Open Access Article
Open Access ArticleRecent advancements in alginate-based films for active food packaging applications†
Chaitanya
Metha
,
Shrutee
Pawar
and
Vasanti
Suvarna
 *
*
Department of Pharmaceutical Analysis & Quality Assurance, SVKM's Dr Bhanuben Nanavati College of Pharmacy, Vile Parle (West), Mumbai 400056, Maharashtra, India. E-mail: vasanti.suvarna@bncp.ac.in; Fax: +91 22 26132905; Tel: +91 22 42332065
First published on 10th July 2024
Abstract
Food packaging possesses a critical role in preserving food quality, increasing food shelf life, and reducing waste. This paper explores the potential of alginate-based food packaging as an environmentally friendly method for food-related issues. Alginate, a naturally occurring polysaccharide extracted from seaweed, has considerable potential as a sustainable packaging material due to its multifaceted properties. These properties enable alginate to encapsulate and preserve a wide range of food products effectively. Alginate food packaging has demonstrated its ability to prolong the shelf life of various food products, including fresh fruits, vegetables, meats, and baked goods. It is beneficial to maintain their moisture content and maintain oxygen levels. Furthermore, it is an effective barrier against microbial growth, while preserving the desired flavor and aroma profiles of the packaged items. Antimicrobial food packaging systems are specifically designed to inhibit microbial growth on surfaces, thus enhancing overall stability and preserving quality during storage periods. However, additional research is necessary to improve performance across various applications within the food industry. Alginate-based edible coatings have attracted significant attention due to their ability to enhance both sensory attributes, such as appearance, and mechanical properties across diverse categories including fruits, vegetables, meat, poultry, seafood, and cheese. These edible films mitigate drying effects on contents by regulating the respiration rate, ensuring optimal conditions for extended freshness and shelf life.
Sustainability spotlightAlginate-based food packaging derived from sustainable production has improved food preservation attributed to special barrier properties that prevent oxygen and moisture penetration. These barriers effectively extend the shelf life of the product, reducing food waste. The versatility of polymer-based food packaging systems facilitates the development of customized solutions while ensuring biocompatibility to guarantee safe food contact. Due to their biodegradable nature, alginate-based films have the potential to replace pollution-causing synthetic polymer-based films in food-packaging applications. The present review discusses alginate-based edible coatings which have gained considerable attention due to their capability in augmenting both sensory attributes such as appearance and mechanical properties across diverse food categories. |
Introduction
The use of plastic packaging, a universal part of modern living, has revolutionized the way food products are stored, transported, and utilized in the food industry. The advantages of plastic packaging are abundant such as durability, economic efficiency, and adaptability for various uses. Despite its advantages, it encounters significant obstacles, particularly in regard to its environmental impact and the generation of non-degradable waste.1,2 Certain materials, due to their long-term nature, can lead to the contamination of various ecosystems, such as marine, freshwater, and terrestrial environments, for extended periods, posing a significant ecological risk. Furthermore, the production of plastic materials is closely linked to the utilization of limited petroleum reserves. This dependence not only increases the strain on these non-renewable resources but also impacts the environment during the extraction and refinement phases.3 The degradation of these materials over time results in the production of microplastics, which could pose a threat to both ecosystems and human health. The incorporation of harmful additives in certain types of plastic can seep into consumer goods, thereby causing concerns regarding public health and safety.4,5 Plastic waste disposal can result in complex problems and significant financial consequences, which often lead to waste accumulation in landfills and pollution from combustion. The use of disposable plastics is widespread, leading to a culture of wastefulness, which in turn leads to more waste generation. Moreover, the production of plastics necessitates a significant energy input that results in the emission of greenhouse gases.6 In response to these challenges, there are global initiatives aimed at reducing plastic usage and fostering the invention of packaging alternatives that are harmless to both the environment and human health. The objective of these initiatives is to minimize the negative consequences associated with plastic packaging.The use of biodegradable polymers in food packaging offers a solution that is both effective and environmentally friendly and can be a viable alternative to traditional plastic packaging materials. Renewable resources like corn starch or sugar cane are often used to derive these substances, such as polylactic acid (PLA) and polyhydroxyalkanoates (PHA). The formation of natural decomposition takes place over a period of months to years, resulting in harmless byproducts like water and carbon dioxide. The sustainability of these materials is a major advantage, which reduces the need for non-renewable fossil fuels.7
The utilization of biodegradable packaging is prevalent in the food industry, particularly for items like fresh produce and takeaway containers. Compared with traditional plastic packaging, biodegradable alternatives contribute to a markedly reduced environmental footprint. This is primarily due to their dependence on renewable resources and their capacity to alleviate the environmental pollution that is typically a byproduct of conventional plastic usage.8,9 Polymers derived from sustainable resources showed a promising alternative for petroleum-based plastics.10
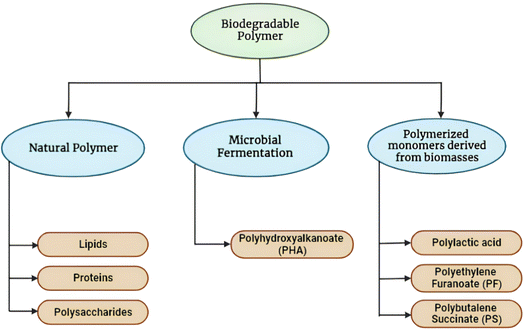
Food packaging systems, which are based on sustainable polymers includes biopolymers such as PLA, polybutylene adipate terephthalate (PBAT), and synthetic polymers such as polyvinyl alcohol (PVA), have brought about a significant transformation in the field of food preservation. The distinct barrier properties of these polymers prevent the ingress of oxygen and moisture, thereby reducing the shelf life of products and reducing the consumption of food. These versatile systems facilitate the fabrication of customized solutions while ensuring biocompatibility for safe food interaction. The environmental awareness is increased and a growing demand for environmentally friendly substitutes, the introduction of polymer-based packaging allows brands to enhance their image as environmentally conscious companies.11 Polypropylene (PP) is known for its good transparency, high melting point, and excellent barrier properties. It offers good resistance to heat, moisture, and chemicals, which make it suitable for food containers, cups, and packaging films that need to withstand high temperatures during packaging processes. High-density polyethylene (HDPE) is a thermoplastic polymer made from petroleum, known for its high strength-to-density ratio, excellent moisture resistance, and exceptional chemical resistance. HDPE provides a good barrier against moisture vapor transmission and is commonly used for packaging milk, juice, and other beverages. Medium-density polyethylene (MDPE) has a density range of 0.926–0.940 g cm−3 and a melting point of 126 °C. It is used in various food packaging applications.12 Chitosan is a polysaccharide derived from chitin, found in the exoskeletons of crustaceans. Chitosan-based films and coatings exhibit antimicrobial properties and can be used for active food packaging. Starch is a polysaccharide extracted from plants like potatoes, corn, and rice. Starch-based films and coatings can be used for food packaging.13 Gelatin is a protein derived from collagen and can be used to make biodegradable food packaging films and coatings.14 PLA is a biodegradable aliphatic polyester derived from renewable resources like corn, sugarcane, or cassava. It is used to make food packaging materials like containers, films, and trays.15 Compared to these synthetic polymers, alginate is a natural polymer derived from brown seaweed. It exhibits superior mucoadhesive strength, allowing it to adhere well to food surfaces. Alginate is also biodegradable and biocompatible, making it suitable for eco-friendly food packaging solutions. Alginate can be easily cross-linked with various cations to form hydrogels with tunable mechanical properties. It can also be chemically modified or blended with other natural polymers like chitosan, gelatin, and carrageenans to form composite materials with enhanced functionality for food packaging applications.12 The versatility and favorable characteristics of alginate make it a promising candidate for developing active and intelligent food packaging systems. Alginate-based films can be integrated with functional additives like antimicrobials, antioxidants, and indicators to extend food shelf-life and monitor quality. Despite the recent advancements, there remains a need for further research and development to address potential shortcomings related to barrier properties and scalability.16 This emphasizes the critical role of continuous innovation in sustainable packaging solutions, ensuring food safety and quality standards,17 and accelerating their incorporation into the packaging industry.18Fig. 1 highlights the classification of biodegradable polymers. Polysaccharides (natural polymer) are complex carbohydrates made up of multiple sugar molecules linked together. Alginate is indeed a type of polysaccharide. It is a natural polysaccharide composed of α-D-mannuronic acid and β-L-guluronic acid derived from seaweed.
Alginate
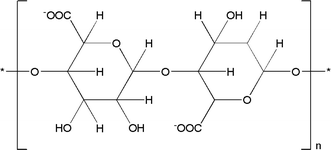
Alginate, a polysaccharide occurring naturally, is derived from a variety of sources, and has gained considerable attention in food packaging field due to its multifaceted properties and environmental benefits.19 Primarily, it originates from diverse species of brown seaweed such as Laminaria hyperborea, Ascophyllum nodosum, and Macrocystis pyrifera.19 These sources are utilized for various applications across food, medicine, and cosmetics.20,21 Additionally, alginate can be obtained from other algal species such as Macrocystis pyrifera and Fucus vesiculosus.22 Certain bacteria like Pseudomonas sp. and Azotobacter sp. produce alginate for research and industrial applications.20,22 Some fungi such as Saprolegnia sp., can also synthesize alginate, although this is less common.23 Furthermore, alginate can be produced synthetically in laboratories through chemical processes.20,21 This multifaceted biomaterial, with its diverse origins and applications, contributes significantly to environmental sustainability. A primary factor facilitating the use of alginates in food packaging is their exceptional biodegradability. Contrasting with traditional plastic packaging, which persists in the environment for extended periods, packaging derived from alginates undergoes natural decomposition. This substantially mitigates the enduring environmental impact of food packaging, aligning with the growing global focus on sustainability and the need for eco-friendly alternatives to combat plastic pollution.24 Alginate possesses excellent barrier characteristics against moisture and gases. It establishes a protective layer around food items, facilitating the retention of freshness and extension of shelf life. This attribute is especially advantageous for perishable goods, as it curtails food wastage and augments the overall quality of the packaged commodities. Furthermore, alginate can be easily moulded into various shapes and sizes, making it suitable for a wide range of food products. Its flexibility and versatility enable its application in innovative and customized packaging solutions.22Fig. 2 depicts structure of alginate.Synthesis
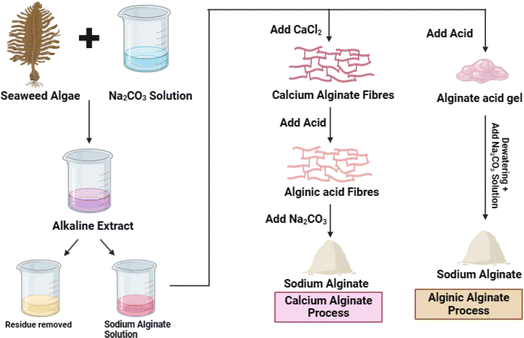
The process of alginate production from brown algae involves a complex sequence of extraction and purification procedures. Initially, brown algae, such as kelp sp., are collected from the ocean. The alginate quality is significantly influenced by kelp sp. and the location of its collection. The harvested seaweed undergoes a thorough cleaning process to remove contaminants like sand, salt, and other residues. The cleaned seaweed is then subjected to maceration, a process where it is chopped into small pieces to eliminate cell structure and facilitate the extraction of alginate. The macerated seaweed is mixed with a mild alkaline solution, typically soda ash or caustic soda, to dissolve the alginate. This process can be expedited by applying heat and agitation. The mixture is subsequently filtered to separate the liquid extract, which contains the dissolved alginate, from the solid algal residue. The liquid extract is acidified, typically with sulfuric acid, which reduces the pH and solidifies the alginate into calcium alginate. Calcium chloride is added to form solid calcium alginate beads, which help in further removal of impurity. These beads are rinsed with clean water to eliminate excess calcium ions and acid, and then dried to yield calcium alginate powder. In the concluding steps, calcium alginate is transformed into sodium alginate by substituting calcium ions with sodium ions, a process typically achieved by treating it with table salt. The resultant sodium alginate is dried and pulverized into a fine powder for diverse applications.23,25 The synthesis of alginate from seaweed algae is illustrated in Fig. 3.Types of food packaging
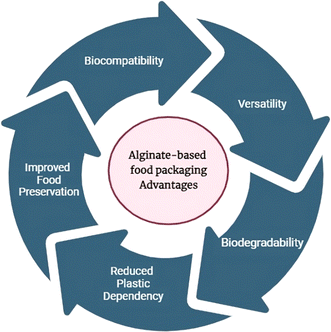 | ||
| Fig. 4 Advantages of alginate-based food packaging.19,24 | ||
Synthesis of edible films
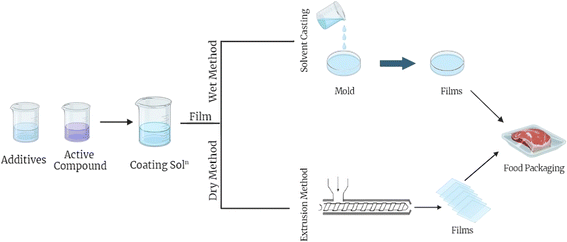
The formation of edible films involves a variety of methods such as coacervation (either straightforward or intricate), gelation or heat-driven coagulation, solvent casting, and extrusion.32 Simple coacervation is a procedure that involves a phase transition or precipitation in a hydrocolloid distributed in water, enabled by diverse methods such as the evaporation of a solvent, the introduction of a water-soluble nonelectrolyte, or the adjustment of pH. Complex coacervation pertains to the precipitation of a polymer complex through the merging of two hydrocolloid solutions bearing contrasting electron charges. Gelation or thermal coagulation is a process of precipitation where a macromolecule undergoes heating to disintegrate it, or a hydrocolloid dispersion is subjected to cooling.33 Solvent casting is a technique utilized to produce flexible, thin, and biodegradable edible films. This is achieved by integrating appropriate edible substances with a solvent that adheres to food safety regulations. The mixture thus formed yields a homogenous, thin film which, upon drying, results in the intended film.34 The film acts as a safeguard for a variety of food items, prolonging their shelf life and aiding in waste minimization.35 The extrusion process produces edible films that are thin, biodegradable, and suitable for consumption. Edible materials are incorporated and then subjected to controlled heat and pressure through extrusion. Post-extrusion, the substance is chilled to harden and subsequently shaped into the preferred configuration. These procedures are similar to those employed for thermoplastic films, particularly solvent casting, and extrusion, although the precise circumstances under which these processes are executed can differ.28,29Fig. 5 summarizes edible film synthesis methods.Coating methods
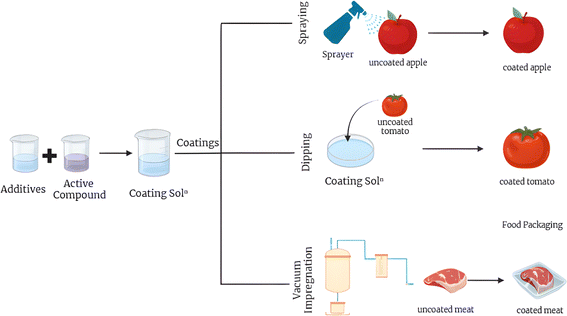
The process of preparing edible coatings and ensuring their adherence to the food surface is vitally significant. These coatings are typically manufactured using three distinct techniques: dipping, spraying, and vacuum impregnation. Dipping, a prevalent method in food packaging, involves submerging the food item in a specially designed solution that often includes additives and edible substances. This combination forms a protective barrier around the food item.36 Post-dipping, the surplus solution is permitted to dissipate, leading to the drying and solidification of the coated items. This technique is frequently utilized in the processing and preservation of an array of products, such as fruits, vegetables, and sweets. The end product exhibits a wide range of alternatives to enhance freshness, visual appeal, and durability. Contingent on the particular requirements of the product, the dipping coating procedure provides a broad spectrum of materials that can be applied either manually or via automated mechanisms.37 The procedure of spray coating involves the application of a uniform protective layer or coating to food items by spraying onto the packaging. This procedure encompasses multiple stages, including the selection of an appropriate coating substance, the formulation of a coating solution, the application of the solution to the food item via spraying, the drying or solidification of the coating, and ultimately, the packaging of the coated food item.37 This technique is adaptable and can be efficiently employed on an extensive range of food products. It fulfils multiple objectives, including enhancing visual appeal, prolonging product longevity, and safeguarding against flavor degradation and moisture intrusion.38 Vacuum impregnation is a technique utilized in food packaging where a particular liquid or solution is introduced into the food via negative pressure. The procedure encompasses several stages: choosing an appropriate coating substance, formulating a solution, positioning the food in a vacuum chamber, infusing the solution into the food, and ultimately distributing the infused food. This technique enables the incorporation of various ingredients into foods, such as flavourings, colorants, or preservatives. It enhances the product's shelf life and improves its sensory attributes. Vacuum impregnation is a prevalent method in the food industry with a multitude of applications for diverse types of products.39Fig. 6 depicts coating application methods.Crosslinking
The process of crosslinking alginate is a prevalent method employed to enhance its characteristics for a range of uses, including but not limited to, food packaging. The crosslinked alginate undergoes a series of transformations, culminating in a material that is both strong and resistant, thereby improving its durability for packaging applications. Alginate crosslinking can be achieved through the use of chemical agents. Ca2+ ions, which are frequently used as crosslinking agents, can be incorporated by immersing the material in a CaCl2 solution. This technique aids in the formation of a robust and long-lasting alginate matrix.40 Physical strategies, including freeze–thaw cycles or the utilization of thermosensitive polymers, can be applied for the crosslinking of alginate. These methodologies can impart beneficial attributes to substances used in food packaging, encompassing the establishment of reversible crosslinks.41 Blending alginate with various polymers, such as starch and PVA, facilitates the production of composites. These composites exhibit improved mechanical strength and enhanced barrier properties.42 The blending process allows properties to be tailored to the precise specifications required for food packaging. The technique, which employs electrostatic forces to facilitate the crosslinking of alginate, is called electrostatic gelation. Alginate can be combined with molecules or polymers that possess opposite charges, such as proteins or chitosan, to form a gel-like structure. This method has the potential to enhance the mechanical strength and barrier properties of alginate films.43 For crosslinking alginate for food packaging, it is crucial to consider specific packaging requirements such as barrier properties, mechanical strength, and biodegradability.42 The process involves incorporating gelling ions into the alginate solution to form a hydrogel suitable for packaging. This can be achieved via two distinct techniques: external and internal gelation. These techniques provide precise control over the gelation process, enabling the formulation of alginate properties to meet specific packaging requirements. This ensures that the final product is not only effective but also environmentally friendly due to its biodegradability.44 In the conventional external gelation method, an alginate solution is exposed to a solution with gelling ions. The carboxyl groups of the guluronic acid residues react immediately with the Ca2+ ions and form an irreversible hydrogel as a result of ion diffusion. In internal gelation, alginate solutions are exposed to an insoluble source of gelling ions.45 In addition, the pH is reduced by the addition of organic acids or by the rapid hydrolysis of lactones, which releases the gelling ions.46 In contrast, internal gelation results in more homogeneous, although less compact, gel matrices with greater pore diameters than external gelation. This is mainly due to the presence of acid, which causes the displacement of Ca2+ ions by H+ ions.47 Khwaldia et al. synthesized a film by crosslinking diphenyl phosphine-ethylene (DPPE) with alginate at varying concentrations (10%, 20%, 30%, and 40% w/w). The DPPE inclusion led to reduced solubility and wettability. The 10% DPPE film exhibited improved water vapor barrier properties, tensile strength, and elongation at break. However, the 40% DPPE film showed the least phenolic content, DPPH (2,2-diphenyl-1-picrylhydrazyl) scavenging activity and FRAP (ferric reduction activity potential) retention after three months of storage.48 Maziyar Makaremi et al., study showed that the bio composite samples were subjected to a two-step crosslinking and rewetting process. Initially, the specimens were crosslinked in a solution containing 2% CaCl2, a process that was accomplished within a span of 2 minutes. Subsequently, the crosslinking was reversed using a 5% sodium citrate solution. However, the duration required to reverse the crosslinking was not constant and varied depending on the unique composition of each sample.49 Parreidt et al. demonstrated a crosslinking method for alginate, significantly influenced the thickness of the resulting films. The technique involved immersing the alginate gel in a crosslinking solution, yielding thinner films than those obtained by adding the crosslinking agent directly to the alginate solution. The crosslinking process, involving the interaction of alginate with Ca2+ ions, resulted in the formation of coatings and films. These crosslinked alginate coatings and films have demonstrated effectiveness in enhancing food product quality and extending shelf life.50 Singh et al. conducted a study on the fabrication of crosslinked polymers, primarily composed of pectin and sodium alginate. The crosslinking process was performed using environmentally friendly acids, specifically citric and tartaric acid. The study evaluated the effectiveness of these materials for food packaging applications, particularly for chocolate and Indian vegetable cakes, with the aim of prolonging their shelf life using innovative packaging materials.51Additives
Li et al. concentrated on fabricating biodegradable food packaging materials utilizing sodium alginate and tannic acid. The study demonstrated that elevated tannic acid concentrations in the films enhanced water vapor barrier properties and antioxidant activity, although with a minor reduction in light transmission. The films demonstrated UV light blocking capabilities and demonstrated enhanced antimicrobial activity against Escherichia coli.67 Chinnaiah et al. engineered a polymer membrane by incorporating leaf extract of Datura metel L. and sodium alginate via the solution casting method. The resultant membrane showed an ionic conductivity of 2.18 × 10−4 S cm−1 and a peak specific capacitance of 131 F g−1 at a current density of 0.2 A g−1, while also demonstrated substantial antimicrobial activity against human pathogens. These findings indicated that the fabricated membranes could be used as solid electrolytes in supercapacitor devices and as antimicrobial components in food packaging.68 Carvalho et al. formulated a polyelectrolyte film by combining sodium alginate and poly(diallyl dimethylammonium chloride), PDDA, to generate a surface possessing antimicrobial properties for food packaging. The films showed a compact structure and exhibited ionic interaction between sodium alginate and PDDA. PDDA enhanced the mechanical robustness and thermal stability of the films due to its hydrophilic nature. The chosen film showcased considerable success in reducing 99.8% of SARS-CoV-2 within a minute of exposure. Additionally, it demonstrated an inhibitory effect against Staphylococcus aureus and Escherichia coli.69 Wei et al. developed an intelligent packaging film capable of real-time food freshness monitoring. Cubic cobalt metal–organic framework (Co-MOF) microcrystals, which are ammonia-sensitive and possess antibacterial properties, were immobilized in a sodium alginate matrix. The sodium alginate-based films, with varying cobalt imidazole content (0.5%, 1.0%, and 2.0%), exhibited enhanced mechanical strength, toughness, oxygen/water barrier, UV-blocking capability, and antibacterial activity. The films, being ammonia sensitive and color stable, are deemed suitable for detecting shrimp spoilage based on observable color alterations.70
Appendini et al. emphasizes the role of packaging technologies in preserving food quality and safety. The research advocates for antimicrobial packaging materials to extend shelf life and limit pathogens. These materials work by inhibiting microorganism growth in the food. Factors like water activity, oxygen consumption, and temperature imbalances can cause food spoilage. The use of engineered biopolymers with antimicrobial properties can help reduce oxygen permeability and inhibit microbial growth on the food surface. Antimicrobials are released over time to maintain control of microbial activity.71 Studies showed that incorporation of natural antimicrobials into alginate-based biofilms for food packaging applications. It showed that incorporating white ginseng extract into alginate films can impart antimicrobial properties. Alginate is used to encapsulate lactic acid bacteria enhancing their ability to inhibit foodborne pathogens in biofilms for ready-to-eat foods, and when combined with garlic oil in films, it improves their natural antibacterial properties.72 Biopolymers mixed with water, ethanol, and other solvents are also used as anti-microbial components in packaging.73 Researchers have demonstrated the antibacterial effectiveness of sodium alginate, which is combined with nanoparticles, against experimental strains. Furthermore, the nanoparticle-infused film demonstrated an improvement in the shelf-life stability of pears and carrots, as demonstrated by the preservation of soluble protein content and a decrease in weight during storage.74
Starch or protein-based biopolymers are recognized for their antibacterial properties in packaging applications.75 Notably, additives such as lysozyme, benzoic acid, propionic acid, lactic acid, ascorbic acid, and nisin enhance the antimicrobial efficacy of biofilms.76 The effectiveness of these materials, however, is contingent upon the storage and distribution systems employed. Recent studies suggested that incorporated alginate with antimicrobial agents into biopolymers may prolong the shelf life of meat products.77 Moreover, biopolymer films containing antibacterial agents have demonstrated the ability to inhibit the growth of pathogens like Listeria monocytogenes and Escherichia coli, thereby safeguarding food from contamination.78 While some biopolymer packaging includes mechanisms to hinder microbial proliferation within the food, it is crucial that these antimicrobial agents do not adulterate the food product. However, the potential migration of these substances from the packaging to the food requires thorough research prior to market release.79 Appendini et al. study concluded that calcium alginates increased the growth of microbes such as coliform bacterial strain on beef and other natural flora.71 The presence of CaCl2 in the biofilm has been attributed to such a possibility.80 Biofilm efficacy in antimicrobial properties varies, with sodium alginate demonstrated greater inhibition than κ-carrageenan due to its superior moisture absorption, facilitating faster antimicrobial agent delivery into the food matrix. This approach is known as “smart packaging”.81 The incorporation of organic acids in films is reported to prevent beef by reducing the growth of microbes like L. monocytogenes, Salmonella typhimurium, and E. coli.82 Biofilms exhibited thermoresistance, attributed to Pediococcus sp. bacteriocins, possess chelating properties effective against L. monocytogenes.83 Alginate films, particularly in combination with PVA and zein, effectively encapsulate and deliver antimicrobials like lysozyme and nisin. This enhances food preservation and extends shelf life by promoting the antimicrobial action of these natural compounds.84,85 Mecitoglu et al., incorporated lactoperoxidase (LPS), an enzyme from bovine milk, into alginate films. Zein films consisting of partially purified lysozyme demonstrated antimicrobial effects on Bacillus subtilis and Lactobacillus plantarum. The study showed that the partially purified lysozyme used in antimicrobial packaging to enhance food safety.86 Researchers used polyelectrolyte packaging with antimicrobial properties using sodium alginate, and cationic starch. These films showed excellent thermal stability and improved antimicrobial effect. The finding revealed that polyelectrolyte sodium alginate packaging has excellent antimicrobial properties and can be used as a suitable food packaging material.87
| Food product | Additives | Advantages | References |
|---|---|---|---|
| Anti-microbial activity | |||
| Fresh-cut apple | CaCl2 + thyme oil | Inhibition of growth of TPC 1, total coliform, LAB 1, yeast, and mold | 105 |
| Fresh-cut watermelon | Calcium lactate + trans-cinnamaldehyde | Effective against yeasts, psychrotrophs, coliforms, and molds | 106 |
| Fresh-cut pineapple | Sunflower oil + lemongrass essential oil | Growth inhibitory effect on yeast and mold, and increased shelf life | 107 |
| Strawberry | CaCl2 + carvacrol + methyl cinnamate | Effective against E. coli and B. cinereal | 108 |
| Capsicum | CaCl2 + pomegranate peel extract | Increased antibacterial and antifungal effects | 109 |
| Peeled and shallot onion | SA-CMC film + gluten blends + onion waste extracts | Improved water barrier property and tensile strength compared to SA-CMC film and reduced microbial load | 110 |
| Tomato | Aloe vera + garlic oil | Enhanced thermal and mechanical sproperties without affecting film transparency and improved antimicrobial properties | 111 |
| Chicken fillet | Galbanum gum + CaCl2 + essential oil of Ziziphora persica | Significant microbial reduction was achieved with composite coating and EO addition to formulation | 112 |
| Chicken breast fillet | Alginate-maltodextrin CaCl2-CMC + lactoperoxidase enzyme | Reduced the microbial loads of Enterobacteriaceae, P. aeruginosa, and aerobic mesophilic bacteria | 113 |
| Chicken meat | Alginate-whey protein + lactoperoxidase enzyme | Increased antimicrobial activity | 114 |
| Abalone | CaCl2 + bamboo leaf extract + rosemary extract | Bacterial inhibition was improved | 115 |
| Chicken fillet | Chitosan + red beet anthocyanin extract | Enhance quality, shelf life, and microbial control | 116 |
| Rainbow trout fillet | CaCl2 + resveratrol | Reduced the growth of bacteria, yeast, and mold | 117 |
| Silver carp fillet | Alginate-CMC + CaCl2 + clove essential oil | Enhancement in antibacterial activity against L. monocytogenes, S. aureus, and E. coli | 118 |
| Bighead carp fillet | CaCl2 + horsemint essential oil | Significant decrease in TVC and TPC growth rates | 119 |
| Sea bass | Tea polyphenols | Significant decrease in TVC growth rates | 120 |
| Sea bass, red sea bream | CaCl2 + e-polylysine + 6-gingerol | Increase in antibacterial activity | 121 and 122 |
| Sea bass, fior di latte, cheese | CaCl2 + reuterin (Lactobacillus reuteri) | Enhancement in antimicrobial activity | 123 and 124 |
| Kashar cheese | Alginate-whey protein + ginger essential oil | Bacteriostatic and bactericidal effect against E. coli and S. aureus | 125 |
| Mozzarella | CaCl2 + potassium sorbate + sodium | Reduced the growth rate of Pseudomonas spp. and Enterobacteriaceae | 126 |
| Benzoate + calcium lactate + calcium ascorbate | |||
| Low-fat cut cheese | Alginate-mandarin fiber + oregano essential oil | Antibacterial activity against S. aureus, psychrophilic bacteria, molds, and yeasts | 127 |
| CaCO3 + microencapsulated lemongrass oil | Decreased the growth rate of E. coli and L. monocytogenes | 128 | |
| Datura metel L. leaf extract | Significant antimicrobial activity against human pathogens | 68 | |
| CuO NPs + CNW | Excellent physicochemical, mechanical, and antimicrobial activity against food pathogens | 129 | |
| White mushrooms | Carvacrol (CAR) + β-cyclodextrin (βCD) | Improved water resistance, mechanical properties, light barrier properties, and heat aging. Films containing 30 g L−1 βCD-CARM were effective against Trichoderma sp. | 130 |
| Cheese | Silver spherical nanoparticles + lemongrass essential oil | Preserved cheese quality for 14 days and changed color to indicate storage conditions for sensitive food items | 131 |
| Poly(diallyl dimethylammonium chloride) | Effectively inactivated 99.8% of SARS-CoV-2 within 1 minute of contact and exhibited inhibitory effects against S. aureus and E. coli bacteria | 69 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Antioxidant activity | |||
| Longan | Saccharomyces cerevisiae + sucrose | Sucrose enhanced SE cell viability, improving the film's antioxidant properties during storage | 132 |
| Meat | Carboxylated cellulose nanocrystals + beetroot extract | High tensile strength and antioxidant capacity | 133 |
| Gelatin-sodium alginate + aqueous beetroot peel extract | Limited microbial deterioration, delayed chemical oxidation, and improved sensory characteristics of the meat | 134 | |
| Gum kondagogu + glycerol | Blend films also exhibited high tensile strength (up to 24 MPa) compared to the pure biopolymer films | 89 | |
| Agar + glycerol + ascorbic acid | Increased tensile strength, transparency, and barrier properties against oxygen and water vapor | 90 | |
| Guava | CaCl2 + pomegranate peel extract | With the addition of pomegranate peel extract, enhanced the antioxidant activity | 135 |
| Date palm pit extract incorporated into alginate-based films | The film with 40% DPPE exhibited the lowest retention of phenolic content, DPPH scavenging activity, and FRAP after a 3 months storage period | 48 | |
| Tannic acid | Increasing tannic acid concentration in the films improved water vapor barrier ability and antioxidant activity while decreasing light transmittance slightly | 67 | |
| Lycopene + β-carotene | Effectively protected sunflower oil from oxidation under accelerated storage conditions | 136 | |
| Grape pomace waste extract + CaCl2 | Protected food from light deterioration while maintaining water resistance and stability | 64 | |
| Chicken fillet | Alginate-galbanum gum + CaCl2 + Ziziphora persica | Due to their high phenolic and flavonoid contents, galbanum gum and Ziziphora persica contributed to significant antioxidant activities | 137 |
| Bream (fish) | CaCl2 + vitamin C + tea polyphenols | Considerable reduction in TBA and lipid peroxidation | 138 |
| Red sea bream | 6-Gingerol | Significant decrease in TBA and lipid peroxidation | 122 |
| Citrus pectin + pterostilbene | Improved moisture resistance and enhanced antioxidant properties | 63 | |
| Bighead carp fillet | CaCl2 + horsemint essential oil | After the eighth day of storage, EC led to decreased oxidation readings; the addition of horsemint EO further enhanced this effect | 139 |
| Silver carp fillet | CaCl2 + clove essential oil | Considerable reduction in lipid peroxidation | 118 |
| Rainbow trout fillet | CaCl2 + resveratrol | Significant decrease in lipid peroxidation | 117 |
| Methyl methacrylate + ammonium persulphate initiators under a nitrogen gas atmosphere | Improved tensile strength, water resistance, thermal stability, and crystallinity | 90 | |
| Alginate-clay nanoparticles + CaCl2 + lycopene | Decrease in FFA | 140 | |
| Sea bass | Alginate + tea polyphenols | Decrease in lipid oxidation | 120 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Antibacterial activity | |||
| Shrimp | Cubic Co-MOF microcrystals with ammonia-sensitivity | Improvement in mechanical strength, toughness, water/oxygen barrier and UV barrier property | 70 |
| Beef | Pectin + cinnamic acid | Exhibited 43.3% soil degradability in 15 days and preserved beef color better than control film during a 5 days test | 65 |
| Pectin + calcium chloride + sodium citrate | Rough surface, exhibit effective antibacterial activity, and possess mechanical properties similar to commercial packaging films | 49 | |
| Cellulose nanocrystals + silver nanoparticles | Exhibited a plasmonic effect at 491 nm and provided excellent ultraviolet (UV) barrier properties | 61 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Combination activity | |||
| (1) Antioxidant and antibacterial activity | |||
| Strawberry | Fish scale-derived gelatin + carvacrol-loaded ZIF-8 nanoparticles | UV protection, increased flexibility, and reduced water vapor permeability. Strong antioxidant properties and long-lasting antibacterial effects against E. coli and S. aureus | 141 |
| Banana | Nitrogen-functionalized carbon dots + layered clay | Increasing UV blocking, antioxidant, and antibacterial activities by up to 70% and enhanced anti-browning activity | 142 |
| Beef and apple | Konjac glucomannan + tea polyphenols (TP) | Exhibited excellent microstructure, hydrogen bonding, improved mechanical and barrier properties, oxidation resistance, antibacterial activity, and stability | 143 |
| Bread | Sulfur quantum dots | Improved UV blocking by 82% and increased tensile strength by 18% | 144 |
| Strong antioxidant and antibacterial effects, and prevented mold growth for 14 days | |||
| Reduced graphene oxide or a mixture of zinc oxide-rGO (ZnO-rGO) + calcium chloride | Films with 50% ZnO-rGO showed high antioxidant and antibacterial activity, and low-temperature food sterilization | 65 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| (2) Antioxidant and antifungal activity | |||
| Banana | Tea tree essential oil nano emulsion + TiO2 nanoparticle | Excellent UV blocking and improved water vapor and oxygen barrier properties | 60 |
| Improved banana postharvest quality and reduced anthracnose | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| (3) Antimicrobial and antioxidant activity | |||
| Cheddar cheese | Carboxymethyl cellulose + Thymus vulgaris purified leaves extract | Good thermal stability, with improved prevention of moisture, acidity, puncture strength | 145 |
| Polymers | Added fillers | Composition | Properties | Application | Reference |
|---|---|---|---|---|---|
| Alginate/CMC/starch | — | — | The enhanced water vapor barrier and mechanical strength are enhanced. Grape shelf life increased during storage, up to several weeks | Blue and green grapes packaging | 146 |
| Alginate | Melanin and zinc oxide/silver nanoparticles | Melanin: 0.10%, 0.25% and 0.50% w/w | Improved water vapor barrier characteristics, mechanical strength, enhanced antioxidant/antimicrobial activity, and improved UV radiation barrier capabilities | Active food packaging | 147 |
| Silver and zinc oxide nanoparticles (10 mM film casting solutions for both metal nanoparticles) | |||||
| Alginate/CMC/starch | Grapefruit seed extract | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (alginate 1 (alginate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC CMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) starch) starch) |
Improved antioxidant/antimicrobial and UV barrier qualities. Green chilies have an extended shelf life of 25 days during storage | Chilli packaging | 148 |
| Alginate | Aloe vera (AV) and garlic oil (GO) | AV gel: 0, 50 and 66.7 wt% | Protective UV protection increased antibacterial activity, and enhanced shelf life of coated tomato | Active packaging | 149 |
| Garlic oil: 0, 1.0%, 3.0% and 5.0% v/w | |||||
| Alginate | Hallocyte (Hal) derived from Dragon and Dunino mines loaded with salicylic acid | — | Improved antimicrobial characteristics than pectin-based films. Antibiotic activities against food spoilage bacteria | Active packaging | 150 |
| Alginate | Sulfur quantum dots | — | Increased UV light barrier characteristics, antioxidant/antibacterial activity, and mechanical strength. Ideal for bread packaging that extends its shelf life for up to two weeks | Bread packaging application | 151 |
| Alginate | Copper sulfide nanoparticles | 0.0, 0.5, 1.0, and 1.5 wt% | Improved UV barrier, hydrophobicity, mechanical strength, and antibacterial resistance to Gram-negative bacteria | Active food packaging | 152 |
| Alginate | Sulfur nanoparticles | 1%, 2%, and 3% w/w | Improved mechanical strength, hydrophobicity, and antimicrobial resistance against Gram-negative bacteria | Active packaging | 153 |
| Alginate | Cottonseed protein. Hydrolysates (CPH) | 0%, 0.15%, 0.30% and 0.60% (w/v) | Produced films containing antimicrobial activity against Gram-positive bacteria and fungi. Sustained release of CPH compounds | Active packaging | 154 |
| Alginate | Cellulose nanocrystal, silver | 1 wt% | High UV barrier properties, increased tensile strength, and decreased water vapor permeation than neat alginate films, have high UV barrier properties | Food packaging | 155 |
| Sodium alginate | Cellulose nanowhisker, copper oxide nanoparticles | CNW (0.5%)–SA (3%)–CuNPs (5 mM) | Excellent antibacterial activity against food pathogens, challenging antioxidant activity | Active food packaging | 156 |
| Alginate | Halloysite nanotubes and zinc oxide nanoparticles | 1, 3, 5, and 7 wt% | Superior mechanical, UV-light, and water vapor barrier properties. Antibiotic activity against foodborne pathogens | Active packaging | 157 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to $500
000 to $500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, highlighting the complexity and expense involved in forming edible films for diverse applications.168,169
000, highlighting the complexity and expense involved in forming edible films for diverse applications.168,169
Conclusion
Alginate-based food packaging materials have significant potential for applications in the food industry. Their inherent biodegradability, compatibility with biological systems, and ability to enhance the longevity of a wide variety of products make them a viable and effective sustainable choice. However, further research and development efforts are needed to optimize their properties and increase production so that they can be widely used. Indeed, given the increasing demand for environmentally sustainable packaging, there is significant potential for alginate-based solutions to help reduce plastic waste and promote a more environmentally conscious approach to food packaging. The future of food packaging looks promising with the emergence of such innovative and sustainable solution.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.References
- C. Relton, M. Strong and M. Holdsworth, BMJ, 2012, 344, e3824 CrossRef PubMed.
- P. Jariyasakoolroj, P. Leelaphiwat and N. Harnkarnsujarit, J. Sci. Food Agric., 2020, 100, 5032–5045 CrossRef CAS PubMed.
- L. Boone, N. Préat, T. T. Nhu, F. Fiordelisi, V. Guillard, M. Blanckaert and J. Dewulf, Sci. Total Environ., 2023, 894, 164781 CrossRef CAS PubMed.
- K. J. Groh, T. Backhaus, B. Carney-Almroth, B. Geueke, P. A. Inostroza, A. Lennquist, H. A. Leslie, M. Maffini, D. Slunge, L. Trasande, A. M. Warhurst and J. Muncke, Sci. Total Environ., 2019, 651, 3253–3268 CrossRef CAS PubMed.
- J. C. Prata, A. L. P. Silva, J. P. da Costa, C. Mouneyrac, T. R. Walker, A. C. Duarte and T. Rocha-Santos, Int. J. Environ. Res. Public Health, 2019, 16, 2411 CrossRef PubMed.
- S. Huang, H. Wang, W. Ahmad, A. Ahmad, N. Ivanovich Vatin, A. M. Mohamed, A. F. Deifalla and I. Mehmood, Int. J. Environ. Res. Public Health, 2022, 19, 4556 CrossRef CAS PubMed.
- A. Iordanskii, Polymers, 2020, 12, 1537 CrossRef CAS PubMed.
- N. Basavegowda and K.-H. Baek, Polymers, 2021, 13, 4198 CrossRef CAS PubMed.
- M. B. Aga, A. H. Dar, G. A. Nayik, P. S. Panesar, F. Allai, S. A. Khan, R. Shams, J. F. Kennedy and A. Altaf, Int. J. Biol. Macromol., 2021, 192, 197–209 CrossRef CAS PubMed.
- A. Nešić, G. Cabrera-Barjas, S. Dimitrijević-Branković, S. Davidović, N. Radovanović and C. Delattre, Molecules, 2019, 25, 135 CrossRef PubMed.
- M. E. González-López, S. d. J. Calva-Estrada, M. S. Gradilla-Hernández and P. Barajas-Álvarez, Front. Sustain. Food Syst., 2023 DOI:10.3389/fsufs.2023.1225371.
- B. Tajeddin and M. Arabkhedri, in Polymer Science and Innovative Applications, Elsevier, 2020, pp. 525–543 Search PubMed.
- K. Y. Perera, A. K. Jaiswal and S. Jaiswal, Foods, 2023, 12, 2422 CrossRef CAS PubMed.
- R. Porta, M. Sabbah and P. Di Pierro, Int. J. Mol. Sci., 2020, 21, 4942 CrossRef PubMed.
- M. Y. Khalid and Z. U. Arif, Food Packag. Shelf Life, 2022, 33, 100892 CrossRef CAS.
- R. Porta, M. Sabbah and P. Di Pierro, Int. J. Mol. Sci., 2022, 23, 3611 CrossRef PubMed.
- C. L. Reichert, E. Bugnicourt, M.-B. Coltelli, P. Cinelli, A. Lazzeri, I. Canesi, F. Braca, B. M. Martínez, R. Alonso, L. Agostinis, S. Verstichel, L. Six, S. De Mets, E. C. Gómez, C. Ißbrücker, R. Geerinck, D. F. Nettleton, I. Campos, E. Sauter, P. Pieczyk and M. Schmid, Polymers, 2020, 12, 1558 CrossRef CAS PubMed.
- C. Su, D. Li, L. Wang and Y. Wang, Crit. Rev. Food Sci. Nutr., 2023, 63, 6923–6945 CrossRef CAS PubMed.
- R. Abka-khajouei, L. Tounsi, N. Shahabi, A. K. Patel, S. Abdelkafi and P. Michaud, Mar. Drugs, 2022, 20, 364 CrossRef CAS PubMed.
- S. Lomartire and A. M. M. Gonçalves, Mar. Drugs, 2022, 20, 141 CrossRef CAS PubMed.
- I. D. Hay, Z. U. Rehman, M. F. Moradali, Y. Wang and B. H. A. Rehm, Microb. Biotechnol., 2013, 6, 637–650 CrossRef CAS PubMed.
- S. Kaidi, F. Bentiss, C. Jama, K. Khaya, Z. Belattmania, A. Reani and B. Sabour, Colloids Interfaces, 2022, 6, 51 CrossRef CAS.
- L. Pereira and J. Cotas, in Alginates – Recent Uses of This Natural Polymer, IntechOpen, 2020 Search PubMed.
- M. G. Kontominas, Foods, 2020, 9, 1440 CrossRef PubMed.
- K. Y. Lee and D. J. Mooney, Prog. Polym. Sci., 2012, 37, 106–126 CrossRef CAS PubMed.
- K. K. Gaikwad, S. Singh and Y. S. Lee, Environ. Chem. Lett., 2018, 16, 523–538 CrossRef CAS.
- H. Li, C. Liu, J. Sun and S. Lv, Foods, 2022, 11, 3044 CrossRef CAS PubMed.
- P. R. Salgado, L. Di Giorgio, Y. S. Musso and A. N. Mauri, Front. Sustain. Food Syst., 2021, 5, 630393 CrossRef.
- E. Poyatos-Racionero, J. V. Ros-Lis, J.-L. Vivancos and R. Martínez-Máñez, J. Cleaner Prod., 2018, 172, 3398–3409 CrossRef.
- P. Müller and M. Schmid, Foods, 2019, 8, 16 CrossRef PubMed.
- Y. Wang, K. Liu, M. Zhang, T. Xu, H. Du, B. Pang and C. Si, Carbohydr. Polym., 2023, 313, 120851 CrossRef CAS PubMed.
- S. Guilbert, N. Gontard and L. G. M. Gorris, LWT–Food Sci. Technol., 1996, 29, 10–17 CrossRef CAS.
- M. Â. Parente Ribeiro Cerqueira, in Encyclopedia of Food Chemistry, Elsevier, 2019, pp. 173–176 Search PubMed.
- F. A. H. Prakoso, R. Indiarto and G. L. Utama, Polymers, 2023, 15, 2800 CrossRef CAS PubMed.
- Md. R. Rahman, J. L. Chang Hui and S. bin Hamdan, in Silica and Clay Dispersed Polymer Nanocomposites, Elsevier, 2018, pp. 1–24 Search PubMed.
- R. Suhag, N. Kumar, A. T. Petkoska and A. Upadhyay, Food Res. Int., 2020, 136, 109582 CrossRef CAS PubMed.
- A. Valdés, M. Ramos, A. Beltrán, A. Jiménez and M. Garrigós, Coatings, 2017, 7, 56 CrossRef.
- D. A. d. S. Rios, M. M. Nakamoto, A. R. C. Braga and E. M. C. da Silva, Appl. Food Res., 2022, 2, 100073 CrossRef.
- R. A. Abalos, E. F. Naef, M. V. Aviles and M. B. Gómez, LWT, 2020, 131, 109773 CrossRef CAS.
- C. Lee, J. Shin, J. S. Lee, E. Byun, J. H. Ryu, S. H. Um, D.-I. Kim, H. Lee and S.-W. Cho, Biomacromolecules, 2013, 14, 2004–2013 CrossRef CAS PubMed.
- T. Senturk Parreidt, K. Müller and M. Schmid, Foods, 2018, 7, 170 CrossRef PubMed.
- L. Aguero, S. Alpdagtas, E. Ilhan, D. Zaldivar-Silva and O. Gunduz, Eur. Polym. J., 2021, 160, 110807 CrossRef CAS.
- J. Tan, Y. Luo, Y. Guo, Y. Zhou, X. Liao, D. Li, X. Lai and Y. Liu, Int. J. Biol. Macromol., 2023, 239, 124275 CrossRef CAS PubMed.
- M. Soazo, G. Báez, A. Barboza, P. A. Busti, A. Rubiolo, R. Verdini and N. J. Delorenzi, Food Hydrocolloids, 2015, 51, 193–199 CrossRef CAS.
- E. Papajová, M. Bujdoš, D. Chorvát, M. Stach and I. Lacík, Carbohydr. Polym., 2012, 90, 472–482 CrossRef PubMed.
- L. W. Chan, H. Y. Lee and P. W. S. Heng, Carbohydr. Polym., 2006, 63, 176–187 CrossRef CAS.
- X. D. Liu, W. Y. Yu, Y. Zhang, W. M. Xue, W. T. Yu, Y. Xiong, X. J. Ma, Y. Chen and Q. Yuan, J. Microencapsulation, 2002, 19, 775–782 CrossRef CAS PubMed.
- K. Khwaldia, Y. M'Rabet and A. Boulila, Food Sci. Nutr., 2023, 11, 555–568 CrossRef CAS PubMed.
- M. Makaremi, H. Yousefi, G. Cavallaro, G. Lazzara, C. B. S. Goh, S. M. Lee, A. Solouk and P. Pasbakhsh, Polymers, 2019, 11, 1594 CrossRef CAS PubMed.
- T. Senturk Parreidt, M. Lindner, I. Rothkopf, M. Schmid and K. Müller, Foods, 2019, 8, 203 CrossRef PubMed.
- P. Singh, P. Baisthakur and O. S. Yemul, Heliyon, 2020, 6, e03026 CrossRef PubMed.
- S. Y. Park, W.-J. Kim, J. B. Choi and S. Kim, Int. J. Precis. Eng. Manuf., 2018, 19, 129–135 CrossRef.
- H. Dysjaland, I. Sone, E. Noriega Fernández, M. Sivertsvik and N. Sharmin, Molecules, 2022, 27, 8356 CrossRef CAS PubMed.
- K. K. Dash, A. Kumar, S. Kumari and M. A. Malik, J. Polym. Environ., 2021, 29, 3649–3659 CrossRef CAS.
- T. Senturk Parreidt, K. Müller and M. Schmid, Foods, 2018, 7, 170 CrossRef PubMed.
- A. Botalo, T. Inprasit, S. Ummartyotin, K. Chainok, S. Vatthanakul and P. Pisitsak, Polymers, 2024, 16, 447 CrossRef CAS PubMed.
- A. Khan, R. Priyadarshi, T. Bhattacharya and J.-W. Rhim, Food Bioprocess Technol., 2023, 16, 2001–2015 CrossRef CAS.
- J. Gubitosa, V. Rizzi, C. Marasciulo, F. Maggi, G. Caprioli, A. M. Mustafa, P. Fini, N. De Vietro, A. M. Aresta and P. Cosma, Int. J. Mol. Sci., 2023, 24, 11462 CrossRef CAS PubMed.
- N. A. S. Abdullah, Z. Mohamad, Z. I. Khan, M. Jusoh, Z. Y. Zakaria and N. Ngadi, Chem. Eng. Trans., 2021, 83, 271–276 Search PubMed.
- M. S. Abdel Aziz and H. E. Salama, Int. J. Biol. Macromol., 2022, 212, 294–302 CrossRef CAS PubMed.
- M. Yadav, Y.-K. Liu and F.-C. Chiu, Nanomaterials, 2019, 9, 1523 CrossRef CAS PubMed.
- V. M. Rangaraj, K. Rambabu, F. Banat and V. Mittal, Food Biosci., 2021, 43, 101251 CrossRef.
- Y. Li, J. Lu, X. Tian, Z. Xu, L. Huang, H. Xiao, X. Ren and Q. Kong, Int. J. Biol. Macromol., 2021, 193, 2093–2102 CrossRef CAS PubMed.
- J. Gubitosa, V. Rizzi, C. Marasciulo, F. Maggi, G. Caprioli, A. M. Mustafa, P. Fini, N. De Vietro, A. M. Aresta and P. Cosma, Int. J. Mol. Sci., 2023, 24, 11462 CrossRef CAS PubMed.
- Z. Alves, N. M. Ferreira, S. Mendo, P. Ferreira and C. Nunes, Int. J. Mol. Sci., 2021, 22, 9943 CrossRef CAS PubMed.
- S.-Y. Sung, L. T. Sin, T.-T. Tee, S.-T. Bee, A. R. Rahmat, W. A. W. A. Rahman, A.-C. Tan and M. Vikhraman, Trends Food Sci. Technol., 2013, 33, 110–123 CrossRef CAS.
- H. Li, C. Liu, J. Sun and S. Lv, Foods, 2022, 11, 3044 CrossRef CAS PubMed.
- K. Chinnaiah, K. Kannan, R. Krishnamoorthy and K. Gurushankar, Int. J. Biol. Macromol., 2023, 242, 125112 CrossRef CAS PubMed.
- G. R. de Carvalho, A. M. Kudaka, R. A. Netto, C. Delarmelina, M. C. T. Duarte and L. M. F. Lona, Int. J. Biol. Macromol., 2023, 244, 125388 CrossRef CAS PubMed.
- D. Wei, S. Feng, Q. Tang, H. Li, D. Peng and Z. Zou, Int. J. Biol. Macromol., 2023, 253, 126607 CrossRef CAS PubMed.
- P. Appendini and J. H. Hotchkiss, Innovative Food Sci. Emerging Technol., 2002, 3, 113–126 CrossRef CAS.
- M. Rinaudo, TIP, 2014, 17, 92–96 CrossRef.
- L. Leistner, Int. J. Food Microbiol., 2000, 55, 181–186 CrossRef CAS PubMed.
- A. Mohammed Fayaz, K. Balaji, M. Girilal, P. T. Kalaichelvan and R. Venkatesan, J. Agric. Food Chem., 2009, 57, 6246–6252 CrossRef CAS PubMed.
- S. A. Valencia-Chamorro, L. Palou, M. A. del Río and M. B. Pérez-Gago, Crit. Rev. Food Sci. Nutr., 2011, 51, 872–900 CrossRef CAS PubMed.
- P. Varela and S. M. Fiszman, Food Hydrocolloids, 2011, 25, 1801–1812 CrossRef CAS.
- K. G. Wanstedt, S. C. Seideman, L. S. Donnelly and N. M. Quenzer, J. Food Prot., 1981, 44, 732–735 CrossRef CAS PubMed.
- L.-F. Wang and J.-W. Rhim, Int. J. Biol. Macromol., 2015, 80, 460–468 CrossRef CAS PubMed.
- F. Şen, İ. Uzunsoy, E. Baştürk and M. V. Kahraman, Carbohydr. Polym., 2017, 170, 264–270 CrossRef PubMed.
- B. Cuq, N. Gontard and S. Guilbert, in Active Food Packaging, Springer US, Boston, MA, 1995, pp. 111–142 Search PubMed.
- V. Jost, K. Kobsik, M. Schmid and K. Noller, Carbohydr. Polym., 2014, 110, 309–319 CrossRef CAS PubMed.
- J. P. Kerry, M. N. O'Grady and S. A. Hogan, Meat Sci., 2006, 74, 113–130 CrossRef CAS PubMed.
- N. Vigneshwaran, D. M. Kadam and S. Patil, in Nanoscience for Sustainable Agriculture, Springer International Publishing, Cham, 2019, pp. 581–600 Search PubMed.
- M. Millette, C. Le Tien, W. Smoragiewicz and M. Lacroix, Food Control, 2007, 18, 878–884 CrossRef CAS.
- N. Natrajan and B. W. Sheldon, J. Food Prot., 2000, 63, 1189–1196 CrossRef CAS PubMed.
- Ç. Mecitoglu, A. Yemenicioğlu, A. Arslanoğlu, Z. S. Elmacı, F. Korel and A. E. Çetin, Food Res. Int., 2006, 39, 12–21 CrossRef.
- F. Şen, İ. Uzunsoy, E. Baştürk and M. V. Kahraman, Carbohydr. Polym., 2017, 170, 264–270 CrossRef PubMed.
- T. Senturk Parreidt, M. Schott, M. Schmid and K. Müller, Int. J. Mol. Sci., 2018, 19, 742 CrossRef PubMed.
- R. K. Ramakrishnan, S. Wacławek, M. Černík and V. V. T. Padil, Int. J. Biol. Macromol., 2021, 177, 526–534 CrossRef CAS PubMed.
- S. Amariei, F. Ursachi and A. Petraru, Membranes, 2022, 12, 576 CrossRef CAS PubMed.
- M. Yang, L. Wang and Y. Xia, Int. J. Biol. Macromol., 2019, 124, 1238–1245 CrossRef CAS PubMed.
- K. Pathakoti, M. Manubolu and H.-M. Hwang, J. Food Drug Anal., 2017, 25, 245–253 CrossRef CAS PubMed.
- K. Frank, C. V. Garcia, G. H. Shin and J. T. Kim, Int. J. Polym. Sci., 2018, 2018, 1–8 CrossRef.
- Z. Yang, M. Li, X. Zhai, L. Zhao, H. E. Tahir, J. Shi, X. Zou, X. Huang, Z. Li and J. Xiao, Int. J. Biol. Macromol., 2022, 213, 145–154 CrossRef CAS PubMed.
- N. Echegaray, N. Guzel, M. Kumar, M. Guzel, A. Hassoun and J. M. Lorenzo, Food Chem., 2023, 404, 134453 CrossRef CAS PubMed.
- B. Deepa, E. Abraham, L. Pothan, N. Cordeiro, M. Faria and S. Thomas, Materials, 2016, 9, 50 CrossRef PubMed.
- X. Ma, R. Li, X. Zhao, Q. Ji, Y. Xing, J. Sunarso and Y. Xia, Composites, Part A, 2017, 96, 155–163 CrossRef CAS.
- M. Abdollahi, M. Alboofetileh, M. Rezaei and R. Behrooz, Food Hydrocolloids, 2013, 32, 416–424 CrossRef CAS.
- Z. Mahcene, A. Khelil, S. Hasni, P. K. Akman, F. Bozkurt, K. Birech, M. B. Goudjil and F. Tornuk, Int. J. Biol. Macromol., 2020, 145, 124–132 CrossRef CAS PubMed.
- M. S. Abdel Aziz, H. E. Salama and M. W. Sabaa, LWT, 2018, 96, 455–460 CrossRef CAS.
- K. Li, J. Zhu, G. Guan and H. Wu, Int. J. Biol. Macromol., 2019, 122, 485–492 CrossRef CAS PubMed.
- H. Wang, X. Gong, Y. Miao, X. Guo, C. Liu, Y.-Y. Fan, J. Zhang, B. Niu and W. Li, Food Chem., 2019, 283, 397–403 CrossRef CAS PubMed.
- J. G. de Oliveira Filho, J. M. Rodrigues, A. C. F. Valadares, A. B. de Almeida, T. M. de Lima, K. P. Takeuchi, C. C. F. Alves, H. A. d. F. Sousa, E. R. da Silva, F. H. Dyszy and M. B. Egea, Food Hydrocolloids, 2019, 92, 267–275 CrossRef.
- S. Iswarya, T. Theivasanthi, K. Chinnaiah and S. C. B. Gopinath, Appl. Nanosci., 2024, 14, 109–122 CrossRef CAS.
- Sarengaowa, W. Hu, K. Feng, Z. Xiu, A. Jiang and L. Ying, Food Biosci., 2019, 32, 100467 CrossRef CAS.
- R. E. Sipahi, M. E. Castell-Perez, R. G. Moreira, C. Gomes and A. Castillo, LWT–Food Sci. Technol., 2013, 51, 9–15 CrossRef CAS.
- N. Azarakhsh, A. Osman, H. M. Ghazali, C. P. Tan and N. Mohd Adzahan, Postharvest Biol. Technol., 2014, 88, 1–7 CrossRef CAS.
- G. Peretto, W.-X. Du, R. J. Avena-Bustillos, J. D. J. Berrios, P. Sambo and T. H. McHugh, J. Agric. Food Chem., 2014, 62, 984–990 CrossRef CAS PubMed.
- M. S. Nair, A. Saxena and C. Kaur, Food Bioprocess Technol., 2018, 11, 1317–1327 CrossRef CAS.
- P. Thivya, Y. K. Bhosale, S. Anandakumar, V. Hema and V. R. Sinija, Food Chem., 2022, 390, 133221 CrossRef CAS PubMed.
- M. S. Abdel Aziz and H. E. Salama, Int. J. Biol. Macromol., 2021, 190, 837–844 CrossRef CAS PubMed.
- H. Hamedi, M. Kargozari, P. M. Shotorbani, N. B. Mogadam and M. Fahimdanesh, Food Hydrocolloids, 2017, 72, 35–46 CrossRef CAS.
- M. Yousefi, M. Farshidi and A. Ehsani, J. Food Saf., 2018, 38, e12449 CrossRef.
- R. Molayi, A. Ehsani and M. Yousefi, Food Sci. Nutr., 2018, 6, 878–883 CrossRef CAS PubMed.
- R. Hao, Y. Liu, L. Sun, L. Xia, H. Jia, Q. Li and J. Pan, LWT–Food Sci. Technol., 2017, 81, 1–9 CrossRef CAS.
- M. Ranjbar, M. H. Azizi Tabrizzad, G. Asadi and H. Ahari, Heliyon, 2023, 9, e18879 CrossRef CAS PubMed.
- B. Bazargani-Gilani, J. Food Saf., 2017, 38, e12395 CrossRef.
- N. Jalali, P. Ariiai and E. Fattahi, J. Food Sci. Technol., 2016, 53, 757–765 CrossRef CAS PubMed.
- R. Heydari, S. Bavandi and S. R. Javadian, Food Sci. Nutr., 2015, 3, 188–194 CrossRef CAS PubMed.
- X. Nie, L. Wang, Q. Wang, J. Lei, W. Hong, B. Huang and C. Zhang, J. Food Sci., 2018, 83, 1695–1700 CrossRef CAS PubMed.
- L. Cai, A. Cao, F. Bai and J. Li, LWT–Food Sci. Technol., 2015, 62, 1053–1059 CrossRef CAS.
- L. Cai, Y. Wang, A. Cao, Y. Lv and J. Li, RSC Adv., 2015, 5, 36882–36889 RSC.
- L. Angiolillo, A. Conte and M. A. Del Nobile, Int. J. Food Microbiol., 2018, 271, 60–66 CrossRef CAS PubMed.
- L. Angiolillo, A. Conte, A. V. Zambrini and M. A. Del Nobile, J. Dairy Sci., 2014, 97, 5345–5355 CrossRef CAS PubMed.
- N. Kavas, G. Kavas and D. Saygili, CyTA–J. Food, 2016, 14, 317–323 CrossRef CAS.
- A. Lucera, M. Mastromatteo, A. Conte, A. V. Zambrini, M. Faccia and M. A. Del Nobile, Food Packag. Shelf Life, 2014, 1, 25–29 CrossRef.
- M. Artiga-Artigas, A. Acevedo-Fani and O. Martín-Belloso, Food Control, 2017, 76, 1–12 CrossRef CAS.
- R. O. Bustos C., F. V. Alberti R. and S. B. Matiacevich, J. Food Sci. Technol., 2016, 53(1), 832–839 CrossRef CAS PubMed.
- K. Saravanakumar, A. Sathiyaseelan, A. V. A. Mariadoss, H. Xiaowen and M.-H. Wang, Int. J. Biol. Macromol., 2020, 153, 207–214 CrossRef CAS PubMed.
- M. Cheng, J. Wang, R. Zhang, R. Kong, W. Lu and X. Wang, Int. J. Biol. Macromol., 2019, 141, 259–267 CrossRef CAS PubMed.
- L. Motelica, D. Ficai, O.-C. Oprea, A. Ficai, V.-L. Ene, B.-S. Vasile, E. Andronescu and A.-M. Holban, Nanomaterials, 2021, 11, 2377 CrossRef CAS PubMed.
- Z. Yang, X. Zhai, M. Li, Z. Li, J. Shi, X. Huang, X. Zou, M. Yan, W. Qian, Y. Gong, M. Holmes, M. Povey and J. Xiao, Int. J. Biol. Macromol., 2022, 223, 673–683 CrossRef CAS PubMed.
- Q. Guo, Y. Yuan, M. He, X. Zhang, L. Li, Y. Zhang and B. Li, Food Chem., 2023, 415, 135799 CrossRef CAS PubMed.
- M. Chaari, K. Elhadef, S. Akermi, B. Ben Akacha, M. Fourati, A. Chakchouk Mtibaa, M. Ennouri, T. Sarkar, M. A. Shariati, M. Rebezov, S. Abdelkafi, L. Mellouli and S. Smaoui, Antioxidants, 2022, 11, 2095 CrossRef CAS PubMed.
- M. S. Nair, A. Saxena and C. Kaur, Food Chem., 2018, 240, 245–252 CrossRef CAS PubMed.
- D. S. Tupuna-Yerovi, H. Schmidt and A. d. O. Rios, Food Sci. Technol. Int., 2023, 108201322311723 CrossRef PubMed.
- H. Hamedi, M. Kargozari, P. M. Shotorbani, N. B. Mogadam and M. Fahimdanesh, Food Hydrocolloids, 2017, 72, 35–46 CrossRef CAS.
- Y. Song, L. Liu, H. Shen, J. You and Y. Luo, Food Control, 2011, 22, 608–615 CrossRef CAS.
- R. Heydari, S. Bavandi and S. R. Javadian, Food Sci. Nutr., 2015, 3, 188–194 CrossRef CAS PubMed.
- A. Ehsani, M. Paktarmani and M. Yousefi, Food Sci. Biotechnol., 2017, 26, 557–562 CrossRef CAS PubMed.
- Y. Li, P. Shan, F. Yu, H. Li and L. Peng, Int. J. Biol. Macromol., 2023, 230, 123192 CrossRef CAS PubMed.
- L. Mao, J. Zuo, Y. Liu, B. Zheng, X. Dai, Z. Bai, Y. Liu and J. Yao, Int. J. Biol. Macromol., 2023, 253, 126653 CrossRef CAS PubMed.
- S. Wang, M. Li, B. He, Y. Yong and J. Zhu, Int. J. Biol. Macromol., 2023, 242, 124732 CrossRef CAS PubMed.
- Z. Riahi, R. Priyadarshi, J.-W. Rhim, E. Lotfali, R. Bagheri and G. Pircheraghi, Colloids Surf., B, 2022, 215, 112519 CrossRef CAS PubMed.
- M. Abdin, M. Mabrouk, L. El-Sebaiy, M. Eissa, M. El-Bana, M. A. Salama, A. E. El-Beltagy and M. A. Naeem, Int. J. Biol. Macromol., 2023, 240, 124474 CrossRef CAS PubMed.
- R. Ramakrishnan, S. V. Kulandhaivelu, S. Roy and V. P. Viswanathan, Ind. Crops Prod., 2023, 193, 116114 CrossRef CAS.
- Ł. Łopusiewicz, S. Macieja, M. Śliwiński, A. Bartkowiak, S. Roy and P. Sobolewski, Materials, 2022, 15, 2381 CrossRef PubMed.
- R. Ramakrishnan, S. V. Kulandhaivelu and S. Roy, Ind. Crops Prod., 2023, 199, 116752 CrossRef CAS.
- M. S. Abdel Aziz and H. E. Salama, Int. J. Biol. Macromol., 2021, 190, 837–844 CrossRef CAS PubMed.
- J. Kurczewska, M. Ratajczak and M. Gajecka, Appl. Clay Sci., 2021, 214, 106270 CrossRef CAS.
- Z. Riahi, R. Priyadarshi, J.-W. Rhim, E. Lotfali, R. Bagheri and G. Pircheraghi, Colloids Surf., B, 2022, 215, 112519 CrossRef CAS PubMed.
- S. Roy and J.-W. Rhim, Int. J. Biol. Macromol., 2020, 164, 37–44 CrossRef CAS PubMed.
- R. Priyadarshi, H.-J. Kim and J.-W. Rhim, Food Hydrocolloids, 2021, 110, 106155 CrossRef CAS.
- J. G. de Oliveira Filho, J. M. Rodrigues, A. C. F. Valadares, A. B. de Almeida, T. M. de Lima, K. P. Takeuchi, C. C. F. Alves, H. A. d. F. Sousa, E. R. da Silva, F. H. Dyszy and M. B. Egea, Food Hydrocolloids, 2019, 92, 267–275 CrossRef.
- M. Yadav, Y.-K. Liu and F.-C. Chiu, Nanomaterials, 2019, 9, 1523 CrossRef CAS PubMed.
- K. Saravanakumar, A. Sathiyaseelan, A. V. A. Mariadoss, H. Xiaowen and M.-H. Wang, Int. J. Biol. Macromol., 2020, 153, 207–214 CrossRef CAS PubMed.
- S. Shankar, S. Kasapis and J.-W. Rhim, Int. J. Biol. Macromol., 2018, 118, 1824–1832 CrossRef CAS PubMed.
- G. T. Bersaneti, S. H. Prudencio, S. Mali and M. A. Pedrine Colabone Celligoi, Food Biosci., 2021, 42, 101173 CrossRef CAS.
- A. A. P. Ribeiro, R. C. Sanfelice, G. R. P. Malpass, M. H. Okura and A. C. G. Malpass, Food Mater. Res., 2023, 3, 26 Search PubMed.
- F. Pavli, I. Kovaiou, G. Apostolakopoulou, A. Kapetanakou, P. Skandamis, G.-J. Nychas, C. Tassou and N. Chorianopoulos, Int. J. Mol. Sci., 2017, 18, 1867 CrossRef PubMed.
- M. Zarandi, M. Hasani, P. M. Shotorbani, A. A. Basti and H. Hamedi, J. Food Meas. Char., 2022, 16, 2556–2570 CrossRef.
- M. d. C. M. Serrano, R. Santos, C. Viegas, M. M. Sapata, R. G. Santos, J. Condeço, A. C. Marques and J. C. Bordado, SSRN Electron. J., 2021 DOI:10.2139/ssrn.3910229.
- J. Lange and Y. Wyser, Packag. Technol. Sci., 2003, 16, 149–158 CrossRef CAS.
- A. Botalo, T. Inprasit, S. Ummartyotin, K. Chainok, S. Vatthanakul and P. Pisitsak, Polymers, 2024, 16, 447 CrossRef CAS PubMed.
- T. Senturk Parreidt, K. Müller and M. Schmid, Foods, 2018, 7, 170 CrossRef PubMed.
- R. Gheorghita, G. Gutt and S. Amariei, Coatings, 2020, 10, 166 CrossRef CAS.
- R. Gheorghita Puscaselu, A. Lobiuc, M. Dimian and M. Covasa, Polymers, 2020, 12, 2417 CrossRef PubMed.
- N. Racmayani and A. Husni, E3S Web Conf., 2020, 147, 03003 CrossRef CAS.
- H. Li, C. Liu, J. Sun and S. Lv, Foods, 2022, 11, 3044 CrossRef CAS PubMed.
- T. Senturk Parreidt, K. Müller and M. Schmid, Foods, 2018, 7, 170 CrossRef PubMed.
- H. P. S. Abdul Khalil, C. K. Saurabh, Y. Y. Tye, T. K. Lai, A. M. Easa, E. Rosamah, M. R. N. Fazita, M. I. Syakir, A. S. Adnan, H. M. Fizree, N. A. S. Aprilia and A. Banerjee, Renewable Sustainable Energy Rev., 2017, 77, 353–362 CrossRef CAS.
- I. D. Ibrahim, Y. Hamam, E. R. Sadiku, J. M. Ndambuki, W. K. Kupolati, T. Jamiru, A. A. Eze and J. Snyman, Polymers, 2022, 14, 4430 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fb00216k |
| This journal is © The Royal Society of Chemistry 2024 |