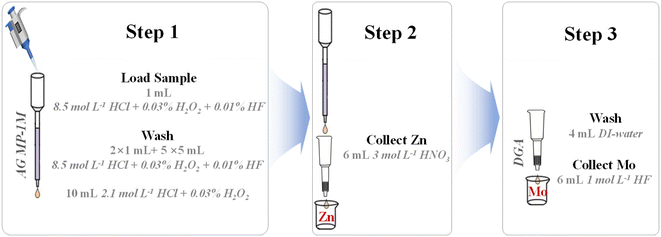
A fast double-stack column chemical separation of Zn and Mo from geological samples for isotopic analysis by MC-ICP-MS
Yan
Han
a,
Lian
Zhou
 a,
Minghui
Shi
ab,
Yating
Hu
a,
Ge
Zhang
a,
Xin
Hou
a and
Lanping
Feng
a,
Minghui
Shi
ab,
Yating
Hu
a,
Ge
Zhang
a,
Xin
Hou
a and
Lanping
Feng
 *a
*a
aState Key Laboratory of Geological Processes and Mineral Resources, China University of Geosciences, Wuhan 430074, China. E-mail: ucg1987@163.com; Fax: +86-27-67885096; Tel: +86-27-67885096
bPetroleum Exploration and Development Institute, Tarim Oil Company, Petrochina, Korla 841000, China
First published on 22nd November 2023
Abstract
A time-saving and highly efficient chemical separation procedure to isolate Zn and Mo from various geological samples is developed, which is based on a single pass of a double-stack column. The first column, filled with AG MP-1M resin (100–200 mesh, Bio-Rad), quantitatively removed matrix and interference elements from sample matrices; while the second column, filled with the DGA spec resin (50–100 μm, Eichrom), further isolated Zn and Mo from sample matrices. The proposed protocol was time-saving (50% column running time) and afforded efficient separation and quantitative recovery (more than 96% and 92% for Zn and Mo, respectively) from complex sample matrices of three geological reference materials W-2a, GSR-1 and GSR-2. Both Zn and Mo isotope ratios in the purified Zn and Mo solutions were determined by multi-collector inductively coupled plasma mass spectrometry (MC-ICP-MS) with the use of the combined standard-sample bracketing and internal normalization (C-SSBIN) method and double-spike method, respectively, for mass bias correction. In addition, the effects of two cone combinations and three mass bias correction methods on Zn isotopic analysis were investigated. The results show that the jet + X cone and the C-SSBIN method with Cu as the internal standard provided high accuracy and precision Zn isotope ratio measurements. Zn and Mo isotope ratios were measured in thirteen geological reference materials for the validation of the feasibility and effectiveness of the proposed method with satisfactory results in this study.
1. Introduction
Zn and Mo are two of the most important mineral elements and essential trace nutrients for biological activity.1 As a moderately volatile transition metal, Zn has five naturally occurring isotopes covering a range of relative abundances, including 64Zn (48.63%), 66Zn (27.90%), 67Zn (4.10%), 68Zn (18.75%) and 70Zn (0.62%), and mainly occurs in metallic form Zn0 or the oxidation state Zn2+.2 Mo is a redox-sensitive element, which exists in two predominant valence states (Mo4+ and Mo6+) in the natural environment. Mo consists of seven natural stable isotopes, 92Mo (14.84%), 94Mo (9.25%), 95Mo (15.92%), 96Mo (16.68%), 97Mo (9.55%), 98Mo (24.13%) and 100Mo (9.63%), respectively.3 Over the past few decades, due to the advent and continuous improvement of instrumental capabilities and the development of new sample preparation techniques, highly precise Zn and Mo isotopic analyses have shown significant isotopic fractionation at the 2 per mil (‰) level at or near the Earth's surface operating at low temperatures and pressures,4,5 and revealed that natural δ66ZnJMC-Lyon is constrained between −1.36 and +1.77‰ (ref. 6) and δ98MoNIST SRM3134 is between −2.3 and +2.5‰.7,8 As a result, Zn and Mo isotopes are rapidly expanding as effective geochemical proxies to understand key processes in various fields of paleoenvironmental reconstruction,9,10 carbon cycle,11,12 and cosmochemistry.13,14 For instance, Zn and Mo are involved in a number of critical enzymes required for marine life and could be removed from the ocean to sediments under sulfidic conditions, which is accompanied by isotope fractionation. As so, the two elements have shown potential to link redox with primary productivity in the paleoenvironmental reconstruction.9,15,16 Combination with Zn and Mo isotopes can be helpful in evaluating the burial amount of organic carbon and tracing the carbon cycle.11,12,17 Additionally, the combined use of two elements with nucleosynthetic isotope anomaly characteristics provides strong evidence for determining the source of a planet's volatile elements and assessing the contribution to a planet's major building blocks.18–20 Therefore, a better understanding of the complex geochemical processes leading to isotopic fractionation in the natural environment can be achieved by applying a combination of Zn isotope and Mo isotope fingerprints.The isotopic compositions of Zn and Mo are commonly analyzed by multi-collector inductively coupled plasma mass spectrometry (MC-ICP-MS), which is susceptible to matrix effects and polyatomic interferences; therefore, in order to achieve high precision and accuracy of the isotopic composition, efficient chemical separation (high/quantitative yields and low blanks) is required prior to the determination. Numerous separation protocols have been successfully developed for the purification of Zn and Mo from geological materials. The majority of published separation methods have used ion exchange chromatography to purify Zn and Mo. For example, the most commonly used protocol for the isolation of Zn uses the Bio-Rad™ AG MP-1 strong basic anion exchange resin, which was originally proposed by Maréchal et al.21 for the separation of Cu, Fe and Zn from the sample matrix. More recently, other resins such as AG1-X8 (ref. 2 and 22) and NOBIAS Chelate-PA1 resin23 have been proposed for the isolation of Zn from various sample types. Two-column ion exchange separation, characterised by a large sample loading tolerance and high purification efficiency of Mo, is widely used for Mo purification. Bio-Rad™ AG1-X8 anion exchange resin was used to separate Zr and most other matrix elements from Mo and then a Bio-Rad™ AG50W-X8 cation exchange resin was used to remove Fe.24 As a two-column ion exchange separation is time-consuming due to tedious elution steps, a single-column separation method using anion exchange resin,25 chelating resin26 and extraction resin27,28 has been reported.
Separation methods for Zn and Mo isotopic analysis are well established and mature. However, the traditional separation methods isolating Zn and Mo usually require two to four independent column separations, which may cause sample loss and isotopic fractionation during the secondary separation with sample evaporation-transfer medium. In addition, these reported separation methods are not suitable for the analysis of rare samples (such as meteorites). Therefore, a single-column separation of Cu, Zn and Mo is proposed by Guedes et al.29 wherein Zn and Mo are eluted together in a nitric acid medium and analyzed separately for isotope ratios. Note that in the study of Guedes et al., only 98Mo and 95Mo were measured with the use of a standard-sample bracketing mass bias correction model. However, in our preliminary experiments, a negative bias in the Mo isotope ratio measured by MC-ICP-MS was found when the Zn to Mo ratio was greater than 25 using the double spike mass bias correction method. As reported, Zn is about 61 times more abundant than Mo in the continental upper crust (Zn at 67 μg g−1 and Mo at 1.1 μg g−1),30 suggesting that Zn and Mo need to be further isolated from each other to achieve accurate isotopic data.
The objective of this study was to establish a rapid and efficient column separation protocol for the quantitative isolation of Zn and Mo from geological materials to obtain high precision and accuracy isotope ratios. An optimized and efficient separation protocol employing double-stack column (a combination of AG MP-1M resin and DGA resin) separation for the purification of Zn and Mo from geological materials was developed, and validated by the Zn and Mo isotopic analyses of various geological reference materials by MC-ICP-MS.
2. Methods
2.1 Reagents and materials
Reagent grade hydrofluoric, nitric and hydrochloric acids (SRC, China) were double purified in a Savillex™ DST-1000 sub-boiling distillation system (Eden Prairie, MN, USA) and diluted to the required molarity with ultra-high purity deionized water (DI-water, 18.2 MΩ cm−1) from a Milli-Q elemental system (Burlington, MA, USA).Four standard solutions, NIST SRM 3114 Cu, NIST SRM 3119 Ga, NIST SRM 683 Zn and NIST SRM 3134 Mo, were purchased from the National Institute of Standards and Technology (Gaithersburg, MD, USA). The 97Mo–100Mo double spike was prepared by mixing enriched 97Mo and 100Mo isotopes, which were purchased from the Oak Ridge National Laboratory (ORNL, USA). The double-spike is prepared and calibrated by Feng et al.,27 and has a wide range of optimal spike ratios.
The anion exchange resin (AG MP-1M, Bio-Rad, 100–200 mesh) and the DGA resin of particle size 50–100 μm were soaked in 2 mol L−1 HNO3 and then washed with 2 mol L−1 HNO3, DI water, 6 mol L−1 HCl and DI water, alternately.
Eight geological reference materials purchased from the United States Geological Survey (USGS) including andesite (AGV-2), diabase (W-2a), shales (SBC-1 and SGR-1b), granodiorite (GSP-2), rhyolite (RGM-2), basalt (BHVO-2) and carbonatite (COQ-1), and five geological reference materials purchased from the Institute of Geophysical and Geochemical Exploration (IGGE) including granite (GSR-1), andesite (GSR-2), basalt (GSR-3), and uraniferous sandstone (GSR-16) as well as plagioclase granulite (GSR-17) were used as test samples for Zn and Mo isotope ratio measurements in this study.
2.2 Sample digestion
Depending on Mo and Zn mass fractions in the sample, sample powder containing at least 50 ng Mo and 300 ng Zn was weighed into a 15 mL PTFE-lined stainless-steel bomb together with the appropriate quantity of the 97Mo–100Mo double spike (97Mo = 51.6%, double spike![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sample = 45%
sample = 45%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55%). All samples were digested with a mixture of 2 mL concentrated HF and 1 mL concentrated HNO3 for 48 hours at 190 °C in an oven. Following evaporation at 120 °C, the fluorides were removed through refluxing twice with 1 mL concentrated HNO3, and the samples were re-digested in 1 mL concentrated HNO3 and 1 mL DI water at 190 °C in an oven for 12 hours to obtain a clear and precipitate-free solution. Then the digest solutions were dried twice in 0.5 mL concentrated HCl to ensure that all cations were converted to chloride species. Finally, sample solutions were taken up in 1 mL 8.5 mol L−1 HCl + 0.03% H2O2 + 0.01% HF in preparation for the purification. A minimum of two aliquots of each of the geological reference samples were subjected to separate digestion and column separation.
55%). All samples were digested with a mixture of 2 mL concentrated HF and 1 mL concentrated HNO3 for 48 hours at 190 °C in an oven. Following evaporation at 120 °C, the fluorides were removed through refluxing twice with 1 mL concentrated HNO3, and the samples were re-digested in 1 mL concentrated HNO3 and 1 mL DI water at 190 °C in an oven for 12 hours to obtain a clear and precipitate-free solution. Then the digest solutions were dried twice in 0.5 mL concentrated HCl to ensure that all cations were converted to chloride species. Finally, sample solutions were taken up in 1 mL 8.5 mol L−1 HCl + 0.03% H2O2 + 0.01% HF in preparation for the purification. A minimum of two aliquots of each of the geological reference samples were subjected to separate digestion and column separation.
2.3 Zinc and molybdenum separation
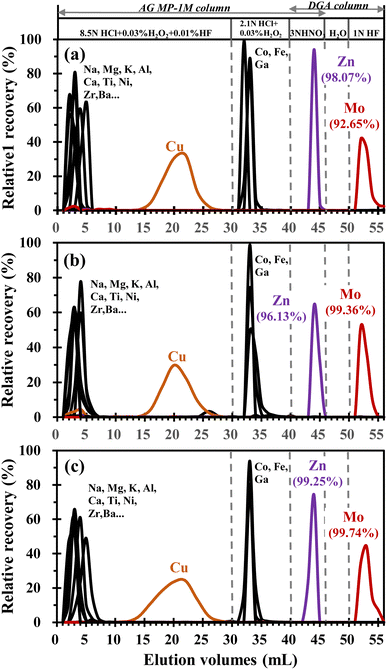
In this study, a single-pass with a double-stack column (using AG MP-1M resin and DGA resin) was employed for the separation of Zn and Mo from the sample matrix (Fig. 1 and Table 1). This protocol permits the sequential separation and purification of Zn and Mo, respectively, from a single sample aliquot. As shown in Table 1, the sample was loaded onto the pre-cleaned AG MP-1M resin column in 1 mL 8.5 mol L−1 HCl + 0.03% H2O2 + 0.01% HF, and was washed with 1 mL 8.5 mol L−1 HCl + 0.03% H2O2 + 0.01% HF twice. The addition of a small amount of H2O2 is to stabilize the redox-sensitive elements (such as Fe and Mo) and prevents the occurrence of multiple elution peaks. Then the column was rinsed again with 5 × 5 mL of the same medium. At this stage, most of the matrix elements (e.g., Na, Mg, Al, K, Ca, Mn, Ti and Zr) were eluted from the column. After that, the remaining interferences, mainly Fe, Ga and Co were stripped from the column by rinsing with 10 mL 2.1 mol L−1 HCl + 0.03% H2O2. Then, the pre-cleaned DGA column was put under the AG MP-1M column in tandem (Fig. 1). Zn and Mo were eluted from the AG MP-1M column using 6 mL of 3 mol L−1 HNO3, and passed through the DGA column. Due to the selective adsorption properties of DGA resin,31 Mo was extracted on the DGA column and Zn was not absorbed. Therefore, the Zn fraction was quantitatively collected in the 6 mL of 3 mol L−1 HNO3 eluent. The columns were decoupled and processed separately. The DGA resin column was rinsed with 4 mL DI-Water to remove any residual interferences, and the Mo fraction was quantitatively collected from the DGA resin using 6 mL 1 mol L−1 HF. The Zn and Mo fractions were then dried separately, taken up in 0.5 mL concentrated HNO3 and 50 μL of 30% (m m−1) H2O2 and left on a hot plate overnight at 120 °C to decompose organic molecules. This step was repeated at least twice to ensure the complete removal of organic material.| Process | Reagent | Volume (mL) | Resin typea |
|---|---|---|---|
| a Column 1(the upper column) is filled with AG MP-1M resin, while the column 2 (the lower column) is filled with DGA resin. | |||
| Clean | 6 mol L−1 HCl | 10 | AG MP-1M |
| DI-water | 5 | ||
| 2 mol L−1 HNO3 | 10 | ||
| 1 mol L−1 HF | 10 | DGA | |
| DI-water | 5 | ||
| 0.05 mol L−1 HCl | 10 | ||
| Condition | 8.5 mol L−1 HCl + 0.03% H2O2 + 0.01% HF | 4 | AG MP-1M |
| 3 mol L−1 HNO3 | 4 | DGA | |
| Load sample | 8.5 mol L−1 HCl + 0.03% H2O2 + 0.01% HF | 1 | AG MP-1M |
| Wash | 8.5 mol L−1 HCl + 0.03% H2O2 + 0.01% HF | 2 × 1 mL + 1 × 5 mL | |
| Wash (Cu) | 8.5 mol L−1 HCl + 0.03% H2O2 + 0.01% HF | 4 × 5 mL | |
| Wash (Fe, Ga) | 2.1 mol L−1 HCl + 0.03% H2O2 | 10 | |
| Collect Zn | 3 mol L−1 HNO3 | 6 | AG MP-1M + DGA |
| Wash | DI-water | 4 | DGA |
| Collect Mo | 1 mol L−1 HF | 6 | |
The total procedure blank is 0.8 ng for Zn and 0.6 ng for Mo measured using three sample blanks which were prepared using the same operations as geological samples. The blank levels for Zn and Mo are negligible as compared to the minimum Zn (300 ng) and Mo (50 ng) contents. Compared to the traditional chemical separation procedure that employed two- or multi-column independent separations, the proposed single-pass with double-stack column separation in this study only takes about 7 hours, which can save at least 50% of the column running time. Prior to MC-ICP-MS analysis, Zn and Mo fractions were diluted to 300 ng g−1 and 50 ng g−1 using 2% (m m−1) HNO3 and 5% (m m−1) HNO3, respectively.
2.4 Mass spectrometry
A quadrupole-base ICP-MS (Model 7700, Agilent Technologies) was used in the semi-quantitative mode for measuring the concentrations of the Mo and Zn and other matrix elements in the test samples. Prior to analysis, the instrument was calibrated using standard solutions of four concentrations of trace elements (1, 10, 25, and 50 ng g−1) and four concentrations of major elements (10, 100, 500, and 1000 ng g−1). Drift corrections were carried out using Indium (In) as an internal standard, and by repeatedly analyzing a quality control solution (QC) as a drift monitor over the duration of a run.Zn and Mo isotope ratios were measured by MC-ICP-MS (Neptune Plus, Thermo Fisher Scientific, Bremen, Germany) at the State Key Laboratory of Geological Processes and Mineral Resources (GPMR), China University of Geosciences, Wuhan. Zn isotope ratios were measured in the wet plasma mode (Table 2). Samples at a concentration of 300 ng g−1 Zn were introduced into the plasma through a self-aspiration nebulizer at a flow rate of 50 μL min−1, connected to a combined Scott spray chamber on top and a cyclonic spray chamber at the bottom. The instrument mass bias was monitored and corrected using a model of combined standard-sample bracketing with internal-normalization (C-SSBIN),32 and copper (NIST SRM 3114) was used as an internal standard which was added to both the sample and standard solutions. Zn isotope ratios were expressed in a standard δ-notation in per mil relative to the JMC-Lyon Zn:
| δ66Zn = [(66Zn/64Zn)sample/(66Zn/64Zn)JMC-Lyon − 1] × 1000 | (1) |
| Neptune plus | Zn isotope | Mo isotope |
|---|---|---|
| RF power | ∼1250W | |
| Cooling gas flow rate | ∼16 L min−1 | |
| Auxiliary gas flow rate | ∼1.0 L min−1 | |
| Sample gas flow rate | ∼1.0 L min−1 | |
| Cones | X-skimmer cone; jet-sample cone | |
| Solution uptake | ∼50 μL min−1 | |
| Mass resolution | Low | |
| Cup configurations | L3(62Ni), L2(63Cu), L1(64Zn), C(65Cu), H1(66Zn), H2(67Zn), H3(68Zn) | L4(91Zr), L2(94Mo), L1(95Mo), C(96Mo), H1(97Mo), H2(98Mo), H3(99Ru), H4(100Mo) |
| Sensitivity | ∼7 V for 64Zn at 300 ng g−1 | ∼8 V for 98Mo at 50 ng g−1 |
| ∼8 V for 63Cu at 200 ng g−1 | ||
| Blocks and cycles | 4 blocks × 10 cycles | 3 blocks × 10 cycles |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Aridus II | ||
| Sweep gas flow rate | — | ∼2.2 L min−1 |
| Nitrogen gas | — | ∼0.06 L min−1 |
Traditionally, JMC-Lyon was used as a primary ‘zero-delta’ Zn isotope standard for Zn isotope analyses by MC-ICP-MS. Because the stocks of JMC-Lyon material are no longer available, a number of reference materials are utilized as ‘zero-delta’ standards for the Zn isotope measurement, including AA-ETH Zn, IRMM-3702, and NIST SRM 683.33–35 In this study, NIST SRM 683 Zn was used as the reference standard for Zn isotope measurement, and the results can be compared with JMC-Lyon based values34:
| δ66ZnJMC-Lyon = δ66ZnNIST SRM 683 + 0.12‰ | (2) |
Mo isotope ratios were measured in the dry plasma mode (Table 2). 50 ng g−1 sample solutions were introduced into the plasma through an Aridus II desolvator with a 50 μL min−1 PFA nebulizer. A sample jet cone and a skimmer X cone combination was used to improve the sensitivity, resulting in a typical Mo intensity of ∼620 V ppm−1. Isobaric interferences from Zr and Ru were monitored by collecting 91Zr and 99Ru in L4 and H3, respectively. A 97Mo–100Mo double-spike was used to correct isotopic fractionation that could occur during ion-exchange chromatography and in combination with standard-sample bracketing for mass bias correction to improve the measurement precision and accuracy.28,36,37 NIST SRM 3134 has been accepted as the ‘zero-delta’ reference material for the Mo isotope measurement by an increasing number of groups,3,36–38 and was used in this study. Mo isotopic compositions are reported in standard delta (δ) notation relative to the mean value of the bracketing NIST SRM 3134 calculated from eqn (3):
| δ98Mo = [(98Mo/95Mo)sample/(98Mo/95Mo)NIST SRM 3134 − 1] × 1000 | (3) |
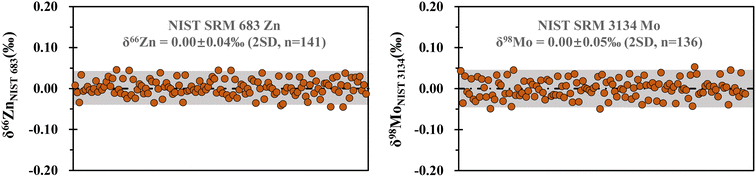
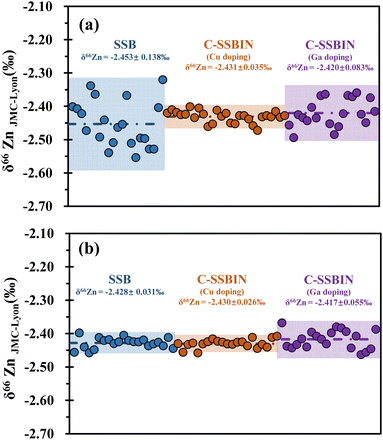
The instrumental measured precision of Zn and Mo isotope ratios was evaluated using the results obtained from the bracketing standards (NIST SRM 683 Zn and NIST SRM 3134 Mo), assuming that the standard deviation (SD) of the sample measurements equals the standard deviation of the bracketing standard measurements. The long-term repeatability of δ66Zn and δ98Mo was better than 0.04‰ and 0.05‰ (2SD), respectively (Fig. 2).
3. Results and discussion
3.1 Matrix evaluation
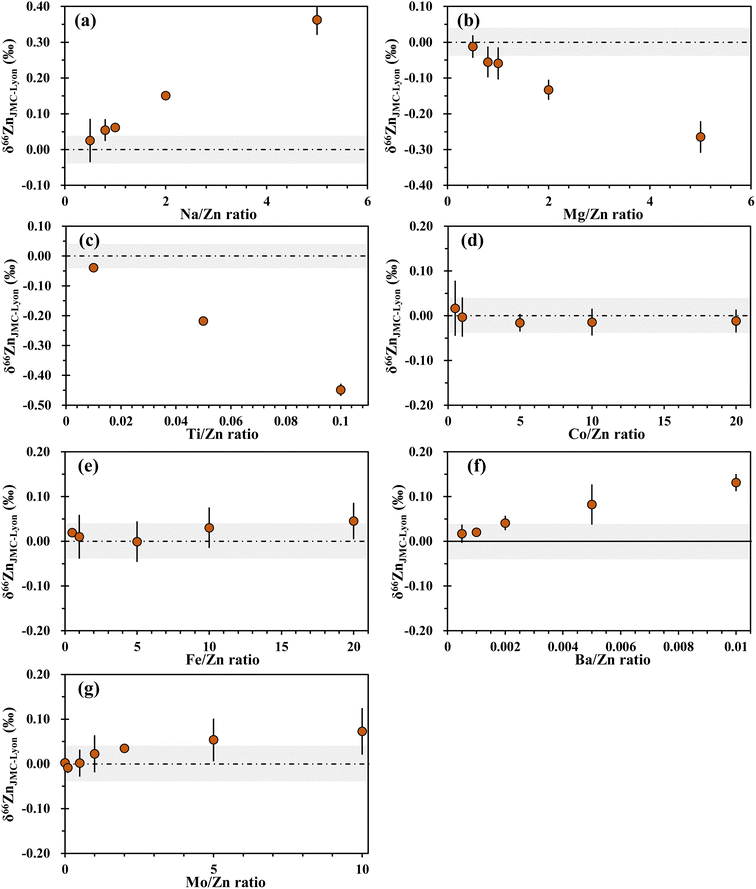
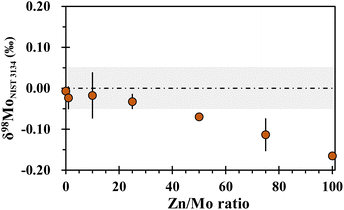
The presence of matrix elements in the sample solution may bring in spectral and non-spectral interferences in Zn and Mo isotope ratio measurements by MC-ICP-MS. Previous studies have investigated the influence of the sample matrix on the precise determination of Zn and Mo isotopic compositions. Generally, the δ66Zn values are most susceptible to the Ti and Ni in the sample solutions, followed by the Mg and Ba contents.34,39 This may be attributed to spectral interferences of these matrix elements, such as 48Ti16O+, 24Mg40Ar+ and 64Ni+ on 64Zn+, and 26Mg40Ar+ and 132Ba2+ on 66Zn+. In addition, elements such as Co, Cu, Na and Al have been found to have non-negligible effects on high-precision Zn isotopic analysis. For instance, significant drifts (>0.07‰) in δ66Zn were observed with Co/Zn, Cu/Zn, Na/Zn and Al/Zn in sample solutions reaching 0.01, 0.5, 1 and 1, respectively.34,39 It should be noted that since Cu is used as an internal standard in the Zn isotope ratio measurements, Cu in the sample itself needs to be separated. The influences of Na, Mg, Ti, Co, Fe, Ba and Mo on Zn isotopic determinations were investigated in this study. Solutions of 300 ng g−1 NIST SRM 683 Zn and 200 ng g−1 NIST SRM 3134 Cu mixed with different matrix elements were measured against the pure solution of 300 ng g−1 NIST SRM 683 Zn and 200 ng g−1 NIST SRM 3134 Cu. As shown in Fig. 3, no significant deviation of δ66Zn was observed for the doped solutions with Ba/Zn ratio up to 0.002, Ti/Zn ratio up to 0.01, Na/Zn and Mg/Zn ratios up to 0.5, Mo/Zn ratio up to 2, and Co/Zn and Fe/Zn ratios up to 20, respectively.For Mo isotopic analysis, when the Ti/Mo,27 Zr/Mo,5 Zn/Mo,5 Sn/Mo,5 Fe/Mo,5,27,36 and Ta/Mo28 ratios of the purified sample are less than 1, 5, 5, 5, 30 and 100, respectively, the accuracy of Mo isotope ratios is not affected (Table 3). The anionic exchange resin AG MP-1 (ref. 21 and 29) is commonly used for the separation of Zn isotopes, wherein Mo is also collected along with Zn by diluted nitric acid. The effect of Zn on Mo isotope ratio measurement has been explored in a previous study,5 which is not negligible considering the very high Zn/Mo ratio in natural samples. In this study, we also performed a Zn doping test and the results are shown in Fig. 4. It is clear that the measured Mo isotope ratio gradually shifted to negative values as the Zn/Mo ratio increased, and biased results occurred when the Zn/Mo ratio exceeded 25. Our result is in agreement with that reported by Zhu et al.5 This could be due to the formation of polyatomic interferences, such as 64Zn15N16O+ on 95Mo, 68Zn14N16O+ on 98Mo and 64Zn36Ar+ and 68Zn14N18O+ on 100Mo. The tested Zn/Mo ratio (25) in the above experiment is much lower than the average Zn/Mo ratio (60.9) in the upper continental crust,30 suggesting that Zn must be effectively separated for Mo isotopic analysis.
| [X]/[Analyte] | Measured | Reported | Sources | ||
|---|---|---|---|---|---|
| W-2a | GSR-1 | GSR-2 | |||
| Na/Zn | 2.9 × 10−3 | 0.016 | 8.5 × 10−3 | 0.5 | This study |
| 1 | Chen et al. (2016)34 | ||||
| Mg/Zn | 0.01 | 4.5 × 10−3 | 7.4 × 10−4 | 0.5 | This study |
| 0.1 | Zhu et al. (2019)39 | ||||
| 0.5 | Chen et al. (2016)34 | ||||
| Al/Zn | <10−5 | <10−5 | 1.7 × 10−3 | 1 | Chen et al. (2016)34 |
| Ti/Zn | 1.1 × 10−3 | 3.2 × 10−3 | 7.8 × 10−4 | 0.01 | This study |
| 0.01 | Zhu et al. (2019)39 | ||||
| 0.005 | Chen et al. (2016)34 | ||||
| V/Zn | 2 × 10−5 | 5 × 10−5 | 3 × 10−5 | 0.5 | Chen et al. (2016)34 |
| Cr/Zn | 4 × 10−5 | <10−5 | 5.6 × 10−5 | 3 | Chen et al. (2016)34 |
| Fe/Zn | 1 | 5.7 | 0.44 | 20 | This study |
| 10 | Chen et al. (2016)34 | ||||
| Co/Zn | <10−5 | <10−5 | <10−5 | 20 | This study |
| 0.1 | Zhu et al. (2019)39 | ||||
| Ni/Zn | 7 × 10−5 | <10−5 | <10−5 | 0.001 | Zhu et al. (2019)39 |
| 0.0005 | Chen et al. (2016)34 | ||||
| Cu/Zn | 5 × 10−5 | 2.4 × 10−4 | 3.5 × 10−4 | 0.5 | Chen et al. (2016)34 |
| Ba/Zn | 2 × 10−5 | 3.2 × 10−4 | 4 × 10−5 | 0.002 | This study |
| 0.1 | Chen et al. (2016)34 | ||||
| Ti/Mo | 0.13 | 0.06 | 0.06 | 1 | Feng et al. (2020)27 |
| Fe/Mo | 2.12 | 1.09 | 0.008 | 5 | Zhu et al. (2022)5 |
| 10 | Feng et al. (2020)27 | ||||
| 30 | Liu et al. (2016)36 | ||||
| Zn/Mo | 0.13 | 0.014 | 0.09 | 25 | This study |
| 5 | Zhu et al. (2022)5 | ||||
| Zr/Mo | 0.12 | 1 × 10−3 | 1.6 × 10−3 | 5 | Zhu et al. (2022)5 |
| Sn/Mo | 0.023 | 0.078 | 3.3 × 10−3 | 5 | Zhu et al. (2022)5 |
| Ta/Mo | 1.8 × 10−3 | 5.2 × 10−4 | 1.3 × 10−4 | 100 | Li et al. (2014)28 |
3.2 Elution curves and recovery check
The AG MP-1M column can quantitatively remove most matrix elements in geological samples such as silicate rocks. The active group of AG MP-1M resin is R-CH2N+(CH3)3, which can easily combine with Cl− in HCl solution. Transition metal ions formed as complex ions (such as CuCl3−, CuCl42−, FeCl4−, ZnCl3−, ZnCl42− and MoO2Cl3−) in concentrated HCl solution can exchange with Cl− on the resin of the exchange column.40,41 The resin does not adsorb and exchange elements that cannot form complex ions with HCl, such as alkali and alkaline earth metal cations (Li, K, Na, Mg, Ca and Ba). According to the affinity of complex ions for the AG MP-1M resin, two different concentrations of hydrochloric acid were used to elute most of the matrix elements in geological samples while retaining Zn and Mo on the resin.40,41 To achieve further separation of Zn and Mo, we investigated the characteristics of DGA resin. The DGA resin consists of 40 wt% N,N,N′N′-tetraoctyldiglycolamide sorbed onto 50–100 μm particle size Amberchrom® CG-71,42 and has been applied for the isotopic analysis of Ca, Sr, Pb, Nd and actinides.43–45 According to the capacity factor of DGA resin, matrix elements like Na, Mg, Al, K, Mn, Co and Zn are eluted from DGA resin in 3 mol L−1 HNO3, while Ca and Mo could be retained. The Ca adsorbed on the DGA column can be eluted by DI-water, while Mo is adsorbed which can be stripped from the resin using hydrofluoric acid rinse.31,44 Therefore, DGA resin is a good choice for further separation of Mo and Zn. Based on the above advantages, it is reasonable to believe that the simultaneous separation of Zn and Mo from a single sample solution can be achieved by employing a tandem column comprising AG MP-1M resin and DGA resin.The efficiency of the proposed single pass with double-stack column protocol was evaluated by analysis of three geological reference materials W-2a, GSR-1 and GSR-2. The collected fractions from the three geological reference materials were analyzed using a quadrupole ICP-MS (7700 ICP-MS, Agilent Technologies, Yokogawa, Japan) to obtain element concentrations. The elution curves of three geological reference materials are presented in Fig. 5. Consistent with previous findings,39,40,46 most of the impurity elements (e.g., Li, Na, Mg, K, Al, Ca, Ba, Zr and REEs) were efficiently removed using a small amount of (c.a. 10 mL) 8.5 mol L−1 HCl after sample loading. Cu can be quantitatively collected in the subsequent 20 mL 8.5 mol L−1 HCl solely, suggesting that this protocol can also be used for Cu separation. Since Fe has a significant impact on both Zn and Mo isotopic analysis and natural samples are usually enriched in Fe, a small amount (c.a. 0.03% m m−1) of H2O2 was added to 10 mL 2.1 mol L−1 HCl keeping Fe in trivalent forms and remove Fe from Zn and Mo on AG MP-1M resin.29,40 As a result, the Fe/Zn and Fe/Mo ratios in the purified samples were typically no more than 6 and 2.2, respectively, which were negligible for isotopic analysis based on the doping experiments (shown in Fig. 3 and Table 3). After switching to 3 mol L−1 HNO3, Zn was eluted in the first 6 mL through a tandem column of the AG MP-1M and the DGA. After decoupling the tandem column, the DGA column was rinsed with 4 mL DI water to remove any residual interferences, and finally, Mo was collected in 6 mL 1 mol L−1 HF.
Ru could pose interference on Mo isotopic analysis and thus requires an efficient removal. No significant amount of Ru in elution fractions from the selected samples (W-2a, GSR-1, and GSR-2) was detected by ICP-MS. Therefore, it was not included in Fig. 5. Importantly, no significant Ru signals were found (collected in H3, monitoring 99Ru) during MC-ICP-MS measurements of geological samples, further confirming the efficient removal of Ru using the proposed separation method.
The ratios of the impurity elements to the target analytes are shown in Table 3. It is clear that the residual impurity elements in the purified samples were far below the threshold values derived from the doping experiments, confirming efficient removal of the interfering elements from the samples. In addition, the recoveries of Zn and Mo for the three samples of W-2a, GSR-1 and GSR-2 were found to be in the range of 96.13% to 99.25%, and 92.65% to 99.74%, respectively (Fig. 5). Although there was a slight loss of Mo for W-2a (Fig. 5a), the double spike technique can correct for isotopic fractionation that occurs during non-quantitative chromatographic purification of Mo. As shown in Fig. 5, consistent elution curves were obtained for three geological reference materials with different lithologies and different Zn–Mo contents. The results of the above elution experiments confirm that the proposed separation procedure is robust for various geological samples for high precision Zn and Mo isotopic composition determination. Note that the proposed separation protocol could also be used for Cu isotopic analysis since quantitative separation of Cu is achieved as shown in Fig. 5.
3.3 Determining the optimum analyte-dopant element (Cu/Ga) of Zn isotopic measurements
The accuracy and precision of isotope ratio measurements by MC-ICP-MS depend on many factors during the chemical separation and mass spectrometry analysis of elements from natural samples. One great challenge is to remove, as much as possible, the matrix elements from the sample, while maintaining a nearly complete recovery of the element of interest. Another critical task of MC-ICP-MS measurements is the correction for instrumental mass bias.47 Several correction methods for instrumental mass bias have been used for Zn isotopic analysis by MC-ICP-MS, including the standard-sample bracketing (SSB) method, combined standard-sample bracketing and internal normalization (C-SSBIN) method and double spike method.22,48,49In this study, we evaluated the SSB and C-SSBIN methods for the correction of Zn isotope ratios measured by using X skimmer cone and two types of sample cones (jet sample and standard sample cone), respectively. Three sets of solutions were prepared, the first one only contained 300 ng g−1 Zn, the second set contained 300 ng g−1 Zn spiked with 200 ng g−1 Cu as the dopant, and the third set, 300 ng g−1 Zn spiked with 200 ng g−1 Ga as the dopant. As shown in Fig. 6, it is evident that measurement precisions of δ66Zn values improved significantly from 0.035–0.138‰ to 0.026–0.055‰ (2SD), when the jet + X cone combination was used as compared to that of the standard + X cone combination. This may be due to the fact that the jet sample cone has a larger orifice diameter than the standard cone, which leads to a higher ion transmission and approximately 1.3 times improvement sensitivity. In addition, the obtained δ66Zn in the NIST SRM 682 by using two cone combinations and three mass bias correction methods, including SSB, C-SSBIN with Cu as the internal standard, and C-SSBIN with Ga as the internal standard is in agreement with the published data (−2.45‰) by John et al.50 and (−2.46‰) Conway et al.51 The above results confirm that the jet + X cone combination provides better precision and accuracy for Zn isotopic analysis, thus it is recommended for the determination of Zn isotope ratios. Furthermore, the instrumental mass bias for the Zn isotope ratio measurements can be better handled by the C-SSBIN method with Cu as the internal standard element.
3.4 Zn and Mo isotope ratios in geological reference materials
In order to demonstrate the validity of the separation protocol, thirteen geological reference materials were digested with at least two independent digestions and measured more than three times on the MC-ICP-MS.| Sample | Zna (μg g−1) | δ66ZnJMC-Lyon (‰, 2SD) | n | References | Moa (μg g−1) | δ98MoNIST SRM 3134 (‰, 2SD) | n | References |
|---|---|---|---|---|---|---|---|---|
| a The Zn and Mo content of each geological reference material is obtained from the certifications issued by USGS and IGGE. b Number of independent digests of each geological reference material and each purified sample solution was measured 3 times by MC-ICP-MS. | ||||||||
| W-2a | 77.7 | 0.25 ± 0.02 | 4 | This study | 0.46 | −0.03 ± 0.05 | 4 | This study |
| 0.22 ± 0.03 | Zhu et al. (2019)39 | −0.07 ± 0.05 | Fan et al. (2020)38 | |||||
| −0.05 ± 0.06 | Burkhardt et al. (2014)56 | |||||||
| −0.04 ± 0.03 | Bezard et al. (2016)57 | |||||||
| −0.03 ± 0.06 | Feng et al. (2020)27 | |||||||
| −0.03 ± 0.03 | Li et al. (2019)58 | |||||||
| AGV-2 | 86.7 | 0.32 ± 0.05 | 3 | This study | 2 | −0.16 ± 0.05 | 3 | This study |
| 0.34 ± 0.02 | Amet and Fitoussi (2020)22 | −0.15 ± 0.01 | Willbold et al. (2016)25 | |||||
| 0.29 ± 0.06 | Araújo et al. (2016)49 | −0.15 ± 0.02 | Zhu et al. (2022)5 | |||||
| 0.28 ± 0.05 | Chen et al. (2016)34 | −0.14 ± 0.05 | Zhao et al. (2016)26 | |||||
| 0.28 ± 0.04 | Zhu et al. (2019)39 | −0.12 ± 0.08 | Feng et al. (2020)27 | |||||
| GSP-2 | 120 | 1.07 ± 0.07 | 3 | This study | 2.1 | −0.07 ± 0.03 | 3 | This study |
| 1.07 ± 0.06 | Chen et al. (2016)34 | −0.17 ± 0.06 | Yang et al. (2017)59 | |||||
| 1.05 ± 0.04 | Zhu et al. (2019)39 | −0.11 ± 0.07 | Guedes et al. (2019)29 | |||||
| RGM-2 | 33 | 0.39 ± 0.03 | 5 | This study | 2.5 | 0.10 ± 0.02 | 5 | This study |
| 0.44 ± 0.02 | Druce et al. (2020)4 | |||||||
| BHVO-2 | 102 | 0.32 ± 0.05 | 6 | This study | 3.8 | −0.06 ± 0.05 | 6 | This study |
| 0.34 ± 0.04 | Rosca et al. (2021)2 | −0.08 ± 0.06 | Bezard et al. (2016)57 | |||||
| 0.33 ± 0.03 | Amet and Fitoussi (2020)22 | −0.08 ± 0.08 | Freymuth et al. (2015)60 | |||||
| 0.32 ± 0.06 | Freymuth et al. (2020)53 | −0.07 ± 0.06 | Gaspers et al. (2020)61 | |||||
| 0.31 ± 0.03 | Chen et al. (2016)34 | −0.07 ± 0.04 | Willbold et al. (2016)25 | |||||
| 0.31 ± 0.04 | Zhu et al. (2019)39 | −0.06 ± 0.03 | Burkhardt et al. (2014)56 | |||||
| 0.27 ± 0.06 | Sossi et al. (2015)52 | −0.03 ± 0.05 | Feng et al. (2020)27 | |||||
| 0.25 ± 0.09 | Araújo et al. (2016)49 | −0.03 ± 0.05 | Zhu et al. (2022)5 | |||||
| 0.01 ± 0.06 | Yang et al. (2015)62 | |||||||
| SBC-1 | 186.8 | 0.42 ± 0.04 | 5 | This study | 2.35 | 0.41 ± 0.04 | 5 | This study |
| 0.47 ± 0.01 | Druce et al. (2020)4 | 0.36 ± 0.08 | Gaspers et al. (2020)61 | |||||
| SGR-1b | 72 | 0.32 ± 0.03 | 4 | This study | 35.5 | 0.43 ± 0.04 | 4 | This study |
| 0.47 ± 0.02 | Druce et al. (2020)4 | 0.44 ± 0.06 | Gaspers et al. (2020)61 | |||||
| 0.44 ± 0.11 | Zhao et al. (2016)26 | |||||||
| 0.42 ± 0.03 | Zhu et al. (2022)5 | |||||||
| 0.38 ± 0.02 | Li et al. (2016)37 | |||||||
| COQ-1 | 87 | 0.24 ± 0.03 | 3 | This study | 7.4 | −0.23 ± 0.02 | 3 | This study |
| 0.27 ± 0.03 | Druce et al. (2020)4 | −0.26 ± 0.10 | Zhao et al. (2016)26 | |||||
| 0.27 ± 0.04 | Lv et al. (2018)54 | −0.20 ± 0.02 | Zhu et al. (2022)5 | |||||
| −0.17 ± 0.02 | Gaspers et al. (2020)61 | |||||||
| GSR-1 | 28 | 0.40 ± 0.04 | 3 | This study | 3.5 | 0.09 ± 0.04 | 3 | This study |
| 0.09 ± 0.01 | Zhu et al. (2022)5 | |||||||
| GSR-2 | 71 | 0.31 ± 0.03 | 3 | This study | 0.54 | −0.32 ± 0.05 | 3 | This study |
| −0.28 ± 0.07 | Zhao et al. (2016)26 | |||||||
| −0.14 ± 0.01 | Zhu et al. (2022)5 | |||||||
| GSR-3 | 150 | 0.44 ± 0.02 | 2 | This study | 2.6 | −0.53 ± 0.09 | 2 | This study |
| 0.44 ± 0.05 | Lv et al. (2016)55 | −0.53 ± 0.06 | Zhu et al. (2022)5 | |||||
| −0.51 ± 0.04 | Zhao et al. (2016)26 | |||||||
| GSR-16 | 33.4 | 0.34 ± 0.03 | 2 | This study | 0.33 | −0.06 ± 0.07 | 2 | This study |
| GSR-17 | 86.2 | 0.29 ± 0.04 | 3 | This study | 0.64 | 0.20 ± 0.07 | 3 | This study |
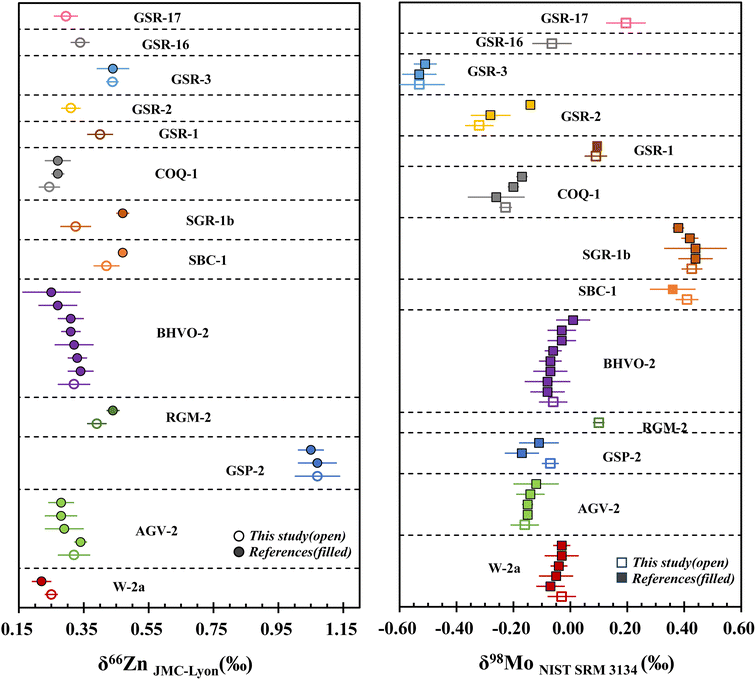
Our δ66Zn values of W-2a and AGV-2 are in good agreement with the previously reported results.22,34,39,49 The average δ66Zn value of 1.07 ± 0.07‰ (2SD, n = 3) in GSP-2 is in good agreement with that reported by Chen et al.34 and Zhu et al.39 The δ66Zn of 0.39 ± 0.03‰ (2SD, n = 5) for RGM-2 is slightly lower than that of 0.44 ± 0.02‰ (2SD) reported by Druce et al.4 δ66Zn for BHVO-2 obtained in this study is indistinguishable from the results reported in previous studies.2,22,34,39,49,52,53 The Zn isotopic results obtained in these reference materials confirm the accuracy of the proposed column separation protocol, which can achieve high precision Zn isotope ratio measurements in geological samples. However, lower values of δ66Zn were obtained in two USGS sedimentary rocks, SBC-1 and SGR-1b, as compared to that reported by Druce et al.4 Due to the limited reference values, we are unable to determine the cause of the discrepancy. The Zn isotopic composition of COQ-1 measured in this study is in agreement with reported values by others,4,54 within the reported uncertainty. Additionally, we also determined the Zn isotopic compositions of five IGGE reference materials, including GSR-1, GSR-2, GSR-3, GSR-16 and GSR-17. Average δ66Zn values of 0.40 ± 0.04‰ (2SD, n = 3), 0.31 ± 0.03‰ (2SD, n = 3), 0.34 ± 0.03‰ (2SD, n = 2) and 0.29 ± 0.04‰ (2SD, n = 3) were obtained for GSR-1, GSR-2, GSR-16 and GSR-17, respectively, and were firstly reported in this study. Our obtained average δ66Zn value of GSR-3 was 0.44 ± 0.02‰ (2SD, n = 2), which well agrees with the published value of 0.44 ± 0.05‰ (2SD) by Lv et al.55
It is evident that the δ98Mo results obtained for W-2a and AGV-2 are in good agreement with the reported values.5,25–27,38,56–58 The δ98Mo value of GSP-2 (−0.07 ± 0.03‰, 2SD, n = 3) obtained in this study is in agreement with the value of −0.11 ± 0.07‰ (2SD) reported by Guedes et al.,29 but in disagreement with results of −0.17 ± 0.05‰ (2SD) reported by Yang et al.59 The observed discrepancies may suggest that the Mo isotopic composition of GSP-2 is heterogeneous. The δ98Mo value of −0.06 ± 0.05‰ (2SD, n = 6) obtained for BHVO-2 in this study is within the reported range of 0.01‰ to −0.08‰.5,25,27,56,57,60–62 Besides the USGS igneous rocks mentioned above, we first reported the Mo isotope ratio of rhyolite RGM-2 (0.10 ± 0.02‰, 2SD, n = 5). The Mo isotopic compositions of shale rocks were determined to be 0.41 ± 0.04‰ (2SD, n = 5) for SBC-1 and 0.43 ± 0.04‰ (2SD, n = 4) for SGR-1b in this study, which are in good agreement with those reported in previous studies.5,26,37,61 The δ98Mo value of carbonatite COQ-1 measured was −0.23 ± 0.02‰ (2SD, n = 3), in accordance with the −0.20 ± 0.02‰ (2SD) reported by Zhu et al.5 and −0.26 ± 0.10‰ (2SD) reported by Zhao et al.,26 but slightly different from the −0.17 ± 0.02‰ (2SD) reported by Gaspers et al.61 The δ98Mo values obtained for GSR-1 and GSR-3 (Table 4) are consistent with those reported by Zhu et al.5 Our GSR-2 (−0.32 ± 0.05‰, 2SD, n = 3) data are in agreement with the −0.28 ± 0.07‰ (2SD) by Zhao et al.,26 but in disagreement with the value of −0.14 ± 0.01‰ (2SD) measured by Zhu et al.,5 who considered that the Mo isotopic composition of GSR-2 is possibly heterogeneous. δ98Mo values for GSR-16 (−0.06 ± 0.07‰, 2SD, n = 2) and GSR-17 (0.20 ± 0.07‰, 2SD, n = 3) were obtained, which have not been reported in previous work.
4. Conclusions
In this study, a single pass with a double-stack column protocol for fast chemical separation of Zn and Mo from various geological reference materials for isotopic analysis by MC-ICP-MS is developed. The proposed protocol is based on the use of AG MP-1M anion exchange resin (100–200 mesh) and DGA resin (50–100 μm). Through employing MC-ICP-MS equipped with jet + X cones, high precision Zn and Mo isotope ratio measurements are obtained. Based on the investigation of different mass bias correction models including SSB and C-SSBIN, and two cone combinations including jet + X and standard + X, jet + X cone combination with the C-SSBIN mass bias correction model (Cu as the internal standard) is found to be preferable for achieving precise and accurate Zn isotope ratio measurements. Through doping experiments, we discovered that it is imperative to further separate and purify Zn and Mo, respectively, from geological samples to ensure the Zn/Mo ratio below 25 for the accurate determination of Mo isotope ratios in geological samples. Importantly, isotopic compositions of Zn and Mo measured in geological reference materials using the proposed method are mostly in agreement with the reported values, validating the proposed stack-column separation protocol. The proposed method is not only effective for purifying Zn and Mo fractions from various geological materials but also reduces the time required for column separation by at least 50% compared to other traditional methods adopting two or more independent columns to separate Zn and Mo.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 42273014 and 41673013) and the open fund of Key Laboratory of Carbonate Reservoirs, CNPC. We thank two anonymous reviewers and Dr Emma Stephen for their constructive comments and the editor for handling, which significantly improved the manuscript.References
- J. Hoefs, Stable Isotope Geochemistry, Springer, 2018 Search PubMed.
- C. Rosca, S. König, M.-L. Pons and R. Schoenberg, Chem. Geol., 2021, 586, 120440 CrossRef CAS.
- H. J. Wen, J. Carignan, C. Cloquet, X. K. Zhu and Y. X. Zhang, J. Anal. At. Spectrom., 2010, 25, 716–721 RSC.
- M. Druce, C. H. Stirling and J. M. Rolison, Geostand. Geoanal. Res., 2020, 44, 711–732 CrossRef CAS.
- H. G. Zhu, J. M. Zhu, D. Tan, X. Lin, K. Lu and W. Yang, J. Anal. At. Spectrom., 2022, 37, 1063–1075 RSC.
- S. Q. Huang, Y. L. Gong, S. H. Tian, Z. W. Liang and C. H. Zhu, Acta Geol. Sin., 2023, 97, 1002–1029 CrossRef.
- B. Kendall, T. W. Dahl and A. D. Anbar, Rev. Mineral. Geochem., 2017, 82, 683–732 CrossRef CAS.
- C. M. Ostrander, S. K. Sahoo, B. Kendall, G. Jiang, N. J. Planavsky, T. W. Lyons, S. G. Nielsen, J. D. Owens, G. W. Gordon, S. J. Romaniello and A. D. Anbar, Geochim. Cosmochim. Acta, 2019, 261, 191–209 CrossRef CAS.
- S. A. Liu, H. C. Wu, S. Z. Shen, G. Q. Jiang, S. H. Zhang, Y. W. Lv, H. Zhang and S. G. Li, Geology, 2017, 45, 343–346 CrossRef CAS.
- C. R. Pearce, A. S. Cohen, A. L. Coe and K. W. Burton, Geology, 2008, 36, 231–234 CrossRef CAS.
- S.-A. Liu, Y.-R. Qu, Z.-Z. Wang, M.-L. Li, C. Yang and S.-G. Li, Earth-Sci. Rev., 2022, 228, 104010 CrossRef CAS.
- Y. Zhang, C. Yuan, M. Sun, J. Li, X. Long, Y. Jiang and Z. Huang, Geochim. Cosmochim. Acta, 2020, 278, 340–352 CrossRef CAS.
- K. R. Bermingham, E. Füri, K. Lodders and B. Marty, Space Sci. Rev., 2020, 216, 133 CrossRef CAS.
- M. Paquet, P. A. Sossi and F. Moynier, Earth Planet. Sci. Lett., 2023, 611, 118126 CrossRef CAS.
- Y. Zhang, H. Wen, C. Zhu, H. Fan, J. Xiao and J. Wen, Chem. Geol., 2021, 575, 120259 CrossRef CAS.
- A. D. Anbar and O. Rouxel, Annu. Rev. Earth Planet. Sci., 2007, 35, 717–746 CrossRef CAS.
- Y. Lü and S.-A. Liu, J. Earth Sci., 2022, 33, 92–99 CrossRef.
- T. Kleine, T. Steller, C. Burkhardt and F. Nimmo, Icarus, 2023, 397, 115519 CrossRef CAS.
- R. Martins, S. Kuthning, B. J. Coles, K. Kreissig and M. Rehkämper, Science, 2023, 379, 369–372 CrossRef CAS PubMed.
- C. Burkhardt, T. Kleine, F. Oberli, A. Pack, B. Bourdon and R. Wieler, Earth Planet. Sci. Lett., 2011, 312, 390–400 CrossRef CAS.
- C. N. Maréchal, P. Télouk and F. Albarède, Chem. Geol., 1999, 156, 251–273 CrossRef.
- Q. Amet and C. Fitoussi, Int. J. Mass Spectrom., 2020, 457, 116413 CrossRef CAS.
- S. Takano, M. Tanimizu, T. Hirata, K.-C. Shin, Y. Fukami, K. Suzuki and Y. Sohrin, Anal. Chim. Acta, 2017, 967, 1–11 CrossRef CAS PubMed.
- J. Barling, G. L. Arnold and A. D. Anbar, Earth Planet. Sci. Lett., 2001, 193, 447–457 CrossRef CAS.
- M. Willbold, K. Hibbert, Y.-J. Lai, H. Freymuth, R. C. Hin, C. Coath, F. Vils and T. Elliott, Geostand. Geoanal. Res., 2016, 40, 389–403 CAS.
- P. P. Zhao, J. Li, L. Zhang, Z. B. Wang, D. X. Kong, J. L. Ma, G. J. Wei and J. F. Xu, Geostand. Geoanal. Res., 2016, 40, 217–226 CrossRef CAS.
- L. Feng, L. Zhou, W. Hu, W. Zhang, B. Li, Y. Liu, Z. Hu and L. Yang, J. Anal. At. Spectrom., 2020, 35, 145–154 RSC.
- J. Li, X. R. Liang, L. F. Zhong, X. C. Wang, Z. Y. Ren, S. L. Sun, Z. F. Zhang and J. F. Xu, Geostand. Geoanal. Res., 2014, 38, 345–354 CrossRef CAS.
- S. d. S. Guedes, V. R. Bellotto, R. V. Santos and E. L. Dantas, Geochim. Bras., 2019, 33, 1–15 CrossRef.
- R. L. Rudnick and S. Gao, in Treatise on Geochemistry, ed. H. D. Holland and K. K. Turekian, Elsevier, Oxford, 2nd edn, 2014, DOI: DOI:10.1016/b978-0-08-095975-7.00301-6, pp. 1–51.
- A. Pourmand and N. Dauphas, Talanta, 2010, 81, 741–753 CrossRef CAS PubMed.
- L. Yang, E. Dabek-Zlotorzynskab and V. Celob, J. Anal. At. Spectrom., 2009, 24, 1564–1569 RSC.
- K. Moeller, R. Schoenberg, P. Rolf-Birger, D. Weiss and S. Dong, Geostand. Geoanal. Res., 2012, 36, 177–199 CrossRef CAS.
- S. Chen, Y. C. Liu, J. Y. Hu, Z. F. Zhang and H. M. Yu, Geostand. Geoanal. Res., 2016, 40, 417–432 CrossRef CAS.
- C. Archer, M. B. Andersen, C. Cloquet, T. M. Conway, S. Dong, M. Ellwood, R. Moore, J. Nelson, M. Rehkämper, O. Rouxel, M. Samanta, K.-C. Shin, Y. Sohrin, S. Takanoh and L. Wasylenkii, J. Anal. At. Spectrom., 2017, 32, 415–419 RSC.
- J. Liu, H. J. Wen, Y. X. Zhang, H. F. Fan and C. W. Zhu, J. Anal. At. Spectrom., 2016, 31, 1287–1297 RSC.
- J. Li, X. K. Zhu, S. H. Tang and K. Zhang, Geostand. Geoanal. Res., 2016, 40, 405–415 CrossRef CAS.
- J. J. Fan, J. Li, Q. Wang, L. Zhang, J. Zhang, X. L. Zeng, L. Ma and Z. L. Wang, Chem. Geol., 2020, 545, 119648 CrossRef CAS.
- Y. T. Zhu, M. Li, Z. C. Wang, Z. Q. Zou, Z. C. Hu, Y. S. Liu, L. Zhou and X. N. Chai, At. Spectrosc., 2019, 40, 206–214 CrossRef CAS.
- C. Maréchal and F. Albarède, Geochim. Cosmochim. Acta, 2002, 66, 1499–1509 CrossRef.
- D. J. Bettinardi, A. Paulenova and P. Tkac, ACS Omega, 2020, 5, 23786–23792 CrossRef CAS PubMed.
- E. P. Horwitz, D. R. McAlister, A. H. Bond and R. E. B. Jr, Solvent Extr. Ion Exch., 2005, 23, 319–344 CrossRef CAS.
- A. Retzmann, T. Zimmermann, D. Pröfrock, T. Prohaska and J. Irrgeher, Anal. Bioanal. Chem., 2017, 409, 5463–5480 CrossRef CAS PubMed.
- L. P. Feng, L. Zhou, L. Yang, D. J. DePaolo, S. Y. Tong, Y. S. Liu, T. L. Owens and S. Gao, Geostand. Geoanal. Res., 2017, 41, 93–106 CrossRef CAS.
- Z. Kazi, N. Guérin, M. Christl, M. Totland, A. Gagné and S. Burrell, J. Radioanal. Nucl. Chem., 2019, 321, 227–233 CrossRef CAS.
- J. Sun, X. K. Zhu, S. H. Tang and Y. L. Chen, Geoscience, 2010, 24, 866–869 CAS.
- A. J. Pietruszka, R. J. Walker and P. A. Candela, Chem. Geol., 2006, 225, 121–136 CrossRef CAS.
- K. Peel, D. Weiss, J. Chapman, T. Arnolda and B. Colesab, J. Anal. At. Spectrom., 2008, 23, 103–110 RSC.
- D. F. Araújo, G. R. Boaventura, J. Viers, D. S. Mulholland, D. Weiss, D. Araújo, B. Lima, I. Ruiz, W. Machado and M. Babinski, J. Braz. Chem. Soc., 2016, 28, 225–235 Search PubMed.
- S. G. John, J. G. Park, Z. T. Zhang and E. A. Boyle, Chem. Geol., 2007, 245, 61–69 CrossRef CAS.
- T. M. Conway, A. D. Rosenberg, J. F. Adkins and S. G. John, Anal. Chim. Acta, 2013, 793, 44–52 CrossRef CAS PubMed.
- P. A. Sossi, G. P. Halverson, O. Nebel and S. M. Eggins, Geostand. Geoanal. Res., 2015, 39, 129–149 CrossRef CAS.
- H. Freymuth, C. D. J. Reekie and H. M. Williams, Geostand. Geoanal. Res., 2020, 44, 407–420 CrossRef CAS.
- Y. W. Lv, S. A. Liu, H. C. Wu, S. V. Hohl, S. M. Chen and S. G. Li, Geochim. Cosmochim. Acta, 2018, 239, 330–345 CrossRef CAS.
- Y. W. Lv, S. A. Liu, J. M. Zhu and S. G. Li, Chem. Geol., 2016, 445, 24–35 CrossRef CAS.
- C. Burkhardt, R. C. Hin, T. Kleine and B. Bourdon, Earth Planet. Sci. Lett., 2014, 391, 201–211 CrossRef CAS.
- R. Bezard, M. Fischer-Gödde, C. Hamelin, G. A. Brennecka and T. Kleine, Earth Planet. Sci. Lett., 2016, 453, 171–181 CrossRef CAS.
- H. Y. Li, J. Li, J. G. Ryan, X. Li, R. P. Zhao, L. Ma and Y. G. Xu, Earth Planet. Sci. Lett., 2019, 520, 105–114 CrossRef CAS.
- J. Yang, J. Barling, C. Siebert, J. Fietzke, E. Stephens and A. N. Halliday, Geochim. Cosmochim. Acta, 2017, 205, 168–186 CrossRef CAS.
- H. Freymuth, F. Vils, M. Willbold, R. N. Taylor and T. Elliott, Earth Planet. Sci. Lett., 2015, 432, 176–186 CrossRef CAS.
- N. Gaspers, T. Magna and L. Ackerman, Geostand. Geoanal. Res., 2020, 44, 363–374 CrossRef CAS.
- J. Yang, C. Siebert, J. Barling, P. Savage, Y.-H. Liang and A. N. Halliday, Geochim. Cosmochim. Acta, 2015, 162, 126–136 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |