Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A comprehensive review on salted eggs: quality formation mechanisms, innovative pickling technologies and value-added applications
Xiaotuo
Wang
ab,
Jingshou
Zhang
b,
Sriram K.
Vidyarthi
c,
Mingqiang
Xu
d,
Ziliang
Liu
b,
Chunjiang
Zhang
e and
Hongwei
Xiao
 *b
*b
aCollege of Intelligent Agriculture, Suzhou Polytechnic Institute of Agriculture, Soochow, Jiangsu 215008, China
bCollege of Engineering, China Agricultural University, Beijing, 100083, China. E-mail: xhwcaugxy@163.com
cDepartment of Biological and Agricultural Engineering, University of California, Davis, One Shields Avenue, Davis, CA 95616, USA
dInstitute of Agro-products Storage and Processing, Xinjiang Academy of Agricultural Sciences, Urumqi, Xinjiang, China
eInstitute of Food Science and Technology, Chinese Academy of Agricultural Sciences, Beijing, China
First published on 29th July 2024
Abstract
Salted eggs are very popular in China for their pleasant flavor and texture. However, the long production cycle of traditional pickling with uncontrollable quality limit their industrialization. The high salt content in salted egg white (SEW) and problems such as hard core and black circle in salted egg yolk (SEY) significantly hinder the sustainable development of the salted egg production industry. This paper reviews the entire process of salted egg production, including salting, post-curing, cooking, and preservation, to fully explore the mechanisms of quality formation. The application of rapid processing, such as physical treatment and chemical additives, to reduce the salt content of SEW and enhance the quality of SEY is elaborated. Besides, the preference for SEY flavor leads to a great waste of SEW with high salt content lacking in foaming ability and low viscosity. Therefore, value-added SEY application as functional ingredients and converting the wastage of higher salt content SEW into valuable products are discussed in this paper. It will provide valuable information to improve processing efficiency, enhance the quality of salted eggs and promote the development of high-value-added and environmentally friendly nutritional egg products.
Sustainability spotlightSalted eggs, especially SEY, have gained immense popularity as a traditional preserved food in China and worldwide owing to their unique texture and delightful flavor. However, the traditional pickling process is too long and results in an excessive salt content in SEW, making it inedible. Therefore, it is necessary to develop rapid processing methods such as physical treatments and chemical additives to reduce the salt content in SEW and improve the quality of SEY. Recently, the preference for SEY flavors has also led to significant wastage of high-salt-content SEW and deterioration of quality, resulting in substantial wastage of high-protein resources and environmental pollution that hinder its industrialization. Hence, exploring value-added technologies for utilizing SEY as functional ingredients becomes essential while converting waste high-salt-content SEW into valuable ingredients through desalting and hydrolysis treatments. This will contribute to the development and mass production of sustainable, high-value-added nutritional egg products that are environmentally friendly. |
1. Introduction
Poultry eggs are widely acknowledged as a highly nutritious food source for a healthy diet, supplying the human body with essential proteins, fats, carbohydrates, minerals, and vitamins. As such, they have gained immense global popularity.1 China has been the leading producer and consumer of fresh poultry eggs since 1985, with an annual yield of over 4 million tons. Approximately 5%–7% of these eggs are allocated for processing purposes, while traditional egg products account for a remarkable 80%.2,3 Salted eggs are a prominent traditional egg product in China that enjoys popularity among consumer groups worldwide.Traditionally, this process involves immersing fresh eggs in a highly concentrated salt solution or wrapping them with a mixture of soil (or plant ash), salt, and water for approximately 20–45 days (Fig. 1).4–8 However, traditional production of salted eggs requires prolonged pickling time owing to natural infiltration and results in high salt content in the products owing to the use of high concentration salt solution, thereby increasing multiple disease risks such as hypertension and arteriosclerosis.9,10 In addition, only in China, over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of SEW with relatively higher salt content (7–12%) and unacceptable odor are discarded annually due to their inedibility, leading to a substantial waste of high-quality protein and also resulting in environmental pollution.9,11
000 tons of SEW with relatively higher salt content (7–12%) and unacceptable odor are discarded annually due to their inedibility, leading to a substantial waste of high-quality protein and also resulting in environmental pollution.9,11
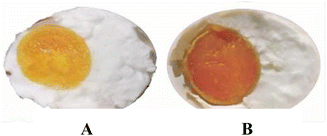
Nowadays, there is a growing concern about shortening the pickling time from the utilization of emerging pickling-assisted technologies, diverse composition and concentration of salt solution, as well as the incorporation of special chemical additives such as alcohol, spices, and acidic or alkaline components.12–14 However, the formation mechanism of salted egg is extremely complicated due to its complex composition. Therefore, there has been a growing emphasis on the investigation of the kinetics and mechanisms of salting in these methods to improve processing efficiency and achieve precise quality control.12 Additionally, appropriate storage and heating processes are necessary to achieve the distinctive characteristics of SEY before consumption (Fig. 2).15 However, in mass production, SEY deterioration may occur in the production process, including vacuum package, leading to phenomena such as “muddy” appearance or hard-core texture along with blackened yolks after cooking (Fig. 3). This downgrades the product quality and its commodity value. However, quality is one of the major factors in food production and consumer acceptability.
Another challenge for salted eggs lies in the higher salt content resulting from the traditional pickling process. The key is to achieve a balance between salt intake and quality. However, reduced salt and high-quality salted eggs is difficult because salt also plays structural and preserving roles in the products. Excessive salt infiltration can lead to an overly salty taste in SEW,9 while insufficient infiltration may fail to showcase the superior characteristics of SEY. The reduction in the content and a substitute of NaCl can be achieved by using alternative additives and improved processing technologies. For example, the salt content of egg white (EW) and egg yolk (EY) decreased to 5% and 1%, respectively, under the combined effect of vacuum and salt, achieving only about half the reduction compared to the traditional processing method without compromising their good quality. This indicates that a combined technology and additive approach offers more potential for producing healthy and attractive salted eggs in a shorter pickling period.16
Focusing on the preference for SEY texture and flavor, two distinct research fields, value-added SEY application as functional ingredients17 and convert wastage higher salt content SEW, commonly found in traditional processing, into valuable ingredients by desalting and hydrolysis treatments, revealed the state-of-the-art technologies in the development of salted egg industry for people with diverse requirements.18 Some researchers have also attempted separate pickling of EY to avoid generating more SEW. However, the resulting product still exhibits inferior taste and odor compared to traditionally processed whole eggs.
The objective of this paper is to systematically and comprehensively review the research progress on the quality formation mechanism, innovative processing technology with shortened pickling time, improved quality and reduced-NaCl, as well as high-value utilization of salted egg. Additionally, it aims to propose future research directions that can enable precise control over the entire production process of preserved eggs. Fig. 4 provides a schematic description briefly introducing salted eggs in this review. Section 2 evaluates the process of mass transfer of water and salt, alteration of protein conformation, and the formation of various small molecules influenced by various factors such as salt and temperature. Changes in textural properties, microstructure, color and flavor of salted eggs were also explored further to elucidate the formation mechanism behind their unique characteristics. Section 3 presents various approaches to enhance the quality and processing efficiency of salted eggs by focusing on how they work and their industrial applicability. The processing technologies of low-salt salted eggs are also discussed in this section. Section 4 investigates value-added techniques applied to salted eggshells, SEW, SEY, and other related potential aspects. Finally, in section 5, the challenges and prospects associated with the whole salted egg processing are also elaborated.
2. Factors influencing quality attributes formation of salted eggs
Eggs are composed of three parts: eggshells with membrane, EW and EY, which produce different effects in the curing process owing to the variation of constituents (Table 1). Generally, high-quality salted eggs should have a smooth, fresh, white, and delicate appearance in SEW while exhibiting an orange-red color, oily texture, sandy consistency, and abundant nutrients in SEY. Table 2 presents the properties of salted eggs. The development of these characteristics is highly associated with the mass transfer of water and salt, alterations in protein conformation, and the synthesis of various small molecules influenced by factors such as salt solution concentration and composition, temperature, and preservation methods, among others.4,26 Details are presented in Table 3.| Major components | Relative percentage (%, W/W) | References | |
|---|---|---|---|
| Eggshell (6%) | Ash | 94.6 | 19 |
| Protein | 3.9 | ||
| Calcium | 34.1 | ||
| Magnesium | 0.3 | ||
| EW (58%) | Water | 88 | 20 |
| Protein | 10.5 | ||
| Ovalbumin: 5.67 | |||
| Ovotransferrin: 1.37 | |||
| Ovomucoid: 1.16 | |||
| Ovomucin: 0.68 | |||
| Lysozyme: 0.36 | |||
| Ash | 0.8 | ||
| Carbohydrates | 0.5 | ||
| Lipids | 0.2 | ||
| EY (36%) | Lipids | 62.5 | 21 and 22 |
| Triglycerides: 38.8 | |||
| Phospholipids: 20.6 | |||
| Cholesterol: 3.1 | |||
| Protein | 33 | ||
| LDL: 7.6 | |||
| HDL: 11.6 | |||
| Livetin: 9.9 | |||
| Phosvitin: 3.6 | |||
| Minerals | 3.5 | ||
| Carbohydrates | 1.2 | ||
| Carotenoids | <0.6 |
| Properties | Content | References |
|---|---|---|
| Textural | Hardness | 23 |
| Adhesiveness | ||
| Gel strength | ||
| Cohesiveness | ||
| Springiness | ||
| Gumminess | ||
| Viscosity elasticity | ||
| Physical–chemical | Weight | 4 and 24–26 |
| Water content | ||
| Salt content pH | ||
| Morphological & dimensional | Hardening ratio | 4, 23 and 24 |
| Microstructure | ||
| Sensory | Color | |
| Nutritional (improved) | Amino acid content | 24 |
| Volatile substances composition | ||
| Phospholipid content | ||
| Cholesterol content | ||
| Functional (improved) | Gelling | 17 and 25 |
| Foaming | ||
| Emulsion |
| Factors | Ingredient | Quality changes | Mechanisms | References |
|---|---|---|---|---|
| Salt penetration rate | EY | Texture | Lipid–water migration | 23 and 27 |
| Viscosity | ||||
| Oil exudation | ||||
| Solidification | ||||
| Granularity | ||||
| Taste | ||||
| Flavor | ||||
| Types of salts | EY | Functional | Ionic characteristics effect on gel strength | 28 |
| MgCl2 and CaCl2 modify visco-elastic properties | ||||
| Salt concentration | EW | 0.1 M NaCl | Performance and appearance of coagulum-type gel formation and protein adsorption at the air–water interface | 12 and 29 |
| ↑Elasticity | ||||
| ↑Gel strength | ||||
| ↑Sensory | ||||
| 0.9 M NaCl | ||||
| EY | ↓Gel consistency | |||
| ↑Foaming with ↑ salt concentration | ||||
| 20% NaCl | ||||
| ↑Sandy texture | ||||
| ↑sensory | ||||
| Prolonged salting time | EY | ↓Hardness | NS | 12 |
| ↓Springiness | ||||
| ↓Gumminess | ||||
| ↓Chewiness | ||||
| Post-curing | EW | ↑Texture | Continued permeation of salt from the SEW into the SEY and water removal from SEY to SEW owing to the concentration difference | 22, 26 and 30 |
| EY | ↑Color | |||
| ↓Salt content in EW | ||||
| Heat | EY | ↑Texture | Reorganize and aggregate lipids separated from lipoproteins and protein released from particles during pickling | 9 and 31–57 |
| ↑Flavor | ||||
| ↑Color | Synergistic reaction among lipid oxidation, lipid migration and Maillard reaction | |||
| ↑Gelling | ||||
| ↑Nutrient content | Synergistic reaction between lipid oxidation and Strecker degradation | |||
| ↑Bioavailability of xanthophylls | ||||
| Preservation after heating | EW | ↓Moisture content | Yolk particles form large aggregates through electrostatic interactions | 22, 30, 49 and 58–61 |
| ↓Salt content in EW | ||||
| ↑Hardness | ||||
| ↓Cohesiveness | ||||
| EY | ↓Springiness | A transition of water molecules in lipoproteins from a bound state to free water | ||
| Structural | ||||
| ↓Flavor |
2.1 Salt effect on the quality formation of salted eggs
Salt, as the most important pickling raw ingredient, plays an indispensable role in the formation of the characteristic quality of salted eggs. During pickling, osmotic pressure, resulting from the concentration difference between the interior and exterior of eggs, enables salt to diffuse and penetrate through both the porous eggshell and inner membrane, facilitating simultaneous moisture removal. The variation in salt content and moisture content in EW and EY during pickling under traditional processing is illustrated in Fig. 5.62During the pickling process, salt penetrated the egg from the eggshell into both the EW and yolk. Salt contents in both EW and EY greatly increased, and coincident moisture content decreased during pickling.9,63,64 However, the NaCl penetration into the EY is comparatively lower than that into the EW, primarily due to the presence of yolk membrane and high-fat content (Fig. 6). Additionally, gel structure formation during pickling also hinders infiltration of excessive NaCl into the EY.4
 | ||
| Fig. 6 A schematic of the behavior of the protein and water molecules in EW during pickling with salt. | ||
During the pickling process with salt, the EW gradually loses viscosity, becomes watery and exhibits shear-thinning behavior due to osmosis (Fig. 7). Moreover, as salt gradually infiltrates into the EY from the EW, interactions and aggregation between protein molecules in EY are accompanied by alterations in spatial structure and the progressive development of a unique EY gel network structure with significant functional properties (Fig. 7).17,65
Specifically, desirable transformations occur in the structure of low-density lipoprotein within the EY. More proteins are released, primarily composed of neutral lipids and phospholipids, as illustrated in Fig. 7, which undergo subsequent interactions and random aggregation to facilitate enhanced polymerization, leading to increased oil exudation and solidification and thereby creating the desired textural granularity.16,23,66,67 The SEY can be categorized into internal and external yolks, which become increasingly distinguishable with longer salting duration. Interestingly, during the salting process, both the internal and external yolks tend to congeal separately, resulting in a progressive solidification starting from the interior region while facilitating an augmented oil flow in the exterior region.4 As the salt was further penetrated, more moisture was removed, and the compactness of the yolk sphere gradually decreased, providing more space for oil exudation.4,26,67,68 The sandy texture and oil leakage of EY gradually developed. However, too much water loss can result in the formation of a “hard heart” within the yolk. Consequently, the oily and sandy properties of salted duck eggs deteriorate, resulting in an overall decline in quality.
Besides, the salt penetration rate significantly affects the texture of salted eggs by influencing lipid and water migration in egg yolks. Changes in the distribution, state and migration of water were further analyzed by LF-NMR and magnetic resonance imaging (MRI) techniques.27 The results revealed significant changes in proton mobility during EY gelation, lipid release, and EW hydration. Furthermore, oil migration within duck egg yolks during pickling enhances their taste and flavor profile.23
In addition, salts employed during the pickling process can modify the ionic characteristics of the formed gel, resulting in variations in the functional properties of salted eggs. However, different salts exhibit varying degrees of ionic character and exert distinct effects on gel strength. For example, the presence of NaCl can result in a weakened gel structure due to excessive coagulation of egg proteins in egg albumen.28 MgCl2 and CaCl2 possess the capability to modify the visco-elastic properties of albumen gel with relatively satisfactory strengthening effects. The presence of Ca2+ and Mg2+ ions induced by these salts leads to less homogeneous gels with randomly aggregated clustered particles. Besides, this novel design also provides good insights for low-sodium salted egg development.
Moreover, the concentration of NaCl also plays a crucial role in determining gel strength. Treatment with 0.9 M NaCl led to a reduction in gel consistency. However, the addition of 0.1 M NaCl slightly enhanced both the elasticity and strength of the gel.69 In addition, NaCl plays a pivotal role in both the performance and appearance of coagulum-type gels formed from cooked salted duck albumen. Higher concentrations of NaCl can lead to the formation of gels with an opaque color and rough texture.70 Furthermore, the foaming properties of salted eggs are significantly influenced by increasing salt concentration due to enhanced protein adsorption at the air–water interface.28,29,71,72 A concentration of 20% NaCl solution was recommended for the rapid salting of hen egg yolks alone, resulting in an enhanced sandy texture and improved sensory quality.12 Moreover, prolonged salting time results in a reduction in the hardness, springiness, gumminess, chewiness, and resilience of salted duck egg gel.
The gelatinization of the yolk is initiated by the release of yolk granules, which are facilitated by fractures in the EY sphere during salting. Consequently, the yolk exhibits a semi-solid appearance with an elevated storage modulus (G′).73 Various constituents present in the yolk, such as granule spheres and LDL suspended in solution, possess the capability to form a gel.66,68 The salting process resulted in the formation of an elastic gel, which was observed through changes in G′ and loss modulus (G′′). Previous studies have reported that NaCl infiltration increases viscosity and modifies the visco-elastic behavior of yolk by delaying the formation of a gel network structure.23,65 Additionally, emulsified native yolk lipid and soil droplets during salting processing influence the rheological properties of SEY.74 Furthermore, adhesiveness and springiness can be modulated by G′ and G′′, indicating that viscosity and elasticity can potentially be controlled during the salting process.23
The quality control of salted eggs is currently challenging due to the reliance on workers' expertise in their preparation. The investigation of dynamics is crucial in this context as it can provide valuable insights into the diffusion and mass transfer characteristics between phases within a system. Xu et al.17 and Wang et al.30 conducted research on the kinetics of mass transfer by monitoring changes in quality, moisture, and salt content during the salting process to gain a better understanding of salt diffusion, water removal, and the resulting changes in quality of salted egg, and models were developed to predict the penetration and diffusion of salt during the salting process of hen egg yolks and the whole egg. This enables effective control of the salt content and moisture content in EW, EY and the whole egg, as well as the quality of final products.75,76
In brief, salt is essential for achieving exceptional quality in salted eggs. During the salting process, the rate of salt penetration, composition and concentration of the pickling solution, and pickling time have an impact on the physical, chemical, sensory, texture, functional, and microstructural characteristics of the salted egg. Moreover, salt penetration inhibits harmful microorganism growth, thereby extending product shelf life.77
2.2 Post-processing effect on the quality formation of salted egg
Post-processing of salted eggs refers to the steps taken after pickling and before consumption, including curing, heating, and preservation. Currently, more attention is being given to studying the continuous effects of cooking and storage methods on SEY after salting, which is in line with modern food consumption needs and the future trend of healthy food development.The composition of EY proteins primarily consists of 68% LDL, 16% high-density lipoproteins (HDL), 4% phosvitin, and 10% livetins (Table 1).33 These abundant protein components play a significant role in the formation of gels,34 which greatly enhance the shape and texture of food by increasing viscosity and water-holding capacity.35 Research has demonstrated that gel formation modifies the texture and taste of various processed foods such as modified meat products, fish balls, imitation crab, and surimi,36 due to improved viscosity and water retention properties during processing for maintaining fat content and stickiness.35 Additionally, the rheological and textural properties of salted eggs are also influenced by gel properties and thermal coagulation of salted egg proteins.37 Xu et al.17 also investigated changes in gelation behavior between raw and cooked salted duck EY during pickling. Differences in secondary structures were observed but no alterations in protein patterns were noticed. During pickling, gelling properties were significantly enhanced, making them highly desirable functional material additives in food products for the development of innovative foods with unique texture, taste, and flavor.9,17,26,38,63 The gel formation in SEY involves multiple interactions between protein molecules as well as between protein and lipid molecules. Various factors such as heating, high-pressure processing, freezing, emulsifying surfactants, and enzymes can induce the formation of EY gels.39–45 However, the main factors affecting the gel properties of proteins in food are salt concentration and temperature.22,46,47
Color is an intuitive indicator that directly influences the consumer acceptance of any food product. Orange-red raw SEY is commonly recognized as a key crucial factor in assessing the quality of salted eggs, to a greater extent, and is a very important attribute in determining the market potential and popularity of salted eggs. According to Liu et al.,12 the color change in SEY was primarily attributed to non-enzymatic browning during pickling. The precipitation of free fatty acids in the yolk leads to an increase in the lightness (L*) value, while oxidation of fat and protein increases the concentration of the pyrrole pigment during curing, resulting in a decrease in both redness (a*) and yellowness (b*) values. In contrast, the reduction in a* value can be attributed to non-enzymatic browning caused by amines present in EY and brain peptides, along with an increase in yellow pigment concentration due to dehydration.48 Additionally, non-enzymatic browning caused by aldol condensation reaction between carbonyl compounds in unsaturated fatty acids and free protein radicals contributes to the decrease in b* value. Furthermore, the polymerization of linolenic acid and lysine also enhances non-enzymatic browning and promotes the formation of fluorescent substances. However, increased salt content and decreased water content may also contribute to darkening surface color through improved pigment concentration and water retention capacity within the yolk. Moreover, after cooking, L*, a*, and b* values tend to increase further with subsequent storage periods. Similarly, the total color difference (ΔE) shows an upward trend after cooking.12,49
The unique composition of the EY lipid, primarily composed of 62% triglycerides, 33% phospholipids, and approximately 5% cholesterol (Table 1),56 rendering it an oily liquid, presents significant potential for its application in food processing.50 Research has demonstrated that the lipid component in SEY plays a crucial role in contributing to its distinctive flavor profile.51 The lipid content of the EY increased, and the oxidation level of the EY lipid varied depending on the conjugated diene acid value and thiobarbituric acid value under high salt conditions and heat treatment.26
The substances generated through the decomposition of fatty acids and the degradation of vitamins caused by lipid oxidation can impact the flavor profile of food, contributing not only to the distinctive taste found in traditional pickled foods but also to a decline in overall flavor quality. Yu, et al.52 investigated 19 types of volatile organic compounds (VOCs) as potential flavor substances contributing to the cooked SEY flavor. Wei et al.53 reported that 1-octen-3-ol, benzaldehyde, 2-pentyl-furan, nonanal, and (E,E)-2,4-decadienal are unique volatile compounds found in SEY. The pickling process-induced lipid oxidation generated volatile flavor compounds such as aldehydes, ketones, esters, and acids, which could lead to oxidative rancidity of lipids, with excessive lipid oxidation significantly impacting the final quality of SEY.54 It has been documented that lipid oxidation and Maillard reaction play a role in promoting the formation of volatile compounds in SEY.55 These reactions interact with each other to produce volatile flavor components.56 Moreover, lipid migration also played a crucial role in the flavor formation of SEY. However, the specific VOCs associated with cooked SEY and their impact on Maillard reaction and lipid oxidation in SEY flavor formation remain unclear.
Some studies have also indicated that the distinctive flavor of SEY may arise from the synergistic reaction between lipid oxidation and Strecker degradation.57 For example, Wang et al.78 investigated the correlation between the oxidation stage and flavor formation, revealing that amino acid metabolism, pentose phosphate pathway, and linoleic acid metabolism occur during the secondary oxidation stage, playing a crucial role in shaping the unique flavor profile. In conclusion, the secondary oxidation stage appears to be more significant for the development of distinct flavors of SEY.
However, the mechanism underlying salt-induced flavor in salted eggs is highly intricate and remains unclear. It is widely recognized that appropriate lipid oxidation can enhance flavor formation; nevertheless, excessive lipid oxidation contributes to food quality deterioration as well.79 Therefore, it is imperative to control lipid oxidation within an optimal range during processing. Nonetheless, comprehensive research on SEY lipids, quality attributes, and flavor formation, particularly regarding volatile and non-volatile flavors accurately induced by different levels of lipid oxidation (primarily primary and secondary oxidations), remains limited.
Furthermore, heat treatment enhanced the bioavailability of xanthophylls such as lutein and zeaxanthin.80 However, it also resulted in the formation of an outer black layer accompanied by an inner hard core that significantly reduces sensory quality and edibility. The specific effects and mechanisms still require exploration in order to achieve desirable oil yield and grittiness while avoiding the occurrence of an outer black layer or inner hard core.
In addition, the most noticeable changes observed in cooked SEY included its tendency to become “muddy” after vacuum packaging, indicating a soft texture, absence of oil droplets, and reduction in sandy texture.59 These alterations ultimately led to a deterioration in the quality of salted eggs. Xue et al.60 reported that the muddiness of SEY was primarily attributed to increased fluidity and re-emulsification of proteins and lipids, as well as lipid oxidation during cooking treatment. Heating led to the denaturation of LDLs, coinciding with the release of lipids, resulting in the disintegration of lipid structures (Fig. 6), further impacting their quality during storage.30,61 The presence of some non-free LDLs in a granular form gives the salted egg a compact sandy-like texture.26
3. Rapid processing and quality improvement technologies of salted eggs
The long production cycle and inconsistent quality of traditional pickled salted eggs have always posed obstacles to the development of the egg processing industry. In order to enhance production efficiency and improve product quality, various emerging technologies and chemical additives have been developed to overcome these limitations, aiming for industrial-scale production. This section will focus on recent research progress in innovative technologies for salted egg pickling, including ultrasound-assisted salting technology,81 magnetoelectric-assisted rapid technology,82 water-cycle technology, vacuum decompression, and pulsed pressure osmotic dehydration technology30,83 chemical additives, among others. Details regarding these technologies can be found in Table 4.| Treatment | Mechanism | Pickling period | Quality changes | Existing problems | References |
|---|---|---|---|---|---|
| Ultrasonic-assisted pickling | Thermal, mechanical and cavitation effects | 20–25 days | ↑Oil yield | Quality deterioration with high-intensity, unclear influences of thermal caused by ultrasound on the quality of salted egg | 61 and 84–88 |
| ↑Color | |||||
| ↓Size of the hard core | |||||
| ↓Viscosity of EW | |||||
| ↑Hardening rate of EY | |||||
| Surface morphology of EW | |||||
| Functional properties | |||||
| Magnetoelectric-assisted pickling | Large-scale directional motion of free ions | 7 days | ↑Oil yield | Device is complicated and expensive | 81 |
| ↑Salt content | |||||
| ↑Physico-chemical properties | |||||
| Water cycle pickling | Enhancement of solute permeability while improving fluid homogeneity | Not be as effective at shortening curing time | ↓Proportion of muddied yolk | Inconspicuous effect on shortening the curing time | 89 |
| Vacuum decompression pickling | Hydrodynamic mechanism and deformation relaxation phenomenon | Reduces pickling time by half | ↑Color | Uniformity of curing | 90–94 |
| ↑Taste | |||||
| Vacuum decompression-citric acid pre-treatment | Citric acid's ability to modify eggshell permeability | Reduction of approximately two-thirds in curing time | Uniformity of curing | 92 | |
| Vacuum-assisted two-stage pickling | ↓Salt content (50%) with good quality | Process complicated | 16 | ||
| Pulsed pressure pickling | Pressure gradients | 2–3 days with post-curing treatment | ↑Oil exudation | Uneven pickling | 82 and 95 |
| ↑Grittiness | |||||
| Microstructure | |||||
| Ultrasonic-pulsed pressure technology | Less than 3 days | ↑Taste | Synergistic mechanism | 96 | |
| ↓Salt content in EW | |||||
| ↑Salt content in EW | |||||
| Enzymatic hydrolysis-microwave irradiation | ↓Salt content with good quality | 97 | |||
| Sodium dihydrogen phosphate, sucrose-phosphate, and carrageenan | ↓Salt content with good quality | 80, 98 and 99 | |||
| CaCl2, ZnCl2, and ferric citrate | Promoted bulk protein aggregation | 32 hours | ↑Hardness | Incomprehensive impact mechanism | 100 |
| ↑Oil exudation | |||||
| ↑Sandiness | |||||
| ↑“Water filling” phenomenon | |||||
| Chemical additives (alcohol) | Shorten pickling time | Quality deterioration with high content of additives | 101 |
3.1 Emerging pickling technologies for salted egg
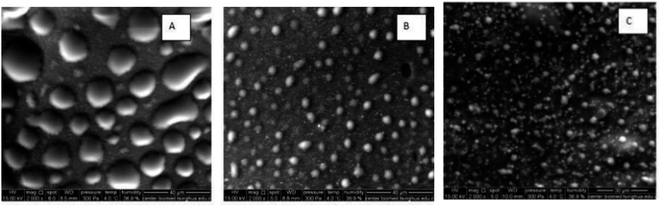
This process also enhances the salt distribution between EW and EY, improving the overall quality of salted eggs with reduced occurrences of hard-core formation by reducing viscosity and surface tension in the EW while increasing the hardening rate of the yolk and protein solubility. Additionally, it increases free sulfhydryl group content and surface hydrophobicity.81,85,87,88,102 Moreover, ultrasonic treatment alters both aggregation and surface morphology of egg whites while inducing LDL aggregation and partial dissociation of yolk granules, thereby directly modifying the functional properties of yolks.84 However, excessive ultrasound intensity may lead to disintegration of the eggshell with air infiltration, resulting in a honeycomb-like structure after heating. Furthermore, the thermal effects caused by ultrasound on the quality of salted eggs remain unclear, and specific parameters as well as mechanisms underlying these quality changes still require further exploration.
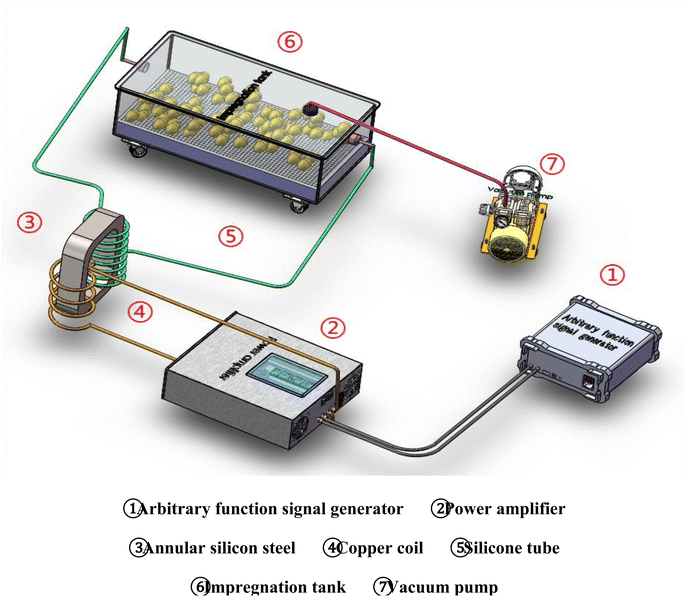
During the curing process, sodium and chlorine ions are mobilized in an alternating electromagnetic field to enhance the mass transfer rate of sodium chloride in EW and yolk, thereby improving the curing rate. Yang et al.82 successfully adjusted parameters such as voltage amplitude, electric field frequency, radial magnetic field rotation frequency, magnetic field strength, and equipment specifications to control the curing time and understand its impact on egg quality. Their findings suggest that optimal salted egg quality can be achieved within approximately 7 days while significantly enhancing yolk oil exudation. Moreover, increasing voltage and magnetic field strength also improve the salt content and oil yield of salted eggs. However, it should be noted that this device is complex and expensive (Fig. 9). Further research is needed to explore its application in egg processing and elucidate its working mechanism.
By incorporating pumps to circulate the pickling liquid within the container, this technology effectively reduces the salinity difference in salted eggs, significantly enhancing the homogeneity of the salt solution and inducing conformational changes in hydrophilic substances present in eggs. These alterations facilitate hydrogen bond formation between these substances and the hydroxyl group or hydrogen of water,89 ultimately reducing resistance to pickling liquid flow and ensuring even and rapid penetration into each egg. Pu et al.103 observed that this technique partially shortens the pickling time for salted eggs. Furthermore, water cycle technology facilitates a more uniform distribution of salt concentration within EW, resulting in diminished disparities in salt content between EW and yolk while substantially decreasing muddied yolk proportion. It is worth noting that compared to other expedited curing techniques, water cycle pickling technology may not be as effective at shortening curing time; therefore, further research is necessary to gain a better understanding of its effects and working mechanism.
However, there are limited reports on the discernible flavor difference between traditional and vacuum pickling methods. Additionally, it is worth noting that both EW and EY in vacuum-assisted two-stage pickling showed only 50% of the salt content found in traditional curing methods without compromising their distinct quality.16 This provides new insights for producing low-sodium salted eggs.
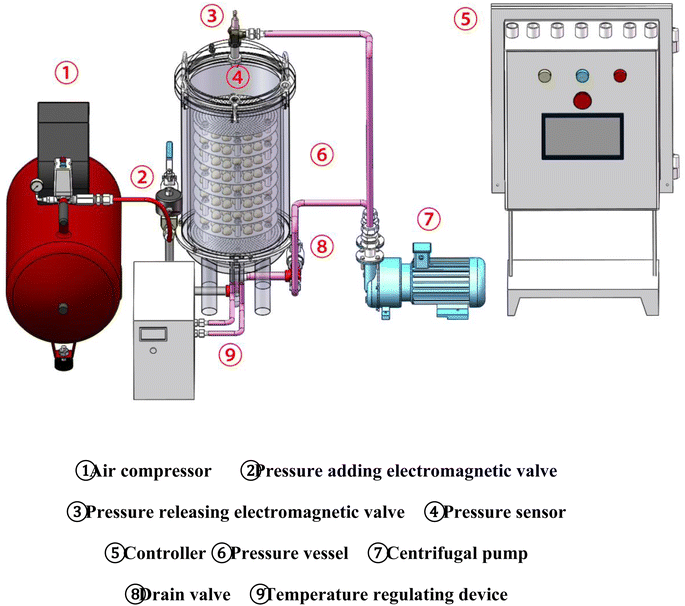
The pulsed pressure pickling technology has successfully reduced the egg moisture content and improved processing efficiency and final product quality compared to the traditional method.30,47,74,83,96 Additionally, oil exudation from EW continues to increase during pickling, while the texture of the EY gradually becomes firmer and its viscosity decreases rapidly over time, resulting in a watery consistency after 48 hours. Furthermore, NMR technology revealed that free water content in the EY progressively diminishes while bound water proportion significantly increases during pickling. The increased salt content causes protein denaturation in the yolk, resulting in reduced free water and increased yolk hardness during pickling. The total water content of EY significantly decreases, while there are no significant changes in the protein water content. The ESEM analysis showed that yolk granules aligned closely and became progressively smaller after pickling. Salted eggs exhibited a higher number of larger holes compared to fresh eggs, which facilitated faster infiltration during the pickling process. Furthermore, pulsed pressure equipment has gained rapid popularity in recent years due to its simple operation and low cost. The operation process of this equipment has been greatly simplified with the introduction of automatic pressure relief control. Oscillation devices have also helped overcome the disadvantages of uneven pickling. Ultrasonic assistance was utilized to obtain SEY after 3 days of salting in a solution with a mass fraction of 24%.101 However, the precise control of pressure addition and relief time, as well as its impact on product processing time and quality, still needs to be strengthened. Additionally, more research is required to investigate the effects of pulsed pressure processing on the final product quality aspects and the underlying mechanisms.
3.2 Chemical additive processing for quality-improved salted eggs
Recently, there has been a growing emphasis on partially replacing salt or chemical additives to produce enhanced reduced-sodium salted eggs due to their suitability for large-scale production at low cost and high efficiency.Alcohol can alter the eggshell microstructure, facilitating increased salt infiltration through the ESM. The free H+ ions in HCl interact with the eggshell to create a water–soluble interface with CO2 and Ca2+, which positively affects the pores of the eggshell and accelerates the infiltration of pickling agents, significantly reducing pickling time.98
Furthermore, sodium dihydrogen phosphate, sucrose-phosphate, and Carrageenan are also used to enhance the gel hardness and improve the water-holding capacity in reduced-salt cured foods, resulting in a shorter pickling period and higher quality.58,99,108 Besides, antioxidants like polyphenols and flavonoids are used to enhance the appearance of low-sodium salted eggs.
In terms of flavor enhancement, various spices were considered as potential additives to the pickling solution for their flavoring properties. However, the most commonly used substitutes are several metal salts, including KCl, CaCl2, and MgCl2. Fan et al.100 found that incorporating divalent or trivalent metal salts like CaCl2, ZnCl2, and ferric citrate into the pickling solution improved the hardness, oil exudation resistance and sandiness of SEY while reducing pickling time to 32 hours. Additionally, these metal salts also improved the “water filling” phenomenon. The impact of metal salts on the SEY quality attributes remains largely unexplored.
The salting kinetics, textural properties, water migration, protein aggregation, and structure of SEY were assessed by Liu et al.109 after heating for 24 hours in the presence of CaCl2 before vacuum packing. The findings demonstrated that CaCl2 significantly promoted bulk protein aggregation, greatly improving the degree of protein polymerization.
The findings showed that CaCl2 significantly promoted protein aggregation, greatly improving protein polymerization. Recent studies revealed that CaCl2-induced changes in lipoprotein structure and increased water loss in EY were the main factors contributing to enhanced oil output and sandy texture during rapid pickling of separated EY.110
Furthermore, the addition of CaCl2 improved HDL structural orderliness and loosened LDL structure, effectively alleviating HDL aggregation behavior. Additionally, the inclusion of CaCl2 resulted in a decrease in the relative content of intermolecular β-sheets within HDL and LDL secondary structures, significantly impacting their tertiary conformation and enhancing SEY quality.111 For instance, adjusting the proportion of metal ions in the brine solution, such as calcium, potassium, magnesium, and zinc, has the potential to enhance low-sodium salted egg products without affecting osmotic pressure. However, limited research exists on how other additive components in the pickling solution impact HDL and LDL structure during salting.
3.3 Other rapid processing technologies of salted eggs
Combining salt and temperature (20–45 °C) during pickling can reduce the production cycle by almost 50%, increasing the likelihood of softening SEY with a declined edible and processing quality. Quantitative proteomics analysis revealed that HDL may be involved in the formation of softness in SEY. The presence of certain antioxidants can effectively inhibit the oxidative degradation of components such as HDL, thereby mitigating the adverse effects of high-temperature curing on both the macro-performance and micro-composition of salted duck egg yolk.112 However, the synergy effect of combined salt temperature still needs to be strengthened to reduce the occurrence of undesirable quality under rapid pickling.4. Value-added applications of salted eggs
The nutritional benefits of eggs are widely acknowledged, and salted eggs, in particular, offer higher levels of proteins, carbohydrates, lipids, and smaller peptides compared to fresh eggs.25,97 Additionally, SEW and SEY possess unique characteristics and functional properties, such as foaming, emulsion, and gelling abilities, making them suitable for various food applications (Fig. 11).17,25,97 The following sections will discuss in detail the use of SEW and SEY, as well as salted eggshells and ESM.4.1 Functional property improvement of salted eggs
The functional properties of proteins include solubility, gelling, foaming and emulsifying (Table 5). Currently, SEY is extensively utilized as a functional material due to its exceptional gelling properties, contributing appealing colour, flavour, and a distinctive grainy texture.18 Nevertheless, the high demand for traditional Chinese foods like mooncakes and glutinous rice dumplings generates significant waste of eggshells and SEW. The potential use of eggshells and SEW is of great interest to the industry.64| Treatment | Functional properties | Mechanism | References | |
|---|---|---|---|---|
| ESM | Enzymolysis-ultrasound | ↑Foaming | Cavitation effect | 113 |
| ↑Emulsifying | ||||
| Adding starch | ↑Emulsifying | ↓Droplet flocculation | 114 | |
| Microbial fermentation | ↑Foaming | ↓Interfacial tension | 115 | |
| ↑Emulsifying | ||||
| SEW | Adding polysaccharide | Gelling | ↑Electrostatic interactions | 116 |
| ↑Foaming | ||||
| ↓emulsifying | ||||
| Desalted and drying | ↑Foaming | ↓Electrostatic repulsion | 117 | |
| Enzymolysis | ↑Foaming | NS | 118 |
Insolubility and numerous disulfide bonds of ESM pose challenges to its effective utilization. The value of ESM can be enhanced through techniques such as enzymatic hydrolysis and microbial fermentation. Enzymolysis combined with ultrasound treatment enhances solubility, foaming, and emulsifying properties with poor emulsion stability due to the cavitation effect of ultrasonic treatment, which reduces the interfacial tension and improves functional properties.113 The addition of resistant starch could improve emulsion stability and extend oil oxidation. Microbial fermentation used in ESM presents good foaming (36.7%), emulsifying activities (94.6 m2 g−1), solubility (90.7%) and biological activities of ESM hydrolysates.114 The addition of resistant starch could enhance its emulsion stability and effectively delay oil oxidation.
The major functional properties of EW are emulsifying, foaming, and gelling. These properties can be influenced by environmental factors such as salt and temperature.117 However, even after desalting and spray drying, the emulsifying property of SEW was significantly lower than that of fresh duck EW. On the other hand, different drying methods had a greater impact on the functional properties of desalted EW powder. Therefore, it is important to consider the effect of drying technology on the functional properties of SEW powder during food processing in order to obtain specialized SEW powder using appropriate techniques. Interestingly, enzymatic hydrolysis also enhanced the functional properties of SEW.118
4.2 Value-added applications of salted eggshell
Eggshells, often discarded for various reasons, have recently found significant applications as adsorbents due to their high calcium carbonate content (94%) (Table 1).119 It can effectively remove contaminants such as carbon dioxide,120 cyanide,121 methylene blue,122 acid mine drainage,123 and for dephosphorization purposes. The adsorption capacity can be further enhanced through calcination due to the conversion of calcium carbonate into calcium oxide. Therefore, the process has the potential to transform eggshell waste into valuable adsorbents. However, further exploration is needed regarding the utilization of salted eggshells.Moreover, due to their unique composition, eggshells have been utilized in other areas, including food additives, organic fertilizers, soil conditioners and calcium supplements.124,125 These diverse uses have greatly enhanced the value of eggshells.
4.3 Value-added applications of SEW
The EW, being a high-quality source of protein, has exceptional functional properties, such as foaming capability and stability, making it extensively utilized in various food processes, especially baking. However, the full utilization of SEW has been hindered by its high salinity.126 To minimize waste and enhance the value of SEW, emerging desalination and utilization methods are employed to convert waste SEW into valuable by-products.127However, the reutilization of SEW remains a significant challenge in the egg product industry due to the production of byproducts and effluents with high salinity, which adversely affects the environment and limits its application scope.70,128,129 Previous studies have demonstrated that desalinated duck SEW and its peptides exhibit bioactivities that improve calcium absorption, promote preosteoclast differentiation, provide anti-osteoporosis benefits, and enhance DPPH radical scavenging activity.11,130 Bioactive peptides have been extracted from enzymatically hydrolyzed SEY protein by Zhang;131 meanwhile, Zheng et al.132 optimized processing parameters to produce high-quality SEW protein. Huang et al.133 obtained SEW protein powder through freeze drying, spray drying, roller drying, and hot-air drying methods while conducting a comparative analysis of their respective qualities. However, there is limited research on the desalination of SEW protein. Desalinated SEW with low salt content may offer broader applications as a new type of high-protein resource while reducing environmental pollution.
A variety of desalination methods, such as ultrafiltration, electrodialysis, and ion-exchange column chromatography, have been used for SEW desalination.63,134–137 However, their industrial application for mass production is hindered by high costs and other disadvantages. Moreover, these techniques only achieve a modest reduction in salt content of 0.25–1%, falling short of the complete elimination of salt in SEW.11 The desalination rate is the lowest in ion-exchange column chromatography, followed by electrodialysis and ultrafiltration. Efficient desalination of SEW is a viable approach to enhance the quality and value utilization of this by-product in food manufacturing. Consequently, several scholars have proposed more efficient methods for desalinating SEW. Wang et al.63 introduced a straightforward and cost-effective approach by immersing the heat-induced gel in water to desalinate SEW. However, the foaming and gelling properties deteriorated rapidly due to severe denaturation of EW protein during thermal processing.138
Zhao et al.61 demonstrated the potential of desalted SEW nanogels as a stabilizer for food-grade pickering emulsions using a heat-induced gel-assisted desalination method while exploring several new technologies combined with the ultrafiltration method to enhance protein desalination efficiency from SEW. Wang et al.139 developed a cost-effective desalination method for SEW derived from microwave hydrothermal treatment, freeze-thawing, and solvent exchange processes. The adsorbent significantly enhanced its adsorption performance, facilitating efficient recycling of SDEW and minimizing by-product waste during mass processing. Du et al.140 employed similar techniques to stabilize O/W pickering emulsion and transform SEW by-products into valuable protein hydrogel ingredients. The effects of ultrasound and microwave treatments as pre-treatments prior to ultrafiltration desalination were investigated, resulting in approximately 10% higher desalination rates with ultrasound treatment and 3% higher rates with microwave treatment compared to the control group without any pre-treatment. Moreover, there was also a notable improvement in product quality. Based on the evaluation of foaming capacity and emulsifying index, ultrasound pre-treatment demonstrated superior outcomes compared to microwave treatment.135
Some technologies, such as enzymatic hydrolysis, have been used for the development and utilization of SEW in food processing.18,140 Yang et al.141 discovered that SEW can serve as a new water-retaining agent to replace phosphate in shrimp treatment after slight hydrolysis by protease or acid, enhancing phosphorus residue without compromising texture. However, the desalination technology has significant drawbacks, including higher cost, high protein loss rate, and low desalting efficiency,142 limiting its suitability for large-scale production. Therefore, there is an urgent need to explore alternative approaches for transforming surplus SEW into valuable ingredients.
Currently, SEW protein is utilized as a food additive to partially replace salt and enhance the gelling properties of various food products.64,143 A recent study has revealed the potential application of SEW in high-protein food production. For example, bioactive compounds like lysozyme can be extracted from SEW to create value-added products such as high-protein noodles.144,145 Furthermore, research has shown that using SEW as an alternative source for producing yellow alkaline noodles is more appealing than fresh duck egg albumen. Products made with SEW have higher protein content and improved color, aroma, and texture compared to yellow alkaline noodles without or with salt (1% of flour weight).64 Moreover, SEW, with a 20% inclusion, has also been used in steamed bread to enhance the rheological properties, volume and texture of bread, suggesting its potential for food industry application. Further research is needed on the nutritional properties, volatile components, and shelf life of SEW-enriched steamed bread. Moreover, the use of SEW shows promise in enhancing the overall quality of bakery products.146 Further research is needed to explore its nutritional properties, volatile components, and shelf life in potential products like SEW-enriched meringue.
Desalted SEW can also serve as a suitable fat substitute in mayonnaise at levels below 30%, according to Wang et al.63 Additionally, combining κ-carrageenan with SEW shows potential in modifying protein structure and gel properties while enhancing the effectiveness of SEW as an additive for surimi and related products.141,147
Therefore, the high-value utilization of SEW should focus on the ultimate effect of salt content on human health and its impact on protein structure and enzymatic hydrolysates. This is because these factors occur before dietary intake and greatly affect the structure and activity of the final product.
4.4 Value-added applications of SEY
The enhanced elevated protein and amino acid content of SEY contribute to nutritious and easily digestible food options.1,148 Furthermore, producers have shown significant interest in utilizing the alluring flavor and aroma characteristics of SEY in various consumables like pasta and noodles.23,149EY protein hydrolysate is an innovative protein source used for culinary advancements, and modifying this ingredient is crucial in developing flavor compounds.150,151 However, limited attention has been given to the similarity in flavor between SEY flavorings and traditional pickled EY within the food industry. Additionally, little research has been conducted on the effects of the Maillard reaction and lipid oxidation on forming SEY flavor from fresh EY. Therefore, exploring new methods for manufacturing high-quality processed SEY flavoring from separated fresh EY with greater efficiency would expand its applications within the food industry.
5. Challenges of salted egg processing and prospects
The technical challenges include prolonged pickling duration, lack of quality control, excessive salt content in SEW, and insufficient knowledge about post-processing techniques. To achieve mass production of salted eggs, it is crucial to have precise control over the entire processing procedure to ensure consistent adherence to acceptable quality standards. Additionally, limitations arise from a limited range of value-added applications. Researchers must further investigate the aspects mentioned above based on the evidence presented.(1) The mechanism underlying the formation of unclear quality in salted egg processing, including the impact of different processes on lipid status and distribution, is still not fully understood. Previous studies have primarily concentrated on the effects of lipid migration during emulsification heating and yolk fat distribution. Appropriate control should also be exercised over lipid oxidation during processing. However, comprehensive research has been limited regarding SEY lipids and their precise influence on quality and flavor formation induced by various levels of oxidation. It is necessary to conduct further investigations to examine mass transfer, explore the effects of salt, temperature, and post-curing methods on salted eggs synergistically, and further develop low-sodium salted egg processing. Furthermore, to meet diverse consumer demands, it is essential to develop various flavors of salted duck egg products with medicinal and healthy benefits, achieving a harmonious blend of delectable nutrition and sustainable well-being. Additionally, the use of advanced techniques, such as isotope labeling in characterizing salted egg flavor, is highly recommended. This will help evaluate the flavor formation process to precise localization of critical points of change in the flavor of salted eggs and provide a foundation for precise flavor control. In addition, the specific salt threshold that affects the texture and flavor of salted eggs is still limited and should be elucidated in the future.
(2) Besides technical challenges, it is crucial to acknowledge the need for theoretical research on quality degradation in relation to safety risks and precise control of salted eggs. A systematic investigation is required to explore the causes of oily and gritty texture, outer black circles, inner hard-cores in the yolk, as well as the “muddy” texture and related techniques.
(3) The limitations in the current research on combined processing technology, key technologies, and equipment for rapid production of salted eggs need to be addressed. Suggestions should also be made to optimize related parameters and further promote the development of mechanization and standardization, ultimately achieving large-scale production of salted eggs. Furthermore, there is an urgent need to develop techniques that ensure the overall high quality of final products. Additionally, it is imperative to investigate post-curing, cooking, preserving mechanisms, and methods for salted eggs in order to prevent any degradation in quality and provide convenient post-processing technologies for extended storage.
(4) Production of reduced-NaCl salted eggs poses challenges in process techniques and equipment. With increasing consumer demand for low-salt foods due to health concerns, further research is necessary on developing low-sodium pickling agents and equipment, establishing a rapid pickling process to meet future requirements. The exploration of a separate production technology of SEY is also a potential research direction.
(5) Moreover, it is imperative to prioritize exploring the high-value utilization of salted eggs, especially SEW. Economical and efficient desalinating technologies are required for SEW to facilitate the mass production of egg by-products. It is essential to explore an efficient extraction method and gain a deeper understanding of the functional properties of bioactive ingredients in order to optimize dietary utilization and further explore potential applications for promoting good health. Additionally, future focus on synergistic effects achieved through combining other bioactive components will be crucial in functional product development, with effectiveness evaluations and clinical applications being necessary steps towards enhancing dietary utilization and advancing the processing capabilities for salted eggs.
(6) Finally, a comprehensive examination of the acceptability, feasibility, stability, and potential safety concerns associated with salted eggs and their derivatives is imperative.
6. Conclusions
In recent years, the increasing market demand for salted eggs, especially SEY, necessitates more precise information to effectively control processing procedures, enhance processing efficiency, improve product quality with a low-sodium concept, and explore value-added applications.The formation mechanism of quality in salted eggs was comprehensively evaluated, including water and salt mass transfer, protein conformation changes, and synthesis of small molecules. This evaluation aimed to further understand the unique characteristics of salted eggs. Additionally, various pickling-assisted physical technologies and chemical additives were developed and assessed for efficient processing and improved quality. Furthermore, potential value-added applications of salted eggshells, SEW, and SEY were extensively summarized.
However, further studies are needed to clarify various aspects of salted egg processing, such as the mechanisms of quality formation, challenges related to reduced-NaCl production techniques and equipment, insufficient theoretical research on quality degradation and safety risks, and precise control over the final product's quality. Additionally, it is recommended that high-value utilization technologies for salted eggs be explored to fully exploit their potential in developing environmentally friendly nutritional egg products with enhanced value-added benefits.
Data availability
The data associated with the paper will be provided on request.Conflicts of interest
The authors declare no conflicts of interest. All authors have read and agreed to the published version of the manuscript.Acknowledgements
This research is supported by the 2115 Talent Development Program of China Agricultural University and Suzhou Polytechnic Institute of Agriculture PhD Promotion Program (No. BS[2022]17).References
- P. Ganesan, T. Kaewmanee, S. Benjakul and B. S. Baharin, Food Sci. Anim. Resour., 2014, 34(1), 1–6 CrossRef CAS PubMed.
- X. Ji, Y. Xu, J. Wang, W. Lyu, R. Li, S. Tan, Y. Xiao, B. Tang, H. Yang and M. Qian, J. Food Sci., 2021, 86(5), 2145–2162 CrossRef CAS PubMed .728914..
- Y. Wang, C. Xiong, W. Luo, J. Li, Y. Tu and Y. Zhao, Poult. Sci., 2021, 100(5), 101051 CrossRef CAS PubMed.
- S. P. Chi and K. H. Tseng, J. Food Sci., 1998, 63(1), 27–30 CrossRef CAS.
- Y. Tu, Y. Zhao, M. Xu, X. Li and H. Du, Food Anal. Methods, 2012, 6(2), 667–676 CrossRef.
- Y. Zhao, Y. Yao, M. Xu, S. Wang, X. Wang and Y. Tu, J. Funct. Foods, 2017, 35, 655–665 CrossRef CAS.
- S. Cheng, T. Zhang, X. Wang, Y. Song, H. Wang, H. Wang, P. Q. Yang and M. Q. Tan, LWT–Food Sci. Technol., 2018, 95, 143–149 CrossRef CAS.
- D. Chen, D. P. Balagiannis and J. K. Parker, Food Chem., 2019, 286, 71–77 CrossRef CAS PubMed.
- L. Xu, Y. Zhao, M. Xu, Y. Yao, X. Nie, H. Du and Y. G. Tu, PLoS One, 2017, 12(8), 0182912 Search PubMed.
- X. Zheng, X. Zhao, Y. Jin, L. Zhou, P. Yang, H. Ahmad and Z. Tian, Biochimie, 2021, 181, 154–161 CrossRef CAS PubMed.
- R. Thamamsena and D. Liu, J. Anim. Sci., 2020, 33(9), 1487–1496 Search PubMed.
- Y. Liu, Y. Ma, Y. Chi and Y. Chi, J. Food Eng., 2022, 329, 111090 CrossRef.
- K. Lai, W. C. Ko and T. Lai, Food Sci. Technol. Int., 1997, 3, 269–273 Search PubMed.
- Z. Liu and Y. Chi, Sci. Technol. Food Ind., 2011, 32(4), 236–239 CAS.
- P. Ganasen and S. Benjakul, J. Food Biochem., 2011, 35(5), 1528–1537 CrossRef CAS.
- L. Tan, M. Zheng, S. Chen, N. Wu, L. Xu and Y. Zhao, LWT–Food Sci. Technol., 2023, 189, 115480 CrossRef CAS.
- L. Xu, Y. Zhao, M. Xu, Y. Yao, X. Nie, H. Du and Y. Tu, Food Hydrocolloids, 2018, 80, 68–77 CrossRef CAS.
- N. Xiao, X. Huang, W. He, Y. Yao, N. Wu, M. Xu, H. Du, Y. Zhao and Y. Tu, Food Res. Int., 2021, 147, 1–58 CrossRef PubMed.
- S. M. Waheed, M. Yousaf, A. Shehzad, M. Inam-Ur-Raheem, M. K. I. Khan, M. R. Khan and R. M. Aadil, Trends Food Sci. Technol., 2020, 106, 78–90 CrossRef.
- S. Moreno-Fernández, M. Garcés-Rimón and M. Miguel, Trends Food Sci. Technol., 2020, 104, 208–218 CrossRef.
- N. Xiao, Y. Zhao, Y. Yao, N. Wu, M. Xu, H. Du and Y. Tu, J. Agric. Food Chem., 2020, 68(7), 1948–1957 CrossRef CAS PubMed.
- J. Camou, J. Sebranek and D. Olson, J. Food Sci., 1989, 54(4), 850–854 CrossRef CAS.
- M. Ai, S. Guo, Q. Zhou, W. Wu and A. Jiang, LWT–Food Sci. Technol., 2018, 88, 119–125 CrossRef CAS.
- K. Lai, Y. Chuang, Y. Chou, Y. Hsu, Y. Cheng, C. Shi, H. Chi and K. Hsu, Poult. Sci., 2010, 89(4), 729–737 CrossRef CAS PubMed.
- J. Sun, W. Zhou, L. Yan, D. Huang and L. Lin, J. Food Eng., 2018, 220, 1–11 CrossRef.
- T. Kaewmanee, S. Benjakul and W. Visessanguan, Food Chem., 2009, 112(3), 560–569 CrossRef CAS.
- X. Xiang, Y. Liu, Y. Liu, X. Wang and Y. Jin, Food Hydrocolloids, 2020, 98, 105257 CrossRef CAS.
- V. Raikos, L. Campbell and S. R. Euston, Food Hydrocolloids, 2007, 21(2), 237–244 CrossRef CAS.
- V. Plancken, L. A. Van and M. E. Hendrickx, J. Agric. Food Chem., 2005, 53(14), 5726–5733 CrossRef PubMed.
- X. Wang, Z. Gao, H. Xiao, Y. Wang and J. Bai, J. Food Eng., 2013, 117(1), 141–150 CrossRef CAS.
- J. Li, C. Wang, X. Li, Y. Su, Y. Yang and X. Yu, Food Biosci., 2018, 23, 115–120 CrossRef CAS.
- N. Yang, N. Yang and Y. Jin, RSC Adv., 2016, 6(99), 97089–97095 RSC.
- Y. Mine, M. Yang and I. Guerrerolegarreta, Handbook of Poultry Science and Technology, 2010, vol. 1, pp. 579–630 Search PubMed.
- A. Laca, B. Paredes, M. Rendueles and M. Díaz, Food Sci. Technol., 2015, 62(1), 7–10 CAS.
- A. H. Martin, E. Bakhuizen, C. Ersch, V. Urbonaite, H. H. J. D. Jongh and L. Pouvreau, Food Hydrocolloids, 2016, 56, 236–244 CrossRef CAS.
- C. C. A. Alleoni, Sci. Agric., 2006, 63(3), 291–298 CrossRef.
- Y. Ren, J. Wu and R. Renema, Handbook of Poultry Science and Technology, 2010, pp. 533–578 Search PubMed.
- P. Ganasen and S. Benjakul, Food Sci. Anim. Resour., 2013, 33(2), 214–220 CrossRef.
- K. Grizzuti and G. E. Perlmann, Biochemistry, 1973, 12(22), 4399–4403 CrossRef CAS PubMed.
- S. Ngarize, A. Adams and N. Howell, Food Hydrocolloids, 2005, 19(6), 984–996 CrossRef CAS.
- C. V. Nikiforidis and V. Kiosseoglou, Food Hydrocolloids, 2007, 21(8), 1310–1318 CrossRef CAS.
- T. Strixner, R. Würth and U. Kulozik, Drying Technol., 2013, 31(13–14), 1485–1496 CrossRef CAS.
- C. Au, N. C. Acevedo, H. T. Horner and T. Wang, J. Agric. Food Chem., 2015, 63(46), 10170–10180 CrossRef CAS PubMed.
- K. Blume, K. Dietrich, S. Lilienthal, W. Ternes and A. M. Drotleff, Food Chem., 2015, 173, 584–593 CrossRef CAS PubMed.
- T. Ulrichs, A. M. Drotleff and W. Ternes, Food Chem., 2015, 172, 909–920 CrossRef CAS PubMed.
- Z. Zhang, Y. Yang, X. Tang and Y. You, Food Chem., 2015, 188(dec.1), 111–118 CrossRef CAS PubMed.
- J. M. S. Renkema, C. M. M. Lakemond, H. H. J. De Jongh, H. Gruppen and T. Vliet, J. Biotechnol., 2000, 79(3), 223–230 CrossRef CAS PubMed.
- D. Cao, F. Feng, C. Xiong, J. Li, H. Xue, Y. Zhao, Y. Wang, Y. Tu and Y. Zhao, Poult. Sci., 2021, 100(7), 101140 CrossRef CAS PubMed.
- S. Lekjing, K. Venkatachalam and P. Noonim, J. King Saud Univ., Sci., 2024, 36(2), 103072 CrossRef.
- M. Schreiner, Int. J. Food Prop., 2006, 9(3), 573–581 CAS.
- S. K. Reinke, S. V. Roth, G. Santoro, J. Vieira, S. Heinrich and S. Palzer, ACS Appl. Mater. Interfaces, 2015, 7(18), 9929–9936 CrossRef CAS PubMed.
- P. Yu, W. Li, W. Wang and M. Ruan, China Condiment, 2017, 42(2), 24–27 Search PubMed.
- H. Wei, M. Li, J. Li and Q. Tong, Chin. Cereals Oils Association, 2013, 28(11), 91–96 CAS.
- L. J. Farmer and D. S. Mottram, J. Sci. Food Agric., 2010, 60(4), 489–497 CrossRef.
- Q. Wang, G. Jin, Y. Jin, M. Ma, N. Wang, C. Liu and L. He, Eur. J. Lipid Sci. Technol., 2014, 116(8), 1044–1053 CrossRef CAS.
- F. B. Whitfield and D. S. Mottram, Crit. Rev. Food Sci. Nutr., 2009, 31(1), 1–58 Search PubMed.
- X. Li, S. Chen, Y. Yao, N. Wu, M. Xu, Y. Zhao and Y. Tu, Foods, 2022, 11(19), 2949 CrossRef CAS PubMed.
- Q. Li, H. Jin, M. Xia, H. Sun, T. Zeng, Y. Wang, L. Lu and Z. Cai, Food Chem., 2024, 445, 138750 CrossRef CAS PubMed.
- H. Xue, H. Liu, G. Zhang, Y. Tu and Y. Zhao, Food Chem., 2023, 413, 135632 CrossRef CAS PubMed.
- H. Xue, M. Xu, M. Liao, W. Luo, G. Zhang, Y. Tu and Y. Zhao, Food Hydrocolloids, 2021, 110, 106181 CrossRef CAS.
- N. Zhao, J. Hu, T. Hou, Z. Ma, C. Wang and H. He, J. Funct. Foods, 2014, 8, 234–242 CrossRef CAS.
- C. Tian, F. Zhang and Y. Wang, J. Jilin Univ., 1992,(2), 74–77 Search PubMed.
- Y. Wang, H. Zheng, Y. Li, B. Li and Y. Chen, Food Hydrocolloids, 2015, 45, 317–326 CrossRef CAS.
- T. C. Tan, T. Phatthanawiboon and A. M. Easa, J. Food Qual., 2016, 39(4), 342–350 CrossRef CAS.
- T. Kaewmanee, S. Benjakul, W. Visessanguan and C. Gamonpilas, Food Bioprocess Technol., 2013, 6(2), 367–376 CrossRef CAS.
- S. A. Woodward and O. J. Cotterill, J. Food Sci., 2010, 52(1), 63–67 CrossRef.
- K. Lai, W. Chung, C. Jao and K. Hsu, Poult. Sci., 2010, 89(4), 738–744 CrossRef CAS PubMed.
- L. Xu, Y. Zhao, M. Xu, Y. Yao, N. Wu, H. Du and Y. Tu, Food Chem., 2019, 275, 600–609 CrossRef CAS PubMed.
- S. A. Woodward, Food Gels, 1990, pp. 175–199 Search PubMed.
- T. Kaewmanee, S. Benjakul and W. Visessanguan, J. Food Sci., 2011, 76(2), S139–S147 CrossRef CAS PubMed.
- V. Karthikeyan, Braz. Arch. Biol. Technol., 2018, 61, e18180134 Search PubMed.
- C. Arzeni, O. E. Perez and A. M. R. Pilosof, Food Hydrocolloids, 2012, 29(2), 308–316 CrossRef CAS.
- H. Peng, J. Lin, D. H. Xiao, J. Huang and H. Zheng, Food Res. Dev., 2011, 32(3), 181–184 Search PubMed.
- V. Kiosseoglou, Curr. Opin. Colloid Interface Sci., 2003, 8(4–5), 365–370 CrossRef CAS.
- M. V. Nguyen, S. Arason, K. A. Thorarinsdottir, G. Thorkelsson and A. Gudmundsdóttir, J. Food Eng., 2010, 100(2), 225–231 CrossRef.
- A. Fuentes, J. M. Barat, I. Fernávdez-Segovia and J. A. Serra, Food Control, 2008, 19(8), 757–763 CrossRef CAS.
- C. A. Lncili, P. Karatepe, M. Akgol, A. Tekin, G. K. Lncili and A. A. Hayalogiu, Meat Sci., 2023, 205, 109305 CrossRef PubMed.
- X. Wang, Y. Huang, B. Zhou, W. Xu, X. Xiang, Q. Huang and S. Li, Ultrason. Sonochem., 2021, 75, 105579 CrossRef CAS PubMed.
- J. Zhang, D. Kang, W. Zhang and J. M. Lorenzo, Trends Food Sci. Technol., 2021, 111(10), 405–425 CrossRef CAS.
- C. Nimalaratne, D. Lopez-Lutz, A. Schieber and J. Wu, J. Agric. Food Chem., 2012, 60, 12547–12552 CrossRef CAS PubMed.
- X. Y. Lin, Y. P. Lai, R. B. Zhu, H. Zhang, B. H. Huang and J. J. Lin, J. Chin. Inst. Food Sci. Technol., 2011, 11, 68–75 Search PubMed.
- N. Yang, Y. Jin, Y. Xu, X. Xu and Z. Jin, Trans. Chin. Soc. Agric. Eng., 2015, 31, 295–300 Search PubMed.
- L. Yuan, J. Zhang, J. Wu, Z. Gao, X. Xie, Z. Wang and X. Wang, J. Food Process. Preserv., 2018, 42(4), e13581 CrossRef.
- Y. Xie, J. Wang, Y. Wang, D. Wu, W. Liang, H. Ye, Z. Cai, M. Ma and F. Geng, Ultrason. Sonochem., 2020, 60(C), 104767 CrossRef CAS PubMed.
- X. Sun, L. He, H. Yang, W. Wu, L. Yue, W. Peng, G. Jin and Y. Jin, Sci. Technol. Food Ind., 2018, 39, 204–211 Search PubMed.
- Z. Yu, H. Guo, C. Liu, R. Wang, L. Zhang, X. Zhang and Y. Chen, Food Res. Int., 2022, 156, 111318 CrossRef CAS PubMed.
- K. L. M. Dang, T. Q. Le and S. Songsermpong, Int. J. Agric. Nat. Resour., 2014, 48(6), 942–953 Search PubMed.
- L. Sheng, Y. Wang, J. Chen, J. Zou, Q. Wang and M. Ma, Food Res. Int., 2018, 108, 604–610 CrossRef CAS PubMed.
- D. Cheng, D. Hou, Y. Shang, J. Du and F. Chen, Sci. Technol. Food Ind., 2009, 30(2), 323–325 CAS.
- J. Yongsawatdigul and S. Gunasekaran, J. Food Process. Preserv., 1996, 20, 145–156 CrossRef.
- Y. Chen, P. Zheng, H. Liu, X. Zhang and Z. Gao, Food Res. Dev., 2022, 43, 6–13 CAS.
- J. Xu, P. Liu, X. Yang, C. Zhang, Y. Wang, F. Zhao and X. Liu, Food Sci. Technol., 2015, 102–107 Search PubMed.
- P. Shao, H. Liu, Q. Zou, L. Tian, Y. Liu and Y. Dong, Sci. Technol. Food Ind., 2017, 38, 8–13 Search PubMed.
- N. Sun, H. Liu, Y. Wen, W. Yuan, Y. Wu, J. Gao and C. Li, J. Food Process. Preserv., 2020, 44(4), 14405 Search PubMed.
- G. Jin, L. He, C. Li, Y. Zhao, C. Chen, Y. Zhang, J. Zhang and M. Ma, LWT–Food Sci. Technol., 2015, 64(2), 1099–1106 CrossRef CAS.
- X. Zhou, S. Shan, J. Zhao, J. Zhang, S. Gu, Z. Pan and Y. Ding, LWT–Food Sci. Technol., 2017, 84, 562–571 CrossRef CAS.
- B. Zhou, M. Zhang, Z. Fang and Y. Liu, Drying Technol., 2014, 32(15), 1840–1847 CrossRef CAS.
- K. M. Lai, W. C. Ko and T. H. Lai, Food Sci. Technol. Int., 1997, 3(3), 269–273 Search PubMed.
- Q. Zhang, W. Fan, Y. Shi, Z. C. Tu, Y. M. Hu and J. Zhang, LWT–Food Sci. Technol., 2023, 188, 115363 CrossRef CAS.
- J. Fan, J. Yan, S. Wen, L. Bin, I. Rui and M. Ma, Food Sci. Technol., 2011, 36(6), 119–122 CAS.
- S. Wang, S. Wang, Y. Zhang and R. Zhang, Trans. Chin. Soc. Agric. Eng., 2013, 29, 286–292 CAS.
- Y. Chen, L. Sheng, M. Gouda and M. Ma, LWT–Food Sci. Technol., 2019, 113, 108303 CrossRef CAS.
- Y. Pu, J. Du, Z. Liang, J. Pi, A. Pan, Y. Wu, J. Shen, Q. Li, J. Xiang and X. Xu, China Poult., 2010, 16, 25–27 Search PubMed.
- N. Ramírez, O. Vega-Castro, R. Simpson, C. Ramirez and H. Nunez, J. Food Process Eng., 2020, 44(3), e13627 CrossRef.
- V. Santarelli, L. Neri, R. Moscetti, C. D. Di Mattia, G. Sacchetti, R. Massantini and P. Pittia, Food Bioprocess Technol., 2021, 14(7), 1326–1340 CrossRef CAS.
- H. Demir, S. Celik and Y. C. Sezer, Food Sci. Technol. Int., 2022, 28(4), 340–352 CrossRef CAS PubMed.
- L. Ji, H. Liu, C. Cao, P. Liu, H. Wang and H. Wang, Food Sci. Technol. Int., 2013, 19(2), 123–131 CrossRef PubMed.
- Z. Liu and Y. Chi, Sci. Technol. Food Ind., 2011, 32(4), 236–239 CAS.
- Y. Liu, M. Qing, J. Zang, Y. Chi and Y. Chi, Food Res. Int., 2023, 163, 112266 CrossRef PubMed.
- Y. Liu, X. Yang, Y. Chi and Y. Chi, Food Res. Int., 2023, 172, 113096 CrossRef CAS PubMed.
- Y. Liu, Y. Chi and Y. Chi, Food Res. Int., 2023, 173(2), 113413 CrossRef CAS PubMed.
- J. Sun, Y. Wu, Q. Zeng, L. Lu, T. Zeng, P. W. Harlina, W. Liu, J. Du, J. Pi and F. Yang, LWT–Food Sci. Technol., 2024, 116237 CrossRef CAS.
- S. Jain and A. K. Anal, LWT–Food Sci. Technol., 2016, 69, 295–302 CrossRef CAS.
- S. Jain and A. K. Anal, J. Food Sci. Technol., 2017, 54(5), 1062–1072 CrossRef CAS PubMed.
- S. Jain and A. K. Anal, Food Res. Int., 2018, 103, 234–242 CrossRef CAS PubMed.
- H. Tang, L. Tan, Y. Chen, J. Zhang, H. Li and L. Chen, J. Sci. Food Agric., 2021, 101(4), 1389–1395 CrossRef CAS PubMed.
- R. Thammasena, C. W. Fu, J. H. Liu and D. C. Liu, Anim. Sci. J., 2020, 91(1), 13339 CrossRef PubMed.
- K. Venkatachalam and M. Nagarajan, J. Food Sci. Technol., 2019, 56(6), 3137–3144 CrossRef CAS PubMed.
- C. Yirong and L. P. Vaurs, J. Environ. Chem. Eng., 2019, 7, 103443 CrossRef CAS.
- T. Witoon, Ceram. Int., 2011, 37, 3291–3298 CrossRef CAS.
- O. A. A. Eletta, O. A. Ajayi, O. O. Ogunleye and I. C. Akpan, Chem. Eng., 2016, 4, 1367–1375 CAS.
- W. T. T. Tsai, J. M. M. Yang, C. W. W. Lai, Y. H. H. Cheng, C. C. C. Lin and C. W. W. Yeh, Bioresour. Technol., 2006, 97, 488–493 CrossRef CAS PubMed.
- G. Kalyoncu Ergüler, Miner. Eng., 2015, 76, 10–19 CrossRef.
- D. A. Oliveira, P. Benelli and E. R. Amante, J. Cleaner Prod., 2013, 46, 42–47 CrossRef CAS.
- C. Yirong and L. P. Vaurs, J. Environ. Chem. Eng., 2019, 7(6), 103443 CrossRef CAS.
- G. Wang and T. Wang, Int. J. Food Sci. Technol., 2009, 44(4), 763–769 CrossRef CAS.
- Z. Chen, B. Cui, X. Guo, B. Zhou, S. Wang, Y. Pei, B. Li and H. Liang, J. Sci. Food Agric., 2021, 102(3), 949–956 CrossRef PubMed.
- T. Quan and S. Benjakul, J. Texture Stud., 2019, 50(5), 434–442 CrossRef PubMed.
- Z. Wang, C. Dai and T. Wang, Int. J. Food Prop., 2017, 20, 1816–1822 CAS.
- D. Guo, H. He, M. Zhao, G. Zhang and T. Hou, J. Food Sci., 2020, 85(3), 834–842 CrossRef CAS PubMed.
- Y. Zhang, Master's thesis, Zhejiang University, Zhejiang, China, 2001.
- H. Zheng, J. Lin and Y. Li, The application prospects of enzymes in egg products processing, 2004 Search PubMed.
- J. Huang, Z. Cai and R. Zhang, Food Sci., 1996, 23(6), 819–829 Search PubMed.
- D. Guo, H. He and T. Hou, Food Chem., 2020, 325, 126919 CrossRef CAS PubMed.
- B. Zhou, M. Zhang, X. Zhong and Y. Liu, Food Bioprod. Process., 2015, 96, 306–313 CrossRef CAS.
- T. Xu and C. Huang, AIChE J., 2008, 54, 3147–3159 CrossRef CAS.
- Y. Wang, M. Zhang, B. Adhikari, A. S. Mujumdar and B. Zhou, Drying Technol., 2013, 31(15), 1826–1836 CrossRef CAS.
- F. Alavi, Z. Emam Djomeh, S. Momen, E. Hosseini and A. A. Moosavi-Movahedi, Food Hydrocolloids, 2020, 99, 105337 CrossRef CAS.
- Y. Wang, Y. Li, Y. Yang, N. Jiang, D. Li, C. liu and Z. Feng, J. Mol. Liq., 2023, 372, 121210 CrossRef CAS.
- M. Du, Z. Sun, Z. Liu, Y. Yang, Z. Liu, Y. Wang, B. Jiang, Z. Feng and C. Liu, LWT–Food Sci. Technol., 2022, 161, 113337 CrossRef CAS.
- J. Yang, C. Cui, W. Feng, H. Zhao, W. Wang and K. Dong, Int. J. Food Sci. Technol., 2017, 52(7), 1623–1631 CrossRef CAS.
- Y. Dai, J. Zhao, H. Liang, Q. Deng, C. Wan, B. Li and B. Zhou, Food Hydrocolloids, 2022, 124, 107260 CrossRef CAS.
- T. Kaewmanee, S. Benjakul and W. Visessanguan, J. Food Sci., 2009, 74(8), S351–S361 CrossRef CAS PubMed.
- M. Liu and S. Zhang, Protein Lysozyme Hormone Effect on the Preservation of Meatballs, China Animal Husbandry Association, 1994, pp. 243–245 Search PubMed.
- Q. Lin, Food Sci., 1996, 23(2), 244–254 Search PubMed.
- A. M. Abker, Z. Xia, G. Hu, X. Fu, Y. Zhang, Y. Jin, M. Ma and X. Fu, Food Chem., 2024, 454, 139609 CrossRef CAS PubMed.
- H. Tang, L. Tan, Y. Chen, J. Zhang, H. Li and L. Chen, J. Sci. Food Agric., 2021, 101(4), 1389–1395 CrossRef CAS PubMed.
- Z. Zhang, T. Xu, W. Qin, B. Huang, W. Chen, S. Li and J. Li, Biochem. Biophys. Res. Commun., 2019, 522(1), 21–25 CrossRef PubMed.
- S. Yang and K. Chen, Poult. Sci., 2001, 80(3), 370–375 CrossRef CAS PubMed.
- M. Garcés-Rimón, M. Sandoval and E. Molina, Int. J. Gastron. Food Sci., 2016, 3, 17–22 CrossRef.
- Y. Su, Z. Chen, J. Li, C. Chang, L. Gu and Y. Yang, Food Chem., 2021, 338, 127913 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |