Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Potential of vacuum impregnation and osmotic dehydration techniques in producing jaggery-fortified apple snacks
Cristina
Barrera
,
Noelia
Betoret
and
Lucía
Seguí
 *
*
Instituto de Ingeniería de Alimentos Food UPV, Universitat Politècnica de Valencia, Camino de Vera s/n, Valencia, 46022, Spain. E-mail: lusegil@upvnet.upv.es
First published on 27th May 2024
Abstract
Fruits are nutrient-rich, highly perishable goods which contribute to postharvest losses and waste. The food industry continues the search for processing methods that allow for the manufacturing of attractive and convenient fortified fruits while extending their shelf life. To meet the present consumer demands for more nutritious and sustainable food products, innovative or revisited food processing techniques need to be explored. In the present work, jaggery is proposed as a non-conventional osmotic agent to produce fortified apple snacks through the combination of vacuum impregnation (VI) and osmotic dehydration (OD) techniques and further stabilization via convective hot air-drying (HAD) or freeze drying (FD). Physicochemical and antioxidant attributes of intermediate and final products were analyzed to evaluate the potential of these techniques to introduce jaggery bioactive constituents in the apple matrix. The results confirmed that the antioxidant properties of jaggery may be incorporated into the tissue by both VI and OD, especially with progressive OD (pOD) in solutions from 30 to 50 Brix degrees. Stabilization through HAD at 60 °C significantly enhanced the antioxidant properties of jaggery-enriched snacks (total phenols: 11.0 ± 0.6 (pOD HAD) and 8.0 ± 0.6 (VI HAD) vs. 6.3 ± 0.12 (HAD) mg GAE per g dry product), whereas FD maintained natural and incorporated antioxidants (total phenols: 10.8 ± 0.4 (pOD FD) and 6.2 ± 0.9 (VI FD) vs. 6.5 ± 0.2 (FD) mg GAE per g dry product). Optical and textural properties were affected by the addition of jaggery and processing techniques. Replacing intercellular air with liquid reduced luminosity, which increased after dehydration, especially through FD. In conclusion, jaggery or non-centrifugal cane sugar is proposed as a healthier osmotic agent to produce more nutritious and sustainable apple snacks by applying matrix engineering techniques such as vacuum impregnation and osmotic dehydration, followed by hot air-drying or freeze-drying stabilization.
Sustainability spotlightTo reduce postharvest losses and waste, the food industry needs to develop sustainable processing methods that allow for the extension of fruit shelf life while manufacturing attractive and convenient nutrient-rich products. A combination of matrix engineering and drying techniques is proposed to produce jaggery-fortified apple snacks. This study addresses Sustainable Development Goal 2 (SDG2: end hunger, achieve food security and improved nutrition and promote sustainable agriculture) by producing fortified apple snacks from perishable fruit, thus reducing food waste, and promoting access to nutrient-dense convenient food. In addition to the mitigation of fruit waste through fruit snack manufacturing, the use of vacuum impregnation and osmotic dehydration techniques reduces the energy requirements of drying processes, thus contributing to more sustainable processes addressing Sustainable Development Goal 12 (SDG12: ensure sustainable consumption and production patterns). |
Introduction
Fruits contain nutrients and bioactive compounds, making them an important part of the human diet. However, they are highly perishable goods and lead to postharvest losses and waste, thus resulting in serious environmental problems1 and nutritional losses.2 Both fresh and processed fruits are considered good preferences from a nutritional point of view. Therefore, the food industry continues the search for processing methods that allow for the manufacturing of attractive and convenient fortified fruits while extending their shelf life.3 In this context, innovative or revisited food processing techniques have gained importance to offer alternatives to meet present consumer demands for more sustainable and healthy diets, as proposed by the FAO.4Osmotic dehydration (OD) and vacuum impregnation (VI) are used to improve the nutritional, sensory and functional values of food products, particularly when used as a pretreatment in preserving operations, such as drying or freezing.5 Among dehydration methods, the combination of osmosis and drying has proven to be an efficient strategy for improving the quality of fruits and vegetables.6 In addition, OD and VI might be used to intentionally introduce bioactive compounds that are not naturally present in the product into the food matrix. Although OD involves a two-way mass transfer process that couples dehydration with an inflow of solutes and other desired nutritional substances into the plant material,3 VI is a process of fluid replacement in porous materials via vacuum pressure and subsequent atmospheric pressure reestablishment, which allows for the direct introduction of solutes and other desired compounds into the porous structure of foodstuffs, thereby enhancing mass transfer rates through hydrodynamic transport.5,7 The ability to control these processes and introduce desired nutritional substances into plant material presents remarkable opportunities for the development of sustainable functional foods.7,8 From a sustainable perspective, OD and VI are non-thermal treatments and are considered low-energy demanding operations. In addition, combined with further drying or freezing, they are known to reduce energy consumption.8,9
A combination of matrix engineering technologies, such as OD and VI, with drying operations is proposed to produce appealing and nutritious functional fruit snacks. This combination not only enhances the flavor and texture of the product but also retains essential nutrients, transforming fruits into convenient on-the-go foods without compromising or ideally increasing their nutritional value. For example, it has been used to produce calcium-rich apple and pineapple snacks and juice-enriched apple snacks, or to introduce and stabilize probiotic microorganisms in the food matrix.10–13 However, jaggery or non-centrifugal sugar is an unconventional natural sweetener that used to be the most common form of sugarcane product before the advent of refined sugar.9 It is still commonly consumed across the tropics and subtropics, where it is produced and used as part of traditional medicine.14 In contrast to refined sugar, jaggery contains several bioactive compounds that make it interesting for functional food development. Apart from being richer in vitamins and minerals, non-refined sugarcane products are particularly rich in phenolic constituents, especially flavonoids, and exhibit antioxidant and antimicrobial effects.14–17 As reported in various studies, various health promoting effects are attributed to sugarcane extracts, including antiproliferative properties against cancer cell lines, stimulation and regulation of the immune system, protection against hepatic damage, recovery of intestinal function, anti-thrombotic and anti-stress properties, protective role against DNA damage, growth stimulation, or prevention from hypertension and diabetes disorders.
Jaggery consists of sugarcane juice concentrated by evaporation. Other fruit or vegetable juices have been proposed as vacuum impregnation solutions to produce functional foods.18–20 In contrast to other fruit juices, jaggery contains sugar mostly in the form of sucrose, and it is presented as a dehydrated powdered product in the case of granulated jaggery.14 This makes granulated jaggery an interesting alternative for the formulation of osmotic solutions. Osmotic solutions to be used in OD and VI processes could benefit from jaggery's healthier properties. However, the use of jaggery as an osmotic agent in VI and OD operations has rarely been exploited in the literature.21 In the present work, granulated jaggery is proposed as an osmotic agent to produce fortified apple snacks by VI and OD with further stabilization by convective hot air-drying (HAD) or freeze-drying (FD). The physicochemical and antioxidant attributes of intermediate and final products are analyzed to evaluate the potential of these techniques to produce more nutritious and sustainable apple snacks.
Materials and methods
Raw materials
Apples (Malus domestica cv. Granny Smith) used in the present study were purchased from a local market in Valencia, Spain. This variety was chosen owing to its high homogeneity and porosity, which present excellent attributes for VI. The apples were washed with tap water and cut into 5 mm thick slices, which were then transformed into rings with internal and external diameters of 20 mm and 65 mm, respectively. Granulated jaggery and white sugar used to prepare the osmotic solutions were obtained from a local market.Snack manufacturing techniques
VI and OD were used alone or combined to obtain intermediate products that were further stabilized by HAD or FD. Details of the conditions of the operations used and the properties analyzed in each case are presented in the subsequent sections. Intermediate VI and OD products were compared to the fresh apple, whereas the controls for dehydrated snacks consisted of HAD and FD samples with no pretreatment.Vacuum impregnation (VI)
VI was carried out by soaking the apple rings in isotonic impregnation solutions (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 mass ratio) and introducing them into a vacuum chamber (Heraeus Vacuum Oven, Thermo Fisher Scientific Inc.) connected to a vacuum pump (Gardner Denver Thomas GmbH Welch Vacuum, Fürstenfeldbruck, Germany), where a pressure of 50 mbar was applied for 10 min. After that, atmospheric pressure was restored, and the samples were soaked in the solutions for 10 more min.
4 mass ratio) and introducing them into a vacuum chamber (Heraeus Vacuum Oven, Thermo Fisher Scientific Inc.) connected to a vacuum pump (Gardner Denver Thomas GmbH Welch Vacuum, Fürstenfeldbruck, Germany), where a pressure of 50 mbar was applied for 10 min. After that, atmospheric pressure was restored, and the samples were soaked in the solutions for 10 more min.
Impregnation solutions consisted of isotonic solutions (∼250 g sugar per L) prepared with combinations of white sugar and jaggery using the following jaggery percentages with respect to total sugar added: 0, 25, 50 and 100% (Table 1). The impregnation properties of the solutions in the apple tissue were evaluated using VI parameters, the procedure, equipment and equations defined by Salvatori et al. (1998).22 Hence, the following parameters were obtained: X1, volumetric fraction occupied by the impregnation liquid after the vacuum step (m3 solution per m3 fresh tissue); X, volumetric fraction occupied by the impregnation liquid after the atmospheric pressure step (m3 solution per m3 fresh tissue); γ1: relative volumetric deformation after the vacuum step; and γ: relative volumetric deformation after the atmospheric pressure step. Experiments were performed at room temperature.
| White sugar (g) | Jaggery (g) | |
|---|---|---|
| 0% GJ | 250 | 0 |
| 25% GJ | 187.5 | 62.5 |
| 50% GJ | 125 | 125 |
| 100% GJ | 0 | 250 |
Osmotic dehydration (OD)
OD was used as an alternative or complementary technique to incorporate the bioactive compounds of interest contained in jaggery into the apple structure. For OD experiments, 100% jaggery solutions were used in all cases, and different dehydration strategies were examined: one step OD in a 50 Brix degrees jaggery solution during 3 h (OD), or progressive OD in a 30, 40 and 50 Brix degrees jaggery solution (pOD), maintaining the samples in each solution for 1 h. Experiments were conducted at room temperature.Convective drying (HAD) and freeze drying (FD)
Convective drying (hot air drying, HAD) was carried out in a CLW 750 TOP+ pilot plant tray dryer (Pol-Eko-Aparatura SPJ, Vladislava, Poland) with cross flow air at 2 m s−1 and 60 °C for 24 h to reduce water activity to values that guarantee snack stability.23 Freeze-drying (FD) was performed by freezing samples at −40 °C for 24 h in a CVN-40/105 Matek freezer and further sublimating at −45 °C (condenser temperature) and 0.1 mbar for 24 h in a 6–80 Telstar Lioalfa pilot plant scale freeze-dryer.Analytical determinations
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 methanol
20 methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solution used as the solvent. Extraction was conducted by stirring the mixture for 1 h and subsequently centrifuging it at 10
water solution used as the solvent. Extraction was conducted by stirring the mixture for 1 h and subsequently centrifuging it at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min in an Eppendorf centrifuge 5804/5804R (Eppendorf SE, Hamburg, Germany) to obtain a clear supernatant, which was used for further measurements.
000 rpm for 5 min in an Eppendorf centrifuge 5804/5804R (Eppendorf SE, Hamburg, Germany) to obtain a clear supernatant, which was used for further measurements.
The total phenolic content was measured using the spectrophotometric method of the Folin–Ciocalteu reagent.25 An aliquot of 0.125 mL of the extract was mixed with 0.5 mL of distilled water and 0.125 of the Folin–Ciocalteau reagent (Sigma Aldrich). The mixture was allowed to react for 7 min in the dark before adding 1.25 mL of a 7% sodium carbonate solution to stop the reaction and 1 mL of distilled water. Absorbance was measured at 760 nm using a spectrophotometer (Helios Zeta UV/vis, Thermo Scientific, UK) after 90 min in the dark. The results were presented in mg of Gallic Acid Equivalents (GAE) per g of fresh or dried sample. The total flavonoid content was obtained using the colorimetric aluminum chloride method described by Luximon-Ramma (2002),26 which consisted of vigorously mixing 1.5 mL of the extract with 1.5 mL of aluminum chloride solution (2% w/v in methanol) and measuring the absorbance at 368 nm using apigenin as the standard (purity ≥ 95%, Sigma-Aldrich). Results were given in mg of apigenin equivalents (AE) per gram of fresh or dried sample.
Antiradical DPPH (1,1-diphenyl-2-picryl hydrazyl, DPPH-) activity assay was based on the method developed by Brand-Williams et al. (1995)27 and consisted of adding 0.05 mL of the extract to 2.95 mL of a 0.06 mM DPPH-methanol solution. The ability to scavenge the ABTS+ cation (2,20-azobis-3-ethyl benzothiazoline-6-sulphonic acid) was measured, as described by Re et al. (1999).28 The radical ABTS+ was released by reacting 7 mM of ABTS with potassium persulfate (2.45 mM) for 16 h at room temperature and in the dark. ABTS+ was mixed with phosphate buffer (pH 7.4) to reach an absorbance of 0.70 ± 0.02 at 734 nm. An aliquot of 100 μL of the sample was added to 2900 μL of the solution ABTS+ in phosphate buffer, and the absorbance of the samples was read after 7 min. Antiradical activities were both expressed in mg of Trolox Equivalent (TE) per gram of fresh or dried sample.
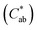 and hue angle
and hue angle 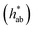 were obtained from the CIE L*a*b* colour coordinates by applying the following equations
were obtained from the CIE L*a*b* colour coordinates by applying the following equations 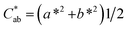 and
and 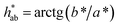 , respectively. Colour differences were calculated using the following equation: ΔE* = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2.
, respectively. Colour differences were calculated using the following equation: ΔE* = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2.
Results
Properties of impregnation solutions
The characterization of the impregnation solutions is summarized in Table 2. As observed, replacing white sugar with jaggery did not significantly affect the general physicochemical attributes. Water activities were similar for the four solutions formulated, which were isotonic with the apples used in the study (aw = 0.989). This implies that the only mechanisms involved in mass transfer during VI are pressure gradients. Other physicochemical parameters, such as Brix degrees, apparent density (ρ), and viscosity (μ), were also similar among the four impregnation solutions prepared. All impregnation liquids showed a Newtonian behaviour and exhibited viscosity values slightly higher than pure water, indicating a certain ease of being impregnated in the cellular tissue. It has been reported that low viscosity values (smaller than 3–3.5 mPa s) favor liquid impregnation in contrast to tissue deformation and further relaxation.31 However, impregnation parameters exhibited certain differences depending on the amount of jaggery participating in the solution. The main differences were found in parameter X, i.e. the volumetric fraction of the impregnation solution that is introduced in the apple matrix at the end of the atmospheric step. The results indicated poorer impregnation properties of solutions containing jaggery. This could be explained by the presence of other compounds different from sucrose, especially insoluble ones, which might accumulate on the surface of the sample and obstruct open pores, thus hindering liquid inflow. However, because both X and X1 presented positive values, the impregnation liquid entered during both the vacuum (X1 > 0) and the atmospheric (X > 0) stages. As for volumetric deformation, the positive values during the vacuum step (γ1 > 0) indicate expansion, whereas the negative values at the end of the atmospheric one (γ1 < 0) suggest a certain contraction of the apple tissue. In any case, no significant differences among the samples were obtained for the deformation parameters. To the best of our knowledge, this is the first time that the VI properties of jaggery-formulated solutions have been reported. Nevertheless, the response of Granny Smith apples to VI has been previously studied for other impregnation solutions. The impregnation parameters of apple cylinders (20 mm diameter × 20 mm height) with sucrose isotonic solutions have been reported as X1 = −0.042 ± 0.003, γ1 = 1.7 ± 0.3, X = 0.19 ± 0.015 and γ = −0.6 ± 1.2,32 whereas the impregnation parameters for apple slices (5 mm thick) immersed in clementine juice enriched with Lactobacillus salivarius were X1 = 0.150 ± 0.010, γ1 = 0.091 ± 0.003, X = 0.19 ± 0.02 and γ = 0.012 ± 0.007.33| 0% GJ | 25% GJ | 50% GJ | 100% GJ | |
|---|---|---|---|---|
| a a, b, c, d different letters in the same row indicate statistically significant differences at the 95% level (p-value ≤ 0.05). | ||||
| a w | 0.986 (0.003)b | 0.984 (0.003)a | 0.985 (0.003)b | 0.985 (0.003)ab |
| Brix | 24.81 (0.12)b | 25.83 (0.12)c | 24.70 (0.10)b | 24.47 (0.06)a |
| ρ (g cm−3) | 1.10157 (0.00004)c | 1.1065 (0.00010)d | 1.10103 (0.00002)b | 1.1002 (0.0002)a |
| μ (mPa s) | 2.57 (0.06)a | 2.57 (0.06)a | 2.57 (0.06)a | 2.57 (0.06)a |
| X | 0.26 (0.02)b | 0.18 (0.02)a | 0.192 (0.0014)a | 0.159 (0.004)a |
| X 1 | 0.04 (0.011)a | 0.033 (0.002)a | 0.05 (0.02)a | 0.04 (0.011)a |
| γ | −0.05 (0.07)a | −0.040 (0.006)a | −0.053 (0.0010)a | −0.07 (0.012)a |
| γ 1 | 0.01 (0.08)a | 0.023 (0.007)a | 0.007 (0.005)a | −0.02 (0.04)a |
| L* | 55.79 (0.05)c | 28.8 (0.11)b | 26.5 (0.2)a | 26.38 (0.02)a |
| a* | −0.61 (0.07)a | 4.17 (0.04)d | 1.94 (0.04)c | 1.00 (0.02)b |
| b* | 0.41 (0.03)a | 6.7 (0.2)d | 2.92 (0.09)c | 2.51 (0.05)b |
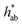
|
146 (5)c | 58.0 (0.7)a | 56.5 (0.7)a | 68.4 (0.5)b |
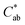
|
0.73 (0.05)a | 7.9 (0.2)d | 3.51 (0.09)c | 2.71 (0.05)b |
| ΔE | — | 28.13 (0.06)a | 29.5 (0.15)b | 29.53 (0.02)b |
| Visual appearance |

|

|

|

|
Jaggery, compared to white sugar, is characterized by its brownish colour, which is provided by the natural constituents of sugarcane, mainly flavonoids, and by Maillard reaction products.14,34 When solubilized in water, jaggery yielded solutions with a marked brown colour (see the visual appearance in Table 2), which could negatively affect the characteristics of the impregnated product and its acceptability. The measurements of optical properties revealed that the addition of granulated jaggery to the impregnation solution significantly decreased the L* coordinate and that this was more determined by the presence of jaggery than by the percentage of replacement. Similarly, an increase in the a* and b* coordinates was evident when jaggery was included in the solution. Hue 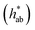 values for jaggery solutions were between red and orange, as opposed to sucrose solution, which was greenish. Chroma or colour saturation
values for jaggery solutions were between red and orange, as opposed to sucrose solution, which was greenish. Chroma or colour saturation 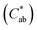 intensified with jaggery addition, as did the calculated colour difference (ΔE) with respect to no jaggery addition. These results agree with those reported by Cervera-Chiner et al. (2021)35 for the optical properties of kiwi fruit and strawberry jams formulated with different percentages of jaggery.
intensified with jaggery addition, as did the calculated colour difference (ΔE) with respect to no jaggery addition. These results agree with those reported by Cervera-Chiner et al. (2021)35 for the optical properties of kiwi fruit and strawberry jams formulated with different percentages of jaggery.
Properties of the VI and OD apple rings
| Fresh | VI0% GJ | VI25% GJ | VI50% GJ | VI100% GJ | |
|---|---|---|---|---|---|
| a a, b, c, d different letters in the same row indicate statistically significant differences at the 95% level (p-value ≤ 0.05). | |||||
| a w | 0.9888 (0.0008)b | 0.987 (0.0012)ab | 0.985 (0.002)a | 0.988 (0.0013)b | 0.9879 (0.0003)b |
| x w | 0.858 (0.005)c | 0.828 (0.011)a | 0.831 (0.004)ab | 0.84 (0.02)bc | 0.830 (0.012)ab |
| °Brix | 10.2 (0.2)a | 16.3 (0.4)c | 14.2 (0.2)b | 14.1 (0.4)b | 13.9 (0.2)b |
| L* | 74 (2)e | 36 (1.5)a | 49 (7)c | 42 (6)b | 54 (3)d |
| a* | −0.1 (0.9)b | −0.7 (0.4)a | 0.3 (0.3)b | 0.5 (0.7)b | 0.5 (0.7)b |
| b* | 22 (3)d | 11 (2)a | 20 (4)c | 16 (2)b | 23(2)d |
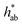
|
90 (2)b | 94 (2)c | 89 (1.0)ab | 88 (3)a | 89 (2)ab |
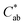
|
23 (3)d | 11 (1.6)a | 20 (4)c | 16 (2)b | 23 (2)d |
| ΔE | — | 40 (2)d | 26 (8)b | 33 (7)c | 21 (4)a |
| Total phenols (mg GAE per gfp) | 1.09 (0.06)bc | 1.11 (0.02)bc | 0.98 (0.04)a | 1.0 (0.11)ab | 1.16 (0.07)c |
| Total flavonoids (mg QE per gfp) | 0.56 (0.03)ab | 0.521 (0.0010)a | 0.58 (0.02)b | 0.643 (0.002)c | 0.783 (0.012)d |
| DPPH AO activity (mg TE per gfp) | 0.118 (0.008)bc | 0.12 (0.018)c | 0.105 (0.007)ab | 0.103 (0.008)a | 0.12 (0.013)c |
| ABTS AO activity (mg TE per gfp) | 8.6 (0.3)c | 5.8 (0.3)b | 4.8 (0.2)a | 4.9 (0.5)a | 6.2 (0.6)b |
Regarding optical properties (Table 3), the replacement of air by the impregnation solutions yielded more translucid samples, which is reflected in the lower L* values. The increased translucence in VI apple slices, resulting in lower L* values, is a known phenomenon that occurs as a result of gas replacement by the impregnation liquid, and the consequent porosity decrease. In line with the results obtained in the present work, Contreras et al. reported a decrease in L* from 71.8 (1.1) to 41.5 (2.6) in VI apple slices.37 Among the impregnated samples, increasing the jaggery proportion implied a higher L*. Although this could be unexpected due to the darker colour of jaggery solutions, it could be explained by a less successful impregnation of jaggery-containing solutions (Table 2). For the other coordinates, a* values moved from green (a* < 0) to red values (a* > 0) when jaggery was added to the impregnation solution. In contrast, changes in b* were more marked in samples impregnated with the solution prepared with white sugar. This result aligns with previous findings in sucrose-impregnated Granny Smith apple slices.37 Colour differences with respect to the fresh apple were higher for the white sugar-impregnated samples, which was consistent with the differences in the L* values. The visual appearance of the impregnated samples is illustrated in Fig. 1.
 | ||
| Fig. 1 Apple rings that are vacuum impregnated with isotonic solutions and formulated with white sugar and jaggery. Percentages (0 to 100%) indicate the amount of white sugar replaced by jaggery. | ||
Regarding antioxidant properties, VI did not significantly increase the antioxidant properties of apple rings; in fact, a decrease was observed in some cases (Table 3). One possible reason for this is the loss of native bioactive constituents, which may occur during VI due to native liquid losses.38 Nevertheless, the total replacement of white sugar with jaggery improved the antioxidant properties of the samples. In this case, the loss of bioactive compounds due to native liquid outflow could have been compensated for by the antioxidant compounds incorporated with jaggery. In contrast, the 25% GJ solution implied a decrease in all the antioxidant properties evaluated, except flavonoids. Total flavonoid content was the only antioxidant parameter that increased in all cases when jaggery was added to the impregnation solution, as flavonoids are among the major phenolic constituents of sugarcane and its derivatives.14,39 Antioxidant activities measured using the DPPH and ABTS methods did not improve in the VI samples. The DPPH inhibition in the VI100% GJ samples was similar to that in the fresh apples. In contrast, ABTS antiradical activity decreased in all cases. One possible reason for this is that antioxidant compounds naturally present in apples could be more sensitive to this method than jaggery constituents. In addition, the shorter reaction times used in the ABTS assay could have contributed to these differences. DPPH radical scavenging activity and total phenolic content obtained for the fresh apple tissue in the present study were slightly lower than those reported by Vega-Gálvez et al. for the same apple variety (0.266 ± 0.004 mg TE per gfp and 1.583 ± 0.007 mg GAE per gfp, respectively).40
| VI100% GJ | Fresh | |||
|---|---|---|---|---|
| OD | pOD | OD | pOD | |
| a a, b, c different letters in the same row indicate statistically significant differences at the 95% level (p-value ≤ 0.05). | ||||
| x w | 0.691 (0.003)a | 0.71 (0.02)a | 0.700 (0.008)a | 0.71 (0.013)a |
| Total phenols (mg GAE per gfp) | 1.07 (0.04)a | 1.18 (0.08)ab | 1.09 (0.04)a | 1.2 (0.2)b |
| Total flavonoids (mg QE per gfp) | 0.698 (0.009)a | 0.73 (0.012)b | 0.70 (0.009)a | 0.72 (0.02)b |
| L* | 44.7 (0.7)a | 48 (5)b | 60 (5)c | 63 (4)c |
| a* | 4.4 (0.5)c | 2 (1.2)b | 3 (2)bc | 3 (2)bc |
| b* | 29 (1.0)b | 25 (3)a | 28 (3)b | 33 (1.7)c |
| h | 81 (1.0)a | 85 (2)b | 84 (3)b | 85 (3)b |
| C | 29 (1.0)b | 25 (3)a | 30 (2)c | 33 (1.9)c |
| ΔE | 31.1 (0.6)c | 27 (5)b | 18 (4)b | 16 (4)a |
In this case, the success of OD treatment for solutes incorporation was evaluated by determining total phenols and flavonoids (Table 4). Combining VI with a subsequent OD treatment did not improve the incorporation of antioxidants into the fruit matrix compared to osmotically dehydrating fresh tissue. Compared to the VI samples, OD succeeded in improving the antioxidant properties of the sample when progressive dehydration was used (pOD), which occurred for both fresh and previous VI apples. This difference between pOD and direct OD could be explained by the fact that direct dehydration in highly concentrated solutions may cause collapsing of the cells next to the osmotic solution, resulting in case hardening and increased resistance to mass transfer in the product surface.41 Contrarily, when osmotic gradients are progressively applied, water and solute diffusion might be facilitated because this layer of increased resistance is avoided. Besides, dehydration through the tissue symplast is partially preserved because its integrity is better maintained with progressive dehydration.42,43 These phenomena promote the migration of water from the inner part of the tissue, and a simultaneous counter-current transfer of solutes and bioactive compounds from the osmotic solution to the sample. In the osmotic solution, decreased resistance to mass transfer can also be achieved by modifying the particle size. Filtration of the juice used as the osmotic agent has been proven to successfully increase antioxidant infusion into the apple tissue during OD as a result of particle size reduction.44 High-pressure homogenization has been reported to be an interesting technique for reducing particle size in fruit juices and stabilizing bioactive compounds, with a positive impact on impregnation properties.18 Combining both approaches, i.e. particle size reduction and progressive dehydration, or VI, could result in a summative effect.
In Table 3, phenols and flavonoid contents are given per gram of fresh product. Considering moisture content reduction between fresh and osmotically dehydrated samples, OD products (Table 4) exhibited better antioxidant properties per gram of consumed products than fresh and VI products (Table 3). In any case, apples subjected to pOD presented the best antioxidant properties among OD ones. Regarding optical properties, VI samples with further OD were characterized by lower luminosity values. In contrast, the OD applied to the fresh tissue implied a luminosity closer to the fresh tissue. The same was applied to the other colour attributes and colour differences, which were more significant when VI was followed by osmotic dehydration (both OD and pOD) than when OD was used alone. As previously discussed, VI decreases luminosity as porosity is reduced, which implies more significant colour differences. However, the increase in the a* coordinate could be due to the inflow of jaggery in the fruit tissue.
| Hot air-dried apple snacks | HAD | HAD VI0% | HAD VI25% | HAD VI50% | HAD VI100% | HAD pOD |
|---|---|---|---|---|---|---|
| a a, b, c different letters in the same row indicate statistically significant differences at the 95% level (p-value ≤ 0.05). | ||||||
| a w | 0.30 (0.02)a | 0.340 (0.004)b | 0.34 (0.014)b | 0.339 (0.009)b | 0.349 (0.008)b | 0.348 (0.002)b |
| x w | 0.014 (0.005)a | 0.027(0.006)b | 0.036 (0.004)bc | 0.04 (0.010)c | 0.058 (0.007)d | 0.074 (0.009)e |
| Total phenols (mg GAE per gdp) | 6.3 (0.12)a | 6 (1.0)a | 7.4 (0.7)b | 7.6 (0.8)b | 8.0 (0.6)b | 11.0 (0.6)c |
| Total flavonoids (mg QE per gdp) | 2.8 (0.10)a | 3.4 (0.5)ab | 3.9 (0.7)bc | 4.1 (0.7)c | 4.7 (0.6)d | 7.33 (0.06)e |
| DPPH AO activity (mg TE per gdp) | 0.72 (0.03)d | 0.59 (0.05)b | 0.7 (0.11)cd | 0.66 (0.09)bcd | 0.63 (0.09)bc | 0.48 (0.03)a |
| ABTS AO activity (mg TE per gdp) | 8.1 (0.3)a | 12.8 (0.8)b | 16 (2)c | 15 (1.2)c | 14.8 (0.8)c | 23 (1.5)d |
| L | 74 (2)c | 55 (3)a | 53 (6)a | 56 (4)a | 55 (5)a | 62 (2)b |
| a* | 8 (1.8)a | 8 (1.3)a | 8 (2)a | 8 (2)a | 9 (2)ab | 11 (1.8)b |
| b* | 38 (4)cd | 35 (2)b | 32 (2)a | 36 (3)bc | 38 (1.1)d | 39 (3)d |
| h | 78 (2)b | 77 (1.7)ab | 76 (4)ab | 77 (3)ab | 76 (3)ab | 75 (1.7)a |
| C | 39 (4)cd | 36 (2)b | 33 (2)a | 37 (4)bc | 40 (1.1)d | 40 (3)d |
| ΔE | — | 19 (3)b | 21 (6)b | 18 (4)b | 19 (5)b | 12 (3)a |
| Freeze-dried apple snacks | FD | FD VI0% | FD VI25% | FD VI50% | FD VI100% | FD pOD |
|---|---|---|---|---|---|---|
| a w | 0.190 (0.005)a | 0.273 (0.006)bc | 0.266 (0.003)bc | 0.261 (0.008)b | 0.261 (0.004)b | 0.285 (0.028)c |
| x w | 0.046 (0.008)b | 0.03 (0.011)a | 0.023 (0.005)a | 0.025 (0.004)a | 0.02 (0.012)a | 0.083 (0.006)c |
| Total phenols (mg GAE per gdp) | 6.5 (0.2)d | 4.8 (0.4)a | 5.2 (0.6)ab | 5.6 (0.7)bc | 6.2 (0.9)cd | 10.8 (0.4)e |
| Total flavonoids (mg QE per gdp) | 2.1 (0.12)a | 2.4 (0.14)b | 2.7 (0.3)c | 3.1 (0.2)d | 3.5 (0.2)e | 6.93 (0.09)f |
| DPPH AO activity (mg TE per gdp) | 0.65 (0.02)b | 0.47 (0.07)a | 0.5 (0.10)a | 0.55 (0.09)a | 0.6 (0.13)ab | 0.50 (0.03)a |
| ABTS AO activity (mg TE per gdp) | 9.1 (0.2)a | 10.3 (0.4)b | 11.3 (0.6)c | 11.9 (0.4)c | 13.0 (0.7)d | 23.8 (0.8)e |
| L | 86.1 (0.8)f | 83 (1.5)e | 81 (1.1)d | 77 (3)c | 74 (1.1)b | 67.2 (0.8)a |
| a* | −1 (1.1)a | 1 (2)b | 3 (1.8)c | 4 (1.5)c | 5.8 (0.8)d | 7 (1)e |
| b* | 21 (1.4)a | 26 (2)b | 28 (3)bc | 29 (1.8)cd | 30.3 (0.5)d | 38 (3)e |
| h | 92 (3)d | 88 (5)c | 85 (2)b | 82 (3)b | 79 (1.6)a | 79.0 (0.9)a |
| C | 21 (1.4)a | 26 (2)b | 28 (3)bc | 29 (1.9)cd | 30.9 (0.5)d | 38 (3)e |
| ΔE | — | 6 (2)a | 9 (3)b | 13 (3)c | 16 (1.1)d | 26 (2)e |
The drying technique used, HAD or FD, had different effects on the properties of VI and pOD jaggery-formulated snacks. When HAD was applied, the use of jaggery in the formulation of apple snacks resulted in a significant improvement in antioxidant properties in all cases. This was evidenced in all the antioxidant parameters assayed. Both phenols and flavonoids increased, especially flavonoids. For antioxidant capacity, ABTS was significantly more sensitive in this case. Among the air-dried VI and OD samples, phenolic constituents increased as the jaggery concentration increased in the impregnation solution; however, this was not observed when comparing VI samples prior to drying (Table 3). This difference suggests a significant effect of HAD on the antioxidant compounds incorporated through VI and OD. It has been previously reported that thermal treatments may improve the antioxidant properties of foods by promoting biochemical reactions that generate new compounds or isomerization reactions that generate more active forms, either by enzyme activation or inactivation.47,48 In particular, for sugarcane products and derivatives, previous research14 showed that moderate thermal treatments imply an increase in the antioxidant capacity of these ingredients. Apple snacks, which were pOD, exhibited the best antioxidant properties. This evidences the capacity of pOD to introduce jaggery bioactive constituents in the apple tissue; additionally, this suggests an ability of OD to better protect native or added bioactive constituents, an effect that has been previously attributed to OD pretreatments.49
The addition of granulated jaggery to the fruit matrix by VI or OD did not always imply an improvement in the antioxidant properties of FD snacks, in contrast to HAD snacks. For total phenolic content, improvement was observed only in samples previously subjected to pOD. However, the total flavonoids exhibited a significant increase in FDVI samples when the jaggery concentration in the impregnation solution was higher, thus representing the abundance of these compounds in jaggery. Results obtained for FD samples agree with those obtained for previous VI in which the use of impregnation solutions of low jaggery content does not compensate for the loss of native antioxidants. In the case of HAD snacks, the FD product that presented the best antioxidant characteristics was subjected to pOD, in which all antioxidant attributes improved significantly. The results for FDpOD apple snacks quite agreed with HADpOD ones, thus reinforcing the idea that OD allows the successful introduction of new antioxidants in the fruit matrix and helps protect native or added antioxidant compounds during further processing.49 DPPH and ABTS antioxidant activities of FD products followed a similar trend to HAD ones although values were generally lower except for pOD ones. The lower temperatures and vacuum conditions used during FD could have better protected temperature-sensitive antioxidant compounds but did not promote the formation of new or more active compounds or influenced enzymatic reactions, as suggested for HAD. Dalmau et al. also reported a significant increase in the antioxidant properties of HAD apple slabs, whereas FD samples exhibited reduced total phenolics and ABTS antioxidant activity compared to the fresh tissue.50
The optical properties of apple snacks are presented in Table 4. As in previous analyses, the L* coordinate was the most affected one. This was especially evidenced in the HADVI samples, which also presented the highest colour differences with respect to HAD ones, due mainly to differences in luminosity. On the contrary, the a* and b* parameters were quite homogeneous among the HAD samples. Compared to the non-dried samples (Table 3), a* and b* values increased significantly after HAD. These changes in the colour coordinates are due to structural and biochemical changes experienced during air drying, such as the contraction of the apple tissue, the concentration of compounds that are responsible for colour (native and incorporated ones), and browning reactions. Similar colour changes have been previously reported for apple slices after convective drying.37
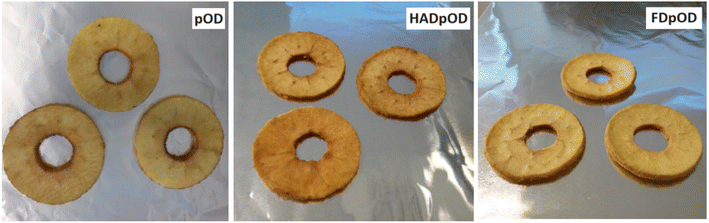
Colour differences among VI samples were reduced after FD and colorimetric properties were quite similar for all FD snacks. Luminosity values slightly increased as the proportion of jaggery incorporated into the product increased, but the differences were not as remarkable as before FD. Therefore, a* and b* were coordinated although less significantly than in HAD snacks. This is due to the use of low temperatures during the process and the reduced exposure to oxygen during sublimation. Among FD samples, colour differences for the FDpOD samples were significantly higher, thus evidencing the incorporation of jaggery-coloured compounds into apple tissue owing to progressive dehydration. Fig. 2 shows the visual appearance of the pOD samples before and after drying by HAD and FD.
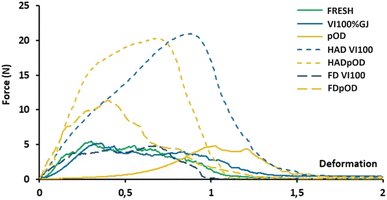
The curves for fresh and vacuum-impregnated samples were quite similar, presenting multiple fractures after some deformations, with low penetration force and a flat peak typical of fresh porous plant tissues51 made up of strongly associated turgent cells. In the pOD samples, deformation prior to rupture prolonged significantly, evidencing a more viscoelastic behaviour due to the loss of cellular turgor. However, dehydrated samples exhibited different behaviours depending on the treatment undergone. Higher and sharper peaks were obtained for HAD, which showed a marked increase in force with deformation, indicating increased tissue compactness. Moreover, FD implied an increased force with deformation but was less marked than HAD. This could be due to the porous structure created in the FD samples after sublimation.52 The trends depicted in Fig. 3 are statistically compared to those in Table 6, where maximum force (Fmax) and deformation at maximum force (dmax/l) values are presented. The maximum force required to penetrate fresh apples is reported as 7.8 ± 0.9 N.37 After vacuum impregnation (VI), followed by air drying at 30 and 50 °C, this force increased to 20.1 and 18.8 N, respectively, which is consistent with the present findings.
| F max (N) | Deformation (dmax/l) | |
|---|---|---|
| a a, b, c different letters in the same column indicate statistically significant differences at the 95% level (p-value ≤ 0.05). | ||
| Fresh | 5.4 (1.2)a | 0.28 (0.04)a |
| IV100% | 5.3 (0.7)a | 0.37 (0.03)a |
| pOD | 4.9 (1.0)a | 0.94 (0.09)c |
| HAD IV100% | 21.4 (5.5)c | 1.4 (0.4)d |
| HAD pOD | 18.8 (2.7)c | 0.58 (0.10)b |
| FD IV100% | 5.7 (1.2)a | 0.48(0.13)ab |
| FD pOD | 11.3 (1.9)b | 0.44 (0.09)ab |
Conclusion
The present work explored jaggery as a non-conventional osmotic agent to obtain nutrient rich apple snacks through vacuum impregnation and osmotic dehydration, followed by air-drying or freeze-drying stabilization. The results confirmed that the antioxidant properties of non-centrifugal sugar may be incorporated into the tissue, thus producing fortified apple snacks, especially when progressive osmotic dehydration was applied. Freeze drying helped preserve the antioxidants present in the jaggery-enriched samples, whereas convective hot air drying intensified the antioxidant properties of the jaggery-enriched products. Optical and textural properties were affected by the addition of jaggery and processing techniques. Further studies should explore the acceptability of the products by consumers, as well as the stability of intermediate products and snacks. In conclusion, jaggery is proposed as a healthier osmotic agent to produce more nutritious and sustainable apple snacks using matrix engineering techniques.Author contributions
Cristina Barrera: conceptualization, investigation, data curation, formal analysis, methodology, writing – review & editing, supervision, funding acquisition. Noelia Betoret: conceptualization, methodology, writing – review & editing. Lucía Seguí: conceptualization, investigation, data curation, formal analysis, methodology, writing – original draft, writing – review & editing, supervision, project administration, funding acquisition.Conflicts of interest
Authors declare no conflict of interest.Acknowledgements
This work was supported by Generalitat Valenciana (Spain) under the project GV/2013/047. Authors would like to acknowledge the bachelor student Tomás Algarra for his instrumental role and contribution to the experiments presented in this paper.References
- M. Ül Kirci, O. Isaksson and R. Seifert, Managing Perishability in the Fruit and Vegetable Supply Chains, Sustainability, 2022, 1(9), 14 Search PubMed.
- I. Vázquez-Rowe, J. Laso, M. Margallo, I. Garcia-Herrero, D. Hoehn and F. Amo-Setién, et al., Food loss and waste metrics: a proposed nutritional cost footprint linking linear programming and life cycle assessment, Int. J. Life Cycle Assess., 2020, 25(7), 1197–1209 CrossRef.
- A. Ciurzyńska, H. Kowalska, K. Czajkowska and A. Lenart, Osmotic dehydration in production of sustainable and healthy food, Trends Food Sci. Technol., 2016, 50, 186–192 CrossRef.
- FAO and WHO, Sustainable Healthy Diets - Guiding Principles, Rome, 2019 Search PubMed.
- Y. Zhao and J. Xie, Practical applications of vacuum impregnation in fruit and vegetable processing, Trends Food Sci. Technol., 2004, 15(9), 434–451 CrossRef CAS.
- R. Pandiselvam, Y. Tak, E. Olum, O. J. Sujayasree, Y. Tekgül and K. G. Çalışkan, et al., Advanced osmotic dehydration techniques combined with emerging drying methods for sustainable food production: Impact on bioactive components, texture, color, and sensory properties of food, J. Texture Stud., 2022, 53(6), 737–762 CrossRef PubMed.
- P. Fito, A. Chiralt, N. Betoret, M. Gras, M. Cháfer and J. Martínez-Monzó, et al., Vacuum impregnation and osmotic dehydration in matrix engineering, J. Food Eng., 2001, 49(2–3), 175–183 CrossRef.
- P. Tiwari, A. Joshi, and M. Thakur, Vacuum impregnation: A novel nondestructive technique for the development of functional foods, in Emerging Technologies in Food Science: Focus on the Developing World, Springer, Singapore, 2020, pp. 187–99 Search PubMed.
- D. Kaur, M. Singh, R. Zalpouri and I. Singh, Osmotic dehydration of fruits using unconventional natural sweeteners and non-thermal-assisted technologies: A review, J. Food Process. Preserv., 2022, 46, e16890 CAS.
- C. Barrera, N. Betoret, P. Corell and P. Fito, Effect of osmotic dehydration on the stabilization of calcium-fortified apple slices (var. Granny Smith): Influence of operating variables on process kinetics and compositional changes, J. Food Eng., 2009, 92(4), 416–424 CrossRef CAS.
- C. G. Burca-Busaga, N. Betoret, L. Seguí, J. García-Hernández, M. Hernández and C. Barrera, Antioxidants Bioaccessibility and Lactobacillus salivarius (CECT 4063) Survival Following the In Vitro Digestion of Vacuum Impregnated Apple Slices: Effect of the Drying Technique, the Addition of Trehalose, and High-Pressure Homogenization, Foods, 2021, 10(9), 2155 CrossRef CAS PubMed.
- J. M. Castagnini, S. Tappi, U. Tylewicz, S. Romani, P. Rocculi and M. Dalla Rosa, Sustainable Development of Apple Snack Formulated with Blueberry Juice and Trehalose, Sustainability, 2021, 13(16), 9204 CrossRef CAS.
- M. M. de Lima, G. Tribuzi, J. A. R. de Souza, I. G. de Souza, J. B. Laurindo and B. A. M. Carciofi, Vacuum impregnation and drying of calcium-fortified pineapple snacks, LWT, 2016, 72, 501–509 CrossRef.
- L. Seguí, L. Calabuig-Jiménez, N. Betoret and P. Fito, Physicochemical and antioxidant properties of non-refined sugarcane alternatives to white sugar, Int. J. Food Sci. Technol., 2015, 50(12), 2579–2588 CrossRef.
- C. Barrera, N. Betoret and L. Seguí, Phenolic Profile of Cane Sugar Derivatives Exhibiting Antioxidant and Antibacterial Properties, Sugar Tech, 2020, 22(5), 798–811 CrossRef CAS.
- J. S. Lee, S. Ramalingam, I. G. Jo, Y. S. Kwon, A. Bahuguna and Y. S. Oh, et al., Comparative study of the physicochemical, nutritional, and antioxidant properties of some commercial refined and non-centrifugal sugars, Food Res. Int., 2018, 109, 614–625 CrossRef CAS PubMed.
- A. Singh, U. Lal, H. Mukhtar, P. Singh, G. Shah and R. Dhawan, Phytochemical profile of sugarcane and its potential health aspects, Pharmacogn. Rev., 2015, 9(17), 45 CrossRef CAS PubMed.
- L. I. Hinestroza-Córdoba, C. Barrera, L. Seguí and N. Betoret, Potential Use of Vacuum Impregnation and High-Pressure Homogenization to Obtain Functional Products from Lulo Fruit (Solanum quitoense Lam.), Foods, 2021, 10(4), 817 CrossRef PubMed.
- M. Kręcisz, J. Kolniak-Ostek, J. Łyczko and B. Stępień, Evaluation of bioactive compounds, volatile compounds, drying process kinetics and selected physical properties of vacuum impregnation celery dried by different methods, Food Chem., 2023, 413, 135490 CrossRef PubMed.
- J. M. Castagnini, N. Betoret, E. Betoret and P. Fito, Vacuum impregnation and air drying temperature effect on individual anthocyanins and antiradical capacity of blueberry juice included into an apple matrix, LWT, 2015, 64(2), 1289–1296 CrossRef CAS.
- M. Pravitha, M. R. Manikantan, V. Ajesh Kumar, S. Beegum and R. Pandiselvam, Optimization of process parameters for the production of jaggery infused osmo-dehydrated coconut chips, LWT, 2021, 146, 111441 CrossRef CAS.
- D. Salvatori, A. Andrés, A. Chiralt and P. Fito, The response of some properties of foods to vacuum impregnation, J. Food Process Eng., 1998, 21(1), 59–73 CrossRef.
- S. Vesterlund, K. Salminen and S. Salminen, Water activity in dry foods containing live probiotic bacteria should be carefully considered: A case study with Lactobacillus rhamnosus GG in flaxseed, Int. J. Food Microbiol., 2012, 157(2), 319–321 CrossRef PubMed.
- B. A. M. AOAC, Association of official analytical chemists, in Official Methods of Analysis, Washington, DC, 1990 Search PubMed.
- V. L. Singleton, R. Orthofer, and R. M. Lamuela-Raventós, Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent, in Methods in Enzymology, 1999, pp. 152–78 Search PubMed.
- A. Luximon-Ramma, T. Bahorun, M. A. Soobrattee and O. I. Aruoma, Antioxidant activities of phenolic, proanthocyanidin, and flavonoid components in extracts of Cassia fistula, J. Agric. Food Chem., 2002, 50(18), 5042–5047 CrossRef CAS PubMed.
- W. Brand-Williams, M. E. Cuvelier and C. Berset, Use of a Free Radical Method to Evaluate Antioxidant Activity, LWT, 1995, 28(1), 25–30 CrossRef CAS.
- R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang and C. Rice-Evans, Antioxidant activity applying an improved ABTS radical cation decolorization assay, Free Radical Biol. Med., 1999, 26(9–10), 1231–1237 CrossRef CAS PubMed.
- P. Talens, N. Martínez-Navarrete, P. Fito and A. Chiralt, Changes in optical and mechanical properties during osmodehydrofreezing of kiwi fruit, Innovative Food Sci. Emerging Technol., 2002, 3(2), 191–199 CrossRef.
- C. M. González, I. Hernando and G. Moraga, Influence of ripening stage and de-astringency treatment on the production of dehydrated persimmon snacks, J. Sci. Food Agric., 2021, 101(2), 603–612 CrossRef PubMed.
- J. M. Barat, P. Fito and A. Chiralt, Modeling of simultaneous mass transfer and structural changes in fruit tissues, J. Food Eng., 2001, 49(2–3), 77–85 CrossRef.
- P. Fito, A. Chiralt, N. Betoret, M. Gras, M. Cháfer and J. Martínez-Monzó, et al., Vacuum impregnation and osmotic dehydration in matrix engineering, J. Food Eng., 2001, 49(2–3), 175–183 CrossRef.
- C. Barrera, C. Burca, E. Betoret, J. García-Hernández, M. Hernández and N. Betoret, Improving antioxidant properties and probiotic effect of clementine juice inoculated with Lactobacillus salivarius spp. salivarius (CECT 4063) by trehalose addition and/or sublethal homogenisation, Int. J. Food Sci. Technol., 2019, 54(6), 2109–2122 CrossRef CAS.
- P. Verma, N. Shah and S. Mahajani, Effect of sodium hydrosulphite treatment on the quality of non-centrifugal sugar: Jaggery, Food Chem., 2019, 299, 125043 CrossRef CAS PubMed.
- L. Cervera-Chiner, C. Barrera, N. Betoret and L. Seguí, Impact of sugar replacement by non-centrifugal sugar on physicochemical, antioxidant and sensory properties of strawberry and kiwifruit functional jams, Heliyon, 2021, 7(1), e05963 CrossRef CAS PubMed.
- M. Gras, N. Vidal-Brotóns, A. Betoret and F. P. Chiralt, The response of some vegetables to vacuum impregnation, Innovative Food Sci. Emerging Technol., 2002, 3(3), 263–269 CrossRef.
- C. Contreras, M. E. Martín-Esparza, A. Chiralt and N. Martínez-Navarrete, Influence of microwave application on convective drying: Effects on drying kinetics, and optical and mechanical properties of apple and strawberry, J. Food Eng., 2008, 88(1), 55–64 CrossRef.
- L. Neri, L. Di Biase, G. Sacchetti, C. Di Mattia, V. Santarelli and D. Mastrocola, et al., Use of vacuum impregnation for the production of high quality fresh-like apple products, J. Food Eng., 2016, 179, 98–108 CrossRef CAS.
- J. M. Duarte-Almeida, A. Salatino, M. I. Genovese and F. M. Lajolo, Phenolic composition and antioxidant activity of culms and sugarcane (Saccharum officinarum L.) products, Food Chem., 2011, 125(2), 660–664 CrossRef CAS.
- A. Vega-Gálvez, K. Ah-Hen, M. Chacana, J. Vergara, J. Martínez-Monzó and P. García-Segovia, et al., Effect of temperature and air velocity on drying kinetics, antioxidant capacity, total phenolic content, colour, texture and microstructure of apple (var. Granny Smith) slices, Food Chem., 2012, 132(1), 51–59 CrossRef PubMed.
- G. Giraldo, P. Talens, P. Fito and A. Chiralt, Influence of sucrose solution concentration on kinetics and yield during osmotic dehydration of mango, J. Food Eng., 2003, 58, 33–43 CrossRef , available from, https://www.elsevier.com/locate/jfoodeng.
- L. Seguí, P. J. Fito and P. Fito, Analysis of structure-property relationships in isolated cells during OD treatments. Effect of initial structure on the cell behaviour, J. Food Eng., 2010, 99(4), 417–423 CrossRef.
- L. Seguí, P. J. Fito and P. Fito, A study on the rehydration ability of isolated apple cells after osmotic dehydration treatments, J. Food Eng., 2013, 115(2), 145–153 CrossRef.
- K. Masztalerz, J. Łyczko and K. Lech, Effect of Filtrated Osmotic Solution Based on Concentrated Chokeberry Juice and Mint Extract on the Drying Kinetics, Energy Consumption and Physicochemical Properties of Dried Apples, Molecules, 2021, 26(11), 3274 CrossRef CAS PubMed.
- P. Sette, D. Salvatori and C. Schebor, Physical and mechanical properties of raspberries subjected to osmotic dehydration and further dehydration by air- and freeze-drying, Food Bioprod. Process., 2016, 100, 156–171 CrossRef.
- P. Verma, N. Shah and S. Mahajani, Drying characteristics of non-centrifugal sugar, Drying Technol., 2020, 38(16), 2162–2171 CrossRef CAS.
- C. Bas-Bellver, C. Barrera, N. Betoret and L. Seguí, Physicochemical, Technological and Functional Properties of Upcycled Vegetable Waste Ingredients as Affected by Processing and Storage, Plant Foods Hum. Nutr., 2023, 78(4), 710–719 CrossRef CAS PubMed.
- M. Chen, D. Yang and S. Liu, Effects of drying temperature on the flavonoid, phenolic acid and antioxidative capacities of the methanol extract of citrus fruit (Citrus sinensis (L.) Osbeck) peels, Int. J. Food Sci. Technol., 2011, 46(6), 1179–1185 CrossRef CAS.
- F. R. Abrahão and J. L. G. Corrêa, Osmotic dehydration: More than water loss and solid gain, Crit. Rev. Food Sci. Nutr., 2023, 63(17), 2970–2989 CrossRef PubMed.
- M. E. Dalmau, G. M. Bornhorst, V. Eim, C. Rosselló and S. Simal, Effects of freezing, freeze drying and convective drying on in vitro gastric digestion of apples, Food Chem., 2017, 215, 7–16 CrossRef CAS PubMed.
- E. Velickova, E. Winkelhausen and S. Kuzmanova, Physical and sensory properties of ready to eat apple chips produced by osmo-convective drying, J. Food Sci. Technol., 2014, 51(12), 3691–3701 CrossRef CAS PubMed.
- C. Contreras, M. E. Martín, N. Martínez-Navarrete and A. Chiralt, Effect of vacuum impregnation and microwave application on structural changes which occurred during air-drying of apple, LWT, 2005, 38(5), 471–477 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |