Heterostructured metal oxides realized by quenching-induced structural transformation†
Changchun
Ye‡
 a,
Zhenghui
Pan‡
*b,
Qinghua
Zhang
c,
Fang
Yin
d,
Yanan
Wang
ce,
Yifei
Li
a,
Guangxu
Chen
a,
Zhenghui
Pan‡
*b,
Qinghua
Zhang
c,
Fang
Yin
d,
Yanan
Wang
ce,
Yifei
Li
a,
Guangxu
Chen
 a,
Jia
Li
*d,
Yongcai
Qiu
a,
Jia
Li
*d,
Yongcai
Qiu
 *a,
Geoffrey I. N.
Waterhouse
*a,
Geoffrey I. N.
Waterhouse
 f,
Lin
Gu
f,
Lin
Gu
 *c,
Zhang
Lin
*c,
Zhang
Lin
 g and
Lin
Guo
g and
Lin
Guo
 *h
*h
aSchool of Environment and Energy, State Key Laboratory of Luminescent Materials and Devices, Guangdong Provincial Key Laboratory of Atmospheric Environment and Pollution Control, South China University of Technology, Guangzhou 510000, Guangdong, China. E-mail: ycqiu@scut.edu.cn
bSchool of Materials Science and Engineering, Tongji University, Shanghai 201804, China. E-mail: zhenghuipan@tongji.edu.cn
cKey Laboratory for Renewable Energy, Beijing Key Laboratory for New Energy Materials and Devices, Laboratory of Advanced Materials and Electron Microscopy, Beijing National Laboratory for Condensed Matter Physics, Institute of Physics, Chinese Academy of Sciences, Beijing, 100190, China. E-mail: l.gu@iphy.ac.cn
dInstitute of Materials Research, Tsinghua Shenzhen International Graduate School, Tsinghua University, Shenzhen 518055, China. E-mail: li.jia@sz.tsinghua.edu.cn
eSongshan Lake Materials Laboratory, Dongguan, Guangdong, 523808, P. R. China
fSchool of Chemical Sciences, The University of Auckland, Auckland 1142, New Zealand
gSchool of Metallurgy and Environment, Central South University, Changsha 410083, P. R. China
hSchool of Chemistry, Beijing Advanced Innovation Center for Biomedical Engineering, Beihang University, Beijing 100191, China. E-mail: guolin@buaa.edu.cn
First published on 21st November 2023
Abstract
Heterostructured metal oxides exhibit outstanding catalytic performance in various chemical/electrochemical reactions, yet still face the bottleneck of synthesis difficulty and insufficient control over the catalyst composition. Herein, a facile synthesis route for heterostructured metal oxides via quenching-induced structural transformation was developed, and the size effect and the promotion mechanism between multiple quenching are also presented. Repeated quenching of hot NiMoO4 powders with a broad range of initial particle size in cold Fe(NO3)3 solution yielded different products depending on the initial NiMoO4 particle size and quenching frequency. Significant disorder and a roughened surface were created on the large-grained NiMoO4 nanoparticles (>27 nm), whilst for smaller NiMoO4 nanoparticles (<27 nm), multiple quenching triggered the structural transformation from NiMoO4 to NiFe2O4 to create a novel NiMoO4/NiFe2O4 heterostructure. We further found that the disordered defect structure generated by pre-quenching can promote the subsequent quenching regulation, and the minimization of particle size was more sensitive to quenching and thus was regulated as a whole, overcoming the thermodynamic bottleneck. The NiMoO4/NiFe2O4 heterostructured nanocatalyst demonstrated remarkable catalytic activity for oxygen evolution and reduction reactions in alkaline media, thus delivering excellent electrochemical performance in rechargeable zinc–air batteries. Our findings provide novel inspiration for the preparation of highly active heterostructured metal oxide nanocatalysts, which can be applied to various oxides, such as CoMoO4/CoFe2O4.
Broader contextHeterostructured metal oxides have excellent properties in many catalytic reactions due to their multi-component active sites and well-defined heterogeneous interface. Although many methods have been used to synthesize heterogeneous catalysts, there are still bottlenecks in terms of synthesis difficulties and insufficient control of catalyst composition. For example, the heterogeneous interface in the core–shell structure formed by surface transformation is in the interior and cannot fully play the catalytic role. Here, we report a facile synthetic route for heterostructured metal oxide via quenching-induced structural transformation, and thus discover the quenching size effect and the promotion effect between multiple quenching. The heterostructured metal oxide catalyst synthesized by this route demonstrates remarkable catalytic activity for oxygen evolution and reduction reactions, delivering excellent electrochemical performance and flexibility in rechargeable zinc–air batteries. |
Introduction
Heterostructured nanocatalysts with multi-component active sites and a well-defined heterojunction interface deliver excellent performance in various catalytic reactions.1–3 The interface between the two components is often rich in high-energy defects and possesses a unique electronic structure due to crystal structure mismatch, thus creating novel properties not observed in the individual component.4–6 Various synthetic approaches have been explored for the construction of heterostructured nanocatalysts.7–9 These typically involve repeating a certain synthesis method several times to introduce different components in a stepwise manner (e.g., hydrothermal–hydrothermal), combining different synthesis methods (e.g., hydrothermal-chemical vapor deposition), or utilizing in situ transformation technologies (e.g., partial sulfurization, nitridation, or selenidation in a specific atmosphere) to create core–shell structures. All these methods have limitations, and the direct and high-yielding synthesis of heterostructured catalysts with an exposed interface remains very challenging.10–12 Moreover, these strategies not only demand very accurate control of temperature, heating rate/time and surrounding atmosphere, but often alter the physicochemical properties of catalyst components in a way that is detrimental to catalytic performance.13,14 For example, the heterogeneous interface formed by sulfurizing or nitriding the oxide-based catalysts is buried inside the heterostructured catalyst and thus is inaccessible or difficult to expose to the catalytic environment.15,16 Therefore, simpler and more reliable synthetic routes need to be developed toward heterostructured metal oxide nano-catalysts.Our previous studies have successfully demonstrated that quenching oxide catalysts in metal salt solution is a universal approach for the rational modulation of the surface texture, composition and electronic structure of metal oxide electrocatalysts, significantly improving the performance for both electrocatalysis and photothermal-catalysis.17–19 Single quenching oxides in metal salt solution can induce surface metal heteroatom doping together with the creation of a disordered stepped surface rich in defects, but cannot penetrate deeply into the bulk structure.17 Previous studies have shown that bulk doping of oxide catalysts is more challenging and can lead to defect and lattice dislocation, which can affect the surface catalytic kinetics via electronic interaction.20–22 Furthermore, a disordered structure created by a bulk doping defect offers more lattice flexibility for structural change (including phase transformation).23–26 This prompts us to explore the effect of multiple quenching on the bulk structural, electronic and catalytic properties of oxide nanoparticles, with particular emphasis on the effect of the initial nanoparticle size on the product.
Experimental and theoretical studies have shown that the in situ phase transformation of oxide induced by high-temperature treatment depends on the temperature, treatment time and initial particle size,27,28 each of which affects the total system energy.29 Atomic rearrangement that occurs under high-temperature annealing leads to changes in the crystal phase of nanomaterials.30 Moreover, the ratio of surface area to volume increases sharply as the particle size decreases to the nanoscale, with the minimization of the surface free energy influencing the exposed crystal planes in nanomaterials.31,32 Therefore, we speculate that by quenching oxide nanoparticles with different sizes in metal salt solution, it should be possible to form heterostructured oxide catalysts containing multiple components (with the smallest nanoparticles being both surface and bulk doped during the quenching, eventually leading to structural transformation, whereas the larger nanoparticles will only be surface modified by the quenching).
Herein, we validated this hypothesis by repeated quenching scheelite-type NiMoO4 nanoparticles initially at 650 °C in cold Fe(NO3)3 solution. Through multiple quenching, the Fe doping level in the surface region of small NiMoO4 nanoparticles (<27 nm) increased dramatically, accompanied by the loss of Mo6+, whilst a thicker disordered and rough surface formed on the larger-grained NiMoO4 particles. The progressive replacement of Mo6+ atom by Fe3+ atom in the small NiMoO4 nanoparticles resulted in structural transformation to form NiFe2O4 nanoparticles, thereby forming a novel NiMoO4/NiFe2O4 heterostructure. Electrochemical tests showed that the NiMoO4/NiFe2O4 heterostructured catalyst prepared by quenching five times (MoNiO4-Fe-5th) exhibited excellent oxygen evolution reaction (OER, 227 mV at 10 mA cm−2) and oxygen reduction reaction (ORR, 408 mV at half-wave potential) performances with a small potential gap (ΔE = 0.635 V), enabling the creation of rechargeable liquid and quasi-solid-state zinc–air batteries with superior performance. Our work demonstrated that repeated quenching of high-temperature oxide nanomaterials in metal salt solution is a very promising approach for the controlled synthesis of heterostructured nanocatalysts for electrocatalysis and other applications. Moreover, we discovered the size effect and the promotion effect between multiple quenching on the bulk phase of nanoparticles, which deepened the understanding of the quenching-induced modification mechanism.
Results and discussion
Theoretical possibility of oxide structure transformation
Our investigation started from the thermodynamic phase space and scanned NiMoxFeyO4 structures with different Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe ratios from NiMoO4 to NiFe2O4, as indicated by the red arrows in Fig. 1(a). Taking the formation energies (Ef) of all NiMoxFeyO4 global minima, we calculated the thermodynamically favored NiMoxFeyO4 intermediate compounds at different Mo
Fe ratios from NiMoO4 to NiFe2O4, as indicated by the red arrows in Fig. 1(a). Taking the formation energies (Ef) of all NiMoxFeyO4 global minima, we calculated the thermodynamically favored NiMoxFeyO4 intermediate compounds at different Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe ratios (Fig. 1(b)). Calculations show that most NiMoxFeyO4 compounds have positive formation energy, indicating that most of the intermediate states are in a thermodynamically unstable crystalline phase.33 As the Fe content increases, the formation energy gradually decreases until reaching the negative minimum point for NiMo1/3Fe4/3O4, and then increases again. NiMo1/3Fe4/3O4 is an octahedral configuration with void channels (Fig. S1, ESI†) and can exist stably (Fig. S2, ESI†). Our previous work confirmed that quenching enabled Fe substitution of Mo in NiMoO4.17 Previous studies also demonstrated that Fe can replace Mo in NiMoO4 to form Fe-doped NiMoxFeyO4.34 We therefore speculate that by quenching small NiMoO4 nanoparticles in Fe(NO3)3 solutions, it should be possible to achieve a high enough Fe-doping content to form NiMo1/3Fe4/3O4, thus overcoming the high formation energy associated with NiMoxFeyO4 intermediate phases (Fig. 1(c)). Moreover, the transformation from scheelite-type NiMoO4 to spinel-type NiFe2O4 might also be achieved by replacing Mo6+ with Fe3+, even though higher energy would be required. This approach relies on the fact that surface energy greatly affects the crystal growth,4 and that abundant defects and disorder will be created in the surface region of NiMoO4 nanoparticles with quenching from high temperature (promoting the leaching of Mo6+ and its replacement by Fe3+).
Fe ratios (Fig. 1(b)). Calculations show that most NiMoxFeyO4 compounds have positive formation energy, indicating that most of the intermediate states are in a thermodynamically unstable crystalline phase.33 As the Fe content increases, the formation energy gradually decreases until reaching the negative minimum point for NiMo1/3Fe4/3O4, and then increases again. NiMo1/3Fe4/3O4 is an octahedral configuration with void channels (Fig. S1, ESI†) and can exist stably (Fig. S2, ESI†). Our previous work confirmed that quenching enabled Fe substitution of Mo in NiMoO4.17 Previous studies also demonstrated that Fe can replace Mo in NiMoO4 to form Fe-doped NiMoxFeyO4.34 We therefore speculate that by quenching small NiMoO4 nanoparticles in Fe(NO3)3 solutions, it should be possible to achieve a high enough Fe-doping content to form NiMo1/3Fe4/3O4, thus overcoming the high formation energy associated with NiMoxFeyO4 intermediate phases (Fig. 1(c)). Moreover, the transformation from scheelite-type NiMoO4 to spinel-type NiFe2O4 might also be achieved by replacing Mo6+ with Fe3+, even though higher energy would be required. This approach relies on the fact that surface energy greatly affects the crystal growth,4 and that abundant defects and disorder will be created in the surface region of NiMoO4 nanoparticles with quenching from high temperature (promoting the leaching of Mo6+ and its replacement by Fe3+).
Promotion effect between multiple quenching
Considering that quenching may have a greater impact on the surface of particles with a smaller size compared with those of a large size, we synthesized the smaller size nickel molybdate precursor and quenched it in different metal salt solutions for verification (Fig. 2(a)). The color of the NiMoO4 nanoparticles changed significantly after repeated quenching in 1 M Fe(NO3)3 solution (Fig. 2(b)), and in particular, the color of the NiMoO4-Fe-5th sample (quenched five times in 1 M Fe(NO3)3 solution) changed to brown while the NiMoO4-NC (natural cooling) sample is light green, which may be attributed to the iron doping induced by quenching, and the doping amount increased with the increase in quenching times (Table S1, ESI†). X-ray diffraction (XRD) patterns show that the main phase of the synthesized NiMoO4-NC is α phase with a small amount of β phase (Fig. 2(c) and Fig. S3a, ESI†). Interestingly, multiple quenching in Fe(NO3)3 solution induces the phase transition from α-NiMoO4 to β-NiMoO4. Furthermore, NiFe2O4 characteristic peaks appear in the NiMoO4-Fe-5th after quenching for five times, indicating that multiple quenching achieves a structural transformation from NiMoO4 to NiFe2O4. The calculated crystallite size indicates that the structural transformation induced by multiple quenching causes a lattice change and increases the size of nanoparticles (Fig. S3b, ESI†), which may affect the OER/ORR dynamics. On the contrary, multiple natural cooling after calcination does not significantly change the crystal structure of NiMoO4 (Fig. S3c, ESI†). Scanning electron microscopy (SEM) shows that the NiMoO4 nanoparticles have particle sizes ranging from 10–120 nm (a mixture of large particles and small particles), with the multiple calcination and quenching cycles causing no obvious change in particle size or morphology (Fig. S4, ESI†). Similarly, samples quenched in Co(NO3)2 (NiMoO4-Co-X) or Cr(NO3)3 (NiMoO4-Cr-X) solution also show significant color changes (Fig. S5 and S6, ESI†), especially in Cr(NO3)3 solution, and the phase transition from α-phase to β-phase occurs (Fig. S7, ESI†). It is worth noting that the samples remain light green after multiple quenching in water, further proving that it is the heteroatom doping that causes the color change. For comparison, NiMoO4-Fe-NC (Fe-doped, where NC denotes natural cooling) nanoparticles were also synthesized without any quenching treatment (Fig. S8, ESI†), which contains more β-phase compared with NiMoO4-NC, suggesting that Fe doping promotes the formation of the β-phase.To further investigate the promotion effect between multiple quenching cycles, we designed a series of controlled experiments involving quenching in Fe(NO3)3 solution (Fig. S9 and S10, ESI†). The final quenching in Fe(NO3)3 solution can be divided into three situations: the first is directly quenching in Fe(NO3)3 solution, and the product is NiMoO4-Fe-1st; the second is pre-quenching in water for 2 or 4 times followed by quenching in 1 M Fe(NO3)3 solution, and the product is NiMoO4-H2O-2nd-Fe-3rd or NiMoO4-H2O-4th-Fe-5th; the third is pre-quenching in 1 M Co(NO3)2 and 1 M Cr(NO2)3 solution, respectively, followed by quenching in 1 M Fe(NO3)3 solution, and the product is NiMoO4-Co-1st-Cr-2nd-Fe-3rd. The experimental results show that compared to the sample quenched directly in Fe(NO3)3 solution (NiMoO4-Fe-1st, Fig. 2(b)), the samples pre-quenched in water and then quenched in Fe(NO3)3 solution have a deeper color (Fig. S9a, ESI†) and more β-NiMoO4 phase structure (Fig. S10a, ESI†). Furthermore, the color of the sample pre-quenched in salt solution and then quenched in Fe(NO3)3 solution is further deepened (Fig. S9b, ESI†), and the phase structure of β-NiMoO4 is further increased (Fig. S10b, ESI†). This comparative experiment proves that there is a promotion effect between multiple quenching, that is, the previous pre-quenching can promote the regulation process of subsequent quenching, such as making it easier to increase the amount of heteroatom doping or defect structure, confirmed by subsequent physical characterization. As a comparison, we also quenched NiMoO4 nanomaterials in a mixed solution of 1 M Co(NO3)2, 1 M Cr(NO2)3, and 1 M Fe(NO3)3 (only quenched once), and found that the color of the sample is still yellowish green, and the phase structure of β-NiMoO4 decreases. This further demonstrates the advantages of multiple quenching for controlling the bulk phase of nanomaterials, as well as the promotion effect between multiple quenching.
Size effect and structural transformation mechanism between multiple quenching
Aberration-corrected high angular annular dark-field scanning transmission electron microscopy (HAADF-STEM) was used to observe the crystal structure and surface morphology changes of quenched samples, and to explore the mechanism of quenching-induced structural transformation. HAADF-STEM images show that the large particles in NiMoO4-NC and NiMoO4-Fe-NC (without quenching) are scheelite-type crystal lattice with a space group of C2/m,35 and both the as-prepared surfaces are smooth (Fig. S11a and b, ESI†). However, the surface of the NiMoO4-Fe-1st and NiMoO4-Fe-3rd catalysts is roughened and disordered after quenching one or three times, respectively (Fig. S11c and d, ESI†), with the thickness of the disordered layer increasing to 2 nm after quenching five times (NiMoO4-Fe-5th, Fig. S11e, ESI†). This disordered structure could also be observed along the [001] axis direction for NiMoO4-Fe-NC and NiMoO4-Fe-5th (Fig. S12, ESI†), further demonstrating that multiple quenching increases the disorder on the surface of large NiMoO4 nanoparticles. Compared with the surface modification of large particles, the bulk structure of the small NiMoO4 particles (<27 nm) changes more dramatically after multiple quenching (Fig. 2(d)–(k)). HAADF-STEM shows that the small particles still maintain the structure of NiMoO4 after quenching once (Fig. 2(f) and (g)). However, the small NiMoO4 particles transform into NiFe2O4 after quenching more than three times (Fig. 2(h)–(k)), confirmed by XRD (Fig. 2(c)) and the detailed characterization studies below.The metal ion content in the filtered quenching solution reveals that the ratio of Mo/Ni increases with the increase in quenching times (Fig. S13, ESI†), indicating that quenching causes the partial dissolution of Mo from the NiMoO4 nanoparticles. The detection of Ni may be attributed to residual fine NiMoO4 particles in the Fe(NO3)3 solution after filtration. The energy-dispersive X-ray (EDX) element maps show that Fe ions are doped in NiMoO4 particles after initial quenching (NiMoO4-Fe-1st, Fig. S14, ESI†), and are mainly concentrated in the near-surface region (Fig. S15, ESI†). However, as the number of quenching times increases to three (NiMoO4-Fe-3rd), the Fe atoms gradually replace the Mo atoms in the small NiMoO4 particles and concentrate in the bulk phase, accompanied by the loss of Mo, which become more obvious in NiMoO4-Fe-5th (Fig. 3(a)). We found that the crystal structure of the small particles is consistent with NiFe2O4 (Fig. S16, ESI†). Based on the fact that Mo atoms dissolve from small particles (EDX and ICP results), we can determine that NiFe2O4 originates from the NiMoO4 small particles rather than the generation of new independent phases. This suggests that multiple quenching induces an in situ structural transformation from NiMoO4 to NiFe2O4, which is consistent with the previous XRD results (Fig. 2(c)). HAADF-STEM also reveals that there are some unstable intermediate structures in which the local atomic arrangement is horizontally reversed during the multiple quenching cycles, forming a lattice dislocation structure (Fig. 3(b)), which likely possesses a unique electronic structure and abundant defects. With further quenching (5 times), more small particles are transformed into NiFe2O4, and finally a novel NiMoO4/NiFe2O4 heterostructure with many unique interfaces is formed (Fig. 2(j) and (k)), consistent with the XRD results and calculations discussed above.
These results indicate that the initial size of NiMoO4 nanoparticles greatly affects the quenching mechanism, with the smaller nanoparticles being more sensitive to quenching, leading to higher Fe doping levels and even the bulk structural transformation. The particle size statistical analysis shows that quenching will transform the bulk structure when the initial size of NiMoO4 nanoparticles is lower than 27 nm, whereas only surface Fe doping occurs when the initial NiMoO4 is larger than 27 nm (Fig. 3(c) and Fig. S14, ESI†), which is defined as the quenching size effect. Fig. 3(d) shows a schematic illustration of the formation mechanism of the NiMoO4/NiFe2O4 heterostructure through quenching multiple times in Fe(NO3)3. Our calculations show that the intermediate state structure (e.g., NiMo1/3Fe4/3O4) from NiMoO4 to NiFe2O4 can be formed by Fe doping at high loading, thus overcoming the normally high formation energy barrier for NiFe2O4 (Fig. 1). For the larger particles (>27 nm), the amount of Fe dopant introduced by multiple quenching remains relatively low and is localized at the surface of the particles, being insufficient to form the intermediate structure or NiFe2O4 and instead forming Fe-doped NiMoO4 with abundant disordered stepped surfaces. Conversely, quenching multiple times allows Fe3+ cations to penetrate into the internal structure of small particles (size <27 nm), and the incorporated Fe content reaches a higher level at the moment of quenching due to the smaller particle size, and it is easier to induce structural transformation to form an intermediate or even connected NiFe2O4 phase. The structural transformation in small particle NiMoO4 is attributed to the following three factors. Firstly, the disordered defect surface generated by pre-quenching (first to fourth time) has a more flexible crystal structure, which is easier to tailor and even causes crystal deformation,24 so it is more sensitive to quenching and can promote the subsequent quenching regulation. Secondly, particles with sufficiently small size have a large surface energy and are more sensitive to the quenching attack,36 resulting in their internal structure also being controlled by quenching as a whole. Thirdly, the high-temperature environment provides great energy for the Fe ion attack at the moment of quenching, and the repeated calcination cycles is conducive to the recrystallization of the reaction intermediates. These factors synergistically promote the in situ structure transformation from NiMoO4 to NiFe2O4 to form a heterostructured catalyst, thus overcoming the theoretical thermodynamic energy barrier of structural transformation. The developed multiple quenching route for synthesizing heterostructured oxides has the advantages of simplicity, rapidity and high efficiency, compared with the current synthesis methods (e.g., multi-step hydrothermal treatments or partial conversions at high temperatures), and can also be applied to other heterostructured structures, such as CoMoO4/CoFe2O4 synthesized by multiple quenching of CoMoO4 in Fe(NO3)3 solution with similar structural transformation (Fig. S17, ESI†).
Surface composition and electronic structure of a NiMoO4/NiFe2O4 heterostructured catalyst
Raman spectroscopy was applied to further verify the NiMoO4/NiFe2O4 heterojunction created by quenching multiple times. The NiMoO4-Fe-1st catalyst quenched once in Fe(NO3)3 shows a similar Raman spectrum to NiMoO4-NC (Fig. 4(a)), with the Raman peaks shifting to lower energy and broadening with each subsequent quenching cycle (especially for NiMoO4-Fe-5th). According to Hooke's law,37,38 the metal–O bond strength can be obtained by Raman shift ω (cm−1), and the larger ω means the stronger metal–O bond. Therefore, we conclude that the multiple quenching reduces the metal–oxygen bond strength in NiMoO4, especially the Mo–O bond, which is beneficial for the subsequent quenching process to destroy the metal–oxygen bond, resulting in a rich defect structure. In addition, Fe doping also results in a lower strength of metal–oxygen bond (Fig. S18, ESI†). Similarly, the Raman spectra for NiMoO4 quenched in Co(NO3)2 or Cr(NO3)3 solution are also red-shifted compared to the data for NiMoO4-NC (Fig. S19b and c, ESI†), which further confirms that multiple quenching in the salt solution contributes to the formation of oxygen defects and the weakening of the metal–O bond strength.39 However, there is no peak shift for NiMoO4 quenched in deionized water (Fig. S19a, ESI†), suggesting that the tailoring of metal–oxygen bond strength is attributed to the quenching-induced doping of metal ions. It is worth noting that the characteristic peaks of NiFe2O4 appear in NiMoO4 samples quenched multiple times in Fe(NO3)3, especially for NiMoO4-Fe-5th (Fig. 4(a), with the Raman spectrum of pure NiFe2O4 shown in Fig. S24a, ESI† for comparison),40 further demonstrating that multiple quenching in Fe(NO3)3 solution induces the in situ structure transformation from NiMoO4 to NiFe2O4.The controlled experiments further validate the promotion effect between multiple quenching cycles. Comparing the sample quenched directly in Fe(NO3)3 solution (NiMoO4-Fe-1st) without a significant Raman red-shift, samples pre-quenched in water and then quenched in Fe(NO3)3 solution have a slight Raman red-shift (Fig. 4(b)). Furthermore, the sample that was pre-quenched in salt solution and then quenched in Fe(NO3)3 solution has a more significant Raman red-shift (Fig. 4(c)). HAADF-STEM has shown that a defect rich structure will be formed on the surface of NiMoO4 after quenching in the salt solution, and the Co and Cr doping induced by pre-quenching reduces the strength of the metal–oxygen bond, which synergistically explains and highlights the role of pre-quenching. Interestingly, the sample quenched once in the mixed metal nitrate solution does not show a red-shift (Fig. S20, ESI†). Combining the visual observations from XRD, TEM and Raman, it can be concluded that the quenching-induced surface defect promotes the subsequent quenching cycles with more Fe uptake, resulting in Fe3+ replacing Mo6+ in small particles (<27 nm) to form a NiMoO4/NiFe2O4 heterostructure with weakened metal–O bond strength.
X-ray photoelectron spectroscopy (XPS) was applied to examine the near-surface region chemical composition and electronic structure of the NiMoO4/NiFe2O4 heterojunction nanocatalysts. The two characteristic peaks at 855.3 eV and 872.9 eV in the Ni 2p spectrum are attributed to the Ni2+ (Fig. 4(d)), whilst the weaker peaks at higher binding energy correspond to Ni3+ species associated with the defect. The Ni 2p core-level signals shift to higher binding energy as the number of quenching procedures increases (Fig. 4(d)), whereas the Mo 3d peaks progressively shift to lower binding energy (Fig. 4(e)). This indicates that Fe3+ incorporation induced by quenching causes a significant charge redistribution in the NiMoO4 surface, with the disorder (i.e. oxygen defect) around the Mo cation leading to a slightly lower overall valency.41 The Ni3+/Ni2+ ratio increases from 0.39 for NiMoO4-NC to 0.66 for NiMoO4-Fe-1st created by single quenching in Fe(NO3)3 solution,42 then increases further to 0.86 for NiMoO4-Fe-5th (Fig. 4(g) and Table S2, ESI†). Conversely, the Fe3+/Fe2+ ratio decreases from 1.13 for NiMoO4-Fe-1st to 0.95 for NiMoO4-Fe-5th (Fig. 3(f)).43 The reference NiMoO4-Fe-NC catalyst has a Fe3+/Fe2+ ratio of 1.36 (Fig. S22, ESI†). These results indicate that quenching multiple times induces the replacement of Mo6+ by Fe3+ and promotes electron transfer from Ni to Fe, leading to the formation of high-valence Ni3+ species. Surface defects created by quenching can capture electrons and promote the transfer of electrons through oxygen bridges, a hypothesis supported by the O 1s data (Fig. S21a, ESI†). The content of oxygen defects in NiMoO4-Fe-5th is 33%, much higher than NiMoO4-NC (12%) and NiMoO4-Fe-1st (22%). This defect was further confirmed by the electron paramagnetic resonance (EPR) spectra. The signal peak with a g-value of 2.003 corresponds to the oxygen vacancy,44 and NiMoO4-Fe-5th has the strongest signal peak (Fig. S21b, ESI†), which means more oxygen vacancies. The significant changes in valence state and defects indicate that quenching multiple times can indeed tailor the surface electronic structure of NiMoO4, which has obvious advantages over single-quenching. For comparison, NiFe2O4 is also synthesized (Fig. S23, ESI†) and contains Fe3+ (some of which is due to surface FeOOH) and Ni3+/Ni2+ species, respectively (Fig. S24, ESI†).
The local chemical and electronic structures of NiMoO4 quenched multiple times in Fe(NO3)3 solution were further studied using soft X-ray absorption spectroscopy (XAS). The Ni L2,3-edge and Fe L2,3-edge spectra involve excitation of the electrons from the metal 2p level into the partially unoccupied 3d orbital, and are very sensitive to the valence state and spin state of the absorbing atoms.45,46 NiMoO4 and NiFe2O4 show similar Ni L2,3-edge spectra (Fig. 4(h) and Fig. S25, ESI†), comprising L2 (2p1/2 → 3d) and L3 (2p3/2 → 3d) absorptions. The L2/L3 intensity ratio is related to the d-band occupancy, and a larger ratio indicates a higher oxidation state of the metal cation.47 The increased intensity of the L2-edge absorption after quenching multiple times indicates an increased oxidation state of Ni, compared to NiMoO4-NC (Fig. 4(h)). The L2 and L3 absorptions are split into t2g and eg components by the octahedral crystal field around the nickel cations.48 The splitting is more obvious at the L3-edge. The peak intensity of the eg feature increases significantly after quenching multiple times, suggesting that the 3d electron occupancy of Ni atoms decreases, with the electron configuration changing from t62ge22g (Ni2+) to t62ge1g (Ni3+).49 It is generally believed that a near-unity average eg occupancy is beneficial to the electron transfer kinetics in oxygen electrocatalysis.50 Therefore, the OER/ORR activity of NiMoO4-Fe-5th is expected to be improved due to the higher Ni3+ content. An opposite trend is observed in the Fe L2,3-edge spectra as the quenching number increases (Fig. 4(i)). The smaller L2/L3 ratio and the enhanced t2g peak for the NiMoO4-Fe-5th sample, compared with NiMoO4-Fe-1st, indicates increased 3d electron occupation, suggesting that some of the Fe3+ cations (t32ge2g) are reduced to Fe2+ cations (t42ge2g). Combining with the XPS and XAS results, there is strong experimental evidence that multiple quenching significantly alters the electronic structure of the NiMoO4 surface. As the number of quenching procedures increases, more Fe ions are doped into the surface region distorting the surface structure and triggering the transition from NiMoO4 to NiFe2O4 to form the NiMoO4/NiFe2O4 heterostructured system rich in Ni3+, Fe2+, and oxygen defects (multiple active species for catalysis). On this basis, the heterostructured NiMoO4-Fe-5th nanocatalyst is expected to efficiently promote the adsorption/desorption of the reaction intermediate during the OER and ORR, leading to efficient bifunctional OER/OR electrocatalysis, which is verified in the following experiments.
Evaluating the electrochemical performance for the OER/ORR
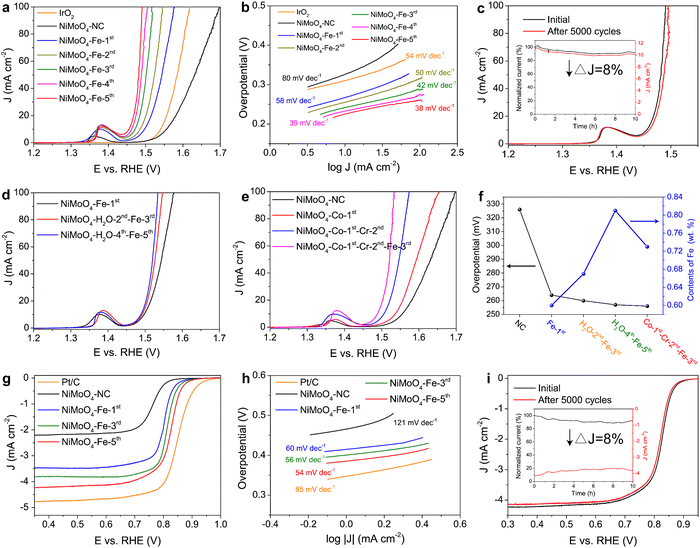
Electrochemical testing shows that multiple quenching significantly improves the OER performance of NiMoO4 with a lower OER overpotential and Tafel slope (Fig. 5(a) and (b)). The overpotential is reduced by 103 mV after quenching five times (from 330 mV for NiMoO4-NC to 227 mV for NiMoO4-Fe-5th), with the data for NiMoO4-Fe-5th being far superior to the data collected for NiMoO4-Fe-NC and NiFe2O4 (Fig. S26, ESI†), and the commercial IrO2 catalyst. The OER polarization curves for NiMoO4-Fe-5th show an oxidation peak from Ni2+ to Ni3+ at ∼1.37 V, with the peak area being much larger than that of NiMoO4-NC (Fig. 5(a)), indicating that more Ni2+ can oxidize to highly active Ni3+ (e.g., NiOOH) in the NiMoO4-Fe-5th catalyst.51 The excellent performance is attributed to the increase in the number of active sites and the improvement of intrinsic activity (Fig. S27, ESI†). Considering that excessive quenching times lead to a complex synthesis dilemma, and the OER performance does not further increase after the seventh quenching incident (Fig. S28, ESI†), we chose quenching five times as the optimal number. The polarization curves after 5000 CV cycles and the chronoamperometric curve confirm that NiMoO4-Fe-5th possesses excellent long-term OER stability (Fig. 5(c)).Similarly, multiple quenching in water, Co(NO3)2 or Cr(NO3)3 solutions also improve the OER activity (Fig. S29, ESI†). For the controlled samples quenched in Fe(NO3)3 solution, samples pre-quenched in water and then quenched in Fe(NO3)3 solution have better OER catalytic activity compared to NiMoO4-Fe-1st (Fig. 5(d)). Furthermore, the sample that was pre-quenched in salt solution and then quenched in Fe(NO3)3 solution (NiMoO4-Co-1st-Cr-2nd-Fe-3rd) has significantly improved OER catalytic performance compared with NiMoO4-Fe-1st (Fig. 5(e)), which is better than that of NiMoO4-Co/Cr/Fe-1st (quenching once in mixed solution, Fig. S30, ESI†). This once again demonstrates that pre-quenching can significantly promote the subsequent quenching. An obvious and convincing piece of evidence is that the doping amount of Fe is increased (Fig. 5(f)), which is beneficial to increasing the active sites. In addition to alkaline electrolyte, the heterostructured catalyst induced by multiple quenching also improves the OER activity in acidic and neutral electrolytes (Fig. S31, ESI†). Moreover, multiple quenching also results in significant OER activity in another system (CoMoO4/CoFe2O4), demonstrating the universality of the multiple quenching procedure in improving the OER performance (Fig. S32, ESI†).
Multiple quenching also improves the ORR performance. The ORR polarization curves show that the half-wave overpotential and Tafel values decrease with the increase of quenching times, while the limiting current density progressively increases (Fig. 5(g) and (h)). NiMoO4-Fe-5th delivers the best ORR activity with a half-wave potential of 0.822 V, close to that of a commercial Pt/C catalyst. The Koutecky–Levich (K–L) curves obtained from the ORR polarization curves at different speeds show good linearity (Fig. S33, ESI†), indicating first-order reaction kinetics.52,53 The calculated electron transfer number for NiMoO4-Fe-5th is about 3.8, consistent with a four-electron reduction process. Stability tests show NiMoO4-Fe-5th offers excellent long-term stability for the ORR (Fig. 5(i)).
The potential gap (ΔE = Ej10 − E1/2) between the OER and ORR for NiMoO4-Fe-5th is the smallest (0.635 V) among the nanocatalysts synthesized in this work (Fig. S34, ESI†), comparable to the benchmark catalysts (IrO2 and Pt/C) and far smaller than most metal catalysts reported to date (Table S3, ESI†). This excellent OER/ORR catalytic activity is attributed to the unique surface/interface structure produced by multiple quenching. First, multiple quenching makes the surface of large NiMoO4 particles disordered and rough, which increases the electrochemical activity area of the catalyst and thus exposes more active sites (Fig. S27, ESI†). Second, the quenching-induced Fe doping introduces additional active sites and constructs dual active sites together with the Ni site (Fig. S35, ESI†), thereby enhancing the intrinsic activity of the catalyst. Third, NiFe2O4 generated by in situ structural transformation has a higher catalytic activity than NiMoO4 (Fig. S26b, ESI†). Fourth, multiple quenching induces a highly active disordered surface/interface with abundant defects, and the unique heterointerface creates novel properties not observed in individual NiMoO4 or NiFe2O4, which promotes surface reconstruction of the catalyst to form more active oxyhydroxide species (NiOOH and FeOOH) on the catalyst surface (Fig. S35 and S36, ESI†). These factors synergistically reduce the energy bandgap and OER Gibbs free energy of the catalyst (Fig. S37 and S38, ESI†), thus ultimately enhancing the catalytic performance of NiMoO4,54,55 in which NiMoO4-Fe-5th is a very promising bifunctional OER/ORR nanocatalyst for application in zinc–air batteries.
Liquid aqueous zinc–air batteries (ZABs) were assembled to evaluate the performance of the NiMoO4/NiFe2O4 heterostructured catalyst. The ZABs with NiMoO4-Fe-5th as the cathode catalyst has an open circuit voltage of 1.56 V with a higher charge–discharge current and peak power density of 123 mW cm−2 at 198 mA cm−2 (Fig. S39, ESI†), which is better than that of ZABs with Pt/C + IrO2 as the cathode catalyst and most oxide-based ZABs reported in the literature (Table S4, ESI†). This excellent performance is explained by the NiMoO4/NiFe2O4 heterostructured catalyst containing a large number of highly active sites and high conductivity that enabled efficient electron transfer (Fig. S39d, ESI†). Galvanostatic charge–discharge tests show that the NiMoO4-Fe-5th based ZABs exhibit excellent durability during cycling over 550 h with a slight round-trip voltage drop (Fig. S40a, ESI†). Moreover, the NiMoO4-Fe-5th based ZABs exhibit an impressive discharge specific capacity of 745 mA h g−1 (Fig. S40b, ESI†), and can drive electric fans as proof of application (Fig. S40c, ESI†). Furthermore, the quasi-solid-state ZABs based on NiMoO4-Fe-5th as the cathode catalyst has a small voltage gap for charge–discharge polarization curves with a power density of 50 mW cm−2, and a low electrochemical impedance (Fig. S41, ESI†). The galvanostatic charge–discharge tests show that the quasi-solid-state ZABs maintain a stable cycling performance when folded at different angles, and can light up LED lamps (Fig. S42, ESI†), proving their excellent flexibility and operational stability. These results demonstrate that the NiMoO4/NiFe2O4 heterostructured catalyst (i.e., NiMoO4-Fe-5th) created by multiple quenching enables the fabrication of ZABs with an excellent output power density, cycle performance and flexibility, thus laying a solid foundation for the application of our quenching technology in catalyst synthesis for energy storage.
Conclusions
For the first time, we have demonstrated a facile synthetic route for heterostructured metal oxide via quenching-induced structural transformation with outstanding catalytic properties, and discovered the size effect and the promotion mechanism between quenching multiple times. The multiple quenching of NiMoO4 nanoparticles in Fe(NO3)3 solutions leads to Mo6+ leaching and Fe3+ doping, introducing significant disorder and a roughened surface on larger nanoparticles whilst triggering structural transformation of smaller NiMoO4 particles (<27 nm) to NiFe2O4. We found that the quenching products strongly depend on the initial NiMoO4 particle size and quenching frequency. The disordered defect structure generated by pre-quenching can promote the subsequent quenching regulation, and the minimization of particle size was more sensitive to quenching and thus was regulated as a whole, overcoming the thermodynamic bottleneck. The created NiMoO4/NiFe2O4 heterostructure was rich in defects and highly active Ni3+ species, thereby promoting electron transfer and improving catalytic performance for the OER/ORR. Liquid and flexible quasi-solid-state ZABs assembled with NiMoO4-Fe-5th exhibited an excellent output power density, cycle performance and flexibility. Our work demonstrated that quenching metal oxides in metal salt aqueous solution is a very effective route for modifying the surface/interface properties (morphology, composition, phase, defect concentration, and electronic structure) for improved catalytic performance, offering an exciting new direction for the synthesis of heterostructured oxide catalysts for energy and other applications.Experimental section
Preparation of NiMoO4 nanoparticles
NiMoO4 nanoparticles were prepared using a sol–gel method.56 Briefly, 0.30 g of ethyl cellulose powder was slowly added to 5 mL of warm water (50 °C) with constant stirring. Next, 1.77 g of (NH4)6Mo7O24 4H2O, 2.91 g of Ni(NO3)2 6H2O and 1 g of citric acid were each dissolved in 5 mL of deionized water, respectively. These solutions were then added into the ethyl cellulose solution under constant stirring at 50 °C to obtain a sol, which was then heated slowly to 90 °C to form a wet gel. The wet gel was then dried in an oven at 100 °C for 12 h, after which it was calcined at 650 °C for 2 h and allowed to naturally cool (NC) to room temperature. The product obtained is denoted herein as NiMoO4-NC.Quenching of NiMoO4 nanoparticles
For the cooling by quenching experiments, the NiMoO4 nanoparticles prepared at 650 °C were removed from the muffle furnace and immediately immersed in an aqueous 1 M Fe(NO3)3 solution at 0 °C to achieve rapid cooling. The quenched samples were then collected by centrifugation, and washed with deionized water and ethanol, before being dried in an oven at 60 °C for 12 h. The obtained sample is denoted herein as NiMoO4-Fe-1st (Fe-1st denotes quenched once in a Fe(NO3)3 solution).For the multiple quenching experiments, NiMoO4-Fe-1st was placed in a muffle furnace and calcined at 650 °C for 2 h, then again quenched in a 1 M Fe(NO3)3 solution at 0 °C. After washing and drying, the obtained sample is denoted herein as NiMoO4-Fe-2nd. Similarly, the samples obtained after the third quenching, fourth quenching and fifth quenching are denoted as NiMoO4-Fe-3rd, NiMoO4-Fe-4th, and NiMoO4-Fe-5th. For comparison, NiMoO4 was also quenched multiple times in H2O, 1 M Co(NO3)2 solution or a 1 M Cr(NO3)3 solution. The products obtained are denoted as NiMoO4-H2O-X, NiMoO4-Co-X, and NiMoO4-Cr-X (where X represents the number of quenching procedures), respectively.
To explore the effect of multiple quenching, further experiments were conducted involving pre-quenching in water before quenching in 1 M Fe(NO3)3 solution. Specifically, NiMoO4 samples pre-quenched two (NiMoO4-H2O-2nd) or four times (NiMoO4-H2O-4th) in water were calcined in a muffle furnace at 650 °C for 2 h, and then rapidly quenched in 1 M Fe (NO3)3 solution. The obtained samples were denoted as NiMoO4-H2O-2nd-Fe-3rd and NiMoO4-H2O-4th-Fe-5th, respectively. Furthermore, NiMoO4 was firstly pre-quenched in 1 M Co(NO3)2 solution, then pre-quenched in 1 M Cr(NO3)3 solution, and finally quenched in 1 M Fe(NO3)3 solution to prepare the NiMoO4-Co-1st-Cr-2nd-Fe-3rd sample. For comparison, we also quenched NiMoO4 in the above mixed solution (containing 1 M Co(NO3)2, 1 M Cr(NO3)3 and 1 M Fe(NO3)3) once, and the resulting sample was denoted as NiMoO4-Co/Cr/Fe-1st.
Preparation of Fe-doped NiMoO4 nanoparticles
4 wt% Fe-doped NiMoO4 nanoparticles were prepared using a method similar to that described for NiMoO4-NC, except that 0.622 g of Fe(NO3)3·9H2O was included in the synthesis. The product is denoted as NiMoO4-Fe-NC.Preparation of NiFe2O4 nanoparticles
NiFe2O4 nanoparticles were prepared using a hydrothermal method.57 Briefly, 0.29 g of Ni(NO3)2·6H2O and 0.81 g of Fe(NO3)3·9H2O and 0.20 g of cetyltrimethylammonium bromide (CTAB) were dissolved in 32 mL of deionized water to form a clear solution. Next, 3 mL of aqueous ammonia was added dropwise into the above solution to achieve a pH of 11. The solution was then transferred to a Teflon-lined stainless autoclave and heated at 180 °C for 12 h. After natural cooling to room temperature, the solid product was collected by centrifugation, then washed three times with deionized water and then ethanol. The sample was then dried in a vacuum oven at 60 °C for 12 h. NiFe2O4 nanoparticles were obtained by calcining the precursor in a muffle furnace at 450 °C for 2 h, followed by natural cooling to room temperature.Preparation and quenching of CoMoO4 nanoparticles
CoMoO4-NC nanoparticles were prepared using a method similar to that described for NiMoO4-NC, except that Co(NO3)2·6H2O was used instead of Ni(NO3)2·6H2O. CoMoO4 nanoparticles at 650 °C are quickly placed in 1 M Fe(NO3)3 solution for repeated quenching to obtain CoMoO4-Fe-5th that has been quenched five times.Author contributions
Y. C. Q. conceived the research project. C. C. Y., Z. H. P. and Y. C. Q. designed the experiments. C. C. Y. carried out the most of experiments. F. Y., J. L. and Y. N. W. conducted the DFT calculations. Q. H. Z. and L. Gu carried out the HAADF-STEM experiments. C. C. Y. drafted the manuscript. Y. C. Q., Z. H. P., Y. F. L., G. X. C., G. I. N. W., Z. L. and L. Guo analyzed the data and revised the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by funding from the Guangdong Innovative and Entrepreneurial Research Team Program (2019ZT08L075), the Foshan Innovative and Entrepreneurial Research Team Program (2018IT100031), the Guangdong Pearl River Talent Program (2019QN01L054, 2019QN01L159), the National Natural Science Foundation of China (22176063, 52000076), the China Postdoctoral Science Foundation (2023M741208); and the Fundamental Research Funds for the Central Universities, conducted at Tongji University. GINW acknowledges funding support from a James Cook Research Fellowship, administered by the Royal Society Te Apārangi, as well as a generous philanthropic donation from Greg and Kathryn Trounson. We acknowledge Beamlines MCD-A and MCD-B (Soochow Beamline for Energy Materials) at NSRL for providing the beam time and helpful discussion.References
- H. Lu, J. Tournet, K. Dastafkan, Y. Liu, Y. H. Ng, S. K. Karuturi, C. Zhao and Z. Yin, Chem. Rev., 2021, 121, 10271–10366 CrossRef CAS PubMed.
- Q. Zhao, Z. Yan, C. Chen and J. Chen, Chem. Rev., 2017, 117, 10121–10211 CrossRef CAS PubMed.
- T. Wu, S. Sun, J. Song, S. Xi, Y. Du, B. Chen, W. A. Sasangka, H. Liao, C. L. Gan, G. G. Scherer, L. Zeng, H. Wang, H. Li, A. Grimaud and Z. J. Xu, Nat. Catal., 2019, 2, 763–772 CrossRef CAS.
- C. Xie, Z. Niu, D. Kim, M. Li and P. Yang, Chem. Rev., 2019, 120, 1184–1249 CrossRef PubMed.
- P. C. Chen, M. H. Liu, J. S. S. Du, B. Meckes, S. Z. Wang, H. X. Lin, V. P. Dravid, C. Wolverton and C. A. Mirkin, Science, 2019, 363, 959–964 CrossRef CAS PubMed.
- L. Lin, W. Zhou, R. Gao, S. Yao, X. Zhang, W. Xu, S. Zheng, Z. Jiang, Q. Yu, Y.-W. Li, C. Shi, X.-D. Wen and D. Ma, Nature, 2017, 544, 80–83 CrossRef CAS PubMed.
- W. X. Huang, A. C. Johnston-Peck, T. Wolter, W. C. D. Yang, L. Xu, J. Oh, B. A. Reeves, C. S. Zhou, M. E. Holtz, A. A. Herzing, A. M. Lindenberg, M. Mavrikakis and M. Cargnello, Science, 2021, 373, 1518–1523 CrossRef CAS PubMed.
- G. Zhao, K. Rui, S. X. Dou and W. Sun, Adv. Funct. Mater., 2018, 28, 1803291 CrossRef.
- M. Li, X. Wang, F. Li, L. Zheng, J. Xu and J. Yu, Adv. Mater., 2020, 32, e1907098 CrossRef PubMed.
- X. Zheng, P. Cui, Y. Qian, G. Zhao, X. Zheng, X. Xu, Z. Cheng, Y. Liu, S. X. Dou and W. Sun, Angew. Chem., Int. Ed., 2020, 59, 14533–14540 CrossRef CAS PubMed.
- M. Luo, W. Sun, B. B. Xu, H. Pan and Y. Jiang, Adv. Energy Mater., 2020, 11, 2002762 CrossRef.
- L. An, Z. Zhang, J. Feng, F. Lv, Y. Li, R. Wang, M. Lu, R. B. Gupta, P. Xi and S. Zhang, J. Am. Chem. Soc., 2018, 140, 17624–17631 CrossRef CAS PubMed.
- W. Gao, Z. D. Hood and M. Chi, Acc. Chem. Res., 2017, 50, 787–795 CrossRef CAS PubMed.
- S. Gbadamasi, M. Mohiuddin, V. Krishnamurthi, R. Verma, M. W. Khan, S. Pathak, K. Kalantar-Zadeh and N. Mahmood, Chem. Soc. Rev., 2021, 50, 4684–4729 RSC.
- B. Zhang, C. Luo, Y. Deng, Z. Huang, G. Zhou, W. Lv, Y.-B. He, Y. Wan, F. Kang and Q.-H. Yang, Adv. Energy Mater., 2020, 10, 2000091 CrossRef CAS.
- H. Ma, Z. Chen, Z. Wang, C. V. Singh and Q. Jiang, Adv. Sci, 2022, 9, e2105313 CrossRef PubMed.
- C. Ye, J. Liu, Q. Zhang, X. Jin, Y. Zhao, Z. Pan, G. Chen, Y. Qiu, D. Ye, L. Gu, G. I. N. Waterhouse, L. Guo and S. Yang, J. Am. Chem. Soc., 2021, 143, 14169–14177 CrossRef CAS PubMed.
- S. Zhao, X. Jin, P. Wu, Y. Zhao, G. Chen, Y. Li, A. Li, D. Ye and Y. Qiu, ACS Appl. Nano Mater., 2020, 3, 10454–10461 CrossRef CAS.
- M. Su, Z. Pan, Y. Chong, C. Ye, X. Jin, Q. Wu, Z. Hu, D. Ye, G. I. N. Waterhouse, Y. Qiu and S. Yang, J. Mater. Chem. A, 2021, 9, 3492–3499 RSC.
- R. G. Mariano, M. Kang, O. J. Wahab, I. J. McPherson, J. A. Rabinowitz, P. R. Unwin and M. W. Kanan, Nat. Mater., 2021, 20, 1000–1006 CrossRef CAS PubMed.
- Y. Qin, Y. Liu, Y. Zhang, Y. Gu, Y. Lian, Y. Su, J. Hu, X. Zhao, Y. Peng, K. Feng, J. Zhong, M. H. Rummeli and Z. Deng, ACS Catal., 2022, 13, 256–266 CrossRef.
- S. Jiao, X. Fu, S. Wang and Y. Zhao, Energy Environ. Sci., 2021, 14, 1722–1770 RSC.
- Y. Duan, Z. Y. Yu, S. J. Hu, X. S. Zheng, C. T. Zhang, H. H. Ding, B. C. Hu, Q. Q. Fu, Z. L. Yu, X. Zheng, J. F. Zhu, M. R. Gao and S. H. Yu, Angew. Chem., Int. Ed., 2019, 58, 15772–15777 CrossRef CAS PubMed.
- J. Gao, C.-Q. Xu, S.-F. Hung, W. Liu, W. Cai, Z. Zeng, C. Jia, H. M. Chen, H. Xiao, J. Li, Y. Huang and B. Liu, J. Am. Chem. Soc., 2019, 141, 3014–3023 CrossRef CAS PubMed.
- T. Guo, L. Li and Z. Wang, Adv. Energy Mater., 2022, 12, 2200827 CrossRef CAS.
- C. Ye, H. Cheng, L. Zheng, J. Lin, Q. Xu, Y. Qiu, Z. Pan and Y. Qiu, Nano Lett., 2023, 23, 1573–1581 CrossRef CAS PubMed.
- Y. Qiu, L. Xin, Y. Li, I. T. McCrum, F. Guo, T. Ma, Y. Ren, Q. Liu, L. Zhou, S. Gu, M. J. Janik and W. Li, J. Am. Chem. Soc., 2018, 140, 16580–16588 CrossRef CAS PubMed.
- J. P. Doye and F. Calvo, Phys. Rev. Lett., 2001, 86, 3570–3573 CrossRef CAS PubMed.
- Q. Guan, C. Zhu, Y. Lin, E. I. Vovk, X. Zhou, Y. Yang, H. Yu, L. Cao, H. Wang, X. Zhang, X. Liu, M. Zhang, S. Wei, W.-X. Li and J. Lu, Nat. Catal., 2021, 4, 840–849 CrossRef CAS.
- J. Liang, F. Ma, S. Hwang, X. Wang, J. Sokolowski, Q. Li, G. Wu and D. Su, Joule, 2019, 3, 956–991 CrossRef CAS.
- X. Han, X. Ling, Y. Wang, T. Ma, C. Zhong, W. Hu and Y. Deng, Angew. Chem., Int. Ed., 2019, 58, 5359–5364 CrossRef CAS PubMed.
- J. M. McHale, A. Auroux, A. J. Perrotta and A. Navrotsky, Science, 1997, 277, 788–791 CrossRef CAS.
- S. C. Ma, S. D. Huang and Z. P. Liu, Nat. Catal., 2019, 2, 671–677 CrossRef CAS.
- Z. Yin, S. Zhang, W. Chen, M. Xinzhi, Y. Zhou, Z. Zhang, X. Wang and J. Li, New J. Chem., 2020, 44, 17477–17482 RSC.
- T. Sun, X. Zhao, B. Li, H. Shu, L. Luo, W. Xia, M. Chen, P. Zeng, X. Yang, P. Gao, Y. Pei and X. Wang, Adv. Funct. Mater., 2021, 31, 2101285 CrossRef CAS.
- Y. Jian, M. Tian, C. He, J. Xiong, Z. Jiang, H. Jin, L. Zheng, R. Albilali and J.-W. Shi, Appl. Catal., B, 2021, 283, 119657 CrossRef CAS.
- Y. Shen, J. Deng, S. Impeng, S. Li, T. Yan, J. Zhang, L. Shi and D. Zhang, Environ. Sci. Technol., 2020, 54, 10342–10350 CrossRef CAS PubMed.
- S. Rong, P. Zhang, F. Liu and Y. Yang, ACS Catal., 2018, 8, 3435–3446 CrossRef CAS.
- Y. Sun, H. Liao, J. Wang, B. Chen, S. Sun, S. J. H. Ong, S. Xi, C. Diao, Y. Du, J.-O. Wang, M. B. H. Breese, S. Li, H. Zhang and Z. J. Xu, Nat. Catal., 2020, 3, 554–563 CrossRef CAS.
- M. B. Askari and P. Salarizadeh, Int. J. Hydrogen Energy, 2020, 45, 27482–27491 CrossRef CAS.
- M. Yu, G. H. Moon, R. G. Castillo, S. DeBeer, C. Weidenthaler and H. Tuysuz, Angew. Chem., Int. Ed., 2020, 59, 16544–16552 CrossRef CAS PubMed.
- J. Kang, X. Qiu, Q. Hu, J. Zhong, X. Gao, R. Huang, C. Wan, L.-M. Liu, X. Duan and L. Guo, Nat. Catal., 2021, 4, 1050–1058 CrossRef CAS.
- L. Jiao, J. Li, L. L. Richard, Q. Sun, T. Stracensky, E. Liu, M. T. Sougrati, Z. Zhao, F. Yang, S. Zhong, H. Xu, S. Mukerjee, Y. Huang, D. A. Cullen, J. H. Park, M. Ferrandon, D. J. Myers, F. Jaouen and Q. Jia, Nat. Mater., 2021, 20, 1385–1391 CrossRef CAS PubMed.
- C. Wang, Y. Tian, Y. Gu, K.-H. Xue, H. Sun, X. Miao and L. Dai, Nano Energy, 2021, 85, 106030 CrossRef CAS.
- C. Tsounis, X. Lu, N. M. Bedford, B. Subhash, L. Thomsen, Q. Zhang, Z. Ma, K. K. Ostrikov, A. Bendavid, J. A. Scott, R. Amal and Z. Han, ACS Nano, 2020, 14, 11327–11340 CrossRef CAS PubMed.
- N. Zhang, X. Feng, D. Rao, X. Deng, L. Cai, B. Qiu, R. Long, Y. Xiong, Y. Lu and Y. Chai, Nat. Commun., 2020, 11, 4066 CrossRef CAS PubMed.
- C. Hu, H. Dong, Y. Li, S. Sinha, C. Wang, W. Xu, L. Song, K. Suenaga, H. Geng, J. Wang, Q. Huang, Y. Tan and X. Huang, Adv. Funct. Mater., 2022, 33, 2211530 CrossRef.
- Q. Ji, Y. Kong, C. Wang, H. Tan, H. Duan, W. Hu, G. Li, Y. Lu, N. Li, Y. Wang, J. Tian, Z. Qi, Z. Sun, F. Hu and W. Yan, ACS Catal., 2020, 10, 5691–5697 CrossRef CAS.
- Y. Lei, T. Xu, S. Ye, L. Zheng, P. Liao, W. Xiong, J. Hu, Y. Wang, J. Wang, X. Ren, C. He, Q. Zhang, J. Liu and X. Sun, Appl. Catal., B, 2021, 285, 119809 CrossRef CAS.
- J. Suntivich, K. J. May, H. A. Gasteiger, J. B. Goodenough and Y. Shao-Horn, Science, 2011, 334, 1383–1385 CrossRef CAS PubMed.
- Q. Wang, X. Huang, Z. L. Zhao, M. Wang, B. Xiang, J. Li, Z. Feng, H. Xu and M. Gu, J. Am. Chem. Soc., 2020, 142, 7425–7433 CrossRef CAS PubMed.
- T. Sun, Y. Li, T. Cui, L. Xu, Y. G. Wang, W. Chen, P. Zhang, T. Zheng, X. Fu, S. Zhang, Z. Zhang, D. Wang and Y. Li, Nano Lett., 2020, 20, 6206–6214 CrossRef CAS PubMed.
- Y. Jiang, Y.-P. Deng, R. Liang, J. Fu, R. Gao, D. Luo, Z. Bai, Y. Hu, A. Yu and Z. Chen, Nat. Commun., 2020, 11, 5858 CrossRef CAS PubMed.
- M. Yu, E. Budiyanto and H. Tuysuz, Angew. Chem., Int. Ed., 2022, 61, e202103824 CrossRef CAS PubMed.
- K. Zeng, X. Zheng, C. Li, J. Yan, J. H. Tian, C. Jin, P. Strasser and R. Yang, Adv. Funct. Mater., 2020, 30, 2000503 CrossRef CAS.
- V. Umapathy, P. Neeraja, A. Manikandan and P. Ramu, Trans. Nonferrous Met. Soc. China, 2017, 27, 1785–1793 CrossRef.
- Z.-Y. Yu, L.-F. Chen and S.-H. Yu, J. Mater. Chem. A, 2014, 2, 10889 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ee03379a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |