Open Access Article
Open Access ArticleInfluence of high hydrostatic pressure treatment on cassava flour's volatile retention performance†
Ladie Anne
Conde
 ab,
Biniam
Kebede
ab,
Biniam
Kebede
 b and
Indrawati
Oey
b and
Indrawati
Oey
 *bc
*bc
aPhilippine Root Crop Research and Training Center (PhilRootcrops), Visayas State University, Baybay City 6521, Leyte, Philippines
bDepartment of Food Science, University of Otago, Dunedin 9054, New Zealand. E-mail: indrawati.oey@otago.ac.nz
cRiddet Institute, Palmerston North 4442, New Zealand
First published on 22nd August 2024
Abstract
In this study, cassava flour structurally modified through high hydrostatic pressure (HHP) was assessed for its headspace volatile profile, when prepared as an aqueous suspension. The headspace profile was used to indirectly evaluate its retention capacity for added mango volatiles. Moreover, the influence of the level of structural modification—dictated by the HHP treatment intensity and mainly characterized by different degrees of gelatinization (54% and 100%)—on the retention stability during storage was also assessed through a chemometrics approach. The new amorphous starch structures in the flour caused by the pressure induced gelatinization led to an 8–13% higher total volatile abundance in the headspace compared to control or untreated cassava flour. A lower headspace abundance of alcohols and a higher abundance of terpenes in HHP-treated flours distinguished them from control samples. This correlated with a 17–20% greater retention of alcohols and a 3–7% reduced retention of terpenes in the HHP treated flour's suspension matrix. During storage, HHP-treated flours exhibited greater retention stability for most volatile compounds compared to control flour. Despite the level of structural modification, defined by a remarkable difference in the degree of gelatinization, results revealed minimal variance in their headspace composition and volatile retention during storage. Nonetheless, the results suggest that careful selection of volatiles is necessary when combining them with HHP-modified cassava flours, as certain volatile classes exhibited higher retention.
Sustainability spotlightCassava is a popular crop in developing countries, valued for its resilience to drought and productivity under marginal land conditions. As a result, it has become a key crop in battling food insecurity and is regarded as a ‘famine reserve crop’. However, the utilization of cassava flour is limited compared to its isolated starch. Modifying cassava flour with high hydrostatic pressure (HHP) may result in alterations to its volatile binding capacity and retention stability. Understanding the volatile retention performance could provide new insights to diversify its applications in the food industry. Additionally, processing cassava flour using HHP can contribute to advancing SDGs 2, 3, and 13, by promoting food security, producing clean-label food products, reducing environmental impact, and fostering sustainable food production. |
1 Introduction
Starch is a major component of most foods, and its interaction ability makes it a widely used material to retain and protect volatile compounds.1,2 Starches retain volatile compounds through non-covalent interactions during capillary and surface sorption or through complex formation.1–5 However, this can also influence the sensory characteristics and flavour stability of food products during storage.6–8 Goubet et al. stated that the retention efficiency of carbohydrates, like starch and its derivatives, depends on the physicochemical properties of the aroma compound, as well as on the molecular weight, conformation, chemical functions, and the physical state of the aroma carrier.9For native starches, granular properties like a large surface area and the presence of channels also enhanced the retention.1,2 For example, the smaller corn starch exhibited significantly higher retention of individually added aroma compounds than the smoother and larger potato starch.10 Similarly, the addition of a volatile mixture in the form of an essential oil into an aqueous solution of native starches revealed that corn starch had the highest sorption of volatiles, followed by tapioca, potato, and least from amylopectin (waxy) corn starch.5 Modified starch and its derivatives have also shown varied binding abilities. For instance, modified (cross-linked and stabilized) waxy corn starch trapped isoamyl acetate better in starch-based dessert cream than waxy corn starch, normal corn starch, and potato starch.11 Meanwhile, other chemically modified starches did not significantly improve the binding of aroma compounds at low concentration12 or the encapsulation of rosemary oil.13
Physical treatment of starches (mostly through thermal gelatinization with or without cryotexturization and high hydrostatic pressure) was also reported to influence the binding properties of starches. The amorphous starch produced from thermal treatments did not show sensitivity to the polymorphic structure or amylose content, when a volatile–starch mixture was prepared either dry10 or as an aqueous suspension.7 Meanwhile, the alteration of the granular structure of corn and sorghum starch caused by high pressure and cryotexturization reduced the retention of ketones, phenols and sesquiterpene hydrocarbons from a volatile mixture.14 Conversely, terpenes were strongly bound by pressurized starches and an increased sorption of alcohols by pressurised waxy maize15 and sorghum starch.14
Of these modified starches, the use of physically modified starches is of current industrial interest, as their production is simple and fast, requires no chemical reagents, creates no residues, and thereby results in “clean-label” products.16 In the gelatinized state, starch proves to be a promising flavour adsorbent or binder that is influenced by granular morphology and structural alterations from the treatment used.14 For HHP in particular, gelatinisation is the main application for starch.17,18
Recently, a HHP-modified cassava flour with highly amorphous starch granules was reported.19 Amorphicity was attributed to pressure-induced gelatinization, as evidenced by granular swelling, reduction in crystallinity, and disruption of short-range order. Compared to pure starches, the volatile retention capability of flour is rather an underexplored area. So far, the commonly reported food products made from modified flours have been baked products like bread and cakes, which utilize a lot of flour and can carry several volatile compounds from intentionally added flavourings (natural or artificial). Moreover, cassava flour is mainly utilised in the production of cassava-based baked products, especially as a gluten-free alternative, and in soup production.20,21 Hence, it is essential to understand how the altered properties of starch in flour influence the retention of these volatiles and its stability when stored. In addition, non-starch components may also influence volatile retention, as citrus fibre was found to bind more terpenes, alcohols, and ketones than native corn starch.22
Although there was a previous effort to understand the impact of HHP treatment of pure starch on the binding of aroma compounds,14,15 none has been on a multicomponent starchy matrix like flour and at different levels of gelatinisation or crystallinity. According to Goubet et al., the amorphous state delivers the highest retention, but structural collapse and recrystallization can lead to the loss of aroma compounds.9 Meanwhile, Somboonchan et al. were able to demonstrate that flavour compounds can interact with starch even in a partially gelatinized state and with incompletely swollen granules.23 Hence, in this study, the general aim is to evaluate the volatile retention performance of a HHP-modified cassava flour. The retention capacity of treated flour was assessed indirectly by measuring the abundance of volatiles in the headspace of the flour suspension. Additionally, the influence of the degree of inherent starch's structural alteration (defined by the degree of gelatinisation) from the intensity of HHP treatment on the volatile binding and its stability during storage was also investigated.
2 Materials and methods
2.1 Preparation and HHP treatment of cassava flour
PhilRootcrops of Visayas State University, Baybay City, Leyte, Philippines, has kindly provided cassava flour from the NSIC Cv13 variety, which is composed of 13.94%db moisture and 81.04%db starch. Aqueous flour suspensions packed in clear vacuum pouches were subjected to high hydrostatic pressure (Multivac HHP 055, Multivac Sepp Haggenmüller GmbH and Co., Wolfertschwenden, Germany) treatment as described by Conde et al.19 The treatment involved a similar compression and decompression rate (100 MPa min−1), and water was used as a pressure-transmitting medium in the 55 L chamber, with an initial temperature of 5 ± 1 °C and a final temperature that did not exceed 31 °C. Afterwards, the treated flour suspensions were freeze dried and stored at −20 °C until further use.From the previously produced flours of Conde et al.,19 samples were selected for volatile retention analysis. This was based on the level of treatment intensity and the magnitude of impact on the macro- and micro-structural properties of the cassava flour's inherent starch. The study focused on the starch fraction as it is the major component of the flour and hence, would greatly contribute to the retention of volatiles compared to other non-starch components. The degree of gelatinisation (%, DG), which was computed as the relative difference in gelatinisation enthalpy to that of untreated flour, was mainly used as a determining factor as it also denotes the microstructural disorder of starch as affected by the observed treatment-induced gelatinisation.24 For this study, a HHP treatment combination with the highest or full and medium degree of gelatinisation was selected. This corresponded to 600–10%-30 or 10% flour concentration held at 600 MPa for 30 min (100% DG) as a high intensity HHP treatment and 600–30%–10 or 30% flour concentration held at 600 MPa for 10 min as a medium intensity treatment (54.25 ± 4.98% DG).19 These flours were also found to be macrostructurally different through polarized microscopy and microstructurally amorphous at different degrees based on long-range order analysis (10.44% and 18.17% relative crystallinity, respectively).19 Untreated and freeze-dried flour suspensions were included for comparative purposes, hereafter referred to as ‘control’.
2.2 Mango flavour preparation and its volatile composition
Artificial mango flavour (Invita NZ Ltd., Auckland, NZ) was used as a volatile source and as a representative fruit flavourant. The mango concentrate was diluted prior to use, first by creating a 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm solution with absolute ethanol. The use of ethanol as solvent helped improve the solubility of flavour concentrate in the following dilution. Then, the ethanol-based solution was finally diluted to 10
000 ppm solution with absolute ethanol. The use of ethanol as solvent helped improve the solubility of flavour concentrate in the following dilution. Then, the ethanol-based solution was finally diluted to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm with water as solvent. From this solution, a 100 ppm aqueous flavour solution was prepared in a 20 mL glass vial by mixing 7.92 mL ultrapure water and 80 μL of the 1000 ppm flavour solution. This was immediately sealed with a silicon-septa lined screw cap, vortexed, covered with foil, and stored at 4 °C until analysis.
000 ppm with water as solvent. From this solution, a 100 ppm aqueous flavour solution was prepared in a 20 mL glass vial by mixing 7.92 mL ultrapure water and 80 μL of the 1000 ppm flavour solution. This was immediately sealed with a silicon-septa lined screw cap, vortexed, covered with foil, and stored at 4 °C until analysis.
The qualitative volatile composition was determined through the headspace solid-phase microextraction technique coupled with gas chromatography-mass spectrometry (HS-SPME GC-MS; Section 2.4) of the 100 ppm aqueous dilution of the flavourant. The peaks from the obtained chromatographs were tentatively identified using the NIST mass spectral library (Version 2.2, National Institute of Standards and Technology) and ascertained by (1) match and reverse match values greater than 90%, (2) comparison of experimental and literature retention indices, and (3) matching retention time with authentic standards for at least one volatile compound per chemical class. Several different volatile chemical classes were identified, including alcohols, alkenes, esters, hydrocarbons, and terpenes (Table 1).
| Peak number in Fig. 1 | Compound | Chemical class |
|---|---|---|
| 1 | Ethyl 2-methylpropanoate | Ester |
| 2 | Tricyclene | Monoterpene |
| 3 | α-Pinene | Monoterpene |
| 4 | Ethyl butanoate | Ester |
| 5 | α-Fenchene | Monoterpene |
| 6 | Camphene | Monoterpene |
| 7 | 1,6-Octadiene, 2,7-dimethyl- | Alkene |
| 8 | β-Pinene | Monoterpene |
| 9 | 3-Methylbutyl acetate | Ester |
| 10 | 3-Carene | Monoterpene |
| 11 | β-Myrcene | Monoterpene |
| 12 | Pseudolimonene | Monoterpene |
| 13 | D-Limonene | Monoterpene |
| 14 | Sabinene | Monoterpene |
| 15 | 1,7-Octadiene, 3,6-dimethylene- | Alkene |
| 16 | γ-Terpinene | Monoterpene |
| 17 | o-Cymene | Monoterpene |
| 18 | α-Terpinolene | Monoterpene |
| 19 | Z-3-Hexenyl acetate | Ester |
| 20 | 1-Hexanol | Alcohol |
| 21 | 3-Hexen-1-ol, (Z)- | Alcohol |
| 22 | Perillene | Monoterpenoid |
| 23 | α-Copaene | Sesquiterpene |
| β-Patchoulene | Sesquiterpene | |
| 24 | Isocaryophyllene | Sesquiterpene |
| α-Bulnesene | Sesquiterpene | |
| 25 | Caryophyllene | Sesquiterpene |
| 26 | 10,10-Dimethyl-2,6-dimethylene bicyclo[7.2.0]undecane | Hydrocarbon |
| 27 | 4,11,11-Trimethyl-8-methylene bicyclo[7.2.0]undec-3-ene | Hydrocarbon |
| 28 | Humulene | Sesquiterpene |
2.3 Preparation of flour-flavour suspension
A flour suspension of 3% (w/v) was prepared by adding 0.2376 g of flour and approximately 7.682 mL of ultrapure water into a 20 mL glass vial. This was then vortexed to disperse the flour and homogenise the flour mixture. The 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm flavour solution was added to the flour mixture at 80 μL to create a 100 ppm flavour suspension. Four vials per replicate were prepared beforehand to allow rapid flavour addition and minimal volatile loss. They were tightly sealed with a silicon-septa lined screw cap and vortexed. The four vials were stored at 4 °C with minimum or no agitation to allow equilibration of headspace. Each vial represented the sampling storage times of 12, 24, 36, and 48 h. Triplicates were prepared for each flour. A similar sampling set-up was made for the 100 ppm flavour solution.
000 ppm flavour solution was added to the flour mixture at 80 μL to create a 100 ppm flavour suspension. Four vials per replicate were prepared beforehand to allow rapid flavour addition and minimal volatile loss. They were tightly sealed with a silicon-septa lined screw cap and vortexed. The four vials were stored at 4 °C with minimum or no agitation to allow equilibration of headspace. Each vial represented the sampling storage times of 12, 24, 36, and 48 h. Triplicates were prepared for each flour. A similar sampling set-up was made for the 100 ppm flavour solution.
2.4 Analysis of headspace volatiles
HS-SPME GC-MS analysis was conducted to analyse the headspace volatile compounds following the studies of Khrisanapant, Kebede, Leong, and Oey25 with modifications. The analysis was performed using an Agilent 6890N gas chromatography system (Agilent Technologies, CA, USA) coupled with a mass selective detector (MSD) system (5975B VL, Agilent Technologies, CA, USA). A polar capillary column (ZB-Wax, 60 m × 0.32 mm i.d., 0.5 μm film thickness, Phenomenex) with a deactivated column as a column guard (6 m × 0.32 mm) was utilized. The process began by exposing the HS-SPME fiber coated with 50/30 μm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) (Supelco, Bellefonte, PA, USA) to the headspace of a vial placed on a cooling tray maintained at 4 °C for 5 minutes. The in-tray sampling was designed to maintain the same cool condition of storage, while no agitation and heating were employed so that the headspace equilibrium was not disturbed and only headspace volatiles were captured. Afterwards, the fibre was immediately desorbed at 230 °C for 2 min and then injected in splitless mode with helium as the carrier gas at 1 mL min−1. For optimal volatile separation, the oven temperature program was as follows: initially held at 50 °C for 5 min, then increased to 210 °C at 5 °C min−1, ramped again to 240 °C at 10 °C min−1 for 5 min, and lastly cooled to 50 °C. The mass spectra were obtained by electronic ionisation (EI) at 70 eV with a scanning range of 29 to 300 m/z. The MS quadrupole and ion source temperatures were set at 150 °C and 230 °C, respectively.The obtained chromatographs from the 100 ppm flavour solutions were processed with Automated Mass Spectral Deconvolution and Identification System (AMDIS) software (Version 2.72, build 140.24, National Institute of Standards and Technology (NIST), Gaithersburg, MD, USA) for deconvolution and peaks were tentatively identified using the NIST mass spectral library (Section 2.2). The peaks of the identified volatiles were integrated using MSD Chemstation F.01.01.2317 (Agilent Technologies, Inc., CA, USA) and extracted peak abundance data were used for further statistical analysis.
2.5 Determination of volatile retention
The volatile retention capacity of aqueous flour-flavour suspensions was evaluated indirectly through measuring the abundance of volatiles in the headspace of the flour suspensions, as a decrease in volatiles' concentration in the headspace entailed an increase in the content of volatile compounds in the aqueous phase.7 An approach patterned from the analysis of aqueous26 and food model27 systems. It is important to accentuate that, in this study, the measurement of volatile compound quantities was done through relative quantification based on their abundance (peak area), rather than determining their absolute quantities. The calculation of the retention percentage26 of a volatile compound is shown below: | (1) |
2.6 Multivariate data analysis and statistical analysis
Multivariate data analysis (MVDA) was performed to investigate the difference in the headspace volatile profile of differently processed flour suspensions, followed by a chemometric approach.25,28 Firstly, Principal Component Analysis (PCA) was performed on Solo (Version 9, Eigenvector Research, Inc., Manson, WA, USA) as an unsupervised exploratory tool to identify any trends or patterns and outliers. Partial Least Squares Discriminant Analysis (PLS-DA) was performed to further investigate the classifications based on flour treatment and storage time as a function of the volatile profile.Discriminant volatiles, compounds that drove the classification during storage, were also selected using a feature or variable selection method. Variable identification (VID) coefficients are correlation coefficients between X-variables (volatiles) and predicted Y-variables (storage time) from the generated multidimensional model of PLS-DA.28,29 Volatiles with an absolute VID value equal to or greater than 0.80 were selected as discriminant volatiles.28,30 Moreover, the abundance of discriminant volatiles at different storage timepoints was subjected to ANOVA testing at a 5% significance level, followed by Tukey's post-hoc test. If assumptions are not met, appropriate nonparametric alternative tests will be used. Biplots containing loading and score plots were constructed via Solo.
3 Results and discussion
3.1 Volatile profile differences as affected by HHP treatment on cassava flours
The volatile composition of the headspace of the untreated and HHP-treated flour-flavour suspensions was analysed by HS-SPME GC-MS. Out of the 30 volatiles detected in the mango flavour solution, only 27 were found in all flour-flavour suspensions. Terpenes were the predominant volatile classes in all flour-flavour suspensions in terms of headspace abundance. Fig. 1 displays a representative headspace total ion chromatogram (TIC) of the mango flavour solution, control, and HHP-treated cassava flour-flavour suspensions after 12 h of storage. The TIC visually displays a reduction in the headspace abundance of major peaks with cassava flour addition regardless of treatment, i.e., sesquiterpenes (peaks 24, 25, and 28), β-myrcene, and limonene. However, control flour had a lower total headspace volatile abundance than HHP-treated flours, which were 8–13% higher. This can indicate a higher retention in the control flour suspension instead. Likewise, Misharina found that native cassava starch effectively adsorbs volatiles in mixture form within the granule, through pores or channels, as well as through partial sorption at the surface.5The PLS-DA biplot shown in Fig. 2, which explains 57.65% of the cumulative variance, visually reveals a grouping between cassava flour samples. HHP-treated flours are positioned close to each other and projected opposite the control along the latent variable (LV) 1 axis. The observation emphasizes that the primary variation in the data is the segregation between control and HHP-treated flour samples. This also suggests that the induced structural modification through HHP on the cassava flour19 primarily discriminates it from the control or untreated flour.
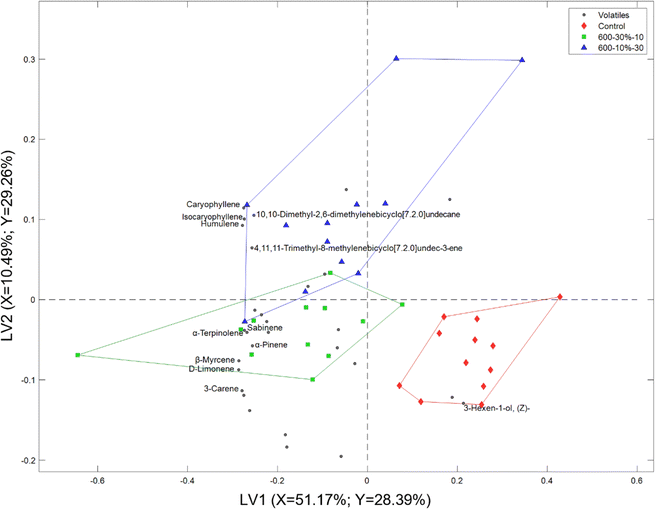 | ||
| Fig. 2 PLS-DA biplot describing the variation in the headspace volatile composition of control and HHP-treated cassava flour-flavour suspensions at all storage points. | ||
Fig. 2 also illustrates the relationship between the volatile compounds (filled small circles) and differently processed flour samples. The majority of the volatiles, with some identified as terpenes and hydrocarbons, were found to be closely associated with HHP-treated samples and positioned opposite the control flour samples. This pattern indicates a higher detected amount of terpenes and hydrocarbons in the headspace of HHP-treated flours compared to the control flour. Moreover, this was evident in the TICs (Fig. 1), where a higher abundance of terpenes, particularly sesquiterpenes, in HHP-treated samples was observed visually compared to the control. As the binding of volatiles by native starch is largely driven by hydrophobic cooperative interactions,31 the retention of highly hydrophobic terpenes was indeed remarkable for the control flour. Meanwhile, 3-hexen-1-ol was strongly associated with the control cassava flour, which indicates a higher abundance in its headspace compared to the HHP-treated flour. This indicates lower retention in the suspension of the control cassava flour. In fact, native cassava starch was found to have a lower affinity for alcohols than potato, wheat, and corn starch.5 Furthermore, pressurised waxy maize15 and sorghum starch14 were reported to have an increased sorption of alcohols than their native counterparts. Accordingly, the headspace pattern indicated a 17–20% higher retention of alcohols and a 3–7% lower terpene retention in HHP-treated flours than in control cassava flour.
There was also a secondary variation between the 600–30%-10 and 600–10%-30 cassava flours along the LV2 axis. Sesquiterpenes were closely associated with 600–10%-30 and monoterpenes with 600–30%-10, which drove the slight delineation between the HHP-treated flours. Likewise, the sesquiterpene abundance was 3.4% higher and monoterpene was 5.5% lower for 600–10%-30 compared to 600–30%-10. This also manifested in a 5% higher average monoterpene retention of the highly structurally modified flour (600–10%-30) compared to the less structurally modified flour (600–30%-10). However, there was minimal difference in the retention percentage for sesquiterpenes.
In summary, the PLS-DA biplot combined with the visual comparison of the TICs revealed two key observations: (1) the incorporation of cassava flour reduces the headspace abundance of volatiles regardless of the treatment, indicating retention in the flour suspension matrix and (2) HHP treatment and processing intensity changed the headspace volatile profile of the suspensions. Moreover, the larger variation within HHP-treated samples compared to control flour (Fig. 2) implies a potential influence of storage time and will be further explored in the subsequent section.
3.2 Trend in volatile profile change during storage
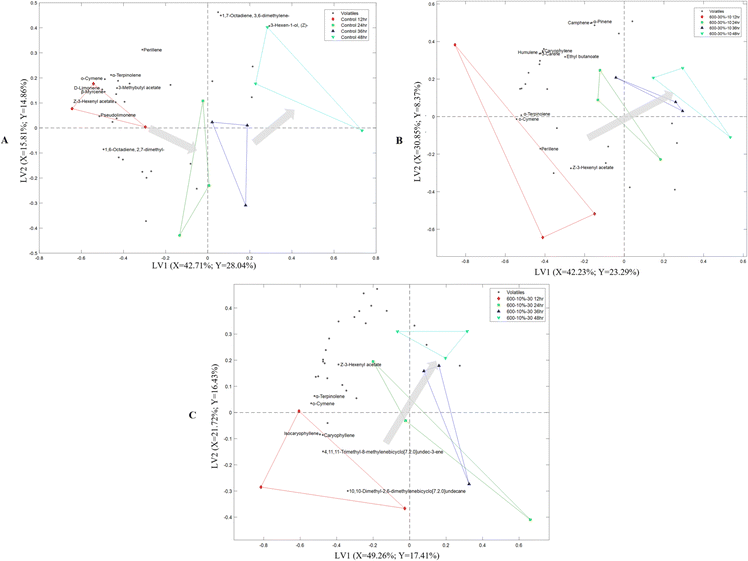
A multidimensional model of PLS-DA was generated for each flour sample to evaluate the volatile changes as a function of storage time. The biplot of the differently processed cassava flour samples (Fig. 3) shows a classification as a function of storage time that was more defined along the LV1 axis. In general, the 12 h and 48 h storage times were farthest from each other, while 24 and 36 h were positioned between the two in a successive manner. The closer the timepoints are to each other the more similar their volatile profiles, while those projected farther apart indicate dissimilar profiles. For the control cassava flour (Fig. 3A), storage timepoints at 24 h and 36 h were positioned close to each other and far off to 12 h and 48 h, respectively. On the other hand, 24–48 h were positioned close to each other and away from 12 h for HHP-treated samples (Fig. 3B and C). Moreover, the control cassava flour showed a V-shaped trend with storage time, while a somewhat linear trend was observed for HHP-treated flour samples. The storage time positioning and non-linear trend of control flour suggest a complex interaction between the flour and volatiles, with potential competition for binding sites,7 taking place at 12–24 h and 36–48 h of storage. In contrast, the linear arrangement of storage time points for HHP-treated samples suggests minor differences in the volatile profile with longer storage of the suspension. Moreover, Fig. 3A also shows that most of the volatiles were positioned close to 12 h for control flour, which implies a greater abundance at the beginning of storage and seems to decrease as a function of time. Volatiles were, however, quite spread out in HHP-treated samples.To better describe the change in the volatile profile and retention stability with storage for control and HHP-treated cassava flour, a chemometric approach through the VID method was performed for each flour sample. VID estimates the correlation between the volatile compounds and storage time points and identifies discriminant compounds that drive the classification (Table 2).
| Flour sample | Storage timepoint | |||||||
|---|---|---|---|---|---|---|---|---|
| 12 h | 24 h | 36 h | 48 h | |||||
| VID | Identity | VID | Identity | VID | Identity | VID | Identity | |
| a Volatile was added because a significant change in abundance was found during storage. b NA = volatiles did not reach a |VID| of 0.80. | ||||||||
| Control | 0.926 | o-Cymene | −0.820 | 1,7-Octadiene, 3,6-dimethylene- | −0.803 | Perillene | 0.803 | 3-Hexen-1-ol, (Z)- |
| 0.916 | Z-3-Hexenyl acetate | −0.817 | o-Cymene | −0.783 | Pseudolimonenea | |||
| 0.909 | 3-Methylbutyl acetate | −0.866 | 3-Hexen-1-ol, (Z)- | −0.924 | Z-3-Hexenyl acetate | −0.871 | 1,6-Octadiene, 2,7-dimethyl- | |
| 0.868 | D-Limonene | −0.937 | 3-Methylbutyl acetate | |||||
| 0.849 | Pseudolimonene | |||||||
| 0.836 | α-Terpinolene | |||||||
| 0.834 | β-Myrcene | |||||||
| 0.822 | 1,6-Octadiene, 2,7-dimethyl- | |||||||
| 600–30%-10 | 0.874 | o-Cymene | 0.899 | Camphene | −0.814 | Perillene | −0.829 | α-Terpinolene |
| 0.820 | α-Terpinolene | 0.882 | Ethyl butanoate | −0.888 | Z-3-Hexenyl acetate | −0.868 | o-Cymene | |
| 0.811 | Perillene | 0.868 | α-Pinene | |||||
| 0.854 | Humulene | |||||||
| 0.853 | 3-Carene | |||||||
| 0.815 | Caryophyllene | |||||||
| 600–10%-30 | 0.877 | o-Cymene | NA | −0.832 | Z-3-Hexenyl acetate | NA | ||
| 0.871 | 4,11,11-Trimethyl-8-methylenebicyclo[7.2.0]undec-3-ene | |||||||
| 0.850 | Isocaryophyllene | |||||||
| 0.828 | Caryophyllene | |||||||
| 0.816 | α-Terpinolene | |||||||
| 0.807 | 10,10-Dimethyl-2,6-dimethylenebicyclo [7.2.0]undecane | |||||||
3.3 Volatile profile and retention stability during storage
Previous studies on native pure starch have proposed that the sorption of the volatiles is both surficial and capillary sorption, with retention related to hydrophobic nonspecific van der Waals interactions.5,14,32 Additionally, a higher sorption of volatiles by native starches was found for hydrophobic compounds (hydrocarbons) and for aliphatic esters,15 with improved sorption as the length of alkyl substituents increases.5 Hence, Z-3-hexenyl acetate and 3-methylbutyl acetate, which had higher hydrophobicity and slightly longer alkyl substituents than the other identified esters, were continuously sorbed in the flour suspension during storage. Likewise, increasing the molecular weight with decreasing polarity enhances retention by carbohydrates.9 This is evident for sesquiterpenes (87–94%) which had a higher retention percentage than monoterpenes (39–84%). However, the volatile abundance of sesquiterpenes remained stable during storage, and hence, they were not selected as discriminant volatiles. That aside, terpenes remained a predominant volatile group in the headspace despite the high retention in the suspension. This is essential, as terpenes are major mango volatiles with some identified as odour-active.33 In addition, the individual contribution of most of the volatiles in both headspace and suspension has changed, and may change the sensory profile of the product.34 It is recommended, therefore, that additional tests should be carried out on its effect on the perceived flavour.
Other discriminant volatile classes, i.e., esters, monoterpenoids, and sesquiterpenes, did not change significantly with storage time for both HHP-treated flours but retained a higher abundance in the headspace—or lower retention in the flour suspension—than control. The observation was similar for high-pressure treated (650–30%-9; 100% DG) corn starch, wherein the observed complete sorption of monoterpenes entailed a reduced binding activity of alcohols, ketones, phenols, and sesquiterpenes compared to the values of native starch.14 Likewise, the extent of volatile binding was also reduced in the HHP-treated (650–30%-9) high amylose and amylopectin starch mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, Hylon VII and waxy maize starch; amylose content of 16.2%; 83.7% DG).15 It has been reported that for both HHP and thermally gelatinised starches, competition for binding sites especially for compounds with distinct classes occurs when added as a multi-component mixture.7,14 This was evident in Fig. 2, as some compounds were found to be highly associated with a certain treatment. However, the majority of the discriminant compounds in HHP-treated flours did not significantly change their headspace abundance with longer storage, showcasing stability in retention. Compared to 4 out of 11 significantly changing discriminant volatiles in control, only 2 out of 10 and 1 of 7 were found for 600–30%-10 and 600–10%-30, respectively. This observation explains the linear trend with storage shown in Fig. 3B and C biplots, and the proximity for 600–30%-10 and overlapping for 600–10%-30 scores at 24–48 h of storage. Meanwhile, the more dynamic headspace of the control showed a non-linear trend. Despite this, only minor differences in overall volatile composition were calculated between HHP-treated flours. Further study is however recommended to check if the sensory perception of mango flavour was different between flour samples of varied starch properties.
3, Hylon VII and waxy maize starch; amylose content of 16.2%; 83.7% DG).15 It has been reported that for both HHP and thermally gelatinised starches, competition for binding sites especially for compounds with distinct classes occurs when added as a multi-component mixture.7,14 This was evident in Fig. 2, as some compounds were found to be highly associated with a certain treatment. However, the majority of the discriminant compounds in HHP-treated flours did not significantly change their headspace abundance with longer storage, showcasing stability in retention. Compared to 4 out of 11 significantly changing discriminant volatiles in control, only 2 out of 10 and 1 of 7 were found for 600–30%-10 and 600–10%-30, respectively. This observation explains the linear trend with storage shown in Fig. 3B and C biplots, and the proximity for 600–30%-10 and overlapping for 600–10%-30 scores at 24–48 h of storage. Meanwhile, the more dynamic headspace of the control showed a non-linear trend. Despite this, only minor differences in overall volatile composition were calculated between HHP-treated flours. Further study is however recommended to check if the sensory perception of mango flavour was different between flour samples of varied starch properties.
The physicochemical properties of the carbohydrate sorbent are also vital for aroma compound retention.9 Błaszczak et al.14,15 reported that the variation in the binding of aroma compounds of pressurised starches was attributed to the significant alteration in the granular structure. Conde et al.19 clearly demonstrated that the granular macro- and micro-structures of the inherent starch granule in the cassava flour samples were significantly modified due to the intensity of the HHP processing conditions.19 The inherent starch granules in 600–10%-30 were enlarged and completely lost their birefringence, while 600–30%-10 was a mixture of granules with different degrees of gelatinisation. The transition to an amorphous state and swelling that occurred due to pressure induced gelatinisation are indicative of weak associative forces that maintain the granular structure,35 and hence, may have caused the loss of the channels or porous surfaces that facilitate the sorption of volatile compounds as seen in native starches. In addition, the binding mechanism in the HHP-treated flours was also hypothesized to be mostly related to physico-chemical interactions rather than the formation of supramolecular complexes, as Błaszczak et al.15 found that the melting enthalpies of pressurised starches, with and without aroma compounds, were not significantly different. Even the mixture of pre-gelatinised and dry starches with aroma compounds did not show high temperature endotherm peaks attributed to complexes.10 Supramolecular complexes begin to form when starch is in the gel solution state36 and then the helix–helix association of the helical segments of amylose containing non-covalently bound aroma compounds is established.3 However, during pressure induced gelatinisation of starch, there is incomplete disintegration and solubilization of amylose is poor.37 Hence, the potential formation of supramolecular complexes is rather low. To verify the retention mechanism in HHP structurally modified cassava flour, additional tests are recommended, including but not limited to DSC, XRD, FTIR, and other chemical tests.
4 Conclusions
The results of this study demonstrated that high pressure treatment can affect the retention capacity of cassava flour for volatile aroma compounds. Compared to control cassava flour, both medium intensity and high intensity treatments of HHP—which represent medium (54% DG) and highly (100% DG) structurally modified flour, respectively—reduced the sorption of most volatiles, as evidenced by the higher level of abundance of volatiles in the headspace. PLS-DA findings revealed that HHP treatment caused increased retention of alcohols and reduced retention of terpenes, leading to distinct classifications of the flour samples. Through a chemometric approach, a clear effect of HHP on the headspace volatile profile and retention was observed during storage. The structural alteration of flour components by HHP—especially starch granules—and potential competition for binding sites reduced the retention of sesquiterpenes and hydrocarbons, but exhibited relatively stable retention during storage for the majority of the volatile compounds compared to control flour. However, despite the remarkable difference in the degree of gelatinisation between the two treated flours, minimal difference was found in their headspace composition and volatile retention during storage. Nonetheless, the capability to bind volatiles in a gelatinized (partial or full) state, albeit at slightly lower capacity, allows for a modified cassava flour-based product to retain flavour and opens research opportunities to maximize the use of the modified flours in binding compounds not limited to volatiles. But to better understand the mechanism of retention in the flour matrix and maximize the use of HHP in tailoring volatile binding in cassava flour, it is suggested to perform microstructural and chemical analysis. Additionally, sensory evaluation can be conducted to assess the effect on perceived flavour.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Ladie Anne Conde: conceptualization, methodology, investigation, formal analysis, visualization, and writing – original draft. Biniam Kebede: conceptualization, methodology, formal analysis, writing – review and editing, and supervision. Indrawati Oey: conceptualization, funding acquisition, methodology, supervision, and writing – review & editing.Conflicts of interest
The authors declare that they have no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.Acknowledgements
Ladie Anne Conde would like to acknowledge the Manaaki New Zealand Scholarship through the Ministry of Foreign Affairs and Trade for her doctoral scholarship. Additionally, the authors would like to thank PhilRootcrops and Visayas State University, Baybay City, Leyte for providing the cassava roots and access to their facilities during flour production. Indrawati Oey is grateful to the Riddet Institute for the project funding.Notes and references
- A. Boutboul, P. Giampaoli, A. Feigenbaum and V. Ducruet, Carbohydr. Polym., 2002, 47, 73–82 CrossRef CAS.
- W. Błaszczak, T. A. Misharina, D. Fessas, M. Signorelli and A. R. Górecki, Food Res. Int., 2013, 54, 338–344 CrossRef.
- B. Conde-Petit, F. Escher and J. Nuessli, Trends Food Sci. Technol., 2006, 17, 227–235 CrossRef CAS.
- I. Buljeta, A. Pichler, I. Ivić, J. Šimunović and M. Kopjar, Molecules, 2021, 26, 4207 CrossRef CAS PubMed.
- T. A. Misharina, Appl. Biochem. Microbiol., 2002, 38, 480–486 CrossRef CAS.
- M. B. Terenina and T. A. Misharina, Appl. Biochem. Microbiol., 2005, 41, 407–412 CrossRef CAS.
- T. A. Misharina, A. L. Samusenko and M. A. Kalinchenko, Appl. Biochem. Microbiol., 2004, 40, 609–612 CrossRef CAS.
- A.-M. Seuvre and A. Voilley, in Springer Handbook of Odor, ed. A. Buettner, Springer International Publishing, Cham, 2017, pp. 35–36, DOI:10.1007/978-3-319-26932-0_13.
- I. Goubet, J. L. Le Quere and A. J. Voilley, J. Agric. Food Chem., 1998, 46, 1981–1990 CrossRef CAS.
- A. D. Jørgensen, S. L. Jensen, G. Ziegler, A. Pandeya, A. Buléon, B. Svensson and A. Blennow, Starch/Staerke, 2012, 64, 461–469 CrossRef.
- N. Cayot, C. Taisant and A. Voilley, J. Agric. Food Chem., 1998, 46, 3201–3206 CrossRef CAS.
- M. Tietz, A. Buettner and B. Conde-Petit, Food Chem., 2008, 108, 1192–1199 CrossRef CAS.
- R. V. d. B. Fernandes, S. V. Borges and D. A. Botrel, Carbohydr. Polym., 2014, 101, 524–532 CrossRef CAS PubMed.
- W. Błaszczak, T. A. Misharina, J. Sadowska, A. R. Górecki and J. Fornal, LWT--Food Sci. Technol., 2014, 55, 657–665 CrossRef.
- W. Błaszczak, T. A. Misharina, V. P. Yuryev and J. Fornal, LWT--Food Sci. Technol., 2007, 40, 1841–1848 CrossRef.
- J. N. BeMiller, in Corn, ed. S. O. Serna-Saldivar, AACC International Press, Oxford, 3rd edn, 2019, pp. 537–549, DOI:10.1016/B978-0-12-811971-6.00019-X.
- D. Zia ud, H. Xiong and P. Fei, Crit. Rev. Food Sci. Nutr., 2017, 57, 2691–2705 CrossRef.
- I. Grgić, Đ. Ačkar, V. Barišić, M. Vlainić, N. Knežević and Z. Medverec Knežević, J. Food Process. Preserv., 2019, 43, e14242 Search PubMed.
- L. A. Conde, B. Kebede, S. Y. Leong and I. Oey, Appl. Sci., 2022, 12, 10043 CrossRef CAS.
- S. M. Chisenga, T. S. Workneh, G. Bultosa and B. A. Alimi, J. Food Sci. Technol., 2019, 56, 2799–2813 CrossRef CAS PubMed.
- K. O. Falade and J. O. Akingbala, Food Rev. Int., 2010, 27, 51–83 CrossRef.
- T. A. Misharina, M. B. Terenina, N. I. Krikunova and I. B. Medvedeva, Appl. Biochem. Microbiol., 2016, 52, 226–232 CrossRef CAS.
- S. Somboonchan, S. Lubbers and G. Roudaut, Food Chem., 2016, 195, 79–86 CrossRef CAS PubMed.
- F. J. Warren, M. J. Gidley and B. M. Flanagan, Carbohydr. Polym., 2016, 139, 35–42 CrossRef CAS PubMed.
- P. Khrisanapant, B. Kebede, S. Y. Leong and I. Oey, Foods, 2019, 8, 651 CrossRef CAS PubMed.
- L. Yeo, D. B. Thompson and D. G. Peterson, Food Chem., 2016, 199, 393–400 CrossRef CAS PubMed.
- M. Keršienė, A. Adams, A. Dubra, N. D. Kimpe and D. Leskauskaitė, Food Chem., 2008, 108, 1183–1191 CrossRef.
- B. T. Kebede, T. Grauwet, G. Tabilo-Munizaga, S. Palmers, L. Vervoort, M. Hendrickx and A. Van Loey, Food Chem., 2013, 141, 1603–1613 CrossRef CAS.
- T. Grauwet, L. Vervoort, I. Colle, A. Van Loey and M. Hendrickx, Trends Biotechnol., 2014, 32, 125–131 CrossRef CAS.
- L. Vervoort, T. Grauwet, B. T. Kebede, I. Van der Plancken, R. Timmermans, M. Hendrickx and A. Van Loey, Food Chem., 2012, 134, 2303–2312 CrossRef CAS.
- T. A. Misharina, M. B. Terenina, N. I. Krikunova and M. A. Kalinchenko, Appl. Biochem. Microbiol., 2006, 42, 111–115 CrossRef CAS.
- T. A. Misharina, A. L. Samusenko and M. A. Kalinchenko, Appl. Biochem. Microbiol., 2006, 42, 224–227 CrossRef CAS.
- J. A. Pino, Int. J. Food Sci. Technol., 2012, 47, 1944–1950 CrossRef CAS.
- R. Vidrih, E. Zlatić and J. Hribar, Czech J. Food Sci., 2009, 27, S58–S61 CrossRef CAS.
- Y. I. Cornejo-Ramírez, O. Martínez-Cruz, C. L. Del Toro-Sánchez, F. J. Wong-Corral, J. Borboa-Flores and F. J. Cinco-Moroyoqui, CyTA--J. Food, 2018, 16, 1003–1017 CrossRef.
- R. V. Golovnya, M. B. Terenina, N. I. Krikunova, V. P. Yuryev and T. A. Misharina, Starch/Staerke, 2001, 53, 269–277 CrossRef CAS.
- D. Knorr, V. Heinz and R. Buckow, Biochim. Biophys. Acta, Proteins Proteomics, 2006, 1764, 619–631 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fb00098f |
| This journal is © The Royal Society of Chemistry 2024 |