Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Porphyrin-containing materials for photodegradation of organic pollutants in wastewaters: a review
Sara R. D.
Gamelas
 a,
João P. C.
Tomé
a,
João P. C.
Tomé
 *b,
Augusto C.
Tomé
*b,
Augusto C.
Tomé
 *a and
Leandro M. O.
Lourenço
*a and
Leandro M. O.
Lourenço
 *a
*a
aLAQV–REQUIMTE, Department of Chemistry, University of Aveiro, 3810-193 Aveiro, Portugal. E-mail: actome@ua.pt; leandrolourenco@ua.pt
bCentro de Química Estrutural, Institute of Molecular Sciences & Departamento de Engenharia Química, Instituto Superior Técnico, Universidade de Lisboa, 1049-001 Lisboa, Portugal. E-mail: jtome@tecnico.ulisboa.pt
First published on 28th February 2024
Abstract
Industrialization and town urbanization have led to an exponential need for clean water and new wastewater treatment strategies. Currently, photocatalysis is the most appealing method to destroy organic pollutants in water, and the use of sunlight, with the assistance of a solid photocatalyst, is considered one of the most sustainable approaches. The use of heterogeneous photocatalysts has unique advantages, namely their recovery and reuse for several cycles without loss of activity. Due to their remarkable optical and photophysical properties, porphyrin (Por) dyes can be used in homogenous and heterogeneous photocatalysis. Different supports can be used to immobilize Pors, allowing the final material to extend its absorption into the white light region since most of the supports only absorb UV light. This review focuses on the photocatalytic performance of non-immobilized and immobilized porphyrins in the photodegradation of organic pollutants for wastewater treatment application.
1. Introduction
Water is the most important natural resource on earth and must be preserved. However, it has been contaminated because of anthropogenic activities. Industrialization and town urbanization have led to enormous problems regarding effluent gathering and treatment. In many countries, most industries discard their wastewater directly into aquatic environments without previous suitable treatment. In India, for instance, most of the industrial effluents are coloured due to the untreated wastewaters from the painting and textile industries. This leads to loss of water quality, which can be hazardous to aquatic life and human health. Because most synthetic dyes are slowly degraded in the environment, it is necessary to remove or degrade those contaminants as much as possible before the effluents are discharged into the rivers to solve or mitigate this problem.1–4Researchers have developed sustainable wastewater treatment strategies in the past few decades.5–10 When developing a new approach for water treatment, its safety and sustainability must be considered. In this context, photocatalysis arises as a green alternative to currently used methods (rapid sand filtration, upflow roughing filters, and slow sand filtration, among others) that show several disadvantages such as high maintenance and expensive costs.11
In the case of photocatalysis, it only requires a photocatalyst and light to produce reactive oxygen species (ROS) that will degrade the pollutant. The use of sunlight, with the assistance of a solid photocatalyst (which may be recycled and reused),12,13 is a valuable and green approach to destroy organic contaminants. During the photocatalytic process with semiconductors, the adsorption process plays an important role since it is necessary for the dye first to be adsorbed into the photocatalyst's surface. This process is based on a surface phenomenon using a solution which has an adsorbable solute (like dyes) that comes in contact with the adsorbent with a highly porous surface.14 Adsorption was already used as an effective separation technique due to its high efficiency in removing dyes. In fact, activated carbon was already used to remove phenols, pesticides, and dyes.15
The efficiency of a photocatalytic process depends on the nature of the photocatalyst as well as the source of light. When irradiated, the catalyst is photo-activated by photons with the same or higher energy than its band gap energy. Typically, higher irradiation intensity leads to higher photocatalytic efficiency.16 Knowing that sunlight is a safe and renewable energy, its use in photodegradation studies offers many advantages, such as cleanness and abundance. However, it requires the development of catalysts that can be activated by sunlight.17
Titanium dioxide has become the most widely used photocatalyst in the past few years due to its high stability, cheapness, non-toxicity, and high efficiency. Furthermore, TiO2 can absorb near-UV light (315–400 nm) and form electron (e−)–hole (h+) pairs which can directly oxidize or reduce substrates. Nonetheless, there are several drawbacks in using bare TiO2 in photocatalysis which include a relatively large band-gap and its wavelength light absorption being restricted to UV light.18,19 Therefore, to improve the use of sunlight, it is necessary to modify TiO2 to extend its wavelength absorption to white light. This modification includes non-/transition-metal ion doping20–22 and dye sensitization.23 Besides TiO2, it is also common to find zinc oxide,24 silicon dioxide,23 polymers25 and g-C3N4 (ref. 26) that can also be modified to increase their absorption spectra to white light.27,28 Among many dye sensitizers, porphyrins (Pors)29,30 have received particular attention due to their valuable features in water treatment. In fact, due to their strong absorption in the visible region and photoelectrical properties, Pors have been extensively used as catalysts for the photooxidation of organic pollutants.23
Pors are aromatic tetrapyrrolic macrocycles with interesting absorption spectra,31 typically displaying a strong absorption band around 400 nm (the Soret band) and other bands with lower intensity in the 500–650 nm region (the Q bands).32,33 Por derivatives can undergo several reaction types, including inner core complexation with metal ions such as Zn(II), Cu(II), Co(II), Sn(III), and Fe(II), among others.34–36 The presence or absence of a metal ion in the macrocycle core can significantly modify its reactivity and photocatalytic performance.37,38 Due to their biological, photophysical, and photochemical properties, tetrapyrrolic macrocycles have also been applied in areas such as sensors,39–41 artificial oxygen transporters,42 biomimetic models,43 semiconductors44 and medicine.45–48 However, considering the water pollution problems, (photo)catalysis is one of the most relevant areas where these dyes can be used.49,50 Moreover, Pors combined with (or supported on) inorganic materials, such as TiO2, ZnO, SiO2, mixtures of two inorganic supports (e.g., TiO2–SiO2, etc.) or nanoparticles, among others, act as photocatalytic materials for wastewater treatment that give rise to higher degradation rates, allow their facile recovery and their reusability without significant loss of activity.51
This review presents several successful examples regarding the photocatalytic degradation of water pollutants using non-immobilized and immobilized porphyrins.
2. Mechanism of the photocatalytic degradation of organic pollutants
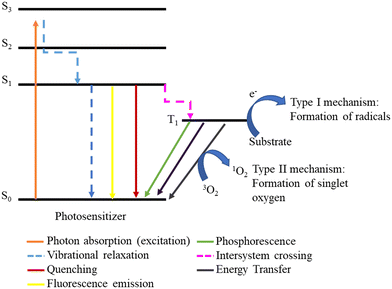
The degradation of pollutants may occur by indirect photolysis in the presence of immobilized or non-immobilized porphyrins that can absorb irradiation and subsequently generate ROS such as singlet oxygen (1O2), hydroxyl (HO·), peroxyl (ROO·), and superoxide anion (O2˙−) radicals.23,52 This methodology requires light, a photosensitizer (PS), and molecular oxygen (3O2). As depicted in Fig. 1, a PS is excited from the ground state (S0) to an excited singlet state (S1 or S3) after irradiation. From this point, the excited molecule can return to the ground state by different processes, including emission (fluorescence) and vibration (heat) or by reacting with substrates. However, an excited triplet state (T1) with a much longer lifetime can be formed if the decay can go through an intersystem crossing. From this point, the PS can perform photoreactions by two competitive mechanisms: type I mechanism, an electron transfer from the PS to the substrate occurs, leading to the formation of reactive radicals, while in the type II mechanism there is an energy transfer from the PS's triplet state to molecular oxygen, generating 1O2.53Some Pors have high quantum yields for triplet state formation, determining their increased ability to generate ROS.54 This quantum yield can be increased by the insertion of some metal ions in their inner core.55
3. Photocatalytic degradation of organic pollutants in water with non-immobilized porphyrins
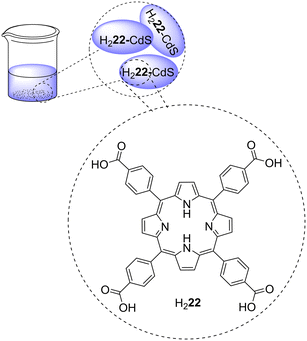
Non-immobilized porphyrins are frequently evaluated as photocatalysts, namely in preliminary assays aiming to study the adequate experimental conditions for the photodegradation of pollutants before their immobilization in organic or inorganic matrixes. In cases where the applied porphyrins are water-insoluble, their recovery and reuse do not necessarily require immobilization in any support. A brief discussion concerning the photodegradation of common organic water pollutants (e.g., dyes, nitroaromatics, phenols, medicines) by non-immobilized porphyrins is presented in this section.Natural nitroaromatic compounds are rare, but they are frequently used as dyes, pesticides, or explosives, for instance. Their use has become so intensive that it led to the contamination of soil and groundwater.56 To find efficient ways to degrade 2,4,6-trinitrotoluene (2,4,6-TNT), Hikal and Harmon57 developed a crystalline structure (Fig. 2) by self-assembly of porphyrins Zn1 and H22, which bear sulfonato and pyridinium groups, respectively. Crystals of Zn1–H22 were characterized by optical microscopy to determine that, depending on the molar ratio of the porphyrins in the starting solution, they may reach a length between 30–55 μm and width of 2–50 μm. Moreover, it was observed that the UV–vis spectrum of the crystalline structure and the spectra of the two monomers are quite different (broadened and blue and/or red-shifted Soret and Q bands). It is essential to highlight that the formation of the crystals led to a dramatic reduction of the intensity of the emission bands accompanied by a red-shift and split of the bands. These materials were able to reduce 80% of 2,4,6-TNT under a tungsten lamp for 3 h, with the photocatalyst being highly photostable under the experimental conditions (Table 1). The authors studied the degradation products and identified trinitrobenzene.
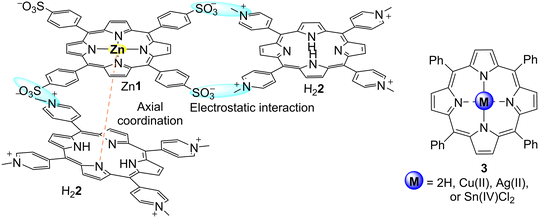 | ||
| Fig. 2 Representation of the interactions between Zn1 and H22 in the solid-state; and structures free-base and metal M3. | ||
Methyl orange (MO) is a water-soluble dye used as a pH indicator in textiles, foodstuffs, farming chemical products, paper, leather industries, etc. However, its release into the environment, even in low quantities, causes serious pollution problems. In this sense, Salker and co-worker58 used meso-tetraphenylporphyrin (H23) and the corresponding metalloporphyrins M3 (M = Cu(II), Ag(II), or Sn(IV)Cl2, Fig. 2 and Table 1) for the photocatalytic degradation of MO. The fluorescent H23 and Sn3Cl2 were revealed to be more efficient for MO photodegradation than the non-fluorescent Cu3 and Ag3.58 Although only small amounts of porphyrin are needed for these photocatalytic studies, the porphyrins used in this work could be recovered, which is a very relevant point for this type of application.
4. Wastewater treatment using polymeric porphyrins or porphyrins immobilized on polymers
In the past few years, due to their low-cost and facile synthesis along with appropriate mechanical properties and excellent electrical and electrochemical activities, organic polymers have attracted increased attention as materials for solar cells, fuel cells, catalysts, adsorption, light emitting diodes, separation processes, magnetism, and rechargeable lithium batteries.25Both polymeric porphyrins and porphyrins immobilized on polymers have been used as photocatalysts to degrade organic pollutants in water. A few examples are discussed in the following sections.
4.1. Polymeric porphyrins
Rahman and co-workers59 described that Congo red (CR), a stable water-soluble anionic azo dye with a benzidine core (a cancer-causing substance), is hard to degrade. This dye is dangerous for human health and has a high ecotoxicity in the environment. There are several methods for removing this dye from water, however adsorbents seem to be the best due to their high efficiency, simplicity, and cheapness.59,60 Thus, the combination of the adsorption of this pollutant from wastewaters with its photodegradation is a trustworthy way to eliminate it. For that reason, Sun and co-workers61 prepared the polymeric dendrite-like nanostructure Fe4 (Fig. 3) and used it as a photocatalyst to degrade Congo red. According to scanning electron microscopy (SEM), each branch of the dendrite-like nanostructure possesses a diameter size of about 30–80 nm. The chiasma and tangle of the nanostructure formed the final microstructure with a size of about 30 μm. This microporous material showed high selectivity (when compared with MO, methyl violet (MV), methyl red (MR) and rhodamine B (RhB)) and efficiency: 88.3% of the CR dye was decomposed in just 120 s under white light irradiation (Table 2). Despite using N,N-dimethylformamide (DMF) in the photocatalytic studies, this material proved to be a good candidate for azo dye photodegradation.RhB has been used for dyeing cotton, silk, paper, bamboo, and other products and consequently has been found in wastewaters produced by these industries. Cationic RhB dyes are water-soluble and can interact with the negatively charged surface of cell membranes and penetrate cells. For that reason, they are considered more toxic than the anionic ones. There are several methods to eliminate this pollutant,62,63 but Zhao and co-workers64 reported a new one where the porphyrin-based porous organic polymer H25 (Fig. 3) is used as a photocatalyst for the degradation of RhB and also methylene blue (MB). After verifying that MB was unstable under white light irradiation, the authors proceeded with the photocatalytic studies only for RhB. The commercially available photocatalyst TiO2 (PC-500), meso-tetrakis(p-aminophenyl)porphyrin (H26), and polymeric porphyrin H27 (prepared from the reaction of H26 and terephthalaldehyde) were used as controls. The experimental results showed that polymer H25 possesses high photocatalytic activity for RhB: 100% photodegradation after white light irradiation for 80 min (Table 2). The authors proposed that linking porphyrin building blocks in an amorphous way contributes positively to a higher degradation effect, prevents aggregation of the porphyrin molecules (π–π stacking), and ensures the porosity and accessibility of the active sites of the porphyrin molecule. One interesting conclusion of this work was the selectivity of H25 for MB degradation in the presence of a mixture of both dyes (MB/RhB 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
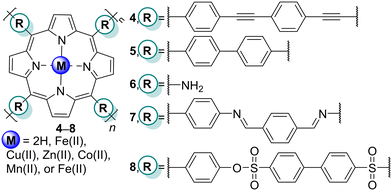
The presence of dyes like acridine orange (AO) in watercourses negatively affects the quality of aquatic and human lives. In this context, Chen and co-workers65 reported the photodegradation of AO in an aqueous solution using polymeric metalloporphyrins M8 (M = Cu, Zn, Co, Mn, or Fe) (Fig. 3 and Table 2). It was observed that Co8 was the best photocatalyst under a high-pressure mercury lamp, and the authors assessed its photocatalytic behaviour under an iodine tungsten lamp, or natural sunlight. Polymer Co8 (Fig. 3) revealed a complete photodegradation of AO under natural sunlight irradiation after 3 h. (Table 2). This work showed that polymeric metalloporphyrins could be successfully employed in wastewater treatment using solar light irradiation.
4.2. Porphyrins immobilized on polymers
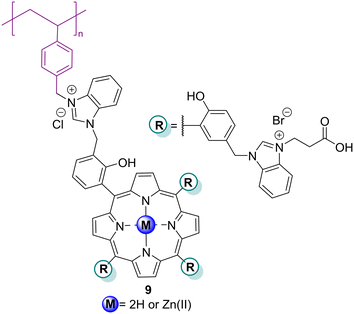
Linking porphyrins to organic polymers can increase their photocatalytic activity and reusability.66–68 Indeed, several organic polymers (e.g., poly(N-isopropylacrylamide), polyacrylonitrile, polystyrene, etc.) incorporating porphyrins, and even self-assembled porphyrin polymers, have been developed for wastewater treatment, namely for the photodegradation of dyes.Bhagat and co-worker69 prepared a polymer-supported Brønsted acid-functionalized Zn9 complex tangled with a benzimidazolium moiety (Fig. 4) and assessed its ability as a photocatalyst for the degradation of MO and CR (Table 3) using atmospheric air or H2O2. After irradiation for 30 min under atmospheric air, a complete degradation of the two dyes was achieved. The catalyst could be reused up to five times without loss of activity.
| Por | Pollutant | Irradiation time (min) | Light source | Light source | Recycle | Ref. |
|---|---|---|---|---|---|---|
| a H2O2. b mg g−1 of Por/material. | ||||||
| Zn9 | MO | 30 | Visible (λ > 400 nm) | 100 | 5 cycles (5% loss) | 69 |
| 25a | ||||||
| CR | 20 | 85 | ||||
| 20a | ||||||
| MR | 30 | 95 | ||||
| 30a | 90 | |||||
| Zn10 | Reactive orange | 250 | 10 | — | 70 | |
| Cu10 | 20 | |||||
| Ni10 | 20 | |||||
| Co10 | 25 | |||||
| Pd10 | 15 | |||||
| Fe10 | 40 | |||||
| Fe11 | 40 | 4 cycles (36% loss) | ||||
| Reactive blue | 180 | UV | 100 | — | ||
| Reactive black | ||||||
| Reactive red | ||||||
| Reactive light yellow | 90 | |||||
| H212 | PCP | 360 | Visible (λ > 400 nm) | 150.31b | 71 | |
| TCP | 30.51b | |||||
| DCP | 46.55b | |||||
| H212 | PCP | 35 | 72 | |||
| TCP | 15 | |||||
| DCP | 0 | |||||
| H213 | PCP | 120 | 100 | |||
| TCP | 360 | |||||
| DCP | 35 | |||||
| H214 | Benzene | — | UV | 95 | 73 | |
| Visible (λ > 400 nm) | ||||||
| Toluene | UV | 90 | ||||
| Visible (λ > 400 nm) | 80 | |||||
| Ethyl benzene | UV | 95 | ||||
| Visible (λ > 400 nm) | 50 | |||||
| Xylene | UV | 95 | ||||
| Visible (λ > 400 nm) | ||||||
| H215 | TCP | 360 | 100 | 74 | ||
| H216 | ||||||
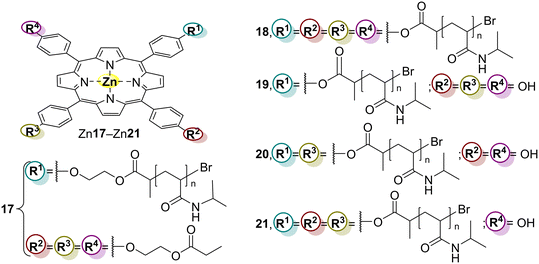
| Zn17 | MB | 180 | 72 | 7 cycles (0% loss) | 75 | |
| Zn18 | 150 | 75 | 7 cycles (30% loss) | 76 | ||
| Zn18 | RhB | 240 | 86 | 5 cycles (5% loss) | 77 | |
| Zn19 | 82 | |||||
| Zn20 | 73 | |||||
| Zn21 | 69 | |||||
 | ||
| Fig. 5 M10 (M = Zn(II), Cu(II), Ni(II), Co(II), Fe(III), and Pd(II)) and Fe11 supported in a polyacrylonitrile or polyacrylonitrile copolymer nanofibre mat. | ||
Lee, Baek, and co-workers71,72 reported the porphyrin star copolymer H212 (Fig. 6) that was able to degrade a series of chlorophenols: 2,4-dichlorophenol (2,4-DCP), 2,4,6-trichlorophenol (2,4,6-TCP), and pentachlorophenol (PCP). In 2013, Lee, Baek, and co-workers73 synthesized the free-base porphyrin star copolymer H214, and in 2018 the same authors74 developed two palladium(II) porphyrins coupled to two different copolymers, H215 and H216, in order to evaluate their photocatalytic activity in the degradation of 2,4,6-TCP in water under white light irradiation. Using low energy and extending the absorption wavelength, H212 could efficiently degrade the chlorophenols in an aqueous solution under white light, reaching removal efficiencies ranging from 30.51 to 150.31 mg g−1 of Por/material (Table 3).71 The same authors72 compared two different types of porphyrin core star copolymers: the same poly(dimethylaminoethyl acrylate)porphyrin H212 and H213, respectively, to efficiently degenerate chlorophenols. Even though they used another block copolymer H213, the degradation was still more efficient when using H212, accomplishing a photodegradation rate of 35% and 100% (Table 3), respectively. This difference can be associated with the higher hydrophobicity of H212 due to the presence of polystyrene polymer in its structure. In this case, the interaction of the hydrophobic contaminants (chlorophenols) with the hydrophobic block (polystyrene) of the material induced a hydrophobic–hydrophobic interaction which could enhance the access to the porphyrin core (photocatalyst), increasing the removal efficiency. The degradation products were identified as hydroquinone and posterior CO2 and H2O.
Baek and co-workers73 firstly reported that the water-soluble amphiphilic star block copolymers H214 could efficiently capture toxic organic compounds such as benzene, toluene, ethylbenzene, and xylene (Table 3) from water due to the hydrophobic–hydrophobic interaction with the inner polystyrene block segments and degrade them by the UV-induced photo-oxidation reaction. They also reported the recovery of the star block copolymers by increasing the temperature above their lower critical solution temperature (LCST) where the thermosensitive H214 forms water insoluble agglomerates.
According to the second study,74 the amphiphilic porphyrin star block copolymers were also homogenously soluble in water. It was also concluded that the water-soluble star block copolymers with hydrophobic core–shell structures, such as H215 and Pd16, were the most effective for the degradation of 2,4,6-TCP in water under white light irradiation when compared with the corresponding homopolymer. The explanation for those results was the effectiveness of the hydrophobic core near the metalloporphyrin in capturing and binding the hydrophobic chlorophenols, contributing to their easier degradation. The authors considered that selecting an inner hydrophobic block to design amphiphilic star block copolymers is vital for the degradation of 2,4,6-TCP in water.
Gao and co-workers77 reported the synthesis of the Zn(II) porphyrin-based multi-arm star polymers Zn18, Zn19, Zn20, and Zn21 (Fig. 7 and Table 3), where n = 1–4. The polymer used was the same as that reported by Duan and co-workers.75,76 All porphyrins were prepared via ATRP and their ability as photocatalysts to degrade RhB under white light irradiation was assessed. The authors were interested in establishing a relationship between the number of arms at the porphyrin and the respective photocatalytic activity and stability. Interestingly, as the number of arms increased, the stability and photocatalytic activity also increased. Thus, the best photocatalyst was Zn18, which can be recycled up to 5 times without activity loss.
4.3. Porphyrins in metal–organic frameworks
Metal–organic frameworks (MOFs) are emerging classes of materials used in photocatalysis, especially those based on metalloporphyrins.78 Nagaraja and co-workers79 used the Ni22 nickel MOF (Fig. 8 and Table 4) in the photodegradation of nitroaromatic compounds under white light for 90 min. When compared with Ni22 alone, the Ni22 MOF revealed an enhanced photocatalytic activity (>90% degradation) which may be attributed to a combined catalytic effect. Also, this MOF was recycled and reused for 3 cycles without loss of catalytic activity and structural rigidity.79 The reusability of the catalysts without loss of stability indicates that they can be applied to treat dye-polluted waters. The degradation products were identified as the respective amino derivatives.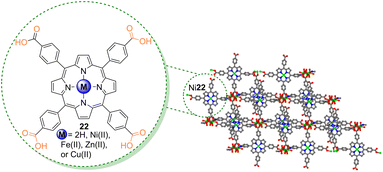 | ||
| Fig. 8 Representation of the Ni22 porphyrin and the Ni22 nickel MOF structure.79 | ||
| MOF | Por | Pollutant | Irradiation time (min) | Light source | Degradation rate (%) | Recycle | Ref. |
|---|---|---|---|---|---|---|---|
| a 6 μM. b 500 nm. c 150 nm. d 300 nm. e pH = 3. f pH = 9. g pH = 7. h pH = 5. i In the presence of H2O2. | |||||||
| NiMOF | Ni22 | Nitrobenzene | 90 | Visible (λ = 420 nm) | 100 | 3 cycles (5% loss) | 79 |
| Nitrotoluene | 60 | — | |||||
| Chloronitrobenzene | 105 | ||||||
| Bromonitrobenzene | 210 | ||||||
| Di-nitrobenzene | 300 | ||||||
| Nitronapthalene | 1440 | 34 | |||||
| ZrMOF | Naphthalene-1,5-diol | 0 | Visible (λ > 420 nm) | 100 | 80 | ||
| PCN-224 | H222 | Tetracycline | 90 | 10a | — | 81 | |
| 50b | |||||||
| 98c | |||||||
| 2 | 100d | 5 cycles (5% loss) | |||||
| Ciprofloxacin | 10 | 90d | |||||
| 90 | 90c | — | |||||
| 25b | |||||||
| 5a | |||||||
| PCN-222 | H222 | RhB | 120 in the dark + 120 min irradiation | UV | 100 | 3 cycles (30% loss) | 83 |
| Ni22 | 3 cycles (10% loss) | ||||||
| H222 | Basic violet 14 | 99 | — | ||||
| Ni22 | 100 | ||||||
| H222 | Crystal violet | 56 | |||||
| Ni22 | 70 | ||||||
| H222 | AB | 47 | |||||
| Ni22 | 83 | ||||||
| Ln-MOF | H222 | Naphthalene-1,5-diol | 20 | Visible λ > 420 nm | 60 | — | 84 |
| Cu-MOF | Cu22 | RhB | 360 | 82e | 85 | ||
| MB | 40f | ||||||
| CR | 89g | ||||||
| Tetracycline | 71h | ||||||
| Norfloxacin | 44f | ||||||
| Oxytocin | 46f | ||||||
| La-MOF | H222 | MO | 125 | 16 | 82 | ||
| 26i | |||||||
| MB | 24 | ||||||
| 61i | |||||||
| Fe22 | MO | 5 | |||||
| 55i | |||||||
| MB | 16 | ||||||
| 93i | |||||||
Wang and co-workers80 synthesized zirconium ultrathin 2D zirconium MOF nanosheets using Ni22 (Fig. 9 and Table 4) and evaluated their photocatalytic activity against naphthalene-1,5-diol. The authors reported good photocatalytic activity and high photostability of these nanosheets when compared with PCN-222, a well-known zirconium metalloporphyrin MOF. These nanosheets' most increased photocatalytic activity might be due to their nanometre thickness, allowing rapid mass transport and a high percentage of exposed catalytically active surfaces.
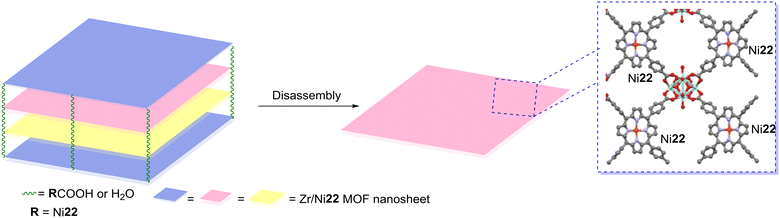 | ||
| Fig. 9 Zirconium ultrathin 2D metal–organic framework nanosheets using Ni22. Adapted from ref. 80. | ||
More recently, Xue, Wang and co-workers81 developed a porphyrin MOF (PCN-224), composed of a Zr6O4(OH)4 cluster and the porphyrin ligand H222 (Table 4), to be used in the photocatalytic degradation, under white light irradiation, of antibiotics such as tetracycline and ciprofloxacin. Three different particle sizes were prepared: 6 μM, 150 nm, 300 nm, and 500 nm. The best photocatalyst was found to be 300 nm-PCN-224 followed by 150 nm-PCN-224, 500 nm-PCN-224 and 6 μM-PCN-224. The best photocatalyst could achieve total degradation of tetracycline after 2 min of irradiation and 90% of ciprofloxacin after 10 min of irradiation. No photocatalytic degradation was observed in the absence of light. Also, no significant changes were observed after 5 cycles of photocatalytic activity.
Kumar and co-workers82 developed a porphyrin H222 and Fe22 La-MOF to be used in the photocatalytic degradation of MO and MB under visible light irradiation. After 125 min of irradiation the H222–La-MOF could degrade 16% of MO and 24% of MB. On the other hand, the Fe22–MOF could achieve a photocatalytic rate of 5 and 16% for MO and MB, respectively. These photocatalytic rates could be enhanced after the addition of H2O2 (Table 4).
B. Zhang, X. Zhang and co-workers83 prepared a bimetallic porphyrin MOF of PCN-222(Ni/Hf) by inserting Ni(II) in the inner core of H222 and coordinating Hf(IV) with the carboxyl groups of the porphyrin. The authors assessed the photocatalytic activity of the MOF for the degradation of four pollutants: RhB, basic violet 14, crystal violet and acid black 210 (AB) – Table 4. The degradation process was not strictly dependent on light since it could reach total degradation of basic violet 14 and RhB after 120 min in the dark. Regarding the degradation of crystal violet and acid black, even under irradiation, it could only reach ∼47% and 60% degradation, respectively. These results were much better than those obtained using PCN-222(Hf) (without Ni(II)). The stability of PCN-222(Ni/Hf) is also enhanced when compared with PCN-222(Hf): after three cycles, the photocatalytic activity decreased by only 10% while a reduction of 30% was observed for PCN-222(Hf).
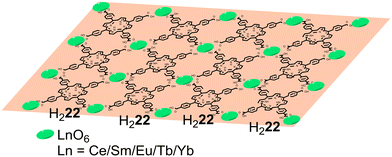
Regarding lanthanides, Huang and co-workers,84 using the same porphyrin, prepared a series of porphyrin-based 2D MOFs (Ln–H222, Ln = Ce, Sm, Eu, Tb, and Yb) (Fig. 10 and Table 4) with different thicknesses and assessed their ability as photocatalysts for the degradation of naphthalene-1,5-diol in order to establish a metal/thickness–activity relationship. The results showed that the thinner the thickness of the nanosheets, the higher the light harvesting ability and the better the photocatalytic activity. Interestingly, the most effective MOF incorporated Yb, which showed outstanding performance due to the effective energy and electron transfer between H222 and Yb3+.
Zhang and co-workers85 prepared a Cu22-based MOF through a rapid microwave hydrothermal route. The resulting nanosheets displayed excellent crystallinity and were used as photocatalysts for the degradation of Congo red, MB and RhB as well as some antibiotics like tetracycline, norfloxacin, and the hormone oxytocin (Table 4). After adsorption of the pollutants and irradiation with white light (λ > 420 nm), the Cu22-MOF showed good photocatalytic activity reaching the highest photodegradation rates for Congo red (89%) and RhB (81.2%) after 6 h.
5. Titanium oxide-supported porphyrins
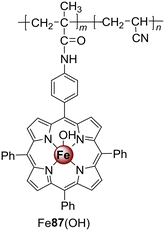
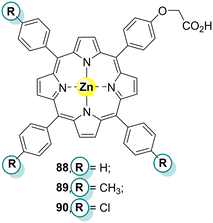
TiO2-based materials are excellent photocatalysts for the oxidation of pollutants in water. They present several advantages: high photocatalytic efficiency, physical and chemical stability, low cost, and low toxicity. However, bare TiO2 can only be excited by UV light and for that reason, it shows no response to white light.86 Considering that UV radiation is less than 5% of natural sunlight and that 43% of the solar spectral energy is in the white light spectrum, it is highly desirable to develop TiO2-based photocatalysts that can absorb visible light in order to improve the use of solar light.86 There are several strategies to increase the light absorption in the visible region, such as doping with noble metals, dye sensitization and even combining TiO2 with other semiconductors. Porphyrin's sensitization is a cheap and efficient method to extend the absorption into the visible region. Despite being a good photocatalyst by itself, the sensitization of TiO2 with porphyrins significantly increases its photocatalytic activity under sunlight and achieves high degradation rates of organic pollutants.18,87 A brief discussion about the photodegradation of organic contaminants by porphyrins immobilized on TiO2 will be considered in the following examples.Chang and co-workers88 and Ji and co-workers89 prepared several TiO2 materials sensitized with M3 (Fig. 2): Ni3–TiO2, Fe3Cl–TiO2, Co3–TiO2, Mn3–TiO2, and Cu3–TiO2 and studied their photoactivity against 2,4-DCP and MO. Chang and co-workers88 proved that under white light (λ = 419 nm) irradiation for 4 h, the Ni3–TiO2 nanocomposite could degrade 81% of 10 ppm 2,4-DCP. The degradation was time and 2,4-DCP concentration-dependent. This study showed that the synthesized nanohybrid could efficiently eliminate pollutants present in wastewaters using sunlight. On the other hand, Ji and co-workers89 found that bare TiO2 could only degrade less than 10% of MO even after 180 min of light irradiation. However, the photocatalytic activity of Cu3 and M3 (M = Ni(II), Fe(III), Co(II), or Mn(II)) impregnated TiO2 could efficiently be enhanced. Moreover, the Cu3–TiO2 composite was the best to degrade MO followed by, in decreasing order, Ni3–TiO2, Mn3–TiO2, Fe3Cl–TiO2, and Co3–TiO2. UV light could increase the photocatalytic activity of all photocatalysts, with Cu3–TiO2 reaching 98% degradation after 1.5 h.
Microemulsions can be used as “reactors” due to their unique properties like ultralow interfacial tension, sizeable interfacial area, thermodynamic stability, and, more importantly, their ability to solubilize otherwise immiscible liquids.90 Having that in mind, Carrero, Madriz, and co-workers91,92 reported the use of TiO2, Ti(IV) citrate, and Zn3 (Fig. 2) as photocatalysts in oil-in-water (O/W) and water-in-oil (W/O) microemulsions to degrade 4-chlorophenol (4-CP). According to the authors, TiO2 and titanium(IV) citrate were at the water phase, while Zn3 was in the oil phase due to its hydrophobic character. The surfactant cetyltrimethylammonium bromide (CTAB) has a positively charged head positioned at the water interface, forming an interphase or pseudophase, attracting the negatively charged Ti(IV) citrate complex when present. The photodegradation results are presented in Table 5.
| Type of microemulsion | Por | Metal complex | Support | Surfactant | Light source | Photodegradation (%) |
|---|---|---|---|---|---|---|
| a Best results obtained by Madriz and co-workers.92 | ||||||
| W/O | — | — | TiO2 | CTAB | UV | 34 |
| O/W | — | — | TiO2 | CTAB | UV | 70 |
| W/O | — | — | TiO2 | CTAB | Visible | 0 |
| O/W | — | — | TiO2 | CTAB | Visible | 0 |
| W/O | Zn3 | — | — | CTAB | UV | 39 |
| O/W | Zn3 | — | — | CTAB | UV | 52 |
| W/O | Zn3 | — | — | CTAB | Visible | 62 |
| O/W | Zn3 | — | — | CTAB | Visible | 26 |
| W/O | — | Ti(IV)-Cit | — | CTAB | UV | 48 |
| O/W | — | Ti(IV)-Cit | — | CTAB | UV | 20 |
| W/O | — | Ti(IV)-Cit | — | CTAB | Visible | 20 |
| O/W | — | Ti(IV)-Cit | — | CTAB | Visible | 20 |
| W/O | Zn3 | — | TiO2 | CTAB | UV | 29 |
| O/W | Zn3 | — | TiO2 | CTAB | UV | 27 |
| W/O | Zn3 | — | TiO2 | CTAB | Visible | 11 |
| O/W | Zn3 | — | TiO2 | CTAB | Visible | 25 |
| O/W | Zn3 | Ti(IV) | — | CTAB | UV | 51 |
| W/O | Zn3 | Ti(IV) | — | — | UV | 75a |
| W/O | Zn3 | Ti(IV) | — | — | Visible | 86a |
In the experiments, after irradiation of the microemulsions with UV light, it was possible to observe that the 4-CP degradation in O/W (Table 5) was much more effective (70%) than in W/O microemulsions (34%). However, there was no 4-CP degradation in the experiments with the TiO2 suspension under white light irradiation. The higher photodegradation rates in O/W can be explained by the fact that the TiO2 particles act individually and do not form aggregates that can decrease the exposed surface and the photoactivation of the particles. As expected, adding Zn3 did not affect the photodegradation rate given the lack of interaction between TiO2 and Zn3 from their different solubilities. Moreover, the Zn3 microemulsion without TiO2 was irradiated with visible light, showing a higher degradation rate in W/O (62%) than in O/W (26%).91 On the other hand, when irradiated with UV light, the O/W showed a higher degradation rate (52%) than W/O (39%). The addition of Ti(IV) to Zn3 microemulsion increased the photodegradation rate of 4-CP. A few years later, the same authors92 used the same porphyrin Zn3, without CTAB, and found that the best conversion percentage of 4-CP (86%) was obtained using W/O microemulsions of titanium citrate mixed with Zn3 and irradiation with white light for 30 min.
Palmisano and co-workers, SŁota and co-workers and Mele and co-workers93,94 reported the synthesis of a polycrystalline TiO2 impregnated with the free-base porphyrin H223 or selected metalloporphyrins M23 (M = Cu(II), Fe(III)Cl, or Mn(III)OAc) (Fig. 11), which were used as catalysts for the photodegradation of 4-nitrophenol (4-NP).
According to the first study, the prepared materials H223 and Cu23 were found to be photostable even after 5 h of light irradiation. Regarding the photocatalytic activity, it is possible to affirm that the TiO2 samples that contain Cu23 are better photocatalysts than the corresponding free-base H223. The photocatalytic activity under UV light for 2 h of bare TiO2 was compared with that of the synthesized hybrids to establish the role of the central metal in M23 (M = 2H, Cu(II), Fe(III), or Mn(III)).94 The obtained results showed that different metals can modulate photocatalyst efficiency. The authors also accessed the stability of the prepared materials where it was possible to observe that all the materials were stable. For example, Cu23–TiO2 and Fe23Cl–TiO2 were more photoactive than bare TiO2 (anatase), whereas Mn23OAc–TiO2 showed a lower photoreactivity. The metal-free porphyrin H223 also showed slightly higher photoactivity than the corresponding bare TiO2, indicating that the porphyrin macrocycle is photoactive even in the absence of metal.94
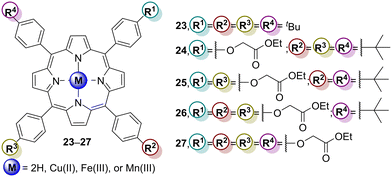
Li and co-workers95 developed four Cu(II) porphyrins (Cu24–Cu27) with one to four peripheral ester groups (Fig. 11) to be used to sensitize TiO2 and to use the composites as photocatalysts for the degradation of 4-NP. The photocatalytic properties of mesoporous TiO2 and Cu24– to Cu27–TiO2 hybrids were evaluated to degrade 4-NP in aqueous media and under white light irradiation. The results showed that the photocatalytic activity order is: Cu27–TiO2 > Cu26–TiO2 > Cu25–TiO2 > Cu24–TiO2 (Table 6). The different number of peripheral groups directly modulates the photocatalytic activity, influencing the electron–hole separation process, and even after 6 cycles, the photocatalytic activity only decreased by 20% for the best photocatalyst. The loss of photodegradation efficiency could be assigned to the in situ generated hydroxyl radicals attacking the porphyrin molecules.
| Por | Irradiation time (min) | Light source | Degradation rate (%) | Recycle | Ref. |
|---|---|---|---|---|---|
| H223 | 300 | Visible (λ = 366 nm) | 100 | — | 93 |
| Cu23 | 240 | ||||
| Cu23 | 120 | 94 | |||
| Fe23 | 180 | ||||
| Mn23 | |||||
| Cu24 | 60 | White light | 6 cycles (50% loss) | 95 | |
| Cu25 | 55 | 6 cycles (45% loss) | |||
| Cu26 | 50 | 6 cycles (40% loss) | |||
| Cu27 | 45 | 6 cycles (30% loss) | |||
| Cu28 | 400 | Visible (λ = 420 nm) | 98 | — | 96 |
| H228 | 90 | ||||
| Cu29 | 99 | 6 cycles (15% loss) | |||
| H229 | 98 | — | |||
| Pt28 | 360 | UV–vis | 99 | 6 cycles (10% loss) | 97 |
| Pt29 | 97 | — | |||
| Pt30 | 96 | ||||
| Cu30 | 400 | 90 | 98 | ||
| Cu31 | 98 | ||||
| Cu32 | 95 | ||||
| Cu33 | 100 | ||||
| Cu34 | Visible (λ > 420 nm) | 90 | 6 cycles (5% loss) | 99 | |
| Cu35 | 89 | ||||
| Cu36 | 87 |
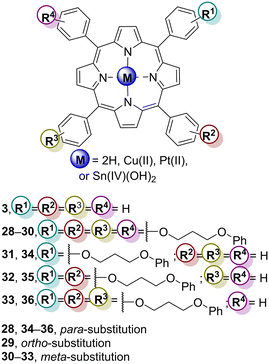
A series of studies using TiO2 impregnated with meso-tetraphenylporphyrin (TPP–H23) derivatives bearing 3-phenoxypropyloxy groups at ortho-, meta- or para-positions (Fig. 12 and Table 6) were performed in order to evaluate the photocatalytic activity of the composites in 4-NP degradation.96,97 The ortho, meta and para tetra-substituted free-base porphyrins H228–H230 (Fig. 12) and the corresponding complexes Cu28–Cu30 and Pt28–Pt30 were used in those studies. Later, Li and co-workers98,99 intended to assess the influence of the number of meta- and para-substituents by synthesizing the mono, di, tri, and tetrasubstituted free-base porphyrins H230–H233 as well as their Cu(II) derivatives Cu30–Cu33.
The first study96 was carried out in aqueous media under visible light (λ = 420 nm) irradiation. Among the synthesized photocatalysts, Cu29–TiO2 was revealed to be the most efficient in 4-NP degradation, followed by Cu28–TiO2, H229–TiO2, and H228–TiO2. TiO2 impregnated with ortho-substituted porphyrins (H229–TiO2 and Cu29–TiO2) exhibited higher photoactivities than TiO2 synthesized with para-substituted porphyrins (Cu28–TiO2 and Pt28–TiO2). This can be due to the lower symmetry of the ortho-substituted structures, which can lead to a minor extent of aggregation and less important intermolecular π–π interactions. All photocatalysts were stable after irradiation for 5–7 h. In this study, TiO2 impregnated with porphyrins could extend the light absorption efficiency and this depends on the amount of porphyrin loaded into the TiO2 surface. When using the best photocatalyst, almost all 4-NP (99%) was degraded after 400 min of irradiation.
According to Li and co-workers,97,98 all Pt(II) and Cu(II) porphyrins (Pt28–Pt30 and Cu30–Cu33, Fig. 12 and Table 6) combined with TiO2 enhanced the photocatalytic efficiency when compared with bare TiO2. Overall, Pt(II) complexes/TiO2 showed better photocatalytic activity in the degradation of 4-NP compared with the Cu(II) porphyrins/TiO2 after 200 min of irradiation. However, when the irradiation time was extended to 300 min, all derivatives induced a complete degradation of the pollutant. Among the ortho-, meta- and para-substituted porphyrins, the ortho derivative Pt29–TiO2 was the best photocatalyst. Regarding the effect of the number of peripheral meta-substitutions on the photocatalysts' efficiency, the best results were obtained with the tetrasubstituted Cu30 followed by the trisubstituted Cu33, disubstituted Cu32 and monosubstituted Cu31.98 Similarly, Li and co-workers99 reached the same results for the para-substituted porphyrins, with the trisubstituted Cu36–TiO2 being the best catalyst followed by disubstituted Cu35–TiO2 and monosubstituted Cu34–TiO2 (Fig. 12 and Table 6). To justify those results, the authors98,99 assumed that the number of substituents does not influence the redox potential of the sensitizers but, instead, it influences the interaction between porphyrins and the porphyrin–TiO2 surface as well as the electron transfer process from the sensitizer to TiO2. Porphyrins with more substituents can adsorb more dissolved oxygen and, consequently, an increased photocatalytic activity is observed. Catalyst recycling studies showed that there is no significant loss of activity after six cycles.
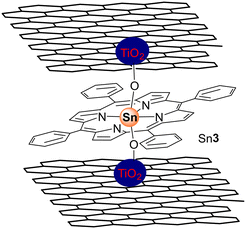
Li and co-workers100 and Mu and co-workers101 used the three isomeric Sn(IV) porphyrin derivatives Sn3(OH)2 (Fig. 2), Sn28(OH)2–Sn30(OH)2 (Fig. 12) to prepare the corresponding TiO2–Sn(IV) porphyrin hybrids via axial functionalization. The ability of the resulting hybrids to photodegrade 4-NP and MO was then evaluated under visible and UV light. Under white light, the three composites showed higher photocatalytic activity than bare TiO2, with the Sn28(OH)2–TiO2 hybrid being the best photocatalyst with a photocatalytic rate of 98.1% after 500 min of light irradiation (Table 7). However, under UV light irradiation, the photocatalytic efficiency of all hybrids was also enhanced when compared with bare TiO2. Regarding the photodegradation efficiencies of 4-NP, an efficient degradation is obtained with the peripheral substituents at the para-positions as well as the presence of axial ligands, which can prevent π–π interactions and decrease the aggregation of the porphyrin molecules, especially for the most planar Sn28(OH)2.
| Por | Pollutant | Irradiation time (min) | Light source | Degradation rate (%) | Recycle | Ref. |
|---|---|---|---|---|---|---|
| Sn28 | 4-NP | 500 | Visible (λ > 400 nm) | 98.1 | — | 100 |
| 450 | UV–vis | 100 | ||||
| Sn29 | 500 | Visible (λ > 400) | 90 | |||
| 450 | UV–vis | 100 | ||||
| Sn30 | 500 | Visible (λ > 400) | ||||
| 450 | UV–vis | |||||
| Sn3 | 180 | 90 | 7 cycles (5% loss) | 101 | ||
| MO | 95 | |||||
| Cu37 | RhB | 30 | 100 | — | 103 | |
| NP | 60 | 90 | 6 cycles (5% loss) | |||
| Cu38 | RhB | 30 | 98 | — | ||
| NP | 60 | 90 | 6 cycles (40% loss) | |||
| Cu39 | RhB | 30 | 100 | — | ||
| NP | 60 | 98 | 6 cycles (5% loss) | |||
| Cu38 | 160 | 90 | — | 104 | ||
| Cu39 | 65 | Visible (λ > 350 nm) | 90 | 106 | ||
| Zn39 | 85 | |||||
| Ni39 | 80 | |||||
| Zn40 | 180 | UV | 94 | 107 | ||
| Cu40 | 98 | |||||
| Co40 | 94 | |||||
| Fe40Cl | 93 | |||||
| Zn41 | 95 | |||||
| Cu41 | 98 | 6 cycles (10% loss) | ||||
| Co41 | 95 | — | ||||
| Cu42 | 160 | Visible (λ > 350 nm) | 100 | 108 | ||
| 120 | UV (λ = 100–400) | |||||
| Zn43 | MO | 150 | UV–vis (λ > 100) | 89 | 6 cycles (10% loss) | 109 |
| Zn44 | 91 | — | ||||
| Zn45 | 84 | |||||
| Zn46 | 73 |
Moreover, it is important to say that the structure of Sn29(OH)2 (Fig. 12) can have more stressed and stereochemically complicated conformations than others. On the other hand, the substituents of porphyrin Sn28(OH)2 have just a small deviation from planarity, which causes a perfect parallel plane between the porphyrin and the TiO2 surface. This means that Sn28(OH)2 can easily approach the TiO2 surface enabling the initial electron injection process from the porphyrin's excited state into the TiO2 CB leading to an effective photocatalytic effect. As expected, the structure of Sn29(OH)2 makes it inaccessible to the surface of TiO2 and blocks the participation of the porphyrin in the photocatalytic effect. Despite the photocatalytic activity being higher than Co(II) and Zn(II) porphyrins, it is still lower than that of Cu(II) porphyrins, which proves that the presence of different metal ions plays an important role in the photocatalytic process.102 In the presence of Sn3(OH)2–TiO2 more than 85% degradation was accomplished with 180 min of irradiation. Also, the characterization studies showed an interaction between the hydroxyl groups of Sn3(OH)2 and TiO2. The authors also established the relevance of the O2 concentration. Recycling studies with the photocatalyst Sn3(OH)2–TiO2 showed that it can be reused seven times without loss of activity.101
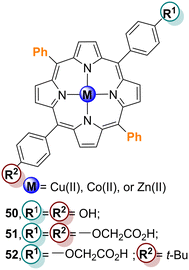
Li and co-workers103,104 evaluated the effect of the different peripheral substituents in porphyrin derivatives in the photodegradation of the pollutants RhB and 4-NP. They found that among Cu37–TiO2, Cu38–TiO2, and Cu39–TiO2 (Fig. 13), the best photocatalyst for RhB degradation was Cu39–TiO2 (Table 7), but for the degradation of 4-NP it was the second best photocatalyst. Their results confirmed that the impregnation of TiO2 with Cu39 enhanced its photocatalytic activity concerning the degradation of both 4-NP and RhB. The photocatalytic activity of Cu38–TiO2 was assessed regarding the photodegradation of 4-NP in an aqueous solution under white light irradiation with an appropriate amount of H2O2. Under those conditions, a remarkable photocatalytic activity was observed, with almost complete degradation of 4-NP after 160 min of irradiation. Cu38–TiO2 showed a catalytic efficiency of 86.7% even after 6 cycles. The superior photocatalytic activity of Cu38–TiO2 is probably due to the presence of the heterojunction along with the higher surface, caused by its mesoporous structure. Cu38–TiO2 was also used as a photocatalyst in 4-NP degradation in another work.105
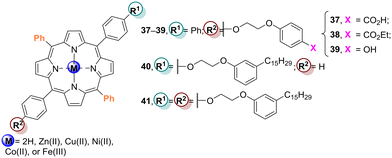 | ||
| Fig. 13 Free-base and Cu(II) porphyrin derivatives 37–39, cardanol-substituted porphyrins 40 and 41 and porphyrin derivatives 50–52. Used to prepare the Por–TiO2 hybrid materials. | ||
Li and co-workers106 evaluated the photocatalytic activity of the TiO2-based materials Cu39–TiO2, Zn39–TiO2 and Ni39–TiO2 (Fig. 13) in aqueous solution under halogen lamp irradiation and compared it with bare TiO2. All porphyrinic materials revealed enhanced photocatalytic efficiency in the photodegradation of 4-NP, with Cu39–TiO2 being the best photocatalyst followed by Zn39–TiO2 and Ni39–TiO2 (Table 7); bare TiO2 was the least active. This result can be justified by the better combination of the excited potential of the CB of both Cu39 and TiO2. Compared with Cu2–TiO2, the higher catalytic activity of Cu39–TiO2 is probably due to the peripheral OH group that can act as an anchoring group allowing a better interaction between porphyrin and TiO2. This is beneficial to the initial electron injection from the porphyrin excited state to the TiO2 CB, leading to an effective photoactivity.
Vasapollo and co-workers107 used cardanol, a by-product of the food industry, for the synthesis of the “cardanol-based” free-base porphyrins H240 and H241 as well as their Zn(II), Cu(II), Co(II), and Fe(III) complexes (Fig. 13). After their immobilization on TiO2, the materials were tested as sensitizers in 4-NP degradation under UV irradiation for 180 min (Table 7). All metallated complexes (Zn40, Cu40, Co40, Fe40Cl, Zn41, Cu41, Co41, and Fe41Cl) showed a slightly better photocatalytic activity (≈98%) than the free-base derivatives H240 and H241 (≈94%). Recycling and stability experiments revealed that all photocatalysts are stable, with the most effective ones being the Cu(II) complexes that could be used for six cycles without loss of activity. The better photoactivity of Cu(II) complexes can be assigned to the Cu(II)–Cu(I) photocatalytic redox cycle. Finally, it was concluded that the oxidizing species responsible for the degradation of 4-NP do not interact with the photocatalyst since the C–C double bond of the cardanol unit appears to be present at the end of the process.
Li and co-workers108 also reported the synthesis of the free-base H242 and its Cu(II) complex Cu42 (Fig. 14). These compounds were used to sensitize TiO2 and the catalytic ability of the resulting hybrids to photodegrade 4-NP was evaluated. The photocatalytic assays were performed in aqueous media under white light and UV irradiation in the presence of H2O2. The addition of H2O2 to the photocatalytic system prompted a high photocatalytic activity of Cu42–TiO2 after white light irradiation for 160 min (Table 7). An enhanced photocatalytic activity was also observed when irradiated with UV light but only a small increase when H2O2 was added.
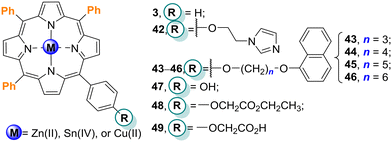 | ||
| Fig. 14 Free-base porphyrin 42, its Cu(II) complex, Zn(II) porphyrins Zn43–Zn46, M3 and mono(substituted-phenyl)porphyrins 47–49 immobilized on TiO2. | ||
Chen and Shen109 prepared TiO2 modified with four Zn(II) porphyrins 43–46 (Fig. 14) in order to study the effects of the spacer length of a peripheral substituent on the photodegradation of MO in an aqueous solution under white light. The results showed that all porphyrins enhanced the photocatalytic activity of bare TiO2 with the following order: Zn44–TiO2 > Zn43–TiO2 > Zn45–TiO2 > Zn46–TiO2 > bare TiO2. The photocatalytic activity of Zn44–TiO2 was much higher than the others (Table 7) because it presented the best match with the CB potential of TiO2. Furthermore, the spacer length of the peripheral substituent can influence the interaction between the Zn(II) porphyrin and TiO2, consequently influencing the initial electron injection process from the porphyrin excited singlet state to the CB of TiO2, essential for an effective photocatalytic effect. The authors performed reusability studies and all the photocatalysts can be reused 6 times without significant loss of activity (10%).
meso-(Hydroxyphenyl)porphyrins and their derivatives are frequently used to sensitize TiO2 and the resulting materials typically show high photocatalytic activity.110–117 For instance, Mu and co-workers110 used porphyrins H247, Zn47 and Sn47Cl2 (Fig. 14) to sensitize TiO2 nanoparticles in order to improve the photocatalytic activity of bare TiO2 nanoparticles. Among the four catalysts (H247, Zn47, Sn47Cl2, bare TiO2), the sensitizer Sn47Cl2–TiO2 showed the highest photocatalytic activity, being able to degrade 86% of MO after 180 min irradiation with white light (Table 8). The results also confirmed that the central metal ion in the porphyrin significantly influenced the photocatalytic activity of the hybrid materials.
| Por | Pollutant | Irradiation time (min) | Light source | Degradation rate (%) | Recycle | Ref. |
|---|---|---|---|---|---|---|
| N.E. – not evaluated. | ||||||
| Sn47Cl | MO | 180 | Visible (λ > 320 nm) | 86 | — | 110 |
| Zn47 | 50 | |||||
| H247 | 60 | |||||
| Cu47 | 4-NP | 300 | UV–vis (λ = 380–780 nm) | 100 | 111 | |
| 60 | UV–vis (λ = 340–780) nm | 90 | 112 | |||
| Cu48 | 300 | UV–vis (λ = 380–780 nm) | 111 | |||
| 60 | UV–vis (λ = 340–780 nm) | 95 | 112 | |||
| Cu49 | 300 | UV–vis (λ = 380–780 nm) | 100 | 111 | ||
| 60 | UV–vis (λ = 340–780 nm) | 112 | ||||
| H250 | Visible λ >350 nm | N.E. | 113 | |||
| Cu50 | 96 | |||||
| Co50 | 81 | |||||
| Zn50 | 86 | |||||
| Cu51 | 300 | 100 | 114 | |||
| Cu52 | 45 | 6 cycles (15% loss) | 115 | |||
| Co52 | 75 | 6 cycles (22% loss) | ||||
| Zn52 | 6 cycles (19% loss) | |||||
| Cu53 | 60 | 97 | — | 116 | ||
| 40 | 100 | 6 cycles (10% loss) | ||||
| Cu 54 | 60 | — | 117 | |||
| 30 | 6 cycles (10% loss) | |||||
Li and co-workers111 immobilized the mono(substituted-phenyl)porphyrins Cu47, Cu48 and Cu49 (bearing hydroxy, ester or carboxy groups, respectively, Fig. 14) on TiO2 and used the resulting hybrids for the photodegradation of 4-NP in aqueous solution under simulated solar irradiation. It was found that porphyrins Cu47–Cu49 enhanced the catalytic activity of bare TiO2 for 4-NP photodegradation after 300 min of irradiation with a xenon lamp (35% with bare TiO2 and almost complete degradation using the hybrids) – Table 8. Cu49–TiO2 was the best photocatalyst followed by Cu47–TiO2, Cu48–TiO2, and bare TiO2. Also, the authors concluded that a larger polarity of the substituent groups and a stronger interaction between Cu47–Cu49 and TiO2 accelerate the electron injection process and improve the electron–hole separation. Cu49 has the largest polarity and its impregnation on TiO2 afforded the highest photocatalytic activity. Therefore, the polarity of the porphyrin substituents is also an important factor to be considered in the photodegradation of organic pollutants. The photodegradation results confirmed that the impregnation of TiO2 with Cu47, Cu48, and Cu49 increased the photodegradation of 4-NP and RhB. It is important to highlight that the different peripheral substituents in the porphyrin macrocycle led to different catalytic activities; porphyrins containing –OH or –COOH groups (Cu47 and Cu49) were the best ones for the degradation of 4-NP and RhB. These results can be justified by the strong interaction of these groups with the surface of TiO2.
Later, the same authors compared the photodegradation efficiency of porphyrins Cu47–Cu49 (Fig. 14) immobilized on “home-prepared” and commercial TiO2 (p-TiO2 and c-TiO2, respectively).112 The results showed that the commercial c-TiO2 caused 84.9% degradation of 4-NP in aqueous solution under metal halide lamp irradiation for 60 min. However, under the same experimental conditions, the “home-prepared” p-TiO2 just exhibited a photodegradation rate of 43.6%. All Cu47–TiO2 hybrids showed higher photocatalytic activity than p-TiO2 in the following order: Cu49–c-TiO2 ≈ Cu49–p-TiO2 > Cu47–c-TiO2 ≈ Cu48–p-TiO2 > Cu47–p-TiO2 > c-TiO2 > Cu48–c-TiO2 > p-TiO2. It is well known that the surface area along with the hydroxylation level of TiO2 could affect the interaction of the sensitizer and TiO2, which, in turn, improves the photocatalytic activity. Moreover, the anchoring group also plays an important role which could explain the higher activity of Cu49; the carboxy group could accelerate the electron transfer from the porphyrin to TiO2. The formation of porphyrin dimers Cu47–Cu47, Cu48–Cu48, and Cu49–Cu49 on the lower and less hydroxylated surface area of c-TiO2 was also detected. It is important to highlight that regardless of the porphyrin chosen to sensitize TiO2, the monosubstituted porphyrin structure is more beneficial for increasing photodegradation than the dimeric structures.
In order to understand the influence of the metal ion in 5,15-bis(4-hydroxyphenyl)porphyrins in the photodegradation of 4-NP, Li and co-workers113 immobilized the free-base H250 along with the metallated porphyrins Cu50, Zn50, and Co50 (Fig. 15) on the surface of TiO2. The four porphyrins and the corresponding TiO2 composites were then evaluated as photocatalysts to degrade 4-NP. The impregnation of porphyrins M50 (M = 2H, Cu(II), Zn(II), or Co(II)) did not influence the particle size of TiO2 but increased its photocatalytic activity. The four photocatalysts enhanced the photoactivity in the following order: Cu50–TiO2 (95.8%) > Zn50–TiO2 (85.6%) > Co50–TiO2 (80.8%) > Cu3–TiO2 (66.8%) > bare TiO2 (60%) after irradiation for 60 min (Table 8). These results showed that the metal ion plays, effectively, an important role in the photodegradation efficiency. Also, the OH anchoring group of the metalloporphyrin is a factor that improves its efficiency as a photocatalyst. Moreover, the results of photoluminescence showed that the three photocatalysts M50 (M = Cu(II), Zn(II), or Co(II)) could increase the separation efficiency of the photoinduced electron and hole. The highest photocatalytic activity was observed with the Cu50–TiO2 hybrid.
Hu and co-workers114 synthesized the dicarboxylic acid Cu51 (Fig. 15 and Table 8), impregnated it on TiO2 and evaluated the photocatalytic activity of the resulting composite on the degradation of 4-NP in aqueous media under xenon lamp irradiation. The results showed that the Cu51–TiO2 composite's photoactivity was enhanced when compared with bare TiO2 due to the interaction between the carboxy group of the porphyrin and the hydroxyls anchored in the TiO2 surface.
As already mentioned, the metalloporphyrins with carboxy groups exhibit higher photocatalytic activity due to the strong interactions between the –COOH groups and TiO2. Also, the role of the central metal is revealed to be crucial, and for that reason, Li and co-workers115 studied the influence of different metals on the photocatalytic activity. Cu52–TiO2 is still the most efficient photocatalyst, followed by Co52–TiO2, Zn52–TiO2 (Fig. 15), and bare TiO2. The Cu(II) ions can easily accept electrons to reach a steady state, causing the isolation of electron holes when compared with Co(II) and Zn(II) in M52–TiO2. All the metalloporphyrin composites could be recycled 6 times with a maximum loss of 22% (Table 8) in the photodegradation efficiency.
The two porphyrinic mesoporous materials Cu53–TiO2 and Cu54–TiO2 (Fig. 16) are efficient photocatalysts for the degradation of 4-NP (Table 8), but the highest efficiency was observed for Cu54–TiO2.116,117 The chemical interaction between the carboxy groups of Cu54 and TiO2 is stronger than the physical interaction of the ester groups of Cu53 with TiO2.116 As the interaction strength increases, the electron transfer efficiency also increases, leading to a better photocatalytic effect. According to the authors, the electron transfer occurs through space, not through the linker group that connects the porphyrin core to the anchoring group. Moreover, when considering the steric constraint of porphyrins, Cu54 bonds to the TiO2 surface by two carboxy groups, with a certain planar deviation, whereas Cu54 places vertical to the surface of TiO2. As a result, the photocatalytic efficiency is higher for Cu54–TiO2 than for Cu53–TiO2. Moreover, the efficiency is maintained at 90% after six cycles.
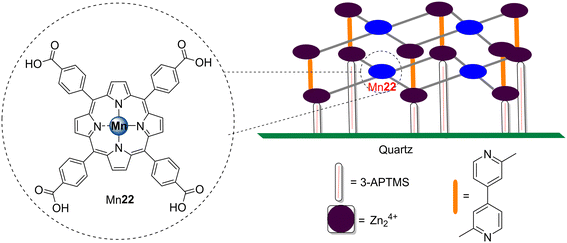
Tasseroul and co-workers118 designed xerogels based on TiO2 modified with the free-base meso-tetrakis(4-carboxyphenyl)porphyrin H222 and its Ni(II) complex Ni22 (Fig. 16) in order to assess their potential to degrade 4-NP (Table 9). An improvement of the photocatalytic activity was observed for the xerogels dried for 10 days, with this effect being more pronounced with Ni22. The high specific surface area of the xerogels allowed the adsorption of 4-NP to be increased and, consequently, the reaction rate to increase.
| Por | Pollutant | Irradiation time (min) | Light source | Degradation rate (%) | Recycle | Ref. |
|---|---|---|---|---|---|---|
| a Dried in 3 days. b Dried in 10 days. c 80 mg of photocatalyst. d 20 mg of photocatalyst. e Presence of SA. | ||||||
| H222 | 4-NP | 360 | Visible (λ > 350 nm) | 0a | — | 118 |
| 30b | ||||||
| Ni22 | 42b | |||||
| H222 | MB | 180 | UV (λ = 254 nm) | 74 | 119 | |
| Mn22Cl | 30 | |||||
| H222 | Acid chrome blue K | 15 | Visible (λ > 400 nm) | 94 | 120 | |
| H256 | 60 | 96 | ||||
| H222 | Famotidine | 180 | 100 | 1 cycle (33% loss) | 123 | |
| 30 | Natural sunlight | 90 | — | |||
| Tamsulosin | 180 | Visible (λ > 400 nm) | 20 | |||
| Solifenacin | 25 | |||||
| H222 | Oxolinic acid | Sunlight simulator | 60 | 124 | ||
| Zn22 | 52 | |||||
| Cu22 | 68 | |||||
| Sn2 | 50 | |||||
| H222 | Oxytetracycline | 60 | ||||
| Zn22 | 35 | |||||
| Cu22 | 62 | |||||
| Sn2 | 60 | |||||
| H222 | Atrazine | 60 | Visible (λ > 420 nm) | 25 | 126 | |
| Fe22Cl | 57 | |||||
| Cu22 | 82 | |||||
| Zn22 | 40 | |||||
| H222 | MB | 30 | 98c | 128 | ||
| 59d | ||||||
| Cu22 | 91c | |||||
| 50d | ||||||
| H222 | 60 | 100c | 129 | |||
| Cu22 | 400 | 90c | 130 | |||
| H222 | 80 | 70 | 10 cycles (10% loss) | 135 | ||
| Zn64 | 84 | |||||
| Fe22 | 120 | UV–visible (λ > 100 nm) | 85e | 5 cycles (10% loss) | 136 | |
| 68 | ||||||
To degrade MB, Viana and co-workers119 modified TiO2 nanoparticles with H222 (Fig. 16) and Mn22Cl (Fig. 16). The carboxylic group, which is chemically linked to the TiO2 nanoparticle, enhances the electronic coupling between the porphyrin and the semiconductor. In the case of the free base H222, the porphyrins could be anchored to TiO2 by one or two of the carboxylic groups that appear to be perpendicular to the TiO2 surface but Mn22Cl was anchored in a non-perpendicular way to TiO2, suggesting that the filling of the TiO2 pores by these molecules occurs, leading to a reduction of the catalyst surface area, in the case of Mn22Cl, decreasing its photocatalytic activity when compared with H222.
Shi and co-workers120 modified TiO2 with H222 or H256 (Fig. 16) and used the composites to degrade acid chrome blue K in aqueous media under visible-light irradiation. The results revealed that both H222–TiO2 and H256–TiO2 could efficiently degrade this dye in 15 min under natural sunlight (94.0% and 96.1% degradation, respectively, Table 9).
The presence of pharmaceuticals in the environment, from wastewater of the pharmaceutical industry, excreted by patients on drug therapy, and drugs flushed down the toilet, is worrying.121 Famotidine, a histamine H2 receptor antagonist used to prevent peptic ulcers,122 can be considered a wastewater pollutant if not destroyed properly. Nolan and co-workers123 prepared a hybrid composed of TiO2 modified with H222 and assessed its capability as a catalyst in the photodegradation of this drug using both visible and solar light irradiation. After reusing the photocatalyst, it could maintain 67% of the original photocatalytic activity. A careful analysis of the photodegradation products of famotidine using the H222–TiO2 composite revealed that it did not lead to complete mineralization of the drug, but instead generated a range of degradation products, mainly the S-oxide of famotidine. The optimized conditions used to degrade famotidine were also used to degrade other pharmaceuticals (tamsulosin and solifenacin) but without success. It was concluded that the H222–TiO2 composite is substrate specific.
D'Urso and co-workers124 studied the ability of TiO2 modified with cationic porphyrins M2 (M = 2H, Zn(II), and Sn(IV)(OH)2) (Fig. 2) and anionic porphyrins H222, Zn22, and Cu22 (Fig. 16 and Table 9) to degrade oxolinic acid and oxytetracycline, two antibiotics used in aquaculture.125 These pharmaceuticals are photodegraded in the presence of bare TiO2 but it would be important to enhance its catalytic activity using porphyrins. Preliminary studies indicated that porphyrin H222 was the best 1O2 generator while Cu22 could not generate 1O2. All cationic porphyrins exhibited similar results. Despite these results, the Cu22–TiO2 composite was considered the best photocatalyst among the hybrid materials, being slightly specific for oxolinic acid. Sn2(OH)2 was chosen for immobilization on TiO2 due to the presence of the hydroxyl axial ligands, but it did not show significant results in the photodegradation of both pharmaceuticals.
In 2009, Chovelon and co-workers126 used porphyrins H222, Fe22Cl, Cu22, and Zn22 (Fig. 16) supported on TiO2 to degrade atrazine127 in aqueous solution under white light irradiation. Atrazine was only degraded after the addition of H2O2 and the best results were obtained with Cu22–TiO2, which achieved 82% degradation after 1 h of irradiation. The photodegradation rates with the remaining composites were much lower: Fe22Cl (57%), Zn22 (40%) and H222 (25%) – Table 9. The authors could identify a series of intermediates after a first allylic oxidation.
Later, Zheng and co-workers128 also used modified TiO2 with H222 and Cu22 (Fig. 16 and Table 9) to degrade MB under white light. The best photocatalyst was also Cu22–TiO2, with a MB photodegradation rate of 59.3% upon 40 min of irradiation (20 mg amount of photocatalyst). This efficiency could be increase to 92% with the increase on the amount of photocatalyst to 80 mg. Wang and co-workers also confirmed that H222–TiO2 and Cu22–TiO2 can efficiently degrade MB under white light irradiation.129,130 Their studies on the photodegradation of MB revealed that the sensitization of TiO2 with H222 and Cu22 enhances its photoactivity and provides good photocatalysts for wastewater treatment.
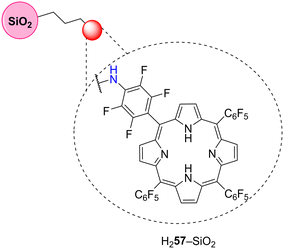
TiO2 nanoparticles modified with H257 (Fig. 17) were prepared and used as photocatalysts for photodegradation of MB by Tsai and co-workers.131 The photocatalytic activity of H257–TiO2 was assessed by performing MB degradation in aqueous media under white light. The absorption spectrum of the H257–TiO2 nanoparticle was extended to 300–500 nm, which greatly enhanced the photocatalytic activity when compared with bare TiO2 reaching 90% degradation of MB after 60 min of solar irradiation (Table 10).
| Por | Pollutant | Irradiation time (min) | Light source | Degradation rate (%) | Recycle | Ref. |
|---|---|---|---|---|---|---|
| a In the presence of H2O2 and TEOA. b 0.001 molar ratio content. c 0.003 molar ratio content. d 0.006 molar ratio content. | ||||||
| H257 | MB | 60 | Natural sunlight | 90 | — | 131 |
| Cu58 | 4-NP | 120 | Visible (λ > 420) | 100 | 132 | |
| Cu59 | 45 | 5 cycles (30% loss) | ||||
| MB | 50 | |||||
| RhB | 120 | |||||
| Cu60 | 4-NP | 80 | — | |||
| Cu61 | 60 | |||||
| Fe62Cl | 180 | Natural sunlight | 100a | 3 cycles (10% loss) | 133 | |
| H263 | MB | 60 | UV | 30 | — | 134 |
| Visible (λ > 400) | 60 | |||||
| Fe63Cl | UV | 85 | ||||
| Visible (λ > 400) | 0 | |||||
| H265 | Acid black 1 | 120 | Natural sunlight | 100 | 137 | |
| H266 | α-Terpinene | 40 | Visible light (λ > 410 nm) | 4 cycles (6% loss) | 138 | |
| H267 | 4-NP | 480 | UV–vis | 52b | 4 cycles (20% loss) | 139 |
| 55c | 4 cycles (25% loss) | |||||
| 61d | 4 cycles (10% loss) | |||||
In 2016, Halder and Bhavana132 studied the role of conformation and the effect of the substituents in the meso-positions of porphyrins derivatives Cu58, Cu59, Cu60, and Cu61 (Fig. 17 and Table 10) adsorbed on TiO2 in the photocatalytic degradation of 4-NP, RhB and MB. The activity of Cu59–TiO2 is significantly higher than that of Cu58–TiO2 and higher than both Cu60–TiO2 and Cu61–TiO2. For instance, the time required to degrade 4-NP with Cu59–TiO2 is 45 min while 2 h are necessary when Cu58–TiO2 is used. With Cu60–TiO2 and Cu61–TiO2 a complete degradation of 4-NP is not achieved in 2 h. Similar results were obtained for RhB and MB. The differences observed in the photocatalytic activity of these compounds were explained considering the position of the substituents and their conformations. The best photocatalyst (Cu59–TiO2) was found to be stable under the experimental conditions and its efficacy was maintained after five cycles.
The photocatalytic activity of hemin anchored TiO2 (Fe62Cl–TiO2, Fig. 18 and Table 10) was investigated by ArunaKumari and Devi133 through the degradation of 4-NP in aqueous solution under solar light irradiation but with the assistance of an appropriate amount of triethanolamine (used as an electron donor). The structural characterization of the catalyst confirmed that the chemisorption of the hemin molecules on the surface of TiO2 is through O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O–Ti bonds which can not only enhance the extent of adsorption but also act as electron transfer channels that facilitate the electron transfer process. The photocatalyst can be reused 3 times without loss of activity. Pal and co-workers134 investigated the potential application of hematoporphyrin-functionalized titania nanohybrids (H263–TiO2 and Fe63Cl–TiO2) (Fig. 18 and Table 10) for the photodegradation of MB in aqueous solution under visible and UV light. Under UV light, Fe63Cl–TiO2 shows higher photocatalytic activity due to the cooperative functions of Fe63Cl and TiO2 in generating reactive species. On the other hand, H263–TiO2 shows higher photocatalytic activity under white light due to the absence of Fe(III) ions that obstruct the electron transfer from the porphyrin macrocycle to TiO2.
C–O–Ti bonds which can not only enhance the extent of adsorption but also act as electron transfer channels that facilitate the electron transfer process. The photocatalyst can be reused 3 times without loss of activity. Pal and co-workers134 investigated the potential application of hematoporphyrin-functionalized titania nanohybrids (H263–TiO2 and Fe63Cl–TiO2) (Fig. 18 and Table 10) for the photodegradation of MB in aqueous solution under visible and UV light. Under UV light, Fe63Cl–TiO2 shows higher photocatalytic activity due to the cooperative functions of Fe63Cl and TiO2 in generating reactive species. On the other hand, H263–TiO2 shows higher photocatalytic activity under white light due to the absence of Fe(III) ions that obstruct the electron transfer from the porphyrin macrocycle to TiO2.
In 2020, Son and co-workers135 reported the synthesis of the porphyrin–squaraine-based sensitizer Zn64 (Fig. 19) that shows broad absorption in the visible and NIR regions, an essential feature in photocatalytic studies. This panchromatic porphyrin derivative was then immobilized on TiO2 and the resulting composite was used as a catalyst for the photodegradation of MB. The photocatalytic studies were performed under white light irradiation and the results were compared with bare TiO2 and H222–TiO2 (a well-established good photocatalyst). After 80 min of irradiation, Zn64–TiO2 exhibited the highest photocatalytic activity (∼80%) followed by H222–TiO2 (∼70%) > TiO2 (∼20%). As predicted, the fast and efficient activity of Zn64 is due to its broad absorption ability. The addition of H2O2 slightly improved the photocatalytic activity. The photocatalyst can be reused up to 10 cycles without significant loss of activity.
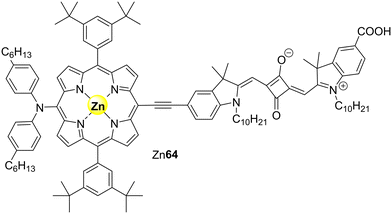 | ||
| Fig. 19 Structure of the porphyrin–squaraine-based panchromatic sensitizer (Zn64) immobilized on TiO2. | ||
In 2015, Yao and co-workers136 bonded porphyrin Fe22Cl to TiO2 particles using 2-hydroxy-5-sulfosalicylic acid (SSA) or salicylic acid (SA) as a bridging molecule (Fig. 20A and B). These materials were then used as photocatalysts in the photodegradation of MB. The main objective was to check the influence of the bridge between the porphyrin and the TiO2 support on the photocatalytic activity. The photocatalytic activity of both derivatives Fe22Cl–SSA–TiO2 and Fe22Cl–SA–TiO2 was evaluated under white light. Adsorption studies showed that Fe22Cl–SSA–TiO2 and Fe22Cl–SA–TiO2 present a higher capacity to adsorb MB than bare TiO2. The FT-IR results showed that the Fe22Cl molecules, rather than being adsorbed onto the surface of TiO2, are linked to TiO2 through stable π-conjugated chemical bonds (Fig. 20A and B). The Fe22Cl–SSA–TiO2 hybrid showed the best photocatalytic activity (99.3%) under visible light when compared with Fe22Cl–SA–TiO2 (85.2%), Fe22Cl–TiO2 (68.9%) and TiO2 (30.7%). Even after being recycled 5 times, the best photocatalyst could degrade ca. 90% of MB. However, a slight decrease of photoactivity was observed when SA was used, suggesting that the bridge between the porphyrin and TiO2 was weaker. Moreover, the degradation efficiency of MB with Fe22Cl–TiO2 seems to decrease rapidly after recycling, which could be due to a bleaching process that is suppressed when SSA is used. The authors suggested that there is a break of the aromatic rings of MB, which was further degraded to CO2 and H2O.
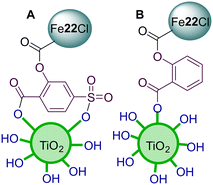 | ||
| Fig. 20 Representation of TiO2 modified with porphyrin Fe22Cl using 2-hydroxy-5-sulfosalicylic acid (A) or salicylic acid (B) bridging molecules. | ||
In 2017, Sobral and co-workers137 prepared and used composite H265–TiO2 (Fig. 21) for the photodegradation of acid black 1 under direct sunlight. The authors found that the photocatalytic activity of bare TiO2 was efficiently improved with its sensitization with H265; the photocatalyst H265–TiO2 could efficiently degrade 90.1% of acid black 1 after 90 min of direct sunlight irradiation, reaching a complete degradation after 120 min.
In 2012, Cai and co-workers138 developed TiO2 microspheres modified with porphyrin H266 (Fig. 21). To sensitize TiO2 microspheres with H266 the authors used allyl bromide and 3-mercaptopropyltrimethoxysilane (MPS) as linkers and studied the capacity of the photocatalyst in α-terpinene degradation under visible light irradiation. The structural characterization of this material revealed that H266–MPS was chemisorbed on the surface of TiO2 through a Si–O–Ti bond (Fig. 21). Regarding the photodegradation assays, when compared with bare TiO2, the presence of the H266–linker–TiO2 greatly enhanced the degradation of α-terpinene under white light. The photocatalyst was reused four times with high efficiency (7% loss).
Mahy and co-workers139 modified TiO2 with three different ratios of a sylilated meso-tetrakis(4-aminophenyl)porphyrin derivative (H267–Si) and evaluated the activity of the resulting hybrids H267–Si–TiO2 (Fig. 22) on the photocatalytic degradation of 4-NP under white light. The hybrid materials proved to be efficient photocatalysts and an improvement in photoactivity is observed when the amount of porphyrin improves.
After 24 h of testing, the photodegradation rate remains constant and no leaching was revealed. However, in the recycling tests, a decrease of photoactivity towards bare TiO2 activity was observed due to bond breaking between H267–Si and TiO2. Remarkably, H267–Si–TiO2 was revealed to be nearly 6 times more photoactive than commercial Evonik P25 for 4-NP degradation under white light.
5.1. Other titanium oxide supports
In 2013, Wan and co-workers141 studied the photocatalytic degradation of some organic dyes with H222 (Fig. 16) modified TiNTs. Within one year apart, Zheng and co-workers,142 and Wan and co-workers143,144 modified TiNTs with metallated porphyrins to obtain Zn22–TiNTs, Fe22Cl–TiNTs and Cu22–TiNTs, respectively, to be used as catalysts in the photodegradation of MB (Table 11).
According to Wan and co-workers,141 a degradation rate of 99.4% was achieved after white light irradiation for 3 h using 5% of H222 loaded into TiO2 nanotubes. Photocatalytic studies showed that the best composite was also efficient in the photodegradation of other organic pollutants, namely RhB.
On the other hand, the studies reported by Zheng and co-workers,142 and Wan and co-workers143,144 revealed that the photocatalysts Fe22Cl–TiNTs, Cu22–TiNTs and Zn22–TiNTs have good activity for MB degradation (higher than 90%) under white light irradiation (Table 11). Moreover, the unique cooperative effect caused by the high surface area, along with the strong adsorption ability and low gap energy, makes Fe22Cl–TiNTs and Zn22–TiNTs good photocatalysts.
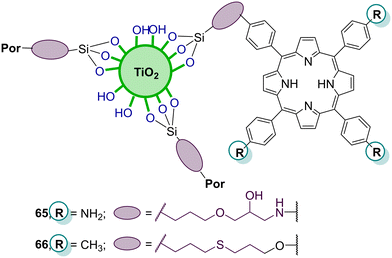
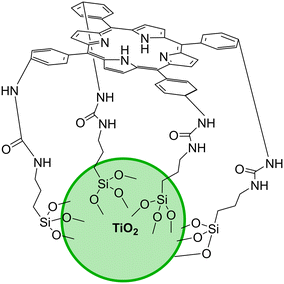
6. Silica-supported porphyrins
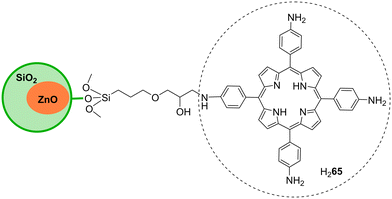
Silica has excellent optical properties, exceptional adsorption and ability of selective separation of chemicals. Moreover, its large surface area, along with its good mechanical and thermal properties, makes silica a good material for active phase-support in catalysis.146 Several types of silica structures can be used for supporting dyes (namely porphyrins) and the resulting hybrids have been frequently used as photocatalysts in water pollutant degradation. The role of those types of silica (nano)structures in photocatalysis is discussed in the following examples.6.1. SiO2 modified with porphyrins
Regarding SiO2 hybrids, Cai and co-workers147 reported the fabrication of silica microspheres functionalized with free-base porphyrin units. The hybrids H268 and H269 (Fig. 23) were used as catalysts for the photodegradation of naphthalene-1,5-diol (Table 12) in water and illumination with an iodine tungsten lamp. Hybrid H269 revealed much higher activity than SiO2 alone or the porphyrin H268. After irradiation for 50 min, the degradation rate was 92.7% with hybrid H269 but only 42.9% in the presence of the unsupported porphyrin. The enhanced catalytic performance of hybrid H269 can be related to the presence of sulfonic groups. The authors also evaluated the reusability of microspheres H269 and found that their catalytic performance has slightly decreased after being reused five times.| Por | Pollutant | Irradiation time (min) | Light source | Degradation rate (%) | Recycle | Ref. |
|---|---|---|---|---|---|---|
| H268 | Naphthalene-1,5-diol | 50 | Visible light (λ > 410 nm) | 43 | — | 147 |
| H269 | 93 | 5 cycles (5% loss) | ||||
| Sn3(OH)2 | Naproxen | 120 | Visible light (λ > 400 nm) | 30 | — | 148 |
| Trimethoprim | 40 | |||||
| Atorvastatin | 70 | |||||
| Tolmetin | 75 | |||||
| Losartan | 100 | |||||
| Propanolol | ||||||
| Cimetidine | ||||||
| Ranitidine | ||||||
| Caffeine | Fluorescent lamp (visible light) | 0 | ||||
| Acetaminophen | ||||||
| Amoxicillin | ||||||
| Naproxen | 50 | |||||
| Trimethoprim | 60 | |||||
| Atorvastatin | 70 | |||||
| Tolmetin | 90 | |||||
| Losartan | 100 | |||||
| Propanolol | ||||||
| Cimetidine | ||||||
| Ranitidine | ||||||
| Fe22Cl | Phenol | 90 | Visible light (λ > 400 nm) | 95 | 150 | |
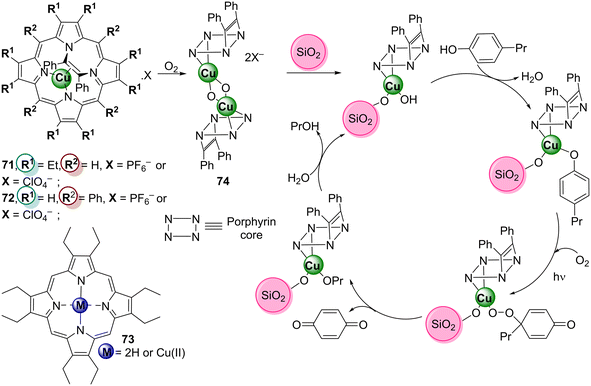
| Cu71 (X = PF6−) | 2,6-DBP | Visible light (λ = 350–800 nm) | 80 | 151 | ||
| Cu71 (X = ClO4−) | 50 | 64 | ||||
| Cu72 (X = PF6−) | 55 | |||||
| Cu74 (X = ClO4−) | 50 | 61 | ||||
| H257 | Metoprolol | 720 | Simulated solar irradiation | 90 | 152 |
Lee and co-workers148 reported the preparation of two photocatalysts consisting of a Sn(IV) porphyrin derivative Sn3(OH)2 supported on silica and the amino-C60 derivative 70 also supported on silica (Fig. 24) and evaluated the influence of the two sensitizers on the photodegradation of a range of phenolic pharmaceutical compounds (Table 12) frequently found as water pollutants. Both photosensitizers were activated under white light to generate 1O2. The study demonstrated that Sn3(OH)2 allowed more effective oxidation of the pharmaceuticals than 70. However, a decrease in the activity of Sn3(OH)2–SiO2 was observed during the recycling tests due to a self-destructive reaction via1O2 induced oxidation of the porphyrin macrocycle. On the other hand, the photocatalytic activity of the aminoC60–SiO270 did not decrease when it was reused for five cycles.
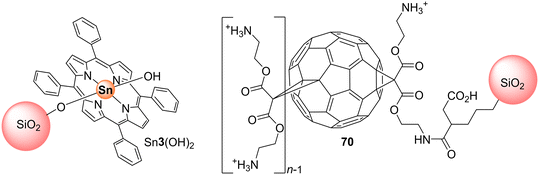 | ||
| Fig. 24 Representation of the photocatalysts Sn3(OH)2–SiO2 and aminoC60–SiO270 used for the degradation of phenolic pharmaceuticals. | ||
In 1993, Bauer and co-workers149 reported that a simple photo-Fenton reaction (Fe2+, H2O2 and light) was adequate to increase the photodegradation of the wastewater pollutant 4-CP when compared with the same reaction in the dark. More recently, Diaz-Uribe and co-workers150 produced a catalyst composed of meso-tetrakis(4-carboxyphenyl)porphyrin iron(III) (Fe22Cl) (Fig. 16) adsorbed on silica and evaluated its efficiency in the oxidation of phenol assisted by white light. In this study, the phenol concentration was reduced from 100 mg L−1 to ca. 5 mg L−1 after 90 min of white light irradiation via oxidation through the photo-Fenton reaction. Hydroquinone, catechol, and p-benzoquinone were identified as intermediate oxidation products but the chain reaction proceeds until the formation of short chain acids that are finally transformed into carbon dioxide and water. The main ROS responsible for the degradation of phenol was OH˙− and its presence during the photooxidation process was corroborated by chemical trapping with terephthalic acid. The study was carried out at four pH values (1.8, 2.4, 2.8, and 3.2), but it was found that the highest rate constant (3.6 ± 0.2 × 10−2 min−1) and the highest yield of the photooxidation process occurred at pH 2.8.
Setsune and co-workers151 synthesized the copper(I) complexes of an N21,N22-etheno bridged octaethylporphyrin (Cu71 (X = PF6− or ClO4−)) and the copper(I) complex of N21,N22-etheno bridged meso-tetraphenylporphyrin (Cu72) with different counter ions (e.g., PF6− and ClO−) (Fig. 25 and Table 12). These complexes were also immobilized on silica gel and used as catalysts for the photooxidation of phenols (2,6-di-t-butylphenol (2,6-DBP) and 4-propylphenol) both under homogeneous and heterogeneous conditions. A plausible mechanism for the heterogeneous photooxidation of 4-propylphenol is shown in Fig. 25.
For the homogenous catalysis, an acetonitrile solution of 2,6-DBP and Cu71 (X = PF6−) was irradiated with white light for 90 min. The only detected oxidation product was 2,6-di-tert-butyl-1,4-benzoquinone, formed in 80% yield (determined by 1H NMR). On the other hand, Cu71 (X = ClO4−) achieved a conversion rate of 64% after 50 min of irradiation; without photoirradiation, only 7% of 2,6-DBP was modified. The efficiency of Cu72 was not very different from the one of OEP derivative Cu71 (X = ClO4−). The N21,N22-etheno bridge porphyrin derivatives could afford higher phenol conversion than the free-base H273 and the tetradentate Cu73 complex. This could be due to their relative photostabilities since a Pd complex of N21,N22-etheno bridge porphyrin was reported to be more photostable than the free-base porphyrin.153,154 The di(μ-hydroxo)dicopper(II) diporphyrin Cu74 (obtained by air-oxidation of complex Cu71 (X = PF6−)) was found to have a similar reactivity in photooxidizing 2,6-DBP (61%) as Cu71 (X = PF6−), which indicated that derivative Cu71 (X = PF6−) acts in the photodegradation reaction through the formation of complex Cu74. Regarding the heterogenous catalysis, the phenol derivative was degraded within 60 min of irradiation with white light when incubated with Cu71 (X = PF6−). Also, this compound was immediately oxidized into a complex Cu74-type which proved to be essential for the photoreaction process. It is important to highlight that, contrary to the homogenous catalysis, the photooxidation of phenol derivatives using Cu71 (X = PF6−) was faster than with its respective free-base in the heterogeneous one. The final composites could be easily recovered and reused up to three times without loss of activity.
Metoprolol is a well-known prescribed β-blocker that is not entirely removed from water in sewage treatment plants. For that reason, Simões and co-workers152 studied the photocatalytic activity of the free-base H257 as a photosensitizer, under homogeneous and heterogeneous conditions, in metoprolol degradation. The promising results led to the study of H257 immobilized on 3-aminopropyl silica (Fig. 26).
Under homogeneous conditions and with simulated solar radiation, H257 was found to be efficient in the photodegradation of metoprolol, with ca. 90% degradation after 12 h. Similarly, the use of the heterogeneous catalyst H257–silica allowed 60% degradation of metoprolol after irradiation for 12 h. Despite the decrease in the photodegradation efficiency, the use of the immobilized porphyrin allows its easy recovery. Studies performed in a sample from the secondary effluent of a wastewater treatment plant fortified with metoprolol revealed that the efficiency of H257–silica was not decreased by the complex matrix of the effluent. It was confirmed that singlet oxygen was the main component responsible for the photodegradation of metoprolol.
6.2. MCM-41 silica modified with porphyrins
Pereira and co-workers155 reported the preparation of MCM-41 with encapsulated porphyrins M75 (M = 2H or Cu(II)) and M76 (M = 2H or Cu(II)) (Fig. 27) in a ship-in-a-bottle way, and the resulting materials were used to degrade 2,4,6-trimethylphenol and two pesticides: fenamiphos and diuron. These pesticides are considered potential groundwater contaminants and it is necessary to find efficient and economical ways to eliminate them. The four immobilized porphyrins M75 (M = 2H or Cu(II)) and M76 (M = 2H or Cu(II)) @MCM-41 were evaluated as photocatalysts for the degradation of 2,4,6-trimethylphenol under polychromatic light (300–460 nm). The best results were obtained with the photocatalyst H276@MCM-41, achieving a degradation of 80%. The studies were extended to the degradation of fenamiphos and diuron in an aqueous solution. The degradation of both pollutants was possible in the presence of O2 under white light irradiation using H276@MCM-41 as a photocatalyst, which was recovered by filtration and reused with no significant decrease of its catalytic activity.7. Immobilized porphyrins on a diversity of supports
7.1. Self-assembly of supramolecular materials
Bhosale and co-workers156 developed a photocatalyst based on a supramolecular nanostructure induced by arginine and the free-base meso-tetrakis(4-carboxyphenyl)porphyrin (H222, Fig. 8) that can degrade RhB. In the presence of L-/D-arginine, H222 forms one-dimensional, self-assembled supramolecular nanobelts with a well-defined morphology that shows an enhanced photocatalytic performance with a maximum RhB degradation up to 90% (when compared with only 15% degradation induced by the H222 monomer) after 3 h under white light irradiation.7.2. Porphyrin–ZnO composites
Zinc oxide is an efficient photocatalyst. In fact, it has been reported157–159 that ZnO can achieve more significant photocatalytic activity than TiO2, the most used inorganic material in catalysis. When modified with porphyrin derivatives, the photocatalytic activity of ZnO is substantially improved, as shown in the next examples concerning the degradation of RhB, MB, MO, and 4-NP.Kang and co-workers160 reported the formation of an inorganic–organic material composed of ZnO microrods and nano-heteroaggregates containing the anionic and cationic porphyrins Co1 and H277 (Fig. 28). The modified ZnO microrods that revealed a photocatalytic activity higher than those of the porphyrin monomers and pure ZnO prompted a total photodegradation of RhB in water, at room temperature, after white light irradiation (λ ≥ 420 nm) for 60 min (Table 13).
| Por | Pollutant | Irradiation time (min) | Light source | Degradation rate (%) | Recycle | Ref. |
|---|---|---|---|---|---|---|
| a In the presence of Cu(II) ions. b In the presence of Fe(III) ions. c In the presence of triethanolamine. | ||||||
| Co1/H277 | RhB | 60 | Visible (λ ≥ 420 nm) | 100 | — | 160 |
| Cu78 | 210 | 63 | 161 | |||
| 36 | UV–vis (λ = 380–780 nm) | 95 | 5 cycles (12% loss) | |||
| H278 | 30 | Sunlight | 98 | 4 cycles (15% loss) | 162 | |
| H279 | ||||||
| H263 | MB | 60 | Visible light (λ > 395 nm) | 55 | — | 37 |
| 80a | ||||||
| 17b | ||||||
| H263 | UV | 25 | ||||
| 90a | ||||||
| 100b | ||||||
| H262 | 50 | Solar | 85 | 4 cycles (0% loss) | 163 | |
| RhB | 80 | |||||
| Eosin yellow | ||||||
| Fe62Cl | 4-NP | 120 | UV | 99 | — | 164 |
| 180 | Solar | 99c | ||||
| Sn22Cl2 | MO | 240 | Visible light (λ > 400 nm) | 85 | 165 | |
Li and co-workers161 reported the synthesis of an organic–inorganic photocatalyst made of Cu78 (Fig. 29) impregnated onto the surface of ZnO. The photocatalytic activity of Cu78–ZnO was evaluated in the photodegradation of RhB under either UV–vis (λ = 380–780 nm) or visible (λ ≥ 420 nm) lights. When irradiated with white light, 63% of RhB was photodegraded with Cu78–ZnO after 210 min (Table 13), but only 23% of RhB was degraded using ZnO (ZnO does not absorb white light). The photocatalyst's reusability was evaluated, and a decrease in the catalytic efficiency from 95% to 83% was observed after five cycles, indicating a slow reduction of the photocatalytic activity.
Mele and co-workers162 accomplished the synthesis of composite nanomaterials based on ZnO impregnated with a lipophilic tetrasubstituted porphyrin bearing hydrogenated cardanol units (H279 and Cu79) (Fig. 29). The FTIR analysis showed that non-covalent interactions occur between H279 and the ZnO support. Sunlight irradiation for 30 min was sufficient to achieve almost complete decomposition (98%) of RhB in the presence of both photocatalysts (H279–ZnO and Cu79–ZnO) – Table 13. These results indicate that these hybrids may be used to eliminate pollutants efficiently from water under ambient conditions. The photocatalysts' reusability was assessed, and it was observed that after four cycles, their photodegradation efficiency was kept above 83%.
As already mentioned, MB is extensively used in the dye industry that can produce hazardous environmental effects in aquatic and human beings.166,167 To remove it from water, Pal and co-workers,37 and more recently Thomas and co-worker,163 reported the formation of light-harvesting nanohybrids composed of ZnO nanorods incorporating organic pigments, namely hematoporphyrin (H263) (ref. 37) and protoporphyrin IX (H262) (ref. 163) (Fig. 18 and Table 13).
Pal and co-workers37 showed that the photoactivation of H263–ZnO nanohybrids with white light leads to the formation of reactive oxygen species and consequently to the photodegradation of MB. Overall, this work37 revealed that this nanohybrid is an effective material for wastewater treatment. Moreover, in another work, they used the nanohybrids Cu62–ZnO and Fe62Cl–ZnO for the photodegradation of MB under white light in the presence of naturally abundant Cu(II) and Fe(III) ions and discovered that these ions could enhance or retard, respectively, the photodegradation of MB. In the presence of Cu(II) ions, 80% degradation of MB was achieved while only 17% degradation was observed in the presence of Fe(III) ions; these results may be correlated with the additional structural stability of the porphyrin macrocycle metallated with Cu(II).37
The photocatalyst composed of protoporphyrin IX–ZnO nanorods (H262–ZnO, Fig. 18) was also reported by Thomas and co-workers.163 The photocatalytic activity of the prepared nanorods was evaluated for several pollutants, such as MB, RhB, and eosin yellow, achieving >80% degradation after 50 or 80 min of solar light irradiation (Table 13). The photocatalyst was found to be stable and reusable for four cycles, and the photocatalytic activity was retained. It is noteworthy that the spectroscopic results revealed that H262 was chemisorbed to the ZnO surface through O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O–Zn bonds. It becomes important to highlight that H262 was not the best photocatalyst despite having a good photocatalytic activity. The best was perylene-3,4,9,10-tetracarboxylic dianhydride–ZnO followed by fluorescein–ZnO and phosphotungstic acid–ZnO.163
C–O–Zn bonds. It becomes important to highlight that H262 was not the best photocatalyst despite having a good photocatalytic activity. The best was perylene-3,4,9,10-tetracarboxylic dianhydride–ZnO followed by fluorescein–ZnO and phosphotungstic acid–ZnO.163
Due to its high stability, 4-NP is a water-soluble highly toxic and hazardous pollutant. Once dissolved in water, its removal by conventional methods is extremely difficult.168–170 However, Devi and co-workers164 reported the preparation of a ZnO-based photocatalyst able to degrade 4-NP under UV and solar light irradiation. According to the authors, almost total degradation of 4-NP occurs after 120 min under UV light irradiation in the presence of ZnO modified with hemin (chloro(protoporphyrinato)iron(III)) (Fe62Cl, Fig. 18 and Table 13) and H2O2. On the other hand, the same degradation rate is achieved after 180 min with triethanolamine as an electron donor under solar light irradiation. The presence of electron donors enhances the efficiency of the degradation process by regenerating neutral hemin from its oxidized form. The neutral hemin can then absorb white light, undergo excitation, and promote a new cyclic process.164
MO, due to its toxicity in water, is frequently used in photodegradation studies.171 Rahimi and co-workers165 reported the photodegradation of 85% of MO using ZnO nanorods with tin(IV) porphyrin derivative Sn22Cl2 after white light irradiation for 240 min. It was found that O2˙− is the principal species responsible for the photodegradation of MO.
7.3. CdS nanocomposites
Cadmium sulphide CdS nanocomposites172,173 are an emerging class of materials used in photocatalysis. Various research groups174 used the free-base H222 (Fig. 30) to prepare CdS nanocomposites used to degrade RhB. It is important to highlight that the photodegradation of RhB with H222–CdS was more efficient under solar light irradiation than with UV light. The photocatalyst was reused five times without loss of its stability.1747.4. Porphyrin–polyoxometalate hybrid materials
Polyoxometalates (POMs) are inorganic metal oxide clusters with remarkable properties. They already have been applied in photocatalysis, electrochemistry, photochromic materials, among others.175,176 Their possible recuperation and reusability make them appealing photocatalysts for the oxidation of organic compounds.175–177 In 2014, Rabbani and co-workers178 reported the synthesis of an organic–inorganic framework where H23 (Fig. 2) was immobilized on the surface of the polyoxometalate H3PMo12O40. The photocatalytic activity of the hybrid material H23–H3PMo12O40 regarding the photodegradation of MB in water under white light irradiation was evaluated and the results confirmed that its efficiency for MB degradation is higher than bare H3PMo12O40. These hybrid materials are, thus, a good alternative for MB photodegradation in an aqueous medium.7.5. Porphyrin–Bi2MoO6 composites
The photocatalytic performance of Bi2MoO6 for water splitting and degradation of organic compounds under visible-light irradiation is well known.179 However, Bi2MoO6 only absorbs white light in the 420–500 nm region. To extend its absorption range to use the sunlight fully, Zhang and co-workers180 introduced different amounts of Cu22 (Fig. 8) into Bi2MoO6 and studied its influence in the photodegradation of RhB. As expected, the resulting composite Cu22/Bi2MoO6 revealed enhanced photocatalytic activity, which can be attributed to the broadened absorption spectrum as well as the increased charge separation rate in the composite. It is important to highlight that the amount of Cu22 loaded into Bi2MoO6 is an important factor for the photocatalytic activity of the composite since above a certain fraction of porphyrin/Bi2MoO6 a decrease of the photocatalytic activity was observed. This result can be explained by the fact that more active reaction sites on the surface of Bi2MoO6 become occupied. The photocatalyst can be reused for five cycles without loss of activity.7.6. Porphyrin–bismuth ferrite hybrids
Condorelli and co-workers181 functionalized multiferroic bismuth ferrite (BFO) with porphyrin H222 (Fig. 8) and the resulting hybrid showed an enhanced photocatalytic activity for the degradation of MB and RhB when compared with bare BFO. The photocatalytic studies were performed in water under simulated solar light irradiation. This hybrid was reused up to four cycles without loss of activity.7.7. Magnetic nanoparticles
Kim and co-workers182 reported the use of water-soluble magnetic nanoparticles functionalized with a Pt(II) 5,15-bis(carboxyphenyl)porphyrin (Pt80, Fig. 31 and 32) as a catalyst for the photodegradation of 2,4,6-TCP in aqueous media under white light irradiation. After immobilization on the magnetic nanoparticles, porphyrin Pt80 maintains its optical and functional properties along with a high singlet oxygen quantum yield. When irradiated with white light, the excited nanoparticles convert 3O2 into 1O2 and other ROS that are responsible for the degradation of 2,4,6-TCP. Despite their high solubility in water, the magnetic property of the microstructure allows its recovery and reuse up to seven cycles by applying a magnetic field.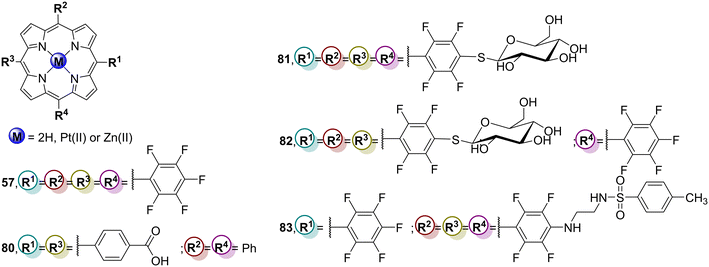 | ||
| Fig. 32 Representation of the silica magnetic nanoparticles functionalized with H257, Pt80 H281, H282 and Zn83. | ||
Tomé and co-workers183 developed silica core–shell magnetic nanoparticles (NP) functionalized with three different porphyrins, H257, H281, and H282 (Fig. 32) and assessed their ability as photocatalysts for the degradation of 17β-estradiol. All non-immobilized porphyrins (H257, H281, and H282) could achieve complete degradation of 17β-estradiol after 8 h of white light irradiation. However, when immobilized in magnetic nanoparticles, H282–NP could induce a complete degradation of the hormone, whereas H257–NP and H281–NP could only achieve 50% degradation after the same time of irradiation.
In 2021, Simões and co-workers184 developed a silica coated nanomagnet porphyrin hybrid and compared the ability of the Zn83–nanomagnet hybrid (Fig. 32) as a photocatalyst for the degradation MO with that of non-immobilized porphyrins H283 and Zn83. The photocatalytic assays were performed under white light irradiation in the presence of hydrogen peroxide. After 270 min of irradiation, complete degradation of the pollutant was achieved by using non-immobilized Zn83 or the Zn83–nanomagnet hybrid. In the absence of H2O2, 60% of MO was degraded when the Zn83–nanomagnet was used, whereas only 10% degradation was observed with the non-immobilized Zn83 and H283.
7.8. Metal-free graphite-like carbon nitride (g-C3N4)
Regarding this type of support, Liu and co-workers28 published a review exploring the ability of graphitic carbon nitride modified with porphyrins, phthalocyanines or other similar dyes as photocatalysts for H2 evolution, CO2 reduction and pollutant degradation. Also, Raizada and co-workers185 reviewed the removal of contaminants from wastewater through photocatalysis by non-noble metals doped on this support. Concerning this last application, they mentioned the work of Zhu and co-workers186 where the hybridization of graphite-like carbon nitride (g-C3N4) with Cu22 (Fig. 8) is explored in the photodegradation of phenol under white light (Table 14). The Cu22 molecules are easily assembled on the surface of g-C3N4 nanosheets through covalent bond and intermolecular interactions, such as electrostatic interaction and π–π stacking interactions between Cu22 and the graphene nanosheets. When compared with pure g-C3N4, Cu22–g-C3N4 showed higher photocatalytic activity under white light irradiation. The enhancement of the photocatalytic activity derives from the efficient electron transfer process from the photoexcited Cu22 molecules to g-C3N4.Cai and co-workers187 adsorbed the metal-free porphyrin H222 (Fig. 8) on g-C3N4 (through π–π stacking interactions) and the resulting hybrid revealed a doubled photocatalytic activity for RhB degradation (Table 14) when compared with bare g-C3N4. The immobilization of the porphyrin on g-C3N4 broadened its absorption spectrum and improved its photocatalytic activity.
Zhu and co-workers188 developed a series of Cu3, Ni3, and Co3 modified g-C3N4 (Fig. 33) in order to assess the photocatalytic degradation of RhB. Co3–g-C3N4 exhibited the highest photocatalytic efficiency reaching a total degradation (99.8%) after 90 min of irradiation, followed by Ni3–g-C3N4, Cu3–g-C3N4, and pristine g-C3N4. The addition of metalloporphyrins, particularly Co3, is favourable for the interfacial charge transfer process, by minimizing the electron–hole recombination, enhancing the photodegradation efficiency of RhB.
Moreover, Li and co-workers189 constructed a visible light-driven PCN-222 g-C3N4 heterojunction using Fe22 (Fig. 8) to decompose RhB and ofloxacin. The composite 1.0 wt% PCN-222–g-C3N4 showed the best photocatalytic activity for ofloxacin and RhB degradation under white light irradiation. The photocatalytic activity of this composite was ca. 3 times higher than that of pristine g-C3N4, reaching ∼98% after irradiation for 120 min. This enhanced photocatalytic activity was attributed to the extended light response range, fast generation, efficient separation of the photogenerated carrier and enhanced adsorption capacity of organic contaminants. The prepared PCN-222–g-C3N4 photocatalyst exhibited excellent recyclability and can be reused four times without activity loss.
7.9. Layered hydroxides
Recently, Liu and co-workers190 constructed an organic–inorganic hybrid palladium porphyrin/NiFe-LDH (Pd22/NiFe-LDH and H222/NiFe-LDH) (Fig. 16) and evaluated its ability as a photocatalyst for the degradation of tetracycline under white light irradiation. The results showed that Pd22/NiFe-LDH is more efficient, achieving a degradation rate of 91% when compared to H222/NiFe-LDH (74%) and bare NiFe-LDH (67%). This enhanced photocatalytic activity is due to the higher production of O2˙− and HO˙.7.10. Lignin
Calvete, Pereira and co-workers191 described the photocatalytic degradation of trimethoprim and sulfamethoxazole, highly prescribed antibiotics, using a white mercury lamp, air as an oxidant and a photocatalyst based on the encapsulation of stable meso-tetra(2,6-dichlorophenyl) porphyrin (H284) (Fig. 34) into acetylated lignin nanoparticles. The authors could achieve almost compete degradation (99 ± 1%) of trimethoprim and sulfamethoxazole after 120 min and 240 min, respectively. The combination of these antibiotics is usually known as cotrimoxazole (also an antibiotic) and for that reason the authors also accessed the ability of this nanoparticle for its photodegradation. After 120 min of irradiation, it was possible to achieve the same photocatalytic rate. It is important to mention that there was no degradation in the dark or just in the presence of the lignin nanoparticles (without porphyrin) for these antibiotics. The photocatalyst could be reused up to 10 times without significant loss of activity (∼5% loss).7.11. Agarose
Hou and co-workers192 developed a Tb-porphyrin (Tb22Cl) (Fig. 16) aerogel using agarose as a building block for the photocatalytic degradation of RhB. After 75 min under visible light irradiation (λ > 420 nm), it was possible to achieve 61% of degradation.8. Immobilized porphyrins on modified inorganic supports
8.1. TiO2–Zr-MOF
In 2017, Feng and co-workers193 developed an efficient catalyst composed of a zirconium-based porphyrin–MOF PCN-222 immobilized onto the surface of TiO2 nanoparticles via 4-mercaptopyridine, which is axially bonded to the metal centre of porphyrin Zn22 (Fig. 35). The composite was effective for RhB degradation under white light irradiation due to the synergistic effect of its components, being able to degrade 75% of RhB after irradiation for 270 min. Fluorescence spectroscopy studies confirmed that the enhanced photocatalytic activity resulted from the electron transfer from PCN-222 to TiO2 and efficient charge separation. Moreover, the composite showed excellent stability and recyclability. | ||
| Fig. 35 Representation of zirconium-based porphyrin Zn22–MOF PCN-222 immobilized onto TiO2 nanoparticles via 4-mercaptopyridine. | ||
8.2. Doped TiO2
Huang and co-workers194 modified La-doped TiO2 with Cu85 (Fig. 36) and assessed its ability as a photocatalyst for MO degradation. Under white light, the photocatalytic activity of La-TiO2 is higher than bare TiO2 and it increases with the sensitization by Cu85. | ||
| Fig. 36 Structure of the porphyrin 85 immobilized on La-doped TiO2 and porphyrin used to prepare triad hybrid multilayers on quartz surfaces 86. | ||
In another study,195 it was observed that the maximum degradation of 4-NP was obtained when the free-base H223 (Fig. 11) was used with TiO2 doped with Fe(III). This catalyst showed an enhanced photocatalytic activity compared with TiO2 or Fe–TiO2, causing a complete degradation of 4-NP after irradiation for 45 min. Finally, the photocatalyst can be recycled at least three times without loss of activity.
The photocatalytic activity of micro and nanostructures of TiO2 impregnated with complexes of metalloporphyrin M23 (M = Mn(III), Fe(III), Ni(II), and Cu(II)) (Fig. 11) under UV light irradiation was also evaluated.196 The highest photocatalytic activity was achieved with impregnated micro-TiO2 catalysts, which indicates an improvement of the electron transfer process between the sensitizer and TiO2 compared with the nano-crystalline structure. Cu23 revealed excellent performance when used in the micro-anatase composite.196
Rahimi and co-workers197 used the free-base porphyrin H222 to sensitize vanadium-doped TiO2. Two photocatalysts were prepared: one containing an ester-like linkage formed between a carboxyl group of H222 and a hydroxyl group of the TiO2 surface (H222–V-TiO2) and the other an amide bond (H222-CO–NHR-V-TiO2) (Fig. 37). These materials were used as catalysts for the photodegradation of MO in an aqueous solution under visible light irradiation. The photocatalytic activity of H28–V-TiO2 was much higher than that of H28-CO–NHR-V-TiO2 with rates of 95% and 62%, respectively, after irradiation for 180 min.
 | ||
| Fig. 37 Representation of vanadium doped TiO2 modified with H222 using a 3-aminopropylsilane bridging unit. | ||
Yao and co-workers198 modified nitrogen doped TiO2 powder with metalloporphyrins Co3, Ni3, Co55, and Ni55 (Fig. 2 and 16) and evaluated the photocatalytic ability of the resulting materials to degrade MB. Nitrogen-doped anatase powder (N-TiO2) modified by each of the four metalloporphyrins exhibited higher degradation efficiency than the unmodified N-TiO2. The results showed that Co3–N-TiO2 and Co55–N-TiO2 exhibited higher degradation efficiency than the corresponding nickel complexes Ni3 and Ni55–N-TiO2, with Co3–N-TiO2 being the best photocatalyst with 94.1% degradation of MB after 180 min irradiation with white light. Additionally, the composites were reused up to four times without significant loss of activity.
Rahimi and co-workers,199 and Abou-Gamra and co-workers200 also used H222 (Fig. 16) on Co-doped TiO2 and regular TiO2 and assessed the ability of the composites to degrade MB and RhB. The results using H222–Co-TiO2 (ref. 199) and H222–TiO2 (ref. 200) showed that these materials are more efficient than bare Co-TiO2 and TiO2, respectively, for the degradation of MB and RhB under white light. The sensitization of metal-doped TiO2 enhances the photocatalytic activity due to the formation of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O–Ti bonds that can function as an electron transfer channel, accelerating the electron injection to the CB of TiO2. Moreover, the composites can directly use sunlight, allowing their use in wastewater treatment.
C–O–Ti bonds that can function as an electron transfer channel, accelerating the electron injection to the CB of TiO2. Moreover, the composites can directly use sunlight, allowing their use in wastewater treatment.
Rabbani and co-workers201 synthesized an Ag-doped mesoporous TiO2 (Ag-TiO2) powder modified with Zn22 (Fig. 16) and the composite was used to degrade 4-NP and MB. The results revealed that Zn22–Ag-TiO2 has a higher activity than Ag-TiO2 under white light but a lower activity under UV light irradiation. The higher activity of Ag-TiO2 under UV light can be justified by the high band gap, being only activated under UV light. The presence of porphyrin molecules on its surface (Zn22–Ag-TiO2) leads to a decrease of active sites for UV light absorption. Regarding the reusability of Zn22–Ag-TiO2, it was verified that there were no significant changes in their photocatalytic activity for four cycles.
Rahimi and co-workers202 used fluorine-doped TiO2 (F-TiO2) nanoparticles modified with H222 or Zn22 (Fig. 16) to photodegrade RhB. The photocatalytic assays were done under UV and white light irradiation. The results showed that, under white light, Zn22–F-TiO2 and H222–F-TiO2 are better catalysts than bare F-TiO2. The O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O–Ti bonds formed between Zn22 or H222 and TiO2 act as an electron transfer channel and accelerate the electron injection to the CB of F-TiO2.
C–O–Ti bonds formed between Zn22 or H222 and TiO2 act as an electron transfer channel and accelerate the electron injection to the CB of F-TiO2.
8.3. TiO2–organic polymers
Considering that photocatalysts resulting from the association of porphyrins with either TiO2 or organic polymers are very effective for the photodegradation of organic pollutants, in 2012, Qi and co-workers203 prepared a fibrous polymer mat-supported Fe3Cl–TiO2 and used it in the photodegradation of azo dyes. The photocatalyst could degrade MO and other azo dyes in up to 98.5% in 5 h. Even after four cycles, the degradation rate remains at 87.0%. These results could contribute to preparing a membrane photo-reactor for continuous wastewater treatment.In 2016, Cantarella and co-workers204 embedded TiO2 modified with H222 (Fig. 16) in poly(methyl methacrylate) (PMMA), aiming to obtain a photocatalyst with no need for recovery after water treatment. The photocatalyst was used in MB degradation and even after 8 cycles, its ability to degrade MB did not decrease. These PMMA materials are stable, cheap and can be prepared by simple methods and, thus, are considered a potential tool for removing organic pollutants from wastewater.
Qi and co-workers205 supported a porphyrin-polyacrylonitrile fibre mat (Fig. 38) in TiO2 and the resulting material (Fe87–PANC–TiO2 mat) was used as a photocatalyst for methyl red degradation in an aqueous solution under white light. A ca. 75% methyl red degradation after irradiation for 120 min was reported. The photocatalyst could be recovered and reused up to 3 times but with a loss of 10% activity after each cycle.
8.4. TiO2–SiO2
Charge-transfer catalysts of TiO2–SiO2, particularly Pt-modified TiO2–SiO2 catalysts,206 have higher activity than bare TiO2. Either TiO2 or SiO2 is widely used as an inorganic support for photocatalytic activity. However, their limitations require the immobilization of a photoactive molecule on the support. Using a TiO2–SiO2 composite modified with a photoactive porphyrin molecule enhances the photocatalytic activity. For that reason, Cai and co-workers207 prepared TiO2–SiO2 modified with Zn(II) carboxyphenyl porphyrins with different para-substituents to be used as photocatalysts for α-terpinene degradation. The photodegradation of α-terpinene with Zn88–, Zn89–, and Zn90–TiO2–SiO2 (Fig. 39) revealed that their photocatalytic activity was much higher than that of bare TiO2–SiO2 under white light irradiation. After irradiation for 50 min, the best photocatalyst was Zn89–TiO2–SiO2 (95.6% degradation), followed by Zn90–TiO2–SiO2 (84.1%) and Zn88–TiO2–SiO2 (76.9%).Qian and co-workers,208 using the layer-by-layer method, constructed triad hybrid multilayers on quartz surfaces formed by zinc meso-tetra(4-pyridyl)porphyrin (Zn86) (Fig. 35) and pyridine-functionalized TiO2 (TiO2-Py) nanoparticles connected by Pd(II) ions. The photocatalytic activity of the nanocomposites for the degradation of MO was assessed in aqueous media under irradiation with visible light (λ > 420 nm) at room temperature. After 48 h, the photocatalyst could degrade 50% of MO, but a complete degradation was achieved only after 8 days. When reused, the photocatalytic activity decreased by about 10%.
8.5. Surface-modified TiO2 nanoparticles
Roques-Carmes and co-workers209 coupled porphyrin derivatives H291 and chlorin H292 to surface-modified TiO2 nanoparticles (Fig. 40) and the photodegradation of MB and MO was used to assess the photocatalytic efficiency of the prepared catalysts under simulated solar and visible light. Porphyrin H291 and chlorin H292 serve as visible–light antennas, thus extending the photoresponse of the hybridized TiO2 nanoparticles towards the visible light region.Enhanced photocatalytic efficiency towards the degradation of MB and MO was observed for the (3-aminopropyl)triethoxysilane (APTES)-modified nanosystems, especially for TiO2–APTES–H292 that lead to 91% degradation of MO under visible light irradiation. Similar nanoparticles prepared using SiO2 instead of TiO2 revealed low photocatalytic activity when compared with those with TiO2.
8.6. TiO2 nanotubes–reduced graphene oxide
Wan and co-workers210 fabricated ternary composites of TiO2 nanotubes (TiNT) with reduced graphene oxide (rGO) and porphyrin H222 and assessed their capability as photocatalysts for the degradation of MB under white light. The experimental results showed more than 92% of MB degradation, about 4.3 times higher than the pure TiNT. The authors suggest a mechanism in which rGO acts as the adsorbent, electron acceptor, and transporter to increase the separation of the electron–hole pairs, accelerating the decomposition of organic pollutants. In turn, porphyrin has a critical role in capturing photons and expanding the absorption wavelength to the visible light region.The same authors also reported the formation of a similar porphyrin/rGO–TiNT nanocomposite but using Cu22.211 In this case, experimental results showed a photodegradation of more than 95% MB, which is about 5 times higher than that of the pure TiNT.
8.7. TiO2–graphene
Rahimi and co-workers212 prepared a TiO2–graphene nanocomposite pillared with porphyrin Sn3(OH)2 (Fig. 41) and used this material as a photocatalyst to degrade MO in an aqueous solution under white light irradiation. This pillared graphene–TiO2–Sn3(OH)2 allowed the complete photocatalytic degradation of MO in 180 min. It was determined that the OH radical is the main active species responsible for the degradation of MO.8.8. TiO2–MOF
Lü, Zhu and co-workers213 published a series of 2D M22 (M = Cu(II), Zn(II), Co(II), and Mn(II))–MOFs/TiO2 (Fig. 16) binary nanocomposites and their ability as photocatalysts for the degradation of RhB under visible light irradiation. After 30 min of irradiation, the best photocatalyst was Cu22–MOF/TiO2 (97%) followed by Zn22–MOF/TiO2 (88%), Co22–MOF/TiO2 (65%) and Mn22–MOF/TiO2 (57%).8.9. Fe3O4@ZrO2
Ghafuri and co-workers214 synthesized a mixture of inorganic supports of Fe3O4@ZrO2 derivatized with H222, Zn22, and Co22 as well as the free-base porphyrin H293, and its metal complexes Zn93 and Co93 (Fig. 42). The photocatalytic properties of materials with carboxy porphyrins H222–, Zn22–, Co22–Fe3O4@ZrO2 and 2-naphthyl esters, H293, Zn93, and Co93–Fe3O4@ZrO2 for the degradation of MB were evaluated and compared. The results showed that carboxy and 2-naphthyl benzoate derivatives lead to different degradation rates, as shown in Fig. 42.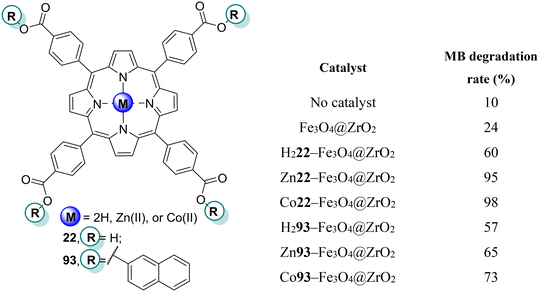 | ||
| Fig. 42 Porphyrins used to sensitize Fe3O4@ZrO2 and the MB photodegradation rates under white light irradiation for 3 h. | ||
The results of the photodegradation of MB allowed us to conclude that: a) the presence of a porphyrin increases the activity of the inorganic support; b) carboxy porphyrin derivatives are more active than the corresponding 2-naphthyl esters; c) Zn(II) and Co(II) porphyrins are more active than the corresponding free-base porphyrins; d) Co(II) porphyrins are more active than Zn(II) porphyrins.
8.10. SiO2–ZnO
Sobral and co-workers215 reported the chemical modification of the inorganic support SiO2–ZnO (prepared by mixing 70% SiO2 and 30% ZnO) with meso-tetrakis(p-aminophenyl)porphyrin (H265) using 3-glycidoxypropyltrimethoxysilane as a coupling agent. This material (Fig. 43) was used as a catalyst for the photodegradation of naphthol black blue under direct sunlight and white light. A 60% degradation of naphthol black blue was achieved with both types of light but at 120 and 180 min of irradiation time, respectively. Moreover, the catalyst was found to be reusable.8.11. SiO2 (quartz)–MOF
Mn22–MOF (Fig. 44) with catalytically active sites was built upon functionalized quartz glass surfaces using a layer-by-layer self-assembly method.216 With a porous reticular structure, the Mn22–MOF films, in the presence of H2O2, could efficiently degrade 90% of MB under visible-light irradiation for 180 min. The catalyst is easily recovered, and after being reused five times, it can still degrade over 85% of MB.8.12. Reduced graphene oxide–Ag nanoparticles
Sun and co-workers217 reported the preparation of organic/inorganic ternary nanohybrids constituted by a Sn(IV) porphyrin (Sn62Cl2), Ag nanoparticles, and reduced graphene oxide (Fig. 45). The nanohybrids revealed superior catalytic activity for the degradation of RhB under white light, reaching 42% degradation in 3 h. Moreover, the ternary nanohybrid was also an active catalyst for reducing 4-NP to 4-aminophenol by NaBH4: 97% of 4-NP was reduced within 17 min.More recently, La, Nguyen and co-workers218 developed the same hybrid (g-C3N4/Ag nanoparticles) using the porphyrin H222 (Fig. 16). The authors evaluated the ability of the hybrid in the photocatalytic degradation of RhB after exposure to sunlight. The authors could achieve a maximum of 97% degradation of RhB using H222-g-C3N4/Ag which was better than using bare g-C3N4/Ag (79%) and H222 (89%). The incorporation of H222 in the hybrid allowed its recovery and reusability for four times with a 12% loss of activity. The authors identified O2˙− and HO˙ as the species responsible for the photodegradation mechanism.
8.13. S or P and N co-doped graphene quantum dots
In 2016, Mahyari and co-workers219 explored the properties of Fe(III) meso-tetra(4-sulfonatophenyl)porphyrin (Fe1Cl) (Fig. 2) supported on S and N co-doped graphene quantum dots (GQDs) as a recyclable heterogeneous photocatalyst for aerobic oxidation of benzyl alcohols under white light irradiation. The synthesized photocatalyst revealed high conversion rates and selectivity for the oxidation of benzyl alcohols to the corresponding aldehydes in water at room temperature. This study provided a promising efficient photocatalyst, recyclable up to 5 cycles without activity loss, to be used in wastewater treatment. The same authors220 extended this study to P and N co-doped graphene quantum dots modified with cobalt(II) porphyrin 1 (Co1). The composite also showed high performance and recyclability as a heterogeneous photocatalyst in the aerobic oxidation of benzyl alcohols under white light irradiation.8.14. BiVO4–graphene nanocomposites
Rahimi and co-workers221 prepared a composite of BiVO4–graphene impregnated with porphyrin H222 (Fig. 16) and evaluated its ability as a photocatalyst to degrade MO in aqueous solution under white light irradiation. The introduction of graphene in the BiVO4 composite enhanced its photocatalytic activity up to 2 times. Incorporation of porphyrin H222 allowed the photocatalytic activity of the resulting nanocomposite to be improved under white light irradiation. After the photosensitization with porphyrin, the photocatalytic activity of the resulting H222–BiVO4–graphene nanocomposite increased 3.5 times and allowed the degradation of 78%, 87%, and 97% of MO at 30 min, 60 min, and 150 min of irradiation, respectively.8.15. Bi2WO6–reduced graphene oxide
Bismuth tungstate-based materials (Bi2WO6) have been widely used as photocatalysts in environmental, energy, and biological fields, including water treatment, air purification, and inactivation of bacteria.222,223Huang and co-workers224 constructed a ternary H222/rGO/Bi2WO6 Z-scheme heterojunction and evaluated its photocatalytic activity. The composite revealed enhanced photocatalytic activity towards the degradation of the antibiotic tetracycline under white light (removal of 83.6%) when compared with pure Bi2WO6, rGO/Bi2WO6, and H222/Bi2WO6. Recycling experiments with the composite showed that the tetracycline removal efficiency remained 79.3% after five cycles.
9. Summary and outlook
This work outlines the significant advances in the photocatalytic oxidation of water pollutants using simple porphyrin derivatives as well as porphyrins linked to a range of organic or inorganic materials, namely polymers, fibres, carbon nanostructures, ZnO, SiO2, and TiO2 (or mixtures of these supports), under UV, visible, and/or solar light irradiation. The presence of different metals in the porphyrin core resulted in different photocatalytic activities, with the highest degradation rates being achieved with Cu(II) porphyrin derivatives.Moreover, by sensitizing TiO2 or SiO2 supports with porphyrins, the photocatalytic activity was greatly enhanced due to the reduction of the material's band gap and the absorption of visible light. This sensitization could be made through covalent or non-covalent interactions. It is important to highlight that the photocatalytic mechanism is based on the excitation of the porphyrin followed by electron transfer to the CB of the support. The photogenerated holes and the excited electrons can react with O2 or H2O to form reactive oxygen species, which will degrade the pollutant. In some cases, the addition of H2O2 is necessary to obtain high rates of pollutant degradation.
The light source (UV, visible, or solar light) is also an important parameter. When solar/white light is used, the support can also be excited along with the porphyrin, but only a small or no excitation of the porphyrin moiety occurs with UV light. The use of solar light is considered a huge advantage in photocatalysis since it reduces costs and takes advantage of all solar spectrum. It is also important to mention that almost all photocatalysts discussed here are reusable, with no significant loss of activity in most cases, and in some cases, the photocatalyst is specific for a selected pollutant. While the presented photocatalytic studies highlighted in this review are promising, there is a noticeable absence of research on identifying the degradation products.
This review shows that the scientific community is closer to finding effective methods for the degradation of pollutants that are difficult to remove from water. However, scale-up studies with the photocatalysts' production and practical applications (in the field) of this technology are (urgently) needed.
Abbreviations
| 2,4,6-TCP | 2,4,6-Trichlorophenol |
| 2,4,6-TNT | 2,4,6-Trinitrotoluene |
| 2,4-DCP | 2,4-Dichlorophenol |
| 2,6-DBP | 2,6-Di-t-butylphenol |
| 4-CP | 4-Chlorophenol |
| 4-NP | 4-Nitrophenol |
| AO | Acridine orange |
| ATRP | Atom transfer radical polymerization |
| BFO | Bismuth ferrite |
| CdS | Cadmium sulphide |
| CB | Conduction band |
| CEES | 2-Chloroethyl ethyl sulfoxide |
| CEESO | 2-Chloroethyl ethyl sulfoxide |
| CTAB | Cetyltrimethylammonium bromide |
| DMF | Dimethylformamide |
| GO | Graphene oxide |
| LCST | Lower critical solution temperature |
| MB | Methylene blue |
| MO | Methyl orange |
| MOF | Metal–organic framework |
| NIR | Near-infrared |
| PAM | Poly(N-isopropylacrylamide) |
| PAN | Polyacrylonitrile |
| PCP | pentachlorophenol |
| PMMA | Poly(methyl methacrylate) |
| POM | Polyoxometalate |
| Por | Porphyrin |
| RhB | Rhodamine B |
| ROS | Reactive oxygen species |
| SA | Salicylic acid |
| SSA | 2-Hydroxy-5-sulfosalicylic acid |
| TiNT | Titanate nanotube |
| TPP | meso-Tetraphenylporphyrin |
| UV | Ultra-violet |
| VB | Valence band |
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Thanks are due to the University of Aveiro and Fundação para a Ciência e a Tecnologia (FCT) for the financial support to LAQV-REQUIMTE (LA/P/0008/2020 DOI: https://doi.org/10.54499/LA/P/0008/2020, UIDP/50006/2020 DOI: https://doi.org/10.54499/UIDP/50006/2020, and UIDB/50006/2020 DOI: https://doi.org/10.54499/UIDB/50006/2020), Centro de Química Estrutural (UIDB/00100/2020 DOI: https://doi.org/10.54499/UIDB/00100/2020 and UIDP/00100/2020 DOI: https://doi.org/10.54499/UIDP/00100/2020), and IMS (LA/P/0056/2020 DOI: https://doi.org/10.54499/LA/P/0056/2020) research units, and to the FCT project P2020-PTDC/QUI-QOR/31770/2017, through national funds and where applicable co-financed by the FEDER-Operational Thematic Program for Competitiveness and Internationalization-COMPETE 2020, within the PT2020 Partnership Agreement. S. Gamelas thanks FCT for her PhD scholarship (SFRH/BD/143549/2019).References
- T. Phani Madhavi, M. Srimurali and K. Nagendra Prasad, J. Environ. Res. Dev., 2014, 8, 890–894 Search PubMed.
- J. Shortle, Water Econ. Policy, 2017, 03, 1771004 CrossRef.
- H. Safardoust-Hojaghan and M. Salavati-Niasari, J. Cleaner Prod., 2017, 148, 31–36 CrossRef CAS.
- A. Tewari, A. Dubey and P. Singh, J. Environ. Res. Dev., 2012, 6, 609–615 Search PubMed.
- X. Zhou, Y. Li and Y. Zhao, RSC Adv., 2014, 4, 15620–15629 RSC.
- V. K. Gupta, I. Ali, T. A. Saleh, A. Nayak and S. Agarwal, RSC Adv., 2012, 2, 6380 RSC.
- J. Schütz, E. Rydin and B. J. Huser, Lake Reservoir Manage., 2017, 33, 152–162 CrossRef.
- X. Yan Xue, R. Cheng, L. Shi, Z. Ma and X. Zheng, Environ. Chem. Lett., 2017, 15, 23–27 CrossRef.
- J. M. Dickhout, J. Moreno, P. M. Biesheuvel, L. Boels, R. G. H. Lammertink and W. M. de Vos, J. Colloid Interface Sci., 2017, 487, 523–534 CrossRef CAS PubMed.
- M. Herraiz-Carboné, S. Cotillas, E. Lacasa, C. Sainz de Baranda, E. Riquelme, P. Cañizares, M. A. Rodrigo and C. Sáez, Sci. Total Environ., 2021, 797, 149150 CrossRef PubMed.
- W. Trösch, Technology Guide: Principles - Applications - Trends, 2009, pp. 394–397 Search PubMed.
- F. Petronella, A. Truppi, C. Ingrosso, T. Placido, M. Striccoli, M. L. Curri, A. Agostiano and R. Comparelli, Catal. Today, 2017, 281, 85–100 CrossRef CAS.
- H. Rajbongshi and D. Kalita, J. Nanosci. Nanotechnol., 2020, 20, 5885–5895 CrossRef CAS PubMed.
- R. K. Vital, N. K. V. Saibaba, K. B. Shaik and R. Gopinath, J. Biorem. Biodegrad., 2016, 7, 1000317 Search PubMed.
- D. Pathania, S. Sharma and P. Singh, Arabian J. Chem., 2017, 10, S1445–S1451 CrossRef CAS.
- P. Fageria, S. Gangopadhyay and S. Pande, RSC Adv., 2014, 4, 24962–24972 RSC.
- Y. Li, T. Li, J. Tian, X. Wang and H. Cui, Part. Part. Syst. Charact., 2017, 34, 1700127 CrossRef.
- B. Cabir, M. Yurderi, N. Caner, M. S. Agirtas, M. Zahmakiran and M. Kaya, Mater. Sci. Eng. B: Solid-State Mater. Adv. Technol., 2017, 224, 9–17 CrossRef CAS.
- K. P. Priyanka, S. Sankararaman, K. M. Balakrishna and T. Varghese, J. Alloys Compd., 2017, 720, 541–549 CrossRef CAS.
- A. Rey, P. García-Muñoz, M. D. Hernández-Alonso, E. Mena, S. García-Rodríguez and F. J. Beltrán, Appl. Catal., B, 2014, 154–155, 274–284 CrossRef CAS.
- J. Sun, L. Qiao, S. Sun and G. Wang, J. Hazard. Mater., 2008, 155, 312–319 CrossRef CAS PubMed.
- Z. Wu, X. Liu, C. Yu, F. Li, W. Zhou and L. Wei, Sci. Total Environ., 2021, 796, 148941 CrossRef CAS PubMed.
- L. Fernández, V. I. Esteves, Â. Cunha, R. J. Schneider and J. P. C. Tomé, J. Porphyrins Phthalocyanines, 2016, 20, 150–166 CrossRef.
- C. Melis, L. Colombo and A. Mattoni, J. Phys. Chem. C, 2011, 115, 18208–18212 CrossRef CAS.
- Q. An, F. Zhang, J. Zhang, W. Tang, Z. Wang, L. Li, Z. Xu, F. Teng and Y. Wang, Sol. Energy Mater. Sol. Cells, 2013, 118, 30–35 CrossRef CAS.
- D. Zeng, C. Yu, Q. Fan, J. Zeng, L. Wei, Z. Li, K. Yang and H. Ji, Chem. Eng. J., 2020, 391, 123607 CrossRef CAS.
- G. Guo, H. Guo, F. Wang, L. J. France, W. Yang, Z. Mei and Y. Yu, Green Energy Environ., 2020, 5, 114–120 CrossRef.
- N. Zhang, L. Wen, J. Yan and Y. Liu, Chem. Pap., 2020, 74, 389–406 CrossRef CAS.
- D. Chen, D. Yang, J. Geng, J. Zhu and Z. Jiang, Appl. Surf. Sci., 2008, 255, 2879–2884 CrossRef CAS.
- Z. Mesgari and J. Saien, Sep. Purif. Technol., 2017, 185, 129–139 CrossRef CAS.
- L. R. Milgrom, The Colours of Life, Oxford University Press, Oxford, UK, 1997 Search PubMed.
- J. E. Falk, Porphyrins and Metalloporphyrins: A new edition based on the original volume, Elsevier Scientific Pub, Liverpool, 1975 Search PubMed.
- X. Huang, K. Nakanishi and N. Berova, Chirality, 2000, 12, 237–255 CrossRef CAS PubMed.
- G. I. Vargas-Zúñiga and J. L. Sessler, in Advances in Inorganic Chemistry, Elsevier Inc., 1st edn, 2018, vol. 71, pp. 327–377 Search PubMed.
- Z. Xu and S. Swavey, Dalton Trans., 2011, 40, 7319 RSC.
- B. B. Beyene and C. H. Hung, Coord. Chem. Rev., 2020, 410, 213234 CrossRef CAS.
- P. Kar, S. Sardar, E. Alarousu, J. Sun, Z. S. Seddigi, S. A. Ahmed, E. Y. Danish, O. F. Mohammed and S. K. Pal, Chem. – Eur. J., 2014, 20, 10475–10483 CrossRef CAS PubMed.
- K. M. Smith, R. Guilard and K. Kadish, The Porphyrin Handbook. Applications: Past, Present and Future, Academic Press, New York, 2000, vol. 1–10 Search PubMed.
- Q. Liu, Y. Ding, Y. Yang, L. Zhang, L. Sun, P. Chen and C. Gao, Mater. Sci. Eng., C, 2016, 59, 445–453 CrossRef CAS PubMed.
- S. R. D. Gamelas, A. T. P. C. Gomes, N. M. M. Moura, M. A. F. Faustino, J. A. S. Cavaleiro, C. Lodeiro, M. I. S. Veríssimo, T. Fernandes, A. L. Daniel-da-Silva, M. T. S. R. Gomes and M. G. P. M. S. Neves, Molecules, 2018, 23, 1–15 CrossRef PubMed.
- C. I. V. Ramos, N. M. M. Moura, S. M. F. Santos, M. A. Faustino, J. P. C. Tomé, F. M. L. Amado and M. G. P. M. S. Neves, Int. J. Mass Spectrom., 2015, 392, 164–172 CrossRef CAS.
- K. B. Ferenz and A. U. Steinbicker, J. Pharmacol. Exp. Ther., 2019, 369, 300–310 CrossRef CAS PubMed.
- S. M. G. Pires, M. M. Q. Simões, I. C. M. S. Santos, S. L. H. Rebelo, M. M. Pereira, M. G. P. M. S. Neves and J. a. S. Cavaleiro, Appl. Catal., B, 2012, 439–440, 51–56 CrossRef CAS.
- C. M. B. Carvalho, T. J. Brocksom and K. T. de Oliveira, Chem. Soc. Rev., 2013, 42, 3302 RSC.
- V. Almeida-Marrero, J. A. González-Delgado and T. Torres, Macroheterocycles, 2019, 12, 8–16 CrossRef CAS.
- S. R. D. Gamelas, J. M. D. Calmeiro, A. T. P. C. Gomes, M. A. F. Faustino, M. G. P. M. S. Neves, A. Almeida, J. P. C. Tomé and L. M. O. Lourenço, Dyes Pigm., 2020, 181, 108476 CrossRef.
- C. P. S. Ribeiro, S. R. D. Gamelas, M. A. F. Faustino, A. T. P. C. Gomes, J. P. C. Tomé, A. Almeida and L. M. O. Lourenço, Photodiagn. Photodyn. Ther., 2020, 31, 101788 CrossRef CAS PubMed.
- S. R. G. Fernandes, R. Fernandes, B. Sarmento, P. M. R. Pereira and J. P. C. Tomé, Org. Biomol. Chem., 2019, 17, 2579–2593 RSC.
- C. Costentin, H. Dridi and J.-M. Savéant, J. Am. Chem. Soc., 2015, 137, 13535–13544 CrossRef CAS PubMed.
- R.-M. Ion, in Phthalocyanines and Some Current Applications, ed. Y. Ylmaz, InTech Open, London, 2017, vol. 9, pp. 189–221 Search PubMed.
- S. N. Ahmed and W. Haider, Nanotechnology, 2018, 29, 342001 CrossRef PubMed.
- J. K. Challis, M. L. Hanson, K. J. Friesen and C. S. Wong, Environ. Sci.: Process. Impacts, 2014, 16, 672–696 CAS.
- C. Schweitzer and R. Schmidt, Chem. Rev., 2003, 103, 1685–1758 CrossRef CAS PubMed.
- S. Mathai, T. A. Smith and K. P. Ghiggino, Photochem. Photobiol. Sci., 2007, 6, 995–1002 CrossRef CAS PubMed.
- A. A. Buglak, M. A. Filatov, M. A. Hussain and M. Sugimoto, J. Photochem. Photobiol., A, 2020, 403, 112833 CrossRef CAS.
- K.-S. Ju and R. E. Parales, Microbiol. Mol. Biol. Rev., 2010, 74, 250–272 CrossRef CAS PubMed.
- W. M. Hikal and H. J. Harmon, Polyhedron, 2009, 28, 113–120 CrossRef CAS.
- S. D. Gokakakar and A. v. Salker, Indian J. Chem. Technol., 2009, 16, 492–498 Search PubMed.
- M. Z. H. b. Zakaria, N. S. A. Zauzi, R. Baini, N. M. Sutan and M. R. Rahman, IOP Conf. Ser.: Mater. Sci. Eng., 2017, 216, 012003 Search PubMed.
- K. Hunger, P. Mischke, W. Rieper and S. Zhang, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2017, pp. 1–24 Search PubMed.
- Z. Xiao, Y. Zhou, X. Xin, Q. Zhang, L. Zhang, R. Wang and D. Sun, Macromol. Chem. Phys., 2016, 217, 599–604 CrossRef CAS.
- N. Bordoloi, M. D. Dey, R. Mukhopadhyay and R. Kataki, Water Sci. Technol., 2018, 77, 638–646 CrossRef CAS PubMed.
- D. K. Surendran, M. M. Xavier, V. P. Viswanathan and S. Mathew, Environ. Sci. Pollut. Res., 2017, 24, 15360–15368 CrossRef CAS PubMed.
- M. Li, H. Zhao and Z. Y. Lu, Microporous Mesoporous Mater., 2020, 292, 109774 CrossRef CAS.
- H. Chen, X. Jin, K. Zhu and R. Yang, Water Res., 2002, 36, 4106–4112 CrossRef CAS PubMed.
- G. Panzarasa and G. Soliveri, Appl. Sci., 2019, 9, 1266 CrossRef CAS.
- B. Muktha, G. Madras, T. N. Guru Row, U. Scherf and S. Patil, J. Phys. Chem. B, 2007, 111, 7994–7998 CrossRef CAS PubMed.
- Y. B. Zhou and Z. P. Zhan, Chem. - Asian J., 2018, 13, 9–19 CrossRef CAS PubMed.
- V. B. Khajone and P. R. Bhagat, Res. Chem. Intermed., 2020, 46, 783–802 CrossRef CAS.
- L. Shao, G. Xing, W. Lv, H. Yu, M. Qiu, X.-M. Zhang and C. Qi, Polym. Int., 2013, 62, 289–294 CrossRef CAS.
- J. W. Choi, S. G. Chung, K. Y. Cho, K. Y. Baek, S. W. Hong, D. J. Kim and S. H. Lee, Water, Air, Soil Pollut., 2012, 223, 1437–1441 CrossRef CAS.
- S. G. Chung, Y. S. Chang, J. W. Choi, K. Y. Baek, S. W. Hong, S. T. Yun and S. H. Lee, Chem. Eng. J., 2013, 215–216, 921–928 CrossRef CAS.
- K. Y. Cho, J. W. Choi, S. H. Lee, S. S. Hwang and K. Y. Baek, Polym. Chem., 2013, 4, 2400–2405 RSC.
- K. Y. Cho, H. J. Kim, X. H. Do, J. Y. Seo, J. W. Choi, S. H. Lee, H. G. Yoon, S. S. Hwang and K. Y. Baek, Res. Chem. Intermed., 2018, 44, 4663–4684 CrossRef CAS.
- N. Qiu, Y. Li, S. Han, T. Satoh, T. Kakuchi and Q. Duan, J. Appl. Polym. Sci., 2014, 131, 1–8 Search PubMed.
- N. Qiu, Y. Li, S. Han, G. Cui, T. Satoh, T. Kakuchi and Q. Duan, J. Photochem. Photobiol., A, 2014, 283, 38–44 CrossRef CAS.
- Y. Ruan and B. Gao, J. Mol. Struct., 2020, 1210, 128050 CrossRef CAS.
- Y. Li, H. Xu, S. Ouyang and J. Ye, Phys. Chem. Chem. Phys., 2016, 18, 7563–7572 RSC.
- M. S. Deenadayalan, N. Sharma, P. K. Verma and C. M. Nagaraja, Inorg. Chem., 2016, 55, 5320–5327 CrossRef CAS PubMed.
- T. He, B. Ni, S. Zhang, Y. Gong, H. Wang, L. Gu, J. Zhuang, W. Hu and X. Wang, Small, 2018, 14, 10–15 Search PubMed.
- Y. Zong, S. Ma, J. Gao, M. Xu, J. Xue and M. Wang, ACS Omega, 2021, 6, 17228–17238 CrossRef CAS PubMed.
- C. Shi, Z. Zhao, L. Zhao, A. Kushwaha, A. Kumar, J. Wang, Y. Pan, M. Muddassir and Q. Lan, Inorg. Chem. Commun., 2023, 154, 110920 CrossRef CAS.
- N. Wang, S. Liu, Z. Sun, Y. Han, J. Xu, Y. Xu, J. Wu, H. Meng, B. Zhang and X. Zhang, Nanotechnology, 2021, 32, 465705 CrossRef CAS PubMed.
- Z. W. Jiang, Y. C. Zou, T. T. Zhao, S. J. Zhen, Y. F. Li and C. Z. Huang, Angew. Chem., Int. Ed., 2020, 59, 3300–3306 CrossRef CAS PubMed.
- S. Zhao, S. Li, Z. Zhao, Y. Su, Y. Long, Z. Zheng, D. Cui, Y. Liu, C. Wang, X. Zhang and Z. Zhang, Environ. Sci. Pollut. Res., 2020, 27, 39186–39197 CrossRef CAS PubMed.
- V. Likodimos, Appl. Catal., B, 2018, 230, 269–303 CrossRef CAS.
- S. Gorduk, O. Avciata and U. Avciata, Inorg. Chim. Acta, 2018, 471, 137–147 CrossRef CAS.
- M. Y. Chang, Y. H. Hsieh, T. C. Cheng, K. S. Yao, M. C. Wei and C. Y. Chang, Thin Solid Films, 2009, 517, 3888–3891 CrossRef CAS.
- X.-T. Zhou, H.-B. Ji and X.-J. Huang, Molecules, 2012, 17, 1149–1158 CrossRef CAS PubMed.
- M. A. Malik, M. Y. Wani and M. A. Hashim, Arabian J. Chem., 2012, 5, 397–417 CrossRef CAS.
- L. Madriz, H. Carrero, J. Herrera, A. Cabrera, N. Canudas and L. Fernández, Top. Catal., 2011, 54, 236–243 CrossRef CAS.
- L. Madriz, H. Carrero, O. Núñez, R. Vargas and J. Herrera, Quim. Nova, 2016, 33, 1714–1719 Search PubMed.
- G. Mele, R. Del Sole, G. Vasapollo, E. García-López, L. Palmisano and M. Schiavello, J. Catal., 2003, 217, 334–342 CrossRef CAS.
- G. Mele, R. del Sole, G. Vasapollo, E. García-López, L. Palmisano, L. Jun, R. SŁota and G. Dyrda, Res. Chem. Intermed., 2007, 33, 433–448 CrossRef CAS.
- X. Liu, M. Yu, Z. Zhang, X. Zhao and J. Li, Res. Chem. Intermed., 2016, 42, 5197–5208 CrossRef CAS.
- C. Wang, J. Li, G. Mele, G. M. Yang, F. X. Zhang, L. Palmisano and G. Vasapollo, Appl. Catal., B, 2007, 76, 218–226 CrossRef CAS.
- M. Y. Duan, J. Li, M. Li, Z. Q. Zhang and C. Wang, Appl. Surf. Sci., 2012, 258, 5499–5504 CrossRef CAS.
- C. Wang, J. Li, G. Mele, M. Duan, X. Lü, L. Palmisano, G. Vasapollo and F. Zhang, Dyes Pigm., 2010, 84, 183–189 CrossRef CAS.
- X. F. Lü, J. Li, C. Wang, M. Y. Duan, Y. Luo, G. P. Yao and J. L. Wang, Appl. Surf. Sci., 2010, 257, 795–801 CrossRef.
- M. Duan, J. Li, G. Mele, C. Wang, X. Lü, G. Vasapollo and F. Zhang, J. Phys. Chem. C, 2010, 114, 7857–7862 CrossRef CAS.
- J. Zhang, L. Zhang, X. Li, S.-Z. Kang and J. Mu, J. Dispersion Sci. Technol., 2011, 32, 943–947 CrossRef CAS.
- P. Pichat, Photocatalysis and Water Purification: From Fundamentals to Recent Applications, 2013 Search PubMed.
- M. Yu, J. Li, W. Sun, M. Jiang and F. Zhang, J. Mater. Sci., 2014, 49, 5519–5528 CrossRef CAS.
- W. J. Sun, J. Li, G. P. Yao, M. Jiang and F. X. Zhang, Catal. Commun., 2011, 16, 90–93 CrossRef CAS.
- M. M. Yu, C. Wang, J. Li, L. Yuan and W. J. Sun, Appl. Surf. Sci., 2015, 342, 47–54 CrossRef CAS.
- W. Sun, J. Li, G. Yao, F. Zhang and J.-L. Wang, Appl. Surf. Sci., 2011, 258, 940–945 CrossRef CAS.
- G. Vasapollo, G. Mele, R. Del Sole, I. Pio, J. Li and S. E. Mazzetto, Molecules, 2011, 16, 5769–5784 CrossRef CAS PubMed.
- G. P. Yao, J. Li, Y. Luo and W. J. Sun, J. Mol. Catal. A: Chem., 2012, 361–362, 29–35 CrossRef CAS.
- S. Chen and F. Shen, J. Inclusion Phenom. Macrocyclic Chem., 2017, 88, 229–238 CrossRef CAS.
- C. Huang, Y. Lv, Q. Zhou, S. Kang, X. Li and J. Mu, Ceram. Int., 2014, 40, 7093–7098 CrossRef CAS.
- X. F. Lü, W. J. Sun, J. Li, W. X. Xu and F. X. Zhang, Spectrochim. Acta, Part A, 2013, 111, 161–168 CrossRef PubMed.
- X. Lü, H. Qian, G. Mele, A. de Riccardis, R. Zhao, J. Chen, H. Wu and N. Hu, Catal. Today, 2017, 281, 45–52 CrossRef.
- W. Sun, J. Li, X. Lü and F. Zhang, Res. Chem. Intermed., 2013, 39, 1447–1457 CrossRef CAS.
- X. Lü, N. Hu, J. Li, H. Ma, K. Du and R. Zhao, Res. Chem. Intermed., 2014, 40, 1911–1922 CrossRef.
- X. Zhao, X. Liu, M. Yu, C. Wang and J. Li, Dyes Pigm., 2017, 136, 648–656 CrossRef CAS.
- X. Q. Su, J. Li, Z. Q. Zhang, M. M. Yu and L. Yuan, J. Alloys Compd., 2015, 626, 252–259 CrossRef CAS.
- C. Dang, Q. Zhang, M. Xu, X. Ruan, P. Xu, J. Yan and J. Li, Inorg. Nano-Met. Chem., 2017, 47, 783–787 CrossRef CAS.
- L. Tasseroul, S. D. Lambert, D. Eskenazi, M. Amoura, C. A. Páez, S. Hiligsmann, P. Thonart and B. Heinrichs, J. Photochem. Photobiol., A, 2013, 272, 90–99 CrossRef CAS.
- C. P. M. de Oliveira, A. L. A. Lage, D. C. da S. Martins, N. D. S. Mohallem and M. M. Viana, Surf. Interfaces, 2020, 21, 100774 CrossRef CAS.
- D. Li, W. Dong, S. Sun, Z. Shi and S. Feng, J. Phys. Chem. C, 2008, 112, 14878–14882 CrossRef CAS.
- K. Kümmerer and K. Kümmerer, Pharmaceuticals in the Environment, Royal Society of Chemistry, Cambridge, 2015 Search PubMed.
- A. S. Taha, C. McCloskey, R. Prasad and V. Bezlyak, Lancet, 2009, 374, 119–125 CrossRef CAS PubMed.
- S. Murphy, C. Saurel, A. Morrissey, J. Tobin, M. Oelgemöller and K. Nolan, Appl. Catal., B, 2012, 119–120, 156–165 CrossRef CAS.
- M. Gaeta, G. Sanfilippo, A. Fraix, G. Sortino, M. Barcellona, G. Oliveri Conti, M. E. Fragalà, M. Ferrante, R. Purrello and A. D'Urso, Int. J. Mol. Sci., 2020, 21, 3775 CrossRef CAS PubMed.
- G. Rigos and G. M. Troisi, Rev. Fish Biol. Fish., 2005, 15, 53–73 CrossRef.
- G. Granados-Oliveros, E. A. Páez-Mozo, F. M. Ortega, C. Ferronato and J. M. Chovelon, Appl. Catal., B, 2009, 89, 448–454 CrossRef CAS.
- H. He, Y. Liu, S. You, J. Liu, H. Xiao and Z. Tu, Int. J. Environ. Res. Public Health, 2019, 16, 5129 CrossRef CAS PubMed.
- J. M. Wan, Z. Z. Wu, H. G. Wang and X. M. Zheng, Adv. Mater. Res., 2012, 441, 544–548 CAS.
- H. Wang, D. Zhou, Z. Wu, J. Wan, X. Zheng, L. Yu and D. L. Phillips, Mater. Res. Bull., 2014, 57, 311–319 CrossRef CAS.
- H. Wang, D. Zhou, S. Shen, J. Wan, X. Zheng, L. Yu and D. Lee Phillips, RSC Adv., 2014, 4, 28978–28986 RSC.
- C.-C. Huang, P. S. Parasuraman, H.-C. Tsai, J.-J. Jhu and T. Imae, RSC Adv., 2014, 4, 6540 RSC.
- S. Halder and P. Bhavana, J. Mol. Struct., 2016, 1120, 62–69 CrossRef CAS.
- M. L. ArunaKumari and L. Gomathi Devi, Environ. Sci.: Water Res. Technol., 2015, 1, 177–187 RSC.
- S. Sardar, S. Sarkar, M. T. Z. Myint, S. Al-Harthi, J. Dutta and S. K. Pal, Phys. Chem. Chem. Phys., 2013, 15, 18562 RSC.
- R. Satish Kumar, K. S. Min, S. H. Lee, N. Mergu and Y. A. Son, J. Photochem. Photobiol., A, 2020, 397, 112595 CrossRef CAS.
- B. Yao, C. Peng, W. Zhang, Q. Zhang, J. Niu and J. Zhao, Appl. Catal., B, 2015, 174–175, 77–84 CrossRef CAS.
- B. Krishnakumar, A. Balakrishna, C. T. Arranja, C. M. F. Dias and A. J. F. N. Sobral, Spectrochim. Acta, Part A, 2017, 176, 134–141 CrossRef CAS PubMed.
- J.-H. Cai, J.-W. Huang, H.-C. Yu and L.-N. Ji, Int. J. Photoenergy, 2012, 2012, 1–10 CrossRef.
- J. G. Mahy, C. A. Paez, C. Carcel, C. Bied, A. S. Tatton, C. Damblon, B. Heinrichs, M. Wong Chi Man and S. D. Lambert, J. Photochem. Photobiol., A, 2019, 373, 66–76 CrossRef CAS.
- X. Li, L. Liu, S. Z. Kang, J. Mu and G. Li, Appl. Surf. Sci., 2011, 257, 5950–5956 CrossRef CAS.
- M. Wei, J. Wan, Z. Hu, B. Wang and Z. Peng, RSC Adv., 2015, 5, 58184–58190 RSC.
- H. G. Wang, Y. Fu, T. Han, J. M. Wan and X. M. Zheng, RSC Adv., 2015, 5, 33570–33578 RSC.
- M. Wei, J. Wan, Z. Hu, Z. Peng and B. Wang, J. Mater. Sci.: Mater. Electron., 2016, 27, 4026–4034 CrossRef CAS.
- M. Wei, J. Wan, Z. Hu, Z. Peng, B. Wang and H. Wang, Appl. Surf. Sci., 2017, 391, 267–274 CrossRef CAS.
- L. Gomathi Devi and P. M. Nithya, Inorg. Chem. Front., 2018, 5, 127–138 RSC.
- N. N. Ilkhechi and B. K. Kaleji, Silicon, 2017, 9, 943–948 CrossRef CAS.
- J. H. Cai, J. W. Huang, H. C. Yu and L. N. Ji, J. Taiwan Inst. Chem. Eng., 2012, 43, 958–964 CrossRef CAS.
- H. Kim, W. Kim, Y. MacKeyev, G. S. Lee, H. J. Kim, T. Tachikawa, S. Hong, S. Lee, J. Kim, L. J. Wilson, T. Majima, P. J. J. Alvarez, W. Choi and J. Lee, Environ. Sci. Pollut. Res., 2012, 46, 9606–9613 CAS.
- G. Ruppert, R. Bauer and G. Heisler, J. Photochem. Photobiol., A, 1993, 73, 75–78 CrossRef CAS.
- C. E. Diaz-Uribe, W. A. L. Vallejo and J. Miranda, J. Photochem. Photobiol., A, 2014, 294, 75–80 CrossRef CAS.
- Y. Takao, F. Matsumoto, K. Moriwaki, T. Mizuno, T. Ohno and J.-I. Setsune, J. Porphyrins Phthalocyanines, 2015, 19, 786–793 CrossRef CAS.
- C. M. B. Neves, O. M. S. Filipe, N. Mota, S. A. O. Santos, A. J. D. Silvestre, E. B. H. Santos, M. G. P. M. S. Neves and M. M. Q. Simões, J. Hazard. Mater., 2019, 370, 13–23 CrossRef CAS PubMed.
- Y. Takao, T. Ohno, K. Moriwaki, F. Matsumoto and J. Setsune, J. Porphyrins Phthalocyanines, 2010, 14, 64–68 CrossRef CAS.
- Y. Takao, T. Takeda, S. Tanikawa and J. Setsune, J. Porphyrins Phthalocyanines, 2003, 7, 521–525 CrossRef CAS.
- M. Silva, M. E. Azenha, M. M. Pereira, H. D. Burrows, M. Sarakha, C. Forano, M. F. Ribeiro and A. Fernandes, Appl. Catal., B, 2010, 100, 1–9 CrossRef CAS.
- D. D. La, S. v. Bhosale, L. A. Jones and S. v. Bhosale, Photochem. Photobiol. Sci., 2017, 16, 151–154 CrossRef CAS PubMed.
- X. Chen, Z. Wu, D. Liu and Z. Gao, Nanoscale Res. Lett., 2017, 12, 4–13 CrossRef PubMed.
- K. M. Lee, C. W. Lai, K. S. Ngai and J. C. Juan, Water Res., 2016, 88, 428–448 CrossRef CAS PubMed.
- F. Chen, C. Yu, L. Wei, Q. Fan, F. Ma, J. Zeng, J. Yi, K. Yang and H. Ji, Sci. Total Environ., 2020, 706, 136026 CrossRef CAS PubMed.
- X. Li, Y. Cheng, S. Kang and J. Mu, Appl. Surf. Sci., 2010, 256, 6705–6709 CrossRef CAS.
- W. Sun, J. Li, G. Mele, Z. Zhang and F. Zhang, J. Mol. Catal. A: Chem., 2013, 366, 84–91 CrossRef CAS.
- V. G. P. Ribeiro, A. M. P. Marcelo, K. T. da Silva, F. L. F. da Silva, J. P. F. Mota, J. P. C. do Nascimento, A. S. B. Sombra, C. da Silva Clemente, G. Mele, L. Carbone and S. E. Mazzetto, Materials, 2017, 10, 1–16 CrossRef PubMed.
- S. Radhika, J. Environ. Chem. Eng., 2017, 5, 4239–4250 CrossRef CAS.
- L. G. Devi, M. L. ArunaKumari, B. G. Anitha, R. Shyamala and G. Poornima, Surf. Interfaces, 2016, 1–3, 52–58 CrossRef CAS.
- R. Rahimi, M. Yaghoubi-Berijani, S. Zargari, M. Rabbani and S. Shariatinia, Res. Chem. Intermed., 2016, 42, 4697–4714 CrossRef CAS.
- M. Shaban, M. R. Abukhadra, A. A. P. Khan and B. M. Jibali, J. Taiwan Inst. Chem. Eng., 2017, 82, 102–116 CrossRef.
- M. Berrios, M. A. Martín and A. Martín, J. Ind. Eng. Chem., 2012, 18, 780–784 CrossRef CAS.
- K. Chakraborty, S. Ibrahim, P. Das, S. Ghosh and T. Pal, in AIP conference proceedings, 2017, vol. 050077, p. 050077 Search PubMed.
- K. Chakraborty, S. Chakrabarty, T. Pal and S. Ghosh, New J. Chem., 2017, 41, 4662–4671 RSC.
- M. Ahmaruzzaman, Adv. Colloid Interface Sci., 2008, 143, 48–67 CrossRef CAS PubMed.
- T. Chen, Y. Zheng, J. Lin and G. Chen, J. Am. Soc. Mass Spectrom., 2008, 19, 997–1003 CrossRef CAS PubMed.
- F. Liu, X. Shao, J. Wang, S. Yang, H. Li, X. Meng, X. Liu and M. Wang, J. Alloys Compd., 2013, 551, 327–332 CrossRef CAS.
- X. Liu, Z. Fang, X. Zhang, W. Zhang, X. Wei and B. Geng, Cryst. Growth Des., 2009, 9, 197–202 CrossRef CAS.
- Q. Liu, R. Zhu, Y. Jiang, Q. Jia, S. Yang, Q. Shao, D. Wang and P. Cui, Mater. Sci. Eng. B: Solid-State Mater. Adv. Technol., 2014, 188, 106–113 CrossRef CAS.
- R. Sivakumar, J. Thomas and M. Yoon, J. Photochem. Photobiol., C, 2012, 13, 277–298 CrossRef CAS.
- Y. Li, M. Liu and L. Chen, J. Mater. Chem. A, 2017, 5, 13757–13762 RSC.
- C. Chen, P. Lei and J. Zhao, Environ. Sci. Technol., 2004, 38, 329–337 CrossRef CAS PubMed.
- R. Rahimi, F. Rafiee and M. Rabbani, Proceedings of The 18th International Electronic Conference on Synthetic Organic Chemistry, 2014, p. b017 Search PubMed.
- H. Yu, L. Jiang, H. Wang, B. Huang, X. Yuan, J. Huang, J. Zhang and G. Zeng, Small, 2019, 15, 1–30 Search PubMed.
- Z. Zhang, H. Huang, S. Sun, J. Xu and N. Zhang, J. Porphyrins Phthalocyanines, 2018, 22, 469–474 CrossRef CAS.
- C. Tudisco, L. Pulvirenti, P. Cool and G. G. Condorelli, Dalton Trans., 2020, 49, 8652–8660 RSC.
- K. H. Choi, K. K. Wang, E. P. Shin, S. L. Oh, J. S. Jung, H. K. Kim and Y. R. Kim, J. Phys. Chem. C, 2011, 115, 3212–3219 CrossRef CAS.
- L. Fernández, W. Borzecka, Z. Lin, R. J. Schneider, K. Huvaere, V. I. Esteves, A. Cunha and J. P. C. Tomé, Dyes Pigm., 2017, 142, 535–543 CrossRef.
- K. A. D. F. Castro, J. M. M. Rodrigues, M. A. F. Faustino, J. P. C. Tomé, J. A. S. Cavaleiro, M. G. P. M. S. Neves and M. M. Q. Simões, J. Organomet. Chem., 2021, 938, 121751 CrossRef CAS.
- V. Hasija, P. Raizada, A. Sudhaik, K. Sharma, A. Kumar, P. Singh, S. B. Jonnalagadda and V. K. Thakur, Appl. Mater. Today, 2019, 15, 494–524 CrossRef.
- D. Chen, K. Wang, W. Hong, R. Zong, W. Yao and Y. Zhu, Appl. Catal., B, 2015, 166–167, 366–373 CrossRef CAS.
- S. Liu, S. Zhou, C. Hu, M. Duan, M. Song, F. Huang and J. Cai, J. Mater. Sci.: Mater. Electron., 2020, 31, 10677–10688 CrossRef CAS.
- J. Zhang, A. Wang, W. Zhao, C. Li, X. Chen, Y. Wang, W. Zhu and Q. Zhong, Dyes Pigm., 2018, 153, 241–247 CrossRef CAS.
- H. Jia, D. Ma, S. Zhong, L. Li, L. Li, L. Xu and B. Li, Chem. Eng. J., 2019, 368, 165–174 CrossRef CAS.
- T. Xiong, M. Chen, M. Li, Q. Chen, G. Liu, Y. Li, Y. He, W. Tang and X. Liu, Mater. Lett., 2023, 352, 135114 CrossRef CAS.
- G. Piccirillo, N. Maldonado-Carmona, D. L. Marques, N. Villandier, C. A. Calliste, S. Leroy-Lhez, M. E. S. Eusébio, M. J. F. Calvete and M. M. Pereira, Catal. Today, 2023, 423, 113903 CrossRef CAS.
- Q. Xu, X. Guo, C. Hou and Z. Wang, Appl. Organomet. Chem., 2023, 37, 1–9 CrossRef.
- Y. Zhao, Y. Dong, F. Lu, C. Ju, L. Liu, J. Zhang, B. Zhang and Y. Feng, J. Mater. Chem. A, 2017, 5, 15380–15389 RSC.
- Z. L. Ma, G. F. Huang, D. S. Xu, M. G. Xia, W. Q. Huang and Y. Tian, Mater. Lett., 2013, 108, 37–40 CrossRef CAS.
- G. Mele, I. Pio, A. Scarlino, E. Bloise, R. del Sole, L. Palmisano and G. Vasapollo, J. Catal., 2013, 2013, 7 Search PubMed.
- R. Słota, G. Dyrda, K. Szczegot, G. Mele and I. Pio, Photochem. Photobiol. Sci., 2011, 10, 361–366 CrossRef PubMed.
- R. Rahimi, M. M. Moghaddas and S. Zargari, J. Sol-Gel Sci. Technol., 2013, 65, 420–429 CrossRef CAS.
- J. Niu, B. Yao, Y. Chen, C. Peng, X. Yu, J. Zhang and G. Bai, Appl. Surf. Sci., 2013, 271, 39–44 CrossRef CAS.
- R. Rahimi, E. Honarvar Fard, S. Saadati and M. Rabbani, J. Sol-Gel Sci. Technol., 2012, 62, 351–357 CrossRef CAS.
- M. A. Ahmed, Z. M. Abou-Gamra, H. A. A. Medien and M. A. Hamza, J. Photochem. Photobiol., B, 2017, 176, 25–35 CrossRef CAS PubMed.
- M. Rabbani, H. Bathaee, R. Rahimi and A. Maleki, Desalin. Water Treat., 2016, 57, 25848–25856 CrossRef CAS.
- R. Rahimi, S. Saadati and E. Honarvar Fard, Environ. Prog. Sustainable Energy, 2015, 34, 1341–1348 CrossRef CAS.
- L. Shao, H. Xie, J. Mo, Z. Yang, Z. Fan and C. Qi, Environ. Eng. Sci., 2012, 29, 807–813 CrossRef CAS.
- M. Cantarella, R. Sanz, M. A. Buccheri, F. Ruffino, G. Rappazzo, S. Scalese, G. Impellizzeri, L. Romano and V. Privitera, J. Photochem. Photobiol., A, 2016, 321, 1–11 CrossRef CAS.
- L. Shao, J. Chen, L. He, G. Xing, W. Lv, Z. Chen and C. Qi, Turk. J. Chem., 2012, 36, 700–708 CAS.
- X. Zhang, H. Yang, F. Zhang and K. Chan, Mater. Lett., 2007, 61, 2231–2234 CrossRef CAS.
- J.-H. Cai, Y.-J. Ye, J.-W. Huang, H.-C. Yu and L.-N. Ji, J. Sol-Gel Sci. Technol., 2012, 62, 432–440 CrossRef CAS.
- X. B. Ren, M. Chen and D. J. Qian, Langmuir, 2012, 28, 7711–7719 CrossRef CAS PubMed.
- Z. Youssef, P. Arnoux, L. Colombeau, J. Toufaily, T. Hamieh, C. Frochot and T. Roques-Carmes, J. Photochem. Photobiol., A, 2018, 356, 177–192 CrossRef CAS.
- J. Wan, M. Wei, Z. Hu, Z. Peng, B. Wang, D. Feng and Y. Shen, Int. J. Hydrogen Energy, 2016, 41, 14692–14703 CrossRef CAS.
- M. Wei, J. Wan, Z. Hu, Z. Peng and B. Wang, Appl. Surf. Sci., 2016, 377, 149–158 CrossRef CAS.
- S. Zargari, R. Rahimi, A. Ghaffarinejad and A. Morsali, J. Colloid Interface Sci., 2016, 466, 310–321 CrossRef CAS PubMed.
- W. Zhu, Z. Xia, B. Shi and C. Lü, Langmuir, 2023, 39, 15665–15675 CrossRef CAS PubMed.
- H. Ghafuri, Z. Movahedinia, R. Rahimi and H. R. Esmaili Zand, RSC Adv., 2015, 5, 60172–60178 RSC.
- B. Krishnakumar, A. Balakrishna, S. A. Nawabjan, V. Pandiyan, A. Aguiar and A. J. F. N. Sobral, J. Phys. Chem. Solids, 2017, 111, 364–371 CrossRef CAS.
- Y. Zhou, W. Yang, M. Qin and H. Zhao, Appl. Organomet. Chem., 2016, 30, 188–192 CrossRef CAS.
- H. Li, Y. Zhang, G. Chang, S. Liu, J. Tian, Y. Luo, A. M. Asiri, A. O. Al-Youbi and X. Sun, ChemPlusChem, 2012, 77, 545–550 CrossRef CAS.
- T. T. Nguyen, H. T. Bui, G. T. Nguyen, T. N. Hoang, C. Van Tran, P. H. Ho, P. T. Hoai Nguyen, J. Y. Kim, S. W. Chang, W. J. Chung, D. D. Nguyen and D. D. La, Environ. Res., 2023, 231, 115984 CrossRef CAS PubMed.
- M. Mahyari, Y. Bide and J. N. Gavgani, Appl. Catal., A, 2016, 517, 100–109 CrossRef CAS.
- M. Mahyari and J. Nasrollah Gavgani, Res. Chem. Intermed., 2018, 44, 3641–3657 CrossRef CAS.
- S. Aghakhaninejad, S. Zargari and R. Rahimi, SN Appl. Sci., 2020, 2, 1–12 Search PubMed.
- H. Yi, L. Qin, D. Huang, G. Zeng, C. Lai, X. Liu, B. Li, H. Wang, C. Zhou, F. Huang, S. Liu and X. Guo, Chem. Eng. J., 2019, 358, 480–496 CrossRef CAS.
- T. Chen, L. Liu, C. Hu and H. Huang, Chin. J. Catal., 2021, 1413–1438 CrossRef CAS.
- K. Hu, C. Chen, Y. Zhu, G. Zeng, B. Huang, W. Chen, S. Liu, C. Lei, B. Li and Y. Yang, J. Colloid Interface Sci., 2019, 540, 115–125 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |