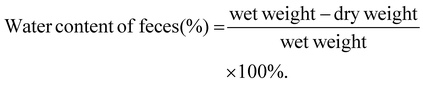Open Access Article
Open Access ArticleSynergistic effects of Ligilactobacillus salivarius Li01 and psyllium husk prevent mice from developing loperamide-induced constipation†
Lvwan
Xu‡
abc,
Bo
Qiu‡
a,
Furong
Ba
a,
Shuobo
Zhang
a,
Shengyi
Han
 d,
Hui
Chen
a,
Youhe
Wu
a,
Wang
Gao
c,
Siyuan
Xie
a,
Yanfei
Chen
a,
Shiman
Jiang
a,
Jingyi
Zhang
b,
Yating
Li
a,
Björn
Berglund
e,
Mingfei
Yao
d,
Hui
Chen
a,
Youhe
Wu
a,
Wang
Gao
c,
Siyuan
Xie
a,
Yanfei
Chen
a,
Shiman
Jiang
a,
Jingyi
Zhang
b,
Yating
Li
a,
Björn
Berglund
e,
Mingfei
Yao
 *abc and
Lanjuan
Li
*abc and
Lanjuan
Li
 *abc
*abc
aState Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, National Medical Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, Zhejiang University School of Medicine, 79 Qingchun Rd., Hangzhou 310003, China. E-mail: mingfei@zju.edu.cn; ljli@zju.edu.cn
bResearch Units of Infectious Disease and Microecology, Chinese Academy of Medical Sciences and Peking Union Medical College, Hangzhou, China
cJinan Microecological Biomedicine Shandong Laboratory, Jinan 250021, China
dZhejiang Provincial People's Hospital, Hangzhou 310014, China
eDepartment of Cell and Molecular Biology, Uppsala University, SE-75123 Uppsala, Sweden
First published on 15th November 2024
Abstract
Constipation is a gastrointestinal (GI) condition marked by difficulty in defecation, abdominal pain and distension, significantly impacting both physical and mental health. Ligilactobacillus salivarius Li01 (Li01) is a probiotic known to prevent constipation in mice, while psyllium husk (PSH) is a dietary fiber with high water retention, acting as an intestinal lubricant. This study investigates the effects of a combined treatment of Li01 and PSH on mice with loperamide-induced constipation. The combination treatment improved GI transit rates, increased the water content of feces, and regulated serum concentrations of GI hormones more effectively than either Li01 or PSH alone. The beneficial effects were linked to higher levels of butyric acid and a greater proportion of non-12-OH bile acids (BAs) in the GI tract. These protective effects were not influenced by changes in gut microbiota. Additionally, Li01 produced butyric acid and fermented PSH in vitro. Our findings suggest that the probiotic Li01 and the prebiotic PSH synergistically protect against constipation in mice, highlighting their potential as functional food components.
1. Introduction
Constipation is a common gastrointestinal (GI) complication that affects about 15% of the global population,1 presenting as difficulty in defecation, abdominal pain, and distension.2,3 This condition significantly impacts both the quality of life and psychological well-being of those affected.4 Constipation results from smooth muscle dysfunction of the colon or neuropathy, causing delayed stool transmission.4 Treatment methods typically include lifestyle changes, dietary adjustments, and medication.5 But laxatives can lead to side effects such as increased abdominal distension and diarrhea.6,7 Hence, microbiota-related therapies are emerging as a preferable alternative.8Gut microbiota, an essential component of the intestinal environment, collaborates with the nervous and immune systems to regulate intestinal movements, thus playing a role in constipation.9,10 Dysregulation of the microbiota is associated with constipation,11 as evidenced by lower levels of Firmicutes in elderly patients with the condition.12 Gut microbiota also influences the metabolism of short-chain fatty acids (SCFAs) and bile acids (BAs),13–16 which are crucial for digestion, absorption, and the interaction between host and microbiome through farnesoid X receptors.17 SCFAs, produced from fermented dietary fiber, provide energy for colonic epithelial cells and regulate intestinal peristalsis.18 Imbalances in SCFAs and BAs are common in those suffering from constipation.19,20
Probiotics, especially those belonging to Bifidobacterium and Lactobacillus have been shown effectiveness in alleviating constipation.2 For instance, Lactiplantibacillus plantarum GUANKE alleviated constipation in mice,21 and clinical studies have demonstrated that Bifidobacterium animalis subsp. lactis HN019, L. plantarum P9, and Bacillus subtilis (BG01-4TM) alleviate constipation.22–24Ligilactobacillus salivarius Li01 (Li01), a probiotic isolated from feces of healthy adults, was previously shown to alleviate constipation in mice by regulating microbiota and altering the 5-HT signaling pathway.25
Psyllium husk (PSH) is a natural dietary fiber with prebiotic potential which mainly consists of soluble fiber in the form of arabinoxylan.26,27 PSH is highly viscous and has a hydrophilic mucopolysaccharide composition,28 facilitating GI motility through considerable water absorptive and retentive properties.26 PSH has been shown to alleviate constipation and lower cholesterol levels.29,30 Some bacteria, such as Lactobacillus spp., can depolymerize dietary fibers, promoting their own growth while also benefiting the health of their host.31 An example being the fermentation of polysaccharides from Momordica charantia by L. plantarum in rats.32 These studies indicate that PSH with probiotics may synergistically facilitate prevention of constipation, however, studies of their combined effects are limited.
In this study, we investigated the potential of a Li01 and PSH combination treatment on loperamide-treated C57BL/6 mice to prevent constipation by evaluating fecal water content, GI transit rates, and concentrations of constipation-related hormones. To further evaluate possible mechanisms of action, we analyzed gut microbiota composition, diversity, and metabolic potential as well as the metabolic and fermentative capacities of Li01. Our study aims to provide new insights for future constipation prevention and treatment strategies.
2. Materials and methods
2.1. Bacterial cultures
Ligilactobacillus salivarius Li01 (CGMCC 7045) was cultivated by using de Man, Rogosa, Sharpe (MRS) Broth (OXOID, Hampshire, UK). Inoculation and incubation of Li01 were carried out under anaerobic conditions at 37 °C for 20 h. Following incubation, the bacterial culture was centrifuged at 6000 rcf for 5 min by using a 5810R benchtop centrifuge (Eppendorf, Hamburg, Germany). The pH of the supernatant was measured with an FE20K pH meter (Mettler Toledo, Zürich, Switzerland). The bacterial pellet was washed with phosphate-buffered saline (PBS) and then resuspended in PBS to achieve a Li01 solution concentration of approximately 1010 CFU per mL. The inactivated Li01 solution was prepared by boiling for 5 min.2.2. Growth curve of Li01
To demonstrate that Li01 can ferment psyllium husk (PSH) and utilize it as an energy source, we compared the growth curves of Li01 in MRS Broth medium and a 2% PSH solution. We used PBS buffer to dissolve PSH and thus acquired 2% PSH solution. Li01 was inoculated into both MRS Broth medium and 2% PSH solution and cultured anaerobically at 37 °C. Samples were taken at 0, 2, 4, 6, 8, 10, 12, 15, 18, and 24 hours post-inoculation. They were then serially diluted and plated on MRS agar (OXOID) plates. After 24 h of incubation at 37 °C, colonies were counted to determine the viable cell count at each time point. This allowed us to assess the growth kinetics of Li01 in both media, confirming its ability to ferment PSH.2.3. Whole-genome sequencing of Li01
Genomic DNA of Ligilactobacillus salivarius Li01 was extracted by using the SDS method.33 Libraries for sequencing were prepared for both the PacBio Sequel and Illumina NovaSeq platforms. For the PacBio Sequel platform, library was constructed by using the single-molecule real-time (SMRT) bell™ template kit (version 1.0). For the Illumina NovaSeq platform, the NEBNext® Ultra™ DNA library prep kit (NEB, USA) was used to construct the sequencing libraries. Preliminary assembly of the genomic data was performed by using SMRT Link v5.0.1. After a series of corrections, gene sequences were screened, and functional predictions were analyzed.2.4. Mouse experiments
2.5. Water content of feces of loperamide-treated mice
Feces were collected from the mice, weighed, and then dehydrated in a drying oven (Boxun, BZF-50) at 80 °C for approximately 24 h until a constant weight was achieved. The water content of the feces was calculated using the following formula:2.6. GI transit rates of loperamide-treated mice
To determine the GI transit rate in mice, a charcoal solution was prepared as follows: first, 10 g of gum arabic (Sigma-Aldrich, St Louis, Missouri, USA) was dissolved by boiling in 80 mL of water until the solution becomes transparent; then 5 g of activated charcoal (Sigma-Aldrich, St Louis, Missouri, USA) was added to the solution and boil three times. Finally, the mixture was diluted in water to 100 mL, cool, and stored at 4 °C for later use. Before sacrificed, all mice were fasted overnight for approximately 12 h. Each mouse was then gavaged with 0.2 mL of the charcoal solution. 20 min after the gavage, the mice were sacrificed and their digestive tracts were removed from the stomach to the cecum. The length of the small intestine and the distance traveled by the charcoal were measured and recorded. GI transit rates were calculated with the following formula:2.7. Quantification of GI hormones and inflammatory factors
The serum concentrations of gastrointestinal (GI) hormones and inflammatory factors in the serum and colon tissue of sacrificed mice were determined by enzyme-linked immunosorbent assay (ELISA). For serum analytes, the following hormones were measured by ELISA kits from Sangon (Shanghai, China): substance P (SP); gastrin (GAS); acetylcholine (ACH); vasoactive intestinal peptide (VIP); 5-hydroxytryptamine (5-HT). Somatostatin (SST) levels in the serum were measured by an ELISA kit from Elabscience (Wuhan, China). Levels of the inflammatory factors TNF-α and IL-6 in colon tissue were determined by using ELISA kits from MultiSciences (Hangzhou, China).2.8. Hematoxylin–eosin (HE) staining of colon tissue
Approximately 0.6 cm of distal colon tissue was collected from the sacrificed mice and placed in 4% paraformaldehyde (Biosharp, Beijing, China) for fixation. The tissue was fixed for 24 h, embedded in paraffin wax, and then sectioned. The sections were stained with hematoxylin and eosin (HE). The tissue structure was observed under a light microscope, and images were processed by using CaseViewer.2.9. Scanning electron microscope (SEM) imaging of PSH gels
Li01 was incubated in PSH for 24 h. The gel-like PSH was then collected and lyophilized for 72 h. After lyophilization, the PSH samples were cut into thin slices and the surfaces were coated with gold. The morphology of the PSH gels was compared by using thermal field emission scanning electron microscopy (Carl Zeiss, G300).2.10. Transmission electron microscope (TEM) imaging of colon tissue
Approximately 0.3 cm of distal colon tissue from the sacrificed mice was fixed in 2.5% glutaraldehyde PBS buffer (Solarbio, Beijing, China). The samples were prepared following a previously described method.34 Briefly, the tissue was fixed with 1% osmic acid, then stained with 2% uranium acetate aqueous solution. After dehydration, embedding, and sectioning, the samples were visualized by using cryo-transmission electron microscopy (Thermo FEI, Tecnai G2 Spirit 120 kV).2.11. 16S rRNA gene sequencing of murine gut microbiota
Total DNA from the bacteria in murine colon contents was extracted by using the DNeasy PowerSoil kit (Qiagen, Hilden, Germany). The V3–V4 variable region of the 16S rRNA gene was amplified by using specific primers.35 The PCR products were purified by using Agencourt AMPure XP beads (Beckman Coulter Co., USA). Sequencing was performed on the Illumina NovaSeq 6000 platform. The raw sequence data in FASTQ format were then analyzed by using bioinformatics tools.2.12. Ultra-performance liquid chromatography-electrospray tandem mass spectrometry (UPLC-ESI-MS/MS) analysis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C. The supernatant was then collected and diluted in 50% acetonitrile aqueous solution, after which quantification of short-chain fatty acids (SCFAs) was performed by using UPLC-ESI-MS/MS. The peak areas obtained from the analysis were used in the regression equation derived from the standard curve to determine the quantities of SCFAs in the samples.
000 rpm for 10 min at 4 °C. The supernatant was then collected and diluted in 50% acetonitrile aqueous solution, after which quantification of short-chain fatty acids (SCFAs) was performed by using UPLC-ESI-MS/MS. The peak areas obtained from the analysis were used in the regression equation derived from the standard curve to determine the quantities of SCFAs in the samples.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, containing IS) solution and 2 steel balls, the beads were ground (60 Hz, 2 min). 500 μL of ice-cold acetonitrile was added and extracted by sonication in an ice water bath. After centrifugation for 10 min (4 °C, 13
1, v/v, containing IS) solution and 2 steel balls, the beads were ground (60 Hz, 2 min). 500 μL of ice-cold acetonitrile was added and extracted by sonication in an ice water bath. After centrifugation for 10 min (4 °C, 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm), the supernatant was dried. Then it was redissolved in MeOH–water. The supernatant was removed after centrifugation. We used UPLC-ESI-MS/MS analysis to quantify them in the samples. Peak area of the them was brought into the regression equation fitted from the standard curve to obtain the quantity.
000 rpm), the supernatant was dried. Then it was redissolved in MeOH–water. The supernatant was removed after centrifugation. We used UPLC-ESI-MS/MS analysis to quantify them in the samples. Peak area of the them was brought into the regression equation fitted from the standard curve to obtain the quantity.
2.13. Quantitative real time polymerase chain reaction (qRT-PCR)
10 mg of liver tissue was used for RNA extraction by fast RNA extraction kit (ABclonal, Wuhan, China). RNA was then reverse transcribed into cDNA using reverse transcription reagents (ABclonal). Finally, universal SYBR green fast qPCR mix (ABclonal) was used to examine the relative expression of genes with QuantStudio 5 real-time PCR systems (Thermo Fisher Scientific). Gapdh was used as the control. The primer sequences used were listed in Table S1.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36
36
2.14. The activity of bacterial bile salt hydrolase (BSH) in feces
According to the ref. 37, we determined the activity of BSH in mice feces. In brief, approximately 50 mg of feces was collected, and the supernatant was removed after centrifugation of the homogenate. Protein concentration was determined by the BCA assay. The protein concentration was diluted to 1 mg ml−1 with 3 mM sodium acetate solution. 10 μl of the protein working solution was taken and 20 μl of 1 mM TCA solution and 170 μl of 3 mM sodium acetate buffer were added to a constant volume of 200 μl. The reaction was stopped after incubation at 37 °C for 30 min. After centrifugation, 20 μl of the supernatant was taken, and 80 μl of pure water and 1.9 ml of ninhydrin working solution were added. The absorbance was measured at 570 nm after cooling.2.15. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis of Li01 metabolites
The levels of metabolites in solutions of 2% PSH were compared before and after inoculation with Li01 by first dissolving PSH in PBS at a concentration of 2%. Li01 was then inoculated in the PSH solution and cultured anaerobically at 37 °C for 24 h. The supernatants were collected before and after fermentation at 0 h and 24 h, respectively. Samples were prepared with methanol and other reagents according to a previously established method.38 Next, the metabolomic profiles of supernatants were determined by an LC-MS system consisting of ACQUITY UPLC I-Class (Waters Corporation, Milford, USA) fitted with a Q-Exactive mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA).2.16. Statistical analysis
One-way ANOVA analysis was used compare sets of normally distributed data with homogeneous variance. For sets not fulfilling these criteria, Kruskal–Wallis testing was used. Correlation between microbiota and metabolites was analyzed by Spearman correlation analysis. P-Values less than 0.05 were considered significant. All hypothesis testing was performed by using IBM SPSS Statistics 27 and GraphPad Prism 8 was used to visualize the data.3. Results
3.1. Li01 and PSH treatment protect mice from constipation
We investigated whether the combination of Li01 and PSH facilitates prevention of constipation in mice compared to Li01 or PSH alone (Fig. 1A). The effects were evaluated by measuring body weight, fecal water content, defecation frequency, GI transit rate, and serum concentrations of GI hormones and neurotransmitters. During the experiment, the body weight change of the mice was recorded (Fig. 1B). On the 12th day, the fecal water content in the LOPLP group was significantly higher than in the LOP group (P = 0.006), LOPP group (P < 0.001), and LOPL group (P = 0.041) (Fig. 1C).Fecal consistency also varied among the groups, with the LOP group having hard and dry fecal granules, while the LOPL and LOPLP groups had relatively softer and wetter feces (Fig. 1D). Defecation frequency within 2 h was significantly higher in both the LOPL (P < 0.001) and LOPLP (P < 0.001) groups compared to the LOP group (Fig. 1E). The GI transit rate in the LOP group was significantly lower than in the Ctrl group (P < 0.001), but it was higher in the LOPL (P = 0.020) and LOPLP (P = 0.003) groups compared to the LOP group (Fig. 1F). Notably, the GI transit rate in the LOPP group was significantly lower than in the LOPLP group (P < 0.001).
Serum concentrations of GI hormones and neurotransmitters were further measured in the sacrificed mice (Fig. 1G–L). SP, GAS, ACH, and 5-HT promote GI motility, whereas VIP and SST function as motility inhibitors.39–42 SP levels were significantly higher in the LOPLP group compared to the LOP group (P = 0.016) (Fig. 1G). GAS levels were lower in the LOP group (P = 0.005) compared to the Ctrl group, but significantly higher in the LOPL (P = 0.047) and LOPLP (P = 0.020) groups compared to the LOP group (Fig. 1H). ACH levels were higher in the LOP group compared to the Ctrl group (P < 0.001), but lower in the LOPLP group compared to the LOPL group (P = 0.034) (Fig. 1I). VIP (P = 0.001) and 5-HT (P = 0.032) levels were lower in the LOPLP group compared to the Ctrl group (Fig. 1J and K). No significant differences were observed in SST levels among any of the groups (Fig. 1L). Additionally, concentrations of inflammatory factors in the colon were measured. TNF-α levels were found to be higher in the LOP group compared to the Ctrl (P = 0.038) and LOPLP groups (P = 0.021) whereas IL-6 levels were lower in the LOPL group compared to the LOP group (P = 0.002) (Fig. 1M and N).
To determine whether viable Li01 are necessary for the effect of prevention of constipation in mice, the experiment was repeated with heat-inactivated Li01. The process and weight changes during the experiment are shown in Fig. 1O and P. Inactivated Li01 did not significantly affect defecation frequency, fecal water content, or GI transit rate in the when comparing the LOPL group to the LOP group (Fig. 1Q–S), indicating that inactivated Li01 does not have a significant protective effect against constipation.
3.2. Effects of Li01 and PSH on colon morphology and gut microbiota composition
HE staining was utilized to visualize the distal colon tissue of sacrificed mice (Fig. 2A) and the colonic muscle thickness was found to be significantly thinner in the LOP group compared to the LOPL (P = 0.003) and LOPLP groups (P = 0.002) (Fig. 2B). TEM imaging revealed disrupted and sparse colonic microvilli structures in the LOP group. In contrast, the colonic microvilli in the LOPL and LOPLP groups were closely and neatly arranged, similar to the Ctrl group (Fig. 2A).Next, the microbiota composition in the colonic contents of the mice was analyzed at the genus level (Fig. 2C). α-Diversity, determined by Shannon index, was significantly lower in the LOP group compared to the Ctrl group (P = 0.002) (Fig. 2D). The relative abundance of Desulfobacterota (P = 0.040) and Deferribacterota (P < 0.001) was significantly lower in the LOP group compared to the Ctrl group, while the relative abundance of Proteobacteria was significantly higher in the LOP group (P = 0.004) (Fig. 2E–G). Compared to the LOPP group, the relative abundance of Lachnospiraceae_NK4A136_group was higher in the LOPLP group (P = 0.066) (Fig. 2H). Both the LOPL and LOPLP groups had significantly higher relative abundances of Bacteroides compared to the Ctrl group (P < 0.001) (Fig. 2I).
3.3. Effects of Li01 and PSH on gut microbiota metabolism
Metabolite production of the gut microbiota was evaluated by quantification of SCFAs and BAs in the cecal contents of the mice. For SCFAs, the levels of acetic acid (P < 0.001), propionic acid (P = 0.019), and butyric acid (P = 0.001) were significantly higher in the LOPLP group compared to the LOPP group (Fig. 3A–C). In contrast, isovaleric acid levels were significantly lower in the LOP group compared to the Ctrl group (P = 0.012) (Fig. 3D). Additionally, isobutyric acid (P = 0.018) and pentanoic acid (P = 0.012) levels were lower in the LOPP group compared to the Ctrl group (Fig. 3E and F). No significant differences in hexanoic acid levels were observed among any of the groups (Fig. 3G).As for BAs (Table S2†), total BAs and secondary BAs were significantly lower in both the LOPL (P = 0.018, P = 0.014) and LOPP groups (P = 0.006, P = 0.007) compared to the Ctrl group (Fig. 3H and I). The proportion of non-12-OH-BAs was significantly higher in the LOPL (P = 0.010) and LOPLP (P = 0.014) groups compared to the LOPP group (Fig. 3J). Levels of hyodeoxycholic acid (HDCA) and murideoxycholic acid (MDCA) were significantly lower in the LOPP group compared to the Ctrl group (P < 0.001 for both), but higher in the LOPLP group compared to the LOPP group (P = 0.030 and P = 0.036, respectively) (Fig. 3K and L).
Using Spearman's correlation, the associations between gut microbiota and metabolites were evaluated (Fig. 3M). Lachnospiraceae_NK4A136_group was found to be positively correlated with butyric acid (P < 0.001), HDCA (P < 0.01), and MDCA (P < 0.05). Additionally, Ruminococcus showed a positive correlation with butyric acid (P < 0.001).
3.4. The influence of gut microbiota disruption on the protective efficacy of Li01 and PSH
To determine if the protective effects of Li01 and PSH against constipation are mediated by gut microbiota, further experiments were conducted on mice with microbiota disrupted by antibiotics (Fig. 4A). Mice were administered antibiotics for two weeks, after which their feces were collected, and total DNA was extracted and subjected to 16S rRNA gene sequencing. The sequencing results confirmed that antibiotics had significantly disrupted the normal gut microbiota (Fig. 4B).Throughout the experiment, changes in body weight of the mice were monitored and are shown in Fig. 4C. The defecation frequency of mice within 2 h before sacrifice was found to be significantly lower in the LOP group compared to the Abx group (P < 0.001) and the LOPLP group (P = 0.006) (Fig. 4D). Furthermore, fecal water content in the LOP group was significantly lower than in the Abx group (P = 0.005) and the LOPLP group (P = 0.005) (Fig. 4E).
Histological examination of the colonic tissue by using HE staining revealed that the colonic muscle layer was thinner in the LOP group compared to the LOPL (P = 0.003) and the LOPLP groups (P = 0.008) (Fig. 4F). The composition of the gut microbiota at the phylum level in the murine colonic contents is shown in Fig. 4G. Analysis of Shannon indices indicated that the LOP group had significantly lower microbial diversity than the Ctrl group (P < 0.001) (Fig. 4H). Furthermore, compared to the LOP group, the LOPLP group exhibited a higher relative abundance of Firmicutes (P = 0.003) and Lactobacillus (P = 0.011), and a lower relative abundance of Proteobacteria (P = 0.026) and Enterobacteriaceae (P = 0.002) (Fig. 4I–L). These findings suggest that the protective effects of Li01 and PSH against constipation are likely mediated by their influence on the gut microbiota composition and function.
3.5. Metabolism-related changes after Li01 and PSH treatment in constipated mice with disrupted microbiota
To investigate the effects of Li01 and PSH on metabolites in mice with their gut microbiota disrupted by antibiotics, concentrations of SCFAs and BAs were measured in the cecal contents.Total SCFA concentrations were significantly lower in the LOP group compared to the Ctrl group (P < 0.001) and the Abx group (P = 0.042) (Fig. 5A). Specifically, cecal levels of acetic acid (P = 0.008), propionic acid (P = 0.012), and butyric acid (P = 0.018) were significantly higher in the LOPLP group compared to the LOP group (Fig. 5B–D).
Levels of total BAs (P < 0.001), primary BAs (P = 0.035), secondary BAs (P < 0.001), and the proportion of non-12-OH BAs (P = 0.005) were found to be significantly higher in the LOPLP group compared to the LOP group (Fig. 5E–H and Table S3†). Additionally, levels of hyocholic acid (HCA) (P = 0.001), α-muricholic acid (MCA) (P = 0.002), β-MCA (P = 0.003), and epiallo-lithocholic acid (LCA) (P = 0.001) were also significantly higher in the LOPLP group than in the LOP group (Fig. 5I–L).
Spearman's correlation analysis was conducted to explore the relationship between gut microbiota and metabolite levels (Fig. 5M). Lactobacillus was found to be positively correlated with butyric acid (P < 0.010), pentanoic acid (P < 0.001), HCA (P < 0.001), α-MCA (P < 0.001), and β-MCA (P < 0.001). Additionally, Clostridium innocuum group (P < 0.050) and Erysipelatoclostridium (P < 0.001) showed positive correlations with butyric acid.
The activity of BSH in LOPLP group was significantly higher than the LOP group (Fig. 5N, P < 0.001 and P = 0.026). However, the relative expressions of bile acid synthesis related enzymes were not changed (Fig. S1†).
These results highlight the significant influence of Li01 and PSH on the metabolic profiles of SCFAs and BAs in constipated mice with depleted gut microbiota, indicating their potential role in mediating the protective effects against constipation.
3.6. In vitro characterization of the Li01 metabolomic profile
Whole-genome sequencing of Li01 was performed to investigate genes related to metabolism carried by the strain. By annotating the predicted metabolic functions of the Li01 genome with the KEGG database, 142 genes related to carbohydrate metabolism and 86 genes related to amino acid metabolism were identified (Fig. 6A). Further analysis with the Carbohydrate-Active enZYmes Database revealed 30 genes encoding glycoside hydrolases, another 30 genes encoding glycosyltransferases, and 13 genes encoding carbohydrate-binding modules (Fig. 6B).To further understand the metabolic changes in mice induced by Li01 treatment, in vitro experiments were conducted to evaluate acid production. After 24 h of Li01 incubation, the pH in the MRS growth medium was observed to have significantly decreased (P < 0.001) (Fig. 6C). Further analysis of the culture medium revealed significant increases in propionic acid (P = 0.016), butyric acid (P = 0.002), isobutyric acid (P = 0.008), and hexanoic acid (P = 0.014) (Fig. 6D) after the incubation period, while no significant changes in concentrations of acetic acid, pentanoic acid, and isovaleric acid were observed. These findings confirmed Li01's capability to independently produce SCFAs.
Furthermore, the growth profiles of Li01 in MRS culture medium and 2% PSH gels were investigated. In MRS medium, Li01 was found to reach the stationary phase after approximately 10 h of growth (Fig. 6E). The growth in PSH indicated that Li01 can ferment PSH for energy. Scanning electron microscopy (SEM) images of lyophilized PSH gels showed an irregular and porous structure (Fig. 6F).
Metabolomic profiling of Li01 during fermentation in PSH gels revealed significant metabolic changes after 24 h of incubation. Of the detected metabolites, 9.46% were fatty acids and conjugates, and 9.24% were amino acids (Fig. 6G). Orthogonal partial least squares discriminant analysis (OPLS-DA) of the two time points showed distinct metabolic profiles (Fig. 6H). A total of 1223 metabolites were found to significantly increase in concentration whereas 776 metabolites significantly decreased (P < 0.050) after Li01 was allowed to grow for 24 h (Fig. 6I). Metabolites with a fold change greater than 2 are highlighted, and the 50 differentially expressed metabolites with highest variable importance in projection (VIP) scores are visualized in Fig. 6J.
4. Discussion
Loperamide, a peripherally acting opioid agonist of μ-opioid receptors in the intestine, inhibits the release of acetylcholine and slows intestinal peristalsis, making it a common model for inducing constipation in research.43,44 In this study, loperamide was employed to establish a murine model of constipation to explore the therapeutic effects of Li01 and PSH. Our findings demonstrate that the combination of Li01 and PSH significantly improved fecal water content, defecation frequency, GI transit rate, and altered levels of GI hormones. In particular, the combination treatment increased levels of SP and GAS, which promote GI motility, and decreased levels of VIP, which inhibits GI motility. Histological analysis further indicated that mice treated with Li01 and PSH had thicker muscle layers and healthier morphology of colonic microvilli compared to mice in the other groups. These findings suggest that the combined treatment of Li01 and PSH yields a superior preventative effect of constipation symptoms compared to treatment with Li01 or PSH, solely.Constipation is often associated with an altered gut microbiota composition.2 In our study, constipated mice showed significantly lower microbial diversity in their gut. However, mice treated with the combination of Li01 and PSH showed a modulated microbiota in their colonic contents. Specifically, the relative abundances of Bacteroides and Lachnospiraceae NK4A136 group were higher in the LOPLP group. Both of these bacterial families are known producers of SCFAs.45,46 Previous studies have shown that the abundance of Bacteroides can be increased by treatment with Lacticaseibacillus rhamnosus VHProbi M15 and dietary fiber inulin, which can relieve constipation.47,48 Similarly, Lachnospiraceae NK4A136 group has been reported to increase in abundance in constipated rats treated with Durio zibethinus rind polysaccharide.49
Our results also revealed that mice in the LOPP group had lower cecal concentrations of SCFAs (Fig. 3A–C), which have been shown to accelerate colonic transit and are typically lower in patients with slow-transit constipation.50,51 Similar results findings have been presented in another study in which PSH was shown to alleviate metabolic syndrome.52 There seems to be a dilution effect. Interestingly, compared to mice in the LOPP group, cecal concentrations of SCFAs, especially butyric acid, were higher among mice in the LOPLP group. Butyrate acid is known to have a potentially beneficial role in the treatment of functional constipation.53 We speculate that Li01 might be essential for the fermentation of PSH in the gut.
In patients suffering from constipation syndrome, reduced levels of total bile acids (BAs) and slower colonic transport are often observed.54 Consistent with this, our study found lower cecal BA levels in loperamide-treated mice. However, mice which were treated with Li01 and PSH showed higher levels of hyodeoxycholic acid (HDCA) and murideoxycholic acid (MDCA). These two BAs are non-12-OH-BAs, which have been shown to alleviate non-alcoholic fatty liver disease and exhibit anti-inflammatory properties.36,55,56 Correlation analysis indicated that the combined treatment of Li01 and PSH reshaped the gut microbiota under loperamide intervention, leading to changes in SCFA and BA concentrations.
Overall, our results demonstrate that the combined treatment of Li01 and PSH provides significant protection against constipation by modulating the composition and metabolic profile of the gut microbiota. Mice receiving the combination treatment exhibited higher defecation frequency, GI transit rates, and cecal concentrations of SCFAs and non-12-OH BAs compared to mice treated with PSH only, that Li01 and PSH together enhances gut motility.
We further investigated the effects of Li01 and PSH treatment on mice with antibiotic-disrupted gut microbiota. The combined treatment altered the microbiota composition in the mice, leading to lower abundance of Proteobacteria and higher abundance of Firmicutes compared to mice in the LOP group. Even after having their microbiota disrupted by antibiotics, mice treated with the Li01 and PSH combination treatment were protected from constipation, suggesting that the therapeutic effect is not solely dependent on the preexisting gut microbiota. Mice treated with Li01 and PSH displayed higher cecal concentrations of some SCFAs and BAs compared to mice in the LOP group, further indicating the beneficial effects of the combination treatment. Despite observed differences in gut microbiota modulation between mice in the experiments with and without antibiotic-disrupted microbiota, the Li01 and PSH combination treatment consistently resulted in elevated cecal concentrations of butyric acid levels and non-12-OH BA proportions and preventing constipation.
Mice in the LOPLP group had higher fecal BSH activity, but their liver genes expressing enzymes related to bile acid synthesis did not change significantly. Therefore, we speculate that the elevation of non-12-OH BA proportions might be more closely related to the microbiota than to the alteration of hepatic bile acid synthesis. However, this speculation needs to be proved by further experiments in the future.
Genome analysis of Li01 revealed functional genes with roles related to lipid and carbohydrate metabolism. Li01, like other Lactobacillus spp., can produce SCFAs57,58 and considerably reduce pH when cultivated in MRS medium. PSH, which is rich in soluble fibers such as arabinoxylans, can be fermented by probiotics to produce SCFAs59 and has shown anti-colitic properties and the ability to alleviate metabolic syndrome.52,60 In this study, we show in vitro that fermentation of PSH by Li01 results in changes in levels of metabolites such as fatty acids and amino acids, suggesting that combination treatment with Li01 and PSH has potential metabolic regulatory benefits.
5. Conclusion
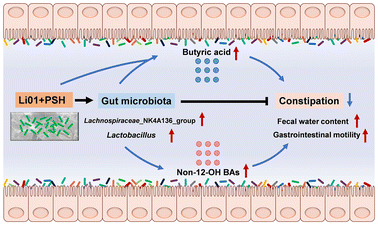
Treatment with Li01 and PSH effectively protects against loperamide-induced constipation in mice and modulates their gut microbiota and metabolism, regardless of whether the original microbiota is intact or disrupted. We propose that Li01 alleviates constipation through two primary mechanisms: (1) by directly fermenting PSH and various nutrients to produce short-chain fatty acids (SCFAs), and (2) by working synergistically with PSH to regulate gut microbiota, thereby increasing the concentration of butyric acid and the proportion of non-12-OH bile acids (BAs) (Fig. 7). Therefore, the combined treatment with Li01 and PSH work together synergistically to prevent constipation.However, the detailed mechanisms underlying this synergy require further exploration. For example, it is essential to identify other crucial metabolites produced when Li01 ferments PSH in vivo and to understand how these metabolites affect gastrointestinal motility in constipation. Additionally, investigating the key enzymes that Li01 utilizes to ferment dietary fiber could provide insights into the processes that facilitate the alleviation of constipation.
In summary, our study supports the concept that the probiotic Li01 and the dietary fiber prebiotic PSH function as a synbiotic, offering a promising approach for the prevention and treatment of constipation. Further research is needed to elucidate the specific metabolic pathways and interactions involved in this beneficial effect.
Author contributions
Lvwan Xu: data curation, investigation, methodology, writing – original draft. Bo Qiu: data curation, investigation, methodology, writing – original draft. Furong Ba: data curation. Shuobo Zhang: investigation. Shengyi Han: investigation. Hui Chen: methodology. Youhe Wu: methodology. Wang Gao: methodology. Siyuan Xie: formal analysis. Yanfei Chen: methodology, funding acquisition. Shiman Jiang: methodology. Jingyi Zhang: methodology. Yating Li: methodology, funding acquisition. Björn Berglund: writing – review and editing. Mingfei Yao: conceptualization, project administration, funding acquisition, writing – review and editing. Lanjuan Li: conceptualization, project administration, funding acquisition, writing – review and editing.Data availability
Data from this study are available to readers upon reasonable requests.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The work was supported by the Fundamental Research Funds for the Central Universities (2022ZFJH003), the National Key Research and Development Program of China (2022YFA1303801; 2022YFC3602003), Zhejiang Provincial Natural Science Foundation of China (LQ22H030013, 2022-KY1-001-039).References
- A. E. Bharucha and B. E. Lacy, Mechanisms, Evaluation, and Management of Chronic Constipation, Gastroenterology, 2020, 158, 1232–1249 CrossRef CAS PubMed.
- M. H. Vriesman, I. J. N. Koppen, M. Camilleri, C. Di Lorenzo and M. A. Benninga, Management of functional constipation in children and adults, Nat. Rev. Gastroenterol. Hepatol., 2020, 17, 21–39 CrossRef PubMed.
- Z. D. Yang, S. M. Ye, Z. M. Xu, H. H. Su, X. Tian, B. Han, B. C. Shen, Q. F. Liao, Z. Y. Xie and Y. J. Hong, Dietary synbiotic ameliorates constipation through the modulation of gut microbiota and its metabolic function, Food Res. Int., 2021, 147, 110569 CrossRef CAS PubMed.
- S. S. Rao, K. Rattanakovit and T. Patcharatrakul, Diagnosis and management of chronic constipation in adults, Nat. Rev. Gastroenterol. Hepatol., 2016, 13, 295–305 CrossRef CAS PubMed.
- M. Camilleri, A. C. Ford, G. M. Mawe, P. G. Dinning, S. S. Rao, W. D. Chey, M. Simren, A. Lembo, T. M. Young-Fadok and L. Chang, Chronic constipation, Nat. Rev. Dis. Primers, 2017, 3, 17095 CrossRef PubMed.
- B. Waclawikova, P. Cesar Telles de Souza, M. Schwalbe, C. G. Neochoritis, W. Hoornenborg, S. A. Nelemans, S. J. Marrink and S. El Aidy, Potential binding modes of the gut bacterial metabolite, 5-hydroxyindole, to the intestinal L-type calcium channels and its impact on the microbiota in rats, Gut Microbes, 2023, 15, 2154544 CrossRef PubMed.
- L. O’Brien, T. J. Wilkinson, C. Frampton, R. B. Gearry and C. Wall, A systematic review and meta-analysis of the dietary fiber menu provision and consumption for older adults living in residential care facilities, Am. J. Clin. Nutr., 2024, 120, 431–441 CrossRef PubMed.
- L. Huang, Q. Zhu, X. Qu and H. Qin, Microbial treatment in chronic constipation, Sci. China: Life Sci., 2018, 61, 744–752 CrossRef PubMed.
- E. Dimidi, S. Christodoulides, S. M. Scott and K. Whelan, Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation, Adv. Nutr., 2017, 8, 484–494 CrossRef CAS PubMed.
- Q. Zhao, Y. Y. Chen, D. Q. Xu, S. J. Yue, R. J. Fu, J. Yang, L. M. Xing and Y. P. Tang, Action Mode of Gut Motility, Fluid and Electrolyte Transport in Chronic Constipation, Front. Pharmacol., 2021, 12, 630249 CrossRef CAS PubMed.
- H. L. Tian, C. Ye, B. Yang, J. Q. Cui, Z. J. Zheng, C. Y. Wu, S. L. Zhou, X. Q. Lv, N. Qin, H. L. Qin, N. Li and Q. Y. Chen, Gut Metagenome as a Potential Diagnostic and Predictive Biomarker in Slow Transit Constipation, Front. Med., 2022, 8, 777961 CrossRef PubMed.
- M. Guo, J. Yao, F. Yang, W. Liu, H. Bai, J. Ma, X. Ma, J. Zhang, Y. Fang, Y. Miao, J. Sun, Y. Zhang and H. Zhao, The composition of intestinal microbiota and its association with functional constipation of the elderly patients, Future Microbiol., 2020, 15, 163–175 CrossRef CAS PubMed.
- D. J. Morrison and T. Preston, Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism, Gut Microbes, 2016, 7, 189–200 CrossRef PubMed.
- L. Mancin, G. D. Wu and A. Paoli, Gut microbiota-bile acid-skeletal muscle axis, Trends Microbiol., 2023, 31, 254–269 CrossRef CAS PubMed.
- Q. Zheng, Y. T. Li, J. L. Ni, Y. Huang, J. J. Wu, X. Xu, G. P. Sheng and L. J. Li, Causality Between Gut Microbiota and Inflammatory Bowel Disease: A Bidirectional Mendelian Randomization Study, Infect. Microbes Dis., 2024, 6, 93–99 CAS.
- F. N. Bian, D. Yan, X. P. Wu and C. Yang, A Biological Perspective of TLR8 Signaling in Host Defense and Inflammation, Infect. Microbes Dis., 2023, 5, 44–55 CAS.
- S. C. James, K. Fraser, W. Young, P. E. Heenan, R. B. Gearry, J. I. Keenan, N. J. Talley, S. A. Joyce, W. C. McNabb and N. C. Roy, Concentrations of Fecal Bile Acids in Participants with Functional Gut Disorders and Healthy Controls, Metabolites, 2021, 11, 612 CrossRef CAS PubMed.
- R. L. Pan, L. L. Wang, X. P. Xu, Y. Chen, H. J. Wang, G. Wang, J. X. Zhao and W. Chen, Crosstalk between the Gut Microbiome and Colonic Motility in Chronic Constipation: Potential Mechanisms and Microbiota Modulation, Nutrients, 2022, 14, 3704 CrossRef CAS PubMed.
- H. Abrahamsson, A. M. Ostlund-Lindqvist, R. Nilsson, M. Simren and P. G. Gillberg, Altered bile acid metabolism in patients with constipation-predominant irritable bowel syndrome and functional constipation, Scand. J. Gastroenterol., 2008, 43, 1483–1488 CrossRef CAS PubMed.
- Y. D. Fan, C. Xu, L. L. Xie, Y. Wang, S. Zhu, J. R. An, Y. W. Li, Z. K. Tian, Y. Q. Yan, S. Yu, H. Z. Liu, B. T. Jia, Y. Y. Wang, L. Wang, L. Yang and Y. H. Bian, Abnormal bile acid metabolism is an important feature of gut microbiota and fecal metabolites in patients with slow transit constipation, Front. Cell. Infect. Microbiol., 2022, 12, 956528 CrossRef CAS PubMed.
- Y. Huang, Y. Guo, X. Li, Y. Xiao, Z. Wang, L. Song and Z. Ren, Effects of Lactiplantibacillus plantarum GUANKE on Diphenoxylate-Induced Slow Transit Constipation and Gut Microbiota in Mice, Nutrients, 2023, 15, 3741 CrossRef CAS PubMed.
- A. Ibarra, M. Latreille-Barbier, Y. Donazzolo, X. Pelletier and A. C. Ouwehand, Effects of 28-day Bifidobacterium animalis subsp. lactis HN019 supplementation on colonic transit time and gastrointestinal symptoms in adults with functional constipation: A double-blind, randomized, placebo-controlled, and dose-ranging trial, Gut Microbes, 2018, 9, 236–251 CrossRef CAS PubMed.
- T. Ma, N. Yang, Y. Xie, Y. Li, Q. Xiao, Q. Li, H. Jin, L. Zheng, Z. Sun, K. Zuo, L. Y. Kwok, H. Zhang, N. Lu and W. Liu, Effect of the probiotic strain, Lactiplantibacillus plantarum P9, on chronic constipation: A randomized, double-blind, placebo-controlled study, Pharmacol. Res., 2023, 191, 106755 CrossRef CAS PubMed.
- C. Patch, A. J. Pearce, M. Cheng, R. Boyapati and J. T. Brenna, Bacillus Subtilis (BG01–4(TM)) Improves Self-Reported Symptoms for Constipation, Indigestion, and Dyspepsia: A Phase 1/2A Randomized Controlled Trial, Nutrients, 2023, 15, 4490 CrossRef PubMed.
- B. Qiu, L. Zhu, S. Zhang, S. Han, Y. Fei, F. Ba, B. Berglund, L. Li and M. Yao, Prevention of Loperamide-Induced Constipation in Mice and Alteration of 5-Hydroxytryotamine Signaling by Ligilactobacillus salivarius Li01, Nutrients, 2022, 14, 4083 CrossRef CAS PubMed.
- M. Belorio and M. Gomez, Psyllium: a useful functional ingredient in food systems, Crit. Rev. Food Sci. Nutr., 2022, 62, 527–538 CrossRef CAS PubMed.
- J. Jalanka, G. Major, K. Murray, G. Singh, A. Nowak, C. Kurtz, I. Silos-Santiago, J. M. Johnston, W. M. de Vos and R. Spiller, The Effect of Psyllium Husk on Intestinal Microbiota in Constipated Patients and Healthy Controls, Int. J. Mol. Sci., 2019, 20, 433 CrossRef PubMed.
- S. K. Gill, M. Rossi, B. Bajka and K. Whelan, Dietary fibre in gastrointestinal health and disease, Nat. Rev. Gastroenterol. Hepatol., 2021, 18, 101–116 CrossRef CAS PubMed.
- S. W. Chey, W. D. Chey, K. Jackson and S. Eswaran, Exploratory Comparative Effectiveness Trial of Green Kiwifruit, Psyllium, or Prunes in US Patients With Chronic Constipation, Am. J. Gastroenterol., 2021, 116, 1304–1312 CrossRef CAS PubMed.
- E. Jovanovski, S. Yashpal, A. Komishon, A. Zurbau, S. Blanco Mejia, H. V. T. Ho, D. Li, J. Sievenpiper, L. Duvnjak and V. Vuksan, Effect of psyllium (Plantago ovata) fiber on LDL cholesterol and alternative lipid targets, non-HDL cholesterol and apolipoprotein B: a systematic review and meta-analysis of randomized controlled trials, Am. J. Clin. Nutr., 2018, 108, 922–932 CrossRef PubMed.
- Y. Sun, S. Zhang, Q. Nie, H. He, H. Tan, F. Geng, H. Ji, J. Hu and S. Nie, Gut firmicutes: Relationship with dietary fiber and role in host homeostasis, Crit. Rev. Food Sci. Nutr., 2023, 63, 12073–12088 CrossRef PubMed.
- H. Gao, J. J. Wen, J. L. Hu, Q. X. Nie, H. H. Chen, T. Xiong, S. P. Nie and M. Y. Xie, Polysaccharide from fermented Momordica charantia L. with Lactobacillus plantarum NCU116 ameliorates type 2 diabetes in rats, Carbohydr. Polym., 2018, 201, 624–633 CrossRef CAS PubMed.
- H. J. Lim, E. H. Lee, Y. Yoon, B. Chua and A. Son, Portable lysis apparatus for rapid single-step DNA extraction of Bacillus subtilis, J. Appl. Microbiol., 2016, 120, 379–387 CrossRef CAS PubMed.
- S. Han, K. Wang, J. Shen, H. Xia, Y. Lu, A. Zhuge, S. Li, B. Qiu, S. Zhang, X. Dong, M. Yao and L. Li, Probiotic Pediococcus pentosaceus Li05 Improves Cholestasis through the FXR-SHP and FXR-FGF15 Pathways, Nutrients, 2023, 15, 4864 CrossRef CAS PubMed.
- J. Xia, W. Guo, M. Hu, X. Jin, S. Zhang, B. Liu, H. Qiu, K. Wang, A. Zhuge, S. Li, Z. Ji, L. Li and K. Xu, Resynchronized rhythmic oscillations of gut microbiota drive time-restricted feeding induced nonalcoholic steatohepatitis alleviation, Gut Microbes, 2023, 15, 2221450 CrossRef PubMed.
- J. Kuang, J. Wang, Y. Li, M. Li, M. Zhao, K. Ge, D. Zheng, K. C. P. Cheung, B. Liao, S. Wang, T. Chen, Y. Zhang, C. Wang, G. Ji, P. Chen, H. Zhou, C. Xie, A. Zhao, W. Jia, X. Zheng and W. Jia, Hyodeoxycholic acid alleviates non-alcoholic fatty liver disease through modulating the gut-liver axis, Cell Metab., 2023, 35, 1752–1766 CrossRef CAS PubMed.
- S. Li, A. Zhuge, H. Chen, S. Han, J. Shen, K. Wang, J. Xia, H. Xia, S. Jiang, Y. Wu and L. Li, Sedanolide alleviates DSS-induced colitis by modulating the intestinal FXR-SMPD3 pathway in mice, J. Adv. Res., 2024, S2090–1232(24)00128-0, DOI:10.1016/j.jare.2024.03.026.
- E. Sinclair, D. K. Trivedi, D. Sarkar, C. Walton-Doyle, J. Milne, T. Kunath, A. M. Rijs, R. M. A. de Bie, R. Goodacre, M. Silverdale and P. Barran, Metabolomics of sebum reveals lipid dysregulation in Parkinson's disease, Nat. Commun., 2021, 12, 1592 CrossRef CAS PubMed.
- L. L. Qin, M. Yu, P. Yang and Z. M. Zou, The rhizomes of Atractylodes macrocephala relieve loperamide-induced constipation in rats by regulation of tryptophan metabolism, J. Ethnopharmacol., 2023, 117637, DOI:10.1016/j.jep.2023.117637.
- A. V. Zholos, M. I. Melnyk and D. O. Dryn, Molecular mechanisms of cholinergic neurotransmission in visceral smooth muscles with a focus on receptor-operated TRPC4 channel and impairment of gastrointestinal motility by general anaesthetics and anxiolytics, Neuropharmacology, 2024, 242, 109776 CrossRef CAS PubMed.
- J. Yu, H. Guo, M. Sun, C. Jiang, S. Jiang, G. Mu, Y. Tuo and P. Gao, Milk fermented by combined starter cultures comprising three Lactobacillus strains exerts an alleviating effect on loperamide-induced constipation in BALB/c mice, Food Funct., 2023, 14, 5264–5276 RSC.
- B. Li, M. Li, Y. Luo, R. Li, W. Li and Z. Liu, Engineered 5-HT producing gut probiotic improves gastrointestinal motility and behavior disorder, Front. Cell. Infect. Microbiol., 2022, 12, 1013952 CrossRef CAS PubMed.
- M. Ali, A. Mujahid, C. P. Bulathsinghala and S. Surani, Cardiac Arrhythmia Secondary to Loperamide Abuse and Toxicity, Cureus, 2020, 12, e6936 Search PubMed.
- O. Yagasaki, H. Suzuki and Y. Sohji, Effects of loperamide on acetylcholine and prostaglandin release from isolated guinea pig ileum, Jpn. J. Pharmacol., 1978, 28, 873–882 CrossRef CAS PubMed.
- D. Lu, Y. Pi, H. Ye, Y. Wu, Y. Bai, S. Lian, D. Han, D. Ni, X. Zou, J. Zhao, S. Zhang, B. Kemp, N. Soede and J. Wang, Consumption of Dietary Fiber with Different Physicochemical Properties during Late Pregnancy Alters the Gut Microbiota and Relieves Constipation in Sow Model, Nutrients, 2022, 14, 2511 CrossRef CAS PubMed.
- C. Yan, S. H. Huang, H. F. Ding, E. Kwek, J. H. Liu, Z. X. Chen, K. Y. Ma and Z. Y. Chen, Adverse effect of oxidized cholesterol exposure on colitis is mediated by modulation of gut microbiota, J. Hazard. Mater., 2023, 459, 132057 CrossRef CAS PubMed.
- S. Cheng, H. Cui, J. Zhang, Q. Wang and Z. Duan, Probiotic potential of Lacticaseibacillus rhamnosus VHProbi M15 on sucralfate-induced constipation in mice, Sci. Rep., 2024, 14, 1131 CrossRef CAS PubMed.
- H. Zou, H. Gao, Y. Liu, Z. Zhang, J. Zhao, W. Wang, B. Ren and X. Tan, Dietary inulin alleviated constipation induced depression and anxiety-like behaviors: Involvement of gut microbiota and microbial metabolite short-chain fatty acid, Int. J. Biol. Macromol., 2024, 259, 129420 CrossRef CAS PubMed.
- H. Jiang, J. Dong, S. Jiang, Q. Liang, Y. Zhang, Z. Liu, C. Ma, J. Wang and W. Kang, Effect of Durio zibethinus rind polysaccharide on functional constipation and intestinal microbiota in rats, Food Res. Int., 2020, 136, 109316 CrossRef CAS PubMed.
- Q. Chen, D. Chen, X. Gao, Y. Jiang, T. Yu, L. Jiang and Y. Tang, Association between fecal short-chain fatty acid levels and constipation severity in subjects with slow transit constipation, Eur. J. Gastroenterol. Hepatol., 2024, 36, 394–403 CrossRef CAS PubMed.
- J. R. Grider and B. E. Piland, The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF, Am. J. Physiol.: Gastrointest. Liver Physiol., 2007, 292, G429–G437 CrossRef CAS PubMed.
- A. Bretin, B. S. Yeoh, V. L. Ngo, L. Reddivari, M. Pellizzon, M. Vijay-Kumar and A. T. Gewirtz, Psyllium fiber protects mice against western diet-induced metabolic syndrome via the gut microbiota-dependent mechanism, Gut Microbes, 2023, 15, 2221095 CrossRef PubMed.
- A. Pituch, J. Walkowiak and A. Banaszkiewicz, Butyric acid in functional constipation, Przegl. Gastroenterol., 2013, 8, 295–298 Search PubMed.
- P. Vijayvargiya, I. Busciglio, D. Burton, L. Donato, A. Lueke and M. Camilleri, Bile Acid Deficiency in a Subgroup of Patients With Irritable Bowel Syndrome With Constipation Based on Biomarkers in Serum and Fecal Samples, Clin. Gastroenterol. Hepatol., 2018, 16, 522–527 CrossRef CAS PubMed.
- J. Zhong, X. He, X. Gao, Q. Liu, Y. Zhao, Y. Hong, W. Zhu, J. Yan, Y. Li, Y. Li, N. Zheng, Y. Bao, H. Wang, J. Ma, W. Huang, Z. Liu, Y. Lyu, X. Ke, W. Jia, C. Xie, Y. Hu, L. Sheng and H. Li, Hyodeoxycholic acid ameliorates nonalcoholic fatty liver disease by inhibiting RAN-mediated PPARalpha nucleus-cytoplasm shuttling, Nat. Commun., 2023, 14, 5451 CrossRef CAS PubMed.
- J. Li, Y. Chen, R. Li, X. Zhang, T. Chen, F. Mei, R. Liu, M. Chen, Y. Ge, H. Hu, R. Wei, Z. Chen, H. Fan, Z. Zeng, Y. Deng, H. Luo, S. Hu, S. Cai, F. Wu, N. Shi, Z. Wang, Y. Zeng, M. Xie, Y. Jiang, Z. Chen, W. Jia and P. Chen, Gut microbial metabolite hyodeoxycholic acid targets the TLR4/MD2 complex to attenuate inflammation and protect against sepsis, Mol. Ther., 2023, 31, 1017–1032 CrossRef CAS PubMed.
- H. Yan, Y. Zhang, X. Lin, J. Huang, F. Zhang, C. Chen, H. Ren, S. Zheng, J. Yang and S. Hui, Resveratrol improves diabetic kidney disease by modulating the gut microbiota-short chain fatty acids axis in db/db mice, Int. J. Food Sci. Nutr., 2024, 1–13, DOI:10.1080/09637486.2024.2303041.
- Z. Liang, Y. Hao, L. Yang, P. Yuan, W. Kang, T. Liang, B. Gu and B. Dong, The potential of Klebsiella and Escherichia-Shigella and amino acids metabolism to monitor patients with postmenopausal osteoporosis in northwest China, BMC Microbiol., 2023, 23, 199 CrossRef CAS PubMed.
- S. C. Choi, B. J. Kim, P. L. Rhee, D. K. Chang, H. J. Son, J. J. Kim, J. C. Rhee, S. I. Kim, Y. S. Han, K. H. Sim and S. N. Park, Probiotic Fermented Milk Containing Dietary Fiber Has Additive Effects in IBS with Constipation Compared to Plain Probiotic Fermented Milk, Gut Liver, 2011, 5, 22–28 CrossRef PubMed.
- A. Bretin, J. Zou, B. San Yeoh, V. L. Ngo, S. Winer, D. A. Winer, L. Reddivari, M. Pellizzon, W. A. Walters, A. D. Patterson, R. Ley, B. Chassaing, M. Vijay-Kumar and A. T. Gewirtz, Psyllium Fiber Protects Against Colitis Via Activation of Bile Acid Sensor Farnesoid X Receptor, Cell. Mol. Gastroenterol. Hepatol., 2023, 15, 1421–1442 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo04444d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |