Importance of a nano-sized molybdenum composite synthesized using a microwave oven in the sorption enhancement of Au(III) from the aqueous phase†
Received
15th September 2023
, Accepted 11th December 2023
First published on 13th December 2023
Abstract
To significantly enhance the unsatisfactory Au(III) sorption performance (2280 mg g−1) by a material designed in our previous work, we ingeniously fabricated a nano-sized and uniform molybdenum composite (MoCOM) using a household microwave oven; it was embedded in a nanofiber mat by electrospinning technique to synthesize a novel material (CS-MoCOM-Th) for effective Au(III) sorption. After embedding MoCOM, an ultrahigh maximum sorption capacity (4090 mg g−1) of Au(III) at high concentrations and a sorption efficiency at low concentrations by CS-MoCOM-Th (99.59%) were achieved at pH 4.0. Mechanistically, the excellent Au(III) sorption performance was mainly driven by electrostatic attraction by protonated C–N+ groups and coordination interaction with N- or S-containing moieties: C–N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C–N+, C
N, C–N+, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, S(−II), and Sn(−II). During sorption, 69.42% of Au(III) was directly reduced to elemental gold on CS-MoCOM-Th, and gold foils precipitated on the nanofiber mat. Notably, excellent sorption selectivity of Au(III) on CS-MoCOM-Th was achieved when heavy metals, including Cu(II), Ni(II), Pb(II), and Zn(II), and noble metal Pt(IV) were co-present at 2 × 10−3 mg mL−1. CS-MoCOM-Th could still sorb 82.15% of Au(III) at 0.01 mg mL−1 after four cycles. Overall, this study highlights that CS-MoCOM-Th is one of the most competitive candidates for Au(III) recovery from the aqueous phase.
S, S(−II), and Sn(−II). During sorption, 69.42% of Au(III) was directly reduced to elemental gold on CS-MoCOM-Th, and gold foils precipitated on the nanofiber mat. Notably, excellent sorption selectivity of Au(III) on CS-MoCOM-Th was achieved when heavy metals, including Cu(II), Ni(II), Pb(II), and Zn(II), and noble metal Pt(IV) were co-present at 2 × 10−3 mg mL−1. CS-MoCOM-Th could still sorb 82.15% of Au(III) at 0.01 mg mL−1 after four cycles. Overall, this study highlights that CS-MoCOM-Th is one of the most competitive candidates for Au(III) recovery from the aqueous phase.
Environmental significance
Given the increasing demand for Au and its decreasing deposits, it is imperative to examine how to effectively recover Au(III) from the aqueous phase, which will have a significant impact on both our daily life and environmental protection. To achieve this, we designed a molybdenum composite-embedded chitosan nanofiber mat (CS-MoCOM-Th) for Au(III) sorption; the maximum sorption capacity reached 4090 mg g−1 at pH 4.0. The excellent sorption performance of Au(III) can be maintained in the presence of multiple interfering metal ions or after several cycles. Au(III) can be directly reduced to elemental gold on the material during sorption. Mechanisms regulating the abovementioned observations are also adequately addressed.
|
1. Introduction
Gold is one of the most widely used metals in currency, jewelry, and electronics owing to its rareness, gorgeous appearance, high ductility, conductivity, and chemical stability.1 In the recent decades, people have found that gold plays an important role in multiple frontier fields, such as catalysis,2 sorption,3 antibody detection,4 and drug delivery.5 Therefore, gold recovery is of great significance in both economic and scientific aspects. The main sources of Au(III)-containing waste include tailing mines, mining wastewater, and the discarded circuit boards of electronic products.6 Over the years, gold has been recovered from these Au-containing wastes mainly by sorption,7 ion exchange,8 metal reduction,9 solvent extraction,10 and electrolytic deposition.11 Among these techniques, researchers particularly favor the sorption method for its simplicity, high efficiency, low secondary pollution, and capability for dilute solution.12,13 Based on the particle size, materials for the sorption method can be divided into two main groups. The materials in the first group include gels,14 granules,15 and powders;16 their particle size ranges from μm to mm. These materials can be readily separated from the aqueous phase after gold sorption; therefore, they are widely used in industries. The particle size of those in the second group ranges from nm to μm, and the materials include nanoparticles,17 nanosheets,18 nanorods,19 and nanofibers.20 Compared to those in the first group, these nanomaterials have a larger surface area and higher surface energy, thereby exhibiting a higher recovery efficiency. However, strong aggregation of nanomaterials in the aqueous phase and the difficulty of separation after gold sorption severely limit their practical applications.
To overcome these shortcomings, researchers have synthesized diverse novel materials, combining a high surface area and separability, such as metal–organic-frameworks (MOFs),21 hydrogels,22 and nanofiber mats.23 Compared to other materials, nanofiber mats have unique advantages: 1) a wide range of fiber material candidates, 2) simple preparation at ambient temperature and pressure, and 3) the uniform surface morphology of the fibers.24,25 Therefore, nanofiber mats have been widely used for sorbing metal ions,26 PM2.5,27 drugs,28 and organic dyes.29 The most representative way to synthesize a nanofiber mat is the electrospinning technique. Among numerous spinnable polymers, investigators prefer biomacromolecules because of their low cost, a wide range of sources, and nontoxicity.30 Particularly, biomacromolecules have abundant nitrogen- or sulfur-containing moieties, which enable them to strongly chelate with Au(III) in the aqueous phase, based on the hard–soft-acid–base (HSAB) theory. Chitosan is a common derivative of chitin by deacetylation, with amino and hydroxyl groups on each molecular chain. Moreover, chitosan is the only natural cationic polysaccharide among biomacromolecules, which theoretically facilitates Au(III) sorption by electrostatic attraction because it is usually present in the form of anions in the aqueous phase.31 However, chitosan is soluble under acidic condition and has poor mechanical strength, which greatly limits its practical applications.
Due to the abovementioned limitations, researchers have made efforts to 1) crosslink chitosan molecular chains by aldehydes,32 acyl chlorides,33 and carboxylic acids34 to form an insoluble three-dimensional body structure; 2) dope nanomaterials like MOFs,35 oxidized graphenes (OGs),36 carbon nanotubes (CNTs),37 and metallic oxides or sulfides38 in chitosan to enhance its mechanical strength. In our previous work, aldehyde was used to crosslink amino groups and hydroxyl groups on chitosan. However, sorption strength improvement of Au(III) by chitosan-based material via molecular level functionalization is still far below expectation. It is expected to physically and chemically reconstruct a novel material by doping nanomaterial in the chitosan-based nanofiber mat thus greatly enhancing its sorption performance. Molybdenum disulfide (MoS2) is one of the popular two-dimensional nanomaterials nowadays, consisting of one layer of Mo atoms and two layers of S atoms. MoS2 has also been proven to be a superior material, thanks to its physical and chemical stability, high surface area, high conductivity, and rich in soft-base moieties.39 Nonetheless, a high-pressure and long time-consuming hydrothermal process is often required to synthesize MoS2, and physical stability of the MoS2-embedded polymer materials is unsatisfactory, which restricts the practical application of the composite material.
Based on the above, it is hypothesized that nano-sized molybdenum composite can be synthesized under normal experimental conditions and tightly embedded in the fiber mat to enhance the physical stability and provide more sites for Au(III) sorption, thus significantly enhancing its sorption performance. To address this knowledge gap, we synthesized a nano-sized molybdenum composite at ambient pressure by a modified microwave method and firmly embedded it inside the chitosan-based nanofiber mat using electrospinning technique. It was further decorated with thiourea to make the finally synthesized nanofiber mat have abundant N- and S-containing functional groups and stable physical makeup, thereby achieving an ultrahigh sorption capacity at high concentrations and excellent recovery efficiency for Au(III) at low concentrations. Overall, our work provides an efficient, feasible, and convenient solution to recover gold from the aqueous phase.
2. Materials and methods
2.1 Materials and reagents
All the materials and reagents were in analytical grade (AR) and were used without any further purification. (NH4)6Mo7O24·4H2O and L-cysteine were purchased from Aladdin. Chitosan (deacetylation degree >99%) and HAuCl4·4H2O were purchased from Sinopharm Chemical Reagent (Shanghai). Acetic acid was provided by Macklin. Polyvinyl alcohol (PVA, type 1799), formaldehyde (37% w/v) solution, thiourea, and triton X-100 were obtained from Xilong Scientific. Ethanol and glycerol were purchased from Beijing Tongguang Fine Chemicals.
2.2 Preparation of the nano-sized molybdenum composite
To prepare a nano-sized molybdenum composite, 0.3 g (NH4)6Mo7O24·4H2O and 0.3 g L-cysteine were added to 30 mL glycerol, respectively, and then dispersed in an ultrasonic bath for 30 min. After that, these two suspensions were mixed and stirred for 10 min and then placed in a microwave oven (M1-231E, Midea) on high energy gear for 3 min. The microwave-treated suspension was centrifuged at 7000 rpm, and the supernatant was then discarded to obtain the resulting molybdenum composite, labelled as MoCOM. MoCOM was rinsed 3 times alternately with deionized (DI) water and ethanol and then dried at 50 °C.
2.3 Preparation of the nanofiber mat
To synthesize nanofiber mat, 0.315 g chitosan and 9.45 mg as-prepared MoCOM were homogeneously mixed and then dispersed in 9 mL of 90% (v/v) acetic acid. Here, the optimal mass ratio of MoCOM to chitosan was determined by our preliminary experiment (Fig. S1†). Two spinning aids, including 6 mL 10% (v/v) PVA solution and 0.3 mL triton X-100, were added to the suspension and mixed well. Electrospinning was performed with a spinning voltage of 23 kV and a spinning rate of 1.0 mL h−1. The humidity was controlled between 20 and 30%. The distance between the syringe needle and the rolling mold for fiber mat creation was about 12 cm. After that, the fiber mat was peeled off from the mold, rinsed, and dried using the same methods mentioned above. The resulting MoCOM-embedded nanofiber mat was labelled as CS-MoCOM. Here, nanofiber refers to the fiber with a dimension (e.g., diameter) on nm-scale. For comparison, a blank nanofiber mat was prepared following the same technical flow but with no MoCOM embedded; it was named as CS.
Chitosan is known to be soluble under acidic conditions, which restricts its practical applications. Using thiourea and formaldehyde as crosslinkers can stabilize the fiber mat CS-MoCOM and CS at low pH values. In addition, thiourea has abundant N- and S-containing functional groups (–NH2 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S). Its assembly on CS-MoCOM and CS is expected to enhance the sorption strength of Au(III). To assemble thiourea on CS-MoCOM and CS, 3 mg CS-MoCOM (or CS) was soaked in 10 mL thiourea solution (2.5% w/v), then adding 5 mL formaldehyde solution and shaking at 140 rpm for 24 h. The thiourea-grafted mat was rinsed and dried with the same method mentioned above, which was named as CS-MoCOM-Th (or CS-Th). The whole methodological roadmap is illustrated in Fig. 1.
S). Its assembly on CS-MoCOM and CS is expected to enhance the sorption strength of Au(III). To assemble thiourea on CS-MoCOM and CS, 3 mg CS-MoCOM (or CS) was soaked in 10 mL thiourea solution (2.5% w/v), then adding 5 mL formaldehyde solution and shaking at 140 rpm for 24 h. The thiourea-grafted mat was rinsed and dried with the same method mentioned above, which was named as CS-MoCOM-Th (or CS-Th). The whole methodological roadmap is illustrated in Fig. 1.
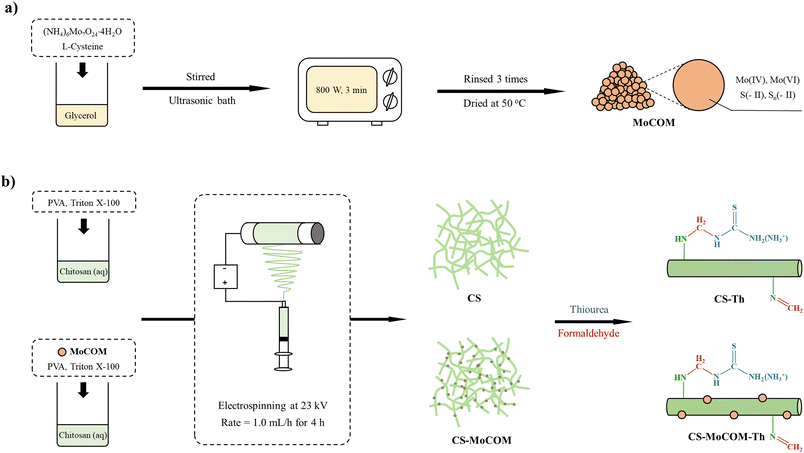 |
| | Fig. 1 A flowchart showing the synthesis of MoCOM (a), CS, CS-Th, and CS-MoCOM-Th (b). | |
2.4 Characterization of the materials
The MoCOM and nanofiber mats CS, CS-MoCOM, CS-Th, and CS-MoCOM-Th were characterized by the state-of-the-art techniques. The surface morphology of the materials was observed by a field emission scanning electron microscope (SEM, Merlin Compact, Zeiss, Germany) and a transmission electron microscope (TEM, Tecnai G2 F30, FEI, USA). The elemental composition of the materials was measured by three instruments for different elements: an elemental analyzer (EA, ECS4024, Costech, USA) for C and N; an inductively coupled plasma optical emission spectrometer (ICP-OES, Prodigy 7, Leeman, USA) and an inductively coupled plasma mass spectrometer (ICP-MS, 7700, Agilent, USA) for S and Mo. The functional groups on the materials were detected by a Fourier transform infrared spectrometer with an attenuated total reflection attachment (ATR-FTIR Spotlight 200, PerkinElmer, USA). The detection wavenumber ranged from 400 to 4000 cm−1. The binding energy for elements or functionalities on the samples was measured by an X-ray photoelectron spectrometer (XPS, K-Alpha, Thermo Fisher Scientific, USA) with Al Kα (1486.6 eV) radiation as an X-ray source. The XPS data were calibrated using the binding energy of the C 1s peak of the adventitious carbon contamination (284.8 eV) as a standard. The crystalline structure of the materials was detected by a powder X-ray diffractometer (XRD, X-Pert3 Powder, Malvern PANalytical, UK) with Cu Kα radiation. The detection diffraction angle (2θ) ranged from 5 to 80°. The zeta potential of the finally synthesized nanofiber mat CS-MoCOM-Th was measured by a Nanosizer (Nano ZS90, Malvern Panalytical, UK). The pH value for zeta potential measurement ranged from 4 to 11.
2.5 Sorption of Au(III) by CS-Th and CS-MoCOM-Th
Given that the uncrosslinked chitosan is soluble under acidic conditions,30,31 CS and CS-MoCOM cannot be used for sorption experiments. Unless otherwise specified, all sorption experiments were carried out by soaking 3.0 mg nanofiber mat in 90 mL of 1 mg mL−1 HAuCl4·4H2O solution (concentration of Au(III) is approximately 0.48 mg mL−1) at 25 °C, then shaking at 180 rpm for 24 h. According to the pH experiment results (Fig. 12c), the solution pH was adjusted to 4.0 using NaOH or HCl solution with concentrations ranging from 0.01 to 5 mol L−1. After sorption, the nanofiber mats were taken out using a ceramic tweezer and then rinsed and dried, as mentioned above, to wash away the remaining solution. The concentrations of metal ions in the aqueous phase before and after sorption were measured by ICP-OES or ICP-MS. All sorption experiments have three replicates to ensure data reliability.
The sorbed amount at equilibrium (Qe, mg g−1), recovery efficiency (RE, %), and sorption rate (vt, mg min−1 g−1) of Au(III) were calculated as follows:
| | | Qe = (C0 − Ce) × V/W × 103 | (1) |
| | | RE = (C0 − Ce)/C0 × 100 | (2) |
where
C0 (mg mL
−1) and
Ce (mg mL
−1) are Au(
III) concentrations in solution before and after sorption at equilibrium, respectively.
V (mL) is the solution volume, and
W (mg) is the mass of the nanofiber mat.
t (min) is the contact time, and
Qt (mg g
−1) is the sorbed amount of Au(
III) at time
t.
2.5.1 Sorption isotherm, kinetics, and thermodynamics experiments.
The sorption isotherm experiment was designed to determine the relationship between Ce and Qe. The initial Au(III) concentration C0 ranged from 2.3 × 10−4 to 1.8 mg mL−1 to fulfill the demand for low concentrations for practical application and high concentrations for exploring the maximum sorption capacity. The experimental data were fitted to Langmuir, Freundlich, and Temkin isotherm models:| | | Ce/Qe = Ce/Qm + 1/KLQm | (4) |
where Qm (mg g−1) is the maximum sorption capacity in theory, and n is the heterogeneity factor. BT is a dimensionless Temkin coefficient. KL (mL mg−1), KF ((mg g−1)/(mg mL−1)1/n), and KT (mL mg−1) are Langmuir, Freundlich, and Temkin partitioning coefficients, respectively.
The sorption kinetics experiment was designed to determine the relationship between Qt and t. The experimental duration ranged from 10 min to 24 h. Independent samples were set up for each sampling time point to keep the solid–liquid ratio unchanged. The experimental data were fitted to pseudo-first-order, pseudo-second-order, and Elovich kinetics models:
| | ln(Qe − Qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − k1t Qe − k1t | (7) |
| | Qt = ln(αβ)/β + ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t/β t/β | (9) |
where
k1 (mg min
−1 g
−1) and
k2 (g min
−1 mg
−1) are model fitting coefficients for pseudo-first-order and pseudo-second-order models, respectively.
α (mg min
−1 g
−1) corresponds to the initial sorption rate, and
β (g mg
−1) is the Elovich sorption constant, which is related to the activation energy for chemisorption.
The sorption thermodynamics experiment was designed to determine the relationship between sorption temperature (T, K) and Qe. T ranged from 15 to 55 °C.
2.5.2 pH-dependent and selective sorption and reusability experiments.
The pH-dependent sorption experiment was designed to determine the optimal pH value for Au(III) recovery by the finally synthesized nanofiber mat CS-MoCOM-Th. The pH value varied from 2 to 9. To obtain the true initial pH value of the sorption system, it was measured as soon as the nanofiber mat was soaked in Au(III) solution rather than before soaking.
The selectivity experiment was designed to evaluate sorption selectivity of Au(III) by the finally synthesized nanofiber mat CS-MoCOM-Th under the interference of other metal ions. Five commonly co-existing metals in Au(III)-containing wastewater: Pt(IV), Pb(II), Zn(II), Cu(II), and Ni(II) were added to the Au(III)-containing solution as disturbances.40 Concentrations of the five elements and Au(III) were all at 2 × 10−3 mg mL−1, and no precipitation was observed in the mixed solution.41
The reusability experiment was designed to evaluate the recyclability of CS-MoCOM-Th for Au(III) sorption. C0 was 0.01 mg mL−1 in all five cycles. After one sorption cycle, the Au(III)-sorbed CS-MoCOM-Th was eluted two times with a mixture of 20 mL of 0.5 mol L−1L-cysteine solution and 20 mL of 0.1 mol L−1 HNO3 in an ultrasonic bath to desorb it completely.7
2.6 Stability test
This test was designed to evaluate the chemical stability of CS-MoCOM-Th under acidic conditions. The test procedures were exactly the same as those described in section 2.5, only replacing Au(III)-containing solution with DI water at pH 4. After sorption, the loss percentage (LP, %) of an element (Mo or S) was calculated as follows:| | | LP = Cs × Vs/(W × elemental composition (%)/100) × 100 | (10) |
where Cs (mg mL−1) is the elemental concentration after sorption. Vs (mL) is the volume of the acidic solution. W (mg) is the mass of CS-MoCOM-Th. Elemental composition (%) represents the mass fraction of a specific element in CS-MoCOM-Th; it was pre-measured, as described in section 2.4. CS-MoCOM-Th is proven to be chemically stable if the LP of both Mo and S is low.
3. Results and discussion
3.1 Characterization of MoCOM
To ensure preparation of the molybdenum composite MoCOM was successful and to further comprehend the synthetic process, the state-of-the-art characterization techniques, including SEM, TEM, XRD, and XPS were applied. The as-prepared MoCOM comprises irregular nanoparticles with a diameter within 20–50 nm (Fig. 2a–c). Compared to the hydrothermal method, a very short reaction duration (3 min) using the microwave method limited the growth of nanoparticles, and particles with smaller sizes were more beneficial to sorption.42 No lattice fringe or XRD characteristic peak was detected on MoCOM (Fig. 2d and 3b), indicating its amorphous structure. It might be attributed to the lack of calcination, which is a critical step in changing the physical makeup of nanomaterials from amorphous to crystalline structure.43 Notably, an amorphous structure means more uncoordinated atoms would be exposed for sorption, hence having a better sorption performance for Au(III) compared to that with a crystalline structure.44
 |
| | Fig. 2 SEM image (a and c), TEM image (b), high-resolution TEM image (d), and TEM-EDS mapping (e–g) of MoCOM. | |
 |
| | Fig. 3 The fully-scanned survey XPS spectra (a), powder XRD spectra (b), high-resolution XPS spectra of Mo 3d (c), S 2p (d) and O 1s (e), and elemental and compound composition (w/w) (f) of MoCOM. | |
Both Mo and S as characteristic elements were detected from the fully scanned survey XPS spectrum of MoCOM (Fig. 3a). They were further confirmed to be evenly distributed on this material, as indicated by the EDS mapping results (Fig. 2e–g). Four peaks at 229.28, 232.13, 232.64, and 235.52 eV were detected from the high-resolution Mo 3d spectrum of MoCOM (Fig. 3c). Here, the peaks at 229.28 and 232.64 eV are the characteristic peaks of Mo(IV), and the remaining two peaks at 232.13 and 235.52 eV are the characteristic peaks of Mo(VI). They represent the oxidation states of Mo, which is commonly produced in the synthetic process of MoS2.45 Four peaks at 161.34, 162.88, 163.42, and 164.63 eV were detected from the S 2p spectrum of MoCOM (Fig. 3d). The first two belong to S(−II) and the last two belong to the polysulfide structure Sn(−II), and all of them are characteristic peaks of MoS2.46 Two peaks at 531.00 and 532.68 eV were detected from the O 1s spectrum of MoCOM (Fig. 3e), representing Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O groups, respectively. The above XPS analysis indicated that MoCOM contained a certain amount of MoS2 and MoO3. According to the results of elemental analysis (Fig. 3f), Mo and S contents (w/w) in MoCOM were 44.87 and 16.43%, respectively. The mass ratio of Mo to S in MoCOM was higher than that in MoS2, which also proved the existence of MoO3. 24.80% of C and 2.20% of N in MoCOM were mainly contributed from the activated carbon that was produced during the microwave process.47 The composition of each component in MoCOM was then calculated using the relative atomic mass (Ar) of each element. It was shown that MoCOM consisted of 41.08% of MoS2, 30.34% of MoO3, and 28.58% of activated carbon (Fig. 3f). The details of the calculation are described in ESI.† Thus, we confirmed that the molybdenum composite MoCOM containing MoS2 and MoO3 was successfully synthesized.
O and C–O groups, respectively. The above XPS analysis indicated that MoCOM contained a certain amount of MoS2 and MoO3. According to the results of elemental analysis (Fig. 3f), Mo and S contents (w/w) in MoCOM were 44.87 and 16.43%, respectively. The mass ratio of Mo to S in MoCOM was higher than that in MoS2, which also proved the existence of MoO3. 24.80% of C and 2.20% of N in MoCOM were mainly contributed from the activated carbon that was produced during the microwave process.47 The composition of each component in MoCOM was then calculated using the relative atomic mass (Ar) of each element. It was shown that MoCOM consisted of 41.08% of MoS2, 30.34% of MoO3, and 28.58% of activated carbon (Fig. 3f). The details of the calculation are described in ESI.† Thus, we confirmed that the molybdenum composite MoCOM containing MoS2 and MoO3 was successfully synthesized.
3.2 Characterization of CS, CS-Th, and CS-MoCOM-Th
The nanofiber mats CS, CS-Th, and CS-MoCOM-Th were systematically characterized to confirm: 1) the original nanofiber mat CS was successfully synthesized; 2) MoCOM was successfully embedded in CS to form CS-MoCOM; 3) thiourea was successfully assembled on CS and CS-MoCOM to synthesize CS-Th and CS-MoCOM-Th. CS was a flat nanofiber mat with diameters ranging from 200 to 600 nm (Fig. 4a). No discernable surface defect or beaded fiber was observed, indicating the appropriate spinning conditions. The edge of the fibers of CS-Th was more obese than that of CS (Fig. 4b), suggesting some thiourea molecules were assembled on the mat to make the fiber smoother. The fibers of CS-MoCOM-Th were significantly thicker than those of CS-Th because they had abundant nanoparticles (Fig. 4c). The appearance of these particles resembles that of MoCOM, verifying that MoCOM was tightly embedded when synthesizing CS-MoCOM-Th. Furthermore, the particles were firmly embedded in the fibers rather than attached to them (Fig. 4d and e). Such an integration mode can strongly enhance the physical stability of the nanoparticles-containing nanofiber mats. The XRD pattern of CS-MoCOM-Th was the same as that of CS and CS-Th (Fig. 5f), indicating that the high electric field force during electrospinning did not crystallize MoCOM. Two broad peaks at about 10 and 21° correspond to the low crystallinity of chitosan.48 From the perspective of morphology characterization, a chitosan-based nanofiber mat with embedded MoCOM was prepared and further thiourea assembly occurred when synthesizing CS-MoCOM-Th. XPS and FT-IR analysis results further evidenced the successful embedment of MoCOM and further assembly of thiourea. According to the XPS analysis results (Fig. 5a–d), peaks for C 1s, N 1s, and O 1s were detected from the fully-scanned survey spectrum of CS (Fig. 5a). Two peaks at 399.39 and 401.49 eV on the N 1s spectrum of CS (Fig. 5b) represented C–N and protonated C–N+ groups, respectively.49 After grafting thiourea, a new peak at 399.81 eV occurred on the N 1s spectrum of CS-Th (Fig. 5b), belonging to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group of Schiff base.50 It was generated by the condensation polymerization of amino groups between chitosan and thiourea in the presence of formaldehyde. Two peaks at 163.49 and 164.63 eV represented the C
N group of Schiff base.50 It was generated by the condensation polymerization of amino groups between chitosan and thiourea in the presence of formaldehyde. Two peaks at 163.49 and 164.63 eV represented the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S group on the S 2p spectrum of CS-Th (Fig. 5c),51 also proving that thiourea was successfully grafted. After embedding MoCOM, new Mo 3d peaks were detected at 229.30, 232.10, 232.63, and 235.49 eV, and new S 2p peaks were detected at 161.86, 162.96, 163.55, and 164.72 eV from the spectra of CS-MoCOM-Th (Fig. 5c and d). The binding energy and peak-width-at-half-height of these new peaks were consistent with those of MoCOM (Fig. 3c and d), indicating that MoCOM was successfully embedded in the nanofiber mat to form CS-MoCOM-Th.
S group on the S 2p spectrum of CS-Th (Fig. 5c),51 also proving that thiourea was successfully grafted. After embedding MoCOM, new Mo 3d peaks were detected at 229.30, 232.10, 232.63, and 235.49 eV, and new S 2p peaks were detected at 161.86, 162.96, 163.55, and 164.72 eV from the spectra of CS-MoCOM-Th (Fig. 5c and d). The binding energy and peak-width-at-half-height of these new peaks were consistent with those of MoCOM (Fig. 3c and d), indicating that MoCOM was successfully embedded in the nanofiber mat to form CS-MoCOM-Th.
 |
| | Fig. 4 SEM images of CS (a), CS-Th (b), and CS-MoCOM-Th (c and d); TEM-EDS mapping of CS-MoCOM-Th (e–g). | |
 |
| | Fig. 5 The fully-scanned survey XPS spectra (a); high-resolution spectra of N 1s (b), S 2p (c), and Mo 3d (d); FT-IR spectra (e); and powder XRD spectra (f) of CS, CS-Th, and CS-MoCOM-Th. | |
As for the FT-IR spectrum of CS (Fig. 5e), the main characteristic peaks and their corresponding functional groups are as follows.52–54 A broad peak at 3300 cm−1 was indicative of the stretching vibration of O–H and N–H groups. The peaks at 2908, 2870, and 897 cm−1 stemmed from the symmetric stretching, asymmetric stretching, and out-of-plane bending vibration of the C–H group, respectively. Two peaks at 1587 and 1375 cm−1 were attributed to the bending vibration of the N–H group and the stretching vibration of the C–N group, respectively. The peak at 1145 cm−1 was the asymmetric stretching vibration of the C–O–C group, which is one of the characteristic peaks of polysaccharides. Two sharp peaks at 1073 and 1037 cm−1 stemmed from the stretching vibration of the C–O group. On the FT-IR spectra of CS-Th and CS-MoCOM-Th, the characteristic peak of primary amine at 1587 cm−1 disappeared, and a new peak representing the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group of Schiff base occurred at 1626 cm−1.55 N–C–N and C
N group of Schiff base occurred at 1626 cm−1.55 N–C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S groups on thiourea were also detected at 1538 and 1236 cm−1, respectively.56,57 Overall, the FT-IR and XPS analysis results consistently confirmed the grafted thiourea on both CS-Th and CS-MoCOM-Th.
S groups on thiourea were also detected at 1538 and 1236 cm−1, respectively.56,57 Overall, the FT-IR and XPS analysis results consistently confirmed the grafted thiourea on both CS-Th and CS-MoCOM-Th.
Elemental analysis was applied to further quantitatively characterize how the two steps of optimization may alter the chemical composition of CS (Table 1). The N content in CS was 4.80%. After grafting thiourea, the N content in CS-Th increased to 8.45%, and 3.83% of S was introduced to this material. Compared to CS-Th, an increased S (5.82%) and Mo (5.88%) content in CS-MoCOM-Th can be attributed to the embedded MoCOM. Both Mo and S in CS-MoCOM-Th were uniformly distributed on its surface, as indicated by the EDS mapping results (Fig. 4e–g and S2†). Notably, the composition of N in CS-MoCOM-Th also increased from 8.45 to 11.60% compared to CS-Th. Given that the N content in MoCOM is only 2.2%, a much higher N content in CS-MoCOM-Th relative to that in MoCOM cannot be fully attributed to embedment of this composite. It is most likely that the embedded MoCOM facilitated the grafting reaction between chitosan in CS-MoCOM-Th and thiourea by promoting dehydration,58 which is the last step to create the Schiff base structure, thus increasing the N content in CS-MoCOM-Th. In summary, the nanofiber mat CS-MoCOM-Th with embedded MoCOM rich in N, S, and Mo was successfully synthesized by the electrospinning technique.
Table 1 Elemental composition of CS, CS-Th, and CS-MoCOM-Th (the composition of O and H was calculated by excluding the total amount of all known elements)
| Nanofiber mats |
Elemental composition (w/w) |
| C (%) |
N (%) |
S (%) |
Mo (%) |
O and H (%) |
| CS |
49.25 |
4.80 |
N.A. |
N.A. |
45.95 |
| CS-Th |
46.25 |
8.45 |
3.83 |
N.A. |
41.47 |
| CS-MoCOM-Th |
43.45 |
11.60 |
5.82 |
5.88 |
33.25 |
3.3 Sorption isotherm of Au(III) by CS-Th and CS-MoCOM-Th
Sorption isotherms of Au(III) by CS-Th and CS-MoCOM-Th were studied to evaluate how additional MoCOM embedment in CS-MoCOM-Th may enhance the sorption strength of Au(III) compared to CS-Th and to find out the maximum Au(III) sorption capacity by these two nanofiber mats. After Au(III) sorption, the appearance of CS-MoCOM-Th changed from light brown to glint golden (Fig. 6d), implying a satisfactory sorption performance. To help further understand sorption behaviors of Au(III) by these two nanofiber mats, the isotherm data were fitted to Langmuir, Freundlich, and Temkin models (Fig. 6c and Table S1†). It appears that compared to the Freundlich model (R2 = 0.895 for CS-Th and 0.923 for CS-MoCOM-Th) and Temkin model (R2 = 0.919 for CS-Th and 0.920 for CS-MoCOM-Th), the data can be better fitted with the Langmuir model (R2 = 0.979 for CS-Th and 0.987 for CS-MoCOM-Th). This suggested that the sorption process was single-layer and homogenous.59 The Langmuir model-fitted maximum sorption capacity of Au(III) by CS-Th was 2960 mg g−1 and that for CS-MoCOM-Th was as high as 4090 mg g−1, and the percentage increase was over 30% (Fig. 6a). The model fitted maximum sorbed amounts of Au(III) by CS-Th and CS-MoCOM-Th were close to the experimental results (2960 vs. 3100 mg g−1 for CS-Th and 4090 vs. 4180 mg g−1 for CS-MoCOM-Th), also indicating that this model was appropriate to describe the sorption process. The Au(III) recovery efficiency of these two nanofiber mats was above 96% when its initial concentration was below 0.033 mg mL−1 (Fig. 6b). When the initial concentration of Au(III) decreased to be as low as 2.3 × 10−4 mg mL−1, the Au(III) recovery efficiency by CS-MoCOM-Th raised to 99.59%. Therefore, CS-Th and CS-MoCOM-Th showed both strong maximum sorption capacity at high concentrations and superior recovery efficiency at low concentrations. The sorbed gold can be obtained by the following two steps: 1) burning CS-MoCOM-Th after sorption at about 600 °C and then collecting the residue. The organic component, MoS2, and Au-containing salts would be oxidized or decomposed at this temperature; 2) calcinating the residue at 1200 °C to remove the oxidized MoO3, whose sublimation point is 795 °C. After the above two steps, elemental gold can be recovered. To objectively appraise the Au(III) recovery performance by the finally-synthesized CS-MoCOM-Th, its maximum sorption capacity was systematically compared with the literature-reported relevant materials (Table 2). The materials for comparison covered those with top performance reported in recent years, either similar to CS-MoCOM-Th in physical makeup like nanofibers or in chemical composition like biopolymers or metal sulfides. The compared results proved that CS-MoCOM-Th performed better than most of the other reported materials, confirming it is a promising solution for Au(III) recovery from the aqueous phase.
 |
| | Fig. 6 The relationship between the sorbed amount (Qe) (a) and recovery efficiency (b) of Au(III) by CS-Th and CS-MoCOM-Th and its equilibrium concentration (Ce), and the corresponding Langmuir isotherm model fitting curves (c); digital photos of CS-MoCOM-Th before and after Au(III) sorption (d). | |
Table 2 A comparison of the isotherm model-fitted maximum sorption capacity (Qm) of Au(III) by CS-MoCOM-Th and other reported materials
| Materials |
Q
m (mg g−1) |
Reference |
| Zr-based metal–organic frameworks (UiO-66-NH2) |
630 |
60
|
| Thiourea-modified chitosan-imprinted resin (IM-TUCS) |
933 |
61
|
| Irreversible amide-linked covalent organic framework (JNU-1) |
1124 |
62
|
| MoS2 nanoflakes |
1133 |
63
|
| Hierarchically flower-like WS2 microcrystals |
1341 |
64
|
| Mn and O dual-doping MoS2 nanoflowers (Mn, O–MoS2) |
1387 |
65
|
| Functional redox-active nanohybrid filter consisting of carbon nanotube and three-dimensional molybdenum disulfide nanoflowers |
2495 |
66
|
| Hyperbranched thiourea-grafted electrospun polyacrylonitrile fibers (HS-PAN) |
3257 |
23
|
| Chitosan-coated commercial MoS2 (CS-MoS2) |
3435 |
67
|
|
Molybdenum compound embedded chitosan electrospinning fiber mat (CS-MoCOM-Th)
|
4090
|
This work
|
| Flexible alkyl amines grafted covalent organic framework (TpTsc COF) |
4366 |
19
|
| N and S-functionalized cellulose microsphere (NS-CM) |
4657 |
68
|
3.4 Sorption kinetics of Au(III) by CS-Th and CS-MoCOM-Th
The sorption kinetics of Au(III) by CS-Th and CS-MoCOM-Th was also investigated to assess the possible increase of its sorption rate by embedding MoCOM and to find out the sorption equilibrium time of Au(III) by these two nanofiber mats. The sorbed amount of Au(III) by both CS-Th and CS-MoCOM-Th steeply increased in the first 3 hours, then began to level off, and finally reached equilibrium at 12 and 10 h, respectively (Fig. 7a). To describe the sorption kinetics of Au(III) by both CS-Th and CS-MoCOM-Th, the sorption kinetics data were fitted to pseudo-first-order, pseudo-second-order, and Elovich models (Fig. 7b; Table S2†). Au(III) sorption by CS-Th and CS-MoCOM-Th is most likely a chemically driven process because the experimental data can be better fitted with pseudo-second-order model (R2 = 0.996 for CS-Th and 0.998 for CS-MoCOM-Th) relative to that with pseudo-first-order model (R2 = 0.682 for CS-Th and 0.935 for CS-MoCOM-Th) or Elovich model (R2 = 0.950 for CS-Th and 0.956 for CS-MoCOM-Th).67 The sorption rates of Au(III) by CS-Th and CS-MoCOM-Th were calculated as 2.99 and 4.87 mg min−1 g−1, respectively, indicating that embedding MoCOM steeply increased the sorption rate by over 60%. Importantly, the sorbed amount of Au(III) by CS-MoCOM-Th reached 50% of its Qe in just 30 min, showing a strong potential for Au(III) recovery. The rapid sorption rate makes CS-MoCOM-Th a reliable candidate for Au(III) recovery in practical applications.
 |
| | Fig. 7 The relationship between contact time (t) and the sorbed amount (Qt) of Au(III) by CS-Th and CS-MoCOM-Th (a), and the corresponding pseudo-second-order kinetic model fitting curves (b). | |
3.5 Sorption thermodynamics of Au(III) by CS-Th and CS-MoCOM-Th
Sorption thermodynamics of Au(III) by CS-Th and CS-MoCOM-Th was explored to find out how Qe would be affected by temperature variation. When temperature increased, Qe of Au(III) by both CS-Th and CS-MoCOM-Th steadily increased (Fig. 8), indicating that its recovery was an endothermic process. Although intended heating can promote Au(III) sorption by these nanofiber mats, the accompanying extra energy and economic cost would considerably increase and even become infeasible when dealing with a large volume of wastewater. Fortunately, Qe of Au(III) by CS-MoCOM-Th remained at 2500 mg g−1 at 15 °C, which is close to the ambient temperature. It means that Au(III) recovery by CS-MoCOM-Th can be completely achieved in the open air, which significantly broadens the application of CS-MoCOM-Th. A wide sorption temperature window with excellent performance means that CS-MoCOM-Th is a promising material for Au(III) recovery with no need for strict temperature control.
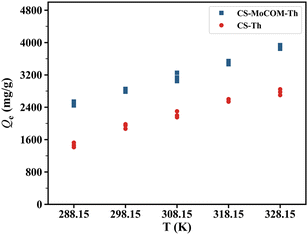 |
| | Fig. 8 The relationship between temperature (T) and the sorbed amount (Qe) of Au(III) by CS-Th and CS-MoCOM-Th at equilibrium. | |
3.6 Sorption and reduction mechanisms of Au(III) by CS-MoCOM-Th
Given that the organic components of CS-Th and CS-MoCOM-Th are same, the sorption and reduction mechanisms of Au(III) by CS-Th are a part of that by CS-MoCOM-Th. Hence, the following discussion on sorption and reduction mechanisms of Au(III) by these two nanofiber mats mainly focused on those by CS-MoCOM-Th. The sorption mechanisms of Au(III) by CS-MoCOM-Th were investigated by XPS analysis. After Au(III) sorption, the characteristic peaks of Au 4d and Au 4f appeared on the fully-scanned survey XPS spectrum of CS-MoCOM-Th (Fig. 9a), confirming that Au(III) was sorbed on this nanofiber mat. Four peaks on the high-resolution Mo 3d spectrum of CS-MoCOM-Th shifted from 229.30, 232.10, 232.63, and 235.49 eV to 229.58, 232.33, 232.86, and 235.75 eV after Au(III) sorption (Fig. 5dvs.Fig. 9c). Among them, the first and third peaks represented Mo(IV) and the second and fourth peaks represented Mo(VI). This indicated that both Mo(IV) and Mo(VI) in CS-MoCOM-Th were involved in Au(III) sorption. Four characteristic peaks for the MoCOM component in CS-MoCOM-Th also shifted from 161.86, 162.96, 163.55, and 164.72 eV to 162.74, 163.57, 164.09, and 165.20 eV on the S 2p spectrum of this material, respectively (Fig. 5cvs.Fig. 9d), confirming that the S-containing moieties in MoCOM also worked for Au(III) sorption. The other two peaks with a lower binding energy on the S 2p spectrum of CS-MoCOM-Th at 163.43 and 164.58 eV belonged to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S group. They shifted to 163.98 and 165.17 eV after Au(III) sorption, respectively, verifying that the C
S group. They shifted to 163.98 and 165.17 eV after Au(III) sorption, respectively, verifying that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S group was also responsible for sorption. Likewise, three peaks representing C–N, C
S group was also responsible for sorption. Likewise, three peaks representing C–N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and C–N+ groups on the N 1s spectrum of CS-MoCOM-Th shifted from 399.20, 399.76, and 401.43 eV to 399.29, 399.99, and 401.82 eV, respectively (Fig. 5bvs.Fig. 9e), highlighting that the N-containing moieties also shared the responsibility for Au(III) sorption. Based on the above, coordination interaction between Au(III) and these N- or S-containing moieties on CS-MoCOM-Th was essential for its sorption. Aside from coordination interaction, whether electrostatic attraction was also a driving force for Au(III) sorption was further investigated. At pH 4, CS-MoCOM-Th was positively charged, as indicated by its zeta potential values (Fig. 12d), mainly attributable to the abundant protonated C–N+ groups on this nanofiber mat. Calculations based on the ionization constants of chloroauric acid suggested that over 99% of Au(III) in solution was present as AuCl4− at pH 4 (Fig. S3†). Hence, electrostatic attraction would occur between AuCl4− and the protonated C–N+ groups on CS-MoCOM-Th at this pH. To briefly sum up, the driving forces for Au(III) sorption by CS-MoCOM-Th were contributed by coordination interaction and electrostatic attraction. Four N- or S-containing groups, C–N, C
N, and C–N+ groups on the N 1s spectrum of CS-MoCOM-Th shifted from 399.20, 399.76, and 401.43 eV to 399.29, 399.99, and 401.82 eV, respectively (Fig. 5bvs.Fig. 9e), highlighting that the N-containing moieties also shared the responsibility for Au(III) sorption. Based on the above, coordination interaction between Au(III) and these N- or S-containing moieties on CS-MoCOM-Th was essential for its sorption. Aside from coordination interaction, whether electrostatic attraction was also a driving force for Au(III) sorption was further investigated. At pH 4, CS-MoCOM-Th was positively charged, as indicated by its zeta potential values (Fig. 12d), mainly attributable to the abundant protonated C–N+ groups on this nanofiber mat. Calculations based on the ionization constants of chloroauric acid suggested that over 99% of Au(III) in solution was present as AuCl4− at pH 4 (Fig. S3†). Hence, electrostatic attraction would occur between AuCl4− and the protonated C–N+ groups on CS-MoCOM-Th at this pH. To briefly sum up, the driving forces for Au(III) sorption by CS-MoCOM-Th were contributed by coordination interaction and electrostatic attraction. Four N- or S-containing groups, C–N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C–N+, and C
N, C–N+, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, on the organic component of CS-MoCOM-Th, were involved in Au(III) sorption. The Mo and S elements introduced by embedding MoCOM also played an important role in further enhancing Au(III) sorption by CS-MoCOM-Th. The electrostatic attraction was an indispensable contributor to Au(III) sorption besides coordination interaction. The protonated C–N+ groups on CS-MoCOM-Th electrostatically attracted Au(III) in the aqueous phase at pH 4, which also enhanced Au(III) sorption.
S, on the organic component of CS-MoCOM-Th, were involved in Au(III) sorption. The Mo and S elements introduced by embedding MoCOM also played an important role in further enhancing Au(III) sorption by CS-MoCOM-Th. The electrostatic attraction was an indispensable contributor to Au(III) sorption besides coordination interaction. The protonated C–N+ groups on CS-MoCOM-Th electrostatically attracted Au(III) in the aqueous phase at pH 4, which also enhanced Au(III) sorption.
 |
| | Fig. 9 The fully-scanned survey XPS spectrum (a), the high-resolution Au 4f (b), Mo 3d (c), S 2p (d), and N 1s (e) spectra of CS-MoCOM-Th after Au(III) sorption. | |
To investigate whether the sorbed Au(III) on CS-MoCOM-Th would be reduced, the state-of-the-art microscopic and spectroscopic techniques, including SEM, TEM, XRD, and EDS mapping, were applied. Four peaks at 38.28, 44.53, 64.78, and 77.69° were detected from the XRD spectra of CS-MoCOM-Th after Au(III) sorption (Fig. 10d). This pattern completely matched with the powder diffraction file (PDF) No. 01-089-3697 for elemental gold, indicating the reduction of Au(III) by CS-MoCOM-Th. Interestingly, a large number of gold foils were observed on the CS-MoCOM-Th surface after Au(III) sorption (Fig. 10a). A small amount of the reduced elemental gold would probably be released from the nanofiber mat to the aqueous phase. However, as far as we know, no applicable method is now available to estimate the released amount of elemental gold after reduction. However, since sorption and reduction occur concurrently, the reduction of Au(III) and the possible release may not influence its sorbed amount on CS-MoCOM-Th. The reduced elemental gold was uniformly distributed inside the nanofibers in the form of nanoparticles with a diameter of 2–10 nm (Fig. 10b, e, and f). Lattice fringes were clearly observed on the high-resolution TEM image of CS-MoCOM-Th after Au(III) sorption (Fig. 10c), and the lattice spacing was 0.237 nm, corresponding to the (111) lattice plane of elemental gold.
 |
| | Fig. 10 SEM image (a), TEM image (b), high-resolution TEM image (c), powder XRD spectra (d), and TEM-EDS mapping (e and f) of CS-MoCOM-Th after Au(III) sorption. | |
The detailed reduction mechanisms of Au(III) sorbed on CS-MoCOM-Th, including the reduced percentage and reducing agent, were further evaluated by XPS analysis. Importantly, both the characteristic peaks for Au(I) and Au(0) were detected from the high-resolution Au 4f spectra of the nanofiber mat after Au(III) sorption (Fig. 9b), indicating that a certain amount of the sorbed Au(III) was reduced. Based on the peak area percentage of Au 4f, 69.42 and 11.31% of Au(III) sorbed on CS-MoCOM-Th was reduced to elemental gold and Au(I), respectively. The peak area ratio of Mo(VI) to Mo(IV) on the Mo 3d spectra of CS-MoCOM-Th almost remained unchanged before and after Au(III) sorption (Fig. 5dvs.Fig. 9c), suggesting that Mo(IV) was not involved in Au(III) reduction. The peak area percentage for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group on CS-MoCOM-Th increased from 35.48 to 52.44% after Au(III) sorption (Fig. 5bvs.Fig. 9e), while that for the C–N group decreased. This indicated that the C–N group on CS-MoCOM-Th acted as a reducing agent, and it was oxidized to the C
N group on CS-MoCOM-Th increased from 35.48 to 52.44% after Au(III) sorption (Fig. 5bvs.Fig. 9e), while that for the C–N group decreased. This indicated that the C–N group on CS-MoCOM-Th acted as a reducing agent, and it was oxidized to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group. A new peak at 168.63 eV belonging to another oxidized product, SOx, was detected from the S 2p spectra of CS-MoCOM-Th after Au(III) sorption (Fig. 5cvs.Fig. 9d). The SOx was derived from the oxidation of the C
N group. A new peak at 168.63 eV belonging to another oxidized product, SOx, was detected from the S 2p spectra of CS-MoCOM-Th after Au(III) sorption (Fig. 5cvs.Fig. 9d). The SOx was derived from the oxidation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S group introduced by grafting thiourea and the S-containing component in the embedded MoCOM by the sorbed Au(III). The chemical formula for Au(III) reduction by CS-MoCOM-Th (eqn (11)) is generalized as shown below:
S group introduced by grafting thiourea and the S-containing component in the embedded MoCOM by the sorbed Au(III). The chemical formula for Au(III) reduction by CS-MoCOM-Th (eqn (11)) is generalized as shown below:
| | C–N(+) + C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S + S(−II) + Sn(−II) + Au(III) → C S + S(−II) + Sn(−II) + Au(III) → C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(+) + SOx + Au(I) + Au(0) N(+) + SOx + Au(I) + Au(0) | (11) |
Based on the discussion above, it can be concluded that: 1) all of the N- and S-containing moieties (C–N, C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif)
N, C–N
+, C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif)
S, S(−
II), and S
n(−
II)) from CS-MoCOM-Th were involved in Au(
III) sorption; 2) reduction of Au(
III) was achieved by the following moieties: C–N, C–N
+, C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif)
S, S(−
II), and S
n(−
II). The sorption and reduction mechanisms of Au(
III) by CS-MoCOM-Th are summarized in
Fig. 11.
 |
| | Fig. 11 Sorption and reduction mechanisms of Au(III) by CS-MoCOM-Th. | |
3.7 Sorption selectivity of Au(III) by CS-MoCOM-Th
Given that the Au(III)-containing wastewater usually contains some impurity elements, sorption selectivity is essential to a specific material for practical use. To evaluate this, the recovery efficiency of Au(III) and the commonly co-existing elements, including Cu(II), Ni(II), Pb(II), Zn(II), and Pt(IV), was tested.40 The concentrations of all six elements were set as 2 × 10−3 mg mL−1.41 Our data showed that the recovery efficiency of Au(III) by CS-MoCOM-Th remained as high as 99.22% (Fig. 12b), highlighting that Au(III) recovery by this material was unaffected by other co-existing elements. The concentrations of the four interfering heavy metals (i.e., Cu(II), Ni(II), Pb(II), and Zn(II)) were almost unchanged after sorption (Fig. 12a), indicating that their sorption strength by CS-MoCOM-Th was quite weak compared to Au(III). This is because all of these four co-existing elements are present as cations, while CS-MoCOM-Th is positively charged at pH 4 (Fig. 12d). The electrostatic repulsion between them may strongly inhibit their sorption to CS-MoCOM-Th, thereby exhibiting a very low recovery efficiency. Notably, the recovery efficiency of Pt(IV) by CS-MoCOM-Th was 86.89%, much higher than that of the other four heavy metals. Unlike those elements, Pt(IV) is present as an anion PtCl62− at pH 4. It would be electrostatically attracted by CS-MoCOM-Th, similar to Au(III). Given that PtCl62− is divalent and AuCl4− is monovalent, the electrostatic attraction of Pt(IV) by CS-MoCOM-Th should have been stronger than Au(III). However, the recovery efficiency of Pt(IV) by CS-MoCOM-Th is still lower than that of Au(III), which is contrary to our observation. Further analysis showed that the coordination interaction between the element S in MoCOM, particularly MoS2, with Pt(IV) was weaker than that with Au(III).63 In addition, the difference in standard electrode potential of AuCl4−/Au (1.002 V) is higher than that of PtCl62−/Pt (0.76 V),69 which means that Au(III) is more readily reduced than Pt(IV) by CS-MoCOM-Th at pH 4, such that Au(III) sorption would be more strongly enhanced. Although the recovery efficiency of Pt(IV) is relatively high, its co-recovery could be acceptable from an economic point of view because platinum is also a noble metal like gold.
 |
| | Fig. 12 Decrease in concentration (a) and recovery efficiency (b) of Au(III) and other co-existing elements, including Cu(II), Ni(II), Pb(II), Zn(II), and Pt(IV) by CS-MoCOM-Th; the relationship between the initial solution pH and sorbed amount (Qe) of Au(III) by CS-MoCOM-Th at equilibrium (c); zeta potential of CS-MoCOM-Th (d); and the relationship between recycle number and recovery efficiency of Au(III) by CS-MoCOM-Th (e). | |
3.8 Effect of pH on Au(III) sorption by CS-MoCOM-Th
The pH value is an important factor that influences Au(III) recovery. To find out the optimal pH value for Au(III) sorption by CS-MoCOM-Th, the relationship between the initial pH and the sorbed amount of Au(III) was investigated. Qe sharply increased with increasing pH from 2 to 4 and then gradually decreased as the pH increased above 8 (Fig. 12c). Notably, Qe remained over 1.20 × 106 mg kg−1 at the initial pH between 2 and 6, showing a wide working pH range for Au(III) sorption by CS-MoCOM-Th. To explore the sorption mechanism of Au(III) by CS-MoCOM-Th at different pH values, its zeta potential was measured (Fig. 12d). At pH 4, CS-MoCOM-Th was positively charged, and most of Au(III) in solution was present as AuCl4− (Fig. S3†). The electrostatic attraction between the nanofiber mat and Au(III) led to a favorable sorption performance. When pH decreased below 4, Cl− concentrations in the aqueous phase increased; hence, an increasing number of sorption sites on CS-MoCOM-Th would be occupied. This would decrease the sorption strength of Au(III) by CS-MoCOM-Th. When the pH increased above 4, AuCl4− intensively hydrolyzed to AuCl3(OH)−, AuCl2(OH)2−, AuCl(OH)3−, and Au(OH)4−, which negatively affected Au(III) sorption by coordination competition.70 Besides, the electrostatic attraction between CS-MoCOM-Th and Au(III) tended to be weaker with increasing pH and even reversed to repulsion when pH was greater than pHpzc (7.29) of this material. Hence, sorption strength of Au(III) by CS-MoCOM-Th gradually decreased. Based on the above, the pH value for all sorption experiments was taken as 4.
3.9 Sorption recyclability and chemical stability of CS-MoCOM-Th
An appropriate material for Au(III) recovery is expected to be efficiently recyclable and chemically stable. The recyclability of CS-MoCOM-Th for Au(III) recovery was investigated by desorbing the Au(III) sorbed on the nanofiber mat with an acidic L-cysteine solution and then repeating the sorption experiment with 0.01 mg mL−1 Au(III)-containing solution. The recovery efficiency of Au(III) gradually decreased from 98.44 to 93.08% in the first three cycles but dropped to 82.15% during the fourth cycle (Fig. 12e). Theoretically, the recovery efficiency reduction of Au(III) can be ascribed to two main reasons: 1) the reduction of Au(III) disabled the sorption sites on CS-MoCOM-Th after each cycle; 2) a small number of Mo or S-containing moieties on CS-MoCOM-Th might drop off and then release into the aqueous phase during sorption. The chemical stability of CS-MoCOM-Th during Au(III) sorption was evaluated. It was shown that the mass loss percentage of Mo and S was as low as 1.11% and 1.71%, respectively, indicating that CS-MoCOM-Th was quite stable during Au(III) sorption. In summary, CS-MoCOM-Th can be recycled a few times (at least four times), while its chemical composition almost remains unchanged.
4. Conclusions
In this study, we presented a novel and simple approach to synthesize a nano-sized molybdenum composite (MoCOM) mainly consisting of MoS2 and MoO3 using a household microwave oven. It was then embedded in a chitosan-based nanofiber mat by electrospinning technique, followed by assembling thiourea on this material to synthesize a composite nanofiber mat (CS-MoCOM-Th) for effective Au(III) recovery by sorption. The embedment of MoCOM greatly enhanced sorption performance of Au(III) by the chitosan-based nanofiber mat from 2980 to 4090 mg g−1, which is almost double that of our previously synthesized material. The recovery efficiency of Au(III) by CS-MoCOM-Th was over 99% when C0 < 0.01 mg mL−1 at pH 4.0, and the sorption reached equilibrium within 10 hours, meeting the requirements of practical application. The sorption strength of Au(III) by CS-MoCOM-Th increased as temperature increased, indicating that the sorption is an endothermic process. The recovery efficiency of Au(III) remained as high as 99.22%, which was not influenced by the co-existing heavy or noble metals (Cu(II), Ni(II), Pb(II), Zn(II), and Pt(IV)). The fiber mat was also proved to be stable and can be reused at least four times. These results imply that CS-MoCOM-Th is one of the most competitive candidates for Au(III) recovery from the aqueous phase. The outstanding sorption performance of Au(III) by CS-MoCOM-Th was verified to be driven by both electrostatic attraction and coordination interaction. 69.42% of the sorbed Au(III) was directly reduced by C–N(+), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, S(−II), and Sn(−II) moieties during sorption. Limited by the experimental conditions, this work was done in the lab. The recovery performance test for Au(III) from real wastewater will be further conducted in future studies. To sum up, the successful synthesis of CS-MoCOM-Th provides a new solution for Au(III) recovery, and this nanofiber mat has a significant application prospect for sustainable development.
S, S(−II), and Sn(−II) moieties during sorption. Limited by the experimental conditions, this work was done in the lab. The recovery performance test for Au(III) from real wastewater will be further conducted in future studies. To sum up, the successful synthesis of CS-MoCOM-Th provides a new solution for Au(III) recovery, and this nanofiber mat has a significant application prospect for sustainable development.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (41821005 and 41991312).
References
- C. R. M. Butt and R. M. Hough, Why Gold is Valuable, Elements, 2009, 5, 277–280 CrossRef CAS.
- C. C. Chintawar, A. K. Yadav and N. T. Patil, Gold-Catalyzed 1,2-Diarylation of Alkenes, Angew. Chem., Int. Ed., 2020, 59, 11808–11813 CrossRef CAS PubMed.
- A. Chmielewski, J. Meng, B. E. Zhu, Y. Gao, H. Guesmi, H. Prunier, D. Alloyeau, G. Wang, C. Louis, L. Delannoy, P. Afanasiev, C. Ricolleau and J. Nelayah, Reshaping Dynamics of Gold Nanoparticles under H2 and O2 at Atmospheric Pressure, ACS Nano, 2019, 13, 2024–2033 CAS.
- N. Ma, T. Zhang, D. W. Fan, X. Kuang, A. Ali, D. Wu and Q. Wei, Triple amplified ultrasensitive electrochemical immunosensor for alpha fetoprotein detection based on MoS2@Cu2O-Au nanoparticles, Sens. Actuators, B, 2019, 297, 126821–126828 CrossRef CAS.
- S. Mignani, X. Y. Shi, V. Cena, J. Rodrigues, H. Tomas and J. P. Majoral, Engineered non-invasive functionalized dendrimer/dendron-entrapped/complexed gold nanoparticles as a novel class of theranostic (radio) pharmaceuticals in cancer therapy, J. Controlled Release, 2021, 332, 346–366 CrossRef CAS PubMed.
- J. X. Wang, F. Faraji, J. Ramsay and A. Ghahreman, A review of biocyanidation as a sustainable route for gold recovery from primary and secondary low-grade resources, J. Cleaner Prod., 2021, 296, 126457–126474 CrossRef CAS.
- X. Yu, J. He, C. Yan, Y. Chu, J. Hu, W. Jia, H. Lv, H. Zhang, P. Wang, D. Werner and X. Wang, Synthesis of a novel fibrous material for effective Au(III) recovery with superior selectivity and one-step reduction, J. Environ. Chem. Eng., 2023, 11, 109176–109187 CrossRef CAS.
- C. Wang, Z. Wang, J. Xu and Y. Nie, Analysis of Highly Efficient Adsorption of Au(S2O3)23− by Calcined Cu/Fe Layered Double Hydroxides, ACS Omega, 2021, 6, 22126–22136 CrossRef CAS PubMed.
- H. J. Wang, Y. L. Feng, H. R. Li and J. X. Kang, Simultaneous extraction of gold and zinc from refractory carbonaceous gold ore by chlorination roasting process, Trans. Nonferrous Met. Soc. China, 2020, 30, 1111–1123 CrossRef CAS.
- H. Mahandra, F. Faraji and A. Ghahreman, Novel Extraction Process for Gold Recovery from Thiosulfate Solution Using Phosphonium Ionic Liquids, ACS Sustainable Chem. Eng., 2021, 9, 8179–8185 CrossRef CAS.
- H. Wang, Y. Wu, N. Deng, B. Li, F. Li and J. B. He, Development of a bipolar electrochemical flow microreactor for recovery of valuable metals from mixed solutions, Chem. Eng. J., 2020, 382, 121907–121911 CrossRef CAS.
- J. S. Xia, R. Marthi, J. Twinney and A. Ghahreman, A review on adsorption mechanism of gold cyanide complex onto activation carbon, J. Ind. Eng. Chem., 2022, 111, 35–42 CrossRef CAS.
- J. Q. Guo, Y. F. Wu, Z. H. Wang, J. M. Yu and J. R. Li, Review: adsorbents for the recovery of precious metals from wastewater, J. Mater. Sci., 2022, 57, 10886–10911 CrossRef CAS.
- F. Hao, X. Miao, M. Zhang, Z. Dong, M. Zhai, Y. Shen, J. Zu, J. Yang and L. Zhao, Efficient and selective adsorption of Au(III), Pt(IV), and Pd(II) by a radiation-crosslinked poly(ionic liquid) gel, New J. Chem., 2022, 46, 22917–22925 RSC.
- Y. Xiang, Y. Liu, M. Li, W. Bai, G. Liu and L. Xu, The recovery of Au(III) by hydrogel-like beads, Hydrometallurgy, 2023, 215, 105964–105972 CrossRef CAS.
- M. Zhao, J. Zhao, Z. Huang, S. Wang and L. Zhang, One pot preparation of magnetic chitosan-cystamine composites for selective recovery of Au(III) from the aqueous solution, Int. J. Biol. Macromol., 2019, 137, 721–731 CrossRef CAS PubMed.
- E. Lakay, S. Hermans, K. Koch and B. Klumperman, The efficient recovery of Au(III) ions from acidic solutions by a novel scavenger based on functionalized poly(styrene-co-maleimide) nanoparticles, Chem. Eng. J., 2021, 414, 128761–128775 CrossRef CAS.
- W. Xu, S. Zhou, B. Wang, P. Zhang and K. Tang, Efficient adsorption of Au(III) from acidic solution by a novel N, S-containing metal–organic framework, Sep. Purif. Technol., 2022, 288, 120646–120657 CrossRef CAS.
- L. Zhang, Q.-Q. Zheng, S.-J. Xiao, J.-Q. Chen, W. Jiang, W.-R. Cui, G.-P. Yang, R.-P. Liang and J.-D. Qiu, Covalent organic frameworks constructed by flexible alkyl amines for efficient gold recovery from leaching solution of e-waste, Chem. Eng. J., 2021, 426, 131865–131873 CrossRef CAS.
- F. Liu, Z. Zhou and G. Li, Persimmon tannin functionalized polyacrylonitrile fiber for highly efficient and selective recovery of Au(III) from aqueous solution, Chemosphere, 2021, 264, 128469–128477 CrossRef CAS PubMed.
- Y. Chen, J. Tang, S. Wang and L. Zhang, Facile preparation of a remarkable MOF adsorbent for Au(III) selective separation from wastewater: Adsorption, regeneration and mechanism, J. Mol. Liq., 2022, 349, 118137–118147 CrossRef CAS.
- K. Sun, W. Peng, H. Li and S. Song, Recovery of Au(CN)2− with magnetic reduced graphene oxide hydrogel in aqueous leach solution, Hydrometallurgy, 2018, 176, 208–215 CrossRef CAS.
- J. Liu, C. Jin and C. Wang, Hyperbranched thiourea-grafted electrospun polyacrylonitrile fibers for efficient and selective gold recovery, J. Colloid Interface Sci., 2020, 561, 449–458 CrossRef CAS PubMed.
- W. B. Huang, Z. Y. Tong, R. Z. Wang, Z. J. Liao, Y. X. Bi, Y. Chen, M. L. Ma, P. Lyu and Y. Ma, A review on electrospinning nanofibers in the field of microwave absorption, Ceram. Int., 2020, 46, 26441–26453 CrossRef CAS.
- Y. Li, J. D. Zhu, H. Cheng, G. Q. Li, H. J. Cho, M. J. Jiang, Q. Gao and X. W. Zhang, Developments of Advanced Electrospinning Techniques: A Critical Review, Adv. Mater. Technol., 2021, 6, 2100410–2100438 CrossRef.
- N. Rosli, W. Z. N. Yahya and M. D. H. Wirzal, Crosslinked chitosan/poly(vinyl alcohol) nanofibers functionalized by ionic liquid for heavy metal ions removal, Int. J. Biol. Macromol., 2022, 195, 132–141 CrossRef CAS PubMed.
- Y. K. Deng, T. Lu, J. X. Cui, W. J. Ma, Q. L. Qu, X. L. Zhang, Y. Y. Zhang, M. M. Zhu, R. H. Xiong and C. B. Huang, Morphology engineering processed nanofibrous membranes with secondary structure for high-performance air filtration, Sep. Purif. Technol., 2022, 294, 121093–121101 CrossRef CAS.
- G. Y. Xing, L. J. Shao, Y. J. Du, H. Y. Tao and C. Z. Qi, Citric acid crosslinked chitosan/poly(ethylene oxide) composite nanofibers fabricated by electrospinning and thermal treatment for controlled drug release, Cellulose, 2021, 28, 961–971 CrossRef CAS.
- L. Jin, J. Ye, Y. Wang, X. Y. Qian and M. D. Dong, Electrospinning Synthesis of ZIF-67/PAN Fibrous Membrane with High-capacity Adsorption for Malachite Green, Fibers Polym., 2019, 20, 2070–2077 CrossRef CAS.
- S. H. Chang, Gold(III) recovery from aqueous solutions by raw and modified chitosan: A review, Carbohydr. Polym., 2021, 256, 117423–117449 CrossRef CAS PubMed.
- D. X. Kong, S. R. Foley and L. D. Wilson, An Overview of Modified Chitosan Adsorbents for the Removal of Precious Metals Species from Aqueous Media, Molecules, 2022, 27, 978–998 CrossRef CAS PubMed.
- G. Martinez-Mejia, N. A. Vazquez-Torres, A. Castell-Rodriguez, J. M. del Rio, M. Corea and R. Jimenez-Juarez, Synthesis of new chitosan-glutaraldehyde scaffolds for tissue engineering using Schiff reactions, Colloids Surf., A, 2019, 579, 123658–123672 CrossRef CAS.
- C. J. Xu, R. E. Xing, S. Liu, Y. K. Qin, K. C. Li, H. H. Yu and P. C. Li, The immunostimulatory effects of hydroxypropyltrimethyl ammonium chloride chitosan-carboxymethyl chitosan nanoparticles, Int. J. Biol. Macromol., 2021, 181, 398–409 CrossRef CAS PubMed.
- P. Suwattanachai, A. Pimkhaokham and S. Chirachanchai, Multi-functional carboxylic acids for chitosan scaffold, Int. J. Biol. Macromol., 2019, 134, 156–164 CrossRef CAS PubMed.
- M. Zhang, G. H. Wang, D. Wang, Y. Q. Zheng, Y. X. Li, W. Q. Meng, X. Zhang, F. F. Du and S. X. Lee, Ag@MOF-loaded chitosan nanoparticle and polyvinyl alcohol/sodium alginate/chitosan bilayer dressing for wound healing applications, Int. J. Biol. Macromol., 2021, 175, 481–494 CrossRef CAS PubMed.
- X. C. Zhou, S. H. Zhang, J. Shi, K. Zhao, A. P. Deng and J. G. Li, An ultrasensitive competitive chemiluminescence immunosensor coupled flow injection cell modified by oxidized graphene-chitosan for the detection of Hg2+, Microchem. J., 2019, 149, 103997–104003 CrossRef CAS.
- S. C. Jang, F. S. Chuang, W. C. Tsen and T. W. Kuo, Quaternized chitosan/functionalized carbon nanotubes composite anion exchange membranes, J. Appl. Polym. Sci., 2019, 136, 47778–47785 CrossRef.
- R. Guo, C. C. Rong, S. Chen, J. A. Zhang, A. Osaka, T. Ikoma, X. N. Li and W. Y. Chen, Synthesis of nanofibrous chitosan/Fe3O4/TiO2@TiO2 microspheres with enhanced biocompatibility and antibacterial property, J. Am. Ceram. Soc., 2023, 106, 2240–2251 CrossRef CAS.
- L. O. Amaral and A. L. Daniel-da-Silva, MoS2 and MoS2 Nanocomposites for Adsorption and Photodegradation of Water Pollutants: A Review, Molecules, 2022, 27, 6782–6813 CrossRef CAS PubMed.
- Y. Geng, J. Li, W. Lu, N. Wang, Z. Xiang and Y. Yang, Au(III), Pd(II) and Pt(IV) adsorption on amino-functionalized magnetic sorbents: Behaviors and cycling separation routines, Chem. Eng. J., 2020, 381, 122627–122635 CrossRef CAS.
- C. Yan, X. Yu, W. Jia, J. He, J. Hu, M. Zhang, J. Wang, L. Tang, J. Liu and X. Wang, Superior sorption capacity and one-step reduction of Au(III) by a novel chitosan-based electrospun fiber mat: A cheap and simple technique, Chem. Eng. J., 2023, 465, 143028–143040 CrossRef CAS.
- Y. J. Zhu and F. Chen, Microwave-Assisted Preparation of Inorganic Nanostructures in Liquid Phase, Chem. Rev., 2014, 114, 6462–6555 CrossRef CAS PubMed.
- Y. Shi, D. Zhang, H. F. Miao, X. K. Wu, Z. C. Wang, T. R. Zhan, J. P. Lai and L. Wang, Amorphous/2H-MoS2 nanoflowers with P doping and S vacancies to achieve efficient pH-universal hydrogen evolution at high current density, Sci. China: Chem., 2022, 65, 1829–1837 CrossRef CAS.
- M. L. Tang, D. C. Grauer, B. Lassalle-Kaiser, V. K. Yachandra, L. Amirav, J. R. Long, J. Yano and A. P. Alivisatos, Structural and Electronic Study of an Amorphous MoS3 Hydrogen-Generation Catalyst on a Quantum-Controlled Photosensitizer, Angew. Chem., Int. Ed., 2011, 50, 10203–10207 CrossRef CAS PubMed.
- M. Krbal, V. Prokop, A. A. Kononov, J. R. Pereira, J. Mistrik, A. V. Kolobov, P. J. Fons, Y. Saito, S. Hatayama, Y. Shuang, Y. Sutou, S. A. Rozhkov, J. R. Stellhorn, S. Hayakawa, I. Pis and F. Bondino, Amorphous-to-Crystal Transition in Quasi-Two-Dimensional MoS2: Implications for 2D
Electronic Devices, ACS Appl. Nano Mater., 2021, 4, 8834–8844 CrossRef CAS.
- M. Krbal, J. Prikryl, I. Pis, V. Prokop, J. Rodriguez Pereira and A. V. Kolobov, Anomalous electrical conductivity change in MoS2 during the transition from the amorphous to crystalline phase, Ceram. Int., 2023, 49, 2619–2625 CrossRef CAS.
- S. H. Choi, B. Stephen, J. H. Park, J. S. Lee, S. M. Kim, W. Yang and K. K. Kim, Water-Assisted Synthesis of Molybdenum Disulfide Film with Single Organic Liquid Precursor, Sci. Rep., 2017, 7, 1983 CrossRef PubMed.
- S. Nazrul, A. Biswal, L. Behera and S. K. Swain, Synthesis of sandwiched chitosan-g-PMMA nanocomposite by layered double hydroxides for packaging applications, Polym. Bull., 2023 DOI:10.1007/s00289-023-04732-6.
- M. Y. Li, J. Liu, Y. M. Hu, X. P. Gao, Q. D. Yuan and F. G. Zhao, Investigation of the specularite/chlorite separation using chitosan as a novel depressant by direct flotation, Carbohydr. Polym., 2020, 240, 116334–116341 CrossRef CAS PubMed.
- Y. H. Dong, J. J. Bi, S. J. Ming, S. T. Zhang, D. J. Zhu, D. Meng and T. Li, Functionalized chitosan as a novel support for stabilizing palladium in Suzuki reactions, Carbohydr. Polym., 2021, 260, 117815–117823 CrossRef CAS PubMed.
- J. Guo, X. Fan, J. Wang, S. Yu, M. Laipan, X. Ren, C. Zhang, L. Zhang and Y. Li, Highly efficient and selective recovery of Au(III) from aqueous solution by bisthiourea immobilized UiO-66-NH2: Performance and mechanisms, Chem. Eng. J., 2021, 425, 130588–130599 CrossRef CAS.
- C. Song, H. Yu, M. Zhang, Y. Y. Yang and G. R. Zhang, Physicochemical properties and antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae, Int. J. Biol. Macromol., 2013, 60, 347–354 CrossRef CAS PubMed.
- M. F. Queiroz, K. R. T. Melo, D. A. Sabry, G. L. Sassaki and H. A. O. Rocha, Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation?, Mar. Drugs, 2015, 13, 141–158 CrossRef PubMed.
- R. Eivazzadeh-Keihan, F. Radinekiyan, H. A. M. Aliabadi, S. Sukhtezari, B. Tahmasebi, A. Maleki and H. Madanchi, Chitosan hydrogel/silk fibroin/Mg(OH)2 nanobiocomposite as a novel scaffold with antimicrobial activity and improved mechanical properties, Sci. Rep., 2021, 11, 650 CrossRef CAS PubMed.
- B. Tahmasbi, M. Nikoorazm, P. Moradi and Y. A. Tyula, A Schiff base complex of lanthanum on modified MCM-41 as a reusable nanocatalyst in the homoselective synthesis of 5-substituted 1H-tetrazoles, RSC Adv., 2022, 12, 34303–34317 RSC.
- Y. C. Li, H. Y. Tian, J. X. Ding, X. Dong, J. Chen and X. S. Chen, Thiourea modified polyethylenimine for efficient gene delivery mediated by the combination of electrostatic interactions and hydrogen bonds, Polym. Chem., 2014, 5, 3598–3607 RSC.
- X. Q. Sun, J. J. Zhang, Y. Chen, Y. Q. Mi, W. Q. Tan, Q. Li, F. Dong and Z. Y. Guo, Synthesis, Characterization, and the Antioxidant Activity of Carboxymethyl Chitosan Derivatives Containing Thiourea Salts, Polymers, 2019, 11, 1810–1825 CrossRef CAS PubMed.
- A. Ramalingam, E. Samaraj, S. Venkateshwaran, S. M. Senthilkumar and G. C. Senadi, 1T-MoS2 catalysed reduction of nitroarenes and a one-pot synthesis of imines, New J. Chem., 2022, 46, 8720–8728 RSC.
- J. Wang and X. Guo, Adsorption isotherm models: Classification, physical meaning, application and solving method, Chemosphere, 2020, 258, 127279–127303 CrossRef CAS PubMed.
- Z. Chang, F. Li, X. Qi, B. Jiang, J. Kou and C. Sun, Selective and efficient adsorption of Au (III) in aqueous solution by Zr-based metal-organic frameworks (MOFs): An
unconventional way for gold recycling, J. Hazard. Mater., 2020, 391, 122175–122184 CrossRef CAS PubMed.
- J. Guo, X. Fan, Y. Li, S. Yu, Y. Zhang, L. Wang and X. Ren, Mechanism of selective gold adsorption on ion-imprinted chitosan resin modified by thiourea, J. Hazard. Mater., 2021, 415, 125617–125623 CrossRef CAS PubMed.
- H. L. Qian, F. L. Meng, C. X. Yang and X. P. Yan, Irreversible Amide-Linked Covalent Organic Framework for Selective and Ultrafast Gold Recovery, Angew. Chem., Int. Ed., 2020, 59, 17607–17613 CrossRef CAS PubMed.
- B. Feng, C. Yao, S. Chen, R. Luo, S. Liu and S. Tong, Highly efficient and selective recovery of Au(III) from a complex system by molybdenum disulfide nanoflakes, Chem. Eng. J., 2018, 350, 692–702 CrossRef CAS.
- L. Wang, K. Wang, R. Huang, Z. Qin, Y. Su and S. Tong, Hierarchically flower-like WS2 microcrystals for capture and recovery of Au (III), Ag (I) and Pd (II), Chemosphere, 2020, 252, 126578–126589 CrossRef CAS PubMed.
- Y. Liang, W. Zhan, Y. Yuan, N. Zamora-Romero, F. Jia, B. Yang, Z. Nasrddinov, J. L. Arauz-Lara and S. Song, Manganese and oxygen dual-doping MoS2 boosts reduction and adsorption activity toward efficient recovery of gold(I) from thiosulfate solutions, J. Alloys Compd., 2022, 928, 167185–167194 CrossRef CAS.
- F. Liu, S. You, Z. Wang and Y. Liu, Redox-Active Nanohybrid Filter for Selective Recovery of Gold from Water, ACS ES&T Engg, 2021, 1, 1342–1350 Search PubMed.
- M. Zhao, Z. Huang, S. Wang and L. Zhang, Ultrahigh efficient and selective adsorption of Au(III) from water by novel Chitosan-coated MoS2 biosorbents: Performance and mechanisms, Chem. Eng. J., 2020, 401, 126006–126016 CrossRef CAS.
- M. Zhang, Z. Dong, F. Hao, K. Xie, W. Qi, M. Zhai and L. Zhao, Ultrahigh and selective adsorption of Au(III) by rich sulfur and nitrogen-bearing cellulose microspheres and their applications in gold recovery from gold slag leaching solution, Sep. Purif. Technol., 2021, 274, 119016–119026 CrossRef CAS.
- J. Kim, K. R. Kim, Y. Hong, S. Choi, C. T. Yavuz, J. W. Kim and Y. S. Nam, Photochemically Enhanced Selective Adsorption of Gold Ions on Tannin-Coated Porous Polymer Microspheres, ACS Appl. Mater. Interfaces, 2019, 11, 21915–21925 CrossRef CAS PubMed.
- C. Wang, G. Lin, J. Zhao, S. Wang and L. Zhang, Enhancing Au(III) adsorption capacity and selectivity via engineering MOF with mercapto-1,3,4-thiadiazole, Chem. Eng. J., 2020, 388, 124221–124228 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  a,
Xuefeng
Yu
a,
Jinlong
Zhang
a,
Xuefeng
Yu
a,
Jinlong
Zhang
 a,
Jinglei
He
a,
Wenyi
Jia
a,
Jianlong
Wang
a,
Jinglei
He
a,
Wenyi
Jia
a,
Jianlong
Wang
 b,
Fuqiang
Liu
c,
Junfeng
Liu
a and
Xilong
Wang
b,
Fuqiang
Liu
c,
Junfeng
Liu
a and
Xilong
Wang
 *a
*a
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C–N+, C
N, C–N+, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, S(−II), and Sn(−II). During sorption, 69.42% of Au(III) was directly reduced to elemental gold on CS-MoCOM-Th, and gold foils precipitated on the nanofiber mat. Notably, excellent sorption selectivity of Au(III) on CS-MoCOM-Th was achieved when heavy metals, including Cu(II), Ni(II), Pb(II), and Zn(II), and noble metal Pt(IV) were co-present at 2 × 10−3 mg mL−1. CS-MoCOM-Th could still sorb 82.15% of Au(III) at 0.01 mg mL−1 after four cycles. Overall, this study highlights that CS-MoCOM-Th is one of the most competitive candidates for Au(III) recovery from the aqueous phase.
S, S(−II), and Sn(−II). During sorption, 69.42% of Au(III) was directly reduced to elemental gold on CS-MoCOM-Th, and gold foils precipitated on the nanofiber mat. Notably, excellent sorption selectivity of Au(III) on CS-MoCOM-Th was achieved when heavy metals, including Cu(II), Ni(II), Pb(II), and Zn(II), and noble metal Pt(IV) were co-present at 2 × 10−3 mg mL−1. CS-MoCOM-Th could still sorb 82.15% of Au(III) at 0.01 mg mL−1 after four cycles. Overall, this study highlights that CS-MoCOM-Th is one of the most competitive candidates for Au(III) recovery from the aqueous phase.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S). Its assembly on CS-MoCOM and CS is expected to enhance the sorption strength of Au(III). To assemble thiourea on CS-MoCOM and CS, 3 mg CS-MoCOM (or CS) was soaked in 10 mL thiourea solution (2.5% w/v), then adding 5 mL formaldehyde solution and shaking at 140 rpm for 24 h. The thiourea-grafted mat was rinsed and dried with the same method mentioned above, which was named as CS-MoCOM-Th (or CS-Th). The whole methodological roadmap is illustrated in Fig. 1.
S). Its assembly on CS-MoCOM and CS is expected to enhance the sorption strength of Au(III). To assemble thiourea on CS-MoCOM and CS, 3 mg CS-MoCOM (or CS) was soaked in 10 mL thiourea solution (2.5% w/v), then adding 5 mL formaldehyde solution and shaking at 140 rpm for 24 h. The thiourea-grafted mat was rinsed and dried with the same method mentioned above, which was named as CS-MoCOM-Th (or CS-Th). The whole methodological roadmap is illustrated in Fig. 1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe = ln
Qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KF + ln
KF + ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce/n
Ce/n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KT + BT
KT + BT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce
Ce![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − k1t
Qe − k1t![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t/β
t/β

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O groups, respectively. The above XPS analysis indicated that MoCOM contained a certain amount of MoS2 and MoO3. According to the results of elemental analysis (Fig. 3f), Mo and S contents (w/w) in MoCOM were 44.87 and 16.43%, respectively. The mass ratio of Mo to S in MoCOM was higher than that in MoS2, which also proved the existence of MoO3. 24.80% of C and 2.20% of N in MoCOM were mainly contributed from the activated carbon that was produced during the microwave process.47 The composition of each component in MoCOM was then calculated using the relative atomic mass (Ar) of each element. It was shown that MoCOM consisted of 41.08% of MoS2, 30.34% of MoO3, and 28.58% of activated carbon (Fig. 3f). The details of the calculation are described in ESI.† Thus, we confirmed that the molybdenum composite MoCOM containing MoS2 and MoO3 was successfully synthesized.
O and C–O groups, respectively. The above XPS analysis indicated that MoCOM contained a certain amount of MoS2 and MoO3. According to the results of elemental analysis (Fig. 3f), Mo and S contents (w/w) in MoCOM were 44.87 and 16.43%, respectively. The mass ratio of Mo to S in MoCOM was higher than that in MoS2, which also proved the existence of MoO3. 24.80% of C and 2.20% of N in MoCOM were mainly contributed from the activated carbon that was produced during the microwave process.47 The composition of each component in MoCOM was then calculated using the relative atomic mass (Ar) of each element. It was shown that MoCOM consisted of 41.08% of MoS2, 30.34% of MoO3, and 28.58% of activated carbon (Fig. 3f). The details of the calculation are described in ESI.† Thus, we confirmed that the molybdenum composite MoCOM containing MoS2 and MoO3 was successfully synthesized.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group of Schiff base.50 It was generated by the condensation polymerization of amino groups between chitosan and thiourea in the presence of formaldehyde. Two peaks at 163.49 and 164.63 eV represented the C
N group of Schiff base.50 It was generated by the condensation polymerization of amino groups between chitosan and thiourea in the presence of formaldehyde. Two peaks at 163.49 and 164.63 eV represented the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S group on the S 2p spectrum of CS-Th (Fig. 5c),51 also proving that thiourea was successfully grafted. After embedding MoCOM, new Mo 3d peaks were detected at 229.30, 232.10, 232.63, and 235.49 eV, and new S 2p peaks were detected at 161.86, 162.96, 163.55, and 164.72 eV from the spectra of CS-MoCOM-Th (Fig. 5c and d). The binding energy and peak-width-at-half-height of these new peaks were consistent with those of MoCOM (Fig. 3c and d), indicating that MoCOM was successfully embedded in the nanofiber mat to form CS-MoCOM-Th.
S group on the S 2p spectrum of CS-Th (Fig. 5c),51 also proving that thiourea was successfully grafted. After embedding MoCOM, new Mo 3d peaks were detected at 229.30, 232.10, 232.63, and 235.49 eV, and new S 2p peaks were detected at 161.86, 162.96, 163.55, and 164.72 eV from the spectra of CS-MoCOM-Th (Fig. 5c and d). The binding energy and peak-width-at-half-height of these new peaks were consistent with those of MoCOM (Fig. 3c and d), indicating that MoCOM was successfully embedded in the nanofiber mat to form CS-MoCOM-Th.


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group of Schiff base occurred at 1626 cm−1.55 N–C–N and C
N group of Schiff base occurred at 1626 cm−1.55 N–C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S groups on thiourea were also detected at 1538 and 1236 cm−1, respectively.56,57 Overall, the FT-IR and XPS analysis results consistently confirmed the grafted thiourea on both CS-Th and CS-MoCOM-Th.
S groups on thiourea were also detected at 1538 and 1236 cm−1, respectively.56,57 Overall, the FT-IR and XPS analysis results consistently confirmed the grafted thiourea on both CS-Th and CS-MoCOM-Th.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S group. They shifted to 163.98 and 165.17 eV after Au(III) sorption, respectively, verifying that the C
S group. They shifted to 163.98 and 165.17 eV after Au(III) sorption, respectively, verifying that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S group was also responsible for sorption. Likewise, three peaks representing C–N, C
S group was also responsible for sorption. Likewise, three peaks representing C–N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and C–N+ groups on the N 1s spectrum of CS-MoCOM-Th shifted from 399.20, 399.76, and 401.43 eV to 399.29, 399.99, and 401.82 eV, respectively (Fig. 5bvs.Fig. 9e), highlighting that the N-containing moieties also shared the responsibility for Au(III) sorption. Based on the above, coordination interaction between Au(III) and these N- or S-containing moieties on CS-MoCOM-Th was essential for its sorption. Aside from coordination interaction, whether electrostatic attraction was also a driving force for Au(III) sorption was further investigated. At pH 4, CS-MoCOM-Th was positively charged, as indicated by its zeta potential values (Fig. 12d), mainly attributable to the abundant protonated C–N+ groups on this nanofiber mat. Calculations based on the ionization constants of chloroauric acid suggested that over 99% of Au(III) in solution was present as AuCl4− at pH 4 (Fig. S3†). Hence, electrostatic attraction would occur between AuCl4− and the protonated C–N+ groups on CS-MoCOM-Th at this pH. To briefly sum up, the driving forces for Au(III) sorption by CS-MoCOM-Th were contributed by coordination interaction and electrostatic attraction. Four N- or S-containing groups, C–N, C
N, and C–N+ groups on the N 1s spectrum of CS-MoCOM-Th shifted from 399.20, 399.76, and 401.43 eV to 399.29, 399.99, and 401.82 eV, respectively (Fig. 5bvs.Fig. 9e), highlighting that the N-containing moieties also shared the responsibility for Au(III) sorption. Based on the above, coordination interaction between Au(III) and these N- or S-containing moieties on CS-MoCOM-Th was essential for its sorption. Aside from coordination interaction, whether electrostatic attraction was also a driving force for Au(III) sorption was further investigated. At pH 4, CS-MoCOM-Th was positively charged, as indicated by its zeta potential values (Fig. 12d), mainly attributable to the abundant protonated C–N+ groups on this nanofiber mat. Calculations based on the ionization constants of chloroauric acid suggested that over 99% of Au(III) in solution was present as AuCl4− at pH 4 (Fig. S3†). Hence, electrostatic attraction would occur between AuCl4− and the protonated C–N+ groups on CS-MoCOM-Th at this pH. To briefly sum up, the driving forces for Au(III) sorption by CS-MoCOM-Th were contributed by coordination interaction and electrostatic attraction. Four N- or S-containing groups, C–N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C–N+, and C
N, C–N+, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, on the organic component of CS-MoCOM-Th, were involved in Au(III) sorption. The Mo and S elements introduced by embedding MoCOM also played an important role in further enhancing Au(III) sorption by CS-MoCOM-Th. The electrostatic attraction was an indispensable contributor to Au(III) sorption besides coordination interaction. The protonated C–N+ groups on CS-MoCOM-Th electrostatically attracted Au(III) in the aqueous phase at pH 4, which also enhanced Au(III) sorption.
S, on the organic component of CS-MoCOM-Th, were involved in Au(III) sorption. The Mo and S elements introduced by embedding MoCOM also played an important role in further enhancing Au(III) sorption by CS-MoCOM-Th. The electrostatic attraction was an indispensable contributor to Au(III) sorption besides coordination interaction. The protonated C–N+ groups on CS-MoCOM-Th electrostatically attracted Au(III) in the aqueous phase at pH 4, which also enhanced Au(III) sorption.


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group on CS-MoCOM-Th increased from 35.48 to 52.44% after Au(III) sorption (Fig. 5bvs.Fig. 9e), while that for the C–N group decreased. This indicated that the C–N group on CS-MoCOM-Th acted as a reducing agent, and it was oxidized to the C
N group on CS-MoCOM-Th increased from 35.48 to 52.44% after Au(III) sorption (Fig. 5bvs.Fig. 9e), while that for the C–N group decreased. This indicated that the C–N group on CS-MoCOM-Th acted as a reducing agent, and it was oxidized to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group. A new peak at 168.63 eV belonging to another oxidized product, SOx, was detected from the S 2p spectra of CS-MoCOM-Th after Au(III) sorption (Fig. 5cvs.Fig. 9d). The SOx was derived from the oxidation of the C
N group. A new peak at 168.63 eV belonging to another oxidized product, SOx, was detected from the S 2p spectra of CS-MoCOM-Th after Au(III) sorption (Fig. 5cvs.Fig. 9d). The SOx was derived from the oxidation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S group introduced by grafting thiourea and the S-containing component in the embedded MoCOM by the sorbed Au(III). The chemical formula for Au(III) reduction by CS-MoCOM-Th (eqn (11)) is generalized as shown below:
S group introduced by grafting thiourea and the S-containing component in the embedded MoCOM by the sorbed Au(III). The chemical formula for Au(III) reduction by CS-MoCOM-Th (eqn (11)) is generalized as shown below:![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S + S(−II) + Sn(−II) + Au(III) → C
S + S(−II) + Sn(−II) + Au(III) → C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(+) + SOx + Au(I) + Au(0)
N(+) + SOx + Au(I) + Au(0)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C–N+, C
N, C–N+, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, S(−II), and Sn(−II)) from CS-MoCOM-Th were involved in Au(III) sorption; 2) reduction of Au(III) was achieved by the following moieties: C–N, C–N+, C
S, S(−II), and Sn(−II)) from CS-MoCOM-Th were involved in Au(III) sorption; 2) reduction of Au(III) was achieved by the following moieties: C–N, C–N+, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, S(−II), and Sn(−II). The sorption and reduction mechanisms of Au(III) by CS-MoCOM-Th are summarized in Fig. 11.
S, S(−II), and Sn(−II). The sorption and reduction mechanisms of Au(III) by CS-MoCOM-Th are summarized in Fig. 11.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, S(−II), and Sn(−II) moieties during sorption. Limited by the experimental conditions, this work was done in the lab. The recovery performance test for Au(III) from real wastewater will be further conducted in future studies. To sum up, the successful synthesis of CS-MoCOM-Th provides a new solution for Au(III) recovery, and this nanofiber mat has a significant application prospect for sustainable development.
S, S(−II), and Sn(−II) moieties during sorption. Limited by the experimental conditions, this work was done in the lab. The recovery performance test for Au(III) from real wastewater will be further conducted in future studies. To sum up, the successful synthesis of CS-MoCOM-Th provides a new solution for Au(III) recovery, and this nanofiber mat has a significant application prospect for sustainable development.




