 Open Access Article
Open Access ArticleAdvancements in food quality monitoring: integrating biosensors for precision detection
Soumitra
Nath
 *
*
Department of Biotechnology, Gurucharan College, Cachar, Silchar, 788004, Assam, India. E-mail: nath.soumitra1@gmail.com; Tel: +91 9401374737
First published on 7th June 2024
Abstract
The integration of biosensors into food quality monitoring systems presents a promising approach to enhance food safety and quality assurance. Biosensors enable rapid, accurate, and on-site detection of contaminants, revolutionizing the management of food safety risks throughout the supply chain. This review provides insights into the current challenges, opportunities and future directions of biosensor technology in ensuring the integrity and safety of our food supply. Electrochemical, optical, and piezoelectric biosensors offer versatile platforms for food quality monitoring, each providing unique advantages in sensitivity, specificity, and detection capabilities. By harnessing these principles, biosensors offer valuable tools for detecting a wide range of contaminants, allergens and adulterants in food samples, thus improving food safety and quality assurance measures. However, biosensor implementation faces challenges such as sensitivity and specificity issues, matrix interference, and shelf-life concerns. Overcoming these challenges requires research and development efforts to improve biosensor design, optimization, and performance. Recent advances in biosensor technology, including nanotechnology integration, multiplexed detection and smartphone-based biosensors, offer exciting opportunities to improve and enhance food quality monitoring. Future perspectives include the development of improved sensing technologies, standardization, regulatory considerations, and integration with the Internet of Things (IoT) for real-time monitoring, paving the way for the revolutionization of food safety practices throughout the global food supply chain.
Sustainability spotlight statementThe integration of biosensors for precision detection in food quality monitoring represents a critical advancement in ensuring the safety and integrity of our food supply chain. Biosensors enable rapid, accurate and on-site detection of contaminants, mitigating the risks associated with foodborne illnesses and enhancing overall food quality assurance. This sustainable advancement aligns with the United Nations Sustainable Development Goal (SDG) 3: Good Health and Well-being, by promoting food safety and reducing the burden of food-related diseases. Additionally, by revolutionizing food safety practices and facilitating real-time monitoring, biosensor technology contributes to SDG 2: Zero Hunger, by ensuring access to safe and nutritious food for all. Through continuous research and development efforts, biosensors hold the potential to significantly improve food safety measures and promote sustainable development in the global food industry. |
1. Introduction
Ensuring the safety and quality of food products is of paramount importance to public health, consumer confidence, and regulatory compliance. Food quality monitoring plays a crucial role in identifying and mitigating risks associated with contaminants, adulterants, and other factors that can compromise the integrity of the food supply chain. Traditional methods of food analysis often involve laboratory techniques that require time, which can delay the detection of hazards and increase the potential of consumer exposure to harmful substances.1In recent years, biosensors have emerged as powerful tools for improving the efficiency and accuracy of food quality monitoring. These analytical devices integrate biological recognition elements with transducer components to detect and quantify specific analytes in food samples.2 By harnessing the inherent specificity and sensitivity of biological interactions, biosensors offer rapid on-site detection capabilities that can replace traditional laboratory-based methods.3 The role of biosensors in enhancing detection accuracy is multifaceted. Unlike conventional assays that rely on complex sample preparation and specialized equipment, biosensors offer simplicity, portability, and real-time monitoring capabilities. Biosensors enable rapid screening of large volumes of food samples, reducing the time and resources required for analysis.3 Furthermore, they can detect target analytes in low concentrations, providing early warning signals of potential food safety hazards.4
This review aims to provide a comprehensive overview of the current state of the art in biosensor technology for food quality monitoring. It discusses the principles and mechanisms underlying biosensor operation, highlighting their ability to achieve high sensitivity and specificity in detecting various contaminants and adulterants.
2. Principles and mechanisms of biosensor operation
The principle of a biosensor revolves around the selective recognition of a target analyte by a biological receptor that initiates a measurable signal indicative of the analyte's concentration or presence. The bioreceptor, also known as the recognition element, is composed of biomolecules or biological entities specifically designed to interact with the target analyte.5 This interaction induces a biochemical or biophysical change, which serves as the basis for detection. The bioreceptor is coupled with a transducer responsible for converting the bioreceptor–analyte interaction into a measurable signal.6 The signal is then amplified by an amplifier, enhancing its detectability and improving the signal-to-noise ratio.7 Finally, the processed signal is analyzed and interpreted by a processor,8 and the results are displayed in a human-readable format, allowing users to interpret the findings.2 Therefore, the integration of the bioreceptor, transducer, amplifier, processor, and display forms the foundation of a biosensor system, enabling accurate and efficient detection of target analytes.The first biosensor was developed by Leland C. Clark, Jr in 1956 for oxygen detection, earning him the title “father of biosensors”. His invention, known as the “Clark electrode,” revolutionized the field. In 1962, Clark demonstrated an amperometric enzyme electrode for glucose detection.9 This was followed in 1969 by the development of the first potentiometric biosensor for urea detection by Guilbault and Montalvo Jr.10 The Yellow Springs Instrument Company (YSI) utilized Clark's technology to create the Model 23A YSI analyzer, which became the first commercially successful glucose biosensor in 1975.11 This device enabled the direct measurement of glucose through the amperometric detection of hydrogen peroxide. In the glucose biosensor, the enzyme glucose oxidase is utilized as a bioreceptor, which interacts with glucose molecules present in the food sample.12 This facilitates the conversion of glucose to gluconic acid and hydrogen peroxide (H2O2). This biochemical process generates a measurable signal, which is detected by a transducer. The transducer translates the signal into a current, with the magnitude of the current directly correlating with the glucose concentration in the sample. The amplified signal is then displayed on a digital screen, providing users with an accurate and real-time measurement of the glucose levels in the food sample (Fig. 1).
 | ||
| Fig. 1 Schematic diagram illustrating biosensor operation principles, highlighting recognition element–analyte interaction and signal transduction. | ||
3. Components of a biosensor for food quality monitoring
Biosensors have become essential in food quality monitoring, combining biological recognition elements with transducer components to detect and measure specific analytes in food samples. A schematic diagram depicting the various components of biosensor operation is presented in Fig. 2, while Table 1 summarizes the principles, uses, and disadvantages associated with these components.| Name of the component | Principle | Uses | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| 1. Bioreceptor | |||||
| Enzymes | Catalyze reactions, generating measurable signals | Detect glucose, cholesterol, metabolites | High specificity, rapid response, stability | Limited to specific reactions, sensitivity affected | 84 |
| Antibodies | Bind antigens, form complexes for detection | Detect proteins, viruses, bacteria | Exceptional specificity, detect low concentrations | Costly production, potential cross-reactivity | 85 |
| Nucleic acids (DNA/RNA) | Hybridize with targets, sequence detection | Identify DNA sequences, pathogens | High specificity, detect SNPs, rapid detection | Requires target sequence, susceptible to degradation | 20 |
| Living cells/cell components | Respond to analytes, monitor changes | Detect toxins, pollutants, biomarkers | Reflect integrated cellular responses, real-time monitoring | Variable responses, maintenance required, complex interpretation | 86 |
| Whole organisms | Exhibit physiological responses to analytes | Monitor environment, detect toxins | Capture broad responses, real-world relevance | Ethical concerns, limited applicability, complex interpretation | 87 |
| Molecularly imprinted polymers (MIPs) | Synthetic polymers with tailored binding | Detect small molecules, drugs, toxins | High selectivity, simple synthesis, versatility | Limited to compatible targets, lower binding affinity | 53 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| 2. Transducer | |||||
| Electrochemical biosensor | Measures changes in electrical properties | Detect ions, molecules, enzymes | High sensitivity, rapid response, low cost | Signal interference, electrode fouling | 84 |
| Optical biosensor | Measures changes in light properties | Detect biomolecules, fluorescence | High sensitivity, real-time monitoring | Limited to transparent or translucent samples | 32a |
| Acoustic biosensor | Measures changes in sound waves | Detect biomolecules, cells | Non-invasive, real-time detection | Limited to liquid-phase samples | 88 |
| Thermal biosensor | Measures changes in heat or temperature | Detect gases, biomolecules | Simple design, rapid response | Sensitivity affected by environmental factors | 89 |
| Gravimetric biosensor | Measures changes in mass or weight | Detect mass changes due to analyte binding | High sensitivity, label-free detection | Requires precise environmental control | 90 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| 3. Amplifier | |||||
| Operational amplifier (Op-Amp) | Amplifies voltage difference between inputs | Signal conditioning, filtering | High gain, low noise, wide bandwidth | Requires external power supply | 91 |
| Transimpedance amplifier | Converts current into voltage for detection | Photodetection, electrochemical sensing | High sensitivity, low noise, wide dynamic range | Limited to current-based transduction | 92 |
| Instrumentation amplifier | Amplifies differential input signal | Bioimpedance measurements | High common-mode rejection, precise gain control | More complex circuitry, higher cost | 93 |
| Low-noise amplifier | Amplifies weak signals with minimal noise | Low-level signal detection | Enhances signal-to-noise ratio, improves sensitivity | Limited to specific frequency ranges | 94 |
| Lock-in amplifier | Amplifies signals at a specific frequency | Phase-sensitive detection | Rejects noise, improves signal detection in noisy environments | Requires reference signal for operation | 95 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| 4. Processor | |||||
| Microcontroller | Integrated circuit with CPU, memory, and I/O | Data analysis, signal processing | Low cost, low power consumption | Limited computational capabilities | 91 |
| Microprocessor | Central processing unit for general-purpose tasks | Complex data analysis, algorithm execution | High computational power, flexibility | Higher cost, higher power consumption | 93 |
| Digital signal processor (DSP) | Specialized processor for signal processing | Noise filtering, signal enhancement | High-speed signal processing, real-time analysis | Limited flexibility for general-purpose tasks | 96 |
| Field-programmable gate array (FPGA) | Programmable logic device for custom logic circuits | Customized data processing, hardware acceleration | High-speed parallel processing, low latency | Steeper learning curve, higher development cost | 96 |
| Application-specific integrated circuit (ASIC) | Custom-designed integrated circuit for specific tasks | Specialized data processing, low power consumption | High performance, optimized for specific applications | Higher development cost, less flexibility for changes | 97 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| 5. Display | |||||
| Digital display | Numerical representation of data | Quantitative analysis, concentration readout | Clear, easy-to-read output | Limited visualization capabilities | 68 and 98 |
| LED/LCD display | Light-emitting diodes or liquid crystal display | Real-time monitoring, data visualization | Bright, energy-efficient, customizable | Limited display size, may require backlight | 99 |
| OLED display | Organic light-emitting diodes | Portable devices, low-power applications | High contrast, wide viewing angles | Limited lifespan, potential burn-in | 100 |
| Graphical display | Graphical representation of data | Trend analysis, pattern recognition | Intuitive visualization, detailed information | Higher cost, more complex interface | 101 |
| Touchscreen display | Interactive display with touch input | User interaction, menu navigation | User-friendly interface, intuitive controls | Susceptible to damage, calibration issues | 68 |
| Analog display | Represents data using continuous variables (e.g., dial) | Continuous monitoring, visual indication | Intuitive representation, immediate feedback | Limited precision, less suitable for precise readings | 98 |
| Indicator display | Provides simple visual indication of status or threshold | Alerting, binary status indication | Easy to understand, low power consumption | Limited information, may require additional interpretation | 102 |
3.1. Recognition element or bioreceptor
Recognition elements are biomolecules that selectively bind to the target analyte, initiating a biological response that is transduced into a measurable signal. Common recognition elements used in biosensors include enzymes, antibodies, nucleic acids (e.g., DNA or RNA), whole cells, and molecularly imprinted polymers (MIPs).5 These biomolecules exhibit high specificity for their target analytes, allowing selective detection of desired compounds. The immobilization of recognition elements on the biosensor surface is crucial for maintaining their stability and activity, ensuring reliable and reproducible detection performance.3.2. Transducer
The transducer in a biosensor converts the bioreceptor–analyte interaction into a measurable signal, facilitating detection and quantification. It serves as the interface between the biological recognition event and the output signal, translating biochemical or biophysical changes into electrical, optical, acoustic, or thermal signals that can be processed and analyzed.6 Various types of transducers are used in biosensors, including electrochemical, optical, acoustic, and thermal transducers, each offering specific advantages depending on the nature of the analyte and the desired sensitivity and selectivity.2 The choice of transducer greatly influences the performance and capabilities of the biosensor system, affecting factors such as detection limit, response time, and compatibility with different sample matrices.3.3. Amplifier
An amplifier serves to increase the strength or magnitude of the signal generated by the transducer in response to the bioreceptor–analyte interaction.7 It plays a critical role in enhancing the detectability and accuracy of the biosensor by amplifying the weak electrical, optical, acoustic, or thermal signals produced by the transducer. Amplifiers can be designed to operate across a wide range of frequencies and can be tailored to suit the specific requirements of the biosensor system.3.4. Processor
The processor serves as the central computational unit responsible for data analysis, signal processing and decision-making. It processes the raw data obtained from the amplifier and converts them into meaningful information. Biosensor processors can range from simple microcontrollers to more powerful microprocessors, depending on the complexity of the biosensing system and computational requirements.8 They may perform tasks such as noise filtering, signal averaging, calibration, pattern recognition and data storage. Additionally, processors can facilitate communication with external devices or networks, enabling real-time monitoring, remote operation, and data sharing.133.5. Display
A display in the context of biosensors serves as an interface for presenting the results of the biosensing process to the user or operator.2 A display can range from simple digital readouts to more sophisticated graphical interfaces, depending on the complexity of the biosensor system and the requirements of the end-user.8 The choice of display is crucial in ensuring effective communication of the biosensor output, allowing quick decision-making and action based on the detected information.4. Applications of biosensors in the detection of food contaminants
Biosensors play a pivotal role in detecting and quantifying various contaminants in food samples, offering rapid, sensitive, and selective analysis capabilities. Biosensor technology is essential for the detection and monitoring of food contaminants in food, such as chemicals, biological agents and physical substances (Table 2). Therefore, this technology plays a pivotal role in maintaining the safety and quality of food throughout the supply chain.| Biosensor type | Application/detection | Analyte | Biological element | Transducer | Display system | References |
|---|---|---|---|---|---|---|
| Enzyme-based biosensor | Pesticide residues | Pesticides | Enzymes (e.g., acetylcholinesterase) | Electrochemical (enzyme-modified electrodes) | Digital display for quantification | 103 |
| Mycotoxins in grains | Mycotoxins | Enzymes (e.g., peroxidase) | Optical (e.g., fluorescence) | Digital display for quantification | 104 | |
| Glucose levels in beverages | Glucose | Glucose oxidase | Electrochemical (glucose sensor) | Digital display for quantification | 105 | |
| Lactose in dairy products | Lactose | β-Galactosidase | Electrochemical (lactose biosensor) | Digital display for quantification | 106 | |
| Alcohol content in beverages | Ethanol | Alcohol dehydrogenase | Electrochemical (alcohol sensor) | Digital display for quantification | 107 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Antibody-based biosensor | Allergen in food products | Allergens | Monoclonal antibodies | Optical (e.g., surface plasmon resonance) or electrochemical (e.g., ELISA) | Graphical display for qualitative/quantitative analysis | 108 |
| Pathogenic bacteria in meat and milk | Bacteria | Polyclonal antibodies | Electrochemical (immunosensors) | Graphical display for qualitative/quantitative analysis | 109 | |
| Gluten in food products | Gluten | Monoclonal antibodies | Optical (e.g., SPR) | Digital display for quantification | 110 | |
| Aflatoxins in spices | Aflatoxins | Monoclonal antibodies | Electrochemical (immunosensors) | Graphical display for qualitative/quantitative analysis | 111 | |
| Foodborne viruses in water | Viruses | Polyclonal antibodies | Optical (e.g., ELISA) or electrochemical (e.g., biosensors) | Graphical display for qualitative/quantitative analysis | 112 | |
| Nucleic acid-based biosensor | Viral contamination in water | Viruses | DNA or RNA probes | Fluorescent or electrochemical | Digital display for quantitative analysis | 113 |
| GMO in food products | Genetically modified organisms | DNA probes | Electrochemical or optical | Digital display for quantitative analysis | 114 | |
| Antibiotic resistance genes | Antibiotic resistance genes | DNA probes | Electrochemical or optical | Digital display for quantitative analysis | 113 | |
| Foodborne pathogens | Bacteria | DNA probes or aptamers | Electrochemical or optical | Digital display for qualitative/quantitative analysis | 115 | |
| Allergen-related gene sequences | Allergens | DNA probes or aptamers | Electrochemical or optical | Digital display for quantitative analysis | 116 | |
| Whole cell-based biosensor | Milk quality | Bacteria | Genetically modified bacterial cells | Optical or electrochemical | Real-time monitoring systems displaying cellular responses | 117 |
| Yeast and mold in beverages | Yeast, mold | Yeast cells | Electrochemical (yeast cell biosensors) | Real-time monitoring systems displaying cellular responses | 118 | |
| Bacterial contamination | Bacteria | Engineered bacterial strains | Optical or electrochemical | Real-time monitoring systems displaying cellular responses | 119 | |
| Chemical detection in solution | Caffeine | Pseudomonas alcaligenes immobilized on a cellophane membrane | Optical or electrochemical | Real-time monitoring systems with short read-time | 87 | |
| Bacterial pathogens in meat | Bacteria | Engineered bacterial strains | Optical or electrochemical | Real-time monitoring systems displaying cellular responses | 24 and 120 | |
| Molecularly imprinted polymer (MIP) based biosensor | Pesticide residues in fruits | Pesticides | Molecularly imprinted polymers | Electrochemical or optical | Graphical display for qualitative/quantitative analysis | 121 |
| Antibiotics in milk | Antibiotics | Molecularly imprinted polymers | Electrochemical or optical | Digital display for quantification | 122 | |
| Aflatoxins in nuts | Aflatoxins | Molecularly imprinted polymers | Optical | Digital display for quantification | 123 | |
| Histamine in fish | Histamine | Molecularly imprinted polymers | Electrochemical | Digital display for quantification | 124 | |
| Melamine in dairy products | Melamine | Molecularly imprinted polymers | Electrochemical or optical | Graphical display for qualitative/quantitative analysis | 121 and 125 | |
4.1. Pesticides and herbicides
Pesticides and herbicides are widely used in agriculture to enhance crop yield and protect against pests and weeds. However, their residues in food products pose significant health risks to consumers. Biosensors offer efficient tools for detecting pesticide and herbicide residues in food samples, ensuring food safety and regulatory compliance. A notable example is the enzyme biosensor, which utilizes acetylcholinesterase enzyme to detect organophosphate pesticides such as chlorpyrifos in fruits and vegetables.14 Scientists have also developed immunosensors that employ specific antibodies to detect glyphosate, a commonly used herbicide, in food matrices.15 These biosensors enable rapid, sensitive and selective pesticide and herbicide residue detection through electrochemical, optical or piezoelectric transduction methods.16 The use of biosensors in real-time monitoring enhances food safety measures, protecting consumers from potential health hazards associated with pesticide and herbicide contamination.4.2. Heavy metals
Heavy metals, such as lead, cadmium, and mercury, pose significant health risks in food products.17 These heavy metals can be detected by biosensors, which offer sensitive and rapid detection methods for heavy metal contamination in food samples. Yuan, et al.18 developed an ultrasensitive electrochemical aptasensor for simultaneous quantitative detection of lead and cadmium in fruit and vegetable samples. In another study, Shakya and Singh19 explored various optical methods, including fiber grating, modal interference, fluorescence, optical absorbance, surface plasmon and surface-enhanced Raman scattering, for heavy metal ion detection in water samples. These biosensors enable real-time monitoring of heavy metal contamination through precise and selective transduction methods.4.3. Pathogenic microorganisms
Rapid and sensitive detection of pathogenic microorganisms is necessary to prevent food spoilage and foodborne diseases. Biosensors, utilizing enzymatic, immunological and molecular recognition elements, are mostly used to specifically detect surface antigens, genetic sequences or metabolic by-products of pathogenic microorganisms. Studies have shown that DNA-based biosensors, employing nucleic acid probes, can detect specific genetic sequences of pathogens such as Salmonella, Escherichia coli, and viral agents like norovirus and hepatitis A virus.20 Immunosensors that use antibodies can be used to recognize surface antigens of bacteria such as Listeria monocytogenes, Salmonella, Campylobacter spp., and Escherichia coli.21 Immunosensors are also used to detect viral pathogens such as influenza virus and rotavirus in food samples.22 Chai, et al.23 developed magnetoelastic detection of Salmonella typhimurium (ME) biosensors, which hold promise for examining pathogens on food surfaces. The effectiveness of this technology was demonstrated by using a coil measurement technique with an E2 phage-coated ME biosensor. This specific biosensor was employed on tomato surfaces. In addition, enzymatic biosensors, whole cell-based biosensors, phage-based biosensors, and nanomaterial-based biosensors offer alternative approaches, each with unique advantages in terms of sensitivity, specificity, and application in food quality assessment.244.4. Allergens
Biosensors offer rapid and accurate detection of allergen and allergenic ingredients, ensuring food safety for individuals with sensitivities. These biosensors utilize various recognition elements, such as antibodies or aptamers, specifically targeting allergic proteins derived from common sources such as nuts, gluten, or shellfish.25 For example, immunosensors can employ antibodies tailored to recognize specific allergenic proteins, allowing rapid and sensitive detection in food samples.26 Additionally, DNA-based biosensors can target specific genetic sequences associated with allergenic ingredients, providing a reliable method for allergen detection.274.5. Mycotoxins
Mycotoxins are toxic compounds produced by certain molds that can contaminate various food products, posing serious health risks to consumers. Studies have shown that an electrochemical immunosensor can be used to detect aflatoxin B1 in pistachios.28 In another study, Jia, et al.29 developed portable chemiluminescence optical fiber aptamer-based biosensors for analysis of multiple mycotoxins. In addition, studies have also shown promising results using immunological biosensors (utilizing antibodies specific to mycotoxins) and DNA-based biosensors (targeting genetic sequences associated with mycotoxin-producing molds).304.6. Food adulterants
Biosensors provide effective tools for detecting food adulterants, such as adulterated ingredients, counterfeit products, or dilution with cheaper substitutes. Electrochemical biosensors have been used to detect the presence of melamine in dairy products.31 Similarly, optical biosensors have been used to detect synthetic colors and dyes in beverages and food products,32 ensuring compliance with regulatory standards and protecting consumer health.4.7. Food quality parameters
Biosensors are also used to assess various quality parameters in food products, such as freshness, ripeness, and nutritional content. Biosensors equipped with enzymatic recognition elements can detect specific biomarkers associated with freshness and spoilage in food products in real time, allowing timely decisions regarding shelf life and product distribution.33 Biosensors play a crucial role in determining the optimal harvesting or processing time for fruits and vegetables.34 Enzymatic biosensors targeting key ripening indicators, such as ethylene or specific enzymes involved in fruit softening, can accurately monitor ripeness levels.35 In addition, whole cell-based biosensors allow the quantification of proteins, fats, carbohydrates, vitamins, and minerals, providing nutritional profiles of food samples.36 A biosensor can also be used to assess food freshness using electronic noses (e-noses), which utilize arrays of chemical sensors to detect volatile compounds emitted during food spoilage.37 These e-noses have been successfully employed to assess the freshness of various perishable food products, including fish, meat, and dairy products, by analyzing changes in odour profiles associated with microbial growth or chemical degradation.5. Challenges and limitations in biosensor implementation
Despite their promising capabilities, the widespread adoption of biosensors in food quality monitoring encounters several challenges and limitations (Fig. 3). A significant challenge is the need for biosensors to maintain high sensitivity and specificity in diverse food matrices, which can vary widely in composition and complexity.38 Furthermore, ensuring the stability and shelf life of biosensor components, particularly biological recognition elements, presents another challenge.39 Environmental conditions, such as temperature and pH, can impact the performance and longevity of biosensors, requiring careful storage and handling protocols.40 In addition, standardization and regulatory considerations pose challenges in ensuring the consistency and reliability of biosensor measurements for regulatory compliance and widespread acceptance in the food industry.41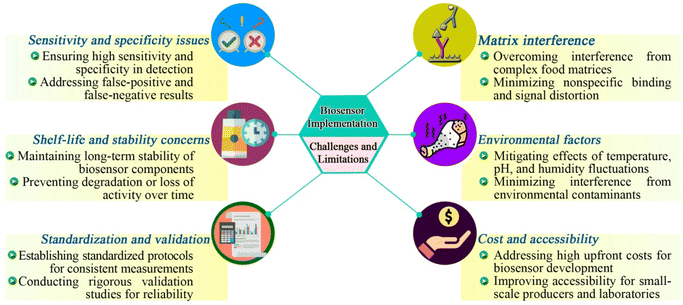 | ||
| Fig. 3 Challenges and limitations encountered in the widespread adoption of biosensors for food quality monitoring. | ||
5.1. Sensitivity and specificity issues
Sensitivity and specificity are fundamental parameters governing the performance of biosensors in food quality monitoring. In various studies, biosensors have faced challenges related to sensitivity and specificity, which have affected their applicability in real-world scenarios. Zachariasova, et al.38 reported cross-reactivity of rapid immunochemical methods for the detection of mycotoxins towards metabolites and masked mycotoxins. In another study by Zhu, et al.,42 a colorimetric biosensor was developed for the detection of aflatoxin B1 (AFB1) in food samples. Although the biosensor demonstrated excellent sensitivity, it showed limited performance in complex food matrices due to interference from matrix components, resulting in reduced specificity. Therefore, enhancing the sensitivity and specificity of biosensors often requires optimization of the recognition elements, immobilization methods, and transduction techniques to minimize false-positive or false-negative results.5.2. Matrix interference
Several studies have reported matrix interference that interferes with biosensor performance. Li, et al.43 developed a surface-enhanced Raman scattering-based lateral flow immunosensor to determine the concentration of colistin in milk within 20 min. However, the biosensor encountered matrix interference from milk proteins, which led to background signal noise and hindered the detection of colistin. Many studies have documented and addressed such analytical challenges for detecting various compounds in food matrix.445.3. Shelf-life and stability concerns
Shelf-life and stability concerns are significant challenges in biosensor development, particularly in the context of food quality monitoring, where long-term performance and reliability are crucial.39 The enzyme-based biosensor developed for detecting various compounds in food samples has stability concerns with the immobilized enzyme, which has decreased activity over time due to enzyme denaturation.45 To address this issue, many studies have investigated different enzyme immobilization techniques, such as cross-linking and encapsulation, to protect the enzyme from degradation and maintain its activity during storage.46 Researchers also developed a DNA-based biosensor for the detection of genetically modified organisms (GMOs) in food products. The biosensor utilized DNA probes immobilized on gold nanoparticle-modified electrodes to capture target DNA sequences from GMO samples. However, concerns arose regarding the stability of immobilized DNA probes, which could undergo degradation or structural changes over time, leading to a decrease in sensitivity and detection efficiency.475.4. Interference from environmental factors
Environmental factors such as temperature, pH, and humidity significantly impact the performance of biosensors. Variations in temperature can alter enzymatic activity in biosensors, affecting their sensitivity. Higher temperatures can increase enzyme activity, leading to higher current signals in glucose sensors, while lower temperatures reduce enzymatic reactions.12 Temperature changes can also affect the refractive index in optical biosensors and alter oscillation frequencies in piezoelectric biosensors, causing inaccurate measurements.48 Fluctuations in pH levels impact the stability and sensitivity of biosensors, particularly those with carbon nanotube-based electrodes for heavy metal detection, as changes in pH can affect the electrochemical properties and response signals of these sensors.40 High humidity levels degrade sensor components, reducing their lifespan and reliability.49 This is especially problematic for optical biosensors, where uncontrolled light exposure and moisture can introduce noise and affect accuracy. Additionally, complex food matrices can introduce chemical interference, reducing the selectivity and accuracy of biosensors.5.5. Standardization and validation
Standardized protocols and validation procedures are of vital importance to ensure the reliability and comparability of biosensor measurements. Biosensor data management comprises the food safety knowledge system, the testing management system, and the dissemination of food safety information. It also integrates data from food factory inspections, government inspections, testing organizations, and consumer purchasing information into a comprehensive food safety and nutrient testing database. Using these data, relevant information can be extracted to address the challenges of risk sharing encountered by food stakeholders. Furthermore, collaborating with various stakeholders can help mitigate food safety issues effectively.50 Regulatory challenges for biosensors include establishing universally accepted protocols for calibration, validation, and performance assessment. Specific challenges include ensuring consistent sensitivity and specificity across diverse food matrices, addressing the lack of standardized procedures for the detection and quantification of various contaminants, and meeting stringent regulatory requirements for biosensor approval and market entry. Additionally, biosensors must undergo rigorous testing to prove their equivalence or superiority to traditional microbiological methods, such as culture-based assays and polymerase chain reaction (PCR).51 Jeyaraman and Eltzov52 developed a 3D-printed, colorimetric biosensor for detecting Bacillus licheniformis in food samples. The sensor uses a casein-based gelatin film that liquefies in response to the pathogen's enzymes, causing a visible dye signal. It detects concentrations as low as 1 CFU per mL within 9.3 hours, offering a cost-effective and efficient alternative to conventional detection methods. In another study, Arreguin-Campos, et al.53 developed screen-printed electrodes (SPEs) functionalized with surface imprinted polymers (SIPs) and combined them with the heat transfer method (HTM) for real-time detection of Escherichia coli in dairy products. The sensor achieved a detection limit of 180 CFU per mL, with high reproducibility and sensitivity. Selectivity tests against C. sakazakii, K. pneumoniae, and S. aureus showed specific responses to E. coli. The sensor also successfully detected E. coli in spiked milk without additional sample preparation, indicating its potential for routine, on-site food safety monitoring. The results were compared against recently reported biosensors for E. coli detection based on antibodies, aptamers, and thermal imprinted polymers, highlighting the common biological receptors employed and demonstrating the effectiveness of the SIP-coated SPEs. Moreover, organizations like the International Organization for Standardization (ISO) and the Food and Drug Administration (FDA) are working on guidelines to ensure the accuracy, sensitivity, and specificity of biosensors. ISO 16140-2 outlines the validation process for alternative methods in food microbiology, which is now being adapted for biosensor technologies.54 These standardization efforts aim to facilitate the wider adoption of biosensors by ensuring their reliability and accuracy in real-world applications, thereby enhancing food safety and quality assurance across the global food supply chain.5.6. Cost and accessibility
The cost of biosensor development, fabrication, and deployment can be prohibitive for small-scale producers, small laboratories, or developing countries with limited resources. High costs associated with biosensor instrumentation, consumables, and maintenance often limit access to these technologies, preventing their widespread adoption in food quality monitoring applications.55 Additionally, the complexity of biosensor operation and data analysis can require specialized training and expertise, further restricting accessibility for end-users.56 Collaborative initiatives by organizations such as the World Health Organization (WHO), Food and Agriculture Organization (FAO), European Food Safety Authority (EFSA), and United Nations (UN) aim to improve training and capacity-building efforts in biosensor technology.41 Several economic models and cost-analysis studies can be considered to justify the investment in biosensor technologies. A Return on Investment (ROI) analysis can help determine the financial benefits relative to costs, such as evaluating savings from reduced food spoilage, lower recall expenses, and decreased labor costs due to testing automation. Patel, et al.57 reported that implementing biosensors for pathogen detection in a mid-sized dairy operation led to a reduction in spoilage-related losses and an increase in consumer satisfaction due to improved safety assurances. Cost-Benefit Analysis (CBA) can compare the total expected costs with benefits derived from improved food safety and quality, monetizing factors like reduced incidences of foodborne illnesses and enhanced consumer confidence.58 Total Cost of Ownership (TCO) analysis, which includes initial purchase costs, operating costs, maintenance, and training expenses over the lifespan of the biosensor technology, can provide a comprehensive view of the long-term financial benefits compared to traditional methods.59 Scaling economies also play a significant role, as larger-scale adoption of biosensors can lead to reduced unit costs. Studies indicate that larger food enterprises can achieve these cost reductions, thereby serving as models for smaller enterprises.60 Additionally, information on grants, subsidies, and financial incentives from government bodies and international organizations can alleviate the financial burden on small and medium-sized enterprises (SMEs), encouraging investment in biosensor technologies. Case studies of successful biosensor implementation in SMEs can highlight tangible benefits and provide a roadmap for biosensor adoption. Pilot programs showing positive investment outcomes can justify broader use. These economic models and case studies effectively demonstrate the financial viability and long-term benefits of biosensor technology, making a strong case for investment by small to medium-sized food enterprises.6. Recent advances in the biosensor implementation for food quality monitoring
Recent advances in biosensor technology have revolutionized food quality monitoring by offering innovative solutions for rapid, sensitive, and on-site detection of various contaminants and quality parameters (Table 3). The integration of nanotechnology, smartphone connectivity, and multiplexing capabilities has enhanced biosensor performance, enabling real-time monitoring and analysis at different stages of the food supply chain.| Biosensor type | Sensitivity | Specificity | Detection limit | Practical deployment challenges | References |
|---|---|---|---|---|---|
| Colorimetric SERS (melamine detection) | Moderate | High | Less than 0.25 ppm | Complexity in the preparation of SERS substrates, potential interference from food matrix, need for specialized equipment | 126 |
| Plasmonic resonance biosensor (allergen detection) | High | High | 0.25 μg mL−1 | Sensitivity to environmental conditions, high cost of plasmonic materials, need for calibration | 127 |
| Electrochemical biosensor (pesticide detection) | High | High | 0.14–2.05 ppb | Electrode fouling, signal interference from food matrix, maintenance of electrode materials | 128 |
| Multiplexed lateral flow immunoassay (pathogen detection) | Moderate to high | High | 1.0–2.0 CFU per mL | Potential cross-reactivity in a multiplex format, difficulty in detecting low concentrations in complex samples | 129 |
| Smartphone-based magnetic nano biosensor (pathogen detection) | High | High | 1.0 CFU per mL | Dependence on smartphone camera quality, variability under ambient light conditions, need for user training | 130 |
| Wearable biosensor (pesticide detection) | High | Moderate to high | 0.48 ppb | Limited battery life, potential skin irritation, need for regular calibration and maintenance | 131 |
| Microfluidic biosensor (multiplex aflatoxin detection) | High | High | 2.7–7.0 ng mL−1 | Complex fabrication process, potential clogging of microchannels, requirement for precise fluid control mechanisms | 132 |
| Enzymatic biosensor (glucose detection) | High | High | 30 ppm | Enzyme stability over time, potential interference from other reducing sugars, need for regular calibration | 133 |
| SERS biosensor (bacterial detection) | High | High | 102–104 CFU per mL | Need for uniform nanostructure fabrication, signal interference from complex food matrices, high cost of substrates | 134 |
6.1. Nanotechnology integration
The integration of nanotechnology into biosensor design has significantly enhanced their performance characteristics. Nanomaterials, such as nanoparticles, nanowires, and nanotubes, offer unique properties, including high surface-to-volume ratios, large surface areas, and tunable surface chemistry, which can be exploited to improve biosensor sensitivity and detection limits. Recent data indicate that these nanotechnology-integrated biosensors exhibit impressive performance characteristics. Liu, et al.61 developed a dual-mode sensing mechanism for the simultaneous colorimetric and surface-enhanced Raman scattering (SERS) detection of melamine in milk. In another study, the colorimetric SERS platform achieved a detection limit as low as 0.05 parts per million (ppm) for melamine, ensuring precise detection of adulterants in food products. In another study, a SERS biosensor based on AuNPs and AgNPs demonstrated the ability to detect and differentiate strains of Bacillus species, and simultaneously screen E. coli, Listeria monocytogenes, and Salmonella typhimurium within 10 seconds of collection time.62 The bimodal single-atom iron nanozyme biosensor exhibited a rapid response time of under 5 minutes for detecting volatile amines, contributing to the real-time assessment of food freshness.63 Moreover, electrochemical biosensors comprising porous hollow cobalt-based oxides encapsulated with bimetallic PdAu nanoparticles for highly sensitive pesticide detection64 offer better signal amplification and signal transduction, thereby enhancing the analytical performance of biosensors for food quality monitoring applications.6.2. Multiplexed detection
Multiplexed biosensors capable of simultaneously detecting multiple analytes in a single assay have gained increasing attention for their ability to provide comprehensive analysis and reduce assay time and cost. A lateral flow immunoassay has been developed to detect Listeria monocytogenes, Salmonella typhimurium and Escherichia coli in food samples using AuNPs as labels, coupled with multiplex PCR and test strips.65 In another study, Shang, et al.66 reported a nucleic acid extraction and real-time recombinase polymerase amplification (RPA) assay in a microfluidic biosensor for multiplex detection of foodborne bacteria. This fully integrated micro-platform (FID-MP) is designed for point-of-care testing (POCT) of multiplex pathogens. It incorporates nucleic acid extraction, RPA, and signal detection functionalities. The FID-MP platform allows for the simultaneous detection of up to eight bacteria, providing shorter testing times and greater portability.6.3. Smartphone-based biosensors
The integration of biosensors with smartphone technology has allowed on-site testing, remote monitoring, and real-time data analysis. Yin, et al.67 have developed a smartphone-based fluorescent sensor to autonomously detect multiple pathogenic bacteria. Additionally, Li, et al.68 demonstrated on-site rapid detection of Listeria monocytogenes in dairy products using smartphone-integrated device-assisted ratiometric fluorescent sensors. Another innovative approach involves a portable smartphone-assisted highly emissive magnetic covalent organic framework-based fluorescence sensor to detect Salmonella typhimurium in food samples.69 These smartphone-based biosensors offer accessibility, affordability, and scalability, making them suitable for widespread deployment and promising on-site detection in food quality monitoring.706.4. Wearable biosensors
Wearable biosensors have the potential to revolutionize food monitoring by enabling continuous tracking of dietary intake and detecting food allergens in real-time. These wearable devices allow individuals to make informed dietary choices, leading to personalized dietary recommendations and improved overall health and well-being.71 A wearable biosensor developed by Wang, et al.72 enables non-invasive sweat analysis to monitor specific biomarkers associated with dietary intake and trace levels of metabolites. This device allows individuals to track multiple metabolites and nutrients throughout the day, including essential amino acids and vitamins, offering valuable insights into their dietary habits and nutritional intake. Furthermore, studies have demonstrated the development of wearable, flexible, glove-embedded non-enzymatic sensors for the multiplexed detection of pesticides in food samples.73 The sensors, printed on three fingers of a rubber glove, exhibit high performance in detecting four classes of pesticides, namely carbendazim (carbamate), diuron (phenylamide), paraquat (bipyridinium), and fenitrothion (organophosphate). These sensors enable the selective detection of pesticides in real samples of apple, cabbage and orange juice, with multidimensional projections showcasing rapid, sensitive and on-site analysis.7. Future perspectives and opportunities
The future of biosensors lies in the development of advanced sensing technologies that offer improved sensitivity, selectivity, and reliability. Continued research into novel recognition elements, such as engineered enzymes, aptamers, and synthetic receptors, holds promise for improving biosensor performance and expanding its applicability to a wider range of analytes. Additionally, advances in nanotechnology, microfluidics, and materials science enable the design of miniaturized, portable biosensors with improved detection limits and reduced sample volumes.3 The integration of cutting-edge techniques, such as surface-enhanced Raman spectroscopy (SERS), nanomaterial-based amplification, and single-molecule detection, further enhances the analytical capabilities of biosensors, paving the way for more sensitive, rapid, and accurate detection of food contaminants and adulterants.74 Furthermore, standardization of biosensor protocols, validation procedures and performance metrics is essential to ensure consistency, comparability, and reliability of results across different platforms and applications.51 Regulatory agencies play a crucial role in establishing guidelines and standards for biosensor development, validation and deployment in food quality monitoring. Collaboration between industry stakeholders, academic researchers, and regulatory authorities is necessary to address regulatory challenges, streamline approval processes, and promote the widespread adoption of biosensors in food safety and quality assurance applications.75Integrating intelligent sensors into Internet of Things (IoT) devices, utilizing wireless sensor networks (WSNs) technologies such as Wi-Fi, Bluetooth, Zigbee, and LoRA, is essential for the early detection of pathogens in plant health monitoring.76 This integration generates extensive data, empowering decision-makers to efficiently oversee food safety and quality, thereby protecting public health. IoT-enabled biosensors deployed throughout the food supply chain enable real-time monitoring and analysis of crucial parameters such as temperature, humidity, pH, and microbial contamination at various stages of production, storage, and transportation.77 Through wireless connectivity and cloud-based platforms, seamless data transmission, remote monitoring, and predictive analytics are facilitated, enabling proactive interventions and risk mitigation strategies. Emerging technologies like blockchain and AI enhance biosensor data integrity and decision-making processes by providing secure, transparent, and tamper-proof data management systems.78 For example, IBM's Food Trust blockchain network has been implemented by companies like Walmart to track food products, ensuring transparency and traceability.79 Blockchain technology ensures that biosensor data remain unaltered and traceable, while AI algorithms, such as those used in IBM Watson, analyze vast datasets to identify patterns and predict potential food safety issues, improving response times and decision accuracy.80 Advancements in the fields of nanotechnology, artificial intelligence (AI), and machine learning (ML) facilitate precise food quality monitoring, thereby enhancing efficiency, minimizing risks, and ensuring regulatory compliance.81 To ensure data privacy and security with the integration of IoT and cloud technologies in biosensor systems, measures such as end-to-end encryption, secure authentication protocols, and compliance with data protection regulations like GDPR are proposed.82 These measures protect sensitive information and maintain the confidentiality and integrity of biosensor data. One such example is Intel's Secure Device Onboard (SDO) technology that automates and secures the onboarding of IoT devices to cloud platforms, ensuring secure communication and management throughout the device lifecycle.83 Additionally, using AI-driven security solutions like those from Palo Alto Networks can detect and respond to unusual activities in real-time, safeguarding against potential breaches.79c These advancements revolutionize food quality control and safety, ushering in a new era of personalized nutrition, autonomous monitoring, and global collaboration, and marking a transformative paradigm shift in the food industry.
8. Conclusions
Biosensors represent a transformative technology with immense potential to improve food safety and quality monitoring. Their ability to provide rapid, accurate, and on-site detection of contaminants offers significant advantages over traditional analytical methods. Although biosensors offer versatile platforms for detecting various contaminants and analytes, their implementation faces challenges such as sensitivity and specificity issues, matrix interference, and regulatory considerations. However, ongoing research and development efforts, along with recent advances in nanotechnology, multiplexed detection, and integration of IoT, offer promising opportunities to overcome these challenges and revolutionize food safety practices. By addressing these opportunities and challenges, biosensors have the potential to enhance food safety, quality assurance, and regulatory compliance efforts, ensuring the integrity and safety of the global food supply chain for years to come.Data availability
No new data were created or analyzed in this study. Data sharing is not applicable to this article.Conflicts of interest
The authors report that there are no competing interests to declare.Acknowledgements
This research did not receive grants from funding agencies in the public, commercial or non-profit sectors.References
- (a) A. Hassoun and R. Karoui, Quality evaluation of fish and other seafood by traditional and nondestructive instrumental methods: advantages and limitations, Crit. Rev. Food Sci. Nutr., 2017, 57(9), 1976–1998 CAS; (b) F. Han, X. Huang and G. K. Mahunu, Exploratory review on safety of edible raw fish per the hazard factors and their detection methods, Trends Food Sci. Technol., 2017, 59, 37–48 CrossRef CAS.
- V. Naresh and N. Lee, A review on biosensors and recent development of nanostructured materials-enabled biosensors, Sensors, 2021, 21(4), 1109 CrossRef CAS.
- B. Srinivasan and S. Tung, Development and applications of portable biosensors, J. Lab. Autom., 2015, 20(4), 365–389 CrossRef CAS.
- J. Zhang, H. Huang, G. Song, K. Huang, Y. Luo, Q. Liu, X. He and N. Cheng, Intelligent biosensing strategies for rapid detection in food safety: a review, Biosens. Bioelectron., 2022, 202, 114003 CrossRef CAS.
- D. Kumar, M. Prasad and H. Mohan, Biological recognition elements, in Electrochemical Sensors, Elsevier, 2022, pp. 213–239 Search PubMed.
- S. Dash and S. Kaushik, Biosensors: Current Trends and Future Perspectives, in Nanomaterials-Based Sensing Platforms, Apple Academic Press, 2022, pp. 1–35 Search PubMed.
- M. M. Shanbhag, G. Manasa, R. J. Mascarenhas, K. Mondal and N. P. Shetti, Fundamentals of bio-electrochemical sensing, Chem. Eng. J. Adv., 2023, 16, 100516 CrossRef CAS.
- T. H. Bui, B. Thangavel, M. Sharipov, K. Chen and J. H. Shin, Smartphone-Based Portable Bio-Chemical Sensors: Exploring Recent Advancements, Chemosensors, 2023, 11(9), 468 CrossRef CAS.
- Mayank and R. Arya, Biosensors, in Advances in Biotechnology, Springer, 2013, pp. 179–194 Search PubMed.
- G. G. Guilbault and J. G. Montalvo Jr, Urea-specific enzyme electrode, J. Am. Chem. Soc., 1969, 91(8), 2164–2165 CrossRef CAS.
- J. Newman and A. Turner, Biosensors for monitoring glucose, Sensors in Medicine and Health Care: Sensors Applications, 2004, 3, 45–78 Search PubMed.
- J. Gupta, S. Arya, S. Verma, A. Singh, A. Sharma, B. Singh and R. Sharma, Performance of template-assisted electrodeposited copper/cobalt bilayered nanowires as an efficient glucose and uric acid senor, Mater. Chem. Phys., 2019, 238, 121969 CrossRef CAS.
- A. Jha, J. Moses and C. Anandharamakrishnan, Smartphone-based detection devices for the agri-food industry, in Smartphone-Based Detection Devices, Elsevier, 2021, pp. 269–310 Search PubMed.
- W. S. T. Tun, A. Saenchoopa, S. Daduang, J. Daduang, S. Kulchat and R. Patramanon, Electrochemical biosensor based on cellulose nanofibers/graphene oxide and acetylcholinesterase for the detection of chlorpyrifos pesticide in water and fruit juice, RSC Adv., 2023, 13(14), 9603–9614 RSC.
- T. Guerrero-Esteban, C. Gutiérrez-Sánchez, E. Martínez-Periñán, M. Revenga-Parra, F. Pariente and E. Lorenzo, Sensitive glyphosate electrochemiluminescence immunosensor based on electrografted carbon nanodots, Sens. Actuators, B, 2021, 330, 129389 CrossRef CAS.
- M. Bakhshpour, I. Göktürk, S. D. Gür, F. Yılmaz and A. Denizli, Sensor applications for detection in agricultural products, foods, and water, in Pesticides Bioremediation, Springer, 2022, pp. 311–352 Search PubMed.
- G. H. Parker, C. E. Gillie, J. V. Miller, D. E. Badger and M. L. Kreider, Human health risk assessment of arsenic, cadmium, lead, and mercury ingestion from baby foods, Toxicol. Rep., 2022, 9, 238–249 CrossRef CAS.
- M. Yuan, S. Qian, H. Cao, J. Yu, T. Ye, X. Wu, L. Chen and F. Xu, An ultra-sensitive electrochemical aptasensor for simultaneous quantitative detection of Pb2+ and Cd2+ in fruit and vegetable, Food Chem., 2022, 382, 132173 CrossRef CAS.
- A. K. Shakya and S. Singh, State of the art in fiber optics sensors for heavy metals detection, Opt Laser. Technol., 2022, 153, 108246 CrossRef.
- M. Pilevar, K. T. Kim and W. H. Lee, Recent advances in biosensors for detecting viruses in water and wastewater, J. Hazard. Mater., 2021, 410, 124656 CrossRef CAS.
- A. Cossettini, J. Vidic, M. Maifreni, M. Marino, D. Pinamonti and M. Manzano, Rapid detection of Listeria monocytogenes, Salmonella, Campylobacter spp., and Escherichia coli in food using biosensors, Food Control, 2022, 137, 108962 CrossRef CAS.
- S. Neethirajan, Recent advances in wearable sensors for animal health management, Sensing and Bio-Sensing Research, 2017, 12, 15–29 CrossRef.
- Y. Chai, S. Horikawa, S. Li, H. C. Wikle and B. A. Chin, A surface-scanning coil detector for real-time, in-situ detection of bacteria on fresh food surfaces, Biosens. Bioelectron., 2013, 50, 311–317 CrossRef CAS.
- L. Xu, X. Bai and A. K. Bhunia, Current state of development of biosensors and their application in foodborne pathogen detection, J. Food Prot., 2021, 84(7), 1213–1227 CrossRef CAS.
- J. Boye, A. Danquah, C. Lam Thang and X. Zhao, Food Allergens. Food Biochemistry and Food Processing, 2012, pp. 798–819 Search PubMed.
- O. Hosu, G. Selvolini and G. Marrazza, Recent advances of immunosensors for detecting food allergens, Curr. Opin. Electrochem., 2018, 10, 149–156 CrossRef CAS.
- M. Zhang, P. Wu, J. Wu, J. Ping and J. Wu, Advanced DNA-based methods for the detection of peanut allergens in processed food, TrAC, Trends Anal. Chem., 2019, 114, 278–292 CrossRef CAS.
- M. D. Kaminiaris, S. Mavrikou, M. Georgiadou, G. Paivana, D. I. Tsitsigiannis and S. Kintzios, An impedance based electrochemical immunosensor for aflatoxin B1 monitoring in pistachio matrices, Chemosensors, 2020, 8(4), 121 CrossRef CAS.
- Y. Jia, S. Zhao, D. Li, J. Yang and L. Yang, Portable chemiluminescence optical fiber aptamer-based biosensors for analysis of multiple mycotoxins, Food Control, 2023, 144, 109361 CrossRef CAS.
- (a) N. S. Jusoh, N. Awaludin, F. Salam, A. Kadir and N. Said, Label-free electrochemical Immunosensor development for mycotoxins detection in grain corn, Malaysian Journal of Analytical Sciences, 2022, 26(6), 1205–1215 Search PubMed; (b) C. Zhu, D. Liu, Y. Li, S. Ma, M. Wang and T. You, Hairpin DNA assisted dual-ratiometric electrochemical aptasensor with high reliability and anti-interference ability for simultaneous detection of aflatoxin B1 and ochratoxin A, Biosens. Bioelectron., 2021, 174, 112654 CrossRef CAS.
- Q.-Q. An, X.-Z. Feng, Z.-F. Zhou, T. Zhan, S.-F. Lian, J. Zhu, G.-C. Han, Z. Chen and H.-B. Kraatz, One step construction of an electrochemical sensor for melamine detection in milk towards an integrated portable system, Food Chem., 2022, 383, 132403 CrossRef CAS.
- (a) S. Zoughi, F. Faridbod, A. Amiri and M. R. Ganjali, Detection of tartrazine in fake saffron containing products by a sensitive optical nanosensor, Food Chem., 2021, 350, 129197 CrossRef CAS; (b) E. S. Okeke, T. P. C. Ezeorba, C. O. Okoye, Y. Chen, G. Mao, W. Feng and X. Wu, Analytical detection methods for azo dyes: a focus on comparative limitations and prospects of bio-sensing and electrochemical nano-detection, J. Food Compos. Anal., 2022, 114, 104778 CrossRef CAS.
- X. Xiong, Y. Tan, E. Mubango, C. Shi, J. M. Regenstein, Q. Yang, H. Hong and Y. Luo, Rapid freshness and survival monitoring biosensors of fish: Progress, challenge, and future perspective, Trends Food Sci. Technol., 2022, 129, 61–73 CrossRef CAS.
- R. K. Pal, Post-Harvest Management of Horticultural Crops: Use of Sensors and New Molecules, in Transformation of Agri-Food Systems, Springer, 2024, pp. 239–253 Search PubMed.
- A. S. More, C. S. Ranadheera, Z. Fang, R. Warner and S. Ajlouni, Biomarkers associated with quality and safety of fresh-cut produce, Food Biosci., 2020, 34, 100524 CrossRef CAS.
- Y.-Z. Wang, J. K. Christopher, Y.-C. Yong and D.-D. Zhai, Nutrient Detection with Whole-Cell Biosensors, Handbook of Cell Biosensors, 2021, pp. 747–766 Search PubMed.
- K. H. Kim, D. Moon, J. E. An, S. J. Park, S. E. Seo, S. Ha, J. Kim, K. Kim, S. Phyo and J. Lee, Wireless portable bioelectronic nose device for multiplex monitoring toward food freshness/spoilage, Biosens. Bioelectron., 2022, 215, 114551 CrossRef CAS.
- M. Zachariasova, P. Cuhra and J. Hajslova, Cross-reactivity of rapid immunochemical methods for mycotoxins detection towards metabolites and masked mycotoxins: the current state of knowledge, World Mycotoxin J., 2014, 7(4), 449–464 CAS.
- A. Sameen, A. Sahar, S. Khan, T. Tariq and B. Ishfaq, Shelf Life of Foods through Nanosensors Application, in Nanotechnology Interventions in Food Packaging and Shelf Life, CRC Press, 2022, pp. 173–202 Search PubMed.
- H. Song, M. Huo, M. Zhou, H. Chang, J. Li, Q. Zhang, Y. Fang, H. Wang and D. Zhang, Carbon nanomaterials-based electrochemical sensors for heavy metal detection, Crit. Rev. Anal. Chem., 2022, 1–20 CrossRef.
- M. Eskola, G. Kos, C. T. Elliott, J. Hajšlová, S. Mayar and R. Krska, Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’of 25, Crit. Rev. Food Sci. Nutr., 2020, 60(16), 2773–2789 CrossRef CAS.
- W. Zhu, L. Li, Z. Zhou, X. Yang, N. Hao, Y. Guo and K. Wang, A colorimetric biosensor for simultaneous ochratoxin A and aflatoxins B1 detection in agricultural products, Food Chem., 2020, 319, 126544 CrossRef CAS.
- Y. Li, S. Tang, W. Zhang, X. Cui, Y. Zhang, Y. Jin, X. Zhang and Y. Chen, A surface-enhanced Raman scattering-based lateral flow immunosensor for colistin in raw milk, Sens. Actuators, B, 2019, 282, 703–711 CrossRef CAS.
- (a) S. Leonardo, G. Gaiani, T. Tsumuraya, M. Hirama, J. Turquet, N. Sagristà, M. Rambla-Alegre, C. Flores, J. Caixach and J. Diogène, Addressing the analytical challenges for the detection of ciguatoxins using an electrochemical biosensor, Anal. Chem., 2020, 92(7), 4858–4865 CrossRef CAS; (b) B. Bucur, C. Purcarea, S. Andreescu and A. Vasilescu, Addressing the selectivity of enzyme biosensors: solutions and perspectives, Sensors, 2021, 21(9), 3038 CrossRef CAS.
- A. Economou, S. K. Karapetis, G. P. Nikoleli, D. P. Nikolelis, S. Bratakou and T. H. Varzakas, Enzyme-based Sensors, Advances in Food Diagnostics, 2017, pp. 231–250 Search PubMed.
- Y. Feng, Y. Xu, S. Liu, D. Wu, Z. Su, G. Chen, J. Liu and G. Li, Recent advances in enzyme immobilization based on novel porous framework materials and its applications in biosensing, Coord. Chem. Rev., 2022, 459, 214414 CrossRef CAS.
- C. Qian, R. Wang, H. Wu, J. Ping and J. Wu, Recent advances in emerging DNA-based methods for genetically modified organisms (GMOs) rapid detection, TrAC, Trends Anal. Chem., 2018, 109, 19–31 CrossRef CAS.
- Y. Chen, J. Liu, Z. Yang, J. S. Wilkinson and X. Zhou, Optical biosensors based on refractometric sensing schemes: A review, Biosens. Bioelectron., 2019, 144, 111693 CrossRef CAS.
- F. Bibi, C. Guillaume, A. Vena, N. Gontard and B. Sorli, Wheat gluten, a bio-polymer layer to monitor relative humidity in food packaging: Electric and dielectric characterization, Sens. Actuators, A, 2016, 247, 355–367 CrossRef CAS.
- G.-J. E. Nychas, E. Z. Panagou and F. Mohareb, Novel approaches for food safety management and communication, Curr. Opin. Food Sci., 2016, 12, 13–20 CrossRef.
- A. Patil-Joshi, B. Rangaswamy and A. Apte-Deshpande, Paper-based PCR method development, validation and application for microbial detection, J. Genet. Eng. Biotechnol., 2021, 19(1), 37 CrossRef.
- M. Jeyaraman and E. Eltzov, Enhancing Food Safety: A Low-cost Biosensor for Bacillus licheniformis Detection in Food Products, Talanta, 2024, 126152 CrossRef CAS.
- R. Arreguin-Campos, M. Frigoli, M. Caldara, R. D. Crapnell, A. G.-M. Ferrari, C. E. Banks, T. J. Cleij, H. Diliën, K. Eersels and B. van Grinsven, Functionalized screen-printed electrodes for the thermal detection of Escherichia coli in dairy products, Food Chem., 2023, 404, 134653 CrossRef CAS.
- Standardization, I. O. f., ISO 16140–2: 2016 Microbiology of the food chain–Method validation–Part 2: Protocol for the validation of alternative (proprietary) methods against a reference method, ISO 2016.
- (a) S. Bansal, A. Singh, M. Mangal, A. K. Mangal and S. Kumar, Food adulteration: Sources, health risks, and detection methods, Crit. Rev. Food Sci. Nutr., 2017, 57(6), 1174–1189 CrossRef CAS; (b) S. Jafari, J. Guercetti, A. Geballa-Koukoula, A. S. Tsagkaris, J. L. Nelis, M.-P. Marco, J.-P. Salvador, A. Gerssen, J. Hajslova and C. Elliott, ASSURED point-of-need food safety screening: a critical assessment of portable food analyzers, Foods, 2021, 10(6), 1399 CrossRef CAS.
- V. Velusamy, K. Arshak, O. Korostynska, K. Oliwa and C. Adley, An overview of foodborne pathogen detection: in the perspective of biosensors, Biotechnol. Adv., 2010, 28(2), 232–254 CrossRef CAS.
- D. Patel, S. P. Oliver, R. A. Almeida and E. R. Vedamuthu, Management Systems for Safety and Quality, Dairy Processing and Quality Assurance, 2015, pp. 553–599 Search PubMed.
- J. Ananthapavan, G. Sacks, L. Orellana, J. Marshall, E. Robinson, M. Moodie, M. Blake, A. Brown, R. Carter and A. J. Cameron, Cost–Benefit and Cost–Utility Analyses to Demonstrate the Potential Value-for-Money of Supermarket Shelf Tags Promoting Healthier Packaged Products in Australia, Nutrients, 2022, 14(9), 1919 CrossRef.
- B. G. Ferrin and R. E. Plank, Total cost of ownership models: an exploratory study, J. Supply Chain Manag., 2002, 38(2), 18–29 CrossRef.
- S. Neethirajan, V. Ragavan, X. Weng and R. Chand, Biosensors for sustainable food engineering: challenges and perspectives, Biosensors, 2018, 8(1), 23 CrossRef.
- S. Liu, A. Kannegulla, X. Kong, R. Sun, Y. Liu, R. Wang, Q. Yu and A. X. Wang, Simultaneous colorimetric and surface-enhanced Raman scattering detection of melamine from milk, Spectrochim. Acta, Part A, 2020, 231, 118130 CrossRef CAS.
- (a) L. He, N.-J. Kim, H. Li, Z. Hu and M. Lin, Use of a fractal-like gold nanostructure in surface-enhanced Raman spectroscopy for detection of selected food contaminants, J. Agric. Food Chem., 2008, 56(21), 9843–9847 CrossRef CAS; (b) H. Chu, Y. Huang and Y. Zhao, Silver nanorod arrays as a surface-enhanced Raman scattering substrate for foodborne pathogenic bacteria detection, Appl. Spectrosc., 2008, 62(8), 922–931 CrossRef CAS.
- G. Song, Z. Zhang, M.-L. Fauconnier, C. Li, L. Chen, X. Zheng and D. Zhang, Bimodal single-atom iron nanozyme biosensor for volatile amine and food freshness detection, Nano Today, 2023, 53, 102025 CrossRef CAS.
- Y. Zhao, L. Liang, W. Guo, X. Lu, C. Zhao and F. Gao, The porous hollow cobalt-based oxides encapsulated with bimetallic PdAu Nanoparticles of electrochemical biosensor for highly sensitive pesticides detection, Nanotechnology, 2023, 34(28), 285501 CrossRef.
- D. Zhao, J. Liu, J. Du, K. Liu and Y. Bai, A highly sensitive multiplex lateral flow immunoassay for simultaneous detection of Listeria monocytogenes, Salmonella Typhimurium and Escherichia coli O157: H7, J. Food Meas. Char., 2023, 17(6), 6577–6587 CrossRef.
- Y. Shang, G. Xing, H. Lin, Y. Sun, S. Chen and J.-M. Lin, Development of nucleic acid extraction and real-time recombinase polymerase amplification (RPA) assay integrated microfluidic biosensor for multiplex detection of foodborne bacteria, Food Control, 2024, 155, 110047 CrossRef CAS.
- P. Yin, J. Wang, T. Li, Q. Pan, L. Zhu, F. Yu, Y.-z. Zhao and H.-B. Liu, A smartphone-based fluorescent sensor for rapid detection of multiple pathogenic bacteria, Biosens. Bioelectron., 2023, 242, 115744 CrossRef CAS.
- H. Li, Y. Ren, R. Jiao, X. Zhang, W. Zhao, H. Chen, D. Zhang, Y. Shen, C. Zhu and Y. Ye, On-site rapid detection of Listeria monocytogenes in dairy products using smartphone-integrated device-assisted ratiometric fluorescent sensors, Int. J. Dairy Technol., 2024, 77(2), 403–414 CrossRef CAS.
- S. Wei, F. Wang, L. Zhang, C. Zhao, J. Li and J. Wang, A portable smartphone-assisted highly emissive magnetic covalent organic framework-based fluorescence sensor for the detection of Salmonella typhimurium, Sens. Actuators, B, 2023, 392, 134076 CrossRef CAS.
- E. M. Khalaf, H. S. Jabbar, R. M. Romero-Parra, G. R. L. Al-Awsi, H. S. Budi, A. S. Altamimi, M. A. Gatea, K. T. Falih, K. Singh and K. A. Alkhuzai, Smartphone-assisted microfluidic sensor as an intelligent device for on-site determination of food contaminants: developments and applications, Microchem. J., 2023, 108692 CrossRef CAS.
- J. R. Sempionatto, V. R.-V. Montiel, E. Vargas, H. Teymourian and J. Wang, Wearable and mobile sensors for personalized nutrition, ACS Sens., 2021, 6(5), 1745–1760 CrossRef CAS.
- M. Wang, Y. Yang, J. Min, Y. Song, J. Tu, D. Mukasa, C. Ye, C. Xu, N. Heflin and J. S. McCune, A wearable electrochemical biosensor for the monitoring of metabolites and nutrients, Nat. Biomed. Eng., 2022, 6(11), 1225–1235 CrossRef CAS.
- P. A. Raymundo-Pereira, N. O. Gomes, F. M. Shimizu, S. A. Machado and O. N. Oliveira Jr, Selective and sensitive multiplexed detection of pesticides in food samples using wearable, flexible glove-embedded non-enzymatic sensors, Chem. Eng. J., 2021, 408, 127279 CrossRef CAS.
- T. Thirugnanasambandan, S. Ramanathan and S. C. Gopinath, Revolutionizing biosensing through cutting-edge nanomaterials: An in-depth exploration of recent technological advances, Nano-Struct. Nano-Objects, 2024, 38, 101128 CrossRef CAS.
- W. Mu, G. A. Kleter, Y. Bouzembrak, E. Dupouy, L. J. Frewer, F. N. Radwan Al Natour and H. Marvin, Making food systems more resilient to food safety risks by including artificial intelligence, big data, and internet of things into food safety early warning and emerging risk identification tools, Compr. Rev. Food Sci. Food Saf., 2024, 23(1), 1–18 CrossRef.
- (a) M. Ataei Kachouei, A. Kaushik and M. A. Ali, Internet of Things-Enabled Food and Plant Sensors to Empower Sustainability, Advanced Intelligent Systems, 2023, 5(12), 2300321 CrossRef; (b) R. K. Dwivedi, R. Kumar and R. Buyya, Gaussian distribution-based machine learning scheme for anomaly detection in healthcare sensor cloud, Int. J. Cloud Appl. Comput. (IJCAC), 2021, 11(1), 52–72 Search PubMed; (c) A. P. Plageras, K. E. Psannis, C. Stergiou, H. Wang and B. B. Gupta, Efficient IoT-based sensor BIG Data collection–processing and analysis in smart buildings, Future Generat. Comput. Syst., 2018, 82, 349–357 CrossRef.
- H. M. R. Abid, N. Khan, A. Hussain, Z. B. Anis, M. Nadeem and N. Khalid, Quantitative and qualitative approach for accessing and predicting food safety using various web-based tools, Food Control, 2024, 110471 CrossRef.
- (a) H. Anwar, T. Anwar and G. Mahmood, Nourishing the Future: AI-Driven Optimization of Farm-to-Consumer Food Supply Chain for Enhanced Business Performance, Innovative Computing Review, 2023, 3(2), 15–29 Search PubMed; (b) J. Lu, J. Shen, P. Vijayakumar and B. B. Gupta, Blockchain-based secure data storage protocol for sensors in the industrial internet of things, IEEE Trans. Ind. Inf., 2021, 18(8), 5422–5431 Search PubMed.
- (a) V. Singh and S. K. Sharma, Application of blockchain technology in shaping the future of food industry based on transparency and consumer trust, J. Food Sci. Technol., 2023, 60(4), 1237–1254 CrossRef CAS; (b) B. Tan, J. Yan, S. Chen and X. Liu, in The Impact of Blockchain on Food Supply Chain: The Case of Walmart, Smart Blockchain, First International Conference, SmartBlock 2018, Tokyo, Japan, December 10–12, 2018, Proceedings 1, Springer, 2018, pp. 167–177 Search PubMed; (c) D. Patel, C. K. Sahu and R. Rai, Security in modern manufacturing systems: integrating blockchain in artificial intelligence-assisted manufacturing, Int. J. Prod. Res., 2024, 62(3), 1041–1071 CrossRef.
- P. Tagde, S. Tagde, T. Bhattacharya, P. Tagde, H. Chopra, R. Akter, D. Kaushik and M. H. Rahman, Blockchain and artificial intelligence technology in e-Health, Environ. Sci. Pollut. Res., 2021, 28, 52810–52831 CrossRef.
- (a) H. Moulahoum and F. Ghorbanizamani, Navigating the development of silver nanoparticles based food analysis through the power of artificial intelligence, Food Chem., 2024, 138800 CrossRef CAS; (b) K. B. Chhetri, Applications of Artificial Intelligence and Machine Learning in Food Quality Control and Safety Assessment, Food Eng. Rev., 2023, 1–21 Search PubMed.
- F. J. Jaime, A. Muñoz, F. Rodríguez-Gómez and A. Jerez-Calero, Strengthening privacy and data security in biomedical microelectromechanical systems by IoT communication security and protection in smart healthcare, Sensors, 2023, 23(21), 8944 CrossRef.
- P. K. Malik, R. Sharma, R. Singh, A. Gehlot, S. C. Satapathy, W. S. Alnumay, D. Pelusi, U. Ghosh and J. Nayak, Industrial Internet of Things and its applications in industry 4.0: State of the art, Comput. Commun., 2021, 166, 125–139 CrossRef.
- Y. Huang, J. Tan, L. Cui, Z. Zhou, S. Zhou, Z. Zhang, R. Zheng, Y. Xue, M. Zhang and S. Li, Graphene and Au NPs co-mediated enzymatic silver deposition for the ultrasensitive electrochemical detection of cholesterol, Biosens. Bioelectron., 2018, 102, 560–567 CrossRef CAS.
- R. Peltomaa, R. Barderas, E. Benito-Peña and M. C. Moreno-Bondi, Recombinant antibodies and their use for food immunoanalysis, Anal. Bioanal. Chem., 2022, 414(1), 193–217 CrossRef CAS.
- L. d'Amone, G. Matzeu, A. Quijano-Rubio, G. P. Callahan, B. Napier, D. Baker and F. G. Omenetto, Reshaping de novo protein switches into bioresponsive materials for biomarker, toxin, and viral detection, Adv. Mater., 2023, 35(11), 2208556 CrossRef.
- V. S. Babu, S. Patra, N. Karanth, M. Kumar and M. Thakur, Development of a biosensor for caffeine, Anal. Chim. Acta, 2007, 582(2), 329–334 CrossRef CAS.
- Z. Xiaobo, H. Xiaowei and M. Povey, Non-invasive sensing for food reassurance, Analyst, 2016, 141(5), 1587–1610 RSC.
- M. Thakur and K. Ragavan, Biosensors in food processing, J. Food Sci. Technol., 2013, 50, 625–641 CrossRef CAS.
- (a) S. Barrias, J. Ibáñez, J. R. Fernandes and P. Martins-Lopes, The role of DNA-based biosensors in species identification for food authenticity assessment, Trends Food Sci. Technol., 2024, 104350 CrossRef CAS; (b) K. Cali, E. Tuccori and K. C. Persaud, Gravimetric biosensors, in Methods in Enzymology, Elsevier, 2020, vol. 642, pp. 435–468 Search PubMed.
- A. Laprovitta, G. Peretti and E. Romero, in Analog Configurability-Test Scheme for An Embedded Op-Amp Module in TI MSP430 Microcontrollers, XIV Argentine Symposium on Technology (AST)-JAIIO 42 (2013), 2013 Search PubMed.
- K. Itakura, K. Kayano, K. Nakazato and K. Niitsu, Theoretical analysis and simulation study of low-power CMOS electrochemical impedance spectroscopy biosensor in 55 nm deeply depleted channel technology for cell-state monitoring, Jpn. J. Appl. Phys., 2017, 57(1S), 01AG02 CrossRef.
- J. Zuo, J. Liang, S. Cheng, Y. Deng, Z. Li, Q. Nie, D. Zhang, X. Zhang, Z. Li and H. Li, Live chicken body fat measurement technology based on bio-electrical impedance, Comput. Electron. Agric., 2024, 220, 108890 CrossRef.
- C. Huang, J. Zhao, R. Lu, J. Wang, S. R. Nugen, Y. Chen and X. Wang, A phage-based magnetic relaxation switching biosensor using bioorthogonal reaction signal amplification for salmonella detection in foods, Food Chem., 2023, 400, 134035 CrossRef CAS.
- J. Devasagayam, C. A. Leclerc, M. B. Richardson, C. G. Ty, A. K. AlSawalhi, S. S. Worthington and C. M. Collier, in A lock-in amplifier biosensor for dairy applications, Organic and Hybrid Sensors and Bioelectronics XVI, SPIE, 2023, pp. 74–80 Search PubMed.
- D. Enériz, N. Medrano and B. Calvo, An FPGA-based machine learning tool for in situ food quality tracking using sensor fusion, Biosensors, 2021, 11(10), 366 CrossRef.
- X. Jin, C. Liu, T. Xu, L. Su and X. Zhang, Artificial intelligence biosensors: challenges and prospects, Biosens. Bioelectron., 2020, 165, 112412 CrossRef CAS.
- V. Pawar, N. Verma and S. Pawar, In Overview of Different Milk and Milk Products Contaminant Detections by Biosensors, Proceedings of the 3rd International Conference on Advances in Science & Technology (ICAST), 2020 Search PubMed.
- A. L. Silva, G. M. d. S. Salvador, S. V. Castro, N. M. Carvalho and R. A. Munoz, A 3D printer guide for the development and application of electrochemical cells and devices, Front. Chem., 2021, 9, 684256 CrossRef CAS.
- E. Daniso, P. Melpignano, S. Susmel and F. Tulli, Optimization of an OLED-based immunosensor for the detection of tetrodotoxin in mussels, Food Control, 2024, 160, 110352 CrossRef CAS.
- N. Khansili, G. Rattu and P. M. Krishna, Label-free optical biosensors for food and biological sensor applications, Sens. Actuators, B, 2018, 265, 35–49 CrossRef CAS.
- H. Alloush, R. Lewis and V. Salisbury, Bacterial bioluminescent biosensors: applications in food and environmental monitoring, Anal. Lett., 2006, 39(8), 1517–1526 CrossRef CAS.
- L. F. Loguercio, A. Thesing, P. Demingos, C. D. de Albuquerque, R. S. Rodrigues, A. G. Brolo and J. F. Santos, Efficient acetylcholinesterase immobilization for improved electrochemical performance in polypyrrole nanocomposite-based biosensors for carbaryl pesticide, Sens. Actuators, B, 2021, 339, 129875 CrossRef CAS.
- M. Majdinasab, S. Ben Aissa and J. L. Marty, Advances in colorimetric strategies for mycotoxins detection: toward rapid industrial monitoring, Toxins, 2020, 13(1), 13 CrossRef.
- P. Krzyczmonik, E. Socha and S. Skrzypek, Electrochemical detection of glucose in beverage samples using poly (3, 4-ethylenedioxythiophene)-modified electrodes with immobilized glucose oxidase, Electrocatalysis, 2018, 9, 380–387 CrossRef CAS.
- E. Loğoğlu, S. Sungur and Y. Yildiz, Development of lactose biosensor based on β-galactosidase and glucose oxidase immobilized into gelatin, J. Macromol. Sci., Part A: Pure Appl. Chem., 2006, 43(3), 525–533 CrossRef.
- A. Samphao, K. Kunpatee, S. Prayoonpokarach, J. Wittayakun, Ľ. Švorc, D. M. Stankovic, K. Zagar, M. Ceh and K. Kalcher, An ethanol biosensor based on simple immobilization of alcohol dehydrogenase on Fe3O4@Au nanoparticles, Electroanalysis, 2015, 27(12), 2829–2837 CrossRef CAS.
- L. Fu, B. J. Cherayil, H. Shi, Y. Wang, Y. Zhu, L. Fu, B. J. Cherayil, H. Shi, Y. Wang and Y. Zhu, Detection and quantification methods for food allergens, Food Allergy: From Molecular Mechanisms to Control Strategies, 2019, 69–91 Search PubMed.
- J. Bhardwaj, S. Devarakonda, S. Kumar and J. Jang, Development of a paper-based electrochemical immunosensor using an antibody-single walled carbon nanotubes bio-conjugate modified electrode for label-free detection of foodborne pathogens, Sens. Actuators, B, 2017, 253, 115–123 CrossRef CAS.
- E. C. Peláez, M.-C. Estevez, R. Domínguez, C. Sousa, A. Cebolla and L. M. Lechuga, A compact SPR biosensor device for the rapid and efficient monitoring of gluten-free diet directly in human urine, Anal. Bioanal. Chem., 2020, 412, 6407–6417 CrossRef.
- F. Azri, Development of a Nanomaterial-Based Electrochemical Immunosensor for Detection of Aflatoxin B1 in Peanuts, Masters thesis, Universiti Putra Malaysia, 2016 Search PubMed.
- S. Neethirajan, S. R. Ahmed, R. Chand, J. Buozis and É. Nagy, Recent advances in biosensor development for foodborne virus detection, Nanotheranostics, 2017, 1(3), 272 CrossRef.
- Y. Chen, C. Qian, C. Liu, H. Shen, Z. Wang, J. Ping, J. Wu and H. Chen, Nucleic acid amplification free biosensors for pathogen detection, Biosens. Bioelectron., 2020, 153, 112049 CrossRef CAS.
- C. L. Manzanares Palenzuela, Electrochemical DNA Biosensors and Sensing Platforms for the Detection and Quantification of Genetically Modified Soybean in Food and Feed, 2018 Search PubMed.
- J. Wang, J. L. Davidson, S. Kaur, A. A. Dextre, M. Ranjbaran, M. S. Kamel, S. M. Athalye and M. S. Verma, Based Biosensors for the Detection of Nucleic Acids from Pathogens, Biosensors, 2022, 12(12), 1094 CrossRef CAS.
- R. Pilolli, L. Monaci and A. Visconti, Advances in biosensor development based on integrating nanotechnology and applied to food-allergen management, TrAC, Trends Anal. Chem., 2013, 47, 12–26 CrossRef CAS.
- Y. Ye, H. Guo and X. Sun, Recent progress on cell-based biosensors for analysis of food safety and quality control, Biosens. Bioelectron., 2019, 126, 389–404 CrossRef CAS.
- M. Gonchar, O. Smutok, M. Karkovska, N. Stasyuk and G. Gayda, Yeast-based biosensors for clinical diagnostics and food control, Biotechnology of Yeasts and Filamentous Fungi, 2017, pp. 391–412 Search PubMed.
- S. A. Ali, D. Mittal and G. Kaur, In situ monitoring of xenobiotics using genetically engineered whole-cell-based microbial biosensors: recent advances and outlook, World J. Microbiol. Biotechnol., 2021, 37, 1–24 CrossRef.
- R. Saini, K. Hegde, S. K. Brar and M. Verma, Advances in whole cell-based biosensors in environmental monitoring, in Tools, Techniques and Protocols for Monitoring Environmental Contaminants, Elsevier, 2019, pp. 263–284 Search PubMed.
- R. Umapathi, B. Park, S. Sonwal, G. M. Rani, Y. Cho and Y. S. Huh, Advances in optical-sensing strategies for the on-site detection of pesticides in agricultural foods, Trends Food Sci. Technol., 2022, 119, 69–89 CrossRef CAS.
- A. Singhal, M. A. Sadique, N. Kumar, S. Yadav, P. Ranjan, A. Parihar, R. Khan and A. K. Kaushik, Multifunctional carbon nanomaterials decorated molecularly imprinted hybrid polymers for efficient electrochemical antibiotics sensing, J. Environ. Chem. Eng., 2022, 10(3), 107703 CrossRef CAS.
- T. Sergeyeva, D. Yarynka, L. Dubey, I. Dubey, E. Piletska, R. Linnik, M. Antonyuk, T. Ternovska, O. Brovko and S. Piletsky, Sensor based on molecularly imprinted polymer membranes and smartphone for detection of Fusarium contamination in cereals, Sensors, 2020, 20(15), 4304 CrossRef CAS.
- M. H. Mahnashi, A. M. Mahmoud, K. Alhazzani, A. Alanazi, M. M. Algahtani, A. M. Alaseem, Y. S. Alqahtani and M. M. El-Wekil, Enhanced molecular imprinted electrochemical sensing of histamine based on signal reporting nanohybrid, Microchem. J., 2021, 168, 106439 CrossRef CAS.
- R. Umapathi, S. Sonwal, M. J. Lee, G. M. Rani, E.-S. Lee, T.-J. Jeon, S.-M. Kang, M.-H. Oh and Y. S. Huh, Colorimetric based on-site sensing strategies for the rapid detection of pesticides in agricultural foods: new horizons, perspectives, and challenges, Coord. Chem. Rev., 2021, 446, 214061 CrossRef CAS.
- T. Lang, S. Pang and L. He, Integration of colorimetric and SERS detection for rapid screening and validation of melamine in milk, Anal. Methods, 2015, 7(15), 6426–6431 RSC.
- R. Pilolli and L. Monaci, Challenging the limit of detection for egg allergen detection in red wines by surface plasmon resonance biosensor, Food Anal. Methods, 2016, 9, 2754–2761 CrossRef.
- M. I. Gaviria, K. Barrientos, J. P. Arango, J. B. Cano and G. A. Peñuela, Highly sensitive fluorescent biosensor based on acetylcholinesterase and carbon dots–graphene oxide quenching test for analytical and commercial organophosphate pesticide detection, Front. Environ. Sci., 2022, 10, 825112 CrossRef.
- D. V. Sotnikov, A. V. Zherdev, E. A. Zvereva, S. A. Eremin and B. B. Dzantiev, Changing cross-reactivity for different immunoassays using the same antibodies: theoretical description and experimental confirmation, Appl. Sci., 2021, 11(14), 6581 CrossRef CAS.
- M. M. A. Zeinhom, Y. Wang, L. Sheng, D. Du, L. Li, M.-J. Zhu and Y. Lin, Smart phone based immunosensor coupled with nanoflower signal amplification for rapid detection of Salmonella Enteritidis in milk, cheese and water, Sens. Actuators, B, 2018, 261, 75–82 CrossRef CAS.
- C. Chen, J. Zhou, Z. Li, Y. Xu, T. Ran and J. Gen, Wearable Electrochemical Biosensors for In Situ Pesticide Analysis from Crops, J. Electrochem. Soc., 2023, 170(11), 117512 CrossRef.
- A. Sheini, Colorimetric aggregation assay based on array of gold and silver nanoparticles for simultaneous analysis of aflatoxins, ochratoxin and zearalenone by using chemometric analysis and paper based analytical devices, Microchim. Acta, 2020, 187, 1–11 CrossRef.
- N. Asghar, G. Mustafa, M. Yasinzai, Y. A. Al-Soud, P. A. Lieberzeit and U. Latif, Real-time and online monitoring of glucose contents by using molecular imprinted polymer-based IDEs sensor, Appl. Biochem. Biotechnol., 2019, 189, 1156–1166 CrossRef CAS.
- W. Zhao, S. Yang, D. Zhang, T. Zhou, J. Huang, M. Gao, Y. Jiang, Y. Liu and J. Yang, Ultrasensitive dual-enhanced sandwich strategy for simultaneous detection of Escherichia coli and Staphylococcus aureus based on optimized aptamers-functionalized magnetic capture probes and graphene oxide-Au nanostars SERS tags, J. Colloid Interface Sci., 2023, 634, 651–663 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |


