Open Access Article
Open Access ArticleA network meta-analysis of the comparative efficacy of different dietary approaches on glycaemic control and weight loss in patients with type 2 diabetes mellitus and overweight or obesity†
Yahui
Yuan‡
abc,
Chun
Chen‡
d,
Qiaoyun
Liu
abc,
Yehao
Luo
abc,
Zhaojun
Yang
abc,
YuPing
Lin
c,
Lu
Sun
c and
Guanjie
Fan
 *abc
*abc
aSchool of Second Clinical Medicine, Guangzhou University of Chinese Medicine, Guangzhou, China. E-mail: fanguanjie@gzucm.edu.cn
bDepartment of Endocrinology, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
cDepartment of Endocrinology, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, China
dWangjing Hospital, China Academy of Chinese Medical Sciences, Beijing, China
First published on 18th November 2024
Abstract
Background: Despite considerable literature supporting the benefit of dietary interventions in individuals with type 2 diabetes mellitus (T2DM) and overweight/obesity, which diet works best is currently unknown. We conducted a systematic review and network meta-analysis (NMA) to evaluate the comparative effectiveness of different dietary approaches in overweight or obese adults with T2DM. Methods: We searched EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL) and PubMed up till July 2023 for controlled studies using different dietary approaches. Next, we updated the literature search to September 2024 but found no new relevant studies. Glycated hemoglobin A1c (HbA1c) levels and body weight were used as primary outcomes. For each outcome, a pooled effect was determined for each intervention compared with other interventions. Mean differences (MDs) and 95% confidence intervals (95% CIs) were computed. The surface under the cumulative ranking curve (SUCRA) was used for ranking the dietary approaches. Moreover, confidence was assessed using the CINeMA (confidence in network meta-analysis) framework. Results: Overall, 31 trials that compared eight diet interventions (Mediterranean, moderate-carbohydrate, low-carbohydrate, vegetarian, low-glycaemic index/load, low-fat, high-protein and control diets) and involved 3096 people were included. In terms of glycemic control, the Mediterranean diet yielded the best ranking (SUCRA: 88.15%), followed by the moderate-carbohydrate diet (SUCRA: 83.3%) and low-carbohydrate (LC) diet (SUCRA: 55.7%). In terms of anthropometric measurements, the LC diet (SUCRA: 74.6%) ranked first, followed by the moderate-carbohydrate diet (SUCRA: 68.7%) and vegetarian diet (SUCRA: 57%). These results also showed that the differences in almost all dietary patterns regarding anthropometric measurements were mostly small and often trivial. Conclusions: In summary, the Mediterranean diet was the most efficient dietary intervention for the improvement of glycaemic control, and the LC diet obtained the highest score for anthropometric measurements in individuals with T2DM and concurrent overweight/obesity.
1. Introduction
The dual epidemic of obesity and type 2 diabetes mellitus (T2DM), affecting all levels of society in almost every country, is a slow-onset disaster and a major global public health issue. According to the International Diabetes Federation, in 2015, there were 415 million people living with diabetes globally, with the total health expenditure attributed to diabetes amounting to US$ 673 billion, and the disease accounted for 12.8% of the worldwide total mortality rate.1 Data from the World Health Organization (WHO) showed that over 1.9 billion adults were overweight in 2016, and of those, over 650 million were obese.2 Overweight and obesity are important accelerators of many diseases, including cardiovascular disease, cancer and T2DM.3–5 Furthermore, obesity accounts for 80% of adults with T2DM in Europe.6 The co-occurrence of obesity and diabetes significantly reduces the quality of life of individuals, simultaneously placing a heavy burden on healthcare resources and systems. Although individuals with T2DM or overweight/obesity alone receive much attention, people with T2DM and coexisting overweight/obesity have been paid limited attention.To diagnose and monitor diabetes, the glycated hemoglobin A1c (HbA1c) level is considered, which is the average blood glucose level over the past 3 months. In T2DM patients, every 1% increment in HbA1c levels is related to an approximate 13% increased risk of cardiovascular events.7–9 Individuals with T2DM who are also overweight were found to be at a higher risk of cardiovascular diseases, with increasing HbA1c levels related to an increased risk of adverse cardiovascular outcomes and all-cause death.10 Increased fasting glucose levels are risk factors for vascular complications in T2DM.11 Weight management is considered an important aspect of treatment in individuals with T2DM. A weight gain of 5 kg and a body mass index (BMI) of 5 kg m−2 were found to be associated with a 52% and 2.13-fold increased risk of T2DM, respectively.12,13 Moreover, a larger waist circumference was strongly and linearly linked with the risk of T2DM.14
Adjusting the diet is an important lifestyle factor that can have a significant impact on the successful management of T2DM and obesity. Dietary interventions have been proven to improve glycemic control and weight, decrease the need for glucose-lowering medications, and promote the sustained remission of diabetes.15–17 In adults who are overweight or who have T2DM, or in obese patients with T2DM, the Mediterranean diet, low-carbohydrate (LC) diet, low-fat (LF) diet, and high-protein (HP) diet are considered as suitable dietary regimes for reducing hyperglycemia and body weight.18–24 However, we still do not have a good understanding of which dietary approaches are optimal for people with T2DM who are overweight or obese. Although patients with T2DM have been studied and different dietary approaches have been compared, patients with T2DM who are overweight or obese have not. Consequently, we decided to perform network meta-analysis (NMA) to rank and compare all the available interventions. Specifically, we aimed to use NMA to compare the effects of various dietary interventions on glycaemic control and anthropometric measurements in overweight/obese patients with T2DM.
2. Methods
This study was registered in PROSPERO International Prospective Register of Systematic Reviews (register number: CRD42023443504). The systematic review and NMA were designed, conducted, and presented in accordance with quality standards for reporting systematic reviews.2.1. Literature search
The EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), and PubMed databases were searched for relevant English-language literature without time restrictions from inception until July 2023. The search term was predefined (ESI Table S1†). Additionally, articles included in the reference lists could also be searched manually. Two authors extracted the data independently. Any disputes were settled by discussion or consultation with an additional third author until consensus was reached. The following information was extracted: first author, publication time, location of the study conducted, study duration, age, total number of participants included in the study, etc.2.2. Eligibility criteria
Randomized controlled trials (RCTs) were included if they included the different dietary approaches. The eligible dietary interventions are listed in ESI Table S2,† while the criteria for inclusion and exclusion in this review are provided in ESI Table S3.†2.3. Outcome
We used the HbA1c level and body weight as the primary outcomes of the study. The fasting glucose level, BMI, and waist circumference were used as the secondary outcomes. Definitions of the outcomes are provided in ESI Table S4.†2.4. Risk of bias assessment
The Cochrane Collaboration Risk of Bias Assessment Tool was used to evaluate the methodological quality of the included trials. We considered the following biases: random-sequence generation, allocation sequence concealment, blinding of participants and clinicians, completeness of outcome data, and selective outcome reporting.25,26If at a minimum of 3 out of the 5 items were considered low risk and no more than one item was considered high risk, the studies were considered to have a low risk of bias; while if at least two of the five items were assessed as high risk, the studies were considered to have a high risk of bias; and the remaining studies were rated as having an unclear risk.
2.5. Data synthesis and analysis
2.6. Transitivity assessment
One of the basic assumptions of NMA and indirect comparisons is transitivity, the violation of which is a threat to the validity of the important results from a network meta-analysis. The distribution of potential effect modifiers across the existing direct comparisons was compared to assess the assumption of transitivity. The following effect modifiers were taken into account: duration of diabetes, mean age, sample size, and duration of research.2.7. Inconsistency assessment
Direct and indirect evidence should be consistent, as valid findings rely on the consistency of the results. Local and global approaches were used to assess inconsistency. The loop-specific approach was used to identify if there were significant inconsistencies, and the side-splitting approach was applied to examine direct and indirect comparisons. The design-by-treatment interaction model, which can be used to examine inconsistency from all the overall possible origins across the network, was used to detect global approaches.2.8. Statistical analysis
For each outcome, the pooled effect of one intervention versus another was determined by carrying out a random effects NMA. The comparison of different interventions and a clinically meaningful ranking can be achieved by an NMA. With regard to each outcome (HbA1c, fasting glucose, body weight, BMI, and waist circumference), a league table was applied to display the mean differences (MDs) with the corresponding 95% confidence intervals (95% CIs). The surface under the cumulative ranking curve (SUCRA) was applied to evaluate the relative rankings of the different interventions. In addition, a frequentist framework was used to perform the calculations with STATA17. The R package “netmeta” was used to generate the network plots.2.9. Subgroup and sensitivity analyses
Subgroup analyses were performed by considering the study duration (<12 months vs. ≥12 months), sample size (<100 vs. ≥100), and age group (<60 years vs. ≥60 years). Sensitivity analyses were run by focusing only on studies judged to have a low risk of bias and by excluding studies with a high risk of bias.2.10. Small-study effects and publication bias
Comparison-adjusted funnel plots were applied to examine the presence of small-study effects when 10 or more studies were available.2.11. Evidence credibility
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system, as extended to NMA, was used to assess the credibility of the evidence. The CINeMA web tool, available at https://cinema.ispm.ch/, was applied to evaluate the results of the NMA. Within-study risk of bias, reporting bias, indirectness, imprecision, heterogeneity, and inconsistency were judged qualitatively.27 The level of concerns for each treatment effect of NMA were judged as “no concerns”, “some concerns”, or “major concerns” for each of the 6 domains.283. Results
3.1. Characteristics
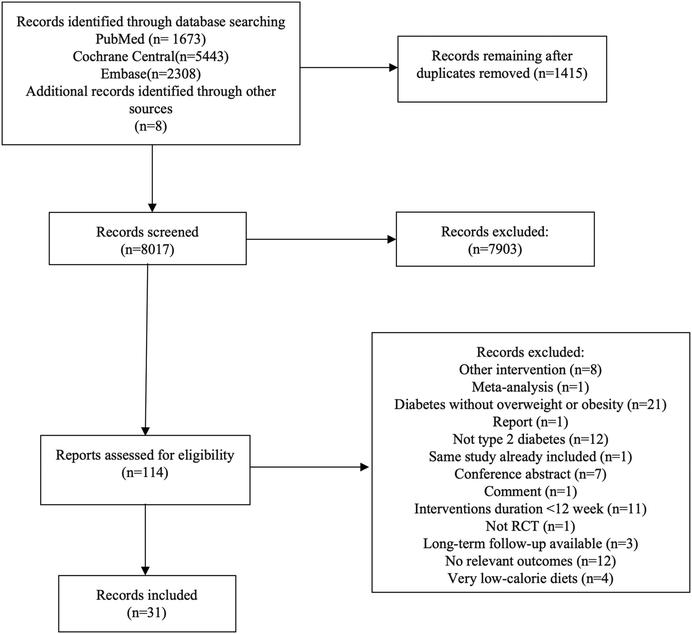
Our literature search retrieved a total of 9432 records, of which 114 full-text articles were evaluated in detail (Fig. 1). Of these, 83 articles were not included in the meta-analysis, with the causes for exclusion provided in ESI Table S5.†Ultimately, 31 trials29–59 met the inclusion criteria and gave enough information to be included in the current study. All the relevant articles included in the present meta-analysis were published between 1999 and 2022 and enrolled a total population of 3096 T2DM patients who were overweight or obese. There were 12 trials carried out in Australia and New Zealand, 9 trials carried out in North America, 5 trials carried out in Europe, 4 trials carried out in Asia, and 1 trial carried out in South Africa. The duration of the interventions varied from 3 to 48 months; the average age of the enrolled patients was 36.5–66.9 years old, and the BMI varied between 25.17 (Asian population) and 41.3 kg m−2. ESI Tables S6 and S7† summarize the characteristics of the studies that were eligible for inclusion.
The included studies used heterogeneous definitions for their intervention diets, which could have introduce bias. For example, the fat intake varied between the LF diets in the included trials. Moreover, the included trials used different protocols for the intervention arms (e.g., group meetings and frequency, dietary consultation, exercise, and intensity). Among the included trials, there was some variation in how the control diet was defined. Three of the six control diets involved were classed as “no intervention”, while the other three control diets involved minimal intervention.
3.2. Risk of bias
Overall, 12 trials were classified as having a low risk of bias, 7 trials as high risk, and 12 trials as an unclear risk of bias. Among the included trials, 58% studies had a low risk of bias for generating random sequences, 19% for concealing allocations, 0% for blinding, 77% for incomplete data, and 84% for selective reporting, according to the specific items of the Cochrane Collaboration risk of bias tool (ESI Fig. S1†).3.3. Network diagrams
Fig. 2 shows the networks of trials used in the meta-analyses for HbA1c, fasting glucose, body weight, BMI, and waist circumference, with the number of included studies proportional to the width of the lines, and the sample number of each intervention approach proportional to the size of the nodes.3.4. Contribution matrix
A contribution matrix was used to show to which extent the direct and indirect comparisons of the dietary interventions contributed to the overall effect. The contribution to these study effects were mainly from indirect comparisons (from ESI Tables S8–S10†).3.5. Transitivity
There was no evidence for transitivity (from ESI Fig. S2–S5†). Thus, we concluded that there was no relevant clinical and methodological heterogeneity across the treatment comparisons (intransitivity) in the current review.3.6. Forest plot
Forest plots for the network meta-analysis of HbA1c, fasting glucose, body weight, BMI, and waist circumference are provided in ESI Fig. S6, S8, S10, S12, and S14.† Direct pairwise meta-analyses are provided and summarized in ESI Fig. S7, S9, S11, S13, and S15.† The data available for directly comparing the glycemic control outcomes demonstrated that the Mediterranean diet was more effective for this outcome. No significant difference was observed in the anthropometric measurements from the direct (head-to-head) evidence.3.7. Results of the NMA
3.7.1.1. Glycemic control. Table 1 shows the results of the NMA for the glycemic control outcomes. The LF diet (MD: 0.50%; 95% CI: 0.13–0.87), Mediterranean (MD: 0.38%; 95% CI: 0.07–0.7), and moderate-carbohydrate diet (MD: 1.48%; 95% CI: 0.43–2.54) were more efficacious than the control diet in reducing HbA1c (moderate to very low confidence of evidence). The Mediterranean diet also lowered HbA1c more effectively compared to the HP diet (MD: 0.45%; 95% CI: 0.16–0.73) and LF diet (MD: 0.34%; 95% CI: 0.16–0.53) (very low confidence of evidence). The moderate-carbohydrate diet (MD: 0.62%; 95% CI: 0.04–1.20) was more effective than the HP diet in reducing HbA1c (low confidence of evidence).
| The values at the upper-right for the dietary approaches correspond to the MD and 95% CI in HbA1c (%) between the row and columns (e.g., the MD in HAb1c between the low-carbohydrate diet and low-fat diet is −0.12%). The values at the bottom-left for the dietary approaches correspond to the MD and 95% CI in fasting glucose (mmol l−1) between the column and the row (e.g., the MD fasting glucose between the low-carbohydrate diet and low-fat diet is 0.31 mmol l−1). HbA1c, glycosylated hemoglobin A1c. GI/GL, glycaemic index/load. | |||||||
|---|---|---|---|---|---|---|---|
| Vegetarian | −0.55 (−1.21, 0.11) | −0.38 (−0.98, 0.23) | 0.35 (−0.84, 1.54) | −0.03 (−0.62, 0.55) | −0.15 (−0.77, 0.47) | 0.07 (−0.56, 0.69) | 0.38 (−0.68, 1.44) |
| −0.30 (−1.77, 1.16) | Moderate carbohydrate | 0.17 (−0.39, 0.73) | 0.90 (−0.27, 2.06) | 0.52 (−0.02, 1.05) | 0.40 (−0.18, 0.97) | 0.62 (0.04, 1.20) | 1.48 (0.43, 2.54) |
| 0.79 (−0.10, 1.68) | 1.10 (−0.34, 2.53) | Mediterranean | 0.73 (−0.33, 1.78) | 0.34 (0.16, 0.53) | 0.23 (−0.06, 0.51) | 0.45 (0.16, 0.73) | 0.38 (0.07, 0.70) |
| 0.46 (−1.45, 2.37) | 0.76 (−1.45, 2.97) | −0.34 (−2.22, 1.55) | Low GI/GL | −0.38 (−1.42, 0.65) | −0.50 (−1.51, 0.51) | −0.28 (−1.34, 0.78) | −0.08 (−0.68, 0.51) |
| 0.03 (−0.62, 0.68) | 0.33 (−0.97, 1.64) | −0.77 (−1.37, −0.16) | −0.43 (−2.22, 1.36) | Low fat | −0.12 (−0.34, 0.11) | 0.10 (−0.11, 0.32) | 0.50 (0.13, 0.87) |
| 0.05 (−0.94, 1.03) | 0.35 (−1.14, 1.85) | −0.75 (−1.69, 0.20) | −0.41 (−2.04, 1.22) | 0.02 (−0.71, 0.75) | Low carbohydrate | 0.22 (−0.09, 0.53) | 0.28 (−0.10, 0.66) |
| −0.16 (−0.91, 0.60) | 0.15 (−1.22, 1.52) | −0.95 (−1.67, −0.23) | −0.61 (−2.45, 1.22) | −0.18 (−0.58, 0.21) | −0.20 (−1.04, 0.63) | High protein | 0.68 (−0.85, 2.21) |
| −2.20 (−4.16, −0.25) | −0.67 (−2.61, 1.27) | −1.77 (−2.57, −0.98) | 1.22 (−0.22, 2.67) | −1.79 (−2.87, −0.71) | −1.59 (−2.48, −0.69) | 0.05 (−1.95, 2.04) | Control |
In terms of fasting glucose, the LC diet (MD: −1.59%; 95% CI: −2.48 to −0.69), LF diet (MD: −1.79%; 95% CI: −2.87 to −0.71), Mediterranean (MD: −1.77%; 95% CI: −2.57 to −0.98), and vegetarian (MD: −2.2%; 95% CI: −4.16 to −0.25) were more efficacious than the control diet in reducing fasting glucose (high to moderate confidence of evidence). The Mediterranean diet intervention was more effective than the HP diet (MD: −0.95%; 95% CI: −1.67 to −1.23) and LF diet (MD: −0.77%; 95% CI: −1.37 to −0.16) in decreasing fasting glucose (low confidence of evidence).
3.7.1.2. Anthropometric measurements. Tables 2 and 3 summarize the estimated effect sizes (MDs and 95% CIs) for comparing the intervention approaches with each other for body weight (kg), BMI (kg m−2), and waist circumference (cm) (high to very low confidence of evidence). The LC diet intervention (MD: −7.67 cm; 95% CI: −14.09 to −1.24) reduced the waist circumference more effectively compared with the control diet (moderate confidence of evidence). No significant difference was noted in body weight, BMI, and waist circumference between the groups.
| The values at the upper-right for the dietary approaches correspond to the MD and 95% CI in body weight (kg) between the row and columns (e.g., the MD in body weight between the low-carbohydrate diet and low-fat diet is −2.51 kg). The values at the bottom-left for the dietary approaches correspond to the MD and 95% CI in BMI (kg m−2) between the row and columns (e.g., the MD in BMI between the low-carbohydrate diet and low-fat diet is 1.39 kg). BMI, body mass index. GI/GL, glycaemic index/load. | |||||||
|---|---|---|---|---|---|---|---|
| Vegetarian | −0.80 (−12.63, 11.03) | 1.71 (−8.97, 12.39) | 1.27 (−14.53, 17.06) | 2.78 (−6.78, 12.35) | 0.27 (−9.76, 10.29) | 4.09 (−6.12, 14.30) | −1.11 (−15.12, 12.89) |
| 0.40 (−3.67, 4.47) | Moderate carbohydrate | 2.51 (−8.40, 13.41) | 2.07 (−13.88, 18.02) | 3.58 (−6.23, 13.40) | 1.07 (−9.20, 11.33) | 4.89 (−5.56, 15.34) | −2.81 (−16.82, 11.19) |
| −1.66 (−5.47, 2.14) | −2.06 (−5.84, 1.71) | Mediterranean | −0.44 (−13.88, 13.00) | 1.07 (−3.67, 5.82) | −1.44 (−7.06, 4.18) | 2.38 (−3.56, 8.33) | 1.62 (−3.28, 6.51) |
| −1.95 (−7.71, 3.82) | −2.35 (−8.09, 3.40) | −0.29 (−5.24, 4.67) | Low GI/GL | 1.51 (−11.06, 14.09) | −1.00 (−13.21, 11.21) | 2.82 (−10.25, 15.89) | 2.58 (−7.10, 12.27) |
| −2.04 (−5.42, 1.34) | −2.44 (−5.79, 0.91) | −0.38 (−2.12, 1.36) | −0.09 (−4.73, 4.55) | Low fat | −2.51 (−5.53, 0.50) | 1.31 (−2.27, 4.89) | 4.13 (−1.61, 9.88) |
| −0.65 (−4.72, 3.42) | −1.05 (−5.09, 2.99) | 1.01 (−1.79, 3.82) | 1.30 (−2.78, 5.38) | 1.39 (−0.81, 3.59) | Low carbohydrate | 3.82 (−0.86, 8.50) | 0.31 (−5.75, 6.37) |
| −2.08 (−6.11, 1.95) | −2.48 (−6.48, 1.52) | −0.41 (−3.21, 2.39) | −0.13 (−5.26, 5.00) | −0.04 (−2.23, 2.16) | −1.43 (−4.54, 1.68) | High protein | 5.39 (−6.96, 17.74) |
| −0.34 (−2.81, 2.14) | −0.05 (−5.04, 4.93) | 0.04 (−1.71, 1.79) | −1.94 (−5.23, 1.35) | −1.35 (−4.21, 1.51) | 0.08 (−2.73, 2.88) | −3.18 (−7.90, 1.54) | Control |
| The values at the bottom-left for the dietary approaches correspond to the MD and 95% CI in waist circumference (cm) between the row and columns (e.g., the MD in waist circumference between the low-carbohydrate diet and low-fat diet is 3.55 cm). GI/GL, glycaemic index/load. | |||||||
|---|---|---|---|---|---|---|---|
| Vegetarian | |||||||
| 1.79 (−6.72, 10.30) | Moderate carbohydrate | ||||||
| 2.18 (−2.47, 6.84) | 0.39 (−8.17, 8.96) | Mediterranean | |||||
| 4.06 (−3.34, 11.46) | 2.27 (−8.05, 12.58) | 1.87 (−5.55, 9.29) | Low GI/GL | ||||
| 1.11 (−2.10, 4.32) | −0.68 (−8.56, 7.20) | −1.08 (−4.43, 2.28) | −2.95 (−9.59, 3.69) | Low fat | |||
| 4.66 (−0.56, 9.88) | 2.87 (−6.01, 11.75) | 2.47 (−2.78, 7.72) | 0.60 (−4.65, 5.85) | 3.55 (−0.53, 7.62) | Low carbohydrate | ||
| 0.54 (−4.03, 5.11) | −1.25 (−9.77, 7.27) | −1.65 (−6.31, 3.02) | −3.52 (−10.90, 3.86) | −0.57 (−3.82, 2.68) | −4.12 (−9.31, 1.08) | High protein | |
| −7.07 (−15.36, 1.23) | 4.75 (−3.46, 12.96) | 4.85 (−4.44, 14.14) | −4.12 (−9.07, 0.83) | 0.22 (−7.57, 8.00) | −7.67 (−14.09, −1.24) | −3.55 (−9.47, 2.37) | Control |
| Rank | HbA1C | SUCRA (%) | Fasting glucose | SUCRA (%) | Summary of HbA1c and fasting glucose | SUCRA (%) |
|---|---|---|---|---|---|---|
| SUCRA, surface under the cumulative ranking curve. HbA1C, HbA1C. GI/GL, glycaemic index/load. | ||||||
| 1 | Moderate carbohydrate | 92.2 | Mediterranean | 90.5 | Mediterranean | 88.15 |
| 2 | Mediterranean | 85.8 | Moderate carbohydrate | 74.4 | Moderate carbohydrate | 83.3 |
| 3 | Low carbohydrate | 63.7 | Low GI/GL | 66.8 | Low carbohydrate | 55.7 |
| 4 | Low fat | 47.1 | Low carbohydrate | 47.7 | Low fat | 46.5 |
| 5 | Vegetarian | 45 | Low fat | 45.9 | Low GI/GL | 45.25 |
| 6 | High protein | 32.9 | Vegetarian | 44.2 | Vegetarian | 44.6 |
| 7 | Low GI/GL | 23.7 | High protein | 30.4 | High protein | 31.65 |
| 8 | Control | 9.6 | Control | 0.2 | Control | 4.8 |
| Rank | Body weight | SUCRA (%) | BMI | SUCRA (%) | Waist circumference | SUCRA (%) | Summary of body weight, BMI and waist circumference | SUCRA (%) |
|---|---|---|---|---|---|---|---|---|
| SUCRA, surface under the cumulative ranking curve. GI/GL, glycaemic index/load. BMI, body mass index. | ||||||||
| 1 | Low carbohydrate | 70.9 | Moderate carbohydrate | 81.1 | Low carbohydrate | 85.3 | Low carbohydrate | 74.6 |
| 2 | Moderate carbohydrate | 69.8 | Vegetarian | 75 | Low GI/GL | 74.3 | Moderate carbohydrate | 68.7 |
| 3 | Vegetarian | 64.6 | Low carbohydrate | 67.6 | Mediterranean | 61.4 | Vegetarian | 57 |
| 4 | Low GI/GL | 53.7 | Mediterranean | 42.5 | Moderate carbohydrate | 55.1 | Low GI/GL | 55.7 |
| 5 | Mediterranean | 53.4 | Low GI/GL | 39 | Low fat | 46.9 | Mediterranean | 52.4 |
| 6 | Low fat | 40.1 | High protein | 32.5 | High protein | 38.6 | Low fat | 39.1 |
| 7 | High protein | 25.1 | Control | 32.1 | Vegetarian | 31.4 | High protein | 32.1 |
| 8 | Control | 22.5 | Low fat | 30.2 | Control | 7.1 | Control | 19.9 |
3.8. Inconsistency
No indication was observed for inconsistency with the side-splitting approach regarding HbA1c, fasting glucose, body weight, BMI, and waist circumference (from ESI Tables S11–S15†). The loop-specific method presented some significant inconsistency in the loop formed by the LF diet versus the moderate-carbohydrate and control diets for HbA1c (ESI Fig. S21†), whereas no important inconsistency was observed in fasting glucose, body weight, BMI, and waist circumference (from ESI Fig. S22–S25†). A notable inconsistency was found in the design-by-treatment model for HbA1c (P = 0.00), but not for fasting glucose (P = 0.403), body weight (P = 0.995), BMI (P = 0.501), and waist circumference (P = 0.929).3.9. Subgroup and sensitivity analyses
We did subgroup analyses to study the effect of the study duration, sample size, and mean age for each outcome (from ESI Tables S16–S30†). The Mediterranean diet and moderate-carbohydrate diet had greater efficacy in lowering HbA1c and fasting glucose in the long run (≥12 months), in patients <60 years old, and in larger-sized studies (≥100), whereas the moderate-carbohydrate diet showed no important difference in patients ≥60 years old and in smaller-sized studies. The LC diet was more effective in reducing body weight in patients <60 years. Moreover, the LC diet showed greater efficacy for the reduction of BMI in the short run.Furthermore, it was confirmed that the Mediterranean diet and moderate-carbohydrate diet showed greater efficacy in HbA1c and fasting glucose decrease in the sensitivity analysis, which excluded studies with a high risk of bias (ESI Tables S31 and S32†). Taken together, the findings suggest that both the Mediterranean and moderate-carbohydrate diets lowered HbA1c and fasting glucose more effectively. In terms of anthropometric measurements (from ESI Tables S33–S35†), although the LC diet and vegetarian diet showed greater efficacy, the differences between the interventions remained small.
3.10. Publication bias
There was no asymmetry in the funnel plots for any of the five outcomes (PHbA1c = 0.446, Pfasting glucose = 0.570, Pbody weight = 0.935, PBMI = 0.602, and Pwaist circumference = 0.737, from ESI Fig. S26–S30†).3.11. Confidence of evidence
The certainty of the evidence evaluated by CINeMA for all the outcomes is presented in the ESI (Fig. S31–S35 and Tables S36–S40†). The confidence of evidence was low or very low for most of the comparisons and moderate for the comparison of the control diet with the moderate-carbohydrate diet regarding HbA1c (ESI Table S36†). The confidence of evidence was low or very low for some of the comparisons and high or moderate for the comparisons of the control diet with LF, HP, LC, Mediterranean, moderate-carbohydrate, and vegetarian diets regarding fasting glucose (ESI Table S37†). The confidence of evidence was low or very low for some of the comparisons and moderate for the comparisons of the control diet with LF, LC, Mediterranean, moderate-carbohydrate diet, low-GI/GL, LC with low-GI/GL, HP, moderate-carbohydrate, and vegetarian diet regarding body weight (ESI Table S38†). The confidence of evidence ranged from high to very low for BMI and waist circumference (ESI Tables S39 and S40†).4. Discussion
4.1. Principal findings
Using NMA, 8 different interventions (Mediterranean, LC, vegetarian, moderate-carbohydrate, low-GI/GL, LF, HP, and control diets) were first ranked for their comparative efficacy for glycaemic control and anthropometric measurements in individuals with T2DM who were also overweight or obese, whereas previous meta-analyses tended to focus on only one of these conditions. The results from this study demonstrated that the Mediterranean diet was the most effective diet for glycaemic control. The summary of the ranking showed that the LC diet was the top-ranked diet in ameliorating anthropometric measurements. Nevertheless, the confidence of the evidence was judged between very low and moderate for many of the comparisons. There is therefore a need for further high-quality studies to enhance credibility.4.2. Comparison with previous studies and reasons for the differences
In this study, we confirmed the favorable effect of the Mediterranean diet on fasting glucose and HbA1c reductions. Also, we found that the glucose-lowering effect of the Mediterranean diet was much more pronounced than the HP and LF diet. T2DM is characterized by elevated blood glucose, insulin resistance, and reduced glucose absorption by tissues, posing a significant threat to human health.60 The Mediterranean diet, which is rich in polyphenol foods, such as whole grains, extra virgin olive oil, red wine, nuts, an assortment of vegetables, and fruits, has been proven to improve insulin resistance, T2DM, and metabolic syndrome. This efficacy stems from the ability of polyphenols to activate the intracellular adenosine monophosphate-activated protein kinase (AMPK) pathway, and delay the oxygen consumption of adenosine diphosphate in isolated mitochondria by upregulating the AMPK-dependent glucose transporter GLUT-4, thereby improving insulin sensitivity and augmenting glucose uptake by tissues.61–63 Olive oil, an important ingredient of the Mediterranean diet, exerts antioxidant and anti-inflammatory effects by reducing the activation of pro-inflammatory mediators and increasing the bioavailability of nitric oxide.64 The glucagon-like peptide (GLP-1) exhibits a favorable effect in the treatment of T2DM. Notably, the Mediterranean diet, being particularly rich in poly-unsaturated fatty acids, augments the efficacy of GLP-1 by binding to and stimulating G protein-coupled receptors, ultimately leading to heightened GLP-1 secretion.65 The Mediterranean diet also boasts an abundance of dietary fiber, which fosters the production of short-chain fatty acids (SCFAs), microbial byproducts of the gut microbiome. These SCFAs play a pivotal role in modulating glucose and lipid metabolism by activating their respective receptors and triggering the secretion of GLP-1 and GLP-2, ultimately enhancing insulin sensitivity and promoting the proliferation of related β cells.63 Thus, the Mediterranean diet maintains diabetic homeostasis and improves blood glucose by improving insulin sensitivity, gut microbiota, and stimulating anti-inflammatory and antioxidant effects.63,66In order to reduce the global prevalence of obese people with T2DM, it is important to identify effective strategies for long-term weight control. The LC diet was found to be effective for weight loss and could be recommended for people who are overweight or obese.67 The LC diet scored the highest SUCRA-value for anthropometrics in our present study, which was inconsistent with the previous study that the caloric restriction diet was rated as the most beneficial dietary intervention and the LC diet was ranked as the second for weight loss and waist circumference.68 The LC diet achieved weight loss by decreasing insulin secretion and fat storage as well as prompting the body to metabolize stored fat for energy. The LC diet typically comprises a greater proportion of protein and fat, two macronutrients known for their slower digestion rate, leading to a prolonged feeling of satiety. This extended feeling of fullness can effectively curb hunger pangs, facilitating better control over overall calorie intake and ultimately supporting weight management. In a LC diet state, the body must convert non-sugar substances into glucose via gluconeogenesis to maintain blood glucose levels,69 as there is an insufficient supply of carbohydrates. Concurrently, proteolytic metabolism becomes more active to provide energy. These processes collectively increase the body's energy expenditure and contribute to weight reduction.
No significant between-groups differences were noted in body weight, BMI, and waist circumference in this NMA, which was consistent with previous studies.70,71 Indeed, a drop in body weight and associated beneficial functional and metabolic changes are favored by any dietary intervention resulting in reduced energy intake.69 In addition, these studies included a control group that received dietary interventions with higher carbohydrate intakes, and consequently the anthropometric measurements in the control groups were similar to those in the other dietary groups, in line with previous studies.72,73 There are two possible reasons for this: too few trials were included to identify a significant difference. Second, the control group was subjected to a series of interventions, including the administration of standard dietary and exercise advice44 or an energy-restricted meal plan.74 Although our present results indicated that the differences in anthropometric measurements were mostly small and often trivial for almost all the dietary patterns, health risks were not reported in the included trials and could vary.75 Therefore, the effects of different diets on health risks, side effects, and patient compliance need further study.
The previous evidence indicated there were no significant differences in body weight and BMI between low glycaemic index/load (low-GI/GL) diets versus high GI/GL diets or any other diet in patients who were overweight or obese.76 Although the low-GI/GL diet showed no significant difference in glycaemic control in our study, the low-GI/GL diet has previously shown a better effect on improving glycaemic control compared with higher-GI diets or control diets in people with T2DM,77,78 which may be due to differences in the comparators and study populations, and so further research is necessary to investigate this. Moreover, the vegetarian diet was more efficient for fasting glucose compared with the control diet in this study, whereas no significant difference was noted with the other between-groups comparisons, which was attributed to the small number of trials included. The vegetarian diet, which contains a higher fiber content, lowers the postprandial glucose response through mechanisms like reducing gastric emptying, thereby slowing down starch digestion and glucose absorption.79 Among the macronutrients, carbohydrates exert the most significant influence on blood glucose and insulin levels, serving as the primary trigger for insulin secretion. A reduction of serum insulin concentrations and enhanced insulin sensitivity occur when adopting a LC diet, which also confers additional benefits such as decreased levels of ghrelin and leptin, along with increased energy expenditure.80 Limiting carbohydrate intake decreases the primary source of blood glucose, thereby directly lowering blood glucose levels. Additionally, it promotes fat metabolism and the production of ketone bodies, which contribute to the stabilization of blood glucose levels. This may partially explain the mechanism of the hypoglycemic effect induced by restrictive carbohydrate diets. Moreover, we did not find a significant glucose-lowering effect of the HP diet. The HP diet decreased fasting glucose and HbA1c.47,81 The inconsistent results may be due to a variety of factors, including variations in protein sources in the HP diet, the population, total energy intake, and other factors.
Consistent with our findings, a previous meta-analysis showed that moderate-carbohydrate diet and Mediterranean diet interventions had better efficiency in HbA1c reduction in the long run (≥12 months) than in the short run (<12 months) in comparison with other diets in patients with T2DM.26,82 In addition, it is worth noting that the Mediterranean diet was more efficient in lowering HbA1c in patients <60 years old, which is similar with previous studies.26,83 Importantly, the Mediterranean and moderate-carbohydrate diets were more effective in lowering HbA1c in larger-sized trials, while the vegetarian and LC diet had better results in smaller-sized studies. However, a previous study found that the Mediterranean diet had a better effect in the short run and in larger-sized trials compared to control diets.84 One possible explanation for this was that Mediterranean diet was effective in both the long and short term and as the number of included trials with the Mediterranean diet was small, leading to inadequate statistical power for assessing these dietary patterns compared with other diets. Whether these differences, as shown in this subgroup analysis, are due to age, the study duration, or sample size remains to be determined. The Mediterranean diet was more efficient than the other diets in fasting glucose reduction, which was consistent with some previous studies84,85 but contradicted another study.86 However, the Mediterranean diet findings should be interpreted with caution since only two trials were available, and there was a low quality of evidence assessed in the study.
Although current trends favor the LC, low-GI/GL, and Mediterranean diets, there is no optimal dietary strategy for patients with obesity and diabetes.87 According to the results of our research on obesity and T2DM in such people, we recommend the clinical use of the Mediterranean diet and the LC diet, but more research is still needed. A review reported that overweight or obese patients with T2DM were more encouraged to adopt the low-carbohydrate Mediterranean diet to control their glycaemic index/load and weight.88 Therefore, future studies should consider the effects of various diets in terms of long-term efficacy, safety, and the health risks.
4.3. Strengths and limitations of the study
NMA methods, which are a combination of direct and indirect comparisons performed simultaneously, were used in this systematic review. Another strength included identifying inconsistencies and assessing the confidence of evidence. To the best of our knowledge, the current study is the first to rank 8 different dietary intervention in terms of their comparative effectiveness in patients with T2DM who were also overweight or obese, whereas previous studies have focused only on people with T2DM or obesity. This study demonstrated that the Mediterranean diet was the most effective intervention diet for improving glycaemic control and the LC diet obtained the highest score for anthropometric measurements in individuals with T2DM who were also overweight or obese. This will have an impact on evidence-based decision-making regarding dietary interventions to manage people with T2DM who are also overweight or obese.The primary limitation of this review was the relatively small number and quality of the included studies. The number of studies was only 31, and 7 of the 31 trials were assessed as having a high risk of bias for quality. However, all the main findings in this study were validated by sensitivity analyses that excluded trials with a high risk of bias. Another important limitation was the different definitions of the different diet interventions and the overlap between some of them. For example, the definitions of the control diets were different, both no intervention and minimal intervention; while the overlap of the LF diets was reflected in the percentage of fat intake: ranging from 10%–30% fat, etc. Another limitation was the statistical inconsistency in HbA1c, which was also presented in the GRADE rating, reducing the confidence in the effect estimates and rankings. Finally, most of the trials focused on the comparisons of the LF diet and LC diet, HP, and moderate-carbohydrate diet, respectively, with fewer trials with other diets.
5. Conclusion
In summary, the Mediterranean diet is the most effective dietary intervention for improving glycaemic control while the LC diet is ranked the highest for anthropometric measurements in individuals with T2DM who are also overweight or obese. Nevertheless, confidence in the evidence was mostly very low to moderate.Author contributions
Y. Y. and C. C. designed the study; Y. Y. and C. C. were responsible for writing; C. C., Y. L. and Q. L. were responsible for the literature collection; Z. Y., Y. L., and L. S. were responsible for data management and statistical analysis; Y. Y. and G. F. is in charge of the overall research design and supervision.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This study was supported by the Guangdong Science and Technology Department (YN2021DB02) and the Special Research Fund for Clinical Studies of Innovative Drugs after Market Launch (WKZX2023CX150007).References
- K. Ogurtsova, J. D. Da Rocha Fernandes and Y. Huang, et al., IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040, Diabetes Res. Clin. Pract., 2017, 128, 40–50 CrossRef CAS PubMed.
- Obesity and overweight[EB/OL], https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- T. M. Powell-Wiley, P. Poirier and L. E. Burke, et al., Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association, Circulation, 2021, 143(21), e984–e1010 CrossRef PubMed.
- A. S. Al-Goblan, M. A. Al-Alfi and M. Z. Khan, Mechanism linking diabetes mellitus and obesity, Diabetes, Metab. Syndr. Obes.: Targets Ther., 2014, 7, 587–591 CrossRef PubMed.
- T. Scully, A. Ettela and D. Leroith, et al., Obesity, Type 2 Diabetes, and Cancer Risk, Front. Oncol., 2020, 10, 615375 CrossRef PubMed.
- C. Tsigos, V. Hainer and A. Basdevant, et al., Management of obesity in adults: European clinical practice guidelines, Obes. Facts, 2008, 1(2), 106–116 CrossRef PubMed.
- Y. Y. Chen, Y. J. Lin and E. Chong, et al., The impact of diabetes mellitus and corresponding HbA1c levels on the future risks of cardiovascular disease and mortality: a representative cohort study in Taiwan, PLoS One, 2015, 10(4), e0123116 CrossRef PubMed.
- S. I. Sherwani, H. A. Khan and A. Ekhzaimy, et al., Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients, Biomark. Insights, 2016, 11, 95–104 CrossRef CAS PubMed.
- J. Chen, D. Yin and K. Dou, Intensified glycemic control by HbA1c for patients with coronary heart disease and Type 2 diabetes: a review of findings and conclusions, Cardiovasc. Diabetol., 2023, 22(1), 146 CrossRef PubMed.
- C. Andersson, L. Van Gaal and I. D. Caterson, et al., Relationship between HbA1c levels and risk of cardiovascular adverse outcomes and all-cause mortality in overweight and obese cardiovascular high-risk women and men with type 2 diabetes, Diabetologia, 2012, 55(9), 2348–2355 CrossRef CAS PubMed.
- M. Tanaka, Relationship between fasting and 2-hour postprandial plasma glucose levels and vascular complications in patients with type 2 diabetes mellitus, J. Int. Med. Res., 2012, 40(4), 1295–1303 CrossRef CAS PubMed.
- Global BMI Mortality Collaboration, E. Di Angelantonio and S. N. Bhupathiraju, et al., Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents, Lancet, 2016, 388(10046), 776–786 CrossRef CAS PubMed.
- S. A. Everson, D. E. Goldberg and S. P. Helmrich, et al., Weight gain and the risk of developing insulin resistance syndrome, Diabetes Care, 1998, 21(10), 1637–1643 CrossRef CAS PubMed.
- A. Jayedi, S. Soltani and S. Z. Motlagh, et al., Anthropometric and adiposity indicators and risk of type 2 diabetes: systematic review and dose-response meta-analysis of cohort studies, Br. Med. J., 2022, 376, e067516 CrossRef PubMed.
- X. Lin and H. Li, Obesity: Epidemiology, Pathophysiology, and Therapeutics, Front. Endocrinol., 2021, 12, 706978 CrossRef PubMed.
- W. Sami, T. Ansari and N. S. Butt, et al., Effect of diet on type 2 diabetes mellitus: A review, Int. J. Health Sci., 2017, 11(2), 65–71 Search PubMed.
- American Diabetes Association, 8. Obesity Management for the Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes-2021, Diabetes Care, 2021, 44(Suppl 1), S100–s110 CrossRef PubMed.
- J. H. Choi, Y. J. Cho and H. J. Kim, et al., Effect of Carbohydrate-Restricted Diets and Intermittent Fasting on Obesity, Type 2 Diabetes Mellitus, and Hypertension Management: Consensus Statement of the Korean Society for the Study of Obesity, Korean Diabetes Association, and Korean Society of Hypertension, Diabetes Metab. J., 2022, 46(3), 355–376 CrossRef PubMed.
- T. A. Apekey, M. J. Maynard and M. Kittana, et al., Comparison of the Effectiveness of Low Carbohydrate Versus Low Fat Diets, in Type 2 Diabetes: Systematic Review and Meta-Analysis of Randomized Controlled Trials, Nutrients, 2022, 14(20), 4391 CrossRef CAS PubMed.
- Z. Yu, F. Nan and L. Y. Wang, et al., Effects of high-protein diet on glycemic control, insulin resistance and blood pressure in type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials, Clin. Nutr., 2020, 39(6), 1724–1734 CrossRef CAS PubMed.
- L. Chiavaroli, D. Lee and A. Ahmed, et al., Effect of low glycaemic index or load dietary patterns on glycaemic control and cardiometabolic risk factors in diabetes: systematic review and meta-analysis of randomised controlled trials, Br. Med. J., 2021, 374, n1651 CrossRef PubMed.
- L. Perin, I. G. Camboim and A. M. Lehnen, Low glycaemic index and glycaemic load diets in adults with excess weight: Systematic review and meta-analysis of randomised clinical trials, J. Hum. Nutr. Diet., 2022, 35(6), 1124–1135 CrossRef PubMed.
- A. D. Termannsen, K. K. B. Clemmensen and J. M. Thomsen, et al., Effects of vegan diets on cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials, Obes. Rev., 2022, 23(9), e13462 CrossRef PubMed.
- L. J. Dominguez, N. Veronese and G. Di Bella, et al., Mediterranean diet in the management and prevention of obesity, Exp. Gerontol., 2023, 174, 112121 CrossRef CAS PubMed.
- J. P. Higgins, D. G. Altman and P. C. Gøtzsche, et al., The Cochrane Collaboration's tool for assessing risk of bias in randomised trials, Br. Med. J., 2011, 343, d5928 CrossRef PubMed.
- L. Schwingshackl, A. Chaimani and G. Hoffmann, et al., A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus, Eur. J. Epidemiol., 2018, 33(2), 157–170 CrossRef CAS PubMed.
- A. Nikolakopoulou, J. P. T. Higgins and T. Papakonstantinou, et al., CINeMA: An approach for assessing confidence in the results of a network meta-analysis, PLoS Med., 2020, 17(4), e1003082 CrossRef PubMed.
- T. Papakonstantinou, A. Nikolakopoulou and J. P. T. Higgins, et al., CINeMA: Software for semiautomated assessment of the confidence in the results of network meta-analysis, Campbell Syst. Rev., 2020, 16(1), e1080 CrossRef PubMed.
- L. K. Heilbronn, M. Noakes and P. M. Clifton, Effect of energy restriction, weight loss, and diet composition on plasma lipids and glucose in patients with type 2 diabetes, Diabetes Care, 1999, 22(6), 889–895 CrossRef CAS PubMed.
- H. Shige, P. Nestel and D. Sviridov, et al., Effect of weight reduction on the distribution of apolipoprotein A-I in high-density lipoprotein subfractions in obese non-insulin-dependent diabetic subjects, Metabolism, 2000, 49(11), 1453–1459 CrossRef CAS PubMed.
- B. Parker, M. Noakes and N. Luscombe, et al., Effect of a high-protein, high-monounsaturated fat weight loss diet on glycemic control and lipid levels in type 2 diabetes, Diabetes Care, 2002, 25(3), 425–430 CrossRef PubMed.
- G. D. Brinkworth, M. Noakes and B. Parker, et al., Long-term effects of advice to consume a high-protein, low-fat diet, rather than a conventional weight-loss diet, in obese adults with type 2 diabetes: one-year follow-up of a randomised trial, Diabetologia, 2004, 47(10), 1677–1686 CrossRef CAS PubMed.
- M. E. Daly, R. Paisey and R. Paisey, et al., Short-term effects of severe dietary carbohydrate-restriction advice in Type 2 diabetes - A randomized controlled trial, Diabetic Med., 2006, 23(1), 15–20 CrossRef CAS PubMed.
- T. Mclaughlin, S. Carter and C. Lamendola, et al., Clinical efficacy of two hypocaloric diets that vary in overweight patients with type 2 diabetes: comparison of moderate fat versus carbohydrate reductions, Diabetes Care, 2007, 30(7), 1877–1879 CrossRef CAS PubMed.
- E. C. Westman, W. S. Yancy Jr. and J. C. Mavropoulos, et al., The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus, Nutr. Metab., 2008, 5(1), 36 CrossRef PubMed.
- T. M. S. Wolever, A. L. Gibbs and C. Mehling, et al., The Canadian Trial of Carbohydrates in Diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: No effect on glycated hemoglobin but reduction in C-reactive protein, Am. J. Clin. Nutr., 2008, 87(1), 114–125 CrossRef CAS PubMed.
- B. J. Brehm, B. L. Lattin and S. S. Summer, et al., One-year comparison of a high-monounsaturated fat diet with a high-carbohydrate diet in type 2 diabetes, Diabetes Care, 2009, 32(2), 215–220 CrossRef PubMed.
- N. J. Davis, N. Tomuta and C. Schechter, et al., Comparative study of the effects of a 1-year dietary intervention of a low-carbohydrate diet versus a low-fat diet on weight and glycemic control in type 2 diabetes, Diabetes Care, 2009, 32(7), 1147–1152 CrossRef CAS PubMed.
- K. Esposito, M. I. Maiorino and M. Ciotola, et al., Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: a randomized trial, Ann. Intern. Med., 2009, 151(5), 306–314 CrossRef PubMed.
- A. Elhayany, A. Lustman and R. Abel, et al., A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: a 1-year prospective randomized intervention study, Diabetes, Obes. Metab., 2010, 12(3), 204–209 CrossRef CAS PubMed.
- N. Iqbal, M. L. Vetter and R. H. Moore, et al., Effects of a low-intensity intervention that prescribed a low-carbohydrate vs. a low-fat diet in obese, diabetic participants, Obesity, 2010, 18(9), 1733–1738 CrossRef CAS PubMed.
- M. L. Vetter, A. Wade and L. G. Womble, et al., Effect of a low-carbohydrate diet versus a low-fat, calorie-restricted diet on adipokine levels in obese, diabetic participants, Diabetes, Metab. Syndr. Obes.: Targets Ther., 2010, 3, 357–361 CrossRef CAS.
- T. P. Wycherley, M. Noakes and P. M. Clifton, et al., A high-protein diet with resistance exercise training improves weight loss and body composition in overweight and obese patients with type 2 diabetes, Diabetes Care, 2010, 33(5), 969–976 CrossRef CAS PubMed.
- R. C. Andrews, A. R. Cooper and A. A. Montgomery, et al., Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial, Lancet, 2011, 378(9786), 129–139 CrossRef CAS PubMed.
- A. N. Fabricatore, T. A. Wadden and C. B. Ebbeling, et al., Targeting dietary fat or glycemic load in the treatment of obesity and type 2 diabetes: a randomized controlled trial, Diabetes Res. Clin. Pract., 2011, 92(1), 37–45 CrossRef CAS PubMed.
- H. Kahleova, M. Matoulek and H. Malinska, et al., Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with Type 2 diabetes, Diabetic Med., 2011, 28(5), 549–559 CrossRef CAS PubMed.
- R. N. Larsen, N. J. Mann and E. Maclean, et al., The effect of high-protein, low-carbohydrate diets in the treatment of type 2 diabetes: a 12 month randomised controlled trial, Diabetologia, 2011, 54(4), 731–740 CrossRef CAS PubMed.
- J. D. Krebs, C. R. Elley and A. Parry-Strong, et al., The Diabetes Excess Weight Loss (DEWL) Trial: a randomised controlled trial of high-protein versus high-carbohydrate diets over 2 years in type 2 diabetes, Diabetologia, 2012, 55(4), 905–914 CrossRef CAS PubMed.
- S. Mishra, J. Xu and U. Agarwal, et al., A multicenter randomized controlled trial of a plant-based nutrition program to reduce body weight and cardiovascular risk in the corporate setting: the GEICO study, Eur. J. Clin. Nutr., 2013, 67(7), 718–724 CrossRef CAS PubMed.
- E. Pedersen, D. R. Jesudason and P. M. Clifton, High protein weight loss diets in obese subjects with type 2 diabetes mellitus, Nutr., Metab. Cardiovasc. Dis., 2014, 24(5), 554–562 CrossRef CAS PubMed.
- C. L. Rock, S. W. Flatt and B. Pakiz, et al., Weight loss, glycemic control, and cardiovascular disease risk factors in response to differential diet composition in a weight loss program in type 2 diabetes: a randomized controlled trial, Diabetes Care, 2014, 37(6), 1573–1580 CrossRef CAS PubMed.
- J. Tay, N. D. Luscombe-Marsh and C. H. Thompson, et al., Comparison of low- and high-carbohydrate diets for type 2 diabetes management: a randomized trial, Am. J. Clin. Nutr., 2015, 102(4), 780–790 CrossRef CAS PubMed.
- X. Li, X. Cai and X. Ma, et al., Short- and Long-Term Effects of Wholegrain Oat Intake on Weight Management and Glucolipid Metabolism in Overweight Type-2 Diabetics: A Randomized Control Trial, Nutrients, 2016, 8(9), 549 CrossRef PubMed.
- N. Watson, K. Dyer and J. Buckley, et al., Effects of Low-Fat Diets Differing in Protein and Carbohydrate Content on Cardiometabolic Risk Factors during Weight Loss and Weight Maintenance in Obese Adults with Type 2 Diabetes, Nutrients, 2016, 8(5), 289 CrossRef PubMed.
- W. F. Mollentze, G. Joubert and A. Prins, et al., The safety and efficacy of a low-energy diet to induce weight loss, improve metabolic health, and induce diabetes remission in insulin-treated obese men with type 2 diabetes: a pilot RCT, Int. J. Diabetes Dev. Countries, 2019, 39(4), 618–625 CrossRef CAS.
- N. A. Alfaris and A. S. Ba-Jaber, Effects of a low-energy diet with and without oat bran and olive oil supplements on body mass index, blood pressure, and serum lipids in diabetic women: A randomized controlled trial, Food Sci. Nutr., 2020, 8(7), 3602–3609 CrossRef CAS PubMed.
- D. J. Jenkins, P. J. Jones and M. M. Abdullah, et al., Low-carbohydrate vegan diets in diabetes for weight loss and sustainability: a randomized controlled trial, Am. J. Clin. Nutr., 2022, 116(5), 1240–1250 CrossRef CAS PubMed.
- S. Li, G. Lin and J. Chen, et al., The effect of periodic ketogenic diet on newly diagnosed overweight or obese patients with type 2 diabetes, BMC Endocr. Disord., 2022, 22(1), 34 CrossRef CAS PubMed.
- P. Weber, M. N. Thomsen and M. J. Skytte, et al., Effects of Carbohydrate Restriction on Body Weight and Glycemic Control in Individuals with Type 2 Diabetes: A Randomized Controlled Trial of Efficacy in Real-Life Settings, Nutrients, 2022, 14(24), 5244 CrossRef CAS PubMed.
- D. A. Striegel, M. Hara and V. Periwal, The Beta Cell in Its Cluster: Stochastic Graphs of Beta Cell Connectivity in the Islets of Langerhans, PLoS Comput. Biol., 2015, 11(8), e1004423 CrossRef PubMed.
- M. Guasch-Ferré, J. Merino and Q. Sun, et al., Dietary Polyphenols, Mediterranean Diet, Prediabetes, and Type 2 Diabetes: A Narrative Review of the Evidence, Oxid. Med. Cell. Longevity, 2017, 2017, 6723931 CrossRef PubMed.
- G. E. Lombardo, S. M. Lepore and V. M. Morittu, et al., Effects of Oleacein on High-Fat Diet-Dependent Steatosis, Weight Gain, and Insulin Resistance in Mice, Front. Endocrinol., 2018, 9, 116 CrossRef PubMed.
- S. Martín-Peláez, M. Fito and O. Castaner, Mediterranean Diet Effects on Type 2 Diabetes Prevention, Disease Progression, and Related Mechanisms. A Review, Nutrients, 2020, 12(8), 2236 CrossRef PubMed.
- S. Bartimoccia and V. Cammisotto, et al., Extra Virgin Olive Oil Reduces Gut Permeability and Metabolic Endotoxemia in Diabetic Patients, Nutrients, 2022, 14(10), 2153, DOI:10.3390/nu14102153.
- H. Oeseburg, R. A. De Boer and H. Buikema, et al., Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A, Arterioscler., Thromb., Vasc. Biol., 2010, 30(7), 1407–1414 CrossRef CAS PubMed.
- T. Milenkovic, N. Bozhinovska and D. Macut, et al., Mediterranean Diet and Type 2 Diabetes Mellitus: A Perpetual Inspiration for the Scientific World. A Review, Nutrients, 2021, 13(4), 1307 CrossRef CAS PubMed.
- T. Hu, K. T. Mills and L. Yao, et al., Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: a meta-analysis of randomized controlled clinical trials, Am. J. Epidemiol., 2012, 176(Suppl 7), S44–S54 CrossRef PubMed.
- B. T. Zeng, H. Q. Pan and F. D. Li, et al., Comparative efficacy of different eating patterns in the management of type 2 diabetes and prediabetes: An arm-based Bayesian network meta-analysis, J. Diabetes Invest., 2023, 14(2), 263–288 CrossRef CAS PubMed.
- F. Brouns, Overweight and diabetes prevention: is a low-carbohydrate-high-fat diet recommendable?, Eur. J. Nutr., 2018, 57(4), 1301–1312 CrossRef CAS PubMed.
- C. Churuangsuk, J. Hall and A. Reynolds, et al., Diets for weight management in adults with type 2 diabetes: an umbrella review of published meta-analyses and systematic review of trials of diets for diabetes remission, Diabetologia, 2022, 65(1), 14–36 CrossRef PubMed.
- C. E. Naude, A. Brand and A. Schoonees, et al., Low-carbohydrate versus balanced-carbohydrate diets for reducing weight and cardiovascular risk, Cochrane Database Syst. Rev., 2022, 1(1), Cd013334 Search PubMed.
- K. A. Meckling, C. O'sullivan and D. Saari, Comparison of a low-fat diet to a low-carbohydrate diet on weight loss, body composition, and risk factors for diabetes and cardiovascular disease in free-living, overweight men and women, J. Clin. Endocrinol. Metab., 2004, 89(6), 2717–2723 CrossRef CAS PubMed.
- P. Dyson, Low Carbohydrate Diets and Type 2 Diabetes: What is the Latest Evidence?, Diabetes Ther., 2015, 6(4), 411–424 CrossRef CAS PubMed.
- W. F. Mollentze, G. Joubert and A. Prins, et al., The safety and efficacy of a low-energy diet to induce weight loss, improve metabolic health, and induce diabetes remission in insulin-treated obese men with type 2 diabetes: a pilot RCT, Int. J. Diabetes Dev. Ctries., 2019, 39, 618–625 CrossRef CAS.
- A. B. Evert, M. Dennison and C. D. Gardner, et al., Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report, Diabetes Care, 2019, 42(5), 731–754 CrossRef PubMed.
- K. Chekima, S. W. Yan and S. W. H. Lee, et al., Low glycaemic index or low glycaemic load diets for people with overweight or obesity, Cochrane Database Syst. Rev., 2023, 6(6), Cd005105 Search PubMed.
- D. Thomas and E. J. Elliott, Low glycaemic index, or low glycaemic load, diets for diabetes mellitus, Cochrane Database Syst. Rev., 2009, 2009(1), Cd006296 Search PubMed.
- O. Ojo, O. O. Ojo and F. Adebowale, et al., The Effect of Dietary Glycaemic Index on Glycaemia in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, Nutrients, 2018, 10(3), 373 CrossRef PubMed.
- D. Pollakova, A. Andreadi and F. Pacifici, et al., The Impact of Vegan Diet in the Prevention and Treatment of Type 2 Diabetes: A Systematic Review, Nutrients, 2021, 13(6), 2123 CrossRef CAS PubMed.
- S. Chawla, F. Tessarolo Silva and S. Amaral Medeiros, et al., The Effect of Low-Fat and Low-Carbohydrate Diets on Weight Loss and Lipid Levels: A Systematic Review and Meta-Analysis, Nutrients, 2020, 12(12), 3774 CrossRef CAS PubMed.
- M. Luger, B. Holstein and K. Schindler, et al., Feasibility and efficacy of an isocaloric high-protein vs. standard diet on insulin requirement, body weight and metabolic parameters in patients with type 2 diabetes on insulin therapy, Exp. Clin. Endocrinol. Diabetes, 2013, 121(5), 286–294 CrossRef CAS PubMed.
- T. Jing, S. Zhang and M. Bai, et al., Effect of Dietary Approaches on Glycemic Control in Patients with Type 2 Diabetes: A Systematic Review with Network Meta-Analysis of Randomized Trials, Nutrients, 2023, 15(14), 3156 CrossRef CAS PubMed.
- B. Pan, Y. Wu and Q. Yang, et al., The impact of major dietary patterns on glycemic control, cardiovascular risk factors, and weight loss in patients with type 2 diabetes: A network meta-analysis, J. Evidence-Based Integr. Med., 2019, 12(1), 29–39 CrossRef PubMed.
- R. Huo, T. Du and Y. Xu, et al., Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis, Eur. J. Clin. Nutr., 2015, 69(11), 1200–1208 CrossRef CAS PubMed.
- M. Zheng, P. Hu and S. Yang, et al., A Review of the Effects of Mediterranean Diet on Prevention of Type 2 Diabetes amongst Overweight Patients, IOP Conf. Ser.: Mater. Sci. Eng., 2018, 394, 022071 CrossRef.
- P. Carter, F. Achana, J. Fau-Troughton, J. Troughton and L. J. Fau-Gray, et al., A Mediterranean diet improves HbA1c but not fasting blood glucose compared to alternative dietary strategies: a network meta-analysis, J. Hum. Nutr. Diet., 2014, 27(3), 280–297 CrossRef CAS PubMed.
- Z. Sandouk and M. C. Lansang, Diabetes with obesity–Is there an ideal diet?, Clevel. Clin. J. Med., 2017, 84(7 Suppl 1), S4–s14 CrossRef PubMed.
- M. Zheng, P. Hu and S. Yang, et al., A Review of the Effects of Mediterranean Diet on Prevention of Type 2 Diabetes amongst Overweight Patients, IOP Conf. Ser.: Mater, 2018, 394, 022071 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo00337c |
| ‡ These authors contributed equally to this study. |
| This journal is © The Royal Society of Chemistry 2024 |