Open Access Article
Open Access ArticleApplication of rosin resin and zinc oxide nanocomposites to chitosan coatings for extending the shelf life of passion fruits
Jailton Ribeiro dos
Santos Junior
a,
Luiz Carlos
Corrêa-Filho
 b,
Vitória Oliveira
Pereira
c,
Henriqueta Talita Guimarães
Barboza
b,
José Carlos Sá
Ferreira
b,
Antônio Gomes
Soares
b,
Renata Valeriano
Tonon
b and
Lourdes Maria Corrêa
Cabral
*b
b,
Vitória Oliveira
Pereira
c,
Henriqueta Talita Guimarães
Barboza
b,
José Carlos Sá
Ferreira
b,
Antônio Gomes
Soares
b,
Renata Valeriano
Tonon
b and
Lourdes Maria Corrêa
Cabral
*b
aFederal Rural University of Rio de Janeiro, 23890-000, Seropédica, RJ, Brazil
bEmbrapa Food Technology, Guaratiba, 23020-470, Rio de Janeiro, RJ, Brazil. E-mail: lourdes.cabral@embrapa.br
cRio de Janeiro State University, Campo Grande, 23070-200, Rio de Janeiro, RJ, Brazil
First published on 26th December 2023
Abstract
Passion fruit (Passiflora edulis Sims f. flavicarpa DEG) is a tropical fruit widespread in Brazil, the largest producer and consumer in the world. As a climacteric fruit, it continues the ripening process after being detached from the plant, resulting in a short shelf life, with post-harvest problems, such as wilting and susceptibility to attack by microorganisms such as fungi. Therefore, this work aimed to develop chitosan-based coatings to be applied on passion fruit to maintain its post-harvest quality. Film forming solutions were prepared using chitosan (C) as the main polymer, carnauba wax (W) or rosin (R) as a hydrophobicity promoting agent and zinc oxide (ZnO) nanoparticles as an antimicrobial agent. The solutions were applied to passion fruit surfaces and the fruits were stored for 10 days at 22.5 °C and 82 RH for continuous evaluation. To determine the coating effect on ripening evolution during storage, the fruits were analysed for mass loss, texture, colour, pH, acidity, total soluble solids, and sugar contents. The post-harvest loss index was also determined during storage. The results showed that C + R coatings were more effective in protecting the fruits against weight loss, injury appearance and microorganism attacks. The visual appearance was also maintained. Increasing the resin concentration in the film forming solution provided better protection for the fruits against excessive weight loss and delayed the physicochemical changes related to maturation (acidity, pH, soluble solids, and firmness). Therefore, rosin-containing coatings provided the best results in postharvest applications to control passion fruit storage problems.
Sustainability spotlightThe application of biodegradable coatings to preserve the quality and extend the shelf life of passion fruit is a significant step towards aligning with the United Nations' Sustainable Development Goals (SDGs). It primarily supports SDG 12, “Responsible Consumption and Production”, by reducing the environmental impact of food packaging. Traditional plastic packaging contributes to pollution and waste, while biodegradable coatings offer an eco-friendly alternative that minimizes plastic waste and promotes responsible production and consumption. Additionally, by preventing food spoilage and waste, it aligns with SDG 15, “Life on Land,” by conserving valuable resources and promoting sustainable land use. Overall, the application of biodegradable coatings for passion fruit exemplifies a commitment to sustainable agriculture, responsible consumption, and environmental stewardship, contributing to the broader agenda of achieving a more sustainable and equitable future as outlined in the UN's SDGs. |
1. Introduction
Brazil stands out as the largest producer of passion fruit, as the soil and climate conditions are favourable to the development of this crop. Among the Passifloraceous species commercially exploited in Brazil, the yellow passion fruit (Passiflora edulis Sims flavicarpa) is the most cultivated due to its greater preference in the internal market, representing 95% of the total production.1 Passion fruit has a strong respiration rate and fast response to ethylene after harvest. This results in post-harvest quality deterioration, such as the loss of moisture and shrinkage of the peel. Phytosanitary problems have affected this crop, causing browning of the bark and fungal rot. Losses in the commercial value of the fruit can also occur due to failures in handling, packaging, storage, and transport.2,3New technologies have been evaluated aiming to interfere in physiological processes, reducing sweating and breathing rates and consequently reducing post-harvest losses, such as the application of edible coatings made from polymeric matrices.4 Coating is a thin layer of polymeric material, formed directly on the product surface that is intended to be protected without altering the visual or sensory characteristics of the fruit.5 In addition, the coatings aim to preserve the physicochemical quality and increase the shelf life of plant products during the storage and commercialization process.6 Previous studies report the application of coatings on a range of fruits, including kiwi, papaya, and guava. These studies highlight the role of chitosan coatings in preserving the post-harvest quality of fruits.7–9
Among the polymers used, polysaccharides and lipids stand out for being biodegradable materials and found in abundance in nature, such as starches, cellulose, gums, pectin, alginate, chitosan, and waxes.4,10 In recent years, the number of studies that seek to combine different polymers and additives in the preparation of films and coatings has grown.11,12
Chitosan is a polysaccharide obtained by deacetylation of chitin, and it has been used in coating preparation. It is considered safe by the Food and Drug Administration (FDA). However, the chitosan coating has low water barrier properties, limiting its use. Seeking to improve these barrier characteristics, several composites have been prepared from mixtures of polysaccharides and lipids.13
Among lipids, carnauba wax has been widely used as an edible coating to reduce water loss and give the fruit a shiny appearance.14,15 High melting and hardness result in durable coatings and improve the adhesion between the coating and fruit, the resistance to water permeation and the visual properties due to their good hydrophobicity and brightness.16,17
To improve coating properties, bioactive compounds with antioxidant and antimicrobial action, flavouring compounds and some other additives can be incorporated into film forming solutions in order to improve the quality, handling and integrity of the coated product. The addition of ZnO nanoparticles has been explored to improve gas barrier properties and provide antimicrobial properties to the coating.18,19
Although several studies have investigated the roles of chitosan in post-harvest processes, questions regarding the use of chitosan composite coatings with rosin resin containing zinc oxide nanoparticles and their application to extend the shelf life of yellow passion fruit are still not completely understood. Therefore, this work aimed to evaluate the application of chitosan-based coatings containing carnauba wax or rosin resin and zinc oxide nanoparticles on the physicochemical properties of passion fruit stored at room temperature.
2. Materials and methods
2.1. Selection of passion fruit
Passion fruits were purchased at the Central Market of Rio de Janeiro, Brazil. They were selected by peel colour, absence of mechanical damage and disease symptoms, to standardize the sample.2.2. Preparation and development of edible coatings
The film forming solutions were prepared using chitosan as the main polymer, glycerol as a plasticizer, carnauba wax or rosin resin as a hydrophobicity promoting agent and zinc oxide nanoparticles as an antimicrobial agent. Acetic acid and Tween 80 were also used to facilitate dissolution and mixing of the components. Carnauba wax or rosin resin was added to the solution at 50% (m/m) in relation to chitosan. The solution was homogenized by means of magnetic stirring at a temperature of 85 °C until the wax/resin softened for 10 minutes, being homogenized with an Ultra Turrax at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm for another 10 minutes.
500 rpm for another 10 minutes.
Zinc oxide nanoparticles were suspended in distilled water and sonicated in an ultrasound bath (Unique USC – 4800, Indaiatuba, Brazil) at 220 W for 15 minutes. Then, the ZnO nanoparticles were incorporated into the filmogenic solutions at a final concentration of 0.05% (w/v), with the aid of the Ultra Turrax for 15 minutes at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm. Table 1 presents the final concentration of compounds in each treatment for 1000 ml of final solution.
500 rpm. Table 1 presents the final concentration of compounds in each treatment for 1000 ml of final solution.
| Treatments | Formulation components | |||||
|---|---|---|---|---|---|---|
| Chitosan (%) | Carnauba wax (%) | Rosin resin (%) | ZnOnano (%) | Tween 80 (%) | Glycerol (%) | |
| a CW: chitosan + wax; CWZ: chitosan + wax + ZnOnano; CR: chitosan + resin; CRZ: chitosan + resin + ZnOnano. 1 – second application of coatings. | ||||||
| CW | 1.2 | 0.6 | — | — | 0.3 | 0.36 |
| CWZ | 1.2 | 0.6 | — | 0.05 | 0.3 | 0.36 |
| CR | 1.2 | — | 0.6 | — | 0.3 | 0.36 |
| CRZ | 1.2 | — | 0.6 | 0.05 | 0.3 | 0.36 |
| CR1 | 1.2 | — | 0.8 | 0.05 | 0.4 | 0.36 |
| CRZ1 | 1.2 | — | 0.8 | 0.05 | 0.4 | 0.36 |
2.3. Application of edible coatings and storage
Before applying the coatings, the fruits were sanitized in a chlorine solution at 80 ppm for 15 minutes and air-dried at room temperature (22.47 ± 1.34 °C). Subsequently, a film-forming solution was applied to each passion fruit using a brush (Atlas, Model 319/5, 21 × 5.2 × 1 cm) and placed on a stainless-steel table at room temperature until the solution completely dried. The passion fruits remained on the stainless-steel table throughout the storage period. Four treatments were carried out in duplicate using the four film-forming solutions, CW, CWZ, CR, and CRZ, shown in Table 1.2.4. Fruit characterization
Fruits were evaluated for physical, chemical, and sensory properties on days 0, 2, 4, 8 and 10. For the analysis, three fruits were considered for each treatment and sampling day, with the analyses conducted in triplicate, resulting in a total of nine fruits per treatment and sampling day. The entire study was replicated twice.2.5. Weight loss
Weight loss was determined by the difference between the initial and final mass of the fruit, with the aid of a semi-analytical scale with a precision of ±0.01 g.202.6. Peel colour
Peel colour was measured according to You et al. (2022)21 using a CR-400 portable colorimeter (Konica Minolta, Tokyo, Japan). The CIELab parameters L* (brightness, 0 – black to 100 – white), a* (green (−a) to red (+a)) and b* (blue (−b) to yellow (+b)) were obtained from two random points on the peel of the fruits.2.7. Firmness
Fruit firmness was determined using a texture analyser TA-XT PLUS (Stable Micro Systems, United Kingdom) according to Zhong et al., (2022).22 Measurements were taken at two equidistant points in the equatorial region of the fruits and the results were expressed in Newtons (N).2.8. Total titratable acidity (TTA), total soluble solids (TSS), TSS/TTA ratio and pH
Total soluble solid (TSS) content was directly read on a digital refractometer Atago PAL-1 and total titratable acidity (TTA) and pH were determined using an automatic titrator (794 Basic Titrino – Metrohm).23 The TSS/TTA ratio was determined by the ratio between the values of total soluble solids and total titratable acidity.2.9. Vitamin C and sugar contents
Vitamin C and sugar contents were quantified by HPLC as described by Da Rosa et al. (2007)24 and Macre (1998),25 respectively.2.10. Determination of external injuries
During storage, fruits with lesions or external damage were identified by visual observation and separated for counting. The results were expressed as a percentage of rotten passion fruit.262.11. Statistical analyses
The results were submitted to analysis of variance (one-way ANOVA). Significant differences between means were analysed using Tukey's test (p < 0.05). Statistical analysis was performed using the STATISTICA® program version 10.0 (Statsoft, Tulsa, USA).3. Results and discussion
3.1. Physical–chemical characterization
The values of TTA, pH, TSS and ratio (TSS/TTA) of the coated passion fruit during storage are shown in Table 2. The pH values ranged from 3.10 to 3.32 which are in line with passion fruit. During storage, it was observed that the pH values of passion fruit increased in all treatments, except for the resin treatment. The pH values of the CR treatment did not differ between storage days. The increase in pH observed during storage may be attributable to the fruit ripening and the consumption of organic acids, especially citric acid, in passion fruit. Citric acid can function as a substrate in the respiration process.27 Furthermore, additional factors, such as the biochemical state of the fruit and a slower respiration rate may have collectively influenced the observed change in pH.28| Parameters | SD | CW | CWZ | CR | CRZ |
|---|---|---|---|---|---|
| a Means followed by the same lowercase letter in the rows and capital letters in the columns do not differ from each other, according to Tukey's test at 5% probability. CW: chitosan + wax; CWZ: chitosan + wax + ZnOnano; CR: chitosan + resin; CRZ: chitosan + resin + ZnOnano; SD: storage day. | |||||
| pH | 0 | 3.10aC ± 0.01 | 3.10aC ± 0.01 | 3.10aA ± 0.01 | 3.10aB ± 0.01 |
| 2 | 3.23aAB ± 0.08 | 3.16aBC ± 0.04 | 3.19aA ± 0.09 | 3.15aB ± 0.03 | |
| 4 | 3.16aBC ± 0.04 | 3.26aAB ± 0.08 | 3.28aA ± 0.11 | 3.20aB ± 0.03 | |
| 8 | 3.24abAB ± 0.06 | 3.30aAB ± 0.07 | 3.26abA ± 0.08 | 3.13bB ± 0.06 | |
| 10 | 3.32aA ± 0.03 | 3.32aA ± 0.06 | 3.28aA ± 0.02 | 3.32aA ± 0.04 | |
| TTA | 0 | 4.96aA ± 0.01 | 4.96aA ± 0.01 | 4.96aA ± 0.01 | 4.96aA ± 0.01 |
| 2 | 3.85aBC ± 0.59 | 4.11aAB ± 0.27 | 4.00aAB ± 0.45 | 4.28aB ± 0.11 | |
| 4 | 4.49aAB ± 0.27 | 3.56aBC ± 0.33 | 3.91aB ± 0.33 | 3.85aBC ± 0.40 | |
| 8 | 3.42aC ± 0.27 | 3.35aC ± 0.05 | 3.50aB ± 0.10 | 3.65aBC ± 0.19 | |
| 10 | 3.20aC ± 0.02 | 3.17aC ± 0.41 | 3.53aB ± 0.14 | 3.23aC ± 0.31 | |
| TSS | 0 | 13.85aA ± 0.10 | 13.85aA ± 0.10 | 13.85aA ± 0.1 | 13.85aA ± 0.10 |
| 2 | 12.70aAB ± 1.30 | 11.68aB ± 0.10 | 12.53aB ± 0.3 | 12.68aB ± 0.20 | |
| 4 | 12.54abAB ± 0.30 | 13.43abA ± 0.10 | 10.88cC ± 1.1 | 11.70bC ± 0.50 | |
| 8 | 10.20dC ± 0.30 | 10.58cdC ± 0.30 | 12.95aAB ± 0.5 | 10.78bcD ± 0.60 | |
| 10 | 12.27aB ± 1.20 | 10.08bC ± 0.50 | 10.83bC ± 0.3 | 12.03aBC ± 0.10 | |
| Ratio | 0 | 2.79aB ± 0.01 | 2.79aB ± 0.01 | 2.79aB ± 0.01 | 2.79aB ± 0.01 |
| 2 | 3.27aB ± 0.27 | 2.83aB ± 0.01 | 2.82aB ± 0.03 | 2.97aB ± 0.04 | |
| 4 | 2.78aB ± 0.25 | 3.58aA ± 0.01 | 2.65aB ± 0.25 | 2.87aB ± 0.19 | |
| 8 | 3.06aB ± 0.20 | 3.15aAB ± 0.03 | 3.70aA ± 0.03 | 2.96aB ± 0.03 | |
| 10 | 4.06aA ± 0.05 | 3.07aB ± 0.32 | 3.08aB ± 0.20 | 3.54aA ± 0.10 | |
Regarding TTA, no significant difference was observed between treatments considering the same day of storage. However, acidity values decreased in all samples throughout storage, and fruits coated with rosin resin (CR) showed the highest acidity values on the last day of storage. Fruit acidity is another important factor affecting consumer acceptance and serves as an indicator of fruit maturity. TTA contributes to the perceived acidity of fruits, and a decrease in TTA may lead to a reduction in the characteristic acidity associated with passion fruit. This can impact the overall flavour profile, potentially resulting in a less tangy taste.29 In general, TTA decreases during post-harvest storage as organic acids are used as primary substrates for respiration and other metabolic processes.30 Silva et al. (2019)31 who applied coatings based on cassava starch to yellow passion fruit observed a decrease in TTA during storage.
TSS values decreased over storage for all samples. The TSS/TTA ratio, representing the balance between sugar and acid content, is closely linked to the aroma and flavour of the fruit. A higher ratio generally signifies a sweeter flavour, whereas a lower ratio indicates higher acidity. Fruit acceptability is closely linked to the TSS/TTA ratio, with consumer preferences often leaning towards fruits with well-balanced sweetness and acidity.32,33 The ratio for passion fruit increased during storage, ranging from 2.79 to 4.06. However, no differences were observed in the TSS/TTA ratio values between the samples on any of the days of storage. According to You et al. (2022),21 the higher the TSS/TTA ratio, the better the sensory quality of the fruit. Rinaldi et al. (2017)34 found an average value of ratio of 5.48 during storage of wild passion fruit; this value being considered for fruits of excellent flavour.
Vitamin C plays a role in various biochemical processes, influencing the development of aroma and flavour compounds throughout the ripening process. Table 3 presents the content of vitamin C and sugars present in passion fruit during storage for all treatments. All samples showed a reduction in vitamin C during storage, indicating that the coatings were not efficient in maintaining this vitamin. The decline in vitamin C content during ripening is attributed, in part, to the oxidation-induced degradation of ascorbic acid. Additionally, as a water-soluble vitamin, the reduction in vitamin C may be associated with the loss of water through perspiration.35 Dulta et al. (2022)11 observed the same behaviour in evaluating the post-harvest quality of oranges coated with film forming solutions based on alginate–chitosan containing ZnO.
| Parameters | SD | CW | CWZ | CR | CRZ |
|---|---|---|---|---|---|
| a Means followed by the same lowercase letter in the rows and capital letters in the columns do not differ from each other, according to Tukey's test at 5% probability. CW: chitosan + wax; CWZ: chitosan + wax + ZnOnano; CR: chitosan + resin; CRZ: chitosan + resin + ZnOnano; SD: storage day. | |||||
| Vitamin C (mg 100 g−1) | 0 | 19.35aA ± 0.39 | 19.35aA ± 0.39 | 19.35aA ± 0.39 | 19.35aA ± 0.39 |
| 2 | 16.49aB ± 0.30 | 12.46aB ± 0.71 | 11.94aB ± 0.42 | 12.67aBC ± 1.06 | |
| 4 | 14.11aC ± 0.57 | 10.98bBC ± 1.13 | 12.49abB ± 1.50 | 13.81aB ± 0.63 | |
| 8 | 10.10bD ± 0.67 | 9.85bc ± 0.90 | 8.31cC ± 0.01 | 11.97aC ± 0.32 | |
| 10 | 10.71aD ± 1.02 | 9.40aC ± 0.40 | 9.21aBC ± 1.58 | 9.29aD ± 0.79 | |
| Fructose (g 100 g−1) | 0 | 1.32aA ± 0.01 | 1.32aAB ± 0.01 | 1.32aAB ± 0.01 | 1.32aA ± 0.01 |
| 2 | 1.41aA ± 0.12 | 1.01aB ± 0.27 | 1.13aB ± 0.28 | 1.16aA ± 0.18 | |
| 4 | 1.33aA ± 0.19 | 1.02aB ± 0.56 | 0.96aB ± 0.46 | 1.06aA ± 0.27 | |
| 8 | 1.41aA ± 0.08 | 1.92aA ± 0.18 | 2.10aA ± 0.51 | 1.66aA ± 0.05 | |
| 10 | 1.46aA ± 0.15 | 1.02aAB ± 0.35 | 1.23aAB ± 0.21 | 1.51aA ± 0.72 | |
| Glucose (g 100 g−1) | 0 | 1.26aA ± 0.02 | 1.26aA ± 0.02 | 1.26aA ± 0.02 | 1.26aAB ± 0.02 |
| 2 | 1.13aA ± 0.26 | 0.97aA ± 0.24 | 1.13aA ± 0.32 | 1.14aAB ± 0.11 | |
| 4 | 1.26aA ± 0.16 | 0.93aA ± 0.60 | 0.74aA ± 0.41 | 0.99aB ± 0.28 | |
| 8 | 1.21aA ± 0.23 | 1.73aA ± 0.38 | 1.56aA ± 0.37 | 1.59aA ± 0.19 | |
| 10 | 1.33aA ± 0.11 | 1.06aA ± 0.36 | 0.94aA ± 0.33 | 1.43aA ± 0.69 | |
| Sucrose (g 100 g−1) | 0 | 4.36aA ± 0.04 | 4.36aA ± 0.04 | 4.36aA ± 0.04 | 4.36aA ± 0.04 |
| 2 | 3.87aAB ± 0.59 | 3.53aAB ± 0.47 | 3.43aA ± 0.78 | 3.95aAB ± 0.14 | |
| 4 | 3.29aAB ± 0.19 | 2.89aB ± 1.67 | 3.01aA ± 0.49 | 2.62aB ± 1.07 | |
| 8 | 2.32aC ± 0.09 | 2.69aAB ± 0.21 | 3.28aA ± 0.85 | 2.44aAB ± 0.26 | |
| 10 | 2.87aBC ± 0.32 | 2.57aAB ± 0.15 | 2.43aA ± 0.33 | 2.19aAB ± 0.54 | |
Regarding the sugar content, the glucose and fructose contents of passion fruit were not affected by the type of coating and storage time. On the other hand, the sucrose content decreased during storage for all coating types (Table 3). Sucrose, a disaccharide composed of glucose and fructose, is a source of energy for the plant. The decline in sucrose content observed in passion fruit during storage is likely associated with the fruit's respiratory processes. Throughout storage, fruits undergo ongoing metabolic processes, including respiration, impacting their chemical composition. Within the respiration process, sucrose is metabolized to supply the energy required to sustain the essential processes of the fruit.36
3.2. Weight loss, firmness, and peel colour
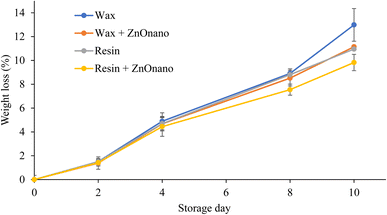
Fruit weight loss occurred continuously during the storage period (Fig. 1). The fruits coated with carnauba wax showed the highest percentage of weight loss on the last day of storage. On the other hand, fruits coated with resin and ZnO showed the lowest percentage of mass loss, with the coating being more efficient in controlling fruit mass loss. The coatings establish a barrier that lowers the transpiration rate, mitigating water loss from the fruit. Additionally, the coatings can influence the maturation rate, aiding in maintaining the turgidity of the passion fruit. Fruit ripening is an active metabolic process in which simple or complex reactions, as well as their combination, make the fruit palatable. However, the ripening of fruits is equally responsible for their deterioration and wilting during storage.37Passion fruit is a climacteric fruit, with a short post-harvest shelf life due to rapid water loss and wrinkling, which contributes to the reduction of its commercialization. Weight loss is mainly attributed to respiration and moisture evaporation through the passion fruit peel, thus resulting in fruit wrinkling and deterioration.38
Fruit firmness is a visual and tactile indicator of quality, playing an important role in consumer acceptability and product shelf life.39 Consumers are inclined to favour fruits that display good firmness, attracted by fresh appearance and tactile experience during the purchasing process.40Table 4 shows the results of fruit firmness during storage. Fruit firmness was influenced by the type of coating used during storage. However, a reduction in firmness during storage was observed. Fruits coated with chitosan, resin and ZnO nanoparticles showed no reduction in firmness over time, showing that the coating was able to effectively delay the loss of passion fruit firmness. Coating application may inhibit dehydration, which leads to resistance to cell wall deterioration.41 Zhou et al. (2022)6 and Zhang et al. (2019)38 also observed a reduction in firmness of purple passion fruit during storage.
| Treatments | Storage day | ||||
|---|---|---|---|---|---|
| Day 0 | Day 2 | Day 4 | Day 8 | Day 10 | |
| a Means followed by the same lowercase letter in the columns and uppercase in the lines do not differ from each other, according to Tukey's test at 5% probability. CW: chitosan + wax; CWZ: chitosan + wax + ZnOnano; CR: chitosan + resin; CRZ: chitosan + resin + ZnOnano. | |||||
| CW | 20.3aA ± 3.3 | 18.3aAB ± 1.5 | 16.9bBC ± 1.7 | 15.3abC ± 1.8 | 14.9aC ± 2.3 |
| CWZ | 20.3aA ± 3.3 | 19.7aA ± 2.7 | 19.5aA ± 2.9 | 16.1abB ± 1.9 | 16.2aB ± 2.2 |
| CR | 20.3aA ± 3.3 | 19.5aAB ± 3.0 | 16.1bB ± 2.9 | 16.3abB ± 2.0 | 15.6aB ± 2.8 |
| CRZ | 20.3aA ± 3.3 | 19.7aA ± 3.9 | 19.5aA ± 2.2 | 17.5aA ± 1.5 | 17.2aA ± 2.8 |
Changes in L*, a*, and b* values offer valuable insights into the colour characteristics of passion fruit. L* values indicate variations in the overall brightness of the passion fruit peel. The a* parameter measures colour along the red-green axis, with negative values indicating greenness. Meanwhile, the b* parameter assesses colour on the yellow-blue axis, with positive values denoting yellow. Ripe passion fruit is characterized by yellow tones. Overall, consumers typically prefer passion fruits displaying vibrant yellow hues, associating such colours with sweetness and maturity.42,43
L* values were not influenced by days of storage and type of coating (Table 5). Green-red a* values showed an increasing trend for all samples throughout storage. However, no differences were observed between treatments on any of the days of storage. For the parameter b*, the fruits showed a significant increase during storage, especially on the eighth day, when the highest values were observed for all samples. This effect is in line with the a* value, where chlorophyll degradation is responsible for increasing the a* value and also increasing the b* value during the late phase of fruit ripening.44 The intensification of the yellow colour results from the degradation of chlorophyll, revealing or synthesizing yellow, orange, and red pigments classified as carotenoids. These pigments are quite common, and their existence is an indicator for both consumers and industry to evaluate the maturity and quality of the fruits.43
| Parameters | SD | CW | CWZ | CR | CRZ |
|---|---|---|---|---|---|
| a Means followed by the same lowercase letter in the columns and uppercase in the lines do not differ from each other, according to Tukey's test at 5% probability. CW: chitosan + wax; CWZ: chitosan + wax + ZnOnano; CR: chitosan + resin; CRZ: chitosan + resin + ZnOnano; SD: storage day. | |||||
| L* | 0 | 70.18aA ± 3.17 | 70.18aA ± 3.17 | 70.18aA ± 3.17 | 70.18aA ± 3.17 |
| 2 | 70.37aA ± 2.05 | 71.57aA ± 3.42 | 71.21aA ± 3.27 | 72.14aA ± 2.53 | |
| 4 | 69.64aA ± 2.99 | 69.51aA ± 5.63 | 69.12abA ± 2.45 | 69.05aA ± 3.39 | |
| 8 | 69.35aA ± 2.88 | 69.53aA ± 3.21 | 69.40bA ± 1.54 | 69.11aA ± 3.50 | |
| 10 | 69.83aA ± 3.67 | 70.13aA ± 3.21 | 70.28abA ± 2.37 | 69.20aA ± 4.03 | |
| a* | 0 | −5.74aA ± 1.09 | −5.74aA ± 1.09 | −5.74aA ± 1.09 | −5.74aA ± 1.09 |
| 2 | −5.06aA ± 2.49 | −5.00abA ± 2.35 | −4.75abA ± 2.54 | −5.30aA ± 2.88 | |
| 4 | −5.03aA ± 3.16 | −3.28abA ± 1.79 | −5.50aA ± 2.70 | −4.38aA ± 2.83 | |
| 8 | −1.96bA ± 1.58 | −3.18bcA ± 1.47 | −1.65cA ± 1.84 | −0.22bA ± 1.72 | |
| 10 | −2.46bA ± 1.88 | −1.69cA ± 1.45 | −1.97bcA ± 1.79 | −1.66bA ± 1.85 | |
| b* | 0 | 38.36bA ± 2.61 | 38.36bA ± 2.61 | 38.36bA ± 2.61 | 38.36bA ± 2.61 |
| 2 | 40.39bA ± 3.19 | 40.65bA ± 3.05 | 39.46bA ± 3.80 | 41.06abA ± 4.64 | |
| 4 | 40.23bA ± 5.48 | 39.75bA ± 2.70 | 41.42bA ± 2.71 | 41.41abA ± 3.47 | |
| 8 | 46.61aA ± 4.14 | 46.77aA ± 3.62 | 45.17aA ± 4.09 | 42.97aA ± 4.03 | |
| 10 | 41.60abA ± 2.40 | 37.48bA ± 1.90 | 40.64bA ± 3.48 | 39.63abA ± 3.11 | |
On the first storage day, the fruits presented a more greenish colour than the passion fruit peel. However, during storage it was noticed that the colour of the fruit peel changed from green to yellow. So that on the last day of storage the fruits were already more yellow. Silva et al. (2019)31 observed changes during storage in the colour of passion fruit peel coated with cassava starch. From the 7th day of storage, the fruits began to show a yellowish colour. According to the authors, this change occurred due to the degradation of chlorophyll and the synthesis of carotenoids.
3.3. Visual appearance of the fruits and post-harvest loss index
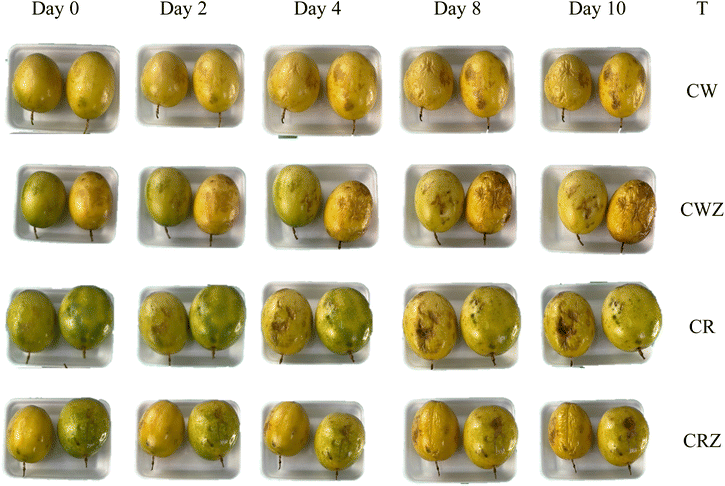
The physiological metabolism of passion fruit increases after harvesting, which easily leads to problems such as water loss and fruit wilting.22Fig. 2 shows the images of the fruits over the 10 days of storage at room temperature. On the first day (D0) the fruits are shiny and have a firm structure. From the fourth day of storage, wrinkling was observed in all treatments. On the last day of storage (10th day), all fruits had a less shiny appearance and wrinkled. The physiological metabolism of passion fruit involves a variety of biochemical processes, intrinsically influencing the changes observed in the fruit's appearance. These changes, ranging from colour variations to modifications in texture and aroma, represent visible manifestations of the underlying metabolic activities associated with ripening of the fruits.The percentages of fruit losses during storage are shown in Table 6. Fruits with external injuries and microbiological deterioration and unfit for consumption were discarded. The treatments CW and CWZ were the ones that lost the most fruits during storage, with a loss of 15.6 and 17.8% respectively. The fruits coated with resin and ZnO nanoparticles showed a lower percentage loss.
| Treatments | Total amount of fruits | Total fruit lost | Percentage of losses (%) |
|---|---|---|---|
| a CW: chitosan + wax; CWZ: chitosan + wax + ZnOnano; CR: chitosan + resin; CRZ: chitosan + resin + ZnOnano. | |||
| CW | 45 | 7 | 15.6 |
| CWZ | 45 | 8 | 17.8 |
| CR | 45 | 6 | 13.3 |
| CRZ | 45 | 5 | 11.1 |
Brazil is one of the countries that loses the most fruits and vegetables during the post-harvest period; most of these losses are associated with the lack of application of efficient conservation methods. The 2021 Food Waste Index report, recently released by the United Nations Environment Program (UNEP), indicated that about 931 million tons of food were discarded as waste in 2019.45 This suggests that 17% of total global food production could be wasted, underscoring the imperative for sustainable post-harvest management practices and innovative coating technologies. Addressing these losses not only enhances economic outcomes for producers but also aligns with worldwide endeavours to diminish food waste and enhance food security.
According to Md Nor & Ding (2020),46 a third of all food production is lost in the post-harvest or wasted, where 45% of this value corresponds to the waste of fruits and vegetables.
3.4. Increasing the resin concentration in the film forming solution
Coatings formulated with rosin resin were more effective in protecting fruits against weight loss and delaying the ripening process, thus allowing the fruits to reach 10 days of storage with adequate consumption conditions. In addition, the coated fruits presented a better visual appearance and less fruit loss during storage. Therefore, a new coating test was carried out to increase the adhesion of the coatings on passion fruit and improve the post-harvest quality of the fruits. For this, the effect of increasing the rosin resin concentration from 0.6 to 0.8% w/v was evaluated compared to uncoated fruits (control). Three treatments were evaluated as follows: CR1, CRZ1 and control.Regarding the physical–chemical characterization, the values of total titratable acidity (TTA), pH, total soluble solids (TSS) and ratio (TSS/TTA) of the fruits during storage are presented in Table 7. The pH value of passion fruit was not influenced by storage time and types of coatings. On the other hand, fruits coated with CR1 showed higher TTA and TSS when compared to uncoated fruits after the 10th day of storage. This fact may be related to the influence of the coating on the conservation and maintenance of fruit quality. In terms of ratio (TSS/TTA), treatments CR1 and CRZ1 did not show differences in their values during storage. Overall, the inclusion of resin in the coatings maintained the physical–chemical properties, signifying control over the metabolic processes of the fruits.
| Parameters | Storage day | CR1 | CRZ1 | Control |
|---|---|---|---|---|
| a Means followed by the same lowercase letter in the rows and capital letters in the columns do not differ from each other, according to Tukey's test at 5% probability. CR1: chitosan + resin; CRZ1: chitosan + resin + ZnOnano; control: no coating. | ||||
| pH | 0 | 3.26aA ± 0.07 | 3.26aA ± 0.07 | 3.26aC ± 0.07 |
| 2 | 3.27aA ± 0.07 | 3.28aA ± 0.06 | 3.21aBC ± 0.05 | |
| 5 | 3.34aA ± 0.09 | 3.33aA ± 0.03 | 3.40aAB ± 0.02 | |
| 7 | 3.35aA ± 0.04 | 3.45aA ± 0.13 | 3.37aAB ± 0.09 | |
| 10 | 3.40aA ± 0.08 | 3.47aA ± 0.07 | 3.45aA ± 0.03 | |
| TTA | 0 | 3.45aA ± 0.37 | 3.45aA ± 0.37 | 3.45aA ± 0.37 |
| 2 | 3.12aA ± 0.51 | 2.98aAB ± 0.06 | 3.36aAB ± 0.27 | |
| 5 | 3.50aA ± 0.15 | 3.06abAB ± 0.24 | 2.73bAB ± 0.14 | |
| 7 | 2.76aA ± 0.07 | 2.42aB ± 0.21 | 2.63aB ± 0.38 | |
| 10 | 2.77aA ± 0.08 | 2.69abAB ± 0.05 | 2.58bAB ± 0.01 | |
| TSS | 0 | 9.32aA ± 0.1 | 9.32aA ± 0.1 | 9.32aA ± 0.1 |
| 2 | 9.02aA ± 1.1 | 9.22aA ± 0.6 | 9.58aA ± 0.9 | |
| 5 | 9.50aA ± 0.2 | 9.58aA ± 1.8 | 9.12aAB ± 0.8 | |
| 7 | 8.83aA ± 0.5 | 7.65bB ± 0.5 | 8.08abB ± 0.6 | |
| 10 | 9.05aA ± 0.4 | 8.58abAB ± 0.5 | 8.18bB ± 0.1 | |
| Ratio | 0 | 2.72aA ± 0.29 | 2.72aA ± 0.29 | 2.72aB ± 0.29 |
| 2 | 2.90aA ± 0.08 | 2.97aA ± 0.17 | 2.90aAB ± 0.02 | |
| 5 | 2.63aA ± 0.12 | 3.17aA ± 0.53 | 3.52aA ± 0.21 | |
| 7 | 3.11aA ± 0.31 | 3.13aA ± 0.03 | 3.33aA ± 0.01 | |
| 10 | 3.15aA ± 0.18 | 3.30aA ± 0.07 | 3.17aAB ± 0.04 | |
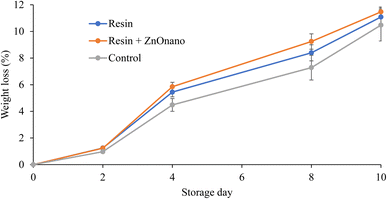
All treatments showed weight loss over storage time, with no significant differences between samples (Fig. 3). However, it was observed that fruit firmness decreased during storage (Table 8). The fruits coated with CR1 showed a higher value until the fifth day of storage, indicating the influence of the coatings on maintaining the firmness of the passion fruit.
| Treatments | Storage day | ||||
|---|---|---|---|---|---|
| D0 | D2 | D5 | D7 | D10 | |
| a Means followed by the same lowercase letter in the columns and capital letters in the rows do not differ from each other, according to Tukey's test at 5% probability. CR1: chitosan + resin; CRZ1: chitosan + resin + ZnOnano; control: no coating. | |||||
| CR1 | 20.9aA ± 2.0 | 18.9aA ± 1.7 | 18.0aA ± 1.9 | 14.1aB ± 1.9 | 12.8aB ± 1.7 |
| CRZ1 | 20.9aA ± 2.0 | 17.7abAB ± 1.2 | 16.1abB ± 2.2 | 15.0aBC ± 1.7 | 12.9aC ± 1.9 |
| Control | 20.9aA ± 2.0 | 16.3bB ± 1.9 | 14.8bBC ± 2.3 | 13.4aCD ± 1.4 | 11.8aD ± 1.2 |
The values of L*, a* and b* of passion fruit during storage are shown in Table 9. The L* parameter, which is related to colour brightness, decreased during storage for all treatments. Even so, CR1 and CRZ1 treatments had the highest L* values compared to the control treatment. For the parameter a*, which is related to the green-red colour, an increase in its values was observed at the end of the 12th day of storage. That is, the intensity of the green colour present in the fruits decreased during storage. CR1 and CRZ1 treatments had the lowest a* values compared to the control treatment. In terms of the b* parameter, all treatments showed an increase during storage. This increase is related to the increase in the yellow colour of the fruits. The visual appearance of coated and uncoated fruits is shown in Fig. 4. At the beginning of storage, the fruits were shiny and with a firm structure. From the 8th day of storage, wrinkling began to appear visibly on all passion fruit.
| Storage day | |||||
|---|---|---|---|---|---|
| 0 | 2 | 5 | 7 | 10 | |
| a Means followed by the same lowercase letter in the columns and capital letters in the rows do not differ from each other, according to Tukey's test at 5% probability. CR1: chitosan + resin; CRZ1: chitosan + resin + ZnOnano; control: no coating. | |||||
| L* | |||||
| CR1 | 71.96aA ± 0.35 | 74.35aA ± 2.00 | 73.76aA ± 2.12 | 73.35aA ± 2.08 | 72.33aA ± 2.46 |
| CRZ1 | 71.96aB ± 0.35 | 75.36aA ± 1.38 | 73.41aAB ± 2.56 | 72.49aB ± 2.15 | 72.28aB ± 1.24 |
| Control | 71.96aA ± 0.35 | 71.50bA ± 2.91 | 69.93bA ± 2.03 | 69.75bA ± 1.66 | 69.85bA ± 1.17 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| a* | |||||
| CR1 | −5.16aA ± 1.30 | −3.15aB ± 0.91 | −2.56aBC ± 0.96 | −2.06aC ± 0.68 | −1.99aC ± 0.88 |
| CRZ1 | −5.16aA ± 1.30 | −3.07aB ± 0.62 | −2.76aBC ± 0.92 | −1.46aC ± 1.98 | −2.10aBC ± 0.53 |
| Control | −5.16aA ± 1.30 | −3.36aB ± 0.72 | −2.71aB ± 0.69 | −1.21aC ± 0.93 | −0.91bC ± 0.94 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| b* | |||||
| CR1 | 38.94aB ± 1.84 | 39.54bB ± 2.26 | 43.92aA ± 1.83 | 44.33aA ± 2.53 | 45.12aA ± 1.97 |
| CRZ1 | 38.94aB ± 1.84 | 41.14bBA ± 2.94 | 43.77aA ± 2.29 | 44.27aA ± 2.76 | 43.43aA ± 1.39 |
| Control | 38.94aB ± 1.84 | 44.75aA ± 2.82 | 44.37aA ± 2.00 | 46.17aA ± 2.73 | 42.66aA ± 0.94 |
 | ||
| Fig. 4 Visual aspects of fruits over time, after the second application of coatings. T: treatment; CR1: chitosan + resin; CRZ1: chitosan + resin + ZnOnano; control: no coating. | ||
At the end of the storage period (10th day), the coated passion fruit exhibited enhanced visual appearance in comparison to the uncoated passion fruit (control). Specifically, the coated fruits displayed reduced wrinkling and fewer dark spots on the peel surface compared to the control treatment. This fact suggests a potential association between the use of resin in coatings and its influence on controlling the release of water vapor.
Table 10 shows the percentages of fruit losses due to external injuries during the 10 days of storage. The CR1 and CRZ1 treatments had the lowest rate of losses due to external injuries, with a value of 11.1% for both treatments. On the other hand, the fruits of the control treatment presented a loss index of 31.1%, being 3 times higher than that by the coated fruits. The decrease in the rate of loss due to the use of coatings shows the great potential of using technology to control this problem in passion fruit post-harvest.
| Treatments | Total amount of fruits | Total fruit lost | Percentage of losses (%) |
|---|---|---|---|
| a CR1: chitosan + resin; CRZ1: chitosan + resin + ZnOnano; control: no coating. | |||
| CR1 | 45 | 5 | 11.1 |
| CRZ1 | 45 | 5 | 11.1 |
| Control | 45 | 14 | 31.1 |
4. Conclusions
Chitosan-based coatings containing rosin resin were efficient in preserving passion fruit against weight loss and delaying the ripening process. The coated passion fruit remained under suitable conditions for consumption until the end of the 10th day of storage. In addition, the fruits had a better visual appearance and a lower rate of fruit loss during storage.The increase in resin concentration in the film forming solution improved the adherence of the coating on the fruit surface and delayed the appearance of lesions when compared to uncoated fruits. This allowed the fruits to reach 10 days of storage under suitable conditions for consumption. The fruits had a shiny appearance, making them more attractive to the consumer.
The results obtained in this study showed the potential use of biodegradable chitosan-based coatings combined with rosin resin and ZnO nanoparticles in the food industry. The use of coatings emerges as a practical solution to decrease losses, preserve product quality, and embrace sustainable practices. Given the demonstrated effectiveness of these coatings in minimizing spoilage, their integration holds the potential to be a valuable innovation for the food supply chain.
Author contributions
Jailton Ribeiro dos Santos Junior: investigation, formal analysis, writing – original draft, data curation. Luiz Carlos Corrêa-Filho: conceptualization, methodology, writing – review & editing, visualization. Vitória Oliveira Pereira: methodology. Henriqueta Talita Guimarães Barboza: methodology. José Carlos Sá Ferreira: methodology. Antônio Gomes Soares: conceptualization, methodology, investigation, formal analysis, writing – original draft, data curation. Renata Valeriano Tonon: conceptualization, methodology, writing – review & editing, visualization. Lourdes Maria Corrêa Cabral: conceptualization, methodology, formal analysis, resources, writing – review & editing, visualization, supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the Coordenação de Aperfeiçoamento Pessoal de Ensino Superior (CAPES, #88887.646273/2021-00) and FAPERJ (E-26/204.362/2021) for financial support and providing the facilities for this study.References
- L. C. R. dos Reis, E. M. P. Facco, M. Salvador, S. H. Flôres and A. de Oliveira Rios, J. Food Sci. Technol., 2018, 55, 2679–2691 CrossRef PubMed.
- F. P. Chen, X. Y. Xu, Z. Luo, Y. Chen, Y. Xu and G. Xiao, J. Food Process. Preserv., 2018, 42, e13749 CrossRef.
- J. Villacís-Chiriboga, K. Elst, J. Van Camp, E. Vera and J. Ruales, Compr. Rev. Food Sci. Food Saf., 2020, 19, 405–447 CrossRef PubMed.
- M. Kouhi, M. P. Prabhakaran and S. Ramakrishna, Trends Food Sci. Technol., 2020, 103, 248–263 CrossRef CAS.
- S. A. A. Mohamed, M. El-Sakhawy and M. A. M. El-Sakhawy, Carbohydr. Polym., 2020, 238, 116178 CrossRef CAS PubMed.
- Y. Zhou, Y. Zhong, L. Li, K. Jiang, J. Gao, K. Zhong, M. Pan and B. Yan, Lebensm.-Wiss. Technol., 2022, 163, 113584 CrossRef CAS.
- W. Batista Silva, G. M. Cosme Silva, D. B. Santana, A. R. Salvador, D. B. Medeiros, I. Belghith, N. M. da Silva, M. H. M. Cordeiro and G. P. Misobutsi, Food Chem., 2018, 242, 232–238 CrossRef PubMed.
- G. L. Dotto, M. L. G. Vieira and L. A. A. Pinto, LWT--Food Sci. Technol., 2015, 64, 126–130 CrossRef CAS.
- E. Fortunati, G. Giovanale, F. Luzi, A. Mazzaglia, J. M. Kenny, L. Torre and G. M. Balestra, Coatings, 2017, 7, 196 CrossRef.
- F. Zhu, Food Chem., 2021, 359, 129871 CrossRef CAS PubMed.
- K. Dulta, G. Koşarsoy Ağçeli, A. Thakur, S. Singh, P. Chauhan and P. K. Chauhan, J. Polym. Environ., 2022, 30, 3293–3306 CrossRef CAS.
- L. Motelica, D. Ficai, O. Oprea, A. Ficai, R. D. Trusca, E. Andronescu and A. M. Holban, Pharmaceutics, 2021, 13, 1020 CrossRef CAS PubMed.
- B. Hassan, S. A. S. Chatha, A. I. Hussain, K. M. Zia and N. Akhtar, Int. J. Biol. Macromol., 2018, 109, 1095–1107 CrossRef CAS PubMed.
- M. S. Butt, M. Akhtar, A. A. Maan and M. Asghar, J. Food Meas. Charact., 2023, 17, 694–705 CrossRef.
- L. Susmita Devi, S. Kalita, A. Mukherjee and S. Kumar, Trends Food Sci. Technol., 2022, 129, 296–305 CrossRef CAS.
- F. Cruces, M. G. García and N. A. Ochoa, Food Bioprocess Technol., 2021, 14, 1244–1255 CrossRef CAS.
- N. Su, C. Fang, H. Zhou, T. Tang, S. Zhang, X. Wang and B. Fei, Polymers, 2021, 13, 3500 CrossRef CAS PubMed.
- B. J. Arroyo, A. C. Bezerra, L. L. Oliveira, S. J. Arroyo, E. A. de Melo and A. M. P. Santos, Food Chem., 2020, 309, 125566 CrossRef CAS PubMed.
- J. Chen, L. Luo, C. Cen, Y. Liu, H. Li and Y. Wang, Int. J. Biol. Macromol., 2022, 220, 462–471 CrossRef CAS PubMed.
- H. Li, W. Li, J. Zhang, G. Xie, T. Xiong and H. Xu, Food Packag. Shelf Life, 2022, 33, 100928 CrossRef CAS.
- M. You, X. Duan, X. Li, L. Luo, Y. Zhao, H. Pan, W. Gong, L. Yang, Z. Xiang and G. Li, Sustainable Chem. Pharm., 2022, 27, 100679 CrossRef CAS.
- Z. Zhong, L. Zhou, K. Yu, F. Jiang, J. Xu, L. Zou, L. Du and W. Liu, Food Bioprocess Technol., 2022, 15, 1836–1850 CrossRef CAS.
- G. W. Latimer, The Association of Official Agricultural (AOAC), Official Method of Analysis, 17th edn, AOAC, Washington, DC, USA, 2000 Search PubMed.
- J. S. Da Rosa, R. L. D. O. Godoy, J. Oiano Neto, R. D. S. Campos, V. M. Da Matta, C. A. Freire, A. S. Da Silva and R. S. De Souza, Food Sci. Technol., 2007, 27, 837–846 CrossRef.
- R. Macre, Food Science and Technology: A Series of Monoghraphys: HPLC in Food Analysis, Editora Academic Press, New York, 2nd edn, 1998 Search PubMed.
- G. Romanazzi, E. Feliziani, M. Santini and L. Landi, Postharvest Biol. Technol., 2013, 75, 24–27 CrossRef CAS.
- K. S. Tumwesigye, A. R. Sousa, J. C. Oliveira and M. J. Sousa-Gallagher, Food Packag. Shelf Life, 2017, 13, 1–14 CrossRef.
- L. S. Devi, A. Mukherjee, D. Dutta and S. Kumar, Sustainable Food Technol., 2023, 1, 415–425 RSC.
- M. Sultan, O. M. Hafez, M. A. Saleh and A. M. Youssef, RSC Adv., 2021, 11, 9572–9585 RSC.
- N. Mahfoudhi and S. Hamdi, J. Food Process. Preserv., 2015, 39, 1499–1508 CrossRef CAS.
- A. C. G. da Silva, N. d. S. e Silva and F. F. de Sousa, Rev. Verde Agroecol. e Desenvolv. Sustentável, 2019, 14, 238–245 CrossRef.
- S. C. F. d. C. Costa, W. d. S. Alves, R. d. L. Erazo, R. M. de Oliveira and W. G. dos Santos, Braz. J. Dev., 2020, 6, 16507–16521 CrossRef.
- C. S. P. Cafieiro, P. P. L. G. Tavares, C. O. de Souza, L. F. S. Cruz and M. E. O. Mamede, An. Acad. Bras. Cienc., 2022, 94, e20211446 CrossRef CAS PubMed.
- M. M. Rinaldi, A. Maria Costa, F. G. Faleiro, N. Tadeu and V. Junqueira, Braz. J. Food Technol., 2017, 20, e2016046 Search PubMed.
- P. Yumbya, J. Ambuko and S. I. Shibairo, J. Post Harvest Technol., 2014, 025–036 Search PubMed.
- S. Durán-Soria, D. M. Pott, S. Osorio and J. G. Vallarino, Front. Plant Sci., 2020, 11, 564917 CrossRef PubMed.
- K. Mondal, S. P. Malhotra, V. Jain and R. Singh, Physiol. Mol. Biol. Plants, 2009, 15, 327–334 CrossRef CAS PubMed.
- R. Zhang, W. Lan, J. Ding, S. Ahmed, W. Qin, L. He and Y. Liu, Molecules, 2019, 24, 3378 CrossRef CAS PubMed.
- L. Aragüez, A. Colombo, R. Borneo and A. Aguirre, Food Packag. Shelf Life, 2020, 25, 100520 CrossRef.
- B. Maringgal, N. Hashim, I. S. Mohamed Amin Tawakkal and M. T. Muda Mohamed, Trends Food Sci. Technol., 2020, 96, 253–267 CrossRef CAS.
- L. Mohammadi, A. Ramezanian, F. Tanaka and F. Tanaka, J. Food Meas. Charact., 2021, 15, 353–362 CrossRef.
- W. C. Schotsmans and G. Fischer, Postharvest Biology and Technology of Tropical and Subtropical Fruits, 2011, vol. 4, pp. 125–143e Search PubMed.
- N. J. D. A. Melo, A. M. P. Negreiros, J. D. A. Sarmento, P. L. D. de Morais and R. S. Júnior, Emir. J. Food Agric., 2020, 32, 897–908 CrossRef.
- K. Hong, J. Xie, L. Zhang, D. Sun and D. Gong, Sci. Hortic., 2012, 144, 172–178 CrossRef CAS.
- UNEP, Food Waste Index Report, https://www.unep.org/resources/report/unep-food-waste-index-report-2021, 2021 Search PubMed.
- S. Md Nor and P. Ding, Food Res. Int., 2020, 134, 109208 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |