Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A highly accurate potential energy surface for carbonyl sulphide (OCS): how important are the ab initio calculations?†
Alec
Owens

Department of Physics and Astronomy, University College London, Gower Street, WC1E 6BT, London, UK. E-mail: alec.owens.13@ucl.ac.uk
First published on 7th June 2024
Abstract
Ab initio quantum chemical methods can produce accurate molecular potential energy surfaces (PESs) capable of predicting the fundamental vibrational wavenumbers to within 1 cm−1. However, for high-resolution applications this is simply not good enough and empirical refinement is necessary, i.e. adjusting the PES to better match laboratory spectroscopic data. Here, the impact of the underlying ab initio calculations is rigorously investigated within the context of empirical refinement. For carbonyl sulphide (OCS), state-of-the-art electronic structure calculations are employed to construct higher- and lower-level ab initio PESs, which are then empirically refined in near-identical procedures. The initial ab initio calculations are shown to considerably affect the accuracy of the final refined PES, with an order-of-magnitude improvement in computed rotation-vibration energy levels achieved for OCS. In demonstrating this, the most accurate PES of the electronic ground state of OCS is produced, reproducing the fundamentals with a root-mean-square error (RMSE) of 0.004 cm−1, and 884 rovibrational energy levels below 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 with an RMSE of 0.060 cm−1.
000 cm−1 with an RMSE of 0.060 cm−1.
1 Introduction
Knowledge of the potential energy surface (PES) is crucial in studies of molecular structure, spectroscopy, and dynamics. The use of wavefunction-based quantum chemical methods, such as coupled cluster or multireference configuration interaction, in conjunction with large basis sets usually of quadruple-zeta quality and above, will produce an accurate ab initio PES, i.e. predicting the fundamental vibrational wavenumbers to within 2–5 cm−1. To improve the accuracy further, one must treat the basis set incompleteness error and smaller additional effects to recover more of the electron correlation energy, known as higher-level corrections.1,2 Numerous highly accurate ab initio PESs have been constructed in this manner, e.g. LiOH,3 H2CS,4 CH3Cl,5 CH4,6 reproducing the fundamentals to within 1 cm−1 on average. Although impressive, purely ab initio PESs are simply not good enough for high-resolution spectroscopic applications where sub-wavenumber accuracy is required.To overcome the limits of ab initio theory, the PES must be empirically refined to high-resolution laboratory spectroscopic data, i.e. adjusting the expansion parameters of the analytic function used to represent the ab initio data to better match experiment. Doing so can lead to orders-of-magnitude improvements in the accuracy of the calculated rotation-vibration (rovibrational) energy levels, better wavefunctions, and more reliable molecular properties as a result. The refinement procedure can be viewed as “pulling” and “pushing” the potential hypersurface in nuclear configuration space to better match the “true” molecular PES, with the original ab initio surface acting as the starting point. This poses the question: how significant are the initial ab initio calculations if the PES is going to be empirically refined?
Anecdotally, there are arguments for and against more sophisticated ab initio calculations of the initial PES. On the one hand, a more accurate ab initio PES should be closer to the “true” surface, thus leading to a smoother refinement and better final product. However, it could be argued that any reasonably accurate ab initio PES can serve as a starting point. The refinement procedure will mask the contribution from higher-level corrections and largely negate the additional computational effort associated with generating a highly accurate ab initio PES.
In this work, the impact of the initial ab initio calculations on the accuracy of a PES that is subsequently empirically refined is rigorously investigated. The carbonyl sulphide molecule (main isotopologue 16O12C32S) is investigated as there is strong interest in its infrared spectrum. Prominent studies of extrasolar planets, known as exoplanets, are actively searching for spectroscopic signatures of OCS.7,8 However, there is currently no infrared OCS molecular line list suitable for the high-temperature environments found on exoplanets, hindering its potential detection. In general, sulphur chemistry is expected to play a key role in the formation of hazes and clouds in the atmospheres of exoplanets9 with OCS an essential atmospheric molecule. On Earth, carbonyl sulphide is one of the most widespread sulphur-containing molecules in the atmosphere with a long atmospheric lifetime (over 2 years).10 OCS may even play a vital role in the prebiotic formation of biomolecules, challenging conventional assumptions about prebiotic chemistry on Earth.11
The purpose of this paper is twofold: (i) to critically evaluate the influence of the underlying ab initio calculations in the context of empirical refinement of the PES, and (ii) to produce the most accurate PES of the electronic ground state of OCS in the literature. There have been several theoretical studies of the PES and rovibrational spectrum of OCS12–19 but none at the accuracy or completeness targeted in this study.
2 Potential energy surface
2.1 Electronic structure calculations
To generate the PES of OCS, a focal-point approach20 has been utilised with the total electronic energy represented as,| Etot = ECBS + ΔECV + ΔEHO + ΔESR + ΔEDBOC. | (1) |
These terms are known as “higher-level” energy corrections to the PES arising from extrapolating the energy to the complete basis set (CBS) limit, core-valence (CV) electron correlation, higher-order (HO) correlation, scalar relativistic (SR) effects, and the diagonal Born–Oppenheimer correction (DBOC).1,2 In principle, their inclusion should lead to a more accurate PES.
The largest contribution is from the energy at the complete basis set (CBS) limit ECBS, computed using the explicitly correlated coupled cluster method CCSD(T)-F12b,21 in conjunction with the F12-optimized correlation consistent basis sets, cc-pVTZ-F12 and cc-pVQZ-F12.22,23 Extrapolation to the CBS limit was done using the two-point formula,24
| ECCBS = FC(ECQZ − ECTZ) + ECTZ, | (2) |
The contribution of CV electron correlation ΔECV was computed at the CCSD(T)-F12b/cc-pCVTZ-F1223,31 level of theory. The same ansatz and ABS were used as before but with a Slater geminal exponent value of β = 1.4 a0−1. The (1s) orbital of sulphur was frozen in all-electron calculations due to the difficulty basis sets have in describing this orbital.
The effect of truncating the coupled cluster expansion, termed HO correlation, was accounted for using the hierarchy of coupled cluster methods such that ΔEHO = ΔET + ΔE(Q). Here, the full triples contribution ΔET = ECCSDT − ECCSD(T), and the perturbative quadruples contribution ΔE(Q) = ECCSDT(Q) − ECCSDT. Calculations using the CCSD(T), CCSDT, and CCSDT(Q) methods were performed in the frozen core approximation using the general coupled cluster approach32,33 implemented in the MRCC code34 interfaced to the CFOUR quantum chemistry program.35 The correlation consistent basis sets cc-pVTZ(+d for S) and cc-pVDZ(+d for S)36,37 were utilised for the full triples and perturbative quadruples calculations, respectively.
The correction from SR effects ΔESR was accounted for using the second-order Douglas–Kroll–Hess approach38,39 at the CCSD(T)/cc-pVQZ-DK40 level of theory employing the frozen core approximation. Lastly, the DBOC ΔEDBOC was determined from all-electron calculations (with the (1s) orbital of S frozen) using the CCSD method41 as implemented in CFOUR with the aug-cc-pCVDZ(+d for S) basis set.42–44 The DBOC arises from the nuclear kinetic energy operator acting on the ground electronic state wavefunction and is dependent on nuclear mass, hence its inclusion means the PES is isotopologue-specific for 16O12C32S. It is worth stating that the natural abundance of the main isotopologue 16O12C32S on Earth is approximately 94% compared to the other OCS isotopologues.
In Fig. 1, the higher-level contributions are illustrated for OCS where one-dimensional cuts of the different corrections have been plotted. They are generally much smaller in magnitude, noticeably around the equilibrium geometry, and vary in a smooth fashion. As the molecule distorts, the higher-level corrections grow in magnitude which has the effect of “pulling” and “pushing” the potential hypersurface closer to its “true” shape.
All terms in eqn (1) were calculated on a grid of 6082 nuclear geometries with energies E up to hc 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1, where h is the Planck constant and c is the speed of light (from here on in we drop the h and c factors when discussing energies in wavenumbers). The grid was constructed in terms of three internal coordinates: the O–C bond length 0.95 ≤ rOC ≤ 1.59 Å the C–S bond length 1.27 ≤ rCS ≤ 2.46 Å and the interbond angle 107.5 ≤ ∠(OCS) ≤ 180.0°. Points were distributed randomly with a higher concentration around the equilibrium region as this is more spectroscopically important. It is possible that fewer points could have been utilised to obtain a satisfactory description of the OCS PES and studies have explored this, for example, in a highly accurate ab initio PES of CH3Cl.45
000 cm−1, where h is the Planck constant and c is the speed of light (from here on in we drop the h and c factors when discussing energies in wavenumbers). The grid was constructed in terms of three internal coordinates: the O–C bond length 0.95 ≤ rOC ≤ 1.59 Å the C–S bond length 1.27 ≤ rCS ≤ 2.46 Å and the interbond angle 107.5 ≤ ∠(OCS) ≤ 180.0°. Points were distributed randomly with a higher concentration around the equilibrium region as this is more spectroscopically important. It is possible that fewer points could have been utilised to obtain a satisfactory description of the OCS PES and studies have explored this, for example, in a highly accurate ab initio PES of CH3Cl.45
The basis sets used to compute the higher-level corrections were chosen pragmatically to ensure timely calculations with less emphasis on tightly converged energies. This was done because the corrections are (i) formed from differences between two absolute energies, and (ii) somewhat cancel each other out when summed up together, further negating the convergence error, for example, the CV and HO contributions along the stretch coordinates in Fig. 1 have similar magnitude but opposing sign. This strategy has been successfully utilised before in calculations of highly accurate ab initio PESs of SiH4,46 CH4,6 and CH3F.47 It is also relevant that the PES of OCS will be empirically refined to laboratory spectroscopic data.
The higher-level corrections were computed at every grid point, which although computationally intensive, was time-effective. An alternative strategy is to design reduced grids for each correction, fit a suitable analytic representation to the ab initio data, and obtain values across the global grid of nuclear geometries by interpolation, for example, as was done in ref. 4,5. While this is less computationally intensive, achieving a satisfactory description of each higher-level correction requires careful consideration and may not be trivial; issues that are avoided in the present approach.
For the purposes of this study, two ab initio PESs were produced. The first (and main) PES of OCS, referred to as CBS-HLai, contained the CBS extrapolated energies plus all the higher-level corrections, i.e. all terms in eqn (1). The second PES, named VQZ-F12ai, was determined from CCSD(T)-F12b/cc-pVQZ-F12 energies and can be regarded as a reference surface. This level of theory is still a very good approximation to the “true” surface and many theoretical studies would regard this as accurate and sufficient. Comparisons between the two surfaces will enable a valuable assessment of the impact of the CBS extrapolation and higher-level corrections on the accuracy of the PES, especially regarding the results of the empirical refinement.
2.2 Analytic representation
Each ab initio dataset was fitted using the analytic expression, | (3) |
| ξ1 = 1 − exp[−a(r1 − req1)], | (4) |
| ξ2 = 1 − exp[−b(r2 − req2)], | (5) |
| ξ3 = sin(π − α) − sin(π − αeq), | (6) |
in terms of the internal stretching coordinates r1 = rOC and r2 = rCS (in Å), the interbond angle α = ∠(OCS) (in radians), the Morse parameters a and b (in Å−1), and the equilibrium structural parameters req1, req2, and αeq, the latter being fixed to 180° as OCS is linear at equilibrium.
The second and third terms in eqn (3) introduce a repulsive contribution to the PES48,49 if the distance between the O and S atoms
 | (7) |
The expansion parameters fi1,i2,i3 were established through a least-squares fitting to the ab initio data, weighted using factors of the form50
 | (8) |
Here, Ẽi(w) = max (Ẽi, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) where Ẽi is the potential energy at the ith geometry above equilibrium and the normalization constant N = 0.0001 (all values in cm−1). The weighting favoured energies below 15
000) where Ẽi is the potential energy at the ith geometry above equilibrium and the normalization constant N = 0.0001 (all values in cm−1). The weighting favoured energies below 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1, producing a more spectroscopically relevant PES. The fit also employed Watson's robust fitting scheme,51 which reduced the weights of outliers and improved the overall description of the PESs.
000 cm−1, producing a more spectroscopically relevant PES. The fit also employed Watson's robust fitting scheme,51 which reduced the weights of outliers and improved the overall description of the PESs.
The ab initio CBS-HLai PES was fitted using a total of 90 parameters (81 expansion parameters, 3 equilibrium parameters, 2 Morse parameters, 4 damping parameters) achieving a weighted root-mean-square error (wRMSE) of 0.011 cm−1 for energies up to 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1. The VQZ-F12ai PES was fitted by 89 parameters (80 expansion parameters, 3 equilibrium parameters, 2 Morse parameters, 4 damping parameters) with a wRMSE of 0.015 cm−1 for energies up to 30
000 cm−1. The VQZ-F12ai PES was fitted by 89 parameters (80 expansion parameters, 3 equilibrium parameters, 2 Morse parameters, 4 damping parameters) with a wRMSE of 0.015 cm−1 for energies up to 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1. The expansion parameters of the CBS-HLai and VQZ-F12ai PESs are provided along with a program to construct them in the ESI.†
000 cm−1. The expansion parameters of the CBS-HLai and VQZ-F12ai PESs are provided along with a program to construct them in the ESI.†
3 Variational calculations
3.1 Rotation-vibration energy level calculations
To assess the accuracy of the PESs, variational calculations of the rovibrational energy level structure of OCS were carried out using the nuclear motion code TROVE.52 TROVE is a well-established program used extensively by the ExoMol database53–55 to generate comprehensive molecular line lists (catalogues of transitions and their probabilities) for exoplanetary science. The methodology of TROVE is well described in the literature52,56–61 and it has been used to compute the spectra of several triatomic molecules including CO2,62 SiO2,63 CaOH,64 KOH and NaOH65 (amongst numerous other larger polyatomics). Full details of the TROVE approach for treating linear and quasi-linear molecules can be found in ref. 60. The key calculation steps for OCS were as follows.The rovibrational Schrödinger equation in the ground electronic state was solved using the exact kinetic energy operator for triatomic molecules60 (based on the bisector embedding66,67) with the potential energy operator represented as a sixth-order power-series expansion. A multi-step procedure59 was employed to build the vibrational basis set from contracted and symmetry-adapted products of one-dimensional basis functions ϕn1, ϕn2 and 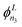 associated with the three vibrational modes of OCS. In TROVE, the two stretching and one bending vibrational mode have the respective quantum numbers n1, n2 and n3, with L being the vibrational angular momentum quantum number associated with the bending mode. These primitive one-dimensional functions were determined numerically through solution of one-dimensional Schrödinger equations for a given mode (stretch or bend) with all other modes set to their equilibrium values. For the stretches, the Numerov–Cooley method68,69 was used on grids of 1000 points each, while the bending mode required the use of Laguerre polynomials as a basis and a grid of 3000 points. The total size of the vibrational basis set was controlled by the polyad number condition 2(n1 + n2) + n3 ≤ 58. Convergence testing was done with respect to the number of basis functions defined through the polyad number condition, and with respect to the number of grid points used to define the primitive one-dimensional basis sets. Vibrational J = 0 states were converged to 10−6 cm−1 (on average) up to 5000 cm−1, and to 10−4 up to 8000 cm−1.
associated with the three vibrational modes of OCS. In TROVE, the two stretching and one bending vibrational mode have the respective quantum numbers n1, n2 and n3, with L being the vibrational angular momentum quantum number associated with the bending mode. These primitive one-dimensional functions were determined numerically through solution of one-dimensional Schrödinger equations for a given mode (stretch or bend) with all other modes set to their equilibrium values. For the stretches, the Numerov–Cooley method68,69 was used on grids of 1000 points each, while the bending mode required the use of Laguerre polynomials as a basis and a grid of 3000 points. The total size of the vibrational basis set was controlled by the polyad number condition 2(n1 + n2) + n3 ≤ 58. Convergence testing was done with respect to the number of basis functions defined through the polyad number condition, and with respect to the number of grid points used to define the primitive one-dimensional basis sets. Vibrational J = 0 states were converged to 10−6 cm−1 (on average) up to 5000 cm−1, and to 10−4 up to 8000 cm−1.
The full rovibrational basis set was constructed from symmetrized products of the symmetry-adapted vibrational basis functions 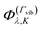 and symmetry-adapted rigid rotor functions |J,k,m〉(Γrot), classified according to the irreducible representations of the Cs(M) molecular group symmetry. That is,
and symmetry-adapted rigid rotor functions |J,k,m〉(Γrot), classified according to the irreducible representations of the Cs(M) molecular group symmetry. That is,
 | (9) |
Here, J is the total angular momentum quantum number, k and m are the rotational quantum numbers associated with the projection of the rotational angular momentum onto the molecular z and laboratory Z axes (in units of ħ), respectively, K = |k|, λ denotes a set of vibrational state quantum numbers, Γvib, Γrot, and Γ denote the symmetry of the vibrational, rotational, and total wavefunctions, respectively. An energy cut-off of E = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 was used to contract the J = 0 eigenfunctions for states up to K ≤ 20.
000 cm−1 was used to contract the J = 0 eigenfunctions for states up to K ≤ 20.
Thus, for a rovibrational state i with total angular momentum J and total symmetry Γ, the total wavefunction Ψ(Γ)i,J is a linear combination of rovibrational basis set functions,
 | (10) |
3.2 Empirical refinement procedure
There is a substantial amount of high-resolution, laboratory spectroscopic data on OCS. Very recently, an exhaustive review was performed71 that resulted in the extraction and analysis of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 071 independently measured and assigned rovibrational transitions. This was carried out using the MARVEL (measured active rotational-vibrational energy levels) procedure,72–75 which takes a user-constructed dataset of assigned spectroscopic transitions with measurement uncertainties and inverts them to yield a consistent set of empirically-derived energy levels with quantum number labelling and uncertainties. Such a dataset is invaluable for refining the PES. For OCS, 13
071 independently measured and assigned rovibrational transitions. This was carried out using the MARVEL (measured active rotational-vibrational energy levels) procedure,72–75 which takes a user-constructed dataset of assigned spectroscopic transitions with measurement uncertainties and inverts them to yield a consistent set of empirically-derived energy levels with quantum number labelling and uncertainties. Such a dataset is invaluable for refining the PES. For OCS, 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 056 of the extracted transitions were validated to produce a list of 5729 rovibrational energy levels up to J = 95 with energies up to 14
056 of the extracted transitions were validated to produce a list of 5729 rovibrational energy levels up to J = 95 with energies up to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 551 cm−1, of which 884 rovibrational energy levels (covering 106 vibrational states) up to J = 10 were utilised in the refinement.
551 cm−1, of which 884 rovibrational energy levels (covering 106 vibrational states) up to J = 10 were utilised in the refinement.
Empirical refinement of the CBS-HLai and VQZ-F12ai PESs was carried out in two steps. Firstly, the equilibrium structural parameters req1 and req2 were adjusted in a nonlinear least-squares fitting procedure to the pure rotational energies up to J = 10 in the ground vibrational state. Two iterations were sufficient to obtain converged parameters. Secondly, the full refinement was performed using an efficient least-squares fitting procedure76 in TROVE. Here, the effect of the refinement was treated as a perturbation ΔV to the original ab initio PES Vai such that the refined surface V′ = Vai + ΔV. Using the same vibrational coordinates, see eqn (4), the perturbation was expanded as
 | (11) |
In rovibrational calculations, computed states are assigned TROVE quantum numbers (n1, n2, n3, L) based on the largest contribution from the vibrational basis functions in the basis set expansion of eqn (10). These need to be correlated with the standard spectroscopic normal mode quantum numbers (v1,vL2,v3) for linear triatomic molecules to enable the computed values to be matched with the empirically-derived values. In OCS, the fundamentals are the C–S stretch ν1 at ≈859 cm−1, the bending mode ν2 at ≈520 cm−1, and the C–O stretch ν3 at ≈2062 cm−1. The additional vibrational angular momentum quantum number L is needed to describe excitation of the ν2 bending mode since motion can occur in two orthogonal planes with different phases. The following correlation rules were used: v1 = n2, vL2 = 2n3 + L, and v3 = n1.
The weighting scheme, i.e. the weights assigned to the empirically-derived values being refined to, is an important aspect of the procedure. Practically speaking, the relative weighting between energies is far more significant than the absolute values. A benefit of the MARVEL OCS dataset is that each energy level possesses a measurement uncertainty and information on the number of transitions that it was established from. Energy levels that are only involved in one transition may not be wholly reliable, whereas an energy level involved in multiple transitions can be deemed more trustworthy and assigned a larger weight in the refinement.
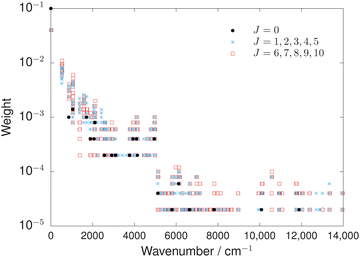
Different weighting schemes were tested that factored in the measurement uncertainty, as was used previously, for example, in ref. 78. However, the most successful scheme was based only on the number of transitions that the energy level was involved in. In Fig. 2, the final weighting scheme used in the refinement of the OCS PESs is illustrated. Pure rotational energies were weighted the largest to ensure rotational band structure was better reproduced. Energies below 5000 cm−1 were weighted an order-of-magnitude larger than energies above 5000 cm−1 to accurately capture the more important spectroscopic region of the PES. Overall, this weighting scheme produced a balanced and highly accurate refinement.
4 Results and Discussion
4.1 Equilibrium geometry and pure rotational energies
In the following, the empirically refined PESs of OCS are referred to as CBS-HLref and VQZ-F12ref, corresponding to the refined versions of the ab initio CBS-HLai and VQZ-F12ai PESs, respectively. In Table 1, the equilibrium bond lengths and rotational constant of OCS determined from the different PESs are listed along with experimental values79–81 derived from measured OCS microwave spectra. The computed values are in close agreement with experiment, with only marginal differences between the ab initio and refined PES bond lengths, especially for the CBS-HL PESs. Comparing the CBS-HL and VQZ-F12 PESs, the difference in equilibrium geometry is approximately 0.001–0.002 Å for the O–C bond length and 0.002–0.003 Å for the C–S bond length. Interestingly, the B rotational constant does not change between the CBS-HL PESs and is close to the refined VQZ-F12ref value.| r(O–C)/Å | r(C–S)/Å | B/cm−1 | |
|---|---|---|---|
| a From microwave spectroscopy. b From laser Stark measurements. | |||
Experiment79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
1.1612 ± 0.0058 | 1.5604 ± 0.0049 | |
Experiment80,81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
1.1543 ± 0.0010 | 1.5628 ± 0.0010 | 0.202857 |
| CBS-HLref | 1.1560 | 1.5619 | 0.203434 |
| CBS-HLai | 1.1561 | 1.5616 | 0.203434 |
| VQZ-F12ref | 1.1576 | 1.5638 | 0.203447 |
| VQZ-F12ai | 1.1577 | 1.5647 | 0.202717 |
Calculations of pure rotational energies up to J = 20 in the ground vibrational state, shown in Table 2, magnifies the seemingly small differences in equilibrium geometries. The CBS-HLref PES shows the closest agreement with the empirically-derived MARVEL energies71 indicating that the equilibrium geometry derived from this PES is the most accurate of the four. The residual errors (observed–calculated) of the computed rotational energies using the CBS-HLai PES are approximately a factor of two larger than the refined CBS-HLref PES values. They are still much more accurate than the VQZ-F12 PES results. Advanced ab initio calculations that treat higher-level corrections can be highly accurate when describing molecular structure82 and the results of Table 1 confirm this.
| J | Observed | CBS-HLai (A) | VQZ-F12ai (B) | CBS-HLref (C) | VQZ-F12ref (D) | o–c (A) | o–c (B) | o–c (C) | o–c (D) |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| 1 | 0.4057 | 0.4057 | 0.4043 | 0.4057 | 0.4046 | 0.0001 | 0.0014 | 0.0000 | 0.0011 |
| 2 | 1.2171 | 1.2170 | 1.2128 | 1.2170 | 1.2139 | 0.0002 | 0.0043 | 0.0001 | 0.0032 |
| 3 | 2.4343 | 2.4339 | 2.4256 | 2.4341 | 2.4278 | 0.0004 | 0.0087 | 0.0002 | 0.0065 |
| 4 | 4.0571 | 4.0565 | 4.0426 | 4.0568 | 4.0463 | 0.0006 | 0.0145 | 0.0003 | 0.0108 |
| 5 | 6.0857 | 6.0848 | 6.0639 | 6.0852 | 6.0695 | 0.0009 | 0.0217 | 0.0005 | 0.0162 |
| 6 | 8.5199 | 8.5186 | 8.4895 | 8.5192 | 8.4973 | 0.0013 | 0.0304 | 0.0007 | 0.0226 |
| 7 | 11.3598 | 11.3581 | 11.3193 | 11.3589 | 11.3297 | 0.0017 | 0.0406 | 0.0009 | 0.0302 |
| 8 | 14.6055 | 14.6033 | 14.5533 | 14.6043 | 14.5667 | 0.0022 | 0.0521 | 0.0012 | 0.0388 |
| 9 | 18.2568 | 18.2540 | 18.1916 | 18.2553 | 18.2083 | 0.0027 | 0.0652 | 0.0014 | 0.0485 |
| 10 | 22.3137 | 22.3104 | 22.2341 | 22.3119 | 22.2545 | 0.0033 | 0.0797 | 0.0018 | 0.0592 |
| 11 | 26.7763 | 26.7723 | 26.6807 | 26.7742 | 26.7053 | 0.0040 | 0.0956 | 0.0021 | 0.0711 |
| 12 | 31.6446 | 31.6399 | 31.5316 | 31.6421 | 31.5606 | 0.0047 | 0.1130 | 0.0025 | 0.0840 |
| 13 | 36.9185 | 36.9130 | 36.7867 | 36.9156 | 36.8205 | 0.0055 | 0.1318 | 0.0029 | 0.0980 |
| 14 | 42.5980 | 42.5916 | 42.4459 | 42.5946 | 42.4849 | 0.0064 | 0.1521 | 0.0034 | 0.1131 |
| 15 | 48.6831 | 48.6758 | 48.5093 | 48.6793 | 48.5539 | 0.0073 | 0.1738 | 0.0039 | 0.1292 |
| 16 | 55.1738 | 55.1656 | 54.9769 | 55.1694 | 55.0274 | 0.0083 | 0.1970 | 0.0044 | 0.1464 |
| 17 | 62.0701 | 62.0608 | 61.8485 | 62.0652 | 61.9054 | 0.0093 | 0.2216 | 0.0049 | 0.1647 |
| 18 | 69.3719 | 69.3615 | 69.1243 | 69.3664 | 69.1878 | 0.0104 | 0.2476 | 0.0055 | 0.1841 |
| 19 | 77.0793 | 77.0678 | 76.8042 | 77.0732 | 76.8747 | 0.0115 | 0.2751 | 0.0061 | 0.2046 |
| 20 | 85.1922 | 85.1794 | 84.8881 | 85.1854 | 84.9661 | 0.0128 | 0.3041 | 0.0068 | 0.2261 |
4.2 Rotation-vibration energy levels
In Table 3, computed J = 0 and J = 1 energy levels using the different PESs are compared against the empirically-derived MARVEL energies.71 For illustrative purposes, only a small selection is given, enough to clearly demonstrate the relative accuracies of the different PESs. Rovibrational energy levels are labelled by the rigorous quantum numbers J and the rotationless parity e/f where states with L = 0 correspond to e parity only, while states with L > 0 can possess both e and f components. The vibrational quantum numbers are approximate and follow the normal mode notation (v1,vL2,v3) for linear triatomic molecules discussed in Section 3.1.| J | e/f | (v1,vL2,v3) | Observed | CBS-HLai (A) | VQZ-F12ai (B) | CBS-HLref (C) | VQZ-F12ref (D) | o–c (A) | o–c (B) | o–c (C) | o–c (D) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | e | (0,11,0) | 520.828 | 521.474 | 522.516 | 520.829 | 520.841 | −0.646 | −1.042 | −0.001 | −0.013 |
| 0 | e | (1,00,0) | 858.967 | 858.320 | 860.309 | 858.961 | 859.020 | 0.647 | −1.989 | 0.006 | −0.053 |
| 0 | e | (0,20,0) | 1047.042 | 1048.281 | 1050.516 | 1047.043 | 1047.125 | −1.239 | −2.236 | −0.001 | −0.083 |
| 1 | e | (1,11,0) | 1372.864 | 1372.927 | 1375.905 | 1372.869 | 1372.880 | −0.063 | −2.978 | −0.004 | −0.016 |
| 1 | e | (0,31,0) | 1573.774 | 1575.576 | 1578.908 | 1573.772 | 1573.772 | −1.803 | −3.332 | 0.001 | 0.001 |
| 0 | e | (2,00,0) | 1710.976 | 1709.598 | 1713.848 | 1710.979 | 1711.018 | 1.378 | −4.250 | −0.003 | −0.042 |
| 0 | e | (1,20,0) | 1892.229 | 1892.878 | 1896.889 | 1892.245 | 1892.168 | −0.649 | −4.011 | −0.017 | 0.060 |
| 0 | e | (0,00,1) | 2062.201 | 2060.822 | 2063.908 | 2062.200 | 2062.238 | 1.379 | −3.086 | 0.002 | −0.036 |
| 0 | e | (0,40,0) | 2104.828 | 2107.081 | 2111.729 | 2104.854 | 2104.718 | −2.254 | −4.647 | −0.026 | 0.109 |
| 1 | e | (2,11,0) | 2218.433 | 2217.832 | 2223.005 | 2218.429 | 2218.470 | 0.601 | −5.173 | 0.004 | −0.038 |
| 1 | e | (1,31,0) | 2412.526 | 2413.729 | 2418.787 | 2412.532 | 2412.519 | −1.203 | −5.058 | −0.006 | 0.007 |
| 0 | e | (3,00,0) | 2555.991 | 2553.796 | 2560.523 | 2556.004 | 2555.922 | 2.195 | −6.728 | −0.013 | 0.069 |
| 1 | e | (0,11,1) | 2575.712 | 2575.003 | 2579.275 | 2575.708 | 2575.753 | 0.708 | −4.272 | 0.003 | −0.041 |
| 1 | e | (2,20,0) | 2731.804 | 2731.792 | 2737.857 | 2731.812 | 2731.725 | 0.012 | −6.065 | −0.008 | 0.079 |
| 0 | e | (1,00,1) | 2918.105 | 2916.240 | 2921.264 | 2918.125 | 2918.156 | 1.865 | −5.024 | −0.020 | −0.051 |
| 0 | e | (0,20,1) | 3095.554 | 3095.482 | 3101.154 | 3095.536 | 3095.667 | 0.072 | −5.672 | 0.018 | −0.112 |
| 1 | f | (1,11,1) | 3424.543 | 3423.398 | 3429.531 | 3424.548 | 3424.380 | 1.145 | −6.133 | −0.005 | 0.163 |
| 1 | e | (0,31,1) | 3615.750 | 3616.278 | 3623.216 | 3615.728 | 3616.024 | −0.528 | −6.938 | 0.022 | −0.274 |
| 0 | e | (2,00,1) | 3768.496 | 3766.931 | 3774.825 | 3768.489 | 3768.584 | 1.565 | −7.894 | 0.007 | −0.088 |
| 0 | e | (1,20,1) | 3937.427 | 3936.898 | 3944.311 | 3937.421 | 3937.371 | 0.530 | −7.413 | 0.006 | 0.056 |
| 1 | e | (1,60,0) | 3990.515 | 3992.934 | 4002.173 | 3990.522 | 3991.784 | −2.420 | −9.239 | −0.007 | −1.269 |
| 0 | e | (0,00,2) | 4101.410 | 4098.179 | 4105.020 | 4101.388 | 4101.400 | 3.232 | −6.841 | 0.022 | 0.010 |
The ab initio CBS-HLai and VQZ-F12ai PESs reproduce the three fundamental wavenumbers with root-mean-square errors (RMSEs) of 0.955 cm−1 and 2.203 cm−1, respectively. The residual errors increase for combination and overtone states, with the CBS-HLai PES noticeably more accurate than the VQZ-F12ai PES. The refined CBS-HLref and VQZ-F12ref PESs reproduce the fundamentals with RMSEs of 0.004 cm−1 and 0.038 cm−1, respectively. This is a substantial improvement over the respective ab initio surfaces but is somewhat expected.
More surprising is the difference in accuracy between the two refined PESs. In Fig. 3 and Table 4, the main results of this study are summarised. The CBS-HLref PES exhibits superior accuracy, reproducing all known empirically-derived rovibrational energy levels of OCS up to J = 10 below 5000 cm−1 with an RMSE of 0.016 cm−1. This is an order-of-magnitude better than the VQZ-F12ref PES, which possesses an RMSE of 0.167 cm−1. The improvement in accuracy extends across all the energy levels used in the refinement up to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1, clearly seen in Fig. 3. Overall, the CBS-HLref PES reproduces the 884 empirically-derived energies with an RMSE of 0.060 cm−1 compared to 0.383 cm−1 of the VQZ-F12ref PES, over a factor of six better.
000 cm−1, clearly seen in Fig. 3. Overall, the CBS-HLref PES reproduces the 884 empirically-derived energies with an RMSE of 0.060 cm−1 compared to 0.383 cm−1 of the VQZ-F12ref PES, over a factor of six better.
 | ||
| Fig. 3 Direct comparison of the residual errors ΔEobs−calc (in cm−1) between the empirically-derived MARVEL energies71 and the corresponding computed values up to J = 10 using the CBS-HLref and VQZ-F12ref PESs of OCS. | ||
| VQZ-F12ref | CBS-HLref | |
|---|---|---|
| RMSE (<5000 cm−1) | 0.167 | 0.016 |
| MAD (<5000 cm−1) | 0.072 | 0.010 |
| RMSE (>5000 cm−1) | 0.511 | 0.083 |
| MAD (>5000 cm−1) | 0.280 | 0.053 |
| RMSE (all) | 0.383 | 0.060 |
| MAD (all) | 0.178 | 0.032 |
A closer inspection of the residual errors between the observed and computed energy levels for the refined PESs is shown in Fig. 4. The overall trend in residual errors is fairly similar between the CBS-HLref and VQZ-F12ref PESs, with larger differences seen between 6000–7000 cm−1 for levels within, e.g. the (v1,vL2,v3) = (4,20,1) vibrational state. Several of these energy levels were only determined from one measured transition in the MARVEL procedure. Although weighted lower in the refinement, both PESs struggle more than expected to reproduce these levels, suggesting possible issues in the underlying spectroscopic experiments used to determine them. The VQZ-F12ref PES performs particularly poorly for the (1,60,0) vibrational state around ≈3991 cm−1 with residual errors around −1.25 cm−1 compared to errors of less than −0.01 cm−1 for the CBS-HLref PES. It is not clear why the VQZ-F12ref exhibits this behaviour.
 | ||
| Fig. 4 Closer inspection of the residual errors ΔEobs−calc (in cm−1) between the empirically-derived MARVEL energies71 and the corresponding computed values up to J = 10 using the CBS-HLref (left panel) and VQZ-F12ref (right panel) PESs of OCS. | ||
In the refinement procedure, the PES expansion parameters are simultaneously fitted to both the empirically-derived energies and the original ab initio dataset. This stops overfitting and unrealistic distortions arising in the PES. However, the fit will be somewhat constrained to the quality of the original ab initio dataset, potentially limiting the accuracy that can be achieved in the refinement.
To test the impact of constraining the refinement to the ab initio dataset, the CBS-HLref PES (defined by the expansion parameters) was refined to the empirical-quality MARVEL energy levels but constrained to the VQZ-F12 ab initio dataset. The 434 empirically-derived rovibrational energy levels of OCS below 5000 cm−1 were reproduced with an RMSE of 0.027 cm−1 (compared to 0.016 cm−1 in the original CBS-HLref PES refinement), while the full dataset of 884 energies was reproduced with an RMSE of 0.071 cm−1 (compared to 0.060 cm−1 in the original CBS-HLref PES refinement). Thus, the results of the refinement do not change significantly upon constraining to a different, less-accurate ab initio dataset.
Both refined PESs used the same analytic representation, and were determined using the same least-squares fitting refinement procedure in the computer program TROVE. This process varied the PES expansion parameters until they converged on an optimum solution in which the computed rovibrational energy levels matched the empirically-derived values as closely as possible for the given PES. The ab initio PES expansion parameters were the starting point of each refinement and seem to dictate the path that the refinement procedure takes to converge on a final solution. It suggests that the accuracy of the starting ab initio PES strongly influences the accuracy that can be achieved for the final refined PES.
5 Conclusions
The purpose of the present work was twofold. Firstly, the influence of the underlying ab initio calculations on the accuracy of a PES refined to experimental data has been critically investigated. Using carbonyl sulphide as an example, two PESs were generated with different levels of ab initio theory and subsequently refined to laboratory spectroscopic data in near-identical procedures. Interestingly, the quality of the underlying ab initio calculations was shown to considerably affect the accuracy of the final refined PES. For OCS, an order-of-magnitude improvement in the accuracy of the computed rovibrational energies was shown. This was achieved using a refined PES based on higher-quality ab initio calculations; a substantial improvement in accuracy that should be factored into future high-resolution theoretical studies.The second outcome of this work was constructing the most accurate PES in the literature for the electronic ground state of carbonyl sulphide. The empirically refined CBS-HLref PES, recommended for future applications, was based on state-of-the-art electronic structure calculations that pushed the limits of ab initio accuracy. The PES was rigorously refined to a comprehensive list of empirically-derived rovibrational energy levels up to J = 10; a list established from an exhaustive analysis of the literature on high-resolution spectra of OCS.71 The CBS-HLref PES reproduced 884 energy levels below 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 with an RMSE of 0.060 cm−1 and 434 energies below 5000 cm−1 with an RMSE of 0.016 cm−1, demonstrating unprecedented accuracy.
000 cm−1 with an RMSE of 0.060 cm−1 and 434 energies below 5000 cm−1 with an RMSE of 0.016 cm−1, demonstrating unprecedented accuracy.
There is strong motivation to study the infrared spectrum of OCS. Major studies of exoplanets are actively searching for spectroscopic signatures of carbonyl sulphide, e.g. in the spectra of the gas giant WASP-39b using the James Webb Space Telescope (JWST),7,8 but a lack of suitable spectroscopic data is hindering its potential detection. Exoplanets can possess temperatures ranging into the thousands of Kelvin but only room-temperature OCS line list data with incomplete coverage is available from the HITRAN spectroscopic database.83 A comprehensive molecular line list of OCS, based on the CBS-HLref PES, has recently been computed84 for the ExoMol database,53–55 which is providing extensive high-temperature spectroscopic data for exoplanets.
Data availability
The data that support the findings of this study are available within the article and its ESI.†Conflicts of interest
The authors have no conflicts to disclose.Acknowledgements
The author is grateful to Sergey Yurchenko for valuable discussions on the TROVE calculations and to Jonathan Tennyson for sharing the MARVEL dataset of empirically-derived energy levels of OCS before publication. The author acknowledges support from the Engineering and Physical Sciences Research Council [grant number EP/S021582/1]. The author also acknowledges the use of the UCL Legion High Performance Computing Facility (Legion@UCL) and associated support services in the completion of this work, along with the Cambridge Service for Data Driven Discovery (CSD3), part of which is operated by the University of Cambridge Research Computing on behalf of the STFC Dirac HPC Facility (https://www.dirac.ac.uk). The Dirac component of CSD3 was funded by BEIS capital funding via STFC capital grants ST/P002307/1 and ST/R002452/1 and STFC operations grant ST/R00689X/1.References
- T. Helgaker, W. Klopper and D. P. Tew, Mol. Phys., 2008, 106, 2107 CrossRef CAS.
- K. A. Peterson, D. Feller and D. A. Dixon, Theor. Chem. Acc., 2012, 131, 1079 Search PubMed.
- J. Koput, J. Chem. Phys., 2013, 138, 234301 CrossRef PubMed.
- A. Yachmenev, S. N. Yurchenko, T. Ribeyre and W. Thiel, J. Chem. Phys., 2011, 135, 074302 CrossRef PubMed.
- A. Owens, S. N. Yurchenko, A. Yachmenev, J. Tennyson and W. Thiel, J. Chem. Phys., 2015, 142, 244306 CrossRef PubMed.
- A. Owens, S. N. Yurchenko, A. Yachmenev, J. Tennyson and W. Thiel, J. Chem. Phys., 2016, 145, 104305 CrossRef PubMed.
- S.-M. Tsai, E. K. H. Lee, D. Powell, P. Gao, X. Zhang, J. Moses, E. Hébrard, O. Venot, V. Parmentier, S. Jordan, R. Hu, M. K. Alam, L. Alderson, N. M. Batalha, J. L. Bean, B. Benneke, C. J. Bierson, R. P. Brady, L. Carone, A. L. Carter, K. L. Chubb, J. Inglis, J. Leconte, M. Line, M. Lopez-Morales, Y. Miguel, K. Molaverdikhani, Z. Rustamkulov, D. K. Sing, K. B. Stevenson, H. R. Wakeford, J. Yang, K. Aggarwal, R. Baeyens, S. Barat, M. de Val-Borro, T. Daylan, J. J. Fortney, K. France, J. M. Goyal, D. Grant, J. Kirk, L. Kreidberg, A. Louca, S. E. Moran, S. Mukherjee, E. Nasedkin, K. Ohno, B. V. Rackham, S. Redfield, J. Taylor, P. Tremblin, C. Visscher, N. L. Wallack, L. Welbanks, A. Youngblood, E.-M. Ahrer, N. E. Batalha, P. Behr, Z. K. Berta-Thompson, J. Blecic, S. L. Casewell, I. J. M. Crossfield, N. Crouzet, P. E. Cubillos, L. Decin, J.-M. Desert, A. D. Feinstein, N. P. Gibson, J. Harrington, K. Heng, T. Henning, E. M.-R. Kempton, J. Krick, P.-O. Lagage, M. Lendl, J. D. Lothringer, M. Mansfield, N. J. Mayne, T. Mikal-Evans, E. Palle, E. Schlawin, O. Shorttle, P. J. Wheatley and S. N. Yurchenko, Nature, 2023, 617, 483 CrossRef CAS PubMed.
- L. Alderson, H. R. Wakeford, M. K. Alam, N. E. Batalha, J. D. Lothringer, J. Adams Redai, S. Barat, J. Brande, M. Damiano, T. Daylan, N. Espinoza, L. Flagg, J. M. Goyal, D. Grant, R. Hu, J. Inglis, E. K. H. Lee, T. Mikal-Evans, L. Ramos-Rosado, P.-A. Roy, N. L. Wallack, N. M. Batalha, J. L. Bean, B. Benneke, Z. K. Berta-Thompson, A. L. Carter, Q. Changeat, K. D. Colon, I. J. M. Crossfield, J.-M. Desert, D. Foreman-Mackey, N. P. Gibson, L. Kreidberg, M. R. Line, M. Lopez-Morales, K. Molaverdikhani, S. E. Moran, G. Morello, J. I. Moses, S. Mukherjee, E. Schlawin, D. K. Sing, K. B. Stevenson, J. Taylor, K. Aggarwal, E.-M. Ahrer, N. H. Allen, J. K. Barstow, T. J. Bell, J. Blecic, S. L. Casewell, K. L. Chubb, N. Crouzet, P. E. Cubillos, L. Decin, A. D. Feinstein, J. J. Fortney, J. Harrington, K. Heng, N. Iro, E. M.-R. Kempton, J. Kirk, H. A. Knutson, J. Krick, J. Leconte, M. Lendl, R. J. MacDonald, L. Mancini, M. Mansfield, E. M. May, N. J. Mayne, Y. Miguel, N. K. Nikolov, K. Ohno, E. Palle, V. Parmentier, D. J. M. Petit dit de la Roche, C. Piaulet, D. Powell, B. V. Rackham, S. Redfield, L. K. Rogers, Z. Rustamkulov, X. Tan, P. Tremblin, S.-M. Tsai, J. D. Turner, M. de Val-Borro, O. Venot, L. Welbanks, P. J. Wheatley and X. Zhang, Nature, 2023, 614, 664 CrossRef CAS PubMed.
- R. Hobbs, P. B. Rimmer, O. Shorttle and N. Madhusudhan, Mon. Not. R. Astron. Soc., 2021, 506, 3186 CrossRef CAS.
- M. A. K. Khalil and R. A. Rasmussen, Atmos. Environ., 1984, 18, 1805 CrossRef CAS.
- L. Leman, L. Orgel and M. R. Ghadiri, Science, 2004, 306, 283 CrossRef CAS PubMed.
- A. Foord, J. Smith and D. Whiffen, Mol. Phys., 1975, 29, 1685 CrossRef CAS.
- K. A. Peterson, R. C. Mayrhofer and R. C. Woods, J. Chem. Phys., 1991, 94, 431 CrossRef CAS.
- J. Martin, J. Francois and R. Gijbels, J. Mol. Spectrosc., 1995, 169, 445 CrossRef CAS.
- Y. Pak and R. C. Woods, J. Chem. Phys., 1997, 107, 5094 CrossRef CAS.
- J. Zuniga, A. Bastida, M. Alacid and A. Requena, J. Chem. Phys., 2000, 113, 5695 CrossRef CAS.
- D. Xie, Y. Lu, D. Xu and G. Yan, Chem. Phys., 2001, 270, 415 CrossRef CAS.
- D. Xie and Y. Lu, Chin. Sci. Bull., 2002, 47, 641 CrossRef.
- L. Shirkov, T. Korona and R. Moszynski, Struct. Chem., 2012, 23, 1425 CrossRef CAS.
- A. G. Csaszar, W. D. Allen and H. F. Schaefer, J. Chem. Phys., 1998, 108, 9751 CrossRef CAS.
- T. B. Adler, G. Knizia and H. J. Werner, J. Chem. Phys., 2007, 127, 221106 CrossRef PubMed.
- K. A. Peterson, T. B. Adler and H.-J. Werner, J. Chem. Phys., 2008, 128, 084102 CrossRef PubMed.
- J. G. Hill and K. A. Peterson, Phys. Chem. Chem. Phys., 2010, 12, 10460 RSC.
- J. G. Hill, K. A. Peterson, G. Knizia and H.-J. Werner, J. Chem. Phys., 2009, 131, 194105 CrossRef PubMed.
- S. Ten-No, Chem. Phys. Lett., 2004, 398, 56–61 CrossRef CAS.
- K. E. Yousaf and K. A. Peterson, J. Chem. Phys., 2008, 129, 184108 CrossRef PubMed.
- F. Weigend, Phys. Chem. Chem. Phys., 2002, 4, 4285–4291 RSC.
- C. Hättig, Phys. Chem. Chem. Phys., 2005, 7, 59–66 RSC.
- H.-J. Werner, P. J. Knowles, G. Knizia, F. R. Manby and M. Schütz, WIREs Comput. Mol. Sci., 2012, 2, 242–253 CrossRef CAS.
- H.-J. Werner, P. J. Knowles, F. R. Manby, J. A. Black, K. Doll, A. Heßelmann, D. Kats, A. Köhn, T. Korona, D. A. Kreplin, Q. Ma, T. F. Miller, A. Mitrushchenkov, K. A. Peterson, I. Polyak, G. Rauhut and M. Sibaev, J. Chem. Phys., 2020, 152, 144107 CrossRef CAS PubMed.
- J. G. Hill, S. Mazumder and K. A. Peterson, J. Chem. Phys., 2010, 132, 054108 CrossRef PubMed.
- M. Kállay and J. Gauss, J. Chem. Phys., 2005, 123, 214105 CrossRef PubMed.
- M. Kállay and J. Gauss, J. Chem. Phys., 2008, 129, 144101 CrossRef PubMed.
- M. Kállay, P. R. Nagy, D. Mester, Z. Rolik, G. Samu, J. Csontos, J. Csóka, P. B. Szabó, L. Gyevi-Nagy, B. Hégely, I. Ladjánszki, L. Szegedy, B. Ladóczki, K. Petrov, M. Farkas, P. D. Mezei and Á. Ganyecz, J. Chem. Phys., 2020, 152, 074107 CrossRef PubMed.
- D. A. Matthews, L. Cheng, M. E. Harding, F. Lipparini, S. Stopkowicz, T.-C. Jagau, P. G. Szalay, J. Gauss and J. F. Stanton, J. Chem. Phys., 2020, 152, 214108 CrossRef CAS PubMed.
- T. H. Dunning Jr., J. Chem. Phys., 1989, 90, 1007–1023 CrossRef.
- T. H. Dunning Jr., K. A. Peterson and A. K. Wilson, J. Chem. Phys., 2001, 114, 9244 CrossRef.
- M. Douglas and N. M. Kroll, Ann. Phys., 1974, 82, 89–155 CAS.
- B. A. Heß, Phys. Rev. A: At., Mol., Opt. Phys., 1986, 33, 3742–3748 CrossRef PubMed.
- W. A. de Jong, R. J. Harrison and D. A. Dixon, J. Chem. Phys., 2001, 114, 48–53 CrossRef CAS.
- J. Gauss, A. Tajti, M. Kallay, J. F. Stanton and P. G. Szalay, J. Chem. Phys., 2006, 125, 144111 CrossRef PubMed.
- R. A. Kendall, T. H. Dunning and R. J. Harrison, J. Chem. Phys., 1992, 96, 6796–6806 CrossRef CAS.
- D. E. Woon and T. H. Dunning, J. Chem. Phys., 1995, 103, 4572–4585 CrossRef CAS.
- K. A. Peterson and T. H. Dunning, J. Chem. Phys., 2002, 117, 10548 CrossRef CAS.
- P. O. Dral, A. Owens, S. N. Yurchenko and W. Thiel, J. Chem. Phys., 2017, 146, 244108 CrossRef PubMed.
- A. Owens, S. N. Yurchenko, A. Yachmenev and W. Thiel, J. Chem. Phys., 2015, 143, 244317 CrossRef PubMed.
- A. Owens, A. Yachmenev, J. Küpper, S. N. Yurchenko and W. Thiel, Phys. Chem. Chem. Phys., 2019, 21, 3496 RSC.
- S. E. Choi and J. C. Light, J. Chem. Phys., 1992, 97, 7031 CrossRef CAS.
- V. G. Tyuterev, S. A. Tashkun and D. W. Schwenke, Chem. Phys. Lett., 2001, 348, 223–234 CrossRef CAS.
- H. Partridge and D. W. Schwenke, J. Chem. Phys., 1997, 106, 4618–4639 CrossRef CAS.
- J. K. G. Watson, J. Mol. Spectrosc., 2003, 219, 326–328 CrossRef CAS.
- S. N. Yurchenko, W. Thiel and P. Jensen, J. Mol. Spectrosc., 2007, 245, 126–140 CrossRef CAS.
- J. Tennyson and S. N. Yurchenko, Mon. Not. R. Astron. Soc., 2012, 425, 21–33 CrossRef CAS.
- J. Tennyson, S. N. Yurchenko, A. F. Al-Refaie, E. J. Barton, K. L. Chubb, P. A. Coles, S. Diamantopoulou, M. N. Gorman, C. Hill, A. Z. Lam, L. Lodi, L. K. McKemmish, Y. Na, A. Owens, O. L. Polyansky, T. Rivlin, C. Sousa-Silva, D. S. Underwood, A. Yachmenev and E. Zak, J. Mol. Spectrosc., 2016, 327, 73–94 CrossRef CAS.
- J. Tennyson, S. N. Yurchenko, A. F. Al-Refaie, V. H. J. Clark, K. L. Chubb, E. K. Conway, A. Dewan, M. N. Gorman, C. Hill, A. E. Lynas-Gray, T. Mellor, L. K. McKemmish, A. Owens, O. L. Polyansky, M. Semenov, W. Somogyi, G. Tinetti, A. Upadhyay, I. Waldmann, Y. Wang, S. Wright and O. P. Yurchenko, J. Quant. Spectrosc. Radiat. Transf., 2020, 255, 107228 CrossRef CAS.
- S. N. Yurchenko, R. J. Barber, A. Yachmenev, W. Thiel, P. Jensen and J. Tennyson, J. Phys. Chem. A, 2009, 113, 11845–11855 CrossRef CAS PubMed.
- A. Yachmenev and S. N. Yurchenko, J. Chem. Phys., 2015, 143, 014105 CrossRef PubMed.
- J. Tennyson and S. N. Yurchenko, Int. J. Quantum Chem., 2017, 117, 92–103 CrossRef CAS.
- S. N. Yurchenko, A. Yachmenev and R. I. Ovsyannikov, J. Chem. Theory Comput., 2017, 13, 4368–4381 CrossRef CAS PubMed.
- S. N. Yurchenko and T. M. Mellor, J. Chem. Phys., 2020, 153, 154106 CrossRef CAS PubMed.
- T. M. Mellor, S. N. Yurchenko and P. Jensen, Symmetry, 2021, 13, 548 CrossRef CAS.
- S. N. Yurchenko, T. M. Mellor, R. S. Freedman and J. Tennyson, Mon. Not. R. Astron. Soc., 2020, 496, 5282–5291 CrossRef CAS.
- A. Owens, E. K. Conway, J. Tennyson and S. N. Yurchenko, Mon. Not. R. Astron. Soc., 2020, 495, 1927–1933 CrossRef CAS.
- A. Owens, V. H. J. Clark, A. Mitrushchenkov, S. N. Yurchenko and J. Tennyson, J. Chem. Phys., 2021, 154, 234302 CrossRef CAS PubMed.
- A. Owens, J. Tennyson and S. N. Yurchenko, Mon. Not. R. Astron. Soc., 2021, 502, 1128–1135 CrossRef CAS.
- S. Carter, N. Handy and B. Sutcliffe, Mol. Phys., 1983, 49, 745–748 CrossRef CAS.
- B. T. Sutcliffe and J. Tennyson, Int. J. Quantum Chem., 1991, 39, 183–196 CrossRef CAS.
- B. V. Noumerov, Mon. Not. R. Astron. Soc., 1924, 84, 592–602 CrossRef.
- J. W. Cooley, Math. Comp., 1961, 15, 363–374 Search PubMed.
- M. Wang, G. Audi, A. Wapstra, F. Kondev, M. MacCormick, X. Xu and B. Pfeiffer, Chin. Phys. C, 2012, 36, 1603 CrossRef.
- E. Xu and J. Tennyson, Mol. Phys., 2023, e2279694 CrossRef.
- T. Furtenbacher, A. G. Császár and J. Tennyson, J. Mol. Spectrosc., 2007, 245, 115–125 CrossRef CAS.
- A. G. Császár, G. Czakó, T. Furtenbacher and E. Mátyus, Annu. Rep. Comput. Chem., 2007, 3, 155–176 Search PubMed.
- T. Furtenbacher and A. G. Csaszar, J. Mol. Struct., 2012, 1009, 123 CrossRef CAS.
- R. Tóbiás, T. Furtenbacher, J. Tennyson and A. G. Császár, Phys. Chem. Chem. Phys., 2019, 21, 3473–3495 RSC.
- S. N. Yurchenko, R. J. Barber, J. Tennyson, W. Thiel and P. Jensen, J. Mol. Spectrosc., 2011, 268, 123–129 CrossRef CAS.
- S. Yurchenko, M. Carvajal, P. Jensen, F. Herregodts and T. Huet, Chem. Phys., 2003, 290, 59 CrossRef CAS.
- A. Owens, A. Mitrushchenkov, S. N. Yurchenko and J. Tennyson, Mon. Not. R. Astron. Soc., 2022, 516, 3995 CrossRef CAS.
- T. W. Dakin, W. E. Good and D. K. Coles, Phys. Rev., 1947, 71, 640 CrossRef CAS.
- A. G. Maki and D. R. Johnson, J. Mol. Spectrosc., 1973, 47, 226 CrossRef CAS.
- A. G. Maki, J. Phys. Chem. Ref. Data, 1974, 3, 221 CrossRef CAS.
- C. Puzzarini, Int. J. Quantum Chem., 2016, 116, 1513 CrossRef CAS.
- I. E. Gordon, L. S. Rothman, R. J. Hargreaves, R. Hashemi, E. V. Karlovets, F. M. Skinner, E. K. Conway, C. Hill, R. V. Kochanov, Y. Tan, P. Wcislo, A. A. Finenko, K. Nelson, P. F. Bernath, M. Birk, V. Boudon, A. Campargue, K. V. Chance, A. Coustenis, B. J. Drouin, J.-M. Flaud, R. R. Gamache, J. T. Hodges, D. Jacquemart, E. J. Mlawer, A. V. Nikitin, V. I. Perevalov, M. Rotger, J. Tennyson, G. C. Toon, H. Tran, V. G. Tyuterev, E. M. Adkins, A. Baker, A. Barbe, E. Canè, A. G. Császár, A. Dudaryonok, O. Egorov, A. J. Fleisher, H. Fleurbaey, A. Foltynowicz, T. Furtenbacher, J. J. Harrison, J.-M. Hartmann, V.-M. Horneman, X. Huang, T. Karman, J. Karns, S. Kassi, I. Kleiner, V. Kofman, F. Kwabia–Tchana, N. N. Lavrentieva, T. J. Lee, D. A. Long, A. A. Lukashevskaya, O. M. Lyulin, V. Y. Makhnev, W. Matt, S. T. Massie, M. Melosso, S. N. Mikhailenko, D. Mondelain, H. S. P. Müller, O. V. Naumenko, A. Perrin, O. L. Polyansky, E. Raddaoui, P. L. Raston, Z. D. Reed, M. Rey, C. Richard, R. Tóbiás, I. Sadiek, D. W. Schwenke, E. Starikova, K. Sung, F. Tamassia, S. A. Tashkun, J. Vander Auwera, I. A. Vasilenko, A. A. Vigasin, G. L. Villanueva, B. Vispoel, G. Wagner, A. Yachmenev and S. N. Yurchenko, J. Quant. Spectrosc. Radiat. Transf., 2022, 277, 107949 CrossRef CAS.
- A. Owens, S. N. Yurchenko and J. Tennyson, Mon. Not. R. Astron. Soc., 2024, 530, 4004 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: See the supplementary material for input files containing the expansion parameters and a program to construct the PESs of OCS, along with a full comparison of computed rovibrational energy levels up to J = 10 using the CBS-HLref and VQZ-F12ref PESs. See DOI: https://doi.org/10.1039/d4cp01205d |
| This journal is © the Owner Societies 2024 |