Absence of superconductivity in I4/mmm-FeH5: experimental evidence†
Yulong
Wang
a,
Su
Chen
a,
Jianning
Guo
a,
Xiaoli
Huang
 *a and
Tian
Cui
*a and
Tian
Cui
 ab
ab
aState Key Laboratory of Superhard Materials, College of Physics, Jilin University, Changchun 130012, China
bSchool of Physical Science and Technology, Ningbo University, Ningbo, 315211, China. E-mail: huangxiaoli@jlu.edu.cn
First published on 7th February 2024
Abstract
The experimentally discovered FeH5 exhibits a structure built of atomic hydrogen that only has bonding between hydrogen and iron atoms [C. M. Pepin, G. Geneste, A. Dewaele, M. Mezouar and P. Loubeyre, Science, 2017, 357, 382]. However, its superconductivity has remained unsolved since its discovery. In this work, we have synthesized I4/mmm-FeH5 at 139 GPa combined with laser-heating conditions. The electrical resistance measurements at ultrahigh pressures indicate that no evidence of superconducting transition of FeH5 is observed in the temperature range of 1.5 K to 270 K. These results indicate that I4/mmm-FeH5 does not exhibit superconductivity within the experimental temperature range, and the introduction of iron atoms is not beneficial to the formation of the superconducting phase.
Introduction
Hydrogen has been predicted to be a superconductor with high critical temperatures (Tc) at high pressures.1 Theoretical calculations indicate that the Tc of hydrogen will exceed 240 K or even close to room temperature with a pressure above 500 GPa.2–4 Although extensive research on hydrogen has been conducted, scientists have not obtained direct data in electrical transport experiments which can prove the metallization and superconductivity of solid hydrogen.5–8 Encouragingly, Ashcroft predicted that the introduction of heavier elements would enhance intermolecular interactions between hydrogen. This process is known as “chemical precompression”, which would have a positive impact and can realize the metallization and superconductivity of hydrogen at lower pressures.9 For example, H3S has a Tc of ∼200 K at ∼150 GPa10–13 and LaH10 has a Tc of ∼250 K at ∼150 GPa.14–17On the other hand, according to the observations in geomagnetism and seismology, iron is the most abundant element in the Earth's core.18–20 However, if the Earth's core consists of pure iron, the density of it is ∼2–5% more than reality at relevant pressures and temperatures.21–25 Therefore, the Earth's core should have elements lighter than iron such as nickel (it is definable exist in the Earth's core), silicon, sulphur, oxygen, carbon and hydrogen.22,26–28 The iron–water reaction, Fe + O2 → FeHx + FeO, could take place at pressures and temperatures lower than the Earth's core, which means that iron hydrides are the prospective components of the Earth's core.29–31 In this regard, conducting measurements of the mechanical, electrical, and magnetic properties of iron hydrides are of significant importance in exploring the Earth's core and gaining further insights into the Earth's structure. For example, FeH has been extensively studied: dhcp-FeH (ε′), which can be stable in excess of 62 GPa, has been synthesised at ∼1000 °C and ∼5 GPa;19,32 two magnetic phase transitions of dhcp-FeH were found theoretically and experimentally at ∼26 GPa and ∼43 GPa, respectively.25,33–36
Concurrently, iron hydrides with a higher hydrogen content have been predicted or synthesized successively. In experiments, Gavriliuk et al. have synthesized I4/mmm-FeH2 at 77 GPa and ∼2000 K;37 Pepin et al. have synthesized I4/mmm-FeH2 and Pm-3m-FeH3 at 67 GPa and 86 GPa after heating to ∼1500 K, respectively,38 and they also first detected I4/mmm-FeH5 at ∼150 GPa after heating to ∼1500 K.39 Gavriliuk et al. have synthesized new iron hydrides with possible Tc of ∼25 K and 27.7 K at ∼178 GPa and 195 GPa respectively; unfortunately, they did not determine their crystal structures.40 Theoretically, several studies have reported new phases of the Fe–H system, including FeH3,41 FeH4,42 FeH643–45 and FeH7.45 Most of these and some other iron hydrides are summarized in a phase diagram.46 Among all the iron hydrides, I4/mmm-FeH5 has the highest hydrogen content in experiments and a very particular structure with puckered hexagonal honeycomb layers. The H–H nearest-neighbour distance of I4/mmm-FeH5 is 1.32 Å at 130 GPa and this phase only has bonding between hydrogen and iron atoms. So, I4/mmm-FeH5 is regarded as a typical example of the “precompression”. Some theoretical works have proposed that I4/mmm-FeH5 is a potential superconductor with a Tc of ∼ 50 K,44,47,48 while others consider that FeH5 is not a superconductor.45,49 Considering the unique structure of FeH5 and controversial calculated results, it is necessary to conduct experiments to explore its superconductivity and provide more clues for the high-temperature superconducting hydrides.
In this work, we have successfully synthesized FeH5 by compression of Fe and NH3BH3 at high pressures with laser heating to ∼1400 K. Electrical transport measurements indicate that FeH5 retains a metallic character without transforming into the superconducting (SC) phase in the temperature range of 1.5–270 K.
Experimental details
We conducted three runs to investigate the superconductivity and structure of the Fe–H system at high pressure. Iron foil (purchased from Alfa Aesar, purity 99.99%) was pre-compressed and loaded into a diamond anvil cell (DAC) together with NH3BH3, which was used as the pressure-transmitting medium and hydrogen source.15,50 For electrical resistance measurements, we used DACs made of NiCrAl alloy, and the diamonds had a culet of 50, 60, and 80 μm in diameter bevelled at 8° to a diameter of about 300 μm. A near-IR laser (YLR-200, 1070 nm) was used in our laboratory to heat the sample in the DAC with a spot size of ∼5 μm.51 The electrical resistance was measured using the four-probe method with the delta model of a Keithley current source (Model 6221) and a voltmeter (Model 2182A).52,53 Mo electrodes were sputtered on the surface of diamond anvils to connect Pt electrodes and the sample, and MgO/epoxy was used as an insulating layer to separate the tungsten gasket and electrodes. The low temperature measurements were carried out in a helium cryostat (1.5–300 K) equipped with a 0–9 T superconducting magnet. The pressure was calibrated by the Raman shift of the diamond anvil.54 All crystal structures were determined by in situ X-ray diffraction (XRD) on the beamline BL15U1 of the Shanghai synchrotron radiation facility (SSRF), with a wavelength of 0.6199 Å and a spot size of ∼3 μm2. The XRD patterns were analysed using Diopatas,55 Jana200656 and GSAS-II.57Results and discussion
Experimental results
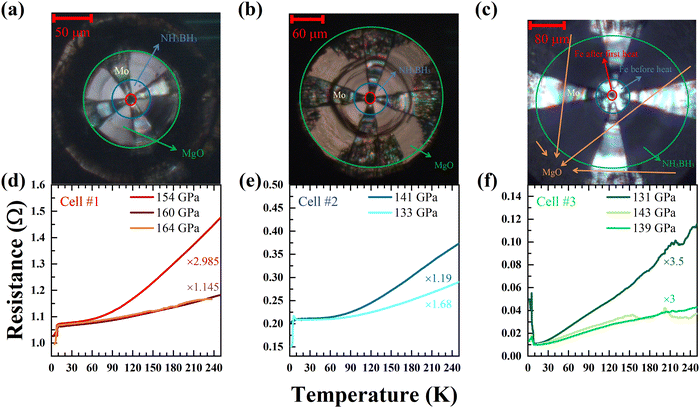
We conducted three runs (cells #1–3) of electrical transport measurements on Fe–H compounds synthesized by laser heating at specific pressures. We have plotted the corresponding electrical resistance data during the cooling process in Fig. 1. In cell #1, three cycles of laser heating were carried out at 154, 160 and 164 GPa, respectively. Unfortunately, no superconducting transition was detected in the electrical resistance measurement apart from the drop of the electrodes (Fig. 1a). In cell #2 and cell #3, after heating the sample at the target pressures, no superconducting transition was detected except the drop of the electrodes as well (Fig. 1b and c).To determine the possible phases produced in the electrical cell, we have performed the synchrotron X-ray diffraction measurements on the same sample. Considering the possibility of synthesising non-superconducting Fe–H phases during the first and second heating in cell #3, we obtained in situ XRD patterns at 143 GPa. The XRD results showed that the sample was still the pure P63/mmc-Fe phase and no FeH compound was generated (Fig. 2a). Therefore, a third laser heating was performed at 139 GPa. After XRD characterization, it was found that the heated sample reacted with a large area (Fig. 2c) and generated the I4/mmm-FeH5 phase (Fig. 2b), which was consistent with the previous work.39 The cell parameters of I4/mmm-FeH5 in this work at a pressure of 139 GPa are a = b = 2.4172 Å, and c = 11.6647 Å. The crystal structures of P63/mmc-Fe and I4/mmm-FeH5 are shown in Fig. 3a and b, and the refined lattice parameters and R-values in this work are shown in Table S1 (ESI†). P63/mmc-Fe is a typical hexagonal close packed (hcp) structure with an ABAB stacking of Fe layers. I4/mmm-FeH5 exhibits a structure consisting of quasi-cubic FeH3 unit layers and four-plane slabs of thin atomic hydrogen, and it can also be considered as a combination of Fe layers and puckered hexagonal honeycomb hydrogen layers. The comparison of the unit cell volume in Fig. 3c also certifies the synthesis of FeH5 in agreement with ref. 39. However, after conducting electrical transport tests, it was found that there was no superconducting transition attributed to FeH5 (Fig. 1c).
 | ||
| Fig. 2 Synchrotron X-ray diffraction (XRD) patterns (λ = 0.6199 Å) of the synthesized sample at high pressures. (a) XRD pattern of the sample before laser-heating at 143 GPa. The cell parameters of P63/mmc-Fe are a = b = 2.2527 Å and c = 3.6053 Å. Grey hollow circles, grey solid circles, red lines and blue lines correspond to the experimental data, Le Bail fitted data, Rietveld fitted data and the difference of experimental and Le Bail fitted data, respectively. The signal of Mo electrodes in the bcc phase is also observed in the XRD pattern.58,59 (b) XRD pattern of synthesized I4/mmm-FeH5 at 139 GPa. (c) XRD patterns of the synthesized sample measured across the sample with a step of 3 μm. The red point indicates the initial laser focus position. | ||
 | ||
| Fig. 3 Structures and pressure–volume data of the Fe–H samples at different pressures in cell #3. (a) and (b) Crystal structures of Fe and FeH5, respectively. Green and red balls represent Fe and H atoms, respectively. (c) Pressure dependence of the unit cell volume (per f.u.). Brown solid squares and red solid and hollow circles represent the experimental data of P63/mmc-Fe from ref. 23 and the experimental and calculation data of I4/mmm-FeH5 from ref. 39, respectively. Experimental data in this study are represented by solid stars with error bars of pressure. | ||
In addition, there is a small drop in 14 K at 143 GPa in cell #3, and to confirm whether the drop was the superconducting transition of FeH5, the second cooling process was made; but unfortunately the result could not be repeated (Fig. S2, ESI†), and other cooling measurements had not detected the similar signal yet. On the other hand, there are obvious some bends in the front of the curves. We attribute it to the superconducting transition of Mo electrodes or MoCx because the diamonds were heated to ∼1000 K to prepare the Mo electrodes in magnetron sputtering equipment. Another reason is that the Mo electrodes may react with diamonds in laser heating of the sample. Two curves, representing the resistance data of Fe and FeH5 at 143 GPa and 139 GPa respectively, are put together to verify whether the bends are from the superconductivity of FeH5 (Fig. S3, ESI†). Vertical reference lines indicate that all the bends of the resistance curve of FeH5 can correspond to the curve of Fe, so they are not evidence of superconducting transitions of FeH5.
In order to further confirm the change of the sample, the electrical resistance as a function of temperature at different pressures was fitted by the form of R(T) = R0 + ATα,60 where R0 is the residual resistance and A and α are the coefficient of power function and the power exponent of temperature, respectively. After the first heating in cell #1 (154 GPa) and second heating in cell #3 (143 GPa), the low temperature resistance is related to the second power of temperature, indicating that the sample may not be reacted with hydrogen and remains pure iron obeying Fermi liquid theory (Fig. 4a and c). But as shown in Fig. 4b, α had a noticeable change after the second heating in cell #1 (160 GPa). Furthermore, the sample colour changed from metallic to black and a distinct Raman signal of hydrogen (see Fig. S1, ESI†) occurred after the third heating. All these phenomena point to the formation of new hydrides.61,62 In Fig. 4a and b, the parameter α is changed from 2.027 to 0.980 after the second heating, probably indicating the strange metal (SM) character in the formed iron hydride.63–65 And, in Fig. 4c and d, the parameter α is changed from 1.960 to 2.122 after the third heating, indicating that FeH5 behaves like a normal metal corresponding to Fermi liquid theory. Future electrical transport measurements will provide more detailed information on various iron hydrides.
Discussion
Because the positions of H atoms cannot be determined by XRD experiments at high pressures, the bonds of H–H atoms are controversial. Pepin et al. consider that the structure of FeH5 is similar to two-dimensional materials, in which Fe–H atoms form bonds while H–H atoms do not form bonds.39 However, in some theoretical work, the structure of FeH5 can be seen as two independent graphene-like puckered hexagonal honeycomb hydrogen atom layers inserted between iron atom layers, with weak electron localization between H atoms.48,49 Furthermore, the high pressure hydrogen phase with the space group Cmca is similar to the I4/mmm-FeH5 structure and the theoretical prediction reveals that Cmca-H2 has a Tc of 242 K at 450 GPa.2,66 And, it has also been predicted in hydrides that SrH10 with the similar structure has a Tc of 259 K. Because the puckered honeycomb layer structures have strong mixing of stretch and bent vibrations, strong electron–phonon coupling easily occurs and can increase the superconducting transition temperature.67 But the superconductivity of I4/mmm-FeH5 is controversial. Some theoretical works predicted that FeH5 has a Tc of ∼50 K at ∼150 GPa,44,47,48 but others consider that FeH5 is not a superconductor.45,49 Although conclusions are different, there are some similarities in their theoretical calculations, and it can be seen from the density of electron states and phonon states.44,45,47–49 The electrons of iron atoms play a major role near the Fermi surface; the only band with appreciable Fe–H hybridization and electron–phonon (e-ph) coupling lies above the Fermi level rather than near the Fermi level; for the phonon modes below 650 cm−1, iron still plays a dominant role and contributes the most to the e-ph coupling interaction. In summary, FeH5 behaves just like elemental metals rather than other high Tc superconducting hydrides in band structure like H3S and LaH10, and exhibits a strong e-ph coupling near the Fermi level and hydrogen plays a dominant role in phonon density of states (PHDOS).12,17 So, it is obvious that the introduction of iron atoms cannot benefit superconductivity with similar structures compared with pure solid hydrogen.Conclusions
In conclusion, we have successfully synthesized FeH5 by compression of Fe and NH3BH3 at 139 GPa with laser heating to about 1400 K. The refined XRD results confirmed the crystal structure of FeH5 as reported in the I4/mmm-FeH5 phase, and no superconductivity evidence is observed in electrical transport measurements within the range of 1.5–270 K. Through function fitting in low temperature resistance, FeH5 behaves as a typical metal in consistent with Fermi liquid theory. Our results indicate that the chemical precompression of iron atoms is not beneficial to the superconductivity even though it has a similar structure to pure atomic hydrogen. We hope that this work can end the debate on the superconductivity of FeH5 and provide more insights into the study of superconducting transition metal hydrides.Data availability
The authors declare that the main data supporting the findings of this study are contained within the paper and its associated ESI.† All other relevant data are available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China under grant numbers 52372257 and 52072188, the Program for Changjiang Scholars and Innovative Research Team in University under the grant number IRT_15R23, the Zhejiang Provincial Science and technology innovation Team under the grant number 2021R01004, and the Fundamental Research Funds for the Central Universities. The authors thank the staff of the Shanghai Synchrotron Radiation Facility for their help during the synchrotron XRD measurements.References
- N. W. Ashcroft, Phys. Rev. Lett., 1968, 21, 1748–1749 CrossRef CAS.
- P. Cudazzo, G. Profeta, A. Sanna, A. Floris, A. Continenza, S. Massidda and E. K. U. Gross, Phys. Rev. Lett., 2008, 100, 257001 CrossRef CAS PubMed.
- J. M. McMahon and D. M. Ceperley, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 144515 CrossRef.
- M. Borinaga, I. Errea, M. Calandra, F. Mauri and A. Bergara, Phys. Rev. B, 2016, 93, 174308 CrossRef.
- P. Loubeyre, F. Occelli and P. Dumas, Nature, 2020, 577, 631–635 CrossRef CAS PubMed.
- M. I. Eremets and I. A. Troyan, Nat. Mater., 2011, 10, 927–931 CrossRef CAS PubMed.
- R. P. Dias and I. F. Silvera, Science, 2017, 355, 715–718 CrossRef CAS PubMed.
- D. Castelvecchi, Nature, 2017, 542, 17 CrossRef CAS PubMed.
- N. W. Ashcroft, Phys. Rev. Lett., 2004, 92, 187002 CrossRef CAS PubMed.
- A. P. Drozdov, M. I. Eremets, I. A. Troyan, V. Ksenofontov and S. I. Shylin, Nature, 2015, 525, 73–76 CrossRef CAS PubMed.
- X. Huang, X. Wang, D. Duan, B. Sundqvist, X. Li, Y. Huang, H. Yu, F. Li, Q. Zhou, B. Liu and T. Cui, Natl. Sci. Rev., 2019, 6, 713–718 CrossRef CAS PubMed.
- D. Duan, Y. Liu, F. Tian, D. Li, X. Huang, Z. Zhao, H. Yu, B. Liu, W. Tian and T. Cui, Sci. Rep., 2014, 4, 6968 CrossRef CAS PubMed.
- M. Einaga, M. Sakata, T. Ishikawa, K. Shimizu, M. I. Eremets, A. P. Drozdov, I. A. Troyan, N. Hirao and Y. Ohishi, Nat. Phys., 2016, 12, 835–838 Search PubMed.
- A. P. Drozdov, P. P. Kong, V. S. Minkov, S. P. Besedin, M. A. Kuzovnikov, S. Mozaffari, L. Balicas, F. F. Balakirev, D. E. Graf, V. B. Prakapenka, E. Greenberg, D. A. Knyazev, M. Tkacz and M. I. Eremets, Nature, 2019, 569, 528–531 CrossRef CAS PubMed.
- M. Somayazulu, M. Ahart, A. K. Mishra, Z. M. Geballe, M. Baldini, Y. Meng, V. V. Struzhkin and R. J. Hemley, Phys. Rev. Lett., 2019, 122, 027001 CrossRef CAS PubMed.
- F. Peng, Y. Sun, C. J. Pickard, R. J. Needs, Q. Wu and Y. Ma, Phys. Rev. Lett., 2017, 119, 107001 CrossRef PubMed.
- H. Liu, I. I. Naumov, R. Hoffmann, N. W. Ashcroft and R. J. Hemley, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 6990–6995 CrossRef CAS PubMed.
- D. J. Stevenson, Science, 1981, 214, 611–619 CrossRef CAS PubMed.
- Q. Williams and R. Jeanloz, J. Geophys. Res., 1990, 95, 19299–19310 CrossRef CAS.
- R. Jeanloz, Annu. Rev. Earth Planet. Sci., 1990, 18, 357–386 CrossRef.
- A. F. Birch, J. Geophys. Res., 1952, 57, 227–286 CrossRef.
- F. Birch, J. Geophys. Res., 1964, 69, 4377–4388 CrossRef CAS.
- H. K. Mao, Y. Wu, L. C. Chen, J. F. Shu and A. P. Jephcoat, J. Geophys. Res., 1990, 95, 21737–21742 CrossRef.
- E. I. Isaev, N. V. Skorodumova, R. Ahuja, Y. K. Vekilov and B. Johansson, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 9168–9171 CrossRef CAS PubMed.
- N. Hirao, T. Kondo, E. Ohtani, K. Takemura and T. Kikegawa, Geophys. Res. Lett., 2004, 31, L06616 CrossRef.
- J.-P. Poirier, Phys. Earth Planet. Inter., 1994, 85, 319–337 CrossRef CAS.
- K. Hirose, S. Labrosse and J. Hernlund, Annu. Rev. Earth Planet. Sci., 2013, 41, 657–691 CrossRef CAS.
- A. E. Ringwood, Proc. Math. Phys. Eng. Sci., 1984, 395, 1–46 CAS.
- Y. Fukai, Nature, 1984, 308, 174–175 CrossRef CAS.
- T. Suzuki, S.-i Akimoto and Y. Fukai, Phys. Earth Planet. Inter., 1984, 36, 135–144 CrossRef CAS.
- E. Ohtani, N. Hirao, T. Kondo, M. Ito and T. Kikegawa, Phys. Chem. Miner., 2005, 32, 77–82 CrossRef CAS.
- J. V. Badding, R. J. Hemley and H. K. Mao, Science, 1991, 253, 421–424 CrossRef CAS PubMed.
- V. E. Antonov, I. T. Belash, E. G. Ponyatovskii, V. G. Thiessen and V. I. Shiryaev, Phys. Status Solidi A, 1981, 65, K43–K48 CrossRef CAS.
- T. Tsumuraya, Y. Matsuura, T. Shishidou and T. Oguchi, J. Phys. Soc. Jpn., 2012, 81, 064707 CrossRef.
- N. Bouldi, P. Sainctavit, A. Juhin, L. Nataf and F. Baudelet, Phys. Rev. B, 2018, 98, 064430 CrossRef CAS.
- J. Ying, J. Zhao, W. Bi, E. E. Alp, Y. Xiao, P. Chow, G. Shen and V. V. Struzhkin, Phys. Rev. B, 2020, 101, 020405 CrossRef CAS.
- A. G. Gavriliuk, V. V. Struzhkin, S. N. Aksenov, A. G. Ivanova, A. A. Mironovich, I. A. Troyan and I. S. Lyubutin, JETP Lett., 2023, 116, 804–816 CrossRef.
- C. M. Pepin, A. Dewaele, G. Geneste, P. Loubeyre and M. Mezouar, Phys. Rev. Lett., 2014, 113, 265504 CrossRef PubMed.
- C. M. Pepin, G. Geneste, A. Dewaele, M. Mezouar and P. Loubeyre, Science, 2017, 357, 382 CrossRef CAS PubMed.
- A. G. Gavriliuk, I. A. Troyan, V. V. Struzhkin, D. N. Trunov, S. N. Aksenov, A. A. Mironovich, A. G. Ivanova and I. S. Lyubutin, JETP Lett., 2023, 118, 742–753 CrossRef CAS.
- Z. G. Bazhanova, A. R. Oganov and O. Gianola, Phys.-Usp., 2012, 55, 489–497 CrossRef CAS.
- F. Li, D. Wang, H. Du, D. Zhou, Y. Ma and Y. Liu, RSC Adv., 2017, 7, 12570–12575 RSC.
- S. Zhang, J. Lin, Y. Wang, G. Yang, A. Bergara and Y. Ma, J. Phys. Chem. C, 2018, 122, 12022–12028 CrossRef CAS.
- A. G. Kvashnin, I. A. Kruglov, D. V. Semenok and A. R. Oganov, J. Phys. Chem. C, 2018, 122, 4731–4736 CrossRef CAS.
- N. Zarifi, T. Bi, H. Liu and E. Zurek, J. Phys. Chem. C, 2018, 122, 24262–24269 CrossRef CAS.
- D. N. Sagatova, P. N. Gavryushkin, N. E. Sagatov, I. V. Medrish and K. D. Litasov, JETP Lett., 2020, 111, 145–150 CrossRef CAS.
- K. M. Skoczylas, A. P. Durajski and R. Szczȩśniak, Phys. B, 2020, 584, 412063 CrossRef CAS.
- A. Majumdar, J. S. Tse, M. Wu and Y. Yao, Phys. Rev. B, 2017, 96, 201107 CrossRef.
- C. Heil, G. B. Bachelet and L. Boeri, Phys. Rev. B, 2018, 97, 214510 CrossRef CAS.
- Y. V. Kondrat’ev, A. V. Butlak, I. V. Kazakov and A. Y. Timoshkin, Thermochim. Acta, 2015, 622, 64–71 CrossRef.
- S. Anzellini and S. Boccato, Crystals, 2020, 10, 459 CrossRef CAS.
- D. Errandonea, E. Bandiello, A. Segura, J. J. Hamlin, M. B. Maple, P. Rodriguez-Hernandez and A. Muñoz, J. Alloys Compd., 2014, 587, 14–20 CrossRef CAS.
- B. Wu, Y. Han, G. Peng, X. Huang, C. Liu, F. Jin and C. Gao, Phys. Status Solidi C, 2011, 8, 1692–1694 CrossRef CAS.
- Y. Akahama and H. Kawamura, J. Appl. Phys., 2006, 100, 043516 CrossRef.
- C. Prescher and V. B. Prakapenka, High Press. Res., 2015, 35, 223–230 CrossRef CAS.
- V. Petříček, M. Dušek and L. Palatinus, Z. Kristallogr. – Cryst. Mater., 2014, 229, 345–352 CrossRef.
- B. H. Toby and R. B. Von Dreele, J. Appl. Crystallogr., 2013, 46, 544–549 CrossRef CAS.
- Y. Akahama, N. Hirao, Y. Ohishi and A. K. Singh, J. Appl. Phys., 2014, 116, 223504 CrossRef.
- J. Wang, F. Coppari, R. F. Smith, J. H. Eggert, A. E. Lazicki, D. E. Fratanduono, J. R. Rygg, T. R. Boehly, G. W. Collins and T. S. Duffy, Phys. Rev. B, 2016, 94, 104102 CrossRef.
- S. Cai, J. Zhao, N. Ni, J. Guo, R. Yang, P. Wang, J. Han, S. Long, Y. Zhou, Q. Wu, X. Qiu, T. Xiang, R. J. Cava and L. Sun, Nat. Commun., 2023, 14, 3116 CrossRef CAS PubMed.
- V. Schettino, R. Bini, M. Ceppatelli, L. Ciabini and M. Citroni, in Adv. Chem. Phys., ed. S. A. Rice, John Wiley & Sons, Inc, Hoboken, NJ, USA, 2005, vol. 131, pp. 105–242 Search PubMed.
- D. Errandonea, J. Phys. Chem. Solids, 2009, 70, 1117–1120 CrossRef CAS.
- S. Martin, A. T. Fiory, R. M. Fleming, L. F. Schneemeyer and J. V. Waszczak, Phys. Rev. Lett., 1988, 60, 2194–2197 CrossRef CAS PubMed.
- S. Martin, A. T. Fiory, R. M. Fleming, L. F. Schneemeyer and J. V. Waszczak, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 846–849 CrossRef CAS PubMed.
- G. S. Boebinger, Science, 2009, 323, 590–591 CrossRef CAS PubMed.
- C. J. Pickard and R. J. Needs, Nat. Phys., 2007, 3, 473–476 Search PubMed.
- K. Tanaka, J. S. Tse and H. Liu, Phys. Rev. B, 2017, 96, 100502 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cp05996k |
| This journal is © the Owner Societies 2024 |