Synergistic effects of Lewis acid–base and Coulombic interactions for high-performance Zn–I2 batteries†
Jiafeng
He‡
ab,
Yongbiao
Mu‡
ab,
Buke
Wu
ab,
Fuhai
Wu
ab,
Ruixi
Liao
ab,
Hongfei
Li
 c,
Tianshou
Zhao
*ab and
Lin
Zeng
c,
Tianshou
Zhao
*ab and
Lin
Zeng
 *ab
*ab
aShenzhen Key Laboratory of Advanced Energy Storage, Southern University of Science and Technology, Shenzhen 518055, P. R. China. E-mail: zhaots@sustech.edu.cn; zengl3@sustech.edu.cn
bDepartment of Mechanical and Energy Engineering, Southern University of Science and Technology, Shenzhen 518055, P. R. China
cSchool of System Design and Intelligent Manufacturing, Southern University of Science and Technology, Shenzhen, 518055, P. R. China
First published on 16th November 2023
Abstract
Zinc–iodine batteries are considered promising energy storage devices due to the presence of non-flammable aqueous electrolytes and intrinsically safe zinc. However, the polyiodide shuttle effect and sluggish reaction kinetics limit their electrochemical performance. Herein, in this work, we synthesized a high-performance host material—the iodine covalent post-functionalized zeolitic imidazolate framework-90 (IL-ZIF-90) with multifunctional nitrogen—to achieve intense adsorption of iodine species. The positively charged nitrogen (N+) can induce Coulombic interactions with negatively charged iodine, while the nitrogen with a lone pair of electrons (Nle) serving as a Lewis base can interact with I2 which acts as a Lewis acid. Density functional theory (DFT) calculations are in accordance with the electrochemical characterization studies, indicating that the Nle species can accelerate the conversion between I2 and I−. Consequently, the cathode enables a capacity of 120.3 mA h g−1 at 4 A g−1, and exhibits an excellent rate capability with a capacity of 86.8 mA h g−1 at a high current density of 20 A g−1. Furthermore, the cathode also demonstrates excellent cyclic stability with a capacity retention of 91.7% at 10 A g−1 after 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. This work provides an effective strategy to realize high-performance Zn–I2 batteries and can be extended to other metal–iodine battery technologies.
000 cycles. This work provides an effective strategy to realize high-performance Zn–I2 batteries and can be extended to other metal–iodine battery technologies.
Broader contextAqueous Zn–I2 batteries have attracted widespread attention owing to the simple redox chemistry between metallic Zn and iodine in mild aqueous electrolytes and their intrinsic safety. Nevertheless, the unchecked/unrestricted migration of soluble polyiodide intermediates could lead to various undesirable side effects, greatly constraining the widespread application of Zn–I2 batteries. Therefore, designing a host material that can simultaneously provide effective confinement and superior electrocatalysis is highly desirable. Hence, a multifunctional host material—the iodine covalent post-functionalized zeolitic imidazolate framework with a highly ordered pore structure to trap iodine species—was proposed. Meanwhile, the host material can facilitate a fast I2 reduction reaction (IRR) through the catalyzation of nitrogen species. As a result, the obtained Zn–I2 batteries exhibit desirable electrochemical stability and reversibility. |
Introduction
Rechargeable aqueous metal-ion batteries with neutral to slightly acidic electrolytes have presented a huge potential for energy storage on a massive scale because of the high safety, extended cycle life, low manufacturing cost, pollution-free operation, and favorable electrochemical characteristics in recent years.1–5 Among the series of aqueous batteries, zinc–iodine (Zn–I2) batteries have attracted significant research attention due to their low cost (75 ppm Zn in Earth's crust and abundant iodine in ocean, 50–60 μg L−1),6 the immanent safety of the aqueous electrolyte, and relatively high theoretical capacities of Zn (820 mA h g−1 or 5855 mA h cm−3) and I2 (211 mA h g−1).7–11 Besides, the intrinsic conversion process of iodine batteries with complete electron exchange produces a flat voltage plateau, which has incomparable advantages compared with that of intercalation-type cathode materials.12–15 Nevertheless, the restricted lifespan of Zn–I2 batteries is far from adequate and is a severe setback to their further progress.In aqueous electrolytes, Zn–I2 batteries exhibit a reversible I2/I− redox reaction with possible intermediary polyiodide species (I3− and I5−) by-products. Whereas, the unchecked/unrestricted migration of these soluble polyiodide intermediates would lead to various undesirable side effects.16 On the one hand, the electrochemical redox reaction between I− and I3− at the cathode exhibits a high energy barrier, revealing the slow reaction rate. Meanwhile, the I3− generated by the process of conversion between I2 and I− promptly diffuses into the electrolyte from the cathode surface, resulting in severe overcharging and poor Coulombic efficiency.17 Besides, the intermediate product I3− should be preserved in the cathode; however, the weak binding force of pure physical adsorption on the cathode side and the quick migration of I3− through the membrane to the Zn anode surface severely reduce the active materials and lead to self-discharge and capacity fading.18 These disadvantages, thus, greatly constrained the widespread application of Zn–I2 batteries.
Up to now, the confinement of polyiodides in inert porous host materials, including functionalized porous carbon, graphene, and carbon cloth, has been regarded as a prevailing strategy for suppressing the shuttling effect in Zn–I2 batteries due to their favorable porosity and high specific surface areas.19,20 Although these host materials can constrain iodine species within the cathode chamber through physical adsorption, such relatively weak interaction is still insufficient for effectively resolving the shuttling effect, especially during long-term cycling. These polyiodides will progressively precipitate out of the matrix material into the electrolyte, resulting in severe self-discharge and the failure to construct the shuttle-free Zn–I2 battery.18 Moreover, the disordered pore structure of the porous carbon host also leads to insufficient iodine utilization and slow ion transport.21,22 What is more, due to the electrochemical inertness of carbon-based frameworks, they cannot promote efficient conversion between I2 and I−.17 To summarize, these carbon host restrictions result in slow kinetics and restricted I2 utilization, leading to poor rate performance and cycle stability of I2-based batteries. Therefore, designing a host material that can simultaneously satisfy effective confinement and superior electrocatalysis is still highly desirable.23,24
In this contribution, we propose a multifunctional host material—the iodine covalent post-functionalized zeolitic imidazolate framework-90 (IL-ZIF-90) with a highly ordered pore structure—to trap iodine species. Meanwhile, IL-ZIF-90 can enable a fast I2 reduction reaction (IRR) by the catalyzation of nitrogen species. The IL-ZIF-90 cathode loaded with iodide ions (IL-ZIF-90-I−) exhibits a lower energy barrier (62.5 kJ mol−1) and lower Tafel slope (65.6 mV dec−1) for the IRR, suggesting the ultra-fast IRR kinetics of IL-ZIF-90-I−. The electrocatalytic activity originates from the synergetic effects of the positively charged nitrogen (N+) and the nitrogen-containing lone pair of electrons (Nle). Accordingly, the IL-ZIF-90-I−//Zn battery shows the best rate performance, with a specific capacity of 86.8 mA h g−1 at a current density of 20 A g−1. Furthermore, benefiting from the robust anchoring of iodine, a record lifespan among the recent Zn–I2 batteries, up to 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, was realized with a capacity retention of 91.7%. These results guide the development of high-performance cathode materials for metal–I2 batteries.
000 cycles, was realized with a capacity retention of 91.7%. These results guide the development of high-performance cathode materials for metal–I2 batteries.
Results and discussion
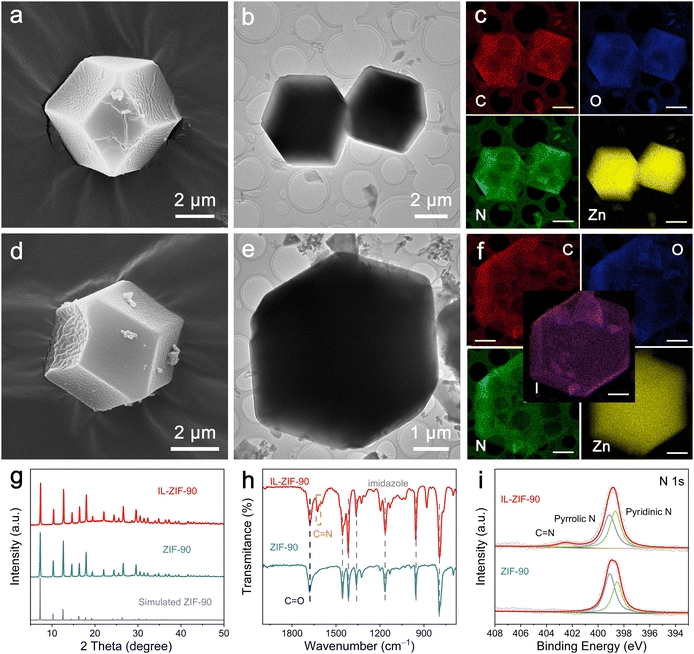
The preparation procedure of IL-ZIF-90 is shown in Fig. S1 (ESI†), which involves two main steps. First, a solvothermal reaction of zinc nitrate and imidazolate-2-formaldehyde was employed to induce the formation of ZIF-90. Then, IL-ZIF-90 was obtained via vigorous reflux, executing the assimilation of IL (1-amino-pyridinium iodide, AmPyI) with ZIF-90.The structure and morphology of the as-prepared ZIF-90 were examined by field-emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM). The FESEM and TEM images (Fig. S2 (ESI†) and Fig. 1a, b) of ZIF-90 exhibit a rhombic dodecahedral structure and a rougher surface with a size of 4–5 μm. Moreover, the energy-dispersive X-ray spectroscopy (EDS) mapping images demonstrate the uniform distribution of C, O, N, and Zn components in the dodecahedral skeleton (Fig. 1c). In addition, after covalent post-functionalization, IL-ZIF-90 maintains the dodecahedral morphology and the same size as ZIF-90 (Fig. S3 (ESI†) and Fig. 1d, e, ESI†). Whereas, the EDS mapping images (Fig. 1f) show the emergence and even distribution of iodine element in the structure along with C, Zn, and N, reflecting the immobilization of iodine functionality within ZIF-90. From the XRD patterns shown in Fig. 1g, it is evident that all the peaks of the synthesized ZIF-90 are entirely consistent with those of the simulated ZIF-90, demonstrating the successful formation of the ZIF-90 crystal structure. Then, the functional groups of ZIF-90 and IL-ZIF-90 were revealed by Fourier transform infrared (FTIR) spectroscopy (Fig. 1h). The characteristic peak of ZIF-90 at 1456, 1416, 1362, 1165, 957, and 767 cm−1 are reasonably attributed to imidazole moieties, and the band at 1682 cm−1 can be ascribed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of aldehyde groups. Upon covalent post-functionalization, a new peak appears at 1624 cm−1 corresponding to the C
O stretching vibration of aldehyde groups. Upon covalent post-functionalization, a new peak appears at 1624 cm−1 corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of imine, indicating that ionic functional groups are partially immobilized on ZIF-90.25 To further analyze the elemental composition and chemical states, X-ray photoelectron spectroscopy (XPS) spectroscopy was performed. As shown in Fig. 1i, the high-resolution N 1s spectrum of ZIF-90 can be deconvoluted into two peaks, corresponding to pyridinic N at ∼398.7 eV and pyrrolic N at ∼399.1 eV. However, an additional peak can be observed at ∼402.5 eV for IL-ZIF-90 originating from the C
N bond of imine, indicating that ionic functional groups are partially immobilized on ZIF-90.25 To further analyze the elemental composition and chemical states, X-ray photoelectron spectroscopy (XPS) spectroscopy was performed. As shown in Fig. 1i, the high-resolution N 1s spectrum of ZIF-90 can be deconvoluted into two peaks, corresponding to pyridinic N at ∼398.7 eV and pyrrolic N at ∼399.1 eV. However, an additional peak can be observed at ∼402.5 eV for IL-ZIF-90 originating from the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of imine, and the intensity of pyridinic N becomes stronger due to the introduction of AmPyI.26 Furthermore, after covalent post-functionalization, the high-resolution spectrum of I 3d in IL-ZIF-90 exhibits two strong peaks at ∼619.0 and ∼630.5 eV, which can be ascribed to I− (Fig. S4, ESI†).27 To sum up, AmPyI interacts with the aldehyde groups in ZIF-90 to generate imine bonds, resulting in the formation of IL-ZIF-90. Moreover, the thermal stability of ZIF-90 and IL-ZIF-90 was determined by thermogravimetric analysis (TGA). The TGA profiles shown in Fig. S5 (ESI†) suggest that both ZIF-90 and IL-ZIF-90 undergo multiple steps in the weight loss process: the initial step occurs in the temperature range of 50–330 °C, corresponding to the elimination of guest molecules from the cavities or unreacted compounds trapped within pores or the framework; the subsequent step from 330–700 °C could probably be owing to the deformation of the framework.28 The above results indicate that ZIF-90 and IL-ZIF-90 possess excellent thermal stability.
N bond of imine, and the intensity of pyridinic N becomes stronger due to the introduction of AmPyI.26 Furthermore, after covalent post-functionalization, the high-resolution spectrum of I 3d in IL-ZIF-90 exhibits two strong peaks at ∼619.0 and ∼630.5 eV, which can be ascribed to I− (Fig. S4, ESI†).27 To sum up, AmPyI interacts with the aldehyde groups in ZIF-90 to generate imine bonds, resulting in the formation of IL-ZIF-90. Moreover, the thermal stability of ZIF-90 and IL-ZIF-90 was determined by thermogravimetric analysis (TGA). The TGA profiles shown in Fig. S5 (ESI†) suggest that both ZIF-90 and IL-ZIF-90 undergo multiple steps in the weight loss process: the initial step occurs in the temperature range of 50–330 °C, corresponding to the elimination of guest molecules from the cavities or unreacted compounds trapped within pores or the framework; the subsequent step from 330–700 °C could probably be owing to the deformation of the framework.28 The above results indicate that ZIF-90 and IL-ZIF-90 possess excellent thermal stability.
After the iodine soak treatment, the FESEM and TEM images in Fig. S6a–c and S7a–c (ESI†) show that the rhombic dodecahedral structures of ZIF-90-I− and IL-ZIF-90-I− persist with no visible iodine species observed. The corresponding EDS elemental mapping images of ZIF-90-I− show a uniform distribution of iodine (Fig. S6d, ESI†). Meanwhile, the high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image and corresponding elemental mapping images of IL-ZIF-90-I− (Fig. S7d and e, ESI†) show an even distribution of C, O, N, Zn, and I elements. Moreover, the crystal structures of ZIF-90-I− and IL-ZIF-90-I− remain unchanged after iodine adsorption, as shown in Fig. S8 (ESI†). Based on the above results, the ZIF-90 and IL-ZIF-90 present similar morphology and crystalline structure stability before/after the introduction of iodine. In addition, the FTIR spectra of ZIF-90-I− display the same characteristic peaks as ZIF-90, while the relative intensity of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond stretching vibration becomes weaker with the addition of adequate iodide ions to the structure (Fig. S9, ESI†). The N 1s spectra in Fig. S10 (ESI†) reveal that ZIF-90-I− and IL-ZIF-90-I− show similar peaks compared with ZIF-90 and IL-ZIF-90, respectively. Moreover, the high-resolution I 3d spectrum of ZIF-90-I− and IL-ZIF-90-I− can also be deconvoluted into two peaks, with the two strongest peaks at ∼619.0 and ∼630.5 eV corresponding to I− derived from soaking ZnI2 (Fig. S4, ESI†).
N bond stretching vibration becomes weaker with the addition of adequate iodide ions to the structure (Fig. S9, ESI†). The N 1s spectra in Fig. S10 (ESI†) reveal that ZIF-90-I− and IL-ZIF-90-I− show similar peaks compared with ZIF-90 and IL-ZIF-90, respectively. Moreover, the high-resolution I 3d spectrum of ZIF-90-I− and IL-ZIF-90-I− can also be deconvoluted into two peaks, with the two strongest peaks at ∼619.0 and ∼630.5 eV corresponding to I− derived from soaking ZnI2 (Fig. S4, ESI†).
N2 adsorption/desorption measurement was performed to investigate the porous characteristic of ZIF-90 and IL-ZIF-90 before and after adsorbing iodine (Fig. S11, ESI†). Both characterization studies reveal typical type-I isotherm characteristics, which imply the presence of micropores in carbon, facilitating the adsorption of iodine and the diffusion of Zn2+. Furthermore, the Brunauer–Emmett–Teller (BET) surface area and pore volume of ZIF-90 were measured to be 1275.99 m2 g−1 and 0.56 cm3 g−1, respectively, which are higher than 70.27 m2 g−1 and 0.11 cm3 g−1 of IL-ZIF-90. The significantly reduced surface area and pore volume of IL-ZIF-90 with the introduction of AmPyI indicate the presence of guest materials attached to ZIF-90. The pore size distributions of ZIF-90 and IL-ZIF-90 are mainly distributed between 2 and 4 nm, suggesting the existence of mesopores (inset in Fig. S11, ESI†). Moreover, after adsorbing iodine species, the BET surface areas of ZIF-90-I− and IL-ZIF-90-I− are calculated to be around 875.04 and 19.99 m2 g−1, respectively. The lower BET surface areas demonstrate that iodine was filled in the cavities of the ZIF, instead of accumulating on the surface.
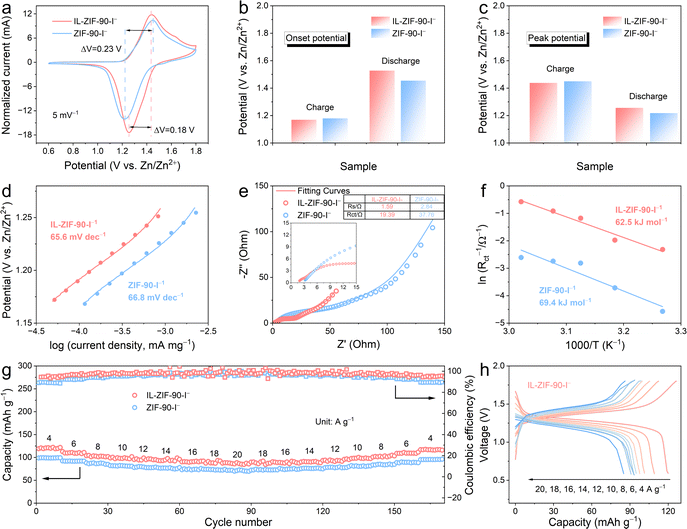
To investigate the redox activity of the IL-ZIF-90-I− cathode, a coin-type battery was assembled with a zinc metal sheet as the anode and 1 M ZnSO4 solution as the electrolyte. Fig. 2a shows the cyclic voltammetry (CV) profiles of the two electrodes at 5 mV s−1 extending from 0.6 to 1.8 V, with the ordinate current normalized for comparability purposes. Both ZIF-90-I− and IL-ZIF-90-I− cathodes exhibit one pair of reversible redox peaks at around 1.43/1.25 V (vs. Zn/Zn2+), reflecting the redox reaction between I2/I−. Compared to ZIF-90-I−, the IL-ZIF-90-I− cathode has a substantially lower polarization voltage, which is evident from the fact that it possesses a lower oxidation peak potential during the cathodic scan but a higher reduction peak potential in the subsequent anodic scan. Additionally, the larger enclosed area and more pronounced peaks of the IL-ZIF-90-I− cathode in the CV curve suggest higher capacity and quicker reaction kinetics. Moreover, the CV curves of ZIF-90 and IL-ZIF-90 cathodes were evaluated, as shown in Fig. S12 (ESI†). The pristine ZIF-90 cathode shows a (quasi)-rectangular shape without redox peaks, while the IL-ZIF-90 cathode displays a pair of peaks at around 1.55/1.26 V (vs. Zn/Zn2+), corresponding to the reaction between I2/I−. Compared to the IL-ZIF-90-I− cathode, the smaller enclosed area, and weaker peaks demonstrate that the initial iodine in the IL-ZIF-90 from AmPyI possesses a poorer capacity and slower reaction kinetics.
At the same time, we monitored the electrochemical potential to calculate the potential difference. Fig. 2b exhibits the onset potentials of two electrodes, derived from the CV curves. The IL-ZIF-90-I− cathode possesses a lower starting charging potential (1.17 V vs. Zn/Zn2+, the following voltages are all relative to the Zn/Zn2+) than the ZIF-90-I− cathode (1.18 V). Meanwhile, the discharge onset potential of the IL-ZIF-90-I− cathode is 1.53 V, which is higher than that of the ZIF-90-I− cathode (1.45 V). Furthermore, the IL-ZIF-90-I− cathode illustrates low charging and high discharging potential peaks at 1.44 and 1.26 V, compared to 1.45 and 1.22 V, respectively (Fig. 2c). Accordingly, the smaller potential difference for the IL-ZIF-90-I− cathode (0.18 V) than that of the ZIF-90-I− cathode (0.23 V) indicates lower polarization (Fig. 2a). Moreover, the IL-ZIF-90-I− cathode exhibits a lower Tafel slope of 65.6 mV dec−1 in comparison to that of the ZIF-90-I− cathode (66.8 mV dec−1), as derived from the CV curves (Fig. 2d). The above results suggest a remarkable enhancement of the redox kinetics of the I2/I− couple through the Lewis acid–base interaction between Nle and elemental I2, where the former serves as a Lewis base and the latter acts as a Lewis acid.29 There will be further evidence to prove this later.
To further understand the redox kinetics of the Zn–I2 battery assembled with the IL-ZIF-90-I− cathode, electrochemical impedance spectroscopy (EIS) of IL-ZIF-90-I− was conducted and compared with ZIF-90-I− (Fig. 2e). The Nyquist plot shows a nearly linear increase in the low-frequency region, which is controlled by zinc-ion diffusion. The IL-ZIF-90-I− cathode reveals fast kinetics, with slightly changed electrode resistance (Rs: ∼1.59 Ω for IL-ZIF-90-I− and ∼2.84 Ω for ZIF-90-I−) and charge transfer resistance (Rct: ∼19.39 Ω for IL-ZIF-90-I− and ∼37.76 Ω for ZIF-90-I−). The smaller Rct value indicates a smaller interfacial resistance of IL-ZIF-90-I−, and the ions in the battery with the IL-ZIF-90-I− cathode can transfer rapidly, contributing to its superior kinetics. Additionally, the activation energy (Ea) can be estimated on the basis of charge-transfer resistances as temperature changes according to the Arrhenius eqn (1):30
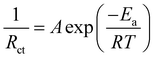 | (1) |
| i = avb | (2) |
Benefiting from the above results, a positive result of the enhanced electrochemical kinetics in IL-ZIF-90-I− is a significant improvement in rate performance. The rate capability of ZIF-90-I− and IL-ZIF-90-I− cathodes was evaluated through the galvanostatic charge/discharge (GCD) tests as shown in Fig. 2g. Impressively, the IL-ZIF-90-I− cathode delivers reversible capacities of 120.3, 110.1, 102.5, 98.8, 95.8, 94.5, 90.8, 89.1, and 86.8 mA h g−1 at 4, 6, 8, 10,12, 14, 16, 18, and 20 A g−1, respectively. When the current density reverts to 4 A g−1, IL-ZIF-90-I− restores a capacity of 116.8 mA h g−1. These results are significantly improved compared to ZIF-90-I−, attributed to the superior IRR kinetics and thermodynamically favorable conversion between I− and I2 in the IL-ZIF-90-I− framework. The corresponding GCD curves of the IL-ZIF-90-I− electrode at various rates are displayed in Fig. 2h. With the increasing current density, IL-ZIF-90-I− consistently exhibits a stable charging and discharging platform, compared with the GCD curves of the ZIF-90-I− electrode (Fig. S14, ESI†). The rate performance of pristine ZIF-90 and IL-ZIF-90 were also measured, as shown in Fig. S15 (ESI†). At an initial specific current of 1 A g−1, a gradual decrease in specific capacity was observed in the IL-ZIF-90 electrode. Due to the lower iodine concentration in pristine IL-ZIF-90 and the low capacity contribution of ZIF-90, their capacities can be considered negligible.
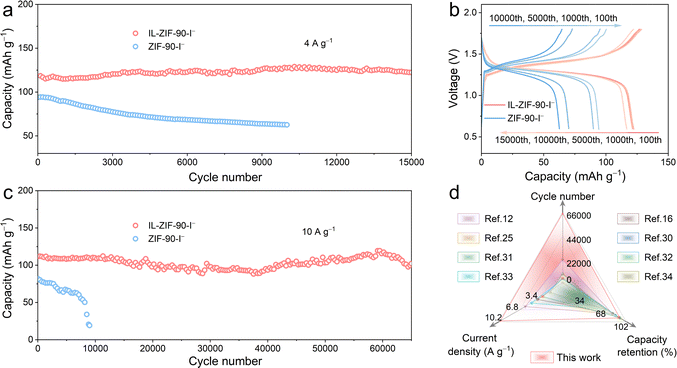
Long-term cycling tests were performed to assess the stability of the two electrodes. The Zn–I2 battery using the IL-ZIF-90-I− cathode displays a superior cycling performance with a high capacity retention of 95.6% and a reversible capacity of 122.4 mA h g−1 after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 4 A g−1, while there is only 66.7% capacity retention after 10
000 cycles at 4 A g−1, while there is only 66.7% capacity retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles for the ZIF-90-I− cathode (Fig. 3a). From the corresponding GCD profiles shown in Fig. 3b, the IL-ZIF-90-I− cathode consistently maintains a low overpotential, significantly lower than that of the ZIF-90-I− cathode. When the current density increases to 10 A g−1, in sharp contrast to the quick battery failure observed for the ZIF-90-I− cathode (merely 23.4% capacity retention after 9000 cycles, Fig. 3c), the Zn–I2 cell with the IL-ZIF-90-I− cathode is ultra-stable with a capacity retention of 91.7%, corresponding to a reversible capacity of 102.2 mA h g−1 after up to 65
000 cycles for the ZIF-90-I− cathode (Fig. 3a). From the corresponding GCD profiles shown in Fig. 3b, the IL-ZIF-90-I− cathode consistently maintains a low overpotential, significantly lower than that of the ZIF-90-I− cathode. When the current density increases to 10 A g−1, in sharp contrast to the quick battery failure observed for the ZIF-90-I− cathode (merely 23.4% capacity retention after 9000 cycles, Fig. 3c), the Zn–I2 cell with the IL-ZIF-90-I− cathode is ultra-stable with a capacity retention of 91.7%, corresponding to a reversible capacity of 102.2 mA h g−1 after up to 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The corresponding GCD profiles are also provided in Fig. S16 (ESI†). For a deeper understanding of this study, various aspects including the cycle number, current density, and capacity retention were further compared with several representative works, as depicted in Fig. 3d (the specific relevant values can be obtained in Table S1, ESI†).13,17,27,32–36 Impressively, the performance of the IL-ZIF-90-I− cathode is exceptional in every aspect, especially in terms of long-term cycling stability. Such superior cyclic performance can be attributed to the firm iodine anchor resulting from the Coulombic interaction between the N+ in IL-ZIF-90-I− and iodine species, preventing the shuttle effect.
000 cycles. The corresponding GCD profiles are also provided in Fig. S16 (ESI†). For a deeper understanding of this study, various aspects including the cycle number, current density, and capacity retention were further compared with several representative works, as depicted in Fig. 3d (the specific relevant values can be obtained in Table S1, ESI†).13,17,27,32–36 Impressively, the performance of the IL-ZIF-90-I− cathode is exceptional in every aspect, especially in terms of long-term cycling stability. Such superior cyclic performance can be attributed to the firm iodine anchor resulting from the Coulombic interaction between the N+ in IL-ZIF-90-I− and iodine species, preventing the shuttle effect.
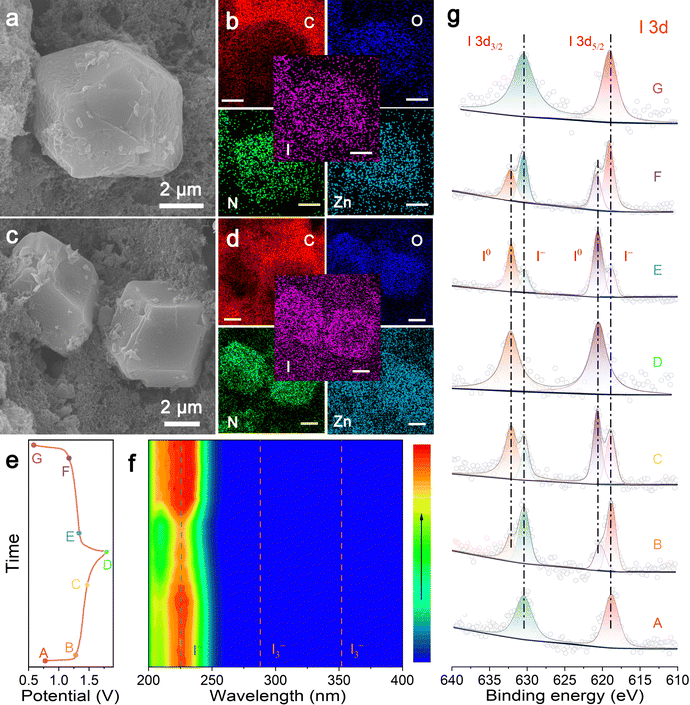
To monitor the microstructure evolution and explore the underlying redox chemistry during cycling, SEM, EDS, in situ ultraviolet-visible (UV-vis) spectroscopy, and XPS were performed. Fig. 4a shows the FESEM image of the fully discharged IL-ZIF-90-I− cathode after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, where the rhombic dodecahedral structure still remains without collapse traces. Furthermore, the corresponding EDS mapping images in Fig. 4b show that element iodine evenly distributes in the structure with C, Zn, and N. When up to 50
000 cycles, where the rhombic dodecahedral structure still remains without collapse traces. Furthermore, the corresponding EDS mapping images in Fig. 4b show that element iodine evenly distributes in the structure with C, Zn, and N. When up to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, the morphology remains intact (Fig. 4c), and the intense iodine signal obtained by EDS mapping matches the SEM image precisely (Fig. 4d). The in situ UV-vis absorption spectra were employed for real-time monitoring of the electrolyte evolution in an open battery utilizing a cuvette as a container. At all voltage states marked on the GCD curve (Fig. 4e), no unexpected signal of I3− ions at 290 and 355 nm is detected in the UV spectra.37 Moreover, one obvious bulge appears at the band of 226 nm corresponding to I− in the electrolyte during discharge to 0.6 V, which fades away during the subsequent charging process (Fig. 4f).30 This phenomenon reflects that the entire redox reaction process involves the direct conversion of I−/I2, which is catalyzed by the Lewis acid–base interaction between Nle and elemental I2, without the sluggish formation of polyiodide products.
000 cycles, the morphology remains intact (Fig. 4c), and the intense iodine signal obtained by EDS mapping matches the SEM image precisely (Fig. 4d). The in situ UV-vis absorption spectra were employed for real-time monitoring of the electrolyte evolution in an open battery utilizing a cuvette as a container. At all voltage states marked on the GCD curve (Fig. 4e), no unexpected signal of I3− ions at 290 and 355 nm is detected in the UV spectra.37 Moreover, one obvious bulge appears at the band of 226 nm corresponding to I− in the electrolyte during discharge to 0.6 V, which fades away during the subsequent charging process (Fig. 4f).30 This phenomenon reflects that the entire redox reaction process involves the direct conversion of I−/I2, which is catalyzed by the Lewis acid–base interaction between Nle and elemental I2, without the sluggish formation of polyiodide products.
Furthermore, XPS was conducted to further investigate the detailed valence state changes of iodine throughout distinct charge–discharge phases (Fig. 4g). Two strong iodine signals around 630 and 620 eV can constantly be produced from the IL-ZIF-90-I− cathode at all stages, corresponding to I 3d3/2 and I 3d5/2, respectively.27 It is worth noting that the whole I 3d spectrum shifts to a higher binding energy during battery charging, implying an increased valence of iodine. Meanwhile, an opposite shift occurs during discharging, indicating the reduction of iodine. Specifically, the main peaks are attributed to I0 at 632.1 and 620.8 eV in a fully charged state, while the fitted peaks energies are 630.3 and 618.9 eV during full discharge, both of which are ascribed to I−.27 In addition, the collective red shift in the binding energy of the nitrogen element indicates an increase in the electron density around N during battery charging to 1.8 V, possibly owing to the interaction with iodine (Fig. S17, ESI†). In this case, I2 acts as a Lewis acid, while Nle functions as a Lewis base, and hence, both exhibit powerful interactions with each other. Moreover, the SEM images with corresponding EDS mapping of IL-ZIF-90-I− cathode were collected at various charge/discharge states as shown in Fig. S18 (ESI†). The IL-ZIF-90-I− cathode maintains a similar morphology during cycling. When charged to 1.2 V, the mapping result shows that iodine species uniformly distribute in the IL-ZIF-90-I− particles (Fig. S18a, ESI†). The generated I2 could also be efficiently retained by IL-ZIF-90-I− when charged to 1.8 V (Fig. S18b, ESI†). Similar iodine species confining in IL-ZIF-90-I− can be observed in the iodine mapping images at the state of discharging to 1.3 V (Fig. S18c, ESI†) and 0.6 V (Fig. S18d, ESI†). The stronger iodine signal on the IL-ZIF-90-I− particles than the surroundings implies the significant capacity of iodine species collected by IL-ZIF-90-I−.
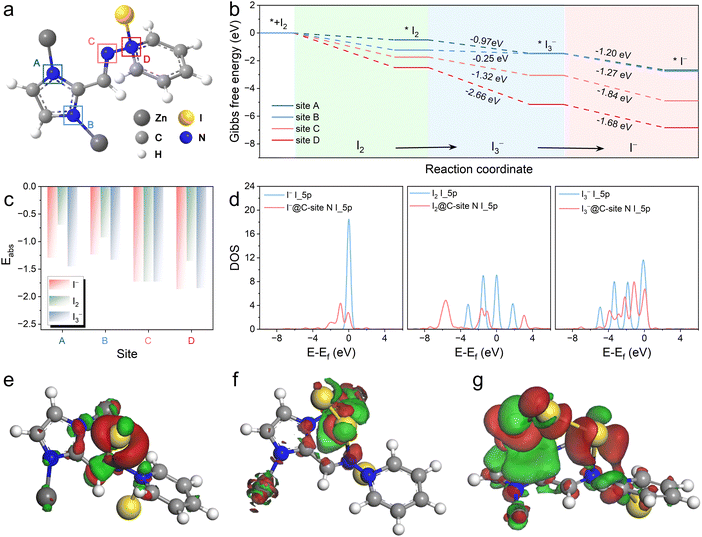
The effective capturing effect and rapid redox kinetics originate from the robust interaction between the nitrogen species of the IL-ZIF-90 host and the iodine species. To reveal the underlying mechanism, density functional theory (DFT) was applied to explore the adsorption behavior and induced electronic structure change of the various N sites on the IL-ZIF-90 cathode. Fig. 5a shows the structure formula of IL-ZIF-90 and four possible adsorption sites, where A, B, C, and D are set directly above pyridinic N, pyrrolic N, N with lone pair of electrons, and positively charged N (pyridinic N), respectively. The Gibbs free energies of I2 reduction pathways in the four N sites of IL-ZIF-90 were calculated as depicted in Fig. 5b. Generally, a low ΔG value indicates a high degree of spontaneity and a rapid response rate. The calculated negative ΔG values of the four N sites suggest that the reduction step of I2 to I− is exothermic and spontaneous. The basic IRR process comprises a reduction reaction from I2 molecules to the I3− intermediate product to the I− ion final product, where the latter phase is the rate-determining step, corresponding to the highest ΔG value. Moreover, the lower ΔG values of site C (−1.68 eV) and site D (−1.84 eV) for this step demonstrate the favorable polyiodide conversion compared with site A (−1.20 eV) and site B (−1.27 eV), consistent with the above experimental results. As summarized in Fig. 5c, for all iodine species including the original active ingredient I− and the reaction products I3−/I2, the four N sites in the IL-ZIF-90 show negative adsorption energies, indicating the spontaneous adsorption behavior. More precisely, site-C N and site-D N hold the lower adsorption energies for all the above iodine species (site C: −1.73 eV for I−, I2, and I3−; site D: −1.87 eV for I−, −1.35 eV for I2, and −1.85 eV for I3−) compared to site-A N and site-B N, which suggests that these two N sites control the immobilization and confinement of iodine species (Fig. S19–S22, ESI†). Also, the DFT calculation results of Ead reveal that site-D N has a stronger Coulombic force than other iodine-based compounds like PVPI.38
Then, the density of states (DOS) near the Femi energy (Ef) around I atoms was further explored. The electronic states of all iodine species are concentrated at different energy eigenvalues. Compared with the isolated iodine species, the partial DOS (PDOS) of iodine species adsorbed on the C/D sites N shows a wider energy band distribution and shifts toward lower energy levels (Fig. 5d and Fig. S23, ESI†). Additionally, we calculated the charge density difference between C/D sites N and iodine species and visualized the results with a colored isosurface (Fig. 5e, f and Fig. S24, ESI†), where the green color (negative values) represents the electron depletion and red color (positive values) stands for electron accumulation. Significant charge transfer can be detected, inducing a strong interaction between the C/D sites N in the IL-ZIF-90 and iodine species, efficiently preventing the active substance from leaking.
Conclusion
In summary, we developed an efficient catalytic iodine host with iodine covalent post-functionalization to IL-ZIF-90 as cathodes for rechargeable Zn–I2 batteries. With this design, the ordered porous structure of the IL-ZIF-90 framework can accommodate a large amount of iodine. Significantly, the nitrogen species in IL-ZIF-90 played important roles, where the Coulombic interaction between the positively charged nitrogen and the negatively charged iodine effectively reduced the leakage of iodine, and the Lewis acid–base interaction between the Nle and elemental iodine enhanced the redox kinetics. Benefiting from the fast redox kinetics of iodine, superior rate capability with a specific capacity of 86.8 mA h g−1 at 20 A g−1 was realized. Moreover, a record cycle lifespan of 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles with 91.7% capacity retention was achieved. The underlying iodine redox mechanism was revealed by in situ UV-vis and ex situ XPS characterization studies. Moreover, the DFT calculations confirmed the high degree of spontaneity and the fast reaction rate of the IRR in IL-ZIF-90-I−. The proposed strategy for the synergistic adsorption of iodine by nitrogen is anticipated to enlighten various advanced metal–iodine batteries.
000 cycles with 91.7% capacity retention was achieved. The underlying iodine redox mechanism was revealed by in situ UV-vis and ex situ XPS characterization studies. Moreover, the DFT calculations confirmed the high degree of spontaneity and the fast reaction rate of the IRR in IL-ZIF-90-I−. The proposed strategy for the synergistic adsorption of iodine by nitrogen is anticipated to enlighten various advanced metal–iodine batteries.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Stable Support Plan Program for Higher Education Institutions (no. 20220815094504001) and the Shenzhen Key Laboratory of Advanced Energy Storage (202204013000060). The authors would also like to acknowledge the TEM resources of the Pico Center at SUSTech Core Research Facilities, which was supported by the Presidential Fund and the Development and Reform Commission of Shenzhen Municipality.References
- C. Li, Q. Li, Z. Wu, Y. Wang, R. Zhang, H. Cui, Y. Hou, J. Liu, Z. Huang and C. Zhi, Adv. Mater., 2023, 2304878 CrossRef PubMed.
- L. Geng, J. Meng, X. Wang, C. Han, K. Han, Z. Xiao, M. Huang, P. Xu, L. Zhang, L. Zhou and L. Mai, Angew. Chem., Int. Ed., 2022, 61, e202206717 CrossRef CAS PubMed.
- B. Tang, L. Shan, S. Liang and J. Zhou, Energy Environ. Sci., 2019, 12, 3288–3304 RSC.
- J. Zheng, Q. Zhao, T. Tang, J. Yin, C. D. Quilty, G. D. Renderos, X. Liu, Y. Deng, L. Wang, D. C. Bock, C. Jaye, D. Zhang, E. S. Takeuchi, K. J. Takeuchi, A. C. Marschilok and L. A. Archer, Science, 2019, 366, 645–648 CrossRef CAS.
- Z. Hou, T. Zhang, X. Liu, Z. Xu, J. Liu, W. Zhou, Y. Qian, H. J. Fan, D. Chao and D. Zhao, Sci. Adv., 8, eabp8960 CrossRef CAS PubMed.
- K. K. Turekian and K. H. Wedepohl, Geol. Soc. Am. Bull., 1961, 72, 175–192 CrossRef CAS.
- A. Konarov, N. Voronina, J. H. Jo, Z. Bakenov, Y.-K. Sun and S.-T. Myung, ACS Energy Lett., 2018, 3, 2620–2640 CrossRef CAS.
- J. Ma, M. Liu, Y. He and J. Zhang, Angew. Chem., Int. Ed., 2021, 60, 12636–12647 CrossRef CAS PubMed.
- N. Zhang, X. Chen, M. Yu, Z. Niu, F. Cheng and J. Chen, Chem. Soc. Rev., 2020, 49, 4203–4219 RSC.
- L. Ma, M. A. Schroeder, O. Borodin, T. P. Pollard, M. S. Ding, C. Wang and K. Xu, Nat. Energy, 2020, 5, 743–749 CrossRef CAS.
- Y. Mu, Z. Li, B.-K. Wu, H. Huang, F. Wu, Y. Chu, L. Zou, M. Yang, J. He, L. Ye, M. Han, T. Zhao and L. Zeng, Nat. Commun., 2023, 14, 4205 CrossRef CAS PubMed.
- W. Deng, Z. Li, Y. Ye, Z. Zhou, Y. Li, M. Zhang, X. Yuan, J. Hu, W. Zhao, Z. Huang, C. Li, H. Chen, J. Zheng and R. Li, Adv. Energy Mater., 2021, 11, 2003639 CrossRef CAS.
- L. Zhang, J. Huang, H. Guo, L. Ge, Z. Tian, M. Zhang, J. Wang, G. He, T. Liu, J. Hofkens, D. J. L. Brett and F. Lai, Adv. Energy Mater., 2023, 13, 2203790 CrossRef CAS.
- X. Li, M. Li, Z. Huang, G. Liang, Z. Chen, Q. Yang, Q. Huang and C. Zhi, Energy Environ. Sci., 2021, 14, 407–413 RSC.
- Y. Yang, S. Liang, B. Lu and J. Zhou, Energy Environ. Sci., 2022, 15, 1192–1200 RSC.
- L. Zhang, M. Zhang, H. Guo, Z. Tian, L. Ge, G. He, J. Huang, J. Wang, T. Liu, I. P. Parkin and F. Lai, Adv. Sci., 2022, 9, 2105598 CrossRef CAS PubMed.
- L. Ma, Y. Ying, S. Chen, Z. Huang, X. Li, H. Huang and C. Zhi, Angew. Chem., Int. Ed., 2021, 60, 3791–3798 CrossRef CAS PubMed.
- S.-J. Zhang, J. Hao, H. Li, P.-F. Zhang, Z.-W. Yin, Y.-Y. Li, B. Zhang, Z. Lin and S.-Z. Qiao, Adv. Mater., 2022, 34, 2201716 CrossRef CAS PubMed.
- H. Pan, B. Li, D. Mei, Z. Nie, Y. Shao, G. Li, X. S. Li, K. S. Han, K. T. Mueller, V. Sprenkle and J. Liu, ACS Energy Lett., 2017, 2, 2674–2680 CrossRef CAS.
- C. Bai, F. Cai, L. Wang, S. Guo, X. Liu and Z. Yuan, Nano Res., 2018, 11, 3548–3554 CrossRef CAS.
- X. Li, T. Gao, F. Han, Z. Ma, X. Fan, S. Hou, N. Eidson, W. Li and C. Wang, Adv. Energy Mater., 2018, 8, 1701728 CrossRef.
- J. J. Hong, L. Zhu, C. Chen, L. Tang, H. Jiang, B. Jin, T. C. Gallagher, Q. Guo, C. Fang and X. Ji, Angew. Chem., Int. Ed., 2019, 58, 15910–15915 CrossRef CAS.
- A. Chen, C. Zhao, J. Gao, Z. Guo, X. Lu, J. Zhang, Z. Liu, M. Wang, N. Liu, L. Fan, Y. Zhang and N. Zhang, Energy Environ. Sci., 2023, 16, 275–284 RSC.
- X. Sun, Y. Qiu, B. Jiang, Z. Chen, C. Zhao, H. Zhou, L. Yang, L. Fan, Y. Zhang and N. Zhang, Nat. Commun., 2023, 14, 291 CrossRef CAS PubMed.
- H. Peng, W. Dong, Q. Chen, H. Song, H. Sun, R. Li, Y. Chang and H. Luo, Appl. Biochem. Biotechnol., 2022, 194, 3527–3540 CrossRef CAS PubMed.
- H. Yuan, Y. Wu, X. Pan, L. Gao and G. Xiao, Catal. Lett., 2020, 150, 3561–3571 CrossRef CAS.
- X. Li, N. Li, Z. Huang, Z. Chen, G. Liang, Q. Yang, M. Li, Y. Zhao, L. Ma, B. Dong, Q. Huang, J. Fan and C. Zhi, Adv. Mater., 2021, 33, 2006897 CrossRef CAS PubMed.
- W. Morris, C. J. Doonan, H. Furukawa, R. Banerjee and O. M. Yaghi, J. Am. Chem. Soc., 2008, 130, 12626–12627 CrossRef CAS PubMed.
- A. Tesfay Reda, D. Zhang, X. Xu and S. Xu, Sep. Purif. Technol., 2022, 292, 120994 CrossRef CAS.
- M. Liu, Q. Chen, X. Cao, D. Tan, J. Ma and J. Zhang, J. Am. Chem. Soc., 2022, 144, 21683–21691 CrossRef CAS PubMed.
- C. Han, H. Li, Y. Li, J. Zhu and C. Zhi, Nat. Commun., 2021, 12, 2400 CrossRef CAS PubMed.
- H. Yang, Y. Qiao, Z. Chang, H. Deng, P. He and H. Zhou, Adv. Mater., 2020, 32, 2004240 CrossRef CAS PubMed.
- Y. Tan, Z. Chen, Z. Tao, A. Wang, S. Lai and Y. Yang, Angew. Chem., Int. Ed., 2023, 62, e202217744 CrossRef CAS PubMed.
- H. Peng, Y. Fang, J. Wang, P. Ruan, Y. Tang, B. Lu, X. Cao, S. Liang and J. Zhou, Matter, 2022, 5, 4363–4378 CrossRef CAS.
- X. Li, S. Wang, T. Wang, Z. Duan, Z. Huang, G. Liang, J. Fan, C. Yang, A. L. Rogach and C. Zhi, Nano Energy, 2022, 98, 107278 CrossRef CAS.
- W. Shang, Q. Li, F. Jiang, B. Huang, J. Song, S. Yun, X. Liu, H. Kimura, J. Liu and L. Kang, Nano-Micro Lett., 2022, 14, 82 CrossRef CAS PubMed.
- Y. Yang, S. Guo, Y. Pan, B. Lu, S. Liang and J. Zhou, Energy Environ. Sci., 2023, 16, 2358–2367 RSC.
- Z. Meng, H. Tian, S. Zhang, X. Yan, H. Ying, W. He, C. Liang, W. Zhang, X. Hou and W.-Q. Han, ACS Appl. Mater. Interfaces, 2018, 10, 17933–17941 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ee03297c |
| ‡ The authors contributed to the work equally. |
| This journal is © The Royal Society of Chemistry 2024 |