Enhancing the photocatalytic efficiency and stability of CsPbBr3 nanocrystals for visible-light driven aerobic diaryl thio/seleno etherification†
Ashis
Mathuri‡
,
Buddhadeb
Pal‡
,
Milan
Pramanik
,
Anupam
Manna
* and
Prasenjit
Mal
 *
*
School of Chemical Sciences, National Institute of Science Education and Research (NISER), An OCC of Homi Bhaba National Institute, PO Bhimpur-Padanpur, Via Jatni, District Khurda, Bhubaneswar, Odisha 752050, India. E-mail: pmal@niser.ac.in
First published on 28th November 2023
Abstract
CsPbBr3 holds promise as a visible light photocatalyst, but its instability in oxygen-rich and polar solvent environments poses a significant challenge for practical utilization. This study demonstrates the effectiveness of orthorhombic CsPbBr3 nanocrystals (NCs) synthesized from dibromoisocyanuric acid. These NCs exhibit high efficiency as heterogeneous visible light photocatalysts (450–470 nm, 5 mol%) in a template-free aerobic thio/seleno etherification reaction involving 1,3,5-trimethoxybenzene, employing diaryl sulfides and diaryl selenides in acetonitrile (ε ∼ 37.5). A novel surface treatment approach is proposed, utilizing electron-rich 1,3,5-trimethoxybenzene as a reactant to stabilize CsPbBr3 NCs in situ and address stability challenges in polar solvents and open-air environments. Additionally, among the four systems, the bromide-enriched orthorhombic CsPbBr3 NCs exhibit markedly enhanced photocatalytic efficiency and stability compared to the cubic systems. The orthorhombic CsPbBr3 NCs, with prolonged excited state lifetime, promote efficient electron transfer, leading to the generation of superoxide radical anions from the conduction band. These findings highlight the potential of perovskite nanocrystals and provide insights into their applications as visible light photocatalysts in organic transformations.
Introduction
In recent years, visible light photocatalysis has emerged as a highly promising field in organic synthesis, opening up new opportunities for efficient and sustainable synthetic methodologies.1 Initially, transition-metal complexes such as Ru(bpy)32+ and Ir(ppy)3 were commonly employed as photocatalysts.2 However, their practical application was limited by their sensitivity to oxygen and moisture, necessitating sophisticated laboratory facilities. Interestingly, significant progress has been made over time, leading to the adoption of organic dyes as exciting alternatives for photocatalytic organic transformations. Organic dyes present numerous advantages as photocatalysts, including their easy availability, tunable absorption properties, and diverse chemical structures.3 These features make them highly versatile and offer great potential for catalyzing a wide range of organic reactions efficiently.4Current research endeavors are focused on expanding and refining the selection of photocatalysts, with the ultimate goal of enhancing the accessibility and applicability of photocatalysis.5 Among the various approaches being explored, heterogeneous photocatalysis stands out as a particularly compelling field of study.6 Heterogeneous photocatalysis offers a multitude of benefits, enabling its successful implementation in diverse areas such as water treatment,7 flow reactors,8 energy production,9etc.10 Notably, perovskite materials have garnered significant attention as a promising research focus within the realm of heterogeneous visible-light photocatalysis in organic synthesis.11
The emergence of CsPbBr3 perovskite nanocrystals (NCs) as potential candidates for photocatalytic applications adds further impetus to the exploration of novel and efficient catalytic processes.12 However, challenges persist concerning CsPbBr3 perovskites, primarily related to their long-term stability.13 Ongoing research is actively addressing these challenges, striving to enhance the efficiency and stability of perovskite-based photocatalysts.13a In the practical application of perovskite NCs as photocatalysts for organic synthesis, key hurdles include their susceptibility to instability caused by light exposure,14 oxygen atmosphere,14a–d and polar solvents.15 Effectively addressing these challenges is crucial for the widespread and successful integration of perovskite NCs across a diverse spectrum of organic synthesis.
In this study, we address the challenge of preserving the stability of CsPbBr3 nanocrystals (NCs) in polar solvents and open air. We employ a tailored synthetic approach, specifically the three-component hot injection method.16 We successfully synthesized four CsPbBr3 NCs with varying crystallinity (orthorhombic and cubic) derived from different bromine sources. These NCs are employed as photocatalysts for the thio/seleno etherification of 1,3,5-trimethoxybenzene. Furthermore, we introduce an innovative surface treatment technique using the electron-rich 1,3,5-trimethoxybenzene reactant, enhancing the in situ stability of the NCs through surface passivation.17 Comparative analysis reveals that orthorhombic CsPbBr3 NCs, enriched with bromide and synthesized from dibromoisocyanuric acid, display significantly improved photocatalytic efficiency compared to their cubic counterparts. These orthorhombic NCs, characterized by an extended excited state lifetime, facilitate efficient electron transfer, leading to the generation of superoxide radical anions from the conduction band.
Results and discussion
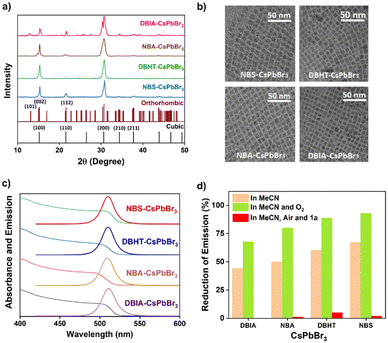
The synthesis of the CsPbBr3 perovskites was achieved by using hot injection methods.18 The DBIA–CsPbBr3 NC was synthesized using dibromoisocyanuric acid as the bromide-precursor (Fig. 1)11f and DBHT–CsPbBr3, NBS–CsPbBr3 and NBA–CsPbBr3 were obtained using bromide precursors 1,3-dibromo-5,5-dimethylhydantoin (DBHT),19N-bromosuccinimide (NBS)20 and N-bromoacetamide (NBA), respectively. The use of the N-bromoacetamide (NBA) precursor for the synthesis of the NBA–CsPbBr3 NC is indeed a relatively novel approach.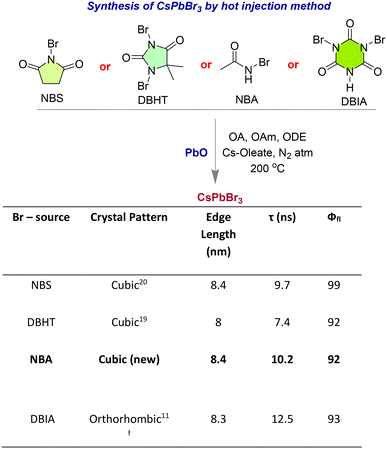 | ||
| Fig. 1 The synthesis of the CsPbBr3 perovskites from the corresponding bromide precursors and their characterization. | ||
The PXRD pattern showed that the crystal structure of the newly synthesized NBA–CsPbBr3 is cubic as DBHT–CsPbBr319 and NBS–CsPbBr3,20 having the characteristic planes of (100), (110), (200), (210), etc. which is nicely coordinated with the reference data (PDF: 00-054-0752) (Fig. 2a). DBIA–CsPbBr3 is orthorhombic, indicating the peaks for (101), (002), (112), (220), (004), etc. with corresponding reference PDF: 01-072-7929 (Fig. 2a). The images from transmission electron microscopy (TEM) demonstrate the morphologies of the CsPbBr3 NCs and average edge length (8–8.4 nm) (Fig. 2b). In the photophysical study, the colloidal suspension of NCs in hexane exhibited significant broad absorption bands in the visible region (up to 525 nm) (Fig. 2c). All the CsPbBr3 NC suspensions displayed intense green photoluminescence with a comparable quantum yield near unity (Φ ∼ 0.92 to 0.99) (Fig. 2c).11f,19,20 The photoluminescence lifetime studies were also conducted for the CsPbBr3 NCs giving the value of τ ∼ 7 to 12.5 ns. (Fig. 1) (spectra and detailed analysis are given in ESI† Fig. S1 and Table S1, respectively).11f,19–21 Redox potentials of the CsPbBr3 NCs are determined through cyclic voltammetry (CV) experiments (oxidation potentials, Eox = +1.6 V and the reduction potentials, Ered = −1.15–−1.25 V) (Fig. S2, ESI†).
In this study, we investigated perovskite stability in acetonitrile and under an O2 atmosphere using photoluminescence studies. Our findings reaffirm that the orthorhombic DBIA–CsPbBr3 perovskite exhibits superior stability compared to its cubic counterparts, as evidenced by a notable reduction in photoluminescence intensity (Fig. 2d and S3–S5, ESI†) in line with the earlier literature.22 The photoluminescence lifetime of DBIA–CsPbBr3 remained consistent at approximately ∼12.6 ns in acetonitrile (Fig. S6, detailed analysis in Table S2, ESI†). It is known in the literature that the efficacy of post-treatment strategies in mitigating surface defects in CsPbBr3 perovskite nanocrystals by employing passivating agents like organic iodides23 and diverse aromatic molecules.24 Specifically, we evaluated the potential of incorporating 1,3,5-trimethoxybenzene, an electron-rich compound, as a capping agent to enhance stability during the reaction process and facilitate surface passivation, attributing the effect to favorable interactions with the perovskite surface and the formation of a protective layer. The importance of bromide ions in the passivation process was underscored through the use of bromide-rich DBIA–CsPbBr3, demonstrating improved reaction efficiency by mitigating amine-induced bromide ion leaching.25 Our study highlights that strategic selection of stabilizing agents, such as 1,3,5-trimethoxybenzene and bromide-rich moieties, significantly impacts CsPbBr3 perovskite nanocrystal stability and performance post-treatment. Remarkably, negligible reduction in photoluminescence was observed in acetonitrile under aerobic conditions, even after 24 h, in the presence of 1,3,5-trimethoxybenzene, suggesting the enhanced stability and catalytic activity of the perovskite nanomaterials (Fig. S7–S10, ESI†). This effect was consistent with DBIA–CsPbBr3 displaying unchanged intensity and a blue-shifted PL peak, attributed to the enhanced carrier concentration by inhibiting trap states, potentially augmenting the stability and catalytic activity of the perovskite nanomaterials (Fig. S7a and b, ESI†).26
In recent times, a notable surge of interest has emerged in harnessing organochalcogen derivatives as crucial foundational elements for the synthesis of natural products, highlighting their substantial promise within the domain of medicinal chemistry applications.27 Notably, Maiti and coworkers have recently introduced a ligand-assisted palladium-catalyzed C–H activation method for synthesizing chalcogens, employing diaryl disulfides and diaryl diselenides as starting materials.28 This process involves the use of costly palladium catalysts, silver salt, and several additives. CsPbBr3, a promising visible light photocatalyst, faces challenges due to its instability under oxygen-rich and polar solvent conditions. Moreover, this study showcases the efficiency of orthorhombic CsPbBr3 nanocrystals (NCs) synthesized from dibromoisocyanuric acid. These NCs demonstrate high efficiency as visible light photocatalysts (450–470 nm, 5 mol%) in template-free aerobic chalcogenation or thio/seleno etherification reactions involving 1,3,5-trimethoxybenzene, utilizing diaryl sulfides and diaryl selenides in acetonitrile (Fig. 3). An innovative surface treatment approach using electron-rich 1,3,5-trimethoxybenzene stabilizes CsPbBr3 NCs, addressing stability challenges in polar solvents and under open-air conditions. Moreover, the bromide-enriched orthorhombic CsPbBr3 NCs exhibit significantly enhanced photocatalytic efficiency and in situ stability compared to their cubic counterparts. Notably, this research marks the first report on the photocatalytic C–S/Se formation reaction utilizing the CsPbBr3 perovskite as a photocatalyst.
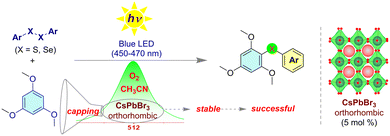 | ||
| Fig. 3 The orthorhombic CsPbBr3 perovskite NCs as photocatalysts for facilitating C–S/Se bond formation via C–H functionalization under an aerobic atmosphere and in acetonitrile. | ||
Based on our comprehensive analysis of the photophysical characteristics, we have explored that these CsPbBr3 NCs hold great promise as effective photocatalysts. The information on the optimized reaction conditions is provided in Table 1. We have used CsPbBr3 (5 mol%) to synthesize phenyl(2,4,6-trimethoxyphenyl)sulfane 3aa from electron-rich 1,3,5-trimethoxybenzene 1a (0.35 mmol) and 1,2-diphenyldisulfane 2a (0.43 mmol) in dry acetonitrile and an O2 (aerobic) atmosphere, under blue LED irradiation (450–470 nm). The results indicate that the yield of compound 3aa using the CsPbBr3 photocatalyst was found to be 88% (entry 10). The reaction's yield was found to be unsatisfactory when 3 mol% of the catalyst was utilized (entry 1). Likewise, employing solvents other than acetonitrile led to either poor yields or no successful outcomes (entries 2–7). Furthermore, employing 0.6 equiv. of substrate 2a resulted in inferior yields of the products (entries 8 and 9). The utilization of either green-LED (entry 11) or normal-air conditions (entry 12) did not yield any noticeable improvement in the results.
| Entry | 2a (equiv.) | CsPbBr3 (mol%) | Solvent | Light source | Yielda3aa (%) |
|---|---|---|---|---|---|
| a Isolated yields after column chromatography; reaction conditions: 1a (60 mg, 0.35 mmol), 2a (94 mg, 0.43 mmol) and CsPbBr3 (5 mol%) in 1.5 mL of CH3CN for 18 h. b In dry CH3CN. c Reaction under air. | |||||
| 1 | 1.2 | 3 | CH3CN | Blue LED | 75 |
| 2 | 1.2 | 3 | DMSO | Blue LED | 0 |
| 3 | 1.2 | 3 | DMF | Blue LED | 0 |
| 4 | 1.2 | 3 | Toluene | Blue LED | 0 |
| 5 | 1.2 | 3 | THF | Blue LED | 0 |
| 6 | 1.2 | 3 | MeOH | Blue LED | 30 |
| 7 | 1.2 | 3 | DCE | Blue LED | 70 |
| 8 | 0.6 | 3 | CH3CN | Blue LED | 40 |
| 9 | 0.6 | 5 | CH3CN | Blue LED | 50 |
| 10 | 1.2 | 5 | CH3CN | Blue LED | 88b |
| 11 | 1.2 | 5 | CH3CN | Green LED | 65 |
| 12 | 1.2 | 5 | CH3CN | Blue LED | 25c |
The reaction efficiency of different disulfides in the presence of electron-rich arenes is shown in Fig. 4a. A variety of disulfides and diselenides were subjected to the reaction under standard conditions. The –Me and –OMe groups at the para- and ortho-positions of disulfides produced the corresponding phenyl sulfanes 3aa–3ae with high yields (88% to 94%). Again, –F containing diaryl disulfides produced thioethers 3af, 3ag, and 3ah with 72%, 69%, and 68% yields, respectively. Next, thioethers 3ai–3ak having a –Cl substituent were synthesized with 65–77% yields. The 4-dibromodiaryl disulfide produced 3al with 79% yield. The yields for the synthesis of compounds 3am and 3an were 61% and 57%, respectively. Furthermore, 1,2-di(thiophen-2-yl)disulfane and 1,2-di(pyridin-2-yl)disulfane reacted well to produce the corresponding thioethers 3ao and 3ap with 50% and 48% yields, respectively. Diaryl sulfide 3aq was produced with a 75% yield from polyaromatics containing disulfides. The coupling reaction between an aliphatic disulfide and trimethoxybenzene failed to produce compound 3ar. On the other hand, the successful coupling of diaryl diselenides (Fig. 4b) with 1,3,5-trimethoxybenzene 1a produces 3as, 3at, 3au and 3av with 56–85% yield, indicating that the reaction conditions were also favorable for this reaction system. The sluggish reaction was observed with anisoles and 1,3,4-trimethoxybenzene (Fig. 4c) under the standard conditions and no products could be isolated.
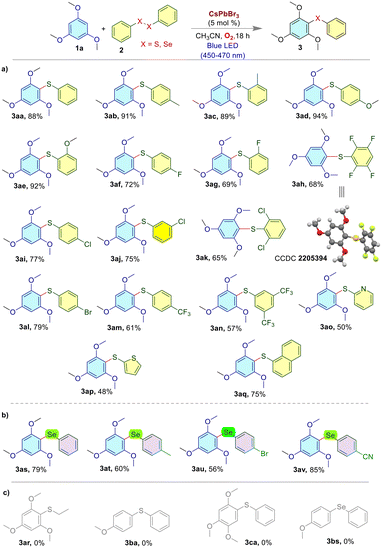 | ||
| Fig. 4 The products from the reactions with a) various disulfides and b) diselenides. c) Unsuccessful attempts. | ||
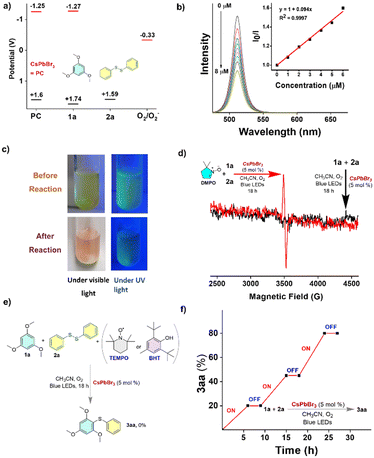
The control experiments (Fig. 5) provided important information about the reaction mechanism. The energy potential diagram shown in Fig. 5a was obtained from CV (cyclic voltammetry) experiments, for the photocatalyst (PC) and reactants 1a and 2a (Fig. S2 and S12, ESI†). The observation of decreasing photoluminescence intensity with increasing addition of disulfide suggests that the disulfide quenches the photoluminescence of CsPbBr3 in acetonitrile with a quenching constant of kq = 7.52 × 1012 s−1 M−1 (Fig. 5c). The observation of a strong signal in EPR in the trapping experiment in the presence of 5,5-dimethyl-1-pyrroline-N-oxide (DMPO, Fig. 5d) provides evidence for a radical-based pathway in the reaction.29 The radical-based mechanism was further supported by the experiments using 1,1-diphenylethylene, 2,2,6,6-tetramethylpiperidine 1-oxyl radical (TEMPO), and butylated hydroxytoluene (BHT) as radical scavengers (Fig. 5e). In the ON–OFF–ON experiment (Fig. 5f), the reaction was conducted under three conditions: light turned on (ON), light turned off (OFF), and light turned on again (ON). Comparing the results of these conditions confirms the essential role of visible light in the reaction.
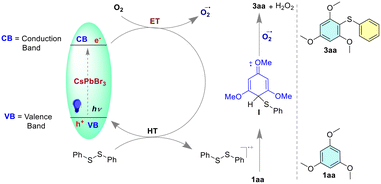
A plausible reaction mechanism is shown in Fig. 6. The presence of a strong absorption band in the visible region of the CsPbBr3 NCs makes them susceptible to photochemical excitation after the absorption of visible light (blue LED). The disulfide radical cation can be generated through the hole transfer (HT) process from the valence band (VB) of the CsPbBr3 perovskite to the disulfide moiety. The disulfide radical cation generated can react with electron-rich arenes to form intermediate I, a radical cation. The superoxide radical anion can be generated through an electron transfer process30 from the conduction band of the perovskite to molecular oxygen. In this process, the electrons in the conduction band of the perovskite are transferred to molecular oxygen (O2), forming superoxide radical anions (O2˙−) [EO(O2/O2˙−) = −0.33 V].31 These superoxide radical anions can then react with intermediate I, leading to deprotonation and led to the formation of product 3aa and by-product hydrogen peroxide (H2O2). The formation of hydrogen peroxide (H2O2) was confirmed by UV-vis spectroscopy through the detection of tri-iodide (I3−) in the reaction medium. The detection of tri-iodide is typically performed by adding potassium iodide (KI) and dilute acid to the reaction mixture, which can then be monitored by UV-vis spectroscopy. The appearance of a peak in the UV-vis spectrum at a wavelength of 359 nm was indicative of the formation of tri-iodide, which confirms the presence of hydrogen peroxide in the reaction medium (Fig. S13, ESI†).32
The turn over number (TON) measures the catalytic efficiency of the catalyst and in this case, the TON of the CsPbBr3 NC is 18.11.33 After the reaction, solid particles containing remaining catalysts (heterogeneous) were easily separated from the reaction mixture using simple centrifugation. Subsequently, the filtrate containing the desired product was employed for the isolation and purification of the compounds. This straightforward separation process facilitates the isolation of the target compounds, streamlining the purification procedure and enhancing the overall efficiency of the synthetic process.
Derivatization of the synthesized compounds (diaryl sulfides) is also shown in Fig. 7. meta-Chloroperoxybenzoic acid (mCPBA) was used for the oxidation of diaryl sulfide 3aa into the corresponding sulfoxide (Fig. 7a).34 The Suzuki (Fig. 7b)35 and Sonogashira (Fig. 7c)36 cross-coupling reactions were performed on compound 3al.
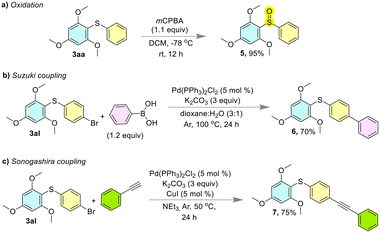 | ||
| Fig. 7 Synthetic utilities of the diaryl sulfides. a) Oxidation of 3aa using mCPBA. b) The Suzuki and c) Sonogashira cross-coupling reactions of 3al. | ||
The orthorhombic CsPbBr3 perovskite, known for its heightened stability and enhanced optoelectronic performance due to minimized crystal defects,37 has garnered significant interest.11f,38 Notably, our study unveils that under aerobic conditions and in the polar solvent acetonitrile, orthorhombic DBIA–CsPbBr3 displays superior stability compared to cubic counterparts (NBS–CsPbBr3, DBHT–CsPbBr3, and NBA–CsPbBr3). Wan-Jian Yin's group conducted comprehensive first-principles density functional theory calculations on 18 halide perovskites (ABX3), affirming the general trend where cubic phases exhibit lower stability while orthorhombic phases manifest higher stability.22a Post-treatment effectively mitigates surface defects in CsPbBr3 perovskite nanocrystals, often using organic iodides and various aromatic molecules as passivating agents. Here, we demonstrate that 1,3,5-trimethoxybenzene, an electron-rich compound, enhances the stability by acting as a capping agent, aiding in surface passivation. Additionally, the use of bromide-rich DBIA–CsPbBr3 highlights bromide ions' importance in passivation, reducing amine-induced bromide ion leaching, and ultimately improving reaction efficiency. Selective utilization of stabilizing agents, including 1,3,5-trimethoxybenzene and bromide-enriched orthorhombic CsPbBr3 NCs, significantly impacts the stability and performance of CsPbBr3 nanocrystals after treatment. This is evidenced by improved photocatalytic efficiency and in situ stability observed in orthorhombic CsPbBr3 NCs when compared to their cubic counterparts. Furthermore, Pb(II) leaching experiments after the reaction using the ICP OES technique reveal that only 32 ppb of Pb(II) was leached out from DBIA–CsPbBr3, while for the other cubic CsPbBr3 the values are more than 180 ppb. This result also confirmed that DBIA–CsPbBr3 is more stable than other CsPbBr3 under the standard reaction conditions. In addition, the orthorhombic CsPbBr3 nanocrystals, characterized by a long excited state lifetime, facilitate effective electron transfer, resulting in the generation of superoxide radical anions from the conduction band, which also helped to improve the efficiency of the reaction.
Conclusions
In summary, this research successfully showcased the efficacy of orthorhombic CsPbBr3 perovskite nanocrystals (NCs) as efficient heterogeneous photocatalysts for visible light, facilitating the synthesis of diaryl sulfides and diaryl selenides. The reactions demonstrated exceptional performance under aerobic conditions and in polar solvents like acetonitrile. The heightened intrinsic stability of orthorhombic CsPbBr3 can be attributed to its minimized crystal defects and elevated bromide ion ratios. Notably, the presence of 1,3,5-trimethoxybenzene among the reactants further contributed to enhancing the stability of DBIA–CsPbBr3 NCs within the reaction system. These findings not only hold significant promise but also offer valuable insights and guidance for the realms of synthetic organic and material chemistry. Leveraging CsPbBr3 NCs as photocatalysts presents an exciting avenue for future development of efficient and sustainable catalytic methodologies.Author contributions
The manuscript has been written through the contribution of all the authors.Conflicts of interest
“There are no conflicts to declare”.Acknowledgements
A. Manna, B. Pal and M. Pramanik thank NISER for the fellowship. A. Mathuri thanks CSIR for fellowship. We also thank the Department of Atomic Energy (DAE, India) for financial support (grant number RIN 4002).Notes and references
- (a) L. Marzo, S. K. Pagire, O. Reiser and B. König, Angew. Chem., Int. Ed., 2018, 57, 10034 CrossRef PubMed; (b) N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075 CrossRef PubMed.
- J. Twilton, C. Le, P. Zhang, M. H. Shaw, R. W. Evans and D. W. C. MacMillan, Nat. Rev. Chem., 2017, 1, 0052 CrossRef CAS.
- (a) J. Vanderghinste and S. Das, Synthesis, 2022, 54, 3383 CrossRef CAS; (b) S. Das, S. K. Parida, T. Mandal, S. K. Hota, L. Roy, S. De Sarkar and S. Murarka, Org. Chem. Front., 2021, 8, 2256 RSC; (c) A. K. Sahoo, A. Rakshit, A. Dahiya, A. Pan and B. K. Patel, Org. Lett., 2022, 24, 1918 CrossRef CAS PubMed; (d) A. K. Bagdi, M. Rahman, D. Bhattacherjee, G. V. Zyryanov, S. Ghosh, O. N. Chupakhin and A. Hajra, Green Chem., 2020, 22, 6632 RSC.
- (a) A. Mathuri, B. Pal, M. Pramanik and P. Mal, J. Org. Chem., 2023, 88, 10096 CrossRef CAS PubMed; (b) R. Bhanja, S. K. Bera and P. Mal, Chem. Commun., 2023, 59, 4455 RSC.
- Y. Zhang, S. Qin, N. Claes, W. Schilling, P. K. Sahoo, H. Y. V. Ching, A. Jaworski, F. Lemière, A. Slabon, S. Van Doorslaer, S. Bals and S. Das, ACS Sustainable Chem. Eng., 2022, 10, 530 CrossRef CAS.
- S. Gisbertz and B. Pieber, ChemPhotoChem, 2020, 4, 456 CrossRef CAS.
- K. N. Heck, S. Garcia-Segura, P. Westerhoff and M. S. Wong, Acc. Chem. Res., 2019, 52, 906 CrossRef CAS PubMed.
- W. Yang, S. Feng, X. Zhang, Y. Wang, C. Li, L. Zhang, J. Zhao, G. G. Gurzadyan and S. Tao, ACS Appl. Mater. Interfaces, 2021, 13, 38722 CrossRef CAS PubMed.
- D. Ricciarelli, W. Kaiser, E. Mosconi, J. Wiktor, M. W. Ashraf, L. Malavasi, F. Ambrosio and F. De Angelis, ACS Energy Lett., 2022, 7, 1308 CrossRef CAS.
- (a) Z. Guo, W. Huo, T. Cao, X. Liu, S. Ren, J. Yang, H. Ding, K. Chen, F. Dong and Y. Zhang, J. Colloid Interface Sci., 2021, 588, 826 CrossRef CAS PubMed; (b) W. Huo, W. Xu, Z. Guo, Y. Zhang and F. Dong, Appl. Catal., B, 2021, 284, 119694 CrossRef CAS; (c) W. Huo, T. Cao, W. Xu, Z. Guo, X. Liu, H.-C. Yao, Y. Zhang and F. Dong, Cuihua Xuebao, 2020, 41, 268 CAS; (d) T. Cao, W. Huo, Z. Guo, C. Jing, Y. Chen, Y. Zhang and Z. Zhou, Appl. Surf. Sci., 2019, 498, 143848 CrossRef CAS.
- (a) Y. Li, T. Wang, Y. Wang, Z. Deng, L. Zhang, A. Zhu, Y. Huang, C. Zhang, M. Yuan and W. Xie, ACS Catal., 2022, 12, 5903 CrossRef CAS; (b) B. Gurung, S. Pradhan, D. Sharma, D. Bhujel, S. Basel, S. Chettri, S. Rasaily, A. Pariyar and S. Tamang, Catal. Sci. Technol., 2022, 12, 5891 RSC; (c) X. Zhu, Y. Lin, Y. Sun, M. C. Beard and Y. Yan, J. Am. Chem. Soc., 2019, 141, 733 CrossRef CAS PubMed; (d) A. Shi, K. Sun, X. Chen, L. Qu, Y. Zhao and B. Yu, Org. Lett., 2022, 24, 299 CrossRef CAS PubMed; (e) B. Pal, A. Mathuri, A. Manna and P. Mal, Org. Lett., 2023, 25, 4075 CrossRef CAS PubMed; (f) A. Manna, T. K. Dinda, S. Ghosh and P. Mal, Chem. Mater., 2023, 35, 628 CrossRef CAS; (g) W.-B. Wu, Y.-C. Wong, Z.-K. Tan and J. Wu, Catal. Sci. Technol., 2018, 8, 4257 RSC; (h) K. Wang, H. Lu, X. Zhu, Y. Lin, M. C. Beard, Y. Yan and X. Chen, ACS Energy Lett., 2020, 5, 566 CrossRef CAS; (i) I. Rosa-Pardo, C. Casadevall, L. Schmidt, M. Claros, R. E. Galian, J. Lloret-Fillol and J. Perez-Prieto, Chem. Commun., 2020, 56, 5026 RSC.
- A. P. Jadhav, S. Y. Park, J.-W. Lee, H. Yan and C. E. Song, Acc. Chem. Res., 2021, 54, 4319 CrossRef CAS PubMed.
- (a) J. A. Steele, T. Braeckevelt, V. Prakasam, G. Degutis, H. Yuan, H. Jin, E. Solano, P. Puech, S. Basak, M. I. Pintor-Monroy, H. Van Gorp, G. Fleury, R. X. Yang, Z. Lin, H. Huang, E. Debroye, D. Chernyshov, B. Chen, M. Wei, Y. Hou, R. Gehlhaar, J. Genoe, S. De Feyter, S. M. J. Rogge, A. Walsh, E. H. Sargent, P. Yang, J. Hofkens, V. Van Speybroeck and M. B. J. Roeffaers, Nat. Commun., 2022, 13, 7513 CrossRef CAS PubMed; (b) G. Sathiyan, R. Ranjan, S. Ranjan, A. Garg, R. K. Gupta and A. Singh, ACS Appl. Energy Mater., 2019, 2, 7609 CrossRef CAS; (c) P. Srivastava, A. P. Parhi, R. Ranjan, S. Satapathi and M. Bag, ACS Appl. Energy Mater., 2018, 1, 4420 CrossRef CAS.
- (a) C. C. Boyd, R. Cheacharoen, T. Leijtens and M. D. McGehee, Chem. Rev., 2019, 119, 3418 CrossRef CAS PubMed; (b) W. R. Mateker and M. D. McGehee, Adv. Mater., 2017, 29, 1603940 CrossRef PubMed; (c) Z. Zhang, X. Tian, C. Wang, J. Jin, Y. Jiang, Q. Zhou, J. Zhu, J. Xu, R. He, Y. Huang, S. Ren, C. Chen, P. Gao, R. Long and D. Zhao, Energy Environ. Sci., 2022, 15, 5274 RSC; (d) R. Wang, M. Mujahid, Y. Duan, Z.-K. Wang, J. Xue and Y. Yang, Adv. Funct. Mater., 2019, 29, 1808843 CrossRef CAS; (e) C. Otero-Martínez, N. Fiuza-Maneiro and L. Polavarapu, ACS Appl. Mater. Interfaces, 2022, 14, 34291 CrossRef PubMed; (f) D. J. Slotcavage, H. I. Karunadasa and M. D. McGehee, ACS Energy Lett., 2016, 1, 1199 CrossRef.
- (a) B. J. Kim, D. H. Kim, S. L. Kwon, S. Y. Park, Z. Li, K. Zhu and H. S. Jung, Nat. Commun., 2016, 7, 11735 CrossRef PubMed; (b) Q. A. Akkerman, G. Rainò, M. V. Kovalenko and L. Manna, Nat. Mater., 2018, 17, 394 CrossRef PubMed.
- (a) S. Bera, R. K. Behera and N. Pradhan, J. Am. Chem. Soc., 2020, 142, 20865 CrossRef PubMed; (b) A. Dutta, R. K. Behera, P. Pal, S. Baitalik and N. Pradhan, Angew. Chem., Int. Ed., 2019, 58, 5552 CrossRef CAS PubMed; (c) N. Pradhan, ACS Energy Lett., 2019, 4, 1634 CrossRef CAS; (d) A. Stelmakh, M. Aebli, A. Baumketner and M. V. Kovalenko, Chem. Mater., 2021, 33, 5962 CrossRef CAS PubMed.
- (a) R. Garai, R. K. Gupta, M. Hossain and P. K. Iyer, J. Mater. Chem. A, 2021, 9, 26069 RSC; (b) J. Shamsi, A. S. Urban, M. Imran, L. De Trizio and L. Manna, Chem. Rev., 2019, 119, 3296 CrossRef CAS PubMed; (c) R. K. Gupta, R. Garai and P. K. Iyer, ACS Appl. Energy Mater., 2021, 4, 10025 CrossRef CAS.
- (a) L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692 CrossRef CAS PubMed; (b) K. Vighnesh, S. Wang, H. Liu and A. L. Rogach, ACS Nano, 2022, 16, 19618 CrossRef CAS PubMed.
- S. K. Mahankudo, A. Das, K. Mishra, M. Barai, P. Mal and S. Ghosh, ACS Appl. Nano Mater., 2022, 5, 4360 CrossRef CAS.
- S. Paul and A. Samanta, ACS Energy Lett., 2020, 5, 64 CrossRef CAS.
- M. Imran, V. Caligiuri, M. Wang, L. Goldoni, M. Prato, R. Krahne, L. De Trizio and L. Manna, J. Am. Chem. Soc., 2018, 140, 2656 CrossRef CAS PubMed.
- (a) F. Shojaei and W.-J. Yin, J. Phys. Chem. C, 2018, 122, 15214 CrossRef CAS; (b) J.-H. Lee, N. C. Bristowe, J. H. Lee, S.-H. Lee, P. D. Bristowe, A. K. Cheetham and H. M. Jang, Chem. Mater., 2016, 28, 4259 CrossRef CAS.
- D.-H. Kang, S.-Y. Kim, J.-W. Lee and N.-G. Park, J. Mater. Chem. A, 2021, 9, 3441 RSC.
- (a) J. Kim, A. J. Yun, B. Gil, Y. Lee and B. Park, Adv. Funct. Mater., 2019, 29, 1905190 CrossRef CAS; (b) V. G. V. Dutt, S. Akhil and N. Mishra, Nanoscale, 2021, 13, 14442 RSC.
- (a) J. Zhuang, J. Wang and F. Yan, Nano-Micro Lett., 2023, 15, 84 CrossRef CAS PubMed; (b) D. P. Nenon, K. Pressler, J. Kang, B. A. Koscher, J. H. Olshansky, W. T. Osowiecki, M. A. Koc, L.-W. Wang and A. P. Alivisatos, J. Am. Chem. Soc., 2018, 140, 17760 CrossRef CAS PubMed; (c) B. Zhang, L. Goldoni, C. Lambruschini, L. Moni, M. Imran, A. Pianetti, V. Pinchetti, S. Brovelli, L. De Trizio and L. Manna, Nano Lett., 2020, 20, 8847 CrossRef CAS PubMed.
- H.-R. Kim, J.-H. Bong, J.-H. Park, Z. Song, M.-J. Kang, D. H. Son and J.-C. Pyun, ACS Appl. Mater. Interfaces, 2021, 13, 29392 CrossRef CAS PubMed.
- (a) P.-L. T. Boudreault, A. Najari and M. Leclerc, Chem. Mater., 2011, 23, 456 CrossRef CAS; (b) R. Qiu, V. P. Reddy, T. Iwasaki and N. Kambe, J. Org. Chem., 2015, 80, 367 CrossRef CAS PubMed; (c) C. Zhao, K. P. Rakesh, L. Ravidar, W.-Y. Fang and H.-L. Qin, Eur. J. Med. Chem., 2019, 162, 679 CrossRef CAS PubMed.
- S. K. Sinha, S. Panja, J. Grover, P. S. Hazra, S. Pandit, Y. Bairagi, X. Zhang and D. Maiti, J. Am. Chem. Soc., 2022, 144, 12032 CrossRef CAS PubMed.
- H. Wang, Y. Li, Z. Tang, S. Wang, H. Zhang, H. Cong and A. Lei, ACS Catal., 2018, 8, 10599 CrossRef CAS.
- G. Liang, J.-H. Wang, T. Lei, Y.-Y. Cheng, C. Zhou, Y.-J. Chen, C. Ye, B. Chen, C.-H. Tung and L.-Z. Wu, Org. Lett., 2021, 23, 8082 CrossRef CAS PubMed.
- (a) P. M. Wood, Biochem. J., 1988, 253, 287 CrossRef CAS PubMed; (b) M. L. Pegis, C. F. Wise, D. J. Martin and J. M. Mayer, Chem. Rev., 2018, 118, 2340 CrossRef CAS PubMed.
- S.-H. Jung, J.-W. Yeon, Y. Kang and K. Song, Asian J. Chem., 2014, 26, 4084 CrossRef.
- X. Zhu, Y. Lin, J. San Martin, Y. Sun, D. Zhu and Y. Yan, Nat. Commun., 2019, 10, 2843 CrossRef PubMed.
- M. Pramanik, A. Mathuri and P. Mal, Chem. Commun., 2021, 57, 5698 RSC.
- R. Martin and S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1461 CrossRef PubMed.
- R. Chinchilla and C. Nájera, Chem. Rev., 2007, 107, 874 CrossRef PubMed.
- S. Bera, A. Saha, S. Mondal, A. Biswas, S. Mallick, R. Chatterjee and S. Roy, Mater. Adv., 2022, 3, 5234 RSC.
- B. Zhang, D. Altamura, R. Caliandro, C. Giannini, L. Peng, L. De Trizio and L. Manna, J. Am. Chem. Soc., 2022, 144, 5059 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2205394. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cy01478a |
| ‡ Equal contributing authors. |
| This journal is © The Royal Society of Chemistry 2024 |