Light-induced degradation of organic pollutants under high salinity conditions using titanium dioxide/ferrocene polymer nanocomposites as photocatalyst and H2O2 activator simultaneously†
Received
13th October 2023
, Accepted 15th December 2023
First published on 15th December 2023
Abstract
In this study, TiO2 nanocomposites immobilized with a ferrocene-containing polymer were facilely prepared according to mussel-inspired biomimetic strategy. The nanocomposites were used as heterogeneous catalysts for photocatalytic oxidation and photo-Fenton-like reactions simultaneously. Notably, mimetic wastewaters containing different organic pollutants could be treated via these synergistic photoreactions in high salinity conditions. A series of organic pollutants such as tetracyclines, bisphenol A, bisphenol S, and rhodamine B could be degraded within 30 minutes in the presence of high-concentration inorganic salts (500 mmol L−1 NaCl or NaNO3). It was possible to recycle the nanocomposites after the reactions for reuse, and the degradation efficiency was maintained stable in repeated consecutive experiments.
Environmental significance
As one of the most commonly used advanced oxidation processes (AOPs), the traditional Fenton reaction is often limited in treating high salinity wastewater. Novel AOPs dominated by singlet oxygen and superoxide radicals could avoid the side effects of inorganic salts and have recently drawn great attention. In this study, we demonstrate that TiO2 nanocomposites immobilized with a ferrocene-containing polymer could act as heterogeneous catalysts for photocatalytic oxidation and photo-Fenton-like reactions simultaneously. Our study contributes to the treatment of wastewater containing different organic pollutants by the synergistic photoreactions based on nano photocatalysts in high salinity conditions.
|
1. Introduction
Owing to the efficient generation of oxidizing free radicals under conditions close to normal temperature and pressure, advanced oxidation processes (AOPs) are widely used to treat non-biodegradable pollutants in water.1,2 Hydrogen peroxide (H2O2), peroxydisulfate (PDS), and peroxymonosulfate (PMS) are inexpensive and efficient oxidants used in the remediation of polluted water. Studies on the degradation of pollutants in water by activating oxidants in different ways to produce living oxygen species, such as hydroxyl radical (·OH), sulfate radical (·SO4−), singlet oxygen (1O2), and superoxide radical (·O2−), have been widely reported.3–9 Among these AOPs, Fenton and Fenton-like reactions are the most common processes for treating wastewater containing complex pollutants.10,11 Industrial wastewater usually contains a large amount of inorganic salts, which quench the ·OH and ·SO4− radicals produced in AOPs, thus affecting the performance of Fenton and Fenton-like reactions.3,12–141O2 is a non-radical reactive oxygen species (ROSs) that can selectively degrade pollutants and is not affected by inorganic salts in water.3,15,16 Metal co-catalysts, such as copper, and organic acid catalysts, such as ascorbic acid, are widely used in AOPs dominated by non-radical ROSs.17–20 However, some co-catalysts are difficult to recycle and can cause potential environmental pollution during application. The application of carbon materials avoided the metal leaching-induced secondary contamination of metal-based heterogeneous catalysts, but the preparation of carbon materials requires high temperatures of hundreds of degrees Celsius.3,7,15 Ferrocene (Fc) and its derivatives can be grafted onto the carrier via a covalent bond under relatively mild conditions. In addition, Fc not only has good reversible redox properties but also exhibits an excellent electron transfer rate, and is widely used as a heterogeneous catalyst.21–23 For example, the heterogeneous catalysts prepared by covalently bonding Fc can avoid the loss of Fe and exhibit excellent catalytic performance.21,24–26 Fc-based heterogeneous catalysts can be prepared under mild conditions while avoiding metal leaching, so they have great prospects in water pollution remediation.
Photo-Fenton has economic advantages and is efficient and environmentally friendly. Therefore, photocatalysis has received widespread attention in recent years.27–32 However, the low utilization of light is a challenge in traditional photo-Fenton reactions.33 The combination of photo-Fenton and photocatalysis has excellent synergistic effects, which can greatly improve the utilization of light and the reaction efficiency.34,35 TiO2, as one of the most common photocatalysts, has the advantages of environmental safety, stability, and affordability.36 It is our hypothesis that grafting ferrocene onto TiO2 can yield a hybrid nanocomposite and hence combine the photo-Fenton reaction and photocatalytic oxidation reaction. However, to the best of our knowledge, there have been very few reports on the fabrication of TiO2/ferrocene polymer hybrid nanocomposites as well as its application as the photocatalysts for AOP.
In this work, TiO2@Fc polymer nanocomposites (TiO2@Fc) were prepared by grafting Fc-containing polymer to TiO2via dopamine-based bonding, which is inspired by the mussel adhesion protein.37,38 The nanocomposites were characterized by XPS, FTIR, TGA, and SEM. Subsequently, TiO2@Fc was used as the photocatalyst for simultaneous Fenton-like and photocatalytic oxidation reactions to degrade pollutants in high-salinity wastewater. The quenching experiment and electron paramagnetic resonance (EPR) were utilized to investigate the mechanism of the photo-Fenton-like reaction. Finally, the repeated experiments and degradation experiments on various organic pollutants demonstrated the potential of the catalyst in the treatment of high-concentration salt-containing organic wastewater.
2. Results and discussion
2.1. Preparation and characterization of TiO2@Fc hybrid nanocomposites
As shown in Scheme 1, a ferrocene-containing polymer (poly(ferrocene)) was first prepared by radical polymerization with AIBN as the initiator and PEGA480, FMMA, and DMA as the monomers. Among them, FMMA serves as an iron source, DMA serves as a chelating agent to graft poly(ferrocene) to TiO2, and PEGA480 is a hydrophilic monomer aiming to enhance the dispersibility of TiO2@Fc in water. After polymerization, TiO2 nanoparticles were directly added into the reaction tube to in situ immobilize poly(ferrocene) onto the surface via biomimetic interaction of catechol groups with TiO2. The final product (named TiO2@Fc) could be separated from the suspension via centrifugation following drying under vacuum.
 |
| | Scheme 1 Synthetic route to TiO2@Fc nanocomposites. | |
TEM, SEM-EDS, FTIR, TGA, and XRD techniques were then used to characterize TiO2@Fc. The TEM image of TiO2@Fc (Fig. 1A) showed that the particle size of the non-agglomerated catalyst was within 20 nm, and the enlarged crystal structure is shown in Fig. 1B. Based on the lattice fringes, the distance between two adjacent planes was estimated to be 0.352 nm, which corresponds to the (101) plane spacing of the anatase phase.36 The surface morphology of the stacked catalyst was displayed in the SEM image (Fig. 1C) and the morphology of the dispersed catalysts showed irregular nanoparticles. The mapping image (Fig. 1D) confirmed the existence of the Fe element and revealed a uniform distribution of Fe on the catalyst. As shown in Fig. 1E, the peaks at 0.7 keV and 6.4 keV came from the Fe element, and the peaks at 2.3 keV and 2.5 keV came from the Ti element in the SEM-EDS data. Compared with TiO2, the FTIR spectrum of TiO2@Fc showed new peaks at 1100, 1731, and 2869 cm−1, which were ascribed to poly(ferrocene) (Fig. 1F). The peaks at 1100 cm−1 belonged to C–O, and the peak of 1731 cm−1 and 2869 cm−1 were because of the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–H, respectively. The TGA analysis (Fig. 1G) showed that 92.5% of poly(ferrocene) was degradable, and the 7.5% weight residual may be due to the presence of iron oxide after the TGA test. When the temperature reached 600 °C, there was 92.00% remaining TiO2 and 87.14% remaining TiO2@Fc, which further proved the presence of degradable poly(ferrocene) in the hybrid nanocomposites. The main peaks appearing in the XRD pattern (Fig. 1H) at 2θ = 25.4(101), 37.9(004), 48.1(200), 54.0(105), 55.1(211), 62.8(204), 69.0(116), 70.4(220), and 75.1(215) were all from anatase TiO2 because Fc was grafted onto the surface of TiO2 in the form of a polymer, which did not affect the crystal structure. Based on these characterizations, TiO2 hybrid nanocomposites with the ferrocene-containing polymer on the surface were successfully prepared.
O and C–H, respectively. The TGA analysis (Fig. 1G) showed that 92.5% of poly(ferrocene) was degradable, and the 7.5% weight residual may be due to the presence of iron oxide after the TGA test. When the temperature reached 600 °C, there was 92.00% remaining TiO2 and 87.14% remaining TiO2@Fc, which further proved the presence of degradable poly(ferrocene) in the hybrid nanocomposites. The main peaks appearing in the XRD pattern (Fig. 1H) at 2θ = 25.4(101), 37.9(004), 48.1(200), 54.0(105), 55.1(211), 62.8(204), 69.0(116), 70.4(220), and 75.1(215) were all from anatase TiO2 because Fc was grafted onto the surface of TiO2 in the form of a polymer, which did not affect the crystal structure. Based on these characterizations, TiO2 hybrid nanocomposites with the ferrocene-containing polymer on the surface were successfully prepared.
 |
| | Fig. 1 TEM (A and B), SEM (C), mapping of Fe (D), EDS (E), FTIR spectra (F), TGA curves (G), and XRD (H) of TiO2@Fc, and TiO2. | |
Then, in order to test the optical and optoelectronic properties of TiO2@Fc, the UV-visible diffuse reflection spectrum and photocurrent response were measured. The photocurrent response of TiO2@Fc (Fig. 2A) showed the rapid and stable photocurrent effect of the catalyst under irradiation, indicating that TiO2@Fc had good electron and hole separation efficiency. Generally, TiO2 had good absorption capacity for light in the range of 200–400 nm, while TiO2@Fc had good light absorption capacity in the range of 200–800 nm, indicating that the addition of Fc greatly increases the absorption range of the catalyst for light (Fig. 2B). The Tauc plots (Fig. 2C) showed that its band gap was approximately 2.02 eV. A smaller band gap indicated that the catalyst had good catalytic activity. Therefore, the UV-visible diffuse reflection spectrum and photocurrent response proved that TiO2@Fc had good photocatalytic performance.
 |
| | Fig. 2 Photocurrent response (A), UV-visible diffuse reflection spectrum (B), and Tauc plots (C) of TiO2@Fc. | |
2.2. Photocatalytic oxidation reaction catalyzed by hybrid nanocomposites
Subsequently, to confirm its photocatalytic properties, the hybrid nanocomposites were used as catalysts for photocatalytic oxidation of organic compounds. The test used RhB as the model compound and simulated sunlight as the light source under different pH. As shown in Fig. 3, when the reaction was stopped at 60 minutes, the removal ratio of RhB with TiO2@Fc as the photocatalysts could reach 40%, 50%, and 25% at pH 3.0 (Fig. 3A), 7.0 (Fig. 3B), and 11.0 (Fig. 3C), respectively. Generally, compared to TiO2, TiO2@Fc showed close or slightly lower catalytic performance, especially when the solution was neutral or alkaline. This is possibly due to the covering of the TiO2 surface by poly(ferrocene) brushes, which will inevitably block the irradiation of TiO2 by sunlight. Nevertheless, the surface coating of the hybrid nanocomposites maintained the photocatalytic activity of TiO2 and could still degrade organic pollutants under the irradiation of simulated sunlight. Which might be caused by eqn (1)–(3).39–41
 |
| | Fig. 3 Removal ratio of RhB solution in the presence of TiO2@Fc or TiO2 with simulated sunlight at pH 3.0 (A), 7.0, (B), and 11.0 (C) (RhB: 20 mg L−1, TiO2@Fc: 1.0 g L−1). | |
2.3. Fenton-like reaction catalyzed by TiO2@Fc under visible light or simulated sunlight
Subsequently, we investigated the possibility of the hybrid nanocomposite acting as the catalyst for the photo-Fenton-like reaction. As shown in Fig. S2,† the adsorption of pollutants by catalysts in the dark can be negligible. In a typical photo-Fenton reaction system, TiO2@Fc was used to activate H2O2 under the irradiation of visible light or simulated sunlight. RhB was used as a model compound during the reaction. As shown in Fig. 4A, the removal ratio of RhB by only TiO2@Fc within 30 min was less than 10%, and the removal ratio increased slightly but was less than 20% after the addition of visible light, proving that adsorption and photocatalysis were very slow to remove RhB. However, under the irradiation of visible light, the removal ratio could reach 90% at 15 min after the addition of H2O2, and almost 100% at 30 min. This showed that the Fenton or Fenton-like reaction was the main process during the degradation of RhB. Without visible light, the removal ratio of RhB by the TiO2@Fc/H2O2 system was less than 20% in 30 min, which further indicated that photo-Fenton was the leading cause of the degradation of RhB. When simulated sunlight was used as the light source (Fig. 4B), the photocatalysis of TiO2@Fc in the absence of H2O2 was still slow, which may be due to the polymer blocking the photocatalytic sites on the surface of TiO2. However, TiO2@Fc/H2O2/simulated sunlight system degraded more than 90% of RhB within 10 min, which was even higher than that using visible light as the light source. This also proved that ultraviolet radiation has a positive effect on the Fenton-like reaction.
 |
| | Fig. 4 Removal ratio of RhB solution in the presence of TiO2@Fc, H2O2 with visible light (A) or simulated sunlight (B) (RhB: 20 mg L−1, H2O2: 8 mmol L−1, TiO2@Fc: 1.0 g L−1, pH = 3.0). | |
2.4. Study of the optimal dose of the photo-Fenton-like reaction
Subsequently, the optimal amounts of H2O2 and TiO2@Fc in the photo-Fenton-like were explored. As shown in Fig. 5A, when TiO2@Fc concentration increases from 0 to 1.0 g L−1, the RhB degradation ratio increases accordingly. In addition, Fig. 5B shows that when the concentration of H2O2 increases from 0 mmol L−1 to 8 mmol L−1, the degradation ratio of RhB increases accordingly. When the concentration of H2O2 was 12 mmol L−1, the removal rate of RhB showed no significant changes. The area of contact between TiO2@Fc and H2O2 increased because of the increase of TiO2@Fc or H2O2, promoting ROS generation. Thus, the optimized concentration of TiO2@Fc was 1.0 g L−1 and the optimal dosage of H2O2 was 8 mmol L−1.
 |
| | Fig. 5 Effects of concentrations of TiO2@Fc (A, H2O2 was 8 mmol L−1, pH = 3.0), H2O2 (B, TiO2@Fc was 1.0 g L−1, pH = 3.0) changes. RhB solution was 20 mg L−1, and the light source was visible light. | |
Under the irradiation of visible light, when the solution was neutral or alkaline, the removal ratio of RhB decreased significantly (Fig. 6A). This was because Fc was more prone to oxidation–reduction reaction under acidic conditions and was more stable under neutral or alkaline conditions; therefore, the photo-Fenton-like reaction should be carried out under acidic conditions. When simulated sunlight was used as the light source (Fig. 6B), the removal ratio of RhB reached 60% under neutral conditions, which is far higher than the removal ratio when visible light was used, indicating that ultraviolet light can still promote the degradation of RhB by TiO2@Fc under neutral conditions.
 |
| | Fig. 6 Effects of different pH with visible light (A) or simulated sunlight (B) (RhB: 20 mg L−1, H2O2: 8 mmol L−1, TiO2@Fc: 1.0 g L−1). | |
2.5. Study of the mechanism of the photo-Fenton-like reaction
We explored the mechanism of the photo-Fenton-like reactions via a series of control experiments. As shown in Fig. 7A, under the irradiation of visible light, the removal ratio of RhB decreased from 100% to ∼80% because of TBA, and to ∼30% because of NaN3, which indicated that 1O2 was the main active ROS in the reaction. After removing the dissolved oxygen in water with N2, the removal ratio of RhB decreased from 100% to 60%, indicating that O2 played an important role in the generation of ROSs. When visible light was replaced by simulated sunlight (Fig. 7B), the removal ratio of RhB decreased from 100% to ∼50% at 30 min because NaN3 is the obligate scavenger. However, TBA as the obligate scavenger and removal of the dissolved oxygen only caused a decrease in the degradation rate; the removal ratio could still reach 100%. This proved that 1O2 is still the main active ROS in photo-Fenton-like reactions in the presence of simulated sunlight.
 |
| | Fig. 7 Effect of TBA, NaN3, and N2 on the removal ratio of RhB with visible light (A) or sunlight (B) (RhB: 20 mg L−1, H2O2: 8 mmol L−1, TiO2@Fc: 1.0 g L−1, pH = 3.0). | |
To further explore the mechanism of the photo-Fenton-like reactions, EPR characterizations were also performed. As shown in Fig. 8, the EPR proved the existence of ·OH (Fig. 8A), ·O2− (Fig. 8B), and 1O2 (Fig. 8C) in this photo-Fenton-like reaction. In order to explore what role dissolved O2 plays in the production of ROSs, a comparative experiment was conducted by removing dissolved O2 from the solution by N2. The signals of ·OH and ·O2− weakened, and the signal of 1O2 had no significant changes after N2 bubbling, which may be caused by eqn (5) and (6).9,17,20
| | ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe2+ + O2 → Fe2+ + O2 → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe3+ + ·O2− Fe3+ + ·O2− | (5) |
| | ·O2− + H2O2 → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O2 + ·OH + OH− O2 + ·OH + OH− | (6) |
| | ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe3+ + e− → Fe3+ + e− → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe2+ Fe2+ | (7) |
| | ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe3+ + H2O2 → Fe3+ + H2O2 → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe2+ + ·O2− + 2H+ Fe2+ + ·O2− + 2H+ | (8) |
| | ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe2+ + H2O2 → Fe2+ + H2O2 → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe3+ + ·OH + OH− Fe3+ + ·OH + OH− | (9) |
 |
| | Fig. 8 DMPO (50 mmol L−1) spin-trapping EPR spectra for ·OH (A), ·O2− (B), and TEMP (50 mmol L−1) spin-trapping EPR spectra for 1O2 (C and D). | |
After removing the simulated sunlight (Fig. 8D), the signal of 1O2 disappeared, indicating that light was a necessary condition for the production of 1O2 in this Fenton-like reaction. Thus, we infer that 1O2 may be caused by eqn (10) and (11).42,43
| | 2![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe3+ + HO2− + hν → 2 Fe3+ + HO2− + hν → 2![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe2+ + 1O2 + H+ Fe2+ + 1O2 + H+ | (11) |
Thus, the mechanism of the TiO2@Fc-catalyzed photo-Fenton-like reaction is described in Scheme 2. Under light irradiation, ferrocene cation (Fc+) generates Fc after receiving electrons, then Fc activates H2O2 to generate ·OH, ·O2−, and 1O2. In addition, ·O2− could also be generated by Fc activating O2. 1O2 was believed to play the most significant role in this photo-Fenton-like reaction.
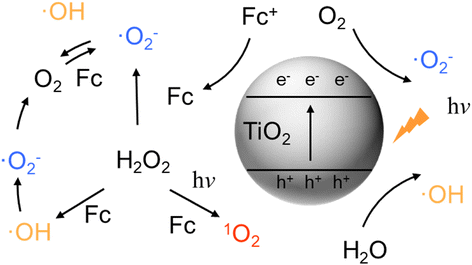 |
| | Scheme 2 Illustration of the TiO2@Fc-catalyzed photo-Fenton-like reaction mechanism. | |
The TOC was then applied to test the mineralization degree of RhB during the photo-Fenton-like reaction, and LC-MS was used to determine the intermediates during the photo-Fenton-like reaction. Taking RhB as an example, the removal ratio of TOC reached 70% during the photo-Fenton-like reaction. As Fig. S1† shows, after 30 min of photo-Fenton-like reaction, the RhB signal (m/z = 443) was almost unobservable and the species of m/z = 166, 240, 317, 331, and 415 were found, indicating that RhB molecules would be attacked by the active species (1O2) and turned into intermediates. The intermediates were then further degraded into small organic molecules by the photo-Fenton-like reaction (Fig. S2†). Finally, some small organic molecules are converted into s CO2 and H2O.
2.6. Studies on high salinity wastewater treatment by photo-Fenton-like reaction
Furthermore, we investigated the effect of ions on this photo-Fenton-like reaction system. As Fig. 9 shows, the RhB solution containing 500 mmol L NaCl or NaNO3 was used to simulate high-salinity wastewater treatment by TiO2@Fc. In traditional Fenton reactions (Fig. 9A), ·OH could react with Cl− and NO3−, and produce weaker radicals, but 1O2 in this photo-Fenton-like system avoided attacks by the anions; therefore, it could degrade RhB effectively (Fig. 9B). Under acidic conditions, NO3− has oxidizing properties, so the degradation rate by adding NO3− is higher than that of adding Cl−. These results further confirmed that 1O2 plays the most important role in this photo-Fenton-like reaction.
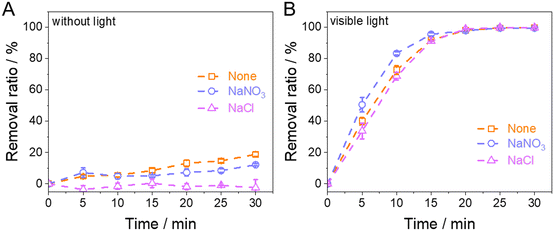 |
| | Fig. 9 Effect of anions on the removal ratio of RhB without light (A) and under visible light (B) (NaCl and NaNO3: 500 mmol L−1, RhB: 20 mg L−1, H2O2: 8 mmol L−1, TiO2@Fc: 1.0 g L−1, pH: 3.0). | |
Afterward, different organic pollutants (BPA, BPS, and TC) were degraded by a photo-Fenton-like reaction to test the degradation effect of TiO2@Fc on other organic pollutants. As shown in Fig. 10A, the Fenton-like reaction had good degradation effects on several organic pollutants. The degradation ratio of BPA and BPS could reach over 90% at 30 min, while the degradation ratio of TC could reach nearly 100% at 15 min. This proved that even just the photo-Fenton-like reaction is efficient in degrading different pollutants.
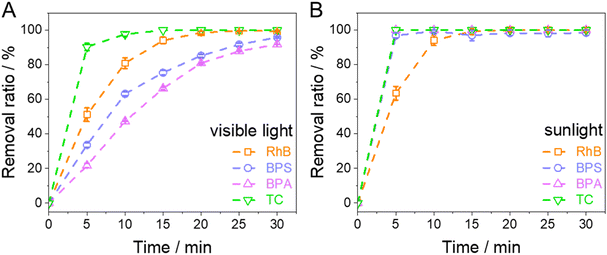 |
| | Fig. 10 Degradation of RhB, BPA, BPS, and TC with visible light (A) or sunlight (B) (RhB: 20 mg L−1, H2O2: 8 mmol L−1, TiO2@Fc: 1.0 g L−1, pH = 3.0). | |
After that, the light source was changed into simulated sunlight in order to combine a photo-Fenton-like reaction and photocatalytic oxidation reaction for the treatment of wastewater. As shown in Fig. 10B, as compared with Fig. 10A, the degradation rate of different pollutants was faster, indicating that photocatalytic oxidation under irradiation of UV light could accelerate the photodegradation rate.
Finally, under optimized experimental conditions, four repeated experiments were conducted to test the reusability of TiO2@Fc. The degradation ratio of RhB after four consecutive Fenton-like reactions could still reach over 95% within 30 min, proving that TiO2@Fc was recovered facilely and reused repeatedly (Fig. 11). Meanwhile, compared with the FTIR spectra of TiO2@Fc before and after the reaction (Fig. S3†), the main peaks did not change, which also proved that TiO2@Fc could maintain its properties during the reaction.
 |
| | Fig. 11 Reusability experiment of TiO2@Fc (RhB: 20 mg L−1, H2O2: 8 mmol L−1, TiO2@Fc: 1.0 g L−1, pH = 3.0, the light source was visible light). | |
3. Conclusions
In conclusion, we used radical polymerization to synthesize ferrocene-containing polymers, and TiO2@Fc nanocomposites were prepared via biomimetic anchoring. This type of nanocomposite combines the catalytic properties of TiO2 and ferrocene, which are used as heterogeneous catalysts for photo-Fenton-like reactions and photocatalytic oxidation simultaneously. In the photo-Fenton-like reaction, 1O2 was identified as the primary reactive oxygen species. Under the irradiation of visible light, the Fenton-like reaction TiO2@Fc as a catalyst can degrade RhB, TC, BPA, and BPS within 30 min under high salinity conditions. When the light source was simulated sunlight, the combination of photocatalytic oxidation could accelerate the degradation rate. It is facile to recover TiO2@Fc from water for repeated use, and the degradation efficiency was maintained stable in multiple consecutive experiments. We hypothesize that hybrid nanocatalysts may have potential applications in treating wastewater containing high concentrations of inorganic salts.
Conflicts of interest
The authors declare no competing financial interests.
Acknowledgements
Financial support from the Fundamental Research Funds for the Central Universities (No. 30922010811) is greatly appreciated.
Notes and references
- X. Cheng, H. Guo, Y. Zhang, X. Wu and Y. Liu, Water Res., 2017, 113, 80–88 CrossRef CAS PubMed.
- Y. Rao, Y. Zhang, J. Fan, G. Wei, D. Wang, F. Han, Y. Huang and J.-P. Croué, Chem. Eng. J., 2022, 435, 134882 CrossRef CAS.
- S. Wu, C. Yang, Y. Lin and J. J. Cheng, J. Environ. Sci., 2022, 115, 330–340 CrossRef CAS PubMed.
- Y. Wen, C. H. Huang, D. C. Ashley, D. Meyerstein, D. D. Dionysiou, V. K. Sharma and X. Ma, Environ. Sci. Technol., 2022, 56, 2626–2636 CrossRef CAS PubMed.
- Y. Li, J. Li, Y. Pan, Z. Xiong, G. Yao, R. Xie and B. Lai, Chem. Eng. J., 2020, 384, 904–914 Search PubMed.
- C. Li, J. Wu, W. Peng, Z. Fang and J. Liu, Chem. Eng. J., 2019, 356, 904–914 CrossRef CAS.
- X. Cheng, H. Guo, Y. Zhang, G. V. Korshin and B. Yang, Water Res., 2019, 157, 406–414 CrossRef CAS PubMed.
- Y. Bu, H. Li, W. Yu, Y. Pan, L. Li, Y. Wang, L. Pu, J. Ding, G. Gao and B. Pan, Environ. Sci. Technol., 2021, 55, 2110–2120 CrossRef CAS PubMed.
- Y. Ahmed, J. Zhong, Z. Yuan and J. Guo, J. Hazard. Mater., 2022, 430, 128408 CrossRef CAS PubMed.
- M. Huang, T. Zhou, X. Wu and J. Mao, Water Res., 2017, 119, 47–56 CrossRef CAS PubMed.
- Y. Liu, W. Jin, Y. Zhao, G. Zhang and W. Zhang, Appl. Catal., B, 2017, 206, 642–652 CrossRef CAS.
- J. Wang and S. Wang, Chem. Eng. J., 2021, 411, 128392 CrossRef CAS.
- M. Li, Y. Yao, W. Zhang, J. Zheng, X. Zhang and L. Wang, Environ. Sci. Technol., 2017, 51, 9252–9260 CrossRef CAS PubMed.
- Y. Lei, X. Lei, P. Westerhoff, X. Zhang and X. Yang, Environ. Sci. Technol., 2021, 55, 689–699 CrossRef CAS PubMed.
- R. Luo, M. Li, C. Wang, M. Zhang, M. A. Nasir Khan, X. Sun, J. Shen, W. Han, L. Wang and J. Li, Water Res., 2019, 148, 416–424 CrossRef CAS PubMed.
- T. Zhang, Y. Chen, Y. Wang, J. Le Roux, Y. Yang and J. P. Croue, Environ. Sci. Technol., 2014, 48, 5868–5875 CrossRef CAS PubMed.
- Y. Pan, H. Su, Y. Zhu, H. Vafaei Molamahmood and M. Long, Water Res., 2018, 145, 731–740 CrossRef CAS PubMed.
- D. Yuan, C. Zhang, S. Tang, X. Li, J. Tang, Y. Rao, Z. Wang and Q. Zhang, Water Res., 2019, 163, 114861 CrossRef CAS PubMed.
- Y. Mao, P. Wang, D. Zhang, Y. Xia, Y. Li, W. Zeng, S. Zhan and J. C. Crittenden, Environ. Sci. Technol., 2021, 55, 13326–13334 CAS.
- Q. Yi, J. Ji, B. Shen, C. Dong, J. Liu, J. Zhang and M. Xing, Environ. Sci. Technol., 2019, 53, 9725–9733 CrossRef CAS PubMed.
- Y. Zhang, S. Zhao, M. Mu, L. Wang, Y. Fan and X. Liu, Chem. Eng. J., 2022, 439, 135605 CrossRef CAS.
- Y. Li, B. Zhang, X. Liu, Q. Zhao, H. Zhang, Y. Zhang, P. Ning and S. Tian, J. Hazard. Mater., 2018, 353, 26–34 CrossRef CAS PubMed.
- Q. Wang, S. Tian, J. Cun and P. Ning, Desalin. Water Treat., 2013, 51, 5821–5830 CrossRef CAS.
- L. Zhang, H. Chen, X. Zhao, Q. Zhai, D. Yin, Y. Sun and J. Li, Appl. Catal., B, 2016, 193, 47–57 CrossRef CAS.
- M. Arshadi, M. K. Abdolmaleki, F. Mousavinia, A. Khalafi-Nezhad, H. Firouzabadi and A. Gil, Chem. Eng. Res. Des., 2016, 112, 113–121 CrossRef CAS.
- L. Li, J.-l. Shi, J.-n. Yan, X.-g. Zhao and H.-g. Chen, Appl. Catal., A, 2004, 263, 213–217 CrossRef CAS.
- C. Du, Y. Zhang, Z. Zhang, L. Zhou, G. Yu, X. Wen, T. Chi, G. Wang, Y. Su, F. Deng, Y. Lv and H. Zhu, Chem. Eng. J., 2022, 431, 133932 CrossRef CAS.
- Z. Cao, Y. Jia, Q. Wang and H. Cheng, Appl. Clay Sci., 2021, 212, 106213 CrossRef CAS.
- W. Shi, W. Sun, Y. Liu, K. Zhang, H. Sun, X. Lin, Y. Hong and F. Guo, J. Hazard. Mater., 2022, 436, 129141 CrossRef CAS PubMed.
- R. Li, M. Zheng, X. Zhou, D. Zhang, Y. Shi, C. Li and M. Yang, Chem. Eng. J., 2023, 464, 142584 CrossRef CAS.
- S. Li, H. Shang, Y. Tao, P. Li, H. Pan, Q. Wang, S. Zhang, H. Jia, H. Zhang, J. Cao, B. Zhang, R. Zhang, G. Li, Y. Zhang, D. Zhang and H. Li, Angew. Chem., Int. Ed., 2023, 62, e202305538 CrossRef CAS PubMed.
- Q. Li, J. Zhao, H. Shang, Z. Ma, H. Cao, Y. Zhou, G. Li, D. Zhang and H. Li, Environ. Sci. Technol., 2022, 56, 5830–5839 CrossRef CAS PubMed.
- C. Lu, J. Wang, D. Cao, F. Guo, X. Hao, D. Li and W. Shi, Mater. Res. Bull., 2023, 158, 112064 CrossRef CAS.
- Y. Liu, X. Wang, Q. Sun, M. Yuan, Z. Sun, S. Xia and J. Zhao, J. Hazard. Mater., 2022, 424, 127387 CrossRef CAS PubMed.
- H. Sun, L. Wang, F. Guo, Y. Shi, L. Li, Z. Xu, X. Yan and W. Shi, J. Alloys Compd., 2022, 900, 163410 CrossRef CAS.
- C. H. Nguyen, C.-C. Fu and R.-S. Juang, J. Cleaner Prod., 2018, 202, 413–427 CrossRef CAS.
- D. Wang, W. Ding, K. Zhou, S. Guo, Q. Zhang and D. M. Haddleton, Biomacromolecules, 2018, 19, 2979–2990 CrossRef CAS PubMed.
- D. Wang, S. Guo, Q. Zhang, P. Wilson and D. M. Haddleton, Polym. Chem., 2017, 8, 3679–3688 RSC.
- A. S. M. Nur, M. Sultana, A. Mondal, S. Islam, F. N. Robel, A. Islam and M. S. A. Sumi, J. Water Process. Eng., 2022, 47, 102728 CrossRef.
- T. Zhang, Y. Liu, Y. Rao, X. Li, D. Yuan, S. Tang and Q. Zhao, Chem. Eng. J., 2020, 384, 123350 CrossRef CAS.
- X. Li, Y. Pi, L. Wu, Q. Xia, J. Wu, Z. Li and J. Xiao, Appl. Catal., B, 2017, 202, 653–663 CrossRef CAS.
- J. Zhang, Y. Wang, M. Hong, B. Peng, C. Bao, X. Xu, D. Li, J. Chen, B. Wang and Q. Zhang, Chem. Eng. J., 2023, 464, 142450 CrossRef CAS.
- S. Yang, P. Wu, J. Liu, M. Chen, Z. Ahmed and N. Zhu, Chem. Eng. J., 2018, 350, 484–495 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, characterization and analysis, experimental procedures, supporting data items, and supporting figures. See DOI: https://doi.org/10.1039/d3en00729d |
|
| This journal is © The Royal Society of Chemistry 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  *ab
*ab
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–H, respectively. The TGA analysis (Fig. 1G) showed that 92.5% of poly(ferrocene) was degradable, and the 7.5% weight residual may be due to the presence of iron oxide after the TGA test. When the temperature reached 600 °C, there was 92.00% remaining TiO2 and 87.14% remaining TiO2@Fc, which further proved the presence of degradable poly(ferrocene) in the hybrid nanocomposites. The main peaks appearing in the XRD pattern (Fig. 1H) at 2θ = 25.4(101), 37.9(004), 48.1(200), 54.0(105), 55.1(211), 62.8(204), 69.0(116), 70.4(220), and 75.1(215) were all from anatase TiO2 because Fc was grafted onto the surface of TiO2 in the form of a polymer, which did not affect the crystal structure. Based on these characterizations, TiO2 hybrid nanocomposites with the ferrocene-containing polymer on the surface were successfully prepared.
O and C–H, respectively. The TGA analysis (Fig. 1G) showed that 92.5% of poly(ferrocene) was degradable, and the 7.5% weight residual may be due to the presence of iron oxide after the TGA test. When the temperature reached 600 °C, there was 92.00% remaining TiO2 and 87.14% remaining TiO2@Fc, which further proved the presence of degradable poly(ferrocene) in the hybrid nanocomposites. The main peaks appearing in the XRD pattern (Fig. 1H) at 2θ = 25.4(101), 37.9(004), 48.1(200), 54.0(105), 55.1(211), 62.8(204), 69.0(116), 70.4(220), and 75.1(215) were all from anatase TiO2 because Fc was grafted onto the surface of TiO2 in the form of a polymer, which did not affect the crystal structure. Based on these characterizations, TiO2 hybrid nanocomposites with the ferrocene-containing polymer on the surface were successfully prepared.






![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe2+ + O2 →
Fe2+ + O2 → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe3+ + ·O2−
Fe3+ + ·O2−![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O2 + ·OH + OH−
O2 + ·OH + OH−![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe3+ + e− →
Fe3+ + e− → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe2+
Fe2+![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe3+ + H2O2 →
Fe3+ + H2O2 → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe2+ + ·O2− + 2H+
Fe2+ + ·O2− + 2H+![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe2+ + H2O2 →
Fe2+ + H2O2 → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe3+ + ·OH + OH−
Fe3+ + ·OH + OH−
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe3+ + HO2− + hν → 2
Fe3+ + HO2− + hν → 2![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe2+ + 1O2 + H+
Fe2+ + 1O2 + H+




