Te-doped-WSe2/W as a stable monolith catalyst for ampere-level current density hydrogen evolution reaction†
Xingchen
Zhang
a,
Dongfang
Zhang
a,
Xinya
Chen
a,
Dingyi
Zhou
a,
Jinying
Zhang
 b and
Zhiyong
Wang
b and
Zhiyong
Wang
 *a
*a
aKey Laboratory of Advanced Light Conversion Materials and Biophotonics, Department of Chemistry, Renmin University of China, Beijing 100872, PR China. E-mail: zhiyongwang@ruc.edu.cn
bState Key Laboratory of Electrical Insulation and Power Equipment, Center of Nanomaterials for Renewable Energy (CNRE), School of Electrical Engineering, Xian Jiaotong University, Xian, Shanxi 710049, PR China
First published on 3rd January 2024
Abstract
The development of efficient electrocatalysts for the hydrogen evolution reaction (HER) holds immense importance in the context of large-scale hydrogen production from water. Nevertheless, the practical application of such catalysts still relies on precious platinum-based materials. There is a pressing need to design high-performing, non-precious metal electrocatalysts capable of generating hydrogen at substantial current levels. We report here a stable monolith catalyst of Te-doped-WSe2 directly supported by a highly conductive W mesh. This catalyst demonstrates outstanding electrocatalytic performance and stability in acidic electrolytes, especially under high current conditions, surpassing the capabilities of commercial 5% Pt/C catalysts. Specifically, at current densities of 10 and 1200 mA cm−2, it exhibits a minimal overpotential of 79 and 232 mV, along with a small Tafel slope of 55 mV dec−1, respectively. The remarkable catalytic activity of Te–WSe2 can be attributed to the exceptional electron transfer facilitated by the stable monolithic structure, as well as the abundant and efficient active sites in the material. In addition, density functional theory calculations further indicate that Te doping adjusts H atom adsorption on various positions of WSe2, making it closer to thermal neutrality compared to the original material. This study presents an innovative approach to develop cost-effective HER electrocatalysts that perform optimally under high current density conditions.
1 Introduction
The remarkable energy density and lack of pollutant emissions make hydrogen an increasingly interesting candidate for renewable and eco-friendly energy.1–8 One promising method for producing hydrogen on a large scale is the combination of renewable energy sources and water splitting.9–13 This has led to the development of high-performing and long-lasting electrocatalysts designed specifically for HER. Presently, catalysts based on Pt and Pt-based materials exhibit the highest activity for the HER, but their limited availability and high cost have hindered widespread adoption of water electrolysis technology.14–19 Considerable efforts are currently being devoted to expediting the development of economically viable and easily accessible catalysts for the hydrogen evolution reaction. This includes the exploration of transition-metal carbides,20–22 nitrides,23,24 sulfides,25–27 oxides28 and phosphides.29–31 Within the category of catalysts that are free of platinum-group metals, transition-metal disulfides (TMDs), such as WS2,32 WSe233 and WTe2,34 have received significant attention and are regarded as promising electrocatalysts for water splitting. Both theoretical predictions and experimental findings suggest that the HER activity of TMDs primarily stems from their edges rather than their basal planes.35,36Various techniques have been devised to improve the catalytic performance of TMDs, such as defect engineering,37 heterostructure fabrication,38 phase conversion39 and foreign atom doping.40 Wang et al. synthesized metallic WSe2 nanoscrolls used as electrocatalysts for the HER, demonstrating much enhanced electrocatalytic performance compared to the semiconducting 2H WSe2 nanoscroll counterparts.41 Zhao et al. synthesized NiMo-doped WSe2 catalyst through a one-step hydrothermal reaction, with overpotentials of 177 and 188 mV at a current density of 10 mA cm−2 in 0.5 M H2SO4 and 1 M KOH, respectively. Theoretical calculations confirmed that NiMo co-doping significantly reduced the potential energy barrier of HER reaction, and thus improved HER performance.42 These studies shed light on the considerable potential of WSe2 as a proficient catalyst for the process of HER, thus revealing its promising prospects in the development of hydrogen production techniques. However, the catalytic efficiency of WSe2 in previous studies does not meet the practical requirements, especially in terms of the high current density needed for industrial production. The objective of this research is to develop an effective approach to enhance the performance of WSe2 beyond that of Pt/C, in order to enable the industrial implementation of non-precious metal catalysts. In order to achieve this, we utilized a Te doping technique to introduce a significant number of electroactive sites into WSe2. The WSe2 material was obtained by selenization of the oxide layer of W meshes. The selenized W mesh can be used as an electrode for HER. Additionally, the W wires play a crucial role in facilitating electron transport. The Te-doped WSe2 prepared in this manner demonstrates outstanding catalytic properties in acidic environments. Notably, its performance at high current densities exceeds that of the commercially available 5% Pt/C catalyst. We conducted first-principles calculations to gain insight into the reasons behind the improved activity.
2 Experimental
2.1 Sample preparation
The synthesis of the Te-doped WSe2 catalyst involved a two-zone chemical vapor deposition (CVD) method, as shown in Fig. 1a. A W mesh (1 × 1 cm2, 200 mesh) was used as the growth supports. First, the W mesh was cleaned ultrasonically with distilled water and ethanol for 1000 s each. Then, in the CVD furnace, 0.1 g of Se and 0.1 g of Te were placed separately in two porcelain boats in the middle of the T1 zone. The W mesh was positioned in the middle of the T2 zone. | ||
| Fig. 1 (a) Schematic illustration of the high-temperature reaction system used for the synthesis of Te–WSe2. (b) Temperature–time profiles for the synthesis of Te–WSe2. | ||
The T1 and T2 zones underwent a gradual heating process, with T1 reaching a temperature of 500 °C and T2 reaching a temperature of 600 °C over a period of 25 min. These temperatures were then maintained for an additional 25 min. Afterwards, both temperature zones were further heated for 15 min until reaching a temperature of 700 °C simultaneously, and this temperature was maintained for 10 min. Throughout the synthesis process, a stream of Ar gas at a flow rate of 100 sccm was continuously passed under ambient pressure until T1 reached a temperature of 400 °C, as shown in Fig. 1b. Following this, a mixture of carrier gases consisting of 100 sccm Ar/H2 (with a ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was used until the furnace cooled down to room temperature. In order to examine the effect of Te doping on the enhancement of catalytic performance of WSe2, both WSe2 and WTe2 were synthesized under identical conditions for comparative analysis.
1) was used until the furnace cooled down to room temperature. In order to examine the effect of Te doping on the enhancement of catalytic performance of WSe2, both WSe2 and WTe2 were synthesized under identical conditions for comparative analysis.
Preparation of Pt/C electrodes involves the following steps: 490 μL of water, 490 μL of ethanol, 20 μL of 5% Nafion solution and 3 mg of Pt/C were mixed together and subjected to ultrasound for 1 hour. Then, 10 μL of the mixture were applied as drops onto a circular glassy carbon electrode with a 5 mm diameter for electrochemical testing.
2.2 Characterization
Scanning electron microscopy (SEM) images were obtained using a Hitachi SU-8010 instrument. High-resolution transmission electron microscopy (HRTEM) images were recorded using an FEI Tecnai G2 F30 microscope under an acceleration voltage of 300 kV. Raman spectra were recorded on a Horiba Xplora spectrophotometer with a 532 nm laser. Structural and chemical analyses of the samples were performed using powder X-ray diffractometer (XRD 7000 X-ray) with Cu Kα radiation (λ = 1.54 Å) and X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha).2.3 Electrochemical measurements
The electrochemical measurements were performed on an electrochemical workstation (CHI660E). The W mesh supporting Te–WSe2 catalysts was used as the working electrode, and Ag/AgCl and Pt electrodes were used as the reference and counter electrodes, respectively. All the potentials mentioned are in reference to the reversible hydrogen electrode (RHE): ERHE = E(Ag/AgCl) + 0.059 × pH + 0.197 V. The electrochemical measurements were carried out in 0.5 M H2SO4. High-purity Ar gas was bubbled into the electrolyte for 30 min before the measurements. Linear sweep voltammetry (LSV) measurements were conducted between 0 and −0.6 V versus Ag/AgCl at a scan rate of 5 mV s−1. All results were corrected by 95% ohmic potential drop (iR) correction. The Tafel curve was obtained from the LSV curves. Electrochemical impedance spectroscopy (EIS) was conducted at an overpotential of 50 mV and in the frequency range of 1000–10 Hz with an amplitude of 5 mV, and then Z-view software was used to select a suitable equivalent circuit for fitting. The value of the double-layer capacitance (Cdl) was calculated by testing typical CV curves in 0 to 0.2 V versus RHE at different scan rates (20, 40, 60, 80, 100, 120, 140, 160, 180, and 200 mV s−1). The electrochemical double layer capacitance (Cdl) values is the slope of plotting ΔJ/2(ΔJ = janodic − jcathodic) at 0.1 V against scan rates. Long-term stability tests were performed using 16 h chronopotentiometry (CP) test under different current densities.2.4 Computational details
First-principles calculations in the framework DFT are performed by using the Vienna ab initio simulation package (VASP)43–45 with the projector–augmented wave method (PAW). The exchange–correlation energy was calculated by using the generalized gradient approximation (GGA) of Perdew–Burke–Ernzerhof (PBE).46 The energy cutoff is set to 400 eV. Spin polarization effects are considered in this study. We performed structural optimization until the residual forces on each ion converged to less than 0.01 eV. The adsorption energy for surface hydrogen adsorbates is defined as follows: ΔEH = Eadsorption − Esurface − 0.5 × EH2(g), where Eadsorption represents the energy of the surface with the adsorbed hydrogen atom, Esurface represents the energy of the pure surface, and EH2(g) represents the energy of the H2 species in the gas phase. The calculation also includes entropy (S) and zero-point energy (ZPE) to obtain the Gibbs free adsorption energy of hydrogen: ΔGH = ΔEH + ΔEZPE − TΔS.3 Results and discussion
The surface morphology of the W mesh was analyzed using scanning electron microscopy (SEM) both before and after the CVD reaction. Initially, the W mesh displayed a fairly even surface with slight surface striations (Fig. 2a). Following the reaction, a significant transformation occurred, resulting in a textured “accordion-like” surface with distinct split stripes (as shown in Fig. 2b). In order to conduct a more detailed examination, the W wire was carefully dissected to reveal its cross section (Fig. 2c). This revealed the growth of layered structures on the surface of the W wire, which corresponded to the Te doped WSe2 layers.HRTEM was utilized to investigate the microstructure and elemental composition of the products. The HRTEM image of Te–WSe2, depicted in Fig. 3a, reveals a layered structure with small grain coverage. Fig. 3b showcases the edge of Te–WSe2, where a crystal lattice spacing of 0.28 nm corresponds to the (100) plane of WSe2. The sample exhibits abundant structural defects, such as irregular edges and internal micro pores, which can potentially act as active sites for catalysis. Fig. 3c displays the selected-area electron diffraction (SAED) pattern of the products, clearly identifying the crystal planes including (011), (013), (008), (023), and (−130) of WSe2. Additionally, the STEM-HAADF images, along with EDS elemental mapping of W, Se, and Te elements (as shown in Fig. 3d), validate the uniform distribution of Te within WSe2, thereby confirming the successful synthesis of Te–WSe2.
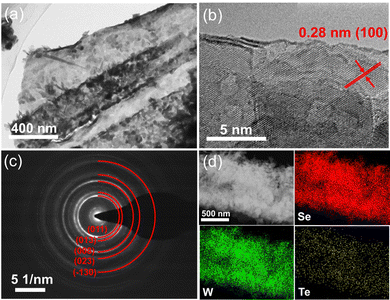 | ||
| Fig. 3 (a) and (b) HRTEM images of Te–WSe2. (c) SAED pattern of Te–WSe2. (d) STEM-HAADF with EDS elemental mapping images of Se, W, Te. | ||
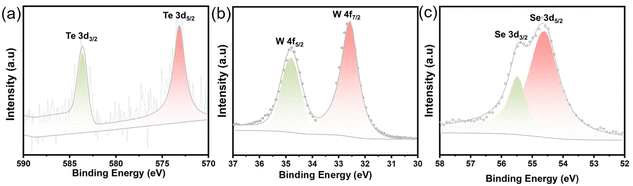
The chemical composition and electronic states of the Te–WSe2 sample were analyzed by using XPS measurements. The XPS spectra reveal the presence of Te, Se, and W elements, confirming their coexistence in the sample. As shown in Fig. 4a, two characteristic peaks observed at 573.20 eV and 583.71 eV corresponded to Te 3d5/2 and Te 3d3/2 states, respectively. The XPS spectrum of Te 3d5/2 and Te 3d3/2 at 573.20 eV and 583.71 eV corresponding to the Te2− state, indicating the successful doping of Te with a doping concentration of 0.65%. The W 4f elemental binding energy profile in Fig. 4b displayed two prominent peaks at 32.63 eV and 34.63 eV, corresponding to W 4f7/2 and W 4f5/2 states in Te–WSe2, respectively. Additionally, the peaks observed at 54.51 eV and 55.33 eV were attributed to Se 3d5/2 and Se 3d3/2 states in Te–WSe2 (as shown in Fig. 4c). As compared to WSe2 (XPS spectra are shown in Fig. S1, ESI†), the Se 3d3/2 and Se 3d5/2 peaks in Te–WSe2 exhibited a shift of −0.4 eV and −0.37 eV, respectively. This shift in Se 3d peaks can be attributed to the reduced electron attraction strength of Te and the enhanced electron attraction strength of Se. Consequently, the electron density surrounding Se increased due to the introduction of Te, which possesses a relatively lower electronegativity compared to Se.
Raman spectrum of Te–WSe2 under 532 nm laser excitation reveals the characteristic in-plane E12g peak of WSe2 at 250 cm−1 (Fig. 5a). With the introduction of Te, the peak shifts to a lower frequency (246.9 cm−1) and the intensity decreases. This frequency shift suggests that Te doping causes soft W–Se vibrations. Additionally, the reduced Raman intensity is likely a result of the change in lattice symmetry, which affects the matrix elements and selection rules for Raman active vibrational modes. XRD spectra were measured to confirm the composition of the products. Fig. 5b illustrates the XRD patterns of Te–WSe2 and WSe2, with the observed peaks for WSe2 aligning well with the standard 2H-WSe2 PDF card (JCPDS no. 38-1388). The XRD spectrum peaks show no significant change upon Te doping, indicating that the original structure of 2H-WSe2 remains unchanged.
 | ||
| Fig. 5 (a) and (b) Raman scattering spectra and XRD pattern of Te–WSe2 and WSe2. The curves denoted with pink and blue colors correspond to signals from Te–WSe2 and WSe2. | ||
To evaluate the electrochemical catalytic potential of Te–WSe2, we conducted a three-electrode cell experiment using an Ar-saturated 0.5 M H2SO4 electrolyte. Additionally, we measured the HER performance of the prepared WSe2 and WTe2 samples to understand the impact of Te doping. The catalytic properties of the samples were evaluated through LSV analysis, and the polarization curves of the different catalysts can be seen in Fig. 6a. By comparing the overpotentials at current densities of 10, 100, 500, and 1200 mA cm−2, we were able to determine the catalyst activities (Fig. 6b). The results indicated a significant improvement in the catalytic performance of WSe2 after Te doping. For the original WSe2, the overpotentials at current density of 10, 100, 500, and 1200 mA cm−2 were measured as 366, 461, 525, and 541 mV, respectively. However, after Te doping, the overpotentials decreased significantly to 79, 170, 207, and 232 mV, respectively. Moreover, as the current density increased, the advantage of Te–WSe2 became increasingly prominent. In fact, the sample achieved a high current density of 1000 mA cm−2 with a low overpotential of 225 mV, surpassing both the commercial 5% Pt/C catalyst and most reported high current density catalysts (Table 1). We have prepared a series of Te-doped WSe2 samples by varying the quantity of Te reactant. It was observed that the sample produced with 0.1 g of Te demonstrated the most favorable catalytic performance (Fig. S2, ESI†).
| Electrocatalyst | Electrolyte | Current density (mA cm−2) | Overpotential (mV) | Tafel slope (mV dev−1) |
|---|---|---|---|---|
| Te–WSe2 (this work) | 0.5 M H2SO4 | 10 | 79 | 55 |
| 100 | 170 | |||
| 500 | 207 | |||
| 1000 | 225 | |||
| CuMo6S8/Cu47 | 1 M KOH | 10 | 172 | 43 |
| 1000 | 320 | |||
| P/Mo–Ni3S2/NF48 | 0.5 M H2SO4 | 1000 | 240 | 75 |
| α-MoB249 | 0.5 M H2SO4 | 10 | 149 | 74.2 |
| 1000 | 334 | |||
| WS2(1−x)Se2x/NiSe250 | 0.5 M H2SO4 | 10 | 88 | 74.2 |
| 400 | ≈175 | |||
| CH4/H2 thermal treatment51 | 0.5 M H2SO4 | 1000 | 412 | 60 |
| MoS2/Mo2C | ||||
| α-MoWSx/N-RGO52 | 0.5 M H2SO4 | 1000 | 349 | 43 |
| CoP/Ni5P4/CoP53 | 0.5 M H2SO4 | 10 | 33 | 43 |
| 500 | ≈130 | |||
| 1000 | 142 | |||
| NiP2/Ni5P4/CoPS/CoP354 | 0.5 M H2SO4 | 10 | 41 | 45.2 |
| 100 | 88 | |||
| 500 | 130 | |||
| 1000 | 150 | |||
| Ni5P4@Cu foam55 | 0.5 M H2SO4 | 10 | 90 | 49 |
| 100 | 164 | |||
| 1000 | ≈230 | |||
| MoS2/Mo2C56 | 0.5 M H2SO4 | 1000 | 227 | 53 |
| MoSx–Fe@UiO-66-(OH)257 | 0.5 M H2SO4 | 10 | 118 | 41 |
| 1000 | 297 | |||
| Co/Se–MoS2-NF36 | 0.5 M H2SO4 | 10 | 104 | 67 |
| 100 | 188 | |||
| 1000 | 382 | |||
| Rh/SiNW58 | 0.5 M H2SO4 | 10 | 180 | 24 |
| 1000 | 950 | |||
| 2H Nb1.35S259 | 0.5 M H2SO4 | 1000 | 370 | 43 |
| Co–Co2P@N,P doped C/rGO60 | 0.5 M H2SO4 | 10 | 130 | 50.64 |
| 1000 | 900 | |||
| MoS2/CNF61 | 0.5 M H2SO4 | 500 | 380 | 69 |
| 1000 | 450 | |||
| MoS2/graphene13 | 0.5 M H2SO4 | 10 | 62 | 43.3 |
| 1000 | 250 | |||
| Ni2P–CuP262 | 0.5 M H2SO4 | 10 | 12 | 41 |
| 100 | 124 | |||
| 1000 | ≈500 | |||
| Co–N doped C63 | 0.5 M H2SO4 | 500 | 272 | 67.6 |
| 1000 | 343 | |||
| α-MoSx64 | 0.5 M H2SO4 | 10 | 68 | 86 |
| 100 | 80 | |||
| 500 | 250 | |||
| 1000 | 322 | |||
| MoSe2/MoO265 | 0.5 M H2SO4 | 10 | 140 | 48.9 |
| 200 | ≈350 |
The relative activity of the catalysts was further compared by the Tafel slope (Fig. 6c). The Tafel slope is an inherent characteristic of electrocatalysts and is indicative of the rate-limiting step in HER. We fitted the experimental data from the polarization curve to the Tafel equation and calculated the Tafel slope (η = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j + a) from the linear portion of the Tafel plot, where “b” represents the Tafel slope. In acidic electrolytes, the typical mechanism of electrochemical hydrogen evolution involves three main steps: Volmer, Heyrovsky, and Tafel reactions, each associated with Tafel slopes of approximately 120, 40, and 30 mV dec−1, respectively.66 Pt/C has the best catalytic performance, with a Tafel slope of 35 mV dec−1. The prepared WSe2 and WTe2 exhibit Tafel slopes of 95 mV dec−1 and 120 mV dec−1, respectively, indicating that both have high initial energy barriers and slow reaction rates in the HER process. After successful incorporation of Te into WSe2, Te–WSe2 exhibits a Tafel slope of 55 mV dec−1, indicating that the Heyrovsky–Volmer reaction is its main pathway in the HER reaction process. The comparison of Te–WSe2 with WSe2 and WTe2 indicates that the introduction of Te effectively accelerates the kinetic process of the catalytic reaction, thereby improving the activity of the catalyst.
j + a) from the linear portion of the Tafel plot, where “b” represents the Tafel slope. In acidic electrolytes, the typical mechanism of electrochemical hydrogen evolution involves three main steps: Volmer, Heyrovsky, and Tafel reactions, each associated with Tafel slopes of approximately 120, 40, and 30 mV dec−1, respectively.66 Pt/C has the best catalytic performance, with a Tafel slope of 35 mV dec−1. The prepared WSe2 and WTe2 exhibit Tafel slopes of 95 mV dec−1 and 120 mV dec−1, respectively, indicating that both have high initial energy barriers and slow reaction rates in the HER process. After successful incorporation of Te into WSe2, Te–WSe2 exhibits a Tafel slope of 55 mV dec−1, indicating that the Heyrovsky–Volmer reaction is its main pathway in the HER reaction process. The comparison of Te–WSe2 with WSe2 and WTe2 indicates that the introduction of Te effectively accelerates the kinetic process of the catalytic reaction, thereby improving the activity of the catalyst.
To elucidate the reason behind the superior hydrogen evolution performance of Te–WSe2, we analyzed the electrochemical active surface areas (ECSA) of the catalysts. The ECSA was evaluated by double-layer capacitance (Cdl) using cyclic voltammetry at various scan rates in the potential range from 0 to 0.2 V versus RHE (Fig. S4 in ESI†). Typically, the ECSA is directly proportional to the Cdl of the electrode, expressed as ECSA = Cdl/Cs, where Cs represents the specific capacitance of the catalysts per unit area under identical electrolyte conditions. The specific capacitance typically falls within a reported range of 0.015–0.110 mF cm−2 in acidic solutions. The general specific capacitance of 0.035 mF cm−2 was used to estimate ECSA in this work.67 The Cdl of WSe2 increased after Te doping (Fig. 6d), indicating a positive effect of Te doping on the electrochemical activity. Te–WSe2 electrode has the largest ECSA of 701.4 cm2, indicating the presence of a greater number of active sites. The order of ECSA among the different samples aligns with the catalytic performance observed in the LSV plots, indicating that the active surface area is an important factor that determines the catalytic properties. It should be noted that the active surface area of the Te–WSe2 electrode is much larger than WSe2 (320.9 cm2) and WTe2 (461.4 cm2), implying that Te doping leads to the formation of more active sites. The calculated ECSA values are summarized in Table 2.
| Cathodes | η 10 (mV) | η 1200 (mV) | Tafel slope (mV dec−1) | R s (Ω cm2) | R ct (Ω cm2) | ECSA (cm2) |
|---|---|---|---|---|---|---|
| Pt/C | 50 | — | 35 | — | — | — |
| Te–WSe2 | 79 | 232 | 55 | 2.008 | 2.68 | 701.4 |
| WSe2 | 366 | 541 | 95 | 2.107 | 9.08 | 320.9 |
| WTe2 | 243 | 476 | 120 | 2.155 | 5.88 | 461.4 |
EIS serves as a valuable technique for investigating the kinetics of electron transfer in the HER. The solution resistance (Rs) signifies the overall resistance within the electrolyte, while the diameter of the semicircle in the high-frequency range reflects the charge transfer resistance (Rct). As depicted in Fig. 6e, all catalysts demonstrate comparable Rs values, implying similar solution resistance. However, when comparing the charge transfer resistance, Te–WSe2 exhibited the lowest Rct at 2.68 Ω cm2, while WSe2 and WTe2 displayed higher resistances of 9.08 Ω cm2 and 5.88 Ω cm2, respectively. The reduction in resistance following Te doping signifies favorable reaction kinetics and effective charge transfer within Te–WSe2.
The stability of a catalyst is a key parameter for evaluating its performance.68,69 In Fig. 6g, a 16 h CP test is illustrated, demonstrating that Te–WSe2 maintains stability across varying current densities: 10, 50, 100, 300, 500, 700, 900, and 1100 mA cm−2. It can be seen that Te–WSe2 exhibits stable operation under diverse current densities without notable overpotential escalation. Notably, at large current densities of 900 and 1100 mA cm−2, the overpotential significantly diminishes with prolonged operation, signifying the successful activation and robust stability of Te–WSe2. Fig. 6h illustrates the 100 h CP tests at current densities of 10 and 100 mA cm−2, further proving the stability of the catalyst. Fig. 4f shows the polarization curves before and after CP test. It can be seen that the electrode exhibits a high stability toward the HER with very small cathodic current loss. After CP testing, Raman and SEM measurements confirmed the retention of the original structure of the catalyst (Fig. S5 and S6, ESI†).
In comparison to previously reported MoX2 (X = S, Se, or Te) catalysts, the Te–WSe2 catalyst featured in this study showcases superior catalytic performance, particularly at elevated current densities. The catalytic performance of Te–WSe2 is related to the electrode's microstructure. Te–WSe2 is cultivated on a metallic W mesh substrate, resulting in a stable monolithic catalyst (Fig. 2c and 7a). The Te–WSe2 layers are directly supported by the highly conductive metal mesh, ensuring a strong supply of electrons to each layer of Te–WSe2. As such, electron transport within the Te–WSe2 layers is facilitated. This arrangement exhibits exceptional overall conductivity, leading to outstanding catalytic performance even at high current densities. Furthermore, the distinct stripes within the “accordion-like” texture reveal a substantial active surface area within the material. Additionally, the specimen exhibits numerous structural defects, including irregular edges and internal microporous structures, all of which can function as highly effective catalytic active sites.
We subsequently explore the potential of Te–WSe2 as a catalyst for the hydrogen evolution reaction in practical settings. The catalytic performance of bipolar catalysts at the cathode and anode plays a pivotal role in the overall efficiency of water hydrolysis. To evaluate this, we employed commercial RuO2 as the anode catalyst and conducted a comparative analysis of Te–WSe2 and commercial Pt/C as cathode catalysts in the water hydrolysis experiment. This experiment was carried out in an acidic solution with a concentration of 0.5 M H2SO4. Significantly, the catalysts Te–WSe2‖RuO2 and Te–WSe2‖Pt/C exhibited remarkably similar performance, as evidenced by their comparable overpotentials at different current densities (as shown in Fig. 7b). This finding highlights the potential of Te–WSe2 as a highly effective catalyst for hydrogen evolution, thus indicating its promising prospects for industrial applications.
To gain deeper insights into the active sites of Te–WSe2 in the context of catalyzing the HER, we conducted DFT calculations on both Te–WSe2 and WSe2. The catalyst's activity can be assessed by the calculated Gibbs free energy (ΔG), which is associated with the adsorption of hydrogen atoms onto the material's surface.70 Effective HER catalysts usually exhibit ΔG values close to zero,71 indicating a balanced hydrogen adsorption strength. If ΔG is excessively positive, hydrogen adsorption becomes challenging, impeding the reaction. Conversely, an excessively negative ΔG results in strong hydrogen adsorption, leading to catalyst poisoning. Thus, an ideal HER catalyst should exhibit a ΔG value close to 0 eV. In our study, we investigated H adsorption at various positions on Te–WSe2, including basal plane (planeSe), doped Te atom at the basal plane (planeTe), metal edge (edgeW), and non-metallic edge (edgeTe, edgeSe). For comparison, we also computed H atom adsorption at the basal plane (planeSe), metal edge (edgeW), and non-metallic edge (edgeSe) of WSe2. The optimized structures and calculated ΔG values are shown in Fig. 8a–c. The pristine WSe2 exhibits ΔG values of 2.12, −0.34, and −0.27 eV for hydrogen atom adsorption at the basal plane, W edge, and Se edge, respectively. In the case of Te–WSe2, the ΔG value for hydrogen atom adsorption at the Se site of the basal plane (planeSe) decreases to 0.89 eV, while the ΔG value for Te site of the basal plane (planeTe) is also calculated to be 0.89 eV. The ΔG value for hydrogen atom adsorption at the metal edge (edgeW) is −0.26 eV, which is closer to zero compared to pristine WSe2. The ΔG value for hydrogen atom adsorption at the Te site of the edge (edgeTe) is −0.27 eV. The calculated ΔG values for edge Se sites in proximity to the Te atom (edgeSe1 and edgeSe2) are −0.28 eV and 0.22 eV, respectively. Based on these findings, Te doping of WSe2 has the potential to promote the adsorption of hydrogen atoms in a reversible manner, thus improving the electrocatalytic properties.
4 Conclusions
In this research, we synthesized WSe2 doped with Te and investigated its electrocatalytic capabilities for HER. To enhance the performance of Te–WSe2, we directly deposited it onto a highly conductive W mesh, resulting in a stable monolith catalyst that greatly enhances electron transport and conductivity. The material exhibits numerous irregular edges and an internal microporous structure, thereby exposing a significant number of active sites. Moreover, our computational analysis demonstrates that Te doping effectively reduces ΔG of the active sites and enhances the catalytic activity of the sites. Consequently, the material exhibits outstanding catalytic performance even at high current densities. Specifically, at current densities of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, 500, and 1000 mA cm−2, the overpotentials are 79, 170, 207 and 225 mV, respectively, with a Tafel slope of 55.0 mV dec−1. This study introduces a novel approach for developing cost-effective HER electrocatalysts that function effectively under high current density conditions.
100, 500, and 1000 mA cm−2, the overpotentials are 79, 170, 207 and 225 mV, respectively, with a Tafel slope of 55.0 mV dec−1. This study introduces a novel approach for developing cost-effective HER electrocatalysts that function effectively under high current density conditions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Public Computing Cloud, Renmin University of China.Notes and references
- Z. Shi, X. Zhang, X. Lin, G. Liu, C. Ling, S. Xi, B. Chen, Y. Ge, C. Tan and Z. Lai, et al., Phase-dependent growth of Pt on MoS2 for highly efficient H2 evolution, Nature, 2023, 621, 300–305 CrossRef CAS PubMed.
- C. Zhang, Z. Xu, N. Han, Y. Tian, T. Kallio, C. Yu and L. Jiang, Superaerophilic/superaerophobic cooperative electrode for efficient hydrogen evolution reaction via enhanced mass transfer, Sci. Adv., 2023, 9, eadd6978 CrossRef PubMed.
- H. Yu, S. Zhu, Y. Hao, Y.-M. Chang, L. Li, J. Ma, H.-Y. Chen, M. Shao and S. Peng, Modulating Local Interfacial Bonding Environment of Heterostructures for Energy-Saving Hydrogen Production at High Current Densities, Adv. Funct. Mater., 2023, 33, 2212811 CrossRef CAS.
- D. Qiao, S. Yun, M. Sun, J. Dang, Y. Zhang, S. Yuan, G. Yang, T. Yang, Z. Gao and Z. Wang, 1D/3D trepang-like N-modified carbon confined bimetal carbides and metal cobalt: boosting electron transfer via dual Mott–Schottky heterojunctions triggered built-in electric fields for efficient hydrogen evolution and tri-iodide reduction, Appl. Catal., B, 2023, 334, 122830 CrossRef CAS.
- L. Wang, Y. Hao, L. Deng, F. Hu, S. Zhao, L. Li and S. Peng, Rapid complete reconfiguration induced actual active species for industrial hydrogen evolution reaction, Nat. Commun., 2022, 13, 5785 CrossRef CAS PubMed.
- S. Sarwar, A. Ali, Y. Wang, M. R. Ahasan, R. Wang, A. J. Adamczyk and X. Zhang, Enhancement of hydrogen evolution reaction activity using metal–rich molybdenum sulfotelluride with graphene support: a combined experimental and computational study, Nano Energy, 2021, 90, 106599 CrossRef CAS.
- Z.-Y. Zhang, H. Tian, L. Bian, S.-Z. Liu, Y. Liu and Z.-L. Wang, Cu–Zn-based alloy/oxide interfaces for enhanced electroreduction of CO2 to C2+ products, J. Energy Chem., 2023, 83, 90–97 CrossRef CAS.
- L. Bian, Z.-Y. Zhang, H. Tian, N.-N. Tian, Z. Ma and Z.-L. Wang, Grain boundary-abundant copper nanoribbons on balanced gas-liquid diffusion electrodes for efficient CO2 electroreduction to C2H4, Chin. J. Catal., 2023, 54, 199–211 CrossRef CAS.
- Q. Fu, L. W. Wong, F. Zheng, X. Zheng, C. S. Tsang, K. H. Lai, W. Shen, T. H. Ly, Q. Deng and J. Zhao, Unraveling and leveraging in situ surface amorphization for enhanced hydrogen evolution reaction in alkaline media, Nat. Commun., 2023, 14, 6462 CrossRef CAS PubMed.
- C. D. Abernethy, G. M. Codd, M. D. Spicer and M. K. Taylor, A highly stable N-heterocyclic carbene complex of trichloro-oxovanadium(V) displaying novel Cl–C(carbene) bonding interactions, J. Am. Chem. Soc., 2003, 125, 1128–1129 CrossRef CAS PubMed.
- S. Zhao, L. Yin, L. Deng, J. Song, Y.-M. Chang, F. Hu, H. Wang, H.-Y. Chen, L. Li and S. Peng, Inheritable Organic-Inorganic Hybrid Interfaces with π–d Electron Coupling for Robust Electrocatalytic Hydrogen Evolution at High-Current-Densities, Adv. Funct. Mater., 2023, 33, 2211576 CrossRef CAS.
- J. Dang, S. Yun, Y. Zhang, G. Yang, J. Yang, D. Qiao and T. Yang, Designing nitrogen-enriched heterogeneous NiS@CoNi2S4 embedded in nitrogen-doped carbon with hierarchical 2D/3D nanocage structure for efficient alkaline hydrogen evolution and triiodide reduction, J. Colloid Interface Sci., 2023, 630, 91–105 CrossRef CAS PubMed.
- S. Sarwar, A. Nautiyal, J. Cook, Y. Yuan, J. Li, S. Uprety, R. Shahbazian-Yassar, R. Wang, M. Park and M. J. Bozack, et al., Facile microwave approach towards high performance MoS2/graphene nanocomposite for hydrogen evolution reaction, Sci. China: Mater., 2020, 63, 62–74 CAS.
- L. Wang, L. Song, Z. Yang, Y.-M. Chang, F. Hu, L. Li, L. Li, H.-Y. Chen and S. Peng, Electronic Modulation of Metal–Organic Frameworks by Interfacial Bridging for Efficient pH-Universal Hydrogen Evolution, Adv. Funct. Mater., 2023, 33, 2210322 CrossRef CAS.
- C. Wan, Z. Zhang, J. Dong, M. Xu, H. Pu, D. Baumann, Z. Lin, S. Wang, J. Huang and A. H. Shah, et al., Amorphous nickel hydroxide shell tailors local chemical environment on platinum surface for alkaline hydrogen evolution reaction, Nat. Mater., 2023, 1–8 Search PubMed.
- Z. Ren, H. Jiang, M. Yuan, Z. Xie, L. Deng, J. Han, K. Lyu, Y. Zhu, X. Li and L. Zhuang, Si regulation of hydrogen adsorption on nanoporous PdSi hybrids towards enhancing electrochemical hydrogen evolution activity, Inorg. Chem. Front., 2023, 10, 1101–1111 RSC.
- J. Zhang, X. Zhang, C. Shi, G. Xia, H. Li, P. Wang and L. Di, Plasma synthesis of defect-rich flexible carbon cloth decorated with PtRu alloyed nanoclusters for highly efficient pH-universal electrocatalytic hydrogen evolution, Nanoscale, 2022, 14, 15942–15949 RSC.
- Y. Wang, S. Yun, J. Shi, Y. Zhang, J. Dang, C. Dang, Z. Liu, Y. Deng and T. Yang, Defect engineering tuning electron structure of biphasic tungsten-based chalcogenide heterostructure improves its catalytic activity for hydrogen evolution and triiodide reduction, J. Colloid Interface Sci., 2022, 625, 800–816 CrossRef CAS PubMed.
- M. Luo, J. Yang, X. Li, M. Eguchi, Y. Yamauchi and Z.-L. Wang, Insights into alloy/oxide or hydroxide interfaces in Ni–Mobased electrocatalysts for hydrogen evolution under alkaline conditions, Chem. Sci., 2023, 14, 3400–3414 RSC.
- C. Yang, R. Zhao, H. Xiang, J. Wu, W. Zhong, W. Li, Q. Zhang, N. Yang and X. Li, Ni-activated transition metal carbides for efficient hydrogen evolution in acidic and alkaline solutions, Adv. Energy Mater., 2020, 10, 2002260 CrossRef CAS.
- K. Liang, A. Tabassum, M. Kothakonda, X. Zhang, R. Zhang, B. Kenney, B. D. Koplitz, J. Sun and M. Naguib, Twodimensional titanium carbonitride MXene as a highly efficient electrocatalyst for hydrogen evolution reaction, Mater. Rep.: Energy, 2022, 2, 100075 CAS.
- Y. Li, S. Zhu, E. Wu, H. Ding, J. Lu, X. Mu, L. Chen, Y. Zhang, J. Palisaitis and K. Chen, et al., Nanolaminated ternary transition metal carbide (MAX phase)-derived core–shell structure electrocatalysts for hydrogen evolution and oxygen evolution reactions in alkaline electrolytes, J. Phys. Chem. Lett., 2023, 14, 481–488 CrossRef CAS PubMed.
- F. Ma, S. Wang, X. Gong, X. Liu, Z. Wang, P. Wang, Y. Liu, H. Cheng, Y. Dai and Z. Zheng, et al., Highly efficient electrocatalytic hydrogen evolution coupled with upcycling of microplastics in seawater enabled via Ni3N/W5N4 janus nanostructures, Appl. Catal., B, 2022, 307, 121198 CrossRef CAS.
- Z. Qi, Y. Zeng, Z. Hou, W. Zhu, B. Wei, Y. Yang, B. Lin and H. Liang, Heterointerface engineering of Ni/Ni3N hierarchical nanoarrays for efficient alkaline hydrogen evolution, Nano Res., 2023, 16, 4803–4811 CrossRef CAS.
- P. Da, Y. Zheng, Y. Hu, Z. Wu, H. Zhao, Y. Wei, L. Guo, J. Wang, Y. Wei and S. Xi, et al., Synthesis of Bandgaptunable Transition Metal Sulfides through Gas-phase Cation Exchange-induced Topological Transformation, Angew. Chem., 2023, 135, e202301802 CrossRef.
- X. Chen, Z. Han, B. Zhang, B. Sun, Y. Wang, Y. Du, X. Han and P. Xu, Construction of plasmonic 1T-WS2/2H-WS2/CdS heterostructures for enhanced solar driven hydrogen evolution, J. Mater. Chem. A, 2022, 10, 24030–24040 RSC.
- S.-F. Chen, T.-S. Wu and Y.-L. Soo, Highly defective graphene quantum dots-doped 1T/2H-MoS2 as an efficient composite catalyst for the hydrogen evolution reaction, Sci. Rep., 2023, 13, 15184 CrossRef CAS PubMed.
- J. Yang, Y. Cao, S. Zhang, Q. Shi, S. Chen, S. Zhu, Y. Li and J. Huang, Interstitial Hydrogen Atom to Boost Intrinsic Catalytic Activity of Tungsten Oxide for Hydrogen Evolution Reaction, Small, 2023, 2207295 CrossRef CAS PubMed.
- Q. Liu, Z. Xue, B. Jia, Q. Liu, K. Liu, Y. Lin, M. Liu, Y. Li and G. Li, Hierarchical nanorods of MoS2/MoP heterojunction for efficient electrocatalytic hydrogen evolution reaction, Small, 2020, 16, 2002482 CrossRef CAS PubMed.
- J. Jin, J. Ge, X. Zhao, Y. Wang, F. Zhang and X. Lei, An amorphous NiCuFeP@Cu3P nanoarray for an efficient hydrogen evolution reaction, Inorg. Chem. Front., 2022, 9, 1446–1455 RSC.
- M. R. Kandel, U. N. Pan, P. P. Dhakal, R. B. Ghising, T. T. Nguyen, J. Zhao, N. H. Kim and J. H. Lee, Unique heterointerface engineering of Ni2P–MnP nanosheets coupled Co2P nanoflowers as hierarchical dual-functional electrocatalyst for highly proficient overall water-splitting, Appl. Catal., B, 2023, 331, 122680 CrossRef CAS.
- L. Sun, M. Gao, Z. Jing, Z. Cheng, D. Zheng, H. Xu, Q. Zhou and J. Lin, 1T-Phase Enriched P doped WS2 nanosphere for highly efficient electrochemical hydrogen evolution reaction, Chem. Eng. J., 2022, 429, 132187 CrossRef CAS.
- C.-H. Chiang, Y.-C. Yang, J.-W. Lin, Y.-C. Lin, P.-T. Chen, C.-L. Dong, H.-M. Lin, K. M. Chan, Y.-T. Kao and K. Suenaga, et al., Bifunctional monolayer WSe2/graphene self-stitching heterojunction microreactors for efficient overall water splitting in neutral medium, ACS Nano, 2022, 16, 18274–18283 CrossRef CAS PubMed.
- D. Xia, Z. Wang, S. Yang, Z. Cai, M. Hu, H. He and K. Zhou, Enhancing electrocatalytic hydrogen evolution of WTe2 by formation of amorphous phosphate nanoshells, Electrochim. Acta, 2021, 385, 138409 CrossRef CAS.
- Y. Tan, J. Feng, H. Dong, L. Liu, S. Zhao, F. Lai, T. Liu, Y. Bai, I. P. Parkin and G. He, The edge effects boosting hydrogen evolution performance of platinum/transition bimetallic phosphide hybrid electrocatalysts, Adv. Funct. Mater., 2023, 33, 2209967 CrossRef CAS.
- Z. Zheng, L. Yu, M. Gao, X. Chen, W. Zhou, C. Ma, L. Wu, J. Zhu, X. Meng and J. Hu, et al., Boosting hydrogen evolution on MoS2 via co-confining selenium in surface and cobalt in inner layer, Nat. Commun., 2020, 11, 3315 CrossRef CAS PubMed.
- M. Wang, R. Song, X. Zhang, G. Liu, S. Xu, Z. Xu, J. Liu and G. Qiao, Defects engineering promotes the electrochemical hydrogen evolution reaction property of phosphorene surface, Int. J. Hydrogen Energy, 2021, 46, 1913–1922 CrossRef CAS.
- X. Shi, X. Zheng, H. Wang, H. Zhang, M. Qin, B. Lin, M. Qi, S. Mao, H. Ning and R. Yang, et al., Hierarchical Crystalline/Amorphous Heterostructure MoNi/NiMoOx for Electrochemical Hydrogen Evolution with Industry-Level Activity and Stability, Adv. Funct. Mater., 2023, 2307109 CrossRef CAS.
- X. Zhang, Z. Zhou, D. Zhang, J. Chen, J. Zhang and Z. Wang, Mixed-phase MoTe2 with exposed edges and rich defects for catalyzing hydrogen evolution reaction with noble-metal-like performance, Electrochim. Acta, 2023, 457, 142455 CrossRef CAS.
- D. P. Jaihindh, P. Anand, R.-S. Chen, W.-Y. Yu, M.-S. Wong and Y.-P. Fu, Cl-doped CuO for electrochemical hydrogen evolution reaction and tetracycline photocatalytic degradation, J. Environ. Chem. Eng., 2023, 11, 109852 CrossRef CAS.
- W. Wang, Y. Li, M. Li, H. Shen, W. Zhang, J. Zhang, T. Liu, X. Kong and H. Bi, Metallic phase WSe2 nanoscrolls for the hydrogen evolution reaction, New J. Chem., 2022, 46, 8381–8384 RSC.
- X. Zhao, K. Liu, F. Guo, Z. He, L. Zhang, S. Lei, H. Li, Y. Cheng and L. Yang, Meta-Position synergistic effect induced by Ni–Mo Co-doped WSe2 to enhance the hydrogen evolution reaction, Dalton Trans., 2022, 51, 11758–11767 RSC.
- G. Kresse and J. Hafner, Ab initio molecular dynamics for liquid metals, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558 CrossRef CAS PubMed.
- G. Kresse, J. Furthmüller and J. Hafner, Theory of the crystal structures of selenium and tellurium: the effect of generalized-gradient corrections to the local-density approximation, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 13181 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized gradient approximation made simple, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- H. Liu, R. Xie, Y. Luo, Z. Cui, Q. Yu, Z. Gao, Z. Zhang, F. Yang, X. Kang and S. Ge, et al., Dual interfacial engineering of a Chevrel phase electrode material for stable hydrogen evolution at 2500 mA cm−2, Nat. Commun., 2022, 13, 6382 CrossRef CAS PubMed.
- Y. Tong, D. Feng and P. Chen, Dual modification strategy of nickel sulfide as pH-universal catalysts for hydrogen production at large current density, ACS Sustainable Chem. Eng., 2021, 9, 10601–10610 CrossRef CAS.
- Y. Chen, G. Yu, W. Chen, Y. Liu, G.-D. Li, P. Zhu, Q. Tao, Q. Li, J. Liu and X. Shen, et al., Highly active, nonprecious electrocatalyst comprising borophene subunits for the hydrogen evolution reaction, J. Am. Chem. Soc., 2017, 139, 12370–12373 CrossRef CAS PubMed.
- H. Zhou, F. Yu, J. Sun, H. Zhu, I. K. Mishra, S. Chen and Z. Ren, Highly efficient hydrogen evolution from edgeoriented WS2(1−x)Se2x particles on three-dimensional porous NiSe2 foam, Nano Lett., 2016, 16, 7604–7609 CrossRef CAS PubMed.
- C. Zhang, Y. Luo, J. Tan, Q. Yu, F. Yang, Z. Zhang, L. Yang, H.-M. Cheng and B. Liu, High-throughput production of cheap mineral-based two-dimensional electrocatalysts for high-current–density hydrogen evolution, Nat. Commun., 2020, 11, 3724 CrossRef CAS PubMed.
- D. Zhang, F. Wang, W. Zhao, M. Cui, X. Fan, R. Liang, Q. Ou and S. Zhang, Boosting hydrogen evolution reaction activity of amorphous molybdenum sulfide under high currents via preferential electron filling induced by tungsten doping, Advanced, Science, 2022, 9, 2202445 CAS.
- I. K. Mishra, H. Zhou, J. Sun, F. Qin, K. Dahal, J. Bao, S. Chen and Z. Ren, Hierarchical CoP/Ni5P4/CoP microsheet arrays as a robust pH-universal electrocatalyst for efficient hydrogen generation, Energy Environ. Sci., 2018, 11, 2246–2252 RSC.
- J. Sun, M. Ren, L. Yu, Z. Yang, L. Xie, F. Tian, Y. Yu, Z. Ren, S. Chen and H. Zhou, Highly efficient hydrogen evolution from a mesoporous hybrid of nickel phosphide nanoparticles anchored on cobalt phosphosulfide/phosphide nanosheet arrays, Small, 2019, 15, 1804272 CrossRef PubMed.
- M. Das, N. Jena, T. Purkait, N. Kamboj, A. De Sarkar and R. S. Dey, Single-phase Ni5P4–copper foam superhydrophilic and aerophobic core–shell nanostructures for efficient hydrogen evolution reaction, J. Mater. Chem. A, 2019, 7, 23989–23999 RSC.
- Y. Luo, L. Tang, U. Khan, Q. Yu, H.-M. Cheng, X. Zou and B. Liu, Morphology and surface chemistry engineering toward pH-universal catalysts for hydrogen evolution at high current density, Nat. Commun., 2019, 10, 269 CrossRef PubMed.
- L. Zhang, Z. Yan, X. Chen, M. Yu, F. Liu, F. Cheng and J. Chen, Facile synthesis of amorphous MoSx–Fe anchored on Zr-MOFs towards efficient and stable electrocatalytic hydrogen evolution, Chem. Commun., 2020, 56, 2763–2766 RSC.
- L. Zhu, H. Lin, Y. Li, F. Liao, Y. Lifshitz, M. Sheng, S.-T. Lee and M. Shao, A rhodium/silicon co-electrocatalyst design concept to surpass platinum hydrogen evolution activity at high overpotentials, Nat. Commun., 2016, 7, 12272 CrossRef CAS PubMed.
- J. Yang, A. R. Mohmad, Y. Wang, R. Fullon, X. Song, F. Zhao, I. Bozkurt, M. Augustin, E. J. Santos and H. S. Shin, et al., Ultrahigh-current–density niobium disulfide catalysts for hydrogen evolution, Nat. Mater., 2019, 18, 1309–1314 CrossRef CAS PubMed.
- G. Li, J. Yu, J. Jia, L. Yang, L. Zhao, W. Zhou and H. Liu, Cobalt–Cobalt Phosphide Nanoparticles@Nitrogen-Phosphorus Doped Carbon/Graphene Derived from Cobalt Ions Adsorbed Saccharomycete Yeasts as an Efficient, Stable, and Large-Current–Density Electrode for Hydrogen Evolution Reactions, Adv. Funct. Mater., 2018, 28, 1801332 CrossRef.
- Z. Zhang, Y. Wang, X. Leng, V. H. Crespi, F. Kang and R. Lv, Controllable edge exposure of MoS2 for efficient hydrogen evolution with high current density, ACS Appl. Energy Mater., 2018, 1, 1268–1275 CrossRef CAS.
- S. Riyajuddin, K. Azmi, M. Pahuja, S. Kumar, T. Maruyama, C. Bera and K. Ghosh, Super-hydrophilic hierarchical Ni-foam-graphene-carbon nanotubes-Ni2P–CuP2 nanoarchitecture as efficient electrocatalyst for overall water splitting, ACS Nano, 2021, 15, 5586–5599 CrossRef CAS PubMed.
- Z. Zhongming, L. Linong, Y. Xiaona, Z. Wangqiang and L. Wei, et al., Design of Aligned Porous Carbon Films with Single-Atom Co-NC Sites for High-Current–Density Hydrogen Generation, Adv. Mater., 2021, 33, 2103533 CrossRef PubMed.
- C. Huang, L. Yu, W. Zhang, Q. Xiao, J. Zhou, Y. Zhang, P. An, J. Zhang and Y. Yu, N-doped Ni–Mo based sulfides for highefficiency and stable hydrogen evolution reaction, Appl. Catal., B, 2020, 276, 119137 CrossRef CAS.
- C. Jian, Q. Cai, W. Hong, J. Li and W. Liu, Edge-riched MoSe2/MoO2 hybrid electrocatalyst for efficient hydrogen evolution reaction, Small, 2018, 14, 1703798 CrossRef PubMed.
- X. Cao, L. Zhang, K. Huang, B. Zhang, J. Wu and Y. Huang, Strained carbon steel as a highly efficient catalyst for seawater electrolysis, Energy Mater., 2022, 2, 200010 CAS.
- C. C. McCrory, S. Jung, J. C. Peters and T. F. Jaramillo, Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction, J. Am. Chem. Soc., 2013, 135, 16977–16987 CrossRef CAS PubMed.
- L. Li, C. Liu, S. Liu, J. Wang, J. Han, T.-S. Chan, Y. Li, Z. Hu, Q. Shao and Q. Zhang, et al., Phase engineering of a ruthenium nanostructure toward high-performance bifunctional hydrogen catalysis, ACS Nano, 2022, 16, 14885–14894 CrossRef CAS PubMed.
- J. Gu, L. Li, Y. Xie, B. Chen, F. Tian, Y. Wang, J. Zhong, J. Shen and J. Lu, Turing structuring with multiple nanotwins to engineer efficient and stable catalysts for hydrogen evolution reaction, Nat. Commun., 2023, 14, 5389 CrossRef CAS PubMed.
- D. Vikraman, S. Hussain, I. Rabani, A. Feroze, M. Ali, Y.-S. Seo, S.-H. Chun, J. Jung and H.-S. Kim, Engineering MoTe2 and Janus SeMoTe nanosheet structures: firstprinciples roadmap and practical uses in hydrogen evolution reactions and symmetric supercapacitors, Nano Energy, 2021, 87, 106161 CrossRef CAS.
- B. Hinnemann, P. G. Moses, J. Bonde, K. P. Jørgensen, J. H. Nielsen, S. Horch, I. Chorkendorff and J. K. Nørskov, Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution, J. Am. Chem. Soc., 2005, 127, 5308–5309 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cp05790a |
| This journal is © the Owner Societies 2024 |