOI:10.1016/j.chemosphere.2004.07.027.
- V. Smith, M. Fegan, D. Pollard, S. Long, E. Hayden and T. P. Ryan, Technetium-99 in the Irish Marine Environment, J. Environ. Radioact., 2001, 56(3), 269–284, DOI:10.1016/S0265-931X(00)00209-5.
- M. García-León, 99Tc in the Environment: Sources, Distribution and Methods, J. Nucl. Radiochem. Sci., 2005, 6(3), 253–259, DOI:10.14494/jnrs2000.6.3_253.
- B. R. Harvey, K. J. Williams, M. B. Lovett and R. D. Ibbett, Determination of Technetium-99 in Environmental Material with Rhenium as a Yield Monitor, J. Radioanal. Nucl. Chem., 1992, 158(2), 417–436, DOI:10.1007/BF02047127.
- K. Tagami, Technetium-99 Behavior in the Terrestrial Environment -Field Observations and Radiotracer Experiments, J. Nucl. Radiochem. Sci., 2003, 4(1), A1–A8, DOI:10.14494/jnrs2000.4.A1.
- K. S. Leonard, D. McCubbin, J. Brown, R. Bonfield and T. Brooks, Distribution of Technetium-99 in UK Coastal Waters, Mar. Pollut. Bull., 1997, 34(8), 628–636, DOI:10.1016/S0025-326X(96)00185-3.
- P. B. du Bois, F. Dumas, C. Voiseux, M. Morillon, P. E. Oms and L. Solier, Dissolved Radiotracers and Numerical Modeling in North European Continental Shelf Dispersion Studies (1982-2016): Databases, Methods and Applications, Water, 2020, 12(6), 1667–1705, DOI:10.3390/w12061667.
- Centre for Environment Fisheries and Aquaculture Science, Radioactivity in Food and the Environment 2021; London, 2022, https://www.foodstandards.gov.scot/downloads/Radioactivity_in_Food_and_the_Environment_%28RIFE%29_28_-_2022.pdf Search PubMed.
- I. L. Pegg, Behavior of Technetium in Nuclear Waste Vitrification Processes, J. Radioanal. Nucl. Chem., 2015, 305(1), 287–292, DOI:10.1007/s10967-014-3900-9.
- Centre for Environment Fisheries and Aquaculture Science, Radioactivity in Food and the Environment 2009; London, 2010, https://www.food.gov.uk/sites/default/files/media/document/rife2009.pdf Search PubMed.
- M. Horstmann, M. Austrup, F. Busch, A. Faust, M. Sperling, U. Karst and D. Clases, Speciation Analysis of Tc Radiopharmaceuticals by HPLC-ICP-MS and HPLC-ESI-HRMS, J. Anal. At. Spectrom., 2023, 38(10), 2038–2045, 10.1039/d3ja00257h.
- Organisation for Economic Co-operation and Development; Nuclear Energy Agency, The Supply of Medical Isotopes: an Economic Diagnosis and Possible Solutions; Paris, 2019, DOI:10.1787/9b326195-en.
- International Atomic Energy Agency, Technetium-99m Radiopharmaceuticals: Manufacture of Kits; Technical Report 466, IAEA, Vienna, 2008 Search PubMed.
- W. Eckelman and P. Richards, Instant 99mTc-DTPA, J. Nucl. Med., 1970, 11(12), 761 CAS.
- G. Crișan, N. S. Moldovean-Cioroianu, D.-G. Timaru, G. Andrieș, C. Căinap and V. Chiș, Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade, Int. J. Mol. Sci., 2022, 23(9), 5023, DOI:10.3390/ijms23095023.
- International Atomic Energy Agency, Nuclear Technology Review 2010 - Annex VII Production and Supply of Molybdenum-99, IAEA, Vienna, 2010 Search PubMed.
- Nuclear Energy Agency, The Supply of Medical Radioisotopes: 2019 Medical Isotope Demand and Capacity Projection for the 2019-2024 Period; Report, NEA, Paris, 2019 Search PubMed.
- K. L. Shi, X. L. Hou, P. Roos and W. S. Wu, Determination of Technetium-99 in Environmental Samples: A Review, Anal. Chim. Acta, 2012, 709, 1–20, DOI:10.1016/j.aca.2011.10.020.
- D. Clases, M. Sperling and U. Karst, Analysis of Metal-Based Contrast Agents in Medicine and the Environment, Trends Anal. Chem., 2018, 104, 135–147, DOI:10.1016/j.trac.2017.12.011.
- Q. J. Chen, H. Dahlgaard and S. P. Nielsen, Determination of 99Tc in Sea Water at Ultra Low Levels, Anal. Chim. Acta, 1994, 285(1–2), 177–180, DOI:10.1016/0003-2670(94)85021-6.
- J. Barrera, A. Tarancón, H. Bagán and J. F. García, A New Plastic Scintillation Resin for Single-Step Separation, Concentration and Measurement of Technetium-99, Anal. Chim. Acta, 2016, 936, 259–266, DOI:10.1016/j.aca.2016.07.008.
- G. Barci-Funel, S. Ballestra, E. Holm, J. J. Lopez and G. Ardisson, Technetium-99 in the Rhône River, Water, 1991, 153(6), 431–438, DOI:10.1007/BF02165897.
- M. Villar, J. Avivar, L. Ferrer, A. Borràs, F. Vega and V. Cerdà, Automatic In-Syringe Dispersive Liquid–Liquid Microextraction of 99Tc from Biological Samples and Hospital Residues Prior to Liquid Scintillation Counting, Anal. Bioanal. Chem., 2015, 407(19), 5571–5578, DOI:10.1007/s00216-015-8761-8.
- H. W. Fischer, S. Ulbrich, D. Pittauerová and B. Hettwig, Medical Radioisotopes in the Environment - Following the Pathway from Patient to River Sediment, J. Environ. Radioact., 2009, 100(12), 1079–1085, DOI:10.1016/j.jenvrad.2009.05.002.
- M. Villar, A. Borràs, J. Avivar, F. Vega, V. Cerdà and L. Ferrer, Fully Automated System for 99Tc Monitoring in Hospital and Urban Residues: A Simple Approach to Waste Management, Anal. Chem., 2017, 89(11), 5857–5863, DOI:10.1021/acs.analchem.7b00184.
- S. Morita, K. Tobita and M. Kurabayashi, Determination of Technetium-99 in Environmental Samples by Inductively Coupled Plasma Mass Spectrometry, Radiochim. Acta, 1993, 63, 63–67, DOI:10.1524/ract.1993.63.special-issue.63.
- D. M. Hill, R. K. Barnes, H. K. Y. Wong and A. W. Zawadzki, The Quantification of Technetium in Generator-Derived Pertechnetate Using ICP-MS, Appl. Radiat. Isot., 2000, 53(3), 415–419, DOI:10.1016/S0969-8043(99)00280-8.
- N. Momoshima, M. Sayad and Y. Takashima, Analytical Procedure for Technetium-99 in Seawater by ICP-MS, Radiochim. Acta, 1993, 63(s1), 73–78, DOI:10.1524/ract.1993.63.special-issue.73.
- K. L. Shi, J. X. Qiao, W. S. Wu, P. Roos and X. L. Hou, Rapid Determination of Technetium-99 in Large Volume Seawater Samples Using Sequential Injection Extraction Chromatographic Separation and ICP-MS Measurement, Anal. Chem., 2012, 84(15), 6783–6789, DOI:10.1021/ac301319a.
- M. Matsueda, K. Yanagisawa, K. Koarai, M. Terashima, K. Fujiwara, H. Abe, A. Kitamura and Y. Takagai, Online Solid-Phase Extraction-Inductively Coupled Plasma-Quadrupole Mass Spectrometry with Oxygen Dynamic Reaction for Quantification of Technetium-99, ACS Omega, 2021, 6(29), 19281–19290, DOI:10.1021/acsomega.1c02756.
- D. Clases, M. Birka, M. Sperling, A. Faust and U. Karst, Isobaric Dilution Analysis as a Calibration Tool for Long Lived Radionuclides in ICP-MS, J. Trace Elem. Med. Biol., 2017, 40, 97–103, DOI:10.1016/j.jtemb.2017.01.002.
- M. Macke, C. D. Quarles, M. Sperling and U. Karst, Fast and Automated Monitoring of Gadolinium-Based Contrast Agents in Surface Waters, Water Res., 2021, 207, 117836, DOI:10.1016/j.watres.2021.117836.
- C. D. Quarles, P. Sullivan, N. Bohlim and N. Saetveit, Rapid Automated Total Arsenic and Arsenic Speciation by Inductively Coupled Plasma Mass Spectrometry, J. Anal. At. Spectrom., 2022, 37(6), 1240–1246, 10.1039/d2ja00055e.
- P. W. J. M. Boumans, Measuring Detection Limits in Inductively Coupled Plasma Emission Spectrometry Using the “SBR-RSDB Approach”-I. A Tutorial Discussion of the Theory, Spectrochim. Acta, Part B, 1991, 46(3), 431–445, DOI:10.1016/0584-8547(91)80040-A.
- M. Horstmann, C. D. Quarles Jr, S. Happel, M. Sperling, A. Faust, K. Rahbar, D. Clases and U. Karst, Quantification of [99Tc]TcO4- in Urine by Means of Anion-Exchange Chromatography-Aerosol Desolvation Nebulization-Inductively Coupled Plasma-Mass Spectrometry, Anal. Bioanal. Chem., 2024, 416, 2849–2858, DOI:10.1007/s00216-024-05149-4.
- P. Rodríguez-González, J. M. Marchante-Gayón, J. I. García Alonso and A. Sanz-Medel, Isotope Dilution Analysis for Elemental Speciation: A Tutorial Review, Spectrochim. Acta, Part B, 2005, 60(2), 151–207, DOI:10.1016/j.sab.2005.01.005.
Footnote |
| † Electronic supplementary information (ESI) available: The Supporting Information is available free of charge on the ACS Pub-lications website and contains common parameters for all instruments used in the on-line ExC-IC-ICP-MS method and recovery data for ExC column, IC-column and chemically regenerated cation-suppressor and ExC filter disk, as well as a compilation of studies quantifying 99Tc by means of ICP-MS with their respective LODs. (PDF). See DOI: https://doi.org/10.1039/d4ja00270a |
| This journal is © The Royal Society of Chemistry 2024 |