Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Potential of cold plasma in enhancing egg white protein for sustainable food applications: a comprehensive review
Ubaida
Akbar
a,
Shivangi
Srivastava
b,
Aamir Hussain
Dar
 *c,
Kshirod Kumar
Dash
*c,
Kshirod Kumar
Dash
 d,
Sabir Ahmad
Mondol
e,
Vinay Kumar
Pandey
f,
Toiba
Majeed
a and
Urba Shafiq
Sidiqi
a
d,
Sabir Ahmad
Mondol
e,
Vinay Kumar
Pandey
f,
Toiba
Majeed
a and
Urba Shafiq
Sidiqi
a
aDepartment of Food Technology and Nutrition, Lovely Professional University, Phagwara, Punjab, India
bDepartment of Food Technology, Harcourt Butler Technical University, Nawabganj, Kanpur, Uttar Pradesh, India
cDepartment of Food Technology, Islamic University of Science and Technology, Kashmir, India. E-mail: daraamirft@gmail.com
dDepartment of Food Processing Technology, Ghani Khan Choudhury Institute of Engineering and Technology, Malda, West Bengal, India
eDepartment of Agricultural Biochemistry, Bidhan Chandra Krishi Viswavidyalaya, Mohanpur, Nadia, West Bengal, India
fRDC, Biotechnology Development, Manav Rachna International Institute of Research and Studies (Deemed to be University) Faridabad, Haryana, India
First published on 9th September 2024
Abstract
The objective of this review is to explore recent insights into the impact of cold plasma treatment on the structural and functional properties of egg white protein and to assess its potential for sustainable food applications. The cold plasma treatment can substantially alter the structural and functional properties of egg white protein. The core of the review lies in the multifaceted effects of cold plasma treatment on egg white proteins, encompassing structural transformations elucidated through SDS-PAGE, Fourier transform infrared spectroscopy, nuclear magnetic resonance, and circular dichroism. Microscopic, rheological, and spectroscopic analyses offer a comprehensive understanding of the various modifications induced by cold plasma treatment. Cold plasma treatment caused alterations in the conformation of the protein structure, changing its solubility, emulsifying, foaming, and gelling properties. These modifications improve protein functioning, rendering them more appropriate for a range of dietary applications. Cold plasma treatment was found to enhance the antibacterial properties of egg white protein by increasing its capacity to suppress the growth of harmful microbes such as Escherichia coli and Staphylococcus aureus. Due to these enhanced properties, cold plasma-treated egg white protein is highly valued as a component in a wide range of food products, such as baked goods, dairy substitutes, meat products, and beverages. However, it is important to note that its use in large-scale production has not been extensively implemented yet. In summary, recent studies indicate that cold plasma treatment can successfully alter the structural and functional characteristics of egg white protein, broadening its potential for use in the food industry and providing new prospects for product formulation and innovation.
Sustainability spotlightEgg white protein offers numerous advantages for sustainable food applications, ranging from its nutritional benefits to its functional versatility and waste reduction potential. By incorporating egg white protein into food formulations and exploring innovative processing techniques, the food industry can contribute to more sustainable food systems. This review provides a thorough examination of the current state of knowledge regarding the use of cold plasma in enhancing egg white protein for sustainable food applications, offering insights into its potential benefits and future directions for research and development in this area. |
1. Introduction
Egg white protein (EWP) stands out as a cost-effective reservoir of premium-quality protein, boasting elevated levels of various amino acids compared to alternative protein sources such as soybean and milk proteins.1 Its retention within the body, coupled with beneficial physical and nutritional properties, renders it a pivotal component in a diverse array of foods, including baked products, meat items, noodles, and meringues.2 Despite its nutritional benefits, EWP is highly prone to oxidation and denaturation during heat processing and storage, leading to undesirable alterations in its composition and properties.3 Conventional processing methods, notably thermal processes like pasteurization, boiling, and drying, have traditionally been used to handle egg white protein (EWP). However, these techniques can negatively impact EWP structural, functional, and nutritional characteristics, compromise its distinct flavor, and introduce the risk of chemical contamination such as the formation of harmful compounds like acrylamide and advanced glycation end-products (AGEs), potentially posing harm to consumers. In response to these challenges, nonthermal processing techniques like High-Pressure Processing (HPP), Pulsed Electric Fields (PEF), Ultrasound Processing, Cold Plasma Treatment, Irradiation and Ozone Treatment have emerged as viable alternatives. This approach, conducted at room temperature, preserves the inherent attributes of food materials with minimal impact on their structure, functionality, and nutritional content. This not only prevents flavour loss but also ensures the retention of essential nutritional constituents, ultimately enhancing the shelf stability of processed food products.4 High-Pressure Processing (HPP), Pulsed Electric Fields (PEF), Ultrasound Processing, Cold Plasma Treatment, Irradiation, and Ozone Treatment offer significant advantages, such as preventing vitamin degradation, altering pigments, producing undesirable compounds, and maintaining food texture and sensory properties. These emerging technologies ensure food safety and quality while minimizing the negative impacts associated with traditional thermal processing methods.2Responding to the demand for high-quality and minimally processed foods, researchers have explored various technologies, including High-Pressure Processing (HPP), Pulsed Electric Fields (PEF), Ultrasound Processing, and Irradiation, with cold plasma standing out as a recent innovation. These methods offer effective alternatives to conventional thermal processing, preserving the nutritional and sensory qualities of food while ensuring safety and extending shelf life. Initially, used for enhancing polymer printing and adhesion properties, elevating material surface energy, and finding numerous applications in electronics,5 cold plasma has now found applications across multiple industries, including textile, medicine, biotechnology, electronics, and air and water purification.6 Cold plasma technology is a versatile tool in the food industry, providing a range of benefits. It helps with microbial decontamination, breaking down toxins, removing pesticide residues, deactivating enzymes, improving food packaging, and modifying ingredients.7 Cold plasma can be divided into atmospheric and low-pressure types based on the pressure conditions. Atmospheric cold plasma (ACP), generated under normal atmospheric conditions without the need for expensive reaction chambers or pumps, is widely used in the food industry due to its cost-effectiveness and convenience, eliminating the need for high temperature and pressure adjustments.8 ACP produces a neutral gas that ionizes and releases reactive species like reactive oxygen species (ROS), reactive nitrogen species (RNS), and ultraviolet (UV) radiation.9 These species interact with food components, mainly proteins, causing reactions such as dimerization, deamidation, sulfoxidation, oxidation, nitration, hydroxylation, or dehydrogenation of amino acids. Notable effects include improved emulsifying and foaming properties of whey protein isolate and the inactivation of enzymes responsible for undesirable reactions.10–15
The mechanisms involved in the modification of secondary protein structures, including the disruption of α-helix and β-plated sheet formations, are being proposed. Reactive species can interact with food allergens, altering conformational and linear epitopes and forming insoluble aggregates through protein crosslinking. Linear epitopes may undergo alteration through fragmentation, while peptide bonds and amino acids can be affected by reactive oxygen species, significantly impacting protein integrity.16–18 Cold plasma treatment (CPT) has shown promising results in reducing the allergenicity of tropomyosin in shrimp and the immunoreactivity of soy proteins.19 This comprehensive review aimed to elucidate the impact of CPT on egg white protein, focusing on its structural and techno-functional characteristics.
2. Egg white protein composition and structure
Eggs stand out as a highly nutritious food source, encompassing proteins, essential lipids, minerals, vitamins, and trace elements within their internal components, including the shell, yolk, and white. This nutritional powerhouse is particularly cost-effective and versatile, serving as an economical animal source for a variety of nutrients while maintaining a moderate caloric content of approximately 140 kcal/100 g.20 The distribution of proteins and lipids within the egg is noteworthy, with proteins present in both the egg white and yolk, while lipids are mostly concentrated in the yolk. Additionally, the eggshell membrane is composed mainly of protein, bone collagen, hyaluronic acid, and chondroitin. The overall composition of an egg includes proteins, water, carbohydrates, fat, ash, and cholesterol.The nutritional richness and sensory attributes of the egg yolk make it a valuable component, consisting of water, protein, lipids, and carbohydrates. Egg white, constituting the majority of the whole egg (58% by volume), is primarily composed of water (88%), protein (10.5%), ash (0.8%), carbohydrates (0.5%), and lipids (0.2%).Within the egg white, water and protein are the predominant elements, featuring major proteins such as ovalbumin (54%), ovomucoid (11%), ovotransferrin (12%), lysozyme (3.5%) and ovomucin (3.5%)21 (Fig. 1). Minor proteins such as ovoglycoprotein, ovoflavoprotein, ovomacroglobulin, ovoinhibitor, cystatin, and avidin also play crucial roles in various food. Renowned for their essential amino acids, high bioavailability, and functionality in food processing, egg white proteins significantly enhance the nutritional profile and versatility of eggs in culinary applications.22
2.1. Major proteins in egg white
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da, comprising 386 amino acid residues. The isoelectric point (pI) of this protein was determined to be 4.5. Ovalbumin, which is linked by a solitary disulfide bond, contains four unbound sulfhydryl groups and is associated with a single carbohydrate moiety that binds to Asn-292.23 An investigation through purification and sequencing revealed that Cys-73 and Cys-120 are likely involved in the formation of the above mentioned disulfide bond.24 The significant role of ovalbumin in augmenting the functional attributes of egg white, including emulsifiability, foamability, and gelation, has been well documented. These desirable characteristics can be attributed to the exceptional thermal and surface properties of ovalbumin.24
000 Da, comprising 386 amino acid residues. The isoelectric point (pI) of this protein was determined to be 4.5. Ovalbumin, which is linked by a solitary disulfide bond, contains four unbound sulfhydryl groups and is associated with a single carbohydrate moiety that binds to Asn-292.23 An investigation through purification and sequencing revealed that Cys-73 and Cys-120 are likely involved in the formation of the above mentioned disulfide bond.24 The significant role of ovalbumin in augmenting the functional attributes of egg white, including emulsifiability, foamability, and gelation, has been well documented. These desirable characteristics can be attributed to the exceptional thermal and surface properties of ovalbumin.24
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da. Its three-dimensional configuration involves three disulfide bonds, and notably, it functions as a trypsin inhibitor. Razi et al. (2024) stated that peptides derived from ovomucoid parasites can bind to IgE and inhibit trypsin, a phenomenon that significantly contributes to egg allergy.21 Moreover, ovomucoid are renowned for their heat resistance, and their considerable carbohydrate content prevents their self-aggregation. This distinctive feature results in its nonparticipation in heat-induced gel formation.28
000 Da. Its three-dimensional configuration involves three disulfide bonds, and notably, it functions as a trypsin inhibitor. Razi et al. (2024) stated that peptides derived from ovomucoid parasites can bind to IgE and inhibit trypsin, a phenomenon that significantly contributes to egg allergy.21 Moreover, ovomucoid are renowned for their heat resistance, and their considerable carbohydrate content prevents their self-aggregation. This distinctive feature results in its nonparticipation in heat-induced gel formation.28
| Substrate | Plasma | Observations | References |
|---|---|---|---|
| Egg shells | After glow corona discharge air plasma | Microbial reductions upto 96–98% | 31 |
| 20 kV, 58 kHz, 12 h | No changes in sensory and physiochemical properties | ||
| Egg surface | Direct or indirect cold atmospheric plasma | S. enterica reduced to 100 cells per egg at 10 min DT and 25 min IDT treatment | 32 |
| He/O2 mixtures | Deactivation due to hydroxyl radicals | ||
| Sinusoidal 25–30 kV, 10–12 kHz | |||
| Chicken eggshells | Higher voltage atmospheric cold plasma |
S. enteritidis reduction of 5.53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU per egg of with no effect on egg quality during DT log CFU per egg of with no effect on egg quality during DT |
33 |
| Dry air | |||
| Modified atmospheric gases | |||
| 85 kV, 15 min |
3. Principles and mechanisms of cold plasma generation
An ionizing gas with a significant amount of energy produces plasma, the fourth state of matter in the cosmos. Plasma is essentially a high-energy system that includes a variety of active substances, including ions, molecules, atoms, electrons, and free radicals. It also promotes various chemical reactions and ultraviolet (UV) radiation.36 When engaging with samples, plasma exhibits not only physical interactions such as etching, collision, and UV radiation but also distinctive chemical reactions with the active species, rendering it a distinctive form of matter.36 Temperature serves as a pivotal determinant in plasma applications, leading to its categorization into thermal plasma (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 K) and nonthermal plasma (300–1000 K).37
000 K) and nonthermal plasma (300–1000 K).37
In recent decades, scholars have explored innovative processing methods aimed at ensuring the safety and extended shelf life of food while enhancing its overall quality and nutritional content. Functionality and impact of cold plasma technology on food products is represented in Fig. 2. A diverse range of thermal and nonthermal methods, such as dielectric heating (including microwave heating and radiofrequency), ohmic heating, infrared (IR) heating, pulsed light (PL), pulsed electric field (PEF), high-pressure processing (HPP), ultrasound, ozone processing, and cold plasma (CP) methods, are being investigated.38 These techniques, which are conducted at or around ambient temperatures, addresses various drawbacks associated with traditional thermal processing. Among the array of emerging nonthermal techniques, cold plasma (CP) is a relatively recent technological intervention dedicated to ensuring food safety and food quality.39 Cold plasma, characterized by temperatures approximating room temperature, offers unique benefits in its application within the food industry like bakery, confectionary, dairy etc.40
In recent years, cold plasma has been used as an efficient method to control microorganisms on food surfaces, including but not limited to S. aureus, Salmonella typhimurium, A. brasiliensis, Listeria monocytogenes, E. coli,41,42 yeasts, and Bacillus subtilis endospores.43 Additionally, it serves as an environmentally friendly and efficient sterilization method for food packaging and food contact surfaces, such as stainless steel and polyethylene surfaces.
Mechanistically in cold plasma a gas is subjected to an electric field—either a continuous, direct current field or an alternating, amplitude field, usually a high-frequency field—between two electrodes, plasma is created.44 The application of various forms of energy, such as microwave or radio frequencies and electric, thermal, or magnetic fields, is required for the induction of the plasma state. These energy sources enhance the kinetic energy of electrons, which increases the number of collisions in the gas. As a result, this process produces plasma constituents such as electrons, radicals, ions, and radiation at different wavelengths, including ultraviolet (UV) radiation.45 The generation of plasma employs a range of methods, including dielectric barrier discharge (DBD), plasma jet (PJ), corona discharge, radio frequency plasma (RFP), and microwave (MW) methods.46
3.1. Dielectric barrier discharge (DBD)
The generation of DBD plasma, which is renowned for its cost-effectiveness and versatility in electrode and dielectric material selection, involves the application of high voltage across metal electrodes coated with dielectric material.47 The operational parameters include gas pressures ranging from 1 × 104 to 1 × 106 Pa, frequencies spanning 10 to 50 MHz, and alternating or pulsed direct current with a voltage amplitude between 1 and 100 kV rms.48 Significantly, DBD has found practical use in in-package food treatment, exemplified by reactors that have achieved notable reductions in microbial counts on fresh produce, underscoring its potential for large-scale decontamination in industrial settings.42,493.2. Corona discharge
When an asymmetric electrode pair arrangement is used, corona discharge (also known as CD) is most observed as a bright glow in a nonuniform electric field close to sharp edges or points. This kind of discharge, shown by the point-plane configuration, produces weakly ionized plasma when an electric field rises above its breakdown threshold in a particular spatial area.30 A multipoint-plate electrode arrangement has become more important for improving the usability of CDs in food-related contexts because it can generate a denser and more energetic plasma than dielectric barrier discharge (DBD).44 This configuration, featuring diffusive discharge, effectively covers the sample surface on a broader scale than does a pin tip.50Venkataratnam et al. (2020)51 conducted an experiment in which a multipoint-plate reactor with a large gap (70 mm) was employed to assess the effectiveness of CDs on major peanut allergens and for pesticide degradation on grapes and strawberries. The reactor was operated at a discharge voltage of 32 kV, a duty cycle of 118 s, a resonant frequency of 52 kHz, and a discharge frequency of 1 kHz. The results revealed noteworthy reductions in pesticide levels, with a 79% reduction in chlorpyrifos on grapes and a 69% reduction on strawberries. Additionally, carbaryl reduction was 73% in strawberries and 86% in grapes.52
In contrast to the typical discharge gap and voltage parameters in cold plasma (CP) applications, the multipoint-plate reactor employed a larger gap (70 mm) and a lower discharge voltage (32 kV). This underscores the role of concurrently developing reactor and generator systems and thoroughly investigating electrode configurations, material properties, and electrical parameters to effectively apply CP in food processing.46
3.3. Plasma jet (PJ)
According to Arif et al., (2024),53 a plasma jet (PJ) is a discharge that can take on a variety of forms, in which the plasma is produced by another plasma source. Of the many possible designs, the most popular ones have two rings or coaxial electrodes with gas passing between them. While the core electrode is energized by RF, usually at 13.56 MHz, the outside electrode is grounded. This causes free electrons to accelerate and interact with gas molecules, forming a multitude of reactive species. The gas flows at a high rate, driving the plasma formed outside the electrode zone as a projected jet and releasing plasma species into the open environment. The gas is frequently a noble gas or a mixture of reactive gases.54 At atmospheric pressure, the PJ produces a more uniform, homogenous, and stable discharge. However, its effectiveness is limited when targeting large areas, necessitating the application of multiple jets in such cases.55,563.4. Radio frequency plasma (RFP)
Inductively coupled plasmas (ICPs), capacitively coupled plasmas (CCPs), and helicon wave sources are the three main types of RFPs. ICP and CCP setups are the most used in industrial applications. In a vacuum chamber, two parallel electrodes are positioned a few centimetres apart as part of the CCP setup.57 Applying an alternating voltage between these electrodes produces radiofrequency (RF).5 An RF source of approximately 1 kW powers the electrodes. It typically operates at a frequency of 13.56 MHz, with a range of 1–500 MHz. The gas can be ionized by an oscillating electromagnetic field, creating a plasma that typically has a density of 1015–1016 m3 when electrons and ions are considered. Typically, two RF power supplies are used in ICP systems. The first one powers a coil, usually placed outside the plasma and divided by a window made of dielectric. The second power supply biases the substrate holder and controls the ion energy.51,58 This approach is unique in that it modifies starch in a novel way and has the potential to produce semiconductors and food packaging made of starch with great thermal stability.59,603.5. Microwave (MW)
In microwave (MW) plasma generators, microwave discharge is generated by electromagnetic waves, typically at 2.45 GHz. The microwave electric field can accelerate the electrons of gas molecules, leading to the formation of cold plasma (CP) without the need for electrodes. This system is capable of producing plasma under both atmospheric and low-pressure conditions.61Via a waveguide, the waves are guided towards a treatment chamber where they interact with the gas electrons. In this mechanism, the microwaves are absorbed by the electrons, which causes inelastic collisions that start ionization reactions and release energy in the form of UV and visible light photons.46 The enhanced electron density in the ionized gas and the effective production of reactive species are two of the main benefits of this method. Although the system is suitable for surface sterilization or decontamination, its limited usage is attributed to its cost and the meticulous care required for its operation.624. Selection of the cold plasma source
Cold plasma sources have been applied in a wide range of fields, including materials processing, surface treatment, biomedical applications, and environmental remediation. The selection of an appropriate cold plasma source is crucial for achieving targeted outcomes specific to applications.4.1. Atmospheric pressure plasma jets
Atmospheric pressure plasma jets (APPJs) are important due to their simplicity, cost-effectiveness, and extensive capabilities for surface treatment and modification.63 APPJs are essential for treating the surfaces of diverse materials because of their capacity to generate a greater flux of various metastable active species. The absence of a vacuum chamber in the APPJ system facilitates the development of small-scale and economical plasma sources.64 Various methods are employed to generate atmospheric pressure plasmas, including microwave discharge, radio frequency (RF) discharge, direct current (DC) discharge and dielectric barrier discharge (DBD). Each of these techniques possesses unique features suitable for specific applications. These have been used to treat EWP, resulting in modifications to protein structures and functionalities. For example, a study by Thirumdas et al. (2018)62 demonstrated that APPJ treatment can improve the foaming and emulsifying properties of EWP by inducing structural changes and increasing surface hydrophobicity. Chronic wounds, stemming from conditions such as venous dysfunction and diabetes mellitus, can pose significant health challenges. APPJs have proven to be remarkably effective in treating a variety of superficial skin infections as well as infected and colonized wounds. Remarkably, patients with chronic infected wounds reported minimal discomfort during plasma jet therapy, and no side effects were observed during the course of treatment.654.2. Dielectric barrier discharges
Dielectric barrier discharge cold atmospheric plasma (DBD-CAP) stands out as a novel technique highly esteemed by contemporary food scientists and engineers as a microbial inactivation technique.4 The key attribute that increases the utility of DBD is its nonthermal equilibrium, which is highly advantageous for material processing. Establishing nonthermal equilibrium plasma conditions in DBDs is notably simpler than using alternative methods such as fast-pulsed high-pressure discharges, low-pressure discharges, or electron beam injection. This technique has been widely used for the decontamination and modification of EWP. Jiang et al. (2020)63 found that DBD treatment led to significant changes in the secondary structure of EWP, such as a decrease in α-helix content and an increase in β-sheet content, which affected the protein's functional properties like gelation and emulsification. DBD is particularly effective due to its ability to generate a high density of reactive species at ambient temperature and pressure.4.3. Low-pressure plasma
While there are a number of methods for creating low-pressure plasma, DC glow discharge is the most often used and conventional method. This method is particularly helpful for sputter deposition of thin metal coatings because of its significant potential drop at the conductive cathode, which results in a strong bombardment of positive ions on the cathode and sputtering materials from the metallic surface.44 However, insulating surfaces are charged in opposition to the applied field, which presents a constraint when using DC discharge for sputtering dielectric materials or creating dielectric films. This challenge could be overcome by employing microwave (MW) or radio frequency (RF) discharges. Although less common due to the need for vacuum systems, low-pressure plasma has been used to achieve specific modifications in EWP. Research by Ji et al. (2019)64 indicated that low-pressure plasma treatment can cause extensive protein cross-linking and oxidation, leading to changes in the protein's solubility and digestibility. This method provides a controlled environment to study the fundamental interactions between plasma and proteins. These alternatives allow for the neutralization of positive charges assembled during one half cycle by electron bombardment in the subsequent half cycle. Low-pressure plasma systems play a pivotal role in modern manufacturing and material processing, providing advantages in terms of precision, uniformity, and versatility across a diverse range of applications.5. Factors of cold plasma affecting the effect of cold plasma on egg white proteins
Numerous factors can influence the impact of cold plasma on egg white proteins. Understanding these factors is crucial for optimizing conditions in applications such as food processing or surface treatment. The following are the key factors to consider.5.1. Distance between the plasma sources
The effectiveness of cold plasma on egg white proteins is significantly affected by the distance from the plasma source. The proximity of the sample to the plasma source plays an essential role in determining the intensity of treatment, whereby shorter distances result in more profound exposure and potential modifications to the proteins. The concentration and energy levels of reactive species produced by the plasma vary based on this distance, impacting the type and extent of protein modifications.66 Moreover, the interaction between reactive species and proteins is directly affected by proximity, with closer distances leading to more direct and concentrated interactions.67 Careful control of this distance parameter is imperative for achieving specific modifications in egg white proteins while considering factors such as uniformity, safety, and the desired treatment outcome.5.2. Treatment time
The efficiency of cold plasma on egg white proteins is significantly influenced by the duration of exposure. The period of treatment plays a pivotal role in shaping both the extent and nature of protein modifications. Prolonged exposure, as indicated by Li et al., (2022),68 tends to have a more profound effect on the structure and functionality of egg white proteins. Researchers meticulously consider treatment duration, recognizing its crucial role in achieving specific modifications such as denaturation or cross-linking.24 It is imperative to identify a potential saturation point where prolonged treatment may yield diminishing returns emphasized the importance of determining optimal treatment times aligned with the specific needs of the application, ensuring efficient attainment of desired protein modifications. Moreover, achieving a balance between treatment duration and energy efficiency is essential, particularly in practical applications where longer treatment times may result in increased energy consumption. To summarize, treatment time has emerged as a strategic parameter for tailoring the effects of cold plasma, facilitating a targeted approach based on the intended application and desired outcomes.695.3. Plasma power
The significance of plasma power is a crucial element in the realm of CPTs. The amount of energy applied to produce cold plasma plays an important role in determining the effectiveness of the treatment. Higher levels of power contribute to greater generation of reactive species, potentially resulting in more significant modifications to proteins.27 The energy transferred to the sample is intricately connected to the plasma power, influencing the localized temperature at the treatment site. The ability to precisely manage energy transfer can be achieved by adjusting the plasma power, providing the opportunity for customized treatment conditions.33The significance of plasma power is a crucial element in the realm of Cold Plasma Technologies (CPTs). The amount of energy applied to produce cold plasma plays an important role in determining the effectiveness of the treatment.27 Higher levels of power contribute to greater generation of reactive species, potentially resulting in more significant modifications to proteins. For example, when treating egg white proteins (EWP) with CPT, specific conditions such as applying 50 W of plasma power for 10 minutes have been shown to enhance the emulsifying properties of the proteins. The energy transferred to the sample is intricately connected to the plasma power, influencing the localized temperature at the treatment site. The ability to precisely manage energy transfer can be achieved by adjusting the plasma power, providing the opportunity for customized treatment conditions.33
5.4. Gas composition
The composition of the gas utilized in CPT plays a pivotal role in determining its impact on egg white proteins. This factor is of utmost importance because it significantly influences the types of reactive species generated, thereby shaping subsequent chemical interactions with egg white proteins. The selection of a specific gas or gas mixture is key for inducing distinct reactions, such as oxidation or cross-linking, ultimately influencing the nature and extent of protein modifications.70,71 Critical consideration of gas composition is essential because it directly governs the dynamic interplay between reactive species and egg white proteins. Moreover, adjusting the gas flow rate is a vital parameter, providing a means for precise control over the concentration of reactive species reaching the egg white sample during the treatment process. In the selection of gas compositions, safety and environmental considerations are imperative to ensure the sustainability and secure application of the treatment method.53,726. Effects of cold plasma treatment on structural analysis
6.1. Structural analysis
Cold plasma treatment (CPT) has proven to be a versatile methodology with substantial impacts on material characteristics.45 The structural attributes of the egg white protein (EWP) include its primary, secondary, tertiary, and quaternary structures.73 These structural dimensions are intricately linked to the rheological and functional properties of EWP. Structural analysis techniques such as nuclear magnetic resonance (NMR), circular dichroism (CD), sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and Fourier transform infrared spectroscopy (FTIR) are essential for comprehending these effects. In the realm of biomedical applications, this treatment contributes to the enhancement of biocompatibility.74A study examine the impact of CPT on EWP, observing significant changes in protein band patterns due to denaturation and cross-linking effects. A research examined the impact of cold plasma on EWP, noting significant changes in protein band patterns due to denaturation and cross-linking effects. This study highlighted how CPT treatment altered the molecular structure of EWP, demonstrating the potential complexities in analysing these proteins post-treatment.78
Furthermore, CPT may prompt protein fragmentation, leading to the emergence of additional bands on the gel or changes in the intensity of existing bands. This fragmentation phenomenon underscores the importance of carefully considering the impact of CPT on protein integrity and structure. CPT can affect various aspects of sample preparation, including buffer conditions, pH, and the presence of contaminants. These alterations can influence protein solubilization,78 SDS binding, and electrophoretic mobility, potentially introducing bias into SDS-PAGE analysis results. Despite these potential challenges, CPT presents opportunities for enhanced resolution in SDS-PAGE analysis. By reducing sample heterogeneity or eliminating interfering contaminants, CPT may improve the clarity of band patterns and facilitate better discrimination between proteins of similar molecular weights. Thus, the effects of CPT on SDS-PAGE analysis are diverse and context dependent.
Mehr et al. (2019)80 observed increased surface charge and protein solubility in grass pea protein isolates with voltage and extended treatment time. Additionally, plasma treatment introduces reactive species onto the surface, leading to biomolecular oxidative modifications81 and affecting CD spectra by altering chromophore chemical environments.
Chaple et al. (2020) and Chen et al. (2020)42 observed the adsorption of amide groups, amino groups, glycoside bonds, and C–C bonds in the ring structure of native and DBD-CAP-treated EWP molecules in FTIR-ATR spectra. This adsorption was separated into three bands, namely, amides I, II, and III, with distinctive peaks such as amide A and amide B revealing N–H or O–H stretching and C–H deformation vibrations due to plasma-induced depolymerization of EWP. Additionally, plasma treatment is effective for surface cleaning and eliminating contaminants and organic residues that may obscure FTIR signals. This dual impact on chemical modification and surface cleaning makes CPT a valuable technique for manipulating material surfaces and enhancing FTIR-based chemical analysis.
| Sources | Cold-plasma conditions | Findings | References |
|---|---|---|---|
| Zein | DBD plasma system: power supply, 1 A electrode spacing, 8 mm; time, 70 s; voltage, 60,70, 80, 90, and 100 V | CPT broke hydrogen bonds in the protein secondary structure causing highly exposed sites of action for water | 84 |
| Flaxseed protein | Jet plasma: voltage and frequency (5 kV; 40 kHz) for 0, 5, 10, 15, 30, 60, 90, 120, and 240 s | Conformation changes are observed in the increased surface hydrophobicity and contents of disulfide bonds with increasing plasma exposure time | 85 |
| Soy protein isolate | DBD system: voltage of 9, 10, and 11 kVpp at a frequency of 3.0 kHz. Treatment times of 1–10 min. Atmospheric air was used | Cross-linkage of free amino acids to the protein and protein-to-protein aggregation led to structural changes in the protein | 18 |
| Soybean glycinin | DBD system: voltage (0 to 50 kV), frequency: 10–20 kHz, time: 2–5 min. Atmospheric air was used as carrier gas | Oxidation of peptide bond amino groups, along with by oxidation of Trp, Tyr, and Phe amino acid residues leading to conformational alteration | 77 |
| Mushroom | DBD system: voltage input: 220 V, output voltage: 0–50 kV, time of 0–10 min, atmospheric air was used as an inducer gas | ACP treatment-induced alterations in secondary structure and disruption of the tertiary structure of the protein. Enhancement of surface hydrophobicity with increasing exposure time | 80 |
| Bovine serum albumin (BSA) | DBD plasma system: voltage used: 20 and 40 kV. Time: 2, 4, 6, and 8 min. Frequency: 13 kHz. Atmospheric air was used as feeding gas | Conformation changes are indicated by the changes in the protein sulfhydryl group and carbonyl contents. The increase in surface hydrophobicity depended on the voltage and time used for exposure | 86 |
| Gelatin | DBD-plasma system connected to an AC voltage source with a frequency of 375 Hz. The gases used include O2, N2, air, Ar, and ethanol–argon | The surface hydrophilicity of the films significantly increased after the cold plasma, while the contact angle significantly reduced | 87 |
| Myofibrillar proteins were extracted from Longissimus dorsi muscle | DBD plasma system: the duty cycle of 10% and a frequency of 7 kHz were applied for 0–20 min using ambient air as the working gas at atmospheric pressure conditions | The changes in the amide I band indicate changes in the secondary structure of proteins. Extensive inter and Intra protein crosslinking and aggregation | |
| Skim milk | Cold plasma jet using 0.9 mol N2 and 0.1 mol O2 per mol gas, two different feed gases were used. The jet generated cold plasma at 20 kV and had an arc drop voltage of 2 kV with frequencies ranging from 15 to 25 Hz | Aggregation and denaturation of proteins caused by the reactive species produced by plasma causing a significant decrease in the protein surface hydrophobicity. Increased α-helix and aggregated β-sheet and a decreased random coil, β-plated sheet, and β-turn with increased time | 75 |
| Soybean protein isolate | DBD system: frequencies used: 80, 100, and 120 Hz, time used 1–10 min | Cold plasma-induced reactive oxygen species-mediated oxidation of soy proteins, causing modifications in soybean protein isolate's secondary and ternary structures | 81 |
Nuclear magnetic resonance (NMR) spectroscopy is another technique that can be used to study the effects of CPT on egg white proteins (EWP). A study investigated the impact of cold plasma on the secondary and tertiary structures of EWP using NMR, revealing significant alterations in protein folding and conformation.84 This highlights how CPT can influence protein structures at a molecular level, which could affect their functional properties.
6.2. Effects of cold plasma treatment on functional parameters
Cold plasma treatment (CPT) is increasingly recognized for its ability to enhance the surface characteristics of diverse materials, rendering them suitable for a broad spectrum of functional applications.CPT offers a nuanced approach to influencing foam stability, depending on factors such as treatment conditions, foam characteristics, and the materials involved.75 Another notable outcome of plasma treatment is the removal of contaminants, the effective purification of foam surfaces and the mitigation of destabilizing factors such as surface heterogeneities or interactions with foreign substances. Additionally, specific plasma treatments may induce cross-linking or polymerization reactions on foam surfaces,18 fortifying the surface layer and enhancing stability by increasing resistance to deformation, collapse, or dissolution. Furthermore, CPT can impart antimicrobial properties to foam surfaces, inhibiting microbial growth and biofilm formation, which is crucial for preventing potential foam destabilization over time. A comprehensive understanding and strategic utilization of these effects are essential for optimizing plasma treatment processes to tailor foam properties for specific applications that demand heightened stability.
| Techno functional properties | Protein sources | Cold plasma system parameters | Inducer gas | Findings | References |
|---|---|---|---|---|---|
| Solubility | Soybean protein isolate | DBD system conditions: input: 220 V, 50 Hz, output: 40 to 60 kV at 50 to 150 Hz. Treatment given to samples: frequencies used: 80, 100, and 120 Hz, time: 1, 2, 5, and 10 min, respectively for each frequency | Atmospheric air | CPT at 80 and 120 Hz was found to increase the solubility of soybean protein isolate | 81 |
| Zein | DBD plasma system: plasma generated with 75 V and 1 A for 1, 3, 5, 7, and 10 min | Atmospheric air | CPT improved the solubility of zein protein at pH 7 and 1.2 compared to the control | 78 | |
| Natural actomyosin from threadfin bream | Jet plasma system: radio-frequency generator with a power of 30 W at a constant current of 0.3 A | Argon | Solubility decreases with increasing CPT time | 96 | |
| Myofibrillar proteins from king prawn | Jet plasma system using a 220 V 136AC single-phase power source with an output voltage of 7 kV DC and a current of 10 A, treatment time: 0–10 min | 98% argon and 2% oxygen | Solubility was found to decrease after plasma treatment | 68 | |
| Emulsifying properties | Soybean protein isolate | DBD system conditions: input: 220 V, 50 Hz, output: 40 to 60 kV at 50 to 150 Hz. Treatment given to samples: frequencies used: 80, 100, and 120 Hz, time: 1, 2, 5, and 10 min, respectively for each frequency | Atmospheric air | Cold plasma frequency of 80 Hz, was found to have high modifying impact on emulsifying properties of soy proteins | 81 |
| Flaxseed protein | Jet plasma: voltage: 5 kV; frequency: 40 kHz, time: 0, 5, 10, 15, 30, 60, 90, 120, and 240 s | Atmospheric air | The treatment was found to increase the emulsifying capacity and stability of flaxseed protein following 5 to 10 s treatment. Then, began to decrease with increasing time | 85 | |
| Actomyosin from king prawn | Jet plasma system: voltage: 220 V | 98% argon and 2% oxygen | The treatment was found to enhance the foaming properties of extracted protein as compared to control | 16 | |
| Egg yolk | Ozone treatment using an ozone generator at an ozone generation rate of 1 g h−1 for different treatment times (0, 10, 20, 30, and 40 min) | Atmospheric air | The emulsion stability and emulsification activity of the egg yolk reached their maximum at 10 min, which was 1.42 and 1.75 times higher than the control | 97 | |
| Foaming properties | Flaxseed protein | Jet plasma: voltage: 5 kV; frequency: 40 kHz, time: 0, 5, 10, 15, 30, 60, 90, 120, and 240 s | Atmospheric air | Foaming capacity was greatly increased when 5 s treatment time was used compared to the control. However, foaming capacity was reduced with augmenting treatment time. Overall, the foaming capacity increased after the treatment as compared to control | 85 |
| Myofibrillar proteins from king prawn | Jet plasma system: voltage: 220 V, time: 0–10 min | 98% argon and 2% oxygen | The foaming properties of extracted myofibrillar protein were increased with increasing treatment time | 68 | |
| Rheology | Little millet flour | DBD plasma system with 230 V, 50 Hz, and a high output voltage of 60 kV for 0–30 min | Atmospheric air | The viscosity of cooked paste was observed to be increased with plasma treatment | 98 |
| Milk | DBD plasma system: voltage at 15 kHz varying voltage of 0, 40, 50, 60, 70, and 80 V, with a duration of 120 s | Atmospheric air | Higher voltage was found to increased viscosity of milk samples whereas low-voltage (60 V) treatment. Was found to decrease the viscosity as compared to control | 99 | |
| Water-holding capacity (WHC) | Parboiled rice flour | Radio frequency (RF) power supply having frequency 13.56 MHz. Different power levels (30 W, 40 W, and 50 W) and durations (5, 10, and 15 min) were used | Atmospheric air | Slight increase in WHC of treated samples compared to untreated counterpart | 100 |
| Myofibrillar proteins were extracted from Longissimus dorsi muscle | DBD plasma system: maximum power, voltage, and current were set to 30 W, 12 kV, and 1 mA, respectively. The duty cycle of 10% and a frequency of 7 kHz applied for 0, 5, 10, 15, 20 min | Atmospheric air | Overall, a significant rise in the amount of bound water in myofibrillar proteins, with rise in the duration of plasma exposure | ||
| Gelation | Pea protein isolate | DBD plasma system: frequency of 3500 Hz, a duty cycle of 70%, a voltage output of 0–30 kV, and a total treatment time of 10 min | Atmospheric air | Pea protein subjected to the combined treatment formed a gel at 14% protein concentration. However, no gel was formed with the control at the same concentration | 101 |
| Myofibrillar protein of Asian sea bass | DBD system: discharge voltage-80 kV (RMS) for 5–15 min | Argon and oxygen (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v) 10 v/v) |
The initial increase in the breaking force and deformation of prepared gel when 5 min treatment time was used compared with the control | 102 |
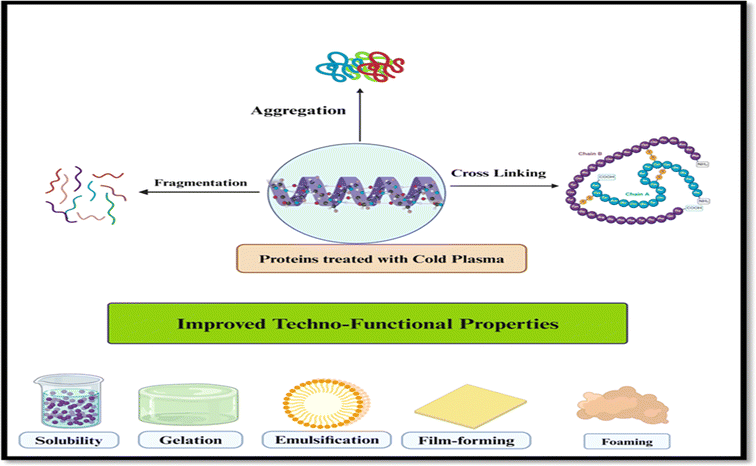 | ||
| Fig. 3 Schematic illustration of the improvement of plant proteins for improving their techno-functional properties. (Modified from ref. 103), created at https://www.biorender.com/. | ||
6.3. Effects of cold plasma treatment on rheological measurements
Rheological properties pertain to the flow characteristics and viscoelastic attributes of egg white proteins (EWPs).104–110 The manner in which proteins are structured and interact plays a pivotal role in determining the rheological behavior of EWP. Treatment with DBD-CAP may disrupt or modify protein–protein interactions, consequently influencing the gelation, viscoelasticity, and gel strength of EWP.111 CPT stands out as a versatile technique with substantial implications for rheological measurements, impacting material behavior during flow or deformation in diverse ways.112 One notable impact of CPT is its capacity to refine surfaces, leading to alterations in properties such as wettability, roughness, and chemical composition. These surface modifications have a profound impact on the interaction between the material and its surroundings, thereby affecting rheological behavior. Furthermore, CPT can induce changes in viscosity by modifying the molecular structure or surface properties of materials, resulting in various flow behaviors, such as shear thinning, shear thickening, or shifts in viscosity magnitude.74 Moreover, CPT has the potential to initiate crosslinking or polymerization reactions in polymers and other materials, influencing rheological properties through alterations in molecular weight distribution, network structure, or chain entanglement behavior.The thermal effects induced by CPT, including localized heating on the material surface, further contribute to changes in rheological behavior, affecting viscosity, elasticity, or relaxation dynamics due to temperature variations. Microstructure modification, as a consequence of CPT, influences rheological properties by altering crystallinity, orientation, and phase distribution within materials.113–117
7. Potential applications of cold plasma treated egg white proteins in the food industry
The CPT of egg white proteins has numerous potential applications in the food industry because of its ability to modify protein structure and improve functional properties.118 One prominent application is foaming and emulsification, where CPT improves the stability and efficiency of egg white proteins, making them ideal for use in various foods such as meringues, cakes, and foam-based desserts. Additionally, CPT can alter the gelation behaviour of egg white proteins, paving the way for novel textures in food items such as gels, custards, and puddings.21,119 Moreover, these treated proteins can be utilized to produce edible films and coatings with enhanced barrier properties, thereby increasing the shelf life of packaged foods and ensuring their quality and safety.120 Modified egg white proteins also serve as functional ingredients in a wide range of food formulations, including meat products, dairy alternatives, bakery goods, and beverages, enhancing their nutritional profile, texture, and sensory attributes.121–123 The antimicrobial properties of cold plasma-treated egg white proteins make them suitable for developing antimicrobial coatings on food surfaces, mitigating the growth of spoilage microorganisms and pathogens, and ultimately enhancing food safety and improving shelf life.124 Furthermore, these proteins can act as carriers for delivering bioactive compounds and nutraceuticals in food products, improving their absorption and bioavailability.125 CPT can reduce the allergenicity of egg white proteins, potentially broadening their application in food products and catering to individuals with egg allergies. Additionally, the treatment effectively inactivates enzymes present in egg white proteins, preventing undesirable changes in foods during processing and storage.126 Modified egg white proteins contribute to enhancing the taste, flavour, and overall sensory characteristics of food items, thereby improving consumer acceptance and marketability. Finally, these proteins can be utilized to fortify foods with essential nutrients, such as vitamins, amino acids, and minerals, thereby enhancing their nutritional value and promoting health and wellness.127,128 When considering potential applications of cold plasma-treated egg white proteins in the food industry, two promising areas stand out: reducing allergenic proteins and processing protein films. Cold plasma treatment has shown the potential to modify the allergenic properties of egg white proteins, making them safer for individuals with egg allergies. This is achieved by altering the protein structure to reduce its allergenic potential without compromising its functional qualities.24 Additionally, cold plasma treatment can be utilized in the production of protein films. These films, derived from modified egg white proteins, can be used as edible coatings or packaging materials that are both functional and environmentally friendly. They offer advantages such as improved barrier properties, enhanced shelf life of packaged goods, and reduced reliance on synthetic additives. Plasma-treated proteins offer notable advantages from a sustainability perspective, reflecting advancements in current research.30 Cold plasma treatment is an eco-friendly technology that operates at low temperatures and pressures, minimizing energy consumption compared to traditional thermal methods. This process effectively enhances the functional and nutritional properties of proteins, such as improving their solubility, emulsifying capacity, and antioxidant activity, without the need for harsh chemicals or excessive heat. Additionally, cold plasma treatment can extend the shelf life of protein-rich food products, thereby reducing food waste and contributing to a more sustainable food system. Current research is focusing on optimizing this technology to maximize its efficiency and scalability, exploring its applications in various food products, and ensuring its commercial viability.29 Studies have demonstrated that plasma treatment can effectively reduce microbial contamination and allergenic properties of proteins, further supporting its potential for sustainable food production. However, there is still a need for more research to address the economic feasibility of large-scale implementation and to establish standardized protocols for its use across different food sectors. These applications highlight the versatility and benefits of cold plasma-treated egg white proteins in creating safer, more sustainable food products. Overall, the application of cold plasma-treated egg white proteins holds great promise for revolutionizing the food sector by enhancing food safety, quality, functionality, and nutritional value, aligning with the evolving needs and preferences of consumers. Potential applications of cold plasma treatment is discussed in Table 4.| Area | Applications | References |
|---|---|---|
| Food safety | Cold plasma-treatment deactivates microorganisms, their spores, enzymes and toxins thereby increasing the safety | 46, 80 and 129–131 |
| Food quality | Cold plasma-treatment can maintain nutritional properties without any effect on the physical and structural integrity and sensory properties of products | 111, 132 and 133 |
| Food shelf life | Enhanced shelf life due to reduced protein and lipid oxidation and microbial inhibition | 134–136 |
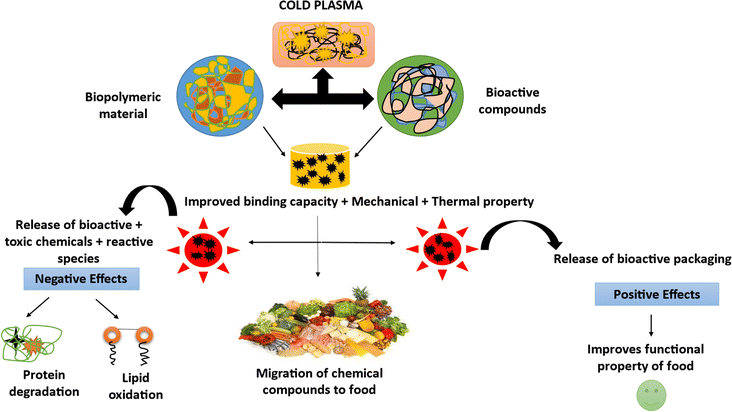
8. Challenges and future directions
Subsequent investigations may delve into refining plasma conditions for optimal efficacy, emphasizing enhanced decontamination efficiency while mitigating potential adverse effects such as plasma-induced lipid oxidation,137 undesirable flavour alterations,138 and changes in color.139 Previous research has demonstrated that employing different plasma technologies (e.g., DBD, glow, or arc) on various poultry products (e.g., chicken meat, eggshell, or chicken skin) results in distinct decontamination outcomes. Therefore, to improve our understanding of the applicability of currently available plasma technologies in the treatment of poultry products, future research may examine the selection of suitable equipment for processing each product. Furthermore, it is advisable to carry out additional genotoxicity and chemical tests on poultry products treated with plasma. Comprehensive physiological and oncological research, together with the required safety procedures, is needed to ensure the safety of plasma equipment operators and investigate potential detrimental impacts, such as exposure to reactive species and free radicals. The future development and commercial application of nonthermal plasma in the poultry sector depend on a number of factors, including industrial equipment, cost-effectiveness, and resolving drawbacks associated with traditional methods. It is imperative that upscaling studies align with the higher-capacity poultry production lines of various factories. The integration of plasma-based equipment into conventional production lines necessitates effective and cost-efficient design, along with exploring benefits and minimizing associated challenges. While recent efforts have focused on continuous plasma treatment for fresh produce,50 comprehensive endeavours related to poultry products are essential. Future studies may consider the incorporation of nonthermal plasma with other preservation techniques. For instance, integrating poultry product decontamination with suitable packaging or essential oils could extend the shelf life and mitigate potential negative impacts on product quality. The antioxidant and antimicrobial properties of essential oils, which act as natural additives, may synergize with nonthermal processes such as plasma, reducing the required processing intensity.140,141 Finally, exploring the applications of plasma-activated water presents an intriguing avenue for future research. Although plasma-activated water has shown efficacy against spoilage bacteria in poultry products,142 its impact on poultry product quality and safety warrants further investigation.143–1579. Conclusion
The nonthermal plasma method has garnered attention from food scientists owing to its potential to increase poultry product safety. Existing academic research has demonstrated its efficacy in decontaminating chicken eggs and meat, paving the way for advanced investigations of cold plasma applications in the poultry industry. The literature highlights cold plasma as a tool capable of bolstering the microbial safety of poultry products, emphasizing the importance of process optimization, such as determining appropriate treatment times. However, it is crucial to acknowledge that uncontrolled plasma treatment may have an adverse impact on the quality parameters of the product, necessitating careful consideration in commercial process design. Additionally, a significant obstacle to the widespread commercial application of this emerging technique is the absence of high-capacity plasma equipment tailored for use with poultry products. To overcome this challenge, food and machinery experts must work together to conduct thorough studies and produce continuous, scaled-up plasma-based equipment at a reasonable cost. It is anticipated that future studies will address these essential aspects for the effective commercialization of no-thermal plasma in the poultry sector, given the state of research and literature available.Abbreviations
| EWP | Egg white protein |
| AGE | Advanced glycation end products |
| HPP | High pressure processing |
| PEF | Pulsed electric field |
| PL | Pulsed light |
| IR | Infrared |
| ACP | Atmospheric cold plasma |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| UV | Ultraviolet |
| CPT | Cold plasma treatment |
| DBD | Dielectric barrier discharge |
| PJ | Plasma jet |
| RFP | Radio frequency plasma |
| MW | Microwave |
| CD | Corona discharge |
| ICP | Inductively coupled plasma |
| CCP | Capacitively coupled plasma |
| APPJ | Atmospheric pressure plasma jets |
| DC | Direct current |
Data availability
As this is a review, no data were used for this study.Conflicts of interest
There is no conflict of interest between the authors.Acknowledgements
No funding was received from any source for conducting this study.References
- Y. Cheng, J. Wang, J. Chi, J. Z. Ma, X. Geng and Y. Chi, Effect of dry heating on egg white powder influencing water mobility and intermolecular interactions of its gels, J. Sci. Food Agric., 2021, 101(2), 433–440 CrossRef CAS PubMed.
- M. Yüceer and C. Caner, Effects of protease-hydrolyzed egg white on the meringue batter properties and meringue textural and sensory properties during storage, Int. J. Gastron. Food Sci., 2021, 25, 100409 CrossRef.
- L. Wu, W. Zhao, R. Yang and X. Chen, Effects of pulsed electric fields processing on stability of egg white proteins, J. Food Eng., 2014, 139, 13–18, DOI:10.1016/j.jfoodeng.2014.04.008.
- M. M. Nasiru, E. B. Frimpong, U. Muhammad, J. Qian, A. T. Mustapha, W. Yan and J. Zhang, et al., Dielectric barrier discharge cold atmospheric plasma: influence of processing parameters on microbial inactivation in meat and meat products, Compr. Rev. Food Sci. Food Saf., 2021, 20(3), 2626–2659 CrossRef CAS PubMed.
- F. G. C. Ekezie, D. W. Sun and J. H. Cheng, A review on recent advances in cold plasma technology for the food industry: current applications and future trends, Trends Food Sci., 2017, 69, 46–58 CrossRef.
- A. Kramer, S. Bekeschus, R. Matthes, C. Bender, M. B. Stope, M. Napp and F. Schauer, et al., Cold physical plasmas in the field of hygiene—relevance, significance, and future applications, Plasma Process. Polym., 2015, 12(12), 1410–1422 CrossRef CAS.
- O. F. Nwabor, H. Onyeaka, T. Miri, K. Obileke, C. Anumudu and A. Hart, A cold plasma technology for ensuring the microbiological safety and quality of foods, Food Eng. Rev., 2022, 14(4), 535–554 CrossRef CAS.
- E. Ozen and R. K. Singh, Atmospheric cold plasma treatment of fruit juices: a review, Trends Food Sci. Technol., 2020, 103, 144–151 CrossRef CAS.
- Y. Han, J. H. Cheng and D. W. Sun, Activities and conformation changes of food enzymes induced by cold plasma: a review, Crit. Rev. Food Sci. Nutr., 2019, 59(5), 794–811 CrossRef CAS PubMed.
- E. Takai, T. Kitamura, J. Kuwabara, S. Ikawa, S. Yoshizawa, K. Shiraki and K. Kitano, et al., Chemical modification of amino acids by atmospheric-pressure cold plasma in aqueous solution, J. Phys. D: Appl. Phys., 2014, 47(28), 285403 CrossRef.
- C. Sarangapani, A. Patange, P. Bourke, K. Keener and P. J. Cullen, Recent advances in the application of cold plasma technology in foods, Annu. Rev. Food Sci. Technol., 2018, 9, 609–629 CrossRef CAS PubMed.
- H. Chutia, D. Kalita, C. L. Mahanta, N. Ojah and A. J. Choudhury, Kinetics of inactivation of peroxidase and polyphenol oxidase in tender coconut water by dielectric barrier discharge plasma, LWT--Food Sci. Technol., 2019, 101, 625–629 CrossRef CAS.
- H. Tolouie, M. A. Mohammadifar, H. Ghomi and M. Hashemi, Cold atmospheric plasma manipulation of proteins in food systems, Crit. Rev. Food Sci. Nutr., 2018, 58(15), 2583–2597 CrossRef CAS PubMed.
- A. Segat, N. N. Misra, P. J. Cullen and N. Innocente, Effect of atmospheric pressure cold plasma (ACP) on activity and structure of alkaline phosphatase, Food Bioprod. Process., 2016, 98, 181–188 CrossRef CAS.
- M. Zheng, Y. Yang, X. Q. Liu, M. Y. Liu, X. F. Zhang, X. Wang and J. G. Tan, et al., Enhanced biological behavior of in vitro human gingival fibroblasts on cold plasma-treated zirconia, PLoS One, 2015, 10(10), e0140278 CrossRef PubMed.
- F. G. C. Ekezie, D. W. Sun and J. H. Cheng, Altering the IgE binding capacity of king prawn (Litopenaeus vannamei) tropomyosin through conformational changes induced by cold argon-plasma jet, Food Chem., 2019, 300, 125143 CrossRef CAS PubMed.
- B. Surowsky, A. Fischer, O. Schlueter and D. Knorr, Cold plasma effects on enzyme activity in a model food system, Innovative Food Sci. Emerging Technol., 2013, 19, 146–152 CrossRef CAS.
- P. Meinlschmidt, E. Ueberham, J. Lehmann, K. Reineke, O. Schlüter, U. Schweiggert-Weisz and P. Eisner, The effects of pulsed ultraviolet light, cold atmospheric pressure plasma, and gamma-irradiation on the immunoreactivity of soy protein isolate, Innovative Food Sci. Emerging Technol., 2016, 38, 374–383 CrossRef CAS.
- E. G. Alves Filho, L. M. A. Silva, F. Oiram Filho, S. Rodrigues, F. A. Fernandes, M. I. Gallão and E. S. de Brito, et al., Cold plasma processing effect on cashew nuts composition and allergenicity, Int. Food Res., 2019, 125, 108621 CrossRef CAS PubMed.
- S. Rehault-Godbert, N. Guyot and Y. Nys, The golden egg: nutritional value, bioactivities, and emerging benefits for human health, Nutr., 2019, 11(3), 684 CAS.
- S. M. Razi, H. Fahim, S. Amirabadi and A. Rashidinejad, An overview of the functional properties of egg white proteins and their application in the food industry, Food Hydrocolloids, 2023, 135, 108183 CrossRef CAS.
- E. D. N. S. Abeyrathne, X. Huang and D. U. Ahn, Advances in the Separation of Functional Egg Proteins–Egg White Proteins, 2019 Search PubMed.
- K. Iwashita, A. Handa and K. Shiraki, Co-aggregation of ovalbumin and lysozyme, Food Hydrocolloids, 2017, 67, 206–215 CrossRef CAS.
- M. M. Nasiru, E. F. Boateng, F. Alnadari, M. Umair, Z. Wang, A. M. Senan and J. Zhang, et al.). Dielectric barrier discharge cold atmospheric plasma treatment of egg white protein: insights into the functional, rheological, and structural properties, Food Bioprocess Technol., 2024, 17(4), 955–976 CrossRef CAS.
- L. Sheng, Y. Wang, J. Chen, J. Zou, Q. Wang and M. Ma, Influence of high-intensity ultrasound on foaming and structural properties of egg white, Int. Food Res., 2018, 108, 604–610 CrossRef CAS PubMed.
- J. Wu and A. Acero-Lopez, Ovotransferrin: structure, bioactivities, and preparation, Int. Food Res., 2012, 46(2), 480–487 CrossRef CAS.
- N. S. Kumar, A. H. Dar, K. K. Dash, B. Kaur, V. K. Pandey, A. Singh and B. Kovács, et al. Recent advances in Cold Plasma Technology for modifications of proteins: a comprehensive review, J. Agric. Food Res., 2024, 101177 Search PubMed.
- E. C. N. Rathnapala, D. U. Ahn and S. Abeyrathne, Functional properties of ovotransferrin from chicken egg white and its derived peptides: a review, Food Sci. Biotechnol., 2021, 30(5), 619–630 CrossRef CAS PubMed.
- C. Liu, P. P. Tang, X. B. Liu, J. X. Liu, X. H. Pan, R. M. Aadil and Z. W. Liu, et al. Cold plasma for enhancing covalent conjugation of ovalbumin-gallic acid and its functional properties, Food Chem., 2024, 454, 139753 CrossRef CAS PubMed.
- S. Kaur, Y. Kumar, V. Singh, J. Kaur and P. S. Panesar, Cold Plasma Technology: Reshaping Food Preservation and Safety, Food Control, 2024, 110537 CrossRef CAS.
- N. Yuno-Ohta, T. Kato, S. Ashizawa, Y. Kimura, N. Maruyama and T. Nishizu, Role of ovomucoid in the gelation of a β-lactoglobulin-ovomucoid mixture, Colloid Polym. Sci., 2016, 294, 1065–1073 CrossRef CAS.
- Y. Shan, D. Tang, R. Wang, A. Tu, Y. Yi, X. Wang and X. Lü, et al., Rheological and structural properties of ovomucin from chicken eggs with different interior quality, Food Hydrocolloids, 2020, 100, 105393 CrossRef CAS.
- Q. Zhao, L. Ding, M. Xia, X. Huang, K. Isobe, A. Handa and Z. Cai, Role of lysozyme on liquid egg white foaming properties: interface behavior, physicochemical characteristics and protein structure, Food Hydrocolloids, 2021, 120, 106876 CrossRef CAS.
- T. Xu, X. Li, C. Wu, G. Fan, T. Li, D. Zhou and X. Hua, et al.). Improved encapsulation effect and structural properties of whey protein isolate by dielectric barrier discharge cold plasma, Int. J. Biol. Macromol., 2024, 257, 128556 CrossRef CAS PubMed.
- J. Legros, S. Jan, S. Bonnassie, M. Gautier, T. Croguennec, S. Pezennec and F. Baron, et al., The role of ovotransferrin in egg-white antimicrobial activity: a review, Foods, 2021, 10(4), 823 CrossRef CAS PubMed.
- A. Shahmohammadi, Lysozyme separation from chicken egg white: a review, Eur. Food Res. Technol., 2018, 244(4), 577–593 CrossRef CAS.
- K. Iwashita, A. Handa and K. Shiraki, Co-aggregation of ovalbumin and lysozyme, Food Hydrocolloids, 2017, 67, 206–215 CrossRef CAS.
- Y. Shimazaki and A. Takahashi, Antibacterial activity of lysozyme-binding proteins from chicken egg white, J. Microbiol. Methods, 2018, 154, 19–24 CrossRef CAS PubMed.
- P. Sarkar and R. K. Duary, Value addition of Samia ricini (Eri) pupae derived proteins: production and characterisation of antioxidant, ACE and DPP-IV inhibitory hydrolysates, J. Insects Food Feed, 2023, 9(7), 935–945 Search PubMed.
- P. Nancy, J. Joy, J. James, B. Joseph, S. Thomas and N. Kalarikkal, Spectroscopic and mass spectrometry analyses of plasma-activated polymeric materials, in Non-Thermal Plasma Technology for Polymeric Materials, Elsevier, 2019, pp. 319–340 Search PubMed.
- H. M. Mehr and A. Koocheki, Effect of atmospheric cold plasma on structure, interfacial and emulsifying properties of Grass pea (Lathyrus sativus L.) protein isolate, Food Hydrocolloids, 2020, 106, 105899 CrossRef.
- Y. Q. Chen, J. H. Cheng and D. W. Sun, Chemical, physical and physiological quality attributes of fruit and vegetables induced by cold plasma treatment: Mechanisms and application advances, Crit. Rev. Food Sci. Nutr., 2020, 60(16), 2676–2690 CrossRef CAS PubMed.
- V. Nehra, A. Kumar and H. K. Dwivedi, Atmospheric non-thermal plasma sources, Int. J. Eng., 2008, 2(1), 53–68 Search PubMed.
- Z. Qu, G. Chen, T. Yang, S. Li and Y. Chen, Mechanisms underlying the formation of non-thermal deamidated zein via synergistic treatment of cold plasma with acid: improved foam performance, Food Hydrocolloids, 2024, 146, 109195 CrossRef CAS.
- B. Aaliya, K. V. Sunooj, M. Navaf, P. P. Akhila, C. Sudheesh, S. A. Mir and J. George, Recent trends in bacterial decontamination of food products by hurdle technology: a synergistic approach using thermal and non-thermal processing techniques, Int. Food Res. J., 2021, 147, 110514 CrossRef CAS PubMed.
- R. Mandal, A. Singh and A. P Singh, Recent developments in cold plasma decontamination technology in the food industry, Trends Food Sci. Technol., 2018, 80, 93–103 CrossRef CAS.
- Y. Ucar, Z. Ceylan, M. Durmus, O. Tomar and T. Cetinkaya, Application of cold plasma technology in the food industry and its combination with other emerging technologies, Trends Food Sci. Technol., 2021, 114, 355–371 CrossRef CAS.
- B. G. Dasan, T. Yildirim and I. H. Boyaci, Surface decontamination of eggshells by using non-thermal atmospheric plasma, Int. J. Food Microbiol., 2018, 266, 267–273 CrossRef CAS PubMed.
- Y. Q. Chen, J. H. Cheng and D. W. Sun, Chemical, physical and physiological quality attributes of fruit and vegetables induced by cold plasma treatment: mechanisms and application advances, Crit. Rev. Food Sci. Nutr., 2020, 60(16), 2676–2690 CrossRef CAS PubMed.
- C. Hertwig, K. Reineke, J. Ehlbeck, D. Knorr and O. Schlüter, Decontamination of whole black pepper using different cold atmospheric pressure plasma applications, Food Control, 2015, 55, 221–229 CrossRef CAS.
- H. Venkataratnam, O. Cahill, C. Sarangapani, P. J. Cullen and C. Barry-Ryan, Impact of cold plasma processing on major peanut allergens, Sci. Rep., 2020, 10(1), 17038 CrossRef CAS PubMed.
- R. Thirumdas, C. Sarangapani and U. S. Annapure, Cold plasma: a novel non-thermal technology for food processing, Food Biophys., 2015, 10, 1–11 CrossRef.
- M. F. Arif, Effects of Atmospheric Cold Plasma Treatment on Canola Protein Structural and Functional Properties, 2024 Search PubMed.
- D. A. Laroque, S. T. Seó, G. A. Valencia, J. B. Laurindo and B. A. M. Carciofi, Cold plasma in food processing: design, mechanisms, and application, Int. J. Food Eng., 2022, 312, 110748 CrossRef CAS.
- N. N. Misra and C. Jo, Applications of cold plasma technology for microbiological safety in meat industry, Trends Food Sci. Technol., 2017, 64, 74–86 CrossRef CAS.
- E. Feizollahi, B. Iqdiam, T. Vasanthan, M. S. Thilakarathna and M. S. Roopesh, Effects of atmospheric-pressure cold plasma treatment on deoxynivalenol degradation, quality parameters, and germination of barley grains, Appl. Sci., 2020, 10(10), 3530 CrossRef CAS.
- D. Ziuzina, N. N. Misra, P. J. Cullen, K. M. Keener, J. P. Mosnier, I. Vilaró and P. Bourke, et al., Demonstrating the potential of industrial scale in-package atmospheric cold plasma for decontamination of cherry tomatoes, Plasma Med., 2016, 6(3–4), 397–412 CrossRef.
- L. Scally, S. Ojha, J. Durek, P. J. Cullen, O. K. Schlüter and M. Oliveira, Principles of non-thermal plasma processing and its equipment, in Non-thermal Food Processing Operations, Woodhead Publishing, 2023, pp. 95–135 Search PubMed.
- C. Sarangapani, L. Scally, M. Gulan and P. J. Cullen, Dissipation of pesticide residues on grapes and strawberries using plasma-activated water, Food Bioprocess Technol., 2020, 13, 1728–1741 CrossRef CAS.
- N. M. Coutinho, M. R. Silveira, R. S. Rocha, J. Moraes, M. V. S. Ferreira, T. C. Pimentel and A. G. Cruz, Cold plasma processing of milk and dairy products, Trends Food Sci. Technol., 2018, 74, 56–68 CrossRef CAS.
- T. M. C. Nishime, Development and Characterization of Extended and Flexible Plasma Jets, 2019 Search PubMed.
- R. Thirumdas, C. Sarangapani and U. S. Annapure, Atmospheric pressure cold plasma for egg white proteins, LWT--Food Sci. Technol., 2018, 89, 48–54, DOI:10.1016/j.lwt.2017.10.048.
- H. Jiang, Y. Hu, Z. Chen and X. Ye, Dielectric barrier discharge (DBD) plasma treatment induces structural changes and improves foaming properties of egg white protein, Food Hydrocolloids, 2020, 101, 105548, DOI:10.1016/j.foodhyd.2019.105548.
- Y. Ji, J. Lee, D. Kim and Y. Choi, Structural and functional changes in egg white proteins induced by low-pressure plasma treatment, Food Chem., 2019, 276, 157–162, DOI:10.1016/j.foodchem.2018.09.175.
- D. Bermudez-Aguirre, Advances in the inactivation of microorganisms and viruses in food and model systems using cold plasma, in Advances in Cold Plasma Applications for Food Safety and Preservation, Academic Press, 2020, pp. 49–91 Search PubMed.
- P. Chabert, T. V. Tsankov and U. Czarnetzki, Foundations of capacitive and inductive radio-frequency discharges, Plasma Sources Sci. Technol., 2021, 30(2), 024001 CrossRef CAS.
- B. Li, L. Peng, Y. Cao, S. Liu, Y. Zhu, J. Dou and C. Zhou, et al. Insights into Cold Plasma Treatment on the Cereal and Legume Proteins Modification: Principle, Mechanism, and Application, Foods, 2024, 13(10), 1522 CrossRef CAS PubMed.
- N. Li, J.-J. Yu, N. Jin, Y. Chen, S.-H. Li and Y. Chen, Modification of the physicochemical and structural characteristics of zein suspension by dielectric barrier discharge cold plasma treatment, J. Food Sci., 2020, 85(8), 2452–2460 CrossRef CAS PubMed.
- A. A. Zamri, M. Y. Ong, S. Nomanbhay and P. L. Show, Microwave plasma technology for sustainable energy production and the electromagnetic interaction within the plasma system: a review, Environ. Res., 2021, 197, 111204 CrossRef CAS PubMed.
- S. Tiwari, A. Caiola, X. Bai, A. Lalsare and J. Hu, Microwave plasma-enhanced and microwave heated chemical reactions, Plasma Chem. Plasma Process., 2020, 40(1), 1–23 CrossRef CAS.
- M. J. Johnson, D. R. Boris, T. B. Petrova and S. G. Walton, Characterization of a compact, low-cost atmospheric-pressure plasma jet driven by a piezoelectric transformer, IEEE Trans. Plasma Sci., 2018, 47(1), 434–444 Search PubMed.
- R. Mongkolnavin, S. Damrongsakkul, O. H. Chin, D. Subedi and C. S. Wong, Cost-Effective Plasma Experiments for Developing Countries, Plasma Science and Technology for Emerging Economies: An AAAPT Experience, 2017, pp. 475–525 Search PubMed.
- G. Daeschlein, S. Scholz, T. von Woedtke, M. Niggemeier, E. Kindel, R. Brandenburg and M. Jünger, In vitro killing of clinical fungal strains by low-temperature atmospheric-pressure plasma jet, IEEE Trans. Plasma Sci., 2010, 39(2), 815–821 Search PubMed.
- M. M. Nasiru, E. F. Boateng, F. Alnadari, M. Umair, Z. Wang, A. M. Senan, W. Yan, H. Zhuang and J. Zhang, Dielectric barrier discharge cold atmospheric plasma treatment of egg white protein: Insights into the functional, rheological, and structural properties, Food Bioprocess Technol., 2024, 17(4), 955–976 CrossRef CAS.
- C. Y. Koga-Ito, K. G. Kostov, F. S. Miranda, N. V. M. Milhan, N. F. Azevedo Neto, F. Nascimento and R. S. Pessoa, Cold atmospheric plasma as a therapeutic tool in medicine and dentistry, Plasma Chem. Plasma Process., 2023, 1–37 Search PubMed.
- D. P. Subedi, U. M. Joshi and C. S. Wong, Dielectric barrier discharge (DBD) plasmas and their applications, Plasma Science and Technology for Emerging Economies: An AAAPT Experience, 2017, pp. 693–737 Search PubMed.
- M. Dharini, S. Jaspin and R. Mahendran, Cold plasma reactive species: generation, properties, and interaction with food biomolecules, Food Chem., 2023, 405, 134746 CrossRef CAS PubMed.
- Z. Li, X. Huang, Q. Tang, M. Ma, Y. Jin and L. Sheng, Functional properties and extraction techniques of chicken egg white proteins, Foods, 2022, 11(16), 2434 CrossRef CAS PubMed.
- (a) M. M. Nasiru, E. F. Boateng, F. Alnadari, A. P. Bassey, W. Yan, K. Masisi, C. Li and J. Zhang, Effect of Cold Atmospheric Plasma Fusion 222 nm UV and PAHP on Cold Pasteurisation of Egg Surface, Food Bioprocess Technol., 2024, 1–16 Search PubMed; (b) M. M. Nasiru, E. F. Boateng, M. Umair, F. Alnadari, A. P. Bassey, J. Qian, W. Yan, C. Li and J. Zhang, Decontamination of egg-associated pathogens by plasma-activated water and hydrogen peroxide, J. Food Saf., 2024, 44(3), e13136 CrossRef CAS; (c) M. M. Nasiru, M. Umair, E. F. Boateng, F. Alnadari, K. U. R. Khan, Z. Wang, J. Luo, W. Yan, H. Zhuang, A. Majrashi and J. Zhang, Characterisation of flavour attributes in egg white protein using HS-GC-IMS combined with E-Nose and E-Tongue: Effect of high-voltage cold plasma treatment time, Molecules, 2022, 27(3), 601 CrossRef CAS PubMed.
- M. E. Mehr, H. Aghamohammadi, S. H. Abbandanak, G. R. Aghamirzadeh, R. Eslami-Farsani and S. M. H. Siadati, Effects of applying a combination of surface treatments on the mechanical behavior of basalt fiber metal laminates, Int. J. Adhes. Adhes., 2019, 92, 133–141 CrossRef CAS.
- B. Zhang, C. Tan, F. Zou, Y. Sun, N. Shang and W. Wu, Impacts of cold plasma technology on sensory, nutritional and safety quality of food: A review, Foods, 2022, 11(18), 2818 CrossRef CAS PubMed.
- B. P. Ismail, L. Senaratne-Lenagala, A. Stube and A. Brackenridge, Protein demand: review of plant and animal proteins used in alternative protein product development and production, Anim. Front., 2020, 10(4), 53–63 CrossRef PubMed.
- M. Henchion, M. Hayes, A. M. Mullen, M. Fenelon and B. Tiwari, Future protein supply and demand: strategies and factors influencing a sustainable equilibrium, Foods, 2017, 6(7), 53 CrossRef PubMed.
- A. Sharifian, N. Soltanizadeh and R. Abbaszadeh, Effects of dielectric barrier discharge plasma on the physicochemical and functional properties of myofibrillar proteins, Innovative Food Sci. Emerging Technol., 2019, 54, 1–8 CrossRef CAS.
- Z.-W. Liu, Y.-X. Zhou, F. Wang, Y.-C. Tan, J.-H. Cheng, A. E.-D. Bekhit, R. M. Aadil and X.-B. Liu, Oxidation induced by dielectric barrier discharge (DBD) plasma treatment reduces IgG/IgE binding capacity and improves the functionality of glycinin, Food Chem., 2021, 363, 130300 CrossRef CAS PubMed.
- L. Wang, J. Xue, H. Zhang and Q. Xu, Effects of cold plasma on the secondary and tertiary structures of egg white protein as revealed by NMR spectroscopy, J. Agric. Food Chem., 2019, 67(5), 1375–1384, DOI:10.1021/acs.jafc.8b05612.
- S. Dong, A. Gao, H. Xu and Y. Chen, Effects of dielectric barrier discharges (DBD) cold plasma treatment on physicochemical and structural properties of zein powders, Food Bioprocess Technol., 2017, 10(3), 434–444 CrossRef CAS.
- M. Huang, Y. Mao, H. Li and H. Yang, Kappa-carrageenan enhances the gelation and structural changes of egg yolk via electrostatic interactions with yolk protein, Food Chem., 2021, 360, 129972 CrossRef CAS PubMed.
- S. Sharma and R. K. Singh, Effect of atmospheric pressure cold plasma treatment time and composition of feed gas on properties of skim milk, LWT--Food Sci. Technol., 2022, 154, 112747 CrossRef CAS.
- K. Itooka, K. Takahashi, Y. Kimata and S. Izawa, Cold atmospheric pressure plasma causes protein denaturation and endoplasmic reticulum stress in Saccharomyces cerevisiae, Appl. Microbiol. Biotechnol., 2018, 102, 2279–2288 CrossRef CAS PubMed.
- N. N. Misra, S. Kaur, B. K. Tiwari, A. Kaur, N. Singh and P. J. Cullen, Atmospheric pressure cold plasma (ACP) treatment of wheat flour, Food Hydrocolloids, 2015, 44, 115–121 CrossRef CAS.
- M. M. Nasiru, E. F. Boateng, F. Alnadari, M. Umair, Z. Wang, A. M. Senan and J. Zhang, et al., Dielectric barrier discharge cold atmospheric plasma treatment of egg white protein: insights into the functional, rheological, and structural properties, Food Bioprocess Technol., 2024, 17(4), 955–976 CrossRef CAS.
- Q. Zhang, Z. Cheng, J. Zhang, M. M. Nasiru, Y. Wang and L. Fu, Atmospheric cold plasma treatment of soybean protein isolate: insights into the structural, physicochemical, and allergenic characteristics, J. Food Sci., 2021, 86(1), 68–77 CrossRef CAS PubMed.
- K. H. Baek, S. H. Ye, G. Y. Dong, E. L. Yee, K. Taemin, K. Hyun-Jun and J. Cheorun, Influence of atmospheric-pressure cold plasma-induced oxidation on the structure and functional properties of egg white protein, Innovative Food Sci. Emerging Technol., 2021, 74, 102869 CrossRef CAS.
- Z. W. Liu, P. P. Tang, C. Liu, Y. C. Tan, T. L. Liu, J. H. Cheng, R. M. Aadil and X. B. Liu, Promoting Ovalbumin Amyloid Fibrils Formation by Cold Plasma Treatment and Improving its Emulsifying Properties, Food Hydrocolloids, 2024, 110531 Search PubMed.
- C. Sarangapani, D. R. Keogh, J. Dunne, P. Bourke and P. J. Cullen, Characterisation of cold plasma treated beef and dairy lipids using spectroscopic and chromatographic methods, Food Chem., 2017, 235, 324–333 CrossRef CAS PubMed.
- X. Yu, S. Huang, C. Nie, Q. Deng, Y. Zhai and R. Shen, Effects of atmospheric pressure plasma jet on the physicochemical, functional, and antioxidant properties of flaxseed protein, J. Food Sci., 2020, 85(7), 2010–2019 CrossRef CAS PubMed.
- K. Subrahmanyam, K. Gul, R. Sehrawat, B. K. Tiwari and S. Sahoo, Cold plasma-mediated inactivation of microorganisms for the shelf-life extension of animal-based foods: efficiency, mechanism of inactivation, and impact on quality attributes, Food Control, 2024, 110464 CrossRef CAS.
- Y. Zhu, M. Elliot, Y. Zheng, J. Chen, D. Chen and S. Deng, Aggregation and conformational change of mushroom (Agaricus bisporus) polyphenol oxidase subjected to atmospheric cold plasma treatment, Food Chem., 2022, 386, 132707 CrossRef CAS PubMed.
- J. A. Purslow, B. Khatiwada, M. J. Bayro and V. Venditti, NMR methods for structural characterization of protein-protein complexes, Front. Mol. Biosci., 2020, 7, 9 CrossRef CAS PubMed.
- Z. W. Liu, M. F. Manzoor, Y. C. Tan, M. Inam-ur-Raheem and R. M. Aadil, Effect of dielectric barrier discharge (DBD) plasma on the structure and antioxidant activity of bovine serum albumin (BSA), Int. J. Food Sci., 2020, 55(7), 2824–2831 CrossRef CAS.
- S. A. Ledari, J. M. Milani and F. S. Lanbar, Improving gelatinbased emulsion films with cold plasma using different gases, Food Sci. Nutr., 2020, 8(12), 6487–6496 CrossRef CAS PubMed.
- D. Geibler, N. Nirmalananthan-Budau, L. Scholtz, I. Tavernaro and U. Resch-Genger, Analyzing the surface of functional nanomaterials—how to quantify the total and derivatizable number of functional groups and ligands, Microchim. Acta, 2021, 188, 1–28 CrossRef PubMed.
- B. S. Murray, Recent developments in food foams, Curr. Opin. Colloid Interface Sci., 2020, 50, 101394 CrossRef CAS.
- D. J. McClements, L. Bai and C. Chung, Recent advances in the utilization of natural emulsifiers to form and stabilize emulsions, Annu. Rev. Food Sci. Technol., 2017, 8, 205–236 CrossRef PubMed.
- J. M. Perez-Andrés, C. Álvarez, P. Cullen and B. K. Tiwari, Effect of cold plasma on the techno-functional properties of animal protein food ingredients, Innovative Food Sci. Emerging Technol., 2019, 58, 102205 CrossRef.
- S. Zhang, W. Huang, E. Feizollahi, M. S. Roopesh and L. Chen, Improvement of pea protein gelation at reduced temperature by atmospheric cold plasma and the gelling mechanism study, Innovative Food Sci. Emerging Technol., 2021, 67, 102567 CrossRef CAS.
- F. Bu, G. Nayak, P. Bruggeman, G. Annor and B. P. Ismail, Impact of plasma reactive species on the structure and functionality of pea protein isolate, Food Chem., 2022, 371, 131135 CrossRef CAS PubMed.
- S. Zhang, W. Huang, M. S. Roopesh and L. Chen, Pre-treatment by combining atmospheric cold plasma and pH-shifting to prepare pea protein concentrate powders with improved gelling properties, Int. Food Res., 2022, 154, 111028 CrossRef CAS PubMed.
- C. Sarangapani, R. Thirumdas, Y. Devi, A. Trimukhe, R. R. Deshmukh and U. S. Annapure, Effect of low-pressure plasma on physico-chemical and functional properties of parboiled rice flour, LWT--Food Sci. Technol., 2016, 69, 482–489 CrossRef CAS.
- O. O. Olatunde, A. Hewage, T. Dissanayake, R. E. Aluko, A. C. Karaca, N. Shang and N. Bandara, Cold atmospheric plasma-induced protein modification: novel nonthermal processing technology to improve protein quality, functionality, and allergenicity reduction, Compr. Rev. Food Sci. Food Saf., 2023, 22(3), 2197–2234 CrossRef CAS PubMed.
- W. Panpipat and M. Chaijan, Effect of atmospheric pressure cold plasma on biophysical properties and aggregation of natural actomyosin from threadfin bream (Nemipterus bleekeri), Food Bioprocess Technol., 2020, 13(5), 851–859 CrossRef CAS.
- F.-G. C. Ekezie, J.-H. Cheng and D.-W. Sun, Effects of atmospheric pressure plasma jet on the conformation and physicochemical properties of myofibrillar proteins from king prawn (Litopenaeus vannamei), Food Chem., 2019, 276, 147–156 CrossRef CAS PubMed.
- Z. Li, Y. Sun, H. Jin, Q. Wang, Y. Jin, X. Huang and L. Sheng, Improvement and mechanism of emulsifying properties of liquid egg yolk by ozonation technology, LWT--Food Sci. Technol., 2022, 156, 113038 CrossRef CAS.
- S. Jaddu, R. C. Pradhan and M. Dwivedi, Effect of multipin atmospheric cold plasma discharge on functional properties of little millet (Panicum miliare) flour, Innovative Food Sci. Emerging Technol., 2022, 77, 102957 CrossRef CAS.
- X. Wu, Y. Luo, F. Zhao, S. M. M and G. Mu, Influence of dielectric barrier discharge cold plasma on physicochemical property of milk for sterilization, Plasma Process. Polym., 2021, 18(1), 1900219 CrossRef CAS.
- S. Zhang, W. Huang, M. S. Roopesh and L. Pretreatment, by combining atmospheric cold plasma and pH-shifting to prepare pea protein concentrate powders with improved gelling properties, Food Res. Int., 2022, 154, 111028 CrossRef CAS PubMed.
- O. O. Olatunde, A. Singh, K. A. Shiekh, P. Nuthong and S. Benjakul, Effect of high voltage cold plasma on oxidation, physiochemical, and gelling properties of myofibrillar protein isolate from Asian sea bass (Lates calcarifer), Foods, 2021, 10(2), 326 CrossRef CAS PubMed.
- F. L. Tabares and I. Junkar, Cold plasma systems and their application in surface treatments for medicine, Molecules, 2021, 26(7), 1903 CrossRef CAS PubMed.
- R. Yang, Y. Liu, D. Meng, D. Wang, C. L. Blanchard and Z. Zhou, Effect of atmospheric cold plasma on structure, activity, and reversible assembly of the phytoferritin, Food Chem., 2018, 264, 41–48 CrossRef CAS PubMed.
- C. Karthik, S. C. Sarngadharan and V. Thomas, Low-Temperature Plasma Techniques in Biomedical Applications and Therapeutics: An Overview, Int. J. Mol. Sci., 2023, 25(1), 524 CrossRef PubMed.
- Y. Devi, R. Thirumdas, C. Sarangapani, R. R. Deshmukh and U. S. Annapure, Influence of cold plasma on fungal growth and aflatoxins production on groundnuts, Food Control, 2017, 77, 187–191 CrossRef CAS.
- K. N. Ho, L. W. Chen, T. F. Kuo, K. S. Chen, S. Y. Lee and S. F. Wang, Surface modification of zirconia ceramics through cold plasma treatment and the graft polymerization of biomolecules, J. Dent. Sci., 2023, 18(1), 73–80 CrossRef PubMed.
- A. S. Chiper and G. Borcia, Stable Surface Modification by Cold Atmospheric-Pressure Plasma: Comparative Study on Cellulose-Based and Synthetic Polymers, Polymers, 2023, 15(20), 4172 CrossRef CAS PubMed.
- L. Di, Z. Fu, M. Dong, A. Zhu, G. Xia and X. Zhang, Cold plasma-prepared Ru-based catalysts for boosting plasma-catalytic CO2 methanation, Chem. Eng. Sci., 2023, 280, 119056 CrossRef CAS.
- R. Kaavya, R. Pandiselvam, M. Gavahian, R. Tamanna, S. Jain, R. Dakshayani and H. A. Hemeg, et al., Cold plasma: a promising technology for improving the rheological characteristics of food, Crit. Rev. Food Sci. Nutr., 2023, 63(32), 11370–11384 CrossRef CAS PubMed.
- S. Amirabadi, J. M. Milani and F. Sohbatzadeh, Effects of cold atmospheric-pressure plasma on the rheological properties of gum Arabic, Food Hydrocolloids, 2021, 117, 106724 CrossRef CAS.
- X. Ge, H. Shen, X. Sun, W. Liang, X. Zhang, Z. Sun and W. Li, et al., Insight into the improving effect on multi-scale structure, physicochemical and rheology properties of granular cold water soluble rice starch by dielectric barrier discharge cold plasma processing, Food Hydrocolloids, 2022, 130, 107732 CrossRef CAS.
- A. M. Trimukhe, K. N. Pandiyaraj, A. Tripathi, J. S. Melo and R. R. Deshmukh, Plasma surface modification of biomaterials for biomedical applications, Advances in Biomaterials for Biomedical Applications, 2017, pp. 95–166 Search PubMed.
- P. Lukes, H. Akiyama, C. Jiang, A. Doria, G. P. Gallerano, A. Ramundo-Orlando, O. Zeni, et al., Special electromagnetic agents: from cold plasma to pulsed electromagnetic radiation, In Bioelectrics, Springer Japan, Tokyo, 2016, pp. 109–154 Search PubMed.
- N. M. Saman, M. H. Ahmad and Z. Buntat, Application of cold plasma in nanofillers surface modification for enhancement of insulation characteristics of polymer nanocomposites: a review, IEEE Access, 2021, 9, 80906–80930 Search PubMed.
- H. Ji, F. Han, S. Peng, J. Yu, L. Li, Y. Liu and Y. Chen, et al., Behavioral solubilization of peanut protein isolate by atmospheric pressure cold plasma (ACP) treatment, Food Bioprocess Technol., 2019, 12, 2018–2027 CrossRef CAS.
- X. Wang, S. C. Huang, S. Hu, S. Yan and B. Ren, Fundamental understanding and applications of plasmon-enhanced Raman spectroscopy, Nat. Rev. Phys., 2020, 2(5), 253–271 CrossRef.
- S. Jaddu, S. Sonkar, D. Seth, M. Dwivedi, R. C. Pradhan, G. Goksen and A. R. Jambrak, et al., Cold plasma: unveiling its impact on hydration, rheology, nutritional, and anti-nutritional properties in food materials–an overview, Food Chem., 2024, 101266 CAS.
- P. Tong, L. Xiong, J. Gao, X. Li, Z. Wu, A. Yang and H. Chen, et al., Influence of heat treatment and egg matrix on the physicochemical and allergenic properties of egg custard, J. Food Sci., 2020, 85(3), 789–799 CrossRef CAS PubMed.
- N. Peng, L. Gu, J. Li, C. Chang, X. Li, Y. Su and Y. Yang, Films based on egg white protein and succinylated casein cross-linked with transglutaminase, Food Bioprocess Technol., 2017, 10, 1422–1430 CrossRef CAS.
- Y. Yu, Y. Guan, J. Liu, W. Hedi, Y. Yu and T. Zhang, Molecular structural modification of egg white protein by pH-shifting for improving emulsifying capacity and stability, Food Hydrocolloids, 2021, 121, 107071 CrossRef CAS.
- N. Gharbi and M. Labbafi, Influence of treatment-induced modification of egg white proteins on foaming properties, Food Hydrocolloids, 2019, 90, 72–81 CrossRef CAS.
- T. Tang, S. Wu, S. Tang, N. Xiao, L. Wu, Y. Tu and M. Xu, Effect of modified egg white powder on the properties of angel cakes, J. Food Eng., 2022, 326, 111012 CrossRef CAS.
- W. Deng, Q. Xu, X. Hu and L. Sheng, Structure and properties of egg white protein films modified by high-intensity ultrasound: an effective strategy, Int. Food Res., 2022, 157, 111264 CrossRef CAS PubMed.
- T. Liu, Y. Zhao, N. Wu, S. Chen, M. Xu, H. Du and Y. Tu, et al., Egg white protein-based delivery system for bioactive substances: a review, Crit. Rev. Food Sci. Nutr., 2024, 64(3), 617–637 CrossRef PubMed.
- Q. Chen, L. Dong, Y. Li, Y. Liu, Q. Xia, S. Sang and L. Liu, Research advance of non-thermal processing technologies on ovalbumin properties: the gelation, foaming, emulsification, allergenicity, immunoregulation and its delivery system application, Crit. Rev. Food Sci. Nutr., 2023, 1–22 Search PubMed.
- H. Zhu, S. Mettu, F. Cavalieri and M. Ashokkumar, Ultrasonic microencapsulation of oil-soluble vitamins by hen egg white and green tea for fortification of food, Food Chem., 2021, 353, 129432 CrossRef CAS PubMed.
- A. Rachman, M. A. Brennan, J. Morton and C. S. Brennan, Effect of egg white protein and soy protein fortification on physicochemical characteristics of banana pasta, J. Food Process. Preserv., 2019, 43(9), e14081 Search PubMed.
- J. Bora, T. Khan and N. K. Mahnot, Cold plasma treatment concerning quality and safety of food: a review, Curr. Res. Nutr., 2022, 10(2), 427–446 Search PubMed.
- X. Yepez, A. E. Illera, H. Baykara and K. Keener, Recent advances and potential applications of atmospheric pressure cold plasma technology for sustainable food processing, Foods, 2022, 11(13), 1833 CrossRef CAS PubMed.
- X. Y. Dong and Y. L. Yang, A novel approach to enhance blueberry quality during storage using cold plasma at atmospheric air pressure, Food Bioprocess Technol., 2019, 12(8), 1409–1421 CrossRef CAS.
- N. U. Sruthi, K. Josna, R. Pandiselvam, A. Kothakota, M. Gavahian and A. M. Khaneghah, Impacts of cold plasma treatment on physicochemical, functional, bioactive, textural, and sensory attributes of food: a comprehensive review, Food Chem., 2022, 368, 130809 CrossRef CAS PubMed.
- S. K. Pankaj, Z. Wan and K. M Keener, Effects of cold plasma on food quality: a review, Foods, 2018, 7(1), 4 CrossRef PubMed.
- H. B. Jadhav and U. Annapure, Consequences of non-thermal cold plasma treatment on meat and dairy lipids–a review, Future Foods, 2021, 4, 100095 CrossRef CAS.
- Y. Pan, J. H. Cheng and D. W. Sun, Cold plasma-mediated treatments for shelf life extension of fresh produce: a review of recent research developments, Compr. Rev. Food Sci. Food Saf., 2019, 18(5), 1312–1326 CrossRef PubMed.
- M. Gavahian, Y. H. Chu and S. Sastry, Extraction from food and natural products by moderate electric field: mechanisms, benefits, and potential industrial applications, Compr. Rev. Food Sci. Food Saf., 2018, 17(4), 1040–1052 CrossRef PubMed.
- K. H. Lee, H. J. Kim, K. S. Woo, C. Jo, J. K. Kim, S. H. Kim and W. H. Kim, et al., Evaluation of cold plasma treatments for improved microbial and physicochemical qualities of brown rice, Lwt, 2016, 73, 442–447 CrossRef CAS.
- M. Rossow, M. Ludewig and P. G. Braun, Effect of cold atmospheric pressure plasma treatment on inactivation of Campylobacter jejuni on chicken skin and breast fillet, LWT--Food Sci. Technol., 2018, 91, 265–270 CrossRef CAS.
- M. Gavahian and A. Farahnaky, Ohmic-assisted hydrodistillation technology: a review, Trends Food Sci. Technol., 2018, 72, 153–161 CrossRef CAS.
- M. Gavahian, Y. H. Chu and S. Sastry, Extraction from food and natural products by moderate electric field: mechanisms, benefits, and potential industrial applications, Compr. Rev. Food Sci. Food Saf., 2018, 17(4), 1040–1052 CrossRef PubMed.
- C. Kang, Q. Xiang, D. Zhao, W. Wang, L. Niu and Y. Bai, Inactivation of Pseudomonas deceptionensis CM2 on chicken breasts using plasma-activated water, J. Food Technol., 2019, 56, 4938–4945 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |