Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Comparison of quality and oxidative stability of pumpkin seed (Cucurbita maxima) oil between conventional and enzymatic extraction methods
Guoqiang
Zhang
 *ab,
Ziqian
Li
c and
Manfei
Fu
*d
*ab,
Ziqian
Li
c and
Manfei
Fu
*d
aDepartment of Food and Nutritional Sciences, University of Reading, PO Box 226, Whiteknights, Reading, RG6 6AP, UK
bSchool of Life Sciences, Faculty of Health and Life Sciences, Coventry University, Coventry, CV1 5FB, UK. E-mail: ae4214@coventry.ac.uk
cCollege of Food Science and Engineering, Nanjing University of Finance and Economics, Nanjing 210023, China
dMedical Research Center, The Affiliated Hospital of Qingdao University, Qingdao 266000, China
First published on 8th April 2024
Abstract
Pumpkin seed oil was obtained from three extraction methods, namely Soxhlet extraction (SE), cold-pressed extraction (CPE), and aqueous enzymatic extraction (AEE). The impact of the extraction method on physicochemical parameters, fatty acids, bioactive compounds, and oxidative stability of the obtained pumpkin seed oil was assessed. Pumpkin seed oil was rich in unsaturated fatty acids (>80%), squalene (174.1–253.6 mg/100 g), β-sitosterol (147.7–208.2 mg/100 g), and tocopherols (53.1–73.3 mg/100 g). Extraction methods affected the physicochemical properties and oxidative stability of pumpkin seed oil. Pumpkin seed oil obtained from SE had a higher content of bioactive compounds than AEE and CPE. After an oxidation storage test, pumpkin seed oil obtained from AEE and SE exhibited better oxidative stability than CPE, with AEE performing the best. Overall, this work could provide comparable information to the oil industry to produce high-quality pumpkin seed oils with appropriate extraction methods.
Sustainability spotlightThe quality and nutritional compound content of oil determine its application and economic value. However, in the oil production process, the extraction method is one of important factors and relates to the quality and nutritional value retention of oil. Compared with conventional extraction methods, aqueous enzymatic extraction is a green, eco-friendly, and sustainable method, since it can eliminate organic solvent consumption and reduce energy demand. Therefore, this work could help the oil industry to produce high quality oil using green and sustainable technology, contributing to SDG 2 (end hunger, achieve food security and improved nutrition and promote sustainable agriculture) and SDG 12 (ensure sustainable consumption and production). |
1. Introduction
Pumpkin (Cucurbita maxima), belonging to the Cucurbitaceae family, is one of the important crops in the world.1 Pumpkin seeds are used as valuable ingredients in the baking industry and are also utilised to produce pumpkin seed oil due to their high oil content (39.7–48.6% w/w).2,3 Pumpkin seed oil is rich in unsaturated fatty acids (over 80%) and contains many bioactive compounds, such as tocopherols, squalene and phytosterols;4–7 in addition, many studies have demonstrated its health benefits, such as anti-inflammatory, diuretic properties, and preventing atherosclerosis as well as prostate disease.8–10 In recent years, pumpkin seed oil requirement and consumption have been increasing and its application has been studied and demonstrated as a promising functional oil source in food, cosmetics, and pharmaceuticals.1,7 This drives oil industries to upgrade pumpkin seed oil quality to meet the customer demand and potentially growing market needs.To some extent, an appropriate extraction method is important in oil extraction processing, which determines oil quality and nutritional value retention.11–13 Soxhlet extraction (SE) and cold-pressed extraction (CPE) are conventional methods in oil extraction.14,15 SE has the advantages of high efficiency and high oil yield, but there are also some significant drawbacks, such as high consumption in organic solvent, toxic solvent residue, and environmental pollution.16,17 Cold-pressed extraction (CPE) is a safe and simple method as compared to Soxhlet extraction (SE), because it avoids use of organic solvent and preserves nutrition value and natural properties.18 However, due to the low degree of processing, adversely, cold-pressed oil could remain more pro-oxidant factors (e.g. metal ions and moisture) and then is more susceptible to oxidation, and thus, the quality and shelf life of oil could be affected.19,20 Aqueous enzymatic extraction (AEE) has received much attention as a novel and green technique in oil extraction.17 The advantages of aqueous enzymatic extraction are being safe, eco-friendly, and sustainable, since the aqueous base solvent (as a green solvent) can eliminate the hazard of organic solvents and enzyme reusability could contribute to a much lower cost and energy consumption.17,21,22 In addition, the quest for producing high quality products through green and sustainable technology is a key to moving towards sustainability.23 Although oil yield is a challenge in AEE, Prommaban et al.24 extracted pumpkin seed oil using AEE and showed that the oil performed well in antioxidant activities and anti-aging activities and could be applied in cosmetics. Besides that, during the processing and storage of oil, lipid oxidation can adversely affect sensory taste and the content of nutritional compounds and ultimately lead to quality deterioration, and thus, the oxidative stability of oil is an important index to reflect oil quality.25,26
However, to date, there have been insufficient available data for comparing the effect of different extraction methods on the nutritional quality of pumpkin seed oil. Therefore, the aim of this study was to investigate and compare three extraction methods, including Soxhlet extraction (SE), cold-pressed extraction (CPE), and aqueous enzymatic extraction (AEE), on physicochemical parameters, fatty acids, bioactive compounds, and oxidative stability of pumpkin seed oil. This study could provide comparable data for further development of high-quality pumpkin seed oil, promote the pumpkin oil industry to explore the green and sustainable technology in oil extraction, and contribute to the realisation of sustainable development goals 2 and 12.
2. Materials and methods
2.1. Chemicals and standards
FAME standard mixture (C4–C24) and isooctane were purchased from Supelco (UK). Protease (from Bacillus amyloliquefaciens), cellulase (from Trichoderma reesei), Folin-Ciocalteu reagent, 5α-cholestan-3β-ol (≥95%), sodium methoxide solution (0.5 M, ACS grade), β-sitosterol (≥95%), cholesterol (≥99%), squalene (≥98%), campesterol (∼65%), stigmasterol (∼95%), α-tocopherol (≥96%, HPLC grade), γ-tocopherol (≥96%, HPLC grade), and δ-tocopherol (≥90%) standards were purchased from Sigma-Aldrich (UK). The Tri-sil HTP reagent was purchased from Thermo Scientific (UK).2.2. The preparation of pumpkin seeds
Whole pumpkin seeds with shells were manually collected from a fresh pumpkin (Cucurbita maxima, purchased from Marks & Spencer; Reading, UK). All collected pumpkin seeds were washed to remove attached flesh and then were dried using a tray dryer (Model No. U0P8, Armfield, England) at 50 °C for 24 h. Afterwards, dried pumpkin seeds were stored at – 20 °C for further oil extraction.2.3. Extraction procedure
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (w/v). The pH of the mixture was adjusted to 6 with 1 M HCl/NaOH and then a 3% (w/w) enzyme mixture was added (protease from bacillus amyloliquefaciens and cellulase from Trichoderma reesei at a 3
5 (w/v). The pH of the mixture was adjusted to 6 with 1 M HCl/NaOH and then a 3% (w/w) enzyme mixture was added (protease from bacillus amyloliquefaciens and cellulase from Trichoderma reesei at a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio). Then, the mixture was incubated in a water bath at 50 °C and 150 rpm for 6 h. After incubation, the mixture was centrifuged at 4000×g for 20 min. The oil (upper layer) was collected and then was stored in a freezer at −20 °C for further analysis.
1 ratio). Then, the mixture was incubated in a water bath at 50 °C and 150 rpm for 6 h. After incubation, the mixture was centrifuged at 4000×g for 20 min. The oil (upper layer) was collected and then was stored in a freezer at −20 °C for further analysis.
2.4. Physicochemical parameters
The acid value, peroxide value, iodine value, and saponification value were determined by using the AOAC method to be 969.17, 965.33, 993.20, and 920.160, respectively.282.5. Determination of fatty acid composition
The fatty acid composition was determined according to the Milinsk et al.29 description with some modifications. 50 mg pumpkin seed oil samples were added to 2 mL of 0.5 M sodium methoxide solution in methanol and then mixed for 5 min for methyl esterification. After this step, 1 mL of isooctane and 5 mL of saturated sodium chloride solution were added and stirred vigorously for 15 min. The upper layer was transferred for gas chromatography (7690B, Agilent, USA) analysis coupled with a flame ionization detector (FID). The analysis of fatty acid methyl esters (FAMEs) was conducted using a fused silica capillary column HP-88 (100 × 0.25 × 0.2). The carrier gas was helium at a constant column flow rate of 1.5 mL min; a split injection system with a splitting ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 was used; the temperature of the injection and detector was kept at 250 °C and 280 °C, respectively. The oven temperature program was held initially at 120 °C (held for 1 min), and then increased up to 175 °C at 10 °C min−1 and held for 10 min, increased to 210 at 5 °C min−1 and held for 5 min, and finally to 230 °C at the same rate and held for 10 min. Each fatty acid was identified by comparing the retention time with the standard FAME mixture. Each fatty acid was quantified using the area normalisation method, the area of each fatty acid to the total area of the total fatty acids, expressed as a relative percentage of each fatty acid to the total fatty acid identified (%).
50 was used; the temperature of the injection and detector was kept at 250 °C and 280 °C, respectively. The oven temperature program was held initially at 120 °C (held for 1 min), and then increased up to 175 °C at 10 °C min−1 and held for 10 min, increased to 210 at 5 °C min−1 and held for 5 min, and finally to 230 °C at the same rate and held for 10 min. Each fatty acid was identified by comparing the retention time with the standard FAME mixture. Each fatty acid was quantified using the area normalisation method, the area of each fatty acid to the total area of the total fatty acids, expressed as a relative percentage of each fatty acid to the total fatty acid identified (%).
2.6. Determination of tocopherol content
The tocopherol content of pumpkin seed oil was determined according to the Martakos et al.30 description with slight modifications. Briefly, 100 μL of oil sample was dissolved into 900 μL of 2-propanol, mixed, filtered, and then was analysed using high performance liquid chromatography (HPLC) with a diode array detector (DAD) (Agilent 1260, Agilent Technologies, Stockport, UK) with a Zorbax SB-C18 column (150 × 4.6 mm). The isocratic mobile phase conditions were followed: methanol and acetonitrile (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; v/v); the flow rate was 1.0 mL min−1; the detection wavelength was set at 295 nm. Each tocopherol compound was quantified according to external standards.
50; v/v); the flow rate was 1.0 mL min−1; the detection wavelength was set at 295 nm. Each tocopherol compound was quantified according to external standards.
2.7. Determination of phytosterol and squalene contents
The phytosterol and squalene contents were determined according to the method described by Liu et al.31 with some modifications. A 0.2 g pumpkin seed oil sample was mixed with 20 mL of 1 M KOH in ethanol and 1 mL of 1 M internal standard (5α-cholestan-3β-ol). The mixture was heated at 90 °C for 1 h and then cooled to room temperature. After that, 10 mL of distilled water was added with 5 mL of n-Hexane, and then was shaken vigorously for 30 s; the upper layer was collected. The extraction was repeated a total of 3 times using 5 mL of n-Hexane. All extracts were combined and evaporated under nitrogen at 40 °C until dryness. The residue was derivatized by using 0.5 mL of Tri-sil HTP reagent at 60 °C for 30 min. Afterwards, the derivatized sample was determined and quantified with a GC (7690B, Agilent, USA) equipped with a HP-5ms column (30 m × 0.25 mm × 0.25 μm; J & W Scientific, Folsom, CA, USA). The carrier gas was helium at a constant column flow rate of 1.0 mL min−1; a split injection system with a splitting ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 was used; the temperature of the injection and FID detector was kept at 280 °C and 290 °C, respectively. The oven temperature program was started at 200 °C and held 0.5 min, and then increased to 270 °C at 10 °C min−1 and held for 25 min. The sterol and squalene were quantified according to external standards.
20 was used; the temperature of the injection and FID detector was kept at 280 °C and 290 °C, respectively. The oven temperature program was started at 200 °C and held 0.5 min, and then increased to 270 °C at 10 °C min−1 and held for 25 min. The sterol and squalene were quantified according to external standards.
2.8. Phenolic extraction from pumpkin oil samples
Pumpkin seed oil samples were extracted to determine the total phenolic acid content according to the He et al.27 description with slight modifications. Briefly, 0.5 g of pumpkin seed oil was mixed with 0.5 mL of 80% (v/v) methanol aqueous solution and vigorously vortexed for 3 min. Afterwards, the mixture was centrifuged at 3000 rpm for 10 min, and then the methanol aqueous layer was collected. It was repeated three times and all pumpkin seed oil phenolic extracts were pooled, and then were used for total phenolic content (TPC) analysis.2.9. Accelerated oxidation storage test
The oxidative stability was measured according to the Kiralan et al.33 description with slight modifications. Briefly, 10 g of pumpkin seed oil were placed in an oven (Genlab classic oven, Gallenkamp, UK) at 60 °C for 30 days. During the storage time, the peroxide value was measured regularly.2.10. Statistical analysis
All samples were analysed in triplicate. The data were analysed using Minitab (version 20) statistical analysis software. One-way analysis of variance (ANOVA) with Tukey's HSD test was used to compare significant differences (p < 0.05) between samples.3. Results and discussion
3.1. Physicochemical parameters
Table 1 shows the physicochemical parameters of pumpkin seed oil samples obtained from the three extraction methods namely Soxhlet extraction (SE), cold-pressed extraction (CPE), and aqueous enzymatic extraction (AEE). The acid value represents free fatty acid content, which relates to the extent of hydrolysis.34 SE extracted pumpkin seed oil showed the highest acid value (6.5 mg KOH per g), followed by AEE (1.5 mg KOH per g) and CPE (0.6 mg KOH per g). The high acid value in SE extracted oil could be associated with the prolonged extraction process at high temperature; the high temperature during the extraction process could cause the decomposition and oxidation of triacylglycerol to glycerol and free fatty acids, resulting in an increase in the acid value.27 The peroxide value represents the oxidation degree.35 The peroxide value of pumpkin seed oil obtained by SE (24.7 meq. O2 per kg) was significantly higher (p < 0.05) than that of CPE (14.5 meq. O2 per kg) and AEE (6.3 meq. O2 per kg) extracted pumpkin seed oil, indicating that the former had a relatively higher oxidation degree. The higher peroxide value in SE extracted pumpkin seed oil could be explained by the longer time of the extraction process including oil extraction and solvent evaporation, resulting in more oxidation of the oil.16 The iodine value indicates the degree of unsaturation in oil.36 Pumpkin seed oil obtained by SE showed the highest iodine value (135.6 I2/100 g) as compared to pumpkin seed oil obtained by CPE (113.3 I2/100 g) and AEE (112.9 I2/100 g), indicating that SE extracted pumpkin seed oil could have more unsaturated fatty acid bonds. In terms of the saponification value, AEE extracted pumpkin seed oil (184.8 mg KOH per g) had a similar value to CPE (184.0 mg KOH per g), but significantly higher (p < 0.05) than SE (110.7 mg KOH per g). According to Chaiyana et al.,37 a high saponification value in oils indicates their suitability for liquid soaps and shampoo production. It was suggested that pumpkin seed oil obtained by CPE and AEE could be used for soap and personal care applications.| Parameters | SE | CPE | AEE |
|---|---|---|---|
| a Mean ± SD values in the same row with different superscripts indicating the significant difference (p < 0.05). SE – Soxhlet extraction; CPE – cold-pressed extraction; AEE – aqueous enzymatic extraction. | |||
| Acid value (mg KOH per g) | 6.5 ± 0.0a | 0.6 ± 0.2c | 1.5 ± 0.2b |
| Peroxide value (meq. O2 per kg) | 24.7 ± 0.5a | 14.5 ± 0.2b | 6.3 ± 0.6c |
| Iodine value (g I2/100 g) | 135.6 ± 3.5a | 113.3 ± 0.8b | 112.9 ± 4.4b |
| Saponification value (mg KOH per g) | 110.7 ± 0.2b | 184.0 ± 6.5a | 184.8 ± 1.3a |
3.2. Fatty acids
Table 2 shows the fatty acid composition of pumpkin seed oil obtained by the three extraction methods namely Soxhlet extraction (SE), cold-pressed extraction (CPE), and aqueous enzymatic extraction (AEE). Pumpkin seed oil was rich in unsaturated fatty acids, where linoleic acid (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; ranging from 47.9–61.9%) was the predominant fatty acid, followed by oleic acid (C18
2; ranging from 47.9–61.9%) was the predominant fatty acid, followed by oleic acid (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; ranging from 21.0–34.1%). On the other hand, palmitic acid (C16
1; ranging from 21.0–34.1%). On the other hand, palmitic acid (C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0; ranging from 10.0–10.5%) and stearic acid (C18
0; ranging from 10.0–10.5%) and stearic acid (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0; ranging from 5.2–6.0%) were the major saturated fatty acids in pumpkin seed oil. The above results are in accordance with previous studies, indicating that pumpkin seed oil is a good source of linoleic acid.5,38,39 Regarding the extraction method, a significant difference was observed in the fatty acid profile, especially in linoleic acid (C18
0; ranging from 5.2–6.0%) were the major saturated fatty acids in pumpkin seed oil. The above results are in accordance with previous studies, indicating that pumpkin seed oil is a good source of linoleic acid.5,38,39 Regarding the extraction method, a significant difference was observed in the fatty acid profile, especially in linoleic acid (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) content. Pumpkin seed oil obtained by SE showed a higher level of linoleic acid (C18
2) content. Pumpkin seed oil obtained by SE showed a higher level of linoleic acid (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) content as compared to pumpkin seed oil obtained by CPE and AEE, which was consistent with our iodine value results (Table 1). Similar results were reported in the Zhang et al.13 study, who extracted filed muskmelon seed oil using pressed and organic solvent (n-hexane) extraction and found that the extraction method affected the fatty acid profile: the organic solvent extracted oil (linoleic acid 17.36% and oleic acid 62.12%) and the pressed oil (linoleic acid 26.60% and oleic acid 49.60%). Other studies also have reported that extraction methods could influence the fatty acid profile, depending on extraction conditions; this could be explained by the properties or structures of fatty acids, which have different responses to different extraction conditions such as pressure, solvent type, and temperature.13,14,40,41
2) content as compared to pumpkin seed oil obtained by CPE and AEE, which was consistent with our iodine value results (Table 1). Similar results were reported in the Zhang et al.13 study, who extracted filed muskmelon seed oil using pressed and organic solvent (n-hexane) extraction and found that the extraction method affected the fatty acid profile: the organic solvent extracted oil (linoleic acid 17.36% and oleic acid 62.12%) and the pressed oil (linoleic acid 26.60% and oleic acid 49.60%). Other studies also have reported that extraction methods could influence the fatty acid profile, depending on extraction conditions; this could be explained by the properties or structures of fatty acids, which have different responses to different extraction conditions such as pressure, solvent type, and temperature.13,14,40,41
| Fatty acid (%) | SE | CPE | AEE |
|---|---|---|---|
| a Mean ± SD values in the same row with different superscripts indicating the significant difference (p < 0.05). SFA – saturated fatty acid; MUFA – monounsaturated fatty acid; PUFA – polyunsaturated fatty acid; SE – Soxhlet extraction; CPE – cold-pressed extraction; AEE – aqueous enzymatic extraction. | |||
Palmitic acid (C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
10.4 ± 0.0b | 10.5 ± 0.0a | 10.0 ± 0.0c |
Stearic acid (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
5.2 ± 0.0c | 6.0 ± 0.0a | 5.6 ± 0.0b |
Behenic acid (C22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
0.2 ± 0.0a | 0.2 ± 0.0a | 0.1 ± 0.0b |
Tricosylic acid (C23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
0.3 ± 0.0a | 0.2 ± 0.0b | 0.2 ± 0.0b |
Oleic acid (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
21.0 ± 0.0c | 34.1 ± 0.1a | 31.6 ± 0.1b |
Linoleic acid (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
61.9 ± 0.1a | 47.9 ± 0.1c | 51.1 ± 0.1b |
ALA (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 n-3) 3 n-3) |
0.4 ± 0.0a | 0.4 ± 0.0a | 0.4 ± 0.0a |
GLA (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 n-6) 3 n-6) |
0.4 ± 0.0a | 0.1 ± 0.0c | 0.2 ± 0.0b |
| SFA | 16.1 | 16.9 | 15.9 |
| MUFA | 21.0 | 34.1 | 31.6 |
| PUFA | 62.7 | 48.4 | 51.7 |
| Unknown | 0.2 | 0.6 | 0.8 |
3.3. Phytosterols and squalene
Phytosterols and squalene, natural bioactive compounds widely present in oil, have beneficial effects on human health, such as anti-inflammatory, cholesterol-lowering, and cardioprotective effects.42,43Table 3 shows the phytosterols and squalene content in the obtained pumpkin seed oil. The phytosterol and squalene contents were influenced by the oil extraction method. β-sitosterol (147.7–208.2 mg/100 g) was the predominant phytosterol in pumpkin seed oil, which was consistent with the Ryan et al.44 report and indicated that β-sitosterol was an identification marker phytosterol in pumpkin seed oil. Meanwhile, cholesterol, stigmasterol, and campesterol were not detected in this study. In contrast, da Silva & Jorge45 reported that pumpkin seed oil contained a relatively low level of stigmasterol (9.9 mg/100 g) and cholesterol (1.3 mg/100 g). These differences could be attributed to the region, pumpkin variety, climate condition, and detection methods.46 Besides, the order of β-sitosterol content in the obtained pumpkin seed oil from the three extraction methods was SE > AEE > CPE.| Compounds | SE | CPE | AEE |
|---|---|---|---|
| a Mean ± SD values in the same row with different superscripts indicating the significant difference (p < 0.05). Cholesterol, stigmasterol, campesterol, α-tocopherol, and δ-tocopherol were not detected in any of the samples; SE – Soxhlet extraction; CPE – cold-pressed extraction; AEE – aqueous enzymatic extraction; TPC – total phenolic content. | |||
| β-sitosterol (mg/100 g) | 208.2 ± 26.8a | 147.7 ± 6.6b | 171.9 ± 6.2a,b |
| Squalene (mg/100 g) | 253.6 ± 33.6a | 174.4 ± 8.1b | 174.1 ± 6.0b |
| γ-tocopherol (mg/100 g) | 72.3 ± 0.5a | 53.1 ± 2.0b | 56.2 ± 2.4b |
| TPC (mg GAE/100 g) | 4.0 ± 0.1a | 1.0 ± 0.1b | 3.8 ± 0.1a |
In terms of squalene, SE extracted pumpkin seed oil showed the highest squalene content (253.6 mg/100 g), whereas CPE and AEE showed similar values, 174.4 mg/100 g and 174.1 mg/100 g, respectively. Compared with other conventional edible oils in terms of squalene content, pumpkin seed oil (174.1–253.6 mg/100 g) was similar to olive oil (116.8–315.4 mg/100 g), but it has a higher content than corn oil (6.8–33.8 mg/100 g), peanut oil (12.1–127.6 mg/100 g), and rapeseed oil (12.4–43.7 mg/100 g).47,48 It was suggested that pumpkin seed oil could be included in the diet as a good source of squalene.
3.4. Tocopherol and total phenolic content (TPC)
Tocopherol and phenolics are important antioxidants in oil, which contribute to the oxidative stability of oil to maintain oil quality and extend shelf life.4,49Table 3 shows the tocopherol content and total phenolic content (TPC) in the obtained pumpkin seed oil. In terms of tocopherol, the representative of tocopherol in pumpkin seed oil was γ-tocopherol (53.1–72.3 mg/100 g), while α-tocopherol and δ-tocopherol were not detected in this study. A similar result was reported by Rabrenović et al.,6 who investigated tocopherol content in six types of pumpkin seed oils and demonstrated that γ-tocopherol was the dominant tocopherol in pumpkin seed oil. In contrast, Fedko et al.50 detected and reported that the pumpkin seed oil contained a low level of α-tocopherol (0.3–7.3 mg/100 g) and δ-tocopherol (0–1.1 mg/100 g). These differences could be attributed to varying growing conditions (e.g. climate and soil) and harvest conditions as well as detection methods. Among the three extraction methods, the γ-tocopherol content in SE extracted pumpkin seed oil (72.3 mg/100 g) was significantly higher (p < 0.05) than that in AEE (56.2 mg/100 g) and CPE (53.1 mg/100 g), whereas there was no significant difference (p > 0.05) between those of AEE and CPE. It was indicated that the tocopherol content was affected by the extraction method. In terms of total phenolic content (TPC), a similar trend to tocopherol was observed; SE (4.0 mg GAE/100 g) and AEE (3.8 mg GAE/100 g) extracted pumpkin seed oil had similar content (p > 0.05), but significantly higher content (p < 0.05) than CPE (1.0 mg GAE/100 g).3.5. Oxidative stability
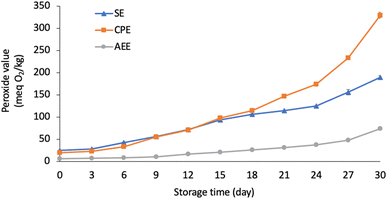
The oxidative stability of pumpkin seed oil obtained by SE, CPE, and AEE was assessed based on peroxide value change under accelerated oxidation conditions at 60 °C (Fig. 1). CPE extracted pumpkin seed oil exhibited a higher peroxide value than SE and AEE at the end of the storage period (day 30), indicating that CPE extracted pumpkin seed oil had the lowest oxidative stability of oil among the three extraction methods. This could be attributed to its lower tocopherol and phenolic content in CPE extracted pumpkin seed oil (Table 3). Many studies have reported that antioxidants play an important role in the oxidative stability of oil, which has a positive effect on preventing the oil oxidation.51,52 In contrast, although SE extracted pumpkin seed oil contained higher tocopherol content than AEE (Table 3), the former had a higher peroxide value than the latter. This could be attributed to the differences in their oxidation rates. According to Mat Yusoff et al.,52 the peroxide value of oil products ranging from 5 (meq. O2 per kg) to 10 (meq. O2 per kg) is considered as having a moderate oxidation rate, while the peroxide value over than 10 (meq. O2 per kg) is considered as having a high oxidation rate; as mentioned earlier (Table 1), the peroxide value of SE and AEE extracted pumpkin oil before the storage test was 24.7 (meq. O2 per kg) and 6.3 (meq. O2 per kg), respectively.Additionally, change in unsaturated fatty acid (UFA) content is also related to oil quality and oxidative stability.26Table 4 shows the fatty acid change between day 0 and day 30. After 30 days of storage at 60 °C, the relative saturated fatty acid content (SFA) increased in all oil samples, while the relative polyunsaturated fatty acid (PUFA) and/or monounsaturated fatty acid (MUFA) content decreased. Other studies have reported similar results after storage tests; the decrease in UFA (including PUFA and MUFA) content and the increase in SFA content could be associated with oil oxidation.53,54 During the oil oxidation process, the PUFA is gradually oxidised to MUFA and subsequently to SFA.26,53 In contrast, CPE extracted pumpkin seed oil had a higher PUFA loss rate than that extracted by SE and AEE, indicating that the former may be more oxidised. Taking together, it was indicated that AEE and SE had relatively higher oxidative stability than CPE, with AEE performing the best.
| Fatty acid (%) | Day 0 | Day 30 |
|---|---|---|
| a SFA – saturated fatty acid; MUFA – monounsaturated fatty acid; PUFA – polyunsaturated fatty acid; UFA – unsaturated fatty acid. | ||
| (a) Soxhlet extraction method (SE) | ||
| SFA | 16.1 | 17.7 |
| MUFA | 21.0 | 18.8 |
| PUFA | 62.7 | 62.6 |
| UFA | 83.7 | 81.4 |
| Unknown | 0.2 | 0.9 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| (b) Cold-pressed extraction method (CPE) | ||
| SFA | 16.8 | 17.8 |
| MUFA | 34.1 | 36.2 |
| PUFA | 48.4 | 45.0 |
| UFA | 82.5 | 81.2 |
| Unknown | 0.6 | 1.0 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| (c) Aqueous enzymatic extraction method (AEE) | ||
| SFA | 15.9 | 17.3 |
| MUFA | 31.6 | 31.3 |
| PUFA | 51.7 | 51.1 |
| UFA | 83.3 | 82.4 |
| Unknown | 0.8 | 0.4 |
5. Conclusions
Pumpkin seed oil is a rich source of linoleic acid, squalene, and tocopherols, suggesting that it has a high potential value as a functional ingredient in food, cosmetics, and pharmaceuticals. The extraction method altered the quality and oxidative stability of pumpkin seed oil. Pumpkin seed oil obtained from SE showed a higher content of bioactive compounds than AEE and CPE. In terms of oil oxidative stability, AEE and SE exhibited better oxidative stability than CPE, whereas AEE performed the best. Overall, AEE is a promising extraction method for producing high quality pumpkin seed oil. This study could provide comparable data to help the oil industry to obtain high quality pumpkin seed oil with appropriate extraction methods. Future work will be conducted on the flavour, acceptability, and bioavailability in vitro to offer a pumpkin seed oil with desirable quality.Author contributions
GZ: conceptualization, methodology, investigation, resources, writing – original draft, and writing – review & editing. ZL: methodology, investigation, writing – original draft, and writing – review & editing. MF: methodology, investigation, writing – original draft, and writing – review & editing.Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.References
- Z. Hu, C. Hu, Y. Li, Q. Jiang, Q. Li and C. Fang, Pumpkin seed oil: a comprehensive review of extraction methods, nutritional constituents, and health benefits, J. Sci. Food Agric., 2024, 104, 572–582 CrossRef CAS PubMed.
- A. Nawirska-Olszańska, A. Kita, A. Biesiada, A. Sokół-Łętowska and A. Z. Kucharska, Characteristics of antioxidant activity and composition of pumpkin seed oils in 12 cultivars, Food Chem., 2013, 139, 155–161 CrossRef PubMed.
- D. Ogrodowska, M. Tańska and W. Brandt, The Influence of Drying Process Conditions on the Physical Properties, Bioactive Compounds and Stability of Encapsulated Pumpkin Seed Oil, Food Bioprocess Technol., 2017, 10, 1265–1280 CrossRef CAS.
- T. Potočnik, M. Rak Cizej and I. J. Košir, Influence of seed roasting on pumpkin seed oil tocopherols, phenolics and antiradical activity, J. Food Compos. Anal., 2018, 69, 7–12 CrossRef.
- G. Procida, B. Stancher, F. Cateni and M. Zacchigna, Chemical composition and functional characterisation of commercial pumpkin seed oil, J. Sci. Food Agric., 2013, 93, 1035–1041 CrossRef CAS.
- B. B. Rabrenović, E. B. Dimić, M. M. Novaković, V. V. Tešević and Z. N. Basić, The most important bioactive components of cold pressed oil from different pumpkin (Cucurbita pepo L.) seeds, LWT – Food Sci. Technol., 2014, 55, 521–527 CrossRef.
- D. Šamec, M. R. Loizzo, O. Gortzi, İ. T. Çankaya, R. Tundis and İ. Suntar, et al., The potential of pumpkin seed oil as a functional food—A comprehensive review of chemical composition, health benefits, and safety, Compr. Rev. Food Sci. Food Saf., 2022, 21, 4422–4446 CrossRef PubMed.
- M. Nishimura, T. Ohkawara, H. Sato, H. Takeda and J. Nishihira, Pumpkin seed oil extracted from Cucurbita maxima improves urinary disorder in human overactive bladder, J. Tradit. Complementary Med., 2014, 4, 72–74 CrossRef PubMed.
- S. Y. Al-Okbi, D. A. Mohamed, E. Kandil, M. A. Abo-Zeid, S. E. Mohammed and E. K. Ahmed, Anti-inflammatory activity of two varieties of pumpkin seed oil in an adjuvant arthritis model in rats, Grasas Aceites, 2017, 68, e180 CrossRef.
- M. C. Morrison, P. Mulder, P. M. Stavro, M. Suárez, A. Arola-Arnal and W. van Duyvenvoorde, et al., Replacement of dietary saturated fat by PUFA-rich pumpkin seed oil attenuates non-alcoholic fatty liver disease and atherosclerosis development, with additional health effects of virgin over refined oil, PLoS One, 2015, 10, e0139196 CrossRef PubMed.
- G. Dąbrowski, S. Czaplicki and I. Konopka, Composition and quality of poppy (Papaver somniferum L.) seed oil depending on the extraction method, LWT – Food Sci. Technol., 2020, 134, 110167 CrossRef.
- R. Y. Khattab and M. A. Zeitoun, Quality evaluation of flaxseed oil obtained by different extraction techniques, LWT – Food Sci. Technol., 2013, 53, 338–345 CrossRef CAS.
- H. Zhang, Y. Yuan, X. Zhu, R. Xu, H. Shen and Q. Zhang, et al., The Effect of Different Extraction Methods on Extraction Yield, Physicochemical Properties, and Volatile Compounds from Field Muskmelon Seed Oil, Foods, 2022, 11, 721 CrossRef CAS PubMed.
- L. B. Gu, G. J. Zhang, L. Du, J. Du, K. Qi and X. L. Zhu, et al., Comparative study on the extraction of Xanthoceras sorbifolia Bunge (yellow horn)seed oil using subcritical n-butane, supercritical CO2, and the Soxhlet method, LWT – Food Sci. Technol., 2019, 111, 548–554 CrossRef CAS.
- G. Melgar-Lalanne, A. J. Hernández-Álvarez, M. Jiménez-Fernández and E. Azuara, Oleoresins from Capsicum spp.: Extraction Methods and Bioactivity, Food Bioprocess Technol., 2017, 10, 51–76 CrossRef CAS.
- C. T. Dursun, T. Dedebas, H. Yalcin and L. Ekici, Extraction method affects seed oil yield, composition, and antioxidant properties of European cranberrybush (Viburnum opulus), Ind. Crops Prod., 2021, 168, 113632 CrossRef.
- D. Xu, J. Hao, Z. Wang, D. Liang, J. Wang and Y. Ma, et al., Physicochemical properties, fatty acid compositions, bioactive compounds, antioxidant activity and thermal behavior of rice bran oil obtained with aqueous enzymatic extraction, LWT – Food Sci. Technol., 2021, 149, 111817 CrossRef CAS.
- A. Puşcaş, A. Mureşan, S. Socaci, F. Dulf, S. Muste and F. Fetea, et al., Cold pressed pumpkin seed oil fatty acids, carotenoids, volatile compounds profiles and infrared fingerprints as affected by storage time and wax-based oleogelation, J. Sci. Food Agric., 2023, 103, 680–691 CrossRef PubMed.
- M. Grajzer, K. Szmalcel, Ł. Kuźmiński, M. Witkowski, A. Kulma and A. Prescha, Characteristics and Antioxidant Potential of Cold-Pressed Oils—Possible Strategies to Improve Oil Stability, Foods, 2020, 9, 1630 CrossRef CAS PubMed.
- S. Siabi, M. Torbati, S. Azadmard-Damirchi, M. Naebi and G. P. Savage, Extraction of Oil from Roman Nettle Seed by Cold Press and Evaluation of its Quality during Storage, Eur. J. Lipid Sci. Technol., 2023, 125, 2200071 CrossRef CAS.
- H. C. Nguyen, D. P. Vuong, N. T. T. Nguyen, N. P. Nguyen, C. H. Su and F. M. Wang, et al., Aqueous enzymatic extraction of polyunsaturated fatty acid–rich sacha inchi (Plukenetia volubilis L.) seed oil: An eco-friendly approach, LWT – Food Sci. Technol., 2020, 133, 109992 CrossRef CAS.
- M. Mat Yusoff, M. H. Gordon, O. Ezeh and K. Niranjan, Aqueous enzymatic extraction of Moringa oleifera oil, Food Chem., 2016, 211, 400–408 CrossRef CAS PubMed.
- H. Shakeela, K. Mohan and P. Nisha, Unlocking a nutritional treasure: health benefits and sustainable applications of spent coconut meal, Sustainable Food Technol., 2024 10.1039/D3FB00247K.
- A. Prommaban, R. Kuanchoom, N. Seepuan and W. Chaiyana, Evaluation of fatty acid compositions, antioxidant, and phar-macological activities of pumpkin (Cucurbita moschata) seed oil from aqueous enzymatic extraction, Plants, 2021, 10, 1582 CrossRef CAS PubMed.
- R. I. Bettaieb, S. Bourgou, P. Detry, W. A. Wannes, T. Kenny and R. Ksouri, et al., Green Extraction of Fennel and Anise Edible Oils Using Bio-Based Solvent and Supercritical Fluid: Assessment of Chemical Composition, Antioxidant Property, and Oxidative Stability, Food Bioprocess Technol., 2019, 12, 1798–1807 CrossRef.
- Y. Huang, X. Xiang, X. Luo, X. Li, X. Yu and S. Li, Study on the emulsification and oxidative stability of ovalbumin-pectin-pumpkin seed oil emulsions using ovalbumin solution prepared by ultrasound, Ultrason. Sonochem., 2021, 78, 105717 CrossRef CAS PubMed.
- Z. He, H. Zhu, W. Li, M. Zeng, S. Wu and S. Chen, et al., Chemical components of cold pressed kernel oils from different Torreya grandis cultivars, Food Chem., 2016, 209, 196–202 CrossRef CAS PubMed.
- AOAC, Official Methods of Analysis of AOAC International, Association of Official Analysis Chemists International, 2005 Search PubMed.
- M. C. Milinsk, M. Matsushita, J. V. Visentainer, C. C. de Oliveira and N. E. de Souza, Comparative analysis of eight esterification methods in the quantitative determination of vegetable oil fatty acid methyl esters (FAME), J. Braz. Chem. Soc., 2008, 19, 1475–1483 CrossRef CAS.
- I. Martakos, M. Kostakis, M. Dasenaki, M. Pentogennis and N. Thomaidis, Simultaneous determination of pigments, tocopherols, and squalene in Greek olive oils: A study of the influence of cultivation and oil-production parameters, Foods, 2020, 9, 31 CrossRef CAS PubMed.
- R. Liu, X. Guo, M. Cheng, L. Zheng, M. Gong and M. Chang, et al., Effects of chemical refinement on the quality of coconut oil, J. Food Sci. Technol., 2019, 56, 3109–3116 CrossRef CAS.
- S. Dudonné, X. Vitrac, P. Coutiére, M. Woillez and J. M. Mérillon, Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays, J. Agric. Food Chem., 2009, 57, 1768–1774 CrossRef PubMed.
- M. Kiralan, G. Özkan, A. Bayrak and M. F. Ramadan, Physicochemical properties and stability of black cumin (Nigella sativa) seed oil as affected by different extraction methods, Ind. Crops Prod., 2014, 57, 52–58 CrossRef CAS.
- S. Zhang, Y. G. Pan, L. Zheng, Y. Yang, X. Zheng and B. Ai, et al., Application of steam explosion in oil extraction of camellia seed (Camellia oleifera Abel.) and evaluation of its physicochemical properties, fatty acid, and antioxidant activities, Food Sci. Nutr., 2019, 7, 1004–1016 CrossRef CAS PubMed.
- H. Wu, J. Shi, S. Xue, Y. Kakuda, D. Wang and Y. Jiang, et al., Essential oil extracted from peach (Prunus persica) kernel and its physicochemical and antioxidant properties, LWT – Food Sci. Technol., 2011, 44, 2032–2039 CrossRef CAS.
- N. Liu, G. Ren, M. Faiza, D. Li, J. Cui and K. Zhang, et al., Comparison of conventional and green extraction methods on oil yield, physicochemical properties, and lipid compositions of pomegranate seed oil, J. Food Compos. Anal., 2022, 114, 104747 CrossRef CAS.
- W. Chaiyana, P. Leelapornpisid, R. Phongpradist and K. Kiattisin, Enhancement of antioxidant and skin moisturizing effects of olive oil by incorporation into microemulsions, Nanomater. Nanotechnol., 2016, 6, 1847980416669488 Search PubMed.
- F. Siano, M. C. Straccia, M. Paolucci, G. Fasulo, F. Boscaino and M. G. Volpe, Physico-chemical properties and fatty acid composition of pomegranate, cherry and pumpkin seed oils, J. Sci. Food Agric., 2016, 96, 1730–1735 CrossRef CAS PubMed.
- V. van Hoed, K. A. Sampaio, B. Felkner, F. Bavec, M. L. Scippo and F. Brose, et al., Tocopherols and Polyphenols in Pumpkin Seed Oil Are Moderately Affected by Industrially Relevant Roasting Conditions, Eur. J. Lipid Sci. Technol., 2017, 119, 1700110 CrossRef.
- P. Shao, Q. Liu, Z. Fang and P. Sun, Chemical composition, thermal stability and antioxidant properties of tea seed oils obtained by different extraction methods: Supercritical fluid extraction yields the best oil quality, Eur. J. Lipid Sci. Technol., 2015, 117, 355–365 CrossRef CAS.
- A. Vilas-Franquesa, B. Juan and J. Saldo, Targeted analysis of sea buckthorn oil extracted by accelerated solvent extraction technique using green and conventional solvents, LWT – Food Sci. Technol., 2022, 164, 113643 CrossRef CAS.
- N. I. Ibrahim and I. N. Mohamed, Interdependence of anti-inflammatory and antioxidant properties of squalene–implication for cardiovascular health, Life, 2021, 103 CrossRef CAS PubMed.
- B. Miras-Moreno, A. B. Sabater-Jara, M. A. Pedreno and L. Almagro, Bioactivity of Phytosterols and Their Production in Plant in Vitro Cultures, J. Agric. Food Chem., 2016, 7049–7058 CrossRef CAS PubMed.
- E. Ryan, K. Galvin, T. P. O'Connor, A. R. Maguire and N. M. O'Brien, Phytosterol, Squalene, Tocopherol Content and Fatty Acid Profile of Selected Seeds, Grains, and Legumes, Plant Foods Hum. Nutr., 2007, 62, 85–91 CrossRef CAS PubMed.
- A. C. da Silva and N. Jorge, Bioactive compounds of the lipid fractions of agro-industrial waste, Food Res. Int., 2014, 66, 493–500 CrossRef CAS.
- C. A. Can-Cauich, E. Sauri-Duch, V. M. Moo-Huchin, D. Betancur-Ancona and L. F. Cuevas-Glory, Effect of extraction method and specie on the content of bioactive compounds and antioxidant activity of pumpkin oil from Yucatan, Mexico, Food Chem., 2019, 285, 186–193 CrossRef CAS PubMed.
- M. Shen, S. Zhao, F. Zhang, M. Huang and J. Xie, Characterization and authentication of olive, camellia and other vegetable oils by combination of chromatographic and chemometric techniques: role of fatty acids, tocopherols, sterols and squalene, Eur. Food Res. Technol., 2021, 247, 411–426 CrossRef CAS.
- C. I. G. Tuberoso, A. Kowalczyk, E. Sarritzu and P. Cabras, Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use, Food Chem., 2007, 103, 1494–1501 CrossRef CAS.
- G. C. G. Carlini, G. G. Roschel, R. A. Ferrari, S. M. Alencar, H. C. Ota and T. F. F. da Silveira, et al., Chemical characterization of Echium plantagineum seed oil obtained by three methods of extraction, J. Food Sci., 2021, 86, 5307–5317 CrossRef CAS PubMed.
- M. Fedko, D. Kmiecik, A. Siger, B. Kulczyński, M. Przeor and J. Kobus-Cisowska, Comparative characteristics of oil composition in seeds of 31 Cucurbita varieties, J. Food Meas. Char., 2020, 14, 894–904 CrossRef.
- N. Mikołajczak, M. Tańska and I. Konopka, Impact of the addition of 4-vinyl-derivatives of ferulic and sinapic acids on retention of fatty acids and terpenoids in cold-pressed rapeseed and flaxseed oils during the induction period of oxidation, Food Chem., 2019, 278, 119–126 CrossRef PubMed.
- M. Mat Yusoff, K. Niranjan, O. A. Mason and M. H. Gordon, Oxidative properties of Moringa oleifera kernel oil from different extraction methods during storage, J. Sci. Food Agric., 2020, 100, 1588–1597 CrossRef CAS PubMed.
- Z. Zhang, L. Zhang, Q. Xie and L. Che, Effect of Accelerated Storage on Fatty Acids, Thermal Properties and Bioactive Compounds of Kenaf Seed Oil, J. Food Sci., 2019, 84, 2121–2127 CrossRef CAS PubMed.
- R.-Y. Zhang, A.-B. Liu, C. Liu, W.-X. Zhu, P.-X. Chen and J.-Z. Wu, et al., Effects of different extraction methods on the physicochemical properties and storage stability of tiger nut (Cyperus esculentus L.) oil, LWT – Food Sci. Technol., 2023, 173, 114259 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |