Open Access Article
Open Access ArticleEffect of carnauba wax nanoemulsion associated with Syzygium aromaticum and Mentha piperita essential oils as an alternative to extend lychee post-harvest shelf life
Conny W. T.
Fukuyama
a,
Larissa G. R.
Duarte
 *b,
Isadora C.
Pedrino
a,
Milene C.
Mitsuyuki
*b,
Isadora C.
Pedrino
a,
Milene C.
Mitsuyuki
 b,
Stanislau Bogusz
Junior
b,
Stanislau Bogusz
Junior
 c and
Marcos D.
Ferreira
c and
Marcos D.
Ferreira
 b
b
aDepartment of Biotechnology, Federal University of São Carlos, São Carlos, SP 13565-905, Brazil. E-mail: conny@estudante.ufscar.br; isadorapedrino@gmail.com
bBrazilian Agricultural Research Corporation, Embrapa Instrumentation, São Carlos, SP 13561-206, Brazil. E-mail: larissagraziele@gmail.com; milene.corso@embrapa.br; marcos.david@embrapa.br
cInstitute of Chemistry (IQSC), University of São Paulo (USP), São Carlos, 13566-590, Brazil. E-mail: stanislau@iqsc.usp.br
First published on 23rd January 2024
Abstract
The demand for tropical fruits worldwide has increased, but their short shelf life poses a challenge. Lychee, a highly perishable fruit used in various ways, lacks effective post-harvest preservation methods, particularly for export. To address this, plant-based edible coatings incorporating nanotechnology and essential oils offer a sustainable solution for maintaining lychee quality. This study aimed to assess the impact of carnauba wax nanoemulsion (CWN) coatings at different concentrations, with or without essential oils of Syzygium aromaticum (CEO) and Mentha piperita (MEO), on lychee preservation after harvesting. Two stages were conducted: first, determining the optimal CWN concentration (4.5%, 9%, and 18%) for storing lychee at 16 °C and 70% relative humidity for five days; second, selecting the 9% CWN concentration with 1% CEO and MEO for storing lychee for eight days under the same conditions. Physical and chemical analyses were performed during storage. GC-MS analysis showed that eugenol (89.73%) and isomenthol (49.46%) were the main components of clove essential oil and peppermint oil, respectively. The treatments with CWN (9%) and CWN (18%) significantly reduced weight loss by approximately 4% compared to the control while maintaining quality indicators such as L* value, pH, and total soluble solids (TSS%). Lychee fruits coated with CWN (9%) combined with CEO and MEO showed a significant reduction in decay incidence and severity after 168 h of storage. Specifically, the CWN–MEO treatment exhibited a 20% incidence and severity compared to the control's 60% and 100%, respectively. Coatings with 9% CWN and 1% MEO have the potential to effectively preserve post-harvest lychee quality, minimize losses, reduce disease severity, extend shelf life, and enhance commercial opportunities.
Sustainability spotlightCurrently, approximately 40% of horticultural food production is lost due to post-harvest diseases. The limited shelf life of these foods presents a significant challenge. Moreover, the excessive use of chemical fungicides to combat these diseases results in problems such as environmental damage and potential harm to humans. By developing plant-based edible coatings that incorporate nanotechnology and essential oils, we are offering a sustainable solution to improving fruit quality and reduce waste in the supply chain. Our research focuses on reducing food waste and post-harvest losses, aligning with the ONU Sustainable Development Goal 12.3, which aims to halve global food waste per capita by 2030. |
1 Introduction
Nowadays, consumers are looking for healthier and natural food, which demands a greater variety of fruits and vegetables. However, most of them tend to have a short shelf-life, especially tropical and subtropical fruits.1Lychee (Litchi chinensis Sonn.) is a non-climacteric fruit2 with high nutritional3 and commercial value,4 commonly cultivated in tropical and subtropical regions.5 Lychees are only available for a few months per year and they have very short shelf-life between 2 and 3 days at room temperature.6 Several problems after harvesting have been reported, such as darkening of the pericarp, desiccation and rot. Some post-harvesting technologies are applied to solve these problems, such as using refrigerated temperatures associated with acid leaching with sulfur treatment, although they are not very sustainable.6,7 Therefore, there are limited options available to extend the shelf life of lychee fruits as these fruits are susceptible to changes in the cold chain. Studies are needed on new fruit preservation alternatives to minimize food waste. Each fruit has its own distinct physiology, highlighting the need to individually evaluate new cost-effective technologies to increase their shelf life.
Edible coatings present an alternative to extend the shelf-life of fruits and vegetables. Most of them are not only biodegradable, but also made of natural compounds that form a layer on the surface that reduces gases and moisture exchange with the environment, thus reducing the respiration rate.8 The respiration rate relates to fruit senescence, and its lower rates allow shelf-life to be increased.9 Edible coatings, in addition to inhibiting respiration, act as a barrier against water loss, inhibiting oxidative reactions. Also, when antimicrobial compounds are added, they reduce the growth of pathogens on the food surface.10
Coating components must be Generally Recognized as Safe (GRAS) and approved by regulatory agencies such as the U.S. Food and Drug Administration – FDA and the European community. Edible coatings are composed of a matrix, and it is possible to incorporate essential oils to obtain their antimicrobial properties.8
Carnauba wax nanoemulsion (CWN) can be used as a matrix for edible coating. Its properties can be complemented by adding plant essential oils with antimicrobial properties.11 The application of carnauba wax nanoemulsion (CWN) associated or not with other components, such as essential oils, has presented promising results for post-harvest conservation in strawberries,12 papayas,13 and tomatoes.14 Those coatings involve the surface of the fruit and are a physical barrier that enables it to reduce the respiration rate and delay senescence.11
Essential oils usually present antibacterial and antifungal activities. Thus, they can be incorporated into edible coatings to improve their properties.15 The essential oil of Syzygium aromaticum (clove) with main component eugenol, has been described as having antibacterial and antifungal activities against post-harvest pathogenic microorganisms in fruits16 and a few foodborne pathogens.17Mentha piperita (peppermint) essential oil has menthol and menthone,18 which are associated with the antifungal activities of these essential oils.19
Furthermore, essential oils can also effectively inhibit enzymatic browning and oxidative reactions in fruits, helping to maintain their appearance, texture, flavor and nutritional value, ensuring a longer shelf life.20 Therefore, research and development of alternatives as suitable essential oil formulations associated with hydrophobic coatings can increase the overall effectiveness of fruit preservation.
The main goal of this research was to evaluate edible coating of carnauba wax nanoemulsion (CWN) in different concentrations associated or not with essential oils of Syzygium aromaticum (CEO) and Mentha piperita (MEO) on the post-harvest conservation of lychee. The study aims at post-harvest preservation of lychee, with visual quality as a physical aspect, delaying senescence and inhibiting the growth of fungal diseases.
2 Materials and methods
2.1. Materials
Carnauba wax type I (99% purity; CAS no. 8015-86-9) was provided by Pontes Indústria de Cera (Parnaíba, PI, Brazil). Syzygium aromaticum and Mentha piperita essential oils were acquired from Laszlo Aromaterapia (Belo Horizonte, MG, Brazil). Lychee fruits, cultivar Bengal, were carefully transported from a local producer to the post-harvest laboratory, Embrapa Instrumentation, São Carlos, SP. Then, they were separated by lack of standard defects, size and stage of maturity.2.2. Essential oil composition
The composition of essential oils was determined by qualitative analysis using gas chromatography with Shimadzu equipment (GC-2010 Plus, Kyoto, Japan) coupled to a quadrupole mass spectrometer (GC-MS). A non-polar DB-5MS capillary column (30 m × 0.25 mm, i.d. × 0.25 μm) was used, and helium was the carrier gas at 1 mL min−1 flow rate. Samples were diluted in dichloromethane (10% v/v) and injected (1 μL) in split mode (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50). Injector temperature: 220 °C, oven temperature: 60 to 240 °C at 3 °C min−1; interface: 240 °C; ion source: +70 eV, m/z: 35–350. Co-injection of authentic standards confirmed sample components. A semi-quantitative analysis of the essential oils (% relative area) was also performed using a flame ionization detector (GC-FID). Qualitative and semi-quantitative analyses were performed in triplicate.
50). Injector temperature: 220 °C, oven temperature: 60 to 240 °C at 3 °C min−1; interface: 240 °C; ion source: +70 eV, m/z: 35–350. Co-injection of authentic standards confirmed sample components. A semi-quantitative analysis of the essential oils (% relative area) was also performed using a flame ionization detector (GC-FID). Qualitative and semi-quantitative analyses were performed in triplicate.
2.3. Edible coating preparation
The synthesis of the carnauba wax nanoemulsion (CWN) was based on the methodology adapted from Hagenmaier & Baker.21 The zeta potential (−43.8 mV), the diameter size (44.1 ± 7.6 nm) and polydispersity indices (0.28) of CWN were measured using a Zetasizer Nano ZS (Malvern Instruments Inc., Westborough, MA, USA).22The experiment was divided into two phases. First, the carnauba wax nanoemulsion (CWN) was applied to lychees randomly divided into four treatments, namely: control, CWN (4.5% solid phase in suspension), CWN (9% solid phase in suspension), and CWN (18% phase suspended solid). The fruits were immersed in the coating solution and stored for five days at 16 °C and 70% relative humidity. In the second phase, after evaluating which concentration of carnauba wax nanoemulsion best preserved the lychees, the fruits were subjected to treatments with essential oil: control, CWN (9% solid phase in suspension), CWN (9%) + CEO (1%), CWN (9%) + MEO (1%), and stored for eight days at 16 °C and 70% relative humidity.
2.4. Physicochemical parameters of lychees
2.5. Decay percentage and severity on lychees
The incidence was visually analyzed through the presence of fungi in lychees during storage. The percentage of rot was determined based on the number of lychees with visible fungus per treatment, with each treatment having five lychees. The severity of the diseases was analyzed using a 4-point scale (0 = no symptoms; 1 = 1% of the affected area; 2 = 2–5%; 3 = 6–100%) to assess the fungal activity of the coatings. Due to the fruit type, on score 3, 6% was considered to have high disease infestation.242.6. Statistical analysis
Parametric analysis of variance and multiple comparisons of Duncan's test or non-parametric ANOVA and multiple comparisons of Kruskal–Wallis were performed at the 5% significance level using the R software version 4.2.2 to assess the differences between treatments.3 Results and discussion
3.1. Essential oil composition
As shown in Table 1, Syzygium aromaticum (clove) essential oil was mainly constituted by eugenol (89.73%) and β-Gurjunene (7.59%). This composition is similar to that obtained by Haro-González et al.25 and Kačániová et al.26 where eugenol was the dominant component of CEO. The antifungal activity of clove essential oil can be associated with the hydroxyl groups in eugenol that can interact with the cellular membrane and damage it.25 Eugenol can also induce oxidative stress by generating reactive oxygen species (ROS) within fungal cells and thus cause damage to DNA, proteins, and lipids.27 Eugenol also impairs fungal growth by interrupting energy production, consequently inhibiting ATP synthesis.28| Compound | Syzygium aromaticum (% area) | Mentha piperita (% area) |
|---|---|---|
| α-Pinene | — | 0.61 |
| Sabinene | — | 0.14 |
| β-Pinene | — | 0.63 |
| 3-Octanol | — | 0.16 |
| p-Cymene | — | 0.15 |
| Limonene | — | 1.76 |
| 1,8-Cineol | — | 0.84 |
| Linalool | — | 0.10 |
| Isopulegol | — | 1.75 |
| Menthone | — | 18.97 |
| Menthol | — | 15.25 |
| Isomenthol | — | 49.46 |
| Neoisomenthol | — | 1.27 |
| γ-Terpineol | — | 0.28 |
| α-Terpineol | — | 0.63 |
| Pulegone | — | 1.80 |
| Piperitone | — | 0.90 |
| Menthyl acetate | — | 0.19 |
| Eugenol | 89.73 | — |
| Neoisomenthyl acetate | — | 4.18 |
| β-Bourbonene | — | 0.19 |
| β-Gurjunene | 7.59 | — |
| Caryophyllene | — | 0.73 |
| α-Humulene | 2.10 | — |
| γ-Selinene | 0.20 | — |
| δ-Cadinene | 0.25 | — |
| Caryophyllene oxide | 0.13 | — |
| Total | 100 | 99.97 |
Mentha piperita (peppermint) essential oil presented isomenthol (49.46%), menthone (18.97%) and menthol (15.25%) as major components, similar to what was described by Desam et al.19 and Kamatou et al.29 These components attribute antifungal activity to the oil, due to its hydrophobicity, which allows interaction with the cellular membrane and leads to its lyses.30 Menthol inhibits the biosynthesis of ergosterol (an important component of the fungal membrane). Thus, it alters membrane fluidity by interrupting the normal functioning of membrane-bound proteins and enzymes, affecting essential cellular processes and cellular integrity.31
Essential oils also possess antioxidant activity crucial for fruit preservation as they eliminate and neutralize free radicals, preventing or delaying oxidative damage.20 It helps maintain the appearance, texture, flavor and nutritional value of the fruits, ensuring that they remain attractive and safe for consumption for longer periods of time.32
The main antioxidant compounds in clove essential oil are eugenol and beta-caryophyllene, while menthol is the main antioxidant compound in peppermint essential oil.33,34 These compounds are capable of donating electrons or hydrogen atoms to free radicals, neutralizing their reactivity and preventing them from causing oxidative damage to cells and tissues.35 Therefore, essential oils in proper concentrations and formulations can safely and effectively preserve food.
Both essential oils presented variations in their percentage of composition compared to other authors, which can be explained due to different factors, such as the geographic origin of the plant, environmental conditions (climatic and seasonal conditions and sunlight), plant organ and age, genetics, nutrition of the plant, and extraction method.25
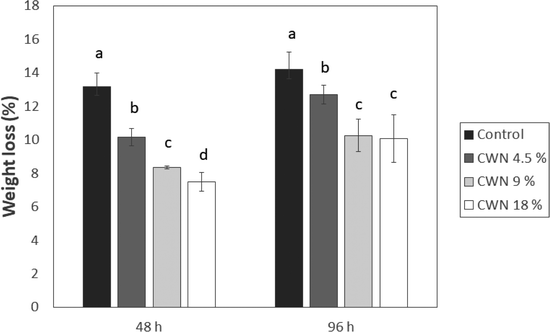
3.2. First phase: control, CWN 4.5%, CWN 9% and CWN 18%
The results presented in Fig. 1 show that lychee lost weight progressively over time during storage at 16 °C and that the control group presented higher weight loss than CWN 4.5%, CWN 9%, and CWN 18% treatment. Therefore, all CWN coatings efficiently prevented weight loss on lychee over time. After 48 h, we observed a reduction of approximately 4 and 5% of weight loss at CWN 9% and 18%, respectively, compared to the control. After 96 h of storage, the difference in weight loss between the control and the CWN 9% and 18% treatments remained at 4%, and between the control and the CWN 4.5% treatment there was only a 2% difference. Thus, CWN 9% and 18% were more effective. Carnauba wax nanoemulsions have already presented promising results on reducing weight loss in tomatoes, as shown by Miranda et al.14 Weight loss is an important quality parameter especially for exporting fruits because their quality and value are related to that.36
| Treatments | pH | TSS (%) | ||||
|---|---|---|---|---|---|---|
| Storage time (h) | 0 | 48 | 96 | 0 | 48 | 96 |
| a Means followed by different letters on the same column indicate significant differences between treatments (p-value < 0.05). | ||||||
| Control | 4.09 ± 0.02a | 4.53 ± 0.01a | 4.94 ± 0.03a | 17.14 ± 0.05a | 18.95 ± 0.15a | 19.87 ± 0.19a |
| CWN 4.5% | 4.09 ± 0.02a | 4.40 ± 0.02 ab | 4.67 ± 0.03b | 17.14 ± 0.05a | 17.55 ± 0.07c | 18.04 ± 0.02c |
| CWN 9% | 4.09 ± 0.02a | 4.28 ± 0.01b | 4.58 ± 0.04c | 17.14 ± 0.05a | 17.31 ± 0.21c | 18.00 ± 0.11c |
| CWN 18% | 4.09 ± 0.02a | 4.50 ± 0.13a | 4.72 ± 0.02b | 17.14 ± 0.05a | 17.87 ± 0.07b | 18.56 ± 0.13b |
The results of total soluble solids (TSS) (Table 2) were that for all treatments with different concentrations of CWN, the TSS value increased over time with significant differences. These results were expected once sugar is synthesized during respiratory processes to be converted into CO2 and water during fruit senescence.38 All treatments with CWN showed little increase in TSS values compared to the control, which can be related to the reduction in the respiration rate on coated lychee and, consequently, a reduction in the fruit metabolic activity.9
After 48 h of storage, the treatment that best lowered the increase in TSS was CWN 9%. After 96 h of storage, the best results were by CWN 4.5% and CWN 9%, showing equal results.
| Treatments | Time (h) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 48 | 96 | |||||||
| L* | C* | a* | L* | C* | a* | L* | C* | a* | |
| a Means followed by different letters on the same column indicate significant differences between treatments (p-value < 0.05). | |||||||||
| Control | 27.70 ± 7.25a | 15.90 ± 4.16ab | 13.10 ± 3.27a | 20.20 ± 4.85b | 12.40 ± 5.67b | 8.50 ± 3.93b | 25.50 ± 2.99a | 15.00 ± 2.13a | 11.90 ± 1.88a |
| CWN 4.5% | 25.90 ± 6.14a | 14.50 ± 4.39b | 11.20 ± 3.92a | 27.10 ± 3.92a | 19.10 ± 3.25a | 13.60 ± 2.02a | 25.40 ± 3.54a | 14.30 ± 3.69a | 10.00 ± 1.99b |
| CWN 9% | 28.90 ± 8.53a | 18.20 ± 5.48a | 12.20 ± 3.49a | 21.20 ± 5.64b | 18.90 ± 4.92a | 5.90 ± 2.76c | 22.00 ± 7.22a | 16.00 ± 6.55a | 10.00 ± 3.64b |
| CWN 18% | 26.40 ± 9.73a | 17.40 ± 3.77ab | 12.60 ± 2.79a | 29.90 ± 3.22a | 19.40 ± 3.69a | 12.70 ± 3.58a | 19.50 ± 7.14a | 14.10 ± 3.51a | 8.10 ± 2.48b |
The L* value represents the lightness,40 and the results obtained showed that after 96 h of storage, the treatments control and CWN 9% had the L* value decreased. The pericarp lightness of lychee coated with CWN 4.5% did not significantly change over the 96 h of storage. The L* value of treatment CWN 18% increased and then decreased, during 96 h of storage. This type of L* value behavior was previously described in lychee by Yang et al.24 Generally, all samples had decreased L values indicating color darkness and fruit senescence for climacteric fruit.41 On the other hand, for lychee, non-climacteric fruit, despite the decreased L values, there was no significant difference between treatments, and the absence of darkness (Fig. 2) can be corroborated with the maintenance of the fruits and with the lowest pH and TSS values during storage (Table 2) of CWN 9% and CWN 18%.
 | ||
| Fig. 2 Uncoated or control treatment lychees (A) and CWN 9% coated lychees (B) after 96 h of storage at 16 °C. | ||
The C* value indicates color saturation42 and although some changes occurred over time for all the treatments, the color saturation can be considered significantly the same at 0 h and 96 h for all the treatments.
The a* value represents the green-red axis,43 and the results show variations over time for all treatments. However, the control treatment showed the highest value, which is significantly different from the CWN treatments, indicating less intensive red color for those treatments, with CWN 18% being the lowest one (Fig. 2).
After all the non-destructive and destructive analyses of the first phase were finished (control, CWN 4.5%, CWN 9% and CWN 18%), the second phase analysis was performed with CWN 9% since it was the treatment with the most promising results in tested parameters to maintain post-harvest quality. Therefore, the second phase analysis compared CWN 9% coating to CWN 9% coating related to 1% clove and 1% peppermint essential oil.
3.3. Second phase: control, CWN 9%, CWN–CEO, and CWN–MEO
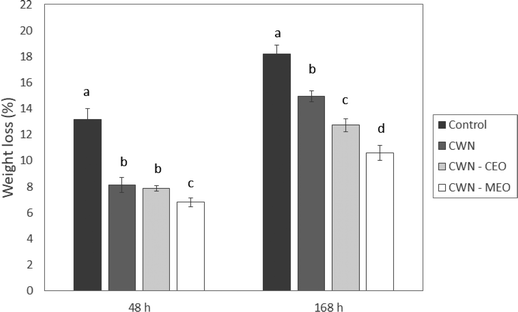 | ||
| Fig. 3 Lychee weight loss during storage time at 16 °C and 70% RH. For each storage period, different letters indicate significant differences between treatments (p < 0.05). | ||
Many studies prove a lower weight loss in fruits with coatings with incorporated essential oils due to the hydrophobic contribution, thus providing protection against moisture loss.15 Sapper et al.44 discovered starch–gellan coatings with thyme essential oil, which prevented water loss from persimmons, and Sarengaowa et al.45 found weight loss inhibition results in potatoes coated with chitosan and cinnamon essential oil.
| Treatments | pH | TA (%) | TSS (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Storage time (h) | 0 | 48 | 168 | 0 | 48 | 168 | 0 | 48 | 168 |
| a Means followed by different letters on the same column indicate significant differences between treatments (p-value < 0.05). | |||||||||
| Control | 3.87 ± 0.05a | 4.44 ± 0.05a | 4.83 ± 0.02a | 0.734 ± 0.006a | 0.724 ± 0.002a | 0.634 ± 0.002c | 16.74 ± 0.08a | 18.28 ± 0.08a | 19.86 ± 0.04a |
| CWN 9% | 3.87 ± 0.05a | 4.23 ± 0.02b | 4.60 ± 0.01b | 0.734 ± 0.006a | 0.706 ± 0.005b | 0.657 ± 0.003b | 16.74 ± 0.08a | 17.85 ± 0.03b | 18.94 ± 0.10b |
| CWN–CEO | 3.87 ± 0.05a | 4.13 ± 0.02c | 4.53 ± 0.02c | 0.734 ± 0.006a | 0.704 ± 0.002b | 0.653 ± 0.001b | 16.74 ± 0.08a | 17.55 ± 0.04c | 18.62 ± 0.01c |
| CWN–MEO | 3.87 ± 0.05a | 4.04 ± 0.02d | 4.46 ± 0.04d | 0.734 ± 0.006a | 0.701 ± 0.001b | 0.675 ± 0.004a | 16.74 ± 0.08a | 17.26 ± 0.01d | 18.16 ± 0.04d |
The titratable acidity (TA) presented a decreasing pattern for all the treatments over time, with the control group having the highest decrease. Therefore, CWN 9%, CWN–CEO, and CWN–MEO treatments were efficient in reducing changes in the TA value. After 168 h of storage, the CWN 9% and CWN–CEO treatments showed significantly equal results in reducing the decrease in TA, the CWN–MEO treatment being the most efficient. As previously mentioned, organic acids are consumed during the senescence process of the fruit, causing a decrease in the TA value. However, the coating reduces the respiration rate and organic acid consumption, such as the TA decrease over time.9,23
The total soluble solids (TSS) has increased over time for all treatments, but the control group had the highest increase in TSS, which evidences that CWN 9%, CWN–CEO, and CWN–MEO treatments reduced TSS changes over time due to delaying the senescence process. CWN–MEO showed a lower increase in TSS and presented the best results, followed by CWN–CEO and CWN 9% treatments. Similar results were found by de Oliveira et al.47 with mangoes coated with chitosan and the essential oil of Mentha piperita and by dos Passos Braga et al.48 with papayas. In both studies, there were not only a few changes in TSS values but also the loss of weight, titratable acidity, and pH, thus indicating the effectiveness of the essential oil in delaying senescence.
| Treatments | Storage time (h) | ||
|---|---|---|---|
| 0 | 48 | 168 | |
| a Means followed by different letters on the same column indicate significant differences between treatments (p-value < 0.05). | |||
| Control | 0.221 ± 0.040a | 0.356 ± 0.042a | 0.383 ± 0.077a |
| CWN | 0.221 ± 0.040a | 0.248 ± 0.043b | 0.289 ± 0.049b |
| CWN–CEO | 0.221 ± 0.040a | 0.226 ± 0.021b | 0.264 ± 0.062b |
| CWN–MEO | 0.221 ± 0.040a | 0.223 ± 0.045b | 0.251 ± 0.055b |
| Treatments | Time (h) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 48 | 168 | |||||||
| L* | C* | a | L* | C* | a | L* | C* | a | |
| a Means followed by different letters on the same column indicate significant differences between treatments (p-value < 0.05). | |||||||||
| Control | 48.20 ± 10.87a | 40.59 ± 5.49a | 31.89 ± 5.63a | 33.27 ± 4.09 ab | 28.98 ± 5.57 ab | 19.98 ± 3.20b | 30.95 ± 6.08a | 27.32 ± 4.85a | 17.71 ± 1.78a |
| CWN | 35.88 ± 11.27b | 35.82 ± 9.08 ab | 26.71 ± 11.44a | 34.87 ± 5.42a | 29.43 ± 5.66 ab | 22.21 ± 3.54a | 31.14 ± 6.71a | 20.97 ± 3.38b | 12.92 ± 2.02b |
| CWN–CEO | 48.62 ± 5.12a | 39.37 ± 4.40 ab | 32.19 ± 4.08a | 31.80 ± 4.39b | 27.40 ± 5.51b | 21.44 ± 4.31ab | 27.95 ± 7.39a | 20.51 ± 3.12b | 13.07 ± 1.77b |
| CWN–MEO | 43.66 ± 6.84 ab | 34.98 ± 5.35b | 27.48 ± 5.45a | 35.31 ± 3.59a | 31.56 ± 4.94a | 22.87 ± 2.57a | 30.82 ± 6.77a | 21.49 ± 4.08b | 13.27 ± 2.18b |
The C* value (saturation) significantly decreased after 168 h of storage for all the treatments. The treatments CWN showed the lowest values for this parameter and for the a* value (green-red), indicating less intensive red color, significantly different from the control. CWN–CEO and CWN–MEO presented final storage results that were significantly equal. The browning rate of lychees was associated with the dehydration rate or weight loss during storage.53 Coated fruits lost less weight than uncoated fruits, the red color was less intense in all coated fruits, and similar results were found in the first step (Fig. 2).
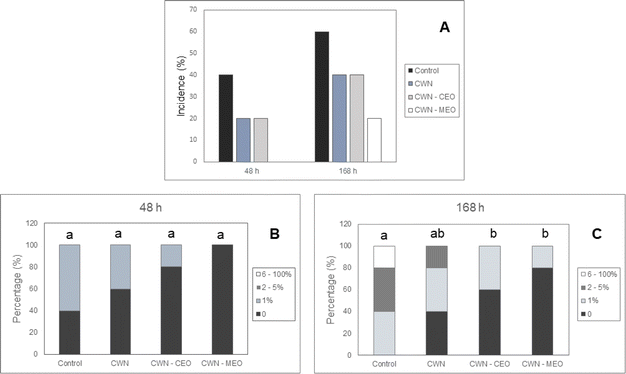
3.4. Decay percentage and severity on lychees
As shown in Fig. 4A, disease incidence in the control group was higher than that in lychee revested with CWN, CWN–CEO, and CWN–MEO. After 48 h of storage, disease incidence was 40% for the control, 20% for CWN and CWN–CEO, and 0% for CWN–MEO. After 168 h of storage, the control group presented 60% of the incidence, CWN and CWN–CEO had 40% of the incidence and CWN–MEO presented 20% of the incidence. The results obtained showed that all treatments reduced the postharvest disease incidence, the CWN–MEO coating led to lower incidence than the others, and CWN and CWN–CEO coatings presented significantly equal results.Lychee coated with CWN, CWN–CEO and CWN–MEO showed lower disease severity compared to control group after 48 h and 168 h of storage (Fig. 4B and C). After 48 h of storage (Fig. 4B), 60% of group control presented 1% of the affected area; 40% of CWN coated lychee had 1% of the area affected; 20% of CWN–CEO coated lychee had 1% of the area affected and none of CWN–MEO coated lychee had the area affected. After 168 h of storage (Fig. 4C), all lychee in the control group had at least 1% of the area affected; CWN coated lychee present 40% of the group with 0% area affected; CWN–CEO coated lychee present 60% of the group with 0% area affected; CWN–MEO coated lychee present 80% of the group with 0% area affected. Therefore, CWN–MEO coating was the most efficient in preventing the increase in disease severity.
It is possible that the superior efficacy of CWN–MEO coatings arises from the synergy between the antimicrobial and antioxidant properties of peppermint essential oil and the barrier-forming abilities of carnauba wax. The bioactive compounds in the oil fortify the wax's defense against microbes and oxidative stress on the lychee surface, resulting in enhanced preservation.
de Oliveira Filho et al.13 report post-harvest decay inhibition in papayas with a coating of carnauba nanoemulsion with clove (Z. aromaticum) and mint essential oil (Mentha spicata), the last association being more effective. Both oils belong to the same Mentha genus, although Mentha spicata and Mentha piperita have differences in their major compounds, which may result in variations in their antifungal properties.54 Similar antifungal efficacy results of coatings with Mentha piperita were obtained in mangoes55 and grapes.56
Essential oils are bioactive compounds of plant origin that have antimicrobial and antioxidant activities due to their phenolic compounds. Recent work has also explored other bioactive compounds of plant origin for food preservation using mango seed grains57 and date seed extracts58–60 due to the presence of polyphenols in these compounds.
Fruits coated with CWN (9%) associated with essential oils CEO and MEO could preserve post-harvest physical–chemical parameters even at temperatures higher than recommended because, in addition to maintaining the physical–chemical parameters such as pH, titratable acidity and total soluble solids, they prolong the shelf life of these fruits as they inhibit the growth of antifungal diseases due to the antimicrobial properties of essential oils. The coating of carnauba wax with essential oils can be an alternative for the conservation of lychees that, when stored at low temperatures, can suffer browning on the skin and pulp and changes in their texture.61
4 Conclusion
The results of this study indicate the effectiveness of carnauba wax nanoemulsion (CWN) coatings, particularly at 9% concentration, in preserving lychee post-harvest quality. At a storage temperature of 16 °C, application of CWN (9%) significantly mitigated the weight loss by 4% compared to untreated fruits, while simultaneously maintaining crucial physicochemical parameters such as pH and soluble solids content during the five-day storage period. Furthermore, the incorporation of Syzygium aromaticum (CEO) and Mentha piperita (MEO) essential oils into CWN demonstrated control over the incidence and severity of diseases in stored lychee fruits. The CWN (9%)–MEO (1%) combination was more effective in reducing the incidence and severity of post-harvest diseases. These findings highlight the potential of using edible coatings based on carnauba wax nanoemulsions (CWNs) at a concentration of 9% with essential oils, particularly Mentha piperita (MEO), in significantly extending the post-harvest shelf life of lychee. This sustainable and safe alternative not only reduces losses, but also ensures greater commercial viability, offering promising prospects for the preservation and commercialization of lychee on a wider scale.Ethical statement
This article does not contain any studies with human or animal subjects.Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.Author contributions
Conny W. T. Fukuyama: writing – original draft, formal analysis, investigation. Larissa G. R. Duarte: conceptualization, data curation, investigation, methodology, project administration, visualization, writing – review & editing. Isadora C. Pedrino: investigation, formal analysis. Milene C. Mitsuyuki: formal analysis, software. Stanislau Bogusz Junior: methodology, project administration. Marcos D. Ferreira: conceptualization, funding acquisition, methodology, project administration, resources, supervision, writing – review & editing.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This research was funded by FAPESP (process 2022/10686-6), Empresa Brasileira de Pesquisa Agropecuária – Embrapa, Rede Agronano, CNPq/MCTI Sisnano (process 442575/2019-0) and an M. D. Ferreira CNPq Research Productivity fellowship (# 307141/2022-5).References
- C. Raherison, I. Baldi, M. Pouquet, E. Berteaud, C. Moesch, G. Bouvier and M. Canal-Raffin, Pesticides Exposure by Air in Vineyard Rural Area and Respiratory Health in Children: A Pilot Study, Environ. Res., 2019, 169, 189–195 CrossRef CAS PubMed.
- V. Deshi, F. Homa, A. Ghatak, M. A. Aftab, H. Mir, B. Ozturk and M. W. Siddiqui, Exogenous methyl jasmonate modulates antioxidant activities and delays pericarp browning in litchi, Physiol. Mol. Biol. Plants, 2022, 28, 1561–1569 CrossRef CAS PubMed.
- Z. Yun, H. Qu, H. Wang, F. Zhu, Z. Zhang, X. Duan, B. Yang, Y. Cheng and Y. Jiang, Comparative transcriptome and metabolome provides new insights into the regulatory mechanisms of accelerated senescence in litchi fruit after cold storage, Sci. Rep., 2016, 6, 19356 CrossRef CAS PubMed.
- J. Yang, X. Zhu, P. Zhang, Y. Wang, Y. Xiao, B. Yang, H. Qu and Y. Jiang, Detection of toxic methylenecyclopropylglycine and hypoglycin A in litchi aril of three Chinese cultivars, Food Chem., 2020, 327, 127013 CrossRef CAS PubMed.
- S. Fan, D. Wang, H. Xie, H. Wang, Y. Qin, G. Hu and J. Zhao, Sugar transport, metabolism and signaling in fruit development of Litchi chinensis Sonn: a review, Int. J. Mol. Sci., 2021, 22(20), 11231 CrossRef CAS PubMed.
- B. Bhushan, A. Pal, R. Narwal, V. S. Meena, P. C. Sharma and J. Singh, Combinatorial approaches for controlling pericarp browning in Litchi (Litchi chinensis) fruit, J. Food Sci. Technol., 2015, 52, 5418–5426 CrossRef CAS PubMed.
- R. Liu, X. Zhu, J. Wang and C. Huang, Activated release of chlorine dioxide gas from polyvinyl alcohol microcapsule (ethylcellulose/sodium-chlorite) hybrid films for active packaging of litchi during postharvest storage, Postharvest Biol. Technol., 2023, 196, 112173 CrossRef CAS.
- F. J. Blancas-Benitez, B. Montaño-Leyva, L. Aguirre-Güitrón, C. L. Moreno-Hernández, Á. Fonseca-Cantabrana, L. del C. Romero-Islas and R. R. González-Estrada, Impact of edible coatings on quality of fruits: a review, Food Control, 2022, 139, 109063 CrossRef CAS.
- N. Maftoonazad and H. S. Ramaswamy, Application and evaluation of a pectin-based edible coating process for quality change kinetics and shelf-life extension of lime fruit (Citrus aurantifolium), Coatings, 2019, 9(5), 285 CrossRef CAS.
- L. G. R. Duarte and C. S. F. Picone, Antimicrobial activity of lactoferrin-chitosan-gellan nanoparticles and their influence on strawberry preservation, Food Res. Int., 2022, 159, 111586 CrossRef CAS PubMed.
- J. G. de Oliveira Filho, M. Miranda, M. D. Ferreira and A. Plotto, Nanoemulsions as Edible Coatings: A Potential Strategy for Fresh Fruits and Vegetables Preservation, Foods, 2021, 10(10), 2438 CrossRef CAS PubMed.
- J. G. de Oliveira Filho, B. R. Albiero, Í. H. Calisto, M. R. V. Bertolo, F. C. A. Oldoni, M. B. Egea, S. Bogusz Junior, H. M. C. de Azeredo and M. D. Ferreira, Bio-nanocomposite edible coatings based on arrowroot starch/cellulose nanocrystals/carnauba wax nanoemulsion containing essential oils to preserve quality and improve shelf life of strawberry, Int. J. Biol. Macromol., 2022, 219, 812–823 CrossRef PubMed.
- J. G. de Oliveira Filho, L. G. R. Duarte, Y. B. B. Silva, E. P. Milan, H. V. Santos, T. C. Moura, V. P. Bandini, L. E. S. Vitolano, J. J. C. Nobre, C. T. Moreira, M. C. Mitsuyuki, S. Bogusz Junior and M. D. Ferreira, Novel Approach for Improving Papaya Fruit Storage with Carnauba Wax Nanoemulsion in Combination with Syzigium aromaticum and Mentha spicata Essential Oils, Coatings, 2023, 13(5), 847 CrossRef.
- M. Miranda, M. D. M. M. Ribeiro, P. C. Spricigo, L. Pilon, M. C. Mitsuyuki, D. S. Correa and M. D. Ferreira, Carnauba wax nanoemulsion applied as an edible coating on fresh tomato for postharvest quality evaluation, Heliyon, 2022, 8(7), e09803 CrossRef CAS PubMed.
- B. Yousuf, S. Wu and M. W. Siddiqui, Incorporating essential oils or compounds derived thereof into edible coatings: effect on quality and shelf life of fresh/fresh-cut produce, Trends Food Sci. Technol., 2021, 108, 245–257 CrossRef CAS.
- N. Hasheminejad and F. Khodaiyan, The effect of clove essential oil loaded chitosan nanoparticles on the shelf life and quality of pomegranate arils, Food Chem., 2020, 309, 125520 CrossRef CAS PubMed.
- M. Radünz, M. L. M. da Trindade, T. M. Camargo, A. L. Radünz, C. D. Borges, E. A. Gandra and E. Helbig, Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil, Food Chem., 2019, 276, 180–186 CrossRef PubMed.
- A. Soltanbeigi, M. Özgüven and M. B. Hassanpouraghdam, Planting-date and cutting-time affect the growth and essential oil composition of Mentha × piperita and Mentha arvensis, Ind. Crops Prod., 2021, 170, 113790 CrossRef CAS.
- N. R. Desam, A. J. Al-Rajab, M. Sharma, M. M. Mylabathula, R. R. Gowkanapalli and M. Albratty, Chemical constituents, in vitro antibacterial and antifungal activity of Mentha × piperita L. (peppermint) essential oils, J. King Saud Univ., Sci., 2019, 31(4), 528–533 CrossRef.
- A. B. Perumal, L. Huang, R. B. Nambiar, Y. He, X. Li and P. S. Sellamuthu, Application of essential oils in packaging films for the preservation of fruits and vegetables: a review, Food Chem., 2022, 375, 131810 CrossRef CAS PubMed.
- R. D. Hagenmaier and R. A. Baker, Edible Coatings from Morpholine-Free Wax Microemulsions, J. Agric. Food Chem., 1997, 45(2), 349–352 CrossRef CAS.
- M. Miranda, X. Sun, C. Ference, A. Plotto, J. Bai, D. Wood, O. B. G. Assis, M. D. Ferreira and E. Baldwin, Nano- and Micro- Carnauba Wax Emulsions versus Shellac Protective Coatings on Postharvest Citrus Quality, J. Am. Soc. Hortic. Sci., 2021, 146(1), 40–49 CAS.
- L. G. R. Duarte, N. C. A. Ferreira, A. C. T. R. Fiocco and C. S. F. Picone, Lactoferrin-Chitosan-TPP Nanoparticles: Antibacterial Action and Extension of Strawberry Shelf-Life, Food Bioprocess Technol., 2023, 16, 135–148 CrossRef CAS.
- C. Yang, F. W. Lee, Y. J. Cheng, Y. Y. Chu, C. N. Chen and Y. C. Kuan, Chitosan coating formulated with citric acid and pomelo extract retards pericarp browning and fungal decay to extend shelf life of cold-stored lychee, Sci. Hortic., 2023, 310, 111735 CrossRef CAS.
- J. N. Haro-González, G. A. Castillo-Herrera, M. Martínez-Velázquez and H. Espinosa-Andrews, Clove Essential Oil (Syzygium aromaticum L. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health, Molecules, 2021, 26(21), 6387 CrossRef PubMed.
- M. Kačániová, L. Galovičová, P. Borotová, V. Valková, H. Ďúranová, P. Ł. Kowalczewski, H. A. H. Said-Al Ahl, W. M. Hikal, M. Vukic, T. Savitskaya, D. Grinshpan and N. L. Vukovic, Chemical composition, in vitro and in situ antimicrobial and antibiofilm activities of Syzygium aromaticum (Clove) essential oil, Plants, 2021, 10(10), 2185 CrossRef PubMed.
- R. Jadimurthy, S. Jagadish, S. C. Nayak, S. Kumar, C. D. Mohan and K. S. Rangappa, Phytochemicals as Invaluable Sources of Potent Antimicrobial Agents to Combat Antibiotic Resistance, Life, 2023, 13(4), 948 CrossRef CAS PubMed.
- M. Hyldgaard, Mechanisms of Action, Resistance, and Stress Adaptation, in Antimicrobials in Food, CRC Press, London, 2020, pp. 735–784 Search PubMed.
- G. P. P. Kamatou, I. Vermaak, A. M. Viljoen and B. M. Lawrence, Menthol: a simple monoterpene with remarkable biological properties, Phytochemistry, 2013, 96, 15–25 CrossRef CAS PubMed.
- I. Camele, D. Grul'ová and H. S. Elshafie, Chemical composition and antimicrobial properties of Mentha × piperita cv. ‘kristinka’ essential oil, Plants, 2021, 10(8), 1567 CrossRef CAS PubMed.
- E. T. d. C. Almeida, G. T. de Souza, J. P. de Sousa Guedes, I. M. Barbosa, C. P. de Sousa, L. R. C. Castellano, M. Magnani and E. L. de Souza, Mentha piperita L. essential oil inactivates spoilage yeasts in fruit juices through the perturbation of different physiological functions in yeast cells, Int. J. Food Microbiol., 2019, 82, 20–29 CrossRef CAS PubMed.
- G. Khaliq, A. Saleh, G. A. Bugti and K. R. Hakeem, Guggul gum incorporated with basil essential oil improves quality and modulates cell wall-degrading enzymes of jamun fruit during storage, Sci. Hortic., 2020, 273, 109608 CrossRef CAS.
- L. R. Ahmadabadi, S. E. Hosseini, S. M. Seyedein Ardebili and A. Mousavi Khaneghah, Application of clove essential oil-loaded nanoemulsions in coating of chicken fillets, J. Food Meas. Charact., 2022, 16, 819–828 CrossRef.
- M. Namiota and R. Bonikowski, The current state of knowledge about essential oil fumigation for quality of crops during postharvest, Int. J. Mol. Sci., 2021, 22(24), 13351 CrossRef CAS PubMed.
- N. Basavegowda and K. H. Baek, Synergistic antioxidant and antibacterial advantages of essential oils for food packaging applications, Biomolecules, 2021, 11(9), 1267 CrossRef CAS PubMed.
- I. Juma, H. Fors, H. P. Hovmalm, A. Nyomora, M. Fatih, M. Geleta, A. S. Carlsson and R. O. Ortiz, Avocado production and local trade in the southern highlands of Tanzania: a case of an emerging trade commodity from horticulture, Agronomy, 2019, 9(11), 749 CrossRef.
- B. H. Cho and S. Koseki, Determination of banana quality indices during the ripening process at different temperatures using smartphone images and an artificial neural network, Sci. Hortic., 2021, 288, 110382 CrossRef.
- L. G. R. Duarte, W. M. P. Alencar, R. Iacuzio, N. C. C. Silva and C. S. F. Picone, Synthesis, characterization and application of antibacterial lactoferrin nanoparticles, Curr. Res. Food Sci., 2022, 5, 642–652 CrossRef CAS PubMed.
- S. R. Sommano, U. Chanasut and W. Kumpoun, Enzymatic browning and its amelioration in fresh-cut tropical fruits, in Fresh-Cut Fruits and Vegetables: Technologies and Mechanisms for Safety Control, Academic Press, Cambridge, 2019, pp. 51–76 Search PubMed.
- A. M. Jiménez, C. A. Sierra, F. J. Rodríguez-Pulido, M. L. González-Miret, F. J. Heredia and C. Osorio, Physicochemical characterisation of gulupa (Passiflora edulis Sims. fo edulis) fruit from Colombia during the ripening, Food Res. Int., 2011, 44(7), 1912–1918 CrossRef.
- A. C. F. Vieira, J. de Matos Fonseca, N. M. C. Menezes, A. R. Monteiro and G. A. Valencia, Active coatings based on hydroxypropyl methylcellulose and silver nanoparticles to extend the papaya (Carica papaya L.) shelf life, Int. J. Biol. Macromol., 2020, 164, 489–498 CrossRef CAS PubMed.
- G. Kannan, B. Kouakou and S. Gelaye, Color changes reflecting myoglobin and lipid oxidation in chevon cuts during refrigerated display, Small Rumin. Res., 2001, 42(1), 67–74 CrossRef.
- W. F. Vieira-Junior, I. Vieira, G. M. B. Ambrosano, F. H. B. Aguiar and D. A. N. L. Lima, Correlation between alteration of enamel roughness and tooth color, J. Clin. Exp. Dent., 2018, 10(8), e815 Search PubMed.
- M. Sapper, L. Palou, M. B. Pérez-Gago and A. Chiralt, Antifungal Starch-Gellan Edible coatings with thyme essential oil for the postharvest preservation of apple and persimmon, Coatings, 2019, 9(5), 333 CrossRef CAS.
- G. Saren, L. Wang, Y. Liu, C. Yang, K. Feng and W. Hu, Screening of Essential Oils and Effect of a Chitosan-Based Edible Coating Containing Cinnamon Oil on the Quality and Microbial Safety of Fresh-Cut Potatoes, Coatings, 2022, 12(10), 1492 CrossRef.
- M. Miranda, X. Sun, A. Marín, L. C. dos Santos, A. Plotto, J. Bai, O. Benedito Garrido Assis, M. D. Ferreira and E. Baldwin, Nano- and micro-sized carnauba wax emulsions-based coatings incorporated with ginger essential oil and hydroxypropyl methylcellulose on papaya: preservation of quality and delay of post-harvest fruit decay, Food Chem.: X, 2022, 13, 100249 CAS.
- K. Á. R. de Oliveira, M. L. da Conceição, S. P. A de Oliveira, M. dos S. Lima, M. de Sousa Galvão, M. S. Madruga, M. Magnani and E. L. de Souza, Postharvest quality improvements in mango cultivar Tommy Atkins by chitosan coating with Mentha piperita L. essential oil, J. Hortic. Sci. Biotechnol., 2020, 95(2), 260–272 CrossRef CAS.
- S. dos Passos Braga, M. Magnani, M. S. Madruga, M. de Souza Galvão, L. L. de Medeiros, A. U. D. Batista, R. T. A. Dias, L. R. Fernandes, E. S. de Medeiros and E. L. de Souza, Characterization of edible coatings formulated with chitosan and Mentha essential oils and their use to preserve papaya (Carica papaya L.), Innovative Food Sci. Emerging Technol., 2020, 65, 102472 CrossRef CAS.
- P. V. Mahajan and T. K. Goswami, Extended storage life of litchi fruit using controlled atmosphere and low temperature, J. Food Process. Preserv., 2004, 28(5), 388–403 CrossRef.
- R. G. Cruz-Monterrosa, A. A. Rayas-Amor, R. M. González-Reza, M. L. Zambrano-Zaragoza, J. E. Aguilar-Toalá and A. M. Liceaga, Application of Polysaccharide-Based Edible Coatings on Fruits and Vegetables: Improvement of Food Quality and Bioactivities, Polysaccharides, 2023, 4(2), 99–115 CrossRef CAS.
- X. Shen, M. Zhang, K. Fan and Z. Guo, Effects of ε-Polylysine/Chitosan Composite Coating and Pressurized Argon in Combination with MAP on Quality and Microorganisms of Fresh-Cut Potatoes, Food Bioprocess Technol., 2020, 13, 145–158 CrossRef CAS.
- Y. Jiang, Role of anthocyanins, polyphenol oxidase and phenols in lychee pericarp browning, J. Sci. Food Agric., 2000, 80(3), 305–310 CrossRef CAS.
- J. Joas, Y. Caro, M. N. Ducamp and M. Reynes, Postharvest control of pericarp browning of litchi fruit (Litchi chinensis Sonn cv Kwaï Mi) by treatment with chitosan and organic acids: I. Effect of pH and pericarp dehydration, Postharvest Biol. Technol., 2005, 38(2), 128–136 CrossRef CAS.
- N. Z. Mamadalieva, H. Hussain and J. Xiao, Recent advances in genus Mentha: phytochemistry, antimicrobial effects, and food applications, Food Front., 2020, 1(4), 435–458 CrossRef.
- K. Á. R. de Oliveira, L. R. R. Berger, S. A. de Araújo, M. P. S. Câmara and E. L. de Souza, Synergistic mixtures of chitosan and Mentha piperita L. essential oil to inhibit Colletotrichum species and anthracnose development in mango cultivar Tommy Atkins, Food Microbiol., 2017, 66, 96–103 CrossRef PubMed.
- I. C. D. Guerra, P. D. L. De Oliveira, M. M. F. Santos, A. S. S. C. Lúcio, J. F. Tavares, J. M. Barbosa-Filho, M. S. Madruga and E. L. De Souza, The effects of composite coatings containing chitosan and Mentha (piperita L. or x villosa Huds) essential oil on postharvest mold occurrence and quality of table grape cv. Isabella, Innovative Food Sci. Emerging Technol., 2016, 34, 112–121 CrossRef CAS.
- R. Krishnamoorthy, A. Hai and F. Banat, Subcritical Water Extraction of Mango Seed Kernels and Its Application for Cow Ghee Preservation, Processes, 2023, 11(5), 1379 CrossRef CAS.
- K. Farousha, V. M. Rangaraj, K. Rambabu, M. A. Haija and F. Banat, Development of date seed extract encapsulated MCM-41: characterization, release kinetics, antioxidant and antibacterial studies, Food Biosci., 2023, 53, 102563 CrossRef CAS.
- V. M. Rangaraj, K. Rambabu, F. Banat and V. Mittal, Effect of date fruit waste extract as an antioxidant additive on the properties of active gelatin films, Food Chem., 2021, 355, 129631 CrossRef CAS PubMed.
- V. M. Rangaraj, S. Devaraju, K. Rambabu, F. Banat and V. Mittal, Silver-sepiolite (Ag-Sep) hybrid reinforced active gelatin/date waste extract (DSWE) blend composite films for food packaging application, Food Chem., 2022, 369, 130983 CrossRef CAS PubMed.
- R. R. Mphahlele, O. J. Caleb and M. E. K. Ngcobo, Effects of packaging and duration on quality of minimally processed and unpitted litchi cv. ‘Mauritius’ under low storage temperature, Heliyon, 2020, 6(1), e03229 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2024 |