Open Access Article
Open Access ArticleBioaccessibility and unravelling of polyphenols, sulforaphane, and indoles biotransformation after in vitro gastrointestinal digestion of a novel lactofermented broccoli beverage†
José Ángel
Salas-Millán
 ab and
Encarna
Aguayo
ab and
Encarna
Aguayo
 *ab
*ab
aPostharvest and Refrigeration Group, Polytechnic University of Cartagena (UPCT), Paseo Alfonso XIII, 48, 30203 Cartagena, Spain. E-mail: encarna.aguayo@upct.es
bFood Quality and Health Group, Institute of Plant Biotechnology (IBV-UPCT), Campus Muralla del Mar, 30202 Cartagena, Spain
First published on 18th November 2024
Abstract
This study assesses the transformation and stability of polyphenols, sulforaphane, and indoles in a fermented beverage made from broccoli leaves during in vitro gastrointestinal digestion (GID). This process was simulated using a dialysis membrane to assess intestinal absorption. The total phenolic compounds (TPC) and antioxidant TEAC assays showed an increase in phytochemical content due to the GID process. The higher TPC and antioxidant activity observed after digestion was likely due to the enzymatic transformation of polyphenols in mildly alkaline conditions. Individual phytochemical analysis revealed that hydroxycinnamic acids, particularly 3CQa, remained stable initially but then decreased significantly during intestinal digestion. Acylated flavonoids exhibited a decrease during intestinal digestion, while deacylated flavonoids initially decreased before stabilising. This indicated the occurrence of enzymatic hydrolysis of more structurally complex flavonoids to glycosylated flavonoids such as kaempferol-3,7-diglucoside, and kaempferol-3-sophoroside-7-glucoside. Consequently, deacylated flavonoids were highlighted for their high bioaccessibility rate after in vitro GID. Glucosinolate-hydrolysis products, including sulforaphane and indoles, exhibited a general decrease during digestion, with sulforaphane showing 51% bioaccessibility. The study highlights the dialysed in vitro GID process, which affects the release and transformation of bioactive compounds, potentially increasing their bioaccessibility and the subsequent health benefits of the lactofermented beverage made from broccoli leaves.
1. Introduction
The fruit and vegetable industry produces by-products that are rich in functional compounds, and which have the potential to both benefit health and also reduce the progression of chronic diseases. Today, the agri-food industry must devise strategies to reduce food loss and waste, promote a circular economy, and enhance the efficiency of the food sector.1 In this context, broccoli leaves represent a novel avenue for the development of functional beverages with potential health benefits.2 Furthermore, fermentation has been proposed as a means of enhancing the value of by-products, not only by improving the sensory qualities of foods, but also by increasing the concentration and bioaccessibility of phytochemicals.3 Previous studies have shown that fermentation can significantly increase the total phenolic content (TPC) and antioxidant capacity of plant foods.4 This enhancement is attributed to the breakdown of complex compounds into simpler forms by microbial enzymes, thereby increasing their bioaccessibility and enhancing their antioxidant activity. Different works have reported the use of the fermentation process in broccoli florets,5–7 and broccoli by-products such as stalk and leaves.2,3 During fermentation, several polyphenols are released from the food matrix and transferred to the liquid medium in which the fermentation takes place. This transfer is due to the enzymatic activity of lactic acid bacteria, which catalyses the hydrolysis of ester groups of polyphenols linked to the plant cell wall.3 Thus, fermentation stands out as an assisted extraction of polyphenols such as hydroxycinnamic acids, and a high diversity of flavonoids, which include deacylated flavonoids, as well as acylated flavonoids. The latter are complex flavonoid groups molecules in which hydroxycinnamic could be found linked to the glycoside moieties in their complex structure.2 The phenolic compounds are recognised for their potential anti-cancer properties and their ability to counteract diseases associated with oxidative stress. Previous research has shown that the health-promoting effects of dietary phenolic compounds are due to their antioxidant, anti-inflammatory and anticarcinogenic activities.8 Other authors reported the biotransformation of glucoraphanin into sulforaphane through fermentation processing, as an alternative to maintain the myrosinase activity.7 This biotransformation also appears in the literature in the development where sulforaphane and indoles were monitored during the shelf life of a fermented beverage made from broccoli leaves.2 Isothiocyanates impact tumour environments, inhibit stem cell renewal, alter metabolism, affect microbiota, and protect against H. pylori.9However, the bioaccessibility of these phytochemicals, referring to the proportion that is absorbed and utilized by the body, remains a critical factor in their effectiveness.10 It is essential to understand the behaviour and dynamic bioaccessibility of these bioactive compounds during gastrointestinal digestion (GID) in order to evaluate their true potential as functional ingredients. In vitro digestion models are widely used to simulate the human GID process11 and enable the study of the stability and bioaccessibility of polyphenols and isothiocyanates. Such models provide insight into how these compounds are released from the food matrix, transformed by digestive enzymes, and potentially absorbed across the intestinal barrier.12 The use of a semipermeable cellulose membrane as a dialysis step could assess the transfer of phytochemicals by passive diffusion and also allow the study of phytochemicals potentially available for further uptake.13 Phenols and isothiocyanates undergo complex changes during digestion that may affect their bioaccessibility and health benefits.14 Thus, some authors have reported the in vitro GID of these phytochemicals from fruits and vegetables, and smoothie-like beverages.15,16 Although the kinetic release of these phytochemicals from the solid food matrix is often reported, relatively few studies have focused on liquid foods such as coffee and tea.17,18 It is proposed that the formation of these phytochemicals during in vitro GID may be due to conversion by digestive enzymes (amylases, lipases, and proteases) under alkaline conditions.19
The present work studies the phytochemical characterisation of a lactofermented beverage made from broccoli leaves during the in vitro GID process. The objective is to find out the dynamic evolution and transformation of hydroxycinnamic acids, acylated and deacylated flavonoids, and the glucosinolate-hydrolysis products sulforaphane and indole compounds during in vitro GID. This research aims to elucidate the bioaccessibility of these phytochemicals in the gastrointestinal tract and explore their potential health benefits upon absorption. The results will contribute to a better understanding of the impact of lactofermentation and GID on the bioaccessibility of bioactive compounds, which will in turn support the development of foods with improved functional properties.
2. Materials and methods
2.1 Fermented beverage elaboration
For the fermentation of broccoli by-products, fresh broccoli leaves (Brassica oleracea L. var. Italica cv. Parthenon) were obtained from the Levante Sur Coop. in south-eastern Spain (La Palma, Cartagena, Spain). Broccoli leaves were immersed in drinking water at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/w) in a Bionet F0-2CC bioreactor (Bionet, Fuente Álamo, Spain) under aseptic conditions. An inoculum (6 log CFU mL−1) of Lactiplantibacillus plantarum CECT 749, isolated from pickled cabbage (Spanish Type Culture Collection, University of Valencia, Spain), was used to initiate the fermentation process. The fermentation was conducted for a period of four days at a temperature of 27.7 °C, in accordance with the results of previous optimisation experiments.2 All details regarding the development and characterization of this beverage can be found in the article Salas-Millán et al.2
10 (w/w) in a Bionet F0-2CC bioreactor (Bionet, Fuente Álamo, Spain) under aseptic conditions. An inoculum (6 log CFU mL−1) of Lactiplantibacillus plantarum CECT 749, isolated from pickled cabbage (Spanish Type Culture Collection, University of Valencia, Spain), was used to initiate the fermentation process. The fermentation was conducted for a period of four days at a temperature of 27.7 °C, in accordance with the results of previous optimisation experiments.2 All details regarding the development and characterization of this beverage can be found in the article Salas-Millán et al.2
2.2 In vitro gastrointestinal digestion, recovery index and bioaccessibility of phytochemicals in lactofermented beverage
A bioaccessibility assessment was conducted via an in vitro gastrointestinal assay in accordance with the INFOGEST protocol.11 This method included different stages of oral, gastric, and intestinal digestion, respectively. The latter stage was carried out by dialysis,20 simulating passive diffusion of phytochemicals after intestinal digestion, using a Spectrum™ 132![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 706 dialysis membrane tube (12–14 kDa; Fisher Scientific, USA). The GID assay was conducted in triplicate, with samples collected at each stage of digestion and at 30-minute intervals during intestinal digestion up to 180 minutes (30, 60, 90, 120, 150, and 180 min). In the current in vitro digestion, no enzyme inhibitors were used after the digestion steps according to the INFOGEST standardised method. For each digestion stage, 10 mL of sample was collected and subsequently frozen until analysis.
706 dialysis membrane tube (12–14 kDa; Fisher Scientific, USA). The GID assay was conducted in triplicate, with samples collected at each stage of digestion and at 30-minute intervals during intestinal digestion up to 180 minutes (30, 60, 90, 120, 150, and 180 min). In the current in vitro digestion, no enzyme inhibitors were used after the digestion steps according to the INFOGEST standardised method. For each digestion stage, 10 mL of sample was collected and subsequently frozen until analysis.
The recovery index, which represents the amount of phytochemicals recovered after the different stages of gastrointestinal digestion and dialysis, was calculated in comparison with the undigested fermented beverage. Additionally, the percentage bioaccessibility was also calculated by comparing the concentration of phytochemicals in the dialysis fraction (which represents what passed through the dialysis membrane) with that in the gastric digestion fraction.15
2.3 Total phenolic content and antioxidant capacity assessment in the digested lactofermented beverage
Total Phenolic Content (TPC) and Trolox Equivalent Antioxidant Capacity (TEAC) were evaluated in the different gastrointestinal steps during the in vitro assay. Additionally, the evolution during the GID process was compared with and without an intestinal dialysis step to assess the progression of digestion. The measurements followed the procedure described by Salas-Millán et al.21 For the TEAC analysis, the ABTS reagent (2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate)) was obtained from Sigma Aldrich (St Louis, USA). The results were expressed as mg gallic acid equivalent (GAE) for TPC and μmol trolox equivalent (TE) per litre in fermented beverage.2.4 Quantification of individual polyphenols, sulforaphane, and indoles by LC-MS analysis
The extraction of the individual polyphenolic compounds, sulforaphane, and indoles was performed in accordance with Gonzales et al.22 and Yu et al.23 methodologies respectively, with minor modifications. A 5 mL sample of each digestion step was previously filtered (0.45 μm polyamide membrane filter) before solid phase extraction (SPE, C18-500 mg, Sigma Aldrich, St Louis, USA). In the case of the dialysate fraction, 250 mL was used to isolate and preconcentrate the phytochemical that had diffused through the dialysis membrane.The individual polyphenolic compounds were identified by LC-MS/MS-ESI-QToF in previous work,2 and their quantification was carried out as per Gonzales et al. (2015)22 using an Agilent 1200 high-pressure liquid chromatograph (HPLC, Santa Clara, USA), equipped with a G1311B quaternary pump, a G1329B standard autosampler and a G1316A column heater, coupled to a 6420 triple quadrupole mass spectrometer (QqQ) with an electrospray ionisation (ESI) source. Individual polyphenolic compounds were separated on a reverse phase Luna Omega C18 column (2.1 × 100 mm; 3 μm). The mobile phase consisted of solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in acetonitrile). The analysis was performed in negative mode, with the autosampler injecting 5 μL of sample; the oven temperature was set at 35 °C and a gradient elution of 0.1–2% B over 15 min, 2–31% B over 22 min, followed by 31–95% B to 47 min with 5 min isocratic conditions at 95% B. The capillary voltage, gas temperature, gas flow, and nebuliser pressure were set at 4000 V, 350 °C, 11 L min−1 and 40 psi, respectively. The quantification of hydroxycinnamic acid derivatives was expressed as mg of chlorogenic acid equivalents (89175, PhytoLab GmbH & Co, Vestenbergsgreuth, Germany), and as mg of rutin (89270, PhytoLab GmbH & Co) for flavonoid derivatives, in both cases per litre of fermented beverage. The transition for identified individual polyphenols is shown in the ESI Section (Table S1†).
The determination of sulforaphane and the indoles indol-3-carbinol, ascorbigen and diindolylmethane was performed according to the methodology described by Hauder et al. (2011),24 with minor modifications. The same HPLC/MS instrument, column and mobile phases used for the quantification of polyphenols were used for the quantification of sulforaphane and indoles compounds. The analysis was performed in positive mode with the mass spectrometer parameters set as follows: capillary voltage of 3500 V, gas temperature at 325 °C, gas flow rate of 10 L min−1 and nebuliser pressure of 45 psi. Only one transition was considered for quantification (Table S1†). The concentration of glucosinolate-hydrolysis products compounds was quantified using authentic standards – sulforaphane (S6317) and indole-3-carbinol (I7256, Sigma Aldrich, St Luis, USA), and ascorbigen (TR-A787038, TRC, Toronto, Canada) – expressed as mg per litre in the fermented beverage. Sulfuraphane conjugation was not considered in the bioaccessibility assessment.
2.5 Statistical analysis
The results are presented as means and the standard error of the means. A two-way analysis of variance (ANOVA) was employed, and means were compared using Fisher's least significant difference (LSD) for mean difference. A p-value of <0.01 was considered significant and all analyses were performed using Prism (GraphPad Software, San Diego, CA, USA). A cluster correlation heatmap was constructed using Pearson r distance to examine the concentration of flavonoids derivatives in the intestinal digestion fraction at different time points. This analysis was conducted using MetaboAnalys 5.0 (https://www.metaboanalys.ca).3. Results and discussion
3.1 TPC and TEAC during in vitro gastrointestinal digestion of lactofermented beverage made from broccoli leaves
The initial values of TPC and TEAC of the fermented beverage from broccoli leaves and their evolution during the dialysed and non-dialysed GID process are shown in Fig. 1. After four days of fermentation at 27.7 °C, the beverage exhibited 259 mg GAE per L of TPC and 192 μmol TE per L of antioxidant capacity. No differences were found in TPC after the oral and gastric digestion stages. On the other hand, there was an increase in TPC in the intestinal digestion process, which could be attributable to either the release of TPC or its biotransformation in both the dialysed and non-dialysed assays.The non-dialysed intestinal digestion process conducted at 30 min intervals up to 180 min showed an increase in the first 30 min, reaching and maintaining a concentration of 641 to 700 mg GAE per L. This was statistically significant (p-value > 0.01) in comparison to the beverage under dialysis intestinal digestion process. On the other hand, the TPC levels in the dialysed intestinal digestion showed a different evolution, with a Gaussian-like curve distribution during the 180 min of the intestinal digestion process. Thus, the increase in TPC was less pronounced compared to the non-dialysed intestinal digestion process, suggesting a passive diffusion of polyphenolic compounds during their release from the food matrix through the dialysis membrane to the dialysate. The peak of TPC occurred at 90 min of intestinal digestion, reaching 520 mg GAE per L; the TPC levels then decreased to 343 mg GAE per L after 180 min.
For the antioxidant capacity, the results followed different trends compared to TPC during the in vitro GID process. For example, no statistical differences were observed between the dialysed and non-dialysed intestinal digestion until 60 min. However, from that point onwards, a notable increase was observed in the TEAC in the non-dialysed intestinal digestion, from 90 to 180 min (209 to 273 μmol TE per L). On the other hand, after 90 min of dialysed intestinal digestion, the TEAC levels decreased, suggesting the importance of passive diffusion through the membrane of antioxidant compounds from fermented beverage.
3.2 Evolution and transformation of individual phytochemicals during dynamic in vitro gastrointestinal digestion
Individual polyphenolic compounds, sulforaphane, and indoles (indol-3-carbinol, ascorbigen and diindolylmethane) were quantified in each stage of the dialysed in vitro GID using a dialysis membrane to assess the passive diffusion of phytochemicals (Fig. 2A). The relative evolution of the phytochemicals was evaluated using the recovery index, which provided a more comprehensive overview of the dynamic process and transformation of each phytochemical through the in vitro GID process. | ||
| Fig. 2 Individual polyphenols (hydroxycinnamic acids, acylated and deacylated flavonoids), sulforaphane, and indoles in the fermented beverage during the in vitro gastrointestinal digestion process. FB: fermented beverage made from broccoli leaves. OD: oral digestion. GD: gastric digestion. ID: intestinal digestion dialysed, conducted at 30 min intervals up to 180 min. DD: dialysate. (A) Chromatograms of LC-QqQ of lactofermented beverage from broccoli leaves and its dialysed intestinal digestion dialysed at 180 min and dialysate. (B) Concentration of grouped hydroxycinnamic acids, acylated and deacylated flavonoids and (C) sulforaphane and indole compounds. Statistical differences are shown in Table 1. (D) Recovery index (%) of each individual compound by the in vitro GID process. (E) Correlation heatmap considering Pearson r distance measure during intestinal digestion from 30 to 180 min of flavonoid derivatives. | ||
Twenty polyphenolic compounds were characterised from the fermented beverage (Table 1) and were classified into three groups. The first group consisted of eight hydroxycinnamic acid (HA) derivatives, including isomers of 3-, 4- and 5-caffeoylquinic acid (3-, 4-, and 5CQa, respectively), isomers of 1- and 5-feruloylquinic acid (1-, and 5FQa, respectively), disinapoyl gentiobioside and sinapic and ferulic acids. The flavonoid derivatives were classified into two categories, depending on their chemical structure: acylated and deacylated flavonoids. Acylated flavonoids (AF) are composed of eight flavonoids which have an acyl bond with a hydroxycinnamic acid (caffeoyl, feruloyl, hydroxyferuloyl or sinapoyl) in their structure. On the other hand, deacylated flavonoids (DF) are composed of four glycosylated flavonoids with no additional structure beyond hexoses or pentoses.
| Compounds | FB | OD | GD | ID30 | ID60 | ID90 | ID120 | ID150 | ID180 | DD |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (n = 3 ± SE). Means followed by different letters in the same row are significantly different (p < 0.05), according to Fisher's LSD. Caffeoylquinic acid (CQa), feruloylquinic acid (FQa), kaempferol (K); quercetin (Q); glucoside (G); sophoroside (Soph); sinapoyl (Sin); feruloyl (Fer); hydroxyferuloyl (hFe); caffeoyl (Caf). FB: lactofermented beverage from broccoli leaves. OD: oral digestion. GD: gastric digestion. ID: dialysed intestinal digestion conducted at 30 min intervals up to 180 min. DD: dialysate fraction. | ||||||||||
| Hydroxycinnamic acids | ||||||||||
| 3CQa | 34.45 ± 4.66a | 25.22 ± 4.78b | 26.54 ± 3.50b | 6.79 ± 1.40c | 4.87 ± 3.52c | 4.30 ± 0.91c | 2.33 ± 0.21c | 1.82 ± 0.17c | 1.40 ± 0.64c | 1.30 ± 0.86c |
| 4CQa | 10.24 ± 0.96a | 6.42 ± 0.82b | 3.04 ± 1.03c | 1.40 ± 0.41d | 2.17 ± 1.16cd | 1.54 ± 0.26cd | 1.72 ± 0.43cd | 1.42 ± 0.65cd | 1.17 ± 0.26d | 1.02 ± 0.10d |
| 5CQa | 4.52 ± 0.86a | 3.18 ± 0.37b | 1.67 ± 0.55c | 1.83 ± 0.08c | 1.72 ± 1.07c | 0.82 ± 0.15cd | 0.90 ± 0.49cd | 0.16 ± 0.09d | 0.13 ± 0.09d | 0.94 ± 0.03cd |
| 1FQa | 14.04 ± 0.64a | 14.67 ± 3.43a | 12.88 ± 1.10a | 5.26 ± 1.15b | 4.88 ± 0.80b | 3.86 ± 0.53bc | 2.98 ± 0.87bcd | 1.04 ± 0.32cd | 0.83 ± 0.22d | 0.53 ± 0.18d |
| 5FQa | 4.26 ± 0.09a | 3.62 ± 1.50a | 4.54 ± 0.78a | 1.70 ± 0.11b | 1.42 ± 0.26bc | 1.10 ± 0.20bc | 1.04 ± 0.13bc | 0.99 ± 0.26bc | 1.02 ± 0.19bc | 0.31 ± 0.04d |
| dSNPGb | 1.35 ± 0.08a | 1.60 ± 0.56a | 1.23 ± 0.15ab | 0.75 ± 0.16bc | 0.60 ± 0.38bc | 0.35 ± 0.18bc | 0.26 ± 0.15bc | 0.14 ± 0.15c | 0.07 ± 0.07c | 0.21 ± 0.01bc |
| Sinapic acid | 0.76 ± 0.10a | 0.56 ± 0.05b | 0.33 ± 0.07c | 0.03 ± 0.00d | 0.02 ± 0.01d | 0.02 ± 0.00d | 0.02 ± 0.01d | 0.01 ± 0.01d | 0.02 ± 0.01d | 0.01 ± 0.00d |
| Ferulic Acid | 0.13 ± 0.02a | 0.10 ± 0.03a | 0.05 ± 0.01bc | 0.00 ± 0.00d | 0.01 ± 0.00cd | 0.00 ± 0.00d | 0.05 ± 0.05d | 0.00 ± 0.00d | 0.00 ± 0.00d | 0.00 ± 0.00d |
| ΣHA | 69.75 ± 1.21 | 55.37 ± 5.25 | 50.27 ± 3.75 | 17.75 ± 0.15 | 15.68 ± 2.97 | 11.99 ± 0.18 | 9.31 ± 0.90 | 5.58 ± 0.49 | 4.63 ± 0.49 | 4.33 ± 0.34 |
| Acylated flavonoids | ||||||||||
| K-3CafSoph-7dG | 0.09 ± 0.01a | 0.08 ± 0.01ab | 0.06 ± 0.01b | 0.01 ± 0.01c | 0.02 ± 0.01c | 0.01 ± 0.00c | 0.01 ± 0.01c | 0.00 ± 0.01c | 0.00 ± 0.00c | 0.01 ± 0.00c |
| K-3CafSoph-7G | 11.93 ± 1.48a | 10.19 ± 0.48a | 5.87 ± 0.67b | 1.09 ± 0.02c | 1.67 ± 1.62c | 2.12 ± 0.24c | 0.76 ± 0.41c | 0.43 ± 0.51c | 0.33 ± 0.33c | 1.50 ± 0.21c |
| K-3SinSoph-7G | 79.65 ± 5.78a | 84.89 ± 2.13a | 64.17 ± 9.64b | 25.81 ± 4.43bc | 29.49 ± 9.51bc | 27.82 ± 5.68bc | 17.40 ± 4.67bc | 16.43 ± 6.89bc | 15.79 ± 6.43bc | 14.51 ± 1.31c |
| K-3CafSoph-7SinG | 17.59 ± 7.00a | 13.38 ± 4.96a | 10.97 ± 3.76ab | 1.47 ± 1.38c | 5.31 ± 2.29bc | 1.24 ± 0.66c | 1.72 ± 1.71c | 1.09 ± 0.10c | 1.09 ± 0.98c | 11.44 ± 1.85ab |
| K-3FeSoph-7G | 53.08 ± 5.25a | 53.26 ± 1.96a | 38.59 ± 5.88b | 18.04 ± 0.75bc | 21.63 ± 5.96bc | 15.97 ± 3.60bc | 15.99 ± 5.17bc | 15.99 ± 7.85bc | 14.03 ± 6.20bc | 5.14 ± 0.33c |
| K-3FeSoph | 1.07 ± 0.71a | 0.24 ± 0.05a | 1.04 ± 0.93ab | 0.27 ± 0.01b | 0.35 ± 0.25b | 0.38 ± 0.24ab | 0.15 ± 0.05ab | 0.47 ± 0.10b | 0.26 ± 0.04b | 2.82 ± 0.46b |
| K-3HFerSoph-7G | 0.20 ± 0.02ab | 0.25 ± 0.11a | 0.12 ± 0.01bc | 0.03 ± 0.03cd | 0.04 ± 0.07cd | 0.01 ± 0.00d | 0.01 ± 0.00d | 0.00 ± 0.00d | 0.01 ± 0.01d | 0.02 ± 0.02d |
| K-3HFerSoph | 1.30 ± 0.69a | 0.39 ± 0.10b | 0.74 ± 0.59ab | 0.16 ± 0.09b | 0.34 ± 0.28b | 0.91 ± 0.43ab | 0.54 ± 0.22ab | 0.13 ± 0.04b | 0.17 ± 0.05b | 0.16 ± 0.03b |
| ΣAF | 165.10 ± 12.10 | 162.92 ± 3.57 | 121.68 ± 9.04 | 46.92 ± 2.78 | 58.89 ± 9.70 | 48.46 ± 4.53 | 36.59 ± 2.92 | 34.54 ± 4.87 | 31.70 ± 5.86 | 35.61 ± 1.54 |
| Deacylated flavonoids | ||||||||||
| Q-3dG | 6.01 ± 1.60a | 4.54 ± 0.46ab | 3.95 ± 1.01bc | 0.93 ± 0.27e | 1.75 ± 0.27de | 2.85 ± 0.05bcd | 1.71 ± 0.44de | 1.45 ± 1.07de | 1.54 ± 0.44de | 2.61 ± 0.13cde |
| K-3Soph-7G | 0.04 ± 0.02c | 0.02 ± 0.03c | 0.03 ± 0.01c | 0.04 ± 0.01c | 0.03 ± 0.05c | 0.07 ± 0.03c | 0.06 ± 0.01c | 0.18 ± 0.03a | 0.08 ± 0.04bc | 0.13 ± 0.02ab |
| K-3,7dG | 64.08 ± 15.55a | 52.10 ± 4.69ab | 37.48 ± 6.86bc | 14.40 ± 5.33c | 35.61 ± 14.89bc | 46.06 ± 13.15ab | 38.00 ± 17.15bc | 46.42 ± 4.22ab | 47.70 ± 6.62ab | 56.33 ± 3.68ab |
| K-3Soph | 0.95 ± 0.14a | 0.83 ± 0.04a | 0.52 ± 0.14b | 0.10 ± 0.02c | 0.10 ± 0.03c | 0.09 ± 0.03c | 0.20 ± 0.09c | 0.13 ± 0.06c | 0.14 ± 0.04c | 0.20 ± 0.00c |
| ΣDF | 71.07 ± 10.00 | 57.49 ± 2.99 | 41.99 ± 4.61 | 15.47 ± 3.13 | 37.50 ± 8.59 | 49.08 ± 7.62 | 39.97 ± 10.12 | 48.19 ± 2.96 | 49.46 ± 3.72 | 59.26 ± 2.19 |
| Sulforaphane and indoles | ||||||||||
| Sulforaphane | 2.54 ± 0.12ab | 2.73 ± 0.18a | 2.15 ± 0.23abc | 1.65 ± 0.00bc | 2.33 ± 0.45abc | 2.00 ± 0.74ab | 1.61 ± 0.55bc | 1.21 ± 0.41c | 0.96 ± 0.51c | 1.12 ± 0.44c |
| Ascorbigen | 0.30 ± 0.02ab | 0.29 ± 0.02a | 0.09 ± 0.02b | 0.00 ± 0.00c | 0.01 ± 0.00c | 0.01 ± 0.00c | 0.00 ± 0.00c | 0.00 ± 0.00c | 0.00 ± 0.00c | 0.00 ± 0.00c |
| Indole-3-carbinol | 0.14 ± 0.01ab | 0.15 ± 0.01a | 0.06 ± 0.01b | 0.12 ± 0.00a | 0.06 ± 0.04b | 0.02 ± 0.01c | 0.01 ± 0.00c | 0.01 ± 0.00c | 0.02 ± 0.01c | 0.01 ± 0.00c |
| Diindolylmethane | 0.03 ± 0.02n.d. | 0.03 ± 0.01n.d. | 0.00 ± 0.00n.d. | 0.00 ± 0.00n.d. | 0.00 ± 0.00n.d. | 0.00 ± 0.00n.d. | 0.00 ± 0.00n.d. | 0.00 ± 0.00n.d. | 0.00 ± 0.00n.d. | 0.00 ± 0.00n.d. |
| ΣSulforaphane and indoles | 3.01 ± 0.04 | 3.20 ± 0.05 | 2.31 ± 0.07 | 1.77 ± 0.03 | 2.40 ± 0.12 | 2.02 ± 0.19 | 1.63 ± 0.14 | 1.23 ± 0.10 | 0.98 ± 0.13 | 1.13 ± 0.11 |
Regarding the in vitro GID process evolution of individual polyphenols, a general view (Fig. 2B) showed a slight decrease after the oral and gastric digestion compared to the first 30 min of intestinal digestion (Fig. 2B, and Table 1) for all polyphenolic compounds. In the fermented beverage, the total amount of HA was found to be 69.7 mg L−1. The major compound in this group was 3CQa, quantified at 34.5 mg L−1, followed by 4CQa and 1FQa, quantified at 10.2 and 14 mg L−1, respectively. The HA content decreased from 55 to 50 mg L−1 after the oral and gastric digestion, respectively. Thus, a significant decrease was observed in the HA level after the first 30 min of intestinal digestion, when it dropped to 17.8 mg L−1 and decreased even further to 4.6 mg L−1 after 180 min.
In terms of the recovery index (Fig. 2D), 3CQa demonstrated greater stability after gastric digestion (77%) in comparison to its other 4- and 5CQa isomers (29 and 37%, respectively). However, we found that the 5CQa isomer exhibited a superior recovery profile, with 37% recovery index after 60 min of intestinal digestion, in comparison to 3- and 4CQa, which exhibited 14 and 21%, respectively. Finally, the recovery index of CQa isomers decreased in a range from 2 to 11% after 180 min of dialysed internal digestion. A superior recovery rate was obtained after oral and gastric digestion for 1- and 5FQa, with a recovery index of 85 to 100% in both isomers (Fig. 2D). Furthermore, in the dialysed intestinal digestion process, 5FQa exhibited a reduction of up to 24% in its recovery index; this was higher than that observed for the rest of the HA. The overall recovery index of HA during the intestinal digestion was low, with only 4.3 mg L−1 of the total HA passing the dialysis membrane, mainly 3- and 4CQa, with 1.3 and 1.0 mg L−1, respectively.
The fermented beverage from broccoli leaves showed a higher concentration of AF relative to DF, at a ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Fig. 2B shows that while the acylated flavonoids exhibit a decline over the intestinal digestion, the deacylated flavonoids initially decreased within the first 30 minutes (15 mg L−1), before increasing and maintaining a concentration of 40 to 50 mg L−1 until 180 min. Among the AF, the triglycosylated kaempferol-3-sinapoylsophoroside-7-glucoside and kaempferol-3-feruloylsophoroside-7-glucoside were the main AF in the fermented beverage, with concentrations of 79.6 and 53.1 mg L−1, respectively. As can be seen in Fig. 2D, the feruloyl and sinapoyl AF derivatives had greater stability during the intestinal digestion process, with a recovery index range of 20 to 26%, compared to the caffeoyl AF derivatives, which decreased from 3 to 6% after 180 min of intestinal digestion. In general, the rest of the diglycosylated AF exhibited a decrease throughout the intestinal digestion, with the exception of kaempferol-3-feruloylsophoroside, which maintained a stable recovery ranging from 20 to 43%. Although the concentration of that compound was relatively low compared to the rest of the AF (0.15 to 0.47 mg L−1) throughout the internal digestion process, the value suggested greater stability of those molecules or a possible low kinetic of transformation to DF with the loss of the feruloyl moiety. Yant et al. (2018)25 reported an increase in the concentration of feruloyl acylated flavonoids after bile acid binding assay in simulated oral digestion using solvents of kale extract, suggesting better bioaccessibility of these compounds after the in vitro GID process.
3. Fig. 2B shows that while the acylated flavonoids exhibit a decline over the intestinal digestion, the deacylated flavonoids initially decreased within the first 30 minutes (15 mg L−1), before increasing and maintaining a concentration of 40 to 50 mg L−1 until 180 min. Among the AF, the triglycosylated kaempferol-3-sinapoylsophoroside-7-glucoside and kaempferol-3-feruloylsophoroside-7-glucoside were the main AF in the fermented beverage, with concentrations of 79.6 and 53.1 mg L−1, respectively. As can be seen in Fig. 2D, the feruloyl and sinapoyl AF derivatives had greater stability during the intestinal digestion process, with a recovery index range of 20 to 26%, compared to the caffeoyl AF derivatives, which decreased from 3 to 6% after 180 min of intestinal digestion. In general, the rest of the diglycosylated AF exhibited a decrease throughout the intestinal digestion, with the exception of kaempferol-3-feruloylsophoroside, which maintained a stable recovery ranging from 20 to 43%. Although the concentration of that compound was relatively low compared to the rest of the AF (0.15 to 0.47 mg L−1) throughout the internal digestion process, the value suggested greater stability of those molecules or a possible low kinetic of transformation to DF with the loss of the feruloyl moiety. Yant et al. (2018)25 reported an increase in the concentration of feruloyl acylated flavonoids after bile acid binding assay in simulated oral digestion using solvents of kale extract, suggesting better bioaccessibility of these compounds after the in vitro GID process.
Five DF were identified in the fermented beverage, one of which belonged to glycosylated quercetin derivatives (quercetin-3-diglucoside) and four belonged to glycosylated kaempferol derivatives: kaempferol-3-sophoroside-7-glucoside (K-3Soph-7G), kaempferol-3,7-diglucoside (K-3,7dG) and kaempferol-3-sophoroside. K-3,7dG was the most abundant of its group, representing 90% with 64 mg L−1 in the fermented beverage (Table 1). In general, the DF group showed a different dynamic during the in vitro GID process compared to the AF, in which some of them increased their concentration throughout the intestinal digestion. Thus, although the total DF decreased to 15.5 mg L−1 after 30 min of dialysed intestinal digestion, they increased and remained stable for 180 min, from 37.5 to 49.5 mg L−1. The recovery index (Fig. 2D) showed this dynamic of each DF during GID, particularly the increase in the production of K-3,7dG and K-3Soph-7G. The correlation chart (Fig. 2E) shows the Pearson's correlation coefficient for each flavonoid compound from 30 to 180 min of dialysed intestinal digestion stages. It showed two differentiated clusters divided into AF and DF. Furthermore, the clusters of DF showed a lower correlation for AF, which supports the hypothesis of conversion of the acyl bonds and glycosides by hydrolysis. The positive correlation between groups of AF compounds showed a similar trend in their concentration during the intestinal digestion process.
With regard to sulforaphane and indoles, their concentration was also monitored during the in vitro GID process (Fig. 2C, and Table 1). These compounds had a lower concentration compared to the rest of the individual polyphenols characterised. Thus, sulforaphane was quantified at 2.5 mg L−1 in the fermented beverage, and indoles such as ascorbigen, indole-3-carbinol, and diindolylmethane were quantified at 0.30, 0.15 and 0.03 mg L−1, respectively. Sulforaphane maintained its concentration through the oral and gastric digestion stages with no significant differences. However, its concentration decreased from 1.65 to 0.96 mg L−1, with a recovery index from 64% to 37% over 180 min of intestinal digestion.
As for the indoles, all of them decreased drastically during the gastric digestion (Fig. 2C) and were quantified below 0.01 mg L−1, for instance ascorbigen and diindolylmethane. This lower stability of the indoles was also shown in the results recovery index (Fig. 2D), where they remained at 5% throughout the intestinal digestion process, with the exception of indole-3-carbinol, whose decrease during the digestion step from 85 to 15% showed greater stability.
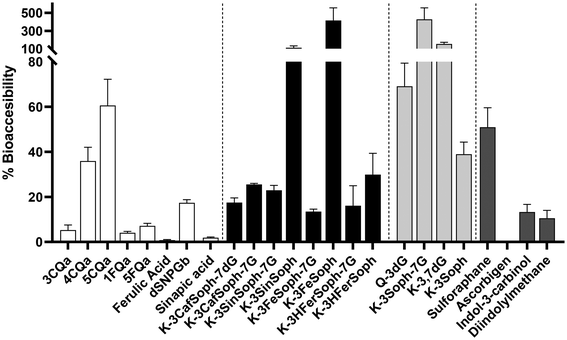
3.3 Bioaccessibility assessment of phytochemicals from lactofermented broccoli beverage
The percentage of bioaccessibility of individual phytochemicals characterised and classified in each of the groups mentioned above is shown in Fig. 3. These values correspond to the proportions of compounds that pass through the dialysis membrane by passive diffusion during the intestinal digestion stage, which simulates the intestinal barrier. The concentration of each compound in the dialysate is therefore considered (Table 1).HA had a bioaccessibility of less than 20%, except for the isomers 4- and 5CQa which had 36 and 60%, respectively. Considering the absolute values of concentration, each isomer of CQa showed a concentration of around 1 mg L−1 in the dialysate (Table 1). Nevertheless, due to the higher concentration of 3CQa in the gastric digestion stage (26.5 mg L−1), its values were below 6% based on the calculation of the percentage of bioaccessibility (section 2.2), rather than having the same concentration as its homologues. 1- and 5FQa were passively diffused 0.53 and 0.31 mg L−1, respectively, with a bioaccessibility of 4 and 7.
With regard to AF, the bioaccessibility of diglycosylated kaempferol-3-O-sinapoylsophoroside and kaempferol-3-O-feruloylsophoroside was highlighted at 110 and 415%, respectively. Both are major precursors of their tetraglycosylated forms: kaempferol-3-O-sinapoylsophoroside-7-diglucoside and kaempferol-3-O-feruloylsophoroside-7-diglucoside, respectively. In the same way, DF also had a higher percentage of bioaccessibility, in K-3-Soph-7G and K-3,7-dG, as a precursor of all kaempferol derivatives, with 430% and 150%, respectively.
Sulforaphane had a bioaccessibility of 51% and its value in the dialysed fraction was 1.12 mg L−1, and 0.96 mg L−1 for the 180 min intestinal digestion process. On the other hand, the indoles had <15% bioaccessibility. Nevertheless, their concentration in the dialysate was 0 mg L−1 for ascorbigen and lower (<0.01 mg L−1) than the gastric digestion stage.
4. Discussion
The TPC and TEAC assays showed a general increase and transformation of the phytochemical content throughout the GID process. Furthermore, a dialysis membrane step could explore the dynamics of an in vitro absorption of this phytochemical through the intestinal barrier.26 This enables better understanding regarding its absorption and utilisation by the body. Several authors have proposed that the observed increase in TPC and TEAC during the GID process is due to the production of polyphenolic and antioxidant compounds from the food matrix, particularly from the plant cell wall and other cell wall macromolecules, such as proteins and polysaccharides.15,27 However, although an increase in TPC and TEAC compared to the initial fermented beverage was observed, the beverage did not contain a food solid matrix, which would not suggest the release of antioxidant compounds. This is due to the fact that only the liquid was collected after the fermentation process whilst the leaves were discarded. Consequently, the dynamics in the content of TPC and TEAC of this fermented beverage could not be compared to other beverages such as fruit juices or smoothies. Moreover, the discrepancies between TPC and TEAC measurements are attributable to the fact that these values do not correlate perfectly. In particular, polyphenolic compounds are not primarily responsible for antioxidant capacity due to differences in reactivity with Folin reagent and among polyphenolic compounds.28 The higher TPC values and TEAC measured after the in vitro intestinal digestion process could be due to the transformation of polyphenolic compounds by digestive enzymes and in mild alkaline conditions.19 Consequently, the characterisation and tracking of individual polyphenolic compounds could provide insight into the evolution of these components during the in vitro GID process, as explained below.In the individual phytochemical assessment, the HA recovery index decreased after gastric digestion, and low drastically along the dialysed intestinal digestion. These results differed from those of previous studies,17 which indicated that the different CQa isomers present in coffee beverages did not undergo a decline after oral or gastric digestion. Furthermore, these authors reported a significant reduction in 4- and 5CQa compared to 3CQa after 60 min of intestinal digestion. On the other hand, recovery indexes of 1- and 5FQa were consistent with those reported by Alongi et al.,29 who compared CQa and FQa isomers in a coffee beverage and reported a decrease in those phytochemicals after the in vitro GID process.
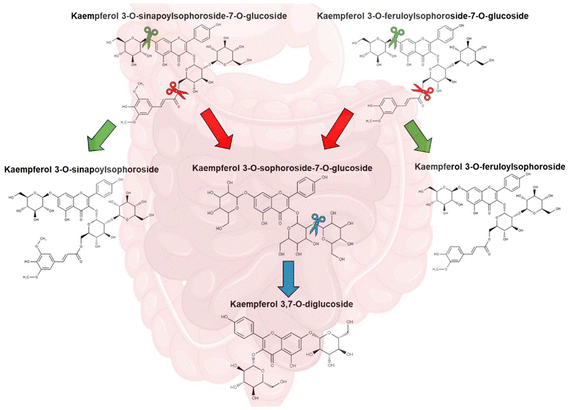
The two deacylated flavonoids K-3,7dG and K-3Soph-7G are the precursors of the acylated flavonoid. Both showed an increase during the in vitro GID process, suggesting a hydrolysis of AF to DF by hydrolytic enzymes from artificial intestinal fluids (amylases, lipases and proteases) at basic pH, as explained below. During this stage, acylated and/or deacylated glycosylated flavonoids may be hydrolysed to flavonoids with a lower amount of glycosylation, to their aglycone forms (kaempferol) or to other undetectable compounds.12
Among the glucosinolate-hydrolysis products (sulforaphane and indoles), the decrease in sulforaphane concentration throughout the intestinal digestion contrasts with that reported by other authors,16 who noted a higher concentration after successive GID steps, mainly due to better extractability from the food matrix. In our case, as our beverage is simply a liquid extract obtained from the fermentation of broccoli leaves, there is no food matrix to support such an increase. Indeed, a decrease was observed due to the dialysed intestinal digestion process. The observed decrease in indoles during gastric digestion is consistent with previous studies evaluating the content of indole compounds in Brassicaceae sprouts, describing similar trends in their concentration.30 Consequently, the observed decrease in indoles during gastric digestion can be attributed to a lower stability by the in vitro GID process.
These results elucidate the stability of phytochemicals such as hydroxycinnamic acids, flavonoids, sulforaphane, and indoles during in vitro GID and passive diffusion through a dialysis membrane. In addition, the conversion of higher flavonoids, such as acylated flavonoids, to deacylated flavonoids has also been studied to understand the release mechanisms of these phytochemicals. Nevertheless, the study of the absorbable fraction across the dialysis membrane could elucidate the bioaccessibility of all these bioactive compounds. Previous works also reported similar values for the percentage of bioaccessibility of hydroxycinnamic acids after an in vitro GID with a dialysis step using fruit juices.31,32 However, the differences between the isomers were not reported as in the present work.
Both acylated and deacylated flavonoids demonstrated a different evolution pattern during the in vitro GID process. Kaempferol-3-O-sinapoylsophoroside and kaempferol-3-O-feruloylsophoroside are major precursors of their tetraglycosylated forms: kaempferol-3-O-sinapoylsophoroside-7-diglucoside and kaempferol-3-O-feruloylsophoroside-7-diglucoside, respectively. These values greater than 100% indicate a release of these compounds from their main derivatives, due to the biotransformation by the hydrolytic enzymes from intestinal fluids that increase the concentration,12 as proposed in Fig. 4. Moreover, this biotransformation improves the bioaccessibility of these flavonoids through the dialysis membrane. Similarly, K-3Soph-7G and K-3,7-dG also had a higher percentage of bioaccessibility as the DF precursor of all kaempferol derivatives. The results suggest that DF diffused passively through the dialysis membrane, with the sequential formation and generation of these structures occurring during the intestinal digestion process, as explained by Kuvow et al. (2016)33 with de- and acylated anthocyanin from purple sweet potato.
The results of the remnant sulforaphane in the intestinal fraction after the in vitro GID process and the content in the dialysate suggested that sulforaphane was not degraded during intestinal digestion, but rather transferred by passive diffusion through the dialysis membrane, resulting in a good bioaccessibility percentage. In accordance with our findings, previous research has also reported sulforaphane bioaccessibility values in in vitro GID with dialysis, ranging from 40 to 60% in fresh broccoli samples.34 The low bioaccessibility and stability of the indoles throughout the in vitro GID have also been reported in cruciferous sprouts,35 with undetected values after gastric digestion.
Furthermore, the biotransformation of major flavonoids to precursor flavonoids through enzymatic activity is highlighted, increasing the bioaccessibility of the latter. The polyphenolic compounds present after intestinal digestion corresponded to the fraction of compounds that were not assimilated and could pass into the colon. These included DF as K-3Soph-7G and K-3,7-dG; and AF as kaempferol-3-sinapoyl-sophoroside-7-glucoside and kaempferol-3-feruloyl-sophoroside-7-glucoside, which could potentially be metabolised by the colon microbiota.36 Therefore, consumption of flavonoids has a positive effect by enriching beneficial species in the gut microbiota, such as Bifidobacterium and Lactobacillus. Additionally, other reports have highlighted the production of degradation products beneficial to human intestinal cells, such as short-chain fatty acids (acetate, propionate, and butyrate)37 which are associated with support of intestinal epithelial cells, improvement of innate and adaptive immunity, increase in mucus thickening and enhancement of tight junction proteins, among others.38 Similarly, the remaining sulforaphane and indol-3-carbinol content in the non-dialysed fraction (180 min intestinal digestion) could also pass through the colon and potentially exert its effect, repairing physiological destruction of the gut barrier and decreasing inflammation.39 Sulforaphane also reversed induced gut dysbiosis in mice and mitigated ulcerative colitis, and increased Butyriciococcus genus species in gut microbiota that produce butyrate.40
In conclusion, the present study unravels the transformation and evolution of polyphenols, sulforaphane, and indoles present in a fermented beverage made from broccoli leaves, focusing on their individual molecules and their correlation with each precursor during the dialysed GID process. The bioaccessibility of phytochemicals, particularly hydroxycinnamic acids, flavonoids, sulforaphane, and indoles, was influenced by their conversion and passive diffusion during digestion, which enhanced increased the availability of certain compounds.
Hydroxycinnamic acid, especially 3CQa, showed stability during the early stages of digestion, but decreased significantly during dialysed intestinal digestion. Acylated flavonoids decreased during intestinal digestion, whereas deacylated flavonoids decreased initially but then stabilised, which would suggest hydrolysis of acylated flavonoids to deacylated flavonoid by digestive enzymes. Glucosinolate-hydrolysis products, such as sulforaphane, and indoles showed a general decrease during digestion. Sulforaphane showed a bioaccessibility of 51%, whereas indole compounds had a lower bioaccessibility (<15% BA).
Overall, the study highlights the dynamic changes and stability of phytochemicals in a fermented broccoli beverage during the GID process. The dialysed GID influences the release and transformation of bioactive compounds, potentially enhancing their bioaccessibility and subsequent health benefits. Further research on individual polyphenolic compounds in more advanced in vitro models, including in vivo assays, would strengthen the mechanism and their bioaccessibility across the intestinal barrier, and could provide deeper insight into their absorption and health effects.
Author contributions
José Ángel Salas-Millán: conceptualization, methodology, formal analysis, investigation, writing – original draft. Encarna Aguayo: conceptualization, investigation, supervision, writing – review & editing, funding acquisition, project administration.Data availability
Data for this article is available from: https://zenodo.org/doi/10.5281/zenodo.12790350.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work is a result of the AGROALNEXT programme and was supported by the Spanish Ministry of Science, Innovation and Universities (MICIU) with funding from the European Union NextGenerationEU (PRTR-C17. I1) and by Fundación Séneca with funding from the Comunidad Autónoma Región de Murcia (CARM). The authors also acknowledge PID2021-123857OB-I00 from the Spanish MICIU- National Research Agency and the ERDF A way of making Europe, of the European Union. José-Ángel Salas-MillÁn acknowledges the financial support received from the MICIU and JimboFresh International SLL through an ‘Industrial PhD’ grant (DIN2019- 010837).References
- E. Gil-Martín, T. Forbes-Hernández, A. Romero, D. Cianciosi, F. Giampieri and M. Battino, Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products, Food Chem., 2022, 378, 131918 CrossRef.
- J.Á Salas-Millán, A. Conesa-Bueno and E. Aguayo, A novel antidiabetic lactofermented beverage from agro-industrial waste (broccoli leaves): Process optimisation, phytochemical characterisation, and shelf-life through thermal treatment and high hydrostatic pressure, Food Biosci., 2024, 59, 103999 CrossRef.
- J.Á Salas-Millán, A. Aznar, E. Conesa, A. Conesa-Bueno and E. Aguayo, Functional food obtained from fermentation of broccoli by-products (stalk): Metagenomics profile and glucosinolate and phenolic compounds characterization by LC-ESI-QqQ-MS/MS, LWT–Food Sci. Technol., 2022, 169, 113915 CrossRef.
- A. Septembre-Malaterre, F. Remize and P. Poucheret, Fruits and vegetables, as a source of nutritional compound, Food Res. Int., 2018, 104, 86–99 CrossRef CAS.
- X. Xu, S. Bi, F. Lao, F. Chen, X. Liao and J. Wu, Induced changes in bioactive compounds of broccoli juices after fermented by animal- and plant-derived Pediococcus pentosaceus, Food Chem., 2021, 357, 129767 CrossRef CAS.
- J. H. Ye, L. Y. Huang, N. S. Terefe and M. A. Augustin, Fermentation-based biotransformation of glucosinolates, phenolics and sugars in retorted broccoli puree by lactic acid bacteria, Food Chem., 2019, 286, 616–623 CrossRef CAS PubMed.
- Y. X. Cai, J. H. Wang, C. McAuley, M. A. Augustin and N. S. Terefe, Fermentation for enhancing the bioconversion of glucoraphanin into sulforaphane and improve the functional attributes of broccoli puree, J. Funct. Foods, 2019, 61, 103461 CrossRef CAS.
- P. G. Anantharaju, P. C. Gowda, M. G. Vimalambike and S. V. Madhunapantula, An overview on the role of dietary phenolics for the treatment of cancers, Nutr. J., 2016, 15(1), 1–16 CrossRef PubMed.
- G. Na, C. He, S. Zhang, S. Tian, Y. Bao and Y. Shan, Dietary Isothiocyanates: Novel Insights into the Potential for Cancer Prevention and Therapy, Int. J. Mol. Sci., 2023, 24(3), 1962 CrossRef CAS PubMed.
- F. J. Blancas-Benitez, J. Pérez-Jiménez, E. Montalvo-González and G. A. González-Aguilar, Sáyago-Ayerdi SG. In vitro evaluation of the kinetics of the release of phenolic compounds from guava (Psidium guajava L.) fruit, J. Funct. Foods, 2018, 43, 139–145 CrossRef CAS.
- A. Brodkorb, L. Egger, M. Alminger, P. Alvito, R. Assunção and S. Ballance, et al., INFOGEST static in vitro simulation of gastrointestinal food digestion, Nat. Protoc., 2019, 14(4), 991–1014 CrossRef CAS PubMed.
- S. Porfírio, P. L. V. Falé, P. J. A. Madeira, M. H. Florêncio, L. Ascensão and M. L. M. Serralheiro, Antiacetylcholinesterase and antioxidant activities of Plectranthus barbatus tea, after in vitro gastrointestinal metabolism, Food Chem., 2010, 122(1), 179–187 CrossRef.
- J. Bouayed, L. Hoffmann and T. Bohn, Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake, Food Chem., 2011, 128(1), 14–21 CrossRef CAS.
- A. C. S. Pais, E. R. Coscueta, M. M. Pintado, A. J. D. Silvestre and S. A. O. Santos, Exploring the bioaccessibility and intestinal absorption of major classes of pure phenolic compounds using in vitro simulated gastrointestinal digestion, Heliyon, 2024, 10(7), e28894 CrossRef CAS.
- A. C. Durán-Castañeda, A. P. Cardenas-Castro, J. Pérez-Jiménez, A. M. Pérez-Carvajal, J. A. Sánchez-Burgos and R. Mateos, et al., Bioaccessibility of phenolic compounds in Psidium guajava L. varieties and P. friedrichsthalianum Nied. after gastrointestinal digestion, Food Chem., 2023, 400, 134046 CrossRef.
- I. Sarvan, E. Kramer, H. Bouwmeester, M. Dekker and R. Verkerk, Sulforaphane formation and bioaccessibility are more affected by steaming time than meal composition during in vitro digestion of broccoli, Food Chem., 2017, 214, 580–586 CrossRef CAS PubMed.
- A. A. Vilas-Boas, A. Oliveira, D. Jesus, C. Rodrigues, C. Figueira and A. Gomes, et al., Chlorogenic acids composition and the impact of in vitro gastrointestinal digestion on espresso coffee from single-dose capsule, Food Res. Int., 2020, 134, 109223 CrossRef CAS.
- S. Porfírio, P. L. V. Falé, P. J. A. Madeira, M. H. Florêncio, L. Ascensão and M. L. M. Serralheiro, Antiacetylcholinesterase and antioxidant activities of Plectranthus barbatus tea, after in vitro gastrointestinal metabolism, Food Chem., 2010, 122(1), 179–187 CrossRef.
- S. Attri, N. Singh, T. R. Singh and G. Goel, Effect of in vitro gastric and pancreatic digestion on antioxidant potential of fruit juices, Food Biosci., 2017, 17, 1–6 CrossRef CAS.
- F. J. Blancas-Benitez, J. Pérez-Jiménez, E. Montalvo-González and G. A. González-Aguilar, Sáyago-Ayerdi SG. In vitro evaluation of the kinetics of the release of phenolic compounds from guava (Psidium guajava L.) fruit, J. Funct. Foods, 2018, 43, 139–145 CrossRef CAS.
- J. Á Salas-Millán, E. Aguayo, A. Conesa-Bueno and A. Aznar, Revalorization of Melon By-Product to Obtain a Novel Sparkling Fruity-Based Wine, Foods, 2023, 12(3), 491 CrossRef.
- G. B. Gonzales, K. Raes, H. Vanhoutte, S. Coelus, G. Smagghe and J. Van Camp, Liquid chromatography–mass spectrometry coupled with multivariate analysis for the characterization and discrimination of extractable and nonextractable polyphenols and glucosinolates from red cabbage and Brussels sprout waste streams, J. Chromatogr. A, 2015, 1402, 60–70 CrossRef CAS PubMed.
- X. Yu, F. Ma, L. Zhang and P. Li, Extraction and Quantification of Sulforaphane and Indole-3-Carbinol from Rapeseed Tissues Using QuEChERS Coupled with UHPLC-MS/MS, Molecules, 2020, 25(9), 2149 CrossRef CAS.
- J. Hauder, S. Winkler, A. Bub, C. E. Rüfer, M. Pignitter and V. Somoza, LC-MS/MS quantification of sulforaphane and indole-3-carbinol metabolites in human plasma and urine after dietary intake of selenium-fortified broccoli, J. Agric. Food Chem., 2011, 59(15), 8047–8057 CrossRef CAS PubMed.
- I. Yang, G. K. Jayaprakasha and B. Patil, In vitro digestion with bile acids enhances the bioaccessibility of kale polyphenols, Food Funct., 2018, 9(2), 1235–1244 RSC.
- C. Dima, E. Assadpour, S. Dima and S. M. Jafari, Bioavailability and bioaccessibility of food bioactive compounds; overview and assessment by in vitro methods, Compr. Rev. Food Sci. Food Saf., 2020, 19(6), 2862–2884 CrossRef PubMed.
- A. S. Stübler, U. Lesmes, V. Heinz, C. Rauh, A. Shpigelman and K. Aganovic, Digestibility, antioxidative activity and stability of plant protein-rich products after processing and formulation with polyphenol-rich juices: kale and kale–strawberry as a model, Eur. Food Res. Technol., 2019, 245(11), 2499–2514 CrossRef.
- G. F. Deng, X. Lin, X. R. Xu, L. L. Gao, J. F. Xie and H. B. Li, Antioxidant capacities and total phenolic contents of 56 vegetables, J. Funct. Foods, 2013, 5(1), 260–266 CrossRef CAS.
- M. Alongi, J. M. Frías Celayeta, R. Vriz, G. K. Kinsella, A. Rulikowska and M. Anese, In vitro digestion nullified the differences triggered by roasting in phenolic composition and α-glucosidase inhibitory capacity of coffee, Food Chem., 2021, 342, 128289 CrossRef CAS.
- Á. Abellán, R. Domínguez-Perles, C. García-Viguera and D. A. Moreno, Evidence on the Bioaccessibility of Glucosinolates and Breakdown Products of Cruciferous Sprouts by Simulated In Vitro Gastrointestinal Digestion, Int. J. Mol. Sci., 2021, 22(20), 11046 CrossRef.
- M. J. Rodríguez-Roque, M. A. Rojas-Graü, P. Elez-Martínez and O. Martín-Belloso, Changes in vitamin C, phenolic, and carotenoid profiles throughout in vitro gastrointestinal digestion of a blended fruit juice, J. Agric. Food Chem., 2013, 61(8), 1859–1867 CrossRef.
- M. J. Rodríguez-Roque, M. A. Rojas-Graü, P. Elez-Martínez and O. Martín-Belloso, In vitro bioaccessibility of health-related compounds as affected by the formulation of fruit juice- and milk-based beverages, Food Res. Int., 2014, 62, 771–778 CrossRef.
- S. Kubow, M. M. Iskandar, K. Sabally, B. Azadi, S. Sadeghi Ekbatan and P. Kumarathasan, et al., Biotransformation of anthocyanins from two purple-fleshed sweet potato accessions in a dynamic gastrointestinal system, Food Chem., 2016, 192, 171–177 CrossRef CAS PubMed.
- M. C. Rodríguez-Hernández, S. Medina, A. Gil-Izquierdo, M. C. Martínez-Ballesta and D. A. Moreno, Broccoli isothiocyanates content and in vitro availability according to variety and origin, Maced. J. Chem. Chem. Eng., 2013, 32(2), 251–264 CrossRef.
- Á. Abellán, R. Domínguez-Perles, C. García-Viguera and D. A. Moreno, Evidence on the Bioaccessibility of Glucosinolates and Breakdown Products of Cruciferous Sprouts by Simulated In Vitro Gastrointestinal Digestion, Int. J. Mol. Sci., 2021, 22(20), 11046 CrossRef.
- O. A. Sánchez-Velázquez, M. Mulero, E. O. Cuevas-Rodríguez, M. Mondor, Y. Arcand and A. J. Hernández-Álvarez, In vitro gastrointestinal digestion impact on stability, bioaccessibility and antioxidant activity of polyphenols from wild and commercial blackberries (Rubus spp.), Food Funct., 2021, 12(16), 7358–7378 RSC.
- M. H. Baky, M. Elshahed, L. Wessjohann and M. A. Farag, Interactions between dietary flavonoids and the gut microbiome: a comprehensive review, Br. J. Nutr., 2022, 128(4), 577–591 CrossRef CAS PubMed.
- T. O. Adebowale, K. Yao and A. O. Oso, Major cereal carbohydrates in relation to intestinal health of monogastric animals: A review, Anim. Nutr., 2019, 5(4), 331–339 CrossRef.
- C. He, L. Huang, P. Lei, X. Liu, B. Li and Y. Shan, Sulforaphane Normalizes Intestinal Flora and Enhances Gut Barrier in Mice with BBN-Induced Bladder Cancer, Mol. Nutr. Food Res., 2018, 62(24), 1800427 CrossRef PubMed.
- Y. Zhang, L. Tan, C. Li, H. Wu, D. Ran and Z. Zhang, Sulforaphane alter the microbiota and mitigate colitis severity on mice ulcerative colitis induced by DSS, AMB Express, 2020, 10(1), 1–9 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo03528c |
| This journal is © The Royal Society of Chemistry 2024 |