Open Access Article
Open Access ArticleElectrospinning of sustainable polymers from biomass for active food packaging
Fuat
Topuz
 *a and
Tamer
Uyar
*a and
Tamer
Uyar
 *b
*b
aDepartment of Chemistry, Faculty of Science and Letters, Istanbul Technical University, Sariyer 34469, Istanbul, Turkey. E-mail: topuzf@itu.edu.tr
bFiber Science Program, Department of Human Centered Design, College of Human Ecology, Cornell University, Ithaca, NY 14853, USA. E-mail: tu46@cornell.edu
First published on 6th July 2024
Abstract
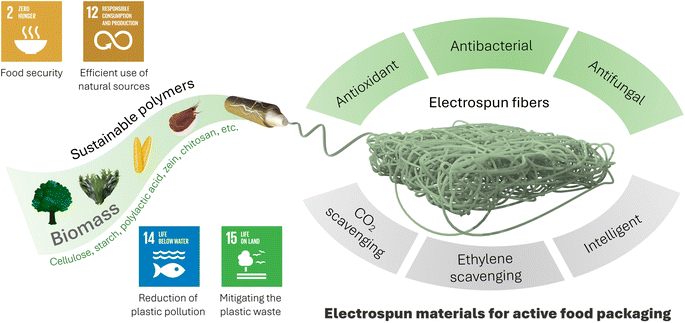
Recent advances in active food packaging have been driven by the integration of electrospun materials, exploiting their inherent advantages. Electrospun materials can be easily functionalized with antioxidant, antibacterial, antifungal, and sensory additives, as well as ethylene scavengers and CO2 emitters making them ideal for active food packaging. However, it's worth noting that certain electrospun materials utilized in this context are derived from petroleum-based synthetic polymers, which may raise environmental concerns post-usage. In this regard, the use of sustainable polymers for electrospun food packaging materials can address problems like waste generation and the environmental impact of traditional synthetic, petroleum-based polymers. Central to this transition is the utilization of biomass-derived polymers sourced from renewable sources like plants, algae, microorganisms, and wastes. Sustainable polymers, such as poly(lactic acid) (PLA), starch, cellulose and derivatives, polyhydroxyalkanoates (PHA), chitosan, gelatin, and zein have emerged as key sustainable players in active food packaging. This review provides a comprehensive overview of electrospun materials of sustainable polymers derived from biomass for the development of active food packaging films. The review begins with a brief description of the fundamentals and process for active food packaging and electrospinning, followed by a detailed examination of the applications of electrospun materials for active food packaging, categorized by polymer type and bioactivity. Finally, the review concludes with current challenges and provides insights into future perspectives in this area.
Sustainability spotlightThis review reports the use of sustainable polymers sourced from biomass for the development of active food packaging materials, in line with the Sustainable Development Goals (SDGs). By contributing to SDG-2 (zero hunger) through improving food security, SDG-12 (responsible consumption and production) via the efficient utilization of natural resources, SDG-14 (life below water) and SDG-15 (life on land) by mitigating plastic pollution from lands and water resources, the integration of sustainable polymers derived from biomass marks a significant step towards environmentally conscious active food packaging. The use of electrospun food packaging materials made from sustainable polymers such as polysaccharides (e.g., cellulose derivatives and starch-based), protein-based (e.g., zein and gelatin), poly(lactic acid) (PLA), and others reduces reliance on petroleum-based polymers by mitigating the environmental impact of plastics. This sustainable approach not only reduces plastic waste generation but also represents a commitment to renewable resources, laying the foundation for a greener future in food packaging innovation. |
1. Introduction
Nowadays, food packaging systems have undergone a remarkable transformation, going beyond traditional barrier films and introducing (bio)active films with functions such as antioxidant, antibacterial, and antifungal properties as well as the ability to monitor changes in packaged foods.1–3 This transformation is further enhanced by the use of biomass-derived sustainable polymers, not only to meet these functional requirements but also to address environmental aspects, thereby reducing the dependence on petroleum-based polymers.4–6 This sustainable approach aims to combat food spoilage, protect packaged foods from oxygen, bacteria, and fungi, and reduce the ecological impact of packaging films. Thus, it can not only overcome the challenge of preservation but also contribute to a more environmentally conscious and responsible packaging solution. In this context, the use of the electrospinning technique has seen an upsurge in the production of cutting-edge food packaging materials, exploiting the beneficial properties of the resulting structures for functionalization and structural customization.7,8The electrospinning process enables the integration of a diverse range of molecules or nanostructured materials with tailored functionalities, providing a versatile platform for active food packaging.9,10 In particular, nanofibers can be equipped with bioactive molecules, transforming them into active food packaging materials that can protect packaged foods from radicals, microorganisms, and fungi. Furthermore, these electrospun materials can be engineered as intelligent systems to monitor changes in food samples to ensure and preserve their freshness.11,12
Active food packaging goes beyond mere protection and incorporates various active ingredients, including oxygen scavengers,13 antimicrobials,14 temperature indicators,15 ethylene scavengers16 and CO2 emitters,17 improving shelf-life, safety, and overall quality of the packaged foods. This proactive approach not only reduces food waste but also meets consumer preferences for fresher and longer-lasting products. While traditional plastic films (e.g., polyethylene (PE), polypropylene (PP), polyethylene terephthalate (PET), etc.) have dominated food packaging in the past and present,18–20 electrospun materials are emerging as a viable option for developing active food packaging materials.8 The increasing environmental concerns surrounding petroleum-based polymers in food packaging have directed research toward sustainable biomass alternatives. Sustainable polymers from biomass are mostly biocompatible and biodegradable, reducing the environmental impact of waste.21 In this context, cellulose and derivatives, poly(lactic acid) (PLA), polyhydroxyalkanoates (PHAs), proteins (e.g., zein and gelatin), chitosan, and starch have emerged as important options for the development of electrospun food packaging materials, reflecting the commitment to environmentally conscious and effective packaging solutions. These polymers have found extensive applications in the production of a wide range of electrospun food packaging materials through the integration of functional molecules, nanostructures, or groups. In this review, we have summarized advances in electrospun food packaging materials produced from sustainable biomass-derived polymers (Fig. 1). First, we provide a concise overview of active food packaging and electrospinning. We then describe the applications of electrospun materials made from sustainable polymers for various food packaging purposes. Finally, we address the existing challenges and offer insights into future perspectives.
2. Active food packaging: an overview
Every year, the world continues to grapple with the persistent issue of global food waste, wherein a notable amount of food produced is lost due to diverse factors, notably including damage inflicted by microbial and oxidation processes.22,23 In addressing this challenge and in line with SDG Goal 2 (zero hunger), there has been a notable development in food packaging focused on improving responsiveness to oxidative and microbial degradation of packaged foods. The core of this packaging innovation lies in its ability to strategically improve the interconnected dynamics of chemical, physical, and biological processes occurring within the packaged food and its environment. This complex optimization aims to ensure the quality and freshness of packaged food and ultimately contribute to a reduction in overall food waste. Therefore, apart from the traditional role of food packaging in containment and protection, active food packaging intervenes in the internal environment of the packaged food to ensure the preservation, safety, and quality of the food.24–28 It uses dynamic mechanisms to protect food from environmental influences and thus preserve the freshness and quality of the packaged food.Food packaging can also be designed as smart packaging to proactively preserve packaged foods and monitor changes.29 In this regard, sensory components, such as pH or temperature indicators, are integrated into the packaging to detect any changes to the food during storage.29–31 Active food packaging can also be employed for the regulation of humidity.32
Incorporating additives with free radical scavenging properties into food packaging materials provides them with an antioxidant effect.33 The polymers can also be modified by introducing radical scavenging groups or molecules to impart antioxidant properties without the need for additional components.34,35 In this regard, various phytochemicals, especially phenolic compounds, are acknowledged for their radical-scavenging abilities. Essential oils (EOs), quaternary groups, and metal nanoparticles have been incorporated into electrospun materials for food packaging, conferring antimicrobial properties.36 Similarly, substances or nanostructures exhibiting antifungal properties have been integrated into food packaging films to enhance their antifungal capabilities.37 Furthermore, electrospun materials can include ethylene scavengers38 and CO2 absorbers39 to enhance the preservation of packaged foods. Additionally, indicators can be embedded in electrospun food packaging films to monitor variations in temperature, pH, and other factors during food storage.40
Key features of active food packaging include:
(1) Improving preservation: the barrier properties of food packaging include the ability of packaging materials to block the passage of gases, moisture, odors, and various external factors that can affect the integrity and safety of the packaged food. A key focus is on preventing the transfer of water vapor, as moisture penetration can lead to spoilage, microbial proliferation, and changes in the texture of food.32,41
(2) Oxygen scavenging: the use of oxygen scavengers is crucial to extend the shelf-life and maintain the quality of packaged foods.13 Oxygen scavengers, either integrated into the packaging material or included as bags in the packaging, interact with oxygen in the packaging environment, reducing its presence. This proactive measure reduces oxygen exposure, preventing oxidative processes that could cause food to spoil, change color and taste, and degrade nutrients.
(3) Antimicrobial activity: antimicrobial components embedded in the packaging material effectively inhibit the proliferation of bacteria, mold, yeast, and other microorganisms responsible for food spoilage and potential health risks to consumers.42 By creating an inhospitable environment for microbial development, these dynamic packaging solutions help extend the shelf life of perishable foods, reduce reliance on chemical preservatives, and reduce the likelihood of foodborne illness.
(4) Ethylene scavenging: ethylene acts as the promoter for ripening of fruits.43 However, its presence can accelerate the decay of fruits and vegetables, resulting in a shorter shelf-life. To extend the freshness of foods, ethylene scavengers can be integrated into food packaging materials.38
(5) CO2 emitters: the antimicrobial effects of absorbed CO2, through carbonic acid formation, acidify the environment, disrupting bacterial cell membranes, cytoplasmic pH, and enzyme activity, thereby inhibiting bacterial growth and enhancing food preservation.44
(6) Temperature indicators: temperature-sensitive indicators can be incorporated into packaging materials to show the temperature history of the packaged food.45
(7) Intelligent sensing: food packaging materials can be functionalized with sensory elements (e.g., pH sensors) to monitor the condition of the packaged food.46
3. Electrospinning: setup and process
Electrospinning represents an electrohydrodynamic technique that employs an electric field to produce micro to nanofibers from a solution containing polymers47,48 and small molecules.49 This method relies on the application of an electric field to transform a viscous solution into nanofibers.50 The fundamental setup comprises (i) a syringe pump, (ii) a high-voltage power source, and (iii) a grounded metal collector.51 In the electrospinning process, the syringe pump propels the solution, forming a spherical droplet at the tip. Under the influence of a high electric field, this droplet transforms into a cone shape, known as the Taylor cone (Fig. 2). When the electric field surpasses the liquid's surface tension, a charged jet is expelled from the needle, accelerating toward the grounded metal collector.50,52,53 Throughout the jet's trajectory, solvent evaporation occurs, resulting in a solidified and elongated nanofiber.54Fig. 2 illustrates the entire electrospinning setup and process, depicting the formation of the Taylor cone and electrospun nanofibers on the collector. As depicted, the electrospinning process is impacted by solution, process, and environmental parameters.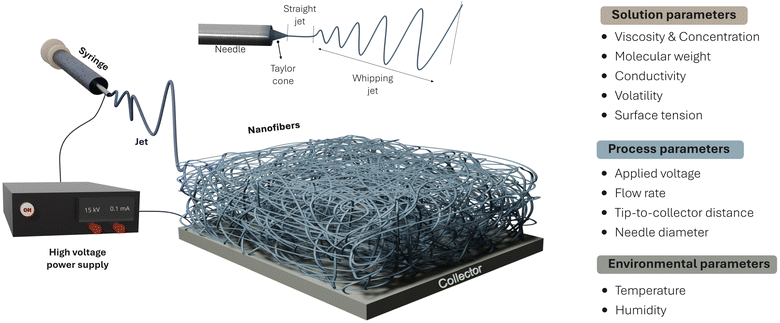 | ||
| Fig. 2 Electrospinning process from a polymer solution to produce electrospun fiber materials. Important parameters affecting the electrospinning process are highlighted. | ||
Electrospinning as a versatile process entails the precise deposition of fibers from a polymer solution or melt under the influence of an electric field. The method has garnered considerable interest in recent times due to its promising potential for generating sophisticated and functional materials tailored for active food packaging applications. The electrospinning technique allows for the development of materials using a diverse range of polymers, selected based on their specific barrier properties, mechanical strength, and compatibility with different types of food. These materials can be enriched with active agents, including antioxidants, antimicrobial agents, and oxygen scavengers, enhancing their functionality to extend the shelf life of packaged food by mitigating oxidative processes and preventing microbial growth. The incorporation and release of active agents can be achieved through various methods, such as core–shell structures or emulsion electrospinning, providing sustained release profiles.55 Additionally, electrospun materials can be applied to traditional food packaging films,56 imparting them with (bio)activity, including antioxidant, antibacterial, antifungal, and sensory functionalities.
4. Electrospun materials from sustainable polymers derived from biomass for active food packaging
In today's world, there is a noticeable trend toward replacing conventional petroleum-based food packaging materials with sustainable alternatives sourced from biomass.5,57–59 This transition is motivated by a shared commitment to tackling environmental risks and promoting sustainable initiatives.59–61 Regarding this matter, electrospun food packaging materials produced from polymers of biomass sources offer several benefits, particularly concerning the environmental footprint of packaging, while also serving as a barrier against light, oxygen, and moisture.8 Another notable aspect is the easy functionalization of electrospun materials with a variety of antioxidants62 and antimicrobial agents.63 This feature contributes significantly to the field of active food packaging as it helps inhibit microbial growth and oxidative reactions that occur in foods. Consequently, the integration of electrospun materials with such properties ensures food safety and helps maintain the high quality of the packaged products. The following section reports the electrospun materials made from sustainable polymers developed for active food packaging. These materials are organized according to both the polymer used and the functionalities of the materials developed.4.1. Polylactic acid (PLA) based electrospun food packaging materials
Poly(lactic acid) (PLA) stands out as an eco-friendly thermoplastic polymer obtained from renewable resources, offering a sustainable substitute for traditional petroleum-derived plastics due to its biodegradable, biocompatible, and environmentally friendly nature.64,65 PLA is mostly produced during the fermentation process of sugars obtained from various biomasses, including corn starch, sugarcane, cassava, and other biomass sources (e.g., algae).66–68 PLA is widely used for the development of a wide range of materials for a broad spectrum of applications, spanning from biomedical to environmental.69 In this context, electrospun fibers of PLA have been also applied to food packaging.70 The integration of PLA fibers with functional molecules can endow them with bioactivity to be employed as antioxidant, antibacterial, and antifungal materials, as well as intelligent packaging materials. For example, for the development of pH-sensing intelligent packaging materials, electrospun PLA films were developed by loading anthocyanins from black carrots.71 The anthocyanin extracted from black carrots loaded PLA fibers exhibited a discernible color transition from reddish pink/dark purple to pinkish gray, suggesting their use as intelligent food packaging materials. Another colorimetric PLA film was developed to monitor cod freshness, utilizing citrated methacrylated urethane (CMU) grafted onto PLA, which was then electrospun into nanofibers.72 This fibrous film undergoes a color change from white to light orange or pink as cod fish deteriorates, at temperatures of 25 °C and 4 °C, respectively.PLA fibers were also functionalized with different bioactive molecules and nanostructures, such as plant extracts, to be employed as antioxidant or antimicrobial materials. In this regard, PLA/hydroxypropyl methylcellulose fibers loaded with pomegranate peel extract (PPE)73 and PLA fibers loaded with ethyl-Nα-dodecanoyl-L-arginate (LAE, ethyl lauroyl arginate)74 were developed as antimicrobial electrospun films. The latter one tested for the shelf-life of strawberries at 25 °C and the results showed no appearance of infections caused by molds for the tested time compared to unwrapped samples. Likewise, PLA nanofibrous films loaded with a butterfly pea flower extract (BPA) and cinnamaldehyde (CIN) were produced and tested for the packaging of beef pieces (Fig. 3a–d).75 Nanofibrous electrospun films showed antioxidative and antibacterial properties while they could detect the freshness of packaged food with a fast color responsiveness under acidic–alkaline conditions (Fig. 3b and c), and the film could be used effectively for visual monitoring of beef spoilage (Fig. 3d). In a study, electrospun PLA films were functionalized with Ag2O-hemp fibers to impart antimicrobial and antifungal properties for fruit preservation against spoilage (Fig. 3e and f).76 Ag2O-hemp fibers modified electrospun PLA nanofibrous film showed high mechanical properties in terms of tensile strength and elongation-at-break, and demonstrated effectiveness against E. coli, S. aureus, A. niger, and Penicillium, while maintaining cytocompatibility and freshness of red grapes at room temperature (Fig. 3e and f). PLA-based nanofibers loaded with Viola odorata petal anthocyanins, combined with carboxymethyl cellulose/cellulose nanocrystals (CNC) films, were used for monitoring the freshness of various foods like Pacific white shrimps, minced lamb meat, chicken fillets, and rainbow trout fillets (Fig. 3g).77 These bilayer films, featuring pH-dependent color changes and slow release of encapsulated extract and CNC during refrigerated storage, demonstrate their potential for food freshness monitoring. Innovatively, recycled keratin from chicken feather waste was blended with PLA or gelatin and electrospun into antibacterial nanofibers.78 The resulting nanofibers showed antibacterial activity against S. aureus ATCC 6538 and E. coli ATCC 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 922 and enhanced thermal properties depending on the keratin loading.
922 and enhanced thermal properties depending on the keratin loading.
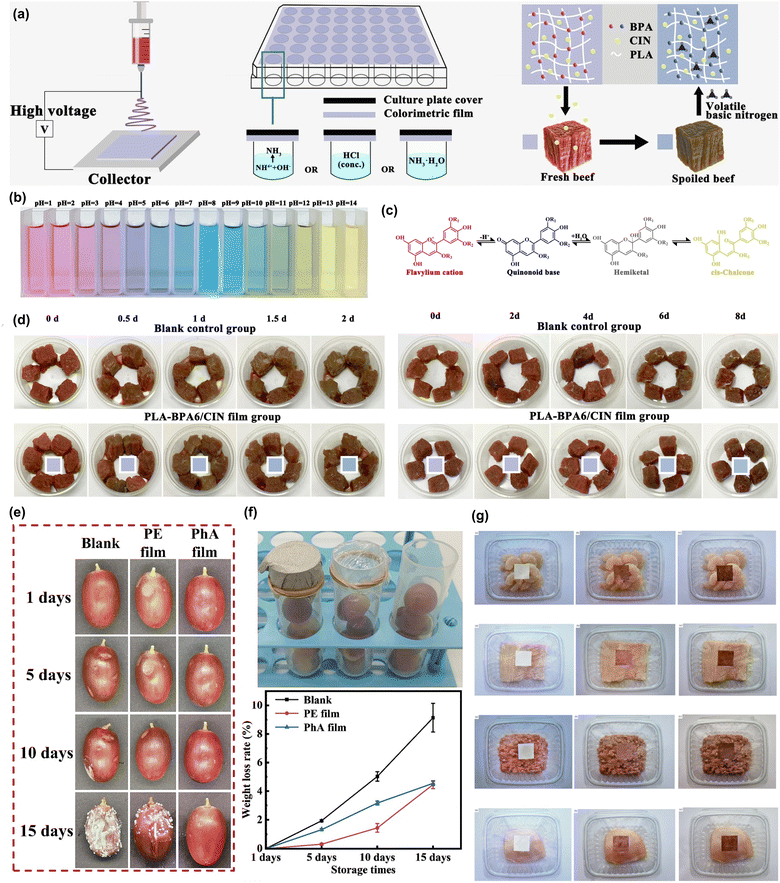 | ||
| Fig. 3 (a) Evaluation of a hydrophobic PLA-based film for beef meat packaging. (b) Monitoring color variations in BPA solutions relative to pH levels. (c) Structural alterations in BPA corresponding to pH shifts. (d) Assessing long-term performance of films in beef meat wrapping. (e) A longitudinal study of electrospun PLA/AgNPs films on hemp fibers for grape packaging. (f) Experimental setup for grape packaging in centrifuge tubes and monitoring weight loss over time. (g) Freshness assessment of shrimp, rainbow trout fillets, minced lamb meat, and chicken fillets utilizing intelligent double-layer films incorporating carboxymethyl cellulose, CNC, PLA, and viola odorata petal anthocyanins nanofibers. The figures were reproduced from ref. 75–77, Elsevier. | ||
Active ingredients have also been incorporated into PLA nanofibers using excipients such as cyclodextrins (CDs). CD molecules are toroidal cyclic oligosaccharides characterized by a characteristic dual nature: a hydrophobic interior and a hydrophilic exterior.79 This unique property allows them to form complexes with molecules that are poorly soluble, thereby rendering them soluble in water.80 In this context, octyl gallate (OG) as an antibacterial molecule was complexed with β-CD, and the resulting inclusion complex (IC) was incorporated into electrospun PLA nanofibers.81 The fibers exhibited bactericidal properties, whereby the OG/β-CD IC can act against bacteria by damaging the membrane, penetrating into cells, and subsequently disrupting the activity of the respiratory electron transport chain, leading to the generation of increased intracellular hydroxyl radicals. The PLA-OG/β-CD IC nanofibrous film was tested for the packaging of a Chinese giant salamander fillet at 4 °C, where the freshness of the fillet was significantly extended. PLA/PCL electrospun films with oregano EO/β-CD ICs were also produced for active food packaging applications.82 The resulting nanofibrous films showed a sustained release profile for the encapsulated oregano EO and showed good biocompatibility and enhanced antibacterial activity, where these electrospun fibers could delay postharvest decay, deterioration, and nutrition loss of blackberries. The Uyar research group also reported CD-ICs of bioactive agents for their higher solubility and enhanced stability. In this regard, α-tocopherol (α-TC)/CD ICs were loaded into PLA nanofibers for potential active food packaging applications.83 The resulting nanofibers showed antioxidant activity that is high enough to inhibit lipid oxidation. The nanofibers underwent direct testing on beef using the thiobarbituric acid reactive substance (TBARS) method, revealing a lower TBARS content compared to the unpackaged meat. Active packaging notably improved the oxidative stability of the meat samples when stored at 4 °C. In another study, a trilayer food packaging material was developed using an extrusion of PLA and electrospun PLA layer containing ethyl lauroyl arginate (ELA), CNC, and chitosan coating.84 The trilayer material demonstrated strong bactericidal activity against L. innocua and S. enterica and degraded over 3 weeks. PLA-based multilayer films were also developed by incorporating ferulic or cinnamic acids using electrospinning.85 Tri-layered films of PLA/starch/PLA with surface-loaded ferulic or cinnamic acid were produced. To promote the compound release of active agents, the active agents were deposited on multilayered films through electrospinning using PLA as a carrier polymer. The multilayer film coated with electrospun fibers demonstrated effective antimicrobial activity against the inhibition of E. coli and L. innocua. The films functionalized with cinnamic acid showed greater antimicrobial activity. Antimicrobial bilayer films based on PLA and Pickering emulsion were produced to enhance the oxygen barrier property of PLA films.86 The addition of thymol in the Pickering emulsion layer endowed them with antimicrobial and antioxidant activity.
In contrast to the aforementioned instances utilizing mono-axial electrospinning, PLA-based electrospun fibrous films were also produced through co-axial electrospinning. In this way, the release of active agents could be controlled using a core–shell fiber structure, where the active agent was embedded in the core while the shell layer was mostly hydrophobic to slow down the release of active agents from the fiber matrix. In this regard, cinnamaldehyde (CMA) and tea polyphenols (TP) were encapsulated as core material, while ZnO NPs/PLA served as the shell layer.87 The synergistic antibacterial efficacy of CMA/TP and ZnO sol induced significant deformation and folding of the cell membrane in Shewanella putrefaciens (S. putrefaciens). Consequently, there was higher permeability of the cell membrane, leading to the release of intracellular contents and disruption of bacteriophage protein expression. A core–shell nanofiber film utilizing PLA and curcumin (CUR) as the active ingredient was created for active food packaging, employing octadecane solution as the core for phase change thermoregulation and PLA/CUR as the shell, demonstrating both antibacterial properties against E. coli and S. aureus and antioxidant activity against DPPH radicals, effectively preserving the freshness of bananas compared to conventional plastic bags.88 In another study, an antioxidant coaxial bionanocomposite was developed using ELA and CNC as active agents.89 The core solution was composed of PLA and ELA while the shell layer was of PLA/CNC. The core–shell structure could slow down the release of ELA from the fibers. The nanofibers showed antibacterial activity against both Gram-positive and Gram-negative bacteria.
Instead of core–shell structured fibers, bioactive agent release was regulated through moisture-triggered mechanisms, exemplified by carvacrol release from electrospun PLA nanofibers in fresh food packaging.90 Blending PEG with PLA before electrospinning allowed for varying encapsulation efficiency and loading capacity of carvacrol, leading to sustained release after an initial burst. Application on strawberries illustrated the efficacy of PLA/PEG/carvacrol in maintaining freshness and reducing microbial counts, highlighting the potential of moisture-triggered carvacrol release from PLA nanofibers for active food packaging. A food packaging system utilizing pectin-coated PLA nanofiber films was created to regulate the release of thymol, employing pectin to safeguard thymol from premature release and pectinase to facilitate controlled release.91 Initially, PLA nanofibers were modified with polyethyleneimine and then coated with pectin to achieve controlled release of thymol, exhibiting potent bactericidal properties against food-related microorganisms, albeit with slightly slower release kinetics compared to thymol-loaded PLA/PEI fibers.
PLA nanofibers were also functionalized with metal–organic frameworks (MOFs) to benefit their strong ability to release highly volatile compounds. In this regard, electrospun PLA/PCL fibers loaded with thymol/MIF-68 (AI) were developed as active nanofibrous film packaging materials.92 The incorporation of thymol/MIF-68 (AI) improved the water vapor barrier performance and UV resistance of the nanofibers, while it caused the weakening of the mechanical properties of the fibers (i.e., tensile and elongation). The MIF-68 (AI) particles improved the residence time of thymol due to the sustained release of thymol. Another PLA-based nanocomposite electrospun films were developed using an MCM-1 mesoporous molecular sieve loaded with phloridzin, and the resulting films were used for the preservation of strawberries.93 In this regard, a blend of PLA, phloridzin, and MCM-41 powder was prepared and electrospun into fibers. The electrospun films showed antibacterial activity against E. coli growth. Phloridzin was used as an antioxidant agent, and the phloridzin-loaded electrospun film was used to wrap strawberries, which could delay lipid oxidation in strawberry packaging, promoting their freshness over 3 weeks. Another nanocomposite PLA electrospun films were produced by incorporating TiO2 nanoparticles and graphene oxide (GO) through an ultrasonic-assisted electrostatic spinning technique.94 The incorporation of TiO2 NPs and GO could significantly boost the tensile strength and stretchability of the nanofibrous films while improving their water barrier properties. The UV-exposed fibrous films showed higher inhibition of both E. coli and S. aureus. The food packaging tests using green peppers through hardness, soluble solids, and chlorophyll content tests revealed that the nanocomposite films could delay the rate of spoilage of green peppers, extending their preservation period.
4.2. Polyhydroxyalkanoates (PHA) based electrospun food packaging materials
Polyhydroxyalkanoates (PHA) are biodegradable polymers produced from microorganisms as intracellular carbon and energy storage compounds.95–97 PHA could also be produced from plant sugars, vegetable oils, and agricultural byproducts through the fermentation of microorganisms using renewable carbon sources. Their physical and mechanical properties could be tailored by tuning the microbial strains and cultivation conditions. PHA has therefore been employed in various food industries including the development of food packaging materials. Most applications of PHA such as poly(3-hydroxybutyrate) (PHB) for food packaging mainly focus on their use in boosting water resistance by the deposition of PHA fibers on nanocellulose-based films.98 Likewise, electrospun PHB or electrospun PHB/bacterial cellulose nanowhiskers (BCNW) fibers were also used for improving the barrier properties of thermoplastic corn starch-based films.99 PHB-BCNW solutions in 2,2,2-trifluoroethanol were prepared and then electrospun onto nanobiocomposites TPCS/BCNW films. The methodology showed good adhesion between the layers and led to higher barrier performance. Such materials with improved barrier properties can be used as food packaging materials. Unlike the above examples, electrospun PHA films were also produced for active food packaging. In one example, a multilayer approach-based electrospun PHA film with/without cellulose nanocrystal (CNC) coatings was reported for active food packaging.100 The design involved creating an antimicrobial hot-tack layer using poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) derived from cheese whey, which was electrospun onto a blown film made of a commercial food contact PHA-based resin. Oregano EO (OEO) and ZnO-NPs were incorporated into the nanofibers of PHVP. The assembly of the multilayer structure took place in a pilot roll-to-roll laminating system, with the blown PHA-based film serving as the outer layers. The electrospun antimicrobial hot-tack PHBV layer and the barrier CNC coating were positioned as interlayers. The resulting multilayers exhibited contact transparency, strong interlayer adhesion, enhanced resistance to water and limonene vapors, and intermediate mechanical properties. Furthermore, these films demonstrated significant antimicrobial and antioxidant activities in both open and closed systems, lasting for up to 15 days. A multilayered electrospun coating with oxygen-scavenging properties was created on conventional cellulose paper through a bilayer electrospinning process.101 In pursuit of this objective, electrospun fibers of PHB and polycaprolactone (PCL) with palladium nanoparticles (PdNPs) were produced. To diminish porosity and enhance barrier properties and interlayer adhesion, the resulting films were exposed to an annealing process. Following annealing, a significant reduction in the oxygen scavenging capacity of the films was observed compared to the non-heated, highly porous electrospun fibers. Another multilayer concept was developed through the electrospinning of PHB, PCL, or PLA onto both sides of a corn starch film.102 The water vapor permeability of the membranes experienced a notable decrease upon electrospun coating, irrespective of the polymer used. Similarly, increasing the electrospun coating led to a reduction in the oxygen permeability of the membrane. Another multilayer concept was developed using PHBV electrospun films with antimicrobial properties.103 Eugenol as a bioactive ingredient was loaded into PHBV fibers, which resulted in the development of antimicrobial fibers. The eugenol-loaded PHBV fibers were used as an interlayer between a structural layer produced through a cast-extruded PHB sheet and a commercial PHBV film as the food contact layer. The resulting multilayer showed hydrophobicity, strong adhesion, mechanical resistance, and higher barrier properties against water vapor and limonene vapors. The antimicrobial efficacy of the multilayer structure was assessed over 15 days in both open and closed environments, revealing notable decreases in the populations of the two food-borne bacterial strains. Another antimicrobial PHB fibrous film was developed through the use of poly(5,5-dimethyl-3-(3′-triethoxysilylpropyl)hydantoin) (PSPH).104 Following the chlorine bleach step, a bactericidal fibrous membrane could be produced, which showed antimicrobial activity against S. aureus and E. coli O157:H7. A similar concept was used for the preparation of antimicrobial polyhydroxybutyrate/poly(butyleneadipate-co-terephthalate) (PHB/PBAT) nanofibrous membranes.105 The fibrous membranes were grafted with 1-allyl hydantoin and perfluorooctyl acrylate and then chlorinated with chlorine bleach. The chlorinated membranes showed antibacterial activity against E. coli O157:H7 (ATCC 43895) and S. aureus (ATCC 6538) with 6.08 and 5.78 log reduction. In another study, PHB of microalgal origin was blended with phenolic compounds and then electrospun into fibers.106 Phenolic compounds of Spirulina were used for antibacterial activity, and the resulting fibers were tested against the growth of S. aureus ATCC 25923. The nanofibers showed good thermal, mechanical, and antibacterial properties, demonstrating their potential for food packaging applications.Nanocomposite PHB-based fibrous membranes were also developed for food packaging applications. In this regard, PHB/PCL fibrous membranes modified by silica composite hydrol for superhydrophobicity were produced.107 First, the fibrous membrane was produced through the electrospinning of the PHB and PCL blend, and then the membrane was modified with silica hydrosol through a dip-pad process. The nanocomposite electrospun films were chlorinated, and the resulting material showed superhydrophobicity with a water contact angle (WCA) of ∼150°. The membranes also showed effective antibacterial activity against E. coli O157:H7 and S. aureus with 99.95% and 99.91% bacterial reduction within 60 min of contact time.
4.3. Starch-based electrospun food packaging materials
Starch, a natural polymer comprised of glucose units, is derived from renewable sources like corn and potato.108 It serves as a sustainable polymer for creating active food packaging materials, incorporating bioactive compounds and sensing elements. In this regard, various antibacterial/antioxidant/antifungal food packaging materials based on starch have been developed. In one example, a phytochemical with antibacterial activity, cinnamaldehyde EO (CEO), was incorporated into electrospun octenyl succinylated starch-pullulan nanofiber mats.109 CEO-loaded electrospun nanofibers showed antibacterial activity against S. aureus and E. coli, and antifungal activity against a saprotrophic and pathogenic fungus, Aspergillus flavus. Such materials with incorporated antibacterial agents hold promise in food packaging applications. Likewise, thyme EO (TEO) loaded antioxidant starch electrospun fibers were developed for active food packaging applications.110 TEO was dissolved in formic acid and blended with starch to produce fibers, where high encapsulation efficiency (>99%) could be reached. The nanofibers showed significant antioxidant activity, which increased with higher TEO loading. Ginger EO (GEO) from avocado seeds was also encapsulated into starch nanofibers.111 The incorporation of the GEO increased the fiber diameter, and increasing the GEO content led to a drastic rise in antibacterial activity against E. coli. Another antimicrobial starch electrospun film was created by incorporating tea polyphenols for active food packaging, with poly(vinyl alcohol) (PVA) added to enhance electrospinnability.112 This nanofibrous film exhibited improved mechanical strength and water vapor barrier properties while gradually releasing encapsulated tea polyphenols through Fickian diffusion. Demonstrating stronger antimicrobial effects against S. aureus compared to E. coli, these fibrous films effectively prolonged the shelf-life of strawberries by maintaining freshness for over six days, likely through mechanisms involving cell membrane disruption, DNA fragment degradation, and the generation of intracellular reactive oxygen species (ROS). Polyfunctional starch nanofiber films loaded with tea polyphenols were developed for food packaging, aiming to improve mechanical strength, antioxidant capacity, and hydrophobicity.113 Fabricated through one-step temperature-assisted electrospinning and cross-linked with glutaraldehyde vapor, the resulting films exhibited altered fiber morphology and a correlation between tea polyphenol concentration and antioxidant properties, albeit with reduced mechanical strength at high polyphenol concentrations.Antibacterial starch/PVA nanofibers for food packaging have been developed using silver sodium zirconium phosphate (Ag-ZrP) (Fig. 4).114 Bead-free electrospun fibers with enhanced mechanical strength were produced. The fibers were cross-linked with glutaraldehyde vapor, which also boosted the hydrophobicity of the fibers. The fibers showed antibacterial activity against E. coli and S. aureus. The antibacterial fibers could prolong the freshness of strawberries over days while a clear sign of rotting was observed for the uncovered strawberries and strawberries covered with Ag-ZrP-free fibers (Fig. 4d). Another silver NP-based starch electrospun fibers were produced for potential food packaging applications.115 Nonuniform spherical AgNPs were synthesized through the use of crude pulp extract of Limonia acidissima and then blended with tapioca starch and guar gum for electrospinning. AgNP loaded electrospun fibers showed antibacterial activity against E. coli and S. aureus, as well as cytocompatibility over NIH3T3 fibroblast cells, which showed bipolar rod-like shapes in the cells due to a contractile structure. Similar to antibacterial starch-based electrospun films, numerous starch-based electrospun fibers with antioxidant properties have been reported. In one study, β-carotene functional corn starch multilayered electrospun structures were produced for food packaging applications.116 β-carotene as an antioxidant derived from plants was incorporated into the exterior PCL layers, while the core layer is composed of cornstarch film. Even though the incorporation of β-carotene did not improve the barrier properties of the films, it gave antioxidant characteristics.
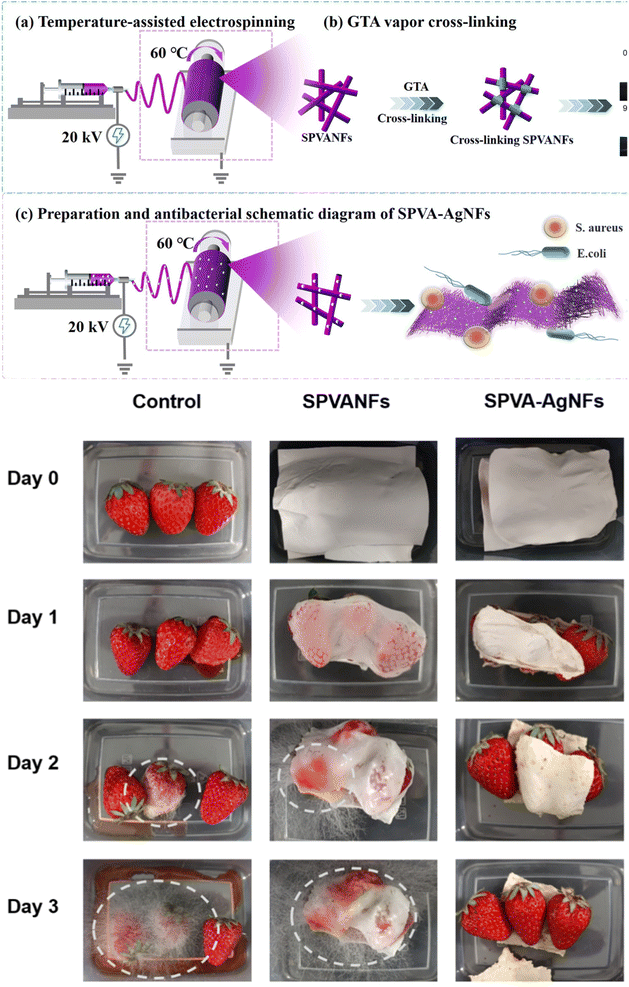 | ||
| Fig. 4 (a) Schematic diagram illustrating the production of SPVA NFs using temperature-assisted electrospinning; (b) cross-linking of SPVA NFs with GTA vapors depicted; (c) schematic representation of the temperature-assisted electrostatic spinning process for creating SPVA-AgNFs and demonstrating its antibacterial properties. (d) Images of strawberries covered with various protective films were observed under room light and maintained at room temperature for 3 days. The figure was reproduced from ref. 114, Elsevier. | ||
In addition to their use as antioxidant and antimicrobial films, starch-based electrospun intelligent food packaging films were also reported. Anthocyanin as a pH sensor was incorporated into the electrospun starch mats for real-time monitoring of food freshness.117 Bead-free fiber morphology could be obtained at different anthocyanin loadings. The fibers were used to monitor the freshness of pork and shrimp during storage and showed a color change due to loss of freshness. Enzyme and humidity-responsive antimicrobial fibers based on starch were developed for active food packaging.118 These fibers, comprising CNCs, starch, and zein, were blended with a combination of nature-derived antimicrobials and CD ICs. They demonstrated the ability to release active ingredients either freely in response to microbial enzymes or in the form of CD-ICs when exposed to high humidity levels. This responsive behavior led to significant reductions in bacterial and fungal populations, with greater efficacy observed at higher relative humidity levels. Starch-based nanofibrous films with self-cleaning action were also reported by tuning the hydrophobicity of the films through the surface decoration with hierarchical flower-like micro/nanostructures.119 To address the extremely low hydrophobicity of starch nanofibrous film, the authors employed a simple and cost-effective solution immersion method to develop a fiber coating using stearic acid (STA). This approach draws inspiration from the superhydrophobic properties observed in biological organisms like lotus leaves. Such films through bioinspired self-assembled coating with self-cleaning ability can be used for the development of self-cleaning packaging materials.
4.4. Chitosan-based electrospun food packaging materials
Chitosan, a biopolymer derived from chitin in crustacean shells, exhibits potent antimicrobial properties, making it ideal for enhancing the shelf life of perishable foods.120,121 By incorporating chitosan into electrospun nanofibers, researchers have developed packaging materials with a superior barrier against microbial growth, effectively extending the freshness of packaged foods and reducing food waste. Moreover, utilizing chitosan sourced from biomass waste promotes sustainability by repurposing residues from the seafood industry and reducing dependence on traditional petroleum-based plastics. In this context, various chitosan-based electrospun fibers were reported for active food packaging applications.122 In one study, chitosan/PCL electrospun fibers incorporated with Chinese yam polysaccharide were developed for active food packaging, demonstrating superior antimicrobial efficacy against E. coli compared to S. aureus.123 Antibacterial testing illustrated that these electrospun membranes effectively prevented water-loss rot in cherry tomatoes, maintaining a weight loss rate of 17.6 ± 0.14%. Thymol, an active ingredient, was encapsulated within chitosan fibers using coaxial electrospinning with PEO/chitosan, facilitated by genipin cross-linking (Fig. 5a).124 These core–shell nanofibers displayed sustained release of thymol, antioxidant properties against DPPH and ABTS radicals, biocompatibility, and antibacterial activity against E. coli and S. aureus, making them promising for active food packaging applications. Cross-linked chitosan fibers containing bioactive extracts have been explored for active food packaging, as illustrated in Fig. 5b.125 A blend of chitosan and gelatin mixed with Zataria multiflora extract was electrospun into fibers for mushroom packaging, then cross-linked with citric acid and heat treatment to produce bead-free fibers with an average diameter of 188 nm. These nanofibers exhibited reduced weight loss and water vapor permeability post-cross-linking, along with antibacterial properties, effectively prolonging mushroom preservation compared to conventional polyethylene films, as depicted in Fig. 5b. In another investigation, chitosan fibers were modified with melissa officinalis extract (MOE) for active food packaging, as depicted in Fig. 5c.126 The resulting nanofibers, formed from a blend of chitosan, PEO, and MOE, exhibited improved mechanical strength through photocrosslinking with benzophenone, while also demonstrating antioxidant and antifungal properties against C. albicans, effectively preserving the freshness of wrapped mushrooms for up to 6 days.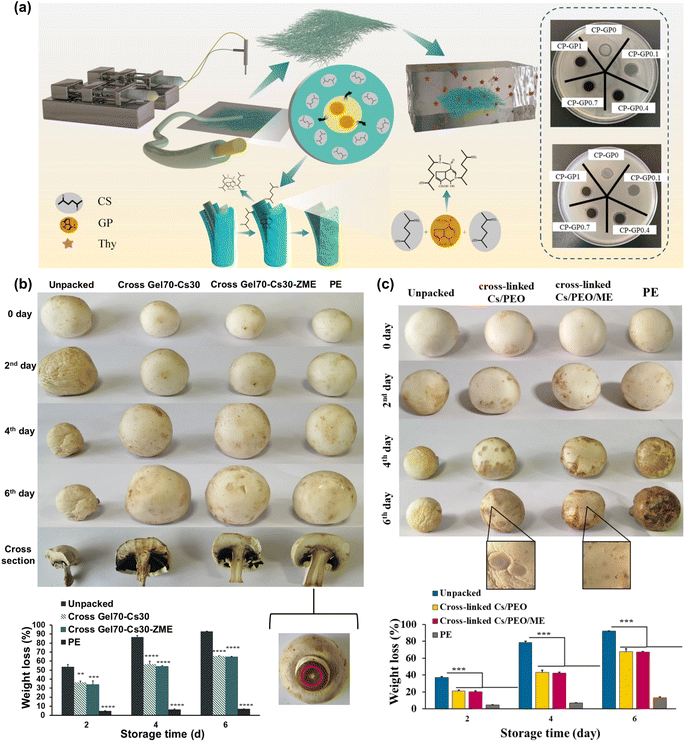 | ||
| Fig. 5 (a) Schematic depiction of the production of thymol-loaded core–shell chitosan fibers and their use as antibacterial packaging materials. (b) The use of the chitosan/gelatin fibers loaded with Zataria multiflora extracts (ZEM) for mushroom packaging over time and the respective changes in weight loss. (c) Appearance changes of mushrooms wrapped by chitosan fibers loaded with MEO during storage and weight loss of mushrooms packed with different samples during storage. The figures were reproduced from ref. 124–126, Elsevier and Springer. | ||
In another study, chitosan fibers were used to wrap dry-aged beef, yielding superior results compared to wet-aging over a 3 weeks period, with reduced microbial counts, including yeasts and molds, and enhanced visual appeal.127 While wet-aged beef showed minimal weight and trimming losses, it also exhibited a significant proliferation of lactic acid bacteria. Likewise, Arkoun et al. studied chitosan-based nanofibers' antibacterial action against meat spoilage and pathogenic bacteria.128 They prepared chitosan fibers via electrospinning of chitosan/PEO blends and evaluated their efficacy against E. coli, Salmonella enterica serovar Typhimurium, S. aureus, and L. innocua, finding that the antibacterial action depended on amino group protonation and was bactericidal rather than bacteriostatic. The susceptibility of bacteria varied by strain rather than Gram classification, with non-virulent strains showing higher susceptibility, achieving a reduction rate of 99.9%. Ultimately, the nanofibers extended the shelf life of fresh meat by one week.
To boost the antimicrobial activity of chitosan, quaternized chitosan-based fibers were also used for active food packaging applications.129 In this regard, the mixture of chitosan, quaternized chitosan, vanillin, and PEO was electrospun into fibers. Vanillin could be encapsulated through imine bonds. The use of quaternized chitosan endowed the fibers with high antioxidant, antibacterial, and antifungal activity against relevant strains, such as E. coli, S. aureus, and C. albicans. The fibers could be used for the preservation of raspberries as model fruits, which could extend their shelf-life to 7 days under atmospheric conditions. In another study, a mixture of quaternized chitosan, organic rectorite (OREC), and PVA was electrospun into fibers, which led to antibacterial films for potential applications in active food packaging.130 The antibacterial activity of the electrospun mats was boosted with increasing amounts of OREC.
Nanocomposite chitosan-based fibers have been developed for active food packaging, such as Fe3O4-chitosan/PVA nanofibrous films, exhibiting bead-free morphology and enhanced antibacterial and mechanical properties.131 Incorporating Fe3O4 nanoparticles at varying concentrations improved bacterial adhesion and inactivation efficacy, with the highest effectiveness observed against E. coli (90%) and S. aureus (66.30%). These films also showed substantial enhancements in tensile strength (up by 46–192%) and elongation at break (increased by 92–141%), along with high biocompatibility, suggesting their potential as active food packaging materials.
CD molecules were utilized for loading bioactive agents into chitosan fibers through inclusion complexation, then blended with chitosan for electrospinning. In one study, 1,8-cineole/CD ICs were incorporated into chitosan/PVA solutions, deteriorating fiber morphology but enabling sustained release of 1,8-cineole, leading to excellent antioxidant and antibacterial activity and extending strawberry shelf-life to 6 days at 25 °C.132 To address chitosan fibers' hydrophilicity, hydrophobic chitosan derivatives were synthesized to develop water-resistant fibers and improve electrospinnability. In another study, pullulan-carboxymethyl chitosan (CMCS)/PEO core–shell nanofibers loaded with nanogels, specifically CMCS-nisin nanogels (CNNGs), were fabricated and confirmed by TEM analysis.133 The bead-free nanofibers exhibited smooth textures and demonstrated high antimicrobial activity against E. coli and S. aureus, effectively extending the shelf-life of bass fish when packed with nisin-loaded core–shell nanofibers from 9 days to 15 days, indicating enhanced stability and bioactivity of the CNNGs. Another intelligent chitosan-based electrospun film was developed through loading shikonin and the use of quaternized chitosan (Fig. 6).134 Bead-free fibers were produced at different loadings of shikonin, which showed hydrophobicity, barrier, and desired mechanical properties. The nanofibers showed high antibacterial and antioxidant activity, and pH-responsive color change in a reversible manner. The electrospun films were used the monitor shrimp freshness by changing color (Fig. 6e).
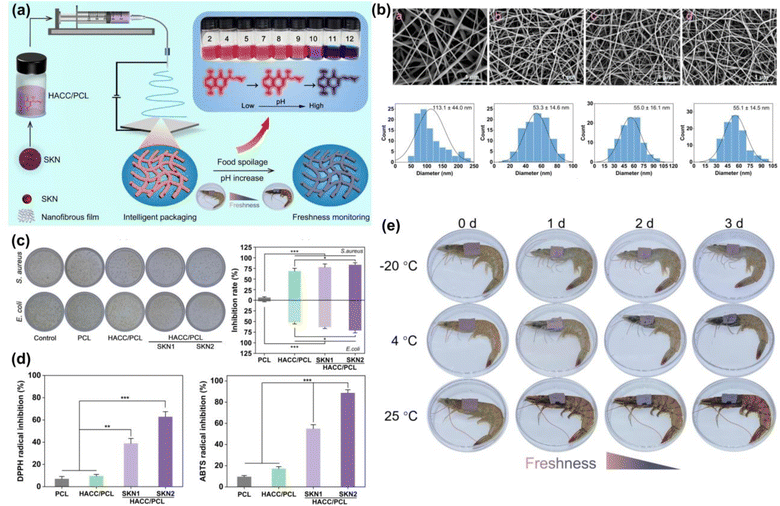 | ||
| Fig. 6 (a) The development of dual-functional shikonin-loaded quaternized chitosan/PCL electrospun film with pH sensitivity. (b) SEM photos of the respective nanofibers. (c) Antimicrobial activity of the nanofibers against E. coli and S. aureus. (d) The antioxidant activity of the nanofibers against DPPH and ABTS radicals. (e) The food packaging applications of the films to monitor shrimp's freshness. The figures were reproduced from ref. 134, Elsevier. | ||
An antimicrobial food packaging based on prodigiosin loaded double layered bacterial cellulose and chitosan composites were produced and their properties were explored for food packaging applications.135 In this regard, two different routes were followed: (i) PVA/chitosan nanofibers were deposited on bacterial cellulose film or (ii) bacterial cellulose/bacterial pigment prodigiosin (PG) blend film was used for the deposition of electrospun film of PVA/chitosan to develop double layered composites. SEM analysis revealed the formation of ribbon-like fibers while the cross-section images revealed the sticking of electrospun fibers on the BC film. WCA analysis revealed the hydrophilic natures of the composites while water vapor transmission rate (WVTR) values BC/PVA-CH-PG and BC-PG/PVA-CH were measured as 1113.71 ± 335.88 g m−2 per day and 888.98 ± 125.12 g m−2 per day, respectively. The composites demonstrated antimicrobial activity against S. aureus and P. aeruginosa, suggesting their potential as active food packaging materials.
CUR was also loaded into chitosan fibers to benefit its pH responsiveness. In this regard, G. Sumnu and colleagues reported the natural halochromic CUR-loaded chitosan/PEO nanofibers as an intelligent packaging material.136 Chitosan/PEO blend was mixed with CUR and electrospun into fibers. The physicochemical properties of the film were notably influenced by the ratio of chitosan to PEO. The nanofilm exhibited substantial color variations correlated with chicken spoilage, as evidenced by changes in pH and total volatile basic nitrogen (TVB-N) levels. Chitosan-based hybrid electrospun nanofiber mats loaded with eugenol were produced and tested for antimicrobial activity.137 The solution of chitosan, cellulose acetate (CA), and gelatin was mixed with eugenol of various concentrations and electrospun into fibers. The antimicrobial activity of the nanofibers was confirmed against Salmonella typhimurium and S. aureus. In addition to the above examples, various chitosan fibers were functionalized with Zataria multiflora (ZEO) and cinnamon (CEO) EOs,138 hordein-quercetin,139 pomegranate peel extract,140 oregano EO (OEO),141 chrysanthemum oil,142 phenolic compounds,143 lauric arginate,144 and clove oil145 for potential food packaging applications. Nanocomposite chitosan electrospun films were also produced using montmorillonite146 and silver nanoparticles (AgNPs)147 for active food packaging applications.
4.5. Cellulose-based electrospun food packaging materials
Cellulose as a natural polymer found in the cell walls of plants, has many intrinsic benefits such as biodegradability and customizable structure.148 Cellulose and its derivatives have been widely used for the fabrication of food packaging materials.149 Numerous studies have utilized cellulose and its derivatives in electrospinning processes to fabricate food packaging films, aiming to enhance barrier properties or boost the mechanical strength of the fibers. This has often been achieved through the incorporation of nanoparticles.150 Likewise, many electrospun cellulose-based active food packaging materials have been developed. In one example, a composite electrospun nanofiber film, comprising ethyl cellulose and soy protein isolate (SPI) blended with bitter orange peel extract, was developed for food packaging.151 Variation in the ethyl cellulose/SPI ratio and bitter orange peel extract content influenced fiber morphology, antioxidant activity, and antibacterial efficacy against E. coli and S. aureus, with a WVTR measured at 657 g m−2 per day. Gliadin-ethyl cellulose fibers loaded with cumin seed oil and reinforced with adipic acid were developed for food packaging.152 Acting as a hydrogen bond cross-linker, adipic acid increased polymer viscosity before electrospinning, resulting in nanofibers with antioxidant properties, strong antibacterial and antifungal activity against various pathogens, and demonstrated biocompatibility with human cells, suggesting their promising potential for food packaging applications. Another ethyl cellulose nanofiber film was produced in the presence of PCL and gelatin and loaded with Zataria multiflora EO (ZEO) and ZnO NPs to provide an ideal food packaging substrate.153 The composite nanofibers were nontoxic and showed the highest antioxidant activity (34.61 ± 1.98%) and antifungal properties against Penicillium notatum and Aspergillus niger. Such fibers are suitable for active packaging materials for foods prone to fungal spoilage. Likewise, ethyl cellulose electrospun food packaging material was developed using pullulan and cinnamaldehyde.154 Bead-free uniform nanofibers were produced with hydrophobicity. The antimicrobial activity of the nanofibers was boosted with cinnamaldehyde content added.Unlike the above example, active agents were not directly released from the fiber matrix, but from the decorated nanogels. Ethyl cellulose/casein nanofibers were modified with ginger essential oil (GEO)-loaded nanogels made of gelatin and carrageenan gum, enabling controlled release of active agents.155 Synthesized via inverse miniemulsion, the gelatin/carrageenan gum aldehyde nanogels measured 95 nm in diameter, increasing to 110 nm after GEO loading, before being blended with the electrospinning solution and spun into nanofibers. These functional nanofibers demonstrated slow release of GEO, antioxidant activity, and antibacterial efficacy against E. coli and S. aureus, with enhanced performance with higher nanogel content, suggesting their potential application in food packaging. CA nanofibers were functionalized with chitosan nanoparticles loaded with Ziziphora clinopodioides essential oil (ZEO) for packaging beef meat samples.156 Synthesized concurrently with CA electrospinning, these nanostructures showed enhanced tensile strength and low vapor barrier, outperforming ZEO-loaded CA fibers alone. They exhibited potent antioxidant activity against E. coli and S. aureus, with a release profile featuring rapid initial release followed by gradual release, effectively inhibiting bacterial growth in refrigerated beef samples. A trilayer bionic nanofibrous membrane, consisting of ethylene-vinyl alcohol copolymer, gelatin, and CA, was electrospun for jerk beef packaging, featuring hierarchical pore networks and asymmetric wettability for moisture control.157 With a high transport index and moisture management capacity, the membrane extended jerk beef shelf-life by 100% under specified storage conditions. Coaxial electrospinning was utilized to produce CA nanofibers with core–shell structures for active food packaging applications.158 These nanofiber films, composed of CA and gelatin with eugenol loaded into the core, demonstrated slow release of eugenol over multiple days, confirmed by transmission electron microscopy analysis. Showing substantial antimicrobial efficacy against E. coli and S. aureus, these findings indicate the potential of these nanofibers as effective food packaging materials.
CA was utilized to create colorimetric nanofibrous films incorporating Perilla frutescens anthocyanins and chamomile EO (Fig. 7).159 These films displayed sensitive and reversible color changes over a pH range of 2–12, sustained release of bioactive agents, antioxidant activity, and significant antimicrobial efficacy against E. coli and S. aureus, particularly effective against the latter. Another colorimetric freshness monitoring system was developed using electrospun ethyl cellulose/gelatin fibers containing purple sweet potato anthocyanin (PSPA) as a pH indicator.160 These fibers exhibited enhanced wettability and pH responsiveness compared to cast films, with pork wrapped in the nanofilm showing an extended shelf life of up to 6 days, characterized by color shifts from light pink to light brown and then to brownish-green as freshness declined.
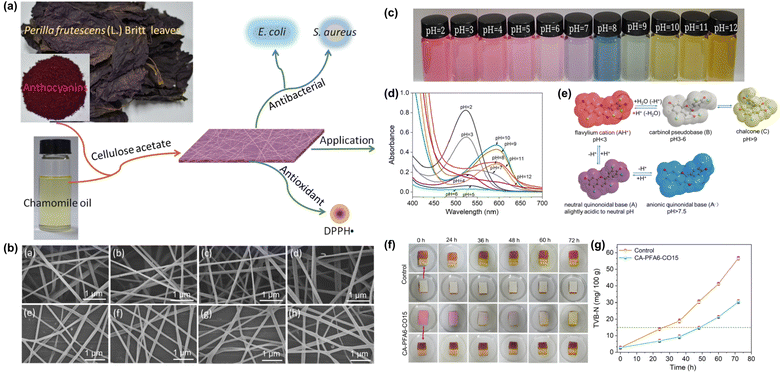 | ||
| Fig. 7 (a) Schematic illustration of the production of antioxidant and antibacterial CA fibers loaded with chamomile oil and anthocyanins extracted from Perilla frutescens (L.) Britt. (b) SEM photos of the CA fibers (CA (a), CA-CO5, 10, 15 (b–d), CA-PFA2, 4, 6 (e–g) and CA-PFA6-CO15 (h)). (c) Color changes and absorption spectra (d) of PFA solutions at pH 2–12, corresponding structural transformation (e). (f) CA-PFA6-CO15 for pork freshness monitoring and (g) corresponding levels of TVB-N. The figures were reproduced from ref. 159, Elsevier. | ||
Cellulose nanofibers provided support for chitosan/tannic acid bilayers, enhancing antibacterial protection.161 The resulting composite mats, fabricated via LbL assembly with electrostatic interactions, exhibited increased antimicrobial activity against S. aureus and E. coli, suggesting their potential application in food packaging due to the biocompatibility of the precursors and antimicrobial efficacy of the materials. Another antifungal packaging was developed through the electrospinning of the blend of CA and poly(vinyl chloride) loaded with AgNPs.162 The incorporation of AgNPs reduced the fiber diameter and decreased the air permeability rates. The hybrid nanofibers could inhibit the growth of yeast and mold because of the AgNPs incorporated. An interesting concept on upcycling blue jeans into fibrous cellulose fibers was reported.163 Carboxylated carbon nanotubes and GO were integrated into cellulose fiber via a wet electrospinning technique. The resulting structures were used for the immobilization of lysozyme enzyme, which could maintain the bioactivity. The fibers were cytocompatible and demonstrated significant antimicrobial activity after immobilization of lysozyme. Another LbL assembly on CA nanofibers was developed using bilayers of chitosan (CS) and epigallocatechin gallate (EGCG) or with bilayers of CS-rectorite (REC) composite (CS-REC) and EGCG.164 Fibrous structure could be maintained after LbL assembly and the fiber diameter was boosted with the addition of REC. The tensile properties of the fibers did not differentiate significantly with LbL deposition. REC increased the encapsulation efficiency and loading capacity of nanofibers and slowed down the release profile of EGCG. The presence of chitosan and EGCG caused antimicrobial activity against S. aureus. A triaxial fiber membrane with CA sheath and PCL as an intermediate a layer and core layer as nisin were developed for long-term antimicrobial activity for potential use in food packaging.165 The antimicrobial tests were done against S. aureus and the antimicrobial activity could be maintained for up to a week. Triaxial electrospun fibers demonstrated excellent antimicrobial activity for up to 5 days and thereafter, they could provide antimicrobial activity for 2 more days. This sustained antimicrobial activity could be attributed to the sustained release of nisin from the fiber core over a long period.
4.6. Zein and gelatin-based electrospun food packaging materials
Utilizing protein-based electrospun materials in food packaging offers a promising path toward sustainable packaging solutions. A number of biomass-derived proteins, including zein and gelatin, have specific properties (e.g., biocompatibility, biodegradability, barrier properties, etc) that are well-suited for packaging purposes. These proteins can be isolated from agricultural residues and food wastes to develop active food packaging materials. In one study, gelatin/zein-based nanofibers were produced by incorporating ZnO and gallic acid to develop nanofibers with antioxidant, antibacterial, and antifungal properties for food packaging applications (Fig. 8a and b).166 The resulting nanofibers were hydrophobic and exhibited antioxidant activity five and nine times higher than that of the zein/gelatin nanofiber film depending on the composition. The food packaging performance of the fibers was explored over cherry samples (Fig. 8b). On the 11th day of the test, the weight loss and firmness were reduced by more than 20 and 60% to the unwrapped samples. The peak of ethylene release was decreased by nearly half, demonstrating the potential of such fibers for active food packaging applications. In another study, linalool-loaded gelatin/zein fibers were cast on the cast film of carboxymethyl chitosan/Oxalis triangularis ssp. Papilionacea (OTA) extract for the development of a colorimetric film.167 The resulting film showed good barrier and water resistance while the outer layer of CO-OTA showed colorimetric sensitivity towards pH stimuli with reversible color changes. The inner part of the membrane showed high antibacterial activity against E. coli and S. aureus, while the encapsulated linalool was released from the fibers in a controlled manner and followed by Fickian diffusion, causing antioxidant activity. The membranes were used for milk freshness monitoring and could double the shelf-life at 25 °C. A gelatin/zein composite nanofiber film loaded with thymol and cinnamaldehyde extended the shelf-life of strawberries by increasing water resistance and reducing the water vapor transmission rate (Fig. 8c and d).168 It effectively shielded against UV light and inhibited the growth of E. coli, S. aureus, and Listeria monocytogenes. Real-time tests showed that packaged strawberries remained fresh for up to 7 days at room temperature. Another gelatin/zein composite electrospun film loaded with ε-polylysine and gallic acid served as an active food packaging film for tuna.169 This film exhibited reduced hydrophobicity and antioxidant properties due to the additives, while also displaying antibacterial activity against Shewanella putrefaciens. Similarly, electrospun gelatin/chitosan nanofibers loaded with betel leaf ethanolic extract were applied as coatings on PLA film for food packaging, showing antioxidant and antibacterial effects, with improved mechanical properties and reduced water vapor permeability.170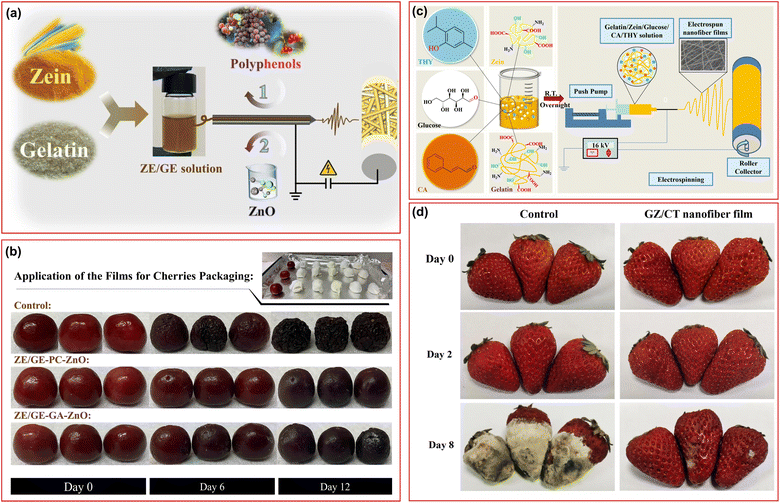 | ||
| Fig. 8 (a) Development of multifunctional electrospun film using zein and gelatin, functionalized with ZnO and polyphenols. (b) Food packaging performance of the fibers using cherries up to 12 days at 25 °C. (c) Schematic illustration of the production of gelatin/zein nanofibers loaded with cinnamaldehyde and thymol and (d) the use of the electrospun film for strawberry preservation. Figures were reproduced from ref. 166 and 168, Elsevier. | ||
Another antimicrobial gelatin electrospun film was developed by incorporating allyl isocyanate (AIC) and supported by pressure-sensitive adhesive (PSA) made of hydroxyethyl cellulose backbone grafted by acrylic acid and methylbutyl acrylate [HEC-g-poly(AA-MBA)].171 The resulting structures with 2% AIC content showed high antimicrobial activity against E. coli O157:H7 and S. aureus ATCC 25923. Cheese samples covered with these electrospun fibers could maintain their freshness over 5 weeks of storage at 4 °C, demonstrating their effectiveness as antimicrobial food packaging. Antimicrobial fibers were also developed using the combination of zein and PLA, with increased carvacrol content enhancing antioxidant activity and leading to sustained release and these fibers, employed in packaging bread, demonstrated better protection against microbial growth with higher carvacrol levels.172 Uyar group also developed CD-IC/carvacrol-loaded fibers with gelatin/pullulan as carrier polymers in which CD-ICs of carvacrol minimize the carvacrol loss during electrospinning and enhance its thermal stability.173 CD molecules were also utilized to form ICs with thymol, enhancing preservation and stability during and after the electrospinning of zein, and zein-thymol/γ-CD ICs nanofibrous mats effectively reduced bacterial growth in beef stored at 4 °C for up to 5 days.174
Electrospun nanofibers can be considered as hydrogels after imbibing a significant amount of water while preserving their fibrous structures. Fish gelatin fiber films were functionalized with different loadings of LAE to create food packaging films.175 These films, produced through electrospinning, could absorb surface water from fish fillets, forming a hydrogel coating. LAE-loaded films exhibited effective antibacterial activity against S. aureus and E. coli, ultimately extending the refrigerated shelf life of large yellow croaker fillets by about three days. Likewise, electrospun hydrogels of gelatin/chitosan blend loaded with 2-phenylacetic acid were developed for food packaging applications.176 The resulting hydrogels showed antibacterial activity against foodborne pathogens; S. aureus and E. coli and could preserve the freshness of chilled chicken for 4 days without spoilage formation. In another study, edible chitooligosaccharide was integrated into a skin gelatin nanofiber-based hydrogel to create antibacterial and antioxidant properties.177 Cross-linking of the nanofibers occurred via the Maillard reaction, facilitated by the addition of glucose, resulting in a highly porous structure upon hydration with a notable swelling ratio of 954%. The incorporation of chitooligosaccharide enhanced both the antioxidant and antibacterial capabilities of the hydrogel, extending the shelf-life of crucian carp by 2 to 4 days, and demonstrating the potential of gelatin-based nanofiber hydrogels for food packaging.
While gelatin-based fibers have been extensively studied for food packaging, their water solubility remains a significant limitation. To address this issue, efforts have focused on developing cross-linked gelatin nanofibers. In one study, water-resistant fish gelatin nanofibers with antioxidant activity were created using chlorogenic acid (CGA) as the active ingredient and citric acid or fructose as cross-linking agents.178 These fibers exhibited rapid CGA release when exposed to food simulants, with citric acid cross-linked fibers demonstrating superior scavenging activity in DPPH assays.
Gelatin-based nanofibers were utilized for monitoring food freshness through various innovative approaches. Functionalized nanofibers with AuNPs, SnO2, and black elderberry extract enabled rapid color changes upon exposure to volatiles, facilitating the detection of freshness in packaged Hake fish fillets.179 Similarly, multifunctional food packaging materials incorporating gelatin/xanthan gum mats containing chitin nanofibers and blackberry anthocyanins were developed for freshness monitoring and extending the shelf-life of Pacific white shrimps, with pH-sensitive color changes indicating microbial growth.180 Another intelligent packaging system, combining gelatin with anthocyanins from red radish, showed color alterations correlating with pH changes, enabling real-time monitoring of meat spoilage through volatile compound release.181
Zein nanofibers loaded with ferulic acid, quercetin, gallic acid, or procyanidin were created for active food packaging, enhancing both antioxidant capacity and hydrophobicity.182 When applied in cherry packaging, the resulting electrospun film significantly improved water loss, hardness, and gas release compared to unwrapped cherries. Antioxidant zein nanofibers were created using a core–shell approach, with ferulic acid loaded in the core with PEO and the shell layer composed of zein.183 This core-loading strategy decelerated the release rate of ferulic acid compared to fibers where it was loaded into the sheath, resulting in enhanced antioxidant activity and reduced weight loss of apple slices compared to blank fibers. Cross-linked zein nanofibers loaded with star anise EO/β-CD ICs were prepared for active food packaging by initially forming the inclusion complexes through mixing, then blending with zein solution for electrospinning.184 These nanofibers displayed enhanced thermal stability, mechanical strength, and water resistance, and exhibited exceptional antioxidant and antimicrobial properties against E. coli and S. aureus. Antimicrobial zein-based electrospun films were also produced by incorporating κ-carrageenan, ZnO NPs, and rosemary EO.185 The resulting electrospun films exhibited satisfactory thermal, mechanical, and surface hydrophobicity while showing both antioxidant and antibacterial activity.
An antiviral multilayer film was developed using cinnamaldehyde-loaded zein fibers and PHB film.186 Cinnamaldehyde-loaded zein fibers were deposited onto the inner sides of PHB films, which were produced through compression molding. The composite films showed antiviral activity against norovirus surrogates, murine norovirus (MNV), feline calicivirus (FCV), and hepatitis. They observed higher activity for norovirus surrogates compared to the virus HAV in antimicrobial biodegradable multilayer systems. Another antimicrobial multilayer film was developed using polyhydroxybutyrate-co-valerate film as a support with cinnamaldehyde-loaded zein fibers and an alginate-based outer layer.187 The multilayer films exhibited antibacterial activity against Listeria monocytogenes and slowly released encapsulated cinnamaldehyde in food simulants, making them promising candidates for active food packaging materials. Food packaging applications of protein-based and other biomass-derived electrospun fibers from various sources are compiled in Table 1.
| Polymer(s) | Active agent(s) | Fiber properties | Activity | Release profile | Description | Ref. |
|---|---|---|---|---|---|---|
| Ethylcellulose, gelatin | ZnO NPs | Bead-free uniform fibers (558–804 nm) | Antimicrobial activity against E. coli and S. aureus | N.D. | Uniform hydrophobic nanofibers with different ZnO NP loadings were produced. The antimicrobial activity of the nanofibers was enhanced by UV irradiation | 188 |
| PLA | Wormwood oil (WO) | Bead-free fibers with a mean diameter of 260 nm | Antimicrobial activity against E. coli and S. aureus | N.D. | The antimicrobial activity of nanofibers was strongly influenced by the added WO content | 189 |
| PLA | AgNPs & Thymus daenensis EO | Beaded-fiber morphology | Antimicrobial activity against E. coli and S. aureus | Sustained and long-term release of AgNPs and EO | An electrospun PLA nanofiber film loaded with AgNPs and EO showed antimicrobial activity due to the sustained release of bioactive agents | 190 |
| PLA | Ferulic acid | The morphology of the fibers showed large differences depending on the solvent | Antimicrobial activity against L. innocua | N.D. | The encapsulation efficiency of ferulic acid into electrospun PLA fibers was in the range of 84–96%. The antimicrobial PLA fibers were easily produced through the incorporation of ferulic acid | 191 |
| PLA | Carvacrol (CRV), nisin (Nis) | Nonuniform fibers | Antimicrobial activity against L. monocytogenes, S. Enteritidis, E. coli, and S. aureus | Sustained release of CRV and Nis from the fibers | The incorporation of CRV and Nis improved the elastic modulus of the fibers. The electrospun fibers loaded with 20% CRV showed antimicrobial activity against different bacteria | 192 |
| PLA, guar gum (GG) | Thyme EO (TEO) | Nonuniform fibers with mean diameters of 395 nm (with 10% TEO) and 347 nm (with 30% TEO) | Antioxidant activity (over DPPH radical assay) and antibacterial activity against E. coli and S. aureus | Sustained release profiles: the lesser content is released as the TWO content increases | The composite fibers of PLA and GG showed both antioxidant and antimicrobial activities. The resulting nanofibers were biocompatible | 193 |
| PLA | Cinnamaldehyde (CMA), tea polyphenol (TP) | Coaxial electrospinning, core shell-fibers with uniform structure | Antibacterial activity | N.D. | The presence of CMA showed synergistic antibacterial activity. CMA destroyed the phospholipid layer of the cell membrane, while TP destroyed the extracellular proteins, resulting in cell membrane perforation and cell death | 194 |
| Zein, PLA, and hydroxypropyl methylcellulose (HPMC) | Zenian (Carum copticum) EO (ZO) | Nonuniform fibers with mean diameters between 718 and 335 nm | Antioxidant activity against DPPH radicals and antimicrobial activity against E. coli and S. aureus | The cumulative release decreased with higher ZO content | Biocompatible composite nanofibers with both antioxidant and antimicrobial activity | 195 |
| PHBV | Fe doped ZnO NPs | Beaded fiber morphology | Antimicrobial activity against E. coli and S. aureus | N.D. | PLA/PHBV/ZnO:Fex electrospun films demonstrate remarkable antibacterial efficacy against Pseudomonas aeruginosa (ATCC-27853) by producing a higher quantity of perhydroxyl (˙OOH) radicals | 196 |
| PLA, PVA | LAE | Core–shell fibers with ribbon-flat morphology | The highest concentrations of LAE released from the fibers to both simulants corresponded to LAE's MIC values against L. innocua | After the initial burst release, sustained release of LAE from the fibers. The release kinetics fit well with Fick's law | LAE-loaded core–shell fibers were prepared for sustained release of LAE from the fibers. The resulting structures showed antimicrobial activity and demonstrated their potential for active food packaging | 197 |
| PLA, chitosan nanoparticles | Cinnamon EO (CEO) | The incorporation of nanoparticles worsened the fiber morphology and resulted in beaded fibers | Antibacterial activity | The sustained release of the CEO was observed | The PLA/CS-CEO fibers showed increased, sustained inactivation rates of E. coli and S. aureus over time, which is attributed to the continuous release of CEO. | 198 |
| PLA | Lignin (from rice husk) | Higher lignin load led to more uniform fibers | The nanofibers showed antioxidant activity through DPPH and ABTS assays | N.D. | Lignin-loaded PLA nanofibers were prepared. The resulting nanofibers exhibited antioxidant activity and demonstrated their potential for food packaging applications | 199 |
| Gelatin, chitosan | 3-Phenyllactic acid | The fiber morphology was deteriorated by the incorporation of 3-phenyllactic acid | Antibacterial activity against S. enterica Enteritidis and S. aureus | N.D. | Depending on the loading of 3-phenylacetic acid, the nanofibers exhibited tunable water vapor permeability and antibacterial activity, demonstrating their potential for active food packaging applications | 200 |
| Chitosan, carrageenan | Malva sylvestris extract (MSE) | Rounded/ribbon-like fibers | Intelligent (pH-based sensing) | N.D. | Fiber mats change color depending on the pH value. When assessing the freshness of silver carp fillets, indicators of total bacterial count, the number of psychrotrophic bacteria, pH (8.10), and total volatile basic nitrogen (40.18 mg N/100 g) were measured, which indicate a high bacterial count and showed increased nitrogen levels | 201 |
| Gelatin, zein, PVA | — | Beaded fiber morphology for zein/gelatin and gelatin fibers, while bead-free fiber morphology for PVA/gelatin fibers | Antioxidant activity | N.D. | The composite nanofiber mat exhibited favorable thermal and mechanical characteristics, coupled with outstanding antioxidant performance. The sweet potatoes and potatoes demonstrated an extended shelf life of 50 days, while the kimchi maintained its quality for 30 days | 202 |
| Gelatin | Cinnamaldehyde EO (CEO), limonene EO (LEO), and eugenol EO (EEO) | Uniform nanofiber structures with different EOs in the presence of β-CD | Antioxidant and antibacterial activity | Sustained release of EOs from gelatin mats | EOs were dissolved with β-CD and the resulting ICs were loaded into electrospun fibers. The resulting nanofibers exhibited both antioxidant and antibacterial activity, demonstrating their potential for active food packaging | 203 |
| Gelatin | Eugenol | Beaded-fiber morphology | Antibacterial activity due to loaded eugenol | N.D. | Eugenol-loaded gelatin fibers can prolong the shelf life of beef samples while preserving their textural properties (such as hardness, gumminess, and chewiness) and sensory qualities throughout the storage period | 204 |
| Gelatin/Plantago psyllium L. seed gum (PPSG) | Cuminum cyminum EO (CCEO) | Bead-free fiber morphology | Antibacterial activity against S. aureus | Sustained release of CCEO from the nanofibers | The first nanoemulsion of CCEO was prepared with the emulsifier TWEEN 20 and then added to the polymer solution for electrospinning. Uniform fibers with antibacterial activity, as well as sustained release profiles, have the potential for active applications in food packaging | 205 |
| Zein | Phycocyanin & Spirulina (AEES) extract | Bead-free fiber morphology for low loading of AEES and bead-free fiber morphology for fibers loaded with phycocyanin | Higher antibacterial activity against S. aureus than E. coli | N.D. | Zein electrospun fibers loaded with AEES or phycocyanin showed antioxidant and antibacterial activity. Both fibers could decrease the peroxide value (PV) and 2-thiobarbituric acid (TBA) values of walnut kernels during 24 weeks of storage, demonstrating their effectiveness against lipid oxidation | 206 |
| Zein | Sakacin | Bead-free fibers were produced at different loading of sakacin | The fibers showed antibacterial activity against Listeria innocua | N.D. | Electrospun zein/sakacin nanofibers showed activity against L. innocua and also extended the refrigerator shelf life of quail breasts. The antimicrobial activity of the fibers could be increased by the zein content | 207 |
| Zein | Phenolic-enriched extracts (PEE) | Bead-free fibers | The fibers showed antioxidant activity because of the loaded PEE. | Sustained release of PPE from the fibers | Antioxidant bead-free zein nanofibers were produced through electrospinning of zein/PEE blends. The cross-linking of zein fibers could improve structural integrity on water contact | 208 |
| Zein | Citronellol-rich Origanum vulgare EO | Bead-free fibers at various EO loadings | The fibers showed both antioxidant and antibacterial activity | N.D. | The encapsulation efficiency and loading capacity of the EO were in the range of 71.56–85.8% and 8.88–40.93%, respectively. Due to the antibacterial and antioxidant activity of the EO, the fibers exhibited both activities | 209 |
| Chitosan, PVA | Catechin | The fiber morphology deteriorated with catechin content added | The nanofibers showed antioxidant activity | Sustained release of catechin | Preservation studies indicated that the electrospun film effectively prolonged the shelf life of strawberries, with the film containing 0.8% catechin concentration demonstrating the most favorable preservation outcomes | 210 |
| Chitosan, flaxseed mucilage | Ziziphora clinopodioides EO (ZEO) and sesame oil (SO) | Bead-free fibers | The fibers showed both antioxidant and antibacterial activity | Sustained release of SO and ZEO | The nanofibers exhibited a continuous release of ZEO and SO over 96 hours, exhibiting notable antioxidant and antimicrobial properties, thereby indicating their promise for use in active food packaging applications | 211 |
| Chitosan, PEO | Anthocyanin | Highly branched fiber morphology | The anthocyanin-loaded fibers showed both antioxidant and antibacterial activity | N.D. | The use of anthocyanin/CS/PEO nanofibers as beef packaging material resulted in a color transition from white or light yellow to yellow-green as the beef decayed over storage time, facilitating visual assessment of beef quality | 212 |
| Gelatin, chitosan | CUR | Bead-free fiber morphology at different loadings of CUR | The CUR-loaded fibers showed both antioxidant and antibacterial activity. The fibers also showed color change depending on ammonia content | N.D. | The addition of CUR significantly enhanced the antioxidant and antimicrobial properties of nanofibers. Additionally, the nanofibers containing 0.2% CUR demonstrated higher colorimetric sensitivity to ammonia, with detection occurring within 3 minutes | 213 |
| Zein | Jaboticaba peel extract (JPE) | The nanofibers showed antibacterial activity | N.D. | Bilayer films of casted chitosan and electrospun zein/JPE fibers were developed. The bilayer film improved the barrier property while endowing the bioactivity | 214 | |
| PLA | AgNPs, vitamin E | Bead-free fibers | Antibacterial and antioxidant activity | N.D. | The tests conducted on fresh apple and apple juice revealed that the PLA/Ag/vitamin E nanofiber membrane effectively decreased the activity of polyphenol oxidase | 215 |
| Alginate | Lactobacillus paracasei KS-199 | Beaded fiber structures resulting from bacterial incorporation | — | N.D. | Encapsulating the bacteria at the nanoscale significantly increased its survival in simulated gastric juice, raising the viability rate from 64.1 to 70.8 log cfu mL−1. Additionally, this nanoencapsulation improved its viability in kefir, with the survival rate increasing from 6.65 to 7.38 log cfu mL−1 | 216 |
4.7. Other sustainable polymers-based electrospun food packaging materials
Except polymers listed above, there are other biobased sustainable polymers including bio-based polyamides, which are synthesized from castor oil, sugar-derived polyethylene, polyesters from vegetable oils, lignin, and pectin. Some of these polymers have been used for the fabrication of electrospun active food packaging materials. In a study, PLA/rice husk lignin fibers were prepared by electrospinning technique for food packaging application.199 The lignin at different concentrations was loaded into ultrafine PLA fibers. Notably, PLA fibers containing 2.5% lignin demonstrated antioxidant activity, displaying approximately 70% reduction for both DPPH and ABTS radicals. SPI-based fibers were also produced for antioxidant food packaging applications. In this regard, antioxidant β-carotene was encapsulated in a mixture of SPI and PVA and then electrospun onto a polyhydroxybutyrate-co-valerate (PHB92/PHV8) film, which was attached to the film through the annealing process.217 The in vitro release assay of the antioxidant in soybean oil, mimicking fatty foods, showed that the heat treatment (annealing) resulted in a reduced release rate and a more prolonged presence of the bioactive compound. Likewise, antimicrobial SPI fibers were produced through encapsulation of Cinnamon Zeylanicum (Cz) and Zataria Multiflora EOs.218 Nanofibers with 20% Cz showed reductions of 72%, 56%, and 42% in S. aureus, B. cereus, and S. Typhimurium, respectively. Like the above example, SPI fibers loaded with ginger EO were produced for the development of antimicrobial fibers.219 Soy protein amyloid fibrils (SAFs) were also used to develop antibacterial food packaging materials. The incorporation of SAFs boosted the mechanical properties of the fibers and increased the hydrophobicity. The composite fibers showed antibacterial activity against E. coli and S. aureus. In another study, antimicrobial electrospun films were also prepared using bio-based polyamide 11 (PA 11) and a nanohybrid of halloysite nanotubes (HNTs) filled with 50 wt% lysozyme as a natural antimicrobial molecule.220 Antimicrobial evaluations using chicken meat stored at 4 °C for 6, 9, and 13 days demonstrated the efficacy of the nanofibers in inhibiting bacterial growth, highlighting their potential suitability for food packaging applications.Pectin, a complex carbohydrate found in the cell walls of plants, especially in fruits like apples, citrus fruits, and berries, is widely utilized in the food industry as a gelling agent, thickener, stabilizer, and in the development of active food packaging materials.221 In one study, pectin/PEO blends were electrospun into fibers in the presence of glycerol.222 Pectin electrospun fibers were used as an interlayer between two external layers of PHBV films by annealing. The resulting multilayer films showed enhanced barrier performance to water vapor and limonene. In another study, composite fibers of pectin with chitosan and PVA were produced as antibacterial electrospun films.223 The disk diffusion test showed that the fibers exhibited significant antibacterial activity against S. aureus but not against E. coli. Another protein-based electrospun food packaging film through an LbL technique was developed using sarcoplasmic protein for the controlled release of CUR.224 The multilayer structure comprised CA as the base and top layers, with CUR-loaded fibers of direct freeze-dried sarcoplasmic protein (DFSP) or chitosan flocculated sarcoplasmic protein (CFSP) as the middle layer. SEM analysis confirmed the formation of the multilayered nanofiber films, exhibiting slow release of CUR from both DFSP and CFSP layers, suggesting promising applications in food packaging.
5. Performance comparison of biomass-derived electrospun food packaging materials
Each biomass-derived polymer presents unique advantages and challenges when used in electrospinning for food packaging applications. The polymer's intrinsic properties, such as hydrophobicity, bioactivity, electrospinnability, mechanical strength, and barrier characteristics, greatly influence their suitability and performance in this field.Hydrophobicity plays a crucial role in determining the direct applicability of electrospun films. Polymers like PLA and PHAs are inherently hydrophobic, which enables their electrospun films to be used directly in food packaging. Their water-repellent nature prevents moisture absorption, maintaining the integrity of the packaging and protecting the food from external humidity. Conversely, hydrophilic biomass polymers such as cellulose, chitosan, and various proteins require additional modifications to render them suitable for food packaging. These modifications include cross-linking with appropriate agents or grafting hydrophobic moieties onto their structure. Cross-linking enhances the polymer's structural stability and reduces its water solubility, while hydrophobic modifications improve water resistance. Without these alterations, hydrophilic polymers may dissolve or degrade in high-moisture environments, compromising their packaging function.
However, some hydrophilic polymers offer unique benefits. Chitosan, for instance, possesses inherent antimicrobial properties, making it an excellent candidate for active food packaging.225 Its ability to inhibit microbial growth can extend food shelf life and maintain food safety without the need for additional antimicrobial agents. This property sets chitosan apart from other biomass-derived polymers in terms of functionality. In this regard, zein or gelatin as biomass-derived polymer is not intrinsically antimicrobial and its applications as antimicrobial films require additional antimicrobial agents/polymers, such as chitosan.226
The process of electrospinning itself varies in efficiency depending on the polymer. Some polymers, like chitosan, pose challenges during electrospinning due to their high viscosity and strong hydrogen bonding, resulting in low throughput.227 To overcome this, chitosan is often blended with more electrospinnable polymers (e.g., PEO, pullulan, PVA) to enhance jet formation and increase productivity.228 The choice of the co-polymer depends on factors such as compatibility, desired final properties, and the intended application.
The mechanical properties of electrospun films are crucial for packaging applications, as they must endure the stresses of handling, transportation, and storage. These properties vary significantly based on factors such as fiber diameter, distribution, the presence of beads, fiber orientation, and the molecular weight of the polymer used.229 Additives can also cause notable variations in mechanical properties. PLA is particularly noteworthy, offering high mechanical strength and stretchability compared to many other biomass-derived polymers. The fibrous structure of electrospun PLA can even exceed the mechanical performance of its bulk form in terms of stretchability, making it an attractive option for robust food packaging. Randomly aligned PLA fibers have a tensile strength of 3.9 MPa and a modulus of 43.8 MPa, with an elongation at break of 87.6%.230 Electrospun CA fibers, on the other hand, exhibit a higher tensile strength (12.1 MPa) and modulus (1170 MPa) but lower stretchability compared to PLA fibers.231 Conversely, electrospun chitosan fibers have much lower values, with a tensile strength of approximately 0.5 MPa and a tensile modulus of about 10 MPa.232 Gelatin fibers show better mechanical properties than chitosan, with a tensile strength of 1.6 MPa and an elongation at break of 17%.233 Fiber alignment also influences mechanical properties. For instance, aligned PLA fibers show higher tensile strength (4.5 MPa) and modulus (62.5 MPa) compared to randomly deposited PLA fibers, although they have a lower elongation at break (27.4%).230
Like the mechanical properties, barrier properties, especially against water vapor and gases, are paramount in food packaging to control moisture content and atmospheric composition inside the package. Hydrophobic polymers like PLA and PHA generally exhibit relatively low oxygen, carbon dioxide, and water vapor permeability.234,235 Whereas, CA has high water vapor permeability compared to PLA and PHA.235 The porous nature of electrospun films, with gaps between fiber strands, can compromise their gas barrier properties. To address this, researchers have developed bilayer or multilayer electrospun films, along with cast films. These composite structures significantly enhance barrier properties, effectively slowing down gas transmission. In this regard, the incorporation of electrospun zein interlayer decreased the water vapor and oxygen permeability of polyhydroxybutyrate-co-valerate films.236 Increasing the deposition time of the zein fibers decreased both permeability due to increased layer thickness.
In summary, the selection of a biomass-derived polymer for electrospun food packaging involves careful consideration of various factors. Hydrophobicity influences direct usability, while hydrophilic polymers may require modifications. Some polymers, like chitosan, offer additional functionalities such as antimicrobial activity.237 Electrospinnability varies among polymers, with some needing co-polymer blending to improve processability.238 Mechanical strength is crucial for package durability, with PLA excelling in this area. Lastly, barrier properties are enhanced through multilayer designs to overcome the inherent porosity of electrospun films. By understanding these aspects, researchers can tailor electrospun films of biomass-derived polymers to create effective, sustainable food packaging solutions.
6. Environmental impact and economic viability of electrospun food packaging materials
6.1. Environmental impact assessment
While the use of biomass-derived polymers inherently suggests a more sustainable approach compared to petroleum-based plastics, a thorough environmental impact assessment is crucial to validate this assumption. This section compares the environmental footprint of electrospun biomass-derived polymers with traditional packaging materials and explores potential challenges in scaling up production.The carbon footprint of packaging materials is a key indicator of their environmental impact. Studies have shown that biomass-derived polymers generally have a lower carbon footprint than petroleum-based plastics.239 For instance, a life cycle assessment (LCA) by Gironi et al. compared PET (poly(ethylene terephthalate)), plastic derived from fossil resources, and PLA, bioplastic derived from sugar cane, and found that global warming kgCO2eq per 1000 bottles for PLA (i.e., 17.202) were much lower than for the PET-based bottles (i.e., 38.186).240 S. Ramakrishna and colleagues reported that the life cycle of PLA involves an energy-intensive process for converting bio-sources to lactic acid and subsequently to PLA, resulting in the release of a substantial amount of CO2 into the atmosphere.241 According to the available data, more than 50% (2.8 kg CO2 per kg PLA) of the released CO2 in the PLA life cycle belongs to its conversion. Thus, more studies should done on optimizing the conversion process of PLA to make PLA a low carbon-material. It's important to note that the carbon footprint can vary depending on the specific biomass source and processing methods. Factors such as land use change, fertilizer use, and transportation distances can impact the overall emissions.242 Therefore, locally sourced and sustainably managed biomass feedstocks are crucial for maximizing carbon benefits. Energy consumption is another critical factor in environmental sustainability.243 G. T. Beckham and his team reported on the energy consumption and greenhouse gas emissions linked to plastic use, revealing that major commodity polymers, each with a global consumption of at least 1 million metric tons annually, contribute to approximately 3.2 quadrillion Btus of energy use and 104 million metric tons of CO2 equivalent emissions each year in the United States alone.244 In another study, carbon emissions of traditional plastic products were compared with biodegradable bioplastic products (BPPs), and they found that carbon emissions of 1000 traditional plastic products (plastic bags, lunch boxes, cups, etc.) were 52.09–150.36 carbon emissions equivalent of per kilogram (kg CO2eq), with the stage of plastic production contributing 50.71–50.77%. In comparison, 1000 similar BPPs topped out at 21.06–56.86 kg CO2eq, approximately 13.53–62.19% lower than traditional plastic products.245
One of the most significant advantages of biomass-derived polymers is their end-of-life disposal options. Many of these polymers, such as PLA,66 polyhydroxyalkanoates (PHAs),97 and cellulose derivatives,246 are biodegradable or compostable under specific conditions. This characteristic reduces the burden on landfills and mitigates issues like microplastic pollution in oceans. In contrast, traditional plastics can persist in the environment for hundreds of years.247 Even with recycling efforts, only 9% of all plastic waste ever produced has been recycled.248 However, it's crucial to note that not all biomass-derived polymers are biodegradable, and even those that may require industrial composting facilities for proper degradation. Therefore, investment in composting infrastructure and clear labeling for consumers are essential. While the environmental benefits of electrospun biomass-derived polymers are evident, scaling up production to meet commercial demand presents challenges that could impact their sustainability.
Some biomass processing methods require significant water, which could strain local water resources.249 Developing water-efficient processes and utilizing wastewater treatment and recycling systems are crucial. While many solvents used in electrospinning are less harmful than those in traditional plastic processing, some are still toxic. Ongoing research into green solvents and solvent recovery systems is essential for minimizing environmental impact.250 To fully understand and improve the environmental impact of electrospun biomass-derived polymers, ongoing LCAs are crucial. These assessments should cover all stages from feedstock production to end-of-life disposal, considering regional variations in energy mix, transportation, and waste management infrastructure. Furthermore, the principles of green chemistry and circular economy should guide process optimization. This includes designing for recyclability, using renewable energy in manufacturing, and developing closed-loop systems where materials and solvents are recycled.
6.2. Economic viability assessment
While the environmental benefits and technical feasibility of electrospun biomass-derived polymers for active food packaging are obvious, their widespread adoption depends on market acceptance and economic feasibility. This section briefly examines key factors that influence the practical implementation of these materials on a larger scale.The cost-effectiveness of electrospun biomass-derived polymers is crucial for their market adoption. At present, these materials are more expensive to produce than traditional plastics because of the need for specialized equipment for production and the higher costs of certain biomass-derived polymers. However, as the technology progresses and economies of scale are realized, these costs are expected to decline. For example, the application of PHAs is constrained by their high production costs, especially due to the expense of substrates.251 Therefore, a wide range of carbon-rich by-products and agro-wastes from industry (e.g., sugar/starch-based,252,253 whey,254 oil/glycerol,255,256 algal257) have been used for PHA production to reduce the cost. Moreover, as governments worldwide introduce more strict regulations on single-use plastics and impose taxes or bans on non-biodegradable materials, the economic landscape is shifting. These policy changes are likely to make conventional plastics more expensive, thereby narrowing the cost gap with biomass-derived polymers. Additionally, increased consumer awareness about environmental issues may drive demand for eco-friendly packaging, creating a larger market that could further reduce production costs. The ability of these polymers, as demonstrated through electrospinning, to extend food shelf life and reduce waste presents a strong economic incentive for food producers and retailers. Adopting this technology could result in substantial long-term savings. In this regard, recent advancements in electrospinning techniques, such as needleless electrospinning258 and multi-jet electrospinning,259 as well as using industrial scale electrospinners,260,261 have demonstrated the potential to enhance production rates and lower costs.
As consumers become more environmentally conscious, the demand for sustainable packaging solutions is on the rise, in line with the United Nations's SDGs. This trend is prompting food companies to explore eco-friendly alternatives. Nonetheless, consumer acceptance relies on the packaging's ability to perform well. For electrospun biomass-derived polymers to be widely accepted, they must match or exceed conventional packaging in terms of food preservation, convenience, and visual appeal. Regulatory approval is vital for the adoption of new food packaging materials. In the United States, the Food and Drug Administration (FDA) ensures that food contact materials are safe for their intended use.262,263 Similarly, in Europe, the European Food Safety Authority (EFSA) imposes equivalent requirements.264,265 While obtaining these approvals can be lengthy and costly, they are necessary for entering the market.
Shifting to electrospun biomass-derived polymers demands considerable investment in research, development, and infrastructure. Nonetheless, the rising interest in sustainable technologies has opened up more funding opportunities. For instance, the European Union's Horizon program has dedicated substantial resources to research in bio-based products.266 Furthermore, collaborations between academia and industry can speed up the development and scaling of these technologies.
7. Current challenges and future perspectives
Despite the numerous benefits they offer, electrospun fibers derived from sustainable polymers face some primary obstacles in their application for food packaging. These include ensuring biocompatibility and compliance with food safety regulations, optimizing barrier properties, and adhesion issues to food surfaces, and addressing scalability and cost-effectiveness issues. Similarly, ensuring the structural stability of these materials is paramount, particularly considering that some sustainable polymers (e.g., chitosan, gelatin, starch, etc.) are hydrophilic, posing challenges in maintaining the integrity and performance of the packaging over time, especially in environments with fluctuating moisture levels. In this regard, hydrophilic fibers could dissolve onto the packaged food. Efficient cross-linking techniques are crucial for improving the structural stability of materials. Nonetheless, this procedure introduces complications that are unfavorable for widespread industrial manufacturing. Addressing this challenge could involve modifying these hydrophilic polymers with hydrophobic groups, offering a rational solution to the issue. This strategic alteration can potentially enhance the structural stability of the packaging material, mitigating concerns associated with moisture-induced degradation. Likewise, a significant concern in using electrospun fibers for food packaging lies in the presence of residual fibers on food samples. This can occur due to the soft nanostructured nature of electrospun mats. Unlike traditional film-based packaging, these fibers may inadvertently adhere to food, leading to contamination. In this regard, bioactive electrospun fibers can be employed as an interlayer between the food contact film as previously reported for eugenol-loaded PHBV fibers between cast-extruded PHB sheet and a commercial PHBV film.103Electrospinning of some biomass-derived polymers presents challenges due to their inherent characteristics like high viscosity, high surface tension, or low solubility. Fine-tuning electrospinning parameters to yield fibers with consistent morphology and desired attributes is crucial yet technically complex. Likewise, future research into novel biomass-derived polymers and blends can result in materials tailored for food packaging needs. This entails investigating nanocomposites, bio-based additives, and functional coatings to enhance electrospun material performance. Further refining the electrospinning process, including innovating new techniques and equipment (e.g., electroblowing), can enhance the efficiency, scalability, and reproducibility of producing biomass-derived electrospun materials for food packaging.
While electrospun materials for food packaging hold promise, a key hurdle is achieving mass production at an economically competitive cost. To rival traditional packaging materials, the expense of sustainable biomass-derived polymers must be addressed. Mechanical strength and barrier properties are vital for effective food preservation. When electrospun films alone don't suffice in barrier properties, combining them with conventional films can enhance (bio)activity while improving barrier properties. Adopting circular principles, such as recycling and biodegradability, can further boost the sustainability of electrospun materials sourced from biomass. Pioneering recycling methods or designing materials easily compostable post-use can reduce environmental impact and foster a more sustainable packaging ecosystem.
Overall, the emerging field of electrospun food packaging using sustainable biomass-derived polymers presents a compelling opportunity for environmentally friendly solutions in the active packaging industry. The development of electrospun packaging using sustainable polymers is an example of the dynamic interaction between science, industry, and environmental protection. It highlights the importance of a multi-faceted approach to mainstreaming environmentally conscious materials into packaging practices. Hence, addressing these challenges necessitates a multidisciplinary approach, engaging chemists, food engineers, and materials scientists.
8. Conclusion
In the field of active food packaging, electrospun materials offer a variety of benefits, ranging from their antioxidant, antibacterial, and antifungal properties to their innovative smart packaging capabilities, all crucial factors in extending the shelf life of various food products. Incorporating functional molecules or groups into electrospun fibers emerges as a crucial strategy to enhance their effectiveness and impart robust antioxidant, antibacterial, and antifungal properties. Furthermore, the emergence of electrospun nanofibers loaded with sensory elements presents an intriguing challenge for smart food packaging. Through this strategic connection, these materials not only protect but also adapt to environmental fluctuations, providing real-time insights into the freshness and quality of packaged food. To this end, a variety of sustainable polymers have been utilized to produce a comprehensive range of electrospun materials loaded with antioxidant, antibacterial, and antifungal agents, and sensory components to detect changes in foods. Examples such as PLA, PHA, starch, cellulose, chitosan, zein, and gelatin highlight the concerted effort to align packaging methods with environmentally conscious principles. While many of these electrospun materials exhibit bioactive properties and sustainability, certain hydrophilic variants dissolve upon contact with water. Despite efforts to stabilize fibers through cross-linking pathways, this process can increase complexity and potentially result in adverse health effects from the cross-linking agents used. Therefore, there is an urgent need for further research focusing on hydrophobic electrospun fibers from sustainable polymers. In conclusion, electrospun nanofibrous materials are being extensively explored in scientific studies in the field of active food packaging, and due to their tailorable structures and biofunctionalities, these innovative nanofibrous materials are poised to emerge as alternatives to traditional plastic film counterparts and possess superior properties. To this end, and in line with SDG 2030, ongoing and future research efforts would likely continue on the use of sustainable polymers from biomass, taking advantage of their sustainability, environmental friendliness, and inherent structural advantages.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
F. T. gratefully acknowledges the financial support from Istanbul Technical University (project number: TGA-2023-43937). F. T. thanks the Turkish Academy of Sciences for financial support under a TUBA-GEBIP program.References
- M. L. Rooney, in Innovations in Food Packaging, ed. J. H. Han, Academic Press, London, 2005, pp. 63–79 Search PubMed.
- J. Wyrwa and A. Barska, Eur. Food Res. Technol., 2017, 243, 1681–1692 CrossRef CAS.
- C. Vilela, M. Kurek, Z. Hayouka, B. Röcker, S. Yildirim, M. D. C. Antunes, J. Nilsen-Nygaard, M. K. Pettersen and C. S. R. Freire, Trends Food Sci. Technol., 2018, 80, 212–222 CrossRef CAS.
- E. G. S. Silva, S. Cardoso, A. F. Bettencourt and I. A. C. Ribeiro, Foods, 2023, 12, 168 CrossRef CAS PubMed.
- M. Asgher, S. A. Qamar, M. Bilal and H. M. N. Iqbal, Food Res. Int., 2020, 137, 109625 CrossRef CAS PubMed.
- F. Wu, M. Misra and A. K. Mohanty, Prog. Polym. Sci., 2021, 117, 101395 CrossRef CAS.
- C. Zhang, Y. Li, P. Wang and H. Zhang, Compr. Rev. Food Sci. Food Saf., 2020, 19, 479–502 CrossRef CAS PubMed.
- F. Topuz and T. Uyar, Food Res. Int., 2020, 130, 108927 CrossRef CAS PubMed.
- Y. Zhang, T. Min, Y. Zhao, C. Cheng, H. Yin and J. Yue, Food Control, 2024, 160, 110291 CrossRef.
- P. Wen, D.-H. Zhu, H. Wu, M.-H. Zong, Y.-R. Jing and S.-Y. Han, Food Control, 2016, 59, 366–376 CrossRef CAS.
- N. Ehsani, H. Rostamabadi, S. Dadashi, B. Ghanbarzadeh, M. S. Kharazmi and S. M. Jafari, Crit. Rev. Food Sci. Nutr., 2022, 1–23 Search PubMed.
- M. Aman Mohammadi, S. M. Hosseini and M. Yousefi, Food Sci. Nutr., 2020, 8, 4656–4665 CrossRef PubMed.
- A. Dey and S. Neogi, Trends Food Sci. Technol., 2019, 90, 26–34 CrossRef CAS.
- A. Mousavi Khaneghah, S. M. B. Hashemi and S. Limbo, Food Bioprod. Process., 2018, 111, 1–19 CrossRef CAS.
- Y. Liu, L. Li, Z. Yu, C. Ye, L. Pan and Y. Song, Packag. Technol. Sci., 2023, 36, 833–853 CrossRef.
- H. Wei, F. Seidi, T. Zhang, Y. Jin and H. Xiao, Food Chem., 2021, 337, 127750 CrossRef CAS PubMed.
- D. S. Lee, H. J. Wang, C. Jaisan and D. S. An, Packag. Technol. Sci., 2022, 35, 213–227 CrossRef CAS.
- S. Ebnesajjad, Plastic Films in Food Packaging: Materials, Technology and Applications, William Andrew, 2012 Search PubMed.
- S. Mangaraj, T. K. Goswami and P. V. Mahajan, Food Eng. Rev., 2009, 1, 133–158 CrossRef CAS.
- J. Boone, F. Lox and S. Pottie, Packag. Technol. Sci., 1993, 6, 277–281 CrossRef CAS.
- Z. Wang, M. S. Ganewatta and C. Tang, Prog. Polym. Sci., 2020, 101, 101197 CrossRef CAS.
- N. Raak, C. Symmank, S. Zahn, J. Aschemann-Witzel and H. Rohm, Waste Manage., 2017, 61, 461–472 CrossRef PubMed.
- D. A. Teigiserova, L. Hamelin and M. Thomsen, Resour., Conserv. Recycl., 2019, 149, 413–426 CrossRef.
- J. Gómez-Estaca, C. López-de-Dicastillo, P. Hernández-Muñoz, R. Catalá and R. Gavara, Trends Food Sci. Technol., 2014, 35, 42–51 CrossRef.
- O. M. Atta, S. Manan, A. Shahzad, M. Ul-Islam, M. W. Ullah and G. Yang, Food Hydrocolloids, 2022, 125, 107419 CrossRef CAS.
- M. Qian, D. Liu, X. Zhang, Z. Yin, B. B. Ismail, X. Ye and M. Guo, Trends Food Sci. Technol., 2021, 114, 459–471 CrossRef CAS.
- M. Soltani Firouz, K. Mohi-Alden and M. Omid, Food Res. Int., 2021, 141, 110113 CrossRef CAS PubMed.
- Z. Ceylan, R. Meral, A. Alav, C. Y. Karakas and M. T. Yilmaz, J. Texture Stud., 2020, 51, 917–924 CrossRef PubMed.
- R. Priyadarshi, P. Ezati and J.-W. Rhim, ACS Food Sci. Technol., 2021, 1, 124–138 CrossRef CAS.
- R. Dobrucka and R. Przekop, J. Food Process. Preserv., 2019, 43, e14194 CAS.
- S. Otles and B. Y. Sahyar, in Comprehensive Analytical Chemistry, ed. V. Scognamiglio, G. Rea, F. Arduini and G. Palleschi, Elsevier, 2016, vol. 74, pp. 377–387 Search PubMed.
- K. K. Gaikwad, S. Singh and A. Ajji, Environ. Chem. Lett., 2019, 17, 609–628 CrossRef CAS.
- V. M. Rangaraj, K. Rambabu, F. Banat and V. Mittal, Food Biosci., 2021, 43, 101251 CrossRef.
- C. Maraveas, I. S. Bayer and T. Bartzanas, Polymers, 2021, 13, 2465 CrossRef CAS PubMed.
- J. Brito, H. Hlushko, A. Abbott, A. Aliakseyeu, R. Hlushko and S. A. Sukhishvili, ACS Appl. Mater. Interfaces, 2021, 13, 41372–41395 CrossRef CAS PubMed.
- S. Ahmed, D. E. Sameen, R. Lu, R. Li, J. Dai, W. Qin and Y. Liu, Crit. Rev. Food Sci. Nutr., 2022, 62, 3088–3102 CrossRef CAS PubMed.
- B. Mishra, A. K. Mishra, S. Kumar, S. K. Mandal, L. Nsv, V. Kumar, K.-H. Baek and Y. K. Mohanta, Metabolites, 2022, 12, 12 CrossRef CAS PubMed.
- K. K. Gaikwad, S. Singh and Y. S. Negi, Environ. Chem. Lett., 2020, 18, 269–284 CrossRef CAS.
- H.-G. Lee, C. H. Cho, H. K. Kim and S. Yoo, Food Packag. Shelf Life, 2020, 26, 100558 CrossRef.
- L. Motelica, D. Ficai, O. C. Oprea, A. Ficai and E. Andronescu, Coatings, 2020, 10, 806 CrossRef CAS.
- P. V. Mahajan, F. A. Rodrigues, A. Motel and A. Leonhard, Postharvest Biol. Technol., 2008, 48, 408–414 CrossRef CAS.
- R. Becerril, C. Nerín and F. Silva, Molecules, 2020, 25, 1134 CrossRef CAS PubMed.
- C. S. Barry and J. J. Giovannoni, J. Plant Growth Regul., 2007, 26, 143–159 CrossRef CAS.
- D. S. Lee, Trends Food Sci. Technol., 2016, 57, 146–155 CrossRef CAS.
- S. Wang, X. Liu, M. Yang, Y. Zhang, K. Xiang and R. Tang, Packag. Technol. Sci., 2015, 28, 839–867 CrossRef CAS.
- H. Cheng, H. Xu, D. Julian McClements, L. Chen, A. Jiao, Y. Tian, M. Miao and Z. Jin, Food Chem., 2022, 375, 131738 CrossRef CAS PubMed.
- A. Luraghi, F. Peri and L. Moroni, J. Controlled Release, 2021, 334, 463–484 CrossRef CAS PubMed.
- X. Li, W. Chen, Q. Qian, H. Huang, Y. Chen, Z. Wang, Q. Chen, J. Yang, J. Li and Y.-W. Mai, Adv. Energy Mater., 2021, 11, 2000845 CrossRef CAS.
- A. Celebioglu and T. Uyar, Nanoscale, 2012, 4, 621–631 RSC.
- Y. Li, J. Zhu, H. Cheng, G. Li, H. Cho, M. Jiang, Q. Gao and X. Zhang, Adv. Mater. Technol., 2021, 6, 2100410 CrossRef.
- A. Greiner and J. H. Wendorff, Angew. Chem., Int. Ed., 2007, 46, 5670–5703 CrossRef CAS PubMed.
- E. Monica, R. Saranya, S. A. Tharifkhan, J. A. Moses and C. Anandharamakrishnan, in Emerging Technologies for the Food Industry, Apple Academic Press, 2024 Search PubMed.
- A. L. Yarin, S. Koombhongse and D. H. Reneker, J. Appl. Phys., 2001, 90, 4836–4846 CrossRef CAS.
- D. H. Reneker and A. L. Yarin, Polymer, 2008, 49, 2387–2425 CrossRef CAS.
- S. R. Falsafi, F. Topuz, Z. Esfandiari, A. Can Karaca, S. M. Jafari and H. Rostamabadi, Food Chem.: X, 2023, 20, 100922 CAS.
- K. Ghosal, A. Chandra, G. Praven, S. Snigdha, S. Roy, C. Agatemor, S. Thomas and I. Provaznik, Sci. Rep., 2018, 8, 5058 CrossRef PubMed.
- A. Hafeez, M. Jabbar, Y. Nawab and K. Shaker, in Plant Biomass Derived Materials, John Wiley & Sons, Ltd, 2024, pp. 537–555 Search PubMed.
- A. Bhardwaj, T. Alam, V. Sharma, M. S. Alam, H. Hamid and G. K. Deshwal, J. Packag. Technol. Res., 2020, 4, 205–216 CrossRef.
- J. Shi, R. Zhang, X. Liu, Y. Zhang, Y. Du, H. Dong, Y. Ma, X. Li, P. C. K. Cheung and F. Chen, Carbohydr. Polym., 2023, 301, 120323 CrossRef CAS PubMed.
- H. Moustafa, A. M. Youssef, N. A. Darwish and A. I. Abou-Kandil, Composites, Part B, 2019, 172, 16–25 CrossRef CAS.
- S. Sid, R. S. Mor, A. Kishore and V. S. Sharanagat, Trends Food Sci. Technol., 2021, 115, 87–104 CrossRef CAS.
- H. Rostamabadi, E. Assadpour, H. S. Tabarestani, S. R. Falsafi and S. M. Jafari, Trends Food Sci. Technol., 2020, 100, 190–209 CrossRef CAS.
- H. Rodríguez-Tobías, G. Morales and D. Grande, Mater. Sci. Eng. C, 2019, 101, 306–322 CrossRef PubMed.
- J. Jacob, U. Lawal, S. Thomas and R. B. Valapa, in Processing and Development of Polysaccharide-Based Biopolymers for Packaging Applications, ed. Y. Zhang, Elsevier, 2020, pp. 97–115 Search PubMed.
- B. K. Lee, Y. Yun and K. Park, Adv. Drug Delivery Rev., 2016, 107, 176–191 CrossRef CAS PubMed.
- M. Singhvi and D. Gokhale, RSC Adv., 2013, 3, 13558–13568 RSC.
- C. H. Hong, S. H. Kim, J.-Y. Seo and D. S. Han, Int. Scholarly Res. Not., 2012, 2012, e938261 Search PubMed.
- A. Ahmad, F. Banat and H. Taher, Environ. Technol. Innovation, 2020, 20, 101138 CrossRef CAS.
- S. Fiori, Chapter 13: Industrial Uses of PLA, in Poly(lactic Acid) Science and Technology, RSC, 2014, ISSN: 978-1-84973-879-8 Search PubMed.
- J. Wu, T. Hu, H. Wang, M. Zong, H. Wu and P. Wen, J. Agric. Food Chem., 2022, 70, 8207–8221 CrossRef CAS PubMed.
- H. Palak and B. Karagüzel Kayaoğlu, Food Packag. Shelf Life, 2023, 39, 101154 CrossRef CAS.
- H. Wang, T. Wan, H. Wang, S. Wang, Q. Li and B. Cheng, Int. J. Biol. Macromol., 2022, 194, 452–460 CrossRef CAS PubMed.
- S. Bodbodak, N. Shahabi, M. Mohammadi, M. Ghorbani and A. Pezeshki, Food Bioprocess Technol., 2021, 14, 2260–2272 CrossRef CAS.
- T. Li, Y. Liu, Q. Qin, L. Zhao, Y. Wang, X. Wu and X. Liao, Food Control, 2021, 130, 108371 CrossRef CAS.
- X. Liu, X. Song, D. Gou, H. Li, L. Jiang, M. Yuan and M. Yuan, Food Chem., 2023, 428, 136784 CrossRef CAS PubMed.
- M. Liao, Y. Pan, X. Fu, S. Wu, S. Gan, Z. Wu, H. Zhao, W. Zheng, Y. Cao, W. Zhou and X. Dong, Int. J. Biol. Macromol., 2023, 253, 126569 CrossRef CAS PubMed.
- F. Rezaei, H. Tajik and Y. Shahbazi, Int. J. Biol. Macromol., 2023, 252, 126512 CrossRef CAS PubMed.
- D. A. Ertek, N. O. Sanli, Y. Z. Menceloglu and S. Avaz Seven, Eur. Polym. J., 2023, 185, 111804 CrossRef CAS.
- É. Fenyvesi, M. Vikmon and L. Szente, Crit. Rev. Food Sci. Nutr., 2016, 56, 1981–2004 CrossRef PubMed.
- F. Topuz and T. Uyar, Carbohydr. Polym., 2022, 297, 120033 CrossRef CAS PubMed.
- Y. Shi, D. Li, Y. Kong, R. Zhang, Q. Gu, M. Hu, S. Tian and W. Jin, Int. J. Food Microbiol., 2022, 361, 109460 CrossRef CAS PubMed.
- C. Shi, A. Zhou, D. Fang, T. Lu, J. Wang, Y. Song, L. Lyu, W. Wu, C. Huang and W. Li, Chem. Eng. J., 2022, 445, 136746 CrossRef CAS.
- Z. Aytac, N. O. S. Keskin, T. Tekinay and T. Uyar, J. Appl. Polym. Sci., 2017, 134(21), 44858 CrossRef.
- C. Patiño Vidal, F. Luzi, D. Puglia, G. López-Carballo, A. Rojas, M. J. Galotto and C. López de Dicastillo, Food Packag. Shelf Life, 2023, 36, 101050 CrossRef.
- R. Ordoñez, L. Atarés and A. Chiralt, Food Chem. Adv., 2023, 2, 100250 CrossRef.
- J.-Y. Zhu, C.-H. Tang, S.-W. Yin and X.-Q. Yang, Carbohydr. Polym., 2018, 181, 727–735 CrossRef CAS PubMed.
- J. Ding, V. Dwibedi, H. Huang, Y. Ge, Y. Li, Q. Li and T. Sun, Int. J. Biol. Macromol., 2023, 237, 123932 CrossRef CAS PubMed.
- T. Dai, Z. Qin, S. Wang, L. Wang, J. Yao, G. Zhu, B. Guo, J. Militky, M. Venkataraman and M. Zhang, Polym. Adv. Technol., 2022, 33, 4062–4071 CrossRef CAS.
- C. Patiño Vidal, E. Velásquez, M. J. Galotto and C. López de Dicastillo, Food Packag. Shelf Life, 2022, 31, 100802 CrossRef.
- C. H. Wong, M. Y. Tan, X. Li and D. Li, J. Food Sci., 2022, 87, 3129–3137 CrossRef CAS PubMed.
- T. Min, L. Zhou, X. Sun, H. Du, X. Bian, Z. Zhu and Y. Wen, Food Res. Int., 2022, 157, 111256 CrossRef CAS PubMed.
- J. Zeng, Q. Ji, X. Liu, M. Yuan, M. Yuan and Y. Qin, J. Mater. Res. Technol., 2022, 18, 5032–5044 CrossRef CAS.
- Y. Xie, G. Cheng, Z. Wu, S. Shi, J. Zhao, L. Jiang, D. Jiang, M. Yuan, Y. Wang and M. Yuan, Nanomaterials, 2022, 12, 1229 CrossRef CAS PubMed.
- X. Dong, X. Liang, Y. Zhou, K. Bao, D. E. Sameen, S. Ahmed, J. Dai, W. Qin and Y. Liu, Int. J. Biol. Macromol., 2021, 177, 135–148 CrossRef CAS PubMed.
- A. Anjum, M. Zuber, K. M. Zia, A. Noreen, M. N. Anjum and S. Tabasum, Int. J. Biol. Macromol., 2016, 89, 161–174 CrossRef CAS PubMed.
- T. V. Ojumu, J. Yu and B. O. Solomon, Afr. J. Biotechnol., 2004, 3, 18–24 CrossRef CAS.
- S. Philip, T. Keshavarz and I. Roy, J. Chem. Technol. Biotechnol., 2007, 82, 233–247 CrossRef CAS.
- A. Cherpinski, S. Torres-Giner, J. Vartiainen, M. S. Peresin, P. Lahtinen and J. M. Lagaron, Cellulose, 2018, 25, 1291–1307 CrossRef CAS.
- M. J. Fabra, A. López-Rubio, J. Ambrosio-Martín and J. M. Lagaron, Food Hydrocolloids, 2016, 61, 261–268 CrossRef CAS.
- K. J. Figueroa-Lopez, S. Torres-Giner, I. Angulo, M. Pardo-Figuerez, J. M. Escuin, A. I. Bourbon, L. Cabedo, Y. Nevo, M. A. Cerqueira and J. M. Lagaron, Nanomaterials, 2020, 10, 1–24 Search PubMed.
- A. Cherpinski, P. K. Szewczyk, A. Gruszczyński, U. Stachewicz and J. M. Lagaron, Nanomaterials, 2019, 9(2), 262 CrossRef CAS PubMed.
- M. J. Fabra, A. López-Rubio, L. Cabedo and J. M. Lagaron, J. Colloid Interface Sci., 2016, 483, 84–92 CrossRef CAS PubMed.
- K. J. Figueroa-Lopez, L. Cabedo, J. M. Lagaron and S. Torres-Giner, Front. Nutr., 2020, 7, 140 CrossRef PubMed.
- X. Fan, Q. Jiang, Z. Sun, G. Li, X. Ren, J. Liang and T. S. Huang, Fibers Polym., 2015, 16, 1751–1758 CrossRef CAS.
- X. Lin, X. Fan, R. Li, Z. Li, T. Ren, X. Ren and T.-S. Huang, Polym. Adv. Technol., 2018, 29, 481–489 CrossRef CAS.
- S. G. Kuntzler, A. C. A. de Almeida, J. A. V. Costa and M. G. de Morais, Int. J. Biol. Macromol., 2018, 113, 1008–1014 CrossRef CAS PubMed.
- X. Lin, M. Yin, Y. Liu, L. Li, X. Ren, Y. Sun and T.-S. Huang, J. Ind. Eng. Chem., 2018, 63, 303–311 CrossRef CAS.
- N. Masina, Y. E. Choonara, P. Kumar, L. C. du Toit, M. Govender, S. Indermun and V. Pillay, Carbohydr. Polym., 2017, 157, 1226–1236 CrossRef CAS PubMed.
- X. Huang, Z. Teng, F. Xie, G. Wang, Y. Li, X. Liu and S. Li, Food Hydrocolloids, 2024, 148, 109426 CrossRef CAS.
- L. M. Fonseca, M. Radünz, H. C. dos Santos Hackbart, F. T. da Silva, T. M. Camargo, G. P. Bruni, J. L. F. Monks, E. da Rosa Zavareze and A. R. G. Dias, J. Sci. Food Agric., 2020, 100, 4263–4271 CrossRef CAS PubMed.
- J. B. Pires, F. N. D. Santos, E. P. D. Cruz, L. M. Fonseca, T. J. Siebeneichler, G. S. Lemos, E. A. Gandra, E. D. R. Zavareze and A. R. G. Dias, Int. J. Biol. Macromol., 2024, 254(1), 127617 CrossRef CAS PubMed.
- L. Chen, F. Wu, M. Xiang, W. Zhang, Q. Wu, Y. Lu, J. Fu, M. Chen, S. Li, Y. Chen and X. Du, Int. J. Biol. Macromol., 2023, 245, 125245 CrossRef CAS PubMed.
- D. Zhang, L. Chen, J. Cai, Q. Dong, Z.-U. Din, Z.-Z. Hu, G.-Z. Wang, W.-P. Ding, J.-R. He and S.-Y. Cheng, Food Chem., 2021, 360, 129922 CrossRef CAS PubMed.
- X. Liu, L. Chen, Q. Dong, Z. Wang, D. Zhang, J. He, Y. Ye, J. Zhou, W. Zhu, Z. Hu, Z.-U. Din, T. Ma, W. Ding and J. Cai, Int. J. Biol. Macromol., 2022, 222, 868–879 CrossRef CAS PubMed.
- T. Alqahtani, Biomass Convers. Biorefin., 2022 DOI:10.1007/s13399-022-03344-w.
- M. J. Fabra, A. López-Rubio, E. Sentandreu and J. M. Lagaron, Starch/Staerke, 2016, 68, 603–610 CrossRef CAS.
- H. Lv, C. Wang, D. He, H. Zhao, M. Zhao, E. Xu, Z. Jin, C. Yuan, L. Guo, Z. Wu, P. Liu and B. Cui, Int. J. Biol. Macromol., 2024, 1, 128384 CrossRef PubMed.
- Z. Aytac, J. Xu, S. K. Raman Pillai, B. D. Eitzer, T. Xu, N. Vaze, K. W. Ng, J. C. White, M. B. Chan-Park, Y. Luo and P. Demokritou, ACS Appl. Mater. Interfaces, 2021, 13, 50298–50308 CrossRef CAS PubMed.
- J. Cai, D. Zhang, R. Zhou, R. Zhu, P. Fei, Z.-Z. Zhu, S.-Y. Cheng and W.-P. Ding, J. Agric. Food Chem., 2021, 69, 5067–5075 CrossRef CAS PubMed.
- R. Priyadarshi and J.-W. Rhim, Innovative Food Sci. Emerging Technol., 2020, 62, 102346 CrossRef CAS.
- H. Wang, J. Qian and F. Ding, J. Agric. Food Chem., 2018, 66, 395–413 CrossRef CAS PubMed.
- M. Ignatova, N. Manolova and I. Rashkov, Macromol. Biosci., 2013, 13, 860–872 CrossRef CAS PubMed.
- C. Zang, Y. Zhang, W. Yang and Y. Hu, Lebensm.-Wiss. Technol., 2024, 198, 115985 CrossRef CAS.
- Z. Zhu, M. Yu, R. Ren, H. Wang and B. Kong, Carbohydr. Polym., 2024, 323, 121381 CrossRef CAS PubMed.
- L. Tayebi, F. Bayat, A. Mahboubi, M. Kamalinejad and A. Haeri, J. Food Meas. Charact., 2024, 18, 3458–3473 CrossRef.
- L. Tayebi, A. Mahboubi, F. Bayat, S. Moayeri-Jolandan and A. Haeri, J. Polym. Environ., 2024 DOI:10.1007/s10924-024-03209-5.
- M. Gudjónsdóttir, M. D. Gacutan, A. C. Mendes, I. S. Chronakis, L. Jespersen and A. H. Karlsson, Food Chem., 2015, 184, 167–175 CrossRef PubMed.
- M. Arkoun, F. Daigle, M.-C. Heuzey and A. Ajji, Molecules, 2017, 22(4), 585 CrossRef PubMed.
- B.-I. Andreica, A. Anisiei, I. Rosca and L. Marin, Food Packag. Shelf Life, 2023, 39, 101157 CrossRef CAS.
- H. Deng, P. Lin, S. Xin, R. Huang, W. Li, Y. Du, X. Zhou and J. Yang, Carbohydr. Polym., 2012, 89, 307–313 CrossRef CAS PubMed.
- W. Yang, Z. Zhang, K. Liu, W. Wang, W. Peng, H. Ma, Q. Wang, X. Shi, H. Sun and X. Duan, Int. J. Biol. Macromol., 2023, 253, 126692 CrossRef CAS PubMed.
- C. Cheng, T. Min, Y. Luo, Y. Zhang and J. Yue, Food Chem., 2023, 418, 135652 CrossRef CAS PubMed.
- M. Duan, J. Sun, S. Yu, Z. Zhi, J. Pang and C. Wu, Int. J. Biol. Macromol., 2023, 233, 123433 CrossRef CAS PubMed.
- Y. Zou, Y. Sun, W. Shi, B. Wan and H. Zhang, Food Chem., 2023, 399, 133962 CrossRef CAS PubMed.
- L. F. A. Amorim, C. Mouro, M. Riool and I. C. Gouveia, Polymers, 2022, 14, 315 CrossRef CAS PubMed.
- E. Yildiz, G. Sumnu and L. N. Kahyaoglu, Int. J. Biol. Macromol., 2021, 170, 437–446 CrossRef CAS PubMed.
- J. Somsap, K. Kanjanapongkul, C. Chancharoonpong, S. Supapvanich and R. Tepsorn, Curr. Appl. Sci. Technol., 2019, 19, 235–247 Search PubMed.
- S. Ebrahimzadeh, M. R. Bari, H. Hamishehkar, H. S. Kafil and L.-T. Lim, Lebensm.-Wiss. Technol., 2021, 144, 111217 CrossRef CAS.
- S. Li, Y. Yan, X. Guan and K. Huang, Food Packag. Shelf Life, 2020, 23, 100466 CrossRef.
- D. Surendhiran, C. Li, H. Cui and L. Lin, Food Packag. Shelf Life, 2020, 23, 100439 CrossRef.
- A. Hasanpour Ardekani-Zadeh and S. F. Hosseini, Carbohydr. Polym., 2019, 223, 115108 CrossRef CAS PubMed.
- L. Lin, X. Mao, Y. Sun, G. Rajivgandhi and H. Cui, Int. J. Food Microbiol., 2019, 292, 21–30 CrossRef CAS PubMed.
- S. G. Kuntzler, J. A. V. Costa and M. G. D. Morais, Int. J. Biol. Macromol., 2018, 117, 800–806 CrossRef CAS PubMed.
- L. Deng, M. Taxipalati, A. Zhang, F. Que, H. Wei, F. Feng and H. Zhang, J. Agric. Food Chem., 2018, 66, 6219–6226 CrossRef CAS PubMed.
- H. Cui, M. Bai, M. M. A. Rashed and L. Lin, Int. J. Food Microbiol., 2018, 266, 69–78 CrossRef CAS PubMed.
- P. Wang, H. Wang, J. Liu, P. Wang, S. Jiang, X. Li and S. Jiang, Carbohydr. Polym., 2018, 181, 885–892 CrossRef CAS PubMed.
- V. K. Pandey, S. N. Upadhyay, K. Niranjan and P. K. Mishra, Int. J. Biol. Macromol., 2020, 157, 212–219 CrossRef CAS PubMed.
- L. Popa, M. V. Ghica, E.-E. Tudoroiu, D.-G. Ionescu and C.-E. Dinu-Pîrvu, Materials, 2022, 15, 1054 CrossRef CAS PubMed.
- Y. Liu, S. Ahmed, D. E. Sameen, Y. Wang, R. Lu, J. Dai, S. Li and W. Qin, Trends Food Sci. Technol., 2021, 112, 532–546 CrossRef CAS.
- C. Pittarate, T. Yoovidhya, W. Srichumpuang, N. Intasanta and S. Wongsasulak, Polym. J., 2011, 43, 978–986 CrossRef CAS.
- M. Rashidi, S. Seyyedi Mansour, P. Mostashari, S. Ramezani, M. Mohammadi and M. Ghorbani, Int. J. Biol. Macromol., 2021, 193, 1313–1323 CrossRef CAS PubMed.
- A. Hosseini, S. Ramezani, M. Tabibiazar, M. Ghorbani and H. Samadi Kafil, Food Packag. Shelf Life, 2021, 30, 100754 CrossRef CAS.
- S. Beikzadeh, S. M. Hosseini, V. Mofid, S. Ramezani, M. Ghorbani, A. Ehsani and A. M. Mortazavian, Int. J. Biol. Macromol., 2021, 191, 457–464 CrossRef CAS PubMed.
- Y. Yang, S. Zheng, Q. Liu, B. Kong and H. Wang, Food Packag. Shelf Life, 2020, 26, 100600 CrossRef.
- M. H. Al-Musawi, A. Khoshkalampour, H. Adnan Shaker Al-Naymi, Z. Farooq Shafeeq, S. Pourvatan Doust and M. Ghorbani, Int. J. Biol. Macromol., 2023, 248, 125969 CrossRef CAS PubMed.
- M. Nazari, H. Majdi, P. Gholizadeh, H. S. Kafil, H. Hamishehkar, A. A. K. Zarchi and A. Khoddami, Int. J. Biol. Macromol., 2023, 235, 123885 CrossRef CAS PubMed.
- L. Xia, L. Li, Y. Xiao, F. Xiao, W. Ji, S. Jiang and H. Wang, Carbohydr. Polym., 2023, 300, 120269 CrossRef CAS PubMed.
- Y. Shi, X. Cao, Z. Zhu, J. Ren, H. Wang and B. Kong, Colloids Surf., B, 2022, 218, 112743 CrossRef CAS PubMed.
- T. Zhang, H. Wang, D. Qi, L. Xia, L. Li, X. Li and S. Jiang, Carbohydr. Polym., 2022, 278, 118914 CrossRef CAS PubMed.
- J. Wu, J. Liao, T. Hu, M. Zong, P. Wen and H. Wu, Int. J. Biol. Macromol., 2024, 265, 130813 CrossRef CAS PubMed.
- J. Huang, Y. Cheng, Y. Wu, X. Shi, Y. Du and H. Deng, Int. J. Biol. Macromol., 2019, 139, 191–198 CrossRef CAS PubMed.
- B. K. Tarus, J. I. Mwasiagi, N. Fadel, A. Al-Oufy and M. Elmessiry, SN Appl. Sci., 2019, 1, 245 CrossRef CAS.
- Y. Liu, J. Vincent Edwards, N. Prevost, Y. Huang and J. Y. Chen, Mater. Sci. Eng. C, 2018, 91, 389–394 CrossRef CAS PubMed.
- J. Tian, H. Tu, X. Shi, X. Wang, H. Deng, B. Li and Y. Du, Colloids Surf., B, 2016, 145, 643–652 CrossRef CAS PubMed.
- D. Han, S. Sherman, S. Filocamo and A. J. Steckl, Acta Biomater., 2017, 53, 242–249 CrossRef CAS PubMed.
- Y. Yuan, H. Tian, R. Huang, H. Liu, H. Wu, G. Guo and J. Xiao, Food Chem., 2023, 418, 135851 CrossRef CAS PubMed.
- L. Li, L. Xia, F. Xiao, Y. Xiao, L. Liu, S. Jiang and H. Wang, Food Chem., 2023, 405, 134994 CrossRef CAS PubMed.
- X. Wu, Z. Liu, S. He, J. Liu and W. Shao, Food Chem., 2023, 426, 136652 CrossRef CAS PubMed.
- Q. Li, W. Zhou, X. Yu, F. Cui, X. Tan, T. Sun and J. Li, J. Sci. Food Agric., 2024, 104, 1942–1952 CrossRef CAS PubMed.
- M. Tagrida, S. Gulzar, K. Nilsuwan, T. Prodpran, B. Zhang and S. Benjakul, Molecules, 2022, 27(18), 5877 CrossRef CAS PubMed.
- M. Al-Moghazy, M. Mahmoud and A. A. Nada, Int. J. Biol. Macromol., 2020, 160, 264–275 CrossRef CAS PubMed.
- A. Altan, Z. Aytac and T. Uyar, Food Hydrocolloids, 2018, 81, 48–59 CrossRef CAS.
- K. Ertan, A. Celebioglu, R. Chowdhury, G. Sumnu, S. Sahin, C. Altier and T. Uyar, Food Hydrocolloids, 2023, 142, 108864 CrossRef CAS.
- Z. Aytac, S. Ipek, E. Durgun, T. Tekinay and T. Uyar, Food Chem., 2017, 233, 117–124 CrossRef CAS PubMed.
- Q. Dong, L. Yang, L. Xiang, Y. Zhao and L. Li, Food Control, 2023, 153, 109959 CrossRef CAS.
- Y. Liu, R. Wang, D. Wang, Z. Sun, F. Liu, D. Zhang and D. Wang, Food Hydrocolloids, 2022, 127, 107546 CrossRef CAS.
- Y. Liu, X. Xia, X. Li, F. Wang, Y. Huang, B. Zhu, X. Feng and Y. Wang, Int. J. Biol. Macromol., 2024, 262, 130033 CrossRef CAS PubMed.
- S. Estevez-Areco, S. Goyanes, M. C. Garrigós and A. Jiménez, Food Hydrocolloids, 2024, 150, 109696 CrossRef CAS.
- T. Cetinkaya, F. Bildik, F. Altay and Z. Ceylan, Food Chem., 2024, 437, 137843 CrossRef CAS PubMed.
- A. Heydarian and N. Shavisi, Food Packag. Shelf Life, 2023, 40, 101219 CrossRef CAS.
- K. Chayavanich, R. Kaneshige, P. Thiraphibundet, T. Furuike, H. Tamura and A. Imyim, Dyes Pigm., 2023, 216, 111331 CrossRef CAS.
- Y. Zhao, G. Guo, B. Xu, H. Liu, H. Tian, J. Li, Y. Ouyang, A. Xiang and R. Kumar, Food Chem., 2023, 405, 134991 CrossRef CAS PubMed.
- X. Huang, W. Jiang, J. Zhou, D.-G. Yu and H. Liu, Polymers, 2022, 14, 4947 CrossRef CAS PubMed.
- Y. Zhang, K. Yang, Z. Qin, Y. Zou, H. Zhong and H. Zhang, Food Packag. Shelf Life, 2022, 34, 100950 CrossRef CAS.
- S. Amjadi, H. Almasi, M. Ghorbani and S. Ramazani, Carbohydr. Polym., 2020, 232, 115800 CrossRef CAS PubMed.
- M. J. Fabra, J. L. Castro-Mayorga, W. Randazzo, J. M. Lagarón, A. López-Rubio, R. Aznar and G. Sánchez, Food Environ. Virol., 2016, 8, 125–132 CrossRef CAS PubMed.
- M. A. Cerqueira, M. J. Fabra, J. L. Castro-Mayorga, A. I. Bourbon, L. M. Pastrana, A. A. Vicente and J. M. Lagaron, Food Bioprocess Technol., 2016, 9, 1874–1884 CrossRef CAS.
- Y. Liu, Y. Li, L. Deng, L. Zou, F. Feng and H. Zhang, J. Agric. Food Chem., 2018, 66, 9498–9506 CrossRef CAS PubMed.
- X. Tang, X. Guo, Y. Duo and X. Qian, Polymers, 2023, 15, 3585 CrossRef CAS PubMed.
- M. Bamian, M. Pajohi-Alamoti, S. Azizian, A. Nourian and H. Tahzibi, Food Meas., 2023, 17, 3450–3463 CrossRef.
- R. Ordoñez, L. Atarés and A. Chiralt, Food Biosci., 2022, 49, 101865 CrossRef.
- F. Lopresti, L. Botta, V. La Carrubba, G. Attinasi, L. Settanni, G. Garofalo and R. Gaglio, eXPRESS Polym. Lett., 2022, 16, 1083–1098 CrossRef CAS.
- L. Yavari Maroufi, M. Ghorbani, M. Mohammadi and A. Pezeshki, Colloids Surf., A, 2021, 622, 126659 CrossRef CAS.
- Y. Han, J. Ding, J. Zhang, Q. Li, H. Yang, T. Sun and H. Li, Int. J. Biol. Macromol., 2021, 184, 739–749 CrossRef CAS PubMed.
- M. Aman Mohammadi, S. Ramezani, H. Hosseini, A. M. Mortazavian, S. M. Hosseini and M. Ghorbani, Food Bioprocess Technol., 2021, 14, 1529–1541 CrossRef CAS.
- M. Râpă, M. Stefan, P. A. Popa, D. Toloman, C. Leostean, G. Borodi, D. C. Vodnar, M. Wrona, J. Salafranca, C. Nerín, D. G. Barta, M. Suciu, C. Predescu and E. Matei, Polymers, 2021, 13, 2123 CrossRef PubMed.
- C. P. Vidal, E. Velásquez, M. J. Galotto and C. López de Dicastillo, Polym. Test., 2021, 93, 106937 CrossRef CAS.
- Y. Liu, S. Wang, R. Zhang, W. Lan and W. Qin, Nanomaterials, 2017, 7(7), 194 CrossRef PubMed.
- M. R. V. Fontes, M. P. da Rosa, L. M. Fonseca, P. H. Beck, E. da Rosa Zavareze and A. R. G. Dias, Braz. J. Chem. Eng., 2021, 38, 133–144 CrossRef CAS.
- Y. Liu, D. Wang, Z. Sun, F. Liu, L. Du and D. Wang, Int. J. Biol. Macromol., 2021, 169, 161–170 CrossRef CAS PubMed.
- N. Shavisi, Int. J. Biol. Macromol., 2024, 266, 131077 CrossRef CAS PubMed.
- S. Ullah, M. Hashmi, J. Shi and I. S. Kim, Polymers, 2023, 15, 2538 CrossRef CAS PubMed.
- K. Mahmood, H. Kamilah, A. A. Karim and F. Ariffin, Food Biophysics, 2023, 18, 186–197 CrossRef.
- M. T. Yilmaz, W. S. Hassanein, A. S. Alkabaa and Z. Ceylan, Food Packag. Shelf Life, 2022, 34, 100968 CrossRef CAS.
- P. Mohajeri, A. Hematian Sourki, A. Mehregan Nikoo and Y. N. Ertas, Int. J. Food Sci. Technol., 2023, 58, 1832–1840 CrossRef CAS.
- M.-T. Golmakani, F. Kiani, M. M. Hajjari, N. Sharif, M. Fazaeli and S. M. H. Hosseini, Lebensm.-Wiss. Technol., 2023, 188, 115408 CrossRef CAS.
- M. Heydari-Majd, M. R. Shadan, H. Rezaeinia, B. Ghorani, F. Bameri, K. Sarabandi and F. Khoshabi, Int. J. Food Microbiol., 2023, 391–393, 110143 CrossRef CAS PubMed.
- M. A. Moreno, M. E. Orqueda, L. G. Gómez-Mascaraque, M. I. Isla and A. López-Rubio, Food Hydrocolloids, 2019, 95, 496–505 CrossRef CAS.
- A. Mayire, Q. Wei, Y. Wang and X. Bai, Food Meas., 2024, 18, 3868–3880 CrossRef.
- C. Fang, F. Xiao, Y. Chen and S. Deng, Sci. Technol. Food Ind., 2024, 45, 10–23 Search PubMed.
- N. Karami, A. Kamkar, Y. Shahbazi and A. Misaghi, Lebensm.-Wiss. Technol., 2021, 140, 110812 CrossRef CAS.
- A. Ahmed, M. Zhang, S. Li and L. Xu, Fibers Polym., 2023, 24, 3075–3084 CrossRef CAS.
- M. Duan, J. Sun, Y. Huang, H. Jiang, Y. Hu, J. Pang and C. Wu, Food Sci. Hum. Wellness, 2023, 12, 614–621 CrossRef CAS.
- L. B. Avila, D. Pinto, L. F. O. Silva, B. S. de Farias, C. C. Moraes, G. S. Da Rosa and G. L. Dotto, Polymers, 2022, 14, 5457 CrossRef CAS PubMed.
- B. S. Munteanu, Z. Aytac, G. M. Pricope, T. Uyar and C. Vasile, J. Nanopart. Res., 2014, 16, 2643 CrossRef.
- M. T. Yilmaz, O. Taylan, C. Y. Karakas and E. Dertli, Carbohydr. Polym., 2020, 244, 116447 CrossRef CAS PubMed.
- G. Pinheiro Bruni, J. P. de Oliveira, L. G. Gómez-Mascaraque, M. J. Fabra, V. Guimarães Martins, E. D. R. Zavareze and A. López-Rubio, Food Packag. Shelf Life, 2020, 23, 100426 CrossRef.
- M. Raeisi, M. A. Mohammadi, V. Bagheri, S. Ramezani, M. Ghorbani, M. Tabibiazar, O. E. Coban, R. Khoshbakht, S. M. H. Marashi and S. M. A. Noori, Biointerface Res. Appl. Chem., 2023, 13(5), 486 CAS.
- F. T. D. Silva, K. F. D. Cunha, L. M. Fonseca, M. D. Antunes, S. L. M. E. Halal, Â. M. Fiorentini, E. D. R. Zavareze and A. R. G. Dias, Int. J. Biol. Macromol., 2018, 118, 107–115 CrossRef CAS PubMed.
- V. Bugatti, L. Vertuccio, G. Viscusi and G. Gorrasi, Nanomaterials, 2018, 8(3), 139 CrossRef PubMed.
- L. L. Wusigale and Y. Luo, Trends Food Sci. Technol., 2020, 97, 391–403 CrossRef CAS.
- B. A. Balik, S. Argin, J. M. Lagaron and S. Torres-Giner, Appl. Sci., 2019, 9(23), 5136 CrossRef CAS.
- J. Safari, S. Esteghlal, M. Keramat and M. Khalesi, Nanosci. Nanotechnol.–Asia, 2020, 10, 134–141 CrossRef CAS.
- Z. Hong, W. Zhou, H. Deng and Q. Huang, Colloids Surf., A, 2023, 672, 131731 CrossRef CAS.
- A. M. Díez-Pascual and A. L. Díez-Vicente, RSC Adv., 2015, 5, 93095–93107 RSC.
- S. Torres-Giner, M. J. Ocio and J. M. Lagaron, Carbohydr. Polym., 2009, 77, 261–266 CrossRef CAS.
- H. Homayoni, S. A. H. Ravandi and M. Valizadeh, Carbohydr. Polym., 2009, 77, 656–661 CrossRef CAS.
- A. Anisiei, F. Oancea and L. Marin, Rev. Chem. Eng., 2023, 39, 31–70 CrossRef CAS.
- T. U. Rashid, R. E. Gorga and W. E. Krause, Adv. Eng. Mater., 2021, 23, 2100153 CrossRef.
- S. Huan, G. Liu, W. Cheng, G. Han and L. Bai, Biomacromolecules, 2018, 19, 1037–1046 CrossRef CAS PubMed.
- C. Sun, Y. Boluk and C. Ayranci, Cellulose, 2015, 22, 2457–2470 CrossRef CAS.
- Z. Chen, B. Wei, X. Mo, C. T. Lim, S. Ramakrishna and F. Cui, Mater. Sci. Eng. C, 2009, 29, 2428–2435 CrossRef CAS.
- J. Lee, G. Tae, Y. H. Kim, I. S. Park, S.-H. Kim and S. H. Kim, Biomaterials, 2008, 29, 1872–1879 CrossRef CAS PubMed.
- D. Plackett and I. Siró, in Multifunctional and Nanoreinforced Polymers for Food Packaging, ed. J.-M. Lagarón, Woodhead Publishing, 2011, pp. 498–526 Search PubMed.
- R. Shogren, J. Environ. Polym. Degrad., 1997, 5, 91–95 CrossRef CAS.
- M. J. Fabra, A. Lopez-Rubio and J. M. Lagaron, Food Hydrocolloids, 2013, 32, 106–114 CrossRef CAS.
- D. Raafat and H.-G. Sahl, Microb. Biotechnol., 2009, 2, 186–201 CrossRef CAS PubMed.
- M. Spasova, N. Manolova, D. Paneva and I. Rashkov, e-Polymers, 2004, 4, 56 Search PubMed.
- R. Mori, RSC Sustainability, 2023, 1, 179–212 RSC.
- F. Gironi and V. Piemonte, Environ. Prog. Sustainable Energy, 2011, 30, 459–468 CrossRef CAS.
- E. Rezvani Ghomi, F. Khosravi, A. Saedi Ardahaei, Y. Dai, R. E. Neisiany, F. Foroughi, M. Wu, O. Das and S. Ramakrishna, Polymers, 2021, 13, 1854 CrossRef PubMed.
- E. Walling and C. Vaneeckhaute, J. Environ. Manage., 2020, 276, 111211 CrossRef CAS PubMed.
- S. Saint Akadiri, A. Adewale Alola, G. Olasehinde-Williams and M. Udom Etokakpan, Sci. Total Environ., 2020, 708, 134653 CrossRef CAS PubMed.
- S. R. Nicholson, N. A. Rorrer, A. C. Carpenter and G. T. Beckham, Joule, 2021, 5, 673–686 CrossRef CAS.
- G. Chen, J. Li, Y. Sun, Z. Wang, G. A. Leeke, C. Moretti, Z. Cheng, Y. Wang, N. Li, L. Mu, J. Li, J. Tao, B. Yan and L. Hou, Engineering, 2024, 32, 152–162 CrossRef CAS.
- Z. Chen, T. Aziz, H. Sun, A. Ullah, A. Ali, L. Cheng, R. Ullah and F. U. Khan, J. Polym. Environ., 2023, 31, 2273–2284 CrossRef CAS.
- A. Chamas, H. Moon, J. Zheng, Y. Qiu, T. Tabassum, J. H. Jang, M. Abu-Omar, S. L. Scott and S. Suh, ACS Sustainable Chem. Eng., 2020, 8, 3494–3511 CrossRef CAS.
- P. Lamba, D. P. Kaur, S. Raj and J. Sorout, Environ. Sci. Pollut. Res., 2022, 29, 86156–86179 CrossRef CAS PubMed.
- K. C. Stone, P. G. Hunt, K. B. Cantrell and K. S. Ro, Bioresour. Technol., 2010, 101, 2014–2025 CrossRef CAS PubMed.
- J. Avossa, G. Herwig, C. Toncelli, F. Itel and R. Michel Rossi, Green Chem., 2022, 24, 2347–2375 RSC.
- K. Y. Sen and S. Baidurah, Curr. Opin. Green Sustainable Chem., 2021, 27, 100412 CrossRef CAS.
- R. Haas, B. Jin and F. T. Zepf, Biosci., Biotechnol., Biochem., 2008, 72, 253–256 CrossRef CAS PubMed.
- N. Poomipuk, A. Reungsang and P. Plangklang, Int. J. Biol. Macromol., 2014, 65, 51–64 CrossRef CAS PubMed.
- S. Povolo and S. Casella, Macromol. Symp., 2003, 197, 1–10 CrossRef CAS.
- F. C. de Paula, S. Kakazu, C. B. C. de Paula, J. G. C. Gomez and J. Contiero, J. King Saud Univ., Sci., 2017, 29, 166–173 CrossRef.
- S. Obruca, S. Petrik, P. Benesova, Z. Svoboda, L. Eremka and I. Marova, Appl. Microbiol. Biotechnol., 2014, 98, 5883–5890 CrossRef CAS PubMed.
- A. Sathish, K. Glaittli, R. C. Sims and C. D. Miller, J. Polym. Environ., 2014, 22, 272–277 CrossRef CAS.
- S.-L. Liu, Y.-Y. Huang, H.-D. Zhang, B. Sun, J.-C. Zhang and Y.-Z. Long, Mater. Res. Innovations, 2014, 18, S833–S837 CAS.
- H. S. SalehHudin, E. N. Mohamad, W. N. L. Mahadi and A. Muhammad Afifi, Mater. Manuf. Processes, 2018, 33, 479–498 CrossRef CAS.
- L. Persano, A. Camposeo, C. Tekmen and D. Pisignano, Macromol. Mater. Eng., 2013, 298, 504–520 CrossRef CAS.
- S. Omer, L. Forgách, R. Zelkó and I. Sebe, Pharmaceutics, 2021, 13, 286 CrossRef CAS PubMed.
- J. Muncke, T. Backhaus, B. Geueke, M. V. Maffini, O. V. Martin, J. P. Myers, A. M. Soto, L. Trasande, X. Trier and M. Scheringer, Environ. Health Perspect., 2017, 125, 095001 CrossRef PubMed.
- J. Muncke, A.-M. Andersson, T. Backhaus, J. M. Boucher, B. Carney Almroth, A. Castillo Castillo, J. Chevrier, B. A. Demeneix, J. A. Emmanuel, J.-B. Fini, D. Gee, B. Geueke, K. Groh, J. J. Heindel, J. Houlihan, C. D. Kassotis, C. F. Kwiatkowski, L. Y. Lefferts, M. V. Maffini, O. V. Martin, J. P. Myers, A. Nadal, C. Nerin, K. E. Pelch, S. R. Fernández, R. M. Sargis, A. M. Soto, L. Trasande, L. N. Vandenberg, M. Wagner, C. Wu, R. T. Zoeller and M. Scheringer, Environ. Health, 2020, 19, 25 CrossRef PubMed.
- K. Grob, Food Addit. Contam.: Part A, 2019, 36, 1895–1902 CrossRef CAS PubMed.
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF), EFSA J., 2016, 14, 4357 Search PubMed.
- G. Philippidis, R. X. Álvarez, L. Di Lucia, H. G. Hermoso, A. G. Martinez, R. M'barek, A. Moiseyev, C. Panoutsou, E. S. Itoiz and V. Sturm, Ecol. Econ., 2024, 219, 108156 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |