Association between dietary patterns and chronic kidney disease combined with hyperuricemia†
Mengrui
Luo‡
a,
Tiancong
Liu‡
b,
Hao
Ju‡
c,
Yang
Xia
 ad,
Chao
Ji
ad,
Chao
Ji
 *ad and
Yuhong
Zhao
*ad and
Yuhong
Zhao
 *ade
*ade
aDepartment of Clinical Epidemiology, Shengjing Hospital of China Medical University, No. 36, San Hao Street, Shenyang, Liaoning 110004, China. E-mail: jichao@cmu.edu.cn
bDepartment of Otorhinolaryngology – Head and Neck Surgery, Shengjing Hospital of China Medical University, China
cDepartment of Ultrasound, Shengjing Hospital of China Medical University, China
dClinical Research Centre, Shengjing Hospital of China Medical University, China
eLiaoning Key Laboratory of Precision Medical Research on Major Chronic Disease, Shengjing Hospital of China Medical University, No. 36, San Hao Street, Shenyang, Liaoning 110004, China. E-mail: zhaoyuhong@sj-hospital.org
First published on 23rd November 2023
Abstract
Background and aims: Chronic kidney disease (CKD) combined with hyperuricemia is a concerning health issue, but the association between this condition and dietary patterns remains poorly understood. The aim of this study was to assess the associations between dietary patterns and CKD combined with hyperuricemia. Methods: This cross-sectional study was conducted involving 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 318 participants aged 18–79 years during 2018–2020. Dietary intake information was collected using a validated 110-item food frequency questionnaire. Factor analysis was used to identify major dietary patterns. CKD was defined as the presence of albuminuria or an estimated glomerular filtration rate <60 mL min−1 1.73 m−2. Hyperuricemia was defined as serum uric acid levels >420 μmol L−1 both in men and women. Logistic regression models were applied to assess the association between dietary patterns and the risk of CKD combined with hyperuricemia. Results: Five major dietary patterns were identified: ‘healthy pattern’, ‘traditional pattern’, ‘animal foods pattern’, ‘sweet foods pattern’, and ‘tea–alcohol pattern’, which together explained 38.93% of the variance in the diet. After adjusting for potential confounders, participants in the highest quartile of the traditional pattern had a lower risk of CKD combined with hyperuricemia (OR = 0.49, 95% CI: 0.32–0.74, Pfor trend < 0.01). Conversely, participants in the highest quartile of the sweet foods pattern had a higher risk compared to those in the lowest quartile (OR = 1.69, 95% CI: 1.18–2.42, Pfor trend < 0.01). However, no significant association was observed between the healthy pattern, animal foods pattern and tea–alcohol pattern and the risk of CKD combined with hyperuricemia. Conclusions: Our results suggest that the traditional pattern is associated with a reduced risk of CKD combined with hyperuricemia, whereas the sweet foods pattern is associated with an increased risk.
318 participants aged 18–79 years during 2018–2020. Dietary intake information was collected using a validated 110-item food frequency questionnaire. Factor analysis was used to identify major dietary patterns. CKD was defined as the presence of albuminuria or an estimated glomerular filtration rate <60 mL min−1 1.73 m−2. Hyperuricemia was defined as serum uric acid levels >420 μmol L−1 both in men and women. Logistic regression models were applied to assess the association between dietary patterns and the risk of CKD combined with hyperuricemia. Results: Five major dietary patterns were identified: ‘healthy pattern’, ‘traditional pattern’, ‘animal foods pattern’, ‘sweet foods pattern’, and ‘tea–alcohol pattern’, which together explained 38.93% of the variance in the diet. After adjusting for potential confounders, participants in the highest quartile of the traditional pattern had a lower risk of CKD combined with hyperuricemia (OR = 0.49, 95% CI: 0.32–0.74, Pfor trend < 0.01). Conversely, participants in the highest quartile of the sweet foods pattern had a higher risk compared to those in the lowest quartile (OR = 1.69, 95% CI: 1.18–2.42, Pfor trend < 0.01). However, no significant association was observed between the healthy pattern, animal foods pattern and tea–alcohol pattern and the risk of CKD combined with hyperuricemia. Conclusions: Our results suggest that the traditional pattern is associated with a reduced risk of CKD combined with hyperuricemia, whereas the sweet foods pattern is associated with an increased risk.
1. Introduction
Chronic kidney disease (CKD) is a progressive condition characterized by structural and functional abnormalities in the kidneys due to various causes.1 Approximately 10% of adults worldwide are affected by some form of CKD which results in 1.2 million deaths.2 In 2019, China had 150.5 million CKD patients, accounting for nearly one-fifth of the global total events.3 CKD is related to an increase in all-cause and cardiovascular mortality,4 and patients who survived morbidities would still have to live for years with disability.5 Instead of treating the illness, it is more crucial to stop CKD from developing.The kidneys play a major role in the extraction of uric acid (UA), with about 70% of the daily UA produced being excreted from the kidneys and the remaining 30% from the gut.6 Therefore, elevated serum UA is common in patients with CKD and worsens as renal function deteriorates. Hyperuricemia is a chronic metabolic disease resulting from high uric acid levels in the blood due to purine metabolism disorders.7 Hyperuricemia was prevalent in the adult population of China at 11.1% in 2015–2016 and 14.0% in 2018–2019.8 Research studies have shown that the prevalence of hyperuricemia exceeds 60% in patients with advanced CKD,9 and about 50% of CKD patients develop hyperuricemia before they require hemodialysis.10 Hyperuricemia is also a risk factor for the onset of CKD, according to earlier meta-analyses.11 For instance, a meta-analysis found that the prevalence of CKD stage ≥3 in gout patients is 24%.12 According to a Japanese cohort study, every 1 mg dL−1 drop in blood urea in male individuals lowered the prevalence of CKD by 23%.13 Therefore, hyperuricemia and CKD have a mutually reinforcing and co-evolving relationship. Patients with both hyperuricemia and CKD also face a higher risk of incident renal replacement therapy and all-cause mortality.14 Hence, there is an urgent need to understand the etiology of these diseases for effective preventive action.
Numerous studies have demonstrated that diet is associated with chronic diseases.15 As people do not consume nutrients or single foods and there are complex interactions between dietary components, dietary patterns that consider the overall characteristics of dietary exposures may better reflect the true association between diet and disease than single nutrients or foods.16,17 Dietary pattern analysis is done in two primary ways: priori dietary patterns and posteriori dietary patterns. Priori dietary patterns, such as the Mediterranean diet (MED) and the Dietary Approaches to Stop Hypertension (DASH), are generally created based on existing dietary guidelines or nutrition recommendations.18 Research studies have shown that higher adherence to a MED or the DASH pattern was linked to a lower risk of CKD and hyperuricemia.19–22 However, high levels of adherence to a priori derived dietary pattern might necessitate changes in food choices and preparation methods, which may present a barrier to adherence.23 Based on relationships between intakes of the various dietary components, posteriori dietary patterns are statistically deduced from the existing dietary consumption data.18 Studies examining posteriori dietary patterns and their association with relevant diseases have been conducted.7,24,25 A dietary pattern characterized by consumption of fresh vegetables, fruits, dairy products, eggs, legumes and their products was found to be associated with a lower risk of hyperuricemia.7 Conversely, higher adherence to a dietary pattern characterized by high intake of poultry, livestock, fish and shrimp, processed meats and nuts was associated with a higher risk of hyperuricemia.7 Similar conclusions can be drawn from studies on CKD and dietary patterns. A dietary pattern characterized by higher intake of plant derived foods such as cereals, tubers, legumes, fruits, and vegetables might benefit kidney function.24 In contrast, higher adherence to a dietary pattern characterized by red meats, poultry and organs, processed and cooked meat, eggs, seafood, cheese, fast foods, snacks, chocolates, alcoholic beverages and coffee was associated with a higher risk of CKD.25
Given the global burden of CKD and the increasing prevalence of hyperuricemia, early detection and the prevention of the development of these two diseases would greatly benefit society and the general population. It is evident that the development of CKD and hyperuricemia is closely linked to dietary factors. However, to date, there is no epidemiological evidence of the association between CKD combined with hyperuricemia and dietary patterns. Therefore, the current study aims to explore the association between dietary patterns and the risk of CKD combined with hyperuricemia in the Northeast China population.
2. Materials and methods
2.1. Study design and participants
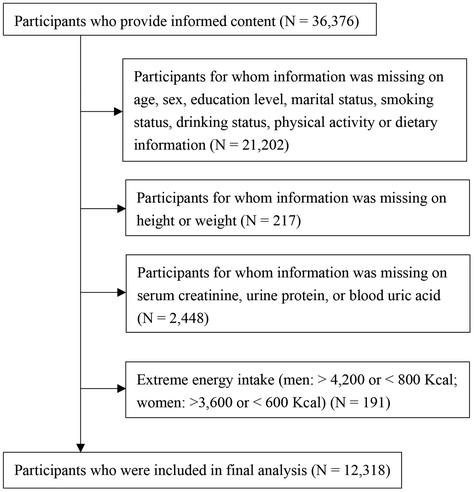
The present study used data from the baseline survey conducted in the regional ethnic cohort of the Northeast Cohort Study of China (NEC-Biobank). The NEC-Biobank focuses on major chronic diseases and risk factors in different populations.26 In the regional ethnic cohort, a total of 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 376 participants aged 18–79 years and who had lived in Northeast China (Shenyang, Fushun, Dalian, Jixi and Xingan league) for more than 3 years were recruited for the baseline survey from July 2018 to June 2020. Participants with lower limb impairment, mental disorders or inability to communicate were excluded. Face-to-face interviews and physical examinations were undertaken by trained interviewers and technicians, respectively. Venous blood and urine samples were gathered from the participants after overnight fasting. The current study was a cross-sectional design based on the baseline survey of the regional ethnic cohort. We enrolled 12
376 participants aged 18–79 years and who had lived in Northeast China (Shenyang, Fushun, Dalian, Jixi and Xingan league) for more than 3 years were recruited for the baseline survey from July 2018 to June 2020. Participants with lower limb impairment, mental disorders or inability to communicate were excluded. Face-to-face interviews and physical examinations were undertaken by trained interviewers and technicians, respectively. Venous blood and urine samples were gathered from the participants after overnight fasting. The current study was a cross-sectional design based on the baseline survey of the regional ethnic cohort. We enrolled 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 318 participants in the final analysis by excluding those with missing information on age, sex, education level, marital status, smoking status, drinking status, physical activity, or dietary information (n = 21
318 participants in the final analysis by excluding those with missing information on age, sex, education level, marital status, smoking status, drinking status, physical activity, or dietary information (n = 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 202); height or weight (n = 217); serum creatinine, urine protein, or blood uric acid (n = 2448); and implausible caloric intake (men: >4200 kcal or <800 kcal; women: >3600 kcal or <600 kcal) (n = 191) (Fig. 1). This study was approved by the Institutional Review Board of Shengjing Hospital of China Medical University (no. 2017PS190K). All participants signed written informed consent forms prior to the study.
202); height or weight (n = 217); serum creatinine, urine protein, or blood uric acid (n = 2448); and implausible caloric intake (men: >4200 kcal or <800 kcal; women: >3600 kcal or <600 kcal) (n = 191) (Fig. 1). This study was approved by the Institutional Review Board of Shengjing Hospital of China Medical University (no. 2017PS190K). All participants signed written informed consent forms prior to the study.
2.2. Outcome variable
Kidney function was evaluated by the estimated glomerular filtration rate (eGFR), which was assessed on serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.27 Albuminuria was defined as the presence of albumin in urine samples. CKD events were defined as an eGFR below 60 mL min−1 1.73 m−2 or the presence of albuminuria. Hyperuricemia is defined as an increased serum UA level above 420 μmol L−1 both in men and women.212.3. Dietary assessment
Dietary intake was assessed at the baseline using a verified 110-item food frequency questionnaire (FFQ). Participants were asked to recall their usual intake of these food items within the past year. The reliability and validity of the FFQ have been proved in previous research.28 The reproducibility coefficients were above 0.5 for most food groups, and the Spearman correlation coefficients between the FFQ and 8 d weighed diet records ranged from 0.3 to 0.7 for most food groups.28 The frequency categories of the FFQ include the following: more than 2 times per day, 1–2 times per day, 4–6 times per week, 2–3 times per week, 1 time per week, 2–3 times per month, and almost never. The consumption of each food item was calculated by multiplying portion sizes by the frequency at which each food item was consumed per day. 110 item foods were categorized into 26 food groups based on the type of food and similar nutrient contents. Factor analysis was conducted to yield dietary patterns and factor loadings for all 26 food groups in grams.17 Varimax rotation was applied to increase data interpretability. Finally, five factors were identified by assessing eigenvalues (>1) and performing the scree test. The factors were named descriptively according to the food groups with high factor loadings (absolute value ≥0.4) with respect to each dietary pattern as follows: healthy pattern, traditional pattern, animal foods pattern, sweet foods pattern, and tea–alcohol pattern.2.4. Covariates
In the current study, data on age (in years), gender (male; female), marital status (married; widowed and others) and education level (low: illiterate or primary school; medium: middle school; high: high school or above) obtained by using a questionnaire were included. The body mass index (BMI) was calculated as a person's weight (in kilograms) divided by height (in meters squared). Overweight was defined as BMI ≥ 24 kg m−2. The categories for smoking status were current or former smokers, and never smokers. The drinking status was categorized as current or former drinkers, and never drinkers. We defined hypertension as average systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or a self-reported history of hypertension. Diabetes was defined as fasting glucose ≥7.0 mmol L−1 or a self-reported history of diabetes. Hyperlipidemia was defined as total cholesterol ≥6.2 mmol L−1 or triglyceride ≥2.26 mmol L−1 or LDL ≥4.14 mmol L−1 or HDL <1.04 mmol L−1 or a self-reported history of hyperlipidemia.292.5. Statistical analysis
The participants were categorized according to the status of CKD combined with hyperuricemia. Normally distributed data are presented as mean ± standard deviation (SD), while data with a skewed distribution are presented as median [interquartile range (IQR)]. Categorical variables are expressed as numbers (percentages). The distribution of participants in terms of demographic and clinical characteristics was examined using the chi-square test for categorical variables, Student's t-test for normally distributed parameters, and the Mann–Whitney U test for parameters with a skewed distribution.The scores for each dietary pattern were categorized into quartiles based on their distributions in all participants. Logistic regression was used to assess the association between dietary patterns and CKD combined with hyperuricemia, yielding crude and multivariable adjusted odds ratios (ORs) and their 95% confidence intervals (CIs). The first quartile (lowest intake) of each exposure variable was used as the reference group. Four stepwise models were used: the crude model did not adjust for variables; model 1 adjusted for age and gender; model 2 further adjusted for the education level, marital status, smoking status, drinking status, physical activity, overweight/obesity, hypertension, diabetes, and hyperlipidemia based on model 1; model 3 further adjusted for the total energy based on model 2.
Additional analyses stratified by age (<50 vs. ≥50 years), gender (male vs. female), BMI (<24 vs. ≥24), hypertension (yes vs. no), diabetes (yes vs. no), hyperlipidemia (yes vs. no), and smoking (current or former vs. never) and drinking status (current or former vs. never) to explore the potential effect modifier. We included multiplicative interaction terms in the regression models to estimate potential interactions. In sensitivity analysis, we also used the Modification of Diet in Renal Disease (MDRD) equation to estimate the eGFR.30 We also estimated the associations of dietary pattern scores with the eGFR and blood UA concentrations by using multivariate linear regression models. All statistical analyses were conducted using SAS version 9.4 for Windows (SAS Institute Inc., Cary, NC, USA). Statistical significance was considered as two-tailed P < 0.05.
3. Results
3.1. Characteristics of the study participants
A total of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 318 participants were included in the present study. The overall prevalence of CKD combined with hyperuricemia was 2.18%. The demographic and clinical characteristics of the participants with and without CKD combined with hyperuricemia are shown in Table 1. Participants with CKD combined with hyperuricemia tended to have higher total energy intake, be male, have a higher educational level, be current or former smokers or alcohol drinkers, be overweight or obese, have hypertension or hyperlipidemia, or have no diabetes (all P < 0.0001).
318 participants were included in the present study. The overall prevalence of CKD combined with hyperuricemia was 2.18%. The demographic and clinical characteristics of the participants with and without CKD combined with hyperuricemia are shown in Table 1. Participants with CKD combined with hyperuricemia tended to have higher total energy intake, be male, have a higher educational level, be current or former smokers or alcohol drinkers, be overweight or obese, have hypertension or hyperlipidemia, or have no diabetes (all P < 0.0001).
| Baseline characteristics | CKD combined with hyperuricemia | P value | |
|---|---|---|---|
| Yes (n = 268) | No (n = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050) 050) |
||
| Abbreviations: CKD, chronic kidney disease; BMI, body mass index; eGFR: estimated glomerular filtration rate. Continuous variables are presented as mean ± SD or median (IQR) according to their distribution; category variables were shown as numbers (percentage). Pvalues are derived from Student's t-tests or Mann–Whitney U tests for continuous variables according to the data distribution, and chi-square tests for the category variables. | |||
| Age (years) | 55 (41, 61) | 55 (46, 60) | 0.8839 |
| Sex, n (%) | <0.0001 | ||
| Male | 226 (84.33) | 3865 (32.07) | |
| Female | 42 (15.67) | 8185 (67.93) | |
| Marital status, n (%) | 0.7656 | ||
| Married | 227 (84.70) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 285 (85.35) 285 (85.35) |
|
| Widowed and others | 41 (15.30) | 1765 (14.65) | |
| Education level, n (%) | |||
| Low | 17 (6.34) | 1939 (16.09) | <0.0001 |
| Medium | 84 (31.34) | 4275 (35.48) | 0.1616 |
| High | 167 (62.31) | 5836 (48.43) | <0.0001 |
| Smoking status, n (%) | <0.0001 | ||
| Current or former | 155 (57.84) | 2974 (24.68) | |
| Never | 113 (42.16) | 9076 (75.32) | |
| Drinking status, n (%) | <0.0001 | ||
| Current or former | 155 (57.84) | 3386 (28.10) | |
| Never | 113 (42.16) | 8664 (71.90) | |
| Physical activity (MET hours per week) | 92.07 (55.10, 153.23) | 93.22 (58.20, 144.92) | 0.9107 |
| BMI | <0.0001 | ||
| <24 | 38 (14.18) | 4749 (39.41) | |
| ≥24 | 230 (85.82) | 7301 (60.59) | |
| Hypertension, n (%) | <0.0001 | ||
| Yes | 165 (61.57) | 4314 (35.80) | |
| No | 103 (38.43) | 7736 (64.20) | |
| Diabetes, n (%) | <0.0001 | ||
| Yes | 51 (19.03) | 1304 (10.82) | |
| No | 217 (80.97) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 746 (89.18) 746 (89.18) |
|
| Hyperlipidemia, n (%) | <0.0001 | ||
| Yes | 184 (68.66) | 4970 (41.24) | |
| No | 84 (31.34) | 7080 (58.76) | |
| eGFR (mL min−1 1.73 m−2) | 92.55 (75.52, 104) | 98.02 (89.07, 105.53) | <0.0001 |
| Urinary protein (g L−1) | 0.20 (0.10, 1.00) | 0.00 | <0.0001 |
| Blood uric acid (μmol L−1) | 461.20 (436.60, 507.70) | 294.51 (247.10, 353.10) | <0.0001 |
| Total energy | 1753.39 (1341.73, 2166.99) | 1533.33 (1220.36, 1903.51) | <0.0001 |
| Healthy pattern | −0.20 (−0.84, 0.43) | −0.16 (−0.72, 0.54) | 0.0947 |
| Traditional pattern | −0.04 (−0.54, 0.67) | −0.16 (−0.71, 0.55) | <0.05 |
| Animal foods pattern | 0.09 (−0.35, 0.69) | −0.22 (−0.48, 0.21) | <0.0001 |
| Sweet foods pattern | −0.17 (−0.58, 0.80) | −0.23 (−0.57, 0.28) | <0.05 |
| Tea–alcohol pattern | 0.30 (−0.30, 1.24) | −0.20 (−0.62, 0.40) | <0.0001 |
3.2. Dietary pattern extraction
We finally extracted five dietary patterns by factor analysis, which totally explained the 38.93% variance of all 26 food groups (Table 2). The first dietary pattern called the healthy pattern was high in vegetables, tubers, fruits, eggs, legumes and legume products, dairy, whole grains, nuts, and sesame paste. The second dietary pattern called the traditional pattern was high in pickled foods, meat, Chinese sauerkraut, ginger and garlic, and refined grains. The third dietary pattern called the animal foods pattern was high in animal organs, animal blood, preserved eggs, and processed meat products. The fourth dietary pattern called the sweet foods pattern was high in sugar-containing beverages, ice cream and candy, cake, and fruit or vegetable juice. The fifth dietary pattern called the tea–alcohol pattern was high in alcohol and alcoholic beverages, tea and tea beverages, and fish.| Food groups | Healthy pattern | Traditional pattern | Animal foods pattern | Sweet foods pattern | Tea–alcohol pattern |
|---|---|---|---|---|---|
| a Factor loadings represent the relative contribution of each food group to the dietary pattern. The five groups with highest factor loadings in each dietary pattern are shown in bold. | |||||
| Refined grain | 0.29 | 0.41 | 0.14 | −0.05 | −0.14 |
| Whole grain | 0.48 | 0.08 | −0.02 | −0.24 | −0.06 |
| Dairy | 0.50 | −0.02 | 0.08 | 0.16 | −0.14 |
| Meat | 0.02 | 0.49 | 0.32 | 0.05 | 0.13 |
| Processed meat products | −0.01 | 0.13 | 0.50 | 0.33 | −0.17 |
| Animal blood | 0.08 | 0.07 | 0.68 | −0.02 | 0.04 |
| Animal organs | 0.04 | 0.12 | 0.68 | 0.06 | 0.12 |
| Fish | 0.39 | −0.25 | 0.32 | 0.13 | 0.41 |
| Egg | 0.54 | 0.10 | 0.09 | −0.04 | −0.10 |
| Preserved eggs | 0.06 | −0.05 | 0.57 | 0.06 | 0.03 |
| Fruit | 0.59 | −0.02 | −0.03 | 0.25 | 0.06 |
| Vegetable | 0.65 | 0.38 | 0.04 | −0.02 | 0.18 |
| Tubers | 0.62 | 0.09 | 0.02 | −0.07 | −0.07 |
| Legumes and legume products | 0.53 | 0.05 | 0.10 | 0.10 | 0.21 |
| Pickled foods | 0.08 | 0.74 | 0.00 | 0.02 | 0.12 |
| Chinese sauerkraut | −0.03 | 0.46 | 0.01 | 0.10 | 0.12 |
| Cake | 0.22 | −0.01 | 0.09 | 0.53 | −0.08 |
| Ginger and garlic | 0.30 | 0.41 | −0.06 | −0.17 | 0.39 |
| Ice cream and candy | 0.01 | 0.39 | 0.00 | 0.55 | −0.12 |
| Nuts | 0.47 | −0.03 | −0.01 | 0.24 | 0.31 |
| Tea and tea beverages | 0.03 | 0.27 | −0.06 | 0.03 | 0.54 |
| Coffee | 0.06 | −0.20 | −0.04 | 0.39 | 0.27 |
| Fruit or vegetable juice | 0.10 | −0.03 | 0.03 | 0.48 | 0.10 |
| Sugar-containing beverages | −0.11 | 0.12 | 0.17 | 0.61 | −0.01 |
| Alcohol and alcoholic beverages | −0.13 | 0.17 | 0.25 | −0.01 | 0.61 |
| Sesame paste | 0.45 | −0.20 | −0.03 | 0.19 | 0.17 |
| Explained variation in food groups, % | 11.79 | 7.54 | 7.19 | 6.86 | 5.55 |
3.3. CKD combined with hyperuricemia
Table 3 reveals the correlation between various dietary patterns and CKD combined with hyperuricemia by logistic regression. The highest quartile of the traditional pattern score was related to lower risk of CKD combined with hyperuricemia compared with the lowest quartiles scores (OR = 0.49, 95% CI = 0.32–0.74, Pfor trend < 0.01), whereas patients with CKD combined with hyperuricemia with highest adherence to the sweet foods pattern had a significantly increased risk compared to the control group (OR = 1.69, 95% CI = 1.18–2.42, Pfor trend < 0.01). The healthy pattern was found to be associated with a reduced risk of CKD combined with hyperuricemia, and the animal foods pattern and the tea–alcohol pattern with an increased risk. However, these associations were not statistically significant after adjusting for confounding factors.| Dietary patterns | Quartile of dietary pattern scores | P trend | |||
|---|---|---|---|---|---|
| Q 1 | Q 2 | Q 3 | Q 4 | ||
| Abbreviations: CKD, chronic kidney disease.a Unadjusted models.b Adjusted only for age and gender.c Further adjusted for education level, marital status, smoking status, drinking status, physical activity, overweight/obesity, hypertension, diabetes, and hyperlipidemia.d Further adjusted for total energy.e Odds ratios and 95% confidence intervals are calculated using multiple logistic regression.f Ptrend for linear trend calculated from category median values. | |||||
| Healthy pattern | <−0.72 | −0.72, −0.16 | −0.16, 0.54 | >0.54 | |
| Cases, N (%) | 78 (2.53) | 61 (1.98) | 76 (2.47) | 53 (1.72) | |
| Crude modela | 1.00 (ref) | 0.78 (0.55, 1.09)e | 0.97 (0.71, 1.34) | 0.67 (0.47, 0.96) | 0.0725 |
| Model 1b | 1.00 (ref) | 0.91 (0.64, 1.28) | 1.22 (0.88, 1.70) | 0.82 (0.57, 1.17) | 0.4844 |
| Model 2c | 1.00 (ref) | 0.96 (0.67, 1.36) | 1.28 (0.91, 1.79) | 0.88 (0.60, 1.27) | 0.7484 |
| Model 3d | 1.00 (ref) | 0.90 (0.63, 1.28) | 1.14 (0.79, 1.63) | 0.68 (0.43, 1.08) | 0.1994 |
| Traditional pattern | <−0.70 | −0.70, −0.16 | −0.16, 0.55 | >0.55 | |
| Cases, N (%) | 50 (1.62) | 66 (2.14) | 76 (2.47) | 76 (2.47) | |
| Crude model | 1.00 (ref) | 1.33 (0.92, 1.93) | 1.53 (1.07, 2.21) | 1.53 (1.07, 2.21) | <0.05 |
| Model 1 | 1.00 (ref) | 0.89 (0.61, 1.31) | 0.83 (0.57, 1.21) | 0.59 (0.41, 0.87) | <0.01 |
| Model 2 | 1.00 (ref) | 0.85 (0.58, 1.25) | 0.81 (0.55, 1.19) | 0.57 (0.39, 0.85) | <0.01 |
| Model 3 | 1.00 (ref) | 0.85 (0.58, 1.26) | 0.77 (0.53, 1.13) | 0.49 (0.32, 0.74) | <0.01 |
| Animal foods pattern | <−0.48 | −0.48, −0.22 | −0.22, 0.22 | >0.22 | |
| Cases, N (%) | 46 (1.49) | 48 (1.56) | 58 (1.88) | 116 (3.77) | |
| Crude model | 1.00 (ref) | 1.05 (0.69, 1.57) | 1.27 (0.86, 1.88) | 2.58 (1.84, 3.68) | <0.0001 |
| Model 1 | 1.00 (ref) | 1.01 (0.67, 1.54) | 1.07 (0.72, 1.60) | 1.50 (1.05, 2.17) | <0.01 |
| Model 2 | 1.00 (ref) | 1.03 (0.68, 1.56) | 1.12 (0.75, 1.69) | 1.44 (1.00, 2.11) | <0.05 |
| Model 3 | 1.00 (ref) | 1.04 (0.68, 1.58) | 1.13 (0.76, 1.70) | 1.42 (0.98, 2.08) | <0.05 |
| Sweet foods pattern | <−0.57 | −0.57, −0.23 | −0.23, 0.28 | >0.28 | |
| Cases, N (%) | 70 (2.27) | 56 (1.82) | 52 (1.69) | 90 (2.92) | |
| Crude model | 1.00 (ref) | 0.80 (0.56, 1.13) | 0.74 (0.51, 1.06) | 1.30 (0.95, 1.78) | <0.05 |
| Model 1 | 1.00 (ref) | 1.10 (0.76, 1.57) | 1.03 (0.71, 1.50) | 1.65 (1.17, 2.32) | <0.01 |
| Model 2 | 1.00 (ref) | 1.15 (0.79, 1.65) | 1.10 (0.75, 1.60) | 1.71 (1.21, 2.43) | <0.01 |
| Model 3 | 1.00 (ref) | 1.15 (0.79, 1.66) | 1.10 (0.75, 1.60) | 1.69 (1.18, 2.42) | <0.01 |
| Tea–alcohol pattern | <−0.62 | −0.62, −0.19 | −0.19, 0.41 | >0.41 | |
| Cases, N (%) | 39 (1.27) | 34 (1.10) | 73 (2.37) | 122 (3.96) | |
| Crude model | 1.00 (ref) | 0.87 (0.55, 1.38) | 1.89 (1.29, 2.83) | 3.22 (2.26, 4.69) | <0.0001 |
| Model 1 | 1.00 (ref) | 0.85 (0.53, 1.37) | 1.41 (0.95, 2.13) | 1.43 (0.98, 2.13) | <0.05 |
| Model 2 | 1.00 (ref) | 0.84 (0.52, 1.35) | 1.29 (0.86, 1.97) | 1.25 (0.83, 1.90) | 0.1478 |
| Model 3 | 1.00 (ref) | 0.86 (0.53, 1.39) | 1.32 (0.88, 2.03) | 1.24 (0.83, 1.90) | 0.1772 |
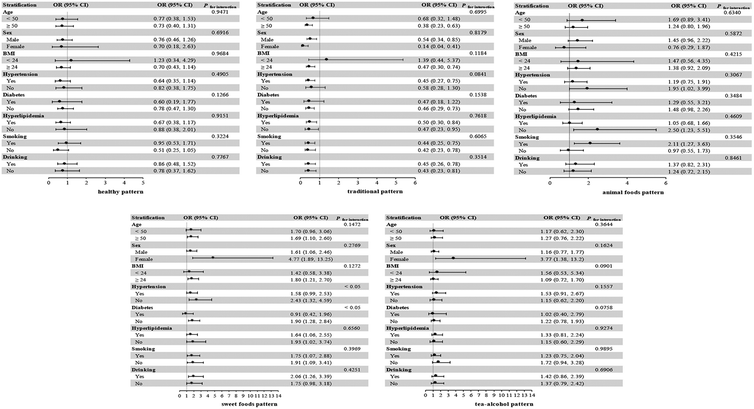
3.4. Subgroup analyses
Subgroup analyses showed the estimated associations between the dietary patterns and CKD combined with hyperuricemia, stratified by age, sex, BMI, hypertension, diabetes, hyperlipidemia, smoking status, and drinking status. The majority of findings of stratified analyses were consistent with the main results (Fig. 2).No discernible differences were observed in associations between the risk of CKD combined with hyperuricemia and the traditional pattern according to strata of sex, hyperlipidemia, smoking status, and drinking status. Significant negative associations were observed among participants aged ≥50 years, with BMI ≥24, with hypertension, and without diabetes. No significant interaction was observed between stratified factors and the traditional pattern (all Pfor interaction > 0.05).
No discernible differences were observed in associations between the risk of CKD combined with hyperuricemia and the sweet foods pattern according to strata of sex, hyperlipidemia, and smoking status. Significant positive associations were observed among participants aged ≥50 years, with BMI ≥24, without hypertension, without diabetes, and current or former drinkers. Moreover, we observed significant interactions between the hypertension status (Pfor interaction < 0.05) and the diabetes status (Pfor interaction < 0.05) with the sweet foods pattern.
3.5. Sensitivity analyses
The sensitivity analysis provided evidence of the relative robustness of our study. We used the MDRD formula to calculate the eGFR and found similar results in line with the main findings. The highest quartile of the traditional pattern score was related to a lower risk of CKD combined with hyperuricemia compared with the lowest quartile score (OR = 0.49, 95% CI = 0.32–0.75, Pfor trend < 0.01). Conversely, patients with CKD combined with hyperuricemia with the highest adherence to the sweet foods pattern had a significantly increased risk compared to the control group (OR = 1.60, 95% CI = 1.11–2.31, Pfor trend < 0.05) (Table 4). The linear regression relationships between the dietary pattern scores and the eGFR and blood UA concentrations are shown in Tables S1 and S2, ESI,† respectively.| Dietary patterns | Quartile of dietary pattern scores | P trend | |||
|---|---|---|---|---|---|
| Q 1 | Q 2 | Q 3 | Q 4 | ||
| Abbreviations: CKD, chronic kidney disease. The eGFR was determined by the Modification of Diet in Renal Disease (MDRD) formula.a Odds ratios and 95% confidence intervals were calculated using multiple logistic regression and adjusted for age, sex, education level, marital status, smoking status, drinking status, physical activity, overweight/obesity, hypertension, diabetes, hyperlipidemia, and total energy.b Ptrend for linear trend calculated from category median values. | |||||
| Healthy pattern | 1.00 (ref) | 0.89 (0.62, 1.28)a | 1.13 (0.78, 1.63) | 0.68 (0.42, 1.08) | 0.2044 |
| Traditional pattern | 1.00 (ref) | 0.88 (0.60, 1.32) | 0.78 (0.53, 1.17) | 0.49 (0.32, 0.75) | <0.01 |
| Animal foods pattern | 1.00 (ref) | 1.09 (0.71, 1.67) | 1.13 (0.75, 1.72) | 1.45 (0.99, 2.15) | <0.05 |
| Sweet foods pattern | 1.00 (ref) | 1.16 (0.80, 1.68) | 1.07 (0.72, 1.57) | 1.60 (1.11, 2.31) | <0.05 |
| Tea–alcohol pattern | 1.00 (ref) | 0.87 (0.54, 1.40) | 1.25 (0.82, 1.93) | 1.19 (0.79, 1.82) | 0.2733 |
4. Discussion
In this cross-sectional study involving 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 318 participants, we found that a higher adherence to the traditional pattern was associated with a lower risk of CKD combined with hyperuricemia, while the sweet foods pattern was associated with the opposite effect. No significant associations were observed between the healthy pattern, animal foods pattern and tea–alcohol pattern and the risk of CKD combined with hyperuricemia after adjusting for confounding factors.
318 participants, we found that a higher adherence to the traditional pattern was associated with a lower risk of CKD combined with hyperuricemia, while the sweet foods pattern was associated with the opposite effect. No significant associations were observed between the healthy pattern, animal foods pattern and tea–alcohol pattern and the risk of CKD combined with hyperuricemia after adjusting for confounding factors.
Previous studies indicated that elevated serum UA was an independent predictor for the development of CKD.31 The underlying mechanism between UA and CKD risk are as follows: (1) UA induces hypertension by affecting endothelial function and reducing nitric oxide production;32 (2) hyperuricemia triggers the activation of the renin–angiotensin–aldosterone system, leading to renal vasoconstriction and reduced renal plasma flow;33 (3) UA may increase oxidative stress, leading to mitochondrial dysfunction, over-secretion of pro-inflammatory cytokines, and proliferation of vascular smooth muscle cells;6 and (4) UA crystals can cause tubular damage through inflammation mediated by direct physical mechanisms.6
Diet has also been implicated in the risk of CKD25,34 and hyperuricemia.7,35,36 The traditional pattern was loaded high with pickled foods, meat, Chinese sauerkraut, ginger and garlic, and refined grains. Studies have shown that consumption of sodium-rich pickled foods,37 as well as red meat and refined grains,38,39 adversely affects kidney function and increases the risk of CKD. Consumption of purine-rich meat also increases the risk of hyperuricemia.40 However, a cross-sectional study with 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 619 participants indicated that adherence to tuber and fermented vegetables could decrease the risk of hyperuricemia (OR = 0.78, 95% CI: 0.69–0.88).29 The inner mechanisms may be attributed to the probiotics and antioxidant potential in fermented vegetables.41 Multiple lines of evidence indicate that dietary probiotics can restore the normal gut microbiome composition, and prevent and alleviate metabolic diseases by enhancing intestinal barrier integrity, reducing gut inflammation, and maintaining insulin sensitivity.42–44 Experimental evidence has shown that Lactobacillus could alleviate hyperuricemia in rats, suggesting its potential therapeutic effect on patients with chronic hyperuricemia.45 While there is a lack of research on the association between diet and CKD, a prospective cohort study with 9229 participants showed that adherence to higher intake of fermented vegetables was associated with lower risks for incident proteinuria (HR = 0.86, 95% CI: 0.75–0.98).46 CKD itself can lead to a dysregulated gut microbiome, and probiotic supplementation has been shown to decrease uremic toxin production and improve kidney function in animals with CKD.47,48 At present, there are few studies on the association between ginger and garlic and CKD or hyperuricemia, but some studies have shown that ginger and garlic have the effects of protecting renal function and reducing uric acid due to their anti-inflammatory and antioxidant effects.49–51 A prospective study with 3052 adults indicated that the habitual intake of garlic was associated with a 32% lower incidence of CKD.52 Thus, the protective effect of the traditional pattern on CKD combined with hyperuricemia may be due to the beneficial effects of Chinese sauerkraut, ginger and garlic, which offset the adverse effects of pickled foods, meat and refined grains on the disease.
619 participants indicated that adherence to tuber and fermented vegetables could decrease the risk of hyperuricemia (OR = 0.78, 95% CI: 0.69–0.88).29 The inner mechanisms may be attributed to the probiotics and antioxidant potential in fermented vegetables.41 Multiple lines of evidence indicate that dietary probiotics can restore the normal gut microbiome composition, and prevent and alleviate metabolic diseases by enhancing intestinal barrier integrity, reducing gut inflammation, and maintaining insulin sensitivity.42–44 Experimental evidence has shown that Lactobacillus could alleviate hyperuricemia in rats, suggesting its potential therapeutic effect on patients with chronic hyperuricemia.45 While there is a lack of research on the association between diet and CKD, a prospective cohort study with 9229 participants showed that adherence to higher intake of fermented vegetables was associated with lower risks for incident proteinuria (HR = 0.86, 95% CI: 0.75–0.98).46 CKD itself can lead to a dysregulated gut microbiome, and probiotic supplementation has been shown to decrease uremic toxin production and improve kidney function in animals with CKD.47,48 At present, there are few studies on the association between ginger and garlic and CKD or hyperuricemia, but some studies have shown that ginger and garlic have the effects of protecting renal function and reducing uric acid due to their anti-inflammatory and antioxidant effects.49–51 A prospective study with 3052 adults indicated that the habitual intake of garlic was associated with a 32% lower incidence of CKD.52 Thus, the protective effect of the traditional pattern on CKD combined with hyperuricemia may be due to the beneficial effects of Chinese sauerkraut, ginger and garlic, which offset the adverse effects of pickled foods, meat and refined grains on the disease.
Conversely, for the sweet foods pattern, a prospective cohort study with 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 766 participants found that greater adherence to the sweet food pattern was significantly positively associated with an increased risk of hyperuricemia (OR = 1.22, 95% CI: 1.12–1.33).35 The presence of fructose in sweet foods may contribute to this association, as fructose consumption can stimulate UA levels through the catabolism of adenine nucleotide.53 In addition, excessive fructose intake has been shown to alter the composition of the gut microbiota, affecting UA metabolism.53 A cross-sectional study with 1521 subjects found that scoring on the western dietary pattern was associated with enhanced odds of CKD (OR = 2.12, 95% CI: 1.19–3.76).34 Sugar-containing beverages in the western diet often use high fructose corn syrup as a sweetener. However, this can lead to hyperuricemia, which is believed to be a contributing factor to kidney damage caused by fructose consumption.54
766 participants found that greater adherence to the sweet food pattern was significantly positively associated with an increased risk of hyperuricemia (OR = 1.22, 95% CI: 1.12–1.33).35 The presence of fructose in sweet foods may contribute to this association, as fructose consumption can stimulate UA levels through the catabolism of adenine nucleotide.53 In addition, excessive fructose intake has been shown to alter the composition of the gut microbiota, affecting UA metabolism.53 A cross-sectional study with 1521 subjects found that scoring on the western dietary pattern was associated with enhanced odds of CKD (OR = 2.12, 95% CI: 1.19–3.76).34 Sugar-containing beverages in the western diet often use high fructose corn syrup as a sweetener. However, this can lead to hyperuricemia, which is believed to be a contributing factor to kidney damage caused by fructose consumption.54
It should be noted that our study has some strengths. First, to our knowledge, our current study is the first to explore the association between the posteriori dietary pattern and the risk of CKD combined with hyperuricemia from an overall diet perspective. Meanwhile, our study includes sufficiently detailed clinical information to make the evaluation more objective and valid. Second, we carefully and comprehensively considered a diverse array of disease-related factors in our statistical analysis, including smoking status, drinking status, overweight/obesity, hypertension, diabetes, and hyperlipidemia, which likely contributed to the reliability of the results. Third, we carried out subgroup analyses and sensitivity analyses, and the findings were consistent, further strengthening the robustness of our results.
However, several caveats are worth being emphasized. First, our analysis is based on self-reported data using an FFQ, which may have led to potential over- or underestimates of actual exposures. Nonetheless, it is important to note that our dietary intake information was based on in-person interviews, which likely enhances the accuracy of the data. In addition, we took measures to reduce these biases by employing trained interviewers and using a validated FFQ. Second, dietary patterns can vary among different populations due to factors such as regional differences, cultural practices, and socioeconomic status. As a result, our findings may not be broadly generalizable to other populations. Third, despite considering many covariates in our statistical analysis, we could not rule out the possibility that residual and unmeasured factors might have contributed to the observed associations. This limitation is inherent in observational studies and calls for cautious interpretation of the results.
5. Conclusions
Our study provides valuable insights into the association between dietary patterns and the risk of CKD combined with hyperuricemia, based on a large population in Northeast China. Specifically, we found that the traditional pattern was associated with a lower risk of CKD combined with hyperuricemia, while the sweet foods pattern was linked to a higher risk. These findings are significant as they shed light on the potential role of dietary choices in the prevention and management of CKD and hyperuricemia. However, it is essential to consider the limitations of our study, and further research is needed to validate and expand on these results in diverse populations and settings.Author contributions
Hao Ju and Chao Ji conceptualized the study; Mengrui Luo and Tiancong Liu analyzed the data and wrote the paper; Yang Xia, Yuhong Zhao and Chao Ji took part in reviewing the paper. All authors have read and approved the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Jie Bang Gua Shuai Project of Liaoning Province (2021JH1/1040050), and the Precise Prevention, Diagnosis, and Treatment for Metabolic Diseases (25129022-YGJC-75). The authors thank all the people who have contributed to this study.References
- K. Kalantar-Zadeh, T. H. Jafar and D. Nitsch, et al., Chronic kidney disease, Lancet, 2021, 398, 786–802 CrossRef CAS.
- GBD Chronic Kidney Disease Collaboration, Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017, Lancet, 2020, 395, 709–733 CrossRef.
- Y. Li, Y. Ning and B. Shen, et al., Temporal trends in prevalence and mortality for chronic kidney disease in China from 1990 to 2019: an analysis of the Global Burden of Disease Study 2019, Clin. Kidney J., 2023, 16, 312–321 CrossRef.
- A. Bayán-Bravo, J. R. Banegas and C. Donat-Vargas, et al., The Mediterranean Diet Protects Renal Function in Older Adults: A Prospective Cohort Study, Nutrients, 2022, 14, 432 CrossRef.
- C. L. Wu, W. H. Tsai and J. S. Liu, et al., Vegan Diet Is Associated with a Lower Risk of Chronic Kidney Disease in Patients with Hyperuricemia, Nutrients, 2023, 15, 1444 CrossRef CAS PubMed.
- H. Yanai, H. Adachi and M. Hakoshima, et al., Molecular Biological and Clinical Understanding of the Pathophysiology and Treatments of Hyperuricemia and Its Association with Metabolic Syndrome, Cardiovascular Diseases and Chronic Kidney Disease, Int. J. Mol. Sci., 2021, 22, 9221 CrossRef CAS PubMed.
- M. Zhou, X. Huang and R. Li, et al., Association of dietary patterns with blood uric acid concentration and hyperuricemia in northern Chinese adults, Nutr. J., 2022, 21, 42 CrossRef CAS.
- M. Zhang, X. Zhu and J. Wu, et al., Prevalence of Hyperuricemia Among Chinese Adults: Findings From Two Nationally Representative Cross-Sectional Surveys in 2015–16 and 2018–19, Front. Immunol., 2021, 12, 791983 CrossRef CAS PubMed.
- H. J. Jeon, J. Oh and D. H. Shin, Urate-lowering agents for asymptomatic hyperuricemia in stage 3–4 chronic kidney disease: Controversial role of kidney function, PLoS One, 2019, 14, e0218510 CrossRef CAS.
- R. J. Johnson, T. Nakagawa and D. Jalal, et al., Uric acid and chronic kidney disease: which is chasing which?, Nephrol., Dial., Transplant., 2013, 28, 2221–2228 CrossRef CAS.
- M. T. James, A. S. Levey and M. Tonelli, et al., Incidence and Prognosis of Acute Kidney Diseases and Disorders Using an Integrated Approach to Laboratory Measurements in a Universal Health Care System, JAMA Network Open, 2019, 2, e191795 CrossRef PubMed.
- M. J. Roughley, J. Belcher and C. D. Mallen, et al., Gout and risk of chronic kidney disease and nephrolithiasis: meta-analysis of observational studies, Arthritis Res. Ther., 2015, 17, 90 CrossRef.
- M. Kuwabara, I. Hisatome and K. Niwa, et al., The Optimal Range of Serum Uric Acid for Cardiometabolic Diseases: A 5-Year Japanese Cohort Study, J. Clin. Med., 2020, 9, 942 CrossRef CAS PubMed.
- C. L. Lee and J. S. Wang, Effects of hyperuricemia on incident renal replacement therapy and all-cause mortality among patients with chronic kidney disease stages 3–5: a retrospective cohort study, Rev. Paul. Med., 2019, 137, 523–529 Search PubMed.
- T. Naber and S. Purohit, Chronic Kidney Disease: Role of Diet for a Reduction in the Severity of the Disease, Nutrients, 2021, 13, 3277 CrossRef CAS PubMed.
- V. K. Sullivan and C. M. Rebholz, Nutritional Epidemiology and Dietary Assessment for Patients With Kidney Disease: A Primer, Am. J. Kidney Dis., 2023, 81, 717–727 CrossRef CAS.
- F. B. Hu, Dietary pattern analysis: a new direction in nutritional epidemiology, Curr. Opin. Lipidol., 2002, 13, 3–9 CrossRef CAS PubMed.
- P. M. Waijers, E. J. Feskens and M. C. Ocké, A critical review of predefined diet quality scores, Br. J. Nutr., 2007, 97, 219–231 CrossRef CAS PubMed.
- M. Guasch-Ferré, M. Bulló and N. Babio, et al., Mediterranean diet and risk of hyperuricemia in elderly participants at high cardiovascular risk, J. Gerontol., Ser. A, 2013, 68, 1263–1270 CrossRef PubMed.
- C. D'Alessandro, D. Giannese and V. Panichi, et al., Mediterranean Dietary Pattern Adjusted for CKD Patients: The MedRen Diet, Nutrients, 2023, 15, 1256 CrossRef.
- K. Yi, S. Cui and M. Tang, et al., Adherence to DASH Dietary Pattern and Its Association with Incident Hyperuricemia Risk: A Prospective Study in Chinese Community Residents, Nutrients, 2022, 14, 4853 CrossRef CAS.
- H. Mozaffari, S. Ajabshir and S. Alizadeh, Dietary Approaches to Stop Hypertension and risk of chronic kidney disease: A systematic review and meta-analysis of observational studies, Clin. Nutr., 2020, 39, 2035–2044 CrossRef.
- E. N. Paterson, C. E. Neville and S. M. Wallace, et al., Dietary patterns associated with renal impairment in the Northern Ireland Cohort for the Longitudinal Study of Ageing (NICOLA), Eur. J. Nutr., 2021, 60, 4045–4054 CrossRef CAS.
- D. Mao and J. Cheng, Two Dietary Patterns From China Might Benefit Kidney Function, as Indicated by Latent Profile Analysis, J. Renal Nutr., 2022, 32, 702–709 CrossRef CAS.
- S. S. Xu, J. Hua and Y. Q. Huang, et al., Association between dietary patterns and chronic kidney disease in a middle-aged Chinese population, Public Health Nutr., 2020, 23, 1058–1066 CrossRef PubMed.
- Q. Chang, Q. Wu and Y. Xia, et al., Cohort Profile: The Northeast China Biobank (NEC-Biobank), Int. J. Epidemiol., 2023, 52, e125–e136 CrossRef PubMed.
- A. S. Levey, L. A. Stevens and C. H. Schmid, et al., A new equation to estimate glomerular filtration rate, Ann. Intern. Med., 2009, 150, 604–612 CrossRef.
- Q. Cui, Y. Xia and Y. Liu, et al., Validity and reproducibility of a FFQ for assessing dietary intake among residents of northeast China: northeast cohort study of China, Br. J. Nutr., 2022, 1–14 Search PubMed.
- Y. Yang, W. Piao and K. Huang, et al., Dietary Pattern Associated with the Risk of Hyperuricemia in Chinese Elderly: Result from China Nutrition and Health Surveillance 2015–2017, Nutrients, 2022, 14, 844 CrossRef CAS PubMed.
- F. Guo, Y. Lin and L. Meng, et al., Association of copper exposure with prevalence of chronic kidney disease in older adults, Clin. Nutr., 2022, 41, 2720–2728 CrossRef CAS.
- J. H. Chen, C. C. Tsai and Y. H. Liu, et al., Sex Difference in the Associations among Hyperuricemia with New-Onset Chronic Kidney Disease in a Large Taiwanese Population Follow-Up Study, Nutrients, 2022, 14, 3832 CrossRef CAS PubMed.
- R. J. Johnson, D. H. Kang and D. Feig, et al., Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease?, Hypertension, 2003, 41, 1183–1190 CrossRef CAS.
- S. G. Mallat, S. Al Kattar and B. Y. Tanios, et al., Hyperuricemia, Hypertension, and Chronic Kidney Disease: an Emerging Association, Curr. Hypertens. Rep., 2016, 18, 74 CrossRef.
- N. Naderinejad, H. S. Ejtahed and G. Asghari, et al., Dietary Patterns and Risk of Chronic Kidney Disease Among Tehranian Adults with High Blood Pressure, Int. J. Endocrinol. Metab., 2020, 18, e89709 Search PubMed.
- T. Zhang, S. Rayamajhi and G. Meng, et al., Dietary patterns and risk for hyperuricemia in the general population: Results from the TCLSIH cohort study, Nutrition, 2022, 93, 111501 CrossRef CAS.
- X. Wu, W. Tang and D. Tang, et al., Two a posteriori dietary patterns are associated with risks of hyperuricemia among adults in less-developed multiethnic regions in Southwest China, Nutr. Res., 2023, 110, 96–107 CrossRef CAS PubMed.
- C. W. Lin, I. W. Chen and Y. T. Lin, et al., Association of unhealthy dietary behaviors with renal function decline in patients with diabetes, BMJ Open Diabetes Res. Care, 2020, 8, e000743 CrossRef PubMed.
- A. L. Kamper and S. Strandgaard, Long-Term Effects of High-Protein Diets on Renal Function, Annu. Rev. Nutr., 2017, 37, 347–369 CrossRef CAS PubMed.
- M. Mazidi, N. Katsiki and D. P. Mikhailidis, et al., A higher ratio of refined grain to whole grain is associated with a greater likelihood of chronic kidney disease: a population-based study, Br. J. Nutr., 2019, 121, 1294–1302 CrossRef CAS.
- S. Aihemaitijiang, Y. Zhang and L. Zhang, et al., The Association between Purine-Rich Food Intake and Hyperuricemia: A Cross-Sectional Study in Chinese Adult Residents, Nutrients, 2020, 12, 3835 CrossRef CAS.
- E. Knez, K. Kadac-Czapska and M. Grembecka, Fermented Vegetables and Legumes vs. Lifestyle Diseases: Microbiota and More, Life, 2023, 13, 1044 CrossRef CAS.
- Y. Cai, X. Yang and S. Chen, et al., Regular consumption of pickled vegetables and fermented bean curd reduces the risk of diabetes: a prospective cohort study, Front. Public Health, 2023, 11, 1155989 CrossRef.
- L. Mayorga Reyes, R. González Vázquez and S. M. Cruz Arroyo, et al., Correlation between diet and gut bacteria in a population of young adults, Int. J. Food Sci. Nutr., 2016, 67, 470–478 CrossRef PubMed.
- F. Bianchi, A. Duque and S. M. I. Saad, et al., Gut microbiome approaches to treat obesity in humans, Appl. Microbiol. Biotechnol., 2019, 103, 1081–1094 CrossRef CAS.
- Y. Xiao, C. Zhang and X. Zeng, et al., Microecological treatment of hyperuricemia using Lactobacillus from pickles, BMC Microbiol., 2020, 20, 195 CrossRef CAS PubMed.
- J. H. Jhee, Y. K. Kee and J. T. Park, et al., A Diet Rich in Vegetables and Fruit and Incident CKD: A Community-Based Prospective Cohort Study, Am. J. Kidney Dis., 2019, 74, 491–500 CrossRef.
- A. Ramezani and D. S. Raj, The gut microbiome, kidney disease, and targeted interventions, J. Am. Soc. Nephrol., 2014, 25, 657–670 CrossRef CAS.
- L. Koppe, D. Mafra and D. Fouque, Probiotics and chronic kidney disease, Kidney Int., 2015, 88, 958–966 CrossRef CAS.
- B. G. Baptista, M. Ribeiro and L. F. Cardozo, et al., Nutritional benefits of ginger for patients with non-communicable diseases, Clin. Nutr. ESPEN, 2022, 49, 1–16 CrossRef PubMed.
- Q. Wang, Q. Yang and X. Cao, et al., Enhanced oral bioavailability and anti-gout activity of [6]-shogaol-loaded solid lipid nanoparticles, Int. J. Pharm., 2018, 550, 24–34 CrossRef CAS.
- M. Ribeiro, L. Alvarenga and L. Cardozo, et al., From the distinctive smell to therapeutic effects: Garlic for cardiovascular, hepatic, gut, diabetes and chronic kidney disease, Clin. Nutr., 2021, 40, 4807–4819 CrossRef CAS.
- Z. Bahadoran, P. Mirmiran and A. A. Momenan, et al., Allium vegetable intakes and the incidence of cardiovascular disease, hypertension, chronic kidney disease, and type 2 diabetes in adults: a longitudinal follow-up study, J. Hypertens., 2017, 35, 1909–1916 CrossRef CAS.
- X. Y. Fang, L. W. Qi and H. F. Chen, et al., The Interaction Between Dietary Fructose and Gut Microbiota in Hyperuricemia and Gout, Front. Nutr., 2022, 9, 890730 CrossRef.
- E. Yuzbashian, G. Asghari and P. Mirmiran, et al., Sugar-sweetened beverage consumption and risk of incident chronic kidney disease: Tehran lipid and glucose study, Nephrology, 2016, 21, 608 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo03354f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |