Sensitivity improvement of laser-induced breakdown spectroscopy to detect heavy metals in water by Tesla coil discharge
Qiuyun
Wang
 ab,
Anmin
Chen
ab,
Anmin
Chen
 *b and
Xun
Gao
*b and
Xun
Gao
 *a
*a
aSchool of Physics, Changchun University of Science and Technology, Changchun 130022, China. E-mail: gaoxun@cust.edu.cn
bInstitute of Atomic and Molecular Physics, Jilin University, Changchun 130012, China. E-mail: amchen@jlu.edu.cn
First published on 23rd November 2023
Abstract
In this paper, Tesla coil discharge (TCD) was combined with laser-induced breakdown spectroscopy (LIBS). The TCD successfully enhanced the emission of Al atomic lines and AlO molecular bands in Al LIBS, and improved the sensitivity of quantitative analysis of Cr and Pb elements detected by LIBS in water. Firstly, the paper explored the effect of the distance from the TCD tip to the sample surface on the emission of Al atomic lines and AlO molecular bands, finding that 5 mm was the optimal distance. Secondly, the paper investigated the effect of laser energy on the emission of Al atomic lines and AlO molecular bands, finding that 25.1 mJ was the optimal laser energy. Finally, the paper constructed the calibration curves of Cr and Pb elements in water and calculated the limit of detection (LoD), finding that compared with surface enhanced LIBS (SELIBS), TCD–SELIBS reduced the LoDs of Cr and Pb elements from 16.7 ng mL−1 and 12.2 ng mL−1 to 7.3 ng mL−1 and 5.5 ng mL−1, respectively. It indicated that the combination of TCD and SELIBS can achieve high-sensitivity detection of Cr and Pb elements in the water. The combination of TCD and LIBS is a simple, low-cost, easy-to-operate, industrialized and commercialized technology, and has a promising application prospect in the high-sensitivity analysis of heavy metals in water.
1. Introduction
Heavy metal elements generally refer to metal elements with a density greater than 4.5 g cm−3, including gold (Au), silver (Ag), copper (Cu), iron (Fe), mercury (Hg), lead (Pb), cadmium (Cd), chromium (Cr), etc. Heavy metal elements exist in nature in the form of chemical compounds. When the environment is polluted by heavy metal elements, they can be enriched in the body and enter the human body through the food chain, which can cause heavy metal poisoning and seriously endanger human life and health.1 Therefore, it is very necessary to increase the detection of heavy metals in the environment.The traditional methods of material composition analysis include atom absorption spectroscopy, spectrophotometry, inductively coupled plasma emission spectroscopy, atom fluorescence spectroscopy, electrochemical analysis, and inductively coupled plasma mass spectrometry.2–6 These traditional methods have the advantages of high detection sensitivity, detection accuracy and stability, but they all require complex chemical pretreatment of the sample to be tested, the single detection time is long and it is easy to cause secondary pollution, so it is only used in laboratory analysis.
With the development of laser spectroscopy, laser-induced breakdown spectroscopy (LIBS) can overcome the above problems well. LIBS technology is an elemental analysis technique with enormous potential for development. It uses the pulse laser as the excitation source to ablate the sample to be tested to generate laser plasma, and detects the emission spectrum of the plasma through a spectrometer, analyzing the spectrum to obtain the element type and content information of the sample to be tested.7–9 It has the unique advantages of simultaneous detection of multiple elements, simple structure, fast detection and analysis speed, and is not affected by the sample state (solid, liquid, and gas),10–12 and has always received a lot of attention.13–20 Especially in water pollution and soil monitoring, nuclear material testing, food testing, deep sea exploration, and other fields, it has great application prospects. However, due to the complex interaction process between the laser and the sample, as well as the large fluctuation of the transient plasma, improving the quantitative performance (detection sensitivity, detection accuracy, and spectral stability) of LIBS technology has always been a hot topic of current research.
To solve the problem of low sensitivity when using LIBS to analyze water samples, researchers used different methods to transfer heavy metal elements in water to solid samples, such as: using pure carbon, graphite, and wood chips to absorb heavy metal aqueous solutions; freezing heavy metal aqueous solutions; dropping heavy metal aqueous solutions on the surface of solid substrates; enriching metal ions in water to other metal surfaces by electrochemical enrichment, etc.21–29, and then the solid samples are analyzed by LIBS. These methods can avoid the problems of direct analysis of water samples, such as poor laser transmission performance of optical components, rapid quenching of atomic emission in laser plasma, and significantly improve the sensitivity and precision of spectral analysis. To this end, surface enhanced LIBS (SELIBS) was selected in this experiment to improve the detection sensitivity and precision of heavy metal elements in water, and reduce the limit of detection (LoD) of heavy metal elements.
To further improve the spectral analysis sensitivity of LIBS technology to analyze heavy metals elements in water, researchers not only made efforts in sample preparation, but also made new explorations in experimental equipment, such as double-pulse LIBS, magnetic confined LIBS, LIBS with laser-induced fluorescence, discharge assisted LIBS, and microwave assisted LIBS.30,31 Among them, discharge assisted LIBS plays an important role in the research field of LIBS enhancement because of its simple device and large enhancement fact. He et al. used femtosecond laser ablation spark-induced breakdown spectroscopy to analyze trace elements in aluminum alloys with an input voltage of 4 kV,32 showing that discharge assistance could enhance the atomic emission intensity of plasma and reduce the LoD of element analysis. Hassanimatin et al. used a combination of electrical spark and LIBS with an input voltage of 5 kV to investigate the effect of experimental parameters on increasing the spectral signal,33 showing that the study could effectively enhance plasma emission and signal-to-noise ratio. Sobral et al. used spark-discharge-assisted laser ablation to study the optical emission of Al plasmas with an input voltage of 12 kV,34 showing that the study achieved stronger signal intensities without increasing the background emission compared to conventional LIBS and spark discharge. Nassef et al. combined LIBS with spark discharge to study the optical emission of Al and Cu plasmas with an input voltage of 10 kV,35 showing that the study significantly enhanced the optical emission and signal-to-background ratio of Al and Cu plasma compared with LIBS alone. The above-mentioned discharge-assisted LIBS technology requires a power supply with a high voltage of several kV, and a high voltage often means a high current. High voltages and high currents are potentially dangerous both in the laboratory and in the industry. Therefore, it is particularly important to find a low-voltage discharge device.
This paper used a Tesla coil discharge (TCD) device instead of a traditional high voltage discharge device to enhance the emission signal of plasma and reduce the LoD of heavy metal elements in the water. The Tesla coil uses the principle of resonance to oscillate and boost the voltage with two-stage coils, and high-frequency and high-voltage alternating current can be obtained from the discharge terminal. The high-voltage breaks down the air to form an arc discharge, that is, a high-voltage generator that uses high-voltage resonance for energy conversion. The Tesla coil used in this experiment is a small discharge circuit with an input voltage of 24 V DC, and the device is easy to combine with LIBS. It has great application prospects in laboratory and industry. Therefore, this paper combined TCD with LIBS (TCD-LIBS) to study the intensities of Al atomic lines and AlO molecular bands in Al plasma. On this basis, the TCD was combined with SELIBS to investigate the detection sensitivity of Cr and Pb elements in the water.
2. Experiment details
2.1 TCD-LIBS system
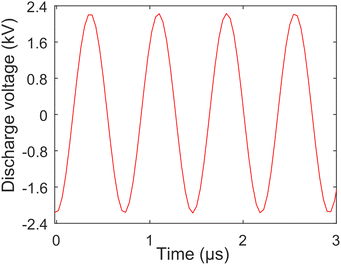
The diagram of the TCD-LIBS experimental setup is displayed in Fig. 1, which mainly includes: laser system (Continuum, Surelite III), TCD system, spectral acquisition system of spectrometer (Princeton Instruments, SP-500I, PI-Acton) + ICCD (Princeton Instruments, PIMAX-4), 3D translation stage (Thorlabs, PT3/M-Z8), and computer. The Q-switched Nd:YAG laser provided the excitation source for the experimental system, and its output wavelength was 1064 nm, the repetition ratio was 10 Hz, the pulse width was 10 ns, the spot diameter was about 400 μm, and the energy range used was from 6.3 mJ to 25.1 mJ. In the TCD system, a high-voltage alternating current with 2.35 kV, 1.35 MHz, 24 W, and 14.5 mA of peak voltage, frequency, power, and current generated a discharge arc between a 0.5 mm diameter tungsten electrode (the electrode was polished into a pointed shape) and the sample surface. The discharge waveform of the TCD was sinusoidal and very stable. The high-voltage alternating current was measured by a high-voltage probe connected to an oscilloscope, and the voltage distribution of the TCD with time is shown in Fig. 2. The distance between the tungsten electrode and the sample surface was 1–6 mm, and the electrode and the sample surface was at 45°. The dimensions of the Tesla discharge device were 8 × 8 × 20 cm3, and the height and diameter of the secondary coil were 10 and 4 cm, respectively. To ensure that the arc position of TCD on the sample surface was consistent with the laser position, the discharge tip of TCD was placed on a manual three-dimensional stage. The distance between the discharge tip and the sample surface was controlled by adjusting the position of the stage, and the laser position was continuously observed through a display screen equipped with an electron microscope. Moreover, TCD did not need to be synchronized with laser pulses. As long as the TCD device was powered on, the discharge arc will be generated and present throughout the entire detection process. The grating and resolution of the spectrometer + ICCD were 1200 lines per mm and 0.04 nm, respectively. A photodiode (PD) triggered the ICCD to synchronize the delay time between the laser pulse and plasma emission. The gate delay and width of the ICCD were set to 0.5 μs and 40 μs, respectively. The 3D translation stage controlled the movement of the sample, which could prevent the laser from hitting the same point of the sample to improve the stability of the detection. In addition, the detection setup also included: iris (I), mirror (M), lenses (L1, focal length 25 cm; L2, focal length 75 mm and diameter 50 mm), dichroic mirror (DM), and fiber. The entire experiment was performed in the atmosphere, and each spectrum was an average of 120 spectral data.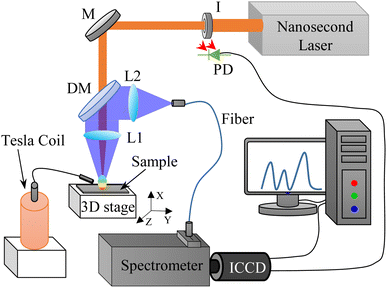 | ||
| Fig. 1 Schematic diagram of TCD-LIBS experimental setup (I: iris; M: 1064 nm mirror; DM: dichroic mirror; L: focusing lens; ICCD: intensified CCD; PD: photodiode). | ||
2.2 Sample preparation
Under laboratory conditions, deionized water was mixed with CrCl3·6H2O and Pb(NO3)2 (Sinopharm Chemical Reagent Co., Ltd, purity greater than 99.0%) powder to prepare stock solutions with a concentration of 10 μg mL−1, and then were diluted to several different concentrations are listed in Table 1.| Sample | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Cr concentration (ng mL−1) | 50 | 100 | 200 | 300 | 400 | 500 |
| Pb concentration (ng mL−1) | 100 | 200 | 400 | 600 | 800 | 1000 |
The schematic diagram of the solid–liquid conversion process is shown in Fig. 3. The specific procedures are as follows:
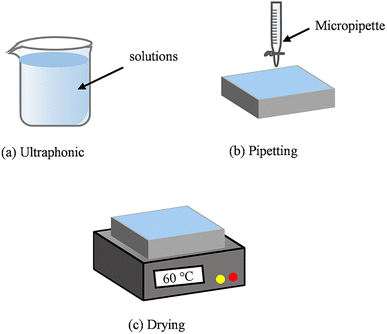 | ||
| Fig. 3 Schematic diagram of the solid–liquid conversion process. (a) Ultraphonic; (b) pipetting; (c) drying. | ||
(a) The Al substrate was placed in alcohol and deionized water for ultrasonic treatment (10 min). This step was essential to remove oil and impurities from the Al substrate surface.
(b) 120 μL microdroplets were deposited on the Al substrate surface using a pipette gun, with an average of 6 drops.
(c) The temperature of the heating table was set to 60 °C to prepare a heavy metal layer on the Al substrate surface. The content of the heavy metal elements in the heavy metal layer corresponded to the content in the standard aqueous solution.
3. Results and discussion
3.1 Emission spectra
Firstly, the experiment selected a highly pure aluminum plate (size 5.0 × 5.0 × 0.12 cm3) as the sample to be analyzed. The measured neutral atomic lines were Al(I) 394.4 nm and 396.1 nm. And the measured radical emission bands were AlO (0–0), (1–1), and (2–2) with the B2Σ+ − X2Σ+ (Δν = 0) transition.36Fig. 4 presents emission spectra of Al(I) and AlO in Al plasmas with and without the TCD at 25.1 mJ laser energy and 5 mm discharge distance. It can be found from the figure that the intensities of the Al(I) and AlO bands with the TCD are stronger than without the TCD. This suggests that the TCD can significantly enhance the intensities of Al(I) and AlO bands. The discharge arc of the TCD partially ionizes the air and enhances carrier collisions. The discharge arc pre-ionizes the sample surface before the pulsed laser reaches the sample surface, and intensifies the lattice vibration, thereby reducing the laser excitation threshold.37 In addition, the TCD can cause an increase in the sample temperature, reduce the reflectivity of the sample surface,38 increase the coupling efficiency between the sample and the pulsed laser, and increase the ablation mass of the sample, resulting in an enhancement in spectral intensity.38,39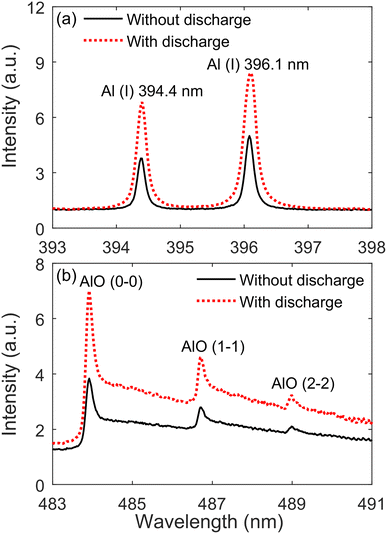 | ||
| Fig. 4 Emission spectra of Al(I) lines (a) and AlO molecular bands (b) in Al plasmas with and without the TCD at 25.1 mJ laser energy and 5 mm discharge distance. | ||
3.2 Effect of discharge length
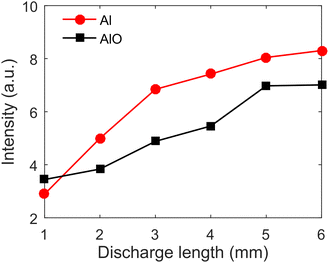
Fig. 5 presents the evolution of Al(I) 396.1 nm and AlO (0–0) peak intensities with the discharge distance. The distance between the tungsten electrode and the sample surface is from 1 mm to 6 mm, and the other emissions of Al(I) lines and AlO molecular bands have the same trend as Al(I) 396.1 nm and AlO (0–0). When the discharge distance is from 1 mm to 6 mm, the emission intensity of Al(I) 396.1 nm and AlO (0–0) increases, and the discharge arc is mainly applied to the sample surface. And, the enhancement amplitude of emission intensity at 5 and 6 mm discharge distance is small. When the discharge distance is longer than 6 mm, the discharge arc becomes unstable or even disconnects from the sample surface, resulting in a decrease in the pre-ionization amount on the sample surface and a decrease in the spectral intensity. Therefore, the discharge distance of 5 mm is used for the following experiments.3.3 Effect of laser energy
Fig. 6 presents the evolution of Al(I) 396.1 nm and AlO (0–0) peak intensities with laser energy at 5 mm discharge distance. The laser energy ranges from 6.3 to 25.1 mJ, and the other emission lines of Al(I) and AlO molecular bands have the same trend as Al(I) 396.1 nm and AlO (0–0). It can be found from the figure that the peak intensity of Al(I) 396.1 nm and AlO (0–0) increases with an increase in laser energy, and the peak intensity with TCD under the same laser is stronger than without TCD. When the laser energy exceeds the ablation threshold of the sample, ablation occurs and a laser plasma is generated. As the laser energy increases, the ablation efficiency of the sample also increases, and the spectral intensity of the laser plasma increases. Therefore, laser energy of 25.1 mJ is chosen for the following experiments.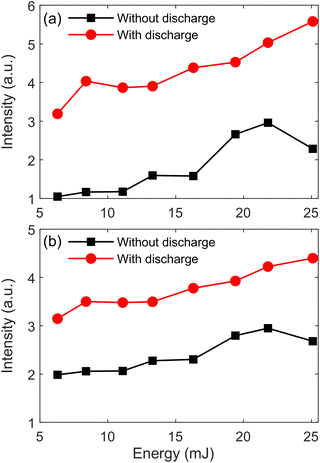 | ||
| Fig. 6 Evolution of Al(I) 396.1 nm (a) and AlO (0–0) (b) peak intensities with laser energy at 5 mm discharge distance. | ||
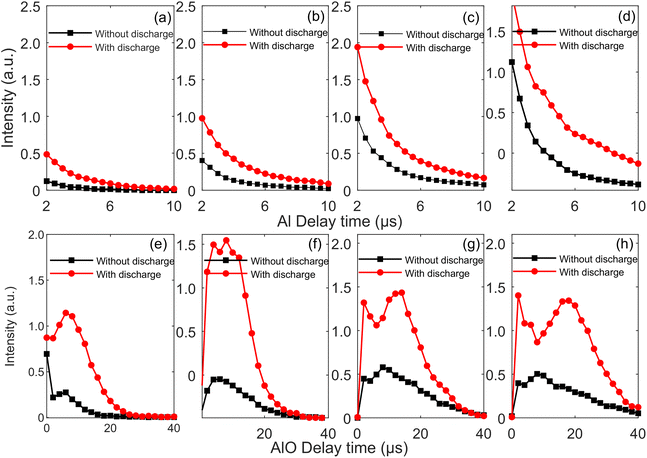
LIBS is a dynamic decay process, and the decay process of Al(I) is different from that of AlO molecular bands. Therefore, it is necessary to discuss the time-resolved spectra of Al(I) and AlO molecular bands under the TCD-LIBS. The gate widths of the ICCD used to measure the time-resolved spectra of Al(I) and AlO molecular bands were set to 0.5 μs and 2 μs, respectively. Fig. 7 presents the time-resolved peak intensities of Al(I) 396.1 nm and AlO (0–0) with and without the TCD. It can be found from Fig. 7(a–d) that the peak intensity of Al(I) decreases monotonically with and without the TCD, the peak intensity of Al(I) is stronger and the plasma lifetime is longer with the TCD. This verifies that the integrated intensity of Al(I) with TCD is stronger than that without the TCD, as shown in Fig. 6(a). It can be found from Fig. 7(e–h) that the peak intensity of AlO (0–0) will have a peak intensity with and without the TCD, the peak intensity of AlO (0–0) is stronger and the plasma lifetime is longer with the TCD. This verifies that the integrated intensity of AlO (0–0) with the TCD is stronger than that without the TCD, as shown in Fig. 6(b). The peak intensity phenomenon of the AlO (0–0) is explained as follows: (1) during early times of the plasma expansion, the shock waves at the plume front act as a barrier, keeping oxygen around the plasma away from the plume; (2) after the collapse of this shock wave, Al particles collide and combine with O2 molecules and O neutral particles in the surrounding air to form AlO molecules; (3) AlO molecules are unstable at high plasma temperatures and soon dissociate into Al and O atoms in the form of AlO → Al + O, and the peak intensity of AlO molecules decreased over time.
3.4 Quantitative analysis of Cr and Pb elements in water
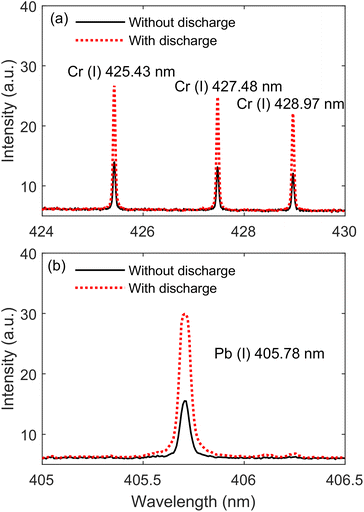
Fig. 8 presents the emission spectra of Cr(I) 425.43 nm, Cr(I) 427.48 nm, Cr(I) 428.97 nm, and Pb(I) 405.78 nm with and without the TCD at 25.1 mJ laser energy and 5 mm discharge distance. The concentrations of Cr and Pb are 500 and 1000 ng mL−1, respectively. It can be found from the figure that the intensities of Cr(I) 425.43 nm, Cr(I) 427.48 nm, Cr(I) 428.97 nm, and Pb(I) 405.78 nm with the TCD are stronger than without the TCD, the result is consistent with that of Al(I) lines and AlO molecular bands, which indicates that the TCD not only enhances the spectral intensity of metal substrates, but also enhances the spectral intensity of heavy metal elements in the water. This also indirectly proves that the enhancement mechanism of the TCD on metal samples is consistent with heavy metal elements in water, that is, the TCD can pre-ionize the sample, reduce the reflectivity of the surface of the sample, and enhance the coupling efficiency between the laser and the sample.To evaluate the analytical sensitivity of TCD–SELIBS technology to Cr and Pb trace heavy metal elements in water, the calibration curves of Cr and Pb heavy metal elements are established in the cases of TCD–SELIBS and SELIBS, respectively, as shown in Fig. 9. The concentration range of element detection in water is generally at the trace level, and the spectral line is not affected by the self-absorption effect, so straight line fitting can be used to obtain the calibration curve of the target element. The calibration curve of the target element can be expressed as I = S·C + b.21 Where I is spectral line intensity, C is the element concentration. S is the slope of the calibration curve, which is usually used to express the sensitivity of the analytical method. b is the intercept of the calibration curve, which is determined by the continuous background intensity of the spectrum.
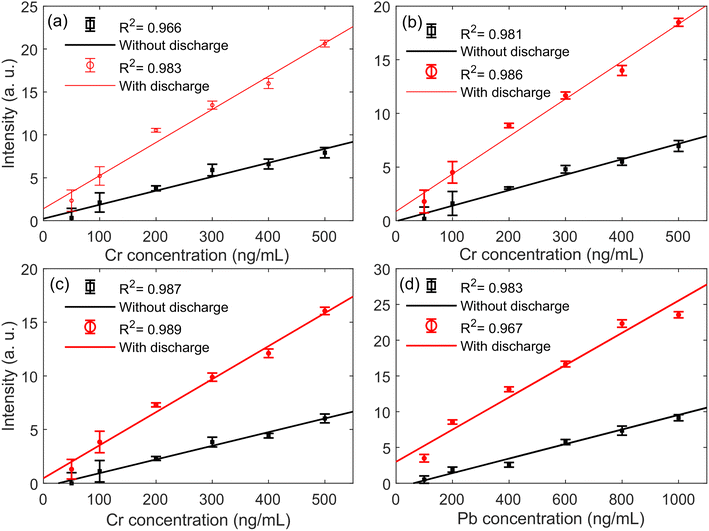 | ||
| Fig. 9 Calibration curves of Cr(I) 425.43 nm (a), Cr(I) 427.48 nm (b), Cr(I) 428.97 nm (c), and Pb(I) 405.78 nm (d) with and without the TCD at 25.1 mJ laser energy and 5 mm discharge distance. | ||
The linear correlation coefficient (R) of the calibration curve is mainly used to describe the correlation between spectral intensity and element concentration. In LIBS analysis, the square value of the correlation coefficient (R2, determination coefficient) is often used to describe the correlativity.40 The closer the value is to 1, the better the correlation between concentration and spectral intensity. According to Fig. 9, the calculated R2 values of Cr and Pb elements without and with the TCD are listed in Table 2. The results show that the TCD can significantly improve the R2 values of Cr and Pb elements, proving that the TCD can improve the correlativity between spectral line intensity and concentration. Spectral intensity is one of the parameters of the quantitative analysis model. The smaller the value fluctuation, the more stable the spectrum. However, in the processes of LIBS acquisition, systematic and accidental errors will cause the spectral emission fluctuation of target elements. For example, the jitter of ambient temperature and laser output energy will affect the characteristic spectral lines of elements. Under the same conditions, the relative standard deviation (RSD) of the spectral intensity obtained by multiple measurements is usually used to evaluate spectral stability. The smaller the value, the more stable the spectral intensity. According to Fig. 9, the calculated RSDs of Cr and Pb elements without and with the TCD are listed in Table 2. The table shows that the TCD can significantly improve the RSDs of Cr and Pb elements, proving that the TCD can improve spectral stability. The LoD of the element is determined by the 3σ principle, that is, LoD = 3σ/S.41σ is the background noise near the characteristic spectral line of the target element, that is, the standard deviation of the background value; S is the slope value of the calibration curve. According to Fig. 9, the calculated LoDs of Cr and Pb elements without and with the TCD are listed in Table 2. The table shows that the TCD can significantly reduce the LoDs of Cr and Pb elements, that is, the LoDs are reduced by about half, proving that the TCD can improve the sensitivity of quantitative analysis.
| Element | With/without discharge | R 2 | LoD (ng mL−1) | RSD (%) |
|---|---|---|---|---|
| Cr(I) 425 43 nm | Without | 0.964 | 16.7 | 7.02 |
| With | 0.983 | 7.3 | 5.87 | |
| Cr(I) 427.48 nm | Without | 0.979 | 18.8 | 7.29 |
| With | 0.987 | 8.1 | 5.79 | |
| Cr(I) 428.97 nm | Without | 0.986 | 21.3 | 7.55 |
| With | 0.989 | 9.1 | 6.40 | |
| Pb(I) 405.78 nm | Without | 0.982 | 23.1 | 13.22 |
| With | 0.964 | 10.5 | 4.7 |
Finally, to evaluate the accuracy of TCD–SELIBS technology for quantitative analysis of Cr and Pb elements in water, we calculated the cross-validation root mean square error (RMSECV) and average relative error (ARE) values of Cr and Pb elements without and with the TCD, as shown in Table 3. The results show that the TCD can significantly decrease RMSECV and ARE, indicating the TCD can significantly improve detection accuracy.
| Spectral line (nm) | With/without TCD | RMSECV (ng mL−1) | ARE (%) |
|---|---|---|---|
| Cr(I) 425.43 | Without | 12.2 | 3.67 |
| With | 8.62 | 2.22 | |
| Cr(I) 427.48 | Without | 9.16 | 2.87 |
| With | 7.68 | 2.13 | |
| Cr(I) 428.97 | Without | 7.52 | 2.07 |
| With | 6.81 | 2.11 | |
| Pb(I) 405.78 | Without | 13.22 | 1.36 |
| With | 4.71 | 3.77 |
4. Conclusions
In this paper, a simple, low-cost, easy-to-operate, industrialized and commercialized technology, namely TCD-LIBS, was developed to enhance the spectral intensity of plasma and improve the detection sensitivity of heavy metal elements in the water. Firstly, the TCD-LIBS could significantly enhance the intensities of Al(I) lines and AlO molecular bands in Al plasmas. The mechanism of spectral intensity enhancement was attributed to the pre-ionization of the sample surface by the discharge arc of the TCD. Moreover, the heating of the sample surface by the discharge arc led to a decrease in the reflectivity of the sample surface, thereby improving the coupling efficiency between the laser and the sample. Subsequently, the Cr and Pb elements in water were quantitatively analyzed using the TCD–SELIBS technique, and the results showed that compared with SELIBS, TCD–SELIBS reduced the LoDs of Cr and Pb elements from 16.74 ng mL−1 and 12.2 ng mL−1 to 7.3 ng mL−1 and 5.5 ng mL−1, respectively. That is, the LoDs were reduced by about half, proving that the TCD can improve the sensitivity of quantitative analysis in LIBS. In conclusion, the TCD-LIBS not only has high safety and operability, but can enhance the spectral intensity to a certain extent and improve the detection sensitivity of heavy metal elements in the water. Therefore, the technique has potential development in the application of LIBS.Author contributions
Qiuyun Wang: investigation, conceptualization, methodology, data curation, software, formal analysis, and writing – original draft. Anmin Chen: investigation, conceptualization, methodology, project administration, formal acquisition, and writing – review & editing, resources, supervision. Xun Gao: project administration, funding acquisition, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the Natural Science Foundation of Jilin province (20220101035JC), National Natural Science Foundation of China (No. 11674128, 11674124, and 11974138) and the Scientific and Technological Research Project of the Education Department of Jilin Province, China (No. JJKH20200937KJ).References
- A. H. Smith and C. M. Steinmaus, Annu. Rev. Public Health, 2009, 30, 107–122 CrossRef PubMed.
- J. A. Salonia, R. G. Wuilloud, J. A. Gásquez, R. A. Olsina and L. D. Martinez, J. Anal. At. Spectrom., 1999, 14, 1239–1243 RSC.
- A. G. Cox, I. G. Cook and C. W. Mcleod, Analyst, 1985, 110, 331–333 RSC.
- D. Larivière, K. M. Reiber, R. D. Evans and R. J. Cornett, Anal. Chim. Acta, 2005, 549, 188–196 CrossRef.
- A. Bidari, E. Zeini Jahromi, Y. Assadi and M. R. Milani Hosseini, Microchem. J., 2007, 87, 6–12 CrossRef CAS.
- W. H. Smith, J. Chem. Educ., 1984, 61, A185 CrossRef.
- F. J. Fortes, J. Moros, P. Lucena, L. M. Cabalín and J. J. Laserna, Anal. Chem., 2013, 85, 640–669 CrossRef CAS PubMed.
- Z. Hou, S. Jeong, Y. Deguchi and Z. Wang, Plasma Sci. Technol., 2020, 22, 070101 CrossRef.
- Z. Wang, F. Dong and W. Zhou, Plasma Sci. Technol., 2015, 17, 617–620 CrossRef CAS.
- G. Nicolas, M. P. Mateo and V. Piñon, J. Anal. At. Spectrom., 2007, 22, 1244–1249 RSC.
- D. W. Hahn and M. M. Lunden, Aerosol Sci. Technol., 2000, 33, 30–48 CrossRef CAS.
- Z. Wang, Y. Deguchi, Z. Zhang, Z. Wang, X. Zeng and J. Yan, Front. Phys., 2016, 11, 231–255 CrossRef.
- D. Rusak, B. Castle, B. Smith and J. Winefordner, Crit. Rev. Anal. Chem., 1997, 27, 257–290 CrossRef CAS.
- L. Sancey, V. Motto-Ros, B. Busser, S. Kotb, J. M. Benoit, A. Piednoir, F. Lux, O. Tillement, G. Panczer and J. Yu, Sci. Rep., 2016, 6, 24377 CrossRef CAS PubMed.
- S. S. Harilal, B. E. Brumfield, N. L. Lahaye, K. C. Hartig and M. C. Phillips, Applied Physics Reviews, 2018, 5, 021301 CrossRef.
- D. Zhang, Y. Chu, S. Ma, S. Zhang, H. Cui, Z. Hu, F. Chen, Z. Sheng, L. Guo and Y. Lu, Anal. Chim. Acta, 2020, 1107, 14–22 CrossRef CAS PubMed.
- D. Chen, Z. Huang, T. Wang, Y. Ma, Y. Zhang, G. Wang and P. Zhang, Anal. Chim. Acta, 2020, 1095, 14–19 CrossRef CAS PubMed.
- J. Yan, Z. Hao, R. Zhou, Y. Tang, P. Yang, K. Liu, W. Zhang, X. Li, Y. Lu and X. Zeng, Anal. Chim. Acta, 2019, 1082, 30–36 CrossRef CAS PubMed.
- G. S. Senesi and N. Senesi, Anal. Chim. Acta, 2016, 938, 7–17 CrossRef CAS PubMed.
- P. Fichet, P. Mauchien, J.-F. Wagner and C. Moulin, Anal. Chim. Acta, 2001, 429, 269–278 CrossRef CAS.
- Z. Chen, H. Li, F. Zhao and R. Li, J. Anal. At. Spectrom., 2008, 23, 871–875 RSC.
- J. O. Cáceres, J. Tornero López, H. H. Telle and A. González Ureña, Spectrochim. Acta, Part B, 2001, 56, 831–838 CrossRef.
- R. Kumar, A. K. Rai, D. Alamelu and S. K. Aggarwal, Environ. Monit. Assess., 2013, 185, 171–180 CrossRef CAS PubMed.
- X. Wen, Q. Lin, G. Niu, Q. Shi and Y. Duan, Appl. Opt., 2016, 55, 6706–6712 CrossRef CAS PubMed.
- D. M. D.-a. Pace, C. A. D. Angelo, D. Bertuccelli and G. Bertuccelli, Spectrochim. Acta, Part B, 2006, 61, 929–933 CrossRef.
- B. Campanella, I. Degano, E. Grifoni, S. Legnaioli, G. Lorenzetti, S. Pagnotta, F. Poggialini and V. Palleschi, Microchem. J., 2018, 139, 230–235 CrossRef CAS.
- X. Yang, Z. Hao, M. Shen, R. Yi, J. Li, H. Yu, L. Guo, X. Li, X. Zeng and Y. Lu, Talanta, 2017, 163, 127–131 CrossRef CAS PubMed.
- M. A. Aguirre, S. Legnaioli, F. Almodóvar, M. Hidalgo, V. Palleschi and A. Canals, Spectrochim. Acta, Part B, 2013, 79–80, 88–93 CrossRef CAS.
- S. Ma, Y. Tang, Y. Ma, Y. Chu, F. Chen, Z. Hu, Z. Zhu, L. Guo, X. Zeng and Y. Lu, Opt. Express, 2019, 27, 15091–15099 CrossRef CAS PubMed.
- X. K. Shen, H. Wang, Z. Q. Xie, Y. Gao, H. Ling and Y. F. Lu, Appl. Opt., 2009, 48, 2551–2558 CrossRef CAS PubMed.
- J.-M. Li, Y.-B. Chu, N. Zhao, R. Zhou, R.-X. Yi, L.-B. Guo, J.-Y. Li, X.-Y. Li, X.-Y. Zeng and Y.-F. Lu, Chin. J. Anal. Chem., 2016, 44, 1042–1046 CAS.
- X. He, B. Chen, Y. Chen, R. Li and F. Wang, J. Anal. At. Spectrom., 2018, 33, 2203–2209 RSC.
- M. M. Hassanimatin and S. H. Tavassoli, Phys. Plasmas, 2018, 25, 053302 CrossRef.
- H. Sobral and A. Robledo-Martinez, Spectrochim. Acta, Part B, 2016, 124, 67–73 CrossRef CAS.
- O. A. Nassef and H. E. Elsayed-Ali, Spectrochim. Acta, Part B, 2005, 60, 1564–1572 CrossRef.
- C. G. Parigger, G. Gautam, A. C. Woods, D. M. Surmick and J. O. Hornkohl, Trends Appl. Spectrosc., 2014, 11, 1–13 Search PubMed.
- X. Wang, A. Li, X. Xu, Y. He, S. Qiu, X. Ma and R. Liu, Spectrochim. Acta, Part B, 2020, 174, 105996 CrossRef CAS.
- K. Ujihara, J. Appl. Phys., 1972, 43, 2376–2383 CrossRef CAS.
- G. Cristoforetti, S. Legnaioli, V. Palleschi, A. Salvetti and E. Tognoni, Spectrochim. Acta, Part B, 2004, 59, 1907–1917 CrossRef.
- L. Zheng, F. Cao, J. Xiu, X. Bai, V. Motto-Ros, N. Gilon, H. Zeng and J. Yu, Spectrochim. Acta, Part B, 2014, 99, 1–8 CrossRef CAS.
- D. W. Hahn and N. Omenetto, Appl. Spectrosc., 2012, 66, 347–419 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |