Single-atom iron doped BiOCl atomic layers to promote efficient CO2 electroreduction towards formate†
Fengwu
Tian
ab,
Tian
Tang
a,
Xixi
Di
a,
Xiaosha
Guo
a,
Dong
Liu
a,
Yixuan
Shi
a,
Zheng
Shen
b,
Xiaohu
Yu
a and
Xianzhao
Shao
 *a
*a
aShaanxi Key Laboratory of Catalysis, School of Chemistry and Environment Science, Shaanxi University of Technology, Hanzhong 723001, Shaanxi, China. E-mail: xianzhaoshao@snut.edu.cn
bCAS Key Laboratory of Science and Technology on Applied Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China
First published on 28th November 2023
Abstract
The electrocatalytic CO2 reduction reaction to valuable fuels is a promising strategy to simultaneously tackle the crises of fossil fuel shortage and carbon emission. Herein, a single-Fe-atom was introduced into the BiOCl catalyst by a facile hydrolysis method, which exhibited a greatly enhanced catalytic performance in the electrochemical CO2RR to formate, exceeding 90% hydrogenation selectivity and maintaining above 90% activity for 10 hours.
The electrocatalytic CO2 reduction reaction (CO2RR) is one of the most promising CO2 resource utilization approaches because of controllable reaction conditions and recyclable raw materials, along with easy modularization.1–5 This technology harnesses renewable energy sources (e.g. solar energy, wind energy) to produce electrical energy, subsequently using electrocatalysis to convert CO2 into high-energy-density fuel substances, like formic acid, for recycling.6–8 The strategy can not only reduce CO2 concentration, but also helps alleviate the strain on energy shortage to a certain extent. Most research on the CO2RR into formic acid has focused on metal electrodes which often possesses a high hydrogen evolution potential (HER) and weak adsorption force for CO, such as Pb, Hg, In, Pa, etc.9–11 Moreover, the high toxicity and cost make them less practical for large-scale industrial applications. To this end, the less toxic and cost-effective metal Sn has attracted attention.12 Although the Sn metal exhibits high formic acid selectivity, it is easily deactivated by carbon deposition thus suffering from poor stability during the electrocatalytic reduction of CO2.13,14 Alternatively, Bi is an easily available, environmentally friendly metal with high selectivity toward formic acid production. Actually, in 1995, the Komatsu group first discovered that the metal Bi is active toward electroreduction of CO2 in aqueous systems, and the efficiency of the product formate reached almost 100%.15 To date, there have been relatively few studies regarding the electrocatalytic reduction of CO2 using either elemental Bi or Bi-based materials. Furthermore, the precedents have shown that Bi-based catalysts still exhibit a high overpotential. Preliminary investigations have indicated that traditional bulk metal electrodes typically possess a low electrochemical active area, leading to low current density, a high reaction overpotential, and susceptibility to electrode deactivation. In contrast, single-atom doping of catalytic materials can significantly increase the number of active sites,16–18 which is beneficial to the CO2RR, allowing the catalyst to exhibit better catalytic activity and target product selectivity.19–23 Notably, the recent emergence of two-dimensional nanocatalytic materials has garnered considerable attention and is considered one of the most promising catalysts in the CO2RR.23
In this study, we have developed an Fe1/BiOCl catalyst (denoted as FBOC) with atomically dispersed Fe on a two-dimensional BiOCl nanocatalyst. The presence of atomically dispersed Fe in BiOCl is verified by a collection of characterization techniques, including X-ray photoelectron spectroscopy (XPS), X-ray absorption spectroscopy and transmission electron microscopy (HAADF-STEM). Furthermore, the introduction of single-atom Fe enriches the electron density of Fe with respect to Bi, resulting in a modification of the electronic structure of Bi within the BiOCl catalyst. This alteration in the electronic structure of Bi influences the reaction pathway for the CO2RR, leading to enhanced catalytic performance. Notably, FBOC not only demonstrates exceptional hydrogenation selectivity (exceeding 90% at −0.8 V vs. RHE) but also exhibits remarkable stability (maintaining above 90% activity at −0.8 V vs. RHE for 10 hours) during the CO2RR.
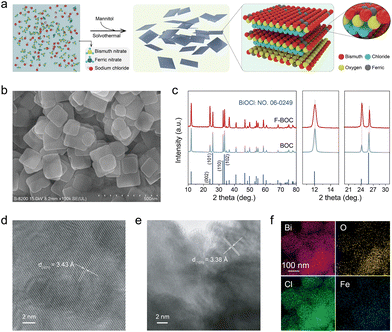
Atomically dispersed Fe on two-dimensional BiOCl (FBOC) and BiOCl nanocatalysts (BOC) were synthesized based on a recently reported method. In brief, a transparent mannitol aqueous solution containing Fe(NO3)3·6H2O, Bi(NO3)3·5H2O and NaBr was transferred to a Teflon-lined stainless steel autoclave to perform a solvothermal process at 180 °C for 3 h. The solid product was collected by centrifugation and washed with deionized water and ethanol five times (Fig. 1a). The FBOC and BOC feature a two-dimensional BiOCl structure, indicating that the introduction of Fe does not change the geometric structure of BiOCl (Fig. 1b and S1†). XRD patterns show that the diffraction peaks of all the products were well indexed to the tetragonal phase BiOCl (JCPDF No. 06-0249) with negligible influence on the phase structures of the BiOCl sample upon the addition of Fe atoms (Fig. 1c). Also, no Fe nanoparticles or clusters were found on the surface of BiOCl, as demonstrated in the TEM and HR-TEM images of FBOC (Fig. 1d, e, S2a and c†). The existence of atomically dispersed Fe is further verified by the associated EDX mapping and line scan, where the Bi, O, Cl, and Fe elements are homogeneously distributed throughout the surface of the material (Fig. 1f, S2b, d, and S3†). The loading amount of Fe on FBOC was determined by inductively coupled plasma optical emission spectrometry (ICP-OES) to be 0.51 wt%.
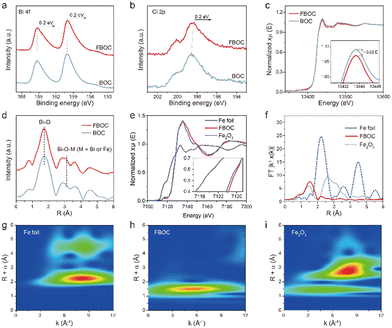
XPS measurement was conducted to explore the states of the elements in FBOC and BOC. No zero valence components related to Fe particles were found in the Fe 2p XPS spectra (Fig. S4†), well in line with the atomic dispersion of Fe. In Bi 4f XPS spectra, two peaks appearing at the binding energies of 159 and 165 eV were observed, which corresponded to Bi 4f7/2 and Bi 4f5/2 of Bi3+ in BiOCl respectively.24–26 Noticeably, the binding energies of Bi 4f have an obvious red shift after the introduction of single atom Fe. Similarly, the red shift of the Cl 2p peak located at 199 eV was also observed in XPS spectra for FBOC and BOC (Fig. 2b). To probe the local atomic structure and cation coordination resulting from the doping effect of single atom Fe, X-ray absorption fine structure (XAFS) was employed. The Bi L3-edge absorption edge position of FBOC shows a slight difference with that of BOC, suggesting a distinct local atomic structure due to Fe incorporation. The R-space spectra show that the peak attributed to Bi–O (around 1.5 Å) broadens and the peak position attributed to Bi–Bi increases slightly with the addition of Fe, indicating that Fe single atoms replace some of the Bi. Additionally, the Fe K-edge absorption edge position of FBOC is located close to Fe2O3 rather than the Fe foil, suggesting that the single Fe atom carries a positive charge with the valence state approaching +3 (Fig. 2e). Fourier transformed (FT) k3-weighted EXAFS spectra (Fig. 2f) show that the main peak at 1.51 Å for FBOC could be assigned to Fe–O coordination in the BiOCl lattice and FBOC does not show the peak of the Fe–Fe bond in reference to standard Fe foil and Fe2O3, confirming that the Fe species are isolated in the form of single atoms. Besides, the wavelet transform (WT) contour plots also demonstrated different Fe features in FBOC from those in the Fe foil and Fe2O3 (Fig. 2g–i) and the intensity maximum at 4.8 Å−1 in FBOC could be ascribed to Fe–O. All above results qualitatively verify the distinct local atomic arrangement of FBOC relative to the BOC.
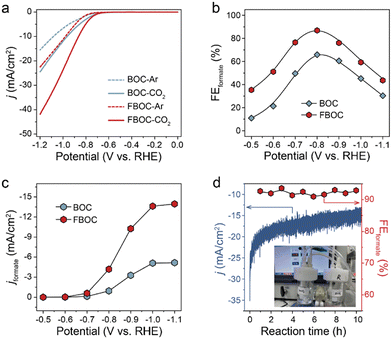
The electrochemical CO2 reduction activity of the as-prepared FBOC and BOC catalysts was assessed on a homemade three-electrode system. As displayed in Fig. 3a and S5,† a remarkable current difference was noticed for both FBOC and BOC in CO2-saturated and Ar-saturated KHCO3 solutions, suggesting that CO2 could be electrochemically reduced on BOC and FBOC. Notably, FBOC possessed the highest catalytic activity regarding the largest CO2 reduction current. The CO2 reduction performance of FBOC and BOC catalysts was further investigated by constant potential electrolysis in CO2-saturated 0.5 M KHCO3 solution. As shown in Fig. 3b and c, FBOC exhibits the best CO2RR activity with ca. 90% FEformate at −0.8 V (vs. RHE). Moreover, FBOC also exhibits excellent catalytic stability with no obvious decay in activity (both current density and FEformate) after 10 h continuous operation (Fig. 3d). All the above results indicate that the introduction of Fe single atoms into BiOCl nanosheets promotes the CO2RR into formate.
To investigate the detailed CO2RR process over FBOC and BOC, the operando attenuated total reflectance surface-enhanced infrared absorption spectroscopy (ATR-SEIRAS) measurements were conducted. Recorded in CO2 saturated electrolyte, the absorption peak centered at 1390 cm−1 can be assigned to O–C–O vibration in bidentate *OCHO, which is detected in the spectra of both FBOC (Fig. 4a) and BOC (Fig. 4b). Notably, the intensity of *C–H stretching between 2852 and 2975 cm−1, a characteristic absorption peak in the CO2RR to generate formic acid/formate, over FBOC is much stronger than that over BOC. On the other hand, the *CO absorption peak appearing at around 1851 cm−1 on FBOC is significantly weaker with respect to that on BOC. Additionally, the *CO absorption peak starts to appear at −0.8 V vs. RHE for the BOC catalyst, while it only shows up when the applied potential is more negative than −0.8 V vs. RHE for the FBOC catalyst, indicating more thermodynamically favorable *CO formation on BOC. Based on the above results, the CO2RR pathways over FBOC and BOC catalysts are proposed (Fig. 4c). Specifically, CO and formate are the main products over the BOC catalyst due to the favorable adsorption of both *CO and *OCHO. In contrast, the single Fe atoms effectively tuned the electronic structure of the BiOCl catalyst, suppressing the adsorption of *CO while favoring the adsorption of *OCHO, and therefore promote the selectivity of the CO2RR toward formate production.
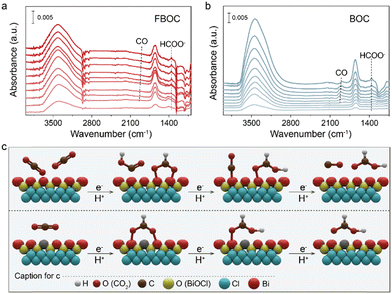 | ||
| Fig. 4 Operando ATR-SEIRAS spectra recorded at different cathodic potentials in CO2 saturated electrolyte: (a) FBOC and (b) BOC. (c) The proposed CO2RR pathways over FBOC and BOC. | ||
To sum up, we have developed a facile hydrolysis method to synthesize single Fe atom doped 2D BiOCl nanosheets at room temperature for the electrochemical CO2RR. The incorporation of single Fe atoms can induce electron transfer from Fe to Bi, which effectively increases the valence state of Bi and as a result promotes the CO2RR to formate, while suppressing the competing CO2RR to CO and HER. This work provides a rational design guidance for single-atom doped 2D nanocatalysts in the application of the electrochemical CO2RR.
Author contributions
Fengwu Tian: investigation, data curation, formal analysis and writing – original draft; Tian Tang: investigation and formal analysis; Xixi Di: investigation; Xiaosha Guo: formal analysis; Dong Liu, Yixuan Shi, Zheng Shen and Xiaohu Yu: writing – original draft and writing – review & editing; Xianzhao Shao: conceptualization, funding acquisition and project administration. All authors discussed the results and contributed to the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key Research and Development Program of China (2021YFB4000700), the National Natural Science Foundation of China (22273053), and the Natural Science Basic Research Program of Shaanxi (2021JCW-20, 2023-JC-YB-107).Notes and references
- H. B. Yang, S.-F. Hung, S. Liu, K. D. Yuan, S. Miao, L. P. Zhang, X. Huang, H.-Y. Wang, W. Z. Cai, R. Chen, J. J. Gao, X. F. Yang, W. Chen, Y. Q. Huang, H. M. Chen, C. M. Li, T. Zhang and B. Liu, Nat. Energy, 2018, 3, 140 CrossRef CAS.
- J. C. Zhang, J. Ding, Y. H. Liu, C. L. Su, H. B. Yang, Y. Q. Huang and B. Liu, Joule, 2023, 8, 1700 CrossRef.
- J. Ding, F. H. Li, J. C. Zhang, Q. Zhang, Y. H. Liu, W. J. Wang, W. Liu, B. B. Wang, J. Cai, X. Z. Su, H. B. Yang, X. Yang, Y. Q. Huang, Y. M. Zhai and B. Liu, J. Am. Chem. Soc., 2023, 145, 11829 CrossRef CAS PubMed.
- H. B. Yang, C.-Q. Xu, S. Baskaran, Y.-R. Lu, C. D. Gu, W. Liu, J. Ding, J. C. Zhang, Q. L. Wang, W. Chen, J. Li, Y. Q. Huang, T. Zhang and B. Liu, EES Catal., 2023, 1, 774 RSC.
- T. Wang, J. D. Chen, X. Y. Ren, J. C. Zhang, J. Ding, Y. H. Liu, K. H. Lim, J. H. Wang, X. N. Li, H. B. Yang, Y. Q. Huang, S. Kawi and B. Liu, Angew. Chem., Int. Ed., 2023, 62, e202211174 CrossRef CAS PubMed.
- Y. Y. Wang, J. K. Zhao, C. Cao, J. Ding, R. Y. Wang, J. Zeng, J. Bao and B. Liu, ACS Catal., 2023, 13, 3532 CrossRef CAS.
- W. J. Zhang, S. S. Liu, Y. Yang, H. F. Qi, S. B. Xi, Y. P. Wei, J. Ding, Z.-J. Wang, Q. X. Li, B. Liu and Z. P. Chen, Angew. Chem., Int. Ed., 2023, 62, e202219241 CrossRef CAS PubMed.
- Q. Zhang, H. J. Tsai, F. H. Li, Z. M. Wei, Q. Y. He, J. Ding, Y. H. Liu, Z.-Y. Lin, X. J. Yang, Z. Y. Chen, F. X. Hu, X. Yang, Q. Tang, H. B. Yang, S.-F. Hung and Y. M. Zhai, Angew. Chem., Int. Ed., 2023, 62, e202311550 CrossRef CAS PubMed.
- Y. Hori, Mod. Aspects Electrochem., 2008, 42, 88 Search PubMed.
- Y. Hori, H. Wakebe, T. Tsukamoto and O. Koga, Surf. Sci., 1995, 335, 258 CrossRef CAS.
- Y. Hori, H. Konishi, T. Futamura, A. Murata, O. Koga, H. Sakurai and K. Oguma, Electrochim. Acta, 2005, 50, 5354 CrossRef CAS.
- A. D. Castillo, M. A. Guerra, J. S. Gullón, A. Sáez, V. Montiel and A. Irabien, J. CO2 Util., 2017, 18, 222 CrossRef.
- W. Luc, C. Collins, S. W. Wang, H. L. Xin, K. He, Y. J. Kang and F. Jiao, J. Am. Chem. Soc., 2017, 139, 1885 CrossRef CAS PubMed.
- Z. M. Wei, J. Ding, X. X. Duan, G.-L. Chen, F.-Y. Wu, L. Zhang, X. J. Yang, Q. Zhang, Q. Y. He, Z. Y. Chen, J. Huang, S.-F. Hung, X. Yang and Y. M. Zhai, ACS Catal., 2023, 13, 4711 CrossRef CAS.
- S. J. Komatsu, T. Yanagihara, Y. K. Hiraga, M. C. Tanaka and A. Kunugi, Electrochemical Reduction of CO2 at Sb and Bi Electrodes in KHCO3 Solution, Japan Science and Technology Agency, 1995, vol. 63, p. 217 Search PubMed.
- L. Liu, P. Zhou, X. Su, Y. Liu, Y. Sun, H. Yang and S. Zheng, Sens. Actuators, B, 2022, 351, 130983 CrossRef CAS.
- L. Liu, T. Xiao, H. Fu, Z. Chen, X. Qu and S. Zheng, Appl. Catal., B, 2023, 323, 122181 CrossRef CAS.
- L. Liu, C. Mao, H. Fu, X. Qu and S. Zheng, ACS Appl. Mater. Interfaces, 2023, 15, 16654–16663 CrossRef CAS PubMed.
- J. Ding, Z. M. Wei, F. H. Li, J. C. Zhang, Q. Zhang, J. Zhou, W. J. Wang, Y. H. Liu, Z. Zhang, X. Z. Su, R. Z. Yang, W. Liu, C. L. Su, H. B. Yang, Y. Q. Huang, Y. M. Zhai and B. Liu, Nat. Commun., 2023, 14, 6550 CrossRef CAS PubMed.
- J. Ding, Z. Y. Teng, X. Z. Su, K. Kato, Y. H. Liu, T. Xiao, W. Liu, L. Y. Liu, Q. Zhang, X. Y. Ren, J. C. Zhang, Z. Y. Chen, O. Teruhisa, A. Yamakata, H. B. Yang, Y. Q. Huang, B. Liu and Y. Zhai, Chem, 2023, 9, 1017 CAS.
- J. Ding, J. Huang, Q. Zhang, Z. M. Wei, Q. Y. He, Z. Y. Chen, Y. H. Liu, X. Z. Su and Y. M. Zhai, Catal. Sci. Technol., 2022, 12, 2416 RSC.
- J. Ding, H. B. Yang, X. L. Ma, S. Liu, W. Liu, Q. Mao, Y. Huang, J. Li, T. Zhang and B. Liu, Nat. Energy, 2023 DOI:10.1038/s41560-023-01389-3.
- J. D. Wu, X. Zhao, X. Q. Cui and W. T. Zheng, Chin. J. Catal., 2022, 43, 2802 CrossRef CAS.
- J. Ding, Z. Dai, F. Tian, B. Zhou, B. Zhao, H. P. Zhao, Z. Q. Chen, Y. L. Liu and R. Chen, J. Mater. Chem. A, 2017, 5, 23453 RSC.
- J. Ding, Z. Dai, F. Qin, H. P. Zhao, S. Zhao and R. Chen, Appl. Catal., B, 2017, 205, 281 CrossRef CAS.
- Z. Dai, F. Qin, H. Zhao, J. Ding, Y. Liu and R. Chen, ACS Catal., 2016, 5, 3180 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cy01523h |
| This journal is © The Royal Society of Chemistry 2024 |