Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Clove essential oil emulsions-loaded arrowroot starch-beeswax-based edible coating extends the shelf life and preserves the postharvest quality of fresh tomatoes (Solanum lycopersicum L.) stored at room temperature†
Nimesh Dileesha
Lakshan
 a,
Chathuri M.
Senanayake
a,
Chathuri M.
Senanayake
 *a,
Thushari
Liyanage
b and
Ahinsa
Lankanayaka
*a,
Thushari
Liyanage
b and
Ahinsa
Lankanayaka
 a
a
aDepartment of Biosystems Technology, Faculty of Technology, University of Sri Jayewardenepura, Homagama, 10200, Sri Lanka. E-mail: chathurisnnk@sjp.ac.lk
bCentral Research Station, Department of Export Agriculture, Matale, 21000, Sri Lanka
First published on 10th June 2024
Abstract
This study assessed the impact of clove essential oil emulsion-loaded arrowroot starch and beeswax-based edible coatings on the physicochemical and microbiological quality characteristics, composition of bioactive compounds, and antioxidant activity of tomatoes stored at 26 ± 2 °C with a relative humidity of 72 ± 2% for 48 days. Nine formulations of edible coatings were prepared by varying the concentrations of arrowroot starch (10, 15, and 20 g L−1) and clove essential oils (0, 2.5, and 5 mL L−1) while keeping the concentration of beeswax constant (5 g L−1). The formulated edible coatings were applied to tomatoes at the mature green stage using the dip coating method. The results indicated that all of the coating treatments improved the postharvest quality attributes and shelf life of tomatoes compared to those of the uncoated control fruits, leading to reduced food waste, increased economic savings, and better sustainability. Fruits coated with the solution containing 15 g L−1 arrowroot starch, 5 g L−1 beeswax, and 5 mL L−1 clove essential oils showed a significant (p < 0.05) delay in changes in weight, firmness, color parameters (L*, a*, b*, and ΔE), total soluble solid content, titratable acidity, pH value, and decay incidence throughout the storage period, and the coating was found to be effective in reducing the microbial load in tomatoes, extending their shelf life to 49 ± 3 days. Furthermore, the application of this coating formulation preserved the bioactive compounds (phenolics, flavonoids, lycopene, and β-carotene) and antioxidant activity of the tomatoes during storage. The results suggest that the application of the coatings formulated with 15 g L−1 arrowroot starch, 5 g L−1 beeswax, and 5 mL L−1 clove essential oil can effectively delay ripening and maintain the postharvest quality attributes of tomatoes during storage at 26 ± 2 °C with a relative humidity of 72 ± 2% for 48 days, demonstrating significant potential for broader food preservation and packaging applications.
Sustainability spotlightAs clove essential oil emulsions-loaded arrowroot starch-beeswax-based edible coatings can extend the shelf life of fresh tomatoes, thereby reducing spoilage and wastage, our study directly addresses the goal of achieving zero hunger by maximizing the use of available resources. By providing an antimicrobial protective barrier that avoids the contamination and deterioration of fruits, this edible coating provides safe food preservation, contributing to safer and healthier food consumption, thus achieving good health and well-being. As a biodegradable, edible, and eco-friendly alternative to plastic packaging, our study helps to reduce plastic waste and therefore minimizes the negative impact of plastic pollution on the marine and terrestrial ecosystems, thus securing responsible consumption and production and life below water and life on land. |
Introduction
Tomato (Solanum lycopersicum L.) belongs to the family Solanaceae and is a climacteric fruit widely consumed owing to its characteristic umami flavor and established health benefits with a significant amount of nutrients and bioactive phytochemicals, including vitamin C and E, β carotene, lycopene, thiamin, riboflavin, and niacin.1,2 Annually, around 170 million tons of fresh and processed tomatoes are produced worldwide, according to the Food and Agriculture Organization (FAO).3 However, their substantial production leads to quality concerns, particularly during the postharvest stage, where tomato decay poses a significant challenge in many developing countries due to its high perishability and improper postharvest practices.4 The short postharvest life of tomatoes is attributed to several factors, including increased ripening, vulnerability to postharvest microbial decay, transpiration, and mechanical damage, accelerating fruit deterioration and consequently leading to a higher postharvest loss of more than 42%.5 Moreover, rapid ripening, accelerated by an increased respiration rate due to high temperature, is the primary aspect contributing to the deterioration of tomatoes in tropical countries such as Sri Lanka, ultimately affecting the economy of the country.6 Accordingly, various studies on low-temperature storage, controlled atmosphere packaging, and modified atmospheric packaging have been undertaken to extend the shelf-life of fresh tomatoes by eliminating the factors responsible for their short postharvest life.6–8 However, their commercial applications are limited by their high cost. Thus, in recent years, edible coatings have attracted significant attention.Edible coatings are composed of a thin layer of edible polymers such as polysaccharides, proteins, and lipids, or their combination, which can be directly applied to fresh or minimally processed fruits or vegetables to create a semipermeable covering material around the surface of the product.9,10 Edible coatings regulate the exchange of gases and water vapor, control microbial contaminations, and improve the aesthetic appearance of fresh commodities.9,11 Starch is a type of polysaccharide widely used in the preparation of edible films and coatings.10 For instance, arrowroot (Maranta arundinacea L.) is an underutilized plant in Sri Lanka, and the native starch obtained from its rhizomes has excellent film-forming ability with better mechanical and thermal properties due to its high amylose content, ranging from 30–35%.12,13 Due to the compact structure of linear amylose, the tensile strength and barrier properties of films and coatings could be improved compared to that of branched amylopectin.14 Regardless of the barrier properties, starch produces films and coatings with a low water resistance due to its hydrophilic nature.15,16 Numerous studies have highlighted the improvement in the water vapor barrier properties of starch films and coatings by the incorporation of hydrophobic components such as fats, oils, and waxes.17–19 Beeswax (BW), which originates from the wax glands of honey bees, is composed of a combination of esters, hydrocarbons, fatty acids, and alcohol, which improves the hydrophobicity of edible films and coatings.19 The incorporation of BW decreased the water vapor permeability of cassava starch-based films.20 In comparison to the uncoated fruits, reduced water loss in Andean blackberry coated with a cassava starch-based coating containing BW was noted by Rodríguez et al.,21 which was attributed to the increased water vapor barrier properties from BW.
In the postharvest stages, the deterioration of tomato is more than 30% primarily due to the fungal decay caused by Rhizopus stolonifer, Alternaria alternata, and Botrytis cinerea.4 Although the application of fungicides such as iprodione, dichloran, and fludioxonil reduces fungal attacks, they ultimately produce toxic compounds, leading to environmental pollution, complications in human health, and the generation of resistant fungal strains. In this case, modified atmosphere packaging, ozone treatment, ultraviolet-C (UV-C) light, and gamma irradiation are some of the existing alternatives to reduce fungal decay in tomatoes. However, their high cost and possible health concerns, particularly with UV-C and gamma irradiation limit their commercial applications.4,5 Additionally, although the use of synthetic additives in active food packaging delays microbial spoilage, their associated health and safety concerns have encouraged the utilization of natural bioactive compounds in recent years. Alternatively, natural bioactive compounds of plant origin are generally recognized as safe (GRAS) food additives by the Food and Drug Administration (FDA).9,22,23
The essential oils (EOs) extracted from the floral buds of clove (Syzygium aromaticum L.) possess various bioactive functions and health benefits, including antimicrobial, antioxidant, analgesic, anesthetic, anticancer, anticoagulant, antidiarrheal, and anti-inflammatory activities, owing to the presence of phenolic compounds, namely eugenol and acetyl eugenol.10,24,25 The hydroxyl groups present in eugenol can interact with the fungal cell membrane, leading to the destabilization of the cell structure, which is the mechanism behind the antifungal activity of clove EOs.26 In addition, by generating reactive oxygen species (ROS), eugenol can trigger oxidative stress within the cells, leading to the modification of the DNA, proteins, and lipids within the cells.25 Additionally, the antibacterial activities of clove EOs in edible packaging have been previously studied. For instance, the application of chitosan coatings enriched with clove EOs on fresh apples inhibited the growth of Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli. Throughout the storage period, uncoated fruits showed a total bacterial count and total aerobic count of 6.72 log CFU g−1 and 5.36 log CFU g−1, respectively. Conversely, the corresponding values were maintained at less than 4 log CFU g−1 and 3 log CFU g−1 in the coated fruits, respectively.27 A biodegradable gelatin and chitosan-based film enriched with clove EOs exhibited antimicrobial effects against Pseudomonas fluorescens, Shewanella putrefaciens, Photobacterium phosphoreum, Listeria innocua, Escherichia coli, and Lactobacillus acidophilus.28 The application of edible coatings containing natural antimicrobial agents such as clove EOs is therefore crucial in the preservation of the postharvest quality of fresh tomatoes. Clove EOs also exhibit antioxidant activities mainly due to the presence of eugenol and β-caryophyllene. These compounds can neutralize free radicals, thus preventing their oxidizing potential in plant cells and tissues.26 Therefore, the antioxidant properties of clove EOs are significant in the preservation of tomatoes by maintaining their appearance, texture, flavor, and nutritional value for safe consumption for a prolonged period.29
The present study aimed to evaluate the effect of edible coatings based on AS and BW loaded with clove essential oil emulsions on the physical, chemical, and microbiological quality attributes, antioxidant activity, and composition of the bioactive compounds in fresh tomatoes stored at 26 ± 2 °C and relative humidity (RH) of 72 ± 2% for 48 days.
Materials and methods
Materials
Freshly harvested tomatoes (Solanum lycopersicum cv. Platinum F1) at the mature green stage, according to the USDA standard tomato color classification chart,30 were collected from a farm in Naula, Sri Lanka. The fruits were visually selected based on uniform size, shape, and absence of physical damage and injuries to prevent variations in the experimental materials and transferred to the laboratory at room temperature (26 ± 2 °C) within 1 h. Arrowroot rhizomes harvested from a farm in Rattota, Sri Lanka, were used to isolate starch. Dried clove buds and food-grade beeswax were provided by the Central Research Station, Department of Export Agriculture, Matale, Sri Lanka. Food-grade soy lecithin (E322) was purchased from Pettah Essence Suppliers (Dam Street, Colombo 12, Sri Lanka). Pharmaceutical-grade glycerol monostearate (GMS) was purchased from Glorchem Enterprise (Bankshall Street, Colombo 11, Sri Lanka). All the other chemicals used in this study were of analytical grade and procured from Sigma-Aldrich Chemical Corporation (St. Louis, MO, USA).Isolation of arrowroot starch
Arrowroot starch was isolated according to the method described by Nogueira et al.31 with a few modifications according to Amaraweera et al.32 Briefly, arrowroot rhizomes were selected, peeled, washed, and cut into small cubes with a size of approximately 1 cm3. The cubes were crushed with distilled water in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 of arrowroot to water using a blender (Mixer Grinder SL-4MIXGR, SISIL, Sri Lanka) for 5 min until a pulp was obtained, followed by suspending the pulp in a volume of 10 times water and stirring for 10 min. Then, the pulp was filtered through a double-fold cotton cloth, and the filtrate was allowed to sediment the starch for 2 h. The starch was collected, followed by drying at 65 °C for 3 h in a hot air oven (YCO-010, Gemmy, Taiwan). The dried starch particles were ground into a fine powder using an analytical grinder (IKA, USA).
2 of arrowroot to water using a blender (Mixer Grinder SL-4MIXGR, SISIL, Sri Lanka) for 5 min until a pulp was obtained, followed by suspending the pulp in a volume of 10 times water and stirring for 10 min. Then, the pulp was filtered through a double-fold cotton cloth, and the filtrate was allowed to sediment the starch for 2 h. The starch was collected, followed by drying at 65 °C for 3 h in a hot air oven (YCO-010, Gemmy, Taiwan). The dried starch particles were ground into a fine powder using an analytical grinder (IKA, USA).
Extraction of clove essential oils
Hydrodistillation was employed for the extraction of clove essential oils. Briefly, dried clove buds were pulverized using an analytical grinder followed by hydrodistilling 25 g of pulverized clove buds mixed with 150 mL of distilled water in a Clevenger apparatus at 40 °C for 2 h. The collected essential oils were dehydrated using anhydrous sodium sulfate and stored at 4 °C for further use.33Preparation of edible coatings
Nine formulations of edible coatings were prepared by varying the concentrations of AS at 10, 15, and 20 g L−1, clove EOs at 0, 2.5, and 5 mL L−1, BW at 5 g L−1, and soy lecithin at 5 g L−1. GMS was added as a plasticizer in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 of AS to GMS for each coating solution (Table 1). AS was dissolved in 100 mL of distilled water at 40 °C for 10 min on a magnetic stirrer (ARE Heating Magnetic Stirrer, Velp Scientifica, Europe). BW was melted on a hot plate at 85 °C for 2 min. The molten BW and GMS were added to the starch solution, and the solution was heated until it reached 85 ± 2 °C on a magnetic stirrer under constant agitation to gelatinize the AS solution.13 Soy lecithin was dissolved as an emulsifier in the solution under constant agitation for 10 min. Then, the solution was allowed to cool to room temperature (26 ± 2 °C) before adding clove EOs. Homogenization of the solution was carried out at 21
0.5 of AS to GMS for each coating solution (Table 1). AS was dissolved in 100 mL of distilled water at 40 °C for 10 min on a magnetic stirrer (ARE Heating Magnetic Stirrer, Velp Scientifica, Europe). BW was melted on a hot plate at 85 °C for 2 min. The molten BW and GMS were added to the starch solution, and the solution was heated until it reached 85 ± 2 °C on a magnetic stirrer under constant agitation to gelatinize the AS solution.13 Soy lecithin was dissolved as an emulsifier in the solution under constant agitation for 10 min. Then, the solution was allowed to cool to room temperature (26 ± 2 °C) before adding clove EOs. Homogenization of the solution was carried out at 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 2 min using a high-performance homogenizing device (MICCRA D-8, Germany) after the addition of clove EOs.
000 rpm for 2 min using a high-performance homogenizing device (MICCRA D-8, Germany) after the addition of clove EOs.
| Materials | Coating formulations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | C | |
a C, control; AS, arrowroot starch (g L−1); clove EOs, clove essential oils (mL L−1); GMS, glycerol monostearate (g L−1); BW, beeswax (g L−1); soy lecithin (g L−1). The ratio of AS to GMS is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5. 0.5.
|
||||||||||
| AS | 10 | 10 | 10 | 15 | 15 | 15 | 20 | 20 | 20 | — |
| Clove EOs | 0 | 2.5 | 5 | 0 | 2.5 | 5 | 0 | 2.5 | 5 | — |
| GMS | 5 | 5 | 5 | 7.5 | 7.5 | 7.5 | 10 | 10 | 10 | — |
| BW | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | — |
| Soy lecithin | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | — |
Application of coating solutions on tomatoes
The fruits were washed with potable water and immersed in a solution of sodium hypochlorite (0.1 g L−1) for 10 min to remove dirt, spray residues, and attached microflora, followed by rinsing with distilled water and air drying at room temperature (26 ± 2 °C). The fruits were randomly divided into 10 groups, each containing 30 fruits, and among the 10 groups, 9 groups were immersed in each coating solution for 2 min followed by air drying at room temperature (26 ± 2 °C) for 30 min. According to the preliminary tests, the amount of coating solution applied on the surface of each fruit was 2 ± 0.5 mL, depending on the surface area. The uncoated fruits were immersed in distilled water, followed by air drying at room temperature (26 ± 2 °C). Both coated and uncoated fruits were separately placed in polypropylene trays and stored under ambient conditions (temperature of 26 ± 2 °C and RH of 72 ± 2%) for 48 days.Physiological weight loss
The weight of the five selected fruits from each treatment was measured from day 0 and at the end of each 4 days storage interval using an analytical balance (Sartorius, Germany) with 0.1 mg accuracy. The total percentage of physiological weight loss on a fresh weight basis was calculated using eqn (1), as discussed by Ali et al.34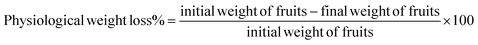 | (1) |
Fruit firmness
Fruit firmness was determined in triplicate by measuring the maximum force (N) required to puncture a 10 mm depth hole in the fruit using a digital fruit firmness tester (FHP-803, USA) with a 7.90 mm plunger tip from day 0 and at the end of each 4 days storage interval according to the method described by Ruelas-Chacon et al.16Fruit color
The CIE color parameters a* (redness), b* (yellowness), and L* (lightness) were directly recorded on the surface of three selected fruits from each treatment using a digital color reader (CR-10, Konica Minolta, Japan) from day 0 and at the end of each 4 days storage interval according to Filho et al.12 The ΔE (total color difference) was determined compared to the color recorded on day 0 using eqn (2).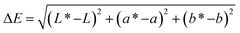 | (2) |
Titratable acidity, pH, and total soluble solid content
The tomatoes from each treatment were homogenized in a blender and the tomato juice was obtained after filtering the resulting pulp. A volume of 10 mL of tomato juice was diluted with 50 mL of distilled water and titrated against a standardized 0.1 N NaOH (sodium hydroxide) solution to the phenolphthalein endpoint. Titratable acidity (TA) was calculated in triplicate as citric acid (%) using eqn (3), according to Sadler and Murphy.35 The pH value of tomato juice was determined in triplicate using a digital pH meter (Ohaus, USA), according to Sadler and Murphy.35 Total soluble solid (TSS) content was measured in triplicate using tomato juice using a digital refractometer (HI 96801, UK) and expressed as °Brix according to the method reported by Kumar et al.36 from day 0 and at the end of each 4 days storage interval.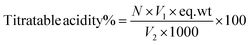 | (3) |
Analysis of bioactive compounds
Extraction of tomato phenolic compounds
The phenolic extract of tomato was obtained by homogenizing 30 g of sample in 10 mL of 80% methanol using a blender. The homogenate was centrifuged at 9000 rpm for 20 min at 4 °C using a benchtop centrifuge (Sorvall ST 8R, ThermoFisher Scientific, Germany), followed by filtering the collected supernatant using Whatman filter paper No. 1 and storage at −20 °C for further analysis.37Total phenolic content
Total phenolic content (TPC) was determined in triplicate at 8 days intervals from day 0 according to the method discussed by Dávila-Aviña et al.37 Briefly, a volume of 50 μL of tomato extract was mixed with 3 mL of deionized water and 250 μL of 1 N Folin–Ciocalteu reagent. After reacting for 5 min, 750 μL of 20% Na2CO3 (sodium carbonate) solution was added. After a 30 min reaction, the absorbance was measured at 760 nm using a UV-vis spectrophotometer (Genesys 10S UV-Vis, ThermoFisher Scientific, USA), and the results were expressed in mg of gallic acid equivalent (GAE) per 100 g of fresh weight.Total flavonoid content
Total flavonoid content (TFC) was determined in triplicate at 8 days intervals from day 0 as discussed by Zhishen et al.38 Briefly, a volume of 1 mL of extract was mixed with 4 mL of deionized water and 300 μL of 5% NaNO2 (sodium nitrite). After 5 min of equilibration, 300 μL of 10% AlCl3 (aluminum chloride) was added and rested for 1 min. A volume of 2 mL of 1 M NaOH was added and the sample volume was increased to 10 mL with deionized water. The absorbance was measured at 415 nm and the results were expressed in mg of rutin equivalent (RE) per 100 g of fresh weight.Antioxidant activity
DPPH radical scavenging activity
The stock solution was prepared by dissolving 2.5 mg of DPPH (2,2-diphenyl-1-picrylhydrazyl) radical in 100 mL of absolute methanol and the solution was adjusted at an absorbance of 0.7 ± 0.02 at 515 nm. A volume of 100 μL of tomato extract was mixed with 3.9 mL of DPPH and kept in the dark for 30 min. The absorbance was recorded at 515 nm and the antioxidant activity was calculated in triplicate samples using eqn (4), as discussed by Dávila-Aviña et al.37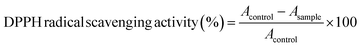 | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. A volume of 50 μL of sample extract was mixed with 950 μL of FRAP reagent and incubated at 37 °C for 30 min in a water bath. The absorbance was measured at 593 nm and the results were expressed as Trolox equivalent (μmol TE) g−1 of fresh weight.
1. A volume of 50 μL of sample extract was mixed with 950 μL of FRAP reagent and incubated at 37 °C for 30 min in a water bath. The absorbance was measured at 593 nm and the results were expressed as Trolox equivalent (μmol TE) g−1 of fresh weight.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (v/v) acetone
6 (v/v) acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-hexane and shaken for 5 min. After allowing the mixture to undergo phase separation for 5 min, the absorbance of the supernatant was determined at 663, 645, 505, and 453 nm using a UV-vis spectrophotometer. Eqn (5)–(8) were used to calculate the pigment contents.
n-hexane and shaken for 5 min. After allowing the mixture to undergo phase separation for 5 min, the absorbance of the supernatant was determined at 663, 645, 505, and 453 nm using a UV-vis spectrophotometer. Eqn (5)–(8) were used to calculate the pigment contents.| Chlorophyll a (mg 100 mL−1) = 0.999A663 − 0.989A645 | (5) |
| Chlorophyll b (mg 100 mL−1) = −0.328A663 + 1.77A645 | (6) |
| Lycopene (mg 100 mL−1) = −0.0458A663 + 0.204A645 + 0.372A505 − 0.0806A453 | (7) |
| β-catotene (mg 100 mL−1) = 0.216A663 − 1.22A645 − 0.304A505 + 0.452A453 | (8) |
Microbial analysis
The yeast and molds and the aerobic plate counts were determined in triplicate at 8 days intervals from day 0 as described in the Bacteriological Analytical Manual.41 Specifically, 1 g of tomato sample was homogenized with 10 mL of sterilized distilled water, which corresponds to a 10−1 dilution. Then, successive dilutions of 10−3 and 10−4 were obtained. The yeast and mold counts were determined by the spread-plate method using potato dextrose agar and incubation in the dark at 25 °C for 5 days. The aerobic plate counts were determined by the spread-plate method using plate count agar and incubation in the dark for 48 ± 2 h at 35 °C. The results were reported in colony forming units per gram (CFU g−1) of tomato.Decay percentage and shelf life
The decay percentage of coated and uncoated fruit was determined in triplicate using eqn (9), according to Ali et al.34 The fruits were stored at room temperature (26 ± 2 °C) until they started to rot. The number of days taken before the first rot appeared on the fruits was recorded as their shelf life, according to Osae et al.42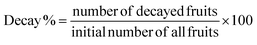 | (9) |
Statistical analysis
The experiment was carried out using a completely randomized design (CRD) with three replications unless otherwise specified. All the data were subjected to analysis of variance (ANOVA) using SAS® Studio statistical software version 3.81 (SAS Institute Inc., Cary, NC, USA), while the mean separation was performed by Duncan's multiple range tests with a significance level of p < 0.05. The results of the study were graphically represented using the OriginPro® 2023 (OriginLab Corporation, Northampton, MA, USA) software.Results and discussion
Physiological weight loss
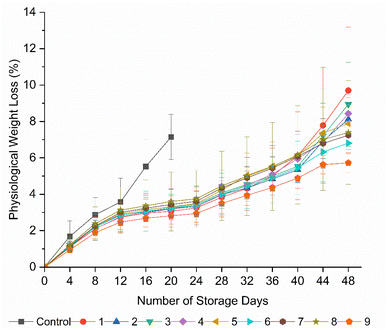
Physiological weight loss is a crucial factor that determines the postharvest storage life and quality attributes of fresh fruits and vegetables. Loss in weight is predominantly associated with a reduction in turgor pressure due to transpiration and loss of carbon reserves during cellular metabolism, with an increased respiration rate.43 Although both the coated and uncoated tomatoes exhibited weight loss over the storage period, the uncoated fruits displayed a significantly (p < 0.05) higher weight loss of 7.14% during 20 days of storage compared to all the coated tomatoes (Fig. 1). The rapid loss in weight as the storage period progressed is attributed to the high transpiration and respiration rates of the uncoated fruits, as discussed by Ruelas-Chacon et al.16All the coated tomatoes showed weight loss from the 4th day to the 48th day of storage without significant differences (p > 0.05) among them. However, at the end of storage, minimum weight losses of 5.75%, 6.81%, and 7.25% were found in formulations 9 (20 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW), 6 (15 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW), and 7 (20 g L−1 AS, 0 mL L−1 EO, 5 g L−1 BW), respectively. This could be explained by the thickness of the coatings with increased starch concentrations, and also the hydrophobicity of BW and clove EOs, given that they provide an obstructive barrier against the movement of moisture and solute between the inside and surrounding environment of the coated fruits compared to other coated fruits and uncoated controls.19,42 In addition to the barrier properties, the reduction in weight loss of the tomatoes coated with coatings containing clove EOs could be attributed to the antimicrobial and antioxidant properties of clove EOs.44 By reducing the microbial activity, clove EOs can maintain the integrity and moisture content of fruits, leading to a reduction in weight loss. Clove EOs help reduce the degradation of food components by preventing oxidative stress on fruit tissues, thus maintaining the fruit quality and reducing weight loss, owing to their antioxidant properties.45 Das et al.43 recorded a 3.53% reduction in weight loss in tomatoes coated with rice starch and coconut oil-based edible coating enriched with tea leaf extract compared to the uncoated fruits during storage at 24 °C for 20 days, demonstrating the moisture barrier properties of lipid-based edible coatings. The effect of the concentration of AS on the coating thickness should be considered given that a similar effect in weight loss was reported by Ali et al.,34 who recorded the minimum weight loss in tomatoes coated with 10% and 15% gum Arabic compared to 5% gum Arabic during 20 days of storage. This result was attributed to the high coating thickness, which sufficiently covered the fruit surface, and is consistent with the present study. Conversely, they reported a higher weight loss in the fruits coated with 20% gum Arabic due to the high thickness of the coating, leading to heat generation and loss of carbon reserves.
In contrast, the tomatoes coated with formulations 1 (10 g L−1 AS, 0 mL L−1 EO, 5 g L−1 BW), 2 (10 g L−1 AS, 2.5 mL L−1 EO, 5 g L−1 BW), and 3 (10 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW) showed an increase in weight loss, although the values were insignificant (p > 0.05) with other treatments, which is probably due to the high transpiration and respiration rates attributed to the low coating thickness with a low starch concentration, as discussed by Donjio et al.46 Nogueira et al.31 recorded a linear correlation between AS concentration and film thickness, which ranged from 0.026 ± 0.008 mm to 0.082 ± 0.011 mm as the AS concentration increased from 2.6% to 5.4%. The results of the present study are also in agreement with the study by Paladugu et al.,47 who reported a reduction in weight loss in tomatoes coated with a 1.5% gum Arabic nanoformulation with a shelf life of 14 days at 32 °C.
Fruit firmness
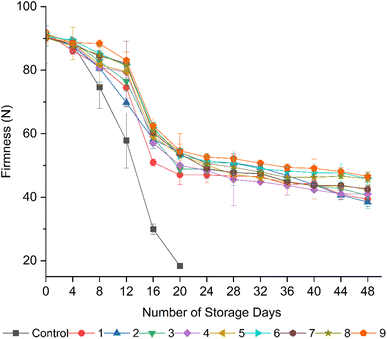
Although both the coated and uncoated tomatoes exhibited a reduction in firmness over the storage period, the uncoated fruits showed a significant (p < 0.05) loss in firmness within 20 days of storage (Fig. 2). This could be attributed to their rapid ripening, which resulted in rapid softening. According to Ali et al.,34 the reduction in firmness with the advancement of fruit ripening is ascribed to the degradation of cell structures, cell wall composition, and intracellular materials. Furthermore, pectinesterase48 and polygalacturonase49 enzymes catalyze the hydrolysis of pectin substances with the advancement of fruit ripening, leading to the depolymerization or shortening of the chain length of pectin substances, which increases the softening of fruits.29,50In contrast to the uncoated fruits, all the coated fruits showed higher retention in firmness. The fruits from formulation 9 (20 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW) maintained a higher firmness, followed by 6 (15 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW), 7 (20 g L−1 AS, 0 mL L−1 EO, 5 g L−1 BW), and 8 (20 g L−1 AS, 2.5 mL L−1 EO, 5 g L−1 BW) from day 8 to 28, but at the end of the storage period, the fruits from formulation 9 (20 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW) exhibited significant (p < 0.05) retention in firmness compared to the other coated fruits. The observed retention in firmness could be credited to the moisture barrier properties of the coating matrix, particularly provided by the incorporation of BW and clove EOs, given that they are hydrophobic in nature.51 Eugenol present in clove EOs possesses strong antimicrobial activities, causing a disruption in the cell membrane, which results in cell death.44 This mechanism helps to reduce the activities of degrading enzymes such as pectinesterase, polygalacturonase, and xylanase, which are secreted on the surface of tomatoes by several microbial species, including Bacillus, Erwinia, Kluyveromyces, Aspergillus, Rhizopus, Trichoderma, Pseudomonas, Penicillium, and Fusarium, leading to a retention in fruit firmness.49,52 On the other hand, oxidative stress on tomato flesh can lead to the breakdown of cell walls and membranes, resulting in softening.4 Clove EOs help maintain the cell wall structure and firmness by scavenging free radicals, which is ascribed to the presence of eugenol.44 A similar mechanism in the retention of firmness was reported by Donjio et al.46 in tomatoes coated with pineapple peel extract and Arabic gum, which was attributed to the antioxidants present in the pineapple peel extract. Moreover, as semipermeable barriers, coating materials alternate the internal atmosphere by reducing the oxygen level and elevating the carbon dioxide level, thus slowing biochemical reactions, which contributes to the preservation of fruit firmness during storage.34 The observations of the current study are consistent with the previous findings by Kumar et al.,36 who reported the maintenance of firmness in tomatoes coated with a chitosan-pullulan composite edible coating enriched with pomegranate peel extract compared to the uncoated control during storage at 23 °C for 15 days.
In contrast, the fruits from formulations 1 (10 g L−1 AS, 0 mL L−1 EO, 5 g L−1 BW), 2 (10 g L−1 AS, 2.5 mL L−1 EO, 5 g L−1 BW), and 3 (10 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW) showed a significant (p < 0.05) loss in firmness at the end of 48 days of storage, which may be traits of the effect of low coating thickness with a low starch content. This leads to an increase in cell wall degrading enzymatic activities associated with an increased respiration rate and due to the low water vapor barrier properties of the coatings.15
Fruit color
Color is a significant determinant of the quality of tomatoes, with predominant redness being an indicator of the presence of lycopene, followed by carotenes (yellow to orange) and xanthophylls (yellow), which contribute to consumer acceptability.53 A gradual reduction in lightness (L*) was recorded in both the coated and uncoated fruits over the storage period (see Tables S1–S4 in the ESI†). However, the uncoated fruits displayed the highest reduction in lightness from the initial 47.37 ± 2.15 to 38.40 ± 6.24 during 20 days of storage. Similarly, the fruits from formulation 1 (10 g L−1 AS, 0 mL L−1 EO, 5 g L−1 BW) showed a rapid decline in lightness from the initial 49.27 ± 0.12 to 40.70 ± 1.05 over the 48 days storage period. Alternatively, the fruits from formulation 6 (15 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW) displayed a significant (p < 0.05) retention in lightness with a reduced decline rate from the initial 49.33 ± 1.53 to 47.33 ± 1.97 over the storage period of 48 days.The results indicated an increased trend in redness (a*) and yellowness (b*), followed by predominantly constant yellowness in both the coated and uncoated fruits over the storage period. At the end of 48 days storage, the fruits from formulations 6 (15 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW) and 8 (20 g L−1 AS, 2.5 mL L−1 EO, 5 g L−1 BW) exhibited the lowest redness values of 19.80 ± 1.44 and 19.47 ± 1.46, respectively, demonstrating a reduced ripening rate. In contrast, the uncoated fruits showed the highest redness value of 26.40 ± 3.64 and yellowness value of 53.37 ± 3.84 at the end of storage, indicating rapid ripening, which was attributed to the degradation of chlorophyll pigments and synthesis of carotenoids, predominantly lycopene.5,54 Moreover, compared to the coated fruits, the uncoated tomatoes rapidly changed their color from green and yellow to red within 4 to 8 days of storage, displaying rapid ripening and the highest total color difference (ΔE) of 34.72 ± 5.13. In agreement with these observations, Ali et al.34 reported that the color of uncoated fruits changed from green to red within 4 to 8 days of storage. According to Pholsin et al.,55 the rapid color change in uncoated tomatoes can be due to increased ethylene production, resulting in the highest redness value of 35.77 ± 0.05 due to the synthesis of lycopene compared to tomatoes coated with a cocoa shell pectin-based coating. However, at the end of 48 days storage, a significant (p < 0.05) reduction in the increment of ΔE was represented in the tomatoes from formulations 8 (20 g L−1 AS, 2.5 mL L−1 EO, 5 g L−1 BW), 9 (20 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW), and 5 (15 g L−1 AS, 2.5 mL L−1 EO, 5 g L−1 BW) with lower ΔE values of 27.17 ± 2.11, 27.20 ± 2.35, and 30.30 ± 1.91, respectively. This could be explained by the reduced respiration rate in the fruits due to the elevated carbon dioxide and decreased oxygen concentrations, as reported by Paul et al.57 According to Paul et al.,57 tomatoes the coated with 2.15% chitosan and 0.05% glycerol exhibited a reduced respiration rate of 21.21 ± 0.06 mg CO2 kg−1 h−1 and ΔE of 2.31 ± 0.01 during storage. This reduction is due to the formation of a thick, and continuous coating, which covered the epidermal openings and altered the internal atmosphere, resulting in a higher carbon dioxide and lower oxygen level. In contrast, the uncoated control tomatoes showed a respiration rate of 42.6 ± 0.98 mg CO2 kg−1 h−1 and ΔE of 3.66 ± 0.07, indicating rapid ripening. An elevated carbon dioxide level decreases ethylene synthesis in tomatoes during ripening, which can delay color changes, as reported in many studies.16,55,57 Furthermore, according to Asiamah et al.56 alterations in tomato color, especially reduction in lightness are possibly related to the mold contaminations on the fruit surface. However, by inhibiting the growth of bacteria and fungi on the tomato surface, clove EOs reduce the production of microbial enzymes and metabolites that can degrade pigments and lead to discoloration.44 In addition, the antioxidants present in clove EOs such as eugenol help prevent the breakdown of tomato pigments, such as lycopene and β-carotene from oxidative degradation by scavenging free radicals.58
Conversely, the fruits coated with formulation 1 (10 g L−1 AS, 0 mL L−1 EO, 5 g L−1 BW) presented the highest ΔE value from the initial 13.78 ± 2.77 to 26.69 ± 4.43, which could be attributed to the high ethylene synthesis due to the low coating thickness with a low starch concentration in the formulation, as discussed by Donjio et al.46 Overall, the results suggested that the application of the AS and BW-based edible coatings delayed the ripening of the tomatoes compared to the uncoated fruits. Kumar et al.36 revealed a reduced increment in a*, b*, and ΔE values and a reduction in L* values in tomatoes coated with chitosan-pullulan composite edible coatings compared to the uncoated fruits during storage at 23 °C and 4 °C. Similar to the uncoated fruits, the tomato coated with different concentrations of cassava starch-chitosan edible coatings enriched with Lippia sidoides EOs and pomegranate peel extract exhibited decreased L*, constant b*, and increased a* values as the storage period progressed.53
Titratable acidity, pH, and total soluble solid content
Although several organic acids are present in tomatoes, TA is a measure of the presence of citric acid, which is the predominant organic acid in tomatoes.11 Regardless of the coating treatments, all the fruits showed a gradual reduction in TA over the storage period (Fig. 3). However, the uncoated fruits exhibited a significant (p < 0.05) drop in TA compared to the coated fruits from day 4, implying a high respiration rate and ethylene synthesis.59 As a climacteric fruit, tomato continues respiration even after harvesting, which utilizes citric acid to supply intermediates to the tricarboxylic acid cycle, resulting in a decline in TA.11,60 The fruits treated with formulation 6 (15 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW) exhibited a significant (p < 0.05) retention in TA throughout the storage period, followed by formulations 9, 8, 7, and 5. However, a significant (p < 0.05) reduction in TA was noted from formulations 1 and 2, where the fruits were coated with coating solutions containing 10 g L−1 of AS, and this trend indicates a reduction in respiration rate with an increase in the concentration of AS in the coating solutions, as discussed by Zhang et al.61 and Dwivany et al.62 In agreement with these findings, Donjio et al.46 recorded a similar trend in the reduction of TA in tomatoes coated with pineapple peel extract and Arabic gum coatings during storage, and they stated the direct correlation between retention in TA and increased concentration of Arabic gum. Adjouman et al.63 reported a significant delay in the changes in TA in tomatoes coated with cassava starch-based composite edible coatings compared to the uncoated fruits and fruits coated with commercial Semperfresh™.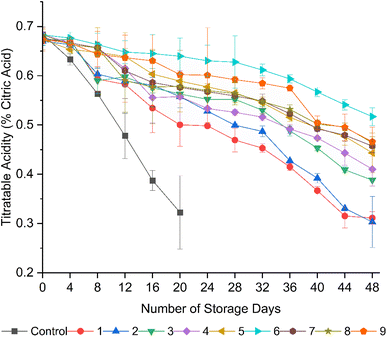 | ||
| Fig. 3 Effect of edible coatings on the titratable acidity (% citric acid) of tomatoes during storage. | ||
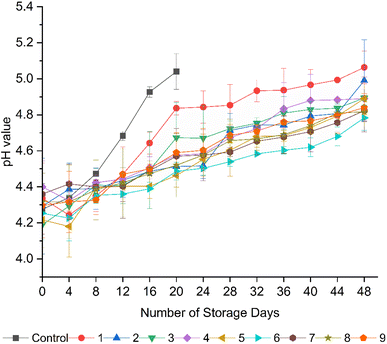
In general, the pH value of fruits increases with ripening due to the consumption of organic acids in cellular metabolism during respiration.54,64 As shown in Fig. 4, an increment in pH value, which was proportional to the decline in TA, was recorded for all the fruits regardless of the coating material but was significantly (p < 0.05) higher in the uncoated fruits (pH 4.28 to 5.04), implying their faster ripening. Similar to TA, the fruits from formulation 6 (15 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW) showed a significant (p < 0.05) retention in pH value, followed by formulations 9, 5, and 7. According to Peralta-Ruiz et al.,4 microbial spoilage in tomatoes is primarily responsible for fungal attacks by Rhizopus stolonifera, Aspergillus niger, Penicillium expansum, and Botrytis cinerea, producing various degrading enzymes and metabolites, which lead to alternations in the pH value and TA in fruits. The addition of clove EOs in coatings could help to retain the pH value and TA during storage by reducing microbial growth and acting as a natural antimicrobial agent.25 An increase in the pH value of tomatoes during their storage is primarily associated with the reduction in TA, which is related to the high respiration rate in uncoated fruits and the restricted respiration rate in coated fruits due to the limited availability of oxygen, as stated in many studies.4,16,29,43 For instance, Ruelas-Chacon et al.16 recorded the highest carbon dioxide production of 10.7 mL kg−1 h−1 in uncoated tomatoes, compared to the lowest carbon dioxide production of 2.8 mL kg−1 h−1 in tomatoes coated with a 1.5% guar gum coating, indicating a delayed respiration rate due to the modification of the internal atmosphere by the coating. Edible coatings act as semi-permeable barriers, which limit the exchange of gases such as oxygen and carbon dioxide between the fruit and the external environment, thereby slowing down the respiration rate.10,56 The reduced oxygen availability and elevated carbon dioxide concentration create a modified atmosphere around the fruit and lead to a reduction in metabolic activities, which are responsible for ripening and senescence.45 Araújo et al.53 reported a slight increase in pH value (4.62 to 5.77) in tomatoes coated with cassava starch-chitosan coatings enriched with Lippa sidoides EOs and pomegranate peel extract during storage at 25 °C for 12 days compared to the uncoated control. The results of pH value in the present study are also in agreement with the study by Firdous et al.,65 who reported a slight increment in pH value from 4.98 to 5.00 in tomatoes coated with 80% Aloe vera gel and 2% calcium chloride edible coating after 30 days of storage.
Several authors reported an increase in the TSS content of tomatoes with the advancement of ripening, and subsequently, a decline toward senescence,2,42,43,66,67 which is consistent with the results of the present study. Regardless of the coating treatment, all the fruits showed a slight increase in TSS content over the storage period (Fig. 5). However, the TSS content was significantly (p < 0.05) increased from day 8 to day 16 in the uncoated fruits, followed by a decrease with senescence within 20 days of storage. Tigist et al.67 also revealed an initial increment in TSS content during the maturation of the fresh commercial tomato varieties, followed by a decline with senescence, which was attributed to the reduced hydrolysis rate of carbohydrates.
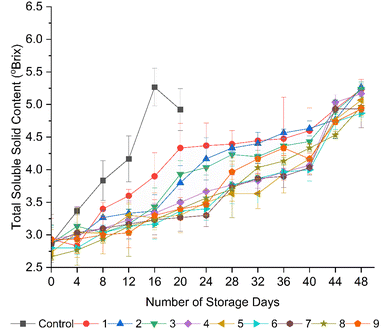 | ||
| Fig. 5 Effect of edible coatings on the total soluble solid content (°Brix) of tomatoes during storage. | ||
At the end of storage, significantly (p < 0.05), the lowest increment in TSS content was found in the tomatoes coated with formulation 6 (15 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW), indicating a reduced respiration rate and ethylene generation, as previously stated by Pholsin et al.55 The results suggest that the application of the AS and BW-based composite edible coatings provided an excellent semipermeable barrier around the fruits, modifying their internal gas composition by reducing the oxygen level and elevating the carbon dioxide level, thus reducing ethylene synthesis, as discussed by Asiamah et al.56 The reduced increment in TSS content in the tomatoes coated with formulation 6 could also be ascribed to the antimicrobial properties of clove EOs, given that they inhibit the growth of spoilage microorganisms and their enzymes, which are responsible for the breakdown of complex carbohydrates into simpler sugars, thereby reducing the increase in TSS.58 In addition, by scavenging free radicals, clove EOs reduce oxidative damage, which prevents cellular breakdown and the release of soluble solids into the tomato juice, leading to a reduced increment in TSS.44 Nevertheless, the tomatoes coated with formulations 1, 2, and 3, which contained 10 g L−1 AS, exhibited significant (p < 0.05) increments in TSS content during their storage, which is probably due to the increase in biochemical reactions that occurred within the cells. This is attributed to the high respiration rate, which is triggered by a low coating thickness, as discussed by Donjio et al.46 An increased TSS content is attributed to the degradation of complex carbohydrates, including starch, hemicellulose, and pectin present in the fruit cells and cell walls, into simple sugars in addition to a reduction in moisture in the fruits during storage.29,54
Total phenolic content and total flavonoid content
Phenols and flavonoids are secondary metabolites, which are synthesized with the advancement of fruit ripening.36,37,40 As shown in Tables 2 and 3, the uncoated control fruits exhibited a significant (p < 0.05) increment in TPC and TFC by the 8th day of storage, and thereafter a rapid decline until the end of storage. Similarly, the fruits coated with the formulations containing 10 g L−1 of AS showed a rapid increment in TPC and TFC, and thereafter a slow decline toward senescence, as previously observed by Dávila-Aviña et al.37 and Pholsin et al.55 This could be explained by the degradation of phenolic compounds due to the high respiration rate and senescence of the uncoated fruits and the coated fruits having a low coating thicknesses, as discussed by Ali et al.34 Similarly, Pholsin et al.55 reported that tomatoes coated with a pectin-based edible coating had the maximum TPC at 6th day of storage, followed by a decrease due to higher respiration rates during storage at 4 °C for 30 days. In contrast, the fruits treated with formulations 6 (15 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW), 7 (20 g L−1 AS, 0 mL L−1 EO, 5 g L−1 BW), and 9 (20 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW) showed a reduced but continuous increment in TPC and TFC throughout the storage period, indicating a deceleration of the maturation process by the applied coating treatments.| Formulation | Number of storage days | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 8 | 16 | 24 | 32 | 40 | 48 | |
| a Values that do not bear the same lowercase letter(s) within a column and the same uppercase letter(s) within a row are significantly different (p < 0.05). Results are mean ± standard deviation of triplicate findings. n.d., not determined. fw, fresh weight. | |||||||
| Control | 7.65 ± 0.13cC | 12.39 ± 0.04aA | 10.85 ± 0.09aB | n.d. | n.d. | n.d. | n.d. |
| 1 | 7.31 ± 0.02deG | 9.45 ± 0.02bE | 9.89 ± 0.05bD | 10.01 ± 0.06dC | 12.25 ± 0.02bA | 10.21 ± 0.03hB | 8.43 ± 0.02hF |
| 2 | 8.01 ± 0.03bE | 9.21 ± 0.11dD | 9.91 ± 0.08bC | 11.02 ± 0.03aB | 13.71 ± 0.02aA | 9.98 ± 0.01iC | 7.21 ± 0.02iF |
| 3 | 7.43 ± 0.11dF | 8.21 ± 0.02hE | 8.31 ± 0.02fD | 10.05 ± 0.03cdC | 11.28 ± 0.03cA | 11.21 ± 0.05fA | 10.85 ± 0.05gB |
| 4 | 8.35 ± 0.12aG | 8.46 ± 0.05fF | 8.82 ± 0.02eE | 9.25 ± 0.05eD | 10.11 ± 0.01gC | 11.38 ± 0.02dB | 12.83 ± 0.01dA |
| 5 | 7.21 ± 0.05eG | 7.91 ± 0.03iF | 8.31 ± 0.06fE | 8.98 ± 0.07gD | 9.41 ± 0.04iC | 10.31 ± 0.01gB | 11.02 ± 0.03fA |
| 6 | 7.98 ± 0.14bG | 8.34 ± 0.04gF | 8.91 ± 0.03deE | 9.12 ± 0.02fD | 9.56 ± 0.04hC | 11.31 ± 0.04eB | 12.9 ± 0.02cA |
| 7 | 6.95 ± 0.09fG | 9.31 ± 0.02cF | 9.72 ± 0.03cE | 10.10 ± 0.02cD | 10.56 ± 0.02eC | 13.35 ± 0.05aB | 13.81 ± 0.04bA |
| 8 | 7.71 ± 0.23cF | 8.85 ± 0.01eE | 8.98 ± 0.02dE | 10.01 ± 0.01dD | 10.31 ± 0.01fC | 11.83 ± 0.02cB | 12.25 ± 0.01eA |
| 9 | 8.21 ± 0.05aF | 8.93 ± 0.05eE | 8.89 ± 0.11deE | 10.56 ± 0.01bD | 10.83 ± 0.02dC | 12.31 ± 0.01bB | 13.88 ± 0.02aA |
| Formulation | Number of storage days | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 8 | 16 | 24 | 32 | 40 | 48 | |
| a Values that do not bear the same lowercase letter(s) within a column and the same uppercase letter(s) within a row are significantly different (p < 0.05). Results are mean ± standard deviation of triplicate findings. n.d., not determined. fw, fresh weight. | |||||||
| Control | 3.13 ± 0.05hC | 6.31 ± 0.28aB | 8.12 ± 0.09aA | n.d. | n.d. | n.d. | n.d. |
| 1 | 3.45 ± 0.02fE | 6.01 ± 0.08aD | 6.91 ± 0.05bC | 6.92 ± 0.02aC | 7.03 ± 0.05cBC | 8.56 ± 0.14aA | 7.21 ± 0.23fB |
| 2 | 3.38 ± 0.01gG | 5.98 ± 0.06aF | 6.71 ± 0.11cE | 6.93 ± 0.03aD | 7.80 ± 0.09bC | 8.42 ± 0.09bA | 7.95 ± 0.05cdB |
| 3 | 4.21 ± 0.01aF | 5.78 ± 0.13abE | 6.81 ± 0.04bcD | 6.85 ± 0.13aD | 7.93 ± 0.11aC | 8.13 ± 0.09cB | 8.30 ± 0.02bA |
| 4 | 3.98 ± 0.03cE | 5.21 ± 0.81cD | 5.35 ± 0.04fD | 6.52 ± 0.11bC | 6.93 ± 0.07cBC | 7.25 ± 0.01eB | 7.98 ± 0.03cdA |
| 5 | 4.02 ± 0.01cE | 5.31 ± 0.16bcD | 5.93 ± 0.10dC | 6.04 ± 0.02cC | 6.75 ± 0.03dB | 7.91 ± 0.12dA | 8.02 ± 0.03cA |
| 6 | 4.01 ± 0.02cF | 4.98 ± 0.08cE | 5.02 ± 0.02gE | 5.56 ± 0.11dD | 6.72 ± 0.03dC | 6.98 ± 0.03fB | 7.52 ± 0.07eA |
| 7 | 3.52 ± 0.02eG | 4.25 ± 0.05dF | 4.91 ± 0.09ghE | 5.08 ± 0.08fD | 5.85 ± 0.05fC | 6.71 ± 0.03gB | 7.81 ± 0.02dA |
| 8 | 3.81 ± 0.05dE | 4.78 ± 0.31cD | 4.82 ± 0.03hD | 5.21 ± 0.02eC | 5.39 ± 0.09gC | 6.91 ± 0.06fB | 8.28 ± 0.04bA |
| 9 | 4.09 ± 0.02bG | 4.80 ± 0.21cF | 5.47 ± 0.04eE | 5.95 ± 0.02cD | 6.27 ± 0.02eC | 7.89 ± 0.01dB | 8.98 ± 0.14aA |
Edible coatings can create abiotic stress on fresh fruits, and thus alter their cellular metabolism. Specifically, they create a semi-permeable barrier, which can limit the exchange of oxygen, carbon dioxide, and water vapor between the fruit and the environment, inducing abiotic stress on fruits.29,68 In addition, the presence of antioxidants in coatings such as clove EOs can induce antioxidative defense mechanisms in fruits, thus promoting abiotic stress, as discussed by Peralta-Ruiz et al.4 This mechanism affects the generation of secondary metabolites such as phenolics and flavonoids.69 Phenolics and flavonoids play a crucial role in the protective mechanism by inhibiting pathogenic infections in tomatoes. A higher phenolic and flavonoid content in plants is closely related to increased resistance to pathogens.29 Furthermore, stimulation of the synthesis of phenolic compounds in tomatoes when exposed to oregano EOs has been reported as a stress response from fruit tissues.70 Similarly, the increased concentrations of phenolics and flavonoids in the tomatoes coated with the formulations containing clove EO emulsions could be explained by the exposure of the fruits to clove EOs in the present study.
The results of the present study indicate a relationship between TPC and TFC and the color and firmness of tomatoes. As the storage period progressed, the increasing trend in redness and yellowness in both the coated and uncoated tomatoes was positively related to the increment in TPC and TFC, indicating fruit ripening. With the advancement of ripening, tomatoes produce more phenolics and flavonoids in response to abiotic stress, which can stimulate the biosynthesis of pigments such as lycopene and β-carotene.4 For instance, the synthesis of flavonoids is crucial in plants to produce yellow and other pigments.29,55 The retention in fruit color in the coated fruits especially with formulation 6 (15 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW) could be attributed to the antioxidant properties of the phenolic and flavonoid compounds and clove EOs, given that they protect tomato pigments such as lycopene and β-carotene from oxidative degradation.44 Moreover, the retention in firmness in the coated tomatoes could be credited to the presence of phenolics and flavonoids given that they increase the microbial resistance of fruits, thus reducing spoilage-causing microorganisms and their enzymes, which leads to the retention in cellular integrity and fruit firmness, as discussed by Kumar et al.29
Antioxidant activity
Tables 4–6 show the antioxidant activity of both the coated and uncoated tomatoes in terms of DPPH radical scavenging activity, ferric reducing antioxidant power, and ABTS radical scavenging activity, respectively. Significantly (p < 0.05) the highest increment in total antioxidant activity was observed in the uncoated control fruits within 8 days of storage, and then decreased drastically as the storage period progressed. In comparison to the other coated fruits, the fruits coated with formulations 1 (10 g L−1 AS, 0 mL L−1 EO, 5 g L−1 BW), 2 (10 g L−1 AS, 2.5 mL L−1 EO, 5 g L−1 BW), and 3 (10 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW) exhibited an elevated increment in antioxidant activity, and thereafter a decline over the storage period. The increase in antioxidant activity observed in the coated and uncoated fruits suggests that they could not delay fruit ripening and the associated metabolic reactions occurring inside the fruits. As a response to high oxidative stress, which is triggered by environmental factors such as exposure to oxygen, the tomatoes exhibited a rapid initial increment in antioxidant activity, indicating a faster ripening process with accelerated respiration rates.4,71 This high increment in antioxidant activity is not desirable given that it leads to premature softening, color changes, and potential flavor loss.5 Rapid ripening can shorten the shelf life of tomatoes, making them less desirable for prolonged storage and marketing.5 In addition, it also affects the balance of other biochemical processes, leading to quality degradation, including changes in texture and taste.29 Conversely, the tomatoes coated with formulations 6 (15 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW), 7 (20 g L−1 AS, 0 mL L−1 EO, 5 g L−1 BW), 8 (20 g L−1 AS, 2.5 mL L−1 EO, 5 g L−1 BW), and 9 (20 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW) showed significantly (p < 0.05) delayed but continuous increment in antioxidant activity during storage, indicating delayed ripening due to the reduced rate of biochemical reactions.29 The addition of clove EOs to the coating solutions drastically improved the antioxidant activity of the tomatoes, which is probably attributed to the induced defense mechanisms, producing phenolic compounds in response to the abiotic stress, as discussed by Bonilla et al.68| Formulation | Number of storage days | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 8 | 16 | 24 | 32 | 40 | 48 | |
| a Values that do not bear the same lowercase letter(s) within a column and the same uppercase letter(s) within a row are significantly different (p < 0.05). Results are mean ± standard deviation of triplicate findings. n.d., not determined. | |||||||
| Control | 18.38 ± 0.02aC | 38.56 ± 0.67aA | 23.21 ± 0.21fB | n.d. | n.d. | n.d. | n.d. |
| 1 | 19.01 ± 1.00aG | 32.78 ± 0.16cD | 34.12 ± 0.12bC | 36.81 ± 0.02bB | 38.71 ± 0.54bA | 31.02 ± 0.05eE | 26.74 ± 0.25fF |
| 2 | 18.21 ± 0.06aE | 31.35 ± 0.11dC | 31.47 ± 0.18cC | 33.58 ± 0.31dB | 38.69 ± 0.38bA | 33.47 ± 0.31cB | 29.56 ± 0.23dD |
| 3 | 18.33 ± 0.03aF | 29.68 ± 0.37eD | 33.87 ± 0.14bB | 34.75 ± 0.16cA | 34.68 ± 0.26eA | 30.02 ± 0.06fC | 27.36 ± 0.08eE |
| 4 | 19.32 ± 1.01aF | 33.65 ± 0.31bD | 35.49 ± 0.23aC | 37.89 ± 0.4aB | 39.69 ± 0.12aA | 37.89 ± 0.11bB | 32.58 ± 0.09cE |
| 5 | 18.56 ± 0.04aG | 25.69 ± 0.21fF | 29.33 ± 0.2dE | 32.58 ± 0.23eD | 36.78 ± 0.27cC | 38.47 ± 0.23aB | 39.89 ± 0.14aA |
| 6 | 18.33 ± 0.98aG | 23.65 ± 0.08hF | 28.74 ± 0.03eE | 32.78 ± 0.14eD | 34.58 ± 0.06eC | 37.98 ± 0.24bB | 39.99 ± 0.14aA |
| 7 | 19.24 ± 0.15aF | 24.36 ± 0.01gE | 31.47 ± 0.26cD | 32.14 ± 0.31fC | 35.89 ± 0.05dB | 38.55 ± 0.02aA | 38.71 ± 0.26bA |
| 8 | 18.91 ± 0.23aF | 19.33 ± 0.03iF | 20.88 ± 0.07gE | 28.96 ± 0.3gD | 34.32 ± 0.51eC | 37.85 ± 0.31bB | 40.12 ± 0.12aA |
| 9 | 18.02 ± 0.11aG | 19.85 ± 0.15jF | 20.45 ± 0.12hE | 29.33 ± 0.12gD | 30.54 ± 0.22fC | 32.65 ± 0.26dB | 40.01 ± 0.13aA |
| Formulation | Number of storage days | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 8 | 16 | 24 | 32 | 40 | 48 | |
| a Values that do not bear the same lowercase letter(s) within a column and the same uppercase letter(s) within a row are significantly different (p < 0.05). Results are mean ± standard deviation of triplicate findings. n.d., not determined. fw, fresh weight. | |||||||
| Control | 0.28 ± 0.01cC | 1.69 ± 0.11aA | 0.84 ± 0.02fB | n.d. | n.d. | n.d. | n.d. |
| 1 | 0.27 ± 0.02cE | 1.59 ± 0.02bA | 1.62 ± 0.12aA | 0.97 ± 0.01fB | 0.82 ± 0.01fC | 0.69 ± 0.03gD | 0.24 ± 0.03eE |
| 2 | 0.29 ± 0.00bcD | 1.58 ± 0.03bA | 1.64 ± 0.09aA | 0.96 ± 0.06fB | 0.74 ± 0.01gC | 0.73 ± 0.02fC | 0.31 ± 0.01dD |
| 3 | 0.35 ± 0.01aD | 0.95 ± 0.01cC | 1.47 ± 0.01bB | 1.78 ± 0.01bA | 0.35 ± 0.02hD | 0.31 ± 0.02hE | 0.27 ± 0.02eF |
| 4 | 0.31 ± 0.01bF | 0.89 ± 0.02cE | 1.36 ± 0.03cB | 1.88 ± 0.03aA | 1.23 ± 0.12eC | 1.10 ± 0.01eD | 0.96 ± 0.01cE |
| 5 | 0.27 ± 0.02cF | 0.58 ± 0.11eE | 0.97 ± 0.03dD | 1.05 ± 0.02deD | 1.47 ± 0.03cC | 1.89 ± 0.02aA | 1.64 ± 0.02bB |
| 6 | 0.31 ± 0.01bG | 0.87 ± 0.02cF | 0.94 ± 0.02deE | 1.25 ± 0.03cD | 1.37 ± 0.02dC | 1.75 ± 0.02cB | 1.82 ± 0.02aA |
| 7 | 0.28 ± 0.01cG | 0.92 ± 0.03cF | 0.99 ± 0.02dE | 1.03 ± 0.01eD | 1.56 ± 0.02bC | 1.74 ± 0.03cB | 1.80 ± 0.01aA |
| 8 | 0.34 ± 0.01aG | 0.74 ± 0.01dF | 0.87 ± 0.01efE | 1.05 ± 0.02deD | 1.54 ± 0.01bcC | 1.68 ± 0.01dB | 1.79 ± 0.01aA |
| 9 | 0.29 ± 0.01bcE | 0.94 ± 0.02cD | 0.96 ± 0.02deD | 1.09 ± 0.02dC | 1.67 ± 0.02aB | 1.84 ± 0.01bA | 1.81 ± 0.02aA |
| Formulation | Number of storage days | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 8 | 16 | 24 | 32 | 40 | 48 | |
| a Values that do not bear the same lowercase letter(s) within a column and the same uppercase letter(s) within a row are significantly different (p < 0.05). Results are mean ± standard deviation of triplicate findings. n.d., not determined. fw, fresh weight. | |||||||
| Control | 60.32 ± 0.12gC | 124.69 ± 0.23aA | 99.65 ± 0.13gB | n.d. | n.d. | n.d. | n.d. |
| 1 | 62.31 ± 0.01dG | 105.47 ± 0.16dC | 116.81 ± 0.03bB | 133.25 ± 0.11aA | 95.11 ± 0.12iD | 81.35 ± 0.02gE | 74.69 ± 0.05hF |
| 2 | 61.25 ± 0.16eF | 102.65 ± 0.22eC | 103.56 ± 0.28fB | 129.13 ± 0.13bA | 98.75 ± 0.22hD | 102.35 ± 0.24eC | 84.69 ± 0.02fE |
| 3 | 60.98 ± 0.11fG | 112.47 ± 0.03cD | 116.95 ± 0.14bC | 120.56 ± 0.21fA | 117.36 ± 0.12gB | 98.35 ± 0.22fE | 79.85 ± 0.19gF |
| 4 | 63.47 ± 0.12aG | 99.87 ± 0.06gF | 115.25 ± 0.81cE | 123.67 ± 0.34dC | 128.96 ± 0.17bA | 127.36 ± 0.16dB | 116.35 ± 0.03eD |
| 5 | 62.58 ± 0.07cG | 101.35 ± 0.14fF | 120.36 ± 0.05aE | 122.54 ± 0.24eD | 126.66 ± 0.17dC | 130.25 ± 0.02bB | 132.27 ± 0.09bA |
| 6 | 62.78 ± 0.06cG | 112.86 ± 0.09bF | 114.99 ± 0.21cE | 118.25 ± 0.17gD | 124.74 ± 0.25eC | 127.19 ± 0.05dB | 131.25 ± 0.08dA |
| 7 | 60.87 ± 0.23fG | 97.36 ± 0.04hF | 108.98 ± 0.14eE | 123.58 ± 0.01dD | 129.87 ± 0.14aC | 130.25 ± 0.31bB | 131.04 ± 0.01dA |
| 8 | 63.05 ± 0.17bG | 97.58 ± 0.02hF | 110.25 ± 0.03dE | 125.54 ± 0.33cD | 127.36 ± 0.11cC | 135.85 ± 0.11aB | 138.95 ± 0.12aA |
| 9 | 62.75 ± 0.09cG | 89.2 ± 0.11iF | 103.27 ± 0.42fE | 114.89 ± 0.51hD | 119.63 ± 0.17fC | 128.74 ± 0.11cB | 131.57 ± 0.45cA |
A direct correlation between TPC and total antioxidant activity has been reported in many studies.37,72,73 The TPC and total antioxidant activity increased with the advancement of fruit ripening mainly due to alterations in lipophilic antioxidant activity.74 Carotenoids, ascorbic acid, and phenolic compounds are the main antioxidants found in tomatoes, although the antioxidant activity of tomatoes can vary depending on their genetics, environmental conditions, maturity stage, and pre- and postharvest conditions.75–77 In addition, the antioxidant activity of tomatoes can also fluctuate due to variations in γ-tocopherol, β-carotene, and vitamin E concentrations.40,75 Maintaining the antioxidant activity in tomatoes provides numerous potential health benefits for consumers, including a reduced risk of chronic diseases such as cardiovascular disease, diabetes, and certain cancers, anti-inflammatory activity, enhanced immune functions, and reduced male and female infertility.78 Additionally, antioxidants help to maintain fruit quality and sensory attributes, while improving the shelf life.5
The results indicated a direct relationship between antioxidant activity and fruit color and firmness. The gradual increment in the antioxidant activity of the coated tomatoes especially from formulation 6 (15 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW) is directly related to the increased trend in redness and yellowness, which is probably attributed to the synthesis of pigments such as lycopene and β-carotene, improving their antioxidant activity with the advancement of fruit ripening.40 Similarly, the coated fruits retained their color, while maintaining their antioxidant activity during storage. In contrast, the uncoated fruits exhibited a rapid increment in redness from the initial −5.00 ± 1.40 to 26.40 ± 3.64, with a high initial increment in antioxidant activity followed by a decline over the storage period. The retention in fruit firmness could also be related to the antioxidant activity of tomatoes, given that antioxidants help to the maintain cell wall integrity and fruit firmness by inhibiting the activity of enzymes such as polygalacturonase and pectinesterase, which break down pectin in the cell walls and lead to fruit softening.29,56
Chlorophyll ‘a’, chlorophyll ‘b’, lycopene, and β-carotene contents
As shown in Fig. 6, a reduction in the concentrations of chlorophyll ‘a’ and chlorophyll ‘b’ and increments in lycopene and β-carotene contents were recorded for both the coated and uncoated tomatoes during storage. However, the rate of the degradation of chlorophyll ‘a’ and ‘b’ pigments and the synthesis of lycopene and β-carotene were recorded to be significantly (p < 0.05) higher for the uncoated fruits, followed by the tomatoes coated with formulations 1 (10 g L−1 AS, 0 mL L−1 EO, 5 g L−1 BW), 2 (10 g L−1 AS, 2.5 mL L−1 EO, 5 g L−1 BW), and 3 (10 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW), implying their elevated ripening as a function of a higher respiration rate and metabolic activity, as discussed by Naeem et al.40 Nevertheless, the tomatoes coated with formulations 6 (15 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW), 8 (20 g L−1 AS, 2.5 mL L−1 EO, 5 g L−1 BW), and 9 (20 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW) exhibited significantly (p < 0.05) delayed rates of production and breakdown of the pigments, which is attributed to the restricted maturation process during storage.71 Javanmardi et al.79 reported that the temperature range and respiration rate are the major factors that affect the synthesis of lycopene in tomatoes during storage. Furthermore, significantly (p < 0.05) delayed rates of production and breakdown of pigments in the fruits with formulations 6, 8, and 9 could also be explained by the effects of clove EOs. Chlorophyll ‘a’ and ‘b’ pigments are highly susceptible to oxidative degradation, and by reducing oxidative stress, clove EOs help to delay pigment breakdown.44 Similarly, as antioxidants of plant origin, clove EOs, particularly eugenol, contribute to reducing the degradation of lycopene and β-carotene pigments.25 Clove EOs are also responsible for the stabilization of lycopene and β-carotene by inhibiting microbial attack and oxidative degradation, thereby maintaining the quality and nutritional benefits of the fruits for a longer period.4 In agreement with the present results, Naeem et al.40 recorded an increment in β-carotene and lycopene contents together with a decrease in the contents of chlorophyll ‘a’ and ‘b’ pigments in tomatoes during storage.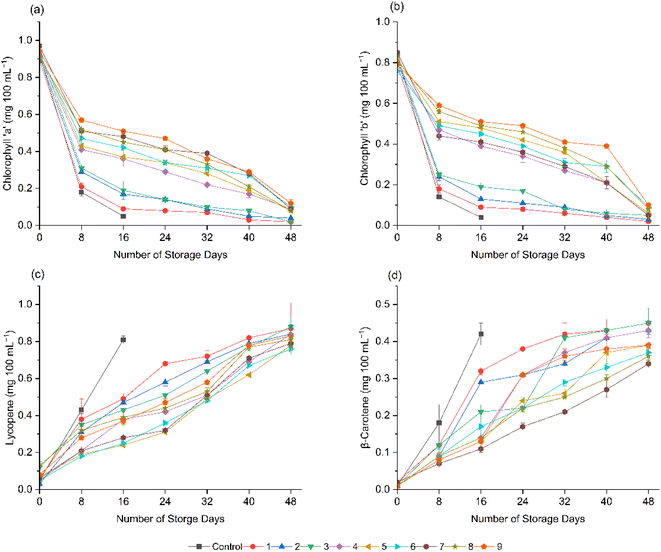 | ||
| Fig. 6 Effect of edible coatings on pigment contents (mg 100 mL−1) of tomatoes during storage: chlorophyll a (a), chlorophyll b (b), lycopene (c), and β-carotene (d). | ||
Maintaining the lycopene and β-carotene contents in tomatoes provides various health benefits, including prevention of cardiovascular diseases, cancer, and diabetes, and protection of skin and eye health.78 In addition, lycopene and β-carotene enhance the nutritional quality and attractiveness of fruits, while improving their shelf life.5
Microbial analysis
The spoilage of tomato is ascribed to fungal and bacterial rot.80 As shown in Table 7, irrespective of the coating material, all the coated fruits maintained the yeast and mold count at <10 CFU g−1 until day 8 of storage. However, the uncoated fruits showed 6.0 × 103 CFU g−1, and after senescence, 3.0 × 104 CFU g−1 of yeast and mold count, indicating fungal contamination. The fruits coated with formulations 3, 6, and 9, which were enriched with 5 mL L−1 clove EOs, maintained the yeast and mold count at <10 CFU g−1 until day 16, and at the end of the storage period, they showed the lowest fungal contamination. This could be credited to the antifungal effect of clove EOs. These findings are supported by the earlier findings of Pinto et al.,81 who investigated the antifungal activity of clove EOs and eugenol against yeast and filamentous fungi, including several foodborne fungal species such as Aspergillus spp. Omidbeygi et al.82 also observed in vitro antifungal effects against Aspergillus flavus in the presence of clove EOs at a concentration of 500 ppm. Conversely, the tomatoes coated with formulations 1, 4, and 7, which were not enriched with clove EOs, showed comparatively higher yeast and mold counts, indicating that the coating materials may have been used as substrates for microbial growth given that they contain carbohydrates.83 However, all the coating treatments maintained the aerobic plate count and the yeast and mold count within the acceptable limits of <5.0 × 105 CFU g−1 and <1.0 × 106 CFU g−1, respectively, for the fresh or frozen fruits, according to Bierhals et al.83| Formulation | Number of storage days | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yeast and mold count (CFU g−1) | Aerobic plate count (CFU g−1) | |||||||||||||
| 0 | 8 | 16 | 24 | 32 | 40 | 48 | 0 | 8 | 16 | 24 | 32 | 40 | 48 | |
| a n.d., not determined. | ||||||||||||||
| Control | <10 | 6.0 × 103 | 1.9 × 104 | 3.0 × 104 | n.d. | n.d. | n.d. | <10 | 3.4 × 104 | 1.1 × 105 | 8.7 × 105 | n.d. | n.d. | n.d. |
| 1 | <10 | <10 | 2.0 × 103 | 1.4 × 103 | 4.0 × 103 | 4.0 × 103 | 4.6 × 103 | <10 | 1.6 × 104 | 5.8 × 104 | 3.2 × 104 | 3.5 × 104 | 4.4 × 104 | 8.6 × 104 |
| 2 | <10 | <10 | 2.3 × 103 | 9.1 × 102 | 2.0 × 103 | 4.0 × 103 | 4.6 × 103 | <10 | <10 | 1.7 × 104 | 2.2 × 104 | 1.6 × 104 | 3.2 × 104 | 2.5 × 104 |
| 3 | <10 | <10 | <10 | 9.1 × 102 | 3.0 × 103 | 2.0 × 103 | 1.4 × 103 | <10 | <10 | <10 | 1.3 × 104 | 1.1 × 104 | <10 | <10 |
| 4 | <10 | <10 | 2.0 × 103 | 3.0 × 103 | 4.0 × 103 | 3.0 × 103 | 3.6 × 103 | <10 | 1.3 × 104 | 1.6 × 104 | 2.6 × 104 | 4.4 × 104 | 3.0 × 104 | 5.6 × 104 |
| 5 | <10 | <10 | 2.3 × 103 | 3.0 × 103 | 2.0 × 103 | 3.0 × 103 | 4.6 × 103 | <10 | <10 | 1.5 × 104 | 1.4 × 104 | 2.7 × 104 | 2.1 × 104 | 1.7 × 104 |
| 6 | <10 | <10 | <10 | 9.1 × 102 | 2.0 × 103 | 4.0 × 103 | 3.0 × 103 | <10 | <10 | <10 | <10 | 1.6 × 104 | 1.4 × 104 | 1.5 × 104 |
| 7 | <10 | <10 | 2.0 × 103 | 2.0 × 103 | 4.0 × 103 | 4.0 × 103 | 6.0 × 103 | <10 | <10 | 2.5 × 103 | 2.3 × 104 | 3.7 × 104 | 2.5 × 104 | 3.0 × 104 |
| 8 | <10 | <10 | 5.0 × 102 | 3.0 × 103 | 4.0 × 103 | 3.2 × 103 | 5.0 × 103 | <10 | <10 | <10 | <10 | 1.2 × 104 | 1.3 × 104 | 2.4 × 104 |
| 9 | <10 | <10 | <10 | 1.4 × 103 | 2.3 × 103 | 3.2 × 103 | 3.0 × 103 | <10 | <10 | <10 | <10 | <10 | 1.4 × 104 | 2.7 × 104 |
The lowest aerobic plate count detected from the fruits coated with formulations 3, 6, and 9, which were enriched with 5 mL L−1 clove EOs, could also be ascribed to the antibacterial effect of clove EOs. The incorporation of clove EOs at a concentration of 5 mL L−1 in the coating solutions is crucial to effectively inhibit microbial growth without negatively altering the sensory attributes of tomatoes, as suggested in the present study and previously reported by Shao et al.84 and Singh et al.85 A biodegradable gelatin and chitosan-based film enriched with 0.75 mL g−1 clove EOs showed an inhibitory effect against six selected microorganisms, including Pseudomonas fluorescens, Shewanella putrefaciens, Photobacterium phosphoreum, Listeria innocua, Escherichia coli and Lactobacillus acidophilus.28 The results of the present study are in consistent with the previous findings of Kumar et al.,29 who reported a higher increment in the total plate count in the control fruits in contrast to the tomatoes coated with edible coatings formulated with whey protein isolate, xanthan gum, glycerol, and clove EOs during 15 days of storage at 20 °C. Das et al.43 also noted an antimicrobial effect in a film prepared from starch, glycerol, coconut oil, and tea leaf extract in reducing the microbial load in tomatoes during 20 days of storage.
Furthermore, the fluctuations in microbial populations noted during storage may be due to the alterations in carbon dioxide and oxygen concentrations in the internal environment around the coated fruits, as discussed by Duran et al.86 They recorded fluctuations in microbial growth in strawberries coated with chitosan-based coatings during storage at 4 °C and 80–85% RH. Valverde et al.87 reported fluctuations in mesophilic aerobic count and yeast and mold count in table grapes coated with Aloe vera gel during 35 days of storage at 1 °C. Fluctuations in mesophilic aerobic plate count and yeast and mold count were also noted by González-Aguilar et al.88 in fresh-cut papaya coated with chitosan during storage at 5 °C.
Decay percentage and shelf life
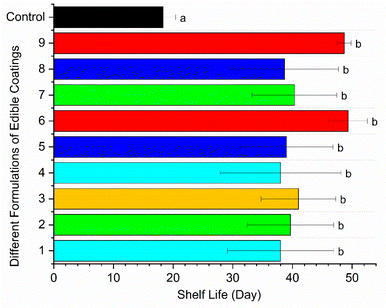
No visible sign of decay was observed in the coated or uncoated fruits until the 13th day of storage, and from the 14th day, the uncoated fruits started to rot. As the storage period extended, the fruits became more susceptible to microbial decay and exhibited 60% decay incidence by the 16th day of storage. Later, on the 20th day, all the uncoated fruits deteriorated, which is probably due to the high respiration rate and ethylene production, resulting in rapid senescence and increased vulnerability to pathogenic infections, as stated by Osae et al.42 and Paul et al.57 In contrast, regardless of the coating formulations, all the coated fruits exhibited no visible sign of decay up to 31 days of storage. Alternatively, the fruits from formulations 6 (15 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW) and 9 (20 g L−1 AS, 5 mL L−1 EO, 5 g L−1 BW) did not rot until the 48th day of storage. The extended shelf life with reduced decay incidence of particular treatments is probably ascribed to the fact that the applied edible coating materials reduced the respiration rate and ethylene synthesis, thus delaying fruit senescence and microbial infections, as discussed by Paul et al.57 and Peralta-Ruiz et al.4After harvesting, the time taken by the fruits to start deteriorating is considered their shelf life.42 The lowest shelf life of 18 days for the uncoated fruits (Fig. 7) is undoubtedly due to the increased physiological changes and metabolic activities that occurred inside the fruit cells with an increase in respiration rate and ethylene biosynthesis over the storage period, leading to fruit senescence.4,29 In the senescence stage, the commodity becomes more susceptible to microbial infections due to the loss of cellular or tissue integrity, resulting in rapid deterioration.29,89 The fruits coated with formulations 6 and 9 showed a remarkably extended shelf life of 49 days at 26 ± 2 °C and 72 ± 2% RH, which is probably ascribed to the reduction in respiration rate, ethylene production, physiological changes, microbial decay, and fruit senescence by the applied edible coatings. According to Osae et al.,42 the application of beeswax, shea butter, and cassava starch edible coatings extended the shelf life of tomatoes by 29, 26, and 23 days, respectively, in contrast to the uncoated fruits as they lasted within 10 days of storage at 20 °C and 80–90% RH due to the increased respiration rate. Extending the shelf life of tomatoes poses significant economic benefits by reducing postharvest losses, enabling broader market access, improving retail efficiency, increasing revenue, enhancing consumer satisfaction, and promoting sustainability. These advantages contribute to a stronger and efficient supply chain, benefiting all stakeholders involved.5,90
Conclusion
This study evaluated the effect of edible coatings based on AS and BW loaded with clove essential oil emulsions on the physical, chemical, and microbiological quality attributes, antioxidant activity, and composition of bioactive compounds in fresh tomatoes stored at 26 ± 2 °C and relative humidity (RH) of 72 ± 2% for 48 days. We hypothesized that the application of edible coatings would significantly improve the quality attributes of fresh tomatoes compared to the uncoated control. The results revealed that the application of the edible coatings extended the shelf life of the fresh tomatoes by preserving their postharvest quality attributes at 26 ± 2 °C and 72 ± 2% RH during 48 days of storage, supporting our hypothesis. The weight, fruit firmness, color, TSS, TA, pH value, antioxidant activity, and composition of bioactive compounds were better maintained in all the coated tomatoes compared to the control sample. Applying the edible coatings based on AS and BW with the incorporation of clove essential oil emulsions at a concentration of 5 mL L−1 was found effective in reducing the microbial load throughout the storage period. The application of the edible coating formulated with 15 g L−1 AS, 5 mL L−1 clove EO, and 5 g L−1 BW (formulation 6) significantly extended the shelf life of the fresh tomatoes to 49 ± 3 days during storage at 26 ± 2 °C and 72 ± 2% RH compared to the uncoated control and other coating treatments, implying reduced food waste, increased economic savings, and mitigated environmental impact by minimizing the need for additional resources used in production and transportation. The fruits exhibited a significant (p < 0.05) delay in changes in weight, fruit firmness, color parameters (L*, a*, b*, and ΔE), TSS content, TA, pH value, and decay incidence, and these fruits exhibited a reduced microbial load during storage. Furthermore, the application of this coating formulation was found to be effective in the preservation of the bioactive compounds (phenolics, flavonoids, lycopene, and β-carotene) and antioxidant activity of the tomatoes, thereby enhancing their nutritional value, providing health benefits such as antioxidant and anti-inflammatory effects, and improving their appeal to health-conscious consumers, potentially leading to higher market demand and better economic returns. Therefore, it is concluded that this coating formulation has potential to be used in future applications as a bioactive and edible food packaging material to extend the shelf life of fresh tomatoes by preserving the postharvest quality attributes. In the future, further investigations need to be carried out to understand the effect of this coating formulation on the respiration rate and ethylene production in fresh fruits and vegetables. In addition, future studies should also be focused on the application of clove EO nanoemulsions in AS and BW-based edible coatings to improve the shelf life of fresh fruits and vegetables by encapsulating bioactive compounds in the coating matrix.Data availability
The datasets used and/or analyzed during the present study are included in this article and its ESI.†Author contributions
Nimesh Dileesha Lakshan: conceptualization, data curation, formal analysis, investigation, methodology, writing – original daft, and writing – review & editing. Chathuri M. Senanayake: supervision, writing – review & final editing, resources. Thushari Liyanage: supervision, writing – review & editing, and resources. Ahinsa Lankanayaka: analysis, writing – review & editing, and resources. The authors read and approved the final version of the manuscript.Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
The authors acknowledge the Department of Biosystems Technology, Faculty of Technology, University of Sri Jayewardenepura, Sri Lanka and the Central Research Station, Department of Export Agriculture, Matale, Sri Lanka for providing the opportunity to conduct the present study. Furthermore, the first author would like to acknowledge support from the Food Research Unit, Horticultural Crop Research and Development Institute, Department of Agriculture, Gannoruwa, Peradeniya, Sri Lanka.References
- I. K. Arah, H. Amaglo, E. K. Kumah and H. Ofori, Int. J. Agron., 2015, 2015, 478041 CrossRef.
- Y. Zhu, D. Li, T. Belwal, L. Li, H. Chen, T. Xu and Z. Luo, Molecules, 2019, 24, 1–16 Search PubMed.
- FAO, https://www.fao.org/faostat/en/#data, accessed May 2024.
- Y. Peralta-Ruiz, C. D. G. Tovar, A. Sinning-Mangonez, E. A. Coronell, M. F. Marino and C. Chaves-Lopez, Polymers, 2020, 12(8), 1822 CrossRef CAS PubMed.
- A. Yadav, N. Kumar, A. Upadhyay, S. Sethi and A. Singh, J. Food Sci., 2022, 87, 2256–2290 CrossRef CAS PubMed.
- G. Bailén, F. Guillén, S. Castillo, M. Serrano, D. Valero and D. Martínez-Romero, J. Agric. Food Chem., 2006, 54, 2229–2235 CrossRef.
- M. I. Gil, M. A. Conesa and F. Artés, Postharvest Biol. Technol., 2002, 25, 199–207 CrossRef CAS.
- H. Majidi, S. Minaei, M. Almassi and Y. Mostofi, J. Food Sci. Technol., 2014, 51, 2155–2161 CrossRef CAS PubMed.
- K. Ncama, L. S. Magwaza, A. Mditshwa and S. Z. Tesfay, Food Packag. Shelf Life, 2018, 16, 157–167 CrossRef.
- S. Pedreiro, A. Figueirinha, A. S. Silva and F. Ramos, Coatings, 2021, 11, 1–26 CrossRef.
- H. T. Duguma, Int. J. Food Sci. Technol., 2022, 57, 1353–1366 CrossRef CAS.
- J. G. de O. Filho, C. C. de O. N. Bezerra, B. R. Albiero, F. C. A. Oldoni, M. Miranda, M. B. Egea, H. M. C. de Azeredo and M. D. Ferreira, Food Packag. Shelf Life, 2020, 26, 100589 CrossRef.
- G. F. Nogueira, B. de O. Leme, G. R. S. dos Santos, J. V. da Silva, P. B. Nascimento, C. T. Soares, F. M. Fakhouri and R. A. de Oliveira, Polysaccharides, 2021, 2, 373–386 CrossRef CAS.
- L. Fu, J. Zhu, S. Zhang, X. Li, B. Zhang, H. Pu, L. Li and Q. Wang, Carbohydr. Polym., 2018, 181, 528–535 CrossRef CAS PubMed.
- T. G. Kawhena, U. L. Opara and O. A. Fawole, Coatings, 2021, 11(4), 442 CrossRef CAS.
- X. Ruelas-Chacon, J. C. Contreras-Esquivel, J. Montañez, A. F. Aguilera-Carbo, M. L. Reyes-Vega, R. D. Peralta-Rodriguez and G. Sanchéz-Brambila, J. Food Qual., 2017, 2017, 8608304 CrossRef.
- M. L. Navarro-Tarazaga, A. Massa and M. B. Pérez-Gago, LWT–Food Sci. Technol., 2011, 44, 2328–2334 CrossRef CAS.
- T. A. Ochoa, B. E. García-Almendárez, A. A. Reyes, D. M. R. Pastrana, G. F. G. López, O. M. Belloso and C. R. González, Food Bioprocess Technol., 2017, 10, 103–114 CrossRef CAS.
- M. O. Reis, J. B. Olivato, A. P. Bilck, J. Zanela, M. V. E. Grossmann and F. Yamashita, Ind. Crops Prod., 2018, 112, 481–487 CrossRef CAS.
- L. D. Pérez-Vergara, M. T. Cifuentes, A. P. Franco, C. E. Pérez-Cervera and R. D. Andrade-Pizarro, NFS J., 2020, 21, 39–49 CrossRef.
- M. C. Rodríguez, C. Villegas Yépez, J. H. Gil González and R. Ortega-Toro, Heliyon, 2020, 6(5), e03974 CrossRef PubMed.
- Y. Pan, Z. Deng and F. Shahidi, Food Prod., Process. Nutr., 2020, 2, 1–19 CrossRef.
- B. Yousuf, S. Wu and M. W. Siddiqui, Trends Food Sci. Technol., 2021, 108, 245–257 CrossRef CAS.
- D. de V. S. Batista, R. C. Reis, J. M. Almeida, B. Rezende, C. A. D. Bragança and F. da Silva, J. Food Sci. Technol., 2020, 57, 274–281 CrossRef PubMed.
- J. N. Haro-González, G. A. Castillo-Herrera, M. Martínez-Velázquez and H. Espinosa-Andrews, Molecules, 2021, 26 Search PubMed.
- C. W. T. Fukuyama, L. G. R. Duarte, I. C. Pedrino, M. C. Mitsuyuki, S. B. Junior and M. D. Ferreira, Sustainable Food Technol., 2024, 2, 426–436 RSC.
- W. Wang, Y. Zhang, Z. Yang and Q. He, Int. J. Biol. Macromol., 2021, 166, 578–586 CrossRef CAS PubMed.
- J. Gómez-Estaca, A. López de Lacey, M. E. López-Caballero, M. C. Gómez-Guillén and P. Montero, Food Microbiol., 2010, 27, 889–896 CrossRef.
- A. Kumar and C. S. Saini, Measurement: Food, 2021, 2, 100005 Search PubMed.
- USDA, United States Standards for Grades of Fresh Tomatoes, US Dep. Agric. Mark. Serv., 1991, pp. 1–13 Search PubMed.
- G. F. Nogueira, F. M. Fakhouri and R. A. de Oliveira, Carbohydr. Polym., 2018, 186, 64–72 CrossRef CAS.
- S. M. Amaraweera, C. Gunathilake, O. H. P. Gunawardene, R. S. Dassanayake, N. M. L. Fernando, D. B. Wanninayaka, S. M. Rajapaksha, A. Manamperi, M. Gangoda, A. Manchanda, C. A. N. Fernando, A. K. Kulatunga and A. Manipura, ACS Omega, 2022, 7, 19579–19590 CrossRef CAS PubMed.
- A. M. Teles, J. V. Silva-Silva, J. M. P. Fernandes, A. L. Abreu-Silva, K. D. S. Calabrese, N. E. Mendes Filho, A. N. Mouchrek and F. Almeida-Souza, J. Evid. Based Complementary Altern. Med., 2021, 2021, 6663255 Search PubMed.
- A. Ali, M. Maqbool, S. Ramachandran and P. G. Alderson, Postharvest Biol. Technol., 2010, 58, 42–47 CrossRef CAS.
- G. D. Sadler and P. A. Murphy, in Food Analysis, Food Science Texts Series, ed. S. S. Nielsen, Springer Cham, Cham, Switzerland, 5th edn, 2017, pp. 219–238 Search PubMed.
- N. Kumar, Neeraj, Pratibha and A.T. Petkoska, ACS Food Sci. Technol., 2021, 1(4), 500–510 CrossRef CAS.
- J. E. Dávila-Aviña, J. A. Villa-Rodríguez, M. A. Villegas-Ochoa, O. Tortoledo-Ortiz, G. I. Olivas, J. F. Ayala-Zavala and G. A. González-Aguilar, J. Food Sci. Technol., 2014, 51, 2706–2712 CrossRef PubMed.
- J. Zhishen, T. Mengcheng and W. Jianming, Food Chem., 1999, 64, 555–559 CrossRef CAS.
- C. Kaur, S. Walia, S. Nagal, S. Walia, J. Singh, B. B. Singh, S. Saha, B. Singh, P. Kalia, S. Jaggi and Sarika, LWT—Food Sci. Technol., 2013, 50, 139–145 CrossRef CAS.
- A. Naeem, T. Abbas, T. M. Ali and A. Hasnain, J. Food Meas. Charact., 2018, 12, 2725–2734 CrossRef.
- Bacteriological Analytical Manual (BAM), https://www.fda.gov/food/laboratory-methods-food/bacteriological-analytical-manual-bam, accessed November 2023.
- R. Osae, M. T. Apaliya, R. N. Alolga, E. Kwaw, P. N. Y. Otu and S. Akaba, Appl. Food Res., 2022, 2, 100041 CrossRef CAS.
- D. K. Das, H. Dutta and C. L. Mahanta, LWT–Food Sci. Technol., 2013, 50, 272–278 CrossRef CAS.
- R. Liñán-Atero, F. Aghababaei, S. R. García, Z. Hasiri, D. Ziogkas, A. Moreno and M. Hadidi, Antioxidants, 2024, 13(4), 488 CrossRef.
- A. do Nascimento, L. C. Toneto, B. M. Lepaus, B. S. Valiati, L. Faria-Silva and J. F. B. de São José, Membranes, 2023, 13(9), 772 CrossRef CAS.
- R. T. Donjio, J. A. Nguemezi, M. Anoumaa, E. T. Phounzong, J. O. Kenfack and T. Fonkou, J. Food Qual., 2023, 2023, 1019310 Search PubMed.
- K. Paladugu and K. Gunasekaran, Int. J. Agric. Sci., 2017, 9, 3866–3870 CAS.
- N. Sharma, M. Rathore and M. Sharma, Rev. Environ. Sci. Biotechnol., 2013, 12, 45–60 CrossRef CAS.
- J. Zeni, K. Cence, C. E. Grando, L. Tiggermann, R. Colet, L. A. Lerin, R. L. Cansian, G. Toniazzo, D. De Oliveira and E. Valduga, Appl. Biochem. Biotechnol., 2011, 163, 383–392 CrossRef CAS PubMed.
- Ö. Yaman and L. Bayoindirli, Lebensm. Wiss. Technol., 2002, 35, 146–150 CrossRef.
- U. Amin, M. U. Khan, Y. Majeed, M. Rebezov, M. Khayrullin, E. Bobkova, M. A. Shariati, I. M. Chung and M. Thiruvengadam, Int. J. Biol. Macromol., 2021, 183, 2184–2198 CrossRef CAS.
- J. G. Xu, T. Liu, Q. P. Hu and X. M. Cao, Molecules, 2016, 21(9), 1194 CrossRef PubMed.
- J. M. S. Araújo, A. C. P. de Siqueira, A. F. Blank, N. Narain and L. C. L. de Aquino Santana, Food Bioprocess Technol., 2018, 11, 1750–1760 CrossRef.
- D. Gierson and A. A. Kader, in The Tomato Crop, ed. J. G. Atherton and J. Rudich, Springer, Dordrecht, 1986, pp. 241–280 Search PubMed.
- R. Pholsin, K. A. Shiekh, S. Jafari, I. Kijpatanasilp, T. Na Nan, I. Suppavorasatit and K. Assatarakul, Food Control, 2024, 155, 110023 CrossRef CAS.
- E. Asiamah, W. Arthur, V. Kyei-Barfour, F. Sarpong and H. K. Ketemepi, Bioact. Carbohydr. Diet. Fibre, 2023, 30, 100373 CrossRef CAS.
- S. K. Paul, S. Sarkar, L. N. Sethi and S. K. Ghosh, J. Food Sci. Technol., 2018, 55, 2446–2456 CrossRef CAS PubMed.
- V. K. Pandey, R. U. Islam, R. Shams and A. H. Dar, Appl. Food Res., 2022, 2, 100042 CrossRef CAS.
- M. A. Taher, E. A. MennatAllah, L. K. Tadros and M. I. Sanad, J. Food Meas. Charact., 2020, 14, 2489–2502 CrossRef.
- W. N. S. M. Azmai, N. S. Abdul Latif and N. Md Zain, Int. Food Res. J., 2018, 25, S185–S194 Search PubMed.
- X. Zhang, X. Zhang, X. Liu, M. Du and Y. Tian, J. Food Process. Preserv., 2019, 43, 1–8 CrossRef.
- F. M. Dwivany, A. N. Aprilyandi, V. Suendo and N. Sukriandi, Int. J. Food Sci., 2020, 2020, 8861610 Search PubMed.
- Y. D. Adjouman, C. Nindjin, K. N. Kouassi, F. A. Tetchi, G. G. Amani and M. Sindic, Int. J. Nutr. Sci. Food Technol., 2018, 4, 1–10 Search PubMed.
- G. E. Anthon, M. Lestrange and D. M. Barrett, J. Sci. Food Agric., 2011, 91, 1175–1181 CrossRef CAS PubMed.
- N. Firdous, M. R. Khan, M. S. Butt and M. Shahid, Pak. J. Agric. Sci., 2020, 57, 245–249 Search PubMed.
- N. Mahfoudhi, M. Chouaibi and S. Hamdi, Food Sci. Technol. Int., 2014, 20, 33–43 CrossRef CAS PubMed.
- M. Tigist, T. S. Workneh and K. Woldetsadik, J. Food Sci. Technol., 2013, 50, 477–486 CrossRef CAS PubMed.
- J. Bonilla, L. Atarés, M. Vargas and A. Chiralt, Procedia Food Sci., 2011, 1, 44–49 CrossRef CAS.
- G. A. Gonzalez-aguilar, J. A. Villa-rodriguez, J. F. Ayala-zavala and E. M. Yahia, Trends Food Sci. Technol., 2010, 21, 475–482 CrossRef CAS.
- I. Rodriguez-Garcia, M. R. Cruz-Valenzuela, B. A. Silva-Espinoza, G. A. Gonzalez-Aguilar, E. Moctezuma, M. M. Gutierrez-Pacheco, M. R. Tapia-Rodriguez, L. A. Ortega-Ramirez and J. F. Ayala-Zavala, J. Sci. Food Agric., 2016, 96, 3772–3778 CrossRef CAS PubMed.
- F. L. Tchouala Tazo, G. Kanmegne, A. Ngotio Tchinda, O. J. Kenfack and E. Tafré Phounzong, J. Food Qual., 2023, 2023, 1–11 CrossRef.
- F. Reyes and L. Cisneros-Zevallos, J. Agric. Food Chem., 2003, 51, 5296–5300 CrossRef PubMed.
- S. H. Mirdehghan and D. Valero, Int. J. Food Prop., 2017, 20, 1798–1806 CAS.
- A. Cano, M. Acosta and M. B. Arnao, Postharvest Biol. Technol., 2003, 28, 59–65 CrossRef CAS.
- Y. Dumas, M. Dadomo, G. Di Lucca and P. Grolier, J. Sci. Food Agric., 2003, 83, 369–382 CrossRef CAS.
- H. S. Erge and F. Karadeniz, Int. J. Food Prop., 2011, 14, 968–977 CrossRef CAS.
- A. Ali, M. Maqbool, P. G. Alderson and N. Zahid, Postharvest Biol. Technol., 2013, 76, 119–124 CrossRef CAS.
- E. J. Collins, C. Bowyer, A. Tsouza and M. Chopra, Biology, 2022, 11 CrossRef CAS PubMed.
- J. Javanmardi and C. Kubota, Postharvest Biol. Technol., 2006, 41, 151–155 CrossRef CAS.
- Z. S. Safari, P. Ding, J. J. Nakasha and S. F. Yusoff, Coatings, 2021, 11(3), 367 CrossRef CAS.
- E. Pinto, L. Vale-Silva, C. Cavaleiro and L. Salgueiro, J. Med. Microbiol., 2009, 58, 1454–1462 CrossRef PubMed.
- M. Omidbeygi, M. Barzegar, Z. Hamidi and H. Naghdibadi, Food Control, 2007, 18, 1518–1523 CrossRef CAS.
- V. S. Bierhals, M. Chiumarelli and M. D. Hubinger, J. Food Sci., 2011, 76, 62–72 CrossRef PubMed.
- X. Shao, B. Cao, F. Xu, S. Xie, D. Yu and H. Wang, Postharvest Biol. Technol., 2015, 99, 37–43 CrossRef CAS.
- A. Singh, R. K. Singh, A. K. Bhunia and N. Singh, LWT—Food Sci. Technol., 2003, 36, 787–794 CrossRef CAS.
- M. Duran, M. S. Aday, N. N. D. Zorba, R. Temizkan, M. B. Büyükcan and C. Caner, Food Bioprod. Process., 2016, 98, 354–363 CrossRef CAS.
- J. M. Valverde, D. Valero, D. Martínez-Romero, F. Guillén, S. Castillo and M. Serrano, J. Agric. Food Chem., 2005, 53, 7807–7813 CrossRef CAS PubMed.
- G. A. González-Aguilar, E. Valenzuela-Soto, J. Lizardi-Mendoza, F. Goycoolea, M. A. Martínez-Téllez, M. A. Villegas-Ochoa, I. N. Monroy-García and J. F. Ayala-Zavala, J. Sci. Food Agric., 2009, 89, 15–23 CrossRef.
- P. S. Tanada-Palmu and C. R. F. Grosso, Postharvest Biol. Technol., 2005, 36, 199–208 CrossRef CAS.
- M. Akhtar, A. Akhtar, W. Nazir and N. Khalid, Prev. Nutr. Food Sci., 2023, 28, 178–188 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fb00033a |
| This journal is © The Royal Society of Chemistry 2024 |