Oxygen vacancy-enriched Zn2SnO4 for aliphatic alcohol sensing and enhanced selectivity towards n-butanol
Reshmi Thekke
Parayil
ab,
B.
Bhagat
c,
Santosh K.
Gupta
 *ab,
K.
Mukherjee
*ab,
K.
Mukherjee
 *c and
Manoj
Mohapatra
ab
*c and
Manoj
Mohapatra
ab
aRadiochemistry Division, Bhabha Atomic Research Centre, Trombay, Mumbai-400085, India. E-mail: santoshg@barc.gov.in
bHomi Bhabha National Institute, Anushaktinagar, Mumbai-400094, India
cDepartment of Chemistry, Pandit Deendayal Energy University, Gandhinagar-382426, Gujarat, India. E-mail: kalisadhanm@yahoo.com
First published on 31st January 2024
Abstract
The sensitive detection of toxic flammable volatile organics using low cost efficient sensors is important for ensuring both indoor and outdoor safety. It is essential for chemical sensors to exhibit a significantly stronger response to target analytes compared to equivalent amounts of analogous competing chemicals. In line with this importance, current work evaluated the performance of Zn2SnO4, a n-type semiconducting metal oxide, for sensing n-butanol in comparison to methanol, ethanol, and propanol vapours. These vapours fall within the category of aliphatic alcohols but vary in characteristics such as molecular weight, vapour pressure, volatility, and diffusivity. In this work we have explored the sensor's performance by adjusting the operating temperature over the range of 225–300 °C while detecting 1000 ppm of each of these vapours. Efforts were made to establish a correlation between the sensor's responses with the interactions of these vapours on the sensor's surface. Prior to assessing the sensing characteristics of the solid-state-route-derived Zn2SnO4, its structural characteristics, including phase purity, crystalline structure, bonding patterns, morphology, and defect characteristics, were studied. This comprehensive analysis sheds light on the potential of Zn2SnO4 as an effective sensor for detecting n-butanol.
1. Introduction
Semiconducting metal oxide (SMO)-based chemiresistive sensors are popular for the sensitive detection of various gases and volatile organic compounds (VOCs) owing to their simple operation, low cost, high response, durability in harsh environments, and possibilities for miniaturization. Various SMOs, such as ZnO, α-Fe2O3, Co3O4, SnO2, and In2O3, are already known for the detection of various toxic and inflammable gases.1–6Research activities are underway to modify the existing SMOs by suitable chemical and electronic sensitizers to improve their response characteristics, such as sensitivity, operating temperature, selectivity, and stability. New SMO compositions are also being tested for exploring their sensing characteristics towards various analytes. Understanding critically the role of various parameters that influence the sensing features remains also very important to researchers. Few recent works are highlighted herein to understand the recent trends in SMO-based sensors. In a recent review, Y. Yoon et al. reported the factors that could enhance the sensitivity of metal oxide sensors and also discussed the opportunities for metal oxides in developing flexible/wearable sensors.7 The influence of various parameters (composition, doping, microstructure, crystalline phases, operating temperature, humidity, homo/hetero-junction, interaction type) in modulating the sensing performances of metal oxide chemiresistive sensors were highlighted by S. Das et al.8 Goel et al. discussed the different parameters that govern the sensitivity and selectivity of SMO gas sensors and also highlighted the techniques to improve the response of SMO sensors.9 SMOs in a 1D architecture are attractive as chemiresistive sensors for the detection of various analytes and B. Yang et al. reviewed the same.5 Si et al. modified Sr@SnO2 materials with zeolite (ZSM-5) catalytic materials and reported that temperature modulation was effective for the selective detection of ethanol vapour.10 For SMO sensors, one of the very fundamental features that influences the receptor and transducer function and alters the overall sensing performance is due to the presence of lattice oxygen vacancies. Al-Hashem et al. described in detail the role of oxygen vacancies in modifying the sensing response of metal oxide sensors.11 The improvement of the gas-sensing performance of a WO3 sensor by modulating the oxygen vacancies was studied by Yu et al.12 Recently, oxygen-vacancy-enriched SnO2–RGO hybrids were reported for the room temperature sensing of NO2.13 In another recent work, Zhou et al. reported H2 sensing by modulating the oxygen vacancies in ZnO nanosheets.14 As reviewed, mostly simple metal oxides and their modified counterparts have been investigated by researchers in sensing studies.
Compared with the aforementioned simple SMOs, binary/ternary metal oxides (with multiple metal ions) have received widespread attention due to their tuneable electrical conductivities and abundant surface active sites.15–20 In this regard, zinc stannate (Zn2SnO4), a ternary, wide-band-gap n-type SMO is attracting the attention of researchers as a promising gas-sensing material. Zinc stannate-based chemiresistive sensors are already being studied for the detection of various gases, including carbon monoxide (CO), nitrogen dioxide (NO2), methane (CH4), and volatile organic compounds (VOCs).21–23 Their promising sensitivity, selectivity to few vapours/gases, and ability to operate in harsh environments make them suitable for gas detection and monitoring. Zn2SnO4 has superior electrical conductivity, electron mobility, optical performance, and stability compared to its counterparts ZnO and SnO2.24–27 Its band gap varies typically between 3.6–4 eV depending on the reaction conditions, particle size, composition, and defects, which ultimately changes its opto-electronic properties as well as performance, not only for chemiresistive gas sensing but also for other applications, such as in photoconductors, photocatalysis, photoluminescence, photodetectors, lithium-ion batteries, and supercapacitors.28–31
The present study explored the possibilities of solid-state-synthesized zinc stannate for the detection of n-butanol, which is a colourless transparent liquid that is extensively used as a solvent, extractant, plasticizer, and reagent in different chemical industries for preparing surfactants, ethylene glycol, butyl ether, rubber products, coating solvents, dibutyl phthalate, etc.32 Prolonged exposure to n-butanol is a health risk as it is corrosive and causes irritation to the human eyes, skin, and respiratory tract. Furthermore, n-butanol is flammable and explosive when mixed with certain organic solvents. Therefore, it would be of great significance to monitor n-butanol in the environment. The properties of n-butanol resemble well modern gasoline and thus the possibility of using this compound as a fuel for combustion engines is being explored by researchers. The presence of 11.5% n-butanol in the air can produce flashes and explosions.33 In this context, the detection of n-butanol seems crucial for the real-time monitoring of n-butanol in industry and laboratories. Existing butanol sensors have limitations, including cross-sensitivity to other volatile organic compounds, high detection thresholds, susceptibility to environmental factors, calibration needs, and potential drift over time. Further their response times may not meet the demands for rapid detection for many sensors. Ongoing research is seeking to address these issues by enhancing the selectivity, sensitivity, and reliability while reducing the costs and improving the performance. Achieving distinguishably higher responses to the target analytes in particular with respect to their equivalent amount of analogous competing chemicals is important for any chemical sensor.20,34
Accordingly, in the present work, the response of Zn2SnO4, an n-type SMO, was assessed towards n-butanol with respect to methanol, ethanol, and propanol vapours. These vapours belong to aliphatic alcohols but differ in their molecular weight, vapour pressure, volatility, and diffusivity. The sensing characteristics were measured by varying the operating temperature within the temperature range of 225–300 °C for the detection of 1000 ppm of each of these vapours. An attempt was made to correlate the sensing response by understanding the interactions of the vapours on the sensor surface. Prior to exploring the sensing characteristics of the solid-state-route-derived Zn2SnO4, its phase purity, crystalline nature, bonding pattern, morphology, defect characteristics were investigated using XRD, FTIR, Raman spectroscopy, XPS, EPR, TEM, and SAED. The present article thus provides comprehensive insights into the structural features of Zn2SnO4 and its potential utilization as an n-butanol sensor.
2. Experimental
2.1. Synthesis
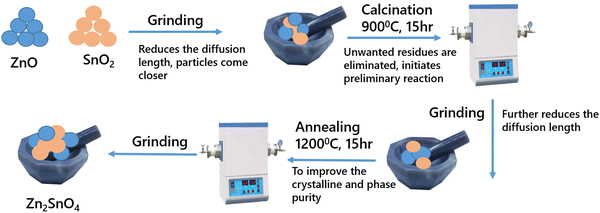
The inverse spinel Zn2SnO4 was synthesized by the conventional solid-state-reaction route. The precursors used were ZnO (SPEX Pure, 99.999%) and SnO2 (SPEX Pure, 99.999%). The required amount of the precursors was weighed and ground in a mortar for complete mixing of the precursors followed by calcining at 900 °C for 15 h in a tubular furnace. Then, it was allowed to cool to room temperature followed by re-grinding. The second stage heating involved sintering at 1200 °C for 15 h. Then, a final grinding was done to obtain the phase-pure compound. The corresponding equation for the solid-state reaction is represented as:| 2ZnO + SnO2 → Zn2SnO4 | (1) |
2.2. Phase and structural characterization
XRD was carried out using a benchtop Proto X-Ray diffractometer to analyze the phase purity of the synthesized material. The system was equipped with a monochromatic X-ray source as CuKα (1.5405 Å). All the measurements were done at an accelerating voltage of 30 kV and tube current of 20 mA. The XRD patterns were recorded within a 2θ range from 15° to 80° at a scan rate of 2° min−1. FTIR was carried out on a Bruker Alpha FTIR spectrometer in the pellet mode. Raman spectral studies were performed on a micro-Raman spectrometer (STR-300, SEKI Technotron, Japan). A 532 nm CW diode pumped solid-state laser (DPSS, gem 532, laser quantum) was used as an excitation source. The XPS measurements were carried out on a KAlpha plus XPS system (Thermo Fisher Scientific Instruments) to determine the elemental content, chemical state, and the oxygen vacancies. The EPR spectra were recorded on an EMX series Bruker instrument in the X band frequency. The emission spectra were recorded on an Edinburgh fluorescence instrument (FLS 1000), with a xenon lamp as the excitation source. The TEM images and SAED patterns of the material were obtained using a transmission electron microscope (FEI TECNAI G2 F20-ST, FEI) after drop-casting a drop of solution on a carbon-coated copper grid. Energy dispersive X-ray spectroscopy (EDX) analysis was performed using an accelerating voltage of 200 kV.2.3. VOC sensing studies
In order to measure the sensing properties, the synthesized powder materials were compacted in the form of thin circular discs (10 mm diameter, 1 mm thick). Ag-paste-based parallel strip electrode was prepared on one surface of the electrode. The sample was kept inside the gas-sensing measurement set-up, which included a hot-plate, temperature-controlled probe station, gas injection points, feed through for the electrical connections, and a programmable source meter (Keithley 2450) interfaced with a computer for automatic data acquisition. A schematic representation of the sensing set-up is shown in Scheme 2.The surface conductance of the sensor was measured by applying fixed DC bias on the electrode. The sensing element was aged at the operating temperature for 2 h in air to ensure a stable electrical contact before starting the sensing measurements. The change in surface conductance of the Zn2SnO4 pellet in air (Ca) and in the target gas (Cg) was measured over time by applying voltage on the strip electrodes. The response (S) was estimated as Ca/Cg. The volume of alcohol (V) [99.99% purity] required achieving a specific vapour concentration in ppm inside the test chamber, as obtained using eqn (2).
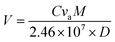 | (2) |
3. Results and discussions
3.1. Phase, vibrational spectroscopy, Raman spectroscopy, and TEM analyses
Fig. 1a presents the XRD pattern of Zn2SnO4. The experimentally observed pattern matched with the standard pattern with PDF no. 00-024-1470, suggesting the pure phase formation of highly crystalline Zn2SnO4. No other precursor peaks were found in the pattern. The main peaks were observed at 17.7°, 29.1°, 34.2°, 36°, 41.6°, 45.6°, 51.6°, 55.1°, 60.4°, 63.5°, 68.5°, 71.4°, 72.3°, 76°, and 78.9°, corresponding to the (111), (220), (311), (222), (400), (331), (422), (511), (440), (531), (620), (533), (622), (444), and (711) planes. The observed diffraction peaks corresponded to the cubic inverse spinel structure of Zn2SnO4.35 The crystallite size was determined using the Debye–Scherrer formula. | (3) |
 | ||
| Fig. 1 (a) Powder XRD pattern, (b) Rietveld pattern, (c) FTIR spectrum, (d) Raman spectrum, (e) TEM image, (f) SAED pattern, and (g) EDX spectrum of Zn2SnO4. | ||
Fig. 1c presents the FTIR spectrum of Zn2SnO4, which was recorded in the range from 480 to 1500 cm−1. The sample was prepared in the form of a pellet using KBr. The spectrum displayed a broad peak at 575 cm−1, corresponding to vibration of the Sn–O–Zn bond in Zn2SnO4,25 and a shoulder peak at 650 cm−1, corresponding to vibration of the Sn–O bond.37
According to Group theory, there are 5 Raman active modes for bulk Zn2SnO4. Theoretically, the peaks are at 143, 227, 377, 532, and 667 cm−1, corresponds to the T2g(1), Eg, T2g(2), T2g(3), and A1g symmetries. As can be seen in Fig. 1d, the Raman peaks here were at 223, 375, 525, and 662 cm−1, which were in agreement with the theoretical values.38 The peak at 662 cm−1 corresponded to the stretching vibration of the short M–O bond in the MO6 octahedron. The peak at 525 cm−1 corresponded to the internal vibration of the oxygen tetrahedron.39,40Fig. 1e and f correspond to the TEM and SAED pattern of the material. TEM suggested that the particles had sizes >300 nm. From the SAED pattern, sharp diffractions spots could be observed, which suggest that the synthesized material possess good crystallinity. The patterns were indexed to the (440) and (400) crystal planes, respectively.35Fig. 1g presents the EDX spectrum of Zn2SnO4 for confirming the elemental composition of the prepared material, which confirmed the material was pure with no impurities.
In order to confirm the surface composition and valence state, XPS was performed. The survey scan of Zn2SnO4 is shown in Fig. 2a. From the survey spectra the presence of Zn, Sn, and O was confirmed. The Zn 2p convoluted spectrum is shown in Fig. 2b, where 2 peaks with binding energy values of 1022 and 1045 eV could be observed, corresponding to Zn 2p3/2 and Zn 2p1/2, respectively. The spin orbit splitting value of 23 eV confirmed that Zn existed in the Zn2+ valence state. Fig. 2c presents the Sn 3d convoluted spectrum, with 2 symmetric peaks at 495 and 486.6 eV, corresponding to Sn 3d3/2 and 3d5/2, respectively, while the peak spacing of 8.4 eV confirmed the Sn4+ valence state.41Fig. 2d presents the O 1s spectrum, which was deconvoluted into 3 peaks with binding energy values of 530, 531, and 532 eV. The first peak corresponded to the lattice oxygen (M–O), the second peak to the oxygen vacancies, and the third peak to the chemisorbed oxygen.42 Thus the XPS analysis confirmed the purity of the prepared material.
 | ||
| Fig. 2 (a) XPS survey scan, core level spectra of: (b) Zn 2p, (c) Sn 3d, (d) O 1s; (e) EPR spectrum; and (f) photoluminescence spectrum of Zn2SnO4. | ||
Fig. 2e presents the EPR spectrum of Zn2SnO4. The spectrum consisted of a resonance signal at g = 1.98, corresponding to the electrons trapped in the oxygen vacancies.43,44 The emission spectrum shown in Fig. 2f resulted from excitation at 320 nm, in which a broad emission could be observed ranging from 500 to 1000 nm, which was mainly contributed by the defect levels between the band gap. The broad emission was composed of green, orange, and red emission, which was mainly due to the presence of oxygen vacancies, and the interaction of oxygen vacancies, oxygen interstitials, Sn interstitials, interstitial Zn, and Sn vacancies respectively. The broad peak could be deconvoluted into 3 peaks with maxima at 562, 639, and 744 nm. The emission spectra are shown in the inset of Fig. 2f. Hu et al.45 reported a broad orange red emission for Zn2SnO4 nanowires, which was due to the interaction of oxygen and tin vacancies and/or interstitial oxygen (orange). The other emission (in the red region) was due to the variation in the Zn/Sn stoichiometry and the disorder in the inverse spinel structure.46 Also Zn2SnO4 micro prisms synthesized by Zhao et al. exhibited an asymmetric broad band emission around 606 nm and 740 nm, which was due to the interactions between the oxygen vacancies and the interfacial tin and/or zinc defects.47
3.2. VOC-sensing studies
The prepared Zn2SnO4 sensing element showed n-type sensing characteristics, i.e. the electrical conductance of the sensor increases in the presence of reducing vapours (e.g. methanol, ethanol, propanol, butanol etc.). The mechanism for chemiresistive sensing by a typical n-type SMO for the detection of reducing vapors, e.g. ethanol, is schematically presented in Fig. 3. As shown in Fig. 3(a), when the semiconducting metal oxide (SMO) sensor is kept at elevated temperature, oxygen is chemically adsorbed on its surface. Such chemi-adsorption of oxygen makes the particles electronically depleted. Particles having enriched oxygen vacancies deplete more than the normal metal oxide particles due to the chemisorption of a higher amount of oxygen. The chemisorption of oxygen on metal oxide followed by the formation of an electron-depleted layer eventually increases the potential barrier (qVs) for interparticle charge transportation, leading to the increase in resistance of the sample.Upon exposure to reducing vapours, the chemi-adsorbed oxygen ions react with the vapours and the released electrons come back to the conduction bands of the SMO particles. As a result, the resistance of n-type sensing materials decreases upon exposure to reducing gases. Fig. 3(b) shows the formation and lowering of the potential barriers when oxygen and reducing gases were exposed respectively on the SMO surface. The respective change in the sensor resistances is shown in Fig. 3(c). The sequential phenomenon of the sensing process over SMO can further be described using the following reactions:
| O2 + SMO → O− + e-depleted SMO (chemisorption) | (4) |
| Rgas + e-depleted SMO → Rad + e-depleted SMO (physisorption) | (5) |
| Rad + O− + e-depleted SMO → ROad + SMO (response process) | (6) |
| ROad + SMO → ROgas + SMO (recovery process) | (7) |
 | (8) |
The formation of oxygen vacancies within a metal oxide crystal lattice can be represented by the following reaction:
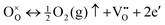 | (9) |
 is the oxygen atom lost from the lattice as oxygen gas [½O2(g)], creating positively charged oxygen vacancies
is the oxygen atom lost from the lattice as oxygen gas [½O2(g)], creating positively charged oxygen vacancies  in the lattice. To maintain the charge neutrality, each
in the lattice. To maintain the charge neutrality, each  traps electrons (e′), which act as donor levels within the band gap.
traps electrons (e′), which act as donor levels within the band gap.
For SMO sensors, the presence of oxygen vacancies modulates both the receptor and transducer functions, which control, respectively, the interaction of the target analyte on the sensing surface and the transportation of the generated electronic signal within the sensor. The oxygen vacancy sites 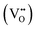 in SMO enhance the chemisorption of oxygen on the sensing surface kept at an elevated temperature. The chemisorbed oxygens help improving the receptor function by oxidizing more of the target gas. The electrons released in the defect state at the conductance band minimum (CBM) act as donor sites and help improving the transduction of the electronic signal within the sensor.
in SMO enhance the chemisorption of oxygen on the sensing surface kept at an elevated temperature. The chemisorbed oxygens help improving the receptor function by oxidizing more of the target gas. The electrons released in the defect state at the conductance band minimum (CBM) act as donor sites and help improving the transduction of the electronic signal within the sensor.
The role of oxygen vacancies in modulating the sensing performance of SMO can further be explained using the width of the electron-depleted layer that is formed due to the chemisorption of oxygen on the SMO surface at elevated temperature. The width (LD) of the electron-depleted layer can be expressed according to the following relations:
 | (10) |
At a fixed temperature, the change of LD will be more prominent for oxygen-vacancy-enriched metal oxide, leading to a superior response of the sensor. The presence of oxygen vacancies in Zn2SnO4 was already confirmed by the EPR and XPS analyses, as shown in Fig. 2. The aforementioned influence of oxygen vacancies is also applicable for alcohol sensing by the n-type Zn2SnO4.
Fig. 4 shows the conductance transients of the Zn2SnO4 sensing element at (a) 225 °C, (b) 250 °C, (c) 275 °C, and (d) 300 °C for the detection of 1000 ppm methanol (MeOH), ethanol (EtOH), n-propanol (PrOH), and n-butanol (BtOH). At each temperature, two conductance transients were recorded for each of the target vapours. At 225 °C, the conductance transients were a bit noisier and the base resistance did not recover fully when the test vapours and oxygen flowed back and forth on the sensing element. However, at higher temperature (250–300 °C), the recorded transients were distinct for each of the test vapours. It was notable that for a fixed test vapour, the conductance transients were identical, which demonstrated that the sensor could identify the target vapour and produce a characteristic chemiresistive sensing signal. Comparing the conductance transients, it seemed that the sensor showed the maximum change in conductance in the presence of butanol vapour.
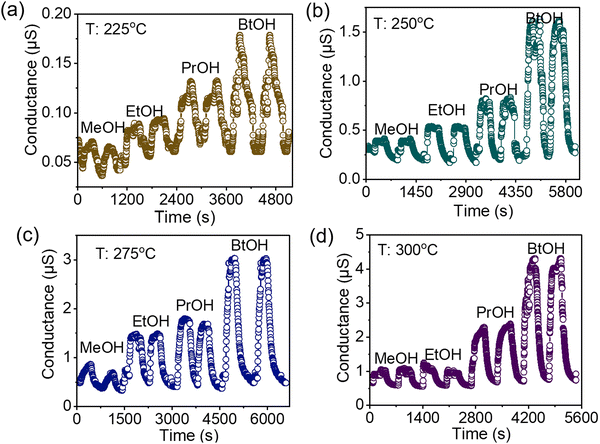 | ||
| Fig. 4 Conductance transients of Zn2SnO4 for sensing 1000 ppm methanol, ethanol, propanol, and butanol at (a) 225 °C, (b) 250 °C, (c) 275 °C, and (d) 300 °C. | ||
The variation of the estimated response with the sensor operating temperature is shown in Fig. 5. As displayed in the figure, the response of the sensor increased with the rise in the operating temperature. This can be explained by the concept of the temperature-dependent chemisorption of oxygen on the metal oxide surface. The chemisorption of oxygen on the metal oxide surface increased with the rise in operating temperature, resulting in an enhanced opportunity to oxidize the target reducing vapours, and thus the response of the sensor also increased with the operating temperature. At lower operating temperature (225 °C and 250 °C), the selectivity towards butanol was not profound compared to the other studied vapours. However, at higher temperature the response of the sensor towards butanol vapour was noticeably higher.
The response of the SMO chemiresistive sensor towards gases and vapours is a complicated process8,49,50 that is governed by two physical functions, namely the receptor and transducer functions, which are influenced by the characteristics of sensing materials (e.g. chemical composition, conductivity, bandgap, surface area, porous nature, morphology), sensor architecture (e.g. thin film, thick film, powder compacted disk), physical and chemical properties of vapours (e.g. mass, diffusivity, boiling point, shape and size of molecules, oxidation probability), and also on the measurement conditions (e.g. temperature, vapour concentration). The entangled effect of all these parameters influences the sensing characteristics, and the critical comparison of the individual sensing responses varying with temperature becomes difficult. However, at a particular temperature, for a fixed sensing element, the sensor transients are mainly the reflection of the adsorption, diffusion, and interaction of the respective vapours with the sensor surface. As envisaged from the table, at a fixed studied operating temperature (225/250/275/300 °C), the sensing response (S) followed the trend: SButanol > SPropanol > SEthanol > SMethanol. In order to understand the sensing responses of the vapours, we took a few physical (molecular weight, diffusivity in air, density, boiling point) and chemical (chemical structure as well as adsorption and oxidation probability) properties into consideration (as summarized in Table 1).
| Analyte | Molar mass (g mole−1) | Mean molecular radius (Å) | Boiling point (°C) | Vapour pressure (kPa) at 25 °C | Diffusion in air coefficient (cm2 s−1) at 25 °C |
|---|---|---|---|---|---|
| Methanol (CH3–OH) | 32.04 | 4.11 | 65 | 11.9 | 0.177 |
| Ethanol (CH3–CH2–OH) | 46.07 | 4.64 | 78 | 5.95 | 0.126 |
| n-Propanol (CH3–CH2–CH2–OH) | 60.1 | 5.10 | 97 | 2 | 0.089 |
| n-Butanol (CH3–CH2–CH2–CH2–OH) | 74.12 | 5.52 | 117 | 0.9 | 0.053 |
The higher molar mass and boiling point of n-butanol led to its low vapour pressure and lower diffusion in air (within the sensing chamber) resulting in the superior adsorption on the sensing surface than the other studied alcohol vapours. The trends for the polarity of the –O–H bond is methanol > ethanol > n-propanol > n-butanol, with the oxidation probability in the same order.
The selectivity towards butanol compared to other vapours was not profound at lower temperature (225/250 °C) due to its higher adsorption but lower oxidation probability. However, the higher oxidation of butanol at increased operating temperature improved its selectivity. In order to understand and correlate the vapour–solid interactions with the chemiresistive sensing signal, the response transients of the sensors for the detection of all the studied vapours were recorded at 275 °C and 300 °C, and are compared in Fig. 6a and b, respectively. The figures clearly show that at 275 °C and 300 °C, the sensor response towards butanol was significantly higher than for the other vapours. The response times for the detection of the vapours were also less than <60 s. The alcohol, in particular butanol, sensing performances of the prepared Zn2SnO4 were further compared with the already reported SMO chemiresistive sensors, and the results are summarized in Table 2. As envisaged from the table, the simple metal oxide and their modified counterparts are known for the sensing of alcohol vapours, including butanol. However, Zn2SnO4-based materials are not very frequently reported for sensing alcohol with a higher response towards butanol. The operative temperature of the present Zn2SnO4 sensor was also comparable to the already reported sensors. Further research studies thus need to explore suitable modifications of primitive Zn2SnO4 for the detection of even lower concentrations of butanol at low operating temperatures.
 | ||
| Fig. 6 Comparison among the sensing responses towards methanol, ethanol, propanol, and butanol vapours at (a) 275 °C and (b) 300 °C. | ||
| S. no. | Material | Targeted analyte/s | Operating temperature | Minimum detection | Ref. |
|---|---|---|---|---|---|
| 1. | Al2O3/ZnO | Propanol, n-butanol, ethanol, acetone, hydrogen, and ammonia | 350 °C | 100 ppm | 51 |
| 2. | In2O3/Pt nanoparticles and Ag nanowires | Ethanol | 125 °C | 200 ppm | 52 |
| 3. | Pd-doped SnO2 nanoparticles | Methanol, ethanol, acetone, or hydrogen | — | 1000 ppm | 53 |
| 4. | CeOx-doped SnO2 nanoparticles | C3H6O, CH4, H2, NO2, H2S, and H2O | 350 °C | 200 ppm | 54 |
| 5. | SnO2 | Ethanol, isopropyl alcohol, acetone, and ammonia | 90 °C | 800 ppm | 55 |
| 6. | Pd–SnO2 | Methanol and ethanol | 200–400 °C | — | 56 |
| 7. | Lanthanum-loaded indium tin oxide (ITO) | Ethanol | 300 °C | 100 ppm | 57 |
| 8. | Soda-lime glass slide was decorated with palladium nanoparticles | Acetone, benzene, ethanol, and toluene | 300–500 °C | 30 ppm | 58 |
| 9. | SnS–ZnS composite | Ethanol | 27 °C | 100 ppb | 59 |
| 10. | ZnO nano-flowers | Methanol and ethanol | 250 °C | 700 ppm | 60 |
| 11. | CuO-decorated Fe2O3 nanoflakes | Ethanol | 250 °C | 100 ppm | 61 |
| 12. | Sm2O3/ZnO/SmFeO3 microspheres | Methanol | 195![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C °C |
5 ppm | 62 |
| 13. | MoS2/TiO2 composite | Methanol ethanol | 300 °C | 5 ppm | 63 |
| 14. | Pd-doped CeO2 nanofibers | Hydrogen, carbon monoxide, ammonia, ethanol, methanol, and benzene. | 200 °C | 100 ppm | 64 |
| 15. | Coating Au nanoclusters onto ZnO and Co3O4 sensors | Ethanol, benzene, toluene, and p-xylene | 450 °C | 100 ppm | 65 |
| 16. | Pt-modified WO3/p-Si film with Al | Methanol | 25 °C | — | 66 |
| 17. | Zn doped NiO samples | Methanol | 260 °C | 100 ppm | 67 |
| 18. | CoxLaFe1−xO3, x = 0.00, 0.01, 0.05, and 0.10 | n-Butanol | — | 10 ppm | 68 |
| 19. | ZnO–In2O3 nanocomposites | n-Butanol | 220![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C °C |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ppm ppm |
69 |
| 20. | Palladium-doped SnO2 | n-Butanol | 100 ppm | 70 | |
| 21. | ZnO@TiO2 | n-Butanol | 200 °C | 133 | 71 |
| 22. | CdO/ZnO nanocomposites | n-Butanol | 300 °C | 72 | |
| 23. | ZnSnO3 hollow spheres | n-Butanol | 200 °C | 300 ppm | 73 |
| 24. | ZnFe2O4 macroporous spheres | n-Butanol | 250 °C | 100 ppm | 74 |
| 25. | Zn2SnO4 | n-Butanol | 225–300 °C | 100 ppm | Present work |
4. Conclusions
The present work evaluated the Zn2SnO4 sensor performance with adjusting the operating temperature in the range of 225–300 °C for different aliphatic alcohols with 1000 ppm concentration. The synthesized material was well characterized by XRD, FTIR, Raman spectroscopy, EDX, TEM, and XPS. The formation of phase-pure Zn2SnO4 with good crystallinity was confirmed through the XRD in conjunction with FTIR and Raman spectra analyses. The defect characterizations as well as photophysical properties analysis were carried out using EPR and photoluminescence measurements, respectively. The XPS, EPR, and PL results in combination suggested the presence of oxygen vacancies in the synthesized materials. The role of oxygen vacancies in modulation of the sensing performances of semiconducting metal oxide sensors was investigated and discussed. The sensing performance for different reducing vapours (methanol, ethanol, propanol, and butanol) was studied in the temperature range of 225–300 °C. The response of the sensor was maximum at 300 °C. Among the four aliphatic alcohols, n-butanol had a superior sensing response due to its higher molar mass and boiling point, which led to its low vapour pressure and lower diffusion in air. The estimated response of the sensor was 4.5 for the detection of 1000 ppm butanol at 300 °C. The response time of the sensor for the detection of 1000 ppm butanol at 300 °C was <60 s. The response and recovery for the detection of reducing vapours over the Zn2SnO4 surface could thus be considered as due to the catalytic oxidation of reducing vapours at elevated temperature where Zn2SnO4 acted as a catalyst. This study reveals the potential of Zn2SnO4 as an effective sensor for detecting n-butanol. This study reveals that oxygen-vacancy-enriched Zn2SnO4 could be an effective sensor for selectively detecting n-butanol. However, the particle size of Zn2SnO4 was found to be a little bigger (400–800 nm), which should be reduced further to improve the response of the sensor. The primitive Zn2SnO4 may also be modified with suitable chemical/electronic sensitizers to make it operative at lower temperature.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors would like to acknowledge Dr Pradip Pachfule, S. N. Bose National Centre for Basic Sciences, Kolkata for help in TEM measurements, Ashmita Biswas, INST Mohali for XPS measurements. Dr Kathi Sudarshan from RCD has been acknowledged for his kind help in Rietveld refinement of the powder XRD pattern.References
- P. M. Bulemo and J. Y. Cheong, ACS Appl. Nano Mater., 2023, 6, 1027–1049 CrossRef CAS
.
- P. Das, B. Mondal and K. Mukherjee, J. Phys. Chem. C, 2017, 121, 1146–1152 CrossRef CAS
.
- M. Khatib and H. Haick, ACS Nano, 2022, 16, 7080–7115 CrossRef CAS PubMed
.
- S. Tyagi, M. Chaudhary, A. K. Ambedkar, K. Sharma, Y. K. Gautam and B. P. Singh, Sens. Diagn., 2022, 1, 106–129 RSC
.
- B. Yang, N. V. Myung and T.-T. Tran, Adv. Electron. Mater., 2021, 7, 2100271 CrossRef CAS
.
- S. K. Gupta, S. Mohan, M. Valdez, K. Lozano and Y. Mao, Mater. Res. Bull., 2021, 142, 111419 CrossRef CAS
.
- Y. Yoon, P. L. Truong, D. Lee and S. H. Ko, ACS Nanosci. Au, 2022, 2, 64–92 CrossRef CAS PubMed
.
- S. Das, S. Mojumder, D. Saha and M. Pal, Sens. Actuators, B, 2022, 352, 131066 CrossRef CAS
.
- N. Goel, K. Kunal, A. Kushwaha and M. Kumar, Eng. Rep., 2023, 5, e12604 CrossRef CAS
.
- R. Si, Y. Xu, C. Shen, H. Jiang, M. Lei, X. Guo, S. Xie, S. Gao and S. Zhang, ACS Sens., 2024 DOI:10.1021/acssensors.3c01831
.
- M. Al-Hashem, S. Akbar and P. Morris, Sens. Actuators, B, 2019, 301, 126845 CrossRef CAS
.
- W. Yu, Z. Shen, F. Peng, Y. Lu, M. Ge, X. Fu, Y. Sun, X. Chen and N. Dai, RSC Adv., 2019, 9, 7723–7728 RSC
.
- Y. Zhang, Y. Xing, Z. Yang, L. Zhao, C. Xin, Z. Wei, T. Fei, S. Liu and T. Zhang, Mater. Today Commun., 2024, 38, 108090 CrossRef CAS
.
- S. Zhou, W. Yan, M. Ling and C. Liang, Inorg. Chem. Front., 2023, 10, 3255–3262 RSC
.
- M. Karmakar, B. Mondal, M. Pal and K. Mukherjee, Sens. Actuators, B, 2014, 190, 627–633 CrossRef CAS
.
- K. Mukherjee and S. B. Majumder, Int. J. Hydrogen Energy, 2014, 39, 1185–1191 CrossRef CAS
.
- A. Šutka and K. A. Gross, Sens. Actuators, B, 2016, 222, 95–105 CrossRef
.
- R. T. Parayil, S. K. Gupta, R. Rohilla, J. Prakash, K. Sudarshan and M. Mohapatra, ACS Appl. Electron. Mater., 2023, 5, 5151–5163 CrossRef CAS
.
- R. T. Parayil, G. D. Patra, B. Modak, K. Sudarshan, M. Sonawane, S. Sen and S. K. Gupta, ACS Appl. Opt. Mater., 2023, 1, 179–192 CrossRef
.
- L. Mao, S. Mohan, S. K. Gupta and Y. Mao, Mater. Chem. Phys., 2022, 278, 125643 CrossRef CAS
.
- N. Joshi, H. Long, P. Naik, A. Kumar, V. R. Mastelaro, O. N. Oliveira, A. Zettl and L. Lin, New J. Chem., 2022, 46, 17967–17976 RSC
.
- S. Ma, L. Shen, S. Ma, J. Wen and J. Xu, Coord. Chem. Rev., 2023, 490, 215217 CrossRef CAS
.
- L. Wang, T. Zhou, R. Zhang, Z. Lou, J. Deng and T. Zhang, Sens. Actuators, B, 2016, 227, 448–455 CrossRef CAS
.
- A. A. Bhat, I. Assadullah, A. Farooq, K. A. Malik, J. H. Malik, R. Tomar, I. Islam, A. M. Ali and S. A. Khandy, Mater. Chem. Phys., 2023, 306, 127993 CrossRef CAS
.
- P. Pratim Das, A. Roy, S. Das and P. S. Devi, Phys. Chem. Chem. Phys., 2016, 18, 1429–1438 RSC
.
- M. Zhang, X. Cui, Y. Wang, B. Wang, M. Ye, W. Wang, C. Ma and Z. Lin, Nano Energy, 2020, 71, 104620 CrossRef CAS
.
- Y. Zhang, X. Xin, H. Sun, Q. Liu, J. Zhang, G. Li, J. Gao, H. Lu and C. Wang, J. Alloys Compd., 2021, 854, 157311 CrossRef CAS
.
- S. K. Gupta, K. Sudarshan, D. Chandrashekhar, A. Balhara and M. Mohapatra, J. Lumin., 2023, 257, 119697 CrossRef CAS
.
- S. K. Gupta, K. Sudarshan and R. M. Kadam, Mater. Lett., 2020, 279, 128511 CrossRef CAS
.
- M. Fakhrzad, A. H. Navidpour, M. Tahari and S. Abbasi, Mater. Res. Express, 2019, 6, 095037 CrossRef CAS
.
-
T. Ivetić, in Recent Advances in Porous Ceramics, IntechOpen London, UK, 2018, pp. 80–81 Search PubMed
.
- W. Guo, Y. Shuai, X. Liu, J. Zhang, J. Wang, K. H. Mahmoud, Z. M. El-Bahy and N. M. Mubarak, Sens. Actuators, B, 2022, 354, 131221 CrossRef CAS
.
- R. Malik, V. K. Tomer, V. Chaudhary, M. S. Dahiya, S. P. Nehra, P. S. Rana and S. Duhan, Sens. Actuators, B, 2017, 239, 364–373 CrossRef CAS
.
- M. Valdez, S. K. Gupta, K. Lozano and Y. Mao, Sens. Actuators, B, 2019, 297, 126734 CrossRef CAS
.
- Y. Zhao, L. Hu, H. Liu, M. Liao, X. Fang and L. Wu, Sci. Rep., 2014, 4, 6847 CrossRef CAS PubMed
.
- S.-H. Wei and S. B. Zhang, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 045112 CrossRef
.
- A. Lanje, S. Sharma, R. Pode and F. N. A. S. R. Ningthoujam, Arch. Appl. Sci. Res., 2010, 2, 127–135 CAS
.
- M. Dimitrievska, T. B. Ivetić, A. P. Litvinchuk, A. Fairbrother, B. B. Miljević, G. R. Štrbac, A. Pérez Rodríguez and S. R. Lukić-Petrović, J. Phys. Chem. C, 2016, 120, 18887–18894 CrossRef CAS
.
- J. Zeng, M. Xin, K. W. Li, H. Wang, H. Yan and W. Zhang, J. Phys. Chem. C, 2008, 112, 4159–4167 CrossRef CAS
.
- Q. Zhao, D. Ju, X. Song, X. Deng, M. Ding, X. Xu and H. Zeng, Sens. Actuators, B, 2016, 229, 627–634 CrossRef CAS
.
- S. Yuvaraj, W. J. Lee, C. W. Lee and R. K. Selvan, RSC Adv., 2015, 5, 67210–67219 RSC
.
- S. K. Gupta, B. Modak, D. Das, A. K. Yadav, P. Modak, A. K. Debnath and K. Sudarshan, ACS Appl. Electron. Mater., 2021, 3, 3256–3270 CrossRef CAS
.
- Y. Yuan, K. Sheng, G. Zhuang, Q. Li, C. Dou, Q.-J. Fang, W.-W. Zhan, H. Gao, D. Sun and X. Han, Chem. Commun., 2021, 57, 8636–8639 RSC
.
- S. K. Gupta, B. Modak, M. Tyagi, N. S. Rawat, P. Modak and K. Sudarshan, ACS Omega, 2022, 7, 5311–5323 CrossRef CAS PubMed
.
- Q. R. Hu, P. Jiang, H. Xu, Y. Zhang, S. L. Wang, X. Jia and W. H. Tang, J. Alloys Compd., 2009, 484, 25–27 CrossRef CAS
.
- J. E. Montenegro, Y. Ochoa-Muñoz and J. E. Rodríguez-Páez, Ceram. Int., 2020, 46, 2016–2032 CrossRef CAS
.
- J.-W. Zhao, L.-R. Qin and L.-D. Zhang, Solid State Commun., 2007, 141, 663–666 CrossRef CAS
.
- A. Kolmakov and M. Moskovits, Annu. Rev. Mater. Res., 2004, 34, 151–180 CrossRef CAS
.
- B. Bhagat, S. K. Gupta, D. Mandal, A. A. Gor, R. Bandyopadhyay and K. Mukherjee, Chem. – Asian J., 2024, 19, e202300841 CrossRef CAS PubMed
.
- J. Rebholz, P. Bonanati, C. Jaeschke, M. Hübner, L. Mädler, U. Weimar and N. Barsan, Sens. Actuators, B, 2013, 188, 631–636 CrossRef CAS
.
- O. Lupan, D. Santos-Carballal, N. Magariu, A. K. Mishra, N. Ababii, H. Krüger, N. Wolff, A. Vahl, M. T. Bodduluri, N. Kohlmann, L. Kienle, R. Adelung, N. H. de Leeuw and S. Hansen, ACS Appl. Mater. Interfaces, 2022, 14, 29331–29344 CrossRef CAS PubMed
.
- S.-Y. Kim, J. Kim, W. H. Cheong, I. J. Lee, H. Lee, H.-G. Im, H. Kong, B.-S. Bae and J.-U. Park, Sens. Actuators, B, 2018, 259, 825–832 CrossRef CAS
.
- J. van den Broek, S. Abegg, S. E. Pratsinis and A. T. Güntner, Nat. Commun., 2019, 10, 4220 CrossRef CAS PubMed
.
- N. Kotchasak, A. Wisitsoraat, A. Tuantranont, S. Phanichphant, V. Yordsri and C. Liewhiran, Sens. Actuators, B, 2018, 255, 8–21 CrossRef CAS
.
- R. Biswas and D. Saha, Appl. Phys. A: Mater. Sci. Process., 2020, 126, 313 CrossRef CAS
.
- H. Liu, R. Wu, Q. Guo, Z. Hua and Y. Wu, ACS Omega, 2021, 6, 30598–30606 CrossRef CAS PubMed
.
- A. Nilabh, S. Sen, M. Narjinary and S. Kundu, Microchem. J., 2020, 158, 105146 CrossRef CAS
.
- J.-Y. Kim, S. S. Kim and M. Tonezzer, Sens. Actuators, B, 2021, 335, 129714 CrossRef CAS
.
- S. K. Lalwani, A. Debnath and Sunny, Nanotechnology, 2022, 33, 505502 CrossRef
.
- N. Banerjee and S. Roy, Emerging Trends in Electronic Devices and Computational Techniques, 2018, pp. 1–4 Search PubMed.
- H. R. Ansari, Z. Kordrostami and A. Mirzaei, IEEE Trans. Instrum.
Meas., 2023, 72, 1–10 Search PubMed
.
- K. Li, Y. Wu, M. Chen, Q. Rong, Z. Zhu, Q. Liu and J. Zhang, Nanoscale Res. Lett., 2019, 14, 57 CrossRef PubMed
.
- S. Singh and S. Sharma, Sens. Actuators, B, 2022, 350, 130798 CrossRef CAS
.
- Q. Hu, B. Huang, Y. Li, S. Zhang, Y. Zhang, X. Hua, G. Liu, B. Li, J. Zhou, E. Xie and Z. Zhang, Sens. Actuators, B, 2020, 307, 127638 CrossRef
.
- Y. K. Moon, S.-Y. Jeong, Y. C. Kang and J.-H. Lee, ACS Appl. Mater. Interfaces, 2019, 11, 32169–32177 CrossRef CAS PubMed
.
- A. Dey, K. Kumari, A. Jana, B. Goswami, P. Nandi and S. K. Sarkar, Singapore, 2021.
- Shailja, K. Singh, R. C. Singh and S. Sharma, J. Phys.: Conf. Ser., 2021, 1849, 012034 CrossRef CAS
.
- J. Gu, B. Zhang, Y. Li, X. Xu, G. Sun, J. Cao and Y. Wang, Sens. Actuators, B, 2021, 343, 130125 CrossRef CAS
.
- D. An, Q. Wang, X. Tong, X. Lian, Y. Zou and Y. Li, Ceram. Int., 2019, 45, 6869–6874 CrossRef CAS
.
- Z. Yao, X. Wang, M. Yang, H. Ma, Y. Sun, H. Zhang, T. Dong and W. Liu, Semicond. Sci. Technol., 2020, 35, 095030 CrossRef CAS
.
- Y. Xu, L. Zheng, C. Yang, W. Zheng, X. Liu and J. Zhang, Sens. Actuators, B, 2020, 310, 127846 CrossRef CAS
.
- M. Poloju, N. Jayababu, E. V. Rao, R. G. Rao and M. V. R. Reddy, Surf. Interfaces, 2020, 20, 100586 CrossRef CAS
.
- G. Feng, Y. Che, C. Song, J. Xiao, X. Fan, S. Sun, G. Huang and Y. Ma, Ceram. Int., 2021, 47, 2471–2482 CrossRef CAS
.
- L. Lv, P. Cheng, Y. Wang, L. Xu, B. Zhang, C. Lv, J. Ma and Y. Zhang, Sens. Actuators, B, 2020, 320, 128384 CrossRef CAS
.
| This journal is © the Owner Societies 2024 |