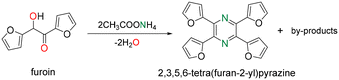Open Access Article
Open Access ArticleSynthesis of amine derivatives from furoin and furil over a Ru/Al2O3 catalyst†
Li
Gao
a,
Massimo
Delle Piane‡
 b,
Marta
Corno
b,
Marta
Corno
 c,
Fan
Jiang
a,
Robert
Raja
c,
Fan
Jiang
a,
Robert
Raja
 d and
M.
Pera-Titus
d and
M.
Pera-Titus
 *ae
*ae
aEco-Efficient Products and Processes Laboratory (E2P2L), UMI 3464 CNRS-Solvay, 3966 Jin Du Road, Xin Zhuang Ind. Zone, 201108 Shanghai, China
bFaculty of Production Engineering and Bremen Center for Computational Materials Science, University of Bremen, Am Fallturm 1, 28359 Bremen, Germany
cDepartment of Chemistry, University of Torino, Via P. Giuria 7, 10125 Torino, Italy
dSchool of Chemistry, University of Southampton, Highfield campus, Southampton SO171BJ, UK
eCardiff Catalysis Institute, School of Chemistry, Cardiff University, Main Building, Park Place, Cardiff CF10 3AT, UK. E-mail: peratitusm@cardiff.ac.uk
First published on 19th March 2024
Abstract
The direct/reductive amination of carbohydrate-based furoin and furil with NH3/H2 was investigated to access amine derivatives. In the sole presence of NH3, cyclic amines, i.e. 2,3,5,6-tetra(furan-2-yl)pyrazine and 2,2′-bipyridine-3,3′-diol, were generated as the main products from furoin and furil, respectively. Over Ru/Al2O3 under NH3/H2, 2-amino-1,2-di(furan-2-yl)ethan-1-ol (i.e. alcohol–amine) was generated as the main product with 47% yield at 140 °C for 2 h starting from furoin. The catalyst could be recycled for at least three consecutive runs. An alcohol–imine was the main intermediate that underwent tautomerization to alcohol–enamine/keto–amine leading to cyclic by-products by self-condensation. DFT calculations, complementing the experimental observations, provided detailed molecular-level insight into the reactivity of the alcohol–imine intermediate. Its preferential adsorption on Ru centers via the NH group, with the OH group pointing away from the surface, was found to direct its hydrogenation towards the alcohol–amine as main product. By combining Ru/Al2O3 and a silica-anchored N-heterocyclic carbene (NHC) catalyst, the alcohol-amine could be accessed with 42% overall yield in a single reactor.
Introduction
Furfural (FF) is a cheap commercial platform molecule (1.0–1.2€ per kg) that can be prepared at a large scale by dehydration of carbohydrates (>200 kT per year).1 FF can be used as a building block for accessing a variety of products and intermediates.1c,2 In particular, FF can be converted into amines by reductive amination, which are valuable intermediates to manufacture polymers, surfactants, biologically active molecules and pharmaceuticals (e.g., furosemide).3 The synthesis of furfurylamines can be conducted from FF and 5-hydroxymethylfurfural (HMF) by direct/reductive amination over homogeneous and heterogeneous catalysts.4 Also, secondary and tertiary tetrahydrofurfurylamines can be accessed with high yield (>90%) from FF over Pd/Al2O3 at room temperature and 1 bar H2.5An upgrading process of FF comprises C–C bond coupling and amination. In this view, it is desirable to design multistep processes with a high degree of intensification and high activity/selectivity to the desired amines. One-pot reactions have been reported for the synthesis of furan- and THF-derived amines combining an aldol condensation reaction of FF with ketones, followed by reductive amination with NH3 and H2, over a mixture of Amberlyst-26 (A26) and Ru/C or Pd/Al2O3 catalysts.6 For example, using Pd/Al2O3 as a catalyst and H2 (2.0 MPa) as a reductant, 98% overall yield of THF–amine was achieved at 120 °C after 20 h.6b
Benzoin condensation is a C–C coupling reaction that can promote the self-condensation of aldehydes using nucleophiles such as cyanides or N-heterocyclic carbenes (NHC) as catalysts.7 FF and HMF can self-condense towards 5,5′-dihydroxymethylfuroin (DHMF) derivatives using NHC organocatalysts.8 The reaction mechanism operates via umpolung condensation as proposed by Breslow.9 In particular, benz-imidazolium (bim) salts with one/two long-chain aliphatic substituents at the N-atoms in the imidazole ring are active catalysts for the reaction.10 Bim catalysts have been supported over silica and polymers with controlled mesoporosity, affording recyclable heterogeneous catalysts.11 In the presence of a base (e.g., 1,8-diazabicylo-[5.4.0]undec-7-ene, DBU), supported bim catalysts can catalyze the self-condensation of FF into furoin with 96–99% yield over three consecutive runs. Sequential reactions have been developed encompassing furoin derivatives targeting the synthesis of branched alkanes. Furoin/furil intermediates derived from FF can be hydrogenated towards THF-derivatives and subjected to ring opening by hydrodeoxygenation over Pd/C + La(OTf)3/Eu(OTf)3 catalysts, accessing C10–C12 diesel fuels.8b,12 Furoin/furil intermediates have also been employed to prepare C12 biofuels by subsequent esterification and etherification reactions.13
Herein we investigated the direct/reductive amination of furoin and furil with NH3/H2 over 5% Ru/Al2O3. DFT calculations were implemented to rationalize the nature and stability of surface species on Ru(0001) in the presence of adsorbed NH3 and ad-H, as well as the formation of 2-amino-1,2-di(furan-2-yl)ethan-1-ol (i.e. alcohol–amine) as main product and the alcohol–imine and its tautomers leading to cyclic by-products. We also engineered a single-reactor tandem process by combining silica-supported bim to catalyze benzoin condensation (C–C coupling), and 5% Ru/Al2O3 to catalyze reductive amination, to access furoin alcohol–amine starting from FF.
Results and discussion
Catalyst-free conversion of furoin/furil under NH3
Furoin was first reacted with both NH3 and ammonium acetate as an NH3 surrogate in N,N-dimethylformamide (DMF). 2,3,5,6-Tetra(furan-2-yl)pyrazine is generated as the main product at a temperature higher than 130 °C (Scheme 1), which is in accordance with an earlier report.14 The yield is about 15–18% at full furoin conversion by reacting furoin at 140 °C for 4 h, using 0.06 g of furoin, 6 mL of DMF and 1 g of ammonium acetate (or 0.22 g NH3). A myriad of minor products are generated with low yield (<5%), including 2-amino-1,2-di(furan-2-yl)ethan-1-ol. The easier operation and higher solubility of ammonium acetate as a masked NH3 reagent compared to gas NH3 prompted us to use this reagent in the forthcoming experiments.We next explored the effect of the operation variables on the yield of 2,3,5,6-tetra(furan-2-yl)pyrazine at 140 °C for 4 h. Among the solvents tested, DMF, THF and 2-methyl-THF afford a yield of 18–21% (Table S1†). The reaction does not proceed in water most likely due to the poor solubility of furoin. We further studied the effect of the temperature using THF for 3 h reaction while keeping the other reaction conditions unchanged (Table S2†). The yield of 2,3,5,6-tetra(furan-2-yl)pyrazine increases monotonously in the range of 130–160 °C up to 30% at full conversion. The highest yield (30%) occurs for an ammonium acetate loading above 0.1 g at 160 °C in THF (Table S3†). The yield of 2,3,5,6-tetra(furan-2-yl)pyrazine reaches 44% at 160 °C for 3 h using dry THF and dry ammonium acetate (Table S4†).
A possible reaction mechanism for 2,3,5,6-tetra(furan-2-yl)pyrazine formation by reaction of furoin with ammonium acetate/NH3 is depicted in Scheme 2. The first step consists of the nucleophilic attack of NH3 to the carbonyl group of furoin to generate an alcohol–imine intermediate with concomitant release of one water molecule. This intermediate can undergo tautomerization to alcohol–enamine/keto–amine by-products (m/z = 191) that can be hardly distinguished by GC-MS. The self-condensation of the keto–amine, followed by dehydrogenation, results in 2,3,5,6-tetra(furan-2-yl)pyrazine.
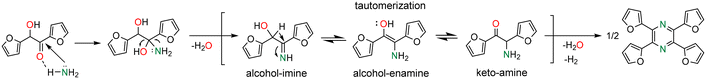 | ||
| Scheme 2 Proposed mechanism for 2,3,5,6-tetra(furan-2-yl)pyrazine formation from the reaction of furoin with ammonium acetate/NH3. | ||
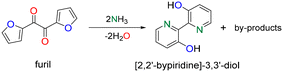
Next, we reacted furil with NH3 in DMF. 2,2′-Bipyridine-3,3′-diol is generated as the main product with a yield of 22% at 170 °C after 4 h at full furil conversion (Scheme 3, Table S5†). A variety of by-products are generated with low yield (<5%), including 2-amino-1,2-di(furan-2-yl)ethan-1-ol and 1,2-di(furan-2-yl)ethane-1,2-diamine (i.e. diamine). The yield of 2,2′-bipyridine-3,3′-diol increases to 36% at 180 °C, but decreases further to 24% at 200 °C (Table S6†). This observation suggests partial product/substrate decomposition at higher temperature. The formation of 2,2′-bipyridine-3,3′-diol can be rationalized on the basis of the mechanism depicted in Scheme 4. Furil reacts fast with NH3 generating a diimine intermediate. This intermediate is unstable and may be easily activated by a proton in solution favoring ring activation and intramolecular rearrangement leading to pyridine rings. The diimine intermediate can also suffer from reversible polymerization, as earlier observed for diformylfuran upon exposure to NH3.15
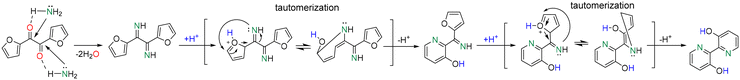 | ||
| Scheme 4 Possible mechanism for 2,2′-bipyridine-3,3′-diol formation from the reaction of furil with NH3. | ||
Furoin and furil amination in the presence of a catalyst
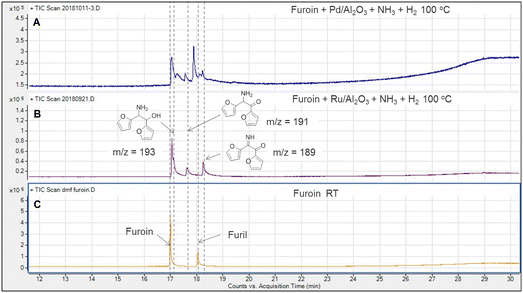
With the results above, we studied the reductive amination of furoin and furil with NH3 and H2. The catalytic tests were first conducted using furoin at 80 °C and 100 °C in DMF over 5% Pd/Al2O3 and 5% Ru/Al2O3 catalysts (Fig. S1† and 1). Pd/Al2O3 provides a broad distribution of products, especially at lower temperature (Fig. 1A). In contrast, the alcohol–amine is formed as the main product over Ru/Al2O3 (Fig. 1B), as confirmed by GC-MS and 1H NMR (presence of two doublets centered at 4.7 ppm and 5.4 ppm) (Fig. S2†). The alcohol–imine and its possible tautomers (keto–amine, alcohol–enamine), and the keto–imine, appear as the main by-products. The detection of the keto–imine suggests the formation of furil from furoin dehydrogenation along the reaction despite the presence of H2 in the reaction medium. Indeed, we confirmed that furil can be generated at room temperature under neat conditions (i.e. without NH3/H2) (Fig. 1C). Additional peaks are observed at higher temperature (not shown) that can be attributed to cyclic by-products, especially 2,3,5,6-tetra(furan-2-yl)pyrazine, and minor formation of oligomers.We measured the yield of alcohol–amine from furoin amination over 5% Pd/Al2O3, 5% Ru/Al2O3, 5% Rh/Al2O3 and 5% Pt/Al2O3, at 160 °C for 2 h (Table 1). The first three catalysts exhibit poor yield (up to 28%) (entries 1–3), whereas the yield over 5% Ru/Al2O3 is the highest (40%) (entry 7) at full furoin conversion. The yield of alcohol–amine is poorly affected by the Ru loading (entries 4–6) and the type of support (i.e. alumina, silica, carbon) at the same Ru loading (5 wt%) (entries 7–9). The alcohol–imine and its tautomers are generated as the main by-products, as well as 2,3,5,6-tetra(furan-2-yl)pyrazine (not quantified).
| Entry | Catalyst | Yield of alcohol–amine (%) |
|---|---|---|
| a Reaction conditions: furoin (60 mg), NH3 (0.45 g), % Ru/Al2O3 (12 mg), DMF (2 mL), 160 °C, 2 h, H2 (2.0 MPa). The catalyst was pre-reduced at 200 °C before the reaction. b Average particle size of Ru nanoparticles measured by HR-TEM. | ||
| 0 | — | 0 |
| 1 | 5% Pd/Al2O3 | 28 |
| 2 | 5% Rh/Al2O3 | 10 |
| 3 | 5% Pt/Al2O3 | 14 |
| 4 | 0.1% Ru/Al2O3 | 30 |
| 5 | 1% Ru/Al2O3 | 35 |
| 6 | 2% Ru/Al2O3 | 40 |
| 7 | 5% Ru/Al2O3 (6.7 nmb) | 40 |
| 8 | 5% Ru/SiO2 | 35 |
| 9 | 5% Ru/C (2.8 nmb) | 40 |
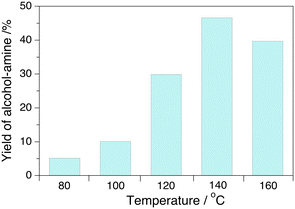
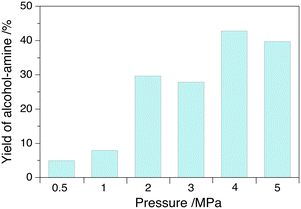
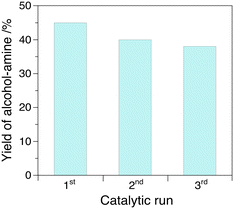
The yield of alcohol–amine increases with the temperature in the range of 80–140 °C at 4.0 MPa H2 pressure with a maximum value of 47%, but decreases slightly further to 40% at 160 °C that we attribute to the formation of oligomers (Fig. 2). The yield of alcohol–amine also increases with the H2 pressure in the range of 0.5–4.0 MPa at constant temperature (160 °C) and keeps unchanged beyond 4.0 MPa (Fig. 3). The alcohol–imine (and possible tautomers) is generated as the main by-product, which can be further hydrogenated to the alcohol–amine product and generate 2,3,5,6-tetra(furan-2-yl)pyrazine and oligomers that were not quantified. However, the keto–imine, which is generated at 80 and 100 °C, vanishes at higher temperature. The catalyst is robust under reaction conditions, and can be reused for three consecutive catalytic runs with a yield of alcohol–amine in the range of 40–45% (Fig. 4).
We also studied the reductive amination of furil with NH3 and H2 over Ru/Al2O3. As in the case of furoin, the alcohol–amine is generated as the main product with 34% yield together with the diimine and 2,2′-bipyridine-3,3′-diol as the main by-products, as well as oligomers. Diamines are generated with less than 5% yield.
Understanding furoin amination over 5% Ru/Al2O3
The results above point out the preferential formation of the alcohol–amine product over Ru/Al2O3 at 140 °C and 2.0 MPa H2 pressure starting from furoin, with ketone–amine/alcohol–imine being the main intermediates. To rationalize the formation of the alcohol–amine and cyclic by-products, we computed the adsorption configurations and energies of the reactants (furoin, furil), end products (alcohol–amine, diamine) and possible intermediates (alcohol–imine + tautomers, diimine). The calculations were carried out both on a bare reduced Ru(0001) surface and on a reduced Ru(0001) surface covered with NH3 and ad-H, at the DFT-PBE level (see the ESI† for computational details).
Table 2 lists the adsorption energies for the optimized configurations of the reactants, products and intermediates on bare and ad-H/NH3-covered Ru(0001). For adsorbed furoin (entries 1–4), four configurations were probed on the bare Ru(0001) interacting via (Scheme 5): i) C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, ii) OH group, iii) both C
O group, ii) OH group, iii) both C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and OH groups, and iv) furan rings. The most stable configuration, i.e. iii), corresponds to a bidentate binding mode with C
O and OH groups, and iv) furan rings. The most stable configuration, i.e. iii), corresponds to a bidentate binding mode with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O showing the shortest Ru–O distance. The adsorption energy on bare Ru(0001) (−156 kJ mol−1) (entry 4) is almost twice the sum of the energies for monodentate adsorption via C
O showing the shortest Ru–O distance. The adsorption energy on bare Ru(0001) (−156 kJ mol−1) (entry 4) is almost twice the sum of the energies for monodentate adsorption via C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (i) and OH (ii) groups, i.e. −59 and −37 kJ mol−1, respectively (entries 1–2). No stable configuration occurs via furan rings as inferred from the positive adsorption energy (+14 kJ mol−1) (entry 3).
O (i) and OH (ii) groups, i.e. −59 and −37 kJ mol−1, respectively (entries 1–2). No stable configuration occurs via furan rings as inferred from the positive adsorption energy (+14 kJ mol−1) (entry 3).
| Entry | Molecule | Interacting groups | ΔEads@bare Ru(0001) | ΔEads@ad-H and NH3 Ru(0001) |
|---|---|---|---|---|
| 1 | Furoin | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
−59 | — |
| 2 | Furoin | OH | −37 | — |
| 3 | Furoin | Furan rings | +14 | — |
| 4 | Furoin | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O + OH O + OH |
−156 | −74 |
| 5 | Furil | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O + C O + C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
−115 | −107 |
| 6 | Alcohol–amine | NH2 | −189 | −36 |
| 7 | Diamine | NH2 | −91 | −24 |
| 8 | Diimine | NH + NH | −252 | −185 |
| 9 | Alcohol–imine | NH | −92 | −31 |
| 10 | Alcohol enamine | NH2 | −45 | −12 |
| 11 | Keto–amine | NH2 | −67 | −23 |
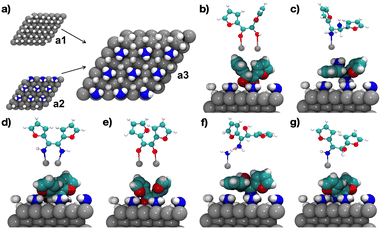
Few studies describe the atomistic details of H2 and NH3 co-adsorption on Ru(0001).16 H2 and NH3 are known to occupy different sites on the Ru(0001) surface, so that ad-H atoms can influence the adsorption pattern of NH3 on Ru(0001).16 Since ad-H atoms occupy mainly fcc sites,17 we simulated the dissociative H2 chemisorption on fcc sites and NH3 adsorption on adjacent top sites. First, we simulated a full monolayer of ad-H on fcc sites without NH3 (Scheme 5a1). The adsorption energy is −111 kJ mol−1 and keeps unchanged when implicit solvation by DMF is included (−112 kJ mol−1). Next, we simulated NH3 chemisorption on only 25% of available top sites to take into account steric constraints (Scheme 5a2). Also, we assumed that no NH3 dissociation occurs at the reaction temperature (up to 160 °C) given its high activation energy (>100 kJ mol−1),18 and that adsorbed NH3 is known to keep undissociated in the presence of ad-H species below 170 °C.17,19 The adsorption energy of NH3 is −75 kJ mol−1 with a limited stabilizing effect of DMF (−5 kJ mol−1), which agrees well with an earlier report.20 The average N–Ru distance is 2.2 Å that is similar to the distance measured for diamine and diimine derivatives. We also computed H2 and NH3 co-adsorption on Ru(0001) (Scheme 5a3). After geometry optimization, half of ad-H atoms move to neighboring hcp sites with a hexagonal arrangement around each adsorbed NH3 molecule. The adsorption energy is −101 kJ mol−1 (−104 kJ mol−1 in DMF).
Using the optimized NH3- and ad-H-covered Ru(0001) surface, we simulated the adsorption of furoin/furil, products and intermediates, and recomputed the adsorption energies (Scheme 5b–g, Table 2). As a rule, although the adsorption energies can show variations when different NH3 and ad-H coverages are considered, the primary effect on the relative stability of the different species and their orientation is expected to be poorly affected.21 With these considerations, all adsorption energies are lower on the NH3- and ad-H-covered Ru(0001) surface than on bare Ru(0001). However, the relative stability between furoin, diamine and diimine is preserved. Furil exhibits stronger adsorption than furoin (−107 kJ mol−1vs. −74 kJ mol−1, compare entries 5 and 4), since the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–Ru interaction is kept when NH3 covers the surface (Scheme 5e). In contrast, in the case of furoin, NH3 bridges the interaction between the OH group and the Ru(0001) surface via H-bonding (Scheme 5b), resulting in lower stability.
O–Ru interaction is kept when NH3 covers the surface (Scheme 5e). In contrast, in the case of furoin, NH3 bridges the interaction between the OH group and the Ru(0001) surface via H-bonding (Scheme 5b), resulting in lower stability.
We also investigated the relative stability of the alcohol–imine and its two tautomers on Ru(0001) in the presence of adsorbed NH3 and ad-H species. The alcohol–imine interacts preferentially with Ru(0001) via the NH group (Scheme 5g), but the adsorption energy is lower compared to the value on the bare surface (−31 vs. −92 kJ mol−1, entry 9) due to steric hindrance. The alcohol–imine is −19 kJ mol−1 more stable than the alcohol–enamine and −8 kJ mol−1 more stable than the keto–amine tautomer over NH3 and ad-H-covered Ru(0001) (entries 10–11) (see also Fig. S3†). This relative stability opposes that observed in bulk solution, where the keto–enamine is the most stable tautomer being 45 kJ mol−1 more stable than the alcohol–imine and 27 kJ mol−1 less stable than the keto–amine.
This body of results points out that the alcohol–imine is a likely intermediate responsible for the formation of the alcohol–amine over Ru(0001). The weak adsorption of the alcohol–imine and its tautomers over Ru(0001) can favor their desorption from the catalyst and the further formation of 2,3,5,6-tetra(furan-2-yl)pyrazine among other cyclic by-products in bulk solution competing with the alcohol–amine proceeding over the catalyst. Besides, preferential interaction of NH or NH2 groups of the alcohol–imine and keto–amine, respectively, on Ru(0001) discourages the formation of the diimine and amine–imine intermediates, and in turn hinders diamine formation. This selectivity shortcoming has also been observed in the amination of isosorbide with NH3 and H2, where the exo-OH group exhibits much lower reactivity than the endo-OH group, resulting in the formation of amino-alcohols with different stereochemistry as the main products with small amounts of diamines.18
Single-reactor tandem benzoin condensation + reductive amination process
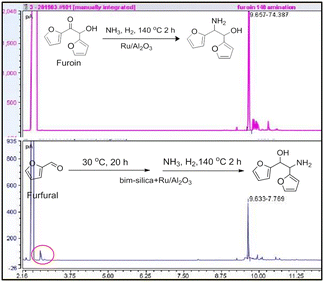
With the results above, we investigated the credentials of a tandem reaction for the synthesis of the alcohol–amine starting from FF by combining supported bim–silica11d and Ru/Al2O3 catalysts in a single reactor (Scheme 6). In this test, both catalysts were loaded in the reactor together with DMF, FF and DBU to activate bim. First, the benzoin condensation reaction was carried out at 30 °C for 20 h. Subsequently, NH3 and H2 were added, and the amination reaction was carried out at 140 °C for 2 h. The results clearly show the formation of the alcohol–amine product (Fig. 5) with 42% overall yield.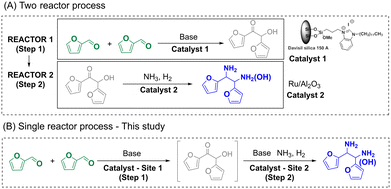 | ||
| Scheme 6 Two-reactor vs. single reactor tandem process for the synthesis of hydrogenated derivatives from FF. | ||
To assess the stability of bim–silica in the presence of NH3 and H2, the catalyst was extracted from the reaction system after the tandem reaction, washed with HCl and DMF and retested in the benzoin condensation of FF. The catalyst was found to be fully active (see the chromatogram in Fig. S4†), pointing out that the bim moiety is not detached from the support during the tandem reaction. TG analyses on the fresh and spent bim–silica catalysts confirm the stability of bim–silica during the tandem reaction (Fig. S5†).
Conclusions
Along this study, we studied the direct/reductive amination of furoin and furil with NH3 and H2. Without a catalyst, furoin reacts with either NH3 or ammonium acetate to generate 2,3,5,6-tetra(furan-2-yl)pyrazine with 39% yield. Furil also reacts with NH3 to generate 2,2′-bipyridine-3,3′-diol with 44% yield. When Ru/Al2O3 and H2 were added to the reaction system, furoin and furil generated 2-amino-1,2-di(furan-2-yl)ethan-1-ol (alcohol–amine) as the main product with 47% and 34% yield, respectively, at 140 °C for 2 h. DFT simulations underscored the significance of the unique adsorption orientation of these intermediates on Ru/Al2O3, with the NH group in proximity to Ru centers and the OH group oriented away from the surface. This orientation, as revealed by our computational analysis, plays a pivotal role in guiding the reaction towards the formation of alcohol–amine, corroborating the experimentally observed product distribution. The alcohol–imine exhibits weak adsorption on ad-H/NH3-covered Ru, allowing the formation of 2,3,5,6-tetra(furan-2-yl)pyrazine in solution competing with alcohol–amine formation on Ru. By combining Ru/Al2O3 and a silica-anchored N-hetero-cyclic carbene (NHC) catalyst, 2-amino-1,2-di(furan-2-yl)ethan-1-ol could be accessed with 42% overall yield in a single reactor.Author contributions
LG: experimental data acquisition, data curation, formal analysis, writing original draft; MDP & MC: DFT calculation, data curation, formal analysis, writing original draft; FJ: investigation, formal analysis, supervision; RR: formal analysis, visualization; MPT: conceptualization, funding acquisition, resources, supervision, validation, visualization, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement No. 720783-MULTI2HYCAT. MDP acknowledges the computational resources of the Bremen Center for Computational Material Science (BCCMS), University of Bremen, Germany.References
- (a) R. Mariscal, P. Maireles-Torres, M. Ojeda, I. Sádaba and M. López Granados, Energy Environ. Sci., 2016, 9, 1144 RSC; (b) X. Li, P. Jia and T. Wang, ACS Catal., 2016, 6, 7621 CrossRef CAS; (c) J.-P. Lange, E. van der Heide, J. van Buijtenen and R. Price, ChemSusChem, 2012, 5, 150 CrossRef CAS PubMed.
- (a) J. Thoen and R. Busch, Industrial Chemicals from Biomass – Industrial Concepts, in Biorefineries-Industrial Processes and Products: Status Quo and Future Directions, ed. B. Kamm, P. R. Gruber and M. Kamm, Wiley, 2005, ch. 12, p. 347 Search PubMed; (b) G. W. Huber, J. N. Chheda, C. J. Barrett and J. A. Dumesic, Science, 2005, 308, 1446 CrossRef CAS PubMed; (c) J. Q. Bond, A. A. Upadhye, H. Olcay, G. A. Tompsett, J. Jae, R. Xing, D. M. Alonso, D. Wang, T. Zhang, R. Kumar, A. Foster, S. M. Sen, C. T. Maravelias, R. Malina, S. R. H. Barrett, R. Lobo, C. E. Wyman, J. A. Dumesic and G. W. Huber, Energy Environ. Sci., 2014, 7, 1500–1523 RSC; (d) M. J. Climent, A. Corma and S. Iborra, Green Chem., 2014, 16, 516 RSC; (e) K. Yan, G. Wu, T. Lafleur and C. Jarvis, Renewable Sustainable Energy Rev., 2014, 38, 663 CrossRef CAS.
- (a) Q. Girka, N. Hausser, B. Estrine, N. Hoffmann, J. Le Bras, S. Marinkovic and J. Muzart, Green Chem., 2017, 19, 4074 RSC; (b) M. Pelckmans, T. Renders, S. Van de Vyver and B. F. Sels, Green Chem., 2017, 19, 5303 RSC; (c) A. Velty, S. Iborra and A. Corma, ChemSusChem, 2022, 15, e202200181 CrossRef CAS PubMed.
- (a) J. J. Martínez, E. Nope, H. Rojas, M. H. Brijaldo, F. Passos and G. Romanelli, J. Mol. Catal. A: Chem., 2014, 392, 235 CrossRef; (b) S. Nishimura, K. Mizuhori and K. Ebitani, Res. Chem. Intermed., 2016, 42, 19 CrossRef CAS; (c) M. Chatterjee, T. Ishizaka and H. Kawanami, Green Chem., 2016, 18, 487 RSC; (d) T. Komanoya, T. Kinemura, Y. Kita, K. Kamata and M. Hara, J. Am. Chem. Soc., 2017, 139, 11493 CrossRef CAS PubMed; (e) A. Dunbabin, F. Subrizi, J. M. Ward, T. D. Sheppard and H. C. Hailes, Green Chem., 2017, 19, 397 RSC; (f) J. A. T. Caetano and A. C. Fernandes, Green Chem., 2018, 20, 2494 RSC; (g) D. Chandra, Y. Inoue, M. Sasase, M. Kitano, A. Bhaumik, K. Kamata, H. Hosono and M. Hara, Chem. Sci., 2018, 9, 5949 RSC; (h) Z. Kuo, C. Bixian, Z. Xiaoting, K. Shimin, X. Yongjun and W. Jinjia, ChemCatChem, 2019, 11, 5562 CrossRef; (i) D. Deng, Y. Kita, K. Kamata and M. Hara, ACS Sustainable Chem. Eng., 2019, 7, 4692 CrossRef CAS; (j) J. He, L. Chen, S. Liu, K. Song, S. Yang and A. Riisager, Green Chem., 2020, 22, 6714 RSC; (k) K. Saini, S. Kumar, H. Li, S. A. Babu and S. Saravanamurugan, ChemSusChem, 2022, 15, e202200107 CrossRef PubMed; (l) C. C. Truong, D. K. Mishra and Y.-W. Suh, ChemSusChem, 2023, 16, e202201846 CrossRef CAS PubMed.
- S. Jiang, E. Muller, F. Jerôme, M. Pera-Titus and K. De Oliveira Vigier, Green Chem., 2020, 22, 1832 RSC.
- (a) S. Jiang, C. Ma, E. Muller, M. Pera-Titus, F. Jerôme and K. De Oliveira Vigier, ACS Catal., 2019, 10, 8893 CrossRef; (b) S. Jiang, E. Muller, M. Pera-Titus, F. Jerôme and K. De Oliveira Vigier, ChemSusChem, 2020, 13, 1699 CrossRef CAS PubMed.
- (a) D. Enders, O. Niemeier and A. Henseler, Chem. Rev., 2007, 107, 5606 CrossRef CAS PubMed; (b) R. S. Menon, A. T. Biju and V. Nair, Beilstein J. Org. Chem., 2016, 12, 444 CrossRef CAS PubMed.
- (a) D. Liu, Y. Zhang and E. Y.-X. Chen, Green Chem., 2012, 14, 2738 RSC; (b) D. Liu and E. Y.-X. Chen, ChemSusChem, 2013, 6, 2236 CrossRef CAS PubMed; (c) H. Zang and E. Y.-X. Chen, Int. J. Mol. Sci., 2016, 16, 7143 CrossRef PubMed; (d) J. Li, B. Wang, Y. Dou and Y. Yang, RSC Adv., 2019, 9, 10825 RSC.
- (a) R. Breslow, J. Am. Chem. Soc., 1957, 79, 1762 CrossRef CAS; (b) R. Breslow, J. Am. Chem. Soc., 1958, 80, 3719 CrossRef CAS.
- (a) K.-I. Iwamoto, H. Kimura, M. Oike and M. Sato, Org. Biomol. Chem., 2008, 6, 912 RSC; (b) K.-I. Iwamoto, M. Hamaya, N. Hashimoto, H. Kimura, Y. Suzuki and M. Sato, Tetrahedron Lett., 2006, 47, 7175 CrossRef CAS.
- (a) L. Wang and E. Y.-X. Chen, ACS Catal., 2015, 5, 6907 CrossRef CAS; (b) J. Wilson and E. Y.-X. Chen, ACS Sustainable Chem. Eng., 2016, 4, 4927 CrossRef CAS; (c) E. Y.-X. Chen, L. Wang and Y. Eguchi, US0346774A1, 2016; (d) I. Miletto, M. Meazza, G. Paul, M. Cossi, E. Gianotti, L. Marchese, R. Rios, M. Pera-Titus and R. Raja, Chem. – Eur. J., 2022, 28, e202202771 CrossRef CAS PubMed.
- (a) D. Liu and E. Y.-X. Chen, ChemSusChem, 2013, 6, 2236 CrossRef CAS PubMed; (b) D. J. Liu and E. Y.-X. Chen, ACS Catal., 2014, 4, 1302 CrossRef CAS; (c) J. Keskiväli, P. Wrigstedt, K. Lagerblom and T. Repo, Appl. Catal., A, 2017, 534, 40 CrossRef.
- E. Y.-X. Chen and D. Liu, US9469626B2, 2016.
- S. Wang, Q. Gu, X. Chen, T. Zhao and Y. Zhang, Eur. J. Chem., 2011, 2, 173–177 CrossRef CAS.
- N.-T. Le, A. Byun, Y. Han, K.-I. Lee and H. Kim, Green Sustainable Chem., 2015, 5, 115–127 CrossRef CAS.
- L. R. Danielson, M. J. Dresser, E. E. Donaldson and J. T. Dickinson, Surf. Sci., 1978, 71, 599 CrossRef CAS.
- I. M. Ciobîcǎ, F. Frechard, R. A. van Santen, A. W. Kleyn and J. Hafner, J. Phys. Chem. B, 2000, 104, 3364 CrossRef.
- H. Hu, M. A. Ramzan, R. Wischert, F. Jerôme, C. Michel, K. de Oliveira Vigier and M. Pera-Titus, ACS Sustainable Chem. Eng., 2023, 11, 8229 CrossRef CAS.
- H. Mortensen, L. Diekhöner, A. Baurichter, E. Jensen and A. C. Luntz, J. Chem. Phys., 2000, 113, 6882 CrossRef CAS.
- X. Hu, M. Yang, D. Xie and H. Guo, J. Chem. Phys., 2018, 149, 044703 CrossRef PubMed.
- N. Gerrits and G.-J. Kroes, J. Phys. Chem. C, 2019, 123, 28291 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cy01605f |
| ‡ Current address: Department of Applied Science and Technology, Politecnico di Torino, Corso Duca degli Abruzzi 24, 10129 Torino, Italy. |
| This journal is © The Royal Society of Chemistry 2024 |