Open Access Article
Open Access ArticleEffects of germination time on the structure, functionality, flavour attributes, and in vitro digestibility of green Altamura lentils (Lens culinaris Medik.) flour
Annalisa
Romano
 *,
Lucia
De Luca
and
Raffaele
Romano
*,
Lucia
De Luca
and
Raffaele
Romano
Department of Agricultural Sciences, University of Naples Federico II, Via Università 100, 80055, Portici (Naples), Italy. E-mail: annalisa.romano@unina.it
First published on 26th February 2024
Abstract
There has been an increase in the use of adoptable bioprocessing methods for the development of high-quality leguminous ingredients. The potential use of germinated green Altamura lentils as a food ingredient is closely related to the resulting properties. The objective of this study was to evaluate the impact of three germination times − 0 (C), 24 (G) and 48 (H) hours − on the physicochemical, microstructural, flavour, functional, and nutritional features of lentil flour samples (CF, GF and HF). Lentil flour samples were obtained by grinding both whole green seeds (C) and germinated seeds (G and H), and then sifting them to obtain a particle size < 300 μm. The germinated samples – GF (24 h) and HF (48 h) – exhibited differences (P < 0.05) in the physicochemical and bioactive properties of CF (control). Similarly, compared with those in the control sample, the total starch, amylose and total phenolic contents in the GF and HF samples decreased, while the protein content increased (p < 0.05). A decrease in the presence of intact starch granules was observed via SEM in the germinated samples. The germination time had a significant (P < 0.05) effect on the colour indices, L*, a*, and b* of the samples. Flavour attributes were significantly influenced by the germination time. Overall, a total of 14 (CF) and 17 (GF and HF) aromatic compounds were identified. The technological characteristics of the CF, GF and HF dough samples were studied using a Brabender farinograph. Germination time affects the flour properties, leading to a significant decrease in farinographic parameters such as water absorption (WA), dough development time (DT), and dough stability (DS) and an increase in the degree of dough weakening (DOS). Differential scanning calorimetry was employed to examine the gelatinization transition of the samples. Germination strongly influenced all the thermal properties of the samples. It also had a significant impact on the in vitro starch digestibility, starch fraction and glycaemic index (eGI) of the samples. In particular, the eGI of germinated lentils was lower than that of the CF. In conclusion, the germination time could be a key factor modulating some crucial lentil flour properties.
Introduction
The value-added processing of lentils (Lens culinaris Medik.) is of growing interest for the development of new food ingredients, owing to their nutritional composition and promising technological properties.1The green Altamura lentils produced in Altamura (Bari, Apulia, Southern Italy) are a Protected Geographical Indication (P.G.I.),2 and are quite rich in components essential for good human health3 such as carbohydrates (50 g per 100 g), proteins (21–26 g per 100 g), dietary fibres (8.4 g per 100 g), minerals, vitamins (mainly vitamin B3/niacin) and phenolic compounds.4 In general, green lentils are very popular in the United States and Europe.5
From lentil seeds, it is possible to produce almost 90% flour yield, a value similar to that of wheat flour yield. Lentil flour has been gradually used in bakery (bread, cake, and crackers), extruded (pasta and snacks) and other products (dressings, soups, and dairy and meat products) in recent years. Its use in food formulations is gaining attention from food industries and popularity among consumers due to its excellent and balanced nutritional composition and the absence of gluten.1,6,7 In particular, food industries are very much interested in the formulation of novel lentil-based ingredients to satisfy the increasing demands of vegetarians and vegans and, more generally, for consumers who are aware of the importance of a healthy diet.
The success of using lentil flour as a food ingredient is closely related to its nutritional, physicochemical, functional and flavour properties. For example, the addition of lentil flour can introduce technological problems, off flavours and novel allergens.1 For these reasons, lentil flour is usually subjected to different pre-processing and processing methods (e.g., cooking, fermentation, soaking, germination, or mechanical methods such as dehulling and milling), ranging from more to less intensive, to obtain the desired functionality and characteristics.8–12
Germination is one of the most common, green and effective processes for efficiently modifying legumes in terms of nutritional and functional properties, processing time, and/or economy13–15 and it is also a great source of nutrients available in the food industry. Therefore, in recent years, the preference for this process has been growing. Germination leads to significant changes in the nutritional and physiological characteristics of legumes by activating endogenous enzymes, removing large amounts of molecular compounds, such as proteins and starch, and consequently improving plant bioavailability.15,16 It also plays an important role in reducing non-nutritive compounds in legumes and increasing the levels of available carbohydrates, dietary fibre, and other components.17,18 Several studies carried out on legumes have shown an increase in antioxidant capacity in relation to their polyphenolic composition, for instance the germination of lupines, peas and edible seeds.19,20
In addition, germination has been linked to the reduction of the formation of off-flavours in legumes.21–24 The study of the change in the aromatic profile of lentils during germination is crucial for the further development of lentil flour as a functional food ingredient, in fact, an acceptable flavour is a necessary feature of any food.23 Like other legumes, lentils may have undesirable flavours (e.g., beany and bitter flavours) due to the presence of some of their volatile constituents.21,22 The beany flavours in raw legume flour samples limit the use of legumes as functional food ingredients.25,26 These flavours are mainly derived from the degradation of amino acids and hydrolytic and oxidative degradation of lipids.27,28 Several studies have reported the positive impact of germination on the distinct beany flavour of some pulse flours (lupin and soybean) and baked food products.29 Therefore, germination could be a green engineering method for changing the composition of lentils and thus modifying/improving the quality of the resulting flour. Details on this approach, however, are scarce. It is thus interesting to understand the impact of germination time on the composition of lentil flour samples to provide additional knowledge on the functionality of these legumes. Such knowledge will contribute to increasing consumption worldwide, as consumers currently seek more natural and healthy products as they are particularly concerned about the effect that foods can have on their health.
The objective of this work was to analyse the impact of germination time on the microstructural, chemical, nutritional, aromatic, and technological properties of whole lentil flour obtained from green Altamura lentils to expand its technological potential for the preparation of high-quality foods (e.g., bakery and gluten-free bakery products).
Materials and methods
Materials
The green lentil seeds (Lens culinaris Medik.) (C) used for this study were obtained directly from producers (Terre di Altamura, Bari, Apulia, Italy) in the beginning of the 2021 crop year.The digestive enzymes: thermostable α-amylase and amyloglucosidase were purchased from Megazyme (Megazyme International, Ireland), and pepsin from porcine gastric mucosa, pancreatin from porcine pancreas and the bile extract were all obtained from Sigma Aldrich (St Louis, MO, USA). The chemicals used in this study were of analytical grade.
Sample preparation
The lentil seeds were washed with tap water and sterilized in a 0.07% (w/v) sodium hypochlorite solution with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (w/v) ratio of seeds to distilled water for 30 min. Then, the seeds were washed several times. Afterwards, they were soaked in a 1
3 (w/v) ratio of seeds to distilled water for 30 min. Then, the seeds were washed several times. Afterwards, they were soaked in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (w/v) ratio of seeds to distilled water overnight in a dark chamber under ambient laboratory conditions (22–24 °C). Finally, the water was drained and the seed samples were ready to germinate under wet cloth under the dark conditions for one (G) or two (H) days. The layer of seeds was sprayed with distilled water at a water-to-seed ratio (w/w) of 1
3 (w/v) ratio of seeds to distilled water overnight in a dark chamber under ambient laboratory conditions (22–24 °C). Finally, the water was drained and the seed samples were ready to germinate under wet cloth under the dark conditions for one (G) or two (H) days. The layer of seeds was sprayed with distilled water at a water-to-seed ratio (w/w) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 every 24 hours.30,31
8 every 24 hours.30,31
Lentil flour samples (CF, GF and HF) were obtained by drying and grinding the undecorticated lentil seeds – C (control, 0 h), G (24 h) and H (48 h), using a laboratory mill (mod. 3100, Perten Instruments Ab, Finland) and then sifted to obtain a particle size <300 μm (Giuliani sifter, Turin, Italy). The flour samples were packed in airtight polypropylene containers at 4 ± 3 °C.
Flour microstructural analysis
The samples were dried at the critical point and coated with gold particles. The microstructure of the samples was examined by scanning electron microscopy (SEM) (LEO EVO 40, Zeiss, Germany) with a 20 kV acceleration voltage and a magnification of ×2000.Chemical characterization
The moisture content of each sample was determined.32 Three samples, weighing approximately 3 g, were dried for 24 h at 105 °C. The samples were removed from the oven and immediately placed in a desiccator within 30 min prior to weighing after cooling. The weight of dried samples was subtracted from the respective initial weight. The results were calculated as the percentage of water per sample weight (%).The total protein content was determined by the Kjeldahl method via the quantification of total nitrogen.33 The factor Nx6.25 was applied to convert total nitrogen into protein content.34
The soluble nitrogen content (%) was determined by following a previous method35 with modifications. Briefly, the sample was weighed (0.4 g) and 30 mL of deionized water was added. The pH was adjusted to 5 by the addition of HCl (0.1 M). The mixture was shaken for 1 h at room temperature to monitor the pH. Subsequently, the mixture was centrifuged at 7000g for 30 min and then filtered using Whatman no. 1 paper. Finally, the soluble nitrogen was determined by following the same procedure used for the determination of total nitrogen.
Total starch (TS) (g per 100 g) was determined using an enzymatic assay kit (Total Starch Assay Kit, Megazyme International Ireland).36 The amylose content in flour samples (CF, GF and HF) was measured using an enzymatic assay kit (Amylose/Amylopectin Assay Kit, Megazyme Ltd, Bray, Ireland) based on the specific precipitation of amylopectin by concanavalin-A lectin. The amylose content (%) was directly calculated by following the specific Megazyme equation based on the measured absorbance values, which were read at 510 nm by means of Jasco, UV-spectrophotometer, V-550 UV/VIS Spectrometer-PerkinElmer, Lecco, Italy. The average values of two measurements were calculated for each flour sample.
Polyphenol extraction was performed by following previous methods.37 Briefly, lentil powder (1 g) was mixed with 20 mL methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (80
water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v). The mixture was left for 2 h at room temperature. Then, the extracts were centrifuged at 6000 rpm for 10 min. The supernatant was filtered through a 0.45 μm membrane filter. To determine the total polyphenol content (TPC) of the lentils, the Folin–Ciocalteu reagent was used. To 400 μL extract, 1 mL Folin–Ciocalteu reagent was added and the mixture was vortexed. Subsequently, 10 mL deionized water and 3 mL 20% sodium carbonate (Na2CO3) were added and incubated for 1 h in the dark. The total phenol content was detected at 750 nm. A calibration curve was generated using gallic acid (0–500 mg mL−1) as the standard. The results are expressed as mg gallic acid equivalent/100 g (mg GAE/100 g).
20, v/v). The mixture was left for 2 h at room temperature. Then, the extracts were centrifuged at 6000 rpm for 10 min. The supernatant was filtered through a 0.45 μm membrane filter. To determine the total polyphenol content (TPC) of the lentils, the Folin–Ciocalteu reagent was used. To 400 μL extract, 1 mL Folin–Ciocalteu reagent was added and the mixture was vortexed. Subsequently, 10 mL deionized water and 3 mL 20% sodium carbonate (Na2CO3) were added and incubated for 1 h in the dark. The total phenol content was detected at 750 nm. A calibration curve was generated using gallic acid (0–500 mg mL−1) as the standard. The results are expressed as mg gallic acid equivalent/100 g (mg GAE/100 g).
Colour characteristics of flour samples
Colorimetric indices (L*, a*, b*, and ΔE) of flour samples were measured with an electronic visual analyser (IRIS visual analyser, Alpha MOS, Toulouse, France). The chromatic coordinates (L, a, and b) are reported as the average of three measurements for each sample.From the parameters determined, the total colour difference (ΔE) was calculated using the following equation:
| ΔE = [(ΔL)2 + (Δa)2 + (Δb)2]1/2 |
Aromatic profile of lentils
Volatile organic compounds (VOCs) were extracted from samples (CF, GF and HF) by following a previous method28 with modifications. Briefly, 2 g of sample was weighed into a 20 mL vial and 5 mL saturated NaCl solution was added. The vial was placed in a thermal bath at a temperature of 60 °C for 10 min. Subsequently, the fibre DVB/CAR/PDMS (50/30 μm layer of divinylbenzene/carboxen/polydimethylsiloxane) was inserted for 50 min in the vials.Subsequently, the SPME fibre was introduced directly into the GC injector, where the thermal desorption of the analytes was performed at 250 °C for 3 min. A 6890 N GC system equipped with a 5973 mass detector was used. VOCs were separated on a 30 m × 0.250 mm capillary column coated with a 0.25 μm polymer of 5% diphenyl 95% dimethylpolysiloxane. Splitless injection was used for the samples.
The oven temperature was held at 40 °C for 5 min and increased from 40 °C to 85 °C at 45 °C min−1, 85 to 200 °C at 9 °C min−1 and 200 to 250 to 45 °C min−1. The temperature was maintained at 250 °C for 3 min. The injection source and ion temperatures were 250 and 230 °C, respectively. Helium was used as the carrier gas at a flow rate of 2 mL min−1. The ionizing electron energy was set to 70 eV and the scanned mass range was set to 40–450 amu in full scan acquisition mode.
Compounds were identified by comparing the mass spectral fragmentation patterns with the spectral data from the NIST Atomic Spectra Database version 1.6 and the retention indices with those reported in the literature. The relative content of VOCs was calculated on the basis of peak area ratios.
Farinographic analysis of samples
The farinograph properties of the control (CF) and germinated (GF and HF) samples were investigated using a Brabender farinograph (Type AT, Brabender OHG, Duisburg, Germany), fitted with a 50 g mixing bowl. Water absorption (WA, percentage of water required to reach a standard dough consistency of 500 ± 20 arbitrary Brabender Units, BU), dough development time (DDT), dough stability (DS) and degree of softening (DOS) were determined and the results are expressed as the average value of three replicates for each sample.38The results are expressed as the means and standard deviations of at least three independent experiments.
In vitro starch digestibility of flour samples
The rapid digestion of starch (RDS), slow digestion of starch (SDS) and the expected glycaemic index (eGI) were determined by different enzymatic methods on flour samples (CF, GF and HF).Specifically, the RDS and SDS contents as well as the eGI were determined using an enzymatic assay kit (Resistant Starch Assay Kit, Megazyme Ltd, Bray, Ireland) with minor modifications.39,40 The RDS was the percentage of total starch hydrolysed within 30 min of incubation in a shaking water bath (200 strokes per min, horizontal agitation) at 37 °C.40 SDS was the percentage of total starch hydrolysed within 30 and 120 min of incubation under the same conditions. The in vitro digestion kinetics was calculated in accordance with the conventional procedure.41
Statistical analysis
All the experimental results are reported as means and standard deviations of at least three independent experiments. All the data obtained were statistically analysed by one-way ANOVA using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). Differences between samples were evaluated by Duncan's test at a significance level of P < 0.05.Results and discussion
Microstructural properties of lentil flour
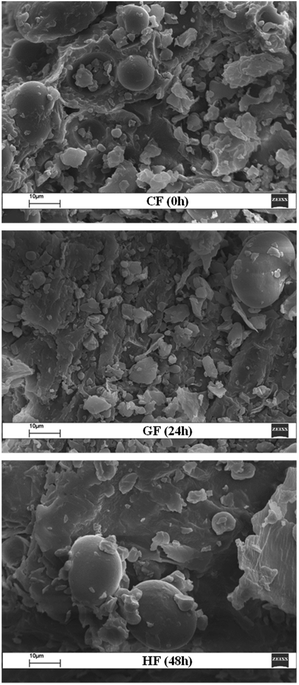
Scanning electron microscopy (SEM) was used to determine the impact of germination time on the microstructure of the lentil flour samples. The SEM images of the flour samples, CF (0 h), GF (24 h) and HF (48 h), are shown in Fig. 1. SEM revealed that the germinated flour samples (GF and HF) had similar microstructures (i.e., starch granule and protein matrix structure) to those of the control (CF), with still visible intact starch granule structures although covered by protein clusters. In particular, the starch granules in all flour samples had an oval shape with heterogeneous sizes ranging from 10 to 30 μm in length and from 10 to 20 μm in width and had a smooth surface, holding bodies or fragments of the protein matrix and/or fibre42 attached to their surface.9,43,44 The size of the protein body granules, which vary from ovoid to spherical, is generally smaller than that of lentil starch granules when observed via SEM, as reported by other workers.13,45 The obvious changes caused by germination included a decrease of intact starch granules, detachment of the granules from the protein and fibre networks, and the presence of free protein wedges in the flour samples (Fig. 1). These results are very important, because other properties and in vitro starch digestibility are significantly influenced by the structure resulting from germination.Chemical composition of the lentil flour samples
Table 1 presents the moisture content, total protein and nitrogen content, soluble nitrogen content, total starch and amylose content, and total phenolic content of the lentil flour samples.| Parameters | CF (0 h) | GF (24 h) | HF (48 h) |
|---|---|---|---|
| a–cDifferent letters in the same row indicate significant differences (P <0.05). | |||
| Moisture content (%) | 7.71 ± 0.51a | 7.91 ± 0.57a | 8.90 ± 0.31b |
| Proteins (%) | 21.19 ± 0.51a | 25.88 ± 0.98b | 26.87 ± 0.01b |
| Soluble nitrogen (%) | 2.65 ± 0.15b | 3.41 ± 0.10c | 1.94 ± 0.10a |
| Total Starch (TS) (%) | 34.83 ± 0.82b | 32.95 ± 0.19a | 32.89 ± 0.53a |
| Amylose (%) | 23.77 ± 0.23c | 22.05 ± 0.54b | 20.02 ± 0.22a |
| Total phenolic content (mgGAE per 100 g) | 190.85 ± 0.75b | 173.3 ± 0.04a,b | 158.9 ± 0.22a |
The moisture content in the control flour (CF) was 7.7%, which is in good agreement with that reported earlier.37 The impact of germination on the moisture content of the samples was significant (P < 0.05) after two days, and moisture content exhibited an upward trend with increasing germination time (Table 1). In fact, in HF, the samples with the longest germination time had the highest moisture content (P < 0.05).
The total protein content of the control and germinated lentil seeds ranged between 21.2% (control) and 26.9% (HF). An increase of the protein percentage depends on a decrease in the total dry weight rather than the absolute protein content. An increase in the protein content is in fact possible if germination is performed with the supplementation of a nitrogen source.46 In this case, only pure water was used for germination. Several authors47–49 have shown an increase in protein content during germination, probably due to the loss of carbohydrates through respiration during germination.50 The soluble nitrogen content was the lowest in HF (1.9%), while the highest value was observed in GF (3.4%). Therefore, after 24 h of germination, an increase in these parameters was observed compared to that in the control (P < 0.05).
The total starch (TS) and amylose contents decreased significantly (P < 0.05) during germination. In particular, the TS content varied between 35% (control) and 33% (germinated lentils), while the amylose content ranged from 24% (CF) to 20% (HF).
These decreases are due to a marked increase in the total spectrum of hydrolytic enzymes during lentil germination. These hydrolytic enzymes are responsible for the conversion of starch into oligosaccharides or monosaccharides, resulting in a reduction in the total starch and amylose contents. Hydrolytic enzymes, including α-amylase, glucosidase, and dextranase are generated from the aleurone layer of pulse seeds and β-amylase in the endosperm is activated during pulse seed germination.51,52 In general, lentils have a high total phenolic content,5 but this content decreased (P < 0.05) during germination from 191 ± 0.75 mg GAE per 100 g for the control and to 159 ± 0.22 mg GAE per 100 g for HF (Table 1). There are few studies on the phenolic components of germinated lentils. It has been reported that the decrease in the quantity of phenolic components is due to the mobilization of stored phenolics by the activation of enzymes such as polyphenol oxidase during the sprouting process.10 Differences in phenolic compound levels after germination depend on the type of seed, the presence or the absence of light, and the processing conditions such as germination time.53 A similar trend has also been reported during the germination of green mung plants.54
Physical and thermal properties
The physical properties, namely colour, and thermal properties of the samples were investigated. The colour parameters recorded for CF, GF and HF are shown in Table 2.| Colour values | CF (0 h) | GF (24 h) | HF (48 h) |
|---|---|---|---|
| a–c Means within the same row with different letters differ significantly at P < 0.05. | |||
| Lightness (L*) | 79.29 ± 0.43b | 79.74 ± 0.81b | 77.95 ± 0.18a |
| Redness (a*) | −2.44 ± 0.06a | −1.82 ± 0.06b | −1.12 ± 0.13c |
| Yellowness (b*) | 29.33 ± 0.69c | 23.24 ± 0.25b | 20.91 ± 0.54a |
| ΔE | 36.00 ± 0.49b | 30.86 ± 0.72a | 30.39 ± 0.53a |
The colour analysis of flour samples revealed significant differences (P < 0.05) between samples with regard to all colour values. L* and b* values decreased significantly as germination progressed, while a* values increased (Table 3). Compared to that of the control flour, the total colour difference (ΔE) decreased from 36.0 (CF) to 30.9 (GF) and −30.4 (HF) after germination. The changes in both the a* and b* parameters are probably related to the increase in the dark colour of the lentil seeds during germination and the change in colour from green to brown yellow due to the growth of the germ. In particular, the decreases in L* and b* and increase in a* could be due to the migration and percolation of pigments, such as uranidins and flavonoids, from the testa of the seeds into the endosperm because of increased enzymatic hydrolysis during germination.55
| Identified VOCs (%) | CF (0 h) | GF (24 h) | HF (48 h) | RI | Column used for RI | Odor descriptor |
|---|---|---|---|---|---|---|
| a–c Different letters in the same row indicate significant differences (P < 0.05). nd: not detected. 1: ZB-Wax 60 m × 0.25 mm × 0.25 μm; 2: ZB-Wax 60 m × 0.32 mm × 0.25 μm; 3: DB-1 30 m × 0.53 mmI × 3.0 μm; 4: ZB-Wax 60 m × 0.32 mm × 0.50 μm; 5: DB-Wax 60 m × 0.25 mm × 0.25 μm; 6: ZB-Wax column 60 m × 0.25 mm i.d., 0.25 μm; ZB-Wax; 7: h, Innowax FSC 60 m × 0.25 mm × 0.25 μm; 8: DB5 30 m × 0.25 mm × 0.25 μm; 9: HP-5MS 30. m/0.25 mm/0.25 μm; 10: OV-101 50 m × 0.25 mm. | ||||||
| Alcohols | ∑15.05 ± 0.45a | ∑17.35 ± 0.74b | ∑20.03 ± 0.39c | |||
| 1-Hexanol | 8.61 ± 0.73a | 8.38 ± 0.86a | 9.49 ± 0.56b | 1364 | 159 | Green, herbal29 |
| 1-Octen-3-ol | nd | 2.88 ± 0.78a | 3.64 ± 0.73b | 1433 | 259 | Mushroom59 |
| Benzyl alcohol | 6.44 ± 0.26a | 6.09 ± 0.68a | 6.90 ± 0.08b | 1027 | 365 | Sweet, floral, fruity64 |
| Aldehydes | ∑53.78 ± 2.21a | ∑64.33 ± 1.98c | ∑61.11 ± 0.78b | |||
| Hexanal | 32.62 ± 4.03b | 43.11 ± 1.93c | 36.59 ± 0.65a | 1127 | 128 | Green grass, fat57 |
| 2-Hexenal | 6.05 ± 0.94a | 8.93 ± 1.08b | 8.22 ± 0.57b | 1238 | 428 | Mild marzipan, floral29 |
| Heptanal | nd | 2.72 ± 0.15a | 4.16 ± 0.10b | 1186 | 528 | Beany66 |
| Benzaldehyde | 7.35 ± 0.74c | 3.10 ± 0.12a | 4.64 ± 0.73b | 1564 | 628 | Almond-flavored66 |
| Nonanal | 7.81 ± 1.23b. | 4.07 ± 0.41a | 4.07 ± 0.39a | 1412 | 428 | Fat, citrus, green beany66 |
| Benzeneacetaldehyde | nd | 2.40 ± 0.79a | 3.43 ± 0.92b | 1663 | 728 | Harsh, green, honey, cocoa57 |
| Esters | ∑2.75 ± 0.71b | ∑1.24 ± 0.33a | ∑2.26 ± 0.32b | |||
| Octyl formate | 2.75 ± 0,71b | 1.24 ± 0.33a | 2.26 ± 0.32b | 1117 | 867 | — |
| Furanoids | ∑4.72 ± 0.23b | ∑2.61 ± 0.52a | ∑2.85 ± 0.92a | |||
| 2-Pentylfuran | 4.72 ± 0.23b | 2.61 ± 0.52a | 2.85 ± 0.92a | 990 | 968 | Fruity,66 green and bean28 |
| Ketones | ∑2.73 ± 0.52a | ∑2.74 ± 0.84a | ∑3.33 ± 0.38b | |||
| 3,5-Octadien-2-one | 2.73 ± 0.52a | 2.74 ± 0.84a | 3.33 ± 0.38b | 1093 | 969 | Creamy, fruity smell, spicy, earthy, green pepper28,70 |
| Others | ∑20.92 ± 1.34b | ∑11.73 ± 1.10a | ∑10.43 ± 1.20a | |||
| Decane | 8.59 ± 1.53c | 5.91 ± 1.36a | 6.76 ± 1.83b | 159.6 | 871 | — |
| Dodecane | 3.13 ± 0.83b | 1.65 ± 0.38a | 1.35 ± 0.48a | 1263 | 872 | — |
| m-Di-tert-butylbenzene | 3.73 ± 0.82b | 1.06 ± 0.14a | 1.05 ± 0.14a | 987.1 | 873 | — |
| Oxirane, heptadecyl- | nd | 3.11 ± 0.63b | 1.27 ± 0.09a | — | — | — |
| 3,3-Dimethyl-hexane | 5.47 ± 0.62 | nd | Nd | 742.9 | 1074 | Tea-type flavor75 |
Volatile organic compounds
The study of the change in the aromatic profile of lentils during germination is an interesting topic for practical purposes.To determine the volatile organic compound (VOC) profiles, G and H were analysed. A total of 13 (C) and 16 (G and H) aromatic compounds were identified through SPME-GC/MS analysis (Table 3). In lentils, various concentrations of aldehydes, alcohols, esters and ketones were found. Aldehydes ranged from 53.78% in the control group to 64.33% in the G group, alcohols ranged from 15.05% in the control group to 20.03% in the HF group, esters ranged from 1.24% in the G group to 2.75% in the control group and ketones ranged from 2.73% in the control group to 3.33% in the HF group. Furthermore, other compounds belonging to the furanoid, alkane and aromatic hydrocarbon classes were found. The most common compounds identified in lentils were aldehydes, alcohols, and ketones as previously reported.22,28
Germination positively influenced the aldehyde concentration, except for nonanal and benzaldehyde which were negatively influenced. Among the aldehydes, hexanal was the most common and ranged from 32.62% in the control group to 43.11% in the GF group, thus, germination influenced the content of this compound at the highest concentration after 24 h of germination. Similar to like what has been previously reported, hexanal is also present in beans, soybeans, and peas56 and influences lentil flavour through the use of odorous notes of green and glass.57 This compound is derived by the oxidation of unsaturated fatty acids, particularly linoleic acid.58 The oxidation reaction increases during pulse germination and lipoxygenase is a family of different enzymes involved in lipid degradation.59,60 The lipoxygenase pathway plays an important role in the formation of volatile organic compounds from unsaturated fatty acids.61 Although lentils have a low fat content, the oxidation of fatty acids is the dominant contributor to the formation of volatile organic compounds during the pulse treatment.62
Heptanal was found only in GF (2.72%) and HF (4.16%), indicating that it was formed during germination as has been reported24 in the faba bean cultivar. Additionally, benzene acetaldehyde was formed during germination and was present in GF at a concentration of 2.40% and in HF at a concentration of 3.43%. This compound was also found in fermented lentil flour as previously reported.63 Furthermore, the benzaldehyde concentration decreased during germination ranging from 7.35% in ungerminated lentils to 3.10% in GF. It can be derived from the amino acid phenylalanine62 and was also found in Pardina lentil flour63 and in germinated pulses influencing the almond flavour.28
Finally, it is interesting to note the reduction in nonanal content during germination (from 7.81% in CF treatment to 4.07% in HF and GF treatment) because this compound normally increases during germination and influences the aroma of the plants.24
Regarding the alcohols, the germination increased the alcohol content in lentils (Table 3). These compounds influence the green, mushroom and fruity aromas of plants.29,59,64 Free fatty acid breakdown and amino acid degradation are involved in the formation of these compounds.62 Among the alcohols, 1-hexanol, 1-octen-3-ol and benzyl alcohol were found. The first compound ranged between 8.38% in CF treatment to 9.49% in HF treatment and its concentration increased with the germination process. It is derived from the oxidation of linoleic acid,28 while 1-octen-3-ol was present only in the germinated sample GF (2.88%) and HF (3.64%). Finally benzyl alcohol ranged between 6.09% in CF and 6.90% in HF, and could be derived from phenylalanine metabolism.62 Interestingly, 1-octen-3-ol was not present in the control group so it was formed during the germination. This compound is generated by the oxidation of unsaturated fatty acids through the lipoxygenase (LOX) pathway and its activity increases with germination in plant seeds.59
Among ketones, 3,5-octadien-2-one was found to be increased during germination, in this way its presence in germinated legumes was also reported.28 Ketones are formed not only by oxidation of fatty acids, but also by amino acid degradation and carotenoid breakdown.62
Furthermore, 2-pentylfuran was found to be a marker of beany aroma, and its concentration was negatively influenced by germination (Table 3).23 From a technological point of view, a decrease in this compound for food applications is needed, as it is among those that influence the unpleasant beany flavour.46
Finally, other compounds such as decane, dodecane, m-di-ter-butylbenzene, oxirane, and heptadecyl- and 3,3-dimethyl-hexane, were found.
Farinographic properties
The use of lentil flour as a food ingredient is generally based on its technological and nutritional properties. Considering that lentil flour samples can be used in the preparation of bakery products,1 it is fundamental to evaluate the effect of germination time on dough mixing properties. For this purpose, the processability of flour samples in the preparation of bakery products was determined using a farinographic instrument. It is very useful in the rheology characterization of dough systems from grains, pseudocereals, and pulses.76 The results for the flour water absorption (WA), dough development time (DDT), dough stability (DS), and degree of dough softening (DOS) are presented in Table 4.| Dough samples | WA (%) | DDT (min) | DS (min) | DOS (BU) |
|---|---|---|---|---|
| a–c Different letters in the same column indicate significant differences (P < 0.05). | ||||
| CF (0 h) | 57.53 ± 0.32c | 1.87 ± 0.12b | 6.12 ± 1.03b | 56.67 ± 7.63a |
| GF (24 h) | 49.87 ± 0.35b | 1.30 ± 0.17a | 1.36 ± 0.16a | 74.33 ± 5.86b |
| HF (48 h) | 46.80 ± 0.30a | 1.50 ± 0.11a | 1.47 ± 0.14a | 86.33 ± 4.16b |
The germination process caused significant changes in the farinograph properties. In fact, the mixing properties of CF (control), GF and HF significantly differed (P < 0.05) with clearer differences between the control (CF) and the two germinated samples (Table 4). In particular, the germination process markedly diminished the water absorption (WA) of the samples, with HF reaching a value of 46.8 g per 100 g. The decrease in WA could be the result of the loss of starch during germination, as well as protein structural changes.77 Similar results were reported by other authors for the WA of germinated samples such as yellow pea77 and highland barley.78
The DDT and DS of the samples exhibited the same trend as that of WA. In particular, the DDT and DS of CF significantly decreased from 1.9 min (CF) to 1.3–1.5 min (GF and HF, respectively) and from 6.1 min (CF) to 1.4–1.5 min in GF and HF, respectively. The decrease in DDT may be correlated with the decrease in WA (Table 4) which indicates that dough absorbs less water and, therefore, requires less mixing time. The DS value is a measure of dough strength (the lower it is the weaker the dough is). The reduction in the DS of the germinated samples (GF and HF) in the present study may have been caused by a reduction in starch content (Table 1) or the presence of proteolytic enzymes.79
The DOS was significantly lower (P < 0.05) for the control dough than for the GF and HF dough samples, which indicates that the germination process may negatively affect the resistance of the dough to mechanical mixing negatively. A higher BU in germinated samples is related to weak dough strength, whereas small values are associated with strong dough strength.80 Thus, germination did not improve the farinograph properties of the samples. However, the potential use of germinated flour samples for bakery products is promising, because even a 10% wheat flour substitution with lentil flour can generally result in a significant improvement in the nutritional profile of the final product.1,81 Currently, only low amounts (usually less than 25%) of lentil flour samples are adequately incorporated into wheat bread formulations without negatively affecting the quality or technological properties.4,81,82
Thermal properties of lentil flour samples
The thermal properties of starch systems such as lentil flour samples include gelatinization. Differential scanning calorimetry (DSC) was employed to examine the gelatinization transition temperature-onset temperature (To), peak temperature (Tp), end temperature (Tend) and enthalpy (ΔH) of the CF (control), GF (24 h), and HF (48 h) samples with the addition of excess water (Table 5).| Parameters | CF (0 h) | GF (24 h) | HF (48 h) |
|---|---|---|---|
| a–cDifferent letters in the same row indicate significant differences (P < 0.05). | |||
| T o (°C) | 62.88 ± 0.3a | 63.34 ± 1.1a,b | 64.05 ± 0.2b |
| T p (°C) | 76.41 ± 0.8a | 77.16 ± 0.16a,b | 77.41 ± 0.15b |
| T end (°C) | 80.46 ± 0.44a | 81.86 ± 0.13b | 82.17 ± 0.43b |
| ΔH (J g−1) | 2.61 ± 0.10b | 2.21 ± 0.11a | 2.18 ± 0.13a |
A single endothermic transition, corresponding mainly to the starch gelatinization transition, was observed in the DSC profiles of all the tested samples. The gelatinization transition temperatures (To, Tp and Tend) and the enthalpy of gelatinization (ΔH) were influenced by the germination time. The Tp and Tend of the samples increased over the course of germination (p < 0.05) (Table 5). Among the samples, HFs after long-term germination (48 h)-presented higher To (64. 1 °C), Tp (77. 4 °C) and Tend (82. 2 °C) values than did the control. The increased gelatinization temperatures could be attributed to the activation of protease and α-amylase, the presence of damaged starch absorbing more water, the decrease in amylose content (Table 1)83,84 and the increase in acid concentrations,11 as also observed by the reduced pH of the lentil samples after germination.12
The ΔH of the CF samples decreased from 2.6 J g−1 to 2.2 J g−1 (GF and HF) after germination, suggesting that a reduction in the energy was required to convert the chemical composition of the samples from an ordered to disordered form. In fact, ΔH can be used to predict the energy required to break down the intermolecular hydrogen bonds of starch granules.85 During germination, the partial hydrolysis of starch by activated endogenous enzymes reduces the intermolecular hydrogen bonds of starch, allowing it to easily detach during heating. Therefore, the highest ΔH value (2.6 J g−1) suggested greater granule structure stability in the control flour samples than in the germinated flour samples (P < 0.05). From a technological point of view, the thermal results obtained in the present work are interesting, because the high gelatinization enthalpy limits the use of leguminous coal.85
In vitro starch digestibility
The effects of germination time on the in vitro starch digestion rate were investigated by measuring the released glucose content during starch digestion. According to the available literature, the hydrolysis kinetics curves of lentil samples are rarely reported. Fig. 2 shows the hydrolysis curves of the lentil flour samples that were compared with those of the reference food (white bread).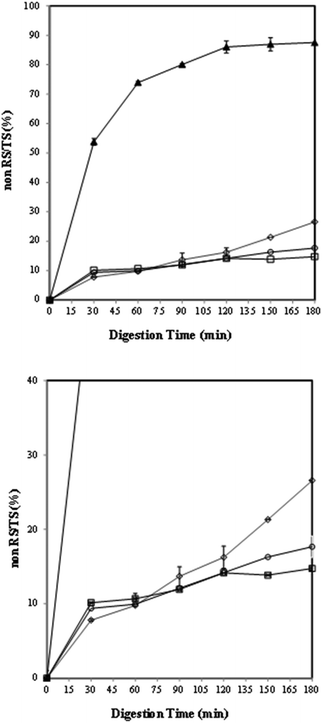 | ||
| Fig. 2 Total starch hydrolysis rate of reference (▲bread) and lentil flour samples: (◊) CF (control), (○) GF (24 h) and (□) HF (48 h). | ||
As expected, all the uncooked lentil samples showed lower starch hydrolysis than the bread that was used as a reference. In particular, the hydrolysis kinetics of the lentil samples is more evident in the enlarged image shown in Fig. 2. The rate and degree of starch digestion were the highest in the flour germinated for 2 days (HF) and 1 day (GF) in comparison to those in the control (CF) within the first 30 min as supported by other authors.86,87 A rapid increase in the percentage of starch hydrolysed from the control was observed during in vitro digestion (60–180 minutes). After 90 minutes of digestion, the overall and endpoint in vitro digestibility values of the germinated samples were lower than those obtained for the control (p < 0.05). An increase in endogenous α-amylase activity and a decrease in phytates which inhibit the amylase activity during germination8,10,88,89 could be possible explanations for the total starch and amylose losses (Table 1), the initial high extent of starch hydrolysis in GF and HF samples (Fig. 2) and the effects of germination on starch fractions (RDS and SDS), resistant starch and the expected glycaemic index (eGI) (Table 6). Specifically, the greater degree of hydrolysis in the germinated samples (within the first 30 min) than in the control could be attributed to their lower amylose content90 and low ΔH in the germinated samples (Table 5).
| Nutritional parameters | CF (0 h) | GF (24 h) | HF (48 h) |
|---|---|---|---|
| a–cDifferent letters in the same row indicate significant differences (P < 0.05). | |||
| RDS (g per 100 g) | 2.72 ± 0.03a | 3.09 ± 0.05b | 3.34 ± 0.01c |
| SDS (g per 100 g) | 2.94 ± 0.40c | 1.58 ± 0.27b | 1.32 ± 0.01a |
| Resistant starch (%) | 29.2 ± 0.27b | 28.3 ± 0.08a | 28.2 ± 0.01a |
| eGI | 50.62 ± 0.35b | 49.12 ± 0.01a | 48.77 ± 0.07a |
Starch and starchy food can be classified according to their digestibility.38 In detail, the amounts of RDS, SDS, resistant starch and eGI in the samples are reported in Table 6.
RDS is the starch fraction that is rapidly and totally digested in the gastrointestinal tract (after 30 minutes of digestion) and is associated with a rapid increase in postprandial plasma glucose, while SDS is more slowly digested in the small intestine.3 As reported in Table 6, the HF samples had the highest RDS content followed by the GF and control samples. This parameter was significantly lower (P < 0.05) for the control and higher for the germinated samples, ranging from 2.7 ± 0.03 to 3.3 ± 0.01 g per 100 g. Unlike SDS, which is the starch fraction that is slowly digested in the gastrointestinal tract, RDS significantly decreased (p < 0.05) with the increasing of germination time, ranging from 1.3 ± 0.1 for HF to 2.9 ± 0.4 g per 100 g for the control. There was also a statistically significant decrease (P < 0.05) in the amount of resistant starch in the germinated samples (GF and HF), which may also be related to both the use of starch as an energy source in the germination process and the larger space within the matrix due to the partial removal of phytic acid and tannins during germination, which increased the susceptibility to the enzymatic attack and consequently improved the digestibility of starch.9
The highest glycaemic index eGI (p < 0.05) was determined for the CF samples (50.6). Although starch digestibility subsequently increased during germination, the eGI significantly decreased (Table 6), as reported10 for the raw and germinated lentil plants. The eGI is affected by the amount of TS, the amylose content (Table 1) and the amount of resistant starch (Table 6). In fact, germination significantly changes the nutritional quality of legumes, including the starch content and the amount of free sugars (e.g., glucose and fructose). This difference is related to the germination process conditions such as time and temperature, which change the content of the induced compounds.10,91
Considering the in vitro digestibility results of the germinated samples, GF and HF might be potential ingredients in the formulation of products for diabetes treatment and weight management and could lead to the formulation of novel foods characterized by the slow release of glucose, that is the low glycaemic index and the prevention of fasting hypoglycaemia.
Conclusions
The effects of germination time on the properties of lentils were investigated. The findings of this study indicated that germination can be a practical and effective treatment for improving the nutritional profiles of lentils, with the potential use of germinated lentil flour as a novel ingredient suitable for people with special nutritional needs (i.e., elderly and diabetic patients). The total starch and amylose contents and the glycaemic index of the germinated samples were in fact lower (P < 0.05) than those of the control flour. The technological and thermal properties of the flour samples were also modified by germination time. Farinographic results revealed that the germination time had a negative impact on dough mixing properties, causing undesirable dough weakening. However, the thermal results of the germinated samples were better than those of the control, particularly concerning gelatinization enthalpies. Moreover, the germination treatment affects the aromatic profile of the samples, with a strategic decrease in 2-pentylfuran and nonanal in the germinated samples.Overall, the results of the present study may help us to better understand and use a sustainable, inexpensive and plant-based protein ingredient such as lentil flour to develop high-quality foods (e.g., bakery products).
Author contributions
A. Romano: conceptualization, methodology, investigation, validation, writing – original draft, writing – review and editing, visualization, project administration, and funding acquisition. L. De Luca: validation, methodology, and investigation. R. Romano: supervision, funding acquisition, writing – review and editing, and conceptualization.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.3 – Call for proposals No. 341 of 15 March 2022 of Italian Ministry of University and Research funded by the European Union – NextGenerationEU; Award Number: Project code PE00000003, Concession Decree No. 1550 of 11 October 2022 adopted by the Italian Ministry of University and Research, CUP D93C22000890001, Project title “ON Foods – Research and innovation network on food and nutrition Sustainability, Safety and Security – Working ON Foods”.References
- A. Romano, V. Gallo, P. Ferranti and P. Masi, Lentil flour: nutritional and technological properties, in vitro digestibility and perspectives for use in the food industry, Curr. Opin. Food Sci., 2021, 40, 157–167, DOI:10.1016/j.cofs.2021.04.003.
- ( https://www.eur-lex.Europa.eu/eli/reg_impl/2017/2362/oj; European Union, 2017; https://www.lenticchiadialtamura.it).
- V. Gallo, A. Romano and P. Masi, Does the presence of fibres affect the microstructure and in vitro starch digestibility of commercial Italian pasta?, Food Struct., 2020, 24, 100139, DOI:10.1016/j.foostr.2020.100139.
- V. Gallo, A. Romano, B. Miralles, M. Santos-Hernández, P. Ferranti, P. Masi and I. Recio, Physicochemical properties, structure and digestibility in simulated gastrointestinal environment of bread added with green lentil flour, LWT-Food Sci. Technol., 2022, 154, 112713, DOI:10.1016/j.lwt.2021.112713.
- A. Manco, C. Gerardi, G. Romano, L. D'Amico, A. Blanco, F. Milano, G. P. Di Sansebastiano, R. Balech and B. Laddomada, Phenolic profile of whole seeds and seed fractions of lentils and its impact on antioxidant activity, Food Biosci., 2023, 54, 102887, DOI:10.1016/j.fbio.2023.102887.
- M. Marchini, E. Carini, N. Cataldi, F. Boukid, M. Blandino, T. Ganino, E. Vittadini and N. Pellegrini, The use of red lentil flour in bakery products: How do particle size and substitution level affect rheological properties of wheat bread dough?, LWT-Food Sci. Technol., 2021, 136, 110299, DOI:10.1016/j.lwt.2020.110299.
- L. Bourré, P. Frohlich, G. Young, Y. Borsuk, E. Sopiwnyk, A. Sarkar, M. T. Nickerson, Y. Ai, A. Dyck and L. Malcolmson, Influence of particle size on flour and baking properties of yellow pea, navy bean, and red lentil flours, Cereal Chem., 2019, 96, 655–667, DOI:10.1002/cche.10161.
- R. A. Ghavidel and J. Prakash, The impact of germination and dehulling on nutrients, antinutrients, in vitro iron and calcium bioavailability and in vitro starch and protein digestibility of some legume seeds, LWT-Food Sci. Technol., 2007, 40(7), 1292–1299, DOI:10.1016/j.lwt.2006.08.002.
- Y. Aguilera, R. M. Esteban, V. Benitez, E. Molla and M. A. Martin-Cabrejas, Starch, functional properties, and microstructural characteristics in chickpea and lentil as affected by thermal processing, J. Agric. Food Chem., 2009, 57(22), 10682–10688, DOI:10.1021/jf902042r.
- M. Świeca, B. Baraniak and U. Gawlik-Dziki, In vitro digestibility and starch content, predicted glycemic index and potential in vitro antidiabetic effect of lentil sprouts obtained by different germination techniques, Food Chem., 2013, 138(2–3), 1414–1420, DOI:10.1016/j.foodchem.2012.09.122.
- S. O. Azeez, C. E. Chinma, S. O. Bassey, U. R. Eze, A. F. Makinde, A. A. Sakariyah, S. S. Okubanjo, N. Danbaba and O. A. Adebo, Impact of germination alone or in combination with solid-state fermentation on the physicochemical, antioxidant, in vitro digestibility, functional and thermal properties of brown finger millet flours, LWT-Food Sci. Technol., 2022, 154, 112734, DOI:10.1016/j.lwt.2021.112734.
- A. Romano, R. Romano, L. De Luca and P. Masi, Potential of Germination to Improve the Properties of Lentils (Lens Culinaris Medik.), Chem. Eng. Trans., 2023, 102, 199–204, DOI:10.3303/CET23102034.
- M. Joshi, Y. Timilsena and B. Adhikari, Global production, processing and utilization of lentil: A review, J. Integr. Agric., 2017, 16(12), 2898–2913, DOI:10.1016/S2095-3119(17)61793-3.
- B. Xu and S. K. C. Chang, Effect of soaking, boiling, and steaming on total phenolic content and antioxidant activities of cool season food legumes, Food Chem., 2008, 110(1), 1–13, DOI:10.1016/j.foodchem.2008.01.045.
- T. Najib, M. H. Mohamad, T. Kaiyang, V. Miranda and M. Venkatesh, Protein structural changes in lentil flour during soaking/germination and thermal treatments: Indication of nutritional and functional properties, Food Chem. Adv., 2023, 3, 100475, DOI:10.1016/j.focha.2023.100475m.
- L. A. Nadtochii, D. V. Kuznetcova, V. Proskura, A. D. Apalko, V. V. Nazarova and M. Srinivasa, Investigation of various factors on the germination of chia seeds sprouts (Salvia hispanica L.), Agron. Res., 2019, 17, 1390–1400, DOI:10.15159/ar.19.128.
- J. Frias, C. Vidal-Valverde, C. Sotomayor, C. Diaz-Pollan and G. Urbano, Influence of processing on available carbohydrate content and antinutritional factors of chickpeas, Eur. Food Res. Technol., 2000, 210, 340–345, DOI:10.1007/s002170050560.
- I. C. Ohanenye, A. Tsopmo, C. E. C. C. Ejike and C. C. Udenigwe, Germination as a bioprocess for enhancing the quality and nutritional prospects of legume proteins, Trends Food Sci. Technol., 2020, 101, 213–222, DOI:10.1016/j.tifs.2020.05.003.
- M. Dueñas, T. Hernández, I. Estrella and D. Fernández, Germination as a process to increase the polyphenol content and antioxidant activity of lupin seeds (Lupinus angustifolius L.), Food Chem., 2009, 117(4), 599–607, DOI:10.1016/j.foodchem.2009.04.051.
- R. Gan, W. Lui, K. Wu, C. Chan, S. Dai, Z. Sui and H. Corke, Bioactive compounds and bioactivities of germinated edible seeds and sprouts: An updated review, Trends Food Sci. Technol., 2017, 59, 1–14, DOI:10.1016/j.tifs.2016.11.010.
- J. I. Ma, J. I. Boye, S. Azarnia and B. K. Simpson, Volatile Flavor Profile of Saskatchewan Grown Pulses as Affected by Different Thermal Processing Treatments, Int. J. Food Prop., 2016, 19(10), 2251–2271, DOI:10.1080/10942912.2015.1121494.
- W. S. Roland, L. Pouvreau, J. Curran, F. van de Velde and P. M. de Kok, Flavor aspects of pulse ingredients, Cereal Chem., 2017, 94(1), 58–65, DOI:10.1094/CCHEM-06-16-0161-FI.
- I. Rajhi, B. Baccouri, F. Rajhi, J. Hammami, Z. Abbes, H. Mhadhbi and G. Flamini, HS-SPME-GC-MS, combined with chemometrics to assess the impact of germination, dehulling, and milling on flavor attributes of brown and green lentils (Lens culinaris subsp. culinaris), S. Afr. J. Bot., 2022, 150, 1102–1110, DOI:10.1016/j.sajb.2022.09.013.
- I. Rajhi, B. Baccouri, F. Rajhi, J. Hammami, M. Souibgui, M. Amri, H. Mhadhbi and G. Flamini, Evaluation of germination effect on volatile compounds of different faba bean cultivars using HS-SPME/GC-MS, J. Food Compos. Anal., 2022, 112, 104692, DOI:10.1016/j.jfca.2022.104692.
- S. Kaneko, K. Kumazawa and O. Nishimura, Studies on the key aroma compounds in soy milk made from three different soybean cultivars, J. Agric. Food Chem., 2011, 59(22), 12204–12209, DOI:10.1021/jf202942h.
- L. Xiang, B. Jiang, Y. L. Xiong, L. Zhou, F. Zhong, R. Zhang, A. B. Tahir and Z. Xiao, Beany flavor in pea protein: Recent advances in formation mechanism, analytical techniques and microbial fermentation mitigation strategies, Food Biosci., 2023, 56, 103166, DOI:10.1016/j.fbio.2023.103166.
- G. MacLeod, J. Ames and N. L. Betz, Soy flavor and its improvement, Crit. Rev. Food Sci. Nutr., 1988, 27(4), 219–400, DOI:10.1080/10408398809527487.
- M. Xu, Z. Jin, Y. Lan, J. Rao and B. Chen, HS-SPME-GC-MS/olfactometry combined with chemometrics to assess the impact of germination on flavor attributes of chickpea, lentil, and yellow pea flours, Food Chem., 2019, 280, 83–95, DOI:10.1016/j.foodchem.2018.12.048.
- K. T. Kaczmarska, M. V. Chandra-Hioe, D. Frank and J. Arcot, Aroma characteristics of lupin and soybean after germination and effect of fermentation on lupin aroma, LWT-Food Sci. Technol., 2018, 87, 225–233, DOI:10.1016/j.lwt.2017.08.080.
- R. Setia, Z. Dai, M. T. Nickerson, E. Sopiwnyk, L. Malcolmson and Y. Ai, Impacts of short-term germination on the chemical compositions, technological characteristics and nutritional quality of yellow pea and faba bean flours, Food Res. Int., 2019, 122, 263–272, DOI:10.1016/j.foodres.2019.04.021.
- T. Najib and M. M. Heydari, V.& Meda, V, Combination of germination and innovative microwave-assisted infrared drying of lentils: effect of physicochemical properties of different varieties on water uptake, germination, and drying kinetics, Appl. Food Res., 2022, 2(1), 100040, DOI:10.1016/j.afres.2021.100040.
- AACC (American Association of Cereal Chemists), International Approved Methods of Analysis, Method 44–15.02. Moisture – Air-Oven Methods. St. Paul, MN, U.S.A., 1999.
- AOAC, Determination of Protein Content in Food, Method 945.18-B, in Official Methods of Analysis, AOAC International Publisher, Gaithersburg, 2005 Search PubMed.
- C. S. Gouveia, G. Freitas, J. H. D. Brito, J. J. Slaski and M. Â. Carvalho, Nutritional and mineral variability in 52 accessions of common bean varieties (Phaseolus vulgaris L.) from Madeira Island, Agric. Sci., 2014, 5(4), 317–329, DOI:10.4236/as.2014.54034.
- M. Á. Sanz, I. Blázquez, I. Sierra, M. Á. Medrano, J. Frias, C. Vidal-Valverde and A. Hernández, Nutritional evaluation of ethanol-extracted lentil flours, J. Agric. Food Chem., 2001, 49(4), 1854–1860, DOI:10.1021/jf001293.
- AACC (American Association of Cereal Chemists), International Approved Methods of Analysis, Method number 76.13, Total starch. St. Paul, MN, U.S., 2009.
- M. S. Alkaltham, M. M. Özcan, N. Uslus, A. M. Salamatullah and K. Hayat, Changes in antioxidant activity, phenolic compounds, fatty acids, and mineral contents of raw, germinated, and boiled lentil seeds, J. Food Sci., 2022, 87(4), 1639–1649, DOI:10.1111/1750-3841.16099.
- A. Raiola, A. Romano, H. Shanakhat, P. Masi and S. Cavella, Impact of heat treatments on technological performance of re-milled semolina dough and bread, LWT-Food Sci. Technol., 2020, 117, 108607, DOI:10.1016/j.lwt.2019.108607.
- AACC (American Association of Cereal Chemists). International Approved Methods of Analysis, Method number 32–40.01. Resistant starch. St. Paul, MN, U.S., 2009.
- A. Romano, C. V. L. Giosafatto, P. Di Pierro, R. Romano, P. Masi and L. Mariniello, Impact of transglutaminase treatment on properties and in vitro digestibility of white bean (Phaseolus vulgaris L.) flour, Food Res. Int., 2016, 88(Part B), 239–246, DOI:10.1016/j.foodres.2016.02.014.
- I. Goñi, A. Garcia-Alonso and F. Saura-Calixto, A starch hydrolysis procedure to estimate glycemic index, Nutr. Res., 1997, 17(3), 427–437, DOI:10.1016/S0271-5317(97)00010-9.
- Z. Ma, J. I. Boye, B. K. Simpson, S. O. Prasher, D. Monpetit and L. Malcolmson, Thermal processing effects on the functional properties and microstructure of lentil, chickpea, and pea flours, Food Res. Int., 2011, 44(8), 2534–2544, DOI:10.1016/j.foodres.2010.12.017.
- S. Liu, H. Yin, M. Pickard and Y. Ai, Influence of infrared heating on the functional properties of processed lentil flours: A study focusing on tempering period and seed size, Food Res. Int., 2020, 136, 109568, DOI:10.1016/j.foodres.2020.109568.
- M. M. Heydari, T. Najib and V. Meda, Investigating starch and protein structure alterations of the processed lentil by microwave-assisted infrared thermal treatment and their correlation with the modified properties, Food Chem. Adv., 2022, 1, 100091, DOI:10.1016/j.focha.2022.100091.
- S. Mahalaxmi, P. Himashree, B. Malini and C. K. Sunil, Effect of microwave treatment on the structural and functional properties of proteins in lentil flour, Food Chem. Adv., 2022, 1, 100147, DOI:10.1016/j.focha.2022.100147.
- M. Xu, Z. Jin, S. Simsek, C. Hall, J. Rao and B. Chen, Effect of germination on the chemical composition, thermal, pasting, and moisture sorption properties of flours from chickpea, lentil, and yellow pea, Food Chem., 2019, 295, 579–587, DOI:10.1016/j.foodchem.2019.05.167.
- A. A. Fouad, F. M. Rehab and F. M. Effect, of germination time on proximate analysis, bioactive compounds and antioxidant activity of lentil (Lens culinaris Medik.) sprouts, Acta Sci. Pol., Technol. Aliment., 2015, 14(3), 233–246, DOI:10.17306/J.AFS.2015.3.25.
- D. Atudorei and S. G. Stroe, G.G Codină, Impact of germination on the microstructural and physicochemical properties of different legume types, Plants, 2021, 10(3), 592, DOI:10.3390/plants10030592.
- B. Oskaybaş-Emlek, A. Özbey and K. Kahraman, Effects of germination on the physicochemical and nutritional characteristics of lentil and its utilization potential in cookie-making, J. Food Meas. Charact., 2021, 15(5), 4245–4255, DOI:10.1007/s11694-021-00958-y.
- V. Uppal and K. Bains, Effect of germination periods and hydrothermal treatments on in vitro protein and starch digestibility of germinated legumes, J. Food Sci. Technol., 2012, 49, 184–191, DOI:10.1007/s13197-011-0273-8.
- D. E. Evans, B. Van Weger, Y. F. Ma and J. Eghinton, The Impact of the Thermostability of alpha-amylase, beta-amylase and limit Dextrinase on Potential work Fermentability, J. Am. Soc. Brew. Chem., 2003, 61, 210–218, DOI:10.1094/ASBCJ-61-0210.
- H. Olaerts, C. Roye, L. J. Derde, G. Sinnaeve, W. R. Meza, B. Bodson and C. M. Courtin, Impact of preharvest sprouting of wheat (Triticum aestivum) in the field on starch, protein, and arabinoxylan properties, J. Agric. Food Chem., 2016, 64, 8324–8332, DOI:10.1021/acs.jafc.6b03140.
- M. L. Lopez-Amoros, T. Hernandez and I. Estrella, Effect of germination on legume phenolic compounds and their antioxidant activity, J. Food Compos. Anal., 2006, 19(4), 277–283, DOI:10.1016/j.jfca.2004.06.012.
- R. Randhir, Y. T. Lin and K. Shetty, Stimulation of phenolics, antioxidant and antimicrobial activities in dark germinated mung bean sprouts in response to peptide and phytochemical elicitors, Process Biochem., 2004, 39, 637–647, DOI:10.1016/S0032-9592(03)00197.
- Y. Liu, M. Xu, H. Wu, L. Jing, B. Gong, M. Gou, K. Zhao and W. Li, The compositional, physicochemical and functional properties of germinated mung bean flour and its addition on quality of wheat flour noodle, J. Food Sci. Technol., 2018, 55, 5142–5152, DOI:10.1007/s13197-018-3460-z.
- P. Khrisanapant, B. Kebede, S. Y. Leong and I. Oey, A comprehensive characterisation of volatile and fatty acid profiles of legume seeds, Foods, 2019, 8(12), 651, DOI:10.3390/foods8120651.
- A. Paucean, O. P. Moldovan, V. Mureşan, S. A. Socaci, F. V. Dulf, E. Alexa, S. M. Man, A. E. Mureşan and S. Muste, Folic acid, minerals, amino-acids, fatty acids and volatile compounds of green and red lentils. Folic acid content optimization in wheat-lentils composite flours, Chem. Cent. J., 2018, 12(1), 1–9, DOI:10.1186/s13065-018-0456-8.
- Z. Ma, J. I. Boye, S. Azarnia and B. K. Simpson, Volatile flavor profile of Saskatchewan grown pulses as affected by different thermal processing treatments, Int. J. Food Prop., 2016, 19(10), 2251–2271, DOI:10.1080/10942912.2015.1121494.
- M. Xu, Z. Jin, Z. Gu, J. Rao and B. Chen, Changes in odor characteristics of pulse protein isolates from germinated chickpea, lentil, and yellow pea: Role of lipoxygenase and free radicals, Food Chem., 2020, 314, 126184, DOI:10.1016/j.foodchem.2020.126184.
- A. J. Taylor and R. S. Linforth, Food flavour technology, John Wiley Sons Inc., USA, 2010 Search PubMed.
- H. Jelen and E. Wasowicz, in Food Flavors, CRC Press, Boca Raton, Florida, 2011, pp. 65–94 Search PubMed.
- A. Karolkowski, E. Guichard, L. Briand and C. Salles, Volatile compounds in pulses: A review, Foods, 2021, 10(12), 3140, DOI:10.3390/foods10123140.
- J. Sánchez-García, S. Muñoz-Pina, J. García-Hernández, A. Heredia and A. Andrés, Volatile profile of quinoa and lentil flour under fungal fermentation and drying, Food Chem., 2024, 430, 137082, DOI:10.1016/j.foodchem.2023.137082.
- M. Yilmaztekin, Characterization of potent aroma compounds of cape gooseberry (Physalis peruviana L.) fruits grown in Antalya through the determination of odor activity values, Int. J. Food Prop., 2014, 17(3), 469–480, DOI:10.1080/10942912.2011.642446.
- E. R. Kuhn, Selectivity vs. polarity: The fundamentals of chromatographic separation, J. Sep. Sci., 2001, 24(6), 473–476, DOI:10.1002/16159314(20010601)24:6<473::AID-JSSC473>3.0.CO;2-Y.
- C. Yi, Y. Li, H. Zhu, Y. Liu and K. Quan, Effect of Lactobacillus plantarum fermentation on the volatile flavors of mung beans, LWT-Food Sci. Technol., 2021, 146, 111434, DOI:10.1016/j.lwt.2021.111434.
- S. Hamm, J. Bleton, J. Connan and A. Tchapla, A chemical investigation by headspace SPME and GC–MS of volatile and semi-volatile terpenes in various olibanum samples, Phytochemistry, 2005, 66(12), 1499–1514, DOI:10.1016/j.phytochem.2005.04.025.
- V. Roussis, M. Tsoukatou, P. V. Petrakis, I. Chinou, M. Skoula and J. B. Harborne, Volatile constituents of four Helichrysum species growing in Greece, Biochem. Syst. Ecol., 2000, 28(2), 163–175, DOI:10.1016/S0305-1978(99)00046-0.
- S. Afsharypuor and M. Suleimany, Volatile oil constituents of Brassica oleracea var. gongylodes seeds, J. Essent. Oil Res., 2002, 14(1), 18–19, DOI:10.1080/10412905.2002.9699748.
- Y. Zhu, H. P. Lv, C. Y. Shao, S. Kang, Y. Zhang, L. Guo, D. Wei-Dong, T. Jun-Feng, P. Qun-Hua and Z. Lin, Identification, of key odorants responsible for chestnut-like aroma quality of green teas, Food Res. Int., 2018, 108, 74–82, DOI:10.1016/j.foodres.2018.03.026.
- P. H. Chen, W. S. Keeran, W. A. Van-Ausdale, D. R. Schindler and D. W. Roberts, Technical paper, Analytical Services Division; Environmental Science and Engineering, 2002 Inc., PO Box 1703, Gainesville, FL 32602, 11.
- V. G. Zaikin and R. S. Borisov, Chromatographic–mass spectrometric analysis of Fischer–Tropsch synthesis products, J. Anal. Chem., 2002, 57(6), 544–551, DOI:10.1023/A:1015754120136.
- C. Song, W. C. Lai, R. Madhusudan, K. Boli Wei and B. Wei, in Analytical advances for hydrocarbon research, Springer, Boston, MA, 2003, 147–210 Search PubMed.
- J. P. Chen, X. M. Liang, Q. Zhang and L. F. Zhang, Prediction of GC retention values under various column temperature conditions from temperature programmed data, Chromatographia, 2001, 53(9), 539–547, DOI:10.1007/BF02491619.
- Y. Gong, A. L. Kerrihard and R. B. Pegg, Characterization of the volatile compounds in raw and roasted Georgia pecans by HS-SPME-GC-MS, J. Food Sci., 2018, 83(11), 2753–2760, DOI:10.1111/1750-3841.14365.
- A. Bresciani, G. A. Annor, M. Gardella and A. Marti, in Woodhead Publishing Series in Food Science, Technology and Nutrition, The Farinograph Handbook, ed. J. E. Bock and C. Don, Woodhead Publishing, 4th edn, 2022, ch. 9, pp. 111–126, DOI:10.1016/B978-0-12-819546-8.00012-1.
- K. Millar, C. Barry-Ryan, R. Burke, S. McCarthy and E. Gallagher, Dough properties and baking characteristics of white bread, as affected by addition of raw, germinated and toasted pea flour, Innovative Food Sci. Emerging Technol., 2019, 56, 102189, DOI:10.1016/j.ifset.2019.102189.
- W. Al-Ansi, Y. Zhang, T. Abdulrazzak, A. Alkawry, A. Al-Adeeb, A. A. Mahdi, Q. A. Al-Maqtari, A. Ahmed, B. S. Mushtaq, M. Fan, Y. Li, H. Qian, L. Yang, Q. Pan and L. Wang, Influence of germination on bread-making behaviors, functional and shelf-life properties, and overall quality of highland barley bread, LWT-Food Sci. Technol., 2022, 159, 113200, DOI:10.1016/j.lwt.2022.113200.
- E. Hallén, Ş. İbanoğlu and P. Ainsworth, Effect of fermented/germinated cowpea flour addition on the rheological and baking properties of wheat flour, J. Food Eng., 2004, 63(2), 177–184, DOI:10.1016/S0260-8774(03)00298-X.
- A. Romano, S. Cavella, G. Toraldo and P. Masi, 2D structural imaging study of bubble evolution during leavening, Food Res. Int., 2013, 50(1), 324–329, DOI:10.1016/j.foodres.2012.10.040.
- K. Kotsiou, G. Palassaros, A. Matsakidou, C.-K. Mouzakitis, C. G. Biliaderis and A. Lazaridou, Roasted-sprouted lentil flour as a novel ingredient for wheat flour substitution in breads: Impact on dough properties and quality attributes, Food Hydrocolloids, 2023, 145, 109164, DOI:10.1016/j.foodhyd.2023.109164.
- D. Portman, C. Blanchard, P. Maharjan, L. S. McDonald, J. Mawson, M. Naiker and J. F. Panozzo, Blending studies using wheat and lentil cotyledon flour—effects on rheology and bread quality, Cereal Chem., 2018, 95(6), 849–860, DOI:10.1002/cche.10103.
- F. Wu, H. Chen, N. Yang, J. Wang, X. Duan, Z. Jin and X. Xu, Effect of germination time on physicochemical properties of brown rice flour and starch from different rice cultivars, J. Cereal Sci., 2013, 58(2), 263–271, DOI:10.1016/j.jcs.2013.06.008.
- L. Xu, L. Chen, B. Ali, N. A. Yang, Y. Chen, F. Wu and X. Xu, Impact, of germination on nutritional and physicochemical properties of adlay seed (Coixlachryma-jobi L.), Food Chem., 2017, 229, 312–318, DOI:10.1016/j.foodchem.2017.02.096.
- R. Hoover, T. Hughes, H. J. Chung and Q. Liu, Composition, molecular structure, properties, and modification of pulse starches: A review, Food Res. Int., 2010, 43(Issue 2), 399–413, DOI:10.1016/j.foodres.2009.09.001.
- A. Negi, P. Boora and N. Khetarpaul, Starch and protein digestibility of newly released moth bean cultivars: Effect of soaking, germination and pressure-cooking, Nahrung / Food, 2001, 45(4), 251–254, DOI:10.1002/15213803(20010801)45:4<251::AID-FOOD251>3.0.CO;2-V.
- J. Frias, J. Fornal and S. G. Ring, Effect of Germination on Physico-chemical Properties of Lentil Starch and its Components, LWT-Food Sci. Technol., 1998, 31(3), 228–236, DOI:10.1006/fstl.1997.0340.
- I. A. Nnanna and R. D. Phillips, Changes in oligosaccharide content, enzyme activities and dry matter during controlled germination of cowpeas (Vigna unguiculata), J. Food Sci., 1998, 53, 1782–1786, DOI:10.1111/j.1365-2621.1988.tb07842.x.
- A. Sumanthi, N. G. Malleshi and S. V. Rao, Elaboration of amylase activity and changes in paste viscosity of some common Indian legumes during germination, Plant Foods Hum. Nutr., 1995, 47, 341–347, DOI:10.1007/BF01088272.
- H. J. Chung, Q. Liu, R. Hoover, T. D. Warkentin and B. Vandenberg, In vitro starch digestibility, expected glycemic index, and thermal and pasting properties of flours from pea, lentil and chickpea cultivars, Food Chem., 2008, 111, 316–321, DOI:10.1016/j.foodchem.2008.03.062.
- G. Zhang, Z. Xu, Y. Gao, X. Huang, Y. Zou and T. Yang, Effects of germination on the nutritional properties, phenolic profiles, and antioxidant activities of buckwheat, J. Food Sci., 2015, 80(5), 1111–1119, DOI:10.1111/1750-3841.12830.
| This journal is © The Royal Society of Chemistry 2024 |