Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent trends in the encapsulation of functional lipids: comprehensive review
Anand
Kumar
a,
Upendra
Singh
b,
Swapnil G.
Jaiswal
c,
Jaydeep
Dave
*d,
Shuai
Wei
*a and
Gebremichael Gebremedhin
Hailu
 *e
*e
aCollege of Food Science and Technology, Guangdong Provincial Key Laboratory of Aquatic Product Processing and Safety, Guangdong Ocean University, Zhanjiang, China. E-mail: weishuaiws@126.com
bDepartment of Agricultural Engineering, Sri Karan Narendra College of Agriculture, Jobner 303329, India
cDepartment of Agricultural Engineering, Maharashtra Institute of Technology, Chhatrapati Sambhajinagar, Maharashtra 431010, India
dFaculty of Medical Technology, Mahidol University, Salaya, Phutthamonthon, Nakhon Pathom 73170, Thailand. E-mail: jdavefst@gmail.com
eDepartment of Food Technology and Process Engineering, Oda Bultum University, Chiro 226, Ethiopia. E-mail: mikialejr@gmail.com
First published on 2nd September 2024
Abstract
Recently, the demand for natural foods with promising health benefits has increased daily. Functional lipids such as omega 3 fatty acids, omega 6 fatty acids, linoleic acid, conjugated linoleic acid, carotenoids, and other functional compounds have many beneficial effects on human health, such as cardiovascular diseases, mental disorders, and metabolic disorders such as diabetes. The application of such substances in food matrices is often hindered by their poor solubility in water, unpleasant flavor, low oral bioavailability and low stability during storage and gastrointestinal interactions. Several encapsulation techniques have been used to address these issues and make these compounds bioaccessible and bioavailable. In the present review, the current knowledge of encapsulation delivery systems with suitable wall materials for functional lipids and their production techniques and the mechanism and behavior of the wall and core matrix are discussed. Additionally, the impact of such encapsulation delivery systems on the stability of encapsulated functional lipids in storage as well as the gastrointestinal environment has been discussed. Furthermore, this review highlights the impact of encapsulated functional lipids on the fortification of staple foods in terms of enhanced physicochemical, functional and nutritional profiles. Finally, the review article concludes with the factors affecting the commercialization of these encapsulated functional lipids.
Sustainability spotlightIn this extensive review, the current knowledge of encapsulation delivery systems with suitable wall materials for functional lipids and their production techniques and the mechanism and behavior of the wall and core matrix are discussed. Additionally, the impact of such encapsulation delivery systems on the stability of encapsulated functional lipids in storage as well as the gastrointestinal environment has been discussed. This work is related to UN's Sustainable Development, end hunger and ensure access by all people, in particular the poor and people in vulnerable situations, including infants, to safe, nutritious and sufficient food all year round as encapsulations have the following advantages: address formulation issues related to restricted chemical or physical stability of active ingredients overcome the incompatibility of active component and food matrix, regulate the release of a sensory active compound, help or enhance nutrition absorption. |
1. Introduction
The increasing demand for safe and sustained nutrition, supported by the rapid growth in nutraceuticals, superfoods, and functional foods, has significantly spurred interest in encapsulation technology.1 The controlled release of functional compounds over time is often preferred by researchers and industry professionals in the nutraceutical field, as it can increase the bioavailability and efficacy of these compounds. This method is particularly beneficial in contexts where sustained delivery is needed to maintain optimal therapeutic levels in the body over an extended period, reducing the need for frequent dosing and improving patient compliance. From a technological perspective, an efficient delivery system should incorporate functional ingredients into food systems with greater physicochemical stability and minimal impact on the sensory attributes of food products.2,3 Moreover, encapsulation techniques should maximize the uptake of encapsulated compounds upon consumption and ensure controlled release in response to specific biological conditions.4 Functional compounds, which are often hydrophobic, tend to degrade during processing or within the body and have rapid clearance rates, leading to poor bioavailability.5 The encapsulation of these compounds is crucial for efficient delivery. Researchers have focused on increasing bioavailability through novel encapsulation techniques.5–7 Among these functional compounds, functional lipids stand out because of their numerous health benefits.Functional lipids such as omega 3 fatty acids, omega 6 fatty acids, linoleic acid, conjugated linoleic acid, carotenoids, and other functional compounds have many beneficial effects on human health, such as cardiovascular diseases, mental disorders, and metabolic disorders such as diabetes.8 These compounds are available in a wide range of natural sources, such as vegetables, seeds, meat, fish, algae and microbes, and have tended to constitute an integral part of the human diet for many years.9 However, several researchers have reported that the direct consumption of such functional lipids still does not satisfy the minimum dietary intake level, which can be a consequence of improper dietary patterns, the geographical distribution of sources, and the limited availability of sources.10–13
Over the last few decades, researchers have developed various techniques and formulations to make functional lipids more accessible and convenient for consumers.14–16 Among these, oils rich in functional lipids have become one of the most widely available and commonly used products. Oils extracted from plant sources such as walnut, linseed, canola and flaxseed are rich in α-linoleic acid.8,17–19 Fish oils are rich sources of ω-3 fatty acids (O3FAs), especially eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and have been used to make dietary supplements.20–22 Various types of nonencapsulated delivery systems are available for the convenient supply of functional lipids as dietary supplements.23 The most commonly used dietary supplements of functional lipids are O3FAs and ω-6 fatty acids (O6FAs). Fish oils entrapped by soft gels, flavored gummies and capsules are the most preferred options for the oral delivery of O3FA, which can mask the odd flavor and odor of the fish oil.24,25 Plant-based oils, including flaxseed oil, primrose oil and pomegranate oils, are also entrapped in soft gels and provide a dietary supply of arachidonic acid (ARA), linoleic acid (LA), α-linoleic acid (ALA), γ-linoleic acid26 and conjugated linoleic acid (CLA).27,28 Recently, several manufacturers have targeted algal oils as sustainable sources of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).29Table 1 summarizes the commercially available functional lipid supplements and their nonencapsulated delivery systems.
| Product name | Source | Functional lipids | Delivery system | Reference |
|---|---|---|---|---|
| a This table provides a selection of commercially available functional lipid supplements from various regions. It highlights key examples rather than offering an exhaustive list of all products available on the market. LA-linoleic acid, ALA-α-linoleic acid, EPA-eicosapentaenoic acid, DHA-docosahexaenoic acid, CLA-conjugated linoleic acid, GLA-γ-linoleic acid, ARA-arachidonic acid. | ||||
| Mar in Oil® | Salmon oil | EPA/DHA | Soft gels | 23 |
| Nature's Bounty® | Herring, anchovy, mackerel, sardine oils | EPA/DHA | Gummies, capsules | 24 |
| Jamieson® | Wild salmon fish oil complex | EPA/DHA | Gummies | 25 |
| CLA One® | — | CLA | Capsules | 23 |
| Nutra Vege® | Algal oil | DHA | Soft gels | 29 |
| Nordic Naturals® | Plant based oil | ALA, ARA, LA | Soft gels | 27 |
| Rx Omega3® | Flaxseed oil | LA, ALA | Soft gels | 28 |
| Neptune Krill 1000® | Krill oil | EPA/DHA | Soft gels | 30 |
| Source Naturals® Phytosterol complex | Plant based oil | β-Sterols and phytosterol complex | Tablets | 31 |
| Clear Muscle® | — | ARA | Liquid caps | 32 |
| Pometane® | Pomegranate oil | Punicic acid | Soft gels | 33 |
| Deep blue® | Shark liver oil | Squalene | Capsules | 34 |
| NOW® by Abbot Pharmaceuticals | Evening prime rose oil | ω-6 fatty acids | Soft gels | 35 |
| Jarrow Formulas, Borage® | — | GLA | Soft gels | 36 |
| NOW foods, astaxanthin | Fish and shellfish | Astaxanthin | Soft gels | 35 |
| Fucothin® | Seaweed | Fucoxanthin | Capsules | 37 |
Generally, marketed functional lipids are entrapped in gelatin-based capsules and soft gels and thus have poor GI stability and a shorter shelf life.38–40 Moreover, functional lipids are unsaturated and hydrophobic in nature, so bioavailability and bioaccessibility can be major obstacles for oral delivery or food fortification.4,5 Furthermore, commercially available dietary supplements contain synthetic antioxidants such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) to prevent the oxidation of functional lipids, which are associated with certain adverse health concerns.41 In addition to these disadvantages, the “burp effect” is inconvenient for the user. Encapsulation techniques are thus designed to protect functional lipids from adverse environmental conditions in foods, enhance water dispersibility, improve food matrix compatibility, reduce unpleasant sensory attributes, and increase GI stability and bioavailability.42–44 This review aims to provide a comprehensive overview of the current state of encapsulation technology for functional lipids by selecting and discussing seminal papers, key studies, and recent developments that have significantly impacted the field. Through this focused selection, we aim to highlight the most relevant and influential research, offering insights into the latest advancements, challenges, and future directions in the encapsulation of functional lipids. By doing so, we intend to support further innovation and application in this promising area of nutraceuticals and functional foods.
2. Conventional encapsulation strategies for functional lipids
During the past few decades, researchers have developed various encapsulation technologies for improving the bioaccessibility of functional lipids.45,46 Various types of wall materials and encapsulation strategies have been developed to ensure the increased oxidative stability of functional lipids either incorporated in food or consumed orally.47,48 The most common encapsulation techniques used for the majority of nutraceutical compounds are drying-based delivery systems for functional compounds.49 Spray drying and freeze drying are the most common drying methods for encapsulating functional lipids.Spray drying is one of the most commonly used encapsulation processes because of its low cost, simplicity, and flexibility. It yields high-quality powders and can preserve various vegetable and animal oils against oxidation as well as external deterioration influences such as humidity, light, and temperature. The processing time is only a few seconds, which is sufficient to preserve heat-sensitive components such as fatty acids.50 Another benefit of encapsulation by spray drying is the capacity to decrease the amount of oil at the particle surface (nonencapsulated oil) and thus increase the encapsulation efficiency (EE).
Spray drying facilitates the preparation of the final product in powder form for better storage and transportation. The aqueous solution or dispersed lipids with wall materials are injected into the spray dryer in the form of sprayed particles, where the water is removed by the hot air in a fraction of time to obtain the powder form of the encapsulated particles. Spray drying provides a wide range of encapsulated functional lipids, including omega 3 fatty acids, EPA-rich oils, ALA-rich oils, and squalene.51,52 Although spray drying is one of the most common methods for the encapsulation of functional lipids, some drawbacks have been linked to this process. For example, a major disadvantage is the use of hot air at high inlet temperatures, which can promote the volatilization and oxidation of some functional lipids. Several authors Encina et al.51 have reported improvements in the oxidative stability of fish oil by spray drying with methanol (MeOH); Goyal et al.53 reported the highest encapsulation efficiency and lowest peroxide values of flaxseed oil encapsulated via the spray drying process.
Recent advances in the encapsulation of essential fatty acids and other functional lipids through spray drying have been extensively reviewed. These reviews discuss challenges such as optimizing wall materials and process conditions to improve encapsulation efficiency and stability.54 The detailed analysis of spray drying parameters highlights the impact of the inlet air temperature, total solids concentration, and wall materials on the encapsulation efficiency of oils.52 Conventional and nanospray-drying technologies emphasize processing variables and their influence on powder characteristics, discussing advantages such as large yields in conventional spray drying and better preservation of active ingredients in nanospray drying.55 Additionally, the encapsulation of various lipids, including essential oils, polyunsaturated fatty acids, and structured lipids, focuses on the selection of suitable encapsulating agents and the increasing trend of combining spray drying with other techniques to increase stability and bioavailability.54
Encapsulation by freeze-drying is achieved by drying an aqueous solution or dispersion containing functional lipids as core and wall materials. This causes the two components to colyophilize, usually resulting in a porous, nonshrunken, complex structure. Minimizing thermal degradation reactions has been shown to be a highly suitable method for drying heat-sensitive substances. Rezvankhah et al.56 and Hasani et al.57 thoroughly reviewed the encapsulation of functional lipids, especially omega 3 fatty acid-rich fish oils, by means of freeze drying. However, the porous structure within the freeze-dried matrix may increase the exposure of the encapsulated core matrix to air if the final product is not packed under vacuum or an inert atmosphere. The major disadvantages of this technology are the high consumption of energy, the long time required for processing, and the higher costs than those of other encapsulation techniques.
3. Recent advancements in delivery systems for the encapsulation of functional lipids
Over the past few decades, research has shifted toward the development of various delivery systems to increase the physiochemical and functional properties of encapsulates for better bioaccessibility and bioavailability of delivered drugs or functional compounds. The delivery systems have been modified by different encapsulation techniques along with food-grade carrier matrices to increase the feasibility of food fortification. Recent advances in delivery systems for the encapsulation of functional lipids are summarized in Table 2.| Techniques of encapsulation | Method used | Core material | Functional lipophilic compound | Carrier material/wall matrix | Encapsulation efficiency (%) | Particle size | In vitro digestion study | Heat stability study | Storage stability study | Remarks | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antisolvent precipitation | Mechanical stirring at | Wakame algae oil | Fucoxanthin | Zein/casein | >85 | 100–130 nm | 29.02% oil release in simulated gastric fluid for 6 h | 100% stability after heating at 75 °C for 60 min | 72.32% retention after 16 days of storage at 25 °C | Static quenching, corresponding to the formation of complexes between fucoxanthin and casein or zein | 58 |
| Mechanical stirring | Egg yolk | Lutein | Zein/soy protein | >80 | 14–200 nm | 33.94% oil release in simulated gastric fluid for 6 h | — | 96.27% retention after 15 days of storage at 25 °C | Zein–lutein complexes can be formed with the help of noncovalent interaction forces | 59 | |
| Sonication from 200–800 W | — | Stigmasterol | Zein | 95.95 | 336.74 nm | — | — | — | Sonication improved the zeta potential of the encapsulated particles which might increase the stability of zein-stigmasterol complex | 60 | |
| Emulsification solvent evaporation | Homogenization at 700 rpm for 15 min at 25 °C | Fish oil | O3FA | Zein | — | 73–265 nm | — | — | — | Oxidative gelation rate is reduced | 61 |
| — | Fish oil | DHA | Zein | 98.8 | 150–200 nm | 3.3% DHA released in Phosphate buffered saline and with 2% Tween 80 as surfactant | — | — | Lower in vitro release proved that the zein and fish oil encapsulation has higher oxidative stability against the GI environment | 62 | |
Homogenization at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min followed by freeze drying to evaporate the solvent 000 rpm for 5 min followed by freeze drying to evaporate the solvent |
Red palm oil | Carotenoids | Carboxy methyl cellulose | 83–96 | 600–2200 μm | >90% oil retained in the GI environment and >20% oil retained in the intestinal digestion | The oil loaded beads have shown lower weight loss up to 150 °C, after with increase in temperature the wight loss is increased | Lowest peroxide value of 25 meq. of O2 per kg of oil was found after 6 days of storage at 25 °C | Freeze-drying had diminished the migration of oil on to the surface of the beads as freezing temperature might have solidified the palm oil | 63 | |
| Coacervation technique &ionic gelation | Emulsion obtained by mechanical stirring at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3 min at 40 °C and pH was shifted to 4 for making coacervation 000 rpm for 3 min at 40 °C and pH was shifted to 4 for making coacervation |
Echium oil | Steariodonic acid and phytosterols | Protein–gelatin | 87 | — | — | — | 96% oil retention after 30 days storage at 37 °C | Gelatin and gum Arabic based coacervation entrapped the echium oil with higher storage stability and less oxidative degradation | 64 |
| Poly saccharide–gum arabica | |||||||||||
Emulsion obtained by mechanical stirring at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3 min followed by pH shifting to 3.8 for coacervation 000 rpm for 3 min followed by pH shifting to 3.8 for coacervation |
Sacha inchi oil | PUFA | Protein–ovalbumin | 99.54 | — | 14.6% of oil release in simulated gastric digestion at pH 2.8 with the presence of pepsin enzyme | — | — | The reduced release under gastric conditions (low pH and presence of proteolytic enzymes) indicates that the ovalbumin and sodium alginate microcapsule protected the acyl in the omega-3 units | 65 | |
| Polysaccharide–sodium alginate | |||||||||||
| Emulsion was made by mechanical stirring at 400 rpm at 40 °C and coacervation was made by pH shifting to 4 | Cod liver oil | PUFA: EPA and DHA | Protein–soy protein isolates | 94 | — | 80.54% oil stability at pH 5.5 | — | 72.24% oil retention at 90 °C for 30 min | Stable emulsion was carried out by the complex coacervation of inulin and soy protein isolates | 66 | |
| Polysaccharide–inulin | |||||||||||
| Emulsion was obtained by stirring at 600 rpm at room temperature and coacervation complex was created by pH shifting at 3 | Algal oil | PUFA: O3FA and O6FA | Protein–soy protein isolates | 90.57 | — | — | — | — | The hexanal peak area is 23.34 which indicated the lowest oxidation | 67 | |
| Polysaccharide–chitosan | |||||||||||
Emulsion was prepared by mechanical stirring at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min at room temperature followed by coacervation at 3.75 pH 000 rpm for 5 min at room temperature followed by coacervation at 3.75 pH |
Pomegranate seed oil | Punicic acid (omega 7 fatty acid) | Protein–whey protein | 67.40 | 8.36–10.96 μm | — | — | — | The complex coacervation provided minimum isomerization of pomegranate seed oil | 68 | |
| Polysaccharide–gum arabica | |||||||||||
| Inclusion complex | Mixing followed by lyophilization | Perilla oil | ALA | γ-Cyclodextrin | — | — | — | 63.3% ALA retention after heating at 60 °C after 4 days | — | The perilla oil was more thermostable when included in the cavity of γ-CD than when placed at interspaces between pseudo rotaxane-type complexes | 69 |
| Kneading method and crystallization method | Anchovy oil | EPA and DHA | β-Cyclodextrin | 74–99 | — | — | — | — | PUFA glycerides from the anchovy oil is poor encapsulated in β-cyclodextrin in controlled crystallization conditions, while the monounsaturated and especially saturated fatty acid glycerides were more appropriate for molecular encapsulation | 70 | |
| Dextrinization method | Fish oil | O3FA | Amylose (maize starch) | 71.22 | — | — | — | — | Dextrinization improved dispersion stability of the complex particles | 71 | |
| Supercritical fluid technique | CO2 pressure-8 M.Pa | Fish oil | EPA and DHA | Polycaprolactone | 38–43 | 6–73 nm | — | — | — | Supercritical fluid extraction successfully developed the nanoparticle s from liquid lipophilic compounds like fish oil | 72 |
| Temperature of extractor 263 K | |||||||||||
| CO2 pressure- 80 bar | Shrimp oil | Astaxanthin | Ethyle cellulose | 84 | 363–370 nm | Almost 70% release of astaxanthin after 10 h in simulated intestinal fluid | — | — | The emulsified shrimp oil gets easily ionized in simulated intestinal fluid showed higher release of astaxanthin in intestinal tissues | 73 | |
| Temperature of the extractor-38 °C. | |||||||||||
| CO2 pressure-9 M.Pa | — | Lycopene | n-Octenyl succinic anhydride (OSA)-modified starch | 64–89 | 345–366 nm | — | — | — | Supercritical extraction emulsion provided the stability of lycopene in aqueous media | 74 | |
| Temperature- 353.15 K | |||||||||||
| Electrostatic nanoencapsulation | Electrospinning | Fish oil | O3FA | Zein fibers | 91 | 190 nm | — | — | Peroxide value of encapsulated complex remains below 200 μmol L−1 for 14 days of storage at 25 °C | The distribution of fish oil in the electrospun materials, revealing that the lipid phase tended to concentrate at the core of the fibers and beads | 75 |
| Coaxial electrospray | — | ARA | Zein | 77–95 | 1–7 μm | — | — | Peroxide value of encapsulated ARA is approximately 8.0 meq. per kg after 30 days of storage at room temperature | Coaxial electrospray technique to produce natural and edible microcapsules with core–shell structures and reduce the unpleasant flavor | 76 | |
| Electro spraying assisted by pressurized gas | Fish oil | DHA | Zein | 84 | 2–3 μm | Peroxide value was 20 meq/kg oil after 30 days of storage at 23 °C | DHA was successfully stabilized in the zein microcapsules due to the low temperature and fast evaporation characteristics of electro spraying technique used | 72 | |||
| Electro spraying at 20–25 kV voltage with flow rates ranging from 0.5 to 1 mL h−1 | Fish oil | O3FA | Kafirin | 94 | 552–861 nm | — | — | — | The kafirin nano capsules loaded with fish oil obtained in this study (average diameter <1 μm) present a high surface-to-volume ratio which is desired for a better release of the encapsulated bioactive compound | 77 | |
| Liposomes | Sonication of liposome suspension at 25 °C for 7 min (1 s on and 1 s off) with nominal frequency of 20 kHz at 80% of full power | Fish oil | EPA & DHA | Soybean lecithin | 73.5 | <200 nm | — | — | The TBA reactivity substance was 0.015 μmol MA equivalent after 90 days of storage in dark at 4 °C | The surface charge, physical stability and oxidative stability of liposomal PUFAs increased as the size of the liposomes decreased | 78 |
| Ultrasonication (10 min; 1 s on and off pulse) at 25 °C using an ultrasonic processor at 80% amplitude | Shrimp oil | EPA & DHA, astaxanthin | Soybean lecithin | 93.64 | 40–284 nm | — | — | The peroxide value was approximately 5 meq. peroxide per kg of oil and TBARS approximately 50 malonaldehyde equivalent after 8 weeks of storage at 30 °C | Nanoliposomes produced using ultrasonication method were more stable, smaller in size and showed better nanoencapsulation efficiency | 79 | |
| Thin film drying prior to ultrasonication for 10 min at 180 W in an ice-cold water bath with a cycle of 2 s sonication and 2 s standing | — | Astaxanthin | Egg yolk lecithin and lactoferrin | 71.92 | 190 nm | — | The rate of thermal degradation rate was approximately 0.7045 during the study from 0–70 °C | — | The lowest rate of thermal degradation was the result of antioxidant effect of lactoferrin coated with the liposomes | 80 | |
| Thin film drying prior to sonication using a frequency of 20 kHz at 90% | Perilla oil | ALA and LA | Soybean lecithin and biopolymers | ALA-79.3 to 89.9, LA-72.6 to 85.6 | 120–300 nm | ∼10% release in simulated gastrointestinal conditions | — | The peroxide value was ∼40 meq peroxides/kg of oil after 30 days of storage at 45 °C | Liposomes crosslinked with biopolymers have more physical as well as gastrointestinal stability | 81 | |
| Solid lipid micro/nanoparticles (SLNs) | Resveratrol-stearate and PUFA mixture were melted at 65 °C followed by cold homogenization at 8000 rpm for 15 min | Fish oil | ALA and DHA | Resveratrol | ALA-77, DHA-100 | ALA-842 nm, DHA-1000 nm | — | — | — | SLNs with resveratrol and PUFA omega-3 acted as anti-tumor for colon cancer and reduce the cell proliferation | 82 |
Oil phase (lipid careers and echium oil) and water phase (WPI solution) were homogenized at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3 min to prepare oil in water microemulsion 000 rpm for 3 min to prepare oil in water microemulsion |
Echium oil | O3FA | Lauric acid, palmitic acid and stearic acid | 78–85 | ∼200 nm | — | — | Sample stabilized by lauric acid have less TBARS values after 21 days of storge as compare to other lipid careers | Different lipid carriers with different chain lengths affected the physicochemical properties of encapsulated echium oil | 83 | |
| Supercritical carbon dioxide with 200 bar expansion pressure, 57 °C, and 50 μm nozzle diameters | Fish oil | O3FA | Fully hydrogenated soybean oil | 97.5 | 5–18 μm | — | — | Anisidine value for the particles with fish oil started to increase on day 9 and reached its maximum on day 15 (2840) while stored at 40 °C | The initial loading concentration of the fish oil have directly proportional to the thermal as well as storage stability of lipid particles | 84 |
3.1. Biopolymer-based delivery systems
Currently, biopolymers, such as chitosan, alginate, whey proteins, gelatins, gum arabic, and zein, have attracted the interest of the scientific community as carrier matrices or wall materials for the encapsulation, immobilization, and controlled release of numerous functional lipids by various delivery systems, such as antisolvent precipitation, complex coacervation, inclusion complexes and solvent evaporation.Fucoxanthin, a functional lipid-soluble algal pigment, was entrapped in the zein and casein wall matrix by mechanical stirring to obtain nanoparticles with a 100–130 nm particle size.86 They reported that static quenching between fucoxanthin and the wall material increased the encapsulation efficiency, i.e., >85%, and increased the heat and storage stability (Table 2).59 developed a nanoencapsulated egg yolk pigment, lutein, via a similar technique with >80% encapsulation efficiency and a 140–200 nm particle size. They reported that the zein–lutein complexes formed noncovalent interactions via mechanical stirring, which increased the storage stability and release profile in gastric fluid (Table 2). Recently, the sonication method replaced mechanical stirring for phase transition, which provides a uniform distribution of encapsulants and increases the encapsulation efficiency of drug delivery systems. Sonication improved the zeta potential of the encapsulated particles, which might increase the stability of the zein–stigmasterol complex.60
The application of nanoparticles in food products is subject to stringent regulations, especially in Europe, where they are classified as novel foods. According to the European Food Safety Authority,87 novel foods must undergo rigorous safety assessments that include evaluations of potential toxicity, absorption, distribution, metabolism, and excretion.87 Products containing nanoparticles must be clearly labeled to inform consumers of their presence.88 The authorization process requires companies to submit a detailed dossier with scientific evidence demonstrating the safety of the nanoparticle for its intended use, as reviewed by the EFSA.89 Additionally, authorized novel foods are subject to ongoing monitoring to ensure safety and traceability, and environmental impact assessments must also be considered.90 This regulatory framework ensures that the nanoparticles used in food products are safe for consumption and that consumers are well informed about their presence.
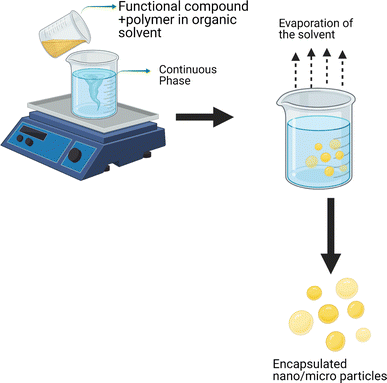
4.1.1.1 Emulsification solvent evaporation. Emulsification followed by solvent evaporation is a technique frequently used for the development of biopolymer-based nanospheres. Encapsulates containing functional compounds have been obtained by homogenizing an organic polymer solution with an aqueous phase, followed by solvent evaporation, which causes the polymer molecules to precipitate and form nanospheres (Fig. 2). Generally, high-pressure homogenization or ultrasonication techniques have been used for nanoparticles. In the case of functional lipids, O/W emulsions are most common when the aqueous phase is water, which acts as an antisolvent and provides more sustainability to the process.91
Recently, scientific research has explored the interaction between functional lipids and biopolymer composites such as zein and carboxy methyl cellulose (CMC) for the production of nanoparticles. For example, zein and fish oil-derived nanocomposites (100–120 nm) have higher encapsulation efficiency (98.8%), and high-pressure homogenization and solvent evaporation methods have been used to develop highly stable nanoparticles with better GI stability.62 Furthermore, Soltani et al.61 reported a reduction in oxidative gelation for zein-fish oil nanocomposites (73–265 nm). Similarly, carotenoids from red palm oil have been immobilized by CMC by high-pressure homogenization followed by freeze drying to achieve higher encapsulation efficiency (83–96%), better storage stability, enhanced GI stability and targeted drug delivery in the intestinal environment (Table 2).
Comunian et al.64 reported that gelatin- and gum Arabic-based coacervation entrapped echium oil with high storage stability and 87% encapsulation efficiency.
The ovalbumin and sodium alginate microcapsule of sachainchi oil protected the acyl group in the omega-3 units, which ultimately reduced the rate of release of functional compounds in the GI tract and provided targeted drug delivery65. Rios-Mera et al.66 developed a stable emulsion (94% encapsulation efficiency) consisting of cod liver oil by the complex coacervation of inulin and soy protein isolates, where they reported increased heat and GI stability at a simulated pH (Table 2). The complex coacervation provided minimum isomerization of pomegranate seed oil in microcapsules (8.36–10.96 μm) developed by using whey protein and gum Arabic as the wall matrix.68
The perilla oil was more thermostable when it was included in the cavity of γ-CD than when it was placed in interspaces between pseudo rotaxane-type complexes.69 However, the chemical affinity of functional compounds influences the encapsulation potential of inclusion complexes. For example, PUFA glycerides from anchovy oil are poorly encapsulated93 in β-cyclodextrin under controlled crystallization conditions, whereas monounsaturated and especially saturated fatty acid glycerides are more appropriate for molecular encapsulation (99% EE). In addition to CDs, chemically modified biopolymers are also used as host compounds for inclusion complexes. Park et al.71 developed a host compound by dextrinization by maize starch, which is used as a wall matrix for fish oil, where dextrinization improved the dispersion stability of the complex particles (Table 2).
This technology has been applied to various functional lipids to form micro- or nanoencapsulations by using different biopolymers as wall materials. Santos et al.74 applied ScCO2 to encapsulate lycopene pigments with n-octenyl succinic anhydride94-modified starch and reported that a supercritical extraction emulsion provided stable lycopene in aqueous media. The importance of supercritical CO2 encapsulation techniques was highlighted by Tirado et al.73 for the emulsification of shrimp oil, where in vitro release profiles in simulated intestinal fluid (SIF) at pH 7.2 and 310 K revealed 70% release of the total encapsulated astaxanthin within 10 hours. Prieto et al.95 successfully developed fish oil nanoparticles 6–73 nm in size from ScCO2 with polycaprolactone as a wall matrix.
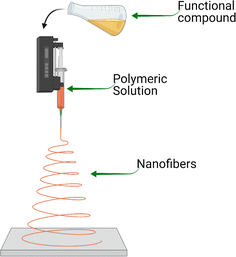
3.2. Electrohydrodynamic processing of encapsulation
Electrohydrodynamic processing of encapsulation refers to the development of nano- or microstructured particles by subjecting a polymeric fluid to a high-voltage electric field.96 Generally, fluid is pumped through a conductive capillary where a voltage is applied. Owing to the higher surface/volume ratio and electric repulsion, the solvent in the fluid evaporates, and the dry material is deposited on the collector. The molecular cohesion of the polymer chains in the polymer fluid determines the final size and shape of the nanomaterial.97 In addition, the functionality of the nanostructures produced by electrohydrodynamic processes can be achieved through the use of blends, coaxial electrospinning (resulting in core–shell structures), the inclusion of other functional molecules, and the adsorption of functional components to the surfaces of the nanomicrostructures.Moomand et al.75 reported the distribution of fish oil in electrospun zein fibers, revealing that the lipid phase tended to concentrate at the core of the fibers and beads. They reported that the applied technique developed spun nanofibers (190 nm) with an increased encapsulation efficiency of O3FA of up to 91% (Table 2).
Hu et al.76 encapsulated (95% EE) ARA with a zein biopolymer via a coaxial spray technique, which produced natural and edible microcapsules (1–7 μm) with core–shell structures and reduced the unpleasant flavor. The electrospray technique provides low-temperature and fast evaporation characteristics and successfully stabilizes fish oil in zein microcapsules (2–3 μm) with an 84% EE of DHA.72 Kafirin-based nanoencapsulated capsules (552–861 nm) loaded with fish oil (94% EE) obtained by electrospinning present a high surface-to-volume ratio, which is desirable for better release of the encapsulated bioactive compound.77
3.3. Lipid-based delivery system for functional lipids
Lipid-based delivery systems are wide-ranging designs for formulations containing active or functional compounds in dissolved or suspended forms in lipidic cores.101 The melting range, solubilizing capacity and miscibility properties of the wall material depend on the fatty acid chain length and degree of unsaturation.102 Lipid-based delivery systems can be developed as simple oils for more complex formulations, such as spontaneous emulsification in aqueous media, and are most suitable for lipid-soluble bioactive compounds and functional lipids.43 Lipid-based delivery systems can be liquid, semisolid, or solid at room temperature; hence, a variety of product formulations are possible, e.g., drinking solutions, filled soft gel capsules and tablets.103 Additionally, they are more desirable for incorporation into fat-based food products such as cheese, butter, mayonnaise, and emulsified meats.104,105Rasti et al.78 developed soybean lecithin-based liposomes containing fish oil by combining thin film hydration and ultrasonication and reported that ultrasonication reduced the size (<200 nm) of the liposomes and made them homogenous, which increased the stability of the nanoliposomes. Gulzar et al.79 studied the impact of ultrasonication and microfluidization on the physicochemical properties of nanoliposomes containing shrimp oil and reported a greater encapsulation efficiency (93.64%) and smaller particle size (40 nm) of the nanoliposomes obtained via ultrasonication than via microfluidization.
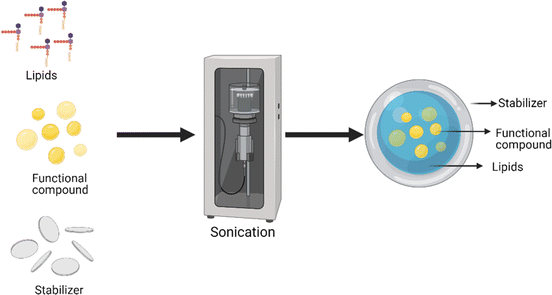 | ||
| Fig. 8 Solid lipid nanoparticles82 developed O3FA-rich resveratrol-based solid lipid nanoparticles by hot homogenization at 65 °C for the delivery of ALA (74% EE; 840 nm) and DHA (100% EE; 1000 nm). The encapsulation efficiency and particle size of solid lipid nanoparticles are affected by the chain length of lipid carriers.83,109 High-pressure homogenization, ultrasonication and supercritical CO2 are the most efficient methods for preparing solid lipid micro/nanoparticles for omega-3-rich oils with high encapsulation efficiency (95–99%) (Table 2). | ||
4. Impact of encapsulation techniques on the stability of functional lipids
Over the past few decades, researchers have focused on the commercialization of fortified food products with functional lipids. Increased consumption of functional lipids can be achieved by fortifying them with staple foods such as breads, milk, fruits, juice, yogurt, cheese, etc. However, the major challenge of the fortification of functional lipids is their unstable nature due to their unsaturated structure, which makes them more prone to lipid oxidation by oxygen, heat and light during processing as well as storage.111 Encapsulation techniques provide oxidative stability by utilizing interfacial technologies to prevent oxygen from contacting functional lipids and incorporating antioxidants and other protective substances, which ultimately increases the bioavailability of functional lipids.42,96,112,113Storage stability and heat stability are proposed considerations for the encapsulation of functional lipids. They are affected by various parameters, including the wall/carrier matrix, emulsifiers, wall matrix properties, glass transition temperature, crystallinity, chemical and physical interaction mechanisms, and processing conditions (temperature, pressure, ratio of wall to core material, particle size and surface area, and oil distribution within the particle).114 Moreover, the amount of free surface oil on the surface of encapsulated particles is the most critical parameter, while considering the encapsulation strategies for functional lipids, as free surface oil is most prone to environmental stress.115 Many researchers have successfully entrapped functional lipids with enhanced storage and heat stability, as shown in Table 2.
Li et al.86 reported 100% stability of fucoxanthin nanoparticles entrapped by a zein–casein wall matrix after heating at 75 °C. Static quenching, corresponding to the formation of complexes between fucoxanthin and casein–zein, also provided an oil retention of up to 72% after 16 days of storage at ambient temperature. Similarly, the zein–lutein complex formed by noncovalent bonding retained approximately 96% of the oil in egg yolk nanoparticles after storage at 25 °C for 15 days59. Sathasivam et al.63 reported that freeze-drying diminished the migration of red palm oil to the surface of microbeads as the freezing temperature solidified the oil in the core of the carboxymethyl cellulose, which resulted in the lowest peroxide values (25 meq. of O2 per kg of oil) after 6 days of storage at room temperature. In contrast, Anwar et al.94 reported that freeze drying produced a porous powder of encapsulates, which allowed more oxygen to interact and generate higher peroxide concentrations.
Gelatin- and gum arabic-based coacervation entraps echium oil with greater storage stability and less oxidative degradation, which retains approximately 96% of the oil after 30 days of storage at 37 °C64. Rios-Mera et al.66 developed a stable emulsion of cod liver oil by complex coacervation of inulin and soy protein, where approximately 72% oil retention was obtained after the emulsion was heated at 90 °C for 30 min. Hexanal is considered an end product of lipid oxidation, which impairs the sensorial attributes of fortified products. Yuan et al.67 reported that hexanal production is reduced when algal oil is encapsulated in the complex coacervation of soy protein and chitosan. Researchers have also reported a decrease in peroxide concentrations during the storage of various encapsulated functional lipids via the use of electrostatic encapsulation techniques.72,75,76 Certain antioxidants and biopolymers provide additional benefits in terms of enhancing the stability of functional lipids when combined with different encapsulation techniques. Liposomal nanoparticles of astaxanthin coated with lactoferrin enhance oxidative stability because of the antioxidant effect of lactoferrin80. Zamani-Ghaleshahi et al.81 reported that perilla oil liposomes crosslinked with biopolymers have greater physical stability. Table 2 summarizes the effects of various encapsulation techniques and wall matrices on the storage and heat stability of encapsulated functional lipids.
5. Influence of encapsulation techniques on the gastrointestinal stability and bioavailability of functional lipids
The bioavailability and bioaccessibility of functional lipids are the only concerns when they are encapsulated in any colloidal system for oral delivery. The ultimate goal of the encapsulation technique is to absorb functional compounds in accessible forms at specific delivery sites, i.e., the aqueous intestinal lumen and intestinal cells. The bioavailability of encapsulated functional compounds is dependent on various factors, such as the solubility of colloidal carriers in aqueous gastrointestinal solution, the physical and biochemical stability of the wall matrix in the gastric environment, and the release efficacy of the core compound in the intestinal environment. In addition to the encapsulation efficiency, the particle size and intermolecular interactions of the core and wall materials also affect the bioavailability and efficiency of the delivery system. Many in vitro studies in a simulated gastrointestinal environment have proven the efficient delivery of functional lipids at targeted sites in micro- or nanoencapsulated forms, and the results are summarized in Table 2.The wakame algae oil entrapped in the zein–casein complex showed approximately 20% oil loss under simulated gastric conditions after 6 h, which might be due to strong static quenching between the zein–casein complex and the core compound.86 Similarly, Li et al.59 reported that the zein–lutein complex provided gastrointestinal stability to lutein nanoparticles through only 33% oil loss in gastric fluid after 6 h, which might be the result of strong noncovalent interactions between the wall and core material. Surfactants used in colloidal delivery systems, such as Tween 80, have also been shown to enhance the GI stability of DHA in fish oil-encapsulated nanoparticles.62 The freeze-dried red palm oil-loaded microbeads retained approximately 90% of the oil in the simulated gastric environment due to the presence of less free surface oil, as discussed earlier.63 The coacervation complex and ionic gelation technique also provided GI stability for various functional lipids by retaining up to 80% of the oil under simulated gastric conditions.65,66 Tirado et al.73 noted that the encapsulated structure of shrimp oil is easily ionized in simulated intestinal fluid, which increases the solubility of astaxanthin in the intestinal environment. Table 2 summarizes the effects of various encapsulation techniques and wall matrices on the gastrointestinal stability of encapsulated functional lipids.
The studies presented in Table 2 offer valuable insights into encapsulation techniques and the release behavior of functional lipids in simulated gastrointestinal environments. However, understanding bioavailability requires more comprehensive investigations, including studies that go beyond in vitro digestion and evaluate the actual absorption and efficacy of these encapsulated compounds in living systems. Several research groups have investigated the bioavailability of encapsulated lipids through cell culture and animal studies. For example, Serini et al.82 investigated the antitumor efficacy of solid lipid nanoparticles (SLNs) encapsulating resveratrol and omega-3 fatty acids (ALA and DHA) in a colon cancer model. These findings demonstrated that these SLNs could reduce cell proliferation and exhibit antitumor activity, suggesting improved bioavailability and therapeutic potential in vivo. Similarly, Barbosa et al.116 studied the stability and bioactivity of encapsulated echium oil in various lipid carriers via animal models. Research has shown that the chain length of lipid carriers affects the physicochemical properties and stability of the encapsulated oil, which in turn influences its bioavailability when it is administered to animals. In another study, Xie et al.117 used supercritical carbon dioxide to encapsulate fish oil in fully hydrogenated soybean oil and evaluated its bioavailability in an animal model. This study revealed that the initial loading concentration of fish oil was directly proportional to its thermal and storage stability, which impacted its absorption and bioavailability in the tested animals.
These studies illustrate that while in vitro digestion studies provide preliminary insights, cell culture and animal studies are crucial for comprehensively evaluating the bioavailability of encapsulated functional lipids. These examples underscore the importance of moving beyond in vitro experiments to assess the true bioavailability and efficacy of encapsulated compounds in living systems.
6. Effect of encapsulation techniques on the fortification of functional lipids
The growing market share of food products with healthier nutrient profiles has attracted the interest of many researchers seeking to fortify staple foods with functional lipids, especially O3FA-rich functional foods. Colloidal systems are interesting platforms for enriching functional lipids in staple foods with increased bioavailability. The motive for selecting staple foods for fortification is to increase the regular dietary intake of such functional lipids, which improves human health and markedly reduces metabolic, cardiovascular and metal disorders. The interaction of the food matrix and encapsulates determines the bioavailability of functional lipids in the food system. Moreover, the encapsulated functional lipids affect the physicochemical, structural and sensorial attributes of fortified food products, which are desirable for increasing the commercial importance of such fortified products. Table 3 summarizes the effects of the fortification of encapsulated functional lipids on the physicochemical and functional properties of selected staple foods.| Fortified food products | Encapsulated functional lipids | Encapsulation technique used | Physicochemical properties of fortified products | Rheological properties of fortified products | Sensorial attributes of fortified products | References |
|---|---|---|---|---|---|---|
| Yogurt | Fish oil powder | Complex coacervate of gelatin/gum acacia | Acidity, and water holding capacity were increased; whey separation was decreased | Gel strength decreased and apparent viscosity increased | Fortified yogurt samples were more yellowish compared to control | 118 |
| Fish oil microcapsules | Complex coacervate of gelatin/gum acacia | Fortified yogurt had higher apparent viscosity | Consistency coefficients of the enriched yogurt was 24.42–28.82 Pa sn | — | 119 | |
| Fish oil nanoliposomes | Liposomes by egg yolk lecithin and fish oil | Whey separation was deceased | — | Fish odor was eliminated | 120 | |
| Cheese | Fish oil powder | Microencapsulation by freeze drying | Whey separation was deceased | Hardness, chewiness and gumminess was increased | — | 121 |
| Fish oil powder | Microencapsulation by spray drying | Milk solid not fat was increased; pH level is maintained up to 30 days of storage | Hardness of enriched cheese is increased after 30 days of storage | Cheese color was changed to yellow after 60 days of storage | 122 | |
| Bread | Fish oil nanoliposomes | Liposomes by sunflower oil and lecithin | Loaf volume was increased, improved crumb characteristics | Harness was reduced, decrease the level of chewiness and gumminess | Light browning in the crumb color | 123 |
| Ice cream | Fish oil powder | Microencapsulation by freeze drying | Saturated fatty acids decreased, PUFA increased | — | — | 124 |
| Flaxseed oil microcapsules | Microencapsulation by freeze drying | Free fatty acid content was increased, melt down rate was decreased | — | Off flavor was masked up to 30 days of storage | 125 | |
| Frankfurter sausages | Fish oil microcapsules | Monolayer microencapsulation by spray drying | Lower down the pH values, MUFA and PUFA increased | — | — | 126 |
| Chicken sausages | Fish oil powder | Microencapsulation by inclusion complex with gelatin wall material | pH was maintained up to 21 days of storage, water binding ability was increased | The sausages with microencapsulated oil showed better ability to accumulate elastic energy (G′); Hardness of sausage was increased | Fortified sausages were rated highest for their consistency (the thickest), especially when they were heated | 127 |
Over the past few decades, there has been a remarkable interest in fortifying milk and dairy products with functional lipids with the aim of increasing the fatty acid profile of such products. Yogurt, cheese and ice creams are the most popular dairy products, and various attempts have been made to fortify such products with various encapsulated functional lipids. The fortification of yogurt with fish oil powder increased its acidity, lowered its pH and increased its water holding capacity, which ultimately increased its shelf-life.119,120 Moreover, yogurt tends to release whey during storage, which is called syneresis. The addition of fish oil powder can control whey separation due to the ability of the wall material to hold water and increase the stability of yogurt during storage118,120. Bermúdez-Aguirre et al.121 reported a similar reduction in whey separation in fish oil microcapsule-fortified cheddar cheese. Moreover, the addition of functional lipids to cheese also increases its textural properties, increasing its shelf stability.121,122 In addition to enhancing the physiochemical properties of emulsified dairy products, the fortification of functional lipids enhances their fatty acid profile by reducing the saturated fatty acids and increasing the PUFAs and MUFAs. The fatty acid profile of ice cream fortified with fish oil powder was greater than that of the control samples.124 Furthermore, Gowda et al.125 reported a lower melt-down rate in ice cream fortified with flaxseed oil microcapsules, which could be attributed to the encapsulated form of the fortified flaxseed oil, which might have increased flocculation and hence improved the structure of the ice cream.
Bread is another staple diet after milk and dairy products and has been popular among scientific communities for fortification with functional bioactive compounds. In addition to enhancing the fatty acid profile of breads, the encapsulated structure of functional lipids also improved the textural and sensorial attributes. Ojagh et al.123 reported that the loaf volume in bread containing fish oil nanoliposomes increased, possibly due to the surface–active properties of lecithin, an emulsifier, and other ingredients within the liposomal system, which improved gas retention, bread volume, and dough stability. Additionally, lecithin reacts with linear amylose and external amylopectin branches and forms a complex that prevents hardening of the bread's crumb. In addition, some ready-to-eat meat products, such as frankfurt and sausages, have recently been fortified with fish oil encapsulates to enhance their fatty acid profile.126,127
7. Factors affecting the industrialization and commercialization of encapsulated functional lipids
The industrialization and commercialization of encapsulated functional lipids are influenced by several factors, each playing a crucial role in determining the success of these products on the market. These factors include the scalability of the encapsulation process, the stability and shelf life of the encapsulated products, regulatory compliance, cost-effectiveness, and consumer acceptance. Below is an exploration of these factors with reference to relevant studies.7.1. Scalability of the encapsulation process
One of the primary challenges in the industrialization of encapsulated functional lipids is the scalability of the encapsulation process. Techniques that work well in the laboratory setting may not always be feasible on an industrial scale owing to complexities in maintaining consistency, controlling process parameters, and ensuring cost efficiency. For example, a study by Xue et al.128 demonstrated that a synthetic surfactant-free technique for encapsulating curcumin into solid lipid nanoparticles (SLNs) could be promising for food-grade applications. However, scaling this technique to an industrial level requires careful consideration of equipment design, process control, and energy consumption to ensure consistent product quality.7.2. Stability and shelf life
The stability and shelf-life of encapsulated functional lipids are critical for their commercialization. Encapsulated lipids must maintain their functional properties over time and under various storage conditions. The stability of these products can be influenced by factors such as the choice of encapsulation material, particle size, and physical and chemical environment. Sun et al.129 highlighted that the use of solid lipid nanoparticles (SLNs) can significantly increase the stability of encapsulated curcumin, leading to improved shelf-life and sustained release, which are vital for commercial products.7.3. Regulatory compliance
For encapsulated functional lipids to be commercialized, they must meet regulatory standards set by health and safety authorities. These regulations can vary by region and include guidelines on the use of encapsulation materials, labeling, and health claims. The study by Shishir et al.130 emphasized the importance of using food-grade materials and processes that comply with regulatory requirements to ensure that the final product is safe for consumption and can be legally marketed in different regions.7.4. Cost-effectiveness
The cost of production is a significant factor that affects the industrialization and commercialization of encapsulated functional lipids. The choice of encapsulation technique, materials, and processing conditions can impact the overall cost of production. Processes that are energy intensive or require expensive materials may not be viable on a large scale. Ezhilarasi et al.131 discussed the economic considerations of using solid lipid nanoparticles (SLNs) for the encapsulation of hydroxycitric acid (HCA), noting that while SLNs offer superior bioavailability, the cost of production must be balanced to ensure commercial viability.7.5. Consumer acceptance
Consumer acceptance is another critical factor that influences the success of encapsulated functional lipids on the market. Although consumers are increasingly seeking functional foods with health benefits, they are also concerned about the safety, naturalness, and sustainability of the ingredients and processes used. A study by Guri et al.132 highlighted the importance of consumer-friendly ingredients and processes in the development of encapsulated functional lipids, suggesting that transparent labeling and education about the benefits of these products can increase consumer acceptance.8. Conclusion and future prospects
Functional lipids such as omega 3 fatty acids, omega 6 fatty acids, linoleic acid, conjugated linoleic acid, carotenoids, and other bioactive lipid compounds have many beneficial effects on human health, such as cardiovascular diseases, mental disorders, and metabolic disorders; hence, they are recommended by medical experts. These compounds are available in a wide range of natural sources, such as vegetables, seeds, meat, fish, algae and microbes, and have tended to constitute integral parts of the human diet for many years. Owing to improper dietary patterns, the geographical distribution of sources, and the short availability of sources, the direct consumption of such functional lipids still does not satisfy the minimum dietary intake. Several commercial concentrated products rich in these functional lipids are also available on the market, but safety and GI stability are the major concerns of these types of products. Moreover, functional lipids are unsaturated and hydrophobic in nature, so bioavailability and bioaccessibility can be major obstacles for oral delivery or food fortification.In recent decades, researchers have developed certain encapsulation techniques involving the selection of suitable wall materials for functional lipids to increase bioavailability during oral delivery as well as the enrichment of food products. The mechanism of encapsulation of functional lipids within the wall/carrier matrix by various physical and chemical interactions affects the heat stability, storage stability and GI stability of encapsulates. Furthermore, encapsulated functional lipids tend to be more bioavailable within food systems and enhance the physicochemical and functional properties of food. Further studies are needed to address food safety concerns regarding fortified foods with encapsulated functional lipids, and a clear research gap was found in the utilization of novel sources of functional lipids such as algae, bacteria and fungi for the fortification of common staple foods by means of encapsulation techniques.
The successful industrialization and commercialization of encapsulated functional lipids depend on careful consideration of factors such as scalability, stability, regulatory compliance, cost-effectiveness, and consumer acceptance. Addressing these factors through research and innovation is essential for bringing effective and commercially viable functional lipid products to the market.
Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Conflicts of interest
There are no conflicts to declare.References
- S. Chew, C. Tan, L. Pui, P. Chong, B. Gunasekaran and K. Nyam, Int. J. Innovative Technol. Explor. Eng., 2019, 8, 154–162 Search PubMed.
- S. Sabet, C. K. Seal, A. Akbarinejad, A. Rashidinejad and D. J. McGillivray, Food Hydrocolloids, 2020, 107, 105922 CrossRef CAS.
- A. Patel and K. P. Velikov, LWT--Food Sci. Technol., 2011, 44, 1958–1964 CrossRef CAS.
- A. Araiza-Calahorra, M. Akhtar and A. Sarkar, Trends Food Sci. Technol., 2018, 71, 155–169 CrossRef CAS.
- A. Sarkar and A. R. Mackie, Curr. Opin. Colloid Interface Sci., 2020, 48, 40–52 CrossRef CAS.
- H. Pool, S. Mendoza, H. Xiao and D. J. McClements, Food Funct., 2013, 4, 162–174 RSC.
- A. Rezaei, M. Fathi and S. M. Jafari, Food Hydrocolloids, 2019, 88, 146–162 CrossRef CAS.
- B. Alabdulkarim, Z. A. N. Bakeet and S. Arzoo, J. King Saud Univ. Sci., 2012, 24, 319–329 CrossRef.
- R. Katiyar and A. Arora, Algal Res., 2020, 46, 101800 CrossRef.
- E. Arab-Tehrany, M. Jacquot, C. Gaiani, M. Imran, S. Desobry and M. Linder, Trends Food Sci. Technol., 2012, 25, 24–33 CrossRef CAS.
- T. F. F. da Silveira, L. M. Cajaíba, L. Valentin, B. Baréa, P. Villeneuve and I. A. Castro, Food Chem., 2020, 309, 125586 CrossRef CAS PubMed.
- P. Karthik and C. Anandharamakrishnan, RSC Adv., 2016, 6, 3501–3513 RSC.
- Y. Wang, C. Li, L. Li, X. Yang, Y. Wu, Y. Zhao and Y. Wei, J. Aquat. Food Prod. Technol., 2018 DOI:10.1080/10498850.2018.1450573.
- D. P. Killeen, S. N. Marshall, E. J. Burgess, K. C. Gordon and N. B. Perry, J. Agric. Food Chem., 2017, 65, 3551–3558 CrossRef CAS PubMed.
- E. C. Rizos, E. E. Ntzani, E. Bika, M. S. Kostapanos and M. S. Elisaf, Jama, 2012, 308, 1024–1033 CrossRef CAS PubMed.
- X. Wan, Y. Zhang, P. Wang and M. Jiang, J. Microbiol., 2011, 49, 151–154 CrossRef CAS PubMed.
- A. N. Carey, D. R. Fisher, D. F. Bielinski, D. S. Cahoon and B. Shukitt-Hale, Inflammation, 2020, 43, 241–250 CrossRef CAS PubMed.
- M. Garcia-Aloy, P. J. M. Hulshof, S. Estruel-Amades, M. C. J. Osté, M. Lankinen, J. M. Geleijnse, J. de Goede, M. Ulaszewska, F. Mattivi, S. J. L. Bakker, U. Schwab and C. Andres-Lacueva, Genes Nutr., 2019, 14, 7 CrossRef PubMed.
- A. Ashkar, S. Laufer, J. Rosen-Kligvasser, U. Lesmes and M. Davidovich-Pinhas, Food Hydrocolloids, 2019, 97, 105218 CrossRef CAS.
- T. Sae-leaw and S. Benjakul, Eur. J. Lipid Sci. Technol., 2017, 119, 1700198 CrossRef.
- N. A. Irvine, B. Ruyter, T. K. Østbye, A. K. Sonesson, K. A. Lillycrop, G. Berge and G. C. Burdge, Lipids, 2019, 54, 725–739 CrossRef CAS PubMed.
- J. A. Emery, F. Norambuena, J. Trushenski and G. M. Turchini, Lipids, 2016, 51, 399–412 CrossRef CAS PubMed.
- F. Alghamdi, Analysis of the Omega-3 Fatty Acids Content in Commercial Omega-3 Supplements in Arab Gulf Countries, Southern Illinois University at Carbondale, 2019 Search PubMed.
- E. M. Tillman, C. M. Crill, D. D. Black, E. B. Hak, L. F. Lazar, M. L. Christensen, E. Y. Huang and R. A. Helms, Pharmacotherapy, 2011, 31, 503–509 CrossRef CAS PubMed.
- M. A. Zulyniak, M. Perreault, C. Gerling, L. L. Spriet and D. M. Mutch, Metabolism, 2013, 62, 1107–1113 CrossRef CAS PubMed.
- A. J. Buglass, Handbook of Alcoholic Beverages: Technical, Analytical and Nutritional Aspects, 2010 Search PubMed.
- A. Zargar and M. K. Ito, Metab. Syndr. Relat. Disord., 2011, 9, 255–271 CrossRef CAS PubMed.
- A. S. Gutstein and T. Copple, J. Am. Assoc. Nurse Pract., 2017, 29, 791–801 CrossRef PubMed.
- L. Oliver, T. Dietrich, I. Marañón, M. C. Villarán and R. J. Barrio, Producing omega-3 polyunsaturated fatty acids: A review of sustainable sources and future trends for the EPA and DHA market, Resources, 2020, 9(12), 148 CrossRef.
- A. F. Cicero, M. Rosticci, M. Morbini, M. Cagnati, E. Grandi, A. Parini and C. Borghi, Arch. Med. Sci., 2016, 12, 507 CrossRef CAS PubMed.
- A. Gupta, C. K. Narkowicz, H. A. Al-Aubaidy, H. F. Jelinek, D. S. Nichols, J. R. Burgess and G. A. Jacobson, Phytosterol supplements do not inhibit dipeptidyl peptidase-4, Diabetes. Metab. Syndr., 2020, 14, 1475–1478 CrossRef PubMed.
- C. J. Mitchell, R. F. D'Souza, V. C. Figueiredo, A. Chan, K. Aasen, B. Durainayagam, S. Mitchell, A. J. Sinclair, I. M. Egner and T. Raastad, J. Appl. Physiol., 2018, 124, 1080–1091 CrossRef CAS PubMed.
- P. Mirmiran, M. R. Fazeli, G. Asghari, A. Shafiee and F. Azizi, Br. J. Nutr., 2010, 104, 402–406 CrossRef CAS PubMed.
- J. P. Dave, A. M. M. Ali and S. C. B. Bavisetty, An overview on recent advances in functional properties of dietary lipids, encapsulation strategies and applications, Nutr. Food Sci., 2022, 52(7), 1158–1180 CrossRef.
- R. R. Ambati, S. M. Phang, S. Ravi and R. G. Aswathanarayana, Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review, Mar. Drugs, 2014, 12(1), 128–152 CrossRef PubMed.
- V. A. Ziboh, S. Naguwa, K. Vang, J. Wineinger, B. M. Morrissey, J. McIntyre, M. Watnik and M. E. Gershwin, Clin. Dev. Immunol., 2004, 11, 13–21 CAS.
- H. V. Chuyen and J.-B. Eun, Crit. Rev. Food Sci. Nutr., 2017, 57, 2600–2610 CrossRef CAS PubMed.
- X. Li, J. Liu, G. Chen, J. Zhang, C. Wang and B. Liu, Algal Res., 2019, 43, 101619 CrossRef.
- S. Liu, N. Low and M. T. Nickerson, J. Am. Oil Chem. Soc., 2010, 87, 809–815 CrossRef CAS.
- M. Ramos, A. Valdes, A. Beltran and M. C. Garrigós, Coatings, 2016, 6, 41 CrossRef.
- A. Augustyniak, G. Bartosz, A. Čipak, G. Duburs, L. U. Horáková, W. Łuczaj, M. Majekova, A. D. Odysseos, L. Rackova and E. Skrzydlewska, Free Radic. Res., 2010, 44, 1216–1262 CrossRef CAS PubMed.
- C. Cheng, Z. Wu, D. J. McClements, L. Zou, S. Peng, W. Zhou and W. Liu, Colloids Surf. B Biointerfaces, 2019, 183, 110460 CrossRef CAS PubMed.
- M. Fathi, A. Martin and D. J. McClements, Trends Food Sci. Technol., 2014, 39, 18–39 CrossRef CAS.
- K. Hu, X. Huang, Y. Gao, X. Huang, H. Xiao and D. J. McClements, Food Chem., 2015, 182, 275–281 CrossRef CAS PubMed.
- Q. Chen, D. McGillivray, J. Wen, F. Zhong and S. Y. Quek, J. Food Eng., 2013, 117, 505–512 CrossRef CAS.
- P. Pourashouri, B. Shabanpour, S. H. Razavi, S. M. Jafari, A. Shabani and S. P. Aubourg, J. Aquat. Food Prod. Technol., 2014, 23, 567–578 CrossRef CAS.
- S. Chatterjee and Z. M. Judeh, Carbohydrate Polym., 2015, 123, 432–442 CrossRef CAS PubMed.
- D. F. Keenan, V. C. Resconi, T. J. Smyth, C. Botinestean, C. Lefranc, J. P. Kerry and R. M. Hamill, Meat Sci., 2015, 107, 75–85 CrossRef CAS PubMed.
- A. L. Charles, A. A. Abdillah, Y. R. Saraswati, K. Sridhar, C. Balderamos, E. D. Masithah and M. A. Alamsjah, Food Hydrocolloids, 2021, 112, 106281 CrossRef CAS.
- C. Chang and M. T. Nickerson, J. Food Sci. Technol., 2018, 55, 2850–2861 CrossRef CAS PubMed.
- C. Encina, C. Vergara, B. Giménez, F. Oyarzún-Ampuero and P. Robert, Trends Food Sci. Technol., 2016, 56, 46–60 CrossRef CAS.
- N. K. Mohammed, C. P. Tan, Y. A. Manap, B. J. Muhialdin and A. S. M. Hussin, Molecules, 2020, 25, 3873 CrossRef CAS PubMed.
- A. Goyal, V. Sharma, M. K. Sihag, S. Arora, A. Singh and L. Sabikhi, Dry. Technol., 2016, 34, 810–821 CrossRef CAS.
- D. M. Sánchez-Osorno, M. C. López-Jaramillo, A. V. Caicedo Paz, A. L. Villa, M. S. Peresin and J. P. Martínez-Galán, Pharmaceutics, 2023, 15, 1490 CrossRef PubMed.
- C. I. Piñón-Balderrama, C. Leyva-Porras, Y. Terán-Figueroa, V. Espinosa-Solís, C. Álvarez-Salas and M. Z. Saavedra-Leos, Processes, 2020, 8, 889 CrossRef.
- A. Rezvankhah, Z. Emam-Djomeh and G. Askari, Dry. Technol., 2020, 38, 235–258 CrossRef CAS.
- M. Hasani, A. E. Rad, M. M. Hosseini and M. S. Noghabi, Biosci., Biotechnol. Res. Asia, 2015, 12, 45–51 CrossRef.
- K. Li, A. J. Sinclair, F. Zhao and D. Li, Nutrients, 2018, 10, 1559 CrossRef PubMed.
- H. Li, Y. Yuan, J. Zhu, T. Wang, D. Wang and Y. Xu, Food Hydrocolloids, 2020, 103, 105715 CrossRef CAS.
- S. Feng, X. Zheng, D. Luan, P. Shao and P. Sun, LWT, 2019, 107, 138–144 CrossRef CAS.
- S. Soltani and A. Madadlou, Food Hydrocolloids, 2015, 43, 664–669 CrossRef CAS.
- J. Zeng, W. Yu, X. Dong, S. Zhao, Z. Wang, Y. Liu, M.-S. Wong and Y. Wang, Nanomed. Nanotechnol. Biol. Med., 2019, 15, 119–128 CrossRef CAS PubMed.
- T. Sathasivam, S. Muniyandy, L. H. Chuah and P. Janarthanan, J. Food Eng., 2018, 231, 10–21 CrossRef CAS.
- T. A. Comunian, M. Nogueira, B. Scolaro, M. Thomazini, R. Ferro-Furtado, I. A. de Castro and C. S. Favaro-Trindade, Food Chem., 2018, 252, 277–284 CrossRef CAS PubMed.
- B. da Silva Soares, R. P. Siqueira, M. G. de Carvalho, J. Vicente and E. E. Garcia-Rojas, Food Chem., 2019, 298, 125045 CrossRef CAS PubMed.
- J. D. Rios-Mera, E. Saldaña, Y. Ramírez, E. A. Auquiñivín, I. D. Alvim and C. J. Contreras-Castillo, LWT, 2019, 116, 108555 CrossRef CAS.
- Y. Yuan, Z.-Y. Kong, Y.-E. Sun, Q.-Z. Zeng and X.-Q. Yang, Lwt, 2017, 75, 171–179 CrossRef CAS.
- A. M. Costa, L. K. Moretti, G. Simões, K. A. Silva, V. Calado, R. V. Tonon and A. G. Torres, LWT, 2020, 131, 109519 CrossRef CAS.
- K. Yoshikiyo, Y. Yoshioka, Y. Narumiya, S. Oe, H. Kawahara, K. Kurata, H. Shimizu and T. Yamamoto, Food Chem., 2019, 294, 56–59 CrossRef CAS PubMed.
- M. Ünlüsayin, N. G. Hădărugă, G. Rusu, A. T. Gruia, V. Păunescu and D. I. Hădărugă, LWT--Food Sci. Technol., 2016, 68, 135–144 CrossRef.
- E. Y. Park, S. M. Choi, S.-T. Lim and J.-Y. Kim, Food Hydrocolloids, 2018, 77, 357–362 CrossRef CAS.
- M. Busolo, S. Torres-Giner, C. Prieto and J. Lagaron, Innovat. Food Sci. Emerg. Technol., 2019, 51, 12–19 CrossRef CAS.
- D. F. Tirado, I. Palazzo, M. Scognamiglio, L. Calvo, G. Della Porta and E. Reverchon, J. Supercrit. Fluids, 2019, 150, 128–136 CrossRef CAS.
- D. T. Santos, Á. Martín, M. A. A. Meireles and M. J. Cocero, J. Supercrit. Fluids, 2012, 61, 167–174 CrossRef CAS.
- K. Moomand and L.-T. Lim, Food Res. Int., 2014, 62, 523–532 CrossRef CAS.
- M. X. Hu, X. L. Chen, L. J. Song and F. He, J. Appl. Polym. Sci., 2020, 50403 Search PubMed.
- T. Cetinkaya, A. C. Mendes, C. Jacobsen, Z. Ceylan, I. S. Chronakis, S. R. Bean and P. J. García-Moreno, LWT, 2021, 136, 110297 CrossRef CAS.
- B. Rasti, S. Jinap, M. Mozafari and A. Yazid, Food Chem., 2012, 135, 2761–2770 CrossRef CAS PubMed.
- S. Gulzar and S. Benjakul, Food Chem., 2020, 310, 125916 CrossRef CAS PubMed.
- M. Qiang, X. Pang, D. Ma, C. Ma and F. Liu, Molecules, 2020, 25, 610 CrossRef CAS PubMed.
- A. Zamani-Ghaleshahi, G. Rajabzadeh, H. Ezzatpanah and M. Ghavami, Food Biophys., 2020, 1–15 Search PubMed.
- S. Serini, R. Cassano, P. A. Corsetto, A. M. Rizzo, G. Calviello and S. Trombino, Int. J. Mol. Sci.c, 2018, 19, 586 CrossRef PubMed.
- M. Azizi, A. Kierulf, M. C. Lee and A. Abbaspourrad, Food Chem., 2018, 246, 448–456 CrossRef CAS PubMed.
- J. Yang and O. N. Ciftci, Food Chem., 2017, 231, 105–113 CrossRef CAS PubMed.
- C. Anandharamakrishnan, Techniques for Nanoencapsulation of Food Ingredients, Springer, 2014 Search PubMed.
- Z. Li, T. Meng, X. Ling, J. Li, C. Zheng, Y. Shi, Z. Chen, Z. Li, Q. Li and Y. Lu, J. Agric. Food Chem., 2018, 66, 5382–5391 CrossRef CAS PubMed.
- E. S. Committee, S. More, V. Bampidis, D. Benford, C. Bragard, T. Halldorsson, A. Hernández-Jerez, S. H. Bennekou, K. Koutsoumanis and C. Lambré, EFSA J., 2021, 19, e06769 Search PubMed.
- M. Correia, E. Verleysen and K. Loeschner, in Nanomaterials for Food Applications, Elsevier, 2019, pp. 273–311 Search PubMed.
- K. Rasmussen, H. Rauscher, S. Gottardo, E. Hoekstra, R. Schoonjans, R. Peters and K. Aschberger, in Nanomaterials for Food Applications, Elsevier, 2019, pp. 381–410 Search PubMed.
- E. Ververis, R. Ackerl, D. Azzollini, P. A. Colombo, A. de Sesmaisons, C. Dumas, A. Fernandez-Dumont, L. F. da Costa, A. Germini and T. Goumperis, Food Res. Int., 2020, 137, 109515 CrossRef CAS PubMed.
- A. F. Esfanjani and S. M. Jafari, Colloids Surf. B Biointerfaces, 2016, 146, 532–543 CrossRef PubMed.
- A. Vaucher, P. C. M. Dias, P. T. Coimbra, I. Costa, R. N. Marreto, G. M. Dellamora-Ortiz, O. De Freitas and M. F. S. Ramos, J. Microencapsul., 2019, 36, 459–473 CrossRef CAS PubMed.
- C. Robert, L. Couëdelo, C. Knibbe, L. Fonseca, C. Buisson, E. Errazuriz-Cerda, E. Meugnier, E. Loizon, C. Vaysse and M. C. Michalski, J. Nutr., 2020, 150, 2900–2911 CrossRef PubMed.
- S. H. Anwar and B. Kunz, J. Food Eng., 2011, 105, 367–378 CrossRef CAS.
- C. Prieto and L. Calvo, J. Supercrit. Fluids, 2017, 128, 227–234 CrossRef CAS.
- C. Anandharamakrishnan, 2017.
- P. Wen, Y. Wen, M.-H. Zong, R. J. Linhardt and H. Wu, J. Agric. Food Chem., 2017, 65, 9161–9179 CrossRef CAS PubMed.
- A. Moreira, D. Lawson, L. Onyekuru, K. Dziemidowicz, U. Angkawinitwong, P. K. Costa and G. R. Williams, Protein encapsulation by electrospinning and electrospraying, J. Controlled Release, 2021, 329, 1172–1197 CrossRef CAS PubMed.
- C. M. Sabliov and C. E. Astete, Polymeric nanoparticles for food applications, Nanotechnology and functional foods: Effective delivery of bioactive ingredients, 2015, p. 272 Search PubMed.
- C. Jacobsen, P. J. García-Moreno, A. C. Mendes, R. V. Mateiu and I. S. Chronakis, Annu. Rev. Food Sci. Technol., 2018, 9, 525–549 CrossRef CAS PubMed.
- S. Chaudhary, T. Garg, R. Murthy, G. Rath and A. K. Goyal, J. Drug Target., 2014, 22, 871–882 CrossRef CAS PubMed.
- C. W. Pouton and C. J. Porter, Adv. Drug Deliv. Rev., 2008, 60, 625–637 CrossRef CAS PubMed.
- H. Bunjes, Curr. Opin. Colloid Interface Sci., 2011, 16, 405–411 CrossRef CAS.
- A. F. Esfanjani, E. Assadpour and S. M. Jafari, Trends Food Sci. Technol., 2018, 76, 56–66 CrossRef.
- A. Gasa-Falcon, I. Odriozola-Serrano, G. Oms-Oliu and O. Martín-Belloso, Foods, 2020, 9, 325 CrossRef CAS PubMed.
- S. Peng, L. Zou, W. Liu, C. Liu and D. J. McClements, J. Agric. Food Chem., 2018, 66, 12421–12430 CrossRef CAS PubMed.
- C. Qian, E. A. Decker, H. Xiao and D. J. McClements, Food Res. Int., 2013, 52, 342–349 CrossRef CAS.
- V. da Silva Santos, A. P. B. Ribeiro and M. H. A. Santana, Food Res. Int., 2019, 122, 610–626 CrossRef PubMed.
- M. Azizi, Y. Li, N. Kaul and A. Abbaspourrad, J. Agric. Food Chem., 2019, 67, 671–679 CrossRef CAS PubMed.
- J. Weiss, E. A. Decker, D. J. McClements, K. Kristbergsson, T. Helgason and T. Awad, Food Biophys., 2008, 3, 146–154 CrossRef.
- F. Shahidi and Y. Zhong, Chem. Soc. Rev., 2010, 39, 4067–4079 RSC.
- F. Chen, G.-Q. Fan, Z. Zhang, R. Zhang, Z.-Y. Deng and D. J. McClements, Food Res. Int., 2017, 100, 387–395 CrossRef CAS PubMed.
- D. J. McClements and S. M. Jafari, in Nanoemulsions, Elsevier, 2018, pp. 3–20 Search PubMed.
- J. Velasco, S. Marmesat, C. Dobarganes and G. Márquez-Ruiz, J. Agric. Food Chem., 2006, 54, 1722–1729 CrossRef CAS PubMed.
- S. Sharma, S.-F. Cheng, B. Bhattacharya and S. Chakkaravarthi, Trends Food Sci. Technol., 2019, 91, 305–318 CrossRef CAS.
- R. de M. Barbosa, L. N. Ribeiro, B. R. Casadei, C. M. Da Silva, V. A. Queiróz, N. Duran, D. R. De Araújo, P. Severino and E. De Paula, Pharmaceutics, 2018, 10, 231 CrossRef PubMed.
- D. Xie, M. Gong, W. Wei, J. Jin, X. Wang, X. Wang and Q. Jin, Compr. Rev. Food Sci. Food Saf., 2019, 18, 514–534 CrossRef PubMed.
- F. Tamjidi, A. Nasirpour and M. Shahedi, Food Sci. Technol. Int., 2012, 18, 381–390 CrossRef CAS PubMed.
- F. Tamjidi, A. Nasirpour and M. Shahedi, J. Agric. Sci. Technol., 2014, 16, 1073–1082 Search PubMed.
- T. Ghorbanzade, S. M. Jafari, S. Akhavan and R. Hadavi, Food Chem., 2017, 216, 146–152 CrossRef CAS PubMed.
- D. Bermúdez-Aguirre and G. V. Barbosa-Cánovas, LWT--Food Sci. Technol., 2011, 44, 1577–1584 CrossRef.
- F. Farbod, A. Kalbasi, S. Moini, Z. Emam-Djomeh, H. Razavi and A. Mortazavi, J. Food Sci. Technol., 2015, 52, 1372–1382 CrossRef CAS PubMed.
- S. M. Ojagh and S. Hasani, J. Food Meas. Char., 2018, 12, 1084–1092 CrossRef.
- P. T. Andajani, H. Purnomo and L. E. Radiati, Int. J. ChemTech Res., 2015, 8, 548–555 CAS.
- A. Gowda, V. Sharma, A. Goyal, A. Singh and S. Arora, J. Food Sci. Technol., 2018, 55, 1705–1715 CrossRef CAS PubMed.
- R. Domínguez, M. Pateiro, R. Agregán and J. M. Lorenzo, J. Food Sci. Technol., 2017, 54, 26–37 CrossRef PubMed.
- J. Stangierski, R. Rezler, K. Kawecki and B. Peplińska, J. Sci. Food Agric., 2020, 100, 2043–2051 CrossRef CAS PubMed.
- J. Xue, T. Wang, Q. Hu, M. Zhou and Y. Luo, Food Hydrocolloids, 2018, 79, 110–116 CrossRef CAS.
- J. Sun, C. Bi, H. M. Chan, S. Sun, Q. Zhang and Y. Zheng, Colloids Surf. B Biointerfaces, 2013, 111, 367–375 CrossRef PubMed.
- M. R. I. Shishir, L. Xie, C. Sun, X. Zheng and W. Chen, Trends Food Sci. Technol., 2018, 78, 34–60 CrossRef CAS.
- P. Ezhilarasi, S. Muthukumar and C. Anandharamakrishnan, RSC Adv., 2016, 6, 53784–53793 RSC.
- A. Guri, I. Gülseren and M. Corredig, Food Funct., 2013, 4, 1410–1419 RSC.
| This journal is © The Royal Society of Chemistry 2024 |