Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enzymatic modification of cold pressed coconut meal protein: nutritional, functional and biological properties
Zahra
Akbarbaglu
a,
Khashayar
Sarabandi
 *b,
Seyed Hadi
Peighambardoust
*a,
Roxana
Sarabandi
c,
Hossein Samadi
Kafil
*b,
Seyed Hadi
Peighambardoust
*a,
Roxana
Sarabandi
c,
Hossein Samadi
Kafil
 d and
Mohammad Ali
Hesarinejad
e
d and
Mohammad Ali
Hesarinejad
e
aDepartment of Food Science, College of Agriculture, University of Tabriz, Tabriz, Iran. E-mail: peighambardoust@tabrizu.ac.ir
bDepartment of Food Chemistry, Research Institute of Food Science and Technology (RIFST), Mashhad, Iran. E-mail: kh.sarabandi@irfst.ac.ir; sarabandi_21@yahoo.com
cDepartment of Basic Science, Faculty of Veterinary Medicine, University of Zabol, Zabol, Iran
dDrug Applied Research Center, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
eDepartment of Food Sensory and Cognitive Science, Research Institute of Food Science and Technology (RIFST), Mashhad, Iran
First published on 1st August 2024
Abstract
Bioactive peptides (BPs) generated by hydrolysis of food proteins exhibit a broad spectrum of biological properties in both in vitro and in vivo models. In this research, variations in amino acid composition, solubility, emulsifying, foaming and water/oil-holding properties and antioxidant capacity of hydrolysates obtained from coconut meal protein (CMP) were investigated. The solubility of hydrolysates was significantly increased (P < 0.05) from 11% (in CMP at pH = 4) to 79.7% (t = 180 min, pH = 4). Emulsifying activity (EAI) and emulsion stability (ESI) indices of different hydrolysates ranged between 43.7 and 70.7 m2 g−1 and 38.7–82.7%, respectively at pH = 3–9. Primary CMP showed the lowest EAI, ESI, and foaming capacity at pH = 5 (near pI). Limited hydrolysis (t = 30 min) of CMP resulted in the highest water-holding (WHC, 6.0 g g−1) and oil-holding (OHC, 5.0 g g−1) capacities. Increasing hydrolysis led to a significant decrease in WHC and OHC values. The extent of hydrolysis significantly increased the amount of essential, antioxidant, hydrophobic, and negatively charged free amino acids in the hydrolysates. Generally, the antioxidant activity of CMP reached the maximum value (DPPH = 74.1%, ABTS = 64.5%, OH = 66.7%, reducing power = 0.87, total antioxidant = 1.29, Fe = 63.7% and Cu = 24.3% chelation) after 90 min of hydrolysis. Overall, improved techno-functional, antioxidant and nutritional indicators of CMP hydrolysates consider these polypeptides as natural sources of antioxidants in the food and pharmaceutical industries.
Sustainability spotlightIn recent years, the growing population (more than 9 billion people worldwide by the next 4 decades) on the one hand and the rise of various diseases (cardiovascular diseases, diabetes, hypertension, various cancers, Alzheimer’s disease, Parkinson's disease, and multiple sclerosis) on the other hand redoubled the need for producing and using functional foods. In the meantime, proteins play a key role in the diet and especially in the production of bioactive peptides. Meanwhile, the production of proteins and peptides from animal sources is associated with environmental challenges, the limitation of energy resources and the increase of greenhouse gases. On the other hand, more than 30% of globally produced foods are discarded during processing, storage, and transportation. The importance of this issue is doubled, especially when considering the use of waste from the food industry to extract proteins and other health-promoting compounds. Therefore, in this study, protein extraction from cold-pressed coconut meal (after oil extraction) and production of bioactive peptides with high techno-functional, nutritional and health-giving properties were investigated with the aim of enriching and producing beneficial food products. Thus, this work meets the Sustainable Development Goals (SDGs) established by the UN. |
1 Introduction
Proteins are a major component of the food pyramid, playing a crucial role in human diet and health. However, in recent years, consumers have shown growing interest in and a tendency to use vegetable proteins.1 On the other hand, more than 30% of globally produced foods are discarded during processing, storage, and transportation.2 The importance of this issue is doubled, especially when considering the use of waste from the food industry to extract proteins and other health-promoting compounds.3 This study aimed to use waste of the coconut oil processing industry to extract its proteins and convert them into added-value hydrolysates as health-promoting compounds. There are numerous research studies on protein extraction from by-products of oilseeds (e.g., pumpkin seed, sesame meal, chia, moringa, tomato seeds, rapeseed meal, and sesame bran), legumes (e.g., soybean meal, peanut cake, and pigeon pea waste), cereals (e.g., bran, wheat, and rice), and various fruits and vegetables (e.g., coconut pulp, tomato pomace, artichoke waste, orange peel, apple pomace and potato peel).4 Meals or cakes resulting from the oil extraction process comprise a major part of the production of waste and by-products.5 These by-products are also rich in natural bioactive compounds including vitamins, carotenoids, polyphenols, flavonoids, fatty acids, salts, phytosterols, polysaccharides, tocopherols, and soluble fibers.6Coconut is a fruit that grows widely in various countries (over 92 countries), with an annual production of more than 60 million tons. Coconut cake, as a by-product of coconut pulp cold-pressing, contains a large amount of protein (about 16–20%).7 Coconut processing waste is mainly consumed as animal feed, whereas it can be considered as a rich source of bioactive compounds with a high nutritional value.1 In addition to the nutritional importance of proteins, their techno-functional properties (such as solubility, emulsification, and water- and oil-holding capacity) play a key role in the stability, taste, texture, and sensory acceptance of emulsions and food products.8 The functional properties of proteins are also affected by their structural changes and denaturation during processing stresses (such as shear or thermal stresses) or the properties and composition of food products (acidic conditions or metal ions).9 Different strategies are used to produce BPs. Microbial fermentation and enzyme-catalyzed proteolysis are the most widely studied and applied, while autolysis and acid hydrolysis are less common. Also, technologies such as high hydrostatic pressure (HHP), microwave, and pulsed electric field (PEF) are recognized as three of the most promising emerging technologies for the production of structurally modified proteins.10
Enzymatic hydrolysis is a way to modify the structure and improve the functional properties of proteins.11 This modification allows the release of some bioactive peptides which are generally composed of 2–20 amino acid residues and inactive in the sequence of their native proteins,10 in addition to changes in polar groups, molecular weight, and the degree of hydrophobicity. Antioxidant, antibacterial, anticancer, antithrombotic, antihypertensive, immunomodulating, anticoagulant and antithrombotic activities are some of the health-promoting properties and physiological effects of bioactive peptides. These advantages have been of core interest to researchers for the extraction and production of these peptides from various plant and animal sources (particularly waste and by-products).12,13
As far as the authors are aware, no comprehensive research has been performed on the production of bioactive peptides from coconut cake protein and investigating its functional and biological properties. An important issue covered in this study is the use of waste from the coconut oil processing industry to extract proteins and convert them into hydrolysates as health-promoting compounds. In this study, we have explored different properties of hydrolysates obtained from enzymatic hydrolysis of coconut meal protein including solubility, emulsification, foaming, water- and oil-holding properties, and antioxidant capacity. Given the importance and advantages of producing bioactive peptides from natural sources and by-products of the food industry, the following steps were the objectives of this study: (1) protein extraction from cold-pressed coconut meal; evaluation of the effect of enzymatic hydrolysis at different times on (2) the DH and composition of amino acids; (3) functional properties (solubility, emulsification, and foaming at different pHs); (4) water- and oil-holding capacities; (5) antioxidant indices (scavenging of free radicals DPPH and ABTS+, Trolox equivalent antioxidant capacity (TEAC), OH, reducing power, total antioxidant activity, and TBARS in the O/W emulsion); (6) chelating pro-oxidant metal ions (Fe2+ and Cu2+), proteins, and hydrolysates. Due to their functional, nutritional and biological characteristics, they can be considered additives, antioxidants or natural preservatives for enriching food formulations and dietary and pharmaceutical supplements.
2 Materials and methods
2.1 Materials
Alcalase® 2.4 L (≥2.4 U g−1) was obtained from Novo Nordisk Co. (Bagsvaerd, Denmark). Chemicals including ABTS (C18H18N4O6S4), DPPH (C18H12N5O6), Coomassie brilliant blue (G250), ferrozine, sodium nitroprusside (SNP), sulphanilamide (SA), Trolox, pyrocatechol violet, and naphthyl-ethylenediamine-dihydrochloride (NEDD) were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Alpha-deoxyribose was supplied by Fluka (Stockholm, Sweden). TCA (trichloroacetic acid), potassium persulphate, ferrous chloride and other chemicals used were purchased from Merck (Darmstadt, Germany).2.2 Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 w/v) for 4 hours. Subsequently, protein extraction was carried out in a 0.1% w/v saline alkaline solution with a pH of 9.5. Following this, the protein was precipitated by adjusting the pH to 4.2 using 0.5 M HCl, then neutralized with 0.5 M NaOH to achieve a pH of 7.0, and finally freeze-dried at −20 °C. The resulting coconut meal protein (CMP) was refrigerated until further use.
5 w/v) for 4 hours. Subsequently, protein extraction was carried out in a 0.1% w/v saline alkaline solution with a pH of 9.5. Following this, the protein was precipitated by adjusting the pH to 4.2 using 0.5 M HCl, then neutralized with 0.5 M NaOH to achieve a pH of 7.0, and finally freeze-dried at −20 °C. The resulting coconut meal protein (CMP) was refrigerated until further use.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v ratio and kept refrigerated at 4 °C for 10 min. After centrifuging the mixture (at 7000 × g for 10 min), the supernatant phase was used to evaluate the concentration of soluble peptides, before and after precipitation using TCA using the Bradford method,14 using a standard curve drawn with bovine serum albumin (BSA). DH (%) was calculated using the following equation:
1 v/v ratio and kept refrigerated at 4 °C for 10 min. After centrifuging the mixture (at 7000 × g for 10 min), the supernatant phase was used to evaluate the concentration of soluble peptides, before and after precipitation using TCA using the Bradford method,14 using a standard curve drawn with bovine serum albumin (BSA). DH (%) was calculated using the following equation: | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 2 min to create an oil-in-water (O/W) emulsion. A 50 μL aliquot of the emulsion was taken from the bottom of the container at 0 and 10 min after homogenization and mixed with 5 mL of 0.1% sodium dodecyl sulphate (SDS) solution. The absorbance of the diluted solution was measured at 500 nm using a spectrophotometer (PJ Instruments, model T80, England). The absorbance values measured immediately (A0) and after 10 min (A10) were used to calculate the emulsifying activity index (EAI) and emulsion stability (ES) as follows:
000 × g for 2 min to create an oil-in-water (O/W) emulsion. A 50 μL aliquot of the emulsion was taken from the bottom of the container at 0 and 10 min after homogenization and mixed with 5 mL of 0.1% sodium dodecyl sulphate (SDS) solution. The absorbance of the diluted solution was measured at 500 nm using a spectrophotometer (PJ Instruments, model T80, England). The absorbance values measured immediately (A0) and after 10 min (A10) were used to calculate the emulsifying activity index (EAI) and emulsion stability (ES) as follows: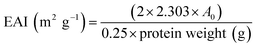 | (2) |
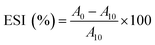 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 × g for 2 min to incorporate air bubbles. The whipped sample was instantly poured into a 25 mL cylinder and the total volume was determined after 45 s. The foaming capacity was calculated on the basis of the following equation:
500 × g for 2 min to incorporate air bubbles. The whipped sample was instantly poured into a 25 mL cylinder and the total volume was determined after 45 s. The foaming capacity was calculated on the basis of the following equation: | (4) |
The whipped sample was kept at 25 °C for 10 min and the volume of that was recorded. The foaming stability was calculated as follows:
 | (5) |
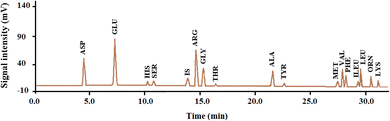
2.2.8.1 Total amino acid analysis. The total amino acid composition of CMP was determined using HPLC (Novapak C18, 4 μm, Waters, Milford, MA) with a reversed-phase column (150 mm × 4.6 mm × 0.5 mm, Teknokroma, RP-C18 ODS-A, Barcelona, Spain) and a fluorescence detector (LC305, Lab Alliance, State College, PA, USA). Acetate buffer was applied as the mobile phase with a flow rate of 1.3 mL min−1. The composition of total amino acids in the samples was evaluated after 24 h of digestion with HCl (6 N, 110 °C) (Fig. 1). The tryptophan content of hydrolysates was measured after alkaline hydrolysis. Total amino acid content was expressed as mg per g dry protein.3
2.2.8.2 Free amino acid analysis. The free amino acid content in the hydrolyzed samples at various time points was determined by precipitating the sample with 10% v/v cold TCA for 2 h and then centrifuging at 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 15 min. The pH of the supernatant was then adjusted to 2.0, and the solution was filtered through a 0.45 μm microfiltration membrane. The filtrate was subsequently analyzed using RP-HPLC to determine the free amino acid compositions, following the method outlined by You et al.17 The free amino acid content was expressed as mg per g dry hydrolysate. Amino acid composition can be used as an indication of nutritional value (e.g., protein efficiency ratio, PER) as well as an indication for the existence of functional groups of AAs in the hydrolysates compared to their native proteins. The following equations were used to calculate the content of functional AAs:
000 × g for 15 min. The pH of the supernatant was then adjusted to 2.0, and the solution was filtered through a 0.45 μm microfiltration membrane. The filtrate was subsequently analyzed using RP-HPLC to determine the free amino acid compositions, following the method outlined by You et al.17 The free amino acid content was expressed as mg per g dry hydrolysate. Amino acid composition can be used as an indication of nutritional value (e.g., protein efficiency ratio, PER) as well as an indication for the existence of functional groups of AAs in the hydrolysates compared to their native proteins. The following equations were used to calculate the content of functional AAs:| Essential amino acids (EAA) = Thr + Met + Val + Leu + Ile + Trp | (6) |
| Antioxidant amino acids (AAA) = Trp + Met + His + Tyr + Lys | (7) |
| Hydrophobic amino acids (HAA) = Ala + Val + Ile + Leu + Tyr + Phe + Trp + Met | (8) |
| Negatively charged amino acids (NCAA) = Asx + Glx | (9) |
| PER = −0.468 + 0.454 (Leu) − 0.105 (Tyr) | (10) |
2.2.9.1 DPPH radical scavenging. A 2 mL portion of CMP/CMP-Hs solution at a concentration of 40 mg mL−1 was blended with 2 mL of 0.2 mM DPPH ethanolic solution. The resulting mixture was then left in the dark for 30 min, followed by centrifugation at 5000 × g for 10 min, and the absorbance of the supernatant was measured at 517 nm. The percentage of DPPH radical scavenging was computed using the following equation:18
 | (11) |
2.2.9.2 ABTS+ radical scavenging. A mixture was created by mixing 7 mM ABTS+ and 2.45 mM potassium persulfate. After incubating in darkness for 12 h, the mixture was diluted with 0.2 M PBS (pH 7.4) until its absorbance at 734 nm reached 0.70. Then, 20 μL of a 10 mg per mL CMP/CMP-Hs solution was added to 2 mL of the ABTS+ solution and vortexed for 10 s. The absorbance of the resulting mixture was measured at 734 nm after reacting in darkness for 6 min. The TEAC of the samples was determined using a standard curve created using standard solutions (ranging from 50 to 1000 μM) of Trolox, after reacting with ABTS in the same manner as mentioned above.9
2.2.9.3 Hydroxyl radical scavenging. A reaction mixture was prepared by combining 0.2 mL of CMP/CMP-Hs at a concentration of 40 mg mL−1, 0.5 mL of 10 mM α-deoxyribose solution, 0.2 mL of 10 mM FeSO4–EDTA solution, 0.9 mL of 0.2 M PBS at pH 7.4, and 0.2 mL of 10 mM hydrogen peroxide solution. The mixture was then incubated at 37 °C for 1 h. After incubation, 1.0 mL of 1.0% TBA and 3% TCA solutions were added. The absorbance of the final mixture, following boiling in water for 15 min and then cooling in an ice bath, was measured at 532 nm.19
2.2.9.4 Reducing power. A solution containing 0.5 mL of 40 mg per mL CMP/CMP-Hs, 0.5 mL of 1% potassium ferricyanide, and 0.5 mL of 0.2 M PBS at pH 6.6 was heated at 50 °C for 20 min. Subsequently, 0.5 mL of 10% TCA solution was added, and the mixture was then centrifuged at 3000 × g for 15 min. The absorbance of a mixture of supernatant (1.0 mL), DW (1.0 mL), and 0.2 mL of 0.1% FeCl3 solution was measured at 700 nm.20
2.2.9.5 Fe2+ chelating activity. A reaction mixture was prepared by combining 1 mL of CMP/CMP-Hs solution at a concentration of 40 mg mL−1, 50 μL of 2 mM iron(II) chloride solution, 1.8 mL of double DW, and 0.1 mL of 5 mM ferrozine solution. The mixture was vortex-mixed and then left at room temperature for 10 min, after which the absorbance was measured at 562 nm.18
2.2.9.6 Cu2+ chelating activity. Initially, 1 mL of CMP/CMP-Hs solution at a concentration of 40 mg mL−1 was combined with 1 mL of 0.2 mM CuSO4 solution and left to incubate at 25 °C for 5 min. Subsequently, 1 mL of 10% TCA solution was added, and the mixture was then centrifuged at 2000 × g for 10 min. Lastly, a mixture of 2 mL of the supernatant, 20 μL of 0.1% pyrocatechol-violet solution, and 1 mL of 10% pyridine solution was vortex-mixed, and its absorbance was measured at 632 nm after 5 min of incubation at room temperature.21
2.2.9.7 Trolox equivalent antioxidant capacity (TEAC). To assess the TEAC, various concentrations of Trolox (ranging from 50 to 1000 μM) were prepared, and a standard curve was constructed based on the reaction with ABTS.
2.2.9.8 Total antioxidant activity (TAA). The total antioxidant capacity was determined following the procedure outlined by Aguilar et al.22 with some slight modifications detailed here. In short, a mixture consisting of 0.2 mL of peptide solution at a concentration of 20 mg mL−1 and 2 mL of reagent (comprising 0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate) was incubated for 90 min in a water bath set to a temperature of 90 °C. After cooling, the absorbance of the mixture was measured at 695 nm. Higher absorbance values in the samples indicate greater total antioxidant capacities.
2.3 Statistical analysis
The SPSS software (ver. 19.0, SPSS Inc., Chicago, IL) was utilized to conduct a one-way analysis of variance. The tests were carried out in triplicate, and Duncan's multiple range test was employed at a significance level of 5% to compare the mean values.3 Results and discussion
3.1 Degree of hydrolysis
The degree of hydrolysis (DH) is an important criterion to evaluate the functional and biological activities of bioactive peptides and hydrolysates. Variation of the DH as a function of proteolysis time using Alcalase is shown in Fig. 2. The DH values were significantly (P < 0.05) increased from 3.0% to 33.2% by an increase in the proteolysis time from 0 to 120 min. Increasing the incubation time allowed extensive proteolysis and cleavage of peptide bonds, thus increasing the peptide solubility and DH. The chain length of the peptides becomes shorter and the amount of free amino acids increases by increasing the degree of hydrolysis.23 However, upon prolonged hydrolysis time (>2 h), the reaction reached a plateau possibly due to saturation of enzyme active sites or reduced amounts of available substrates for the enzymatic reaction.24 There was no significant (P > 0.05) increase in the DH of hydrolysates after 120 min. Similar results were obtained in research studies performed on pea protein isolates,25 lentil proteins,26 black bean proteins,27 and Bunium persicum press cake,24 where an increase in DH values of hydrolysates was confirmed as a function of proteolysis time using different enzymes.3.2 Solubility of protein hydrolysates
Protein solubility is an important measure influencing peptide techno-functional properties i.e., surface activity and gel forming ability. Protein solubility is mainly affected by protein–water interactions or surface hydrophilicity.28 The increase in protein solubility is the most distinguished effect of proteolysis on protein functional properties.29 The results of protein solubility as a function of hydrolysis time using Alcalase are shown in Fig. 3. The results indicated that proteolysis time has a substantial impact on the solubility of primary coconut meal protein (CMP) and its hydrolysates. At a lower degree of hydrolysis shown by shorter incubation time, there was a minimum solubility at pH values 4–5, which is considered the coconut protein isoelectric point, where the net charge of protein molecules is zero.30 It is reported that proteins are positively and negatively charged at a pH below and above their pIs, respectively,31 which on both occasions positively influences their solubility. | ||
| Fig. 3 Solubility of primary CMP (t = 0 min) and its different hydrolysates as a function of pH and hydrolysis time. Data are means of triplicate measurements. Error bars indicate SD values. | ||
It can be seen from Fig. 3 that primary CMP exhibited significantly lower solubility compared to hydrolysates at different incubation times. The minimum solubility of primary proteins is related to low net surface charges and the electrostatic repulsions in the protein structure.32 Increasing the proteolysis degree significantly increased the solubility of hydrolysates at any pH value. Specifically, at the pH of the isoelectric point of primary CMP, hydrolysates did not show a different solubility when compared to other pHs. The Alcalase treated sample at 120 min and 180 min had a solubility range of 75–85%, regardless of pH changes. Protein solubility is related to hydrophilicity and, thereby, electrostatic repulsion forces in the third structure of the protein. The effect of enzymatic hydrolysis can be attributed to breaking disulfide bonds of the protein into low-Mw amino acids, exposing their charged hydrophilic or hydrophobic groups, that would eventually result in increased ionizable amino and carboxyl groups favoring the formation of more hydrogen bonds that increase solubility.11 It is reported that enzymatic hydrolysis can improve protein solubility, which presents a close relationship with the decrease in surface hydrophobicity.29 Our results are consistent with those reported for hydrolysis of pea protein isolates25 and lentil,26 black bean,27 legume,33 poppy-pollen23 and walnut20 proteins demonstrating an increase in protein solubility as a function of enzymatic hydrolysis time.
3.3 Emulsifying and foaming properties
Hydrolysis can directly affect the functional properties of proteins such as solubility, emulsifying and foaming abilities in food systems. Emulsifying and foaming properties of primary CMP and its hydrolysates were investigated as a function of hydrolysis time and the results are shown in Fig. 4a–d. Variation in the emulsifying activity index (EAI, Fig. 4a) and emulsion stability index (ESI, Fig. 4b) is shown for primary CMP and its hydrolysates obtained as a function of different hydrolysis times and varying pH. These indices are helpful for preliminary testing of the effects of enzymatic modification of proteins as potential emulsifying agents.30Primary CMP showed the lowest emulsifying (EAI and ESI) and foaming (FC and FS) abilities at a pH point near pI (pH = 5). Decreasing or increasing pH below or above this point increased emulsifying and foaming properties. This is due to the fact that the CMP molecule has positive and negative charges at pHs below and above its pI, respectively, in which both conditions can positively influence its solubility. Hydrolysates obtained from the partial proteolysis conditions (t = 30 min) exhibited the highest EAI, ESI, FC, and FS values.
EAI and ESI ranged between 50 and 70 m2 g−1 and 40–80%, respectively for different hydrolysates. Foaming capacity (FC) ranged between 60 and 120%, while it was not significantly different as a function of proteolysis time (P > 0.05), and significantly declined as pH increased (P < 0.05). Foaming stability (FS) varied in a range of 30–70%, with the variation being significant as a function of hydrolysis time at each pH point.
Looking at emulsifying and foaming properties of the obtained hydrolysates as a function of hydrolysis time one could conclude that low DHs and solubility correspond to enhanced functional properties of the obtained hydrolysates. Improvement in emulsifying/foaming properties of hydrolysates with lower DH and solubility can be ascribed to an increase in their ability to rearrange and re-orientate at the interface by weakening the interfacial tension forces.34 In another study, enzymatic hydrolysis led to an improvement in the emulsifying activity of grass turtle protein. In this way, all the emulsions produced with hydrolyzed ones had more stability (51–81.33%).11 Our results are in line with findings in other studies on the effect of hydrolysis time on the techno-functional properties of soybean,35 lentil,26 black bean,27 and legume33 proteins.
3.4 Water- and oil-holding capacities
The extent of the proteolysis can affect water- and oil-holding (WHC and OHC) properties of protein hydrolysates. OHC is also a measure of oil quantity that can be directly bound by the protein and is regarded as an important functional criterion, especially in meat and confectionary products.32 The results in Fig. 5 indicate that limited hydrolysis (t = 30 min) of CMP by Alcalase resulted in the highest WHC (6.0 g water per g hydrolysate) and OHC (5.0 g oil per g hydrolysate) values. Increasing hydrolysis time led to a significant decrease in WHC and OHC values of hydrolysates (P > 0.05). It is possible that the partial hydrolysis conditions result in the exposure of hydrophilic groups and hydrophobic amino acids, which were hidden in the protein structure.36 This can eventually lead to increased WHC and OHC of the hydrolysates. However, extensive hydrolysis can promote the above mechanism in the opposite direction by breaking down the protein tertiary structures leading to reduced capacity to hold water and oil molecules.33The decrease in the WHC and OHC by an increase in the DH might have been due to the hydrolytic degradation of the protein structure. It has been reported that protein hydrolysates from grass carp skin with the lowest DH had significantly higher OHC due to the larger protein particle sizes.37 A decline in OHC of shark muscle protein and red salmon head hydrolysates with a DH increase has also been reported.38 In a number of investigations, the values of WHC (3.9–5.3 g g−1) and OHC (3.2–3.4 g g−1) for legume peptides,33 OHC (2.6–9.7 g g−1) for wheat glutenin hydrolysates34 and WHC (1.2–5.4 g g−1) and OHC (5.3–7.0 g g−1) for Spirulina protein hydrolysates21 under the influence of the primary protein, type of the enzyme and hydrolysis conditions were emphasized.
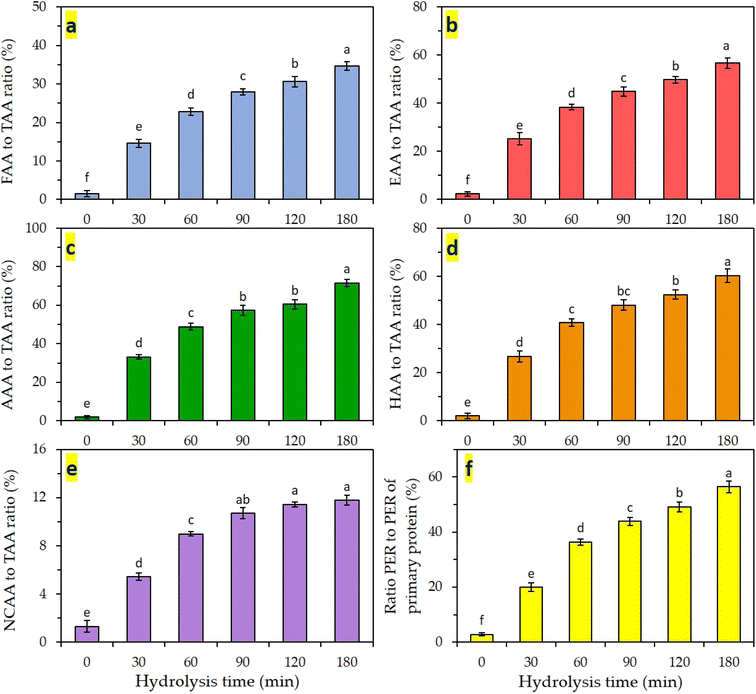
3.5 Amino acid composition
The amino acid composition of proteins or polypeptides plays an important role in their nutritional, biological, health-promoting, and functional properties.10 Certain polypeptides have functional groups (e.g., hydrophilic, hydrophobic, and positively or negatively charged amino acids) in their structure that possess techno-functional properties i.e., antioxidant, free radical scavenging, emulsifying, foaming, and water- or oil-binding properties. Therefore, amino acid composition of hydrolysates was characterized as a function of hydrolysis time. Table 1 shows changes in amino acid composition of primary CMP and the hydrolysates obtained at different hydrolysis times. The content of free amino acids (FFA) in hydrolysates increased as a function of hydrolysis time indicating substantial cleavage of the protein structure and liberation of small peptides and amino acids in the hydrolysates. Different groups of functional amino acids, including essential (EEA/TAA ratio), antioxidant (AAA/TAA ratio), hydrophobic (HAA/TAA ratio), and negatively charged (NCAA/TAA ratio) amino acids were characterized as a function of hydrolysis time and the results are shown in Table 1 and Fig. 6a–e. EAA content, and more specifically, the ratio of EAA/TAA, as well as the protein efficiency ratio (PER) are among the most important indicators of polypeptide nutritional value. As can be seen in Fig. 6a, the ratio of FAA/TAA significantly increased from 1.5% in non-digested CMP to 34.6% in the 180-min hydrolyzed sample (P < 0.05). This can explain that at the early stage of enzymatic digestion, the proteins were mainly broken into small fragments. After the digestion was completed, these peptides were more hydrolyzed to produce free amino acids. A similar trend was observed when comparing the nutritional and functional amino acid groups (EAA, AAA, HAA, and NCAA) in the samples hydrolyzed at different times when compared to non-digested CMP (Fig. 6b–e). The ratio of EAA/TAA significantly increased from 2.3% in non-digested CMP to 56.6% in the 180-min hydrolyzed sample (P < 0.05).| Amino acids | CMP (TAA) | Free amino acids (mg per g dry matter) at different proteolysis times | |||||
|---|---|---|---|---|---|---|---|
| 0 min | 30 min | 60 min | 90 min | 120 min | 180 min | ||
| a Data are means of duplicate measurements. TAA = total amino acids; * denotes EAA = essential amino acids; AAA = antioxidant amino acids; HAA = hydrophobic amino acids; NCAA = negatively charged amino acids; PER = protein efficiency ratio. Data are displayed as the mean ± standard deviation. Different superscripts in the same row are notable differences (p < 0.05). Different functional groups of amino acids were measured according to eqn (6)–(10) described in Section 2.2.8.1. | |||||||
| Aspartic acid (Asp) | 82.1 ± 1.8 | 0.4 ± 0.02e | 5.2 ± 0.15d | 8.4 ± 0.3c | 10.5 ± 0.4b | 10.9 ± 0.3b | 11.5 ± 0.2a |
| Glutamic acid (Glu) | 161.9 ± 2.7 | 2.0 ± 1.5e | 8.1 ± 0.15d | 13.6 ± 0.9c | 15.7 ± 0.7b | 16.9 ± 0.4ab | 17.5 ± 0.8a |
| Histidine (His)* | 20.1 ± 0.7 | 0 ± 0f | 7.5 ± 0.2e | 10.5 ± 0.5d | 12.2 ± 0.3c | 13.5 ± 0.4b | 15.5 ± 0.6a |
| Serine (Ser) | 39.8 ± 1.6 | 1.1 ± 0.1f | 3.2 ± 0.1e | 6.7 ± 0.4d | 8.3 ± 0.4c | 9.1 ± 0.3b | 10.4 ± 0.4a |
| Arginine (Arg) | 112.1 ± 3.6 | 0.4 ± 0.02f | 8.9 ± 0.2e | 14.1 ± 0.9d | 23.5 ± 2.1c | 26.1 ± 1.3b | 30.4 ± 2.4a |
| Glycine (Gly) | 41.2 ± 1.8 | 0.8 ± 0.1f | 3.7 ± 0.15e | 5.9 ± 0.3d | 7.3 ± 0.3c | 8.4 ± 0.4b | 9.4 ± 0.5a |
| Threonine (Thr)* | 24.5 ± 1.3 | 0 ± 0f | 3.6 ± 0.2e | 5.1 ± 0.1d | 6.2 ± 0.2c | 7.9 ± 0.3b | 8.3 ± 0.2a |
| Alanine (Ala) | 38.1 ± 1.6 | 0 ± 0f | 10.1 ± 0.4e | 15.3 ± 0.5d | 19.5 ± 1.2c | 21.2 ± 0.9b | 23.3 ± 0.8a |
| Tyrosine (Tyr) | 21.1 ± 0.6 | 0.3 ± 0.02e | 7.9 ± 0.3d | 11.8 ± 0.4c | 13.1 ± 0.5b | 13.2 ± 0.3b | 16.9 ± 0.9a |
| Methionine (Met)* | 13.3 ± 0.5 | 0.2 ± 0.01f | 5.3 ± 0.2e | 7.2 ± 0.2d | 9.5 ± 0.4c | 9.9 ± 0.3b | 11.5 ± 0.3a |
| Valine (Val)* | 36.5 ± 2.1 | 0 ± 0e | 8.3 ± 0.2d | 12.1 ± 0.4c | 12.4 ± 0.5c | 14.1 ± 0.8b | 15.8 ± 0.7a |
| Phenylalanine (Phe)* | 36.5 ± 1.4 | 0.7 ± 0.1f | 12.6 ± 0.7e | 18.7 ± 0.8d | 22.3 ± 1.9c | 24.8 ± 0.9b | 28.4 ± 1.2a |
| Isoleucine (Ile)* | 24.4 ± 0.9 | 1.1 ± 0.1f | 3.1 ± 0.2e | 5.4 ± 0.2d | 5.8 ± 0.2c | 6.2 ± 0.2b | 7.6 ± 0.2a |
| Leucine (Leu)* | 47.3 ± 1.2 | 2.3 ± 0.15f | 10.6 ± 0.7e | 17.9 ± 0.8d | 20.9 ± 1.4c | 23.6 ± 1.4b | 27.2 ± 1.2a |
| Lysine (Lys)* | 32.1 ± 1.5 | 1.2 ± 0.1e | 7.8 ± 0.2d | 13.2 ± 0.4c | 15.2 ± 0.6b | 15.9 ± 0.7b | 18.1 ± 0.6a |
| Tryptophan (Trp)* | 4.7 ± 0.1 | 0 ± 0e | 1.8 ± 0.2d | 2.1 ± 0.1c | 2.7 ± 0.2b | 2.8 ± 0.2b | 3.3 ± 0.2a |
| TAA in primary CMP | 735.8 ± 16.4 | ||||||
| FAA | — | 9.6 ± 1.4f | 107.4 ± 3.8e | 167.8 ± 7.2d | 204.8 ± 10.9c | 224.6 ± 9.1b | 243.8 ± 13.1a |
| EAA | 230.9 ± 8.2 | 5.5 ± 0.3f | 60.4 ± 2.6e | 91.7 ± 2.7d | 107.2 ± 3.8c | 119.7 ± 5.8b | 135.8 ± 5.7a |
| AAA | 90.4 ± 5.3 | 1.7 ± 0.1e | 30.7 ± 2.6d | 44.3 ± 3.5c | 52.4 ± 4.2b | 54.8 ± 4.3b | 65.2 ± 3.8a |
| NCAA | 244.3 ± 5.8 | 3.2 ± 0.1d | 13.3 ± 0.6c | 22.1 ± 2.7b | 26.4 ± 2.8a | 28.1 ± 3.5a | 28.8 ± 3.2a |
| HAA | 221.1 ± 9.8 | 4.5 ± 0.25e | 59.6 ± 2.6d | 90.4 ± 3.2c | 106.1 ± 4.5b | 106.5 ± 5.2b | 134.2 ± 5.3a |
| PER | 2.1 ± 0.1 | ||||||
The nutritional value (EAA/TAA ratio) of the latter hydrolysate was in accordance with the values recommended by FAO/WHO (32–36%).3 In our study, however, more than 65% of the final digests still remained in the form of peptides, which may contribute to their techno-functional properties i.e., antioxidant, emulsifying, and foaming activities.17 The existence of substantial amounts of HAA, AAA and NCAA in the hydrolysate samples (especially at t > 120 min) has a stronger reactivity with lipophilic free radicals due to their greater solubility in lipid phases.39 Antioxidant amino acids can play a role in the antioxidant capacity of the peptides by donating electrons to free radicals.40
The variation of the protein efficiency ratio (PER) in hydrolysates as compared to that of primary CMP is shown in Fig. 6f as an indication of the nutritional value of the obtained hydrolysates. The ratio of PER/PER of CMP strongly and significantly (P < 0.05) increased from 2.8% in non-digested CMP to 56.5% in the 180-min digest (Fig. 6f) indicating the liberation of essential amino acids during hydrolysis. Proteolytic action of different enzymes including Alcalase as a function of hydrolysis time in liberating functional amino acids in hydrolysates has been reported in other studies.41 It was shown that progressive proteolysis leads to higher contents of EAA, AAA, and charged AAs in the obtained hydrolysates compared to the primary protein. Because of the chemical structure and functional groups present in these amino acids, they are able to eliminate free radicals and serve as potent antioxidants within biological cells.11 Consequently, it is anticipated that these hydrolysates will exhibit strong antioxidant activity against free radicals.
3.6 Antioxidant characterization
In this study the effect of different hydrolysis times on inhibiting anionic (DPPH) and cationic (ABTS+) free radicals, hydroxyl radicals (as initiators of lipid peroxidation), reducing power (RP), Fe2+ and Cu2+ ion chelating activity, TEAC, and total antioxidant activity of Alcalase hydrolysates was investigated and the results are shown in Fig. 7a–h. Enzymatic hydrolysis, especially at prolonged times, in general, led to a significant increase in antioxidant activity of hydrolysates assessed by different protocols. However, different antioxidant activity measurement protocols exhibited different results, which will be discussed below.The reducing power is an index to determine the ability of peptides to donate electrons or hydrogen.46 The same behavior was also observed in reducing power (RP) assay. Fig. 7d demonstrates that RP antioxidant activities of hydrolysates significantly increased (from 0.62 to 0.87) until t = 90 min (P > 0.05), followed by a decrease after this point. In another research study, hydrolysates from Diplodus proteins were prepared using Alcalase and Savinase enzymes under optimal conditions and their antioxidant and antibacterial activities were evaluated. The hydrolysate generated by Savinase (DPH-S) generally exhibited a greater antioxidant activity across all the considered methods, in terms of ferrous chelating activity (IC50 = 2.19 mg mL−1), DPPH radical scavenging (IC50 = 3.76 mg mL−1) and reducing power (1.92 ± 0.12) activity.47
4 Conclusions
Bioactive peptides and hydrolysates derived from the enzymatic action of plant proteins exhibit numerous functional properties, including antioxidant, nutritional, techno-functional, and health-promoting effects. This study focused on the extensive proteolysis of coconut meal protein using the Alcalase enzyme to produce hydrolysates with varying molecular weights. The conditions of Alcalase hydrolysis significantly influenced the degree of hydrolysis and the techno-functional properties (solubility, emulsification, foaming, and water- and oil-holding capacity) as well as the antioxidant activities of the hydrolysates. All these factors were affected by the composition and types of specific amino acids (hydrophobic, antioxidant, and charged). Given their positive nutritional characteristics and favorable techno-functional and antioxidant indicators, coconut meal protein peptides can be regarded as a natural source of antioxidant peptides, making them suitable for applications in food and pharmaceuticals. However, further studies are necessary to identify the specific peptides and amino acid sequences that could enhance the functionality of the hydrolysates.Abbreviations
| BPs | Bioactive peptides |
| CMP | Coconut meal protein |
| EAI | Emulsifying activity index |
| ESI | Emulsion stability index |
| WHC | Water-holding capacity |
| OHC | Oil-holding capacity |
| TEAC | Trolox equivalent antioxidant capacity |
| FC | Foaming capacity |
| FS | Foaming stability |
| EAA | Essential amino acids |
| AAA | Antioxidant amino acids |
| HAA | Hydrophobic amino acids |
| PER | Protein efficiency ratio |
| HHP | High hydrostatic pressure |
| PEF | Pulsed electric field |
Data availability
All data generated or analyzed during this study are included in this article.Author contributions
Zahra Akbarbaglu: methodology, resources, investigation. Khashayar Sarabandi: supervision, project administration, data curation. Seyed Hadi Peighambardoust: writing-original draft. Roxana Sarabandi: methodology, resources. Hossein Samadi Kafil: data curation, review & editing. Mohammad Ali Hesarinejad: review & editing.Conflicts of interest
The authors declare that they have no known conflict of interests or personal relationships that could have appeared to influence the work reported in this paper.References
- P. Rodsamran and R. Sothornvit, Physicochemical and functional properties of protein concentrate from by-product of coconut processing, Food Chem., 2018, 241, 364–371 CrossRef CAS PubMed
.
- T. A. Comunian, M. P. Silva and C. J. F. Souza, The use of food by-products as a novel for functional foods: their use as ingredients and for the encapsulation process, Trends Food Sci. Technol., 2021, 108, 269–280 CrossRef CAS
.
- J. Ge, C.-X. Sun, A. Mata, H. Corke, R.-Y. Gan and Y. Fang, Physicochemical and pH-dependent functional properties of proteins isolated from eight traditional Chinese beans, Food Hydrocolloids, 2021, 112, 106288 CrossRef CAS
.
- A. Görgüç, E. Gençdağ and F. M. Yılmaz, Bioactive peptides derived from plant origin by-products: biological activities and techno-functional utilizations in food developments-a review, Food Res. Int., 2020, 136, 109504 CrossRef PubMed
.
- X. Luo, S. Wu, J. Xue, H. Hu, Z. He, X. Liu and F. Wu, The bioactive peptide screening from Torreya grandis meal protein hydrolysates, Food Biosci., 2021, 44, 101419 CrossRef CAS
.
- K. Kumar, A. N. Yadav, V. Kumar, P. Vyas and H. S. Dhaliwal, Food waste: a potential bioresource for extraction of nutraceuticals and bioactive compounds, Bioresour. Bioprocess., 2017, 4, 1–14 CrossRef PubMed
.
- S. Thaiphanit and P. Anprung, Physicochemical and emulsion properties of edible protein concentrate from coconut (Cocos nucifera L.) processing by-products and the influence of heat treatment, Food Hydrocolloids, 2016, 52, 756–765 CrossRef CAS
.
- Y. Chen, J. Chen, C. Chang, J. Chen, F. Cao, J. Zhao, Y. Zheng and J. Zhu, Physicochemical and functional properties of proteins extracted from three microalgal species, Food Hydrocolloids, 2019, 96, 510–517 CrossRef CAS
.
- Z. Akbarbaglu, A. Ayaseh, B. Ghanbarzadeh, K. Sarabandi, M. S. Kharazmi and S. M. Jafari, Chemical structure and bio-functional properties of Arthrospira platensis peptides produced by ultrasonication-enzymolysis: their emulsification capabilities, Process Biochem., 2023, 132, 191–199 CrossRef CAS
.
- A. Marciniak, S. Suwal, N. Naderi, Y. Pouliot and A. Doyen, Enhancing enzymatic hydrolysis of food proteins and production of bioactive peptides using high hydrostatic pressure technology, Trends Food Sci. Technol., 2018, 80, 187–198 CrossRef CAS
.
- M. S. Islam, W. Hongxin, H. Admassu, A. Noman, C. Ma and F. An Wei, Degree of hydrolysis, functional and antioxidant properties of protein hydrolysates from grass turtle (Chinemys reevesii) as influenced by enzymatic hydrolysis conditions, Food Sci. Nutr., 2021, 9, 4031–4047 CrossRef CAS PubMed
.
- J. Tkaczewska, Peptides and protein hydrolysates as food preservatives and bioactive components of edible films and coatings-a review, Trends Food Sci. Technol., 2020, 106, 298–311 CrossRef CAS
.
- K. Sarabandi, A. Dashipour, Z. Izadi, Z. Akbarbaglu, I. Katouzian and S. M. Jafari, Nutritional, biological, and structural properties of bioactive peptides from bellflower, Persian-willow, and bitter-orange pollens, J. Food Sci., 2023, 88(7), 3119–3133 CrossRef CAS PubMed
.
- M. M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed
.
- S. N. Jamdar, V. Rajalakshmi, M. D. Pednekar, F. Juan, V. Yardi and A. Sharma, Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysate, Food Chem., 2010, 121, 178–184 CrossRef CAS
.
- V. Klompong, S. Benjakul, D. Kantachote and F. Shahidi, Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type, Food Chem., 2007, 102, 1317–1327 CrossRef CAS
.
- L. You, M. Zhao, J. M. Regenstein and J. Ren, Changes in the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates during a simulated gastrointestinal digestion, Food Chem., 2010, 120, 810–816 CrossRef CAS
.
- B. M. Kimatu, L. Zhao, Y. Biao, G. Ma, W. Yang, F. Pei and Q. Hu, Antioxidant potential of edible mushroom (Agaricus bisporus) protein hydrolysates and their ultrafiltration fractions, Food Chem., 2017, 230, 58–67 CrossRef CAS PubMed
.
- P. Ambigaipalan and F. Shahidi, Bioactive peptides from shrimp shell processing discards: antioxidant and biological activities, J. Funct. Foods, 2017, 34, 7–17 CrossRef CAS
.
- M. Moghadam, M. Salami, M. Mohammadian, Z. Emam-Djomeh, R. Jahanbani and A. A. Moosavi-Movahedi, Physicochemical and bio-functional properties of walnut proteins as affected by trypsin-mediated hydrolysis, Food Biosci., 2020, 36, 100611 CrossRef CAS
.
- Z. Akbarbaglu, A. Ayaseh, B. Ghanbarzadeh and K. Sarabandi, Techno-functional, biological and structural properties of Spirulina platensis peptides from different proteases, Algal Res., 2022, 102755 CrossRef
.
- J. G. dos S. Aguilar, V. Granato Cason and R. J. S. de Castro, Improving antioxidant activity of black bean protein by hydrolysis with protease combinations, Int. J. Food Sci. Technol., 2019, 54, 34–41 CrossRef CAS
.
- K. Sarabandi, Z. Akbarbaglu, S. H. Peighambardoust, A. ayaseh and S. M. Jafari, Physicochemical, antibacterial and bio-functional properties of persian poppy-pollen (Papaver bracteatum) protein and peptides, J. Food Meas. Charact., 2023, 1–12 Search PubMed
.
- Z. Shahi, S. Z. Sayyed-Alangi and L. Najafian, Effects of enzyme type and process time on hydrolysis degree, electrophoresis bands and antioxidant properties of hydrolyzed proteins derived from defatted Bunium persicum Bioss. press cake, Heliyon, 2020, 6, e03365 CrossRef PubMed
.
- V. G. Arteaga, M. A. Guardia, I. Muranyi, P. Eisner and U. Schweiggert-Weisz, Effect of enzymatic hydrolysis on molecular weight distribution, techno-functional properties and sensory perception of pea protein isolates, Innovative Food Sci. Emerging Technol., 2020, 65, 102449 CrossRef
.
- A. Rezvankhah, M. S. Yarmand, B. Ghanbarzadeh and H. Mirzaee, Generation of bioactive peptides from lentil protein: degree of hydrolysis, antioxidant activity, phenol content, ACE-inhibitory activity, molecular weight, sensory, and functional properties, J. Food Meas. Charact., 2021, 15, 5021–5035 CrossRef
.
- J. A. do Evangelho, N. L. Vanier, V. Z. Pinto, J. J. De Berrios, A. R. G. Dias and E. da Rosa Zavareze, Black bean (Phaseolus vulgaris L.) protein hydrolysates: physicochemical and functional properties, Food Chem., 2017, 214, 460–467 CrossRef PubMed
.
- G. Leni, L. Soetemans, A. Caligiani, S. Sforza and L. Bastiaens, Degree of Hydrolysis Affects the Techno-Functional Properties of Lesser Mealworm Protein Hydrolysates, Foods, 2020, 9(4), 381 CrossRef CAS PubMed
.
- O. L. Tavano, Protein hydrolysis using proteases: an important tool for food biotechnology, J. Mol. Catal. B: Enzym., 2013, 90, 1–11 CrossRef CAS
.
- S. Thaiphanit, G. Schleining and P. Anprung, Effects of coconut (Cocos nucifera L.) protein hydrolysates obtained from enzymatic hydrolysis on the stability and rheological properties of oil-in-water emulsions, Food Hydrocolloids, 2016, 60, 252–264 CrossRef CAS
.
- A. A. Tokmakov, A. Kurotani and K.-I. Sato, Protein pI and intracellular localization, Front. Mol. Biosci., 2021, 1179 Search PubMed
.
- Y.-N. Du, S. Xue, J.-R. Han, J.-N. Yan, W.-H. Shang, J.-N. Hong and H.-T. Wu, Simultaneous extraction by acidic and saline solutions and characteristics of the lipids and proteins from large yellow croaker (Pseudosciaena crocea) roes, Food Chem., 2020, 310, 125928 CrossRef CAS PubMed
.
- X. Xu, Y. Qiao, B. Shi and V. P. Dia, Alcalase and bromelain hydrolysis affected physicochemical and functional properties and biological activities of legume proteins, Food Struct., 2021, 27, 100178 CrossRef CAS
.
- F. Bozkurt, H. Bekiroglu, K. Dogan, S. Karasu and O. Sagdic, Technological and bioactive properties of wheat glutenin hydrolysates prepared with various commercial proteases, LWT, 2021, 149, 111787 CrossRef CAS
.
- M. Islam, Y. Huang, S. Islam, B. Fan, L. Tong and F. Wang, Influence of the Degree of Hydrolysis on Functional Properties and Antioxidant Activity of Enzymatic Soybean Protein Hydrolysates, Molecules, 2022, 27, 6110 CrossRef CAS PubMed
.
- A. Noman, Y. Xu, W. Q. AL-Bukhaiti, S. M. Abed, A. H. Ali, A. H. Ramadhan and W. Xia, Influence of enzymatic hydrolysis conditions on the degree of hydrolysis and functional properties of protein hydrolysate obtained from Chinese sturgeon (Acipenser sinensis) by using papain enzyme, Process Biochem., 2018, 67, 19–28 CrossRef CAS
.
- J. Wasswa, J. Tang, X. Gu and X. Yuan, Influence of the extent of enzymatic hydrolysis on the functional properties of protein hydrolysate from grass carp (Ctenopharyngodon idella) skin, Food Chem., 2007, 104, 1698–1704, DOI:10.1016/j.foodchem.2007.03.044
.
- S. Sathivel, S. Smiley, W. Prinyawiwatkul and P. J. Bechtel, Functional and nutritional properties of red salmon (Oncorhynchus nerka) enzymatic hydrolysates, J. Food Sci., 2005, 70, c401–c406 CAS
.
- C. C. Udenigwe and R. E. Aluko, Food protein-derived bioactive peptides: production, processing, and potential health benefits, J. Food Sci., 2012, 77, R11–R24 CrossRef CAS PubMed
.
- F.-F. Liu, Y.-Q. Li, C.-Y. Wang, Y. Liang, X.-Z. Zhao, J.-X. He and H.-Z. Mo, Physicochemical, functional and antioxidant properties of mung bean protein enzymatic hydrolysates, Food Chem., 2022, 133397 CrossRef CAS PubMed
.
- X. Zhao, X. Zhang, H. Liu, G. Zhang and Q. Ao, Functional, nutritional and flavor characteristic of soybean proteins obtained through reverse micelles, Food Hydrocolloids, 2018, 74, 358–366 CrossRef CAS
.
- C.-B. Ahn, J.-Y. Je and Y.-S. Cho, Antioxidant and anti-inflammatory peptide fraction from salmon byproduct protein hydrolysates by peptic hydrolysis, Food Res. Int., 2012, 49, 92–98 CrossRef CAS
.
- Y. Song, J. Yu, J. Song, S. Wang, T. Cao, Z. Liu, X. Gao and Y. Wei, The antihypertensive effect and mechanisms of bioactive peptides from Ruditapes philippinarum fermented with Bacillus natto in spontaneously hypertensive rats, J. Funct. Foods, 2021, 79, 104411 CrossRef CAS
.
- F. Shahidi and Y. Zhong, Measurement of antioxidant activity, J. Funct. Foods, 2015, 18, 757–781 CrossRef CAS
.
- M. M. Aondona, J. K. Ikya, M. T. Ukeyima, T. J. A. Gborigo, R. E. Aluko and A. T. Girgih, In vitro antioxidant and antihypertensive properties of sesame seed enzymatic protein hydrolysate and ultrafiltration peptide fractions, J. Food Biochem., 2021, 45, e13587 CrossRef CAS PubMed
.
- K. O. Lima, C. da C. de Quadros, M. da Rocha, J. T. J. G. de Lacerda, M. A. Juliano, M. Dias, M. A. Mendes and C. Prentice, Bioactivity and bioaccessibility of protein hydrolyzates from industrial byproducts of stripped weakfish (Cynoscion guatucupa), LWT, 2019, 111, 408–413 CrossRef CAS
.
- F. Hamed, I. Elgaoud, S. Eljoudi, B. Deracinois, C. Flahaut, N. Nedjar and A. Barkia,
Diplodus Protein Hydrolysates: Antioxidant and Antibacterial Properties and Identification of Biopeptides, Waste Biomass Valorization, 2024, 1–15 Search PubMed
.
- Z. Zhou, Y.-Y. Zhang, R. Xin, X.-H. Huang, Y.-L. Li, X. Dong, D. Zhou, B. Zhu and L. Qin, Metal ion-mediated pro-oxidative reactions of different lipid molecules: revealed by nontargeted lipidomic approaches, J. Agric. Food Chem., 2022, 70, 10284–10295 CrossRef CAS PubMed
.
- C. Fan, X. Wang, X. Song, R. Sun, R. Liu, W. Sui, Y. Jin, T. Wu and M. Zhang, Identification of a Novel Walnut Iron Chelating Peptide with Potential High Antioxidant Activity and Analysis of Its Possible Binding Sites, Foods, 2023, 12, 226 CrossRef CAS PubMed
.
- C. Torres-Fuentes, M. Alaiz and J. Vioque, Affinity purification and characterisation of chelating peptides from chickpea protein hydrolysates, Food Chem., 2011, 129, 485–490, DOI:10.1016/j.foodchem.2011.04.103
.
- Z. Zheng, J. Li, J. Li, H. Sun and Y. Liu, Physicochemical and antioxidative characteristics of black bean protein hydrolysates obtained from different enzymes, Food Hydrocolloids, 2019, 97, 105222 CrossRef CAS
.
- S. Phongthai, S. D'Amico, R. Schoenlechner, W. Homthawornchoo and S. Rawdkuen, Fractionation and antioxidant properties of rice bran protein hydrolysates stimulated by in vitro gastrointestinal digestion, Food Chem., 2018, 240, 156–164 CrossRef CAS PubMed
.
- J. Xie, M. Du, M. Shen, T. Wu and L. Lin, Physico-chemical properties, antioxidant activities and angiotensin-I converting enzyme inhibitory of protein hydrolysates from mung bean (Vigna radiate), Food Chem., 2019, 270, 243–250 CrossRef CAS PubMed
.
- A. I. Akinyede, T. N. Fagbemi, O. F. Osundahunsi and R. E. Aluko, Amino acid composition and antioxidant properties of the enzymatic hydrolysate of calabash nutmeg (Monodora myristica) and its membrane ultrafiltration peptide fractions, J. Food Biochem., 2021, 45, e13437 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2024 |